Abstract
Introduction
Primary Sjögren’s syndrome (pSS) is a systemic autoimmune disorder that primarily affects the exocrine glands such as the lacrimal and the salivary glands. Dry eye disease (DED) is one of the most prevalent manifestations of pSS and is usually classified into aqueous-deficient dry eye and evaporative dry eye. Sjögren’s syndrome dry eye (SSDE) is generally described as aqueous-deficient dry eye. However, as the leading pathophysiological mechanism of evaporative dry eye, meibomian gland dysfunction (MGD) also has influence on SSDE, which has been shown in recent studies. We speculate that SSDE is more than just an aqueous-deficient dry eye. While no related systematic review and meta-analysis has been published, the present study is designed to derive a better understanding of the association between MGD and SSDE.
Methods and analysis
The Preferred Reporting items for Systematic Reviews and Meta-Analysis for Protocols 2015 statement was used to prepare this protocol. PubMed, Embase, Web of Science, Cochrane Database, China National Knowledge Infrastructure and Wan Fang Database will be searched from their inception to 31 October 2021, with restrictions to publications in English or Chinese. Two reviewers will independently carry out data extraction and quality assessment. The diagnosis of pSS will meet the standard diagnostic criteria, such as American College of Rheumatology/European League against Rheumatism Classification Criteria (ACR/EULAR) or American-European Consensus Group Classification criteria (AECG), and the definition of MGD and DED will differ between studies. The quality of included studies will be judged using the Newcastle-Ottawa Quality Scale. We will carry out this meta-analysis using RevMan V.5.4.1. The incidence of MGD in patients with SSDE will be indicated as OR with 95% CI.
Ethics and dissemination
Ethical approval is not required as this meta-analysis is performed based on published studies and does not involve human participants. The results will be published in a peer-reviewed journal.
PROSPERO registration number
CRD42021226017.
Keywords: rheumatology, ophthalmology, protocols & guidelines, immunology
Strengths and limitations of this study.
This is the first systematic review and meta-analysis to evaluate the relationship between meibomian gland dysfunction and primary Sjögren’s syndrome dry eye.
Different types of design, diagnostic criteria for primary Sjögren’s syndrome and various evaluation tools for meibomian gland dysfunction and dry eye disease are limitations to this study and may reduce the quality of evidence.
Subgroup analysis may reduce these restrictions.
Introduction
Primary Sjögren’s syndrome (pSS) is a female-dominated, systemic autoimmune disorder characterised by wide clinical manifestations, extending from exocrine gland symptoms to extraglandular involvement. As a systemic disease, multidisciplinary cooperation is required, especially from the departments of rheumatology, immunology and ophthalmology. Dry eye disease (DED) is usually classified into aqueous-deficient dry eye and evaporative dry eye.1 The mechanism of Sjögren’s syndrome dry eye (SSDE) currently remains unclear. In majority of the literature, SSDE is classified as aqueous-deficient dry eye, with much attention being paid to the lack of aqueous tear production.2–5 However, meibomian gland dysfunction (MGD) has been diagnosed in patients with pSS.6
The International Workshop on Meibomian Gland Dysfunction defines MGD as a chronic, diffuse abnormality of the meibomian glands, commonly characterised by terminal duct obstruction or qualitative or quantitative changes in glandular secretion.7 As the leading pathophysiological mechanism of evaporative dry eye,8 MGD causes a lesion to the tear film lipid layer, affecting the rate of tear evaporation and tear hyperosmolarity, eventually triggering onset of dry eye.9
Currently, evidence has shown that MGD has influence on SSDE.10–13 On the basis of these premises, we speculate that SSDE is more than just an aqueous-deficient dry eye. In this protocol, we aim to perform a comprehensive review of the association between MGD and SSDE.
Methods
Registration
The Preferred Reporting Items for Systematic Review and Meta-Analysis for Protocols (PRISMA-P) 2015 statement was used to perform this protocol.14
Patient and public involvement
No patients were involved in this study.
