Abstract
Background:
Alzheimer’s (AD) and Parkinson’s diseases (PD) show deposits of improperly folded modified proteins. Protein expression mechanisms are involved since the early stages. Several studies evaluated epigenomics and proteomics profiles in these patients, with promising results. In general, they focused on early, specific, and minimally invasive biomarkers for the diagnosis and prognosis of AD and PD.
Objectives
This review aimed at summarizing results to find the most reliable evidence in the field.
Results
Among epigenomics studies, there is a focus on microRNAs (miRNAs) as candidate diagnostic biomarkers for AD or PD from blood samples like miR-342-3p, miR-107, miR-106a-5p, miR-106b-5p, miR-195, and miR-19b. In addition, DNA methylation has been tested in a few works, obtaining significant differences in some genes (NCAPH2/LMF2 COASY, SPINT1, BDNFTREM1, TREM2, NPAS2, PDE4D), which could be useful for evaluating the disease progression as well as potential risk factors. Regarding proteomics, most of the studies were untargeted and used plasma or serum samples. In general, they highlighted the importance of coagulation, inflammation pathways, and oxidative stress. Among targeted studies, some proteins (phosphorylated tau, C reactive protein (CRP), interleukins, necrosis factors, transferrin, glial fibrillary acidic protein (GFAP), and neurofilaments) showed different plasma levels in AD and PD patients in comparison with healthy participants. Finally, a few studies have identified specific-AD and PD epigenetic and proteomic biomarkers (ApoE and oxidized DJ-1) in comparison with other similar pathologies.
Conclusion
In general, there is a common lack of clinical validation of these potential biomarkers because of which its use in clinical practice is still limited.
Keywords: Alzheimer’s disease, Parkinson’s disease, epigenomics, proteomics, biomarkers, diagnosis, early
1. INTRODUCTION
Alzheimer’s (AD) and Parkinson’s diseases (PD) are the most prevalent neurodegenerative diseases in the world. They are age-associated and show a long preclinical phase preceding the clinical manifestations. Also, AD usually presents mild cognitive impairment (MCI) in the early stages [1]. The overlap of cognitive impairment across AD and PD and their heterogeneity suggests complex physiopathology that may be interconnected [2], complicating their accurate and early diagnosis. In general, both diseases show deposits of improperly folded modified proteins in specific brain areas [3] (β-amyloid (Aβ), total tau (t-tau), and phosphorylated (p-tau) in AD, and α-synuclein in PD) [4, 5], as well as neuroinflammation [6]. Dementia with Lewy Bodies (DLB) shares clinical features with AD and PD [7], leading to remarkable difficulties in obtaining an accurate diagnosis. The current gold standard diagnosis is post-mortem brain examination [8, 9].
Nowadays, diagnosis of AD is based on cognitive assessments and neuroimaging and cerebrospinal fluid (CSF) biomarkers [10]. Sensitivity and specificity are variable for these biomarkers, with magnetic resonance imaging (MRI) being the less sensitive and specific one [11]. High cost and time-requirement techniques and unusual and unpleasant investigations are the characteristics of the current, accurate diagnosis of early AD. Nevertheless, some blood biomarkers correlate with CSF biomarkers and positron emission tomography (PET) imaging data [12]. Regarding PD diagnosis, it continues to be based on clinical criteria, with some motor and non-motor symptoms [13, 14], which can precede the motor dysfunction by several years [15]. The identification of early, specific and minimally-invasive biomarkers for diagnosis and prognosis of AD and PD is of great relevance in improving their treatment [16]. In general, AD and PD result from pathological imbalance affecting different types of neuronal cells at multiple varied levels. This imbalance may be detected by associated changes in the epigenome and proteome. Epigenetic mechanisms include DNA methylation, which controls gene expression and repression [17], and non-coding RNAs such as microRNAs (miRNAs) that regulate gene expression at the post-transcriptional level [18]. Epigenetic regulation is critical for the normal development and functioning of the human brain [19]. Indeed, epigenetic abnormalities have been detected in AD or PD [20] (e.g., CpG methylation of α-synuclein in blood, proposed as PD biomarker [21], and epigenetically deregulation of Aβ peptide [22] and tau-protein pathways [23, 24]). Also, abnormal expression of miRNAs in the blood can be associated with deregulated specific protein synthesis, leading to their deposition in the brain, which makes miRNAs optimal candidates biomarkers for AD and PD diagnosis [25, 26]. However, few studies have evaluated the diagnostic capacity of miRNAs expression in peripheral fluids [26, 27]. Regarding proteomic, the molecular exchange between signalling proteins in the brain and biological fluids has allowed the identification of preliminary proteomic signatures specific for AD [28] and PD [29] in plasma. Therefore, proteomic studies in accessible samples are also promising. Epigenomics and/or proteomics impairment under AD/PD conditions could trigger potential biomarkers. This review aimed to summarize the main results of the epigenomic and proteomic studies applied to (1) the search of minimally invasive biomarkers for early AD or PD diagnosis, and (2) the identification of physiological mechanisms involved in the development of AD and PD.
2. METHODS
A PubMed search was carried out on September 30, 2020. It was limited to the last 6 years, using a combination of search terms related to AD, PD, and epigenetics or proteomics (see Appendix A for complete search terms). In addition, original research articles in the English language that used minimally invasive human samples were included. Regarding exclusion criteria, reviews, meta-analysis, clinical trials, and studies with animals and invasive samples were not included.
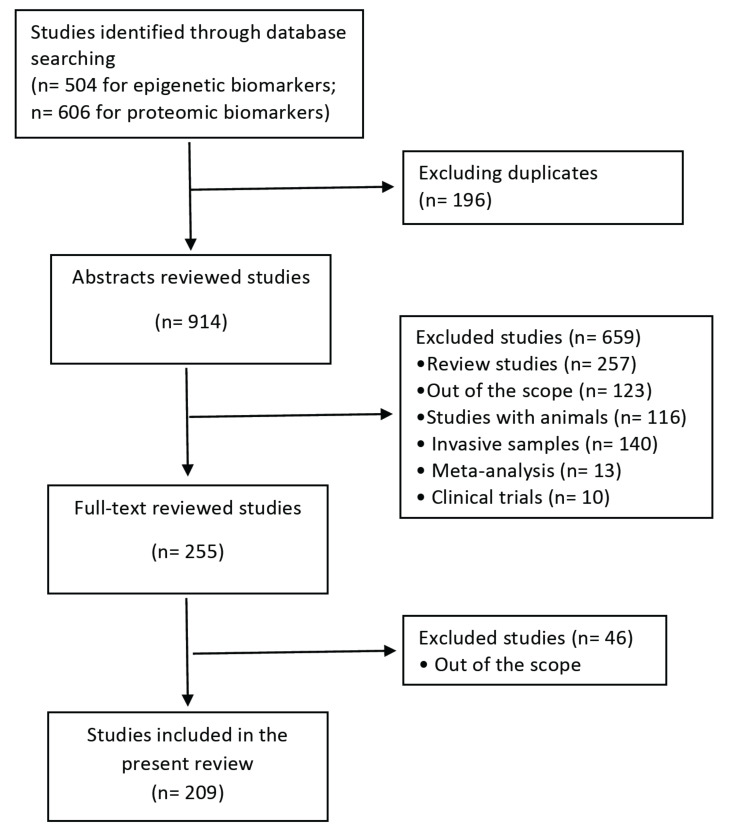
The search identified a total of 504 potential articles for epigenetic biomarkers and 606 articles for proteomic biomarkers. We screened the titles and abstracts to check if they fell within the scope of this review. In some cases, when abstracts were not available or more information was required to decide, a quick review of the whole article was carried out. At this stage, a total of 209 original articles containing data dealing with AD/PD and epigenetics or proteomic biomarkers on human minimally invasive samples were obtained (Fig. 1).
Fig. (1).
Flow diagram summarizing the systematic search, screening, and studies selection for this review.
3. RESULTS AND DISCUSSION
3.1. Epigenetic Biomarkers
Most of the studies were focused on miRNA analysis in serum, plasma, or blood samples [27, 30-82]. Screenings of miRNAs signatures are mainly based on the use of array and Next Generation Sequencing (NGS) techniques [30, 32, 38, 40-42, 49, 52, 54, 63, 74, 75, 77, 79-81, 83], while target studies for candidate miRNAs are based on quantitative polymerase chain reaction [27, 31, 35-37, 43-48, 50, 51, 53, 55-62, 64-73, 76, 82, 84]. In this sense, some single miRNAs have been proposed as candidate biomarkers for AD or PD, but the combination of multi-biomarkers could increase the sensitivity and specificity of diagnosis. However, miRNAs are not the only epigenetic mechanisms that have recently been investigated. In fact, a few studies mostly focused on specific DNA methylated regions associated with AD and PD [85-98]. Finally, most of these studies were designed as transversal case-control studies for specific populations. Table 1 summarizes the miRNA studies reviewed in this study.
Table 1.
Studies of miRNAs as potential biomarkers for AD and PD.
| Disease | Sample | Participants | Biomarkers (has-miR) and Results |
Analytical
Method |
Refs. |
|---|---|---|---|---|---|
| AD | Serum | Japanese population Discovery cohort: AD (511) VaD (46), DLB (85), HC (144) Validation cohort: AD (510) VaD (45), DLB (84), HC (144) |
78-miRNAs signature | Untargeted (array) | Shigemizu 2019 [30] |
| USA population Discovery cohort:AD(10), MCI (16), HC (14) Validation cohort: AD (1), MCI (4), HC (4) |
5-miRNAs signature (miR-455-3p, miR-4668-5p, miR-3613-3p, miR-4674 and miR-6722) miR-455-3p: candidate biomarker for AD |
Kumar 2017 [41] | |||
| Chinese population mild AD (30), moderate AD (30), HC (30) |
miR-222: significantly decreased in the mild and moderate AD groups | Zeng 2017 [52] | |||
| Chinese population Discovery cohort: AD(19), HC (9) Validation Cohort: AD (121), HC(86) |
9-miRNAs signature (miR-26a-5p, miR-181c-3p, miR-126-5p, miR-22-3p, miR-148b-5p, miR-106b-3p, miR-6119-5p, miR-1246 and miR-660-5p) miR-22-3p: best miRNA |
Untargeted (NGS) | Guo 2017 [63] | ||
| Chinese population Discovery cohort:AD (20), HC (20) Validation cohort: AD (45), HC (40) |
9-miRNAs signature (miR-146a-5p, miR-106b-3p, miR-195-5p, miR-20b-5p, miR-497-5p, miR-125b-3p, miR-29c-3p, miR-93-5p and miR-19b-3p) miR-29c-3pandmiR-19b-3p: contribute to the cognitive function |
Wu 2017 [74] | |||
| Chinese population Discovery cohort: AD (10), HC (7) Validation cohort: AD (127), MCI (30), VaD (30), HC (123) |
4-miRNAs signature (miR-31, miR-93, miR-143 and miR-146a) | Dong 2015 [78] | |||
| Japanese population Discovery cohort: AD (27), HC (18) Validation cohort: AD (36), HC (22) |
3-miRNAs signature (miR-501-3p, let-7f-5p and miR-26b-5p) miR-501-3p: candidate biomarker for AD progression |
Hara 2017 [80] | |||
| Australian population Discovery cohort:AD (23), MCI (3), HC (23) Validation cohort: AD(16), MCI(8), HC (36) |
16-miRNAs signature | Cheng 2015 [81] | |||
| Italian population AD (18), MCI-AD (18); HC (30) |
Four miRNAs desregulated (miR-5588-5p, miR-3658, miR-567 miR-3908) miR567: Candidate biomarkers for MCI-AD progression to AD. |
De Felice 2020 [77] | |||
| German Population AD (47), FTD (48), HC (38) |
29-miRNAs signature | Targeted (96 miRNAs) | Denk 2018 [82] | ||
| Chinese population AD (84), HC (62) |
miR-223, miR-519, miR-29 and miR-125b miR-223: candidate biomarker for AD |
Targeted (4 miRNAs) | Jia 2016 [31] | ||
| Chinese population AD (105), HC (98) |
miR-133b: significantly downregulated in AD patients. Candidate biomarker for AD. | Targeted (1 miRNA) | Yang 2019 [65] | ||
| Iranian population AD (56; 33 responders and 23 no responders to rivastigmine), HC (50) |
miR-106b-5p: candidate biomarker for AD | Targeted (1 miRNA) | Madadi 2020 [72] | ||
| Iranian population AD (20), HC (15) |
miR4422: candidate biomarker for AD | Targeted (2 miRNA) | Hajjri 2020 [73] | ||
| Plasma | USA population AD (35), HC (35) |
20-miRNAs signature miR-342-3p: best candidate |
Untargeted (NGS) | Lugli 2015 [32] | |
| Polish population Discovery cohort:AD (7), MCI-AD (7),HC (6) Validation cohort: AD (13), MCI-AD (8),HC (9) |
23 AD miRNAs confirmed, 26 novel differential miRNAs. 15 miRNAs validated. miR-483-5p, miR-486-5p, miR-30b-5p, miR-200a-3p, miR-502-3p and miR-142-3p: candidate biomarkers for AD |
Untargeted (qRT-PCR) | Nagaraj 2017 [33] | ||
| Danish population AD (10), VaD (4), FTD (4), DLB (2) |
miR-590-5p,miR-142-5p and miR-194-5p: differentially expressed between AD patients and the other pathologies | Untargeted (qRT-PCR) | Sorensen 2016 [34] | ||
| Disease | Sample | Participants | Biomarkers (has-miR) and Results |
Analytical
Method |
Refs. |
| AD | Plasma | Spanish population AD (56), MCI (26), FTD (27), HC (38) |
3-miRNAs signature (miR-92a-3p, miR-181c-5p and miR-210-3p) | Targeted (NA) | Siedlecki-Wullich 2019 [35] |
| Italian population AD (20), FTD (54), HC (53) |
miR-29b-3p, miR-34a-5p, miR-16-5p, miR-17-5p, miR-107, miR-19b-3p, let-7b-5p, miR-26b-5p and miR-127-3p miR-127-3p: downregulated in FTD and upregulated in AD |
Targeted (9 apoptosis miRNAs) | Piscopo 2018 [36] | ||
| Chinese population AD (97), aMCI (116), HC (81) |
miR-107: capability to discriminate between patients with amnestic mild cognitive impairment and healthy controls | Targeted (1miRNA) | Wang 2015 [37] | ||
| Blood (PBMC) | Chinese population AD (55), HC (49) |
9 miRNAs selected for validation (miR-425-5p, miR-130a-3p, miR-3607-3p, miR-339-5p, miR-4297, miR-639, miR-25-5p, miR-5699 and miR-5000-5p) miR-339 and miR-425: candidate biomarkers for AD |
Untargeted (array) | Ren 2016 [38] | |
| Blood | Brazilian population Discovery cohort:AD (4),HC (4) Validation cohort:AD (21),HC (17) |
21 differential expressed miRNAs were validated miR-144-5p, miR-221 and miR-374: downregulated in AD |
Untargeted (qRT-PCR) | Manzine 2018 [39] | |
| USA population (previously reported) AD (54), HC (22) German population AD (49), MCI (20), MS (90), HC (55) |
68 dysregulated miRNAs in both cohorts miR-151 a-3p: lower p-value in both cohorts (AUC 0.74) |
Untargeted (NGS) | Keller 2016 [40] | ||
| Australian population AD (40), HC (31) |
miR-146b-5p and miR-15b-5p: candidate biomarker for AD | Wu 2020 [75] | |||
| Italian population AD (18), MCI-AD(18); HC (30) |
Four miRNAs desregulated (miR-5588-5p, miR-3658, miR-567 miR-3908) miR567: Candidate biomarkers for MCI-AD progression to AD. |
De Felice 2020 [77] | |||
| Turkish population AD (172), HC (109) |
miR-9-5p, miR-29a-3p, miR-106a-5p, miR-106b-5p, miR-107, miR-125a-3p and miR-125b-5p miR-9-5p, miR-106a-5p, miR-106b-5p and miR-107: expression reduced in AD patients miR-106a-5p: candidate biomarker for AD |
Targeted (7 miRNAs) | Yilmaz 2016 [27] | ||
| European population MCI converted to AD within 2 years (19) and stable MCI(26) |
miR-146a and miR-181a: AD progression | Targeted (9 miRNAs) | Ansari 2019 [64] | ||
| Brazilian population AD (36), HC (38) |
miR-9: candidate biomarker for AD | Targeted (25 miRNA) | Souza 2020 [69] | ||
| Tears | Spanish population AD (9), MCI (8), HC (15) |
miR-200b-5p: candidate biomarker fo AD | Untargeted (array) | Kenny 2019 [83] | |
| AD + PD | Serum | Scandinavian population Discovery cohort: PD drug naïve (16), HC (8) Verification cohort: PD drug naïve (164), HC (182) Validation cohort: PD drug naïve (42), AD (48), HC (22+182) |
3-miRNAs signature for PD (miR-335-5p, miR-3613-3p, miR-6865-3p) | Untargeted (array) | Patil 2019 [42] |
| Chinese population DAT (107), PDD (30), VaD (20), MCI (101) |
miR-135a, miR193b and miR-384 combined are better than a particular miRNA for early AD diagnosis. miR-384: best among the three miRNAs to discriminate AD, VaD, and PDD. |
Targeted (3 exosomal miRNAs) | Yang 2018 [43] | ||
| Chinese population PD (80), AD (30), HC (110) |
miR-29: significantly downregulated in PD patients. The expression of serum miR-29a and miR-29c expression tended to decrease with disease severity. | Targeted (1 miRNA family) | Bai 2017 [44] | ||
| Chinese population PD (46), AD(40), MSA(35),HC (46) |
miR-520d-5p: elevated in PD patients but not in AD nor MSA cases | Targeted (1 miRNA) | Jin 2018 [45] | ||
| Disease | Sample | Participants | Biomarkers (has-miR) and Results |
Analytical
Method |
Refs. |
| AD + PD | Serum | Italian population AD (30), PD (30), VaD (24), VP (25), HC (30) |
3-miRNAS signature for AD-like (miR-34b, miR-125b, miR-130b) 6-miRNAS signature for PD-like (let-7d, miR-15b, miR-24, miR-142-3p, miR-181c, miR-222) 3 miRNAs altered in all four neurodegenerative diseases (miR-23a, miR-22*, miR-29a) |
Targeted (26 miRNA) | Barbagallo 2020 [265] |
| Plasma | USA population AD(50), FTD(50), PD(50), ALS(50),HC (50) |
miRNA pairs combinations AD (22 pairs, 18 miRNAs) PD (18 pairs, 18miRNAs) AD vs PD (36 pairs, 18miRNAs) 5 Common selected miRNAs: miR-128a, miR-146a, miR-29a, miR-9*and miR-99b |
Targeted (37 miRNAs) | Sheinerman 2017 [46] | |
| Spanish population Cohort 1 (20-21/group): AD, PAD, PD, HC Cohort 2 for validation (15/group): AD, PAD, HC |
miR-34a-5p and miR-545-3p: candidate biomarkers for AD not confirmed in an independent cohort (cohort 2) | Targeted (10 miRNAs) | Cosin-Tomas 2017 [47] | ||
| Chinese population PD (319), NDC (305: 69 epilepsy, 57 CerebroVD, 49 AD, 47 parkinsonism no PD, 22 ET, 14 MG, 14 MND, 11 PNP, 7 dementia, 6 RLS, 3 migraine, 3 MS, 2 myelopathy, 1 SC), HC (273) |
miR-105-5p: candidate biomarkers for PD | Targeted (1 miRNA) | Yang 2019 [48] | ||
| PD | Serum | Chinese population Discovery cohort: PD (15), HC (15) Training cohort: PD (45), HC (36) Validation cohort: PD (61), HC (55) |
15-miRNAs signature miR-195, miR-185, miR-15b, miR-221 and miR-181a: candidate biomarkers for PD |
Untargeted (NGS) | Ding 2016 [49] |
| Chinese population Discovery cohort: PD (77), HC (106) Training cohort: PD (30), HC (30) Validation cohort: PD (92), HC (74) |
12 miRNAs differentially expressed selected for validation miR-141, miR-214, miR-146b-5p and miR-193a-3p: candidate biomarkers for early diagnosis of PD |
Dong 2016 [79] | |||
| Chinese population PD (109), HC (40) |
miR19b, miR24 and miR195: candidate biomarkers for PD | Targeted (24 miRNAs) | Cao 2017 [50] | ||
| Chinesepopulation PD (138) HC (112) |
miR-221: candidate biomarker for PD | Targeted (16 miRNAs) | Ma 2016 [51] | ||
| Spanish population RBD converted to PD/DLB (28: before conversion 28, after 20: 8 to PD and 12 to BLB), RBD disease free (28), HC (28) |
miR-19b, miR-29a, and miR-29c miR-19b: candidate biomarker for prodromal stage of PD and DLB |
Targeted (3 miRNAs) | Fernandez-santiago 2015 [53] | ||
| Chinese population PD (50), HC (50) |
miR-204-5p: candidate biomarker for PD. | Targeted (45 miRNA) | Chiu 2019 [66] | ||
| Chinese population PD (80), HC (60) |
miR-150: candidate biomarker for PD. | Targeted (1 miRNA) | Haiting 2020 [71] | ||
| Turkish population PD (51), HC (20) |
miR-29c: candidate biomarker for PD | Targeted (7 miRNA) | Ozdilek 2020 [76] | ||
| Plasma | Chinese population Discovery cohort: PD (3), HC (5) Validation cohort: PD (20), HC (20) Validation cohort for miR-4639-5p: PD (169), ET (60), HC (170) |
7 miRNAs with differential expression were selected for validation (miR-34c-3p, miR-148b-5p, let-7i-3p, miR-4639-5p, miR-34a-3p, miR-181a-5pand miR-30a-5p) miR-4639-5p: candidate biomarkers for early diagnosis of PD |
Untargeted (array) | Chen 2017 [54] | |
| Italian population PD (99+10 drug-naïve), HC (109) |
miR29a-3p, miR29b-3p, miR30a-5p, miR30b-5p, miR103a-3p, miR191-5p miR30a-5p: candidate biomarker for PD |
Targeted (6 miRNAs) | Schwienbacher 2017 [55] | ||
| Chinese population PD (269), NCD (176: 38 epilepsy, 38 cerebroVD, 25 PNP, 20 ET 16 MG, 15 migraine, 14 MND, 10 MS), HC ((222) |
miR-132: candidate biomarker for diagnosis and progression of PD | Targeted (1 miRNA) | Yang 2019 [56] | ||
| Chinese population PD (25), HC (25) |
15 miRNAs with a differential expression pattern 5-miRNAs with AUC value > 0.8 (miR-27a, let-7a, let-7f, miR-142-3p and miR-222) | Targeted (91 miRNAs) | Chen 2018 [57] | ||
| Disease | Sample | Participants | Biomarkers (has-miR) and Results |
Analytical
Method |
Refs. |
| PD | Plasma | Chinesepopulation PD(46), HC (49) |
miR-433, miR-133b, miR-34b, miR-34c, miR-153, and miR-7 miR-433 and miR-133b: candidate biomarkers for PD |
Targeted (6 miRNAs) | Zhang 2017 [58] |
| Chinese population PD (60; 24 with depression and 36 without), HC (60) |
miR-137, miR-124, and miR-184 miR-137 and miR-124: candidate biomarkers for PD |
Targeted (3 miRNAs) | Li 2017 [59] | ||
| Greek population PD (152: 99 idiopathic, 27 with GBA mutated; 26 with SNCA mutated), HC (101) |
miRNAs signatures for idiopatic PD (8: miR-7-5p, miR-22-3p, miR-124-3p, miR-136-3p, miR-139-5p, miR-330-5p, miR-433-3p, miR-495-3p) and genetic (14) PD miR-433-3p: significantly upregulated in the three PD cohorts |
Targeted (20 miRNA) | Ravanidis 2020 [67] | ||
| Greek population PD (108), HC (92) |
4 miRNAs are significant differentially expressed: (miR-22-3p, miR-139-5p, miR-154-5p, and miR-330-5p) | Targeted (12 miRNA) | Ravanidis 2020 [68] | ||
| Blood (PBMC) |
Italian population PD (37), HC (43) |
miR-155, miR-26a, miR-146a and miR-132 miR-155: up-regulated in PD patients and expression modified with levodopa treatment. Candidate biomarker for disease progression. miR-146a: down-regulated in PD patients |
Targeted (4 miRNAs) | Caggiu 2018 [60] | |
| Italian population PD (46: 36 dopa-treated and 10 drugs naïve); HC (46) |
miR-30a-5p, miR-30b-5p, miR29a-3p, miR29b-3p and miR-103a-3p miR-30b-5p, miR29a-3p and miR-103a-3p:candidate biomarker for treated PD |
Targeted (5 miRNAs) | Serafin 2015 [61] | ||
| Iranian population PD (33), HC (25) |
miR-376a: candidate biomarker for PD | Targeted (1 miRNA) | Baghi 2020 [70] | ||
| Blood | Turkish population PD (102), HC (102) |
miR-4671-3p, miR-335-3p, miR-561-3p, miR-579-3p and miR-3143 miR-335-3p, miR-561-3p and miR-579-3p: significantly associated with PD susceptibility miR-561-3p: candidate biomarker for PD |
Targeted (5 miRNAs) | Yilmaz 2016 [62] | |
| Saliva | Chinese population PD (30), HC (30) |
miR-874 and miR-145-3p: candidate biomarker for PD. | Targeted (2 miRNA) | Chen 2020[272] | |
| Canadian population PD (83), HC (77) |
miR-153 and miR-223: candidate biomarkers of idiopathic PD | Targeted (2 miRNA) | Cressatti 2020[84] |
3.1.1. DNA Methylation
Most of the studies on DNA methylation were focused on specific genes associated with these diseases, and blood was the preferential sample used.
