Abstract
A traumatic brain injury (TBI) initiates an inflammatory response with molecular cascades triggered by the presence of necrotic debris, including damaged myelin, hemorrhages and injured neuronal cells. Molecular cascades prominent in TBI-induced inflammation include the release of an excess of proinflammatory cytokines and angiogenic factors, the degradation of tight junctions (TJs), cytoskeletal rearrangements and leukocyte and protein extravasation promoted by increased expression of adhesion molecules. The brain-gut axis consists of a complex network involving neuroendocrine and immunological signaling pathways and bi-directional neural mechanisms. Importantly, modifying the gut microbiome alters this axis, and in turn may influence brain injury and neuroinflammatory processes. In recent years it has been demonstrated that the activity and composition of the gastrointestinal (GI) microbiome population influences the brain through all of above-mentioned pathways affecting homeostasis of the central nervous system (CNS). The GI microbiome is involved in the modulation of cellular and molecular processes which are fundamental to the progression of TBI-induced pathologies, including neuroinflammation, abnormal blood brain barrier (BBB) permeability, immune system responses, microglial activation, and mitochondrial dysfunction. It has been postulated that interaction between the brain and gut microbiome occurs mainly via the enteric nervous system and the vagus nerve through neuroactive compounds including serotonin or dopamine and activation by bacterial metabolites including endotoxin, neurotransmitters, neurotrophic factors, and cytokines. In recent years the multifactorial impact of selected immunomodulatory drugs on immune processes occurring in the CNS and involving the brain-gut axis has been under intensive investigation.
Keywords: Traumatic brain injury, secondary brain damage, trauma, cells interactions, microbiome, immunomodulation
1. INTRODUCTION
A brain injury is the common cause of death among young male adults [1]. In survivors, mechanical external forces that cause the primary cause of brain injury, often involving the white matter, initiate a severe destructive and very protracted inflammation recently elucidated in the rat spinal cord injury model [2, 3]. While primary brain injury is irreversible as it occurs in an accident, the inflammatory damage, with neuronal dysfunction related to trauma-induced oxidative stress, apoptotic cell death, microglial activation and mitochondrial dysfunction, ischemia, edema and phagocytic macrophage activation and invasion, is amenable to treatment [4, 5]. Permanent neuronal loss with apoptosis of neurons and oligodendrocytes occurs along a very protracted course of severe destructive inflammation and may still be observed 1 year after brain trauma [6, 7]. Neural cell death involves impairment of function with increased calcium level in the cytosol [8]. Damage to the blood-brain barrier (BBB) in the CNS around the site of inflammation can result in an uncontrolled release of metalloproteinases, inflammatory cytokines, and proteases and the inappropriate activation and dysfunction of endothelial cells and extravasation of oedema fluid into the interstitial space [9]. The first peak of increased BBB permeability is noted within the initial few hours after injury and persists for 3-4 days, and a second peak may occur after 5 days as a result of inflammatory response and microglial activation [10]. Damage to the BBB may offer an opportunity for the therapeutic access of anti-inflammatory drugs administered parenterally, however, a “leaky” BBB may still be a barrier to large anti-inflammatory molecules such as Serp-1 and M-T7, immunomodulatory proteins isolated from Myxoma virus, indicating the need for pre-clinical testing of each candidate anti-inflammatory compound [11]. Although subdural infusion of Serp-1 resulted in lowering of the numbers of macrophages in the COI in the rat model of SCI, intraperitoneal infusion resulted in no effect [11]. Secondary axotomy and axonal degeneration are the result of destructive neuroinflammatory processes and involve proteolysis, excitotoxicity and mitochondrial dysfunction [12]. The site of trauma and supervening inflammation are localized by increasingly severe astrocytic reaction that not only walls off the site but also appears to actively transfer edema fluid from surrounding CNS tissue thus contributing to the formation of the COI [2]. Progressively severe astrogliosis around the COI may also participate in anti-inflammatory mechanisms that are water soluble and ultimately lead to inhibition and elimination of macrophage infiltration in the COI and result in a mature syrinx following the SCI [2, 13].
