Abstract
The aim of this work is to review tacrine analogues from the last three years, which were not included in the latest review work, donepezil and galantamine hybrids from 2015 and rivastigmine derivatives from 2014. In this account, we summarize the efforts toward the development and characterization of non-toxic inhibitors of cholinesterases based on mentioned drugs with various interesting additional properties such as antioxidant, decreasing β-amyloid plaque aggregation, nitric oxide production, pro-inflammatory cytokines release, monoamine oxidase-B activity, cytotoxicity and oxidative stress in vitro and in animal model that classify these hybrids as potential multifunctional therapeutic agents for Alzheimer’s disease. Moreover, herein, we have described the cholinergic hypothesis, mechanisms of neurodegeneration and current pharmacotherapy of Alzheimer’s disease based on the restoration of cholinergic function through blocking enzymes that break down acetylcholine.
Keywords: Tacrine, donepezil, galantamine, rivastigmine, multifunctional cholinesterase inhibitors, alzheimer’s disease
1. INTRODUCTION
1.1. The Cholinergic Hypothesis and Therapy of Alzheimer’s Disease
In the mid-1970s, studies based on a brain autopsy of patients with Alzheimer’s disease (AD) revealed a significant deficit of the choline acetyltransferase (ChAT) in the limbic system and cerebral cortex, which is responsible for the acetylcholine (ACh) synthesis [1-3]. In a further study, it has been determined that the nucleus basalis of Meynert in the basal forebrain, which is the source of cortical cholinergic innervation, undergoes neurodegeneration in Alzheimer’s disease [4, 5]. The aforementioned studies, as well as the demonstration that cholinergic antagonists impair memory [6], formed the basis for the cholinergic hypothesis of Alzheimer’s Disease.
ACh is a major neurotransmitter in the brain, with activity through the cortex, basal forebrain and basal ganglia [7]. Cholinergic projection neurons are found in nuclei such as the medial habenula, the pedunculopontine and laterodorsal tegmental areas [8]. ACh acts through two classes of receptors: ionotropic nicotinic receptors (nAChRs) and metabotropic muscarinic receptors (M1-M5) [9]. They are located both pre- and postsynaptically throughout the brain, being responsible for diverse brain activity. Cholinergic neurotransmission is involved in a number of psychic processes, including walking and sleep, learning, memory, stress response, and affectivity [10, 11]. Furthermore, its impact on other neurotransmitters greatly expands the scope of the activity. For instance, stimulation of M1/M5 receptors releases neurotransmitter, dopamine, from striatal synaptosomes [12]. Moreover, nAChRs stimulate the release of glutamate, gamma-aminobutyric acid (GABA), norepinephrine or serotonin [13]. Many studies have shown that the cholinergic lesion in Alzheimer's disease is associated with nicotinic and muscarinic receptor changes. Nordberg and Winblad [14] have determined a decrease in postsynaptic nicotinc receptors on cortical neurons. Mash et al. [15] have shown a decrease in M2 receptors (mostly presynaptic) in the cerebral cortex, however, the level of M1 receptors remained unchanged. On the other hand, it has been suggested that M1 receptors may be dysfunctional in the cerebral cortex [16]. Interestingly, the gene expression level of α7 nicotinc receptor is increased in Alzheimer’s disease patients compared to healthy controls, however, its impact on pathogenesis remains to be elucidated [17]. Current pharmacotherapy is based on the restoration of cholinergic function through blocking enzymes that break down acetylcholine. Suppression of acetylcholine breakdown by cholinesterase inhibitors sustains ACh activity at cholinergic synapses. Tacrine was the first cholinesterases inhibitor [both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)] approved by the FDA for the treatment of Alzheimer's disease [18]. Tacrine was shown to have efficacy in mild to moderately impaired Alzheimer's patients, however, it did not affect the ultimate course of the disease [19]. Moreover, tacrine exhibited significant hepatotoxicity (in approximately 25% of patients) and quite low bioavailability after oral administration (about 17%) [19]. For the above reasons, the use of tacrine was limited soon after its inception in therapeutic application [20].
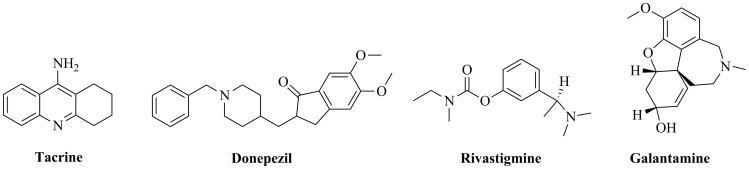
However, tacrine structure is still widely used in medicinal chemistry for designing hybrid or multitarget compounds without toxic side effects [21, 22]. Medicines currently used in AD therapy include three cholinesterase inhibitors: donepezil, rivastigmine and galantamine [23]. Chemical structures of tacrine, donepezil, rivastigmine and galantamine are given in (Fig. 1). In 1996, donepezil was approved for mild to moderate AD therapy as a non-competitive, rapidly reversible acetylcholinesterase (AChE) inhibitor [24]. Rivastigmine is another drug, which was approved in 2000 for AD treatment [25]. Galantamine was approved in 2001 as a competitive, rapidly reversible potent AChE inhibitor [26]. Rivastigmine (the same as tacrine) is a non-competitive, pseudo-irreversible inhibitor of both AChE and BChE [25]. BChE regulates extracellular acetylcholine concentration and is mainly located on glial cells, whereas AChE plays a major role in cholinergic transmission and is located in pre- and postsynaptic membranes [27]. The activity of AChE rapidly declines with the death of cholinergic neurons, with a simultaneous increase in the role of BChE role in the regulation of cholinergic transmission [27]. Therefore, inhibition of both enzymes is a significant treatment benefit for rivastigmine. However, donepezil also not only acts at the neurotransmitter level, it inhibits various aspects of glutamate-induced excitotoxicity, induces a neuroprotective isoform of AChE as well as reduces early expression of inflammatory cytokines and oxidative stress effects [28]. Furthermore, some recent studies indicate that treatment based on the promotion of cholinergic function may also have more durable beneficial biological effects. It has been shown that the use of donepezil is associated with substantially less regional cortical thinning and basal forebrain atrophy over time [29, 30]. Moreover, study on the same population has found a 45% reduction in the rate of hippocampal atrophy after one-year donepezil treatment [31]. In another study, after 18 months of treatment with donepezil and galantamine, a decrease in regional cerebral blood flow in the parietal cortex as well as an increase in the frontal and limbic cortices has been observed [32].
Fig. (1).
Chemical structures of tacrine, donepezil, rivastigmine and galantamine.
Although the current therapy of AD is based on the inhibition of cholinesterase, clinical limitations of its use should be mentioned. Serious side effects of tacrine are hepatotoxicity, nausea, vomiting, loss of appetite, diarrhoea and clumsiness. A limitation of use is also its short half-life, which requires multiple application by each patient [33]. Side effects that occur with rivastigmine are nausea, vomiting, diarrhea, anorexia, weight loss and abdominal pain. Some neurological side effects are possible as well like headache, and syncopes [34]. Also, a donepezil revealed some lack of tolerance in patients manifested by dizziness, gastrointestinal symptoms (for example a nausea, vomiting, or constipation), insomnia, fatigue, sinus bradycardia and toxic effect toward the liver [35]. Any side effect of galantamine has not been proved. Its use is well tolerated and safe [36].
Unfortunately, there are also some limitations of cholinesterase inhibitor studies. Many investigations of neuropathology and cognitive impairment in multiple species have shown that Alzheimer's disease is a uniquely human disease [37]. Therefore, commonly used experimental animal models contribute to a very poor success rate of AD clinical trials, which can be partially explained by the premature translation of successful pathology reduction in transgenic mice to humans [37]. Furthermore, the complexity of pathomechanisms of Alzheimer disease, and its incomplete understanding, makes it difficult to develop an effective therapeutic strategy [38-40].
The aim of this work is to review tacrine analogues from 2017, donepezil and galantamine from 2015, as well as rivastigmine derivatives from the last six years, taking into account their potential multifunctional activity against AD under in vitro and in vivo condition.
1.2. Other Mechanisms of Neurodegeneration in Alzheimer's Disease
Unfortunately, the current pharmacotherapeutic approach based on cholinesterase inhibitors has positive results only for a short period of time, usually 1-3 years, and is not able to completely arrest the progression of the disease [41]. This is due to the multifaceted, progressive and interactive pathophysiology of AD. Besides, impairment of cholinergic neurotransmission, AD pathology includes an aggregation of β-amyloid that leads to tau protein hyperphosphorylation, excess production of nitric oxide (NO) and reactive oxygen species (ROS), microglia and astrocyte activation and ultimately to neurotoxicity and cognitive dysfunction [42].
However, the complete mechanism of AD pathogenesis is still unclear, which has a major impact on the failure of clinical trials in AD treatment. Therefore, the development of multi-target drugs to inhibit multiple factors involved in AD seems to be a promising therapeutic approach.
Senile plaques are one of the neuropathological AD hallmarks present extracellularly [43]. They consist mainly of amyloid-β protein (Aβ), which is produced from its precursor called amyloid precursor protein (APP) [44]. APP is cut by β- and γ-secretases forming Aβ, which is mainly generated from neurons, however, γ-secretase-dependent cleave occurs in several cell types, and generates heterogeneity in Aβ, mainly producing Aβ40 and Aβ42 [44]. Aβ42 is the predominant species deposited in AD brains as well as the most toxic one [45]. According to the Amyloid Cascade Hypothesis, the accumulation of Aβ plaques in the brain of AD patients leads to a number of secondary pathological changes, including altered ionic homeostasis, inflammation (microglial and astrocytic activation), accumulation of the hyperphosphorylated tau protein forming neurofibrillary tangles (NFTs), synaptic degeneration and neuronal cell death [46].
Oxidative stress is a condition of imbalance between reactive oxygen species (ROS) production and antioxidant defences, resulting in excessive accumulation of ROS [47]. It has been suggested that oxidative imbalance may play an important role in the initiation and progression of Alzheimer’s Diseases [48]. It has been determined that the accumulation of Aβ increases oxidative stress and leads to mitochondrial dysfunction and energy failure [49]. Moreover, Aβ-induced oxidative imbalance may increase the levels of the products related to lipid peroxidation, protein oxidation and DNA/RNA oxidation [50]. It has been found that decreased levels of antioxidants (uric acid, vitamin C and E) or antioxidant enzymes (e.g. superoxide dismutase, catalase) in patients with AD [51]. Besides, disturbances in metal levels, such as iron, zinc, and copper have been identified in AD patients [52]. There is evidence to support that these alterations lead to neuronal death through enhancing the ROS production, affecting cellular redox homeostasis (the depletion of glutathione), disruption of mitochondrial function as well as inducing intracellular free calcium, which is part of a signalling pathway [53, 54]. Metal ions may also accelerate protein aggregation and their high micromolar concentrations are found in amyloid plaques [55]. Moreover, overproduction of the nitric oxide (NO) inducing by Aβ (through the activation of microglia) also contributes to the production of highly reactive oxidative species and secondary components of nitroxidative stress [56].
Many studies have shown that neuroinflammation plays a fundamental role in the progression of Alzheimer's disease [57]. Chronically activated microglia release many proinflammatory and toxic products, including cytokines, reactive oxygen species and nitric oxide [58]. One of the more important proinflammatory cytokines in AD is tumour necrosis factor α (TNF-α), which plays both the initiation and regulation role of the cytokine cascade during inflammation [59]. Mice lacking TNFR1 (TNF-α receptor) crossed with the transgenic AD model exhibit mitigated hippocampal microglial activation, reduced plaque deposition and improved performance of incognitive tasks [60]. Cyclooxygenase 2 (COX-2) is upregulated in many inflammatory disorders [61]; however, it is not clear in the case of AD. Increased levels of COX-2 mRNA and protein staining in AD tissue have been detected [62, 63]. On the other hand, a decreased COX-2 expression has been found particularly in the late stage AD [64, 65]. In the case of COX-1, which is prominently expressed by microglia in human brain [66], contribution in neuroinflammation in AD has also not been established. Nevertheless, 5-lipoxygenase (5-LOX), producing pro-inflammatory leukotrienes, as well as, COX enzymes, is considered to be potential novel targets for AD therapy [67]. The family of phosphodiesterase-4 (PDE4) enzymes regulating cyclic adenosine monophosphate (cAMP) signalling in neurons and glial cells represents another underexploited therapeutic targets [68]. PDE4B (subtype of PDE4) is induced in macrophages, monocytes, and microglial cells by inflammatory stimulators including Aβ [69] and represents a novel therapeutic target, which may reduce neuroinflammation in AD [68].
Monoamine oxidase (MAO) is one of the main enzymes, which catabolize catecholamines and serotonin and is widely distributed in the central nervous system in 2 main isoforms: A and B [70]. It has been determined that both A and B forms of MAO are altered in Alzheimer’s disease [71]; however, the mechanism by which they affect AD pathogenesis is not fully understood. A recent study has shown that MAO-B levels are increased in astrocytes as well as in pyramidal neurons in the AD brain [72]. Moreover, the study also suggested that MAO-B regulates Aβ production in neurons via γ-secretase [72]. It has been shown that MAO-B inhibitor may be a useful and reliable tool for the treatment of AD patients improving their cognitive functions and reducing behavioral alterations, without frequent or severe side effects [73].
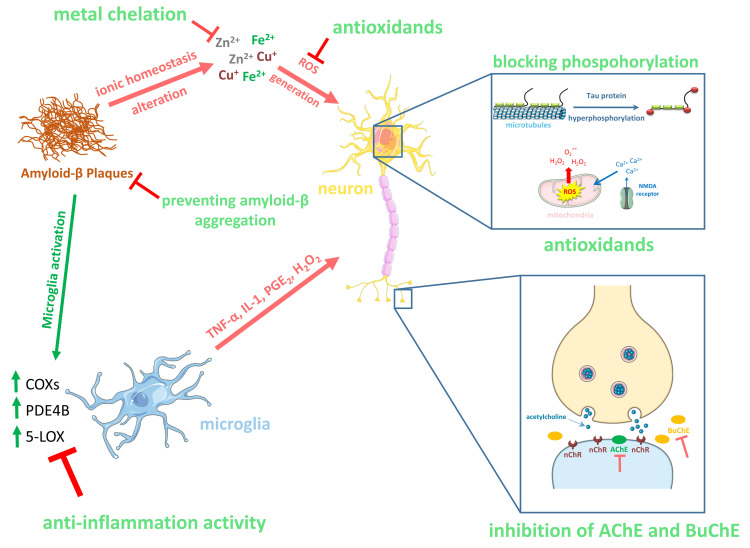
Some metals like iron or copper play a significant role in human health, because they have an impact on the proper course of the homeostasis process. Their excess or deficiencyis essential for an occurrence of several commonly known neurodegenerative disorders like AD. Hence, metal chelating activity is valuable and desirable property during searching for AD efficient treatment therapy [46]. Multitarget therapeutic strategies for AD are summarized in (Fig. 2).
Fig. (2).
Therapeutic strategies of multifunctional compounds for Alzheimer's disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2. MULTIFUNCTIONAL DERIVATIVES OF TACRINE, DONEPEZIL, GALANTAMINE AND RIVASTIGMINE
In the work published in 2019 [74], authors described new multifunctional tacrine derivatives. This article presents the latest analogues, which were synthesized and investigated in a span of the last three years. Moreover, herein we described donepezil and galantamine hybrids which were reported in the last five years, as well as rivastigmine derivatives from the last six years. All structures showing multifunctional properties were included in Table (1).
Table 1.