3.1.1.1. Alzheimer’s Disease
Among AD studies, different papers were centered on the differential methylation of specific loci. In fact, using a methylation chip for a genome-wide screening, the best candidate genes for differential DNA methylation in AD were NCAPH2/ LMF2, RERG, COASY, and SPINT1, showing correlation with the Mini-Mental State Examination (MMSE) score [99]. Moreover, NCAPH2/LMF2 methylation levels were considered a potential biomarker for AD and diagnosis [99]. A subsequent analysis of the same group suggested that methylation in this region could be associated with hippocampal atrophy through apoptosis [96]. However, the corresponding mechanism remains largely unknown [96, 99]. The same authors measured DNA methylation by methylation-sensitive high resolution melting in the promoter regions of two genes (COASY, SPINT1). They detected significant hypermethylation in AD and amnestic MCI (aMCI) vs. healthy controls (HC) and proposed COASY methylation as a diagnostic biomarker due to its high sensitivity and specificity (>96%) [93]. Although these studies were performed in a small cohort (30 AD patients, 28 aMCI patients, and 30 HC), this group validated these results this year in a larger cohort (AD (151), vascular dementia (VaD) (21), MCI (22), HC (200)) [100]. Moreover, this recent study has also pointed out that COASY methylation levels were significantly higher in AD patients without cardiovascular diseases compared to AD patients with them [100]. However, as far as we know, these results have not been subsequently corroborated by other authors. In another study (46 AD patients and 61 HC), significant hypermethylation of the promoter of
DRD4 (dopaminergic receptor) in male AD patients resulted in a higher risk of AD among men [95]. In addition, a longitudinal analysis revealed significant hypermethylation in specific CpG islands of BDNF promoter in the aMCI group, which converted to AD after 5 years, indicating that DNA methylation status of these regions could be used as a potential epigenetic biomarker for predicting the progression from aMCI to AD [94]. BDNF is a brain-derived neurotrophic factor with a key role in the growth, development, differentiation, and regeneration of various types of neurons in the central nervous system (CNS); its DNA methylation was already associated with AD in previous studies [101, 102]. Moreover, Sao et al. [86] proposed TREM1 as a biomarker for AD, based on its differential hypomethylation in AD patients vs. HC and its function in the immune response. On the other hand, Ozaki et al. [92] detected hypomethylation of TREM2 intron 1 in AD patients vs. HC, which was responsible for a higher TREM2 mRNA expression in leukocytes. Based on it, TREM2 methylation was proposed as a potential AD biomarker [92]. Therefore, TREM2 and TREM1 may be associated with the immune responses in AD [86]. In addition, the methylation levels of two opioid receptor genes associated with AD (OPRM1, OPRL1) were analyzed, showing significant hypermethylation of both in AD [85]. Although the methylation status of the APOE gene had been previously described [103-106], Shao et al. [91] found an association between the methylation of an extended region of regulatory elements that span across the extended APOE locus (TOMM40-APOE-APOC2) and AD. However, some differences were found between blood and brain samples. GRP50, a G protein receptor, was significantly hypomethylated in male AD patients [88]. Previous studies have pointed out the relevant role of GPCRs in the pathogenesis of AD [107]. A significant hypomethylation of BIN1 in a specific assay for late-onset AD (LOAD) was also detected (LOAD (50), HC (50)) [89]. BIN1 had functions relevant to several aspects of AD pathogenesis [108], and was identified as the second major genetic risk factor for LOAD after APOE-ɛ4 by the AlzGene database [109]. Hypomethylation of BIN1 might play an important role in its expression and therefore was proposed as a candidate biomarker for LOAD [89]. Finally, two untargeted DNA methylation studies based on the array have been recently reported. In a cohort of North American population from the Alzheimer’s Disease Neuroimaging Initiative (AD (94), MCI (336), HC (223)), FAM8A1 was the most significant differentially methylated for AD [110]. FAM8A1 encodes a protein that is associated with endoplasmic reticulum degradation of proteins with roles in Alzheimer’s disease pathogenesis. Also, differentially methylated loci were enriched near the brain and neurodegeneration-related genes (e.g., BDNF, BIN1, APOC1); however, additional studies to detect the blood-brain overlap in DNA methylation are required [110]. On the other hand, Roubroeks et al. [111] proposed a methylation signature specific for AD and MCI based on their results from a cohort from the cross-European AddNeuroMed study (AD (86), MCI (109), HC (89)). Besides, HOXB6, which encodes for a homeobox protein implicated in the hematopoietic development of granulocutes and monocytes [112], was significantly hypermethylated for AD [111].
3.1.1.2. Parkinson’s Disease
Related to PD, few studies have shown satisfactory results considering DNA methylation as a potential biomarker. According to Pihlstrom et al. [87], SNCA promoter is significantly hypomethylated in PD patients, despite the small number of participants (36 PD patients and 36 HC). However, previous case-control studies of the methylation of this locus in PD showed conflicting results [21, 113-115], and no other recent works have supported these promising results. Moreover, although in DLB patients, the mean methylation of SNCA was lower compared to HC, no significant differences were observed between the expression of SNCA in DLB and HC [98]. In this sense, a recent study in a small cohort (PD (37), DLB (23), HC (60)) has shown that DRD2 DNA methylation was significantly increased in DLB patients and significantly decreased in PD patients [90]. DRD2 is a dopamine receptor that might regulate the efficacy [116, 117] and side effects [118, 119] of medication in PD. On the other hand, considering the evidence of circadian alterations as PD features [120], Mao et al. [97] analyzed the DNA methylation of clock genes (NPAS2, CRY1), comparing methylation levels of leukocyte DNA (80 medicated PD patients, 30 drug-naive PD patients, and 80 HC). They concluded that NPAS2 hypomethylation occurred at early PD stages and that it could be a potential biomarker [97]. Regarding untargeted studies, only two DNA methylation studies have been reported. First, Kaut et al. [121] evaluated the DNA methylation pattern in PD patients compared to brothers or twins without PD in a cohort of 62 discordant siblings (24 monozygotic twins, 17 male PD patients, and 21 healthy male brothers) with a 450K methylation array. From this, 62 differentially methylated CpGs in 51 genes were selected. These results were validated using two independent cohorts (one with 221 PD and 227 HC; and other with 472 PD and 487 HC). A signature of CpGs in blood cells with a reasonable discriminatory power was proposed. Also, PDE4D was found to be significantly hypermethylated in PD in all cohorts and other risk loci for PD, such as MAPT, GPX4 and GPX1, were differentially methylated too [121]. Second, Henderson-Smith et al. [122] performed a longitudinal epigenome-wide methylation analysis with a two-year window, using a methylation array with over 850.000 CpG sites (400 PD patients and HC). Longitudinal methylation changes in PD with the highest statistical significance were detected in proteins of potential importance to nervous system function (ABAT, EDC3), multiple cytoskeletal and extracellular matrix-associated proteins (TRTAP5, ELMO1, BCAN) and epigenetic factors (NCOR2, HDAC4). Also, the comparison between medicated vs. non-medicated PD patients showed larger changes in methylation longitudinally, suggesting that medication can modify the epigenome. Therefore, these preliminary results presented blood DNA methylation as a potential epigenetic biomarker for disease progression and response to dopaminergic medication [122].
In general, DNA methylation could be a promising approach to early AD/PD diagnosis. However, additional studies to detect the blood-brain overlap in DNA methylation are required. Furthermore, medication effects on DNA methylation should be considered. Among these biomarkers, the DNA methylation of genes COASY, SPINT1, and BDNF are more related to AD. It could be explained by the relationship with neuropsychological impairment, as well as with neuron regeneration. However, different methylation of genes NPAS2, PDE4D, and MAPT is more related to PD, constituting potential biomarkers, although the relationship with underlying physiopathological aspects is not clear. In both diseases, dopaminergic receptors (DRD4 and DRD2) also have significant differences in DNA methylation levels. Anomalies in dopaminergic transmission may lead to the disturbance of synaptic plasticity [95] and may even be related to drug response [116-119].
3.1.2. miRNA
3.1.2.1. Alzheimer’s Disease
In AD studies, participants were classified into AD and HC, as well as MCI, and other neurodegenerative or non-neurodegenerative diseases, such as VaD, frontotemporal dementia (FTD), and dementia with Lewy bodies (DLB)) [30, 33-37, 40, 41, 64, 77, 78, 81-83] (Table 1).
Serum was the most used sample for miRNAs assays in AD. Most of the studies were untargeted on the Asiatic population. Different miRNAs have been proposed as candidate biomarkers for AD. Kumar et al. [41] detected a significant up-regulation of miR-455-3p in the serum of AD patients, post-mortem brains, and AD mice. The downregulation of serum miR-501-3p levels has also been associated with its upregulation in AD brains, which may be involved in the pathogenesis of AD through an aberrant neuronal cell cycle [80]. Jia et al. [31] found that miR-223, an inflammation-associated miRNA, which may participate in CNS regeneration, was significantly down-regulated, and it was strongly correlated with MMSE scores. De Felice et al. [77] proposed miR567 as a candidate biomarker for MCI which will progress to AD, according to its differential expression not only in serum but also in blood and cerebrospinal fluid (also, taking into account that its target genes might be functionally active in neuronal cells). miR4422, which targets GSAP and BACE1, was significantly deregulated in the Iranian population and could lead to an increase in the formation of Aβ plaque [73]. However, despite the promising results, no other group has confirmed any of these results. On the other hand, in the Chinese population, miRNA-22-3p was proposed as the best candidate for AD [63], meanwhile, miR29c-3p, an apoptosis miRNA, and miR-19b-3p were considered important factors for cognitive function downregulation in AD [74]. One year later, these three miRNAs were included within a proposed 29-miRNAs signature for AD on the German population [82]. Finally, other proposed miRNAs for AD in serum, such as miR-222 [52] or miR-133b [65], have been included in miRNA-signatures for PD [49, 57, 58, 123].
Plasma is also a recurrent sample used for target and untargeted miRNAs assays in AD, mostly on the Caucasian population. Based on these studies, other miRNAs candidates have been proposed for biomarkers of AD. Wang et al. [37] focused their assay on plasma miR-107 expression on the Chinese population from previous studies. This miRNA regulates the expression of β-APP cleaving enzyme 1 (BACE1), an enzyme involved in the Aβ generation [124]. They concluded that this miRNA expression in plasma had a high capability to discriminate between MCI and HC [37]. This is one of the most studied miRNAs in AD. However, other studies have selected this miRNA as a candidate biomarker in blood assays, which will be mentioned in the next section. Lugli et al. [32] proposed miR-342-3p as the most interesting single miRNA for AD from an untargeted assay on patients recruited from Chicago (IL, USA). This miRNA was also pointed out one year before by Tan et al. [125] as an AD biomarker in Chinese population. Besides, other untargeted assay in serum included miR-342-3p in its specific 16-miRNA signature for AD in the Australian population [81]. On the other hand, Sorensen et al. [34] reported a differential upregulation of miR590-5p and miR142-5p and a downregulation of mir-194-5p in AD patients compared with patients with other types of dementias in plasma from a small Danish cohort. However, these results were not consistent with the CSF results from the same patients. Even more, as far as we know, these miRNAs have not been proposed as AD biomarkers for any other group. Finally, a recent publication has proposed a 3-miRNAs signature (miR-92a-3p, miR-181c-5p, and miR-210-3p) for early AD in the Spanish population, suggesting that it could be a good prognostic signature for the progression from MCI to AD [35].
Regarding whole blood samples, Ren et al. [38] identified two specific differential expressed miRNAs, miR-339 and miR-425, as potential diagnostic biomarkers for AD in peripheral blood mononuclear cells (PBMC) from the Chinese population. Even more, BACE1 was considered to be a potential target of these two miRNAs, in silico and in cell culture. Therefore, miR-339 and miR-425 may be involved in the pathogenesis of AD through inhibition of BACE1 protein expression [38]. Also, miR-9-5p implicated in amyloidogenesis was proposed as a candidate biomarker for AD [69]. In addition, Manzine et al. [39] found that miR-144-5p, miR-221, and miR-374 were down-regulated in AD subjects, compared to HC in the Brazilian population. These authors proposed that miR-221 targets ADAM10, an enzyme implicated in the Aβ protein precursor processing in AD [39]. However, two other groups proposed miR-221 as a candidate biomarker for PD [49, 51] (Table 1). In this sense, Wu et al. [75] selected two miRNAs differentially expressed in AD from an untargeted assay. They were: miR-146b-5p, involved in the innate immune system, and miR-15b-5p, implicated in the regulation of the cell cycle. However, miR-146b-5p was previously included in a four miRNAs signature proposed as a candidate biomarker for early PD [79]. Ansari et al. [64] pointed out the implication of miR-146a and miR-181a in AD progression since these miRNAs were up-regulated in patients with MCI who later converted to AD. However, these two miRNAs were also associated with PD progression [49, 60]. As mentioned before, miR-107 has been proposed as a candidate biomarker for AD due to its differential expression and its role in the regulation of BACE1 expression. This miRNA is among the 68 dysregulated miRNA in two different cohorts, one from the USA and another from Germany [40]. It was also included in the 21 differential expressed miRNAs that were validated for AD by Manzine et al. [39] and in a 7-targeted miRNA assay from the Turkish population [27]. This last group observed that the expression of miR-107 and three more miRNAs (miR-9-5p, miR-106a-5p, and miR-106b-5p) was reduced in AD patients. However, according to their results, miR-106a-5p was the best candidate biomarker for AD [27]. Also, miR-106a-5pand miR-106b-5p were included within the 68 dysregulated miRNAs in two different populations of AD vs. HC [40]. Furthermore, these two miRNAs were included in two different serum-miRNAs signatures from distinct populations, one made of 16 miRNAs [81] and another of 29 [82]. Recently, miR106b-5p has also been proposed as a candidate biomarker for AD in the Iranian population [72].
3.1.2.2. Parkinson’s Disease
Most of the studies are focused only on PD, although a few of them include other neurological disorders such as essential tremor (ET) [54, 56]. Also, one study focused on rapid eye movement sleep behavior disorder as a prodromal stage for PD and DLB [53] (Table 1).
In serum samples, miR-221, also mentioned in AD, has been proposed as a candidate biomarker for PD in the Chinese population [49, 51]. In the study of 16 miRNAs, Ma et al. [51] observed a significant downregulation of miR-221 in PD-patient serum. Ding et al. [49] also included this miRNA in a 5-miRNA panel for PD. In this study, miR-195 was up-regulated, and miR-221, miR-185, miR-15b, and miR-181a were down-regulated [49]. In addition, miR-195 was proposed as a candidate biomarker for PD along with miR-24 and miR-19b, and some of their predicted target genes might be involved in neuronal apoptosis, regeneration, and the neurodegenerative process [50]. Interestingly, miR-24 was included in the 15 differentially-expressed miRNA signature, from which Ding et al. [49] validated their 5 candidate miRNAs. Also, miR-19b was proposed out of three down-regulated miRNAs in PD as a candidate biomarker for the prodromal stage of PD and DLB in the Spanish population. Chiu et al. [66] proposed that upregulated expression of miR-294-5p in PD might lead to the death of dopaminergic cells by targeting DYRK1A-mediated ER stress and apoptotic signaling cascade. Also, miR-150, down-regulated in PD patients, might be involved in the inflammatory pathogenesis of the disease by targeting AKT3 [71].
In plasma samples, most of the publications are based on the Chinese population, and there is a lack of correlation among most of the studies. Chen et al. [54] proposed miR-4639, which regulates negatively DJ-1 expression, as a potential early biomarker for PD. Yang et al. [56] proposed miR-132 as a candidate biomarker for diagnosis and progression of PD since its expression was significantly increased in plasma of patients compared to HC and neurological disorder controls, and it correlated with the disease progression and severity. However, similar expression was observed for this miRNA between PD patients and HC (Italian cohort) [60]. The expression levels of miR-433 were significantly lower in plasma of PD patients compared with the controls, and therefore it was proposed as a candidate biomarker for PD by Zhang et al. [58]. miR-433 regulates fibroblast growth factor 20 (FGF20) levels and subsequent α-synuclein expression [126]. Even more, in a recent study of idiopathic and genetic PD, miR-433 was the only miRNA significantly upregulated in all the PD cohorts [67]. However, in the validation essay performed by the same group for the eight brain-enriched miRNAs differentially expressed between healthy controls and idiopathic PD patients, miR-433 was no longer differentially expressed and showed reverse expression [68]. In this case, gathering the results of both series, the authors stated a 5-miRNAs signature for PD (miR-22-3p, miR-124-3p, miR-136-3p, miR-154-5p, and miR-323a-3p) [68]. Other candidate biomarkers obtained from targeted studies in small PD cohorts, not confirmed by other groups, were miR-137, miR-124, and mir-133b [58, 59]. Finally, based on previous results of blood analysis [61] (Table 1), miR-30a-p5 was proposed as a potential biomarker for PD in plasma, although the expression profile from both types of samples was different [55].
In whole blood samples, a few studies have been carried out, mainly from the European population. As mentioned before, miR-30b-5p was proposed as a candidate biomarker for the treatment of PD in PBMC, along with miR-29a-3p and miR-103a-3p [61]. The association of these last two miRNAs with PD was not confirmed later in plasma by the same research group [55]. Yilmaz et al. [62] proposed miR-561-3p, which targets LRRK2, as a candidate biomarker for PD. It should be recalled that LRRK2 gene mutations are the most known cause of late-onset autosomal dominant and sporadic PD [127, 128]. Caggiu et al. [60], in the analysis of 4 miRNAs commonly studied in neurodegenerative diseases, found that miR-146a was down-regulated and miR-155 was up-regulated in PD patients. Moreover, this group proposed that miR-155 could be a biomarker for PD progression [60]. However, miR-146a was also included in a 4-miRNA signature for AD [78] and was recently proposed as a candidate biomarker for progression from MCI to AD [64]. Finally, this year miR-376a has been proposed as a candidate biomarker for PD, probably through regulation of PGC1α and TFAM, in an assay from a small cohort of the Iranian population [70].
In general, there is some evidence that miRNAs are promising AD/PD biomarkers. However, there is a lack of consistency among studies. So, further validation studies are required. Specifically, some miRNAs (miR-455-3p, miR-501-3p, miR-223, miR-29c-3p, miR-19b-3p, miR-15b-5p, and miR-107) are more related to AD, probably due to the relationship with aberrant neuronal cell cycle and Aβ generation, as well as with clinical correlation and inflammation process. In PD, other miRNAs (miR-195, miR-24, miR-19b) might be related to neuronal apoptosis, regeneration, and the neurodegenerative process of the disease and could also be used in diagnosis.
3.2. Protein Biomarkers
Recent studies have focused on the study of proteins as potential early biomarkers for minimally-invasive diagnosis of AD and/or PD. Regarding the design, most of the studies were carried out at a single point in time and in a single cohort, but few of them focused on disease progression [129, 130] or the use of a second cohort to validate or confirm the results obtained [131-133]. As can be seen in Table 2, plasma is the most used sample [129, 134-137], followed by serum [12, 138-142] or other blood derivatives [143, 144]. Few of them used saliva [145-148] or urine samples [149-151], as well as tears [83, 152, 153].
Table 2.
Studies of proteins as potential biomarkers for AD and PD.
