Abstract
Oxidative stress, which results in the damage of diverse biological molecules, is a ubiquitous cellular process implicated in the etiology of many illnesses. The sulfhydryl-containing tripeptide glutathione (GSH), which is synthesized and maintained at high concentrations in all cells, is one of the mechanisms by which cells protect themselves from oxidative stress. N-acetylcysteine (NAC), a synthetic derivative of the endogenous amino acid L-cysteine and a precursor of GSH, has been used for several decades as a mucolytic and as an antidote to acetaminophen (paracetamol) poisoning. As a mucolytic, NAC breaks the disulfide bonds of heavily cross-linked mucins, thereby reducing mucus viscosity. In vitro, NAC has antifibrotic effects on lung fibroblasts. As an antidote to acetaminophen poisoning, NAC restores the hepatic GSH pool depleted in the drug detoxification process. More recently, improved knowledge of the mechanisms by which NAC acts has expanded its clinical applications. In particular, the discovery that NAC can modulate the homeostasis of glutamate has prompted studies of NAC in neuropsychiatric diseases characterized by impaired glutamate homeostasis. This narrative review provides an overview of the most relevant and recent evidence on the clinical application of NAC, with a focus on respiratory diseases, acetaminophen poisoning, disorders of the central nervous system (chronic neuropathic pain, depression, schizophrenia, bipolar disorder, and addiction), cardiovascular disease, contrast-induced nephropathy, and ophthalmology (retinitis pigmentosa).
Keywords: Antioxidant, glutathione, N-acetylcysteine, mucolytic, oxidative stress, acetaminophen, treatment, psychiatry
1. INTRODUCTION
Oxidative stress is a ubiquitous cellular process implicated in the etiology of many illnesses [1-5]. Several endogenous mechanisms exist to protect cells against oxidative stress [6]. The sulfhydryl (thiol)-containing tripeptide glutathione (GSH) is synthesized and maintained at high concentrations in virtually all cells and is one of the principal mechanisms by which reactive oxygen species (ROS) are eliminated [3, 7, 8].
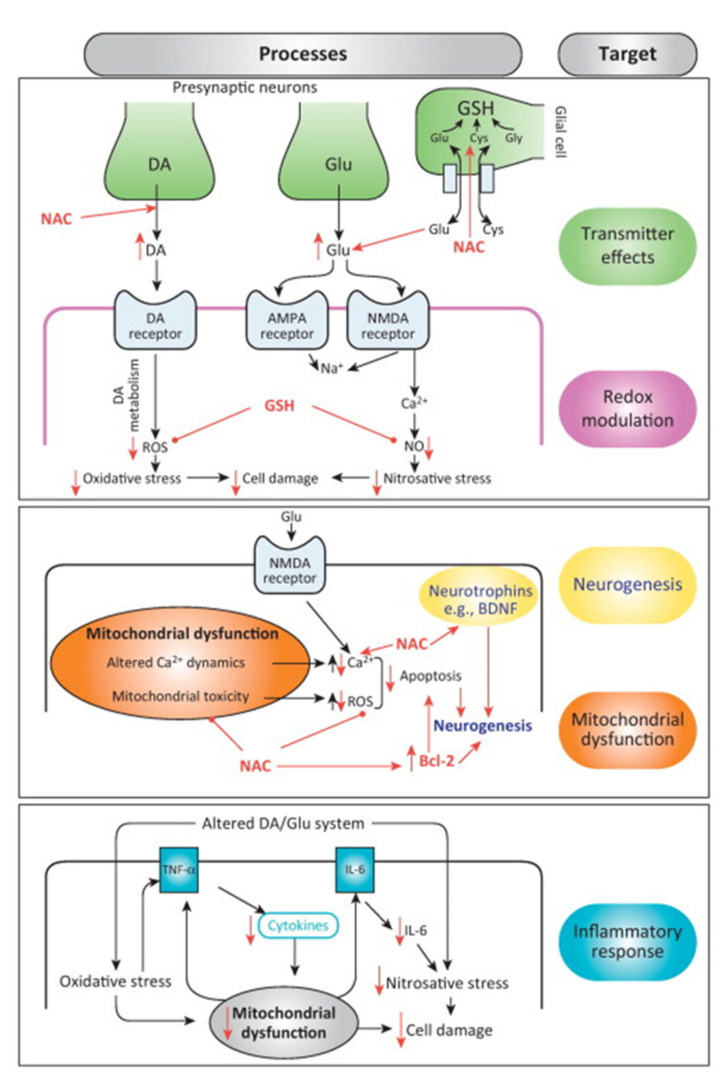
N-acetylcysteine (NAC) is a synthetic derivative of the endogenous amino acid L-cysteine (Fig. 1), and a precursor of GSH. NAC not only modulates oxidative stress but other pathophysiologic processes implicated in disease. These include mitochondrial dysfunction, apoptosis, and inflammation, as well as indirect effects on neurotransmitters such as glutamate and dopamine [9, 10]. The proposed pathophysiologic targets of NAC are presented in (Fig. 2), and will be covered further in subsequent sections.
Fig. (1).
Chemical structures of cysteine, N-acetylcysteine and glutathione.
Fig. (2).
Proposed pathophysiologic targets of N-acetylcysteine (NAC). The various mechanisms by which NAC acts as a neurotransmitter, modulates redox reactions, promotes neurogenesis, corrects mitochondrial dysfunction, and dampens the inflammatory response are described in the text.
Adapted from Berk M, et al. Trends. Pharmacol. Sci., 2013, 34(3), 167-177. ® Elsevier Inc.
AMPA, 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate; BDNF, brain-derived neurotrophic factor; Bcl-2, B cell lymphoma 2; Ca, calcium; Cys, cysteine; DA, dopamine; Glu, glutamate; Gly, glycine; GSH, glutathione; IL, interleukin; NAC, N-acetylcysteine; NMDA, N-methyl-D-aspartate; NO, nitrous oxide; ROS, reactive oxygen species; TNF, tumor necrosis factor. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Whether antioxidant molecules, including cysteine and GSH, given exogenously have a therapeutic effect has long attracted interest [11]. The therapeutic activity of NAC has been extensively investigated in in vitro and in vivo studies and, consequently, it has been used clinically for more than 50 years [12]. The clinical benefit of NAC as a mucolytic agent was first described in the 1960s in patients with cystic fibrosis (CF) [13]. In the 1970s, NAC was used to treat acetaminophen (paracetamol) overdose [14, 15].
Since the 1980s, in addition to its licensed indications as a mucolytic and an antidote to acetaminophen overdose, the therapeutic potential of NAC has been investigated in a wide range of conditions where oxidative stress is thought to be a driving factor in disease onset and/or progression [9]. In January 2019, a small expert group met to discuss the state-of-the-art of the use of NAC in clinical practice. This narrative and consensus-based review summarizes the main conclusions of that meeting, as well as providing an overview of the relevant current literature about the clinical applications of NAC. In the following sections, we will briefly describe the pharmacology of NAC and its role in counteracting oxidative stress. Next, we focus on the current clinical use of NAC in respiratory diseases and acetaminophen poisoning. Finally, we consider newer data about the potential of NAC in selected therapeutic areas, including disorders of the central nervous system (CNS; chronic neuropathic pain, depression, bipolar disorder, schizophrenia, and addiction), cardiovascular disease, contrast-induced nephropathy, and ophthalmology (retinitis pigmentosa [RP]).
2. METHODS
A literature search was carried out through PubMed/MEDLINE for all studies, randomized controlled trials, systematic reviews, meta-analyses, and review articles of NAC published up to February 2019. Published articles that addressed the review objective were discussed and reviewed, and selected papers were supplemented by the clinical experience of the panel. The bibliographies of retrieved papers identified in the search were manually searched for additional relevant articles, and recent recommendations and treatment guidelines were also considered for inclusion. The literature was monitored as the manuscript was developed, and papers identified reviewed for relevance.
3. PHARMACOLOGY OF NAC
GSH is synthesized in two steps from cysteine, glutamate, and glycine. Of these precursors, cysteine has the lowest intracellular concentration, limiting the rate of GSH synthesis in the presence of oxidative stress [8]. NAC is more stable than L-cysteine, which is rapidly oxidized in solution to the disulfide cysteine. Thus, NAC is a more efficient source of antioxidant sulfhydryl moieties than cysteine. Once inside cells, NAC is rapidly hydrolyzed to cysteine, which can be incorporated into GSH [8]. Furthermore, unlike GSH, NAC can cross the phospholipid bilayer of the plasma membrane of cells.
NAC is available as oral, inhalation, and intravenous (IV) formulations. In respiratory diseases, NAC is usually administered orally at a daily dose of 600 mg [16], although it may be more effective as a mucolytic agent when given by inhalation, as nebulized NAC may be able to reach a higher concentration in the airways [17]. However, available data do not support the efficacy of inhaled NAC in terms of lung function and disease exacerbations, probably due to the low intrinsic reducing activity and short half-life of NAC in the airway environment [17].
After oral administration, NAC is rapidly absorbed, with peak plasma concentrations reached between 30 minutes and 1 hour [8, 16]. Orally administered NAC is absorbed in the small intestine and undergoes first-pass hepatic metabolism to cysteine, which is used by the liver to synthesize GSH [8]. The hepatic pool of GSH is replenished before GSH is released into the plasma via a membrane transporter [8].
The oral bioavailability of NAC is low (< 5%), probably due to gut-wall metabolism and high first-pass metabolism [3, 8]. Low plasma detection may also be due to rapid diffusion into cells and conversion to GSH [8]. Intravenous NAC formulations bypass first-pass and gut-wall metabolism and rapidly provide sufficient concentrations in acute acetaminophen overdose [8].
3.1. NAC and Oxidative Stress
Oxidative stress, which arises from an imbalance between the levels of pro- and antioxidants, can lead to oxidation, peroxidation, and damage of vital cellular macromolecules, such as DNA, proteins, and lipids by ROS and by reactive nitrogen species (RNS) [18, 19]. ROS include the superoxide radical (O2– •), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH), and are mostly produced by the mitochondria in the pathway of oxidative phosphorylation during normal cell metabolism when molecular O2 is converted into H2O [8]. H2O2 is also produced by peroxisomes when fatty acids are degraded. In addition, oxidative bursts of ROS are used by phagocytes to kill microorganisms. Such reactions occur very rapidly, which means that markers of oxidative stress are usually indirect, and no validated biomarkers for oxidative stress are currently available [9, 20]. Various markers of oxidative stress include analysis of the activities of antioxidant enzymes glutathione peroxidase (GPx), glutathione reductase, catalase (CAT), glutathione-S- transferase, and superoxide dismutase (SOD), measuring levels of GSH or the GSH/oxidized GSH [GSH/GSSG] ratio, or monitoring markers of lipid peroxidation, oxidative DNA or protein damage [9, 20].
Intracellular H2O2 is mostly detoxified by the enzymes GPx and CAT. However, some molecules escape degradation. Oxidative stress occurs when metabolically derived oxidants prevail over antioxidant defenses. Cellular antioxidant defense mechanisms include vitamin C and vitamin E, enzymes such as SOD, CAT, and GPx, and thiols or sulfhydryl-containing compounds such as GSH and thioredoxin [6]. GSH is the most thoroughly investigated. It exerts a protective effect on cells not only as an antioxidant defense mechanism, but also because of a number of other effects, including protein thiolation, drug detoxification, and regulation of signal transduction modulated by oxidation-reduction reactions [8]. The antioxidant effect of GSH is provided by the free sulfhydryl group, which constitutes a source of reducing equivalents to scavenge harmful ROS [8]. GSH also plays a crucial role in the antioxidant activity of GPx, a defense mechanism against peroxides, by providing reducing equivalents.
In vivo, NAC mainly acts as an indirect antioxidant via its effect as a GSH precursor (Fig. 2), its contribution to scavenging superoxide and peroxides being negligible compared to that of antioxidant enzymes [8, 10, 21]. Nevertheless, the nucleophilic free sulfhydryl group of NAC enables it to act as a direct antioxidant against a number of electrophilic groups of oxidant radicals [10]. For example, NAC protects α1-antitrypsin from inactivation by hypochlorous acid, a potent oxidant produced by the myeloperoxidase enzyme of activated phagocytes [16].
The mechanisms usually thought to be important in the licensed indications for NAC, mucolysis, and detoxification of acetaminophen overdose, are fairly well understood, and both rely on the delivery of sulfhydryl moieties [8]. As a mucolytic, NAC breaks the disulfide bonds of heavily cross-linked mucus glycoproteins (mucins), thereby reducing mucus viscosity [21]. As an antidote to acetaminophen poisoning, NAC restores the hepatic GSH pool depleted in the drug detoxification process [8, 21]. GSH, in turn, reacts with and neutralizes the electrophilic and harmful metabolite of acetaminophen, N-acetyl-p-benzoquinone imine (NAPQI) [21] and scavenges reactive oxygen and reactive nitrogen species [22].
4. NAC FOR RESPIRATORY DISEASES
Aside from its ability to break disulfide bonds and change mucus rheology [9, 23], NAC has other actions that have the potential to treat pulmonary diseases. For example, NAC increases the secretion activity of alveolar type II cells, leading to increased alveolar surfactant [24]. In addition, antimicrobial and anti-biofilm properties against several respiratory pathogens [25], as well as interference with inflammatory pathways [4, 17], have also been reported. Thus, the mechanisms by which NAC acts in respiratory disease are complex and not limited to its antioxidant activity [4].
There are a number of large and well-designed trials reporting the efficacy and safety of NAC for the treatment of chronic obstructive pulmonary disease (COPD) [26-28] and idiopathic pulmonary fibrosis (IPF) [29-32]. These provide clinically valuable information but do not clarify which mechanisms are most important in explaining the NAC effect.
4.1. Chronic Obstructive Pulmonary Disease
COPD is a common, preventable and treatable disease caused by a mixture of small airways disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contribution of which varies from person to person [33]. Oxidative stress in response to endogenous and exogenous oxidants, including cigarette smoke and other inhaled oxidants leads to the chronic inflammation characteristic of COPD [4, 9, 33, 34]. Extracellular and intracellular levels of GSH are frequently abnormal in COPD, and the inability to maintain normal GSH levels may contribute to disease progression [17]. Lung function declines with age but more rapidly in COPD sufferers, and this excess decline likely reflects the cumulative effects of prolonged oxidative stress. Thus, COPD may be regarded as a disease of accelerated aging of the lungs, characterized by loss of elasticity, which leads to respiratory impairment from the age of 30 years, raising the possibility that, in younger patients, protection from oxidative stress could prevent disease progression. The time to progress to serious ill-health in COPD is unpredictable but is usually slow, so that preventive interventions, such as with NAC, do have a chance to be effective.