Structures of tacrine, donepezil and rivastigmine derivatives and their biological activities.
| S. No. | Structure | Additional Functions | References |
|---|---|---|---|
| 1 |
|
Antioxidant activity, protective properties against the injury caused by H2O2 | [81] |
| 2 |
|
2a-d: excellent metal chelating properties. 2a,b: inhibitory potency against AChE-induced Aβ aggregation | [82] |
| 3 |
|
Promising in vivo AChE inhibition profile |
[83] |
| 4 |
|
Inhibitory potency against PAS and AChE-induced Aβ aggregation, ability to induce S-phase post-treatment | [84] |
| 5 |
|
Inhibitory potency against Aβ self-aggregation at the level of 47% at 20 µM, antioxidant properties towards PC12 cells from CoCl2-damage | [85] |
| 6 |
|
Neuroprotective activity towards SH-SY5Y cells against rotenone plus oligomycin-A or okadaic acid | [86] |
| 7 |
|
7b,c,f: neuroprotective activity on SH-SY5Y cell line | [88] |
| 8 |
|
Antioxidant properties, inhibition of Aβ1-42 aggregation, neuroprotection of SH-SY5Y in the presence of H2O2 | [89] |
| 9 |
|
Inhibition of self-/Cu-induced Aβ aggregation, radical scavenging capacity and metal chelating properties (Fe, Cu, Zn), neuroprotection in SH-SY5Y cells treated with Aβ1-42 ascorbate/iron stressors | [96] |
| 10 |
|
Inhibition of Aβ self-aggregation amelioration, the impairment of memory in mice models treated with scopolamine in the Morris water maze test | [97] |
| 11 |
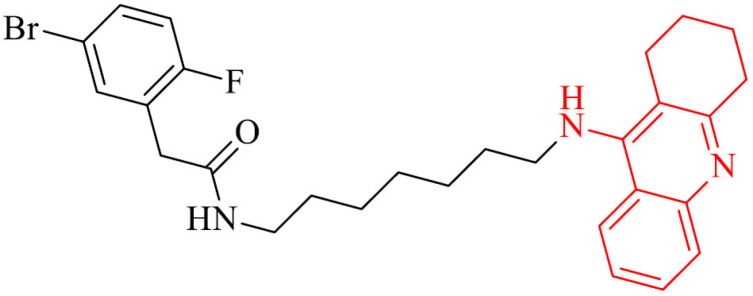
|
Inhibition of Aβ aggregation | [100] |
| 12 |
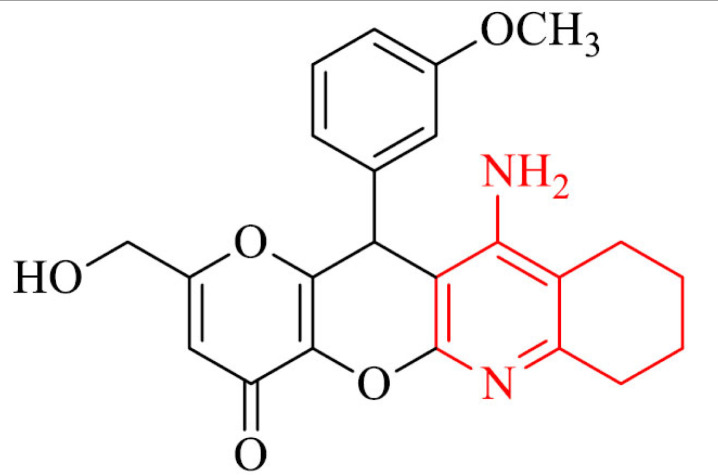
|
Antioxidant activity and neuroprotection against Aβ1-40 in SH-SY5Y cells | [101] |
| 13 |
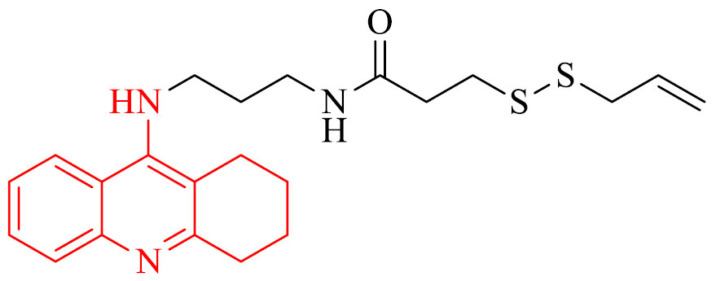
|
Improvement of cognitive and locomotor activity in a mouse model with AD in the step-through test and open field test, an increase of the amount of H2S in hippocampus, decrease mRNA expression of the proinflammatory cytokines, TNF-α, IL-6, and IL-1β and increase synapse-associated in the hippocampus of tested mice | [108] |
| 14 |
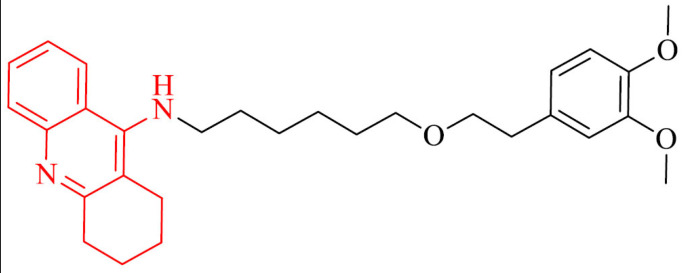
|
Inhibition of Aβ42 self-aggregation, neuroprotection in rats neurons in a serum and K+ deprivation model | [111] |
| 15 |
|
Ability to cross the BBB in vitro | [120] |
| 16 |
|
Inhibition of hMAO A, and MAO-B, strong antioxidant properties |
[127] |
| 17 |
|
Strong antioxidant effect with the oxygen radical absorbance capacity | [129] |
| 18 |
|
Inhibition of MAO-B and Aβ1−42 aggregation, antioxidant effect, ability to chelat Cu2+ ions, to protect PC12 cells from oxidative stress caused by H2O2, rotenone, and oligomycin-A, to protect BV-2 cells from LPS-Stimulated Inflammation, improvement of cognition and spatial memory against scopolamine-induced acute memory deficit, D-galactose (D-gal) and AlCl3 induced chronic oxidative stress in mice model without acute toxicity and hepatotoxicity, ability to cross the BBB in vitro |
[130] |
| 19 |
|
Inhibitory activity against hMAO-B, self-induced and hAChE-induced Aβ aggregation, antioxidant properties, the neuroprotective effects on PC12 cells treated with H2O2 and against LPS-stimulated inflammation on BV-2 cells, ability to cross the BBB in vitro, good liver metabolic stability in RLM, dose-dependently reversed scopolamine-induced memory deficit in mice model but without acute toxicity | [131] |
| 20 |
|
Anti-inflammatory properties in vivo in mice model, antioxidant activity in SH-SY5Y cell line and ability to chelate Cu2+ and Fe2+ ions, protection of SH-SY5Y cells against the late neuronal death caused by Aβ1-42 oligomers | [132] |
| 21 |
|
ADME profile in silico, anti-inflammatory and neuroprotective activity against AβO-induced neuroinflammation and neurodegeneration in vitro in THP-1 cells and in vivo in mice model | [135] |
| 22 |
|
Inhibitory activity against BACE1 and Aβ1−42 aggregation, neuroprotective properties against Aβ1−42-induced damage in SH-SY5Y cells |
[139] |
| 23 |
|
Inhibitory activity against Aβ self-aggregation and Cu2+-induced Aβ aggregation, ability to protect SH-SY5Y cells from the Aβ-induced toxicity | [141] |
| 24 |
|
Inhibitory potency against MAO-B, ability to cross the BBB in vitro | [143] |
| 25 |
|
Selective Cu2+ chelator in a 1:1 ratio, ability to cross the BBB in vitro, activity against MAO-B | [144] |
| 26 |
|
Inhibition of PDE9A | [153] |
| 27 |
|
Ability to inhibit self-induced and Cu2+-induced Aβ1-42 aggregation, inhibitory activity against MAO-B, antioxidative properties, ability to chelate metals and to cross the BBB in vitro | [154] |
| 28 |
|
Prevention from ROS in SH-SY5Y cell line | [155] |
| 29 |
|
Anti-inflammatory and antioxidant activity in BV-2 cells, the induction of proteins expression (i.e. GSH) involved in the antioxidant defense in SH-SY5Y cell line, a decrease of ROS levels and NO-release in BV-2 cells | [156] |
| 30 |
|
Antioxidative activity, selective metal chelator and neuroprotector against H2O2-induced PC12 cell injury, ability to pass the BBB in vitro, relevant neuroprotective effects in scopolamine-induced cognitive impairment in mice model | [157] |
| 31 |
|
31a-c: pleiotropic activities 31d-f: protection from the self-mediated Aβ aggregation, 31e,f: neuroprotective effect in HT22 cells against glutamate-índuced neuronal death |
[158] |
| 32 |
|
Prevention of Aβ self-aggregation in TEM observation, ABTS+ scavenging, and ability to chelat Cu2+ ions | [159] |
| 33 |
|
Prevention of Aβ self-aggregation, protection of HT22 cells from glutamate and H2O2 induced cell death, scavenging free radicals and Cu2 ions chelation | [160] |
| 34 |
|
Antioxidant activity, neuroprotection of H2O2- induced PC12 cells and Aβ1-42- induced SH-SY5Y cells, hepatoprotection of H2O2- induced LO2 cells, selective metal chelator, activity against Cu2+/hAChE/self-induced Aβ1-42 aggregation, disaggregation of Cu2+-induced Aβ1-42 aggregation, ability to pass the BBB in vitro | [161] |
2.1. Tacrine Analogues
It has been proven that introduction of chlorine atom to the structure of tacrine and its derivatives has an effect on the increase of anti-cholinesterase activity and decrease in hepatotoxicity [75, 76]. Ragab et al. [77] designed, synthesized and biologically evaluated a series of new 4- (chlorophenyl)tetrahydroquinoline derivatives. The results of the biological investigation showed that all analogues had equivalent or much higher activity against cholinesterase and were less hepatotoxic than referenced drug tacrine. Moreover, in silico drug-likeness of the obtained derivatives were predicted and their practical logP was estimated, showing the ability of all of them to be promising hits/leads. In addition, the docking study was performed for the most potent cholinesterase inhibitor in the series, showing its binding mode is similar to that representing by tacrine.
The first step against the main toxic effects of nerve agents is simultaneous administration of anticholinergic agents and oxime reactivators, such as pralidoxime (2- PAM), which can reactivate inhibited AChE [78, 79]. 2- PAM is not active against every nerve agent, thus there is a need to look for more efficient compound. Novel series of tacrine-pyridinium hybrid reactivators linked by carbon chain were designed, synthesized and tested for their eeAChE inhibitory activity. The highest obtained results for AChE activity were 95%, 92% and 90% and 1 μM concentrations of the oximes [80].
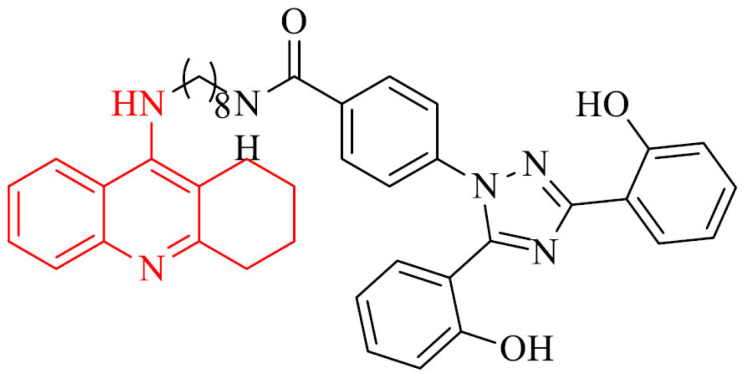
Wang et al. [81] synthesized a series of hybrids of tacrine and deferasirox as potential multifunctional drugs used in AD treatment. The most derivatives in the series were potent inhibitors of AChE from bovine serum (bAChE) metal ions chelators. The most effective compound, 1, showed in addition an antioxidant activity, low cytotoxicity and protective properties against the injury caused by H2O2. Type of inhibition, based on molecular modeling, for all of compounds, was mixed with the ability to bind to the catalytic anionic site (CAS) and the periphelar anionic site (PAS) of Torpedo californica variant of AChE (TcAChE).
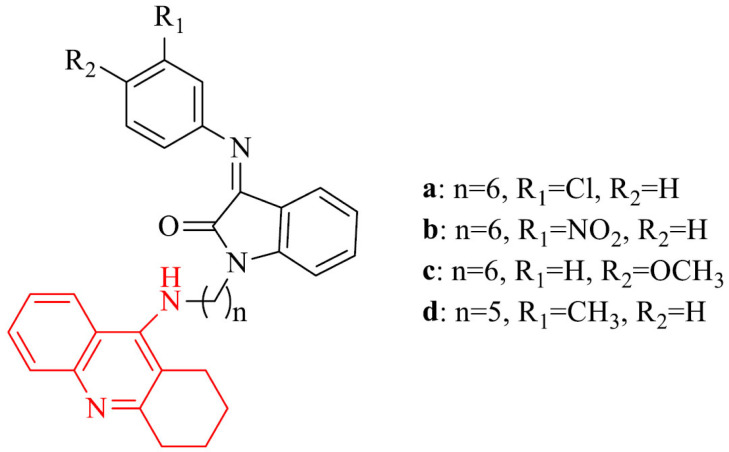
Tacrine-isatin Schiff base hybrids displayed potent activity against AChE and BChE with the selectivity for AChE over BChE. The most potent inhibitors of AChE were derivatives 2a-c with IC50 values of 0.42 nM, 0.62 nM, 0.95 nM, respectively. They were 92-, 62- and 41-fold stronger than referenced tacrine (IC50 = 38.72 nM). The most active derivative against BChE was 2d with an IC50 value of 0.11 nM, which was 56 times stronger than tacrine (IC50 = 6.21 nM). All compounds in the series showed excellent metal chelating properties. Moreover, 2a,b were good inhibitors of AChE-induced amyloid-beta (Aβ) aggregation. Studies of kinetic and molecular modeling exhibited mixed-type inhibition for 2a [82].
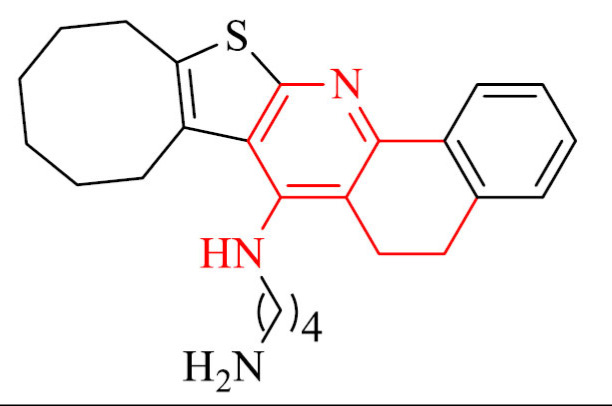
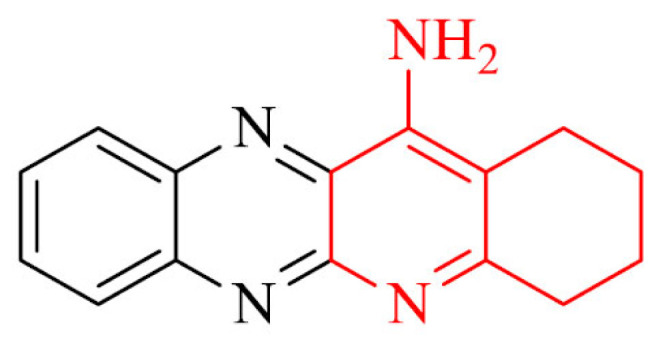
El-Malah et al. [83] designed and synthesized new thienopyridine-tacrine analogues as potential cholinesterase inhibitors. Obtained derivatives have been evaluated for their in vivo brain AChE inhibitory activity. For the most promising compounds, in vitro AChE inhibition was investigated. The most potent in the series was hybrid 3 with an IC50 value of 172 nM.
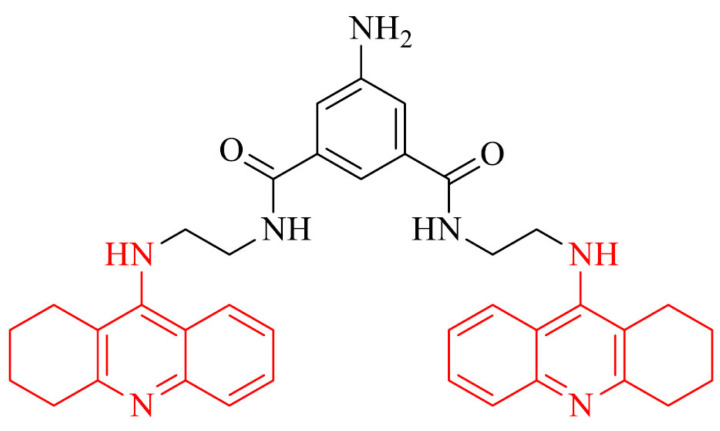
An example of bivalent reversible inhibitor of AChE is 5-amino-N1,N3-bis(2-(1,2,3,4-tetrahydroacridin-9-ylamino)ethyl)isophthalamide compound 4. It was designed using molecular modeling and showed high affinity and ability to bind to CAS and PAS. The synthesis included using 9-alkyl(1,2,3,4-tetrahydroacridine) pharmacophore with appended functionality. Biological evaluation revealed high inhibition of AChE and BChE with IC50 values of 0.54 ± 0.06 nM and 32.49 ± 1.2 nM, respectively (the value of selectivity ratio was 60.16 toward AChE). Moreover, compound 4 was able to inhibit PAS and aggregation of Aβ induced by AChE. Rat hippocampal neuron studies showed low cytotoxic effect and ability to induce S-phase post-treatment. Therefore, a further detailed evaluation of this derivative as a potential drug for AD treatment is proposed [84].
Li et al. [85] synthesized a series of novel tacrine-phenolic acid dihybrids and tacrine-phenolic acid-ligustrazine trihybrids and evaluated them towards cholinesterase inhibitory activity. The most interesting in the series was compound 5, which inhibited eeAChE, hAChE, eqBChE and hBChE with IC50 values of 3.9 nM, 65.2 nM; 24.3 nM and 48.8 nM, respectively. It was stronger towards AChE (mixed-type dual site inhibitor) and simultaneously weaker against BChE than referenced drug tacrine. It was not hepatotoxic and exhibited inhibition of Aβ self-aggregation at a level of 47% at 20 µM, antioxidant properties towards PC12 cells from CoCl2-damage, which makes it a promising multifunctional drug for patients with AD.
Quinoxalines are compounds showing anti-neuroinflammation, antitumoral, antomycobacterial and antifungal properties. Therefore, a series of novel quinoxalinetacrines were synthesized and their biological activity was investigated. Derivative 6 showed lower activity against AChE and BChE with IC50 values of 22.0 ± 1.3 μM and 6.79 ± 0.33 μM, respectively, but it was selective toward BChE. Furthermore, this compound exhibited lower hepatotoxicity than tacrine and neuroprotective activity against rotenone plus oligomycin-A or okadaic acid [86].
Also, a series of tacrine analogues containing pyranopyrazole moiety were synthesized. These derivatives were investigated for their AChE and BChE inhibition. Most compounds in the obtained group showed high activity against AChE with IC50 values for the two strongest analogues of 0.044 µM and 0.058 µM [87].
