Abstract
Both preclinical and clinical studies have pointed that aerobic exercise, at moderate doses, is beneficial at all stages of life by promoting a range of physiological and neuroplastic adaptations that reduce the anxiety response. Previous research about this topic has repeatedly described how the regular practice of aerobic exercise induces a positive regulation of neuroplasticity and neurogenesis-related genes, as well as a better control of the HPA axis function. However, limited progress has been carried out in the integration of neuroendocrine and neuroplastic changes, as well as in introducing new factors to understand how aerobic exercise can promote resilience to future stressful conditions. Resilience is defined as the ability to adapt to stress while maintaining healthy mental and physical performance. Consistent findings point to an important role of FKBP5, the gene expressing FK506-binding protein 51 (FKBP51), as a strong inhibitor of the glucocorticoid receptor (GR), and thus, an important regulator of the stress response. We propose that aerobic exercise could contribute to modulate FKBP5 activity acting as a potential therapeutic approach for mood disorders. In this sense, aerobic exercise is well known for increasing the growth factor BDNF, which by downstream pathways could affect the FKBP5 activity. Therefore, our manuscript has the aim of analyzing how FKBP5 could constitute a promising target of aerobic exercise promoting resilient-related phenotypes.
Keywords: Anxiety, depression, exercise, glucocorticoid receptor, HPA, resilience
1. AEROBIC EXERCISE AS A HEALTHY AVENUE TO PROMOTE RESILIENCE
Overwhelming evidence exists that lifelong exercise is associated with a longer healthspan, whereas physical inactivity is the fourth leading contributor to death worldwide [1]. Among its benefits, aerobic exercise causes not only positive effects on physical health but also on psychological well-being. Hence, those subjects who perform exercise regularly suffer from less depression [2], anxiety [3] and cognitive impairments [4]. Similar results have been found in preclinical studies in which, although running exercise is comparable to other forms of stress in terms of corticosterone release, it induces patterns of neuronal activity that correspond to predictable, controllable, and rewarding stimuli, in contrast to negative stressors, such as social isolation or electric shocks [5].
Among the biological mechanisms related to the positive impact of aerobic exercise, several studies have pointed to structural (e.g. increased neurogenesis, synaptogenesis, gliogenesis and angiogenesis) and cellular/molecular (e.g. altered central monoamine neurotransmission and increased growth factor expression) changes that could promote enhanced neuroplasticity and may be capable of buffering the detrimental effects of chronic stress [6]. Hence, the increase of local and systemic expression of growth factors, notably the brain-derived neurotrophic factor (BDNF) has been commonly associated with improvements in cognitive functioning, as well as in anxiety and depression-related behaviors [7]. Accordingly, the ability of aerobic exercise to enhance BDNF release and function in the synapse promotes dendritic spine integrity and activates other cellular pathways is a cornerstone for brain processes that are necessary to repair and reorganize neuroplasticity altered during the course of mood disorders [8, 9]. In addition, other growth factors, such as the insulin-like growth factor-1 and the vascular endothelial growth factor, have been shown to play an important role in BDNF-induced effects on neuroplasticity, as well as to exert neuroprotective effects of their own contributions to the beneficial effects of exercise on the brain [10]. On the other hand, exercise appears to have a blunting effect on the hypothalamic-pituitary–adrenal axis (HPA axis) and the sympathetic nervous system. This blunting impact on stress responsiveness seems to contribute to reducing emotional, physiological and metabolic reactivity, as well as increase positive mood and psychological well-being [6]. Finally, another biological target of aerobic exercise is the immune system. Thus, higher physical activity has been associated with lower inflammatory cytokine responses to a mental stressor, along with a greater parasympathetic control [11]. Moreover, regular exercisers also showed attenuated leucocyte trafficking and adhesion molecule expression in response to a mental stressor compared with less physically active individuals [12].