The inflammatory response after TBI involves both the resident microglia and infiltrating inflammatory cells, primarily phagocytic, CD68+/CD163- macrophages apparently stimulated by large quantities of damaged myelin and markedly elevated inflammatory cytokines including IL1, IL-6, IFN and chemokines with degradation of tight junctions (TJs), cytoskeletal rearrangements and protein promoted by adhesion molecules [14]. The inflammatory response is histologically evident on day 3 post-injury in the rat model of the SCI when large numbers of phagocytic macrophages begin to infiltrate the necrotic areas, become sequestered in the forming COI where they remove the myelin-rich necrotic debris and haemorrhage within 2 weeks post-SCI [2]. Large numbers of phagocytic macrophages persist in the COI for over 4 weeks apparently sustained by a mechanism of a vicious cycle where damaged myelin is potently chemotactic and chemokines elevated, resulting in activation of infiltrating macrophages that coincides with additional damage to the surrounding CNS with damage to oligodendrocytes and myelin resulting in more macrophage chemotaxis [15, 16]. The extraordinarily destructive and protracted inflammation initiated by trauma involving the white matter is therefore of the primary interest to therapeutic efforts leading to neuroprotection. The inflammatory response involves the release of DAMPS (danger associated molecular patterns): HMGB1, heat-shock, S100 proteins, ATP, which are bound by PRRs (Pattern Recognition Receptors) such as TLR [17]. PRRs recognise ligands and triggers, directly or by oligomerisation to inflammasomes (NLRP1 and NLRP3), with subsequent production of proinflammatory cytokines. Inflammasomes' activation of caspase-1 catalyses the cleavage of pro-interleukins into the active forms of interleukin-18 and IL-1β [18]. Kimbler et al. documented that blockade of P2X7 receptor with NLRP3 activating properties, extends the lesion and increases brain oedema, directly at 12 and 24 hours post injury in an animal model [19]. NADPH oxidase 2 (NOX2) is a main contributor to oxidative stress and NOX2-dependent inflammasome activation contributes to TBI pathology. Importantly inhibition of NADPH oxidase enzymes provides neuroprotective effects and reduces superoxide production [20]. Although increased synthesis of pro-inflammatory cytokines such as IL-1β, IL-6, IL-18 and TNFα, accompanied by rising levels of anti-inflammatory cytokines including IL-8, IL-10 and production of chemokines such as MCP1 and CCL5 has been attributed to activate blood-borne and CNS-resident immune cells, notably neutrophils, microglia and T-cells [21]. Recent systematic study on the progression of inflammation initiated by the SCI indicates that the CNS response, specifically astrogliosis plays an important role in the inhibition and elimination of infiltrating macrophages, thus eliminating the inflammation [2]. With this in mind, effective anti-inflammatory therapies should result in inhibiting destructive inflammation leading to its accelerated elimination and neuroprotection also reducing edema, neurodegeneration, cognitive deficits and improving overall neurological recovery [22]. It needs to be emphasized that TBI is a pro-inflammatory condition that affects not only the brain, but also impairs functions of other organs including eyes, lung, intestines, myocardium, and vascular circulation with long lasting effects and complications which need to be considered in medical practice [23].
2. BRAIN-GUT AXIS DURING TBI
In recent years the relationship between gut microbiome and brain has been an important topic. Gut microbiome plays a fundamental role in the functionality of the immune system. Over the past few years, some data have been presented that communities of microbes play a pivotal role in controlling aspects of host physiology [24]. The nervous system is strictly connected with the gut microbiome. This axis consists of a complex network involving neuroendocrine and immunological signalling pathways and direct neural mechanisms [25, 26]. It has been demonstrated that the activity and composition of the GI microbiome population influence the brain through a variety of pathways, including homeostasis of the CNS.
In mouse models, the gut microbiome is associated with changes in permeability of the BBB [27]. Braniste et al., demonstrated decreased expression of occludin by brain endothelial cells in reference to sensitive changes in the intestinal gut microbiota. In this study bacterial metabolites, including short fatty acids; butyrate, acetate or propionate, have affected the permeability of the BBB [27].