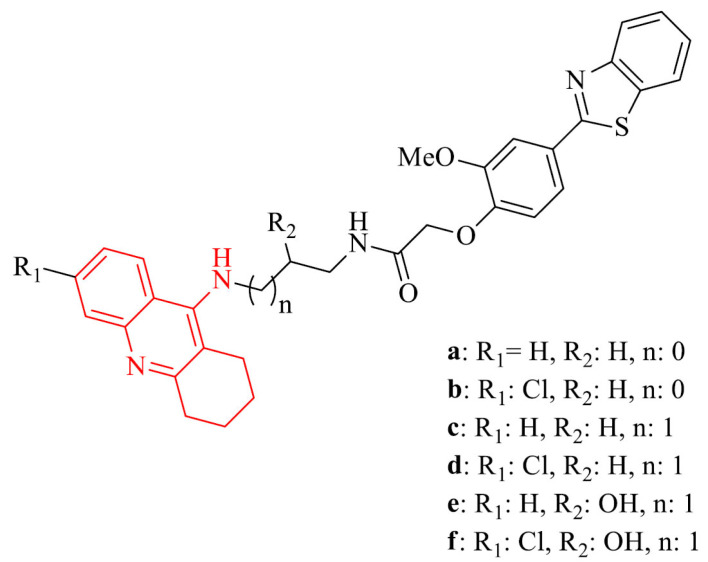
Due to multifunctional nature of AD, it is important to design structures that could display multi-target-directed role. Example of this approach was described by Rajeshwari et al. [88]. Six selected hybrids containing two pharmacophores: tacrine and 2-phenylbenzothiazole exhibited, based on molecular studies, the ability to bind to CAS and PAS of AChE. All compounds in the series proved excellent activity against AChE with IC50 values in the range of 0.06-0.27 nM. Studies carried out on SH-SY5Y (neuroblastoma cell line) cells treated with Aβ1-42 peptides revealed neuroprotective activity against Aβ-induced cell toxicity for three compounds: 7b,c,f, whereas 7c was the most potent. Results were expressed as the percentage of SH-SY5Y untreated cells and for mentioned compounds were p < 0.05.
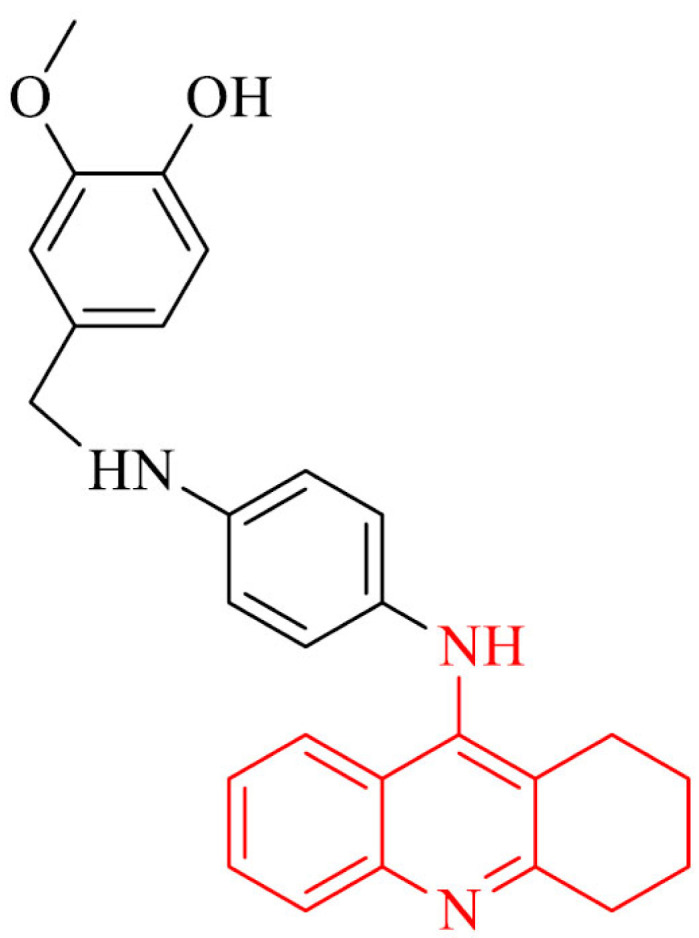
A promising strategy in the treatment of Alzheimer’s disease is the combination of vanillina and tacrine. Because of the various biological properties of vanillin, e.g: antioxidant activity, Aβ-amyloid inhibitory activity and capacity to inhibit AChE, it is used to obtain multi-target-directed ligands (MTDL). Antioxidant properties of novel series of tacrine- vaniline hybrids were tested by Scipioni et al. [89] using DPPH assay, achieving IC50 value of 19.5 µM, as well as FRAP and ORAC tests with results up to 1.54 and 6.4 Trolox equivalents, respectively. The kinetic studies revealed inhibition of AChE at µM range. Moreover, compound 8 inhibited Aβ1-42 amyloid similar to referenced curcumin. The investigation of neuroprotection for derivative mention above at 1 µM proved it to protect cells of SH-SY5Y in the presence of hydrogen peroxide at 400 µM.
Medrasi et al. [90] synthesized new pyrrolotacrines in Friedländer-type of reaction and investigated their ability to inhibit AChE. All compounds in the series showed potent or moderate anti-AChE activity with IC50 value of 0.2 µM for the most potent derivative. Interestingly, the replacement of a fused cyclohexane ring by a fused cyclopenatne ring didn’t have an impact on the improvement of AChE inhibition. Furthermore, the effect of the presence of chlorine atom on amelioration cholinesterase inhibitory, which is observed by some authors is not observed in results reported by Medrasi et al. [91-93].
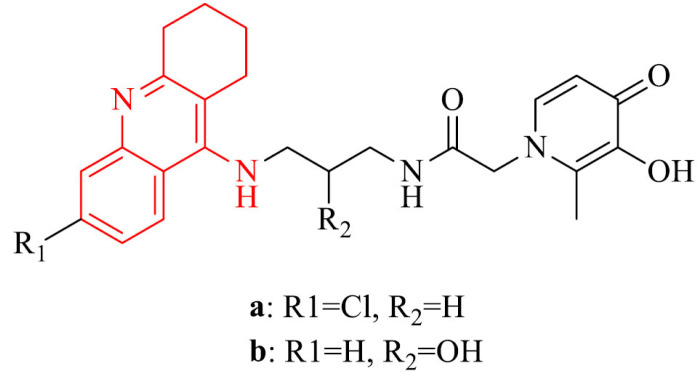
Deferiprone is well known metal chelating drug, which binds to iron in a 3:1 ratio; thus it decreases the content of this element in the body of animals and humas [94]. Some research proved that it attenuated the increase in AChE activity and decreased Aβ and iron deposition, as well as neuroprotective and memory enhancing effects for deferiprone in rats treated with scopolamine which might be attributed to its iron chelating action and anti-oxidative effect [95]. A combination of tacrine and deferiprone provides good AChE inhibitory activity, inhibition of self-/Cu-induced Aβ aggregation, radical scavenging capacity and metal chelating properties (Fe, Cu, Zn). Chand et al. [96] synthesized a series of tacrine-deferiprone hybrids. Besides advantages mentioned above, obtained analogues are characterized by neuroprotection in SH-SY5Y cells treated with Aβ1-42 ascorbate/iron stressors (9a and 9b). The best results displayed derivatives with chloro-substitution, containing 2-hydroxypropyl linkers with IC50 values in the range of 0.64-1.01 µM. One can include them in the potential of leading compounds in the search for effective therapy in Alzheimer’s disease treatment.
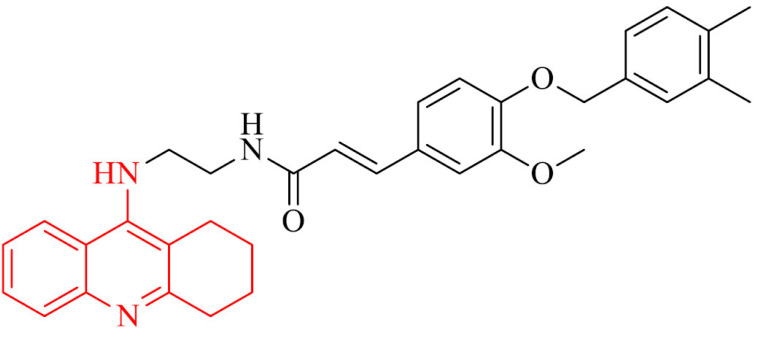
Also, compound 10 is a promising multifunctional anti-Alzheimer’s disease candidate. It was the most active in the series of tacrine-ferulic acid hybrids with IC50 against eeAChE and BChE of 37.02 nM and 101.4 nM, respectively. Moreover it didn’t show relevant hepatotoxicity and it was able to inhibit Aβ self-aggregation by 65.49% at 25µM and ameliorated the impairment of memory in mice models treated with scopolamine in the Morris water maze test [97].
Ökten et al. [98] designed and bioevaluated three disubstituted six or seven hydrocycle membered tacrine derivatives. Substitutents were bromine atom or silyl. All of obtained compounds effectively inhibited four enzymes: AChE, BChE, Carbonic anhydrase isoenzymes I and II (hCA I and II) with IC50 values of 30.26 ± 6.71–117.54 ± 42.22 nM, 22.45 ± 5.81–77.41 ± 4.02 nM, 57. 28 ± 22.16– 213.41 ± 82.75 nM and 46.95 ± 11.32–274.94 ± 62.15 nM, respectively.
Also a series of eleven tacrine-1,2,3-triazole hybrids were synthesized through Cu(i)-catalyzed alkyne-azide 1,3-dipolar cycloaddition (CuAAC) reaction as potential drugs using in Alzheimer’s disease treatment. The most potent compound inhibited eeAChE and BChE in micromolar range, which gave slightly lower result than the referenced tacrine. Molecular docking studies revealed it can bind to both, CAS and PAS sites [99].
Cheng et al. [100] designed and synthesized a series of brominated phenylacetic acid-tacrine derivatives and evaluated their AChE and BChE inhibitory activity, capacity to inhibit self-induced and AChE-induced Aβ aggregation and hepatotoxic effect. Among all, compound 11 exhibited the highest AChE/BChE inhibition, lack of hepatotoxicity in comparison to referenced drugs tacrine and donepezil, as well as inhibition of mentioned above Aβ aggregation.
Multitarget small molecules (MTSM) are able to interact simultaneously with the different enzymatic systems or receptors playing important role in the pathology of AD, thus MTSM are one of the most promising approaches in searching for new anti-AD drugs. A novel series of tacrine and kojic acid hybrids, which are MTSM, were synthesized and their biological activity was evaluated. The most active compound in the series, 12, proved to be less hepatotoxic than tacrine and selective inhibitor of hAChE with IC50 value of 4.52 µM. Moreover it exhibited antioxidant activity and neuroprotection against Aβ1-40 at 3 µM and 10 µM concentrations in SH-SY5Y cells [101].
Next interesting group of tacrine analogues are tacrine-H2S donor hybrids (THS). Hydrogen sulfide (H2S) is an endogenous signaling gasotransmitter molecule. It is produced in the liver, as well as in brain by cystathionine betasynthase, 3-mercaptopyruvate-sulfurtransferase, cysteine aminotransferase and cystathionine γ-lyase [102]. The role of H2S in brain is regulation of synaptic activity of neurons and glia by increasing the intracellular calcium ions, as well as it acts significant function in the development of long-term potentiation [103, 104]. The decrease of level of H2S promotes the progression of depression and it plays relevant role in the development of learning and memory deficits [105, 106]. Some studies also proved that it slows down the progression of Alzheimer's disease in experimental models by targeting multiple pathophysiological mechanisms [107]. H2S also has neuroprotective, hepatoprotective, and anti-inflammatory properties. From that perspective, the hybrids of H2S-donors and tacrine could be efficient strategy in Alzheimer’s disease treatment. An example of this approach is the connection of tacrine and H2S-releasing moieties (ACS81) to give compound 13. Authors reported that this hybrid, besides excellent AChE inhibition, improved cognitive and locomotor activity in a mouse model with AD in the step-through test and open field test, respectively. Moreover, it induced the increase in the amount of H2S in hippocampus, decreased mRNA expression of the proinflammatory cytokines, TNF-α, IL-6, and IL-1β and increased synapse-associated in the hippocampus of tested mice. Regarding the fact, tacrine was withdrawn from use due to its hepatotoxicity, it is important that obtained results for compound 13 showed its safety for the liver. Without a doubt, this hybrid is a promising candidate against AD [108].
Gniazdowska et al. [109] designed and synthesized a group of radioconjugates – tacrine derivatives labeled with technetium-99m and gallium-68, which are potential diagnostic radiopharmaceuticals. Authors tested lipophilicity, stability in the presence of an excess of standard amino acids cysteine or histidine, human serum and in cerebrospinal fluid. Based on investigation of lipophilicity, Gniazdowska et al. selected for molecular docking and biodistribution studies, as well as cholinesterases inhibition assays only two compounds, but both of them were good BChE and AChE inhibitors. Unfortunately, they showed the ability to cross the blood-brain barrier into the brains of rats, but in insufficient level, which may be due to insufficient lipophilicity related to used hydrophilic chelators (Hynic and DOTA). Enhanced utility of PET and SPECT imaging for both conjugates may be reached after some modifications in administration procedure like change into the central nervous system injection or by insertion though the catheter (for PET) and proper adjustment of detection system for applied activity dose (for SPECT).
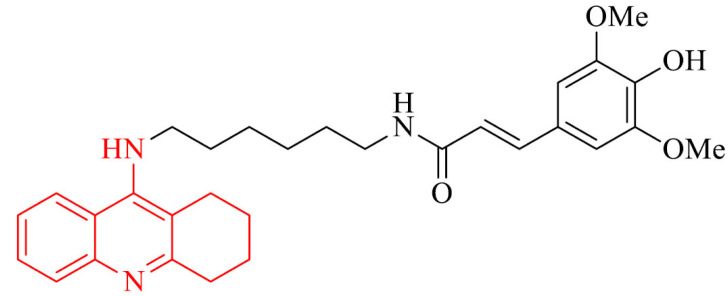
BChE, besides AChE, plays an important role in AD. In the central nervous system, it is mainly present in glial cells and neurons, and it regulates extracellular acetylcholine concentration. With the decrease of AChE activity which occurs with the death of cholinergic neurons, the role of BChE in cholinergic transmission increases [110]. Due to this fact, a series of tacrine-phenolic heterodimers were designed and synthesized as potent BChE inhibitors using in Alzheimer’s disease treatment [111]. A series includes different modifications of the nature (imino, amino, ether), various length of the linker, as well as number and position of the substituents on the aromatic ring. All of them proved to be strong BChE inhibitors from the nanomolar to subnanomolar range. The most active was compound 14 and it showed IC50 value of 0.52 nM against human BChE, what was 85-fold more active than referenced drug tacrine. What is important for multifunctional nature of AD, this compound can inhibit the self-aggregation of Aβ42 with lack of neurotoxicity up to 5 mM, low hepatotoxicity and stability under physiological conditions. Moreover, it exhibited neuroprotection in rats neurons in a serum and K+ deprivation model.
Lopes et al. [112] designed, synthesized and evaluated cholinesterase inhibitory activity of tacrine and carbohydrate-based moieties hybrids as potential agents against AD. The synthesis was based on the reaction between tacrine and the appropriate sugar-based tosylates according to the mechanism of nucleophilic substitution. Most of the D-xylose analogues showed potent AChE and BChE inhibitory activity with IC50 values of 2.2 nM and 4.93 nM, respectively, for the most active compound. Most hybrids in the series were selective for AChE over BChE (the highest IC50 ratio that was noticed was 7.6). Only two derivatives showed selectivity for BChE. Mostly, described derivatives showed a lack of hepatotoxicity according to ProTox-II. Furthermore, on the basis of molecular modeling studies, it was found that designed hybrids are able to interact with the entire binding cavity and the main contribution of the linker is to enable the most favorable positioning of the two moieties with CAS, PAS, and hydrophobic pocket to provide optimal interactions with the binding cavity.
Cheng et al. [113] designed and synthesized a series of dual AChE and BChE inhibitors for AD treatment by connecting tacrine with indole-3-acetic acid (IAA). Some compounds in the series exhibited dual inhibitory activity in nanomolar range. Based on molecular modeling analysis for these derivatives, the capacity to bind to catalytic active site and peripheral anionic site of cholinesterase was found. The preliminary SAR analysis suggested the optimal length of diaminoalkyl linker between tacrine and IAA is six atoms of carbon.