The hypothesis that treatment with NAC can prevent COPD exacerbations, (periods of symptomatic deterioration usually associated with increased pulmonary inflammation) was tested in the BRONCUS, HIACE, and PANTHEON trials, with somewhat mixed results reflecting the different doses, studied and different target populations Table (1) [26-28]. In the randomized, placebo-controlled BRONCUS trial, oral NAC 600 mg/day for 3 years did not reduce the rate of decline in forced expiratory volume in 1 second (FEV1) or the number of exacerbations per year [26], at least in patients who used inhaled corticosteroids (ICS) Table (1), possibly because the NAC concentration used was too low. In subsequent trials, a higher NAC dose of 1200 mg/day (oral NAC 600 mg, twice daily), was tested. In the 1-year HIACE trial in Chinese patients with stable COPD Table (1), high-dose NAC significantly improved lung function and decreased exacerbation frequency compared with placebo [27]. A post-hoc analysis of the HIACE trial showed that the benefits of high-dose NAC treatment in terms of reduced exacerbation frequency and prolonged time to first exacerbation were significant in the subgroup of patients at high risk of exacerbations, but not in those at low risk [35].
Table 1.
Summary of randomized, placebo-controlled controlled trials of oral N-acetylcysteine in chronic obstructive pulmonary disease.
| First Author | Study | Sample Size & Population | Intervention | Primary Outcomes | Comments |
|---|---|---|---|---|---|
| Decramer 2005 [26] |
BRONCUS | Smoking-related stable COPD (n = 523) Age 40–75 years FEV1 40–70% predicted Mean age 62 years Women 21% Current smokers 46% Mean predicted FEV1 57% |
600 mg of NAC daily or matching placebo for 3 years | No difference in FEV1 in pts (54 mL vs. 47 mL; 95% CI –25, 10); No overall difference in exacerbations per year (HR 0.99, p = 0.85) Potential reduction in exacerbation rate in pts not treated with ICS |
Dose only 600 mg once daily |
| TSE 2013 [27] |
HIACE | Smoking-related stable COPD (n = 120) Age 50–80 years FEV1 < 70% predicted value over 1 year Mean age 71 years Women 7% Current smokers 23% Mean predicted FEV1 54% |
600 mg of NAC twice daily or matching placebo for 1 year | FEF improved 25% to 75% (p = 0.037) Reduction in exacerbation frequency (0.96 vs. 1.71 times per year, p = 0.019) |
Trend towards a reduction in hospital admission rates |
| Zheng 2014 [28] |
PANTHEON | Moderate-to-severe COPD (n = 1006) Age 40–80 years FEV1 30–70% of predicted value over 1 year Mean age 66 years Women 18% Current smokers 18% Ex-smokers 58% Non-smokers 24% Mean predicted FEV1 49% |
600 mg of NAC twice daily or matching placebo for 1 year | Reduction in exacerbation frequency (1.16 vs. 1.49 per patient-year, p = 0.0011; RR 0.78, 95% CI 0.67–0.90; p = 0.0011) | Time to second exacerbation and time to third exacerbation extended No significant difference in treatment effect and ICS use |
Abbreviations: CI confidence interval, COPD chronic obstructive pulmonary disease, FEF forced expiratory flow, FEV1 forced expiratory volume in one second, HR hazard ratio, ICS inhaled corticosteroids, NAC N-acetylcysteine, pts patients, RR risk ratio.
In the large, randomized, double-blind, placebo-controlled PANTHEON trial, high-dose NAC for 1 year reduced the rate of acute exacerbations of COPD (AECOPD) by 22% [28]. Like the HIACE trial, PANTHEON involved Chinese patients. One-year treatment with high-dose NAC reduced the risk of AECOPD by 22% Table (1), independent of corticosteroid use. NAC was effective from 6 months onwards, suggesting that the preventive effects of NAC are slow to develop but then progress, and are sustained with regular treatment. Thus, early intervention might be essential to prevent the progression of COPD in terms of exacerbations [28].
Post-hoc analysis of the PANTHEON study confirmed that NAC reduces the rate of COPD exacerbations defined using conventional criteria, compared with placebo, particularly in patients with a history of smoking or not treated with ICS [36]. NAC may thus represent an alternative to ICS-containing therapies in these subgroups. A meta-analysis clarified the role of NAC in preventing exacerbations of chronic bronchitis or COPD and evaluated the differences between the responses induced by low (≤ 600 mg/day) and high (> 600 mg/day) doses of NAC [37]. The data from 13 studies in a total of 4,155 patients in a variety of patient populations showed that patients treated with NAC had consistently and significantly fewer exacerbations of chronic bronchitis or COPD (relative risk 0.75, 95% CI 0.89–0.97, p < 0.01). The beneficial effect of NAC was more marked in patients with no evidence of airway obstruction. However, high-dose NAC was also effective in patients with airway obstruction. These findings suggest that NAC should be used at a dose ≥ 1200 mg/day to prevent exacerbations in patients with a chronic bronchitis phenotype of COPD and airway obstruction, while regular doses (600 mg/day) may be sufficient in the absence of airway obstruction [37].
Based on the evidence emerging from the recent trials showing that NAC can reduce exacerbations in patients with COPD, including those taking ICS, the 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report includes NAC, as well as other thiol-based drugs, as additional therapies to consider in the treatment of COPD [33].
4.2. Idiopathic Pulmonary Fibrosis
IPF is a chronic, fibroproliferative interstitial pneumonia of unknown etiology, occurring primarily in the elderly and resulting in a progressive loss of lung function and a poor prognosis. Oxygen radicals and decreased GSH levels are implicated in the pathogenesis and progression of IPF [38]. Therefore, NAC and other thiol-based drugs may be useful for the treatment of IPF. Preclinical studies have revealed that NAC inhibits several profibrotic mechanisms in a number of experimental models [39]. Glutathione has antifibrotic effects on lung fibroblasts in vitro [38]. Decreased lung glutathione levels have been documented in bronchoalveolar lavage obtained from patients with IPF, and its level is augmented by oral NAC and associated with improved lung function in patients with IPF [40]. The randomized, placebo- controlled IFIGENIA trial in 182 patients with IPF investigated the effectiveness of 1-year treatment with high-dose oral NAC (600 mg, three times daily) added to standard therapy with prednisone plus azathioprine [29]. NAC was shown to preserve lung function (vital capacity and single-breath carbon monoxide diffusing capacity) more than standard therapy plus placebo, with no significant differences in the safety profile of the two treatments. However, subsequent well-designed, randomized, placebo-controlled clinical trials with NAC 600 mg three times daily, combined with other medications or in monotherapy failed to provide evidence supporting the use of NAC in patients with IPF [31] and identified safety issues with the combination of prednisone, azathioprine, and NAC [30]. Interestingly, the analysis of single-nucleotide polymorphisms (SNPs) within the genes TOLLIP and MUC5B, which are involved in lung host defense, suggested that the response to NAC could be influenced by the genotype of IPF patients (i.e., the TOLLIP rs3750920 genotype) [41]. The SNP analysis was performed on the data of the PANTHER-IPF trial evaluating the safety of the combination of prednisone and azathioprine with NAC [30].
The recent phase 2, placebo-controlled PANORAMA trial was designed to assess the safety and tolerability of NAC combined with pirfenidone in patients with IPF (n = 123) [32]. The study also included exploratory efficacy measurements (forced vital capacity, carbon monoxide diffusing capacity, and 6-minutes' walk distance). The addition of NAC to pirfenidone did not significantly affect the safety and tolerability profile of pirfenidone, but it did not appear to confer further benefits in terms of forced vital capacity, although it must be acknowledged that the study was underpowered to demonstrate a meaningful difference. Therefore, the debate over the therapeutic potential of NAC in subgroups of IPF patients is evident, and it is hoped that the studies in carriers of the TOLLIP rs3750920 genotype will confirm the reported benefits of NAC in this subgroup before the potential of NAC in IPF is excluded [42]. In this regard, a multicenter clinical trial in this specific subgroup is currently underway.
4.3. Current Use of NAC in Clinical Practice of Pulmonary Medicine
The implementation of NAC and other thiol-based drugs in pulmonary medicine remains limited [17], and there is a need to examine further the implications of recent findings to fully define the potential of NAC in respiratory diseases, including COPD [43].
Current treatment guidelines for COPD support the use of mucolytics, including NAC, to reduce the risk of exacerbations [33, 44]. Although there are limited published data on its potential benefits as a mucolytic agent in bronchiectasis (CF and non-CF bronchiectasis), our experience suggests it is being used in clinical practice in lung diseases associated with tenacious and copious sputum production, such as in allergic bronchopulmonary aspergillosis and bronchiectasis regardless of cause, and there is anecdotal evidence for the use of oral NAC as an antioxidant for IPF.
5. USE OF NAC IN TOXICOLOGY
The most extensively investigated application of NAC in toxicology is its use as an antidote to acetaminophen poisoning. ROS and RNS play a crucial role also in acute liver injury induced by acetaminophen overdose [22]. Although some damage to proteins, lipids, and mitochondrial DNA occurs, mitochondrial oxidative stress and mitochondrial membrane permeability transition activation are the key events in acetaminophen-induced hepatic cell necrosis [45, 46].
5.1. NAC as an Antidote to Acetaminophen Poisoning
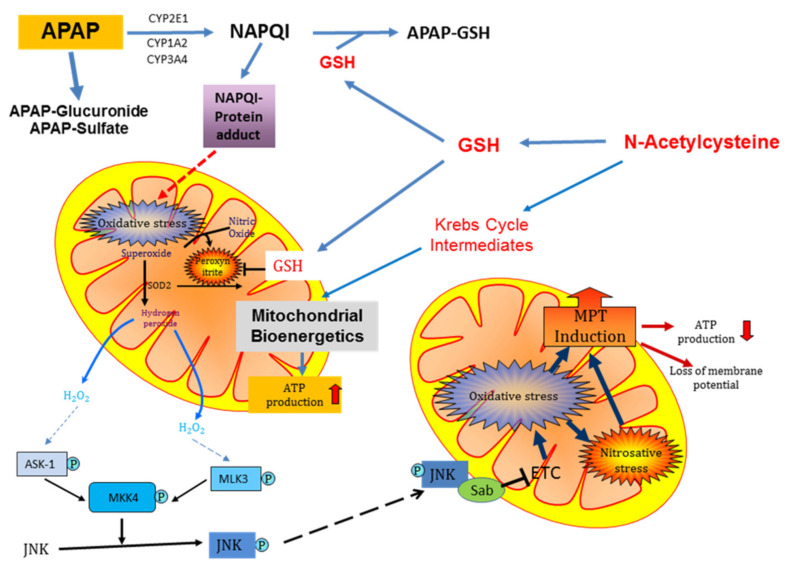
Acetaminophen at therapeutic doses is considered to be a safe drug. Under these conditions, more than 90% of acetaminophen is eliminated through phase II conjugation reactions, and only less than 10% is metabolized by cytochrome P450 2E1, forming the reactive metabolite NAPQI [47]. NAPQI is effectively detoxified by conjugation with cellular GSH, and only a very limited amount covalently binds to proteins [47, 48]. However, in the case of an overdose of acetaminophen, NAPQI formation is substantially increased, resulting in the depletion of hepatic GSH levels and increased protein adduct formation, including the formation of mitochondrial protein adducts [48] (Fig. 3). Importantly, protein adducts on mitochondria are most critical for toxicity because they trigger initial oxidative stress, which is amplified through a mitogen-activated protein (MAP) kinase cascade with the ultimate activation of c-jun N-terminal kinase (JNK). The mitochondrial translocation of phospho-JNK causes the enhanced leakage of electrons from the electron transport chain and formation of superoxide, which reacts with nitric oxide (NO) to form the highly reactive oxidant peroxynitrite in the mitochondria [49]. Peroxynitrite is considered the ultimate oxidant responsible for the mitochondrial membrane permeability transition pore (MPTP) opening, potentially leading to liver cell death [50-52]. The MPTP opening causes cessation of ATP production and matrix swelling [45] (Fig. 3), which results in the rupture of the outer mitochondrial membrane and the release of intermembrane proteins, including endonuclease G and apoptosis-inducing factor (AIF). Both endonuclease G and AIF translocate to the nucleus and cause DNA fragmentation [53]. Together, these events trigger cellular necrosis [54]. Importantly, these mechanisms of cell death demonstrated in mice and mouse hepatocytes also apply to acetaminophen-induced cell death in human hepatocytes and in overdose patients [55-57].
Fig. (3).
Schematic of the metabolism of acetaminophen by the hepatocyte and mechanism of action of N-acetylcysteine as an antidote to acetaminophen-induced hepatotoxicity in overdose.
APAP, acetaminophen; ASK, apoptosis signal-regulating kinase; ATP, adenosine triphosphate; CYP, cytochrome P450; GSH, glutathione; JNK, c-jun N-terminal kinase; MLK, mixed-lineage kinase; MPT, mitochondrial permeability transition; NAPQI, N-acetyl-p-benzoquinone imine; Sab, SH3 domain-binding protein that preferentially associates with Btk; SOD, superoxide dismutase. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Based on the early, limited mechanistic understanding of acetaminophen hepatotoxicity, which included reactive metabolite formation and hepatic GSH depletion [58, 59], various antidotes with the capacity to replenish hepatic GSH levels were tested. Several antidotes worked in animal models and in patients [15]. However, NAC was quickly adopted as the preferred antidote for acetaminophen overdose in patients because of its superior efficacy and limited side effects [14, 15, 60].
NAC is most effective in patients when given within the first 8-10 hours after an overdose [14, 61] but still shows some beneficial effects when administered later [61, 62]. Among patients presenting with plasma acetaminophen concentrations that put them at risk for hepatotoxicity, 6–7% of patients treated with NAC within 10 hours after the overdose developed liver injury, as indicated by a transient increase of plasma alanine aminotransferase levels; 26–29% showed hepatotoxicity when NAC was given between 10–16 hours, and 40–60% developed liver injury when NAC was started between 16–24 hours [61, 62]. These results were all significantly better than those of historical controls.
To ensure the appropriate use of NAC to prevent liver injury from acetaminophen toxicity, a nomogram that plots acetaminophen plasma concentrations against the time of overdose was developed [63] and subsequently modified [60, 61]. Although the current nomogram is conservative and has proven to be a reliable guide for NAC treatment after acetaminophen poisoning [64], treatment decisions based on a single measurement may not assess the risk completely, as patients who were initially stratified as below the treatment line, can cross the treatment threshold following overdose [65].
Animal studies indicated that early NAC treatment protects by scavenging NAPQI and by preventing protein adduct formation [66]. However, NAC does not react directly with NAPQI but provides the essential amino acid cysteine for the synthesis of GSH, which reacts with NAPQI [67, 68] (Fig. 3). GSH also directly scavenges peroxynitrite and functions as a co-factor for the detoxification of hydrogen peroxide by GPx [50]. Furthermore, excess NAC not used for GSH synthesis is converted to Krebs cycle intermediates and supports mitochondrial bioenergetics leading to higher ATP levels in hepatocytes [69] (Fig. 3).
NAC also protects against mitochondrial dysfunction [50, 51, 69], a key factor in acetaminophen-induced liver injury [52]. Thus, NAC has multiple modes of action against acetaminophen-induced hepatotoxicity, which helps explain why NAC is beneficial, even if administered with significant delay after the overdose in patients [62] and human hepatocytes [55].