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
|---|---|---|---|---|---|
| AD | Serum | AD (43), HC (43) | Proteomics: 61 peptides differentially expressed, 9 of them derived from Pregnancy zone protein (PZP). Increased levels of PZP were found in presymptomatic AD compared to HC. | Untargeted (LC-MS) | Ijsselstijn, 2011 [218] |
| AD (15), MCI (15), HC (15) | 195 proteins: Some identified proteins were associated with AD-related plaque particle formation. | Untargeted (Flow cytometer- MS) | Madasamy, 2015 [185] | ||
| AD (58), HC (55) Confirmatory study 68 AD (68), HC (57) |
Proteomics: A peptide, 2 phosphatidylcholine and a glycerophosphatidylcholine were confirmed as potential biomarkers for AD diagnosis. | Untargeted (Capillary liquid chromatography-MS) | Shah, 2016 [217] | ||
| AD (15), HC (15) | Proteomics: Zinc-alpha-2-glycoprotein, fibulin-1 (FBLN1), platelet basic protein, thrombospondin-1, S100 calcium-binding protein A8, and S100 calcium-binding protein A9 showed different levels between AD patients and HC. | Untargeted (iTRAQlabeling and high-pH RPLC fractionation NanoLC-MS/MS) | Shen, 2017 [216] | ||
| DLB (30), AD (30), HC (28) | 146 peptides: A model including 4 peptides could discriminate between DLB group from the non-DLB (AD and HC) with a sensitivity of 93.3% and specificity of 87.9%. | Untargeted (HPLC-matrix assisted laser desorption/ionization time-of-flight mass spectrometer) | Suzuki, 2015 [215] | ||
| High neocortical amyloid-β burden (NAB) (107), Low NAB (91) | Proteomics: Pancreatic polypeptide and IgM showed a significant association with NAB (Neocortical Amyloid-β Burden). | Untargeted (SOMAscan) | Voyle, 2015 [158] | ||
| AD (45), HC (20) | Proteomics: 6 proteins differentially expressed: actin, apolipoprotein A-IV (Apo A-IV), inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), alpha-1-antitrypsin (AAT), antithrombin-III (AT-III) and activity-dependent neuroprotector homeobox protein (ADNP) | Untargeted (Two-dimensional gel electrophoresis, nano-HPLC-ESI-MS/MS, ELISA) | Yang, 2012 [179] | ||
| 6 sMCI (6), MCI-AD (6), AD (6), HC (6) | Proteomics: Complement C3 and alpha-2-macroglobulin were potential biomarkers for AD but not ready to be used in clinical practise. | Untargeted (LC-MS/MS, ELISA) | Zabel, 2012 [139] | ||
| Longitudinal, within-person analysis of serum NfL dynamics (196) preclinical AD | Neurofilament Light (NfL) has potential utility as a clinically useful biomarker. | Targeted (Array immunoassay technology) | Preische 2019 [219] | ||
| Pre-MCI (11), AD (4), HC (2) | Oligomeric proteins and variants are good AD biomarkers since early stages and they could serve as progression biomarkers | Targeted (Phage capture ELISA system) | Williams, 2017 [138] | ||
| AD (26), HC (24) | sAβPPα did not show differences between groups | Targeted (ELISA) | Taverna, 2013 [221] | ||
| AD (284), MCI (225), HC (557) | CRP Lower levels of CRP in AD compared to MCI and HC and reduced of this protein levels were found in MCI compared to HC. |
Targeted (multiplexedimmunoassay) | O’Bryant, 2013 [222] | ||
| Probable AD (192), HC (174) | Lower levels of CRP in AD than HC. | Targeted (multiplexed immunoassay) | O’Bryant, 2010 [180] | ||
| Probable AD (12), MCI (18), HC (13) | Leptin, hsCRP, IL-6 and TNF-α levels. TNF-α is associated with cognitive and functional decline at early clinical stages of dementia. |
Targeted (Leptin, IL-6 and TNF-a (ELISA), hsCRP (immunoturbidimetric method)) | Magalhães, 2018 [225] | ||
| AD (28), MCI (30), HC (77) | IL-18 and T-lymphocyte-secreted protein I-309 levels were different between groups. | Targeted (NA) | Villareal, 2016 [223] | ||
| Memantine-sensitive AD (90), memantine-insensitive AD (87) | A logistic regression model based on VEGF, BDNF, IL-6 and IL-1βfor diagnosing and choosing the best course of treatment for moderate AD | Targeted (ELISA) | Wei, 2018 [224] | ||
| AD (100), MCI (45), HC (100) | beta2-microglobulin could play a role in AD | Targeted (Solid-phase, two sites chemiluminescent immunometric assay on Immulite 2000 Automatic analyzer) | Dominici, 2018 [184] | ||
| AD (20), ALS (12), MS (42), tick-borne encephalitis (TBE) (12), HC (20). | Higher concentrations of free γc-globulin in patients suffering from neurodegenerative diseases | Targeted (ELISA) | Kułakowska, 2018 [162] | ||
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| AD | Serum | AD (920), MCI (277, HC (819) | Vitamin D binding protein (VDBP)is correlated with a score (inversely correlated with cognitive performance) | Targeted (Multiplex fluorescent immunoassay 177 utilizing coloured microspheres with protein-specific 178 antibodies) |
Bishnoi, 2015 [226] |
| AD (32), sMCI (13), other dementias (15) | Insulin-like growth factor-I (IGF-I) showed higher levels in AD and other dementias compared to HC and IGF-binding protein-3 (IGFBP-3) was increased in AD and sMCI compared to HC. In addition, IGF-I and IGFBP-3 correlated negatively with CSF β-amyloid. | Targeted (ELISA) | Johansson, 2013 [190] | ||
| MCI (3), AD (3), HC (3) | Proproteinconvertasesubtilisin/kexin type 9 (PCSK9), coagulation factor XIII, A1 polypeptide (F13A1), and dermcidin (DCD)are differentially expressed in MCI and AD. They could reflect PiB-PET imaging for MCI and AD | Targeted (NA) | Kang, 2016 [12] | ||
| AD (40), MCI (9), young controls (22), elderly controls (22) | Sirtuin1 (SIRT1) showed reduced levels with the age but this reduction is even more pronounced in MCI and AD. | Targeted (Surface Plasmon Resonance (SPR), Western-blot, ELISA) | Kumar, 2013 [167] | ||
| AD (156), HC (156) | 33 proteins: an eight-protein-based algorithm was the most robust with a sensitivity of 97.7%, specificity of 88.6%, and AUC of 99% | Targeted (Chemiluminescence) | Yu, 2018 [172] | ||
| AD (68), HC (52) | Peptides from Anthrax toxinreceptor 1, Nuclear protein 1, Glycogen phosphorylase, andOlfactory receptor 8J1 showed a statistically significant ability to discriminatebetween AD and HC | Untargeted (Biopanning and microarrays) | San Segundo-Acosta, 2019 [176] | ||
| AD (64), HC (45) | Increased concentrations of the presynaptic protein beta-synuclein in AD | Targeted (MS) | Oeckl, 2020 [177] | ||
| Plasma | Discovery cohort: 19 HC, 31 subjective memory concerns (SMC), 23 MCI, 6 AD. Replication cohort: 3 HC, 2 SMC, 25 AD, 49 FTD |
Proteomics: α2-Macroglobulin, fibrinogen γ-chain (FGG), and complement factor H-related protein 1 were associated with NAB. A model including FGG levels and age predicted NAB with a sensitivity of 59% and specificity of 78%. | Untargeted (LC-MS/MS) | Ashton, 2015 [191] | |
| AD (25), HC (25) | Proteomics: Several proteins showed differences between groups. Complement 4a plasma protein showed higher levels in AD. | Untargeted (LC-MS/MS, iTRAQ) | Bennett, 2012 [198] | ||
| MCI (10), AD (10), HC (10) | Redox proteomics: Haptoglobin was downregulated or increasingly oxidized in AD and MCI compared to HC. In addition, α2-macroglobulin, was selectively oxidized in AD compared with HC | Untargeted (Western-blot, MS) | Cocciolo, 2012 [192] | ||
| AD (207), HC (754) Validation: HC (58), AD (112) |
Proteomics: Panel of proteins differentially expressed between both groups. The validation with an external cohort showed an accuracy of 80%. | Untargeted (Plasma screening of 151 multiplexed analytes) | Doecke, 2012 [202] | ||
| AD (109), HC (58) | Proteomics: Set of 5 proteins differentiated between the AD and HC with a sensitivity of 89.36% and a specificity of 79.17%. | Untargeted (190-analyte multiplex immunoassay panel) |
Guo, 2013 [203] | ||
| AD (7), HC (7) | Proteomics: Twenty differentially expressed proteins between groups. Half of them are implied in amyloid/Aβ-peptide processing pathway | Untargeted (SDS-gel electrophoresis, ESI-MS) | Henkel, 2012 [156] | ||
| MCI-AD (163), HC (54) | Proteomics: Developed 11 signatures that discriminate between groups with sensitivity and specificity between 65-86%. | Untargeted (Fluorescent immunoassay) | Johnstone, 2012 [200] | ||
| Discovery: 195 subjects (93 twin pairs and 9 individuals). Replication: HC (91), MCI (81), AD (82). |
1129 proteins: Mitogen-activated protein kinase (MAPK) and MAPKAPK5 protein were associated with change in 10 years, and MAP2K4 were negatively correlated with volume of the left entorhinal cortex. These proteins could be potential biomarkers for AD conversion. | Untargeted (Slow Off-rate Modified Aptamer (SOMAmer)-based capture array called ‘SOMAscan’) | Kiddle, 2015 [159] | ||
| AD (9), HC (10) | Proteomics: Differential expression for proteins such as A-1, alpha-2-HS-glycoprotein, afamin, apolipoprotein A-4 and fibrinogen gamma chain between AD and HC. | Untargeted (2D-DIGE MALDI-TOF/TOF/MS) |
Kitamura, 2017 [28] | ||
| MCI (50), HC (50) | Proteomics: Keratin type-2 was up regulated, and albumin was down regulated in MCI | Untargeted (2D-PAGE MALDI-TOF/MS-MS) |
Kumar, 2018 [157] | ||
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| AD | Plasma | AD (109), MCI (360), HC (58) | 146 analytes: 4 signatures using 4-5 proteins. Apolipoprotein A-II, apolipoprotein E, serum glutamic oxaloacetic transaminase, α-1-microglobulin, and brain natriuretic peptide were present in the 4 signatures. They differentiate AD from HC with a specificity of 85% and a sensitivity of 74%. None of them predict AD progression with a great accuracy. | Untargeted (Fluorescent immunoassay) | Llano, 2013 [193] |
| Cohort 1: MCI (261), AD (24), HC (411). Cohort 2: MCI (180), HC (153) |
Proteomics: Complement regulators C1 inhibitor and factor H, fibronectin, ceruloplasmin, vitamin D-binding, apolipoproteins AIV, B-100 and H showed lower levels in MCI | Untargeted (iTRAQ 2D LC-MSMS) |
Muenchhoff, 2015 [133] | ||
| AD (85), MCI (300), HC (49) | Proteomics: 5 proteins associated to brain structural changes could discriminate between AD and HC with a specificity of 57% and a sensitivity of 89%. | Untargeted (190 analyte multiplex immunoassay panel) | Nazeri, 2014 [186] | ||
| AD (90), stable MCI (37), MCI-AD (39), HC (69). | Proteomics: DC-SIGNR, Aurora kinase A, MIP-1α, RGM-A, BMP-RII, CD39, Kallikrein 14, Ck-β-8-1, VEGF, EphA1 and HCC-1 proteins were differentially expressed among groups and could be useful as biomarkers for AD progression. | Untargeted (Slow Off-rate Modified Aptamer (SOMAmer)-based capture array called ‘SOMAscan’) | Sattlecker, 2016 [160] | ||
| AD (331), MCI (149), HC (211) | Proteomics: 13 protein predicted AD with an AUC of 0.70 | Untargeted (Slow Off-rate Modified Aptamer (SOMAmer)–based capture array called “SOMAscan”) |
Sattlecker, 2014 [201] | ||
| MCI (396), AD (112), HC (58) | Proteomics: Pancreatic polypeptide and N-terminal protein B-type brain natriuretic peptide showed higher levels in AD and MCI. ApoE ε3/ε4 or ε4/ε4 alleles showed distinct profiles | Untargeted (Multiplex Immunoassay Panel) | Soares, 2012 [196] | ||
| aMCI (147), nMCI (114), AD (19), HC (411) | Proteomics: 30 proteins were differentially expressed between (AD or MCI) and HC. These proteins include inflammatory response, cholesterol transport and blood coagulation pathways | Untargeted (iTRAQ Two-dimensional liquid chromatography and MS/MS) |
Song, 2014 [197] | ||
| AD (79), MCI (88), HC (95) | Proteomics: Complement components C3 and C3a, complement factor-I, γ-fibrinogen and alpha-1-microglobulin with age and sex explain 35% of variance in whole brain volume in AD patients | Untargeted (2DGE and LC/MS/MS) | Thambisetty, 2011 [194] | ||
| Baltimore Longitudinal Study of Aging (57) | Proteomics: 18 proteins differentiate between individuals with high and low brain Aβ. ApoE protein was related to brain changes in AD. | Untargeted (2DGE and (LC/MS/MS)) | Thambisetty, 2010 [195] | ||
| non-demented older individuals (157) | Proteomics: A2M, Apo-A1, and multiple complement proteins could be potential pre-clinical biomarkers for AD. | Untargeted (2DGE and LC-MS/MS) | Westwood, 2016 [130] | ||
| MCI (119) | Proteomics: 60 discriminant proteins between groups predicted progressive MCI with an accuracy of 79%. | Untargeted (LC-MS/MS) | Yang, 2014 [135] | ||
| Cohort 1: AD (77), HC (50) Validation: AD (10), MCI (30), HC (10) |
1129 proteins: A diagnose model with 5 proteins was developed. It diagnoses AD with sensitivity of 100.0%, specificity of 80.0% and accuracy of 90.0%. MCI and pre-dementia were diagnosed with sensitivity of 96.7%, specificity of 80.0% and accuracy of 92.5% | Untargeted (The SOMAscan assay is based on protein capture slow off-rate modified aptamers) | Zhao, 2014 [131] | ||
| MCI (161), HC (378) | Higher levels of Tau in MCI than in HC, and it was also related to memory and cortical thickness | Targeted (ELISA) | Dage, 2016 [209] | ||
| MCI (29), mild AD (21), HC (23) | Phosphorylated tau protein (threonine 181), denoted p-tau181level is correlated more to AD severity than T-tau |
Targeted (Immunoassay) | Yang, 2018 [165] | ||
| AD (80), HC (37), behavioural variant frontotemporal dementia (bvFTD) (14) | Soluble amyloid precursor protein (sAPP)α and β. sAPPβ showed lower levels in AD compared to HC and bvFTD. | Targeted (ELISA) | Perneczky, 2013 [189] | ||
| MCI-AD (21), Dementia-AD (44), HC (27) | Further evidence for the potential of sAPPβ in plasma as an AD biomarker candidate | Targeted (ELISA) | Alexopoulos, 2018 [204] | ||
| Dementia due to AD (23), Dementia due to other reasons (17) | Aβ38, Aβ40 and Aβ42. the Aβ42/Aβ40 ratio in blood plasma as a promising AD biomarker candidate which correlates significantly with the validated core biomarkers of AD | Targeted (Chemiluminescenceimmunomultiplex assay) | Shahpasand-Kroner, 2018 [173] | ||
| AD (58), HC (61) | Aβ levels, and platelet count. Correlation indicates that platelets may be involved in the pathogenesis of AD and become a potential peripheral biomarker for AD | Targeted (ELISA) | Sun 2018 [205] | ||
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| AD | Plasma | AD (24), HC (37) | Aβ oligomers. Higher levels in patients with AD than in HC, and they correlated well with conventional AD biomarkers | Targeted (ELISA) | Wang, 2017 [206] |
| AD (70), MCI (50), non-demented subjects with white matter hyperintensity (nd-WMH) (68), HC (33) | Protein-conjugated acrolein (PC-Acro) and amyloid-β40/42ratio detected MCI, AD and nd-WMH | Targeted (ELISA) | Waragai, 2012 [207] | ||
| Mild to moderate AD (10), MCI (20), patients who transitioned within 36 months from MCI to AD (20), HC (10) | P-tau, Aβ1-42, neurogranin (NRGN), and repressor element 1-silencing transcription factor (REST). Neuronally derived blood exosome proteins P-tau, Aβ1-42, NRGN, and REST could predict MCI to AD progression. | Targeted (ELISA) | Winston, 2016 [208] | ||
| AD (25), HC (20) | Neurogranin (Ng). There were identified 16 endogenous Ng peptides, but their levels were similar between both groups. | Targeted (nanoflow liquid chromatography- MS/MS) | Kvartsberg, 2015 [211] | ||
| MCI (13), AD (24), VaD (10), Mixed (8), other (5), HC (20) | Brain-derived neurotrophic factor (BDNF), Heart-type fatty acid-binding protein (FABP), Glial fibrillary acidic protein (GFAP), Interleukin-6 (IL6), Neuron-specific enolase (NSE), Neutrophil gelatinase-associated lipocalin (NGAL), Soluble tumor necrosis factor receptor I (TNFRI), D-dimer (DDMER), Thrombomodulin (TM), C-reactive protein (CRP). Some metabolites showed differences between groups. | Targeted (Arrays) | Rosén, 2011 [188] | ||
| AD (203), MCI (58), HC (117) | Lower levels of CRP in AD compared to HC and MCI | Targeted (ELISA) | Yarchoan, 2013 [210] | ||
| Dementia (80), aMCI (89), HC (133) | SUMO1 protein could be associated with AD as it showed increased levels in dementia patients and its levels correlate with MMSE | Targeted (ELISA) | Cho, 2015 [212] | ||
| MCI (49), AD (61), HC (35) | Higher levels of Chitinase 3-like 1 (CHI3L1) were found in AD compared to MCI and HC, while not differences were found between HC and MCI. | Targeted (ELISA) | Choi, 2011 [134] | ||
| AD (63), MCI (59), HC (63) | Cystatin C (CysC)levels were different among groups at baseline. | Targeted (ELISA) | Ghidoni, 2010 [214] | ||
| AD (126), vascular dementia (VaD)(96), non-AD neurodegenerative dementias (NND) (30), HC (98) | Soluble low-density lipoprotein receptor-related protein-1 (sLRP) showed lower levels in AD than the other groups, its sensitivity was 77.8% and its specificity was 93.3% for NND, 85.7% for HC and 58.3% for those with VaD. The soluble form of the receptor for advanced glycation end products (sRAGE)showed lower levels in AD compared with VaD or HC. AUC 0.88 with both protein levels. |
Targeted (ELISA) | Liang, 2013 [187] | ||
| AD (84) | Plasma prion protein (PrP)is not useful for AD progression monitorization | Targeted (EIA) | Schmidt, 2014 [129] | ||
| AD (40), non-demented (ND) (80) | Apolipoproteins. ApoA-I showed lower levels in AD and discriminated AD from ND with an AUC of 0.93. | Targeted (ELISA) | Shih, 2014 [213] | ||
| AD (257), HC (137) | Angiotensin-converting enzyme (ACE)levels were different between genotypes | Targeted (ELISA) | Yang, 2011 [163] | ||
| HC (92), MCI (527), AD (202) | A panel of proteins (n = 44), age and apolipoprotein E (APOE) ε4, predicted AD with good accuracy | Untargeted (SOMAscan assay) | Shi, 2020 [181] | ||
| AD (52), amnestic mild cognitive impairment (98), HC (114) | Level of sAPPβ was significantly increased in AD patients than in HC | Targeted (ELISA) | Yun, 2020 [182] | ||
| HC (408),MCI (400), AD (192) | A 7-protein panel could significantly discriminate between individuals with high and low amyloid pathology | Targeted (Luminex, ELISA, meso-scale discovery (MSD) assays) | Westwood, 2020 [183] | ||
| Early AD (33), Late AD (30), HC (36) | Elevation in markers GFAP and NfLin early AD | Targeted (SIMOA immunoassay) | Elahi, 2020 [220] | ||
| HC (186), MCI (50), AD (42)) | Proinflammatory endophenotypeidentify a specific subsetof adults with Down Syndromeand high risk of AD | Targeted (Meso-scale discovery assay) | Petersen, 2020 [199] | ||
| AD (186), HC (485), Validation cohort: AD (242), HC (199) |
CDH6 and HAGH may be new biomarkers in pre-symptomatic AD | Untargeted (affinity-based assay) | Ahmad, 2020 [178] | ||
| Blood | AD (106), HC (51) | 6-biomarker panel (A1Micro, A2Macro, AAT, ApoE, complement C3 and PPP) that discriminate between AD patients and HC with a sensitivity of 85.4% and specificity of 78.6%. | Untargeted (immunoassay platform and reference LC/MSMS(ADNI1)) | Jammeh, 2016 [228] | |
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| AD | Blood | mild to moderate AD (72), MCI (33), HC (113). | Alpha-defensins 1 and 2levels were increased in AD | Untargeted (SELDI-TOF MS) | Watt, 2015 [155] |
| probable AD (7), HC (7). | Proteomics: Secretory (alpha) granule proteins showed lower levels in AD. Pathways as glycoprotein synthesis, lipid homeostasis, amyloidogenic proteins, and regulators of protease activity were altered in AD platelets. | Untargeted (SDS-PAGE, LC-MS/MS) | Donovan, 2013 [154] | ||
| MCI (5), AD (5), HC (5) | Proteomics: Thioredoxin-dependent peroxide reductase, myosin light polypeptide 6, and ATP synthase subunit β showed differential levels among groups | Untargeted (2D–PAGE, ESI-MS) | Sultana, 2013 [227] | ||
| MCI (34), AD (45), HC (28) | APP, β-APP cleaving enzyme 1 (BACE1), presenilin 1 (PS1) and a disintegrin and metalloproteinase-10 (ADAM-10). APP and ADAM-10 showed reduced levels in MCI and AD compared with HC. The ratio ADAM-10/BACE1 was higher for the MCI compared to AD |
Targeted (Western blot) | Bermejo-Bescós, 2013 [229] | ||
| MCI-AD (30), HC (23) | Amyloid-β protein precursor (AβPP) isoform (115 kDa) was increased in AD/MCI group compared to HC. | Targeted (Western-blot) | Jelic, 2013 [168] | ||
| AD (68), MCI (19), Old control (33), young control (11) | sAPP-α and sAPP-b. sAPP-β showed higher levels in MCI and AD compared to HC. |
Targeted (ELISA) | Marksteiner, 2013 [164] | ||
| AD (25), HC (26) | Amyloid protein precursor (APP), tau protein, clusterin, α-synuclein and immunoglobulin (Ig). Slightly elevated Ig levels in AD subjects (not significative) and APP-N measures were negatively correlated with cognitive scores. |
Targeted (ELISA) | Mukaetova-Ladinska, 2012 [232] | ||
| AD (65), HC (809) | Changes in amyloid structure biomarker indicates prodromal AD and correlates with CSF AD biomarkers and amyloid PET imaging | Targeted (ATR-FTIR analyses (attenuated total reflection Fourier transform infrared)) | Nabers, 2018 [233] | ||
| MCI (34), AD (21) | APP ratio (percentage of 120-130 kDa to 110 kDa isoforms of the amyloid precursor protein). MCI who converted to dementia showed lower levels for the ratio. The accuracy was 0.74 and sensitivity and specificity were 75% | Targeted (Western blot) | Zainaghi, 2012 [231] | ||
| MCI (398) | ApoE, BDNF, clusterin, IL-6R, IL-13, and TNF-α. Age, sex, education, hippocampal volume, APOE genotype, and plasma identify patients with brain amyloidosis with a sensitivity of 68% and a specificity 78%. |
Targeted (immunoassay platform and reference LC/MSMS(ADNI1)) | Apostolova, 2015 [234] | ||
| AD (38), HC (34) | Ischemia-modified albumin (IMA), advanced oxidation protein products (AOPP), ferric reducing antioxidant power (FRAP) and the prooxidant-antioxidant balance (PAB). AOPP, IMA and PAB showed elevated levels in AD group while FRAP showed lower levels. |
Targeted (Colorimetric assays) | Altunoglu, 2015 [235] | ||
| AD (30), MCI (30), HC (60) | Platelet monoamine oxidase-B, frequently described to be increased in platelets and brains of AD patients, shows a gender-independent but stage-related increase since it is unaltered in MCI subjects | Targeted (SDS-PAGE and western-blot) | Reumiller, 2018 [236] | ||
| AD (13), HC (27) | Platelet amyloid precursor protein (APP) ratio (120-kD and 130-kD isoforms compared to that of the 110-kD isoform) was lower in AD than HC. | Targeted (electrophoresis and immunoblot, densitometry) |
Srisawat, 2013 [230] | ||
| Late AD (27), late control (26), early AD (13), early control (17) | GLUT1 transporter and the insulin receptor (INSR). INSR from red blood cells (RBC) membrane are increased in AD compared to HC | Targeted (Flow citometry) | Várady, 2015 [175] | ||
| 303 participants and replicated in ADNI cohort (566 participants) | Alpha-2 macroglobulin is correlated with tau and p-tau and higher levels of this protein are related to AD progression. | Targeted (NA) | Varma, 2017 [132] | ||
| Platelets and leukocytes | AD (20), HC (20) | Combining the protein level of ADAM10, BACE1, and PSEN1 in platelets, yielded a good accuracy to discriminate AD from controls | Targeted (Western blot) | Bram, 2019 [169] | |
| aMCI (53), HC (45) | In aMCI the AβPP ratio level was significantly lower and levels of P-tau231 and Ser396/404 phosphorylated tau were significantly higher | Targeted (Western blot) | Shi, 2020 [166] | ||
| Tears | AD (14), HC (9) | Proteomics. Levels of lipocalin-1, dermcidin, lysozyme-C and lacritin could constitute a diagnosis tool with a sensitivity of 81% and a specificity of 77%. | Untargeted (SDS-PAGE, LC-MS/MS) | Kalló, 2016 [271] | |
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| AD | AD (9), MCI (8), HC (15) | Elongation initiation factor 4E (eIF4E) as a unique protein present in AD | Untargeted (RP-LC-MS/MS) | Kenny 2019 [83] | |
| Saliva | AD (7), HC (27) | Amyloid-β Protein 42 salivary levels were elevated in AD compared to HC. This biomarker could be useful as AD diagnosis. | Targeted (ELISA) | Lee, 2017 [145] | |
| AD (15), HC (7). | Salivary beta amyloid 42 (Aβ42) levels were significantly higher in AD patients than in HC | Targeted (ELISA) | Sabbagh, 2018 [146] | ||
| Urine | AD (45), MCI (60), HC (65) | Alzheimer-associated neuronal thread protein (AD7c-NTP) were higher in AD and MCI compared to HC. | Targeted (ELISA) | Ma, 2015 [149] | |
| aMCI(23), naMCI group (23). | aMCI has higher levels of urinary AD7c-NTP | Targeted (ELISA) | Ku, 2019 [150] | ||
| AD (97), aMCI (50), HC (84) | MCP-1 levels weresignificantly higher in patients with AD and aMCI than in CN controls | Targeted (ELISA) | Xu, 2020[151] | ||
| PD | Serum | PD (81), other neurodegenerative diseases (39), HC (40) | Proteomics: 17 variables showed differences between groups. A model based on 5 biomarkers differentiate between PD and HC. | Untargeted (Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDITOF-MS)) | Li, 2011 [141] |
| Slight PD (8), middle to serious PD (8), HC (6) | Proteomics: 26 proteins were differentially expressed among groups | Untargeted (iTRAQ labelling, 2D-LC-MS/MS) | Zhang, 2012 [142] | ||
| PD (18), HC (7) | Proteomics: 15 differentially expressed proteins were found. Some of them are implied in antioxidation, lipid metabolism, intracellular transport, cell proliferation and immunoregulation pathways. | Untargeted (2-DE, ESI-Q-TOF-MS/MS) | Zhao, 2010 [239] | ||
| PD (51), HC (50) | High-sensitivity C-reactive protein (hs-CRP) and carcinoembryonic antigen (CEA) showed higher levels in PD compared to HC. | Targeted (hs-CRP nephelometric assay CEA: chemiluminescence immunoassay method) | Akıl, 2015 [174] | ||
| Early PD (63), HC (117) | High-sensitivity C-reactive protein (hs-CRP) showed higher levels in early PD. | Targeted (NA) | Song, 2011 [246] | ||
| Early PD (58), HC (20). | Interleukin (IL)-1β, IL-2, IL-6, IL-10, tumor necrosis factor-α, and high-sensitivity C-reactive protein. There is an increased peripheral inflammation in the early stage of PD, but the role of inflammation in motor and non-motor symptoms is unclear. | Targeted (ELISA) | Kim, 2018 [245] | ||