It is postulated that brain-gut microbiome communication occurs mainly via the enteric nervous system and the vagus nerve through neuroactive compounds and activation by bacterial metabolites, neurotransmitters, neurotrophic factors, cytokines, and endotoxins [28, 29]. Sympathetic and parasympathetic nervous systems have a pivotal role in the modulation of gut functions as regional motility, intestinal permeability, immune response of the mucosa, epithelial fluid maintenance and production of gastric acid, mucus, bicarbonate, gut peptides and antimicrobial peptides [30]. Therefore the autonomic nervous system mediates communication between the CNS and viscera. The HPA (hypothalamus-pituitary-adrenal) axis activity, stimulated in response to environmental factors such as stress, reacts to microbiota-related energy sources production related to gut microbiota metabolic activity, which also results in restoration of absorbable nutrients and energy [31]. Bacteria stimulate gut-microbiota axis by synthesizing neuroregulators and neurotransmitters, which connect via Meissner’s plexus with antigen presenting cells [32]. In addition, dysbiosis predisposes to adult stress responsiveness [33]. Previous research showed association between gut microbiome with autism, major depressive disorder, and Guillain-Barre syndrome [34]. Gut microbiota composition maintains homeostasis of the immune system. Different bacteria promote or antagonize production of pro- or anti-inflammatory cytokines, and importantly regulate the differentiation of T cells. Therefore, changes in the gut microbiome may result in dysregulation in the immune system and in the nervous system. Per example, in multiple sclerosis patients Acinetobacter calcoaceticus and Akkermansia muciniphila increased production of proinflammatory Th1 phenotype of peripheral mononuclear cells [35]. The GI microbiome is involved in the modulation of cellular and molecular processes which are fundamental to the progression of TBI-induced pathologies, including neuroinflammation, BBB permeability, immune system responses, microglial activation, and mitochondrial dysfunction, as well as intestinal motility and permeability [25, 36].
The brain injury generates microbiome disruptions through neuroendocrine and immunological pathways. These perturbations contribute to bi-directional imbalance even greater in the microbiome composition and induce secondary damage [37]. In experimental models, brain injury has induced disruption of the motility and permeability of intestinal wall, and finally changed the gut microbiome composition [38].
Theories about how TBI alters intestinal permeability are still under investigation. Some authors have indicated that increased NF-kB, leading to up-regulation of ICAM-1 and production of cytokines including IL-6 predispose to declined expression of tight junction proteins [39]. Another theory focuses on reduced vagal stimulation of the enteric system after brain trauma [40]. Holulden et al. have documented that damage to the CNS can lead to gut dysbiosis [41]. Brain injury predisposes to changes of cecal microbiota composition with specific changes involving Peptococcaceae and Prevotellaceae and may be affected by altered autonomic activity [41]. Expanded brain lesion correlates with increased levels of Firmicutes sp. which is composed of more than 200 different genera such as Lactobacillus sp., Bacillus sp., Clostridium sp., Enterococcus sp., and Ruminicoccus sp. [39, 42, 43]. It is important to note that this imbalance correlates with the extent of injury and the impact of the pathophysiology of brain injury [44-46]. Lesion volume in MRI and loss of behavioural function correlates with philogenic changes and alpha diversity [37]. In the rat model of post-ischemic stroke, intestinal dysbiosis is linked to worse neurological outcomes [47]. Kigerl et al. demonstrated in the post-TBI period disruption of microbiome evident as suppression in Bacteroides sp. and proliferation of Firmicutes sp. [48]. Authors observed changes even 1 month after injury [48]. This disruption of the physiological balance resulted in elevation of Th1 cells, Th17 cells and in increased expression of IFN-γ, and IL-17 in mouse model of stroke [25]. In recent years experimental animal data have demonstrated remarkable changes in gut microbiota, even 2 hours after brain injury, which correlated with TBI lesion volume and predicted the degree of locomotor impairment [37, 49, 50]. Howard et al. reported an imbalance in gut microbiome in injured patients within 72 h of TBI, resulting in the decrease in Bacteroidales sp., Fusobacteriales sp., Verrucomicrobiales sp., and increase in Clostridiales sp., and Enterococcus sp. [51]. Changes in the GI microbiome can affect the BBB given the part of bacterial translocation, LPS exposure or activation of gdT-cells with other parallel mechanisms such neutrophil induced release of TNF alpha and MMP 9 [52]. Moreover, dysbiosis predisposes to alterations of the integrity and permeability of the BBB initiated by the inflammatory response [27]. Imbalances in intestinal content can be associated with increased severity of neuroinflammation, increased microglial activity and intensified neuropathology processes [53-56]. Total absence of intestinal bacteria in axenic animal model, results in impaired immune response of the microglia, in amplifying of myelination, in hyperactivity of the HPA axis, in changes in brain neurochemistry and in decreased anxiety and social behaviors [53, 57-60]. Additionally mitochondrial dysfunction after TBI is probably also impacted by microbiome disruptions [61-64].