Search strategy
PubMed, Embase, Web of Science, Cochrane Database, China National Knowledge Infrastructure and Wan Fang Database will be searched from their inception to 31 October 2021 by two reviewers (TH and YR) independently. Combination of medical subject headings and free terms will be used to search for potentially qualified publications (box 1). The reference lists of original and review articles will be screened manually.
Box 1. Search strategy for PubMed.
Search items
#1 “Sjogren’s Syndrome” OR “Sjogrens Syndrome” OR “Syndrome, Sjogren’s” OR “Sjogren Syndrome” OR “Sicca Syndrome” OR “Syndrome, Sicca”.
#2 “Meibomian Gland Dysfunction” OR “Dysfunction, Meibomian Gland” OR “Meibomian Gland Dysfunctions” OR “MG Dysfunction” OR “MG Dysfunctions”.
#3 #1 AND #2.
#4 “Dry Eye Syndromes” OR “Dry Eye Syndrome” OR “Dry Eye Disease” OR “Dry Eye Diseases” OR “Dry Eye” OR “Dry Eyes” OR “Evaporative Dry Eye Disease” OR “Evaporative Dry Eye Syndrome” OR “Evaporative Dry Eye” OR “Dry Eye, Evaporative” OR “Evaporative Dry Eyes.
#5 #3 AND #4.
Eligibility criteria
Study design
Prospective and retrospective cohort studies and case–control studies published in English or Chinese will be included in this meta-analysis. In case of similar research delivered by the same authors and published in different journals, the latest study or the study with the largest number of participants will be included.
Participants
Participants will be patients with clinical diagnosis of pSS associated with dry eye and MGD, regardless of age, gender and race. The diagnosis of pSS will meet the AECG criteria in 200215 or the ACR criteria in 201216 or the ACR/EULAR criteria in 2016.17 The diagnostic tools for DED and MGD will differ between studies and will be grouped into three categories: symptom questionnaires, objective ocular testing and biomarkers or other emerging diagnostic techniques.18 19
Outcomes
The primary outcome is incidence of MGD in patients with SSDE. The secondary outcomes mainly include the following aspects: tear film break-up time, Schirmer I test, corneal fluorescein staining and ocular surface disease index.
Study selection and data extraction
Study selection
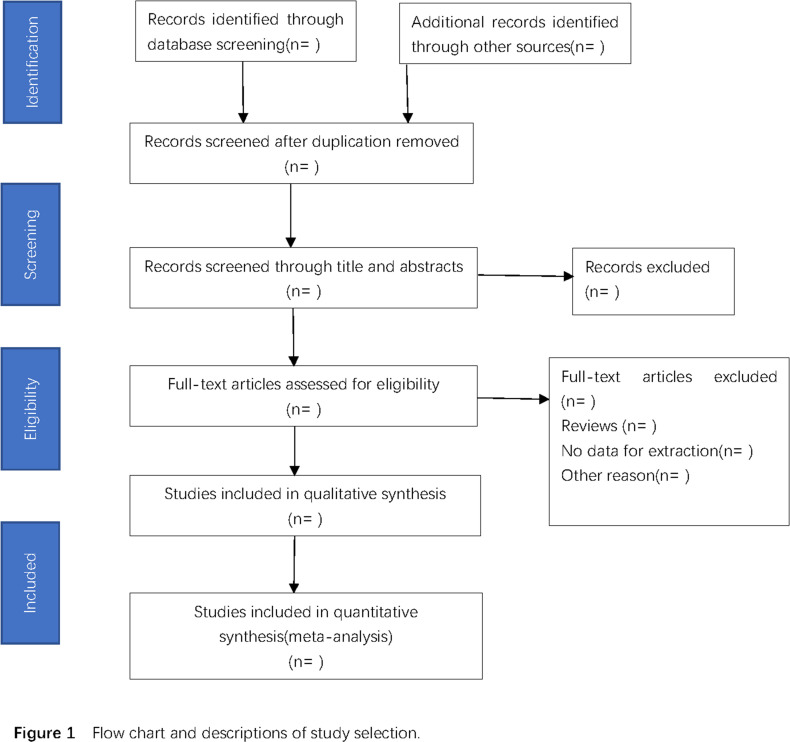
Two reviewers (TH and YR) will independently conduct the study selection. Title and abstract screening of the literature will eliminate studies that do not meet the inclusion criteria. During full-text screening, all potentially eligible studies will be retrieved for inclusion and any disagreement will be resolved by discussion. A third reviewer (YG) will be consulted in order to reach a consensus in case an agreement still cannot be reached by discussion. The study selection process is demonstrated as a PRISMA flow diagram in figure 1. Studies that meet the standards will be imported into EndNote V.X9.