| PD (60), HC (45) | Higher levels of advanced oxidized protein products were observed in PD compared to HC | Targeted (LC/MS/MS) | García-Moreno, 2013 [140] | ||
| PD (18), HC (7) | DJ-1 protein may not be a biomarker of PD. In addition, there may be differences in the serum DJ-1 protein levels between Chinese and Japanese patients | Targeted (ELISA) | An, 2018 [237] | ||
| Sporadic idiopathic PD (213), progressive supranuclear palsy (PSP) (46), multiple system atrophy (MSA) (80), HC (177) | Expression of Rab35 was increased in PD compared to HC and other parkinsonian disorders, and it is also related to age at onset of PD. | Targeted (ELISA) | Chiu, 2016 [251] | ||
| PD (108), HC (31) | Caffeine, Theophylline, Theobromine, Paraxanthine, 1,7-Dimethyluric acid, 1,3,7-Trimethyluric acid, 1-Methylxanthine, 3-Methylxanthine, 1-Methyluric acid, 7-Methylxanthine, 5-acethylamino-6-formylamino-3-methyluracil, 5-acethylamino-6-amino-3-methyluracil. Lower levels of caffeine and caffeine metabolite profiles are promising diagnostic biomarkers for early PD. This is consistent with the neuroprotective effect of caffeine. | Targeted (LC-MS) | Fujimaki, 2018 [252] | ||
| PD (23), schizophrenia (17), HC (12) | Apolipoprotein E levels are higher in PD patients as compared with schizophrenic patients | Targeted (ELISA) | Gupta, 2018 [249] | ||
| PD (43), HC (40) | Serum levels of Tumor necrosis factor-α-induced protein-8 like-2(TIPE2) and its gene expression might be important prognostic biomarkers of PD. | Targeted (ELISA) | Kouchaki, 2018 [238] | ||
| PD (23), acute cerebral infarction (ACI, 30), HC (29) | Glial fibrillary acidic protein (GFAP) and neurofilament proteins (NFs) showed higher levels in PD and ACI than HC. Not differences were observed between AD and ACI. | Targeted (ELISA) | Su, 2012 [248] | ||
| Disease | Sample | Participants (n) | Biomarker and Results | Analytical Method | Refs. |
| PD | Early PD (106), HC (146) | CystatinClevels may predict the severities of sleep-disordered breathing problems in early PD patients | Targeted (Immunoturbidimetry assay) | Xiong, 2018 [171] | |
| PD (20), HC (10) | The expression levels of some proteins (complement C1q, protein Immunoglobulin Lambda Variable 1-33 (IGLV1-33)Cluster -33) were decreased in PD | Untargeted (MS) | Jiang, 2019 [242] | ||
| Plasma | PD (36), HC (16) | Proteomics: IgGκL and human serum amyloid P component (SAP) were found differentially expressed between groups. | Untargeted (2-DE analysis, LC/MS/MS) | Chen, 2011 [253] | |
| PD (58), HC (38) | Alpha-synuclein level is not valuable marker for AD | Targeted (ELISA) | Malec-Litwinowicz, 2018 [254] | ||
| PD (313) | CRP levels correlate with death risk in PD patients and it could serve as prognosis biomarker. | Targeted (NA) | Sawada, 2015 [136] | ||
| PD (375) | CRP levels could serve as prognosis biomarker for motor deterioration in PD. | Targeted (NA) | Umemura, 2015 [137] | ||
| tremor-dominant PD (TD-PD) (33), non-tremor dominant PD (NT-PD) (43) | The elevated plasma transferrin level, combining with decreased plasma iron level might be given considerable weight in the recognition of parkinsonian tremor. | Targeted (Scatter turbidimetry) | Si, 2018 [255] | ||
| PD (145), HC (45) | N8-acetylspermidine and N-acetylputrescine levels were significantly and mildly elevated in PD, respectively | Untargeted (Capillary Electrophoresis Time-of-Flight MS) | Saiki, 2019 [241] | ||
| PD (12), HC (12) | Proteins CCDC154, TRIM3, DHH, NRP2 and CLIC1 were detected with high specificity and sensitivity | Untargeted (liquid chromatography-mass spectrometry (LC-MS)) | Dong 2019 [250] | ||
| PD (96), HC (45) | Proteins (BSP, OMD, ACY1, GHR) robustly associated with PD | Untargeted (SOMAscanassay) | Posavi, 2019 [243] | ||
| PD (64), HC (30) | Aβ42 and tau protein inPD may be useful marker for cognitive impairments | - | Chojdak-Łukasiewicz, 2020 [244] | ||
| Blood | PD (9), HC (9) | Proteomics. Diagnosis model-based peptides from a protein signature achieved an AUC of 0.877. | Untargeted (LC-MS/MS) | Alberio, 2014 [256] | |
| PD (43), progressive supranuclear palsy (PSP, 13), MSA (8), HC (16) | Oxidized DJ-1 protein levels in erythrocytes can be used as a marker for the differential diagnosis of PD | Targeted (ELISA) | Yamagishi, 2018 [258] | ||
| PD (16), HC (8) | Clusterin, complement C1r subcomponent, and apolipoprotein A1. The expression levels of apolipoprotein A1 in exosomes may be useful for tracking the progression of PD. | Targeted (2D-DIGE analysis, MALDI-TOF/TOF/MS) | Kitamura, 2018 [257] | ||
| PD (53), HC (53) | heat shock cognate (hsc) 70 protein and lysosomal-associated membrane protein (lamp) 2A. Hsc70 levels were reduced in PD patients. | Targeted (Western blot) | Sala, 2014 [143] | ||
| Early PD (103), HC (156) | Mitochondrial ribosomerecycling factor (MRRF) and ribosomal protein S18 (RPS18), distinguished betweenPD and HC | Untargeted (Microarray) | Wu, 2020 [247] | ||
| Blood derived extracellular vesicles | PD (60), HC (37) | Proteomics: Proteomic analysis further revealed that there is a specific signature of proteins that could reliably differentiate HC from mild and moderate PD patients | Untargeted (nanoLCMS/MS) | Lamontagne-Proulx, 2019 [144] | |
| Tears | PD (36), HC (18) | Proteins from S100 superfamily (i.e. [S100A7], [S100A8] and [S100A11]), Peroxiredoxin-6 [PRDX6], Annexin-A5 [ANXA5] and Glutathione S-transferase-A1 [GSTA1] were upregulated in PD | Untargeted (Bottom-up liquid chromatography electrospray ionization tandem mass spectrometry (BULCMS)) | Boerger, 2019 [153] | |
| Saliva | PD (20), HC (20) | α-synuclein could serve as PD diagnosis biomarker showing lower levels in PD compared to HC. | Targeted (ELISA) | Al-Nimer, 2014 [147] | |
| PD (16), HC (22) | DJ-1and total proteins showed elevated levels in PD patients. Saliva could be useful for protein biomarker research. | Targeted (Western blot) | Masters, 2015 [148] | ||
| Urine | PD (28), HC (22) | SNAP23 and calbindin were the most elevated in PD | Untargeted (Mass spectrometry) |
Wang, 2019 [240] |
Untargeted studies were carried out mostly using mass spectrometry for different protein identification [139, 154]. This technique could be coupled to liquid chromatography [130, 135], matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) [141], or surface-enhanced laser desorption/ionization (SELDI-TOF) [155]. Electrophoresis was also commonly used in proteomic studies [156, 157]. However, few proteomic studies used the slow off-rate modified aptamer (SOMA) scan technology [158-160]. This technology is a multiplexed, aptamer-based assay that allows the simultaneous quantification of a large amount of proteins [161]. Targeted studies were carried out mostly by Enzyme-Linked Immuno-Sorbent Assay (ELISA) [162-164] or other immunoassays [129, 165]. The second group of analytical tools used for protein targeted determinations is based on western blot [166-170]. In addition, few of them used immunoturbidimetric, chemiluminiscent methods [171-174], or flow cytometry [175].
3.2.1. Alzheimer’s Disease
In AD studies, participants were commonly divided into two groups (AD and HC) [172, 176-180], and some of them also included a third group with AD in early stages (MCI or incipient AD) [181-186], while few studies included other neurological or neurodegenerative pathologies, such as vascular disease (VD), FTD or other types of dementia [187-190]. Cases were not defined in all the studies using the same criteria and similar methodology for their inclusion and exclusion.
Proteomic studies were carried out mostly using plasma samples, and most of them were untargeted studies (Table 2). These studies revealed that different globulins are altered during the pathology course showing in α2-macroglobulin [130, 191, 192] and α1-microglobulin [193, 194] differential levels between AD, MCI, and HC. Proteins from the amyloid pathway also showed altered expression in AD [156, 166, 182]. Apolipoproteins (APO), playing an important role in AD, showed differential expression in the pathology [28, 133, 193, 195], and individuals showed a differential protein profile depending on their APOE genotype [195, 196]. Also, plasmatic proteomic studies revealed the importance of coagulation and the complement cascade in AD [28, 133, 197, 198], and inflammatory pathways showed alterations too [197, 199]. Among other proteins, it is important to highlight different kinases such as mitogen-activated protein kinase and aurora kinase A [159, 160], or keratin type 2 and albumin [167]. In addition, untargeted studies allowed the development of different multivariate protein signatures [169, 181, 183, 200] that predicted AD with accuracies above 70% [131, 135, 169, 201, 202]. Among these multivariable studies, sensitivities around 90% with lower specificities were obtained [186, 203]. Amyloid and tau pathologies are closely related to AD. For that reason, several targeted studies have been focused on the determination of different proteins implied in these pathways. Thus, soluble amyloid precursor protein β (sAPPβ) was studied [182, 189, 204], as well as the pathological peptides generated from APP, such as Aβ38, Aβ40, Aβ42 [173, 205-208]. Moreover, the levels of phosphorylated tau, which are other hallmarks of the pathology, were studied, showing differences in plasma from HC and different stages of AD pathology [165, 209]. Related to inflammation pathways, proteins such as C reactive protein (CRP), interleukins, or necrosis factors showed elevated levels in AD patients [188, 210]. Neurogranin peptides did not seem to be good biomarkers for AD since their levels were similar between AD and HC [211]. In addition, proteins, such as SUMO1 and Chitinase 3-like 1, showed higher levels in AD than in non-AD groups. Apolipoprotein A1 (soluble low-density lipoprotein-related protein 1) and soluble form of the receptor for advanced glycation end products showed reduced levels in AD. They were postulated as potential biomarkers for this disease [134, 187, 212, 213]. Other proteins related to AD were Cystatin C and the angiotensin-converting enzyme (ACE) [163, 214], while plasma prion protein was not considered a good biomarker for AD progression [129]. In serum samples, untargeted protein research revealed some proteins associated with AD-related plaque particle formation and neocortical amyloid-β burden [158, 185]. Multivariate models that included different proteins, as well as ApoE, age, sex and education level as co-variables discriminated between AD and HC [180]. In addition, the 4-peptide model developed by Suzuki et al. [215] could discriminate between AD and non-AD groups. Among differentially expressed proteins, it is relevant to highlight APOs, globulins and components from coagulation and complement [139, 179, 216]. In addition, lipid metabolism biomarkers are altered in AD [217]. Ijsselstijn et al. [218] found that pregnancy zone protein (PZP) levels were increased in AD since pre-symptomatic stages, so they could act as early AD biomarker. Moreover, serum samples were extensively used in AD protein biomarkers search. Neurofilament light (Nfl), and proteins or peptides from amyloid and tau pathways were studied as potential diagnosis and progression biomarkers [138, 219, 220]. However, Taverna et al. [221] did not find differences for the soluble amyloid precursor protein α peptide between AD and HC. Inflammation biomarkers, such as CRP were studied in AD, showing a relationship with the disease [180, 222]. In addition, other inflammatory proteins, such as interleukins or TNF-α, seemed to be good biomarkers for AD diagnosis, showing a correlation with cognitive alterations since early AD stages [223-225]. Also, globulins such as β2-microglobulin and γc-globulin have been related to AD [162, 184]. Moreover, a panel of 33 proteins, mainly related to inflammation, permitted the development of a multivariate diagnosis model for AD with an AUC close to 100% [172]. Similarly, vitamin D binding protein was associated with cognitive decline [226] and different growing factors, such as IGF-I and IGFBP-3, showed correlation with Aβ [190]. In addition, age was closely related to AD and Sirtuin 1, which was negatively correlated with age. It was more reduced in AD since its early stages [167].
Regarding blood samples, few proteomic studies have been carried out, probably due to a large number of components present in the blood, making the untargeted analysis more difficult. These studies found defensins, thioredoxin-dependent peroxide reductase, or ATP synthase as potential AD biomarkers [155, 227]. Meanwhile, lipid and amyloid pathways were postulated as important pathways in disease development [154]. In addition, a panel of 6 proteins, including globulins, complement proteins, and APOs, permitted AD discrimination with sensitivity and specificity around 80% [228]. In addition, amyloid and tau-related pathway proteins were extensively studied in blood samples [169]. Specifically, amyloid precursor protein (APP) has been determined in blood samples [229-232], but also soluble amyloid precursor protein β (sAPPβ) derived from APP was studied in blood samples [164]. In addition, the enzyme BACE1, which contributes to the generation of pathological peptides, was studied [229]. Moreover, blood amyloid structure correlates with PET imaging and typical CSF biomarkers of AD in prodromal stages [233], and α2-macroglobulin showed correlation with tau and p-tau being a satisfactory biomarker for AD progression [132]. Inflammation is an important pathway in AD that allows brain amyloidosis identification in a multivariate model, which includes ApoE genotype and demographic variables [234]. Also, oxidative stress is increased in AD patients [235]. In addition, glut transporter insulin receptor, tropomiosin-1, and monoamine oxidase-B protein showed increased levels in AD compared to HC [175, 236].
3.2.2. Parkinson’s Disease
Most of the proteomics studies in PD are focused on differential expression between PD and HC [147, 237-244]. Few of them included early PD patients [245–247], while other studies included acute cerebral infarction [248] or schizophrenia [249], trying to differentiate these diseases from PD. Proteomic studies carried out in PD used predominantly serum samples (Table 2). Untargeted studies found signatures with differential protein levels between PD and HC [141, 142, 250]. Antioxidation, lipid metabolism, or proliferation are implied in PD [239]. Targeted studies are mainly focused on inflammation pathways. In this sense, CRP showed higher levels in PD compared to HC, which could be a good biomarker of this disease since its early stages [174, 245, 246]. Also, oxidative stress seems to be involved in the disease since high levels of advanced oxidized protein products were found in PD [140]. Other potential biomarkers were Rab35, glial fibrillary acidic protein (GFAP), and neurofilament proteins (NFs) [248, 251]. Caffeine metabolites, which have neuroprotective effects, were also differentially expressed in early PD [252]. In addition, Cystatin C was able to predict the severity of sleep-disordered breathing associated with PD [171]. Tumor necrosis factor-α-induced protein-8 like-2 (TIPE2) has been postulated as a good prognosis biomarker [238]. However, other proteins such as DJ-1 were not considered good biomarkers since their levels did not differ between PD and HC [237].
Regarding plasma samples from PD patients, untargeted studies revealed IgGκL and amyloid P components, which were differentially expressed in comparison to HC [253]. Among targeted proteomic studies in PD, α-synuclein was studied, but its levels were not evaluable [254]. The inflammatory pathway was also evaluated by means of CRP that has been postulated as a potential prognosis biomarker for motor deterioration [136, 137]. In addition, transferrin decreased levels found in PD patients seemed to be important in associated tremors [255]. In whole blood samples, untargeted proteomic studies allowed the development of protein signatures that could differentiate between PD and HC with great accuracy [144, 247, 256]. From blood targeted analysis, Kitamura et al. [257] found ApoA1 as a potential progression biomarker. In addition, heat shock cognate protein 70 kDa showed reduced levels in PD [143]. Meanwhile oxidized DJ-1 protein allowed the differential diagnosis of PD [258].
In general, some proteins have been identified as promising biomarkers for AD/PD diagnosis and prognosis, as well as different related physiological mechanisms involved in disease development. Nevertheless, further studies are required to validate some proteins and advance physiological knowledge.
Some proteins (sAPPβ, Aβ38, Aβ40, aβ42, CRP, interleukins) are more related to AD, probably due to their relationship with amyloid pathologies, as well as with inflammation pathways, and they could be developed as diagnostic biomarkers. However, other proteins (Rab35, GFAP, NFs, transferrin) are more related to PD, and they could show some relationship with associated tremors.
3.3. Specific Biomarkers for AD or PD Diagnosis
Some miRNAs and proteins have been postulated as potential diagnostic tools. However, most of these studies compared AD or PD patients with HC. There is a lack of studies about differential diagnosis among similar pathologies to identify specific AD or PD biomarkers.
Nevertheless, some studies (mostly targeted) analyzed the specificity of the proposed miRNA biomarkers for one of these specific neurodegenerative diseases. Sheinerman et al. [46] studied 37 brain-enriched miRNAs in different neurological disorders, including AD and PD, analyzing miRNA pairs for detection and differentiation of these diseases, but no specific miRNA was found for AD or PD. As mentioned before, miR-107 has been proposed as one of the most promising miRNAs associated with AD. Sheinerman et al. [46] included this miRNA within its miRNAs selection, although no conclusive results about its specificity for AD were presented. Even more, on a targeted assay for 9 apoptosis miRNAs, including miR-107, Piscopo et al. [36] studied potential biomarkers for differential diagnosis in FTD in the Italian population. However, only miR127-3p showed significant differences between FTD and HC, so only this miRNA was determined in samples from AD patients, proposing this miRNA as a potential biomarker for differential diagnosis in FTD [36]. Therefore, if miR-107 could be a good candidate for differential diagnosis in AD vs. FTD remains to be determined. Cosin-Tomas et al. [47] analyzed a set of 10 plasma miRNAs for AD using PD samples as specificity control and proposed miR34a and miR-545-3p as candidate biomarkers. However, these results were not confirmed in the validation cohort [47]. Also, miR-384, which regulates the expression of APP and BACE1 [259], might discriminate between AD and other neurological diseases, such as VaD and Parkinson’s disease with dementia [43]. However, these results were not confirmed by other authors. The miRNA 29 family (miR-29a, miR-29b-1, miR-29b-2, miR-29c) regulates neuronal maturation [260] and dendritic spine morphology [261], and its deregulation has been related to various neurological disorders, including AD [262] and PD [61, 76, 263, 264]. In this sense, Sheinerman et al. [46] included miR-29a in different miRNAs pairs for both AD and PD. Conversely, Bai et al. [44] compared the expression profile of 29 miRNAs families in serum from patients with PD, AD, and HC. In conclusion, the serum levels of miR-29a and miR-29c were significantly decreased in PD patients, reducing with disease severity. Furthermore, changes of miR-29s in PD serum were specific, as the miR-29 levels in AD serum did not differ from HC [44]. However, further studies are required to confirm miR-29s specificity for PD. Barbagallo et al. [265] define two specific miRNAs signatures for AD-like and PD-like disorders: one for AD and VaD (miR-34b, miR-125b, miR-130b) and another for PD and Vascular parkinsonism (VP) (let-7d, miR-15b, miR-24, miR-142-3p, miR-181c, miR-222). However, according to different authors, miR-34b [51, 58], miR-125b and miR-130b [57] were included in PD miRNAs signatures, and let-7d [40, 82], miR142-3p [33], miR-181c [35] and miR-222 [52] were differentially expressed in AD patients. Patil et al. [42] proposed a 3 miRNA signature (miR-335-5p, miR-3613-3p, mir-6865-3p) specific for PD in an untargeted study since they exhibited different characteristics in AD serum samples. Also, miR-335-5p was predicted to target α-synuclein [42], and it was also included in another three miRNA signature for PD focused on miRNAs that target LRRK2 [62]. However, although miR-3613-3p was predicted to target LRRK2 [42], it was also selected, in combination with other miRNAs, for AD signatures [32, 41]. Similarly, mir-6865-3p was included in a 78 miRNA signature for AD [30]. Therefore, the specificity of these miRNAs for PD remains unclear. On the other hand, different authors have analyzed the specificity of a proposed biomarker for PD [45, 48]. Jin et al. [45] proposed miR-520d-5p, which regulates ceruloplasmin expression, as a specific biomarker for PD in a cohort with other neurodegenerative diseases (AD and multiple system atrophy (MSA)). However, its expression did not correlate with disease severity or the motor phenotype [45]. Based on human miRNA-mRNA network, Yang et al. [48] selected miR-105-5p as a putative biomarker for PD and evaluated its specificity in plasma samples from different neurological disorders, concluding that the plasma miR-105-5p expression level may be able to differentiate idiopathic PD from other types of parkinsonism, ET and other neurodegenerative diseases, such as AD [48]. Besides, no other authors have confirmed miR-520d-5p nor miR-105-5p as specific biomarkers for PD. In conclusion, it has not been demonstrated in a reliable manner that any of the proposed miRNAs are specific for AD or PD.
Regarding proteins, a few studies focused on differences between AD and PD. In this sense, tubulin and tau proteins showed differences between these pathologies in serum [266]. Moreover, brain- and heart-type fatty acid-binding proteins were found to be increased in 35% of PD patients, 29% of AD patients, and 24% of other cognitive disorders [267]. An untargeted blood analysis showed monoamine oxidase B (MAO-B) and tropomyosin 1 as good diagnosis biomarkers for AD [268]. However, in spite of the fact that MAO-B may be useful in distinguishing AD from non-demented PD and healthy individuals, its levels were similar between AD and demented PD patients [170]. Also, PD could be differentiated from schizophrenic patients by means of serum ApoE levels [249]. Meanwhile, oxidized DJ-1 protein allowed a differential diagnosis for PD [258]. In addition, Rab35 is differentially expressed in PD and HC and in other parkinsonian disorders [251]. However, GFAP and NfL levels were higher in PD compared to HC, but they did not allow differentiation from acute cerebral infarction [248]. In the same sense, ApoE levels could discriminate PD from schizophrenia [249].
Most of the potential biomarkers identified in the reviewed studies do not show specificity for AD or PD. However, further studies, with participants clearly identified, are required to assay some miRNAs and proteins as promising specific biomarkers.
3.4. Non-invasive Samples for AD and PD Diagnosis
Recently, an important research field in new diagnosis tools from minimally non-invasive samples is increasing. It constitutes an interesting alternative for AD since it would avoid current invasive diagnosis tools. In this sense, some studies have focused on the identification of potential biomarkers from urine, saliva and tears samples, especially in AD. Targeted studies found higher levels of Aβ42 [145, 146, 269] and reduced levels of lactoferrin [270] in saliva from AD patients compared to HC. In addition, α-synuclein and DJ-1 were found to be elevated in PD patients [147, 148]. Also, Alzheimer-associated neuronal thread protein has been postulated as a good biomarker for AD and MCI in urine samples [149, 150], as well as MCP-1 [151]. Moreover, tears could be a promising sample to study neurodegenerative diseases [83]. Specifically, an untargeted study found different proteins, such as lipocalin-1, lysozyme-C, and lacritin, as potential diagnosis biomarkers [271], as well as some proteins from the S100 superfamily [153].
Regarding miRNAs, miR-153 and miR-223, regulators of α-synuclein [84], and miR-874 and miR-145-3p, which might regulate DJ-1 [272], were proposed as salivary candidate biomarkers for PD. However, as mentioned before, miR-223 was previously associated with AD in serum [31]. Also, miR-200b-5p and elongation initiation factor 4E have been proposed as candidate biomarkers for AD from an untargeted assay in tears [83]. In addition, Youn et al. [273] found different levels of urinary neural thread protein between AD and HC groups, which permitted differentiation of AD from PD.
Among non-invasive samples, it is important to highlight that recent research is focusing on tears and urine as potential samples for early AD/PD diagnosis, as well as for the disease follow-up. It would advance disease progression assessment and personalized medicine development.