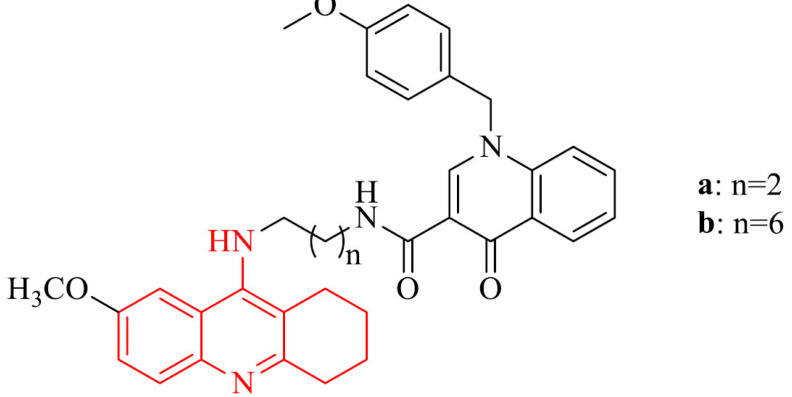
M1 muscarinic acetylcholine receptors (M1 mAChRs) play important, selective role for verbal memory mechanisms [114, 115]. Stimulation of them is able to improve cognitive deficit and M1 agonists can advance verbal memory, enhance cholinergic transmission, as well as have an impact on amyloid precursor protein (APP) processing, what in results prevents Aβ-aggregation and slows the progression of Alzheimer’s disease [116-119]. The series of tacrine-benzyl quinolone carboxylic acid (tacrine-BQCA) derivatives was synthesized and their hAChE/hBChE inhibitory activity was evaluated. The indirect aim was increase of cholinergic transmission by inhibiting of cholinesterase and the direct aim was supporting cholinergic transmission via M1 mAChR activation. The series consisted of three groups of compounds that differed in tacrine moiety (7-methoxytacrine, 6-chlorotacrine or unsubsituted tacrine). After the introduction of BQCA, a positive modulator of M1 muscarinic acetylcholine receptors the impact on M1 mAChRs was investigated via Fluo-4 NW assay on the Chinese hamster ovarian (CHO-M1WT2) cell line. All of the derivatives in the series were potent hAChE and hBChE inhibitors with activity in micromolar and nanomolar range. Unfortunately, the agonist effect towards M1 mAChRs, which should be provide by introduction of BQCA moiety wasn’t revealed. Investigation of permeation BBB in vitro was positive for compounds 15a,b [120].
Phosphodiesterase 4 (PDE4) is the primary cAMP-specific hydrolase which is involved in the process of memory consolidation and a PDE4D inhibitor can affect the hippocampus and dependent on it memory tasks [121, 122]. Pan et al. [123] designed, synthesized and bioevaluated a novel series of tacrine-pyrazolo[3,4-b]pyridine hybrids as potential cholinesterase and phosphodiesterase 4D (PDE4D) inhibitors. The inhibitory activity depended on the length of carbon chain between tacrine and pyrazolo[3,4-b]pyridine. The most active compounds in the series inhibited AChE with IC50 value of 0.125 µM, as well as it showed a desired balance of AChE and BChE and PDE4D inhibition activities, with IC50 values of 0.449 and 0.271 μM, respectively.
Also, a novel series of phosphorus and thiophosphorus tacrine analogues was designed and synthesized in reaction between appropriate chlorophosphates or chlorothiophosphates and N1-(1,2,3,4-tetrahydroacridin-9-yl)propane-1,3- diamine. Their inhibitory activity and modeling studies were investigated. Two compounds in the series proved to be more active than tacrine, whereas the most active was 3- times stronger than referenced drug. All of the synthesized derivatives proved to be less toxic against SH-SY5Y cell line than tacrine [110].
2.2. Donepezil Analogues
Łozińska et al. [124] synthesized a novel series of donepezil-melatonin hybrids as selective inhibitors of BChE. The synthesis was based on coupling reaction of alkylamine-substituted N-benzylpiperidine derivatives and with a carbonate analogue of N-acetylserotonin. The modification of the linker between two main moieties has been carried out by introduction of carbamate bond connected to the aromatic system of the melatonin. It led to improvement of the affinity toward BChE, as it was observed in the case of tacrine and melatonin derivatives [125].
Also, a novel series of donepezil-hydrazinonicotinamide hybrids was synthesized and bioevaluated. All of the obtained compounds revealed a higher affinity to AChE in comparison to BChE, higher selectivity toward AChE than to BChE and they were less active against AChE and more active against BChE than referenced drug donepezil [126].
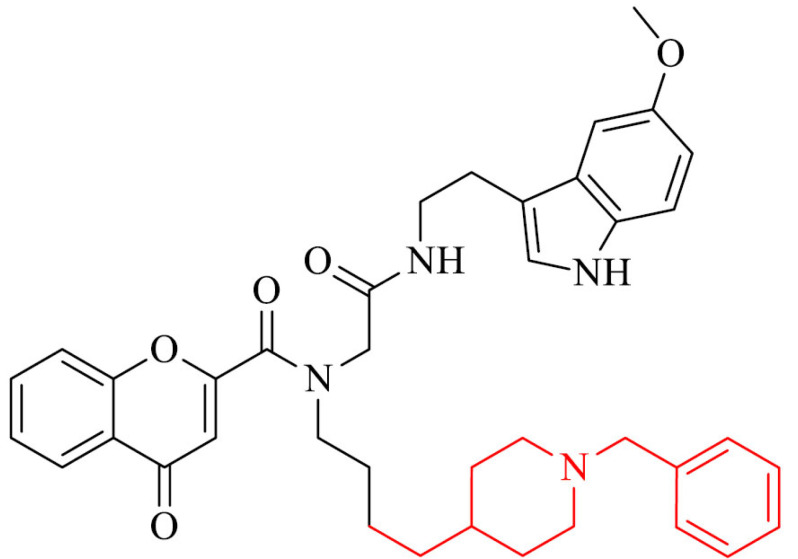
Next interesting group of donepezil derivatives are hybrids of donepezil, chromone and melatonin as potential multifunctional drugs against AD. The most promising compound in the series, 16, exhibited potent hBChE inhibition with IC50 values of 11.90 ± 0.05 nM, but also a moderate inhibition of hAChE, human monoamine oxidase A (hMAO A), and monoamine oxidase B (MAO-B) with IC50 of 1.73 ± 0.34 µM, 2.78 ± 0.12 µM, 21.29 ± 3.85 µM, respectively. This analogue revealed also strong antioxidant properties (3.04 TE) [127]. It should be mentioned, it is a selective BChE inhibitor, what is significant in the case of patients with moderate to severe forms of AD [128].
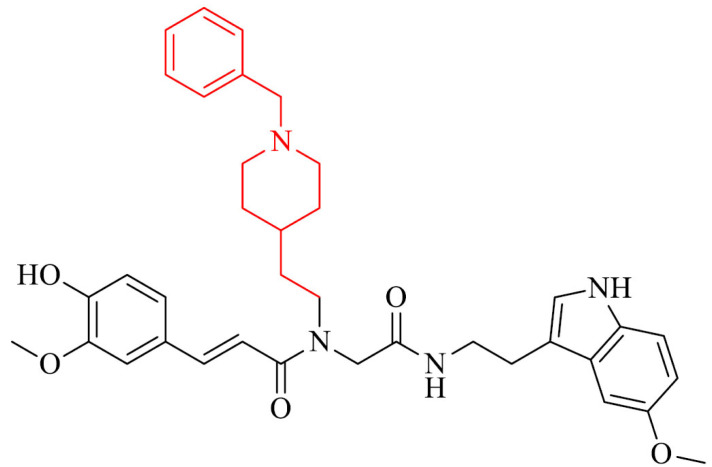
Benchekroun et al. [129] a novel series of donepezil and ferulic acid derivatives as potential multitarget anti-AD agents. All of the obtained compounds were characterized by strong antioxidant effect with the oxygen radical absorbance capacity values in the range 4.80–8.71 TE, what is much higher than results observed for referenced melatonin and ferulic acid. One of the most active hybrids, selective against eqBChE with strong antioxidant properties proved to be compound 17.
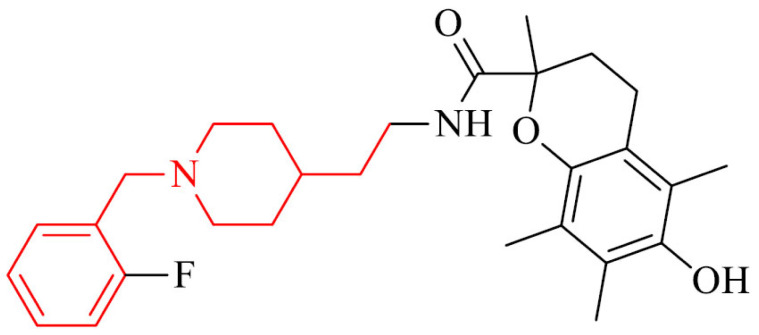
Donepezil – trolox hybrids were designed, synthesized and bioevaluated as multifunctional drugs for AD treatment. They exhibited moderate to good inhibition of hAChE and MAO-B with IC50 values of 0.54 μM and 4.3 μM, respectively, for the most active compound in the series, 18. Moreover, it showed Aβ1−42 inhibitory activity, great antioxidant effect (IC50 = 41.33 μM by DPPH method, 1.72 and 1.79 TE by ABTS and ORAC methods) and ability to chelat Cu2+ ions. It did not show significant toxicity in HepG2, PC12, and BV-2 cells (murine microglia cell line), as well as it is able to protect cells from oxidative stress caused by H2O2, rotenone, and oligomycin-A. Furthermore, oral administration of this analogue improved cognition and spatial memory against scopolamine-induced acute memory deficit, D- galactose (D-gal) and AlCl3 induced chronic oxidative stress in mice model without acute toxicity and hepatotoxicity [130]. This derivative revealed ability to cross BBB in vitro.
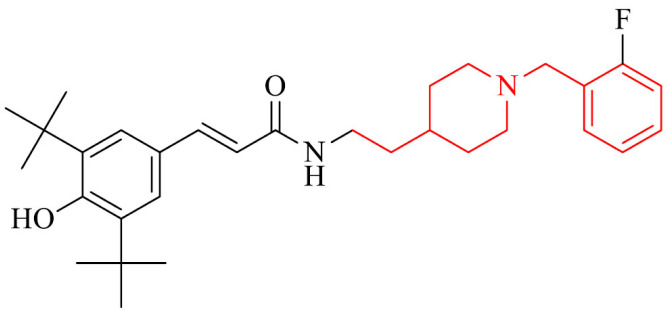
A promising strategy in searching for new drugs for AD is represented by a novel series of donepezil – butylated hydroxytoluene (BHT) hybrids. Most of the compounds displayed moderate to good inhibition of AChE/MAO-B and excellent antioxidant activities. The most active in the series was compound 19 and it proved to be a multifunctional agent with cholinergic, antioxidant and neuroprotective activity. It inhibited eeAChE and hAChE with IC50 values of 0.075 μM and 0.75 μM, respectively. Moreover, it exhibited inhibitory activity against hMAO-B with IC50 value of 7.4 μM, ability to inhibit self-induced and hAChE-induced Aβ aggregation, antioxidant properties with IC50 value of 71.7 μM using DPPH method, as well as 0.82 and 1.62 TE by ABTS method and ORAC method, respectively. Also, the neuroprotective effects on PC12 cells treated with H2O2 and against Lipopolysaccharides (LPS)-stimulated inflammation on BV-2 cells were tested. In addition, compound 19 could cross BBB in vitro and showed good liver metabolic stability in RLM (rat liver microsomes) and the passive avoidance test showed for this derivative dose-dependently reversed scopolamine-induced memory deficit in mice model but without acute toxicity [131].
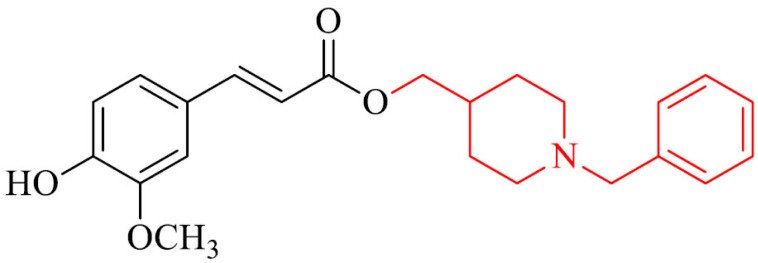
Dias et al. [132] designed, synthesized and evaluated biological activity of novel series of feruloyl – donepezil hybrids as potential multifunctional drugs using in AD. The most active analogue in the series was compound 20. It inhibited eeAChE with IC50 value of 0.46 µM. Moreover, it proved its multifunctional profile in the investigation of anti-inflammatory properties in vivo in mice model, antioxidant activity in SH-SY5Y cell line and ability to chelat Cu2+ and Fe2+ ions. Also, a neuroprotection studies revealed it protected SH-SY5Y cells against the late neuronal death caused by Aβ1-42 oligomers. Molecular docking studies showed non-competitive mechanism of inhibition that can bind to PAS of AChE.
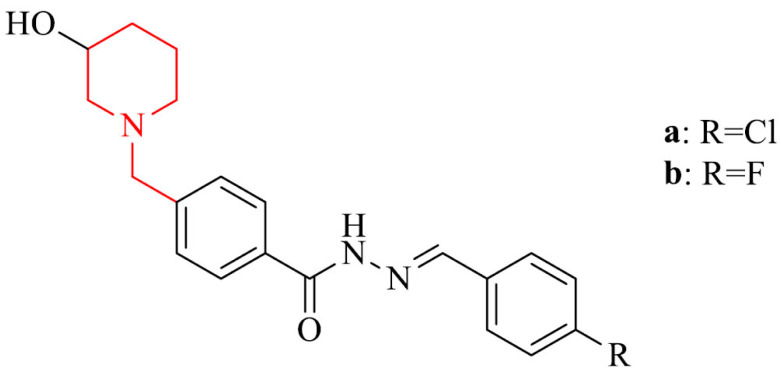
Several studies suggest that Aβ deposits and neurofibrillary tangles provide cause neuroinflammation, which contribute to early and late stage of the AD neurodegeneration [133, 134]. Therefore, drugs designed to treat the different neuroinflammations are needed. Dias Viegas et al. [135] reported synthesis and biological evaluation of a series of N-benzyl-piperidine-arylacylhydrazone derivatives as donepezil hybrids, that can possess anti-inflammatory properties, as well as can be active against neurodegeneration in patients brains with AD. Two compounds in the series, 21a and 21b, were good AChE inhibitors, exhibited the best ADME (Absorption, Distribution, Metabolism, Excretion) profile in silico, anti-inflammatory and neuroprotective activity against AβO-induced neuroinflammation and neurodegeneration in vitro in THP-1 cells (human acute monocytic leukemia cell line) and in vivo in mice model. They inhibited tumor necrosis factor alpha (TNF-α), which is secreted by activated microglia cells and as a result it makes a progression of AD. It suggests, they could prevent he signal transduction pathway mediated by TNF type 1 receptor (TNFR1) at brain level, what is important regarding the fact that TNFR1 also is involved in cognitive decline [136, 137]. Also, they showed inhibitory activity against cyclooxygenase-1 and -2 (COX-1 and COX-2, respectively), which play significant role in the mechanisms of CNS inflammation [138].
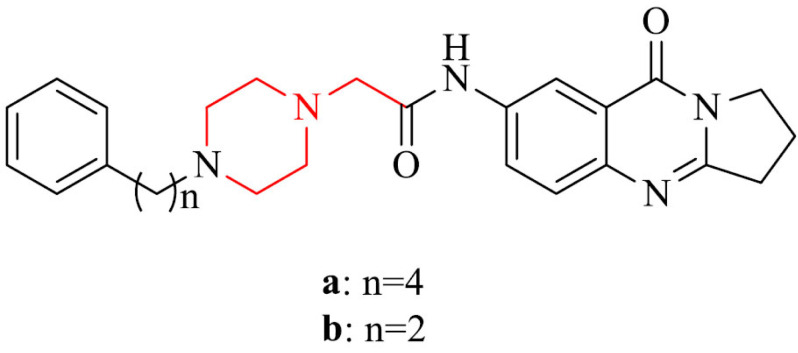
Next interesting group are deoxyvasicinone-donepezil hybrids. They exhibited from moderate to excellent inhibitory activity against hAChE, BACE1, and Aβ1−42 aggregation. Among all, compounds 22a and 22b revealed low cytotoxicity and neuroprotective properties against Aβ1−42-induced damage in SH-SY5Y cells. Derivative 22a inhibited hAChE, BACE1 and Aβ1−42 aggregation with IC50 values of 56.14 nM, 0.834 µM and 13.26 µM, respectively. In the same investigation, the results obtained for compound 22b are 3.29 nM, 0.129 µM and 9.26 µM, respectively. Moreover, these compounds should be able to penetrate the blood-brain barrier [139].
Valencia et al. [140] described neurogenic and neuroprotective hybrids by connecting flavonoid-related structures and a donepezil fragment to obtain new 4-chromone- and 4-quinolone – N-benzylpiperidine hybrids (DFHs). They exhibited nanomolar affinities for the sigma-1 receptor (σ1R). Also, they inhibited hAChE, 5-lipoxygenase (5-LOX), MAO-A and MAO-B. Based on kinetic studies, the new DFHs can bind with the two main sites of the AChE gorge (CAS and PAS). Hybrids derived from 4-chromone series inhibited hAChE with IC50 value in the range from the nanomolar to the low micromolar. What is interesting, derivatives from 4-oxo-1Hquinoline series were not as potent hAChE inhibitors as 4-chromone analogues. It may be caused by existence of a tautomeric equilibrium in the azaheterocycle what can impede the interaction with the enzyme. Moreover, most of the compounds in the series can cross BBB, based on in vitro PAMPA-BBB model. Also, they can scavenge free radical species and protect neuronal cells against mitochondrial oxidative stress.