In summary, these findings are consistent with the concept of physiological toughening as a mechanism by which regular exercise can improve stress tolerance by optimizing neuroendocrine and physiological responses [6]. Obviously, the expression of growth factors and neuroplasticity are promising avenues of research with the potential to elucidate the mechanisms of how aerobic exercise works. Concerning this, new lines of research have started to focus on the relationship between the FK506-binding protein 5 (FKBP5) gene and BDNF because both are expressed and affect the functioning of brain areas, such as the hippocampus, amygdala and the prefrontal cortex (PFC), involved in the control of the stress response [13, 14]. Genetic studies revealed associations between stressful life events and alterations in the HPA axis that were mediated, in part, by gene × environment interactions involving FKBP5 and BDNF polymorphisms [9, 15, 16]. Consequently, would it also be possible that interactions between genes and eustresors, such as aerobic exercise, could improve the HPA functioning and promote resilient-related behaviors by epigenetic mechanisms?
The present CN perspective has the aim of analyzing the synergistic effects of BDNF and FKBP5, as a still unknown target of aerobic exercise, in promoting resilience to cope with stressful situations.
2. THE UNKNOWN ROLE OF FKBP5 PROTEIN IN THE POSITIVE EFFECTS INDUCED BY AEROBIC EXERCISE
In the past decade, FKBP5 (OMIM 602623) has emerged as a promising genetic candidate for investigations of vulnerability to mood and anxiety disorders owing to its involvement in regulating the sensitivity of GR [17]. Elevated FKBP5 levels lead to a decreased negative feedback regulation of the HPA axis and GR resistance, which is probably responsible for a dysregulated stress response [18]. Besides, the expression of FKBP51 correlates with plasma BDNF levels in depressed patients [19]. More precisely, the inhibition of GR negatively affects BDNF-induced TrkB phosphorylation and its downstream signaling pathways, whereas a short activation of GR is associated with the long-lasting BDNF-delivered mechanisms required for memory consolidation [20].
Additionally, and as we mentioned before, BDNF is involved in the regulation of synaptic plasticity by pre-and post-synaptic mechanisms. Potential downstream targets of BDNF are the Synapsins, a family of presynaptic phosphoproteins, which affect the proportion of vesicles that are available for release [21]. Several studies have found that BDNF increases Synapsin phosphorylation, thus enhancing the availability of vesicles and facilitating neurotransmitter release [22, 23]. Interestingly, the presynaptic vesicle protein Synapsin has shown to be an important molecule candidate to modulate FKBP5 reducing the stress responsiveness [24]. It has been found that the expression of FKBP51 and Synapsin is regulated in opposite directions not only in the mouse PFC, but also in the PFC of schizophrenic patients, who are generally known for exhibiting an altered stress-coping behavior [24]. On the other hand, a recent study revealed a critical role of FKBP51 in mBDNF secretion and suggested the involvement of mBDNF in the performance of stress-coping behavior after the administration of the antidepressant S-ketamine [25]. Specifically, and contrary to our expectations, these authors found that the enhancement of BDNF in the extracellular space after S-ketamine administration was absent in FKBP51 deficient mice. This effect could be possible if we consider that this protein plays a double role in mediating responses to stimuli with both positive (eustresors) and negative (stressors) characteristics [26]. Likewise, the antidepressant effect of paroxetine was related to an enhancement of both BDNF and FKBP5 [19]. Nevertheless, although the mechanism by which FKBP5 is able to modulate mBDNF levels is still unknown, it has been proposed that its interaction with NMDA receptors, as well as with inhibitory synapses in brain regions such as the hippocampus, could affect the neuronal activity and consequently BDNF levels [27, 28].