CONCLUSION
Some studies evaluated epigenomics and proteomics profiles in AD and PD minimally invasive samples, focusing on the search for early biomarkers for diagnosis. Among epigenomics studies, most of them focused on miRNAs as candidate biomarkers, such as miR-342-3p, miR-107, miR-106a-5p, miR-106b-5p, miR-195, and miR-19b. In fact, some AD and PD-related miRNAs could be involved in neuronal impairment. However, few of them may be AD or PD specific. Among proteomics studies, they highlighted the importance of coagulation, inflammation, and oxidative stress pathways (CRP, interleukins, necrosis factors, transferrin, GFAP, neurofilaments). Some AD-related proteins showed a relationship with amyloid pathologies, and they could be developed as diagnostic biomarkers. However, other proteins could show some relationship with associated tremors in PD. Nevertheless, there is a general lack of clinical validation studies to allow the use of these potential biomarkers in clinical practice. In addition, recent research focusing on the use of non-invasive samples is required in order to improve the assessment of disease progression.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- aMCI
Amnestic mild cognitive impairment
- APO
Apolipoprotein
- AUC
Area under the ROC (receiver operating characteristic) curve
- Aβ
β-amyloid protein
- BACE1
β-APP cleaving enzyme 1
- CNS
Central Nervous System
- CRP
C Reactive Protein
- CSF
Cerebrospinal fluid
- DAT
Dementia of Alzheimer-type
- DLB
Dementia with Lewy Bodies
- ELISA
Enzyme-Linked Immuno-Sorbent Assay
- ET
Essential tremor
- FTD
Frontotemporal dementia
- GFAP
Glial fibrillary acidic protein
- HC
Healthy control
- MALDI-TOF
Matrix-assisted laser desorption/ionization-time of flight
- MCI
Mild cognitive impairment
- MG
Myasthenia gravis
- miRNA
Micro ribonucleic acid
- MMSE
Mini-Mental State Examination
- MND
Motor neuron disease
- MS
Multiple sclerosis
- MSA
Multiple system atrophy
- NA
Not available
- NfL
Neurofilament Light
- NGS
Next generation sequencing
- p-tau
Phosphorylated tau protein
- PAD
Preclinical Alzheimer’s disease
- PBMC
Peripheral blood mononuclear cells
- PD
Parkinson’s disease
- PDD
Parkinson disease with dementia
- PZP
Pregnancy zone protein
- RBD
Rapid eye movement sleep behavior disorder
- RLS
Restless legs syndrome
- SELDI-TOF
Surface-enhanced laser desorption/ionization-time of flight
- SOMA
Slow off-rate modified aptamer
- t-tau
Total tau protein
- TNF-α
Tumor necrosis factor-α
- VaD
Vascular Dementia
- VD
Vascular disease
- VP
Vascular parkinsonism
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
CC-P acknowledges a postdoctoral “Miguel Servet” grant CP16/00082, and a FIS PI19/00570 grant from the Health Institute Carlos III (Spanish Ministry of Economy, Industry and Innovation).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
APPENDIX A
Epigenomics
((Alzheimer[Title/Abstract] OR alzheimer's[Title/Abstract] OR Parkinson[Title/Abstract] OR Parkinson's[Title/Abstract]) AND (biomarker OR biomarkers) AND (miRNA OR DNA methylation OR histone OR chromatin) AND (“2015”[Date - Publication]: “3000”[Date - Publication]) AND (english[Language] OR spanish[Language]))
Proteomics
((Alzheimer[Title/Abstract] OR alzheimer's[Title/Abstract] OR Parkinson[Title/Abstract] OR Parkinson's[Title/Abstract]) AND (biomarker OR biomarkers) AND (protein[Title] OR proteomics)) AND (“2010”[Date - Publication]: “3000”[Date - Publication]) AND (english[Language] OR Spanish [Language])).
REFERENCES
- 1.Häggström J., Rosenhall U., Hederstierna C., Östberg P., Idrizbegovic E. A Longitudinal Study of Peripheral and Central Auditory Function in Alzheimer’s Disease and in Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. Extra. 2018;8(3):393–401. doi: 10.1159/000493340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen M-C., Chan L.L., Tan L.C.S., Tan E.K. Mild cognitive impairment in Parkinson’s disease: a distinct clinical entity? Transl. Neurodegener. 2017;6:24. doi: 10.1186/s40035-017-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe D.J. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004;6(11):1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 4.Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi K., Tanji K., Odagiri S., Miki Y., Mori F., Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013;47(2):495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 6.Kempuraj D., Thangavel R., Natteru P.A., Selvakumar G.P., Saeed D., Zahoor H., Zaheer S., Iyer S.S., Zaheer A. Neuroinflammation Induces Neurodegeneration. . J. Neurol. Neurosurg. 2016. [PMC free article] [PubMed]
- 7.Haider A., Sanchez-Manso J.C. Dementia. Treasure Island, FL: Lewy Body; 2018. [Google Scholar]
- 8.Kansal K., Irwin D.J. The use of cerebrospinal fluid and neuropathologic studies in neuropsychiatry practice and research. Psychiatr. Clin. North Am. 2015;38(2):309–322. doi: 10.1016/j.psc.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beach T.G., Adler C.H., Sue L.I., Serrano G., Shill H.A., Walker D.G., Lue L., Roher A.E., Dugger B.N., Maarouf C., Birdsill A.C., Intorcia A., Saxon-Labelle M., Pullen J., Scroggins A., Filon J., Scott S., Hoffman B., Garcia A., Caviness J.N., Hentz J.G., Driver-Dunckley E., Jacobson S.A., Davis K.J., Belden C.M., Long K.E., Malek-Ahmadi M., Powell J.J., Gale L.D., Nicholson L.R., Caselli R.J., Woodruff B.K., Rapscak S.Z., Ahern G.L., Shi J., Burke A.D., Reiman E.M., Sabbagh M.N. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35(4):354–389. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel H., Bürger K., Teipel S.J., Bokde A.L.W., Zetterberg H., Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4(1):38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Schmand B., Huizenga H.M., van Gool W.A. Meta-analysis of CSF and MRI biomarkers for detecting preclinical Alzheimer’s disease. Psychol. Med. 2010;40(1):135–145. doi: 10.1017/S0033291709991516. [DOI] [PubMed] [Google Scholar]
- 12.Kang S., Jeong H., Baek J.H., Lee S.J., Han S.H., Cho H.J., Kim H., Hong H.S., Kim Y.H., Yi E.C., Seo S.W., Na D.L., Hwang D., Mook-Jung I. PiB-PET Imaging-Based Serum Proteome Profiles Predict Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimers Dis. 2016;53(4):1563–1576. doi: 10.3233/JAD-160025. [DOI] [PubMed] [Google Scholar]
- 13.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., Halliday G., Goetz C.G., Gasser T., Dubois B., Chan P., Bloem B.R., Adler C.H., Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 14.Berg D., Postuma R.B., Adler C.H., Bloem B.R., Chan P., Dubois B., Gasser T., Goetz C.G., Halliday G., Joseph L., Lang A.E., Liepelt-Scarfone I., Litvan I., Marek K., Obeso J., Oertel W., Olanow C.W., Poewe W., Stern M., Deuschl G. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015;30(12):1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 15.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017:18. doi: 10.1038/nrn.2017.91. [DOI] [PubMed] [Google Scholar]
- 16.Boyko A.A., Troyanova N.I., Kovalenko E.I., Sapozhnikov A.M. Similarity and Differences in Inflammation-Related Characteristics of the Peripheral Immune System of Patients with Parkinson’s and Alzheimer’s Diseases. Int. J. Mol. Sci. 2017;18(12):E2633. doi: 10.3390/ijms18122633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim, Derek H K, Maher E.R. DNA methylation: a form of epigenetic control of gene expression. Obstet. Gynaecol. 2010;12:37–42. doi: 10.1576/toag.12.1.037.27556. [DOI] [Google Scholar]
- 18.Sadakierska-Chudy A., Filip M. A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotox. Res. 2015;27(2):172–197. doi: 10.1007/s12640-014-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado-Morales R., Agís-Balboa R.C., Esteller M., Berdasco M. Epigenetic mechanisms during ageing and neurogenesis as novel therapeutic avenues in human brain disorders. Clin. Epigenetics. 2017;9:67. doi: 10.1186/s13148-017-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berdasco M., Esteller M. Clinical epigenetics: seizing opportunities for translation. Nat. Rev. Genet. 2018 doi: 10.1038/s41576-018-0074-2. [DOI] [PubMed] [Google Scholar]
- 21.Ai S.X., Xu Q., Hu Y.C., Song C.Y., Guo J.F., Shen L., Wang C.R., Yu R.L., Yan X.X., Tang B.S. Hypomethylation of SNCA in blood of patients with sporadic Parkinson’s disease. J. Neurol. Sci. 2014;337(1-2):123–128. doi: 10.1016/j.jns.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Marques S.C.F., Lemos R., Ferreiro E., Martins M., de Mendonça A., Santana I., Outeiro T.F., Pereira C.M.F. Epigenetic regulation of BACE1 in Alzheimer’s disease patients and in transgenic mice. Neuroscience. 2012;220:256–266. doi: 10.1016/j.neuroscience.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Mut J.V., Aso E., Heyn H., Matsuda T., Bock C., Ferrer I., Esteller M. Promoter hypermethylation of the phosphatase DUSP22 mediates PKA-dependent TAU phosphorylation and CREB activation in Alzheimer’s disease. Hippocampus. 2014;24(4):363–368. doi: 10.1002/hipo.22245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolia V., Fuso A., Cavallaro R.A., Di Luzio A., Scarpa S. B vitamin deficiency promotes tau phosphorylation through regulation of GSK3beta and PP2A. J. Alzheimers Dis. 2010;19(3):895–907. doi: 10.3233/JAD-2010-1284. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y., Liu R., Terpstra E., Wang Y., Qiao F., Wang J., Tong Y., Pan B. Dysregulation and diagnostic potential of microRNA in Alzheimer’s disease. J. Alzheimers Dis. 2016;49(1):1–12. doi: 10.3233/JAD-150451. [DOI] [PubMed] [Google Scholar]
- 26.Roser A.E., Caldi Gomes L., Schünemann J., Maass F., Lingor P. Circulating miRNAs as Diagnostic Biomarkers for Parkinson’s Disease. Front. Neurosci. 2018;12:625. doi: 10.3389/fnins.2018.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yılmaz S.G., Erdal M.E., Özge A.A., Sungur M.A. Can Peripheral MicroRNA Expression Data Serve as Epigenomic (Upstream) Biomarkers of Alzheimer’s Disease? OMICS. 2016;20(8):456–461. doi: 10.1089/omi.2016.0099. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura Y., Usami R., Ichihara S., Kida H., Satoh M., Tomimoto H., Murata M., Oikawa S. Plasma protein profiling for potential biomarkers in the early diagnosis of Alzheimer’s disease. Neurol. Res. 2017;39(3):231–238. doi: 10.1080/01616412.2017.1281195. [DOI] [PubMed] [Google Scholar]
- 29.Alberio T., Bucci E.M., Natale M., Bonino D., Di Giovanni M., Bottacchi E., Fasano M. Parkinson’s disease plasma biomarkers: an automated literature analysis followed by experimental validation. J. Proteomics. 2013;90:107–114. doi: 10.1016/j.jprot.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Shigemizu D., Akiyama S., Asanomi Y., Boroevich K.A., Sharma A., Tsunoda T., Matsukuma K., Ichikawa M., Sudo H., Takizawa S., Sakurai T., Ozaki K., Ochiya T., Niida S. Risk prediction models for dementia constructed by supervised principal component analysis using miRNA expression data. Commun. Biol. 2019;2:77. doi: 10.1038/s42003-019-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia L-H., Liu Y-N. Downregulated serum miR-223 servers as biomarker in Alzheimer’s disease. Cell Biochem. Funct. 2016;34(4):233–237. doi: 10.1002/cbf.3184. [DOI] [PubMed] [Google Scholar]
- 32.Lugli G., Cohen A.M., Bennett D.A., Shah R.C., Fields C.J., Hernandez A.G., Smalheiser N.R. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS One. 2015;10(10):e0139233. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagaraj S., Laskowska-Kaszub K., Dębski K.J., Wojsiat J., Dąbrowski M., Gabryelewicz T., Kuźnicki J., Wojda U. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer’s disease patients from non-demented subjects. Oncotarget. 2017;8(10):16122–16143. doi: 10.18632/oncotarget.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørensen S.S., Nygaard A-B., Christensen T. miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia - an exploratory study. Transl. Neurodegener. 2016;5:6. doi: 10.1186/s40035-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siedlecki-Wullich D., Català-Solsona J., Fábregas C., Hernández I., Clarimon J., Lleó A., Boada M., Saura C.A., Rodríguez-Álvarez J., Miñano-Molina A.J. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2019;11(1):46. doi: 10.1186/s13195-019-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piscopo P., Grasso M., Puopolo M., D’Acunto E., Talarico G., Crestini A., Gasparini M., Campopiano R., Gambardella S., Castellano A.E., Bruno G., Denti M.A., Confaloni A. Circulating miR-127-3p as a Potential Biomarker for Differential Diagnosis in Frontotemporal Dementia. J. Alzheimers Dis. 2018;65(2):455–464. doi: 10.3233/JAD-180364. [DOI] [PubMed] [Google Scholar]
- 37.Wang T., Chen K., Li H., Dong S., Su N., Liu Y., Cheng Y., Dai J., Yang C., Xiao S. The feasibility of utilizing plasma MiRNA107 and BACE1 messenger RNA gene expression for clinical diagnosis of amnestic mild cognitive impairment. J. Clin. Psychiatry. 2015;76(2):135–141. doi: 10.4088/JCP.13m08812. [DOI] [PubMed] [Google Scholar]
- 38.Ren R-J., Zhang Y-F., Dammer E.B., Zhou Y., Wang L-L., Liu X-H., Feng B-L., Jiang G-X., Chen S-D., Wang G., Cheng Q. Peripheral Blood MicroRNA Expression Profiles in Alzheimer’s Disease: Screening, Validation, Association with Clinical Phenotype and Implications for Molecular Mechanism. Mol. Neurobiol. 2016;53(8):5772–5781. doi: 10.1007/s12035-015-9484-8. [DOI] [PubMed] [Google Scholar]
- 39.Manzine P.R., Pelucchi S., Horst M.A., Vale F.A.C., Pavarini S.C.I., Audano M., Mitro N., Di Luca M., Marcello E., Cominetti M.R. microRNA 221 Targets ADAM10 mRNA and is Downregulated in Alzheimer’s Disease. J. Alzheimers Dis. 2018;61(1):113–123. doi: 10.3233/JAD-170592. [DOI] [PubMed] [Google Scholar]
- 40.Keller A., Backes C., Haas J., Leidinger P., Maetzler W., Deuschle C., Berg D., Ruschil C., Galata V., Ruprecht K., Stähler C., Würstle M., Sickert D., Gogol M., Meder B., Meese E. Validating Alzheimer’s disease micro RNAs using next-generation sequencing. Alzheimers Dement. 2016;12(5):565–576. doi: 10.1016/j.jalz.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S., Vijayan M., Reddy P.H. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum. Mol. Genet. 2017;26(19):3808–3822. doi: 10.1093/hmg/ddx267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patil K.S., Basak I., Dalen I., Hoedt E., Lange J., Lunde K.A., Liu Y., Tysnes O-B., Forsgren L., Aarsland D., Neubert T.A., Larsen J.P., Alves G., Møller S.G. Combinatory microRNA serum signatures as classifiers of Parkinson’s disease. Parkinsonism Relat. Disord. 2019;64:202–210. doi: 10.1016/j.parkreldis.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Yang T.T., Liu C.G., Gao S.C., Zhang Y., Wang P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018;31(2):87–96. doi: 10.3967/bes2018.011. [DOI] [PubMed] [Google Scholar]
- 44.Bai X., Tang Y., Yu M., Wu L., Liu F., Ni J., Wang Z., Wang J., Fei J., Wang W., Huang F., Wang J. Downregulation of blood serum microRNA 29 family in patients with Parkinson’s disease. Sci. Rep. 2017;7(1):5411. doi: 10.1038/s41598-017-03887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin L., Wan W., Wang L., Wang C., Xiao J., Zhang F., Zhao J., Wang J., Zhan C., Zhong C. Elevated microRNA-520d-5p in the serum of patients with Parkinson’s disease, possibly through regulation of cereloplasmin expression. Neurosci. Lett. 2018;687:88–93. doi: 10.1016/j.neulet.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Sheinerman K.S., Toledo J.B., Tsivinsky V.G., Irwin D., Grossman M., Weintraub D., Hurtig H.I., Chen-Plotkin A., Wolk D.A., McCluskey L.F., Elman L.B., Trojanowski J.Q., Umansky S.R. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimers Res. Ther. 2017;9(1):89. doi: 10.1186/s13195-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosín-Tomás M., Antonell A., Lladó A., Alcolea D., Fortea J., Ezquerra M., Lleó A., Martí M.J., Pallàs M., Sanchez-Valle R., Molinuevo J.L., Sanfeliu C., Kaliman P. Plasma miR-34a-5p and miR-545-3p as Early Biomarkers of Alzheimer’s Disease: Potential and Limitations. Mol. Neurobiol. 2017;54(7):5550–5562. doi: 10.1007/s12035-016-0088-8. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z., Li T., Cui Y., Li S., Cheng C., Shen B., Le W. Elevated Plasma microRNA-105-5p Level in Patients With Idiopathic Parkinson’s Disease: A Potential Disease Biomarker. Front. Neurosci. 2019;13:218. doi: 10.3389/fnins.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding H., Huang Z., Chen M., Wang C., Chen X., Chen J., Zhang J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Parkinsonism Relat. Disord. 2016;22:68–73. doi: 10.1016/j.parkreldis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Cao X-Y., Lu J-M., Zhao Z-Q., Li M-C., Lu T., An X-S., Xue L-J. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci. Lett. 2017;644:94–99. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 51.Ma W., Li Y., Wang C., Xu F., Wang M., Liu Y. Serum miR-221 serves as a biomarker for Parkinson’s disease. Cell Biochem. Funct. 2016;34(7):511–515. doi: 10.1002/cbf.3224. [DOI] [PubMed] [Google Scholar]
- 52.Zeng Q., Zou L., Qian L., Zhou F., Nie H., Yu S., Jiang J., Zhuang A., Wang C., Zhang H. Expression of microRNA-222 in serum of patients with Alzheimer’s disease. Mol. Med. Rep. 2017;16(4):5575–5579. doi: 10.3892/mmr.2017.7301. [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Santiago R., Iranzo A., Gaig C., Serradell M., Fernández M., Tolosa E., Santamaría J., Ezquerra M. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann. Neurol. 2015;77(5):895–901. doi: 10.1002/ana.24384. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., Gao C., Sun Q., Pan H., Huang P., Ding J., Chen S. MicroRNA-4639 Is a Regulator of DJ-1 Expression and a Potential Early Diagnostic Marker for Parkinson’s Disease. Front. Aging Neurosci. 2017;9:232. doi: 10.3389/fnagi.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwienbacher C., Foco L., Picard A., Corradi E., Serafin A., Panzer J., Zanigni S., Blankenburg H., Facheris M.F., Giannini G., Falla M., Cortelli P., Pramstaller P.P., Hicks A.A. Plasma and White Blood Cells Show Different miRNA Expression Profiles in Parkinson’s Disease. J. Mol. Neurosci. 2017;62(2):244–254. doi: 10.1007/s12031-017-0926-9. [DOI] [PubMed] [Google Scholar]
- 56.Yang Z., Li T., Li S., Wei M., Qi H., Shen B., Chang R.C-C., Le W., Piao F. Altered Expression Levels of MicroRNA-132 and Nurr1 in Peripheral Blood of Parkinson’s Disease: Potential Disease Biomarkers. ACS Chem. Neurosci. 2019;10(5):2243–2249. doi: 10.1021/acschemneuro.8b00460. [DOI] [PubMed] [Google Scholar]
- 57.Chen L., Yang J., Lü J., Cao S., Zhao Q., Yu Z. Identification of aberrant circulating miRNAs in Parkinson’s disease plasma samples. Brain Behav. 2018;8(4):e00941. doi: 10.1002/brb3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X., Yang R., Hu B-L., Lu P., Zhou L-L., He Z-Y., Wu H-M., Zhu J-H. Reduced Circulating Levels of miR-433 and miR-133b Are Potential Biomarkers for Parkinson’s Disease. Front. Cell. Neurosci. 2017;11:170. doi: 10.3389/fncel.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N., Pan X., Zhang J., Ma A., Yang S., Ma J., Xie A. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017;38(5):761–767. doi: 10.1007/s10072-017-2841-9. [DOI] [PubMed] [Google Scholar]
- 60.Caggiu E., Paulus K., Mameli G., Arru G., Sechi G.P., Sechi L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci. 2018;13:1–4. doi: 10.1016/j.ensci.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serafin A., Foco L., Zanigni S., Blankenburg H., Picard A., Zanon A., Giannini G., Pichler I., Facheris M.F., Cortelli P., Pramstaller P.P., Hicks A.A., Domingues F.S., Schwienbacher C. Overexpression of blood microRNAs 103a, 30b, and 29a in L-dopa-treated patients with PD. Neurology. 2015;84(7):645–653. doi: 10.1212/WNL.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 62.Yılmaz S.G., Geyik S., Neyal A.M., Soko N.D., Bozkurt H., Dandara C. Hypothesis: Do miRNAs Targeting the Leucine-Rich Repeat Kinase 2 Gene (LRRK2) Influence Parkinson’s Disease Susceptibility? OMICS. 2016;20(4):224–228. doi: 10.1089/omi.2016.0040. [DOI] [PubMed] [Google Scholar]
- 63.Guo R., Fan G., Zhang J., Wu C., Du Y., Ye H., Li Z., Wang L., Zhang Z., Zhang L., Zhao Y., Lu Z.A. 9-microRNA Signature in Serum Serves as a Noninvasive Biomarker in Early Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2017;60(4):1365–1377. doi: 10.3233/JAD-170343. [DOI] [PubMed] [Google Scholar]
- 64.Ansari A., Maffioletti E., Milanesi E., Marizzoni M., Frisoni G.B., Blin O., Richardson J.C., Bordet R., Forloni G., Gennarelli M., Bocchio-Chiavetto L. PharmaCog Consortium. miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging. 2019;82:102–109. doi: 10.1016/j.neurobiolaging.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Yang Q., Zhao Q., Yin Y. miR-133b is a potential diagnostic biomarker for Alzheimer’s disease and has a neuroprotective role. Exp. Ther. Med. 2019;18(4):2711–2718. doi: 10.3892/etm.2019.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiu C.C., Yeh T.H., Chen R.S., Chen H.C., Huang Y.Z., Weng Y.H., Cheng Y.C., Liu Y.C., Cheng A.J., Lu Y.C., Chen Y.J., Lin Y.W., Hsu C.C., Chen Y.L., Lu C.S., Wang H.L. Upregulated Expression of MicroRNA-204-5p Leads to the Death of Dopaminergic Cells by Targeting DYRK1A-Mediated Apoptotic Signaling Cascade. Front. Cell. Neurosci. 2019;13:399. doi: 10.3389/fncel.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravanidis S., Bougea A., Papagiannakis N., Maniati M., Koros C., Simitsi A.M., Bozi M., Pachi I., Stamelou M., Paraskevas G.P., Kapaki E., Moraitou M., Michelakakis H., Stefanis L., Doxakis E. Circulating Brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson’s disease. Mov. Disord. 2020;35(3):457–467. doi: 10.1002/mds.27928. [DOI] [PubMed] [Google Scholar]