Figure 1.
Flow chart and descriptions of study selection.
Data extraction
Detailed data will be extracted in an Excel spreadsheet by two researchers (HY and QH) and will be checked for accuracy by YG. These will include the following: first author’s name, year of publication, country of publication, study design, sample size, age, gender, duration of pSS, duration of follow-up, diagnostic criteria, evaluation tools for MGD and DED, outcomes and quality assessment (Newcastle-Ottawa Scale). The authors of primary studies will be contacted to obtain missing data.
Quality assessment
The quality of the included studies will be estimated by two reviewers (HY and QH) using the Newcastle-Ottawa Quality Scale adapted for observational studies. The scale awards 0–9 stars based on selection, comparability and outcome (cohort) or exposure (case–control). Studies with 0–3 stars, 4–6 stars or 7–9 stars are considered to be of low, moderate or high quality. Disagreements between two reviewers will be settled by discussion or consultation with YG.
Data synthesis and statistical analysis
At least five studies of the same outcomes in similar populations will be pooled to perform the meta-analysis using RevMan V.5.4.1. Otherwise, a systematic review will be implemented. For categorical variables, OR with 95% CI will be used. For continuous variables, we will choose mean difference or standardised mean difference with 95% CI, depending on whether the measurement scale is consistent or not.
Assessment of heterogeneity
χ2 statistics and the I2 test will be used to estimate the statistical heterogeneity among the included studies. P<0.1 is considered to be representative of statistical heterogeneity.20 I2 values of <25%, 25%–50% and >50% represent low, medium and high heterogeneity.21 For a significant heterogeneity (I2>50%, p<0.1), a random-effect model will be selected to synthesise the data. Otherwise, a fixed-effect model will be used.
Subgroup analysis
Subgroup analysis will be conducted among publications with high heterogeneity including the following aspects: diagnostic criteria for pSS, study design and different evaluation tools for MGD and DED.
Sensitivity analysis
Sensitivity analysis will be conducted, eliminating studies one by one. Possible major source of heterogeneity may be found.
Assessment of publication bias
Visual assessment of funnel plot will be applied to appraise publication bias if more than 10 articles are included.22
Quality of evidence
The Grading of Recommendations Assessment, Development and Evaluation will be used to assess the strength of evidence for each outcome and will be divided into high, moderate, low and very low level of evidence.23
Discussion
Symptoms of MGD have a significant impact on quality of life, causing not only ocular irritation, but also sequelae of ocular surface inflammation and resultant deficits in visual function.24 SSDE is a complicated disease that needs multidisciplinary participation. More attention should be paid to MGD. To our knowledge, this is the first systematic review and meta-analysis to evaluate the relationship between MGD and pSS dry eye. Different types of design, diagnostic criteria for pSS and various evaluation tools for MGD and DED will give a limit to this study, which may restrict the quality of the evidence. Subgroup analysis may reduce these restrictions.
Supplementary Material
Footnotes
Contributors: CZ designed the study protocol and registered the protocol in the PROSPERO database. CZ and QH drafted the manuscript. TH and YR will search and select studies independently, and HY with QH will extract the data and assess the quality of studies included. YG will be the third reviewer for study selection, data extraction and quality assessment. CZ and QH revised the final study. All authors reviewed and approved the final manuscript for submission.