A key to recovery after acetaminophen-induced liver injury is the onset of regeneration and replacement of necrotic hepatocytes [70], which is critically dependent on mitochondrial biogenesis [71]. Although early NAC treatment is beneficial in limiting the injury, continuous NAC treatment well beyond the injury phase can delay regeneration [72] mainly through impairment of mitochondrial biogenesis, an adaptive response whose role is to maintain or restore homeostasis following mitochondrial dysfunction [71]. This is an underappreciated problem of prolonged NAC treatment.
Despite the widely accepted use of NAC as an antidote against acetaminophen poisoning, for ethical reasons, no randomized clinical trials were conducted in establishing the efficacy and optimal dose of NAC [60, 73]. The initial IV treatment of a limited number of acetaminophen overdose patients with an empirical dose of NAC showed complete protection of all patients that were treated within 10 hours of the overdose [15]. In a follow-up study, only one patient out of 62 treated with NAC within 10 hours developed severe liver injury as compared to a retrospective control group, which showed that 33 out of 57 patients (58%) developed severe liver injury after an acetaminophen overdose [14]. In a similar study in the US, over 600 patients were treated with an oral dose of NAC [61]. Only 7% of patients with plasma acetaminophen levels in the toxic range developed transient liver injury when treated within 10 hours after the overdose. However, the efficacy of NAC declined when treatment was initiated later [61, 62]. The high efficacy after early treatment and the limited side effects compared to cysteamine and other compounds established NAC as the antidote of choice for acetaminophen overdose [14, 61]. In addition, even late presenting patients with acetaminophen-induced fulminant hepatic failure showed a survival benefit when treated with NAC [74].
Due to the lack of randomized clinical trials, there is no universally accepted NAC treatment protocol. In the 1970s, Prescott and coworkers in the UK used an IV protocol [15], while Rumack and coworkers in the US developed a 72-hour oral treatment protocol because the US Food and Drug Administration did not approve the use of the mucolytic drug by the IV route [60]. In 2004, an IV preparation was approved in the US. Retrospective comparison of cohorts treated with a 20-hour IV and a 72-hour oral protocol showed a slightly better outcome with IV treatment for early presenting patients (< 12 hours), no difference when treated between 12 hours and 18 hours, and lower risk with the 72-hour oral regimen [75]. The limitations of this study have been discussed [76, 77]; a more recent comparison between shorter IV versus longer oral treatments did not find any significant difference in adverse outcomes when patients were treated within 8 hours of the overdose [78]. In addition, in low-risk patients, an abbreviated 12-hour IV NAC dosing regimen was as effective as treatment with the same dose over 20 hours but with fewer adverse effects [79, 80]. Thus, the various NAC treatment protocols are highly effective for most patients presenting early (< 8 hours) after an overdose and at least partially effective for late-presenting patients. However, there is some concern that patients with massive acetaminophen overdoses will need more than the standard NAC treatment [81-83]. Given the potential increased adverse effects of high doses of NAC, including anaphylactic reactions and fluid overload [84], mechanistically complementary interventions such as 4-methylpyrazole may be beneficial in supporting standard-of-care NAC treatment in patients with high overdoses [85-87].
Challenges for treating acetaminophen overdose patients with the optimal NAC dosing regimen remain because of the variations in acetaminophen dose, use of different preparations of acetaminophen, and co-ingestion of various other drugs or alcohol. Interestingly, since the adoption of IV NAC as the mainstay for the treatment of acetaminophen poisoning, the overall case-fatality has decreased from approximately 3% in the early 1970s to less than 1% in the 2008–2012 period [73, 88].
5.2. Role of NAC in Non-acetaminophen Poisonings
The antidote effects of NAC to other toxic compounds are less well established than those to acetaminophen, and this is an area of toxicology still in development [9]. Investigated toxins include the herbicide paraquat [89], mushroom toxins [90], essential oils [91], and hydrocarbons (chloroform, carbon tetrachloride) [92, 93]. As with acetaminophen overdose, NAC exerts an antidote action predominantly by replenishing the cellular GSH pool; antioxidant effects and scavenging of free radicals have also been reported [9]. Furthermore, NAC has also been investigated for heavy-metal poisoning and has the potential of acting as an antidote for heavy metal ions such as lead [94-96], cadmium, chromium, and mercury [97]. According to the prevailing hypothesis about the mechanisms of lead toxicity, Pb(II) oxidizes GSH, leading to increased levels of free radicals [95].
6. NAC FOR NEUROLOGIC AND PSYCHIATRIC DISORDERS
NAC has been investigated in many neurologic and psychiatric diseases, including neurodegenerative diseases, chronic and neuropathic pain, mood disorders, psychoses, and addiction [5, 9, 98, 99]. Cognitive dysfunction lies at the core of most of these conditions, and oxidative stress has been implicated in cognitive impairment [100]. A recent systematic review provides an overview of the available data on the effects of NAC on human cognition in healthy individuals and in patients with Alzheimer’s, bipolar disorder, schizophrenia, and other diseases of the CNS [100]. The available data, albeit early, suggest that NAC as an intervention provides significant cognitive improvements. However, the clinical relevance of its effects is currently unclear, given the limitations of the studies identified, including the heterogeneity of studies, the number of studies with insufficient power, and other methodologic considerations [100].
Preclinical data support a positive effect of NAC on cognition in animal models of mitochondrial dysfunction, inherited metabolic disorders, heavy metal neurotoxicity, and Alzheimer’s disease [101-105]. Such data has helped dissect the mechanisms underlying the effects of NAC on the CNS. For example, a study in mice lacking glutamate transporter type 3 (excitatory amino acid transporter-3 [EAAT3]), an animal model that recapitulates brain changes and cognitive impairment associated with aging, showed that NAC was able to reverse learning and memory impairment [106]. Besides taking up glutamate, EAAT3 also transports cysteine, the rate-limiting substrate in the synthesis of glutathione. Mice lacking EAAT3 have decreased levels of GSH and increased oxidative stress in neurons, and are characterized by premature brain aging. Although the cognitive impairment of the EAAT3 knockout mice might be due also to impaired glutamate uptake, the authors concluded that NAC treatment reversed cognitive impairment, at least in part, by increasing neuronal GSH levels.
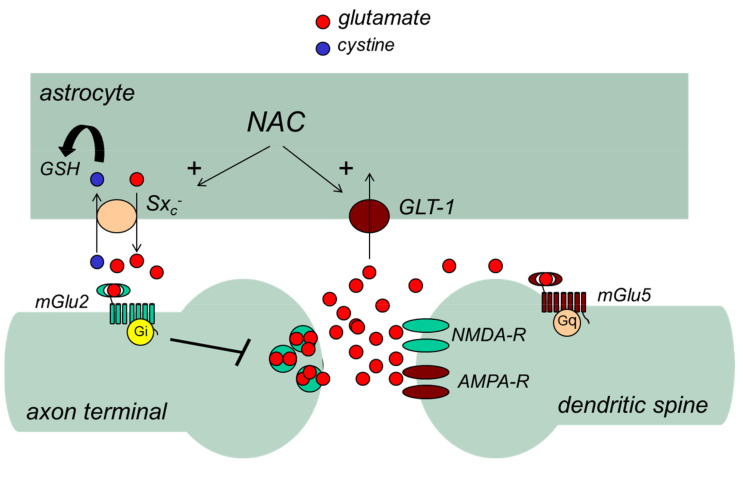
A large body of preclinical evidence has shown that NAC can act on the CNS by modulating glutamate homeostasis [107] (Fig. 2). Glutamate is the major excitatory neurotransmitter in the CNS and is involved in mechanisms of activity-dependent synaptic plasticity underlying learning and memory processes, such as long-term potentiation (LTP) and long-term depression (LTD) [108]. However, excessive excitatory action of glutamate may cause excitotoxic neuronal damage as a result of a sustained influx of extracellular calcium ions (Ca2+) [109, 110]. For this reason, the homeostasis of extracellular glutamate is tightly controlled by the combined activity of neuronal and glial membrane transporters, such as glutamate transporter-1 (GLT1), glutamate aspartate transporter (GLAST), and the cysteine/glutamate antiporter, or system xc– (Sxc–), present in astrocytes, and EAAT3 present in neurons. GLT1, GLAST, and EAAT3 clear synaptic and extra-synaptic glutamate, whereas Sxc– mediates the exchange of extracellular cysteine and intracellular glutamate across the cellular plasma membrane [111]. Sxc– is a fundamental component for the control of extracellular glutamate and feedback regulation of glutamate release [107]. The uptake of cysteine provides the intracellular cysteine required for the synthesis of GSH [111]. Notably, NAC has been shown to activate Sxc– [111, 112] and to induce the expression of GLT1 [113].
Glutamate receptors are subdivided into ionotropic receptors (i.e., α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate [AMPA], N-methyl-D-aspartate, and kainate receptors), which form ligand-gated ion channels, and metabotropic glutamate (mGlu) receptors, which are coupled to G proteins (Fig. 2). By activating Sxc–, NAC enhances the endogenous activation of presynaptic mGlu2 receptors, thereby inhibiting glutamate release from excitatory nerve endings [114]. This action, combined with the induction of GLT1 in astrocytes, may at least in part account for the beneficial effects of NAC in conditions associated with excessive release of glutamate and impairment of glutamate homeostasis, and characterized by maladaptive synaptic plasticity and excitotoxic neuronal death [113]. Conditions associated with altered glutamate homeostasis include drug addiction, chronic pain, depression, schizophrenia, and neurodegenerative disorders [107], suggesting potential applications for NAC as adjuvant therapy in these disorders. (Fig. 4) summarizes the actions of NAC on Sxc– and its effects on glutamate homeostasis.
Fig. (4).
Regulation of glutamate homeostasis by N-acetylcysteine (NAC). NAC stimulates glutamate efflux by activating the cysteine:glutamate antiporter (Sxc–) thereby enhancing the endogenous activation of mGlu2 receptors. In addition, NAC enhances the clearance of synaptic glutamate by inducing GLT-1.
GLT, glial membrane transporter; GSH, glutathione; mGlu, metabotropic glutamate; NMDA, N-methyl-d-aspartate. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6.1. NAC and Addiction
In preclinical studies, mGlu receptors are promising candidate pharmacotherapeutic targets in the management of substance use disorders [115]. The role of NAC in glutamate homeostasis has prompted research into the use of NAC for the treatment of addiction; most preclinical and clinical trials of NAC in neurologic and psychiatric diseases relate to addiction [9].
Studies in animal models suggest that vulnerability to relapse reflects an alteration in glutamate homeostasis in the nucleus accumbens [114, 116]. In particular, cocaine treatment in mice has been shown to cause a bidirectional loss of activity-dependent synaptic plasticity in the nucleus accumbens core, which is a defect in both LTP and LTD of excitatory synaptic transmission [114]. This was associated with reduced expression of xCT (the catalytic subunit of Sxc–) and mGlu2 receptors [116], suggesting that a defective endogenous activation of mGlu2 receptors in the nucleus accumbens underlies the altered glutamate homeostasis that is ultimately responsible for the loss of control in drug-seeking behavior and relapse. Systemic treatment of the cocaine-addicted mice with NAC was able to normalize both LTP and LTD in the nucleus accumbens; furthermore, NAC action was inhibited by the potent mGlu2/3 receptor antagonist, LY341495 [116].
More recent findings suggest that NAC corrects the alterations in glutamate homeostasis associated with drug addiction by inducing GLT1, a glial transporter that clears extracellular glutamate and inhibits the activation of extrasynaptic receptors that are involved in the induction and expression of maladaptive synaptic plasticity, such as mGlu5 receptors [117]. This mechanism might be at least as important as the activation of xCT for the ability of NAC to inhibit drug- and cue-induced reinstatement of cocaine, heroin, and nicotine seeking [112, 118-125]. Interestingly, NAC suppressed D9-tetrahydrocannabinol and cannabidiol self-administration and cue-induced reinstatement in a rodent model of cannabis relapse [126].
Clinical data provide some support for the efficacy of NAC in preventing relapse and normalizing glutamate homeostasis. In a 4-week, open-label pilot study in 23 treatment-seeking cocaine-dependent subjects, NAC at doses of 1.2, 2.4, and 3.6 g/day improved retention rates and was well tolerated [127]. Most subjects who completed the study (n = 16) either discontinued the use of cocaine or significantly reduced the use of cocaine during treatment. In an open-label, randomized, cross-over study, cocaine-dependent patients showed higher glutamate levels in the dorsal anterior cingulate cortex compared with healthy controls, as assessed by proton magnetic resonance spectroscopy [128]. In the cocaine-dependent patients, glutamate levels were normalized by a single oral administration of NAC (2.4 g) [128]. In a recent randomized, placebo-controlled trial conducted to investigate the effect of NAC and working memory-training to reduce cocaine use and craving and to improve inhibition, a 25-day treatment with NAC (2.4 g/day) was associated with fewer cocaine problems and cocaine-positive urines compared with placebo [129]. In this study, NAC had, however, no effects on cocaine craving.
The efficacy of NAC in addiction is not always apparent. For example, NAC consistently reduced cannabis use in adolescents but not in adults [130-133]. There are data suggesting efficacy in smoking cessation [134]. A systematic review of nine studies (165 patients) evaluating various addictions (cocaine, cannabis, nicotine, methamphetamine, pathologic gambling), confirmed the potential of NAC for the treatment of addiction, especially to cocaine and cannabis [135]. The involvement of glutamatergic pathways in the pathophysiology of addiction was also confirmed. However, another systematic review concluded that the evidence for NAC as a treatment for addiction is rather limited and pointed out that although several controlled studies have reported significant results for cocaine, the largest and best-designed trial was positive versus placebo only in a small subgroup of participants, who were abstinent at the beginning of the trial [5]. Finally, a recent meta-analysis of clinical studies with NAC in substance use disorders showed that NAC is superior to placebo in reducing drug craving [136].
In spite of the inconsistencies in currently available clinical evidence, the prevailing view in the field is that the safety and tolerability profile of NAC encourages further study of the use of the drug in the treatment of addiction and associated psychiatric disorders. Large scale studies in methamphetamine addiction and other addictive disorders are underway [137].
6.2. NAC for the Treatment of Depression
Chronic pain and depression often coexist, and activation of specific brain regions that are associated with the so- called “pain matrix,” including the insula, cingulate cortex, and amygdala, play a key role in the regulation of mood and in the mechanisms of resilience to stress. Interestingly, the mGlu2 receptor, the endogenous activation of which is enhanced by NAC via Sxc– activation, as discussed in the previous sections, has also been linked to resilience to stress. Accordingly, Nasca and coworkers found that chronic unpredictable stress causes large reductions in hippocampal mGlu2 receptors exclusively in mice that are not resilient to stress, and that mice lacking mGlu2 receptors are more susceptible to stress [138]. A follow-up study prompted by these findings showed that chronic stress caused a decrease in mGlu2 receptors and xCT in the ventral hippocampus via an epigenetic mechanism [139]. A short treatment period (3 days) with either NAC or L-acetylcarnitine, but not fluoxetine, corrected the depressive-like phenotype in these mice by inducing resilience to stress [139]. There are experimental data in mice that the antidepressant effects of NAC are blocked by AMPA antagonists [140], suggesting the involvement of AMPA receptors in the antidepressant-like effects of NAC. The use of NAC in depression is supported by the presence of oxidative stress in depression, suggesting glutathione is a feasible treatment target [141].