- 68.Ravanidis S., Bougea A., Papagiannakis N., Koros C., Simitsi A.M., Pachi I., Breza M., Stefanis L., Doxakis E. Validation of differentially expressed brain-enriched microRNAs in the plasma of PD patients. Ann. Clin. Transl. Neurol. 2020;7(9):1594–1607. doi: 10.1002/acn3.51146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Souza V.C., Morais G.S., Jr, Henriques A.D., Machado-Silva W., Perez D.I.V., Brito C.J., Camargos E.F., Moraes C.F., Nóbrega O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients With Late-Onset Alzheimer Disease. Am. J. Alzheimers Dis. Other Demen. 2020;35:1533317520911573. doi: 10.1177/1533317520911573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baghi M., Rostamian Delavar M., Yadegari E., Peymani M., Pozo D., Hossein Nasr-Esfahani M., Ghaedi K. Modified level of miR-376a is associated with Parkinson’s disease. J. Cell. Mol. Med. 2020;24(4):2622–2634. doi: 10.1111/jcmm.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H., Yu L., Li M., Chen X., Tian Q., Jiang Y., Li N. MicroRNA-150 serves as a diagnostic biomarker and is involved in the inflammatory pathogenesis of Parkinson’s disease. Mol. Genet. Genomic Med. 2020;8(4):e1189. doi: 10.1002/mgg3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madadi S., Saidijam M., Yavari B., Soleimani M. Downregulation of serum miR-106b: a potential biomarker for Alzheimer disease. Arch. Physiol. Biochem. 2020;0:1–5. doi: 10.1080/13813455.2020.1734842. [DOI] [PubMed] [Google Scholar]
- 73.Hajjri S.N., Sadigh-Eteghad S., Mehrpour M., Moradi F., Shanehbandi D., Mehdizadeh M. Beta-Amyloid-Dependent miRNAs as Circulating Biomarkers in Alzheimer’s Disease: a Preliminary Report. J. Mol. Neurosci. 2020;70(6):871–877. doi: 10.1007/s12031-020-01511-0. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y., Xu J., Xu J., Cheng J., Jiao D., Zhou C., Dai Y., Chen Q. Lower Serum Levels of miR-29c-3p and miR-19b-3p as Biomarkers for Alzheimer’s Disease. Tohoku J. Exp. Med. 2017;242(2):129–136. doi: 10.1620/tjem.242.129. [DOI] [PubMed] [Google Scholar]
- 75.Wu H.Z.Y., Thalamuthu A., Cheng L., Fowler C., Masters C.L., Sachdev P., Mather K.A. the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Differential blood miRNA expression in brain amyloid imaging-defined Alzheimer’s disease and controls. Alzheimers Res. Ther. 2020;12(1):59. doi: 10.1186/s13195-020-00627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozdilek B., Demircan B. Serum microRNA expression levels in Turkish patients with Parkinson’s disease. Int. J. Neurosci. 2020;7454:1–9. doi: 10.1080/00207454.2020.1784165. [DOI] [PubMed] [Google Scholar]
- 77.De Felice B., Montanino C., Oliva M., Bonavita S., Di Onofrio V., Coppola C. MicroRNA Expression Signature in Mild Cognitive Impairment Due to Alzheimer’s Disease. Mol. Neurobiol. 2020;57(11):4408–4416. doi: 10.1007/s12035-020-02029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong H., Li J., Huang L., Chen X., Li D., Wang T., Hu C., Xu J., Zhang C., Zen K., Xiao S., Yan Q., Wang C., Zhang C.Y. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers. 2015;2015:625659. doi: 10.1155/2015/625659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong H., Wang C., Lu S., Yu C., Huang L., Feng W., Xu H., Chen X., Zen K., Yan Q. A panel of four decreased serum microRNAs as a novel biomarker for early Parkinson’s disease. . Biomarkers Biochem. Indic. Expo. response, susceptibility to Chem. . 2016;21:129–137. doi: 10.3109/1354750X.2015.1118544. [DOI] [PubMed] [Google Scholar]
- 80.Hara N., Kikuchi M., Miyashita A., Hatsuta H., Saito Y., Kasuga K., Murayama S., Ikeuchi T., Kuwano R. Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017;5(1):10. doi: 10.1186/s40478-017-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng L., Doecke J.D., Sharples R.A., Villemagne V.L., Fowler C.J., Rembach A., Martins R.N., Rowe C.C., Macaulay S.L., Masters C.L., Hill A.F. Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry. 2015;20(10):1188–1196. doi: 10.1038/mp.2014.127. [DOI] [PubMed] [Google Scholar]
- 82.Denk J., Oberhauser F., Kornhuber J., Wiltfang J., Fassbender K., Schroeter M.L., Volk A.E., Diehl-Schmid J., Prudlo J., Danek A., Landwehrmeyer B., Lauer M., Otto M., Jahn H., FTLDc study group Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS One. 2018;13(5):e0197329. doi: 10.1371/journal.pone.0197329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kenny A., Jiménez-Mateos E.M., Zea-Sevilla M.A., Rábano A., Gili-Manzanaro P., Prehn J.H.M., Henshall D.C., Ávila J., Engel T., Hernández F. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci. Rep. 2019;9(1):15437. doi: 10.1038/s41598-019-51837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cressatti M., Juwara L., Galindez J.M., Velly A.M., Nkurunziza E.S., Marier S., Canie O., Gornistky M., Schipper H.M. Salivary microR-153 and microR-223 Levels as Potential Diagnostic Biomarkers of Idiopathic Parkinson’s Disease. Mov. Disord. 2020;35(3):468–477. doi: 10.1002/mds.27935. [DOI] [PubMed] [Google Scholar]
- 85.Xu C., Liu G., Ji H., Chen W., Dai D., Chen Z., Zhou D., Xu L., Hu H., Cui W., Chang L., Zha Q., Li L., Duan S., Wang Q. Elevated methylation of OPRM1 and OPRL1 genes in Alzheimer’s disease. Mol. Med. Rep. 2018;18(5):4297–4302. doi: 10.3892/mmr.2018.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sao T., Yoshino Y., Yamazaki K., Ozaki Y., Mori Y., Ochi S., Yoshida T., Mori T., Iga J-I., Ueno S-I. TREM1 mRNA Expression in Leukocytes and Cognitive Function in Japanese Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018;64(4):1275–1284. doi: 10.3233/JAD-180418. [DOI] [PubMed] [Google Scholar]
- 87.Pihlstrøm L., Berge V., Rengmark A., Toft M. Parkinson’s disease correlates with promoter methylation in the α-synuclein gene. Mov. Disord. 2015;30(4):577–580. doi: 10.1002/mds.26073. [DOI] [PubMed] [Google Scholar]
- 88.Chen W., Ji H., Li L., Xu C., Zou T., Cui W., Xu S., Zhou X., Duan S., Wang Q. Significant association between GPR50 hypomethylation and AD in males. Mol. Med. Rep. 2019;20(2):1085–1092. doi: 10.3892/mmr.2019.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salcedo-Tacuma D., Melgarejo J.D., Mahecha M.F., Ortega-Rojas J., Arboleda-Bustos C.E., Pardo-Turriago R., Arboleda H. Differential Methylation Levels in CpGs of the BIN1 Gene in Individuals With Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2019;33(4):321–326. doi: 10.1097/WAD.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 90.Ozaki Y., Yoshino Y., Yamazaki K., Ochi S., Iga J.I., Nagai M., Nomoto M., Ueno S.I. DRD2 methylation to differentiate dementia with Lewy bodies from Parkinson’s disease. Acta Neurol. Scand. 2020;141(2):177–182. doi: 10.1111/ane.13186. [DOI] [PubMed] [Google Scholar]
- 91.Shao Y., Shaw M., Todd K., Khrestian M., D’Aleo G., Barnard P.J., Zahratka J., Pillai J., Yu C-E., Keene C.D., Leverenz J.B., Bekris L.M. DNA methylation of TOMM40-APOE-APOC2 in Alzheimer’s disease. J. Hum. Genet. 2018;63(4):459–471. doi: 10.1038/s10038-017-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozaki Y., Yoshino Y., Yamazaki K., Sao T., Mori Y., Ochi S., Yoshida T., Mori T., Iga J-I., Ueno S-I. DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J. Psychiatr. Res. 2017;92:74–80. doi: 10.1016/j.jpsychires.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi N., Shinagawa S., Nagata T., Shimada K., Shibata N., Ohnuma T., Kasanuki K., Arai H., Yamada H., Nakayama K., Kondo K. Usefulness of DNA Methylation Levels in COASY and SPINT1 Gene Promoter Regions as Biomarkers in Diagnosis of Alzheimer’s Disease and Amnestic Mild Cognitive Impairment. PLoS One. 2016;11(12):e0168816. doi: 10.1371/journal.pone.0168816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie B., Xu Y., Liu Z., Liu W., Jiang L., Zhang R., Cui D., Zhang Q., Xu S. Elevation of Peripheral BDNF Promoter Methylation Predicts Conversion from Amnestic Mild Cognitive Impairment to Alzheimer’s Disease: A 5-Year Longitudinal Study. J. Alzheimers Dis. 2017;56(1):391–401. doi: 10.3233/JAD-160954. [DOI] [PubMed] [Google Scholar]
- 95.Ji H., Wang Y., Jiang D., Liu G., Xu X., Dai D., Zhou X., Cui W., Li J., Chen Z., Li Y., Zhou D., Zha Q., Zhuo R., Jiang L., Liu Y., Shen L., Zhang B., Xu L., Hu H., Zhang Y., Yin H., Duan S., Wang Q. Elevated DRD4 promoter methylation increases the risk of Alzheimer’s disease in males. Mol. Med. Rep. 2016;14(3):2732–2738. doi: 10.3892/mmr.2016.5560. [DOI] [PubMed] [Google Scholar]
- 96.Shinagawa S., Kobayashi N., Nagata T., Kusaka A., Yamada H., Kondo K., Nakayama K. DNA methylation in the NCAPH2/LMF2 promoter region is associated with hippocampal atrophy in Alzheimer’s disease and amnesic mild cognitive impairment patients. Neurosci. Lett. 2016;629:33–37. doi: 10.1016/j.neulet.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 97.Mao W., Zhao C., Ding H., Liang K., Xue J., Chan P., Cai Y. Pyrosequencing analysis of methylation levels of clock genes in leukocytes from Parkinson’s disease patients. Neurosci. Lett. 2018;668:115–119. doi: 10.1016/j.neulet.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 98.Funahashi Y., Yoshino Y., Yamazaki K., Mori Y., Mori T., Ozaki Y., Sao T., Ochi S., Iga J-I., Ueno S-I. DNA methylation changes at SNCA intron 1 in patients with dementia with Lewy bodies. Psychiatry Clin. Neurosci. 2017;71(1):28–35. doi: 10.1111/pcn.12462. [DOI] [PubMed] [Google Scholar]
- 99.Kobayashi N., Shinagawa S., Nagata T., Shimada K., Shibata N., Ohnuma T., Kasanuki K., Arai H., Yamada H., Nakayama K., Kondo K. Development of Biomarkers Based on DNA Methylation in the NCAPH2/LMF2 Promoter Region for Diagnosis of Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. PLoS One. 2016;11(1):e0146449. doi: 10.1371/journal.pone.0146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobayashi N., Shinagawa S., Niimura H., Kida H., Nagata T., Tagai K., Shimada K., Oka N., Shikimoto R., Noda Y., Nakajima S., Mimura M., Shigeta M., Kondo K. Increased blood COASY DNA methylation levels a potential biomarker for early pathology of Alzheimer’s disease. Sci. Rep. 2020;10(1):12217. doi: 10.1038/s41598-020-69248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagata T., Kobayashi N., Ishii J., Shinagawa S., Nakayama R., Shibata N., Kuerban B., Ohnuma T., Kondo K., Arai H., Yamada H., Nakayama K. Association between DNA methylation of the BDNF promoter region and clinical presentation in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra. 2015;5(1):64–73. doi: 10.1159/000375367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma W., Zhou X., Ji H., Luo M., Liu G., Li J., Wang Q., Duan S. Population difference in the association of BDNF promoter methylation with mild cognitive impairment in the Xinjiang Uygur and Han populations. Psychiatry Res. 2015;229(3):926–932. doi: 10.1016/j.psychres.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Larsen F., Solheim J., Prydz H. A methylated CpG island 3′ in the apolipoprotein-E gene does not repress its transcription. Hum. Mol. Genet. 1993;2(6):775–780. doi: 10.1093/hmg/2.6.775. [DOI] [PubMed] [Google Scholar]
- 104.Yu C.E., Cudaback E., Foraker J., Thomson Z., Leong L., Lutz F., Gill J.A., Saxton A., Kraemer B., Navas P., Keene C.D., Montine T., Bekris L.M. Epigenetic signature and enhancer activity of the human APOE gene. Hum. Mol. Genet. 2013;22(24):5036–5047. doi: 10.1093/hmg/ddt354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma Y., Smith C.E., Lai C.Q., Irvin M.R., Parnell L.D., Lee Y.C., Pham L., Aslibekyan S., Claas S.A., Tsai M.Y., Borecki I.B., Kabagambe E.K., Berciano S., Ordovás J.M., Absher D.M., Arnett D.K. Genetic variants modify the effect of age on APOE methylation in the Genetics of Lipid Lowering Drugs and Diet Network study. Aging Cell. 2015;14(1):49–59. doi: 10.1111/acel.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foraker J., Millard S.P., Leong L., Thomson Z., Chen S., Keene C.D., Bekris L.M., Yu C.E., Fischer A. The APOE Gene is Differentially Methylated in Alzheimer’s Disease. J. Alzheimers Dis. 2015;48(3):745–755. doi: 10.3233/JAD-143060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thathiah A., De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12(2):73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- 108.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bertram L., McQueen M.B., Mullin K., Blacker D., Tanzi R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 110.Vasanthakumar A., Davis J.W., Idler K., Waring J.F., Asque E., Riley-Gillis B., Grosskurth S., Srivastava G., Kim S., Nho K., Nudelman K.N.H., Faber K., Sun Y., Foroud T.M., Estrada K., Apostolova L.G., Li Q.S., Saykin A.J. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Harnessing peripheral DNA methylation differences in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to reveal novel biomarkers of disease. Clin. Epigenetics. 2020;12(1):84. doi: 10.1186/s13148-020-00864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roubroeks J.A.Y., Smith A.R., Smith R.G., Pishva E., Ibrahim Z., Sattlecker M., Hannon E.J., Kłoszewska I., Mecocci P., Soininen H., Tsolaki M., Vellas B., Wahlund L.O., Aarsland D., Proitsi P., Hodges A., Lovestone S., Newhouse S.J., Dobson R.J.B., Mill J., van den Hove D.L.A., Lunnon K. An epigenome-wide association study of Alzheimer’s disease blood highlights robust DNA hypermethylation in the HOXB6 gene. Neurobiol. Aging. 2020;95:26–45. doi: 10.1016/j.neurobiolaging.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giampaolo A., Felli N., Diverio D., Morsilli O., Samoggia P., Breccia M., Lo Coco F., Peschle C., Testa U. Expression pattern of HOXB6 homeobox gene in myelomonocytic differentiation and acute myeloid leukemia. Leukemia. 2002;16(7):1293–1301. doi: 10.1038/sj.leu.2402532. [DOI] [PubMed] [Google Scholar]
- 113.Richter J., Appenzeller S., Ammerpohl O., Deuschl G., Paschen S., Brüggemann N., Klein C., Kuhlenbäumer G. No evidence for differential methylation of α-synuclein in leukocyte DNA of Parkinson’s disease patients. Mov. Disord. 2012;27(4):590–591. doi: 10.1002/mds.24907. [DOI] [PubMed] [Google Scholar]
- 114.Tan Y.Y., Wu L., Zhao Z.B., Wang Y., Xiao Q., Liu J., Wang G., Ma J.F., Chen S.D. Methylation of α-synuclein and leucine-rich repeat kinase 2 in leukocyte DNA of Parkinson’s disease patients. Parkinsonism Relat. Disord. 2014;20(3):308–313. doi: 10.1016/j.parkreldis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Song Y., Ding H., Yang J., Lin Q., Xue J., Zhang Y., Chan P., Cai Y. Pyrosequencing analysis of SNCA methylation levels in leukocytes from Parkinson’s disease patients. Neurosci. Lett. 2014;569:85–88. doi: 10.1016/j.neulet.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 116.McDonell K.E., van Wouwe N.C., Harrison M.B., Wylie S.A., Claassen D.O. Taq1A polymorphism and medication effects on inhibitory action control in Parkinson disease. Brain Behav. 2018;8(7):e01008. doi: 10.1002/brb3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller N.S., Chou K.L., Bohnen N.I., Müller M.L.T.M., Seidler R.D. Dopaminergic polymorphisms associated with medication responsiveness of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 2018;48:54–60. doi: 10.1016/j.parkreldis.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rieck M., Schumacher-Schuh A.F., Altmann V., Callegari-Jacques S.M., Rieder C.R.M., Hutz M.H. Association between DRD2 and DRD3 gene polymorphisms and gastrointestinal symptoms induced by levodopa therapy in Parkinson’s disease. Pharmacogenomics J. 2018;18(1):196–200. doi: 10.1038/tpj.2016.79. [DOI] [PubMed] [Google Scholar]
- 119.Rieck M., Schumacher-Schuh A.F., Altmann V., Francisconi C.L.M., Fagundes P.T.B., Monte T.L., Callegari-Jacques S.M., Rieder C.R.M., Hutz M.H. DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson’s disease patients. Pharmacogenomics. 2012;13(15):1701–1710. doi: 10.2217/pgs.12.149. [DOI] [PubMed] [Google Scholar]
- 120.Willis G.L. Parkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev. Neurosci. 2008;19(4-5):245–316. doi: 10.1515/REVNEURO.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- 121.Kaut O., Schmitt I., Tost J., Busato F., Liu Y., Hofmann P., Witt S.H., Rietschel M., Fröhlich H., Wüllner U. Epigenome-wide DNA methylation analysis in siblings and monozygotic twins discordant for sporadic Parkinson’s disease revealed different epigenetic patterns in peripheral blood mononuclear cells. Neurogenetics. 2017;18(1):7–22. doi: 10.1007/s10048-016-0497-x. [DOI] [PubMed] [Google Scholar]
- 122.Henderson-Smith A., Fisch K.M., Hua J., Liu G., Ricciardelli E., Jepsen K., Huentelman M., Stalberg G., Edland S.D., Scherzer C.R., Dunckley T., Desplats P. DNA methylation changes associated with Parkinson’s disease progression: outcomes from the first longitudinal genome-wide methylation analysis in blood. Epigenetics. 2019;14(4):365–382. doi: 10.1080/15592294.2019.1588682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao N., Jin L., Fei G., Zheng Z., Zhong C. Serum microRNA-133b is associated with low ceruloplasmin levels in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20(11):1177–1180. doi: 10.1016/j.parkreldis.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 124.Wang W.X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28(5):1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan L., Yu J.T., Tan M.S., Liu Q.Y., Wang H.F., Zhang W., Jiang T., Tan L. Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer’s disease. J. Alzheimers Dis. 2014;40(4):1017–1027. doi: 10.3233/JAD-132144. [DOI] [PubMed] [Google Scholar]
- 126.Wang G., van der Walt J.M., Mayhew G., Li Y.J., Züchner S., Scott W.K., Martin E.R., Vance J.M. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of α-synuclein. Am. J. Hum. Genet. 2008;82(2):283–289. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berg D., Schweitzer K.J., Leitner P., Zimprich A., Lichtner P., Belcredi P., Brüssel T., Schulte C., Maass S., Nägele T., Wszolek Z.K., Gasser T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease*. Brain. 2005;128(Pt 12):3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 128.Ozelius L.J., Senthil G., Saunders-Pullman R., Ohmann E., Deligtisch A., Tagliati M., Hunt A.L., Klein C., Henick B., Hailpern S.M., Lipton R.B., Soto-Valencia J., Risch N., Bressman S.B. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. . N. Engl. J. Med. . 2006;354(4):424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 129.Schmidt C., Becker H., Peter C., Lange K., Friede T., Zerr I. Plasma prion protein concentration and progression of Alzheimer disease. Prion. 2014;8(2):210–214. doi: 10.4161/pri.27964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Westwood S., Leoni E., Hye A., Lynham S., Khondoker M.R., Ashton N.J., Kiddle S.J., Baird A.L., Sainz-Fuertes R., Leung R., Graf J., Hehir C.T., Baker D., Cereda C., Bazenet C., Ward M., Thambisetty M., Lovestone S. Blood-Based Biomarker Candidates of Cerebral Amyloid Using PiB PET in Non-Demented Elderly. J. Alzheimers Dis. 2016;52(2):561–572. doi: 10.3233/JAD-151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao X., Lejnine S., Spond J., Zhang C., Ramaraj T.C., Holder D.J., Dai H., Weiner R., Laterza O.F. A candidate plasma protein classifier to identify Alzheimer’s disease. J. Alzheimers Dis. 2015;43(2):549–563. doi: 10.3233/JAD-141149. [DOI] [PubMed] [Google Scholar]
- 132.Varma V.R., Varma S., An Y., Hohman T.J., Seddighi S., Casanova R., Beri A., Dammer E.B., Seyfried N.T., Pletnikova O., Moghekar A., Wilson M.R., Lah J.J., O’Brien R.J., Levey A.I., Troncoso J.C., Albert M.S., Thambisetty M. Alpha-2 macroglobulin in Alzheimer’s disease: a marker of neuronal injury through the RCAN1 pathway. Mol. Psychiatry. 2017;22(1):13–23. doi: 10.1038/mp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muenchhoff J., Poljak A., Song F., Raftery M., Brodaty H., Duncan M., McEvoy M., Attia J., Schofield P.W., Sachdev P.S. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease across two independent cohorts. J. Alzheimers Dis. 2015;43(4):1355–1373. doi: 10.3233/JAD-141266. [DOI] [PubMed] [Google Scholar]
- 134.Choi J., Lee H-W., Suk K. Plasma level of chitinase 3-like 1 protein increases in patients with early Alzheimer’s disease. J. Neurol. 2011;258(12):2181–2185. doi: 10.1007/s00415-011-6087-9. [DOI] [PubMed] [Google Scholar]
- 135.Yang H., Lyutvinskiy Y., Herukka S-K., Soininen H., Rutishauser D., Zubarev R.A. Prognostic polypeptide blood plasma biomarkers of Alzheimer’s disease progression. J. Alzheimers Dis. 2014;40(3):659–666. doi: 10.3233/JAD-132102. [DOI] [PubMed] [Google Scholar]
- 136.Sawada H., Oeda T., Umemura A., Tomita S., Kohsaka M., Park K., Yamamoto K., Sugiyama H. Baseline C-Reactive Protein Levels and Life Prognosis in Parkinson Disease. PLoS One. 2015;10(7):e0134118. doi: 10.1371/journal.pone.0134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Umemura A., Oeda T., Yamamoto K., Tomita S., Kohsaka M., Park K., Sugiyama H., Sawada H. Baseline Plasma C-Reactive Protein Concentrations and Motor Prognosis in Parkinson Disease. PLoS One. 2015;10(8):e0136722. doi: 10.1371/journal.pone.0136722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williams S.M., Schulz P., Rosenberry T.L., Caselli R.J., Sierks M.R. Blood-Based Oligomeric and Other Protein Variant Biomarkers to Facilitate Pre-Symptomatic Diagnosis and Staging of Alzheimer’s Disease. J. Alzheimers Dis. 2017;58(1):23–35. doi: 10.3233/JAD-161116. [DOI] [PubMed] [Google Scholar]
- 139.Zabel M., Schrag M., Mueller C., Zhou W., Crofton A., Petersen F., Dickson A., Kirsch W.M. Assessing candidate serum biomarkers for Alzheimer’s disease: a longitudinal study. J. Alzheimers Dis. 2012;30(2):311–321. doi: 10.3233/JAD-2012-112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.García-Moreno J-M., Martín de Pablos A., García-Sánchez M-I., Méndez-Lucena C., Damas-Hermoso F., Rus M., Chacón J., Fernández E. May serum levels of advanced oxidized protein products serve as a prognostic marker of disease duration in patients with idiopathic Parkinson’s disease? Antioxid. Redox Signal. 2013;18(11):1296–1302. doi: 10.1089/ars.2012.5026. [DOI] [PubMed] [Google Scholar]
- 141.Li Y-H., Wang J., Zheng X-L., Zhang Y-L., Li X., Yu S., He X., Chan P. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry combined with magnetic beads for detecting serum protein biomarkers in parkinson’s disease. Eur. Neurol. 2011;65(2):105–111. doi: 10.1159/000323427. [DOI] [PubMed] [Google Scholar]