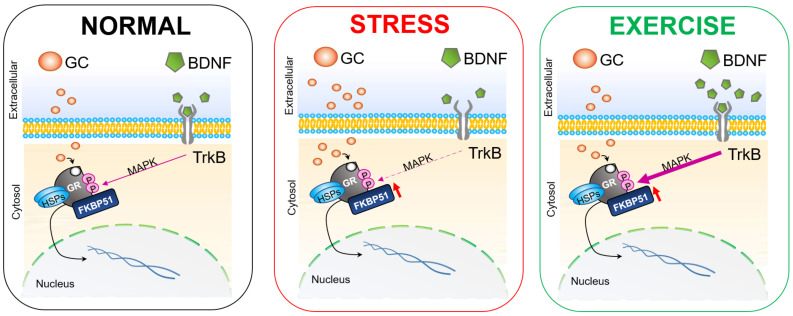
Regarding the direct modulation of FKBP5 by aerobic exercise, we have found in the literature that is a research field scarcely explored. A recent study has found an increase in the gene expression of FKBP5 in relevant limbic areas (e.g. mPFC, insular cortex and hippocampus) after a protocol of wheel-running [29, 30]. It is possible that the enhancement of FKBP5 after running is induced by glucocorticoids (GCs) increase owing to the stressful but positive nature of the aerobic exercise. Previous studies have found that FKBP5 expression can be produced by GCs and has been shown to be a very accurate measure of GR regulation and signaling constituting an appropriate marker of HPA flexibility [31, 32]. Thus, when GCs enter the cytoplasm, they bind to the GR-chaperone complex, favoring the exchange of FKBP5 for FKBP4, which allows GR translocation to the nucleus and promotes the transcriptional activity of many genes involved in the feedback regulation of the HPA axis. Hence, the greater release of GCs caused by the exercise is compensated by the increase of FKBP5 complexes whose exchange to FKBP4 favors the regulation of the stress axis. Therefore, the increased expression of FKBP5 mediated by GCs is considered as an ultrashort, intracellular negative feedback loop that regulates intracellular GR sensitivity [33] (Fig. 1). In addition, to understand the paradoxical increase of GCs by exercise, it has also been described that GCs released into the blood eventually reach the mPFC, elevating dopamine release, upregulating BDNF [34] and inducing control and coping-related behaviors [35, 36]. Additionally, exercise has rewarding effects related to the dopaminergic striatal circuitry activation and the induction of stress resistance [37]. In contrast, chronic stress and depression have been associated with an overall reduction in dopamine neurotransmission in areas such as PFC, VTA and nucleus accumbens [38]. In this sense, region-specific effects have been reported by previous research on FKBP5. For example, mice lacking the Fkbp5 gene show stress-induced decline in synapsin expression in the prefrontal cortex but not in the hippocampus, and selective Fkbp5 silencing in the amygdala was shown to confer resilience to restraint stress exposure [32].
Fig. (1).
Hypothetical mechanisms of the action of stress and exercise in the BDNF- and FKBP5-mediated GR phosphorylation signaling pathway. Aerobic exercise increases BDNF expression, which in turn promotes GR phosphorylation through the serine residues by mitogen-activated protein kinase (MAPK) signaling pathway [39]. Hence, exercise could boost MAPK signaling enhancing the level of the activated form of this transcription factor as previous studies have found after only a week of voluntary running [40]. In consequence, activation of a TrkB-MAPK pathway could trigger GR phosphorylation and the expression of genes involved in resilience-related neuroplasticity. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
CONCLUSION
Most people are confronted with stressful situations at some point in their lives and do not developmental disorders as a result. This ability to deal with and overcome adversity involves the complex construct of resilience. Several resilience-promoting avenues have been described, being the performance of regular physical activity one of them. It exerts antidepressant and anxiolytic-like effects by toughening the physiological and neuroendocrine mechanisms involved in the negative feedback of the HPA axis. Hence, we propose a scarcely explored pathway mediated by increased exercise-induced GCs and BDNF, which through its action on the FKBP5 chaperone could result in the transcription of genes involved in resilient behavior to cope with future stressors. Thereby, studies with animal models carrying mutations targeting BDNF-sensitive GR phosphorylation sites could be an adequate approach to analyze the physiological and behavioral importance of these modifications, as well as the pleiotropic effects of FKBP5 depending on the stressor applied.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kohl H.W., III, Craig C.L., Lambert E.V., Inoue S., Alkandari J.R., Leetongin G., Kahlmeier S. The pandemic of physical inactivity: Global action for public health. Lancet. 