Data from clinical trials are in line with the evidence from preclinical research and suggest the potential of NAC as adjunctive treatment to antidepressant therapy [142, 143]. A placebo-controlled trial in 252 patients with major depressive disorder who were randomized to 12-weeks’ NAC or placebo added to standard antidepressant treatment, and followed up to 16 weeks, found no significant differences in depressive symptoms assessed using the Montgomery-Ǻsberg Depression Rating Scale (MADRS) between the two groups at week 12 (primary endpoint) [144]. However, other between-group differences were significant and in favor of NAC at the 16-week post discontinuation endpoint. This was true in other situations. In the smoking cessation trial of Prado and colleagues, there was a significant reduction in depression [134], a secondary outcome also seen in the post- traumatic stress disorder trial of Kalivas and colleagues [99]. Lastly, the IPF trial conducted by the Idiopathic Pulmonary Fibrosis Clinical Research Network reported significant benefits of NAC on mental health quality of life, a measure highly associated with mood measured psychometrically [31]. A recent meta-analysis including five studies evaluating NAC and 574 patients with depression (291 randomized to NAC and 283 to placebo) with a follow-up of 12–24 weeks revealed significantly greater improvements in depressive symptoms (MADRS) and functionality with NAC than with placebo [143]. NAC was associated with a favorable safety and tolerability profile, overlapping with that of placebo.
6.3. NAC in Schizophrenia and Bipolar Disorder
Several clinical trials have investigated the utility of adjunctive NAC in schizophrenia, with moderate benefits suggested, particularly for negative symptoms [5]. Specifically, one double-blind trial in 140 patients with chronic schizophrenia, described by the Cochrane Collaboration as a “high quality” trial that measured clinically-useful outcomes (Positive and Negative Syndrome Scale [PANSS] and Clinical Global Impression [CGI]) over a longer period of time [145], compared adjunctive NAC versus placebo [146]. Patients were randomized to oral NAC 1 g twice daily or placebo as an add-on to maintenance therapy. After 24 weeks, there were significantly greater improvements in the NAC group in PANSS total, negative, and general qualitative measures (p = 0.009, 0.018, and 0.035, respectively) and CGI- Severity (CGI-S) and CGI-Improvement (CGI-I) scores (p = 0.004, and 0.025, respectively) [146]. No significant change in the PANSS positive subscale was seen. A qualitative analysis follow-up to this study showed improved mental state for patients receiving NAC [147].
A benefit in terms of negative symptoms of schizophrenia was also shown in another, shorter-term study [148], and NAC has been shown to have beneficial effects in experimental models of schizophrenia through the regulation of glutamate homeostasis [107]. While there are negative studies, a recent 1-year study showed benefits for NAC over placebo at months 9 and 12 in negative but not positive symptoms, replicating the findings of previous studies [149, 150]. This finding was upheld by subsequent meta-analysis [151]. However, further adequately-powered real-world studies with appropriate follow-up periods are needed to confirm the role of adjunctive antioxidants in schizophrenia; in this respect, NAC is considered to be a promising intervention that should have priority in the design of such trials [145].
Data from several controlled and uncontrolled studies of adjunctive oral NAC 1 g twice daily have shown beneficial effects on the depressive component of bipolar disorder during maintenance phase treatment [142, 152-157]. In one randomized controlled trial, significant improvements compared with placebo were observed on the MADRS and Bipolar Depression Rating Scale (BDRS) and on secondary outcome measures of clinical status, quality of life, and functioning [142]. Effect sizes at study endpoint were medium- to-high for improvements in MADRS and for 9 of 12 secondary measures. There was no significant difference between NAC and placebo in the other primary outcome measure, time to a new mood episode [142]. Data from various subgroup analyses further support the benefits of NAC in terms of symptom severity, functioning, response rate, symptom remission, and quality of life [155-158].
A subsequent 2-month open-label phase of a randomized placebo-controlled trial of adjunctive oral NAC 1 g twice daily in 149 patients with moderate bipolar depression showed robust improvement in BDRS, quality of life, and functioning [152]. Latency to a new mood episode was not improved in the controlled phase of the study [153], in which very few participants in either arm relapsed, suggesting that NAC may not affect cycling between mood states. However, more recent, but shorter, studies have not confirmed this result [151]. Whether this reflects a true null effect, or whether NAC takes longer to work, as suggested by the two initial studies (6 months) and the Brieier study in schizophrenia (9 months), remains to be determined.
6.4. NAC in Obsessive-compulsive Disorder and Autism
The antioxidant properties of NAC and its ability to modulate neurotransmitters such as glutamate and dopamine have also led to adjunctive NAC being investigated in obsessive-compulsive disorder (OCD), where excessive glutamatergic activity and altered dopamine signaling are thought to play a role [9]. There were benefits (improvements in the Yale-Brown Obsessive Compulsive Scale and CGI-S after 12 weeks), compared with placebo, although not in CGI-I [159]. However, systematic reviews of trials in OCD have found limited robust data for the treatment effects of NAC in OCD, and further studies are needed [5, 98, 160-162].
Although parameters of oxidative stress may be elevated in children with an autism spectrum disorder, suggesting a role for NAC in the domain of irritability, the evidence is inconsistent and limited by studies with small sample size or small effect size [163]. Decreased levels of GSH, GPx, cysteine, and methionine, and increases in GSSG concentrations, compared with controls, have been shown in a number of studies, whereas levels of homocysteine, SOD, and cystathionine have not been shown to be consistently different between children with autism and controls [163]. A role for NAC in supporting mitochondrial metabolism has been shown in preclinical models of autism spectrum disorder, reviewed in Frye and Berk, 2019 [9], and promising, if mixed, results have been seen in some aspects of autism spectrum disorder in the limited number of studies conducted to date [5, 9]. Larger clinical trials are needed to determine whether core autism symptoms may respond to treatment with NAC.
6.5. NAC for the Treatment of Chronic Pain
All types of chronic pain, such as nociceptive, inflammatory, neuropathic, and neurovascular pain, are characterized by the development of nociceptive sensitization. This is the amplification of pain transmission along the entire pain neuraxis, from peripheral nociceptors to the upper CNS regions that form the “pain matrix” and encode the perceptive, cognitive, and emotional aspects of pain [164]. mGlu2 receptors are found in many stations of the pain neuraxis and negatively modulate pain transmission in the first pain synapse between primary afferent fibers and second-order neurons in the dorsal horns of the spinal cord [164]. In a study performed to dissect the role of mGlu2 and mGlu3 receptors in the endogenous control of inflammatory pain, mice lacking mGlu2 receptors showed amplification of pain in the second phase of the formalin test, which reflects the development of nociceptive sensitization in the spinal cord [165].
The established role of mGlu2 receptors in the modulation of pain transmission prompted investigations about the effects of NAC in animal models of inflammatory and neuropathic pain. Bernabucci and coworkers found that NAC induced analgesia in models of inflammatory pain and in the chronic constriction injury model of neuropathic pain [166]. Furthermore, NAC-induced analgesia was abrogated by pharmacologic inhibition of Sxc– or by the mGlu2/3 receptor antagonist, LY341495, suggesting that NAC alleviates chronic pain by amplifying the endogenous activation of mGlu2 receptors [166]. Other potential mechanisms underlying the action of NAC on neuropathic pain include the activation of type-9 matrix metalloprotease and the decreased phosphorylation of p38 MAP kinase in the spinal cord [167, 168].
Preclinical evidence supporting a beneficial effect of NAC on chronic pain is also provided by a model of diabetic neuropathy. A study investigating the effects of NAC on the dorsal root ganglion TRPM2 channel in diabetic rats showed that NAC prevented the enhancement of channel activity (calcium influx) and oxidative stress associated with streptozotocin-induced diabetes [169].
Taken together, the findings from preclinical studies in animal models suggest that NAC has the potential for the treatment of chronic neuropathic pain, including diabetic neuropathy. Clearly, these data need to be confirmed in clinical trials. Currently, studies in humans are extremely limited [170, 171]. A recent study in healthy volunteers assessing the effects of NAC on thermal-pain thresholds and laser-evoked potentials showed that the acute oral administration of NAC was able to inhibit pain transmission, providing support for the therapeutic potential of NAC for patients with chronic pain [171].
7. NAC FOR CARDIOVASCULAR DISEASE
Cardiac mitochondrial dysfunction is implicated in cardiovascular diseases such as cardiomyopathies, heart failure, and myocardial infarction. Accordingly, NAC has been investigated in numerous experimental models, and human studies encompassing a wide range of cardiovascular diseases, comprehensively reviewed in Frye and Berk, 2019 [9]. However, the application of NAC in the management of these conditions in clinical practice remains limited, and evidence from clinical trials is often conflicting.
Several mechanisms of action of NAC have been proposed across different cardiovascular diseases, including enhancement of NO metabolism by supplying free sulfhydryl groups needed for the activation of guanylate cyclase, inhibition of the angiotensin-converting enzyme, prevention of the oxidation of nitrate compounds, reduction of ROS and other free radicals, anti-inflammatory activity, and antiplatelet effects [9, 172, 173].
A systematic review of data from patients with heart failure, atrial fibrillation, myocardial infarction, hypertension, atherosclerosis, ischemic heart disease, or undergoing cardiothoracic surgery identified a variety of beneficial cardiovascular effects of NAC [174]. For example, in patients undergoing cardiothoracic surgery, NAC significantly decreased the risk of developing postoperative atrial fibrillation; it also decreased ischemia-reperfusion injury, potentiated the vasodilator effects of nitroglycerin and angiotensin-converting enzyme inhibitors, and reduced myocardial oxidative stress. In ischemic heart disease, the concomitant administration of intravenous nitroglycerin and NAC was able to increase the systemic and coronary hemodynamic effects related to nitroglycerin. In addition, NAC prevented and reversed nitrate tolerance. In patients with myocardial infarction, NAC combined with streptokinase significantly decreased oxidative stress, improved ventricular function, and reduced acute myocardial infarction episodes; it also prevented cardiac remodeling. In atherosclerosis, NAC therapy appeared to inhibit extracellular degradation and to improve vascular stability in both the early and late stages of atherosclerosis. In hemodialysis patients with heart failure, NAC was associated with a reduction of heart failure symptoms and other cardiovascular events [174].
In a randomized, placebo-controlled study in 251 patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary angioplasty, high-dose NAC (1200 mg IV bolus before angioplasty and 1200 mg IV bolus twice daily for 48 hours following angioplasty) reduced oxidative stress [172]. Clinical benefits as compared with placebo in terms of myocardial reperfusion injury were not apparent. More encouraging results have emerged from a recent study in patients with STEMI undergoing primary angioplasty [173]. The randomized, placebo-controlled study in 112 patients evaluated the effects of IV high-dose NAC (29 g over 2 days) versus placebo on infarct size assessed by early cardiac magnetic resonance imaging. All patients received concomitant low-dose nitroglycerin (7.2 mg over 2 days). The study showed an absolute 5.5% smaller myocardial infarct size in NAC-treated patients compared with placebo-treated patients (16.5% versus 11.0% size reduction, p = 0.02). Myocardial salvage with NAC was more than doubled compared with placebo (60% vs. 27%, p < 0.01) [173].
The ability of NAC to protect against the cardiovascular complications associated with diabetes was addressed in a recent systematic review that identified 49 papers reporting on in vitro and in vivo studies investigating the cardiac effects of NAC, mostly in animal models of diabetes [175]. A large body of preclinical data suggesting a cardioprotective effect of NAC in diabetes was highlighted, most likely related to the ability of NAC to reduce oxidative stress. In diabetes, as in other therapeutic areas, there is a need for clinical trials to confirm the benefits of NAC.
8. NAC FOR CONTRAST-INDUCED NEPHROPATHY
The intravascular administration of contrast media for radiologic imaging techniques can result in acute renal dysfunction [176]. Prevention of contrast-induced nephropathy (CIN), usually defined as an increase of ≥ 25% in the serum creatinine level from baseline, is crucial because even small changes in renal function are associated with increased morbidity and mortality [176]. The incidence of CIN in the general population ranges from 1% to 6%, while it can reach 50% in special populations, including patients with diabetes, pre-existing renal impairment, and multiple comorbidities [177]. Widely-accepted preventive strategies for CIN include volume expansion with intravenous saline or sodium bicarbonate and the use of low-osmolar or iso-osmolar contrast media [176].
Owing to its antioxidant and renal vasodilator properties, NAC has been extensively investigated for CIN prevention in patients undergoing coronary diagnostic and interventional procedures, with mixed results [9]. Because of these uncertainties, the evidence supporting a generalized use of NAC to prevent CIN is currently considered insufficient [178]. As a consequence, a universally accepted protocol for the use of NAC in the prevention of CIN is not available.
However, promising results emerged from a study involving 354 consecutive patients with STEMI undergoing primary angioplasty that investigated NAC for the prevention of CIN [179]. Patients were randomized to standard dose NAC (600 mg IV bolus before primary angioplasty and 600 mg orally, twice daily for 48 hours following angioplasty), double dose NAC (1200 mg IV bolus before primary angioplasty and 1200 mg orally, twice daily for 48 hours following angioplasty), and placebo. CIN was reported in 33% of patients treated with placebo and in 15% and 8% of patients treated with standard- and double-dose NAC, respectively (p < 0.001). Hospital outcomes were significantly better in patients treated with NAC compared with those treated with placebo. For example, hospital mortality was 11% in patients treated with placebo and 3.4% in those treated with NAC (p = 0.005) [179].
In contrast, in the randomized, placebo-controlled study in 251 patients with STEMI undergoing primary angioplasty discussed in the previous section, high-dose NAC (1200 mg IV bolus twice daily intravenously for 48 hours) was associated with a numerically lower incidence of CIN in patients treated with NAC versus placebo (14% vs. 20%), but the difference between the two treatment groups was not statistically significant [172], possibly because the study was clearly underpowered (1200 patients would have been required to achieve statistical significance with respect to this endpoint). A large randomized study in 720 patients with STEMI undergoing primary angioplasty compared four CIN prophylactic regimens: hydration with sodium chloride alone, and hydration with sodium chloride plus either NAC (oral), sodium bicarbonate (infusion), or NAC plus sodium bicarbonate [180]. The overall rate of CIN was 21.9%. In this study, the addition of NAC, sodium bicarbonate, or NAC plus sodium bicarbonate to hydration with sodium chloride did not reduce the incidence of CIN.