- 142.Zhang X., Yin X., Yu H., Liu X., Yang F., Yao J., Jin H., Yang P. Quantitative proteomic analysis of serum proteins in patients with Parkinson’s disease using an isobaric tag for relative and absolute quantification labeling, two-dimensional liquid chromatography, and tandem mass spectrometry. Analyst (Lond.) 2012;137(2):490–495. doi: 10.1039/C1AN15551B. [DOI] [PubMed] [Google Scholar]
- 143.Sala G., Stefanoni G., Arosio A., Riva C., Melchionda L., Saracchi E., Fermi S., Brighina L., Ferrarese C. Reduced expression of the chaperone-mediated autophagy carrier hsc70 protein in lymphomonocytes of patients with Parkinson’s disease. Brain Res. 2014;1546:46–52. doi: 10.1016/j.brainres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 144.Lamontagne-Proulx J., St-Amour I., Labib R., Pilon J., Denis H.L., Cloutier N., Roux-Dalvai F., Vincent A.T., Mason S.L., Williams-Gray C., Duchez A.C., Droit A., Lacroix S., Dupré N., Langlois M., Chouinard S., Panisset M., Barker R.A., Boilard E., Cicchetti F. Portrait of blood-derived extracellular vesicles in patients with Parkinson’s disease. Neurobiol. Dis. 2019;124:163–175. doi: 10.1016/j.nbd.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 145.Lee M., Guo J-P., Kennedy K., McGeer E.G., McGeer P.L. A Method for Diagnosing Alzheimer’s Disease Based on Salivary Amyloid-β Protein 42 Levels. J. Alzheimers Dis. 2017;55(3):1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 146.Sabbagh M.N., Shi J., Lee M., Arnold L., Al-Hasan Y., Heim J., McGeer P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC Neurol. 2018;18(1):155. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Al-Nimer M.S., Mshatat S.F., Abdulla H.I. Saliva α-synuclein and a high extinction coefficient protein: A novel approach in assessment biomarkers of Parkinson′s disease. N. Am. J. Med. Sci. 2014;6(12):633–637. doi: 10.4103/1947-2714.147980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masters J.M., Noyce A.J., Warner T.T., Giovannoni G., Proctor G.B. Elevated salivary protein in Parkinson’s disease and salivary DJ-1 as a potential marker of disease severity. Parkinsonism Relat. Disord. 2015;21(10):1251–1255. doi: 10.1016/j.parkreldis.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 149.Ma L., Chen J., Wang R., Han Y., Zhang J., Dong W., Zhang X., Wu Y., Zhao Z. The level of Alzheimer-associated neuronal thread protein in urine may be an important biomarker of mild cognitive impairment. J. Clin. Neurosci. 2015;22(4):649–652. doi: 10.1016/j.jocn.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 150.Ku B.D., Kim H., Kim Y.K., Ryu H.U. Comparison of Urinary Alzheimer-Associated Neural Thread Protein (AD7c-NTP) Levels Between Patients With Amnestic and Nonamnestic Mild Cognitive Impairment. Am. J. Alzheimers Dis. Other Demen. 2019 doi: 10.1177/1533317519880369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu Y., Shen Y.Y., Zhang X.P., Gui L., Cai M., Peng G.P., Pan X.D., Zhang J., Gan D., Li B., Cheng H.P., Deng J., Li W.W., Zeng G.H., Shi A.Y., Zhou Z.H., Luo B.Y., Chen X.C., Wang Y.J. Diagnostic potential of urinary monocyte chemoattractant protein-1 for Alzheimer’s disease and amnestic mild cognitive impairment. Eur. J. Neurol. 2020;27(8):1429–1435. doi: 10.1111/ene.14254. [DOI] [PubMed] [Google Scholar]
- 152.Maass F., Rikker S., Dambeck V., Warth C., Tatenhorst L., Csoti I., Schmitz M., Zerr I., Leha A., Bähr M., Lingor P. Increased alpha-synuclein tear fluid levels in patients with Parkinson’s disease. Sci. Rep. 2020;10(1):8507. doi: 10.1038/s41598-020-65503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boerger M., Funke S., Leha A., Roser A.E., Wuestemann A.K., Maass F., Bähr M., Grus F., Lingor P. Proteomic analysis of tear fluid reveals disease-specific patterns in patients with Parkinson’s disease - A pilot study. Parkinsonism Relat. Disord. 2019;63:3–9. doi: 10.1016/j.parkreldis.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Donovan L.E., Dammer E.B., Duong D.M., Hanfelt J.J., Levey A.I., Seyfried N.T., Lah J.J. Exploring the potential of the platelet membrane proteome as a source of peripheral biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2013;5(3):32. doi: 10.1186/alzrt186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watt A.D., Perez K.A., Ang C-S., O’Donnell P., Rembach A., Pertile K.K., Rumble R.L., Trounson B.O., Fowler C.J., Faux N.G., Masters C.L., Villemagne V.L., Barnham K.J. Peripheral α-defensins 1 and 2 are elevated in Alzheimer’s disease. J. Alzheimers Dis. 2015;44(4):1131–1143. doi: 10.3233/JAD-142286. [DOI] [PubMed] [Google Scholar]
- 156.Henkel A.W., Müller K., Lewczuk P., Müller T., Marcus K., Kornhuber J., Wiltfang J. Multidimensional plasma protein separation technique for identification of potential Alzheimer’s disease plasma biomarkers: a pilot study. J. Neural Transm. (Vienna) 2012;119(7):779–788. doi: 10.1007/s00702-012-0781-3. [DOI] [PubMed] [Google Scholar]
- 157.Kumar A., Singh S., Verma A., Mishra V.N. Proteomics based identification of differential plasma proteins and changes in white matter integrity as markers in early detection of mild cognitive impaired subjects at high risk of Alzheimer’s disease. Neurosci. Lett. 2018;676:71–77. doi: 10.1016/j.neulet.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 158.Voyle N., Baker D., Burnham S.C., Covin A., Zhang Z., Sangurdekar D.P., Tan Hehir C.A., Bazenet C., Lovestone S., Kiddle S., Dobson R.J. AIBL research group. Blood Protein Markers of Neocortical Amyloid-β Burden: A Candidate Study Using SOMAscan Technology. J. Alzheimers Dis. 2015;46(4):947–961. doi: 10.3233/JAD-150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kiddle S.J., Steves C.J., Mehta M., Simmons A., Xu X., Newhouse S., Sattlecker M., Ashton N.J., Bazenet C., Killick R., Adnan J., Westman E., Nelson S., Soininen H., Kloszewska I., Mecocci P., Tsolaki M., Vellas B., Curtis C., Breen G., Williams S.C., Lovestone S., Spector T.D., Dobson R.J. Plasma protein biomarkers of Alzheimer’s disease endophenotypes in asymptomatic older twins: early cognitive decline and regional brain volumes. Transl. Psychiatry. 2015;5:e584–e584. doi: 10.1038/tp.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sattlecker M., Khondoker M., Proitsi P., Williams S., Soininen H., Kłoszewska I., Mecocci P., Tsolaki M., Vellas B., Lovestone S., Dobson R.J. Longitudinal Protein Changes in Blood Plasma Associated with the Rate of Cognitive Decline in Alzheimer’s Disease. J. Alzheimers Dis. 2016;49(4):1105–1114. doi: 10.3233/JAD-140669. [DOI] [PubMed] [Google Scholar]
- 161.Candia J., Cheung F., Kotliarov Y., Fantoni G., Sellers B., Griesman T., Huang J., Stuccio S., Zingone A., Ryan B.M., Tsang J.S., Biancotto A. Assessment of Variability in the SOMAscan Assay. Sci. Rep. 2017;7(1):14248. doi: 10.1038/s41598-017-14755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kułakowska A., Tarasiuk J., Kapica-Topczewska K., Chorąży M., Pogorzelski R., Kulczyńska-Przybik A., Mroczko B., Bucki R. Pathophysiological implications of actin-free Gc-globulin concentration changes in blood plasma and cerebrospinal fluid collected from patients with Alzheimer’s disease and other neurological disorders. Adv. Clin. Exp. Med. 2018;27(8):1075–1080. doi: 10.17219/acem/70441. [DOI] [PubMed] [Google Scholar]
- 163.Yang Y-H., Lai C-L., Tyan Y-C., Chou M-C., Wang L-C., Yang M-H., Liu C-K. Angiotensin-converting enzyme gene and plasma protein level in Alzheimer’s disease in Taiwanese. Age Ageing. 2011;40(2):238–242. doi: 10.1093/ageing/afq179. [DOI] [PubMed] [Google Scholar]
- 164.Marksteiner J., Humpel C. Platelet-derived secreted amyloid-precursor protein-β as a marker for diagnosing Alzheimer’s disease. Curr. Neurovasc. Res. 2013;10(4):297–303. doi: 10.2174/15672026113109990022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang C-C., Chiu M-J., Chen T-F., Chang H-L., Liu B-H., Yang S-Y. Assay of Plasma Phosphorylated Tau Protein (Threonine 181) and Total Tau Protein in Early-Stage Alzheimer’s Disease. J. Alzheimers Dis. 2018;61(4):1323–1332. doi: 10.3233/JAD-170810. [DOI] [PubMed] [Google Scholar]
- 166.Shi Y., Gu L., Wang Q., Gao L., Zhu J., Lu X., Zhou F., Zhu D., Zhang H., Xie C., Zhang Z. Platelet amyloid-β protein precursor (AβPP) ratio and phosphorylated tau as promising indicators for early Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(4):664–670. doi: 10.1093/gerona/glz005. [DOI] [PubMed] [Google Scholar]
- 167.Kumar R., Chaterjee P., Sharma P.K., Singh A.K., Gupta A., Gill K., Tripathi M., Dey A.B., Dey S. Sirtuin1: a promising serum protein marker for early detection of Alzheimer’s disease. PLoS One. 2013;8(4):e61560. doi: 10.1371/journal.pone.0061560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jelic V., Hagman G., Yamamoto N.G., Teranishi Y., Nishimura T., Winblad B., Pavlov P.F. Abnormal platelet amyloid-β protein precursor (AβPP) metabolism in Alzheimer’s disease: identification and characterization of a new AβPP isoform as potential biomarker. J. Alzheimers Dis. 2013;35(2):285–295. doi: 10.3233/JAD-122122. [DOI] [PubMed] [Google Scholar]
- 169.Bram J.M.F., Talib L.L., Joaquim H.P.G., Sarno T.A., Gattaz W.F., Forlenza O.V. Protein levels of ADAM10, BACE1, and PSEN1 in platelets and leukocytes of Alzheimer’s disease patients. Eur. Arch. Psychiatry Clin. Neurosci. 2019;269(8):963–972. doi: 10.1007/s00406-018-0905-3. [DOI] [PubMed] [Google Scholar]
- 170.Zellner M., Baureder M., Rappold E., Bugert P., Kotzailias N., Babeluk R., Baumgartner R., Attems J., Gerner C., Jellinger K., Roth E., Oehler R., Umlauf E. Comparative platelet proteome analysis reveals an increase of monoamine oxidase-B protein expression in Alzheimer’s disease but not in non-demented Parkinson’s disease patients. J. Proteomics. 2012;75(7):2080–2092. doi: 10.1016/j.jprot.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 171.Xiong K-P., Dai Y-P., Chen J., Xu J-M., Wang Y., Feng P., You S-J., Liu C-F. Increased Serum Cystatin C in Early Parkinson’s Disease with Objective Sleep Disturbances. Chin. Med. J. (Engl.) 2018;131(8):907–911. doi: 10.4103/0366-6999.229902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu S., Liu Y-P., Liu H-L., Li J., Xiang Y., Liu Y-H., Jiao S-S., Liu L., Wang Y., Fu W. Serum Protein-Based Profiles as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Mol. Neurobiol. 2018;55(5):3999–4008. doi: 10.1007/s12035-017-0609-0. [DOI] [PubMed] [Google Scholar]
- 173.Shahpasand-Kroner H., Klafki H-W., Bauer C., Schuchhardt J., Hüttenrauch M., Stazi M., Bouter C., Wirths O., Vogelgsang J., Wiltfang J. A two-step immunoassay for the simultaneous assessment of Aβ38, Aβ40 and Aβ42 in human blood plasma supports the Aβ42/Aβ40 ratio as a promising biomarker candidate of Alzheimer’s disease. Alzheimers Res. Ther. 2018;10(1):121. doi: 10.1186/s13195-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akıl E., Bulut A., Kaplan İ., Özdemir H.H., Arslan D., Aluçlu M.U. The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol. Sci. 2015;36(3):423–428. doi: 10.1007/s10072-014-1976-1. [DOI] [PubMed] [Google Scholar]
- 175.Várady G., Szabó E., Fehér Á., Németh A., Zámbó B., Pákáski M., Janka Z., Sarkadi B. Alterations of membrane protein expression in red blood cells of Alzheimer’s disease patients. Alzheimers Dement. (Amst.) 2015;1(3):334–338. doi: 10.1016/j.dadm.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.San Segundo-Acosta P., Montero-Calle A., Fuentes M., Rábano A., Villalba M., Barderas R. Identification of Alzheimer’s Disease Autoantibodies and Their Target Biomarkers by Phage Microarrays. J. Proteome Res. 2019;18(7):2940–2953. doi: 10.1021/acs.jproteome.9b00258. [DOI] [PubMed] [Google Scholar]
- 177.Oeckl P., Halbgebauer S., Anderl-Straub S., von Arnim C.A.F., Diehl-Schmid J., Froelich L., Grimmer T., Hausner L., Denk J., Jahn H., Steinacker P., Weishaupt J.H., Ludolph A.C., Otto M. Targeted Mass Spectrometry Suggests Beta-Synuclein as Synaptic Blood Marker in Alzheimer’s Disease. J. Proteome Res. 2020;19(3):1310–1318. doi: 10.1021/acs.jproteome.9b00824. [DOI] [PubMed] [Google Scholar]
- 178.Ahmad S., Milan M.D.C., Hansson O., Demirkan A., Agustin R., Sáez M.E., Giagtzoglou N., Cabrera-Socorro A., Bakker M.H.M., Ramirez A., Hankemeier T., Stomrud E., Mattsson-Carlgren N., Scheltens P., van der Flier W.M., Ikram M.A., Malarstig A., Teunissen C.E., Amin N., van Duijn C.M. CDH6 and HAGH protein levels in plasma associate with Alzheimer’s disease in APOE ε4 carriers. Sci. Rep. 2020;10(1):8233. doi: 10.1038/s41598-020-65038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang M-H., Yang Y-H., Lu C-Y., Jong S-B., Chen L-J., Lin Y-F., Wu S-J., Chu P-Y., Chung T-W., Tyan Y-C. Activity-dependent neuroprotector homeobox protein: A candidate protein identified in serum as diagnostic biomarker for Alzheimer’s disease. J. Proteomics. 2012;75(12):3617–3629. doi: 10.1016/j.jprot.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 180.O’Bryant S.E., Waring S.C., Hobson V., Hall J.R., Moore C.B., Bottiglieri T., Massman P., Diaz-Arrastia R. Decreased C-reactive protein levels in Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010;23(1):49–53. doi: 10.1177/0891988709351832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shi L., Westwood S., Baird A.L., Winchester L., Dobricic V., Kilpert F., Hong S., Franke A., Hye A., Ashton N.J., Morgan A.R., Bos I., Vos S.J.B., Buckley N.J., Kate M.T., Scheltens P., Vandenberghe R., Gabel S., Meersmans K., Engelborghs S., De Roeck E.E., Sleegers K., Frisoni G.B., Blin O., Richardson J.C., Bordet R., Molinuevo J.L., Rami L., Wallin A., Kettunen P., Tsolaki M., Verhey F., Lleó A., Alcolea D., Popp J., Peyratout G., Martinez-Lage P., Tainta M., Johannsen P., Teunissen C.E., Freund-Levi Y., Frölich L., Legido-Quigley C., Barkhof F., Blennow K., Zetterberg H., Baker S., Morgan B.P., Streffer J., Visser P.J., Bertram L., Lovestone S., Nevado-Holgado A.J. Discovery and validation of plasma proteomic biomarkers relating to brain amyloid burden by SOMAscan assay. Alzheimers Dement. 2019;15(11):1478–1488. doi: 10.1016/j.jalz.2019.06.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yun S.M., Cho S.J., Jo C., Park M.H., Han C., Koh Y.H. Elevation of plasma soluble amyloid precursor protein beta in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2020;87:103995. doi: 10.1016/j.archger.2019.103995. [DOI] [PubMed] [Google Scholar]
- 183.Westwood S., Baird A.L., Anand S.N., Nevado-Holgado A.J., Kormilitzin A., Shi L., Hye A., Ashton N.J., Morgan A.R., Bos I., Vos S.J.B., Baker S., Buckley N.J., Ten Kate M., Scheltens P., Teunissen C.E., Vandenberghe R., Gabel S., Meersmans K., Engelborghs S., De Roeck E.E., Sleegers K., Frisoni G.B., Blin O., Richardson J.C., Bordet R., Molinuevo J.L., Rami L., Wallin A., Kettunen P., Tsolaki M., Verhey F., Lléo A., Sala I., Popp J., Peyratout G., Martinez-Lage P., Tainta M., Johannsen P., Freund-Levi Y., Frölich L., Dobricic V., Legido-Quigley C., Bertram L., Barkhof F., Zetterberg H., Morgan B.P., Streffer J., Visser P.J., Lovestone S. Validation of plasma proteomic biomarkers relating to brain amyloid burden in the EMIF-Alzheimer’s disease multimodal biomarker discovery cohort. J. Alzheimers Dis. 2020;74(1):213–225. doi: 10.3233/JAD-190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dominici R., Finazzi D., Polito L., Oldoni E., Bugari G., Montanelli A., Scarpini E., Galimberti D., Guaita A. Comparison of β2-microglobulin serum level between Alzheimer’s patients, cognitive healthy and mild cognitive impaired individuals. Biomarkers. 2018;23(6):603–608. doi: 10.1080/1354750X.2018.1468825. [DOI] [PubMed] [Google Scholar]
- 185.Madasamy S., Chaudhuri V., Kong R., Alderete B., Adams C.M., Knaak T.D., Ruan W., Wu A.H.B., Bigos M., Amento E.P. Plaque array method and proteomics-based identification of biomarkers from Alzheimer’s disease serum. Clin. Chim. Acta. 2015;441:79–85. doi: 10.1016/j.cca.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 186.Nazeri A., Ganjgahi H., Roostaei T., Nichols T., Zarei M. Alzheimer’s Disease Neuroimaging Initiative. Imaging proteomics for diagnosis, monitoring and prediction of Alzheimer’s disease. Neuroimage. 2014;102(Pt 2):657–665. doi: 10.1016/j.neuroimage.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liang F., Jia J., Wang S., Qin W., Liu G. Decreased plasma levels of soluble low density lipoprotein receptor-related protein-1 (sLRP) and the soluble form of the receptor for advanced glycation end products (sRAGE) in the clinical diagnosis of Alzheimer’s disease. J. Clin. Neurosci. 2013;20(3):357–361. doi: 10.1016/j.jocn.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 188.Rosén C., Mattsson N., Johansson P.M., Andreasson U., Wallin A., Hansson O., Johansson J-O., Lamont J., Svensson J., Blennow K., Zetterberg H. Discriminatory Analysis of Biochip-Derived Protein Patterns in CSF and Plasma in Neurodegenerative Diseases. Front. Aging Neurosci. 2011;3:1. doi: 10.3389/fnagi.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perneczky R., Guo L-H., Kagerbauer S.M., Werle L., Kurz A., Martin J., Alexopoulos P. Soluble amyloid precursor protein β as blood-based biomarker of Alzheimer’s disease. Transl. Psychiatry. 2013;3:e227–e227. doi: 10.1038/tp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johansson P., Åberg D., Johansson J-O., Mattsson N., Hansson O., Ahrén B., Isgaard J., Åberg N.D., Blennow K., Zetterberg H., Wallin A., Svensson J. Serum but not cerebrospinal fluid levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) are increased in Alzheimer’s disease. Psychoneuroendocrinology. 2013;38(9):1729–1737. doi: 10.1016/j.psyneuen.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 191.Ashton N.J., Kiddle S.J., Graf J., Ward M., Baird A.L., Hye A., Westwood S., Wong K.V., Dobson R.J., Rabinovici G.D., Miller B.L., Rosen H.J., Torres A., Zhang Z., Thurfjell L., Covin A., Hehir C.T., Baker D., Bazenet C., Lovestone S., AIBL Research Group Blood protein predictors of brain amyloid for enrichment in clinical trials? Alzheimers Dement. (Amst.) 2015;1(1):48–60. doi: 10.1016/j.dadm.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cocciolo A., Di Domenico F., Coccia R., Fiorini A., Cai J., Pierce W.M., Mecocci P., Butterfield D.A., Perluigi M. Decreased expression and increased oxidation of plasma haptoglobin in Alzheimer disease: Insights from redox proteomics. Free Radic. Biol. Med. 2012;53(10):1868–1876. doi: 10.1016/j.freeradbiomed.2012.08.596. [DOI] [PubMed] [Google Scholar]
- 193.Llano D.A., Devanarayan V., Simon A.J. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Evaluation of plasma proteomic data for Alzheimer disease state classification and for the prediction of progression from mild cognitive impairment to Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2013;27(3):233–243. doi: 10.1097/WAD.0b013e31826d597a. [DOI] [PubMed] [Google Scholar]
- 194.Thambisetty M., Simmons A., Hye A., Campbell J., Westman E., Zhang Y., Wahlund L-O., Kinsey A., Causevic M., Killick R., Kloszewska I., Mecocci P., Soininen H., Tsolaki M., Vellas B., Spenger C., Lovestone S. AddNeuroMed Consortium. Plasma biomarkers of brain atrophy in Alzheimer’s disease. PLoS One. 2011;6(12):e28527. doi: 10.1371/journal.pone.0028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thambisetty M., Tripaldi R., Riddoch-Contreras J., Hye A., An Y., Campbell J., Sojkova J., Kinsey A., Lynham S., Zhou Y., Ferrucci L., Wong D.F., Lovestone S., Resnick S.M. Proteome-based plasma markers of brain amyloid-β deposition in non-demented older individuals. J. Alzheimers Dis. 2010;22(4):1099–1109. doi: 10.3233/JAD-2010-101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Soares H.D., Potter W.Z., Pickering E., Kuhn M., Immermann F.W., Shera D.M., Ferm M., Dean R.A., Simon A.J., Swenson F., Siuciak J.A., Kaplow J., Thambisetty M., Zagouras P., Koroshetz W.J., Wan H.I., Trojanowski J.Q., Shaw L.M. Biomarkers Consortium Alzheimer’s Disease Plasma Proteomics Project. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch. Neurol. 2012;69(10):1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Song F., Poljak A., Kochan N.A., Raftery M., Brodaty H., Smythe G.A., Sachdev P.S. Plasma protein profiling of Mild Cognitive Impairment and Alzheimer’s disease using iTRAQ quantitative proteomics. Proteome Sci. 2014;12(1):5. doi: 10.1186/1477-5956-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bennett S., Grant M., Creese A.J., Mangialasche F., Cecchetti R., Cooper H.J., Mecocci P., Aldred S. Plasma levels of complement 4a protein are increased in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2012;26(4):329–334. doi: 10.1097/WAD.0b013e318239dcbd. [DOI] [PubMed] [Google Scholar]
- 199.Petersen M., Zhang F., Krinsky-McHale S.J., Silverman W., Lee J.H., Pang D., Hall J., Schupf N., O’Bryant S.E. Proteomic profiles of prevalent mild cognitive impairment and Alzheimer’s disease among adults with Down syndrome. Alzheimers Dement. (Amst.) 2020;12(1):e12023. doi: 10.1002/dad2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnstone D., Milward E.A., Berretta R., Moscato P. Alzheimer’s Disease Neuroimaging Initiative. Multivariate protein signatures of pre-clinical Alzheimer’s disease in the Alzheimer’s disease neuroimaging initiative (ADNI) plasma proteome dataset. PLoS One. 2012;7(4):e34341. doi: 10.1371/journal.pone.0034341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sattlecker M., Kiddle S.J., Newhouse S., Proitsi P., Nelson S., Williams S., Johnston C., Killick R., Simmons A., Westman E., Hodges A., Soininen H., Kłoszewska I., Mecocci P., Tsolaki M., Vellas B., Lovestone S., Dobson R.J. AddNeuroMed Consortium. Alzheimer’s disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 2014;10(6):724–734. doi: 10.1016/j.jalz.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 202.Doecke J.D., Laws S.M., Faux N.G., Wilson W., Burnham S.C., Lam C.P., Mondal A., Bedo J., Bush A.I., Brown B., De Ruyck K., Ellis K.A., Fowler C., Gupta V.B., Head R., Macaulay S.L., Pertile K., Rowe C.C., Rembach A., Rodrigues M., Rumble R., Szoeke C., Taddei K., Taddei T., Trounson B., Ames D., Masters C.L., Martins R.N. Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarker and Lifestyle Research Group. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch. Neurol. 2012;69(10):1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo L-H., Alexopoulos P., Wagenpfeil S., Kurz A., Perneczky R. Alzheimer’s Disease Neuroimaging Initiative. Plasma proteomics for the identification of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2013;27(4):337–342. doi: 10.1097/WAD.0b013e31827b60d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alexopoulos P., Gleixner L-S., Werle L., Buhl F., Thierjung N., Giourou E., Kagerbauer S.M., Gourzis P., Kübler H., Grimmer T., Yakushev I., Martin J., Kurz A., Perneczky R. Plasma levels of soluble amyloid precursor protein β in symptomatic Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268(5):519–524. doi: 10.1007/s00406-017-0815-9. [DOI] [PubMed] [Google Scholar]