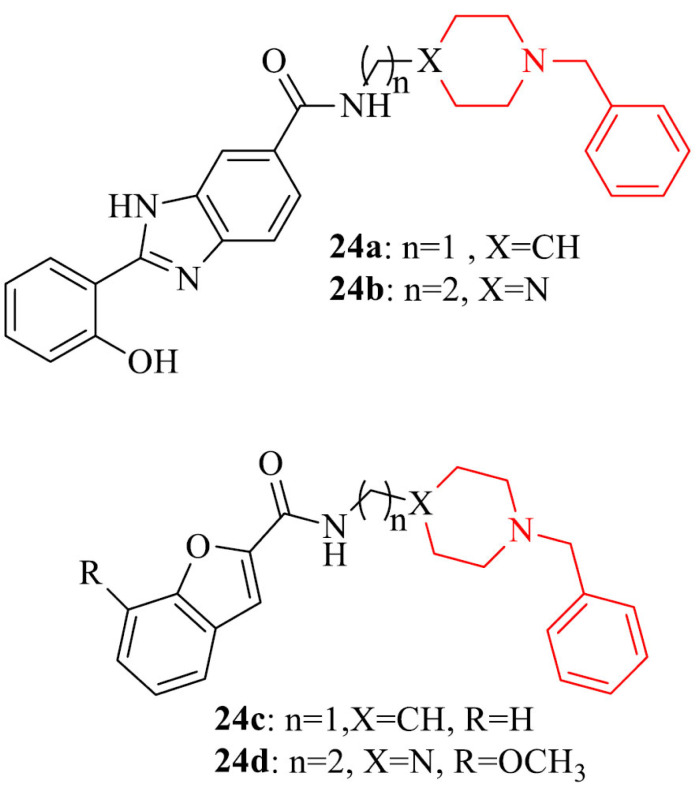
Also, a novel series of donepezil derivatives obtained by connecting donepezil by a 1–2 methylene bridge with a substituted planar heterocyclic structure were designed, synthesized and bioevaluated as potential drugs for patients with AD. AChE inhibitory activity of obtained compounds, as it was anticipated, was lower in comparison to referenced drug, donepezil. The reason for this was the weak binding of the benzylpiperazine or N-benzylpiperidine portion with the CAS of AChE. Introduction of a heterocyclic planar group contributed to a moderate inhibitory activity against Aβ self- aggregation, while the inhibition of the Cu2+-induced Aβ aggregation was particularly enhanced for compounds with metal chelation capacity. Four compounds in the series, 23a-d, were able to protect SH-SY5Y cells from the Aβ-induced toxicity [141].
Chandrika et al. [142] described synthesis and biological tests of two series of bifunctional donepezil derivatives against AD. They contained 1,3- or 1,4-chalcone-donepezil hybrids. The second group possessed higher eeAChE and efBChE inhibitory activity than 1,3-chalcone-donepezil hybrids, with several exceptions. Some compounds showed better inhibition of bioAβ42 oligomer assembly in comparison to donepezil, but any of them was able to dissociate preformed oligomers. Moreover, combination studies proved a covalent linkage between chalcones and donepezil is needed for the prevention of bioAβ42 oligomerization.
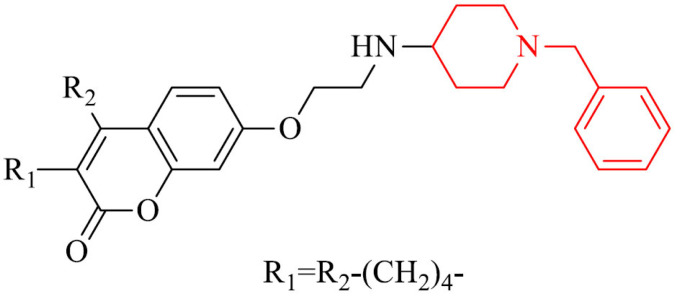
Coumarin-donepezil hybrids were synthesized and their biochemical properties were tested as potent ChE and MAO-B inhibitors. Most of the compounds displayed potent inhibition of AChE, BChE and selective activity against MAO-B. The most active derivative in the series, 24, inhibited eeAChE, eqBChE, hAChE, hBChE and hMAO-B with IC50 values of 0.87 μM, 0.93 μM, 1.37 μM, 1.98 μM and 2.62 μM, respectively. Cytotoxicity investigation did not reveal toxic effect on SH-SY5Y cell line. Furthermore, molecular modeling studies proved it to be mixed-type inhibitor, able to bind to CAS, PAS and mid-gorge site of AChE, and it revealed a competitive mechanism of inhibition of MAO-B. In addition it was able to penetrate BBB in vitro [143].
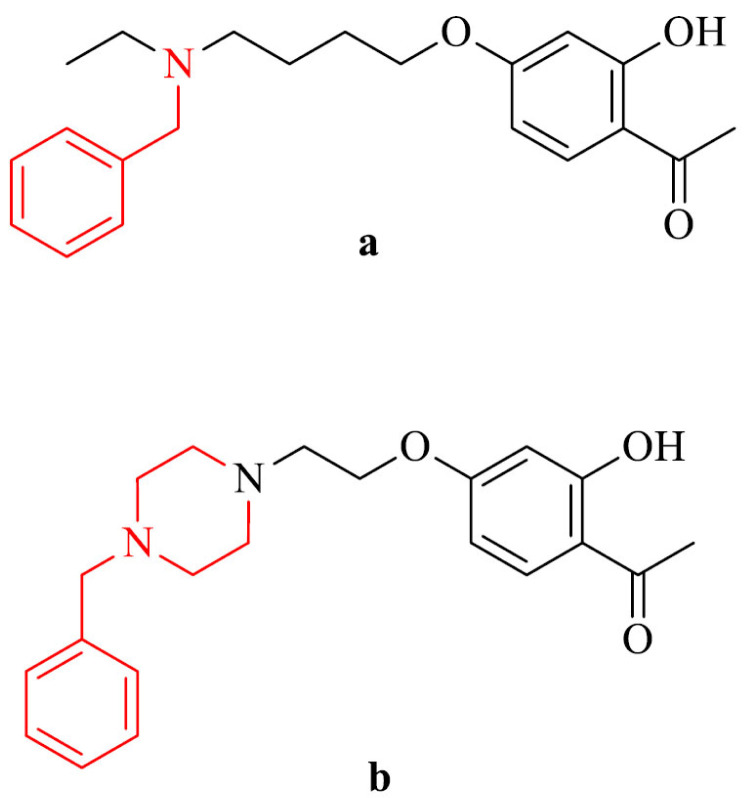
Synthesis of 2-acetylphenol-donepezil hybrids is an another approach in searching for multi-target-directed molecules against AD. reported by Zhu et al. [144]. They evaluated their inhibitory activity against AChE, BChE, MAO-A and MAO-B. Compound 25a revealed the highest inhibition of eeAChE with IC50 value of 2.9 µM and is able to bind to CAS and PAS of AChE. In addition, it was a selective Cu2+ chelator in a 1:1 ratio and can pass the BBB in vitro. The most active against MAO-B was derivative 25b with IC50 value of 6.8 µM., also with ability to cross the BBB. The structure-active-relationship indicated that the presence of O-alkylamine moiety contributed decrease potency of hMAO-B inhibition.
2.3. Galantamine Analogues
The binding site of AChE is deep and narrow. PAS is located at the entrance of the binding gorge and takes part in an interaction with Aβ, forming as a result the amyloid plaques. That is why blocking of PAS is important for protecting from AChE-induced Aβ aggregation. Galantamine fits perfectly to the binding gorge of AChE, but unfortunately it is too short to fully fill it, so galantamine derivatives that can bind to both CAS and PAS are needed [145-149]. Stavrakov et al. [150] synthesized a series of galantamine and camphane hybrids as dual-site binding AChE inhibitors. Camphane is a bulky fragment that can be located on the wide gorge entrance, what short galantamine is not able to fill. Some compounds in the series exhibited 191- and 369- times higher AChE inhibition than referenced drug galantamine. Moreover, they bind to PAS of AChE, do not show neurotoxic effect and can pass the BBB.
2.4. Rivastigmine Analogues
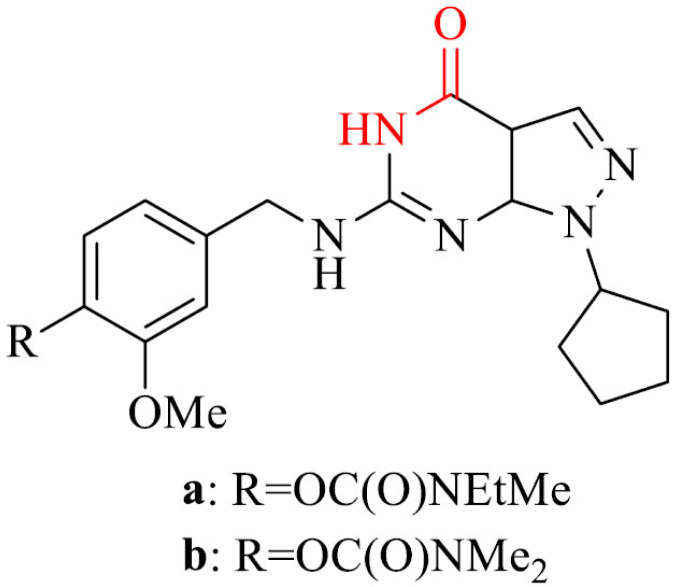
Phosphodiestrases 9 (PDE9) are group of enzymes that can hydrolyze the intracellular second messenger cyclic guanosine monophosphate (cGMP). cGMP can regulate pleiotropic cellular functions in health and disease [151, 152]. One of the eleven isoforms of PDE9 is phosphodiesterase 9A (PDE9A). Its concentration is high in the cortex, basal ganglia, hippocampus, and cerebellum of brain. It has been proved that the inhibition of phosphodiestrases (PDEs) increase the performance of cognition functions in animals organisms. The inhibition of PDE9A is relevant for AD, because of restoring proteins associated with memory and attenuating neuronal injuries in hippocampal area by the reduction of amount of cGMP in brain to active NO/cGMP/PKG/CREB signaling, with simultaneous increase of brain-derived neurotrophic factor (BDNF) [151]. Yu et al. [153] as the first one designed, synthesized and evaluated biological activity of series of pyrazolopyrimidinone-rivastigmine hybrids to inhibit simultaneously PDE9A and BChE. Most of the analogues in the series proved to be inhibitors of both PDE9A and BChE. The most potent were compounds 26a and 26b and inhibited PDE9A with IC50 values of 14 nM and 17 nM, respectively and BChE with IC50 values of 3.3 μM and 0.97 μM, respectively. The results obtained for BChE proved these two derivatives to be stronger inhibitors than referenced rivastigmine. In addition, they were not toxic.
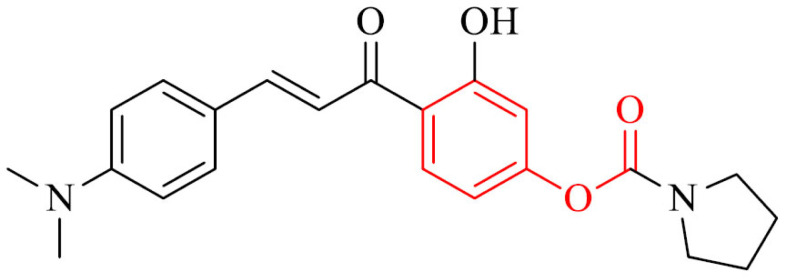
Also, a series of novel 4’-aminochalcone-revastigmine hybrids were synthesized and their biological properties were evaluated as multitarget drugs against AD. In the whole series, the highest multifunctional activity exhibited compound 27. It inhibited AChE with mixed type of inhibition with IC50 value of 4.91 µM, binding both CAS and PAS. In addition, it was able to inhibit self-induced and Cu2+-induced Aβ1-42 aggregation by 89.5% and 79.7% at 25 μM, respectively, MAO-B with IC50 value of 0.29 µM. The antioxidative properties of 27 were at the level of 2.83-fold of Trolox. Morover, it could chelat metals and cross the BBB in vitro [154].
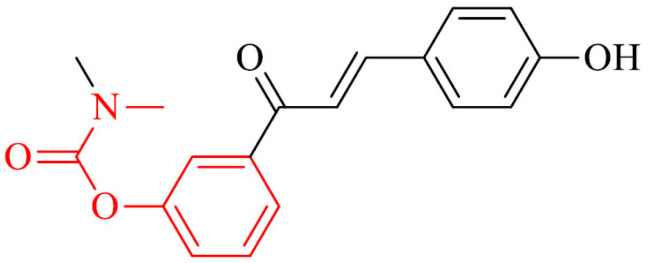
A novel series of chalcone and tacrine derivatives were reported by Wang et al. [155] for AD treatment. Most of the analogues in the series, exhibited selective hBChE inhibition. The most potent in the series was compound 28, which inhibited hAChE and hBChE with IC50 values of 0.87µ and 0.36 µM, respectively, what was better or similar to results obtained for referenced rivastigmine. Due to multifunctional nature of AD, the series were designed as molecules with wide spectrum of biological activity. Compound 28 prevented as well from reactive oxygen species (ROS) in SH-SY5Y cell line, possessed low cytotoxicity and is in the required 10 druggability ranges from in silico ADMET prediction.
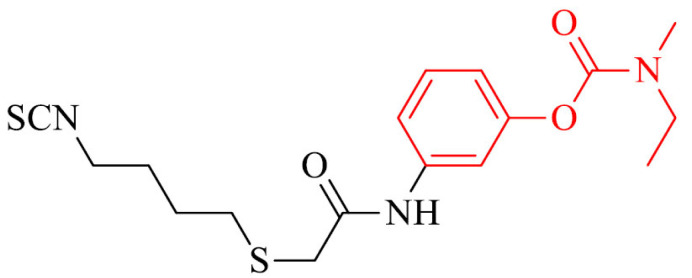
As it was mentioned above, a relevant decrease of amount of H2S is observed in brains of patients with AD. Also, a series of rivastigmine connected with ulforaphane (SFN) and erucin (ERN) were synthesized as H2S donors with antioxidant and neuroprotective activity. Compound 29 proved to be a new well-balanced anti-inflammatory and antioxidant agent in BV-2 cells. Moreover, it could induce the expression of proteins (i.e. GSH) involved in the antioxidant defense in SH-SY5Y cell line, decrease ROS levels and NO-release in microglia BV-2 cells what was not observed for referenced drug, rivastigmine [156].
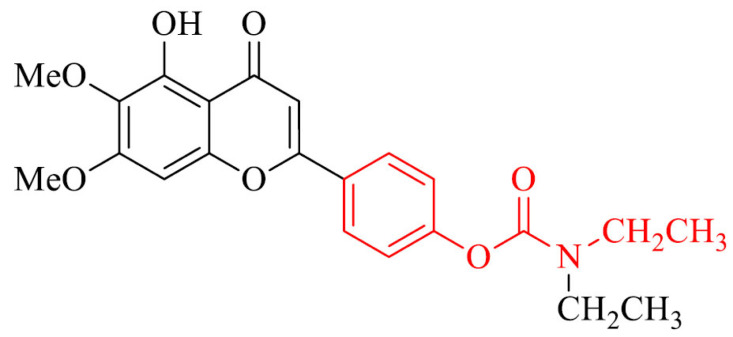
Another approach is synthesis and evaluation of AChE/BChE inhibitory potential, metal chelating activity and neuroprotection against hydrogen peroxide (H2O2)-induced PC12 cell injury of novel scutellarein rivastigmine derivatives. The most interesting compound in the series was mixed-type inhibitor, compound 30 which inhibited AChE and BChE with IC50 values of 0.57 and 22.6 µM, respectively and could bind to both CAS and PAS. It showed antioxidative activity at the level of 1.3-fold of Trolox. In addition, it was selective metal chelator and neuroprotector against mentioned above H2O2-induced PC12 cell injury. Moreover it could pass the BBB in vitro and showed relevant neuroprotective effects in scopolamine-induced cognitive impairment in mice model [157].
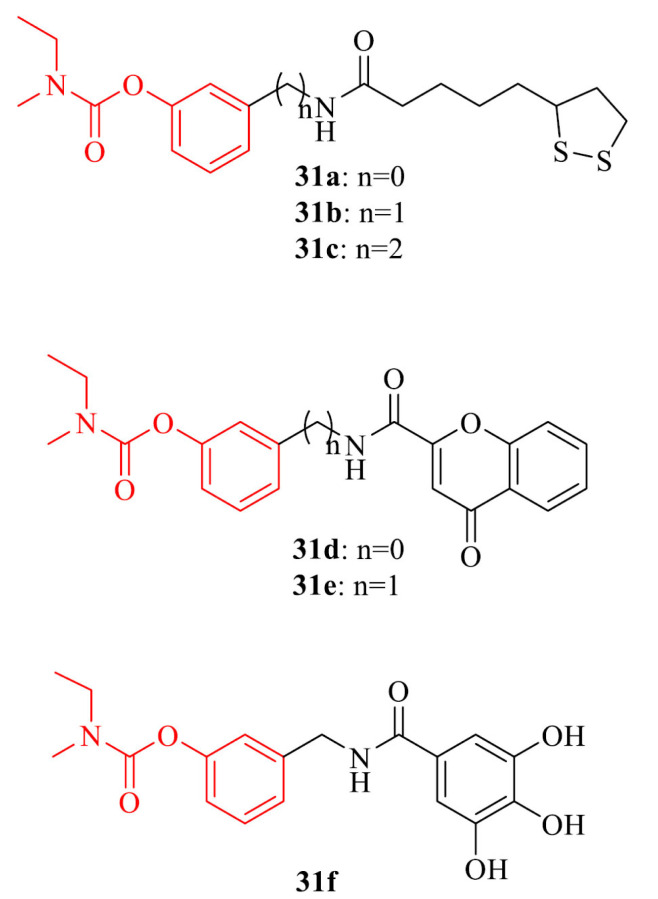
Nesi et al. [158] combined a rivastigmine with natural antioxidant moieties such as gallic acid (GA), lipoic acid (LA) and 2-chromonecarboxylic acid (CCA) to get potential cholinesterase inhibitors with neuroprotective activity. LA derivatives 31a-c revealed the highest BChE inhibitory activity with IC50 values in the range of 340 – 378 nM and pleiotropic activities. Analogues 31d-f were able to protect from the self-mediated Aβ aggregation with percentages of inhibition from 53% to 59%. Compounds 31e,f showed neuroprotective effect in HT22 cells against glutamate-induced neuronal death.