Systematic reviews of the literature and meta-analyses assessing NAC for CIN prevention in angioplasty interventions are also available [9, 181, 182]. A meta-analysis of five randomized, controlled clinical trials involving a total of 643 patients with impaired renal function undergoing cardiovascular procedures found that the addition of NAC to IV saline hydration reduced CIN by 20% compared with hydration alone [181]. More recently, a meta-analysis that included 48 randomized-controlled trials was performed to compare different strategies of CIN prevention versus hydration in patients with chronic kidney disease undergoing coronary angiography [182]. Five strategies significantly reduced the odds of CIN and, among these, NAC (evaluated in 27 trials with a total of 5694 patients) was the most effective (odds ratio 0.77, 95% CI 0.65–0.91, p = 0.002). According to subgroup analysis, the benefit of NAC was most evident in patients receiving higher contrast doses [182]. A previously published meta-analysis evaluating the efficacy of high-dose NAC for the prevention of CIN seems to support this concept [183]. High-dose NAC was a priori defined as a daily dose greater than 1200 mg or a single peri-procedural dose greater than 600 mg, peri-procedural being defined as immediately or within 4 hours of planned contrast agent exposure. Sixteen prospective studies of patients (total sample size of 1677 subjects) randomized to NAC administered either orally or intravenously versus a control group (842 assigned to high-dose NAC, 835 to the control arm) were included in this meta-analysis. The overall effect size revealed an odds ratio of 0.46 (95% CI 0.33–0.63, p < 0.0001) for the occurrence of CIN with the use of high-dose NAC, suggesting a significant protective effect.
A recent systematic review pointed out that the outcomes related to the effects of NAC on renal function are very variable, with a rate of positive results from the analyzed trials of 39% for oral NAC and 29% for IV NAC [9]. Of note, no trial reported detrimental effects associated with NAC use, and NAC-related adverse events were rare.
9. USE OF NAC IN OPHTHALMOLOGY
The effect of topical and oral NAC in various ophthalmologic conditions has been investigated in a number of small trials, with promising results [9, 184-186]. Currently, most evidence supporting the use of NAC in ophthalmology comes from studies in RP.
9.1. NAC for the Treatment of Retinitis Pigmentosa
In RP, a large number of mutations in many different genes lead to rod photoreceptor death [187, 188]. Once widespread rod cell death has occurred, cone photoreceptors gradually degenerate, causing visual loss and complete blindness. Rod cells are characterized by very high oxygen consumption [189]. As rod cells die, oxygen consumption diminishes while oxygen supply remains unchanged, leading to hyperoxia in the retina [190].
In a transgenic pig model of RP, there was progressive oxidative damage of cone photoreceptors following the death of rod cells [191]. Studies in multiple murine models of RP showed that a mixture of antioxidants prevented oxidative damage to cone cells and promoted cone cell function and survival [192, 193]. Oral NAC promoted cone cell function and survival in a murine model of RP [194].
The results from preclinical studies in animal models are, at least in part, reflected in the findings from studies in human subjects. Patients with RP show a significant reduction in the GSH/GSSG ratio in the aqueous humor, and a significant increase in protein carbonyl content, suggesting oxidative stress and oxidative damage in the eyes of these patients [195].
The FIGHT RP study was a dose-ranging study aimed at testing the safety of oral NAC and its effect on several parameters of visual function in patients with RP [196]. There were three cohorts with 10 participants in each. Cohort 1 received 600 mg NAC twice daily for 3 months and then 600 mg three times daily for 3 months, cohort 2 received 1200 mg twice daily for 3 months and then 1200 mg three times daily for 3 months, and cohort 3 received 1800 mg twice daily for 3 months and then 1800 mg three times daily for 3 months. The primary outcome measure was safety, and key secondary outcomes were mean change from baseline best- corrected visual acuity (BCVA), mean change from baseline macular sensitivity measured by microperimetry, change from baseline in ellipsoid zone width at weeks 12 and 24, and NAC levels in the aqueous humor and in plasma. There were no severe adverse events and 11 drug-related adverse events, nine of which involved the gastrointestinal tract. Eight were mild and resolved spontaneously and three were moderate, occurring during three times daily dosing, and resolving after dose reduction to twice daily administration. The maximum tolerated dose was determined to be 1800 mg twice daily. While on NAC, participants showed evidence of improved cone function. The strongest evidence was an improvement in macular sensitivity, which was greatest in cohort 3. Since 1800 mg NAC twice daily was very well tolerated and cohort 3 showed the greatest improvement in macular sensitivity, it has been selected as the dose for a multicenter trial aimed at determining the long-term safety of NAC and whether long-term NAC can promote cone survival in patients with RP.
In FIGHT RP, good intraocular levels of NAC were obtained with oral dosing. The mean aqueous level was in the range of 100 ng/ml in cohort 1 and 300 ng/ml in cohorts 2 and 3. Mean baseline BCVA was 72 letters (20/40 Snellen) in cohort 1 and 74 and 75 letters in cohorts 2 and 3, respectively, which is approximately 20/32 Snellen. With this good baseline vision, it was surprising to see a gradual, steady increase in BCVA during the treatment period in each cohort that was statistically significant (linear mixed effects models). As random changes tend to fluctuate, a steady progressive increase is unlikely to be due to chance. Macular sensitivity was measured with the Macular Integrity Assessment microperimetry instrument, which measures sensitivity at 68 loci and calculates the mean sensitivity from those 68 measurements. There was a steady increase in mean sensitivity during the treatment period in each cohort, which was greatest in cohort 3 at 0.15 dB/month (p = 0.008). The improvement in mean sensitivity between baseline and week 24 was statistically significant in cohorts 2 and 3. There was no change from baseline in ellipsoid zone width in any of the cohorts during treatment; this is a promising result, but is not definitive because 6 months may be too short to detect a change in the absence of treatment.
Thus, the FIGHT RP study showed that oral NAC is safe in patients with RP, with a maximum tolerated dose of 1800 mg twice daily that resulted in good intraocular levels. Gradual improvements in visual acuity and macular sensitivity suggest the possibility of improvement in cone function during a 24-week treatment period. A large, placebo-controlled multicenter clinical trial is being planned to determine if long term oral NAC can promote cone survival and function and reduce visual disability in patients with RP.
10. PRECAUTIONS OF USE
Data from clinical studies and extensive use in clinical practice and as over-the-counter supplementation have demonstrated that oral NAC is generally well-tolerated and safe, even at high doses [9, 12].
NAC has been rarely associated with clinically significant adverse events (SmPC (Zambon) oral NAC). The most frequently reported adverse event is gastrointestinal discomfort (emesis, diarrhea) [9]. In very rare cases, severe skin reactions in response to IV NAC have been reported, with a temporal relationship with NAC administration [12]. A reduction in platelet aggregation during NAC administration has been observed in some studies, but the clinical relevance of these findings is unclear [16]. NAC also has a favorable profile in terms of pharmacologic interactions, with few described drug-drug interactions. These include antitussive medicines, oral antibiotics, and nitroglycerin [16]. In particular, antitussive agents should not be given concomitantly with NAC, because a decrease in the cough reflex may result in the accumulation of bronchial secretions. The administration of oral antibiotics and NAC should preferably be spaced by at least 2 hours, as their coadministration may be associated with decreased antibiotic activity [16]. This should be set against the evidence from studies in biofilms that NAC may enhance the activity of antibiotics in respiratory airway infections by inhibiting and/or disrupting biofilm formation (reviewed in [25]).
The concomitant administration of NAC and nitroglycerin should be avoided because it induces hypotension. If concomitant administration cannot be avoided, patients should be monitored for hypotension and warned about the possible occurrence of hypotension and headache [16]. There is also some suggestion that inhibition of ROS, for example, by the administration of NAC, may attenuate adaptations to exercise [197].
Despite the overall safety and tolerability of NAC, some precautions are advised [16]. Patients with bronchial asthma should be closely monitored during treatment with NAC, which should be discontinued as soon as bronchospasm occurs. NAC is contraindicated in children aged < 2 years because mucolytics can cause bronchial obstruction in young children. Caution is advised in patients with peptic ulcer or a history of this condition. NAC is also contraindicated in patients with hypersensitivity to the active substance or any of the excipients, and during pregnancy and breastfeeding.
Finally, it should be remembered that redox reactions play a key role in signaling pathways involved in cell damage prevention and cell maturation. It has been suggested that a biphasic dose-response (hormesis effect) may occur with NAC, whereby high-dose NAC and a complete block of ROS production may not always be beneficial [10]. Furthermore, as NAC is readily oxidized to its disulfide di-NAC, which has oxidative rather than reductive effects, the source and form of NAC taken may cause great variability in the response to treatment and should be taken into account [9].
CONCLUSION
NAC is a small, synthetic derivative of L-cysteine that has been extensively investigated and used for more than 50 years for the treatment of several conditions. The therapeutic activity of NAC relies on its ability to provide reducing sulfhydryl moieties and to act as a precursor of GSH to replenish the intracellular GSH pool when this has been depleted in conditions of oxidative stress, in processes of drug detoxification, or in other conditions leading to GSH deficit. Several other mechanisms of actions of NAC, including modulation of mitochondrial dysfunction, apoptosis, and inflammatory processes, have emerged from the intense research program involving this molecule, with the modulatory action of NAC on glutamate homeostasis perhaps the best characterized. By activating Sxc–, a key component in the control of extracellular glutamate, and inducing GLT1, NAC prevents excessive and neurotoxic glutamate release from excitatory nerve endings.
These multiple actions of NAC have prompted studies addressing the potential of NAC in a wide range of therapeutic areas, including disease driven by oxidative stress as well as conditions characterized by impaired glutamate homeostasis. In the present review, we have focused on the six areas where we work (lung diseases, poisoning, CNS disorders, cardiovascular disease, CIN, and ophthalmologic conditions), while we invite the readers to consult a recently published systematic review of the literature concerning the therapeutic use of NAC in medicine [9], for a comprehensive view of the topic.
With regard to NAC and respiratory diseases, besides the established role as a mucolytic in chronic bronchitis, NAC has proven effective in reducing the risk of exacerbation in patients with COPD. Along with other sulfhydryl-containing drugs, NAC is currently mentioned as adjunctive therapy by the 2020 GOLD guidelines for the management of COPD. The licensed NAC oral dose of 600 mg/day appears to be sufficient for preventing COPD exacerbations, although patients presenting with airway obstruction may benefit from doses ≥ 1200 mg/day. By contrast with COPD, evidence from randomized clinical trials has shown that monotherapy with NAC may not, in general, be recommended for patients with IPF, another respiratory condition in which NAC has been extensively investigated. However, analysis of SNPs has shown that patients with a specific genotype (TOLLIP rs3750920) have a better response to NAC compared with the general IPF population. These findings, and the potential anti-fibrotic effects in vitro, when confirmed in larger trials, highlight the potential of patient genotyping and precision medicine in identifying patients with IPF who may benefit from NAC, in pulmonary medicine as well as in other therapeutic areas such as CF and non-CF bronchiectasis.
Management of drug poisoning is another therapeutic area where NAC has long had an established position. The available evidence shows that current protocols are effective for most patients presenting early (< 8 hours) following intake of acetaminophen overdose. An interesting area of current research concerns the use of NAC for the detoxification of other compounds, including heavy metals.
Due to its properties as an antioxidant and modulator of neurotransmission (glutamatergic transmission in particular), NAC has long attracted considerable interest as an adjunctive treatment to therapies for a wide range of CNS disorders. In the present article, we have focused on four main areas: chronic pain (neuropathic), depressive symptoms, schizophrenia, and drug addiction. Preclinical studies with NAC are promising across all these areas. Clinical trials addressing the role of NAC in the treatment of chronic pain are currently lacking or just beginning to emerge. Clinical evidence from studies with NAC in depression is overall in line with preclinical findings and suggests a role of NAC as an adjunctive treatment to antidepressant therapy for symptom improvement. The role of NAC in the treatment of addiction has been extensively investigated, especially in patients addicted to cocaine or cannabis, with mixed results in terms of relapse prevention. There is a need for larger studies, considering the current lack of effective approaches to addiction and the social relevance of this condition.
NAC has also been investigated in a wide range of cardiovascular conditions, with conflicting results emerging from clinical trials. Overall, the use of NAC in cardiovascular clinical practice is limited. A promising area seems to be the use of NAC for the prevention of cardiovascular complications in patients with diabetes. In patients undergoing primary angioplasty, NAC may play a role in the prevention of kidney damage induced by CIN. However, clinical evidence from trials evaluating the efficacy of NAC in this indication is mixed and considered insufficient for inclusion in current guidelines. In this area subgroup analysis has identified patients who may benefit from the addition of NAC to recommended preventive measures, highlighting the importance of stratifying patients to optimize treatment outcomes.
As for the use of NAC in ophthalmology, the results of the FIGHT RP trial demonstrated improvement in cone function in patients with RP during a 24-week treatment period with NAC. This suggests that some of the visual dysfunction is due to cones that are functioning suboptimally due to oxidative stress. It is reasonable to hypothesize that long term reduction of oxidative stress with oral NAC could promote survival of cones and thereby stop or slow the inexorable reduction in visual fields that leads to blindness. A large phase III, multicenter, placebo-controlled, double- masked clinical trial is being planned to test this hypothesis.
In conclusion, more than 50 years of clinical use have shown that NAC is effective, safe, and generally well tolerated. Furthermore, recent clinical trials, despite great variability in efficacy outcomes, have consistently confirmed the favorable safety profile of NAC across a wide range of therapeutic areas. Although much remains to be done to definitively establish NAC in some of the conditions considered in this review, this molecule has a number of properties that make it an ideal adjunctive treatment to established therapies for complex diseases, including a favorable pharmacokinetic profile with few relevant drug-drug interactions. NAC is an inexpensive drug that has already proven easy to repurpose. Finally, precision medicine and an increased understanding of the pharmacogenetics of SNPs may ultimately prove useful for improving the efficacy of treatment with NAC by identifying patients likely to benefit.
ACKNOWLEDGEMENTS
We thank Lorenza Lanini and Ray Hill, independent medical writers acting on behalf of Springer Healthcare Italy, who provided medical writing support, and Tracy Harrison, of Springer Healthcare Communications, who provided editorial assistance post submission. This was funded by Zambon Pharma SpA (Italy).
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Michael Berk is supported by an NHMRC Senior Principal Research Fellowship (1059660 and 1156072).
CONFLICT OF INTEREST
Ganesh Raghu reports consultation services to Zambon Pharma SpA and research grant support from National Institutes of Health, Bethesda, Maryland, USA for IPF studies with NAC.
Hartmut Jaeschke reports the receipt of funding by National Institutes of Health grants R01 NIDDK102142 and R01 NIDDK070195.
Michael Berk has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Lundbeck, Merck, Pfizer, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Lundbeck Merck, Pfizer and Servier.
Peter M. A. Calverley reports funding from the Medical Research Council, EU HORIZON 2020 programme, Wellcome Trust, GSK, Boehringer Ingelheim and Novartis. He has spoken at meetings organized by GSK, Boehringer Ingelheim, Recipharm, Zambon and Phillips Respironics. He has advised Recipharm, Phillips Respironics and Zambon on study conduct and product development.
Luca Richeldi reports the receipt of funding of research supports from Roche and Boehringer Ingelheim. He additionally reports the receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen, Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant, Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics, Zambon, Veracyte, Acceleron, CSL Behring.
The other authors declare no conflict of interest, financial or otherwise.