- 205.Sun H-L., Li W-W., Zhu C., Jin W-S., Liu Y-H., Zeng F., Wang Y-J., Bu X-L. The Correlations of Plasma and Cerebrospinal Fluid Amyloid-Beta Levels with Platelet Count in Patients with Alzheimer’s Disease. BioMed Res. Int. 2018;2018:7302045. doi: 10.1155/2018/7302045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang M.J., Yi S., Han J.Y., Park S.Y., Jang J-W., Chun I.K., Kim S.E., Lee B.S., Kim G.J., Yu J.S., Lim K., Kang S.M., Park Y.H., Youn Y.C., An S.S.A., Kim S. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res. Ther. 2017;9(1):98. doi: 10.1186/s13195-017-0324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Waragai M., Yoshida M., Mizoi M., Saiki R., Kashiwagi K., Takagi K., Arai H., Tashiro J., Hashimoto M., Iwai N., Uemura K., Igarashi K. Increased protein-conjugated acrolein and amyloid-β40/42 ratio in plasma of patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012;32(1):33–41. doi: 10.3233/JAD-2012-120253. [DOI] [PubMed] [Google Scholar]
- 208.Winston C.N., Goetzl E.J., Akers J.C., Carter B.S., Rockenstein E.M., Galasko D., Masliah E., Rissman R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. (Amst.) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dage J.L., Wennberg A.M.V., Airey D.C., Hagen C.E., Knopman D.S., Machulda M.M., Roberts R.O., Jack C.R., Jr, Petersen R.C., Mielke M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12(12):1226–1234. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yarchoan M., Louneva N., Xie S.X., Swenson F.J., Hu W., Soares H., Trojanowski J.Q., Lee V.M-Y., Kling M.A., Shaw L.M., Chen-Plotkin A., Wolk D.A., Arnold S.E. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J. Neurol. Sci. 2013;333(1-2):9–12. doi: 10.1016/j.jns.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kvartsberg H., Portelius E., Andreasson U., Brinkmalm G., Hellwig K., Lelental N., Kornhuber J., Hansson O., Minthon L., Spitzer P., Maler J.M., Zetterberg H., Blennow K., Lewczuk P. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimers Res. Ther. 2015;7(1):40. doi: 10.1186/s13195-015-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cho S-J., Yun S-M., Lee D.H., Jo C., Ho Park M., Han C., Ho Koh Y. Plasma SUMO1 Protein is Elevated in Alzheimer’s Disease. J. Alzheimers Dis. 2015;47(3):639–643. doi: 10.3233/JAD-150103. [DOI] [PubMed] [Google Scholar]
- 213.Shih Y-H., Tsai K-J., Lee C-W., Shiesh S-C., Chen W-T., Pai M-C., Kuo Y-M. Apolipoprotein C-III is an amyloid-β-binding protein and an early marker for Alzheimer’s disease. J. Alzheimers Dis. 2014;41(3):855–865. doi: 10.3233/JAD-140111. [DOI] [PubMed] [Google Scholar]
- 214.Ghidoni R., Benussi L., Glionna M., Desenzani S., Albertini V., Levy E., Emanuele E., Binetti G. Plasma cystatin C and risk of developing Alzheimer’s disease in subjects with mild cognitive impairment. J. Alzheimers Dis. 2010;22(3):985–991. doi: 10.3233/JAD-2010-101095. [DOI] [PubMed] [Google Scholar]
- 215.Suzuki I., Noguchi M., Arito M., Sato T., Omoteyama K., Maedomari M., Hasegawa H., Suematsu N., Okamoto K., Kato T., Yamaguchi N., Kurokawa M.S. Serum peptides as candidate biomarkers for dementia with Lewy bodies. Int. J. Geriatr. Psychiatry. 2015;30(12):1195–1206. doi: 10.1002/gps.4274. [DOI] [PubMed] [Google Scholar]
- 216.Shen L., Liao L., Chen C., Guo Y., Song D., Wang Y., Chen Y., Zhang K., Ying M., Li S., Liu Q., Ni J. Proteomics Analysis of Blood Serums from Alzheimer’s Disease Patients Using iTRAQ Labeling Technology. J. Alzheimers Dis. 2017;56(1):361–378. doi: 10.3233/JAD-160913. [DOI] [PubMed] [Google Scholar]
- 217.Shah D.J., Rohlfing F., Anand S., Johnson W.E., Alvarez M.T.B., Cobell J., King J., Young S.A., Kauwe J.S.K., Graves S.W. Discovery and Subsequent Confirmation of Novel Serum Biomarkers Diagnosing Alzheimer’s Disease. J. Alzheimers Dis. 2016;49(2):317–327. doi: 10.3233/JAD-150498. [DOI] [PubMed] [Google Scholar]
- 218.Ijsselstijn L., Dekker L.J.M., Stingl C., van der Weiden M.M., Hofman A., Kros J.M., Koudstaal P.J., Sillevis Smitt P.A.E., Ikram M.A., Breteler M.M.B., Luider T.M. Serum levels of pregnancy zone protein are elevated in presymptomatic Alzheimer’s disease. J. Proteome Res. 2011;10(11):4902–4910. doi: 10.1021/pr200270z. [DOI] [PubMed] [Google Scholar]
- 219.Preische O., Schultz S.A., Apel A., Kuhle J., Kaeser S.A., Barro C., Gräber S., Kuder-Buletta E., LaFougere C., Laske C., Vöglein J., Levin J., Masters C.L., Martins R., Schofield P.R., Rossor M.N., Graff-Radford N.R., Salloway S., Ghetti B., Ringman J.M., Noble J.M., Chhatwal J., Goate A.M., Benzinger T.L.S., Morris J.C., Bateman R.J., Wang G., Fagan A.M., McDade E.M., Gordon B.A., Jucker M. Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat. Med. 2019;25(2):277–283. doi: 10.1038/s41591-018-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Elahi F.M., Casaletto K.B., La Joie R., Walters S.M., Harvey D., Wolf A., Edwards L., Rivera-Contreras W., Karydas A., Cobigo Y., Rosen H.J., DeCarli C., Miller B.L., Rabinovici G.D., Kramer J.H. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 2020;16(4):681–695. doi: 10.1016/j.jalz.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Taverna M., Straub T., Hampel H., Rujescu D., Lichtenthaler S.F. A new sandwich immunoassay for detection of the α-secretase cleaved, soluble amyloid-β protein precursor in cerebrospinal fluid and serum. J. Alzheimers Dis. 2013;37(4):667–678. doi: 10.3233/JAD-130509. [DOI] [PubMed] [Google Scholar]
- 222.O’Bryant S.E., Johnson L., Edwards M., Soares H., Devous M.D., Ross S., Rohlfing G., Hall J. Texas Alzheimer’s Research & Care Consortium. The link between C-reactive protein and Alzheimer’s disease among Mexican Americans. J. Alzheimers Dis. 2013;34(3):701–706. doi: 10.3233/JAD-122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Villarreal A.E., O’Bryant S.E., Edwards M., Grajales S., Britton G.B. Panama Aging Research Initiative. Serum-based protein profiles of Alzheimer’s disease and mild cognitive impairment in elderly Hispanics. Neurodegener. Dis. Manag. 2016;6(3):203–213. doi: 10.2217/nmt-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wei H., Zhu X., Li Y. Application value of serum biomarkers for choosing memantine therapy for moderate AD. J. Neurol. 2018;265(8):1844–1849. doi: 10.1007/s00415-018-8926-4. [DOI] [PubMed] [Google Scholar]
- 225.Magalhães C.A., Ferreira C.N., Loures C.M.G., Fraga V.G., Chaves A.C., Oliveira A.C.R., de Souza L.C., Resende E.P.F., Carmona K.C., Guimarães H.C., Cintra M.T.G., Lanna I.N., Zauli D.A.G., Bicalho M.A., Carvalho M.G., Sousa L.P., Caramelli P., Gomes K.B. Leptin, hsCRP, TNF-α and IL-6 levels from normal aging to dementia: Relationship with cognitive and functional status. J. Clin. Neurosci. 2018;56:150–155. doi: 10.1016/j.jocn.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 226.Bishnoi R.J., Palmer R.F., Royall D.R., Vitamin D. Vitamin D binding protein as a serum biomarker of Alzheimer’s disease. J. Alzheimers Dis. 2015;43(1):37–45. doi: 10.3233/JAD-140042. [DOI] [PubMed] [Google Scholar]
- 227.Sultana R., Baglioni M., Cecchetti R., Cai J., Klein J.B., Bastiani P., Ruggiero C., Mecocci P., Butterfield D.A. Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic. Biol. Med. 2013;65:595–606. doi: 10.1016/j.freeradbiomed.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jammeh E., Zhao P., Carroll C., Pearson S., Ifeachor E. Identification of blood biomarkers for use in point of care diagnosis tool for Alzheimer’s disease.; Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2016. pp. 2415–2418. [DOI] [PubMed] [Google Scholar]
- 229.Bermejo-Bescós P., Martín-Aragón S., Jiménez-Aliaga K., Benedí J., Felici E., Gil P., Ribera J.M., Villar Á.M. Processing of the platelet amyloid precursor protein in the mild cognitive impairment (MCI). Neurochem. Res. 2013;38(7):1415–1423. doi: 10.1007/s11064-013-1039-7. [DOI] [PubMed] [Google Scholar]
- 230.Srisawat C., Junnu S., Peerapittayamongkol C., Futrakul A., Soi-ampornkul R., Senanarong V., Praditsuwan R., Assantachai P., Neungton N. The platelet amyloid precursor protein ratio as a diagnostic marker for Alzheimer’s disease in Thai patients. J. Clin. Neurosci. 2013;20(5):644–648. doi: 10.1016/j.jocn.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 231.Zainaghi I.A., Talib L.L., Diniz B.S., Gattaz W.F., Forlenza O.V. Reduced platelet amyloid precursor protein ratio (APP ratio) predicts conversion from mild cognitive impairment to Alzheimer’s disease. J. Neural Transm. (Vienna) 2012;119(7):815–819. doi: 10.1007/s00702-012-0807-x. [DOI] [PubMed] [Google Scholar]
- 232.Mukaetova-Ladinska E.B., Abdel-All Z., Dodds S., Andrade J., Alves da Silva J., Kalaria R.N., O’Brien J.T. Platelet immunoglobulin and amyloid precursor protein as potential peripheral biomarkers for Alzheimer’s disease: findings from a pilot study. Age Ageing. 2012;41(3):408–412. doi: 10.1093/ageing/afr171. [DOI] [PubMed] [Google Scholar]
- 233.Nabers A., Perna L., Lange J., Mons U., Schartner J., Güldenhaupt J., Saum K.U., Janelidze S., Holleczek B., Rujescu D., Hansson O., Gerwert K., Brenner H. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med. 2018;10(5):e8763. doi: 10.15252/emmm.201708763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Apostolova L.G., Hwang K.S., Avila D., Elashoff D., Kohannim O., Teng E., Sokolow S., Jack C.R., Jagust W.J., Shaw L., Trojanowski J.Q., Weiner M.W., Thompson P.M. Alzheimer’s Disease Neuroimaging Initiative. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology. 2015;84(7):729–737. doi: 10.1212/WNL.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Altunoglu E., Guntas G., Erdenen F., Akkaya E., Topac I., Irmak H., Derici H., Yavuzer H., Gelisgen R., Uzun H. Ischemia-modified albumin and advanced oxidation protein products as potential biomarkers of protein oxidation in Alzheimer’s disease. Geriatr. Gerontol. Int. 2015;15(7):872–880. doi: 10.1111/ggi.12361. [DOI] [PubMed] [Google Scholar]
- 236.Reumiller C.M., Schmidt G.J., Dhrami I., Umlauf E., Rappold E., Zellner M. Gender-related increase of tropomyosin-1 abundance in platelets of Alzheimer’s disease and mild cognitive impairment patients. J. Proteomics. 2018;178:73–81. doi: 10.1016/j.jprot.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 237.An C., Pu X., Xiao W., Zhang H. Expression of the DJ-1 protein in the serum of Chinese patients with Parkinson’s disease. Neurosci. Lett. 2018;665:236–239. doi: 10.1016/j.neulet.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 238.Kouchaki E., Daneshvar Kakhaki R., Tamtaji O.R., Dadgostar E., Behnam M., Zaribaf A., Nikoueinejad H., Akbari H., Asemi Z. Correlation of serum levels and gene expression of tumor necrosis factor-α-induced protein-8 like-2 with Parkinson disease severity. Metab. Brain Dis. 2018;33(6):1955–1959. doi: 10.1007/s11011-018-0302-7. [DOI] [PubMed] [Google Scholar]
- 239.Zhao X., Xiao W-Z., Pu X-P., Zhong L-J. Proteome analysis of the sera from Chinese Parkinson’s disease patients. Neurosci. Lett. 2010;479(2):175–179. doi: 10.1016/j.neulet.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 240.Wang S., Kojima K., Mobley J.A., West A.B. Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine. 2019;45:351–361. doi: 10.1016/j.ebiom.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Saiki S., Sasazawa Y., Fujimaki M., Kamagata K., Kaga N., Taka H., Li Y., Souma S., Hatano T., Imamichi Y., Furuya N., Mori A., Oji Y., Ueno S.I., Nojiri S., Miura Y., Ueno T., Funayama M., Aoki S., Hattori N. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019;86(2):251–263. doi: 10.1002/ana.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jiang R., Rong C., Ke R., Meng S., Yan X., Ke H., Wu S., Azim A. Differential proteomic analysis of serum exosomes reveals alterations in progression of Parkinson disease. Medicine (Baltimore) 2019;98(41):e17478. doi: 10.1097/MD.0000000000017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Posavi M., Diaz-Ortiz M., Liu B., Swanson C.R., Skrinak R.T., Hernandez-Con P., Amado D.A., Fullard M., Rick J., Siderowf A., Weintraub D., McCluskey L., Trojanowski J.Q., Dewey R.B., Jr, Huang X., Chen-Plotkin A.S. Characterization of Parkinson’s disease using blood-based biomarkers: A multicohort proteomic analysis. PLoS Med. 2019;16(10):e1002931. doi: 10.1371/journal.pmed.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chojdak-Łukasiewicz J., Małodobra-Mazur M., Zimny A., Noga L., Paradowski B. Plasma tau protein and Aβ42 level as markers of cognitive impairment in patients with Parkinson’s disease. Adv. Clin. Exp. Med. 2020;29(1):115–121. doi: 10.17219/acem/112058. [DOI] [PubMed] [Google Scholar]
- 245.Kim R., Kim H-J., Kim A., Jang M., Kim A., Kim Y., Yoo D. Im, J.H.; Choi, J.H.; Jeon, B. Peripheral blood inflammatory markers in early Parkinson’s disease. J. Clin. Neurosci. 2018;58:30–33. doi: 10.1016/j.jocn.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 246.Song I-U., Chung S-W., Kim J-S., Lee K-S. Association between high-sensitivity C-reactive protein and risk of early idiopathic Parkinson’s disease. Neurol. Sci. 2011;32(1):31–34. doi: 10.1007/s10072-010-0335-0. [DOI] [PubMed] [Google Scholar]
- 247.Wu Y., Yao Q., Jiang G.X., Wang G., Cheng Q. Identification of distinct blood-based biomarkers in early stage of Parkinson’s disease. Neurol. Sci. 2020;41(4):893–901. doi: 10.1007/s10072-019-04165-y. [DOI] [PubMed] [Google Scholar]
- 248.Su W., Chen H.B., Li S.H., Wu D.Y. Correlational study of the serum levels of the glial fibrillary acidic protein and neurofilament proteins in Parkinson’s disease patients. Clin. Neurol. Neurosurg. 2012;114(4):372–375. doi: 10.1016/j.clineuro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 249.Gupta A.K., Rani K., Swarnkar S., Kumar G.K., Khan M.I., Pokhriyal R., Kumar D.R., Goyal V., Tripathi M., Gupta R., Chadda R.K., Vanamail P., Hariprasad G. Evaluation of Serum Apolipoprotein E as a Potential Biomarker for Pharmacological Therapeutic Efficacy Monitoring in Dopamine Dictated Disease Spectrum of Schizophrenia and Parkinson’s disease: A Preliminary Study. J. Cent. Nerv. Syst. Dis. 2018;10:1179573518803585. doi: 10.1177/1179573518803585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dong W., Qiu C., Gong D., Jiang X., Liu W., Liu W., Zhang L., Zhang W. Proteomics and bioinformatics approaches for the identification of plasma biomarkers to detect Parkinson’s disease. Exp. Ther. Med. 2019;18(4):2833–2842. doi: 10.3892/etm.2019.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chiu C-C., Yeh T-H., Lai S-C., Weng Y-H., Huang Y-C., Cheng Y-C., Chen R-S., Huang Y-Z., Hung J., Chen C-C., Lin W.Y., Chang H.C., Chen Y.J., Chen C.L., Chen H.Y., Lin Y.W., Wu-Chou Y.H., Wang H.L., Lu C.S. Increased Rab35 expression is a potential biomarker and implicated in the pathogenesis of Parkinson’s disease. Oncotarget. 2016;7(34):54215–54227. doi: 10.18632/oncotarget.11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fujimaki M., Saiki S., Li Y., Kaga N., Taka H., Hatano T., Ishikawa K-I., Oji Y., Mori A., Okuzumi A., Koinuma T., Ueno S.I., Imamichi Y., Ueno T., Miura Y., Funayama M., Hattori N. Serum caffeine and metabolites are reliable biomarkers of early Parkinson disease. Neurology. 2018;90(5):e404–e411. doi: 10.1212/WNL.0000000000004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen H-M., Lin C-Y., Wang V. Amyloid P component as a plasma marker for Parkinson’s disease identified by a proteomic approach. Clin. Biochem. 2011;44(5-6):377–385. doi: 10.1016/j.clinbiochem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 254.Malec-Litwinowicz M., Plewka A., Plewka D., Bogunia E., Morek M., Szczudlik A., Szubiga M., Rudzińska-Bar M. The relation between plasma α-synuclein level and clinical symptoms or signs of Parkinson’s disease. Neurol. Neurochir. Pol. 2018;52(2):243–251. doi: 10.1016/j.pjnns.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 255.Si Q-Q., Yuan Y-S., Zhi Y., Tong Q., Zhang L., Zhang K. Plasma transferrin level correlates with the tremor-dominant phenotype of Parkinson’s disease. Neurosci. Lett. 2018;684:42–46. doi: 10.1016/j.neulet.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 256.Alberio T., McMahon K., Cuccurullo M., Gethings L.A., Lawless C., Zibetti M., Lopiano L., Vissers J.P.C., Fasano M. Verification of a Parkinson’s disease protein signature in T-lymphocytes by multiple reaction monitoring. J. Proteome Res. 2014;13(8):3554–3561. doi: 10.1021/pr401142p. [DOI] [PubMed] [Google Scholar]
- 257.Kitamura Y., Kojima M., Kurosawa T., Sasaki R., Ichihara S., Hiraku Y., Tomimoto H., Murata M., Oikawa S. Proteomic Profiling of Exosomal Proteins for Blood-based Biomarkers in Parkinson’s Disease. Neuroscience. 2018;392:121–128. doi: 10.1016/j.neuroscience.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 258.Yamagishi Y., Saigoh K., Saito Y., Ogawa I., Mitsui Y., Hamada Y., Samukawa M., Suzuki H., Kuwahara M., Hirano M., Noguchi N., Kusunoki S. Diagnosis of Parkinson’s disease and the level of oxidized DJ-1 protein. Neurosci. Res. 2018;128:58–62. doi: 10.1016/j.neures.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 259.Liu C.G., Wang J.L., Li L., Wang P.C. MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int. J. Mol. Med. 2014;34(1):160–166. doi: 10.3892/ijmm.2014.1780. [DOI] [PubMed] [Google Scholar]
- 260.Kole A.J., Swahari V., Hammond S.M., Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25(2):125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lippi G., Steinert J.R., Marczylo E.L., D’Oro S., Fiore R., Forsythe I.D., Schratt G., Zoli M., Nicotera P., Young K.W. Targeting of the Arpc3 actin nucleation factor by miR-29a/b regulates dendritic spine morphology. J. Cell Biol. 2011;194(6):889–904. doi: 10.1083/jcb.201103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hébert S.S., Horré K., Nicolaï L., Papadopoulou A.S., Mandemakers W., Silahtaroglu A.N., Kauppinen S., Delacourte A., De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Margis R., Margis R., Rieder C.R.M. Identification of blood microRNAs associated to Parkinsonĭs disease. J. Biotechnol. 2011;152(3):96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 264.Botta-Orfila T., Morató X., Compta Y., Lozano J.J., Falgàs N., Valldeoriola F., Pont-Sunyer C., Vilas D., Mengual L., Fernández M., Molinuevo J.L., Antonell A., Martí M.J., Fernández-Santiago R., Ezquerra M. Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson’s disease. J. Neurosci. Res. 2014;92(8):1071–1077. doi: 10.1002/jnr.23377. [DOI] [PubMed] [Google Scholar]
- 265.Barbagallo C., Mostile G., Baglieri G., Giunta F., Luca A., Raciti L., Zappia M., Purrello M., Ragusa M., Nicoletti A. Specific Signatures of Serum miRNAs as Potential Biomarkers to Discriminate Clinically Similar Neurodegenerative and Vascular-Related Diseases. Cell. Mol. Neurobiol. 2020;40(4):531–546. doi: 10.1007/s10571-019-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Salama M., Shalash A., Magdy A., Makar M., Roushdy T., Elbalkimy M., Elrassas H., Elkafrawy P., Mohamed W., Abou Donia M.B. Tubulin and Tau: Possible targets for diagnosis of Parkinson’s and Alzheimer’s diseases. PLoS One. 2018;13(5):e0196436. doi: 10.1371/journal.pone.0196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Teunissen C.E., Veerhuis R., De Vente J., Verhey F.R.J., Vreeling F., van Boxtel M.P.J., Glatz J.F.C., Pelsers M.A.L. Brain-specific fatty acid-binding protein is elevated in serum of patients with dementia-related diseases. Eur. J. Neurol. 2011;18(6):865–871. doi: 10.1111/j.1468-1331.2010.03273.x. [DOI] [PubMed] [Google Scholar]
- 268.Veitinger M., Oehler R., Umlauf E., Baumgartner R., Schmidt G., Gerner C., Babeluk R., Attems J., Mitulovic G., Rappold E., Lamont J., Zellner M. A platelet protein biochip rapidly detects an Alzheimer’s disease-specific phenotype. Acta Neuropathol. 2014;128(5):665–677. doi: 10.1007/s00401-014-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bermejo-Pareja F., Antequera D., Vargas T., Molina J.A., Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol. 2010;10:108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Carro E., Bartolomé F., Bermejo-Pareja F., Villarejo-Galende A., Molina J.A., Ortiz P., Calero M., Rabano A., Cantero J.L., Orive G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimers Dement. (Amst.) 2017;8:131–138. doi: 10.1016/j.dadm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kalló G., Emri M., Varga Z., Ujhelyi B., Tőzsér J., Csutak A., Csősz É. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS One. 2016;11(6):e0158000. doi: 10.1371/journal.pone.0158000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen Y., Zheng J., Su L., Chen F., Zhu R., Chen X., Ye Q. Increased Salivary microRNAs That Regulate DJ-1 Gene Expression as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2020;12:210. doi: 10.3389/fnagi.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Youn Y.C., Park K-W., Han S-H., Kim S. Urine neural thread protein measurements in Alzheimer disease. J. Am. Med. Dir. Assoc. 2011;12(5):372–376. doi: 10.1016/j.jamda.2010.03.004. [DOI] [PubMed] [Google Scholar]