2012;380(9838):294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 2.Rethorst C.D., Wipfli B.M., Landers D.M. The antidepressive effects of exercise: A meta-analysis of randomized trials. Sports Med. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wipfli B.M., Rethorst C.D., Landers D.M. The anxiolytic effects of exercise: A meta-analysis of randomized trials and dose-response analysis. J. Sport Exerc. Psychol. 2008;30(4):392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- 4.Cassilhas R.C., Tufik S., de Mello M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016;73(5):975–983. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stranahan A.M., Lee K., Mattson M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromol. Med. 2008;10(2):118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman M.N., Deuster P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi: 10.1098/rsfs.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotman C.W., Berchtold N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Luikart B.W., Birnbaum S., Chen J., Kwon C.H., Kernie S.G., Bassel-Duby R., Parada L.F. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 10.Llorens-Martín M., Torres-Alemán I., Trejo J.L. Growth factors as mediators of exercise actions on the brain. Neuromol. Med. 2008;10(2):99–107. doi: 10.1007/s12017-008-8026-1. [DOI] [PubMed] [Google Scholar]
- 11.Hamer M., Steptoe A. Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosom. Med. 2007;69(7):660–666. doi: 10.1097/PSY.0b013e318148c4c0. [DOI] [PubMed] [Google Scholar]
- 12.Hong S., Johnson T.A., Farag N.H., Guy H.J., Matthews S.C., Ziegler M.G., Mills P.J. 2005. [DOI] [PubMed]
- 13.Hernaus D., van Winkel R., Gronenschild E., Habets P., Kenis G., Marcelis M., van Os J., Myin-Germeys I., Collip D. Brain-derived neurotrophic factor/FK506-binding protein 5 genotype by childhood trauma interactions do not impact on hippocampal volume and cognitive performance. PLoS One. 2014;9(3):e92722. doi: 10.1371/journal.pone.0092722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirakawa H., Akiyoshi J., Muronaga M., Tanaka Y., Ishitobi Y., Inoue A., Oshita H., Aizawa S., Masuda K., Higuma H., Kanehisa M., Ninomiya T., Kawano Y. FKBP5 is associated with amygdala volume in the human brain and mood state: A voxel-based morphometry (VBM) study. Int. J. Psychiatry Clin. Pract. 2016;20(2):106–115. doi: 10.3109/13651501.2016.1144772. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie C.F., Phifer J., Bradley B., Ressler K.J. Risk and resilience: Genetic and environmental influences on development of the stress response. Depress. Anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M., Chen L., Yang J., Han D., Fang D., Qiu X., Yang X., Qiao Z., Ma J., Wang L., Jiang S., Song X., Zhou J., Zhang J., Chen M., Qi D., Yang Y., Pan H. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J. Affect. Disord. 2018;227:226–235. doi: 10.1016/j.jad.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Hauger R.L., Olivares-Reyes J.A., Dautzenberg F.M., Lohr J.B., Braun S., Oakley R.H. Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology. 2012;62(2):705–714. doi: 10.1016/j.neuropharm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassen N.C., Fries G.R., Zannas A.S., Hartmann J., Zschocke J., Hafner K., Carrillo-Roa T., Steinbacher J., Preißinger S.N., Hoeijmakers L., Knop M., Weber F., Kloiber S., Lucae S., Chrousos G.P., Carell T., Ising M., Binder E.B., Schmidt M.V., Rüegg J., Rein T. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 2015;8(404):ra119. doi: 10.1126/scisignal.aac7695. [DOI] [PubMed] [Google Scholar]
- 20.Begni V., Riva M.A., Cattaneo A. Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin. Sci. (Lond.) 2017;131(2):123–138. doi: 10.1042/CS20160009. [DOI] [PubMed] [Google Scholar]