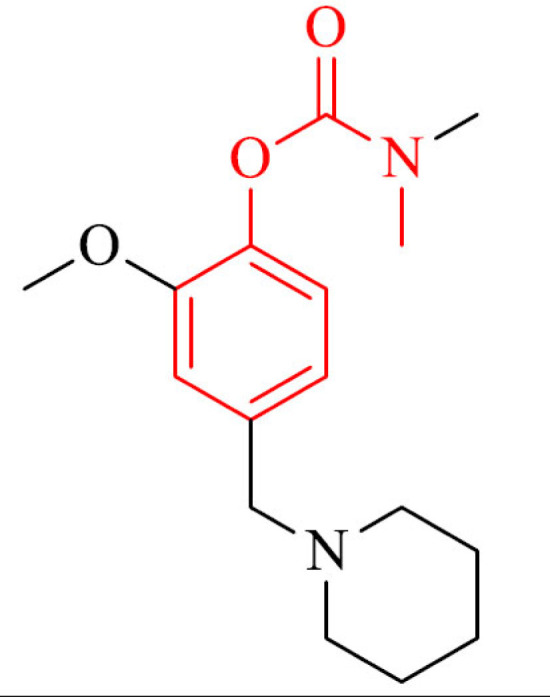
Rivastigmine-curcumin hybrids showed high AChE/ BChE inhibitory activity with sub-micromolar IC50 values. The most active compound 32 was 20-times stronger (IC50 = 0.097 µM) than referenced rivastigmine and prevented the Aβ self-aggregation in TEM assay. In addition, the hydrolysate of 32 revealed potent ABTS+ scavenging and and was able to chelat Cu2+ ions in moderate range [159].
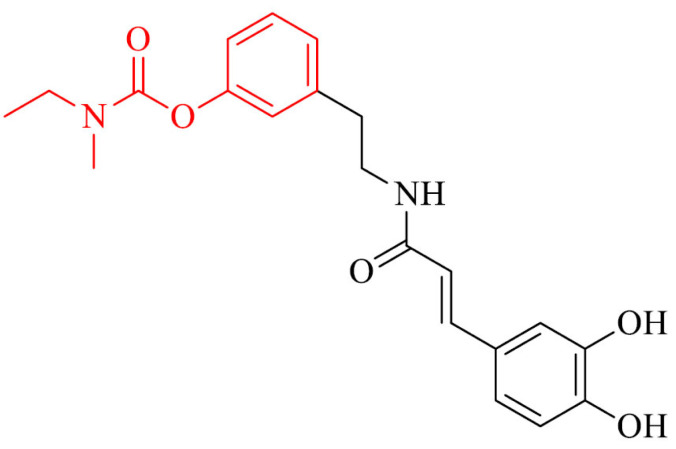
On the basis of rivastigmine and caffeic or ferulic acid, a novel group of compounds was designed, synthesized and evaluated as MTDLs. The most interesting compound in the series was 33 and inhibited cholinesterase, prevented Aβ self-aggregation, protected HT22 cells from glutamate and H2O2 induced cell death, scavenged free radicals and chelated Cu2+ ions [160].
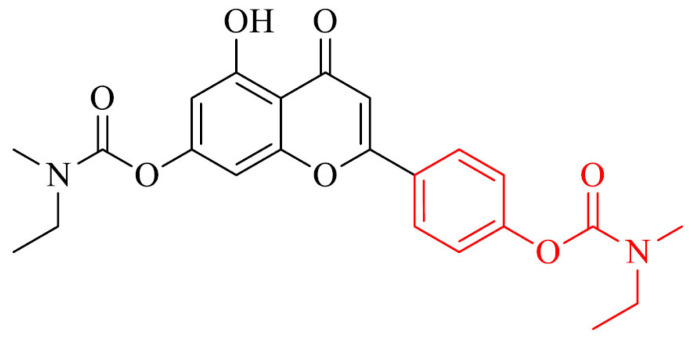
A reversible hAChE/hBChE inhibitor was an analogue of apigenin and rivastigmine, compound 34, which inhibited these enzymes with IC50 values of 6.8 mM and 16.1 mM, respectively. Moreover, it demonstrated remarkable antioxidant activity (ORAC = 1.3 eq), it exhibited neuroprotection of H2O2- induced PC12 cells and Aβ1-42- induced SH-SY5Y cells, hepatoprotection of H2O2- induced LO2 (normal human hepatocytes cell line) cells and it was selective metal chelator. It also inhibited Cu2+-induced Aβ1-42 aggregation (78.9%), hAChE-induced Aβ1-40 aggregation (73.6%) and self-induced Aβ1-42 aggregation (77.9%). It could also disaggregate Cu2+-induced Aβ1-42 aggregation (64.6%) and pass the BBB in vitro. Compound 34 was selected to in vivo studies and improved dyskinesia recovery ratio and response efficiency on AlCl3-mediated AD zebrafish, and showed remarkable neuroprotective activity against Aβ1-40-induced vascular injury. It also did not exhibit acute toxicity at dose up to 2000 mg/kg, and could improve scopolamine-induced memory impairment. The metabolism of 34 in vitro revealed that 4 metabolites in rat liver microsome metabolism, 2 metabolites in human liver microsome metabolism, and 4 metabolites in intestinal flora metabolism, which offered supports for the preclinical study of this derivative, what makes it promising MTDL against AD [161].
Krátký et al. [162] reported synthesis of series of 4-chlorophenyl N-substituted carbamates 5 from isocyanates and evaluated obtained compounds towards eeAChE/eqBChE inhibitory activity. They demonstrated not selective inhibition. Only some of them were more active than referenced drug, rivastigmine. Based on SAR analysis, it was found out neither the halogenation of the salicylanilide nor the presence of 2-phenylcarbamoyl moiety is required for the potent inhibition of AChE.
CONCLUSION
Despite extensive knowledge of AD, the etiology and cause of it are still not clear, which has an impact on the lack of effective therapy that could stop the progression of the disease. Many years of work on obtaining an active, multifunctional and free of side effects substance that could treat AD has resulted in the publication of numerous scientific papers that are a source of valuable knowledge for the world of science.
Herein, we have supplied the previous review article with the latest tacrine analogues from 2017, as well as with donepezil and galantamine from 2015. Moreover, we have reviewed rivastigmine derivatives from the last six years. We clearly described tacrine conjugates with e.g. deferasirox, pyridinium, thienopyridine, phenolic acid, vanillina, deferiprone, or also indole-3-acetic acid. Many of them exhibited promising additional therapeutic properties, like protection against Aβ-aggregation, oxidative stress, or the ability to chelate metal ions. Described donepezil hybrids include e.g. multitarget donepezil-ferulic acid agents or connection with a natural moiety, such as melatonin with chromone, which showed activity against hAChE, hBChE, hMAO-A and MAO-B. Among galantamine derivatives must be mentioned compounds that can bind to both CAS and PAS. The binding site of AChE is deep and narrow and galantamine is too short to fully fill the binding gorge of AChE, so its analogues must be able to bind to both CAS and PAS. In the literature, one can find alantamine and camphane hybrids as dual-site binding AChE inhibitors. Among the rivastigmine hybrids, we described were connections with e.g. pyrazolopyrimidinone, 4’-aminochalcone or with natural antioxidant moieties such as GA, LA and CCA to get potential cholinesterase inhibitors with neuroprotective activity.
Conclusively, the results reported by many research groups, mentioned by us in this work, support a role of tacrine, donepezil, galantamine and rivastigmine as chemical scaffolds for further structural modification and the design of new drugs for AD treatment. Due to the multifactorial nature of AD, multi-target compounds are of the greatest importance at this point. Current science focuses not only on the inhibition of cholinesterase, but also on other factors responsible for the pathogenesis and symptoms of AD. It is desirable that the proposed substances among others could inhibit Aβ aggregation, would have neuroprotective and antioxidant properties and would be able to chelate metal ions.
As the most interesting anti-AD candidates, that target most of the therapeutic strategies showed in the (Fig. 2), we type compounds 2, 4 and 7 among tacrine analogs, 18, 19, 22a and 22b among donepezil derivatives and 27, 30, 31a-c among rivastigmine hybrids. They revealed their multifunctional nature and an excellent cholinesterase inhibitory activity, which is required for potential anti-AD agents. The compounds mentioned above are worth expanded research and could lead to a strong cholinesterase inhibitor with interesting biological properties in the future.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Bowen D.M., Smith C.B., White P., Davison A.N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 2.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2(8000):1403. doi: 10.1016/S0140-6736(76)91936-X. [DOI] [PubMed] [Google Scholar]
- 3.Perry E.K., Perry R.H., Blessed G., Tomlinson B.E. Neurotransmitter enzyme abnormalities in senile dementia: CAT and GAD activities in necropsy tissue. J. Neurol. Sci. 1977;34(2):247–265. doi: 10.1016/0022-510X(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam M. A horseradish peroxidase method for the identification of the efferents of acetyl cholinesterase-containing neurons. J. Histochem. Cytochem. 1976;24(12):1281–1285. doi: 10.1177/24.12.826585. [DOI] [PubMed] [Google Scholar]
- 5.Whitehouse P.J., Price D.L., Clark A.W., Coyle J.T., DeLong M.R. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981;10(2):122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 6.Drachman D.A., Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch. Neurol. 1974;30(2):113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013;521(18):4124–4144. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J., Qin C., Hu F., Tan J., Qiu L., Zhao S., Feng G., Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69(3):445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Nordberg A., Alafuzoff I., Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J. Neurosci. Res. 1992;31(1):103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 10.Haense C., Kalbe E., Herholz K., Hohmann C., Neumaier B., Krais R., Heiss W.D. Cholinergic system function and cognition in mild cognitive impairment. Neurobiol. Aging. 2012;33(5):867–877. doi: 10.1016/j.neurobiolaging.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Robinson L., Platt B., Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011;221(2):443–465. doi: 10.1016/j.bbr.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Yamada M., Gomeza J., Basile A.S., Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J. Neurosci. 2002;22(15):6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGehee D.S., Heath M.J., Gelber S., Devay P., Role L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269(5231):1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 14.Nordberg A., Winblad B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci. Lett. 1986;72(1):115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- 15.Mash D.C., Flynn D.D., Potter L.T. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer’s disease and experimental cholinergic denervation. Science. 1985;228(4703):1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- 16.Jiang S., Li Y., Zhang C., Zhao Y., Bu G., Xu H., Zhang Y.W. M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neurosci. Bull. 2014;30(2):295–307. doi: 10.1007/s12264-013-1406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyohara J., Hashimoto K. α7 nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer’s disease. Open Med. Chem. J. 2010;4:37–56. doi: 10.2174/1874104501004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farlow M., Gracon S.I., Hershey L.A., Lewis K.W., Sadowsky C.H., Dolan-Ureno J. The Tacrine Study Group. A controlled trial of tacrine in Alzheimer’s disease. JAMA. 1992;268(18):2523–2529. doi: 10.1001/jama.1992.03490180055026. [DOI] [PubMed] [Google Scholar]
- 19.Crismon M.L. Tacrine: first drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994;28(6):744–751. doi: 10.1177/106002809402800612. [DOI] [PubMed] [Google Scholar]
- 20.Watkins P.B., Zimmerman H.J., Knapp M.J., Gracon S.I., Lewis K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA. 1994;271(13):992–998. doi: 10.1001/jama.1994.03510370044030. [DOI] [PubMed] [Google Scholar]
- 21.Oset-Gasque M.J., Marco-Contelles J.L. Tacrine-natural-product hybrids for Alzheimer’s disease therapy. Curr. Med. Chem. 2018;••• doi: 10.2174/0929867325666180403151725. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Spilovska K., Korabecny J., Nepovimova E., Dolezal R., Mezeiova E., Soukup O., Kuca K. Multitarget tacrine hybrids with neuroprotective properties to confront Alzheimer’s disease. Curr. Top. Med. Chem. 2017;17(9):1006–1026. doi: 10.2174/1568026605666160927152728. [DOI] [PubMed] [Google Scholar]
- 23.Stanciu G.D., Luca A., Rusu R.N., Bild V., Beschea Chiriac S.I., Solcan C., Bild W., Ababei D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules. 2019;10(1):40. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith D.R., Scott L.J. Donepezil: in vascular dementia. Drugs Aging. 2003;20(15):1127–1136. doi: 10.2165/00002512-200320150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Onor M.L., Trevisiol M., Aguglia E. Rivastigmine in the treatment of Alzheimer’s disease: an update. Clin. Interv. Aging. 2007;2(1):17–32. doi: 10.2147/ciia.2007.2.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser M.D., Davies J.R., Chang X. New gold in them thar hills: Testing a novel supply route for plant-derived galanthamine. J. Alzheimers Dis. 2017;55(4):1321–1325. doi: 10.3233/JAD-160791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartorelli L., Giraldi C., Saccardo M., Cammarata S., Bottini G., Fasanaro A.M., Trequattrini A. Upgrade Study Group. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer’s disease. Curr. Med. Res. Opin. 2005;21(11):1809–1818. doi: 10.1185/030079905X65655. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson S.A., Sabbagh M.N. Donepezil: potential neuroprotective and disease-modifying effects. Expert Opin. Drug Metab. Toxicol. 2008;4(10):1363–1369. doi: 10.1517/17425255.4.10.1363. [DOI] [PubMed] [Google Scholar]
- 29.Cavedo E., Dubois B., Colliot O., Lista S., Croisile B., Tisserand G.L., Touchon J., Bonafe A., Ousset P.J., Rouaud O., Ricolfi F., Vighetto A., Pasquier F., Galluzzi S., Delmaire C., Ceccaldi M., Girard N., Lehericy S., Duveau F., Chupin M., Sarazin M., Dormont D., Hampel H. Hippocampus Study Group. Reduced regional cortical thickness rate of change in donepezil-treated subjects with suspected prodromal Alzheimer’s disease. J. Clin. Psychiatry. 2016;77(12):e1631–e1638. doi: 10.4088/JCP.15m10413. [DOI] [PubMed] [Google Scholar]
- 30.Cavedo E., Grothe M.J., Colliot O., Lista S., Chupin M., Dormont D., Houot M., Lehéricy S., Teipel S., Dubois B., Hampel H., Hampel H. Hippocampus Study Group. Reduced basal forebrain atrophy progression in a randomized Donepezil trial in prodromal Alzheimer’s disease. Sci. Rep. 2017;7(1):11706. doi: 10.1038/s41598-017-09780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois B., Chupin M., Hampel H., Lista S., Cavedo E., Croisile B., Louis Tisserand G., Touchon J., Bonafe A., Ousset P.J., Ait Ameur A., Rouaud O., Ricolfi F., Vighetto A., Pasquier F., Delmaire C., Ceccaldi M., Girard N., Dufouil C., Lehericy S., Tonelli I., Duveau F., Colliot O., Garnero L., Sarazin M., Dormont D. “Hippocampus Study Group”; Hippocampus Study Group. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1041–1049. doi: 10.1016/j.jalz.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Shirayama Y., Takahashi M., Oda Y., Yoshino K., Sato K., Okubo T., Iyo M. rCBF and cognitive impairment changes assessed by SPECT and ADAS-cog in late-onset Alzheimer’s disease after 18 months of treatment with the cholinesterase inhibitors donepezil or galantamine. Brain Imaging Behav. 2019;13(1):75–86. doi: 10.1007/s11682-017-9803-y. [DOI] [PubMed] [Google Scholar]
- 33.Sharma K., Lai M.S., Lu C.J., Chen R.C. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019;20(2):1479–1487. doi: 10.3892/mmr.2019.10374. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury R., Rajamanickam J., Grossberg G.T. An update on the safety of current therapies for Alzheimer’s disease: focus on rivastigmine. Ther. Adv. Drug Saf. 2018;9(3):171–178. doi: 10.1177/2042098617750555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N., Gordon M.L. Clinical efficacy and safety of donepezil in the treatment of Alzheimer’s disease in Chinese patients. Clin. Interv. Aging. 2018;13:1963–1970. doi: 10.2147/CIA.S159920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa R., Ohnishi T., Kobayashi H., Yamaoka T., Yajima T., Tanimura A., Kato T., Yoshizawa K. Long-term effect of galantamine on cognitive function in patients with Alzheimer’s disease versus a simulated disease trajectory: an observational study in the clinical setting. Neuropsychiatr. Dis. Treat. 2017;13:1115–1124. doi: 10.2147/NDT.S133145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond E., Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133(2):155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naseri N.N., Wang H., Guo J., Sharma M., Luo W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordone S., Annarumma L., Rossini P.M., De Gennaro L. Sleep and β-Amyloid Deposition in Alzheimer Disease: Insights on Mechanisms and Possible Innovative Treatments. Front. Pharmacol. 2019;10:695. doi: 10.3389/fphar.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao K., Zu H.B. Microglial polarization: novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology. 2020;28(1):95–110. doi: 10.1007/s10787-019-00613-5. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y., Lai M.S., Lu C.J., Chen R.C. How long can patients with mild or moderate Alzheimer’s dementia maintain both the cognition and the therapy of cholinesterase inhibitors: a national population-based study. Eur. J. Neurol. 2008;15(3):278–283. doi: 10.1111/j.1468-1331.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A., Singh A., Ekavali A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol. Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Strooper B., Vassar R., Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010;6(2):99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of A β 42(43) and A β 40 in senile plaques with end-specific A β monoclonals: evidence that an initially deposited species is A β 42(43). Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 46.Awasthi M., Singh S., Pandey V.P., Dwivedi U.N. Alzheimer’s disease: An overview of amyloid beta dependent pathogenesis and its therapeutic implications along with in silico approaches emphasizing the role of natural products. J. Neurol. Sci. 2016;361:256–271. doi: 10.1016/j.jns.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Sies H., Berndt C., Jones D.P. Oxidative Stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi S., Abramov A.Y. Oxidative medicine and cellular longevity. Hindawi Publishing Corporation; 2012. Mechanism of oxidative stress in neurodegeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radi E., Formichi P., Battisti C., Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014;42(s3) Suppl. 3:S125–S152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y., Zhao B. Oxidative medicine and cellular longevity. Hindawi Publishing Corporation; 2013. Oxidative stress and the pathogenesis of Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnham K.J., Bush A.I. Metals in Alzheimer’s and Parkinson’s diseases. Curr. Opin. Chem. Biol. 2008;12(2):222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mundy W.R., Freudenrich T.M. Sensitivity of immature neurons in culture to metal-induced changes in reactive oxygen species and intracellular free calcium. Neurotoxicology. 2000;21(6):1135–1144. [PubMed] [Google Scholar]