3. MODULATION OF MICROBIOME AND IMMUNITY AFTER BRAIN INJURY
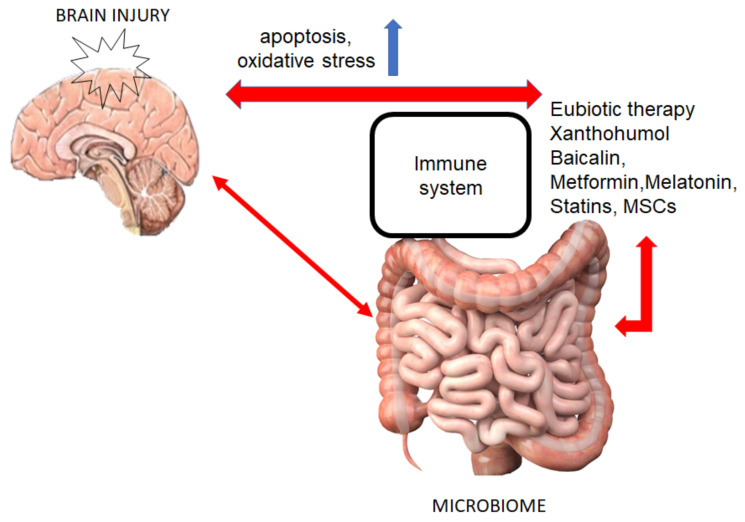
Alterations to the gut microbiome affect the brain-gut axis, and in turn brain injury impacts inflammatory processes that may alter the gut microbiome (Fig. 1) [25].
Fig. (1).
Connection between gut microbiome -brain and selected therapeutic interventions. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Eubiotic Therapies
Eubiotic therapies as transplants and administration of pre/probiotics are novel proposition for patients after brain trauma [65]. These eubiotic therapies probably have the potential to shift the gut microbiome composition to a balanced, beneficial state, especially in a period of TBI-induced inflammatory progression. The window of 24-72 hours following the TBI is considered ideal for administration for an eubiotic therapy [51]. Preclinical studies showed that an effective eubiotic therapy reduces lesion volume and microglial activation, inhibits neuroinflammation, improves mitochondrial activity, and stabilises the immune system processes and intestinal functions [47].
The probiotic supplementation leads to recovery of the basic synaptic transmission in diabetic rats as well as to enhanced activation of superoxide dismutase [66]. Recent data suggest that the administration of probiotics may modulate mitochondrial homeostasis via production of short-chain fatty acid (SCFAs) products such as butyrate, propionate, and acetate [67]. Microbial-derived SCFAs have beneficial effects on the host energy metabolism [68]. Furthermore, supplementation with Lactobacillus sp. probiotic before myocardial infarction reclaims myeloid cell proportions to normal, shifts SCFAs balance and has cardioprotective effects [69]. Probiotic treatment improves the intestinal and peripheral tissue environment since it upregulates fatty acid oxidation in the muscle and liver [68]. In addition, it improves sympathetic activity, gut brain neural circuit gluconeogenesis and thermogenesis [68]. Gut microbiome also produces alternative energy sources, including dietary ketones, important metabolites to regenerate bioenergetic status after TBI [70]. The butyrate is an important mediator of host-microbe crosstalk that promotes histone acetylation and up-regulates gene expression in host cells [71]. These effects are generated via the β-oxidation pathway and because butyrate is an inhibitor of histone deacetylases (HDACs) that can also ameliorate cognitive functions in neuropsychiatric disease and play a crucial role in neuroprotection [72, 73]. Li et al. have shown in mouse models that butyric acid produced by Clostridum butyricum improved neurological deficits and BBB impairment in TBI and in stroke [3, 56, 74]. These treatments significantly upregulated the expression of TJ proteins such as occluding and zonula occludens-1 but also p-Akt and Bcl-2, as well as down-regulated expression of Bax [74]. They also augmented the secretion of intestinal GLP-1 and induced expression of cerebral GLP-1R [74]. The propionic acid, a prominent microbiome metabolite, modulates mitochondrial functions in cells line [64]. Frye et al. have demonstrated that increased ROS levels lead to a detrimental effect of propionic acid resulting in mitochondrial dysfunction in individuals with inflammatory processes in the gut [64].