Funding: This work was supported by the Science and Technology Development Fund of the Hospital of Chengdu University of Traditional Chinese Medicine (19LW19, 18MZ12).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:276–83. 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2. Akpek EK, Bunya VY, Saldanha IJ. Sjögren's syndrome: more than just dry eye. Cornea 2019;38:658–61. 10.1097/ICO.0000000000001865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vijmasi T, Chen FYT, Chen YT, et al. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis 2013;19:1957–65. [PMC free article] [PubMed] [Google Scholar]
- 4. Liew MSH, Zhang M, Kim E, et al. Prevalence and predictors of Sjögren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol 2012;96:1498–503. 10.1136/bjophthalmol-2012-301767 [DOI] [PubMed] [Google Scholar]
- 5. Jung HH, Ji YS, Sung MS, et al. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjögren's syndrome. Chonnam Med J 2015;51:26–32. 10.4068/cmj.2015.51.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menzies KL, Srinivasan S, Prokopich CL, et al. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjögren's syndrome patients and nondry eye controls. Invest Ophthalmol Vis Sci 2015;56:836–41. 10.1167/iovs.14-13864 [DOI] [PubMed] [Google Scholar]
- 7. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930–7. 10.1167/iovs.10-6997b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vehof J, Utheim TP, Bootsma H. Advances, limitations and future perspectives in the diagnosis and management of dry eye in Sjögren’s syndrome. Clinical and Experimental Rheumatology 2020;38:S301–9. [PubMed] [Google Scholar]
- 9. Chan TCY, Chow SSW, Wan KHN, et al. Update on the association between dry eye disease and meibomian gland dysfunction. Hong Kong Med J 2019;25:38–47. 10.12809/hkmj187331 [DOI] [PubMed] [Google Scholar]
- 10. Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology 1998;105:1485–8. 10.1016/S0161-6420(98)98033-2 [DOI] [PubMed] [Google Scholar]
- 11. Zang S, Cui Y, Cui Y, et al. Meibomian gland dropout in Sjögren's syndrome and non-Sjögren's dry eye patients. Eye 2018;32:1681–7. 10.1038/s41433-018-0149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Qin Q, Liu B, et al. Clinical analysis: Aqueous-deficient and meibomian gland dysfunction in patients with primary Sjogren's syndrome. Front Med 2019;6:291. 10.3389/fmed.2019.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goto E, Matsumoto Y, Kamoi M, et al. Tear evaporation rates in Sjögren syndrome and non-Sjögren dry eye patients. Am J Ophthalmol 2007;144:81–5. 10.1016/j.ajo.2007.03.055 [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiboski SC, Shiboski CH, Criswell LA, et al. American College of rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s international collaborative clinical alliance cohort. Arthritis Care Res 2012;64:475–87. 10.1002/acr.21591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiboski CH, Shiboski SC, Seror R. American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol 2016;2017:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren's syndrome in patients with dry eye. Clin Ophthalmol 2016;10:43–53. 10.2147/OPTH.S80043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoo Y-S, Na K-S, Kim DY, et al. Morphological evaluation for diagnosis of dry eye related to meibomian gland dysfunction. Exp Eye Res 2017;163:72–7. 10.1016/j.exer.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 20. Pandis N. The chi-square test. Am J Orthod Dentofacial Orthop 2016;150:898–9. 10.1016/j.ajodo.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 21. Melsen WG, Bootsma MCJ, Rovers MM, et al. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 2014;20:123–9. 10.1111/1469-0691.12494 [DOI] [PubMed] [Google Scholar]
- 22. Debray TPA, Moons KGM, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: a comparison of new and existing tests. Res Synth Methods 2018;9:41–50. 10.1002/jrsm.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt G, Oxman AD, Akl EA, et al. Grade guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 24. Sabeti S, Kheirkhah A, Yin J, et al. Management of meibomian gland dysfunction: a review. Surv Ophthalmol 2020;65:205–17. 10.1016/j.survophthal.2019.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.