- 55.Pithadia A.S., Lim M.H. Metal-associated amyloid-β species in Alzheimer’s disease. Curr. Opin. Chem. Biol. 2012;16(1-2):67–73. doi: 10.1016/j.cbpa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Malinski T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimers Dis. 2007;11(2):207–218. doi: 10.3233/JAD-2007-11208. [DOI] [PubMed] [Google Scholar]
- 57.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heneka M.T., O’Banion M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Fillit H., Ding W.H., Buee L., Kalman J., Altstiel L., Lawlor B., Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991;129(2):318–320. doi: 10.1016/0304-3940(91)90490-K. [DOI] [PubMed] [Google Scholar]
- 60.He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid β generation and prevents learning and memory deficits in Alzheimer’s mice. J. Cell Biol. 2007;178(5):829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–1073. doi: 10.1096/fasebj.12.12.1063. [DOI] [PubMed] [Google Scholar]
- 62.Ho L., Purohit D., Haroutunian V., Luterman J.D., Willis F., Naslund J., Buxbaum J.D., Mohs R.C., Aisen P.S., Pasinetti G.M. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch. Neurol. 2001;58(3):487–492. doi: 10.1001/archneur.58.3.487. [DOI] [PubMed] [Google Scholar]
- 63.Kitamura Y., Shimohama S., Koike H., Kakimura Ji., Matsuoka Y., Nomura Y., Gebicke-Haerter P.J., Taniguchi T. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-γ in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 1999;254(3):582–586. doi: 10.1006/bbrc.1998.9981. [DOI] [PubMed] [Google Scholar]
- 64.O’Banion M.K., Chang J.W., Coleman P.D. Eicosanoids and other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury. Springer: Boston, MA; 1997. Decreased expression of prostaglandin G/H synthase-2 (PGHS-2) in Alzheimer’s disease brain. [Google Scholar]
- 65.Hoozemans J.J., Veerhuis R., Rozemuller A.J., Arendt T., Eikelenboom P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol. Dis. 2004;15(3):492–499. doi: 10.1016/j.nbd.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 66.Hoozemans J.J.M., Rozemuller A.J.M., Janssen I., De Groot C.J.A., Veerhuis R., Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001;101(1):2–8. doi: 10.1007/s004010000251. [DOI] [PubMed] [Google Scholar]
- 67.Manev H., Chen H., Dzitoyeva S., Manev R. Cyclooxygenases and 5-lipoxygenase in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(2):315–319. doi: 10.1016/j.pnpbp.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurney M.E., D’Amato E.C., Burgin A.B. Phosphodiesterase-4 (PDE4) molecular pharmacology and Alzheimer’s disease. Neurotherapeutics. 2015;12(1):49–56. doi: 10.1007/s13311-014-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliva A.A., Jr, Kang Y., Furones C., Alonso O.F., Bruno O., Dietrich W.D., Atkins C.M. Phosphodiesterase isoform-specific expression induced by traumatic brain injury. J. Neurochem. 2012;123(6):1019–1029. doi: 10.1111/jnc.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shih J.C. Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. Neurotoxicology. 2004;25(1-2):21–30. doi: 10.1016/S0161-813X(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 71.Reinikainen K.J., Paljärvi L., Huuskonen M., Soininen H., Laakso M., Riekkinen P.J. A post-mortem study of noradrenergic, serotonergic and GABAergic neurons in Alzheimer’s disease. J. Neurol. Sci. 1988;84(1):101–116. doi: 10.1016/0022-510X(88)90179-7. [DOI] [PubMed] [Google Scholar]
- 72.Schedin-Weiss S., Inoue M., Hromadkova L., Teranishi Y., Yamamoto N.G., Wiehager B., Bogdanovic N., Winblad B., Sandebring-Matton A., Frykman S., Tjernberg L.O. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res. Ther. 2017;9(1):57. doi: 10.1186/s13195-017-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mangoni A., Grassi M.P., Frattola L., Piolti R., Bassi S., Motta A., Marcone A., Smirne S. Effects of a MAO-B inhibitor in the treatment of Alzheimer disease. Eur. Neurol. 1991;31(2):100–107. doi: 10.1159/000116655. [DOI] [PubMed] [Google Scholar]
- 74.Przybyłowska M., Kowalski S., Dzierzbicka K., Inkielewicz-Stepniak I. Therapeutic Potential of Multifunctional Tacrine Analogues. Curr. Neuropharmacol. 2019;17(5):472–490. doi: 10.2174/1570159X16666180412091908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erdélyi M. Halogen bonding in solution. Chem. Soc. Rev. 2012;41(9):3547–3557. doi: 10.1039/c2cs15292d. [DOI] [PubMed] [Google Scholar]
- 76.Gregor V.E., Emmerling M.R., Lee C., Moore C.J. The synthesis and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity of tacrine (Cognex®) derivaties. Bioorg. Med. Chem. Lett. 1992;2(8):861–864. doi: 10.1016/S0960-894X(00)80545-4. [DOI] [Google Scholar]
- 77.Ragab H.M., Teleb M., Haidar H.R., Gouda N. Chlorinated tacrine analogs: Design, synthesis and biological evaluation of their anti-cholinesterase activity as potential treatment for Alzheimer’s disease. Bioorg. Chem. 2019;86:557–568. doi: 10.1016/j.bioorg.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 78.Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J. Toxicol. Clin. Toxicol. 2002;40(6):803–816. doi: 10.1081/CLT-120015840. [DOI] [PubMed] [Google Scholar]
- 79.Jokanović M. Structure-activity relationship and efficacy of pyridinium oximes in the treatment of poisoning with organophosphorus compounds: a review of recent data. Curr. Top. Med. Chem. 2012;12(16):1775–1789. doi: 10.2174/1568026611209061775. [DOI] [PubMed] [Google Scholar]
- 80.Kim J., Malpani Y.R., Lee J., Shin J.S., Han S.B., Jung Y.S. Novel tacrine-pyridinium hybrid reactivators of organophosphorus-inhibited acetylcholinesterase: Synthesis, molecular docking, and in vitro reactivation study. Bioorg. Med. Chem. Lett. 2018;28(23-24):3784–3786. doi: 10.1016/j.bmcl.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Yang Y., Hong K.H., Ning Y., Yu P., Ren J., Ji M., Cai J. Design, synthesis and evaluation of a novel metal chelator as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Chem. 2019;87:720–727. doi: 10.1016/j.bioorg.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 82.Riazimontazer E., Sadeghpour H., Nadri H., Sakhteman A., Tüylü Küçükkılınç T., Miri R., Edraki N. Design, synthesis and biological activity of novel tacrine-isatin Schiff base hybrid derivatives. Bioorg. Chem. 2019;89:103006. doi: 10.1016/j.bioorg.2019.103006. [DOI] [PubMed] [Google Scholar]
- 83.El-Malah A., Abouelatta A.I.Y., Mahmoud Z., Salem H.H. New cyclooctathienopyridine derivatives in the aim of discovering better Anti-Alzheimer’s agents. J. Mol. Struct. 2019;1196:162–168. doi: 10.1016/j.molstruc.2019.06.071. [DOI] [Google Scholar]
- 84.Meena V.K., Chaturvedi S., Sharma R.K., Mishra A.K., Hazari P.P. Potent Acetylcholinesterase Selective and Reversible Homodimeric Agent Based on Tacrine for Theranostics. Mol. Pharm. 2019;16(6):2296–2308. doi: 10.1021/acs.molpharmaceut.8b01058. [DOI] [PubMed] [Google Scholar]
- 85.Li G., Hong G., Li X., Zhang Y., Xu Z., Mao L., Feng X., Liu T. Synthesis and activity towards Alzheimer’s disease in vitro: Tacrine, phenolic acid and ligustrazine hybrids. Eur. J. Med. Chem. 2018;148:238–254. doi: 10.1016/j.ejmech.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 86.Ramos E., Palomino-Antolín A., Bartolini M., Iriepa I., Moraleda I., Diez-Iriepa D., Samadi A., Cortina C.V., Chioua M., Egea J., Romero A., Marco-Contelles J. QuinoxalineTacrine QT78, a Cholinesterase Inhibitor as a Potential Ligand for Alzheimer’s Disease Therapy. Molecules. 2019;24(8):1503. doi: 10.3390/molecules24081503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derabli C., Boualia I., Abdelwahab A.B., Boulcina R., Bensouici C., Kirsch G., Debache A. A cascade synthesis, in vitro cholinesterases inhibitory activity and docking studies of novel Tacrine-pyranopyrazole derivatives. Bioorg. Med. Chem. Lett. 2018;28(14):2481–2484. doi: 10.1016/j.bmcl.2018.05.063. [DOI] [PubMed] [Google Scholar]
- 88.Rajeshwari R., Chand K., Candeias E., Cardoso S.M., Chaves S., Santos M.A. New Multitarget Hybrids Bearing Tacrine and Phenylbenzothiazole Motifs as Potential Drug Candidates for Alzheimer’s Disease. Molecules. 2019;24(3):587. doi: 10.3390/molecules24030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scipioni M., Kay G., Megson I.L., Kong Thoo Lin P. Synthesis of novel vanillin derivatives: novel multi-targeted scaffold ligands against Alzheimer’s disease. MedChemComm. 2019;10(5):764–777. doi: 10.1039/C9MD00048H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Medrasi H.Y., Salaheldin A.M., Hafez E.A. Synthesis and Biological Evaluation of New Pyrrolotacrines for the Treatment of Alzheimer’s Disease. International Journal of Modern Organic Chemistry. 2019;6(1):1–13. [Google Scholar]
- 91.Recanatini M., Cavalli A., Belluti F., Piazzi L., Rampa A., Bisi A., Gobbi S., Valenti P., Andrisano V., Bartolini M., Cavrini V. Structure-activity relationships of 9-amino-1, 2, 3, 4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity. J. Med. Chem. 2000;43:2007–2018. doi: 10.1021/jm990971t. [DOI] [PubMed] [Google Scholar]
- 92.Gao X.H., Tang J.J., Liu H.R., Liu L.B., Liu Y.Z. Structure-activity study of fluorine or chlorine-substituted cinnamic acid derivatives with tertiary amine side chain in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev. Res. 2019;80(4):438–445. doi: 10.1002/ddr.21515. [DOI] [PubMed] [Google Scholar]
- 93.Imramovsky A., Stepankova S., Vanco J., Pauk K., Monreal-Ferriz J., Vinsova J., Jampilek J. Acetylcholinesterase-inhibiting activity of salicylanilide N-alkylcarbamates and their molecular docking. Molecules. 2012;17(9):10142–10158. doi: 10.3390/molecules170910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saliba A.N., Harb A.R., Taher A.T. Iron chelation therapy in transfusion-dependent thalassemia patients: current strategies and future directions. J. Blood Med. 2015;6:197–209. doi: 10.2147/JBM.S72463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fawzi S.F., Menze E.T., Tadros M.G. Deferiprone ameliorates memory impairment in Scopolamine-treated rats: the impact of its iron-chelating effect on -amyloid disposition. Behav. Brain Res. 2019;••• doi: 10.1016/j.bbr.2019.112314. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 96.Chand K., Rajeshwari, Candeias E., Cardoso S.M., Chaves S., Santos M.A. Tacrine-deferiprone hybrids as multi-target-directed metal chelators against Alzheimer’s disease: a two-in-one drug. Metallomics. 2018;10(10):1460–1475. doi: 10.1039/C8MT00143J. [DOI] [PubMed] [Google Scholar]
- 97.Zhu J., Yang H., Chen Y., Lin H., Li Q., Mo J., Bian Y., Pei Y., Sun H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018;33(1):496–506. doi: 10.1080/14756366.2018.1430691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ökten S., Ekiz M., Koçyiğit U.M., Tutar A., Çelik I., Akkurt M., Gökalp F., Taslimi P., Gülçin I. Synthesis, Characterization, Crystal Structures, Theoretical Calculations and Biological Evaluations of Novel Substituted Tacrine Derivatives as Cholinesterase and Carbonic Anhydrase Enzymes Inhibitors. J. Mol. Struct. 2019;1175:906–915. doi: 10.1016/j.molstruc.2018.08.063. [DOI] [Google Scholar]
- 99.Wu G., Gao Y., Kang D., Huang B., Huo Z., Liu H., Poongavanam V., Zhan P., Liu X. Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors. MedChemComm. 2017;9(1):149–159. doi: 10.1039/C7MD00457E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng Z.Q., Song J.L., Zhu K., Zhang J., Jiang C.S., Zhang H. Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors. Mar. Drugs. 2018;16(9):293. doi: 10.3390/md16090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dgachi Y., Martin H., Malek R., Jun D., Janockova J., Sepsova V., Soukup O., Iriepa I., Moraleda I., Maalej E., Carreiras M.C., Refouvelet B., Chabchoub F., Marco-Contelles J., Ismaili L. Synthesis and biological assessment of KojoTacrines as new agents for Alzheimer’s disease therapy. J. Enzyme Inhib. Med. Chem. 2019;34(1):163–170. doi: 10.1080/14756366.2018.1538136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26(3):243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 103.Kimura H. Physiological Roles of Hydrogen Sulfide and Polysulfides. Handb. Exp. Pharmacol. 2015;230:61–81. doi: 10.1007/978-3-319-18144-8_3. [DOI] [PubMed] [Google Scholar]
- 104.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013;63(5):492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Tian Q., Chen L., Luo B., Wang A.P., Zou W., You Y., Zhang P., Tang X.Q. Hydrogen Sulfide Antagonizes Chronic Restraint Stress-Induced Depressive-Like Behaviors via Upregulation of Adiponectin. Front. Psychiatry. 2018;9:399. doi: 10.3389/fpsyt.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He J.T., Li H., Yang L., Mao C.Y. Role of hydrogen sulfide in cognitive deficits: Evidences and mechanisms. Eur. J. Pharmacol. 2019;849:146–153. doi: 10.1016/j.ejphar.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 107.Giuliani D., Ottani A., Zaffe D., Galantucci M., Strinati F., Lodi R., Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 108.Cheng X.J., Gu J.X., Pang Y.P., Liu J., Xu T., Li X.R., Hua Y.Z., Newell K.A., Huang X.F., Yu Y., Liu Y. Tacrine-Hydrogen Sulfide Donor Hybrid Ameliorates Cognitive Impairment in the Aluminum Chloride Mouse Model of Alzheimer’s Disease. ACS Chem. Neurosci. 2019;10(8):3500–3509. doi: 10.1021/acschemneuro.9b00120. [DOI] [PubMed] [Google Scholar]
- 109.Gniazdowska E., Koźmiński P., Halik P., Bajda M., Czarnecka K., Mikiciuk-Olasik E., Masłowska K., Rogulski Z., Cheda Ł., Kilian K., Szymański P. Synthesis, physicochemical and biological evaluation of tacrine derivative labeled with technetium-99m and gallium-68 as a prospective diagnostic tool for early diagnosis of Alzheimer’s disease. Bioorg. Chem. 2019;91:103136. doi: 10.1016/j.bioorg.2019.103136. [DOI] [PubMed] [Google Scholar]
- 110.Przybyłowska M., Inkielewicz-Stepniak I., Kowalski S., Dzierzbicka K., Demkowicz S., Daśko M. Synthesis and Cholinesterase Inhibitory Activity of N-Phosphorylated / N-Tiophosphorylated Tacrine. Med. Chem. 2019;••• doi: 10.2174/1573406415666190716115524. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Roldán-Peña J.M., Romero-Real V., Hicke J., Maya I., Franconetti A., Lagunes I., Padrón J.M., Petralla S., Poeta E., Naldi M., Bartolini M., Monti B., Bolognesi M.L., López Ó., Fernández-Bolaños J.G. Tacrine-O-protected phenolics heterodimers as multitarget-directed ligands against Alzheimer’s disease: Selective subnanomolar BuChE inhibitors. Eur. J. Med. Chem. 2019;181:111550. doi: 10.1016/j.ejmech.2019.07.053. [DOI] [PubMed] [Google Scholar]