References
- 1.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344(8925):796–798. doi: 10.1016/S0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- 3.Bavarsad Shahripour R., Harrigan M.R., Alexandrov A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4(2):108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santus P., Corsico A., Solidoro P., Braido F., Di Marco F., Scichilone N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD. 2014;11(6):705–717. doi: 10.3109/15412555.2014.898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deepmala S, J., Kumar N., Delhey L., Berk M., Dean O., Spielholz C., Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Yu B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 8.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Frye R.E., Andrus J.P., Lemley K.V., De Rosa S.C., Ghezzi P., Holmgren A., Jones D., Jahoor F., Kopke R., Cotgreave I., Bottiglieri T., Kaplowitz N., Nakamura H., Staal F., Ela S.W., Atkuri K.R., Tirouvanziam R., Heydari K., Sahaf B., Zolopa A., Mantovani J.J., Herzenberg L.A., Herzenberg L.A. In: The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Frye R.E., Berk M., editors. Singapore: Springer Nature; 2019. Pharmacology, Formulations, and Adverse Effects. p. 392. [DOI] [Google Scholar]
- 10.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K. Anti-oxidants for therapeutic use: Why are only a few drugs in clinical use? Adv. Drug Deliv. Rev. 2009;61(4):287–289. doi: 10.1016/j.addr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Dodd S., Dean O., Copolov D.L., Malhi G.S., Berk M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008;8(12):1955–1962. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 13.Hurst G.A., Shaw P.B., LeMaistre C.A. Laboratory and clinical evaluation of the mucolytic properties of acetylcysteine. Am. Rev. Respir. Dis. 1967;96(5):962–970. doi: 10.1164/arrd.1967.96.5.962. [DOI] [PubMed] [Google Scholar]
- 14.Prescott L.F., Illingworth R.N., Critchley J.A., Stewart M.J., Adam R.D., Proudfoot A.T. Intravenous N-acetylcystine: The treatment of choice for paracetamol poisoning. BMJ. 1979;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott L.F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A.T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2(8035):432–434. doi: 10.1016/S0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency (EMA) Fluimicil Mucolytic (N-acetylcysteine): Summary of product characteristics. 2017. Available from: http://www.ema.europa.eu/ (accessed 14 February, 2019)
- 17.Cazzola M., Calzetta L., Page C., Rogliani P., Matera M.G. Thiol-based drugs in pulmonary medicine: Much more than mucolytics. Trends Pharmacol. Sci. 2019;40(7):452–463. doi: 10.1016/j.tips.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Beckman K.B., Ames B.N. Oxidative decay of DNA. J. Biol. Chem. 1997;272(32):19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 19.Galle J., Heermeier K., Wanner C. Atherogenic lipoproteins, oxidative stress, and cell death. Kidney Int. Suppl. 1999;71:S62–S65. doi: 10.1046/j.1523-1755.1999.07116.x. [DOI] [PubMed] [Google Scholar]
- 20.de Andrade K.Q., Moura F.A., dos Santos J.M., de Araújo O.R., de Farias Santos J.C., Goulart M.O. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of N-acetylcysteine. Int. J. Mol. Sci. 2015;16(12):30269–30308. doi: 10.3390/ijms161226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 22.Du K., Ramachandran A., Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calzetta L., Matera M.G., Rogliani P., Cazzola M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2018;12(8):693–708. doi: 10.1080/17476348.2018.1495562. [DOI] [PubMed] [Google Scholar]
- 24.Mereto G.C., Balestra L., Henriquet F. Alveolar surfactant in lungs of operated patients after acetylcysteine treatment. Eur. J. Respir. Dis. Suppl. 1980;111:160–161. [PubMed] [Google Scholar]
- 25.Blasi F., Page C., Rossolini G.M., Pallecchi L., Matera M.G., Rogliani P., Cazzola M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016;117:190–197. doi: 10.1016/j.rmed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Decramer M., Rutten-van Mölken M., Dekhuijzen P.N., Troosters T., van Herwaarden C., Pellegrino R., van Schayck C.P., Olivieri D., Del Donno M., De Backer W., Lankhorst I., Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet. 2005;365(9470):1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 27.Tse H.N., Raiteri L., Wong K.Y., Yee K.S., Ng L.Y., Wai K.Y., Loo C.K., Chan M.H. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144(1):106–118. doi: 10.1378/chest.12-2357. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J.P., Wen F.Q., Bai C.X., Wan H.Y., Kang J., Chen P., Yao W.Z., Ma L.J., Li X., Raiteri L., Sardina M., Gao Y., Wang B.S., Zhong N.S., PANTHEON study group Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014;2(3):187–194. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 29.Demedts M., Behr J., Buhl R., Costabel U., Dekhuijzen R., Jansen H.M., MacNee W., Thomeer M., Wallaert B., Laurent F., Nicholson A.G., Verbeken E.K., Verschakelen J., Flower C.D., Capron F., Petruzzelli S., De Vuyst P., van den Bosch J.M., Rodriguez-Becerra E., Corvasce G., Lankhorst I., Sardina M., Montanari M., IFIGENIA Study Group High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 30.Raghu G., Anstrom K.J., King T.E., Jr, Lasky J.A., Martinez F.J., Idiopathic Pulmonary Fibrosis Clinical Research Network Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez F.J., de Andrade J.A., Anstrom K.J., King T.E., Jr, Raghu G., Idiopathic Pulmonary Fibrosis Clinical Research Network Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370(22):2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behr J., Bendstrup E., Crestani B., Günther A., Olschewski H., Sköld C.M., Wells A., Wuyts W., Koschel D., Kreuter M., Wallaert B., Lin C.Y., Beck J., Albera C. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2016;4(6):445–453. doi: 10.1016/S2213-2600(16)30044-3. [DOI] [PubMed] [Google Scholar]
- 33.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstrtuctive Pulmonary Disease: 2020 Report. 2020. Available from: https://goldcopd.org/gold-reports/ (accessed 05 November, 2020)
- 34.Repine J.E., Bast A., Lankhorst I., Oxidative Stress Study Group Oxidative stress in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997;156(2 Pt 1):341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 35.Tse H.N., Raiteri L., Wong K.Y., Ng L.Y., Yee K.S., Tseng C.Z.S. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146(3):611–623. doi: 10.1378/chest.13-2784. [DOI] [PubMed] [Google Scholar]
- 36.Papi A., Zheng J., Criner G.J., Fabbri L.M., Calverley P.M.A. Impact of smoking status and concomitant medications on the effect of high-dose N-acetylcysteine on chronic obstructive pulmonary disease exacerbations: A post-hoc analysis of the PANTHEON study. Respir. Med. 2019;147:37–43. doi: 10.1016/j.rmed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Cazzola M., Calzetta L., Page C., Jardim J., Chuchalin A.G., Rogliani P., Matera M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015;24(137):451–461. doi: 10.1183/16000617.00002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantin A.M., Larivée P., Bégin R.O. Extracellular glutathione suppresses human lung fibroblast proliferation. Am. J. Respir. Cell Mol. Biol. 1990;3(1):79–85. doi: 10.1165/ajrcmb/3.1.79. [DOI] [PubMed] [Google Scholar]
- 39.Myllärniemi M., Kaarteenaho R. Pharmacological treatment of idiopathic pulmonary fibrosis - preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur. Clin. Respir. J. 2015;2:2. doi: 10.3402/ecrj.v2.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behr J., Maier K., Degenkolb B., Krombach F., Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am. J. Respir. Crit. Care Med. 1997;156(6):1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 41.Oldham J.M., Ma S.F., Martinez F.J., Anstrom K.J., Raghu G., Schwartz D.A., Valenzi E., Witt L., Lee C., Vij R., Huang Y., Strek M.E., Noth I., IPFnet Investigators IPFnet Investigators. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015;192(12):1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghu G., Noth I., Martinez F. N-acetylcysteine for idiopathic pulmonary fibrosis: The door is still open. Lancet Respir. Med. 2017;5(1):e1–e2. doi: 10.1016/S2213-2600(16)30327-7. [DOI] [PubMed] [Google Scholar]
- 43.Matera M.G., Calzetta L., Cazzola M. Oxidation pathway and exacerbations in COPD: The role of NAC. Expert Rev. Respir. Med. 2016;10(1):89–97. doi: 10.1586/17476348.2016.1121105. [DOI] [PubMed] [Google Scholar]
- 44.Criner G.J., Bourbeau J., Diekemper R.L., Ouellette D.R., Goodridge D., Hernandez P., Curren K., Balter M.S., Bhutani M., Camp P.G., Celli B.R., Dechman G., Dransfield M.T., Fiel S.B., Foreman M.G., Hanania N.A., Ireland B.K., Marchetti N., Marciniuk D.D., Mularski R.A., Ornelas J., Road J.D., Stickland M.K. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147(4):894–942. doi: 10.1378/chest.14-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kon K., Kim J.S., Jaeschke H., Lemasters J.J. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 46.Ramachandran A., Lebofsky M., Baines C.P., Lemasters J.J., Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011;45(2):156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGill M.R., Jaeschke H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013;30(9):2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran A., Jaeschke H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019;39(2):221–234. doi: 10.1055/s-0039-1679919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cover C., Mansouri A., Knight T.R., Bajt M.L., Lemasters J.J., Pessayre D., Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315(2):879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 50.Knight T.R., Ho Y.S., Farhood A., Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: Protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303(2):468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 51.James L.P., McCullough S.S., Lamps L.W., Hinson J.A. Effect of N-acetylcysteine on acetaminophen toxicity in mice: Relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 2003;75(2):458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- 52.Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44(1):88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bajt M.L., Cover C., Lemasters J.J., Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94(1):217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 54.Gujral J.S., Knight T.R., Farhood A., Bajt M.L., Jaeschke H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67(2):322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 55.Xie Y., McGill M.R., Dorko K., Kumer S.C., Schmitt T.M., Forster J., Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014;279(3):266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGill M.R., Sharpe M.R., Williams C.D., Taha M., Curry S.C., Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGill M.R., Staggs V.S., Sharpe M.R., Lee W.M., Jaeschke H., Acute Liver Failure Study Group Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60(4):1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell J.R., Jollow D.J., Potter W.Z., Gillette J.R., Brodie B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187(1):211–217. [PubMed] [Google Scholar]
- 59.Jollow D.J., Mitchell J.R., Potter W.Z., Davis D.C., Gillette J.R., Brodie B.B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187(1):195–202. [PubMed] [Google Scholar]
- 60.Rumack B.H., Bateman D.N. Acetaminophen and acetylcysteine dose and duration: Past, present and future. Clin. Toxicol. (Phila.) 2012;50(2):91–98. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- 61.Rumack B.H., Peterson R.C., Koch G.G., Amara I.A. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch. Intern. Med. 1981;141(3 Spec No):380–385. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- 62.Smilkstein M.J., Knapp G.L., Kulig K.W., Rumack B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 63.Rumack B.H., Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55(6):871–876. [PubMed] [Google Scholar]
- 64.Fisher E.S., Curry S.C. Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol. 2019;85:263–272. doi: 10.1016/bs.apha.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Mutsaers A., Green J.P., Sivilotti M.L.A., Yarema M.C., Tucker D., Johnson D.W., Spyker D.A., Rumack B.H. Changing nomogram risk zone classification with serial testing after acute acetaminophen overdose: A retrospective database analysis. Clin. Toxicol. (Phila.) 2019;57(6):380–386. doi: 10.1080/15563650.2018.1529320. [DOI] [PubMed] [Google Scholar]
- 66.Corcoran G.B., Racz W.J., Smith C.V., Mitchell J.R. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J. Pharmacol. Exp. Ther. 1985;232(3):864–872. [PubMed] [Google Scholar]
- 67.Corcoran G.B., Wong B.K. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: Studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 1986;238(1):54–61. [PubMed] [Google Scholar]
- 68.Lauterburg B.H., Corcoran G.B., Mitchell J.R. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J. Clin. Invest. 1983;71(4):980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saito C., Zwingmann C., Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51(1):246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhushan B., Apte U. Liver regeneration after acetaminophen hepatotoxicity: Mechanisms and therapeutic opportunities. Am. J. Pathol. 2019;189(4):719–729. doi: 10.1016/j.ajpath.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du K., Ramachandran A., McGill M.R., Mansouri A., Asselah T., Farhood A., Woolbright B.L., Ding W.X., Jaeschke H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food. Chem. Toxicol. 2017;108(Pt A):339–350. doi: 10.1016/j.fct.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang R., Miki K., He X., Killeen M.E., Fink M.P. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit. Care. 2009;13(2):R55. doi: 10.1186/cc7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park B.K., Dear J.W., Antoine D.J. Paracetamol (acetaminophen) poisoning. BMJ. Clin. Evid. 2015;2015:2101. [PMC free article] [PubMed] [Google Scholar]
- 74.Keays R., Harrison P.M., Wendon J.A., Forbes A., Gove C., Alexander G.J., Williams R. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: A prospective controlled trial. BMJ. 1991;303(6809):1026–1029. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yarema M.C., Johnson D.W., Berlin R.J., Sivilotti M.L., Nettel-Aguirre A., Brant R.F., Spyker D.A., Bailey B., Chalut D., Lee J.S., Plint A.C., Purssell R.A., Rutledge T., Seviour C.A., Stiell I.G., Thompson M., Tyberg J., Dart R.C., Rumack B.H. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann. Emerg. Med. 2009;54(4):606–614. doi: 10.1016/j.annemergmed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Bond G.R. Is the oral acetylcysteine protocol the best treatment for late-presenting acetaminophen poisoning? Ann. Emerg. Med. 2009;54(4):615–617. doi: 10.1016/j.annemergmed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 77.Buckley N.A., Eddleston M., Li Y. IV versus oral acetylcysteine. Ann. Emerg. Med. 2010;55(4):393–394. doi: 10.1016/j.annemergmed.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 78.Williamson K., Wahl M.S., Mycyk M.B. Direct comparison of 20-hour IV, 36-hour oral, and 72-hour oral acetylcysteine for treatment of acute acetaminophen poisoning. Am. J. Ther. 2013;20(1):37–40. doi: 10.1097/MJT.0b013e318250f829. [DOI] [PubMed] [Google Scholar]