- 21.Cesca F., Baldelli P., Valtorta F., Benfenati F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010;91(4):313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Jovanovic J.N., Czernik A.J., Fienberg A.A., Greengard P., Sihra T.S. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat. Neurosci. 2000;3(4):323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 23.Valente P., Casagrande S., Nieus T., Verstegen A.M., Valtorta F., Benfenati F., Baldelli P. Site-specific synapsin I phosphorylation participates in the expression of post-tetanic potentiation and its enhancement by BDNF. J. Neurosci. 2012;32(17):5868–5879. doi: 10.1523/JNEUROSCI.5275-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt U., Buell D.R., Ionescu I.A., Gassen N.C., Holsboer F., Cox M.B., Novak B., Huber C., Hartmann J., Schmidt M.V., Touma C., Rein T., Herrmann L. A role for synapsin in FKBP51 modulation of stress responsiveness: Convergent evidence from animal and human studies. Psychoneuroendocrinology. 2015;52:43–58. doi: 10.1016/j.psyneuen.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Anderzhanova E., Hafner K., Genewsky A.J., Soliman A., Pöhlmann M.L., Schmidt M.V., Blum R., Wotjak C.T., Gassen N.C. The stress susceptibility factor FKBP51 controls S-ketamine-evoked release of mBDNF in the prefrontal cortex of mice. Neurobiol. Stress. 2020;13:100239. doi: 10.1016/j.ynstr.2020.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matosin N., Halldorsdottir T., Binder E.B. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: The FKBP5 model. Biol. Psychiatry. 2018;83(10):821–830. doi: 10.1016/j.biopsych.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Qiu B., Xu Y., Wang J., Liu M., Dou L., Deng R., Wang C., Williams K.E., Stewart R.B., Xie Z., Ren W., Zhao Z., Shou W., Liang T., Yong W. Loss of FKBP5 affects neuron synaptic plasticity: An electrophysiology insight. Neuroscience. 2019;402(402):23–36. doi: 10.1016/j.neuroscience.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Zorumski C.F., Izumi Y., Mennerick S. Ketamine: NMDA receptors and beyond. J. Neurosci. 2016;36(44):11158–11164. doi: 10.1523/JNEUROSCI.1547-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T.Y., Gao Z., Liang N.C. Sex-dependent wheel running effects on high fat diet preference, metabolic outcomes, and performance on the barnes maze in rats. Nutrients. 2020;12(9):2721. doi: 10.3390/nu12092721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T.Y., Gardner J.C., Gao Z., Pan Y.X., Liang N.C. Role of glucocorticoid signaling in exercise-associated changes in high-fat diet preference in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318(3):R515–R528. doi: 10.1152/ajpregu.00288.2019. [DOI] [PubMed] [Google Scholar]
- 31.Rein T. FK506 binding protein 51 integrates pathways of adaptation: FKBP51 shapes the reactivity to environmental change. BioEssays. 2016;38(9):894–902. doi: 10.1002/bies.201600050. [DOI] [PubMed] [Google Scholar]
- 32.Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology. 2016;41(1):261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmer C., Taff C.C., Ardia D., Ryan T.A., Winkler D., Vitousek M. On again, off again: Acute stress response and negative feedback together predict resilience to experimental challenges. Funct. Ecol. 2019;33:619–628. doi: 10.1111/1365-2435.13281. [DOI] [Google Scholar]
- 34.Perreault M.L., Jones-Tabah J., O’Dowd B.F., George S.R. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int. J. Neuropsychopharmacol. 2013;16(2):477–483. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butts K.A., Weinberg J., Young A.H., Phillips A.G. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc. Natl. Acad. Sci. USA. 2011;108(45):18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter A., Al-Bayati M., Paraskevopoulou F., Krämer B., Pruessner J.C., Binder E.B., Gruber O. Interaction of FKBP5 variant rs3800373 and city living alters the neural stress response in the anterior cingulate cortex. Stress. 2021;8:1–9. doi: 10.1080/10253890.2020.1855420. [DOI] [PubMed] [Google Scholar]
- 37.Herrera J.J., Fedynska S., Ghasem P.R., Wieman T., Clark P.J., Gray N., Loetz E., Campeau S., Fleshner M., Greenwood B.N. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur. J. Neurosci. 2016;43(9):1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belujon P., Grace A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20(12):1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arango-Lievano M., Jeanneteau F. Timing and crosstalk of glucocorticoid signaling with cytokines, neurotransmitters and growth factors. 2016. [DOI] [PubMed]
- 40.Shen H., Tong L., Balazs R., Cotman C.W. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107(2):219–229. doi: 10.1016/S0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]