- 112.Lopes J.P.B., Silva L., da Costa Franarin G., Antonio Ceschi M., Seibert Lüdtke D., Ferreira Dantas R., de Salles C.M.C., Paes Silva-Jr F., Roberto Senger M., Alvim Guedes I., Emmanuel Dardenne L. Design, synthesis, cholinesterase inhibition and molecular modelling study of novel tacrine hybrids with carbohydrate derivatives. Bioorg. Med. Chem. 2018;26(20):5566–5577. doi: 10.1016/j.bmc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 113.Cheng Z.Q., Zhu K.K., Zhang J., Song J.L., Muehlmann L.A., Jiang C.S., Liu C.L., Zhang H. Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BChE inhibitors. Bioorg. Chem. 2019;83:277–288. doi: 10.1016/j.bioorg.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 114.Borghans L., Sambeth A., Blokland A. Biperiden Selectively Impairs Verbal Episodic Memory in a Dose- and Time-Dependent Manner in Healthy Subjects. J. Clin. Psychopharmacol. 2020;40(1):30–37. doi: 10.1097/JCP.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 115.Fisher A., Michaelson D.M., Brandeis R., Haring R., Chapman S., Pittel Z. M1 muscarinic agonists as potential disease-modifying agents in Alzheimer’s disease. Rationale and perspectives. Ann. N. Y. Acad. Sci. 2000;920:315–320. doi: 10.1111/j.1749-6632.2000.tb06941.x. [DOI] [PubMed] [Google Scholar]
- 116.Davis A.A., Fritz J.J., Wess J., Lah J.J., Levey A.I. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J. Neurosci. 2010;30(12):4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang S., Wang Y., Ma Q., Zhou A., Zhang X., Zhang Y.W. M1 muscarinic acetylcholine receptor interacts with BACE1 and regulates its proteosomal degradation. Neurosci. Lett. 2012;515(2):125–130. doi: 10.1016/j.neulet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 118.Tarr J.C., Turlington M.L., Reid P.R., Utley T.J., Sheffler D.J., Cho H.P., Klar R., Pancani T., Klein M.T., Bridges T.M., Morrison R.D., Blobaum A.L., Xiang Z., Daniels J.S., Niswender C.M., Conn P.J., Wood M.R., Lindsley C.W. Targeting selective activation of M(1) for the treatment of Alzheimer’s disease: further chemical optimization and pharmacological characterization of the M(1) positive allosteric modulator ML169. ACS Chem. Neurosci. 2012;3(11):884–895. doi: 10.1021/cn300068s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shirey J.K., Brady A.E., Jones P.J., Davis A.A., Bridges T.M., Kennedy J.P., Jadhav S.B., Menon U.N., Xiang Z., Watson M.L., Christian E.P., Doherty J.J., Quirk M.C., Snyder D.H., Lah J.J., Levey A.I., Nicolle M.M., Lindsley C.W., Conn P.J. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 2009;29(45):14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hepnarova V., Korabecny J., Matouskova L., Jost P., Muckova L., Hrabinova M., Vykoukalova N., Kerhartova M., Kucera T., Dolezal R., Nepovimova E., Spilovska K., Mezeiova E., Pham N.L., Jun D., Staud F., Kaping D., Kuca K., Soukup O. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018;150:292–306. doi: 10.1016/j.ejmech.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 121.Kandel E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blokland A., Heckman P., Vanmierlo T., Schreiber R., Paes D., Prickaerts J. Phosphodiesterase Type 4 Inhibition in CNS Diseases. Trends Pharmacol. Sci. 2019;40(12):971–985. doi: 10.1016/j.tips.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 123.Pan T., Xie S., Zhou Y., Hu J., Luo H., Li X., Huang L. Dual functional cholinesterase and PDE4D inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and evaluation of tacrine-pyrazolo[3,4-b]pyridine hybrids. Bioorg. Med. Chem. Lett. 2019;29(16):2150–2152. doi: 10.1016/j.bmcl.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 124.Łozińska I., Świerczyńska A., Molęda Z., Hartman A.M., Hirsch A.K.H., Czarnocki Z. Donepezil-melatonin hybrids as butyrylcholinesterase inhibitors: Improving binding affinity through varying mode of linking fragments. Arch. Pharm. (Weinheim) 2018;351(11):e1800194. doi: 10.1002/ardp.201800194. [DOI] [PubMed] [Google Scholar]
- 125.Fernández-Bachiller M.I., Pérez C., Campillo N.E., Páez J.A., González-Muñoz G.C., Usán P., García-Palomero E., López M.G., Villarroya M., García A.G., Martínez A., Rodríguez-Franco M.I. Tacrine-melatonin hybrids as multifunctional agents for Alzheimer’s disease, with cholinergic, antioxidant, and neuroprotective properties. ChemMedChem. 2009;4(5):828–841. doi: 10.1002/cmdc.200800414. [DOI] [PubMed] [Google Scholar]
- 126.Zurek E., Szymański P., Mikiciuk-Olasik E. Synthesis and biological activity of new donepezil-hydrazinonicotinamide hybrids. Drug Res. (Stuttg.) 2013;63(3):137–144. doi: 10.1055/s-0033-1333735. [DOI] [PubMed] [Google Scholar]
- 127.Pachón-Angona I., Refouvelet B., Andrýs R., Martin H., Luzet V., Iriepa I., Moraleda I., Diez-Iriepa D., Oset-Gasque M.J., Marco-Contelles J., Musilek K., Ismaili L. Donepezil + chromone + melatonin hybrids as promising agents for Alzheimer’s disease therapy. J. Enzyme Inhib. Med. Chem. 2019;34(1):479–489. doi: 10.1080/14756366.2018.1545766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arendt T., Brückner M.K., Lange M., Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease resemble embryonic development--a study of molecular forms. Neurochem. Int. 1992;21(3):381–396. doi: 10.1016/0197-0186(92)90189-X. [DOI] [PubMed] [Google Scholar]
- 129.Benchekroun M., Ismaili L., Pudlo M., Luzet V., Gharbi T., Refouvelet B., Marco-Contelles J. Donepezil-ferulic acid hybrids as anti-Alzheimer drugs. Future Med. Chem. 2015;7(1):15–21. doi: 10.4155/fmc.14.148. [DOI] [PubMed] [Google Scholar]
- 130.Cai P., Fang S.Q., Yang X.L., Wu J.J., Liu Q.H., Hong H., Wang X.B., Kong L.Y. Rational Design and Multibiological Profiling of Novel Donepezil-Trolox Hybrids against Alzheimer’s Disease, with Cholinergic, Antioxidant, Neuroprotective, and Cognition Enhancing Properties. ACS Chem. Neurosci. 2017;8(11):2496–2511. doi: 10.1021/acschemneuro.7b00257. [DOI] [PubMed] [Google Scholar]
- 131.Cai P., Fang S.Q., Yang H.L., Yang X.L., Liu Q.H., Kong L.Y., Wang X.B. Donepezil-butylated hydroxytoluene (BHT) hybrids as Anti-Alzheimer’s disease agents with cholinergic, antioxidant, and neuroprotective properties. Eur. J. Med. Chem. 2018;157:161–176. doi: 10.1016/j.ejmech.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 132.Dias K.S., de Paula C.T., Dos Santos T., Souza I.N., Boni M.S., Guimarães M.J., da Silva F.M., Castro N.G., Neves G.A., Veloso C.C., Coelho M.M., de Melo I.S., Giusti F.C., Giusti-Paiva A., da Silva M.L., Dardenne L.E., Guedes I.A., Pruccoli L., Morroni F., Tarozzi A., Viegas C., Jr Design, synthesis and evaluation of novel feruloyl-donepezil hybrids as potential multitarget drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;130:440–457. doi: 10.1016/j.ejmech.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 133.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hung A.S., Liang Y., Chow T.C., Tang H.C., Wu S.L., Wai M.S., Yew D.T. Mutated tau, amyloid and neuroinflammation in Alzheimer disease-A brief review. Prog. Histochem. Cytochem. 2016;51(1):1–8. doi: 10.1016/j.proghi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 135.Dias Viegas F.P., de Freitas Silva M., Divino da Rocha M., Castelli M.R., Riquiel M.M., Machado R.P., Vaz S.M., Simões de Lima L.M., Mancini K.C., Marques de Oliveira P.C., Morais É.P., Gontijo V.S., da Silva F.M.R., D’Alincourt da Fonseca Peçanha D., Castro N.G., Neves G.A., Giusti-Paiva A., Vilela F.C., Orlandi L., Camps I., Veloso M.P., Leomil Coelho L.F., Ionta M., Ferreira-Silva G.Á., Pereira R.M., Dardenne L.E., Guedes I.A., de Oliveira Carneiro Junior W., Quaglio Bellozi P.M., Pinheiro de Oliveira A.C., Ferreira F.F., Pruccoli L., Tarozzi A., Viegas C., Jr Design, synthesis and pharmacological evaluation of N-benzyl-piperidinyl-aryl-acylhydrazone derivatives as donepezil hybrids: Discovery of novel multi-target anti-alzheimer prototype drug candidates. Eur. J. Med. Chem. 2018;147:48–65. doi: 10.1016/j.ejmech.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 136.Medeiros R., Figueiredo C.P., Pandolfo P., Duarte F.S., Prediger R.D., Passos G.F., Calixto J.B. The role of TNF-alpha signaling pathway on COX-2 upregulation and cognitive decline induced by beta-amyloid peptide. Behav. Brain Res. 2010;209(1):165–173. doi: 10.1016/j.bbr.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 137.Cheng X., Shen Y., Li R. Targeting TNF: a therapeutic strategy for Alzheimer’s disease. Drug Discov. Today. 2014;19(11):1822–1827. doi: 10.1016/j.drudis.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 138.Choi S.H., Aid S., Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 2009;30(4):174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hiremathad A., Keri R.S., Esteves A.R., Cardoso S.M., Chaves S., Santos M.A. Novel Tacrine-Hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018;148:255–267. doi: 10.1016/j.ejmech.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 140.Estrada Valencia M., Herrera-Arozamena C., de Andrés L., Pérez C., Morales-García J.A., Pérez-Castillo A., Ramos E., Romero A., Viña D., Yáñez M., Laurini E., Pricl S., Rodríguez-Franco M.I. Neurogenic and neuroprotective donepezil-flavonoid hybrids with sigma-1 affinity and inhibition of key enzymes in Alzheimer’s disease. Eur. J. Med. Chem. 2018;156:534–553. doi: 10.1016/j.ejmech.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 141.Piemontese L., Tomás D., Hiremathad A., Capriati V., Candeias E., Cardoso S.M., Chaves S., Santos M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzyme Inhib. Med. Chem. 2018;33(1):1212–1224. doi: 10.1080/14756366.2018.1491564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thamban Chandrika N., Fosso M.Y., Tsodikov O.V., LeVine Iii H., Garneau-Tsodikova S. Combining Chalcones with Donepezil to Inhibit Both Cholinesterases and Aβ Fibril Assembly. Molecules. 2019;25(1):77. doi: 10.3390/molecules25010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie S.S., Lan J.S., Wang X., Wang Z.M., Jiang N., Li F., Wu J.J., Wang J., Kong L.Y. Design, synthesis and biological evaluation of novel donepezil-coumarin hybrids as multi-target agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2016;24(7):1528–1539. doi: 10.1016/j.bmc.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 144.Zhu G., Wang K., Shi J., Zhang P., Yang D., Fan X., Zhang Z., Liu W., Sang Z. The development of 2-acetylphenol-donepezil hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019;29(19):126625. doi: 10.1016/j.bmcl.2019.126625. [DOI] [PubMed] [Google Scholar]
- 145.Johnson G., Moore S.W. The peripheral anionic site of acetylcholinesterase: structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006;12(2):217–225. doi: 10.2174/138161206775193127. [DOI] [PubMed] [Google Scholar]
- 146.Radić Z., Pickering N.A., Vellom D.C., Camp S., Taylor P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry. 1993;32(45):12074–12084. doi: 10.1021/bi00096a018. [DOI] [PubMed] [Google Scholar]
- 147.Ordentlich A., Barak D., Kronman C., Ariel N., Segall Y., Velan B., Shafferman A. Functional characteristics of the oxyanion hole in human acetylcholinesterase. J. Biol. Chem. 1998;273(31):19509–19517. doi: 10.1074/jbc.273.31.19509. [DOI] [PubMed] [Google Scholar]
- 148.Ordentlich A., Barak D., Kronman C., Flashner Y., Leitner M., Segall Y., Ariel N., Cohen S., Velan B., Shafferman A. Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J. Biol. Chem. 1993;268(23):17083–17095. [PubMed] [Google Scholar]
- 149.Harel M., Schalk I., Ehret-Sabatier L., Bouet F., Goeldner M., Hirth C., Axelsen P.H., Silman I., Sussman J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA. 1993;90(19):9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stavrakov G., Philipova I., Zheleva-Dimitrova D., Valkova I., Salamanova E., Konstantinov S., Doytchinova I. Docking-based design and synthesis of galantamine-camphane hybrids as inhibitors of acetylcholinesterase. Chem. Biol. Drug Des. 2017;90(5):709–718. doi: 10.1111/cbdd.12991. [DOI] [PubMed] [Google Scholar]
- 151.Schlossmann J., Schinner E. cGMP becomes a drug target. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385(3):243–252. doi: 10.1007/s00210-012-0730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dorner-Ciossek C., Kroker K.S., Rosenbrock H. Role of PDE9 in Cognition. Adv. Neurobiol. 2017;17:231–254. doi: 10.1007/978-3-319-58811-7_9. [DOI] [PubMed] [Google Scholar]
- 153.Yu Y.F., Huang Y.D., Zhang C., Wu X.N., Zhou Q., Wu D., Wu Y., Luo H.B. Discovery of Novel Pyrazolopyrimidinone Derivatives as Phosphodiesterase 9A Inhibitors Capable of Inhibiting Butyrylcholinesterase for Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2017;8(11):2522–2534. doi: 10.1021/acschemneuro.7b00268. [DOI] [PubMed] [Google Scholar]
- 154.Xiao G., Li Y., Qiang X., Xu R., Zheng Y., Cao Z., Luo L., Yang X., Sang Z., Su F., Deng Y. Design, synthesis and biological evaluation of 4′-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017;25(3):1030–1041. doi: 10.1016/j.bmc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 155.Wang L., Wang Y., Tian Y., Shang J., Sun X., Chen H., Wang H., Tan W. Design, synthesis, biological evaluation, and molecular modeling studies of chalcone-rivastigmine hybrids as cholinesterase inhibitors. Bioorg. Med. Chem. 2017;25(1):360–371. doi: 10.1016/j.bmc.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 156.Sestito S., Pruccoli L., Runfola M., Citi V., Martelli A., Saccomanni G., Calderone V., Tarozzi A., Rapposelli S. Design and synthesis of H2S-donor hybrids: A new treatment for Alzheimer’s disease? Eur. J. Med. Chem. 2019;184:111745. doi: 10.1016/j.ejmech.2019.111745. [DOI] [PubMed] [Google Scholar]
- 157.Sang Z., Li Y., Qiang X., Xiao G., Liu Q., Tan Z., Deng Y. Multifunctional scutellarin-rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015;23(4):668–680. doi: 10.1016/j.bmc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 158.Nesi G., Chen Q., Sestito S., Digiacomo M., Yang X., Wang S., Pi R., Rapposelli S. Nature-based molecules combined with rivastigmine: A symbiotic approach for the synthesis of new agents against Alzheimer’s disease. Eur. J. Med. Chem. 2017;141:232–239. doi: 10.1016/j.ejmech.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 159.Li Y., Peng P., Tang L., Hu Y., Hu Y., Sheng R. Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer’s disease therapy. Bioorg. Med. Chem. 2014;22(17):4717–4725. doi: 10.1016/j.bmc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 160.Chen Z., Digiacomo M., Tu Y., Gu Q., Wang S., Yang X., Chu J., Chen Q., Han Y., Chen J., Nesi G., Sestito S., Macchia M., Rapposelli S., Pi R. Discovery of novel rivastigmine-hydroxycinnamic acid hybrids as multi-targeted agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017;125:784–792. doi: 10.1016/j.ejmech.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 161.Sang Z., Wang K., Shi J., Cheng X., Zhu G., Wei R., Ma Q., Yu L., Zhao Y., Tan Z., Liu W. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020;187:111958. doi: 10.1016/j.ejmech.2019.111958. [DOI] [PubMed] [Google Scholar]
- 162.Krátký M., Štěpánková Š., Vorčáková K., Vinšová J. Investigation of salicylanilide and 4-chlorophenol-based N-monosubstituted carbamates as potential inhibitors of acetyl- and butyrylcholinesterase. Bioorg. Chem. 2018;80:668–673. doi: 10.1016/j.bioorg.2018.07.017. [DOI] [PubMed] [Google Scholar]