- 79.Wong A., Homer N., Dear J.W., Choy K.W., Doery J., Graudins A. Paracetamol metabolite concentrations following low risk overdose treated with an abbreviated 12-h versus 20-h acetylcysteine infusion. Clin. Toxicol. (Phila.) 2019;57(5):312–317. doi: 10.1080/15563650.2018.1517881. [DOI] [PubMed] [Google Scholar]
- 80.Wong A., McNulty R., Taylor D., Sivilotti M., Greene S., Gunja N., Koutsogiannis Z., Graudins A. The NACSTOP trial: A multicenter, cluster-controlled trial of early cessation of acetylcysteine in acetaminophen overdose. Hepatology. 2019;69(2):774–784. doi: 10.1002/hep.30224. [DOI] [PubMed] [Google Scholar]
- 81.Bateman D.N., Dear J.W. Should we treat very large paracetamol overdose differently? Br. J. Clin. Pharmacol. 2017;83(6):1163–1165. doi: 10.1111/bcp.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cairney D.G., Beckwith H.K., Al-Hourani K., Eddleston M., Bateman D.N., Dear J.W. Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin. Toxicol. (Phila.) 2016;54(5):405–410. doi: 10.3109/15563650.2016.1159309. [DOI] [PubMed] [Google Scholar]
- 83.Hendrickson R.G. What is the most appropriate dose of N-acetylcysteine after massive acetaminophen overdose? Clin. Toxicol. (Phila.) 2019;57(8):686–691. doi: 10.1080/15563650.2019.1579914. [DOI] [PubMed] [Google Scholar]
- 84.Pakravan N., Waring W.S., Sharma S., Ludlam C., Megson I., Bateman D.N. Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin. Toxicol. (Phila.) 2008;46(8):697–702. doi: 10.1080/15563650802245497. [DOI] [PubMed] [Google Scholar]
- 85.Akakpo J.Y., Ramachandran A., Duan L., Schaich M.A., Jaeschke M.W., Freudenthal B.D., Ding W.X., Rumack B.H., Jaeschke H. Delayed treatment with 4-methylpyrazole protects against acetaminophen hepatotoxicity in mice by inhibition of c-Jun n-terminal kinase. Toxicol. Sci. 2019;170(1):57–68. doi: 10.1093/toxsci/kfz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akakpo J.Y., Ramachandran A., Kandel S.E., Ni H.M., Kumer S.C., Rumack B.H., Jaeschke H. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum. Exp. Toxicol. 2018;37(12):1310–1322. doi: 10.1177/0960327118774902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kiernan E.A., Fritzges J.A., Henry K.A., Katz K.D. A case report of massive acetaminophen poisoning treated with a novel “triple therapy”: N-acetylcysteine, 4-methylpyrazole, and hemodialysis. Case Rep. Emerg. Med. 2019;2019:9301432. doi: 10.1155/2019/9301432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Major J.M., Zhou E.H., Wong H.L., Trinidad J.P., Pham T.M., Mehta H., Ding Y., Staffa J.A., Iyasu S., Wang C., Willy M.E. Trends in rates of acetaminophen-related adverse events in the United States. Pharmacoepidemiol. Drug Saf. 2016;25(5):590–598. doi: 10.1002/pds.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gawarammana I.B., Buckley N.A. Medical management of paraquat ingestion. Br. J. Clin. Pharmacol. 2011;72(5):745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karvellas C.J., Tillman H., Leung A.A., Lee W.M., Schilsky M.L., Hameed B., Stravitz R.T., McGuire B.M., Fix O.K., United States Acute Liver Failure Study Group Acute liver injury and acute liver failure from mushroom poisoning in North America. Liver Int. 2016;36(7):1043–1050. doi: 10.1111/liv.13080. [DOI] [PubMed] [Google Scholar]
- 91.Janes S.E., Price C.S., Thomas D. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur. J. Pediatr. 2005;164(8):520–522. doi: 10.1007/s00431-005-1692-1. [DOI] [PubMed] [Google Scholar]
- 92.Dell’Aglio D.M., Sutter M.E., Schwartz M.D., Koch D.D., Algren D.A., Morgan B.W. Acute chloroform ingestion successfully treated with intravenously administered N-acetylcysteine. J. Med. Toxicol. 2010;6(2):143–146. doi: 10.1007/s13181-010-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valles E.G., de Castro C.R., Castro J.A. N-acetyl cysteine is an early but also a late preventive agent against carbon tetrachloride-induced liver necrosis. Toxicol. Lett. 1994;71(1):87–95. doi: 10.1016/0378-4274(94)90202-X. [DOI] [PubMed] [Google Scholar]
- 94.Aykin-Burns N., Franklin E.A., Ercal N. Effects of N-acetylcysteine on lead-exposed PC-12 cells. Arch. Environ. Contam. Toxicol. 2005;49(1):119–123. doi: 10.1007/s00244-004-0025-0. [DOI] [PubMed] [Google Scholar]
- 95.Chen W., Ercal N., Huynh T., Volkov A., Chusuei C.C. Characterizing N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) binding for lead poisoning treatment. J. Colloid Interface Sci. 2012;371(1):144–149. doi: 10.1016/j.jcis.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Penugonda S., Mare S., Lutz P., Banks W.A., Ercal N. Potentiation of lead-induced cell death in PC12 cells by glutamate: Protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol. Appl. Pharmacol. 2006;216(2):197–205. doi: 10.1016/j.taap.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Blanusa M., Varnai V.M., Piasek M., Kostial K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr. Med. Chem. 2005;12(23):2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 98.Minarini A., Ferrari S., Galletti M., Giambalvo N., Perrone D., Rioli G., Galeazzi G.M. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017;13(3):279–292. doi: 10.1080/17425255.2017.1251580. [DOI] [PubMed] [Google Scholar]
- 99.Back S.E., McCauley J.L., Korte K.J., Gros D.F., Leavitt V., Gray K.M., Hamner M.B., DeSantis S.M., Malcolm R., Brady K.T., Kalivas P.W. A double-blind, randomized, controlled pilot trial of N-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders. J. Clin. Psychiatry. 2016;77(11):e1439–e1446. doi: 10.4088/JCP.15m10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skvarc D.R., Dean O.M., Byrne L.K., Gray L., Lane S., Lewis M., Fernandes B.S., Berk M., Marriott A. The effect of N-acetylcysteine (NAC) on human cognition - A systematic review. Neurosci. Biobehav. Rev. 2017;78:44–56. doi: 10.1016/j.neubiorev.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 101.Prakash A., Kumar A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharmacol. Toxicol. 2009;105(2):98–104. doi: 10.1111/j.1742-7843.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 102.Otte D.M., Sommersberg B., Kudin A., Guerrero C., Albayram O., Filiou M.D., Frisch P., Yilmaz O., Drews E., Turck C.W., Bilkei-Gorzó A., Kunz W.S., Beck H., Zimmer A. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology. 2011;36(11):2233–2243. doi: 10.1038/npp.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsiao Y.H., Kuo J.R., Chen S.H., Gean P.W. Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiol. Dis. 2012;45(3):1111–1120. doi: 10.1016/j.nbd.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 104.Scaini G., Teodorak B.P., Jeremias I.C., Morais M.O., Mina F., Dominguini D., Pescador B., Comim C.M., Schuck P.F., Ferreira G.C., Quevedo J., Streck E.L. Antioxidant administration prevents memory impairment in an animal model of maple syrup urine disease. Behav. Brain Res. 2012;231(1):92–96. doi: 10.1016/j.bbr.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 105.Rodrigues F.S., Souza M.A., Magni D.V., Ferreira A.P., Mota B.C., Cardoso A.M., Paim M., Xavier L.L., Ferreira J., Schetinger M.R., Da Costa J.C., Royes L.F., Fighera M.R. N-acetylcysteine prevents spatial memory impairment induced by chronic early postnatal glutaric acid and lipopolysaccharide in rat pups. PLoS One. 2013;8(10):e78332. doi: 10.1371/journal.pone.0078332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cao L., Li L., Zuo Z. N-acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience. 2012;220:85–89. doi: 10.1016/j.neuroscience.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tardiolo G., Bramanti P., Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules. 2018;23(12):3305. doi: 10.3390/molecules23123305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bliss T.V., Collingridge G.L., Morris R.G. Synaptic plasticity in health and disease: Introduction and overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;369(1633):20130129. doi: 10.1098/rstb.2013.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi D.W. Excitotoxic cell death. J. Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 110.Zipfel G.J., Babcock D.J., Lee J.M., Choi D.W. Neuronal apoptosis after CNS injury: The roles of glutamate and calcium. J. Neurotrauma. 2000;17(10):857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]
- 111.Bridges R.J., Natale N.R., Patel S.A. System xc⁻ cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012;165(1):20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baker D.A., McFarland K., Lake R.W., Shen H., Tang X.C., Toda S., Kalivas P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 113.Spencer S., Kalivas P.W. Glutamate transport: A new bench to bedside mechanism for treating drug abuse. Int. J. Neuropsychopharmacol. 2017;20(10):797–812. doi: 10.1093/ijnp/pyx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kalivas P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 115.Tomko R.L., Jones J.L., Gilmore A.K., Brady K.T., Back S.E., Gray K.M. N-acetylcysteine: A potential treatment for substance use disorders. Curr. Psychiatr. 2018;17(6):30–36, 41-42, 55. [PMC free article] [PubMed] [Google Scholar]
- 116.Moussawi K., Pacchioni A., Moran M., Olive M.F., Gass J.T., Lavin A., Kalivas P.W. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reichel C.M., Moussawi K., Do P.H., Kalivas P.W., See R.E. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J. Pharmacol. Exp. Ther. 2011;337(2):487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker D.A., McFarland K., Lake R.W., Shen H., Toda S., Kalivas P.W. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann. N. Y. Acad. Sci. 2003;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 119.Madayag A., Lobner D., Kau K.S., Mantsch J.R., Abdulhameed O., Hearing M., Grier M.D., Baker D.A. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 2007;27(51):13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knackstedt L.A., Melendez R.I., Kalivas P.W. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amen S.L., Piacentine L.B., Ahmad M.E., Li S.J., Mantsch J.R., Risinger R.C., Baker D.A. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou W., Kalivas P.W. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry. 2008;63(3):338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramirez-Niño A.M., D’Souza M.S., Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: Comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl.) 2013;225(2):473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reissner K.J., Gipson C.D., Tran P.K., Knackstedt L.A., Scofield M.D., Kalivas P.W. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict. Biol. 2015;20(2):316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Powell G.L., Leyrer-Jackson J.M., Goenaga J., Namba M.D., Piña J., Spencer S., Stankeviciute N., Schwartz D., Allen N.P., Del Franco A.P., McClure E.A., Olive M.F., Gipson C.D. Chronic treatment with N-acetylcysteine decreases extinction responding and reduces cue-induced nicotine-seeking. Physiol. Rep. 2019;7(1):e13958. doi: 10.14814/phy2.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spencer S., Neuhofer D., Chioma V.C., Garcia-Keller C., Schwartz D.J., Allen N., Scofield M.D., Ortiz-Ithier T., Kalivas P.W. A model of delta(9)-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biol. Psychiatry. 2018;84(8):601–610. doi: 10.1016/j.biopsych.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mardikian P.N., LaRowe S.D., Hedden S., Kalivas P.W., Malcolm R.J. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(2):389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Schmaal L., Veltman D.J., Nederveen A., van den Brink W., Goudriaan A.E. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: A randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schulte M.H.J., Wiers R.W., Boendermaker W.J., Goudriaan A.E., van den Brink W., van Deursen D.S., Friese M., Brede E., Waters A.J. The effect of N-acetylcysteine and working memory training on cocaine use, craving and inhibition in regular cocaine users: Correspondence of lab assessments and Ecological Momentary Assessment. Addict. Behav. 2018;79:24–31. doi: 10.1016/j.addbeh.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 130.Squeglia L.M., Baker N.L., McClure E.A., Tomko R.L., Adisetiyo V., Gray K.M. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict. Behav. 2016;63:172–177. doi: 10.1016/j.addbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gray K.M., LaRowe S.D., Watson N.L., Carpenter M.J. Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addict. Behav. 2011;36(1-2):140–143. doi: 10.1016/j.addbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gray K.M., Sonne S.C., McClure E.A., Ghitza U.E., Matthews A.G., McRae-Clark A.L., Carroll K.M., Potter J.S., Wiest K., Mooney L.J., Hasson A., Walsh S.L., Lofwall M.R., Babalonis S., Lindblad R.W., Sparenborg S., Wahle A., King J.S., Baker N.L., Tomko R.L., Haynes L.F., Vandrey R.G., Levin F.R. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249–257. doi: 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomko R.L., Gilmore A.K., Gray K.M. The role of depressive symptoms in treatment of adolescent cannabis use disorder with N-Acetylcysteine. Addict. Behav. 2018;85:26–30. doi: 10.1016/j.addbeh.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prado E., Maes M., Piccoli L.G., Baracat M., Barbosa D.S., Franco O., Dodd S., Berk M., Vargas Nunes S.O. N-acetylcysteine for therapy-resistant tobacco use disorder: A pilot study. Redox Rep. 2015;20(5):215–222. doi: 10.1179/1351000215Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Asevedo E., Mendes A.C., Berk M., Brietzke E. Systematic review of N-acetylcysteine in the treatment of addictions. Br. J. Psychiatry. 2014;36(2):168–175. doi: 10.1590/1516-4446-2013-1244. [DOI] [PubMed] [Google Scholar]
- 136.Duailibi M.S., Cordeiro Q., Brietzke E., Ribeiro M., LaRowe S., Berk M., Trevizol A.P. N-acetylcysteine in the treatment of craving in substance use disorders: Systematic review and meta-analysis. Am. J. Addict. 2017;26(7):660–666. doi: 10.1111/ajad.12620. [DOI] [PubMed] [Google Scholar]
- 137.McKetin R., Dean O.M., Turner A., Kelly P.J., Quinn B., Lubman D.I., Dietze P., Carter G., Higgs P., Baker A.L., Sinclair B., Reid D., Manning V., Te Pas N., Liang W., Thomas T., Bathish R., Kent M., Raftery D., Arunogiri S., Cordaro F., Hill H., Berk M. A study protocol for the N-ICE trial: A randomised double-blind placebo-controlled study of the safety and efficacy of N-acetyl-cysteine (NAC) as a pharmacotherapy for methamphetamine (“ice”) dependence. Trials. 2019;20(1):325. doi: 10.1186/s13063-019-3450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nasca C., Bigio B., Zelli D., Nicoletti F., McEwen B.S. Mind the gap: Glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol. Psychiatry. 2015;20(6):755–763. doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nasca C., Bigio B., Zelli D., de Angelis P., Lau T., Okamoto M., Soya H., Ni J., Brichta L., Greengard P., Neve R.L., Lee F.S., McEwen B.S. Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron. 2017;96(2):402–413.e5. doi: 10.1016/j.neuron.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 140.Linck V.M., Costa-Campos L., Pilz L.K., Garcia C.R., Elisabetsky E. AMPA glutamate receptors mediate the antidepressant-like effects of N-acetylcysteine in the mouse tail suspension test. Behav. Pharmacol. 2012;23(2):171–177. doi: 10.1097/FBP.0b013e3283512c3a. [DOI] [PubMed] [Google Scholar]
- 141.Anderson G., Berk M., Dean O., Moylan S., Maes M. Role of immune-inflammatory and oxidative and nitrosative stress pathways in the etiology of depression: Therapeutic implications. CNS Drugs. 2014;28(1):1–10. doi: 10.1007/s40263-013-0119-1. [DOI] [PubMed] [Google Scholar]
- 142.Berk M., Copolov D.L., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Bush A.I. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol. Psychiatry. 2008;64(6):468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 143.Fernandes B.S., Dean O.M., Dodd S., Malhi G.S., Berk M. N-Acetylcysteine in depressive symptoms and functionality: A systematic review and meta-analysis. J. Clin. Psychiatry. 2016;77(4):e457–e466. doi: 10.4088/JCP.15r09984. [DOI] [PubMed] [Google Scholar]
- 144.Berk M., Dean O.M., Cotton S.M., Jeavons S., Tanious M., Kohlmann K., Hewitt K., Moss K., Allwang C., Schapkaitz I., Robbins J., Cobb H., Ng F., Dodd S., Bush A.I., Malhi G.S. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: A double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry. 2014;75(6):628–636. doi: 10.4088/JCP.13m08454. [DOI] [PubMed] [Google Scholar]
- 145.Magalhães P.V., Dean O., Andreazza A.C., Berk M., Kapczinski F. Antioxidant treatments for schizophrenia. Cochrane Database Syst. Rev. 2016;2:CD008919. doi: 10.1002/14651858.CD008919.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Judd F., Katz F., Katz P., Ording-Jespersen S., Little J., Conus P., Cuenod M., Do K.Q., Bush A.I. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol. Psychiatry. 2008;64(5):361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 147.Berk M., Munib A., Dean O., Malhi G.S., Kohlmann K., Schapkaitz I., Jeavons S., Katz F., Anderson-Hunt M., Conus P., Hanna B., Otmar R., Ng F., Copolov D.L., Bush A.I. Qualitative methods in early-phase drug trials: Broadening the scope of data and methods from an RCT of N-acetylcysteine in schizophrenia. J. Clin. Psychiatry. 2011;72(7):909–913. doi: 10.4088/JCP.09m05741yel. [DOI] [PubMed] [Google Scholar]
- 148.Farokhnia M., Azarkolah A., Adinehfar F., Khodaie-Ardakani M.R., Hosseini S.M., Yekehtaz H., Tabrizi M., Rezaei F., Salehi B., Sadeghi S.M., Moghadam M., Gharibi F., Mirshafiee O., Akhondzadeh S. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 2013;36(6):185–192. doi: 10.1097/WNF.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 149.Breier A., Liffick E., Hummer T.A., Vohs J.L., Yang Z., Mehdiyoun N.F., Visco A.C., Metzler E., Zhang Y., Francis M.M. Effects of 12-month, double-blind N-acetyl cysteine on symptoms, cognition and brain morphology in early phase schizophrenia spectrum disorders. Schizophr. Res. 2018;199:395–402. doi: 10.1016/j.schres.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 150.Conus P., Seidman L.J., Fournier M., Xin L., Cleusix M., Baumann P.S., Ferrari C., Cousins A., Alameda L., Gholam-Rezaee M., Golay P., Jenni R., Woo T.W., Keshavan M.S., Eap C.B., Wojcik J., Cuenod M., Buclin T., Gruetter R., Do K.Q. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr. Bull. 2018;44(2):317–327. doi: 10.1093/schbul/sbx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cho M., Lee T.Y., Kwak Y.B., Yoon Y.B., Kim M., Kwon J.S. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust. N. Z. J. Psychiatry. 2019;53(8):742–759. doi: 10.1177/0004867419835028. [DOI] [PubMed] [Google Scholar]
- 152.Berk M., Dean O., Cotton S.M., Gama C.S., Kapczinski F., Fernandes B.S., Kohlmann K., Jeavons S., Hewitt K., Allwang C., Cobb H., Bush A.I., Schapkaitz I., Dodd S., Malhi G.S. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. J. Affect. Disord. 2011;135(1-3):389–394. doi: 10.1016/j.jad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 153.Berk M., Dean O.M., Cotton S.M., Gama C.S., Kapczinski F., Fernandes B., Kohlmann K., Jeavons S., Hewitt K., Moss K., Allwang C., Schapkaitz I., Cobb H., Bush A.I., Dodd S., Malhi G.S. Maintenance N-acetyl cysteine treatment for bipolar disorder: a double-blind randomized placebo controlled trial. BMC Med. 2012;10:91. doi: 10.1186/1741-7015-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dean O.M., Bush A.I., Copolov D.L., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. Effects of N-acetyl cysteine on cognitive function in bipolar disorder. Psychiatry Clin. Neurosci. 2012;66(6):514–517. doi: 10.1111/j.1440-1819.2012.02392.x. [DOI] [PubMed] [Google Scholar]
- 155.Magalhães P.V., Dean O.M., Bush A.I., Copolov D.L., Malhi G.S., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. N-acetyl cysteine add-on treatment for bipolar II disorder: a subgroup analysis of a randomized placebo-controlled trial. J. Affect. Disord. 2011;129(1-3):317–320. doi: 10.1016/j.jad.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 156.Magalhães P.V., Dean O.M., Bush A.I., Copolov D.L., Malhi G.S., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. N-acetylcysteine for major depressive episodes in bipolar disorder. Br. J. Psychiatry. 2011;33(4):374–378. doi: 10.1590/S1516-44462011000400011. [DOI] [PubMed] [Google Scholar]
- 157.Magalhães P.V., Dean O.M., Bush A.I., Copolov D.L., Weisinger D., Malhi G.S., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. Systemic illness moderates the impact of N-acetyl cysteine in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;37(1):132–135. doi: 10.1016/j.pnpbp.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 158.Magalhães P.V., Dean O.M., Bush A.I., Copolov D.L., Malhi G.S., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. A preliminary investigation on the efficacy of N-acetyl cysteine for mania or hypomania. Aust. N. Z. J. Psychiatry. 2013;47(6):564–568. doi: 10.1177/0004867413481631. [DOI] [PubMed] [Google Scholar]
- 159.Afshar H., Roohafza H., Mohammad-Beigi H., Haghighi M., Jahangard L., Shokouh P., Sadeghi M., Hafezian H. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2012;32(6):797–803. doi: 10.1097/JCP.0b013e318272677d. [DOI] [PubMed] [Google Scholar]
- 160.Smith L., Tracy D.K., Giaroli G. What future role might N-acetyl-cysteine have in the treatment of obsessive compulsive and grooming disorders? A systematic review. J. Clin. Psychopharmacol. 2016;36(1):57–62. doi: 10.1097/JCP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 161.Oliver G., Dean O., Camfield D., Blair-West S., Ng C., Berk M., Sarris J. N-acetyl cysteine in the treatment of obsessive compulsive and related disorders: a systematic review. Clin. Psychopharmacol. Neurosci. 2015;13(1):12–24. doi: 10.9758/cpn.2015.13.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Couto J.P., Moreira R. Oral N-acetylcysteine in the treatment of obsessive-compulsive disorder: A systematic review of the clinical evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:245–254. doi: 10.1016/j.pnpbp.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 163.Frustaci A., Neri M., Cesario A., Adams J.B., Domenici E., Dalla B.B., Bonassi S. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic. Biol. Med. 2012;52(10):2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 164.Chiechio S. Modulation of chronic pain by metabotropic glutamate receptors. Adv. Pharmacol. 2016;75:63–89. doi: 10.1016/bs.apha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 165.Zammataro M., Chiechio S., Montana M.C., Traficante A., Copani A., Nicoletti F., Gereau R.W., IV mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Mol. Pain. 2011;7:6. doi: 10.1186/1744-8069-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bernabucci M., Notartomaso S., Zappulla C., Fazio F., Cannella M., Motolese M., Battaglia G., Bruno V., Gradini R., Nicoletti F. N-Acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors. Mol. Pain. 2012;8:77. doi: 10.1186/1744-8069-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li J., Xu L., Deng X., Jiang C., Pan C., Chen L., Han Y., Dai W., Hu L., Zhang G., Cheng Z., Liu W. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain. 2016;157(8):1711–1723. doi: 10.1097/j.pain.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 168.Horst A., de Souza J.A., Santos M.C., Riffel A.P., Kolberg C., Ribeiro M.F., de Fraga L.S., Partata W.A. N-acetylcysteine downregulates phosphorylated p-38 expression but does not reverse the increased superoxide anion levels in the spinal cord of rats with neuropathic pain. Braz. J. Med. Biol. Res. 2017;50(2):e5801. doi: 10.1590/1414-431x20165801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sözbir E., Nazıroğlu M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab. Brain Dis. 2016;31(2):385–393. doi: 10.1007/s11011-015-9769-7. [DOI] [PubMed] [Google Scholar]
- 170.Perez R.S., Zuurmond W.W., Bezemer P.D., Kuik D.J., van Loenen A.C., de Lange J.J., Zuidhof A.J. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003;102(3):297–307. doi: 10.1016/S0304-3959(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 171.Truini A., Piroso S., Pasquale E., Notartomaso S., Di Stefano G., Lattanzi R., Battaglia G., Nicoletti F., Cruccu G. N-acetyl-cysteine, a drug that enhances the endogenous activation of group-II metabotropic glutamate receptors, inhibits nociceptive transmission in humans. Mol. Pain. 2015;11:14. doi: 10.1186/s12990-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thiele H., Hildebrand L., Schirdewahn C., Eitel I., Adams V., Fuernau G., Erbs S., Linke A., Diederich K.W., Nowak M., Desch S., Gutberlet M., Schuler G. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J. Am. Coll. Cardiol. 2010;55(20):2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 173.Pasupathy S., Tavella R., Grover S., Raman B., Procter N.E.K., Du Y.T., Mahadavan G., Stafford I., Heresztyn T., Holmes A., Zeitz C., Arstall M., Selvanayagam J., Horowitz J.D., Beltrame J.F. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation. 2017;136(10):894–903. doi: 10.1161/CIRCULATIONAHA.117.027575. [DOI] [PubMed] [Google Scholar]
- 174.Talasaz A.H., Khalili H., Fahimi F., Mojtaba S. Potential role of N-acetylcysteine in cardiovascular disorders. Therapy. 2011;8(3):237–245. doi: 10.2217/thy.11.12. [DOI] [Google Scholar]
- 175.Dludla P.V., Dias S.C., Obonye N., Johnson R., Louw J., Nkambule B.B. A systematic review on the protective effect of N-acetyl cysteine against diabetes-associated cardiovascular complications. Am. J. Cardiovasc. Drugs. 2018;18(4):283–298. doi: 10.1007/s40256-018-0275-2. [DOI] [PubMed] [Google Scholar]
- 176.Weisbord S.D., Palevsky P.M. Radiocontrast-induced acute renal failure. J. Intensive Care Med. 2005;20(2):63–75. doi: 10.1177/0885066604273503. [DOI] [PubMed] [Google Scholar]
- 177.Parfrey P. The clinical epidemiology of contrast-induced nephropathy. Cardiovasc. Intervent. Radiol. 2005;28(Suppl. 2):S3–S11. doi: 10.1007/s00270-005-0196-8. [DOI] [PubMed] [Google Scholar]
- 178.Fliser D., Laville M., Covic A., Fouque D., Vanholder R., Juillard L., Van Biesen W., Ad-hoc working group of ERBP A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol. Dial. Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marenzi G., Assanelli E., Marana I., Lauri G., Campodonico J., Grazi M., De Metrio M., Galli S., Fabbiocchi F., Montorsi P., Veglia F., Bartorelli A.L. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N. Engl. J. Med. 2006;354(26):2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 180.Thayssen P., Lassen J.F., Jensen S.E., Hansen K.N., Hansen H.S., Christiansen E.H., Junker A., Ravkilde J., Thuesen L., Veien K.T., Jensen L.O. Prevention of contrast-induced nephropathy with N-acetylcysteine or sodium bicarbonate in patients with ST-segment-myocardial infarction: a prospective, randomized, open-labeled trial. Circ. Cardiovasc. Interv. 2014;7(2):216–224. doi: 10.1161/CIRCINTERVENTIONS.113.000653. [DOI] [PubMed] [Google Scholar]
- 181.Misra D., Leibowitz K., Gowda R.M., Shapiro M., Khan I.A. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: a meta-analysis. Clin. Cardiol. 2004;27(11):607–610. doi: 10.1002/clc.4960271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sharp A.J., Patel N., Reeves B.C., Angelini G.D., Fiorentino F. Pharmacological interventions for the prevention of contrast-induced acute kidney injury in high-risk adult patients undergoing coronary angiography: a systematic review and meta-analysis of randomised controlled trials. Open Heart. 2019;6(1):e000864. doi: 10.1136/openhrt-2018-000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trivedi H., Daram S., Szabo A., Bartorelli A.L., Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am. J. Med. 2009;122(9):874.e9–874.e15. doi: 10.1016/j.amjmed.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 184.Akyol-Salman I., Azizi S., Mumcu U., Baykal O. Efficacy of topical N-acetylcysteine in the treatment of meibomian gland dysfunction. J. Ocul. Pharmacol. Ther. 2010;26(4):329–333. doi: 10.1089/jop.2010.0001. [DOI] [PubMed] [Google Scholar]
- 185.Kruh J.N., Kruh-Garcia N.A., Foster C.S. Evaluation of the effect of N-acetylcysteine on protein deposition on contact lenses in patients with the Boston keratoprosthesis type I. J. Ocul. Pharmacol. Ther. 2015;31(6):314–322. doi: 10.1089/jop.2015.0010. [DOI] [PubMed] [Google Scholar]
- 186.Yalçin E., Altin F., Cinhüseyinoglue F., Arslan M.O. N-acetylcysteine in chronic blepharitis. Cornea. 2002;21(2):164–168. doi: 10.1097/00003226-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 187.Daiger S.P., Sullivan L.S., Bowne S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013;84(2):132–141. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Q. Retinitis pigmentosa: progress and perspective. Asia Pac. J. Ophthalmol. (Phila.) 2016;5(4):265–271. doi: 10.1097/APO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 189.Campochiaro P.A., Mir T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 190.Yu D-Y., Cringle S.J., Su E-N., Yu P.K. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41(12):3999–4006. [PubMed] [Google Scholar]
- 191.Shen J., Yang X., Dong A., Petters R.M., Peng Y.W., Wong F., Campochiaro P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005;203(3):457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 192.Komeima K., Rogers B.S., Campochiaro P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213(3):809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 193.Komeima K., Rogers B.S., Lu L., Campochiaro P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee S.Y., Usui S., Zafar A.B., Oveson B.C., Jo Y.J., Lu L., Masoudi S., Campochiaro P.A. N-Acetylcysteine promotes long-term survival of cones in a model of retinitis pigmentosa. J. Cell. Physiol. 2011;226(7):1843–1849. doi: 10.1002/jcp.22508. [DOI] [PubMed] [Google Scholar]
- 195.Martínez-Fernández de la Cámara C., Salom D., Sequedo M.D., Hervás D., Marín-Lambíes C., Aller E., Jaijo T., Díaz-Llopis M., Millán J.M., Rodrigo R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One. 2013;8(9):e74223. doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Campochiaro P.A., Iftikhar M., Hafiz G., Akhlaq A., Tsai G., Wehling D., Lu L., Wall G.M., Singh M.S., Kong X. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J. Clin. Invest. 2020;130(3):1527–1541. doi: 10.1172/JCI132990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Petersen A.C., McKenna M.J., Medved I., Murphy K.T., Brown M.J., Della Gatta P., Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol. (Oxf.) 2012;204(3):382–392. doi: 10.1111/j.1748-1716.2011.02344.x. [DOI] [PubMed] [Google Scholar]