Probiotics rich in bacteria producing lactic acid decrease levels of circulating TNF-α, attenuate cerebral monocyte infiltration and reduce microglial activation [75, 76]. D’Mello et al. suggested that probiotics reduce microglial activation and monocyte infiltration and alter behavior [75]. In recent animal studies, probiotic supplementation after the SCI improved locomotor recovery and shifted immune response to protective mode by elevation in the Treg cell numbers (CD4+CD25+FoxP3+ T cells and CD11c+ DCs) [48]. In a randomized study by Min Tan et al. probiotic administation resulted in increased levels of IL-12p70 and IFN-γ in patients after brain trauma [77]. In addition, levels of IL-4 and IL-10 were reduced and these patients had decreased incidence of nosocomial infections, and lowered mortality rate within the 28-day period [77]. Daily probiotic prophylaxis can restore deviated TH1/TH2 response, reduce ventilator-associated pneumonia involving infections caused by P.aeruginosa, decreased GI dysfunction, and shorten the length of stay in the ICU [78-80].
A transplant of a normal fecal microbiota can prevent dysbiosis after SCI, and has a neuroprotective effect [81]. Anti-inflammatory effects of the normal microbiome are demonstrated in an animal model of ischemic stroke. Dysbiosis influences proinflammatory T cell activation in the immune compartment and in brain with lymphocyte migration to the CNS [47]. Thus, fecal transplantation can reduce the brain lesion and markedly improve stroke outcome via its anti-inflammatory effect.
Approximately one quarter of TBI patients develop a progressive neurodegenerative syndrome related to neurodegenerative proteinopathy or to neuroinflammation [82, 83]. Both processes initiated by brain trauma are the subject of ongoing studies [84]. The immune response to brain injury may be both a destructive contributor to outcomes and also a therapeutically tractable target mechanism. The impact of selected immunomodulatory drugs on inflammatory processes occurring in the neurotrauma with their role in affecting the brain-gut axis is a novel topic in recent years (Table 1).
Table 1.
Medications tested for the microbiome modulation.
| Agents/Therapy | Immune System Effect | Microbiome Interactions | Others |
|---|---|---|---|
| Eubiotic therapie | -inhibit inflammation -improve mitochondrial -stabilize immune system functions |
-shift the microbiome composition -reduce lesion volume -reduce microglial activation |
-improve locomotion recovery -reduce ventilator associated pneumonia -decrease gastrointenstinal dysfunction -short leigh of stay in ICU |
| Xanthohumol | -inhibit oxidative stress -improve anti-inflammatory reactions |
- decrease intenstinal microbiota diversity - alters bile acid metabolism |
-regulate fat metabolism -modulate triglyceride and cholesterol levels -control glucose and insulin levels -inhibits Notch signaling and induces apoptosis in carcinoma (anticancer activity) |
| Baicalin | --inhibit oxidative stress -improve anti-inflammatory reactions |
-increase of Proteobacteria, Euryarchaeota, and Fusobacteria -reduce of Firmicutes, Actinobacteria, and Bacteroidetes |
-antitumor and antiviral properties -neuroprotective effects via Akt/Nrf2 and PI3K/AKT/FoxO1 pathway |
| Metformin | --inhibit oxidative stress -improve proinflammatory reactions |
-adjust abundance of microbiome -decrease Intestinibacter, Bacteroides fragilis -increase bile acid glycoursodeoxycholic acid (GUDCA |
-hypoglycemic effect -reduce cerebral edema -reduce neuronal apoptosis -improve neurological deficits |
| Melatonine | -reduce inflammation -modulate superoxide dismutase,gluthione, gluthione peroxidase -inhibit apoptotic pathways |
-reduce dysbiosis in mice after spinal injury -ameliorate intenstinal integrity |
-attenuate brain edema and hyperpermeability -ameliorate locomotion recovery |
| Ketamine | -inhibit neuronal apoptosis | -reduce Deferrribacteres Mollicutes -increase Turicibacterales |
-reduce neurological deficits -inhibit neuronal apoptosis -reduce brain edema |
| Statins | -immunomodulatory and anti-inflammatory effects -reduce apoptosis |
-increase the abundance of Escherichia/Shigella,Ruminococcaceae UCG 014, Sutterella, Bacteroides, Butyricimonas, and Mucispirillum -decrease short chain fatty acids production |
-reduce triglyceride levels -raise HDL levels -reduce risk of blood clots, heart attack and stroke |
| Mesenchymal cells | -augments transcription of immunomodulatory genes -inhibit inflammation -inhibit apoptosis -regulate IgA production |
-restore gut functions -induce gut microbiome diversity |
3.2. Xanthohumol
Xanthohumol is a natural flavonoid present in hops (Humulus lupulus L), which possesses antioxidant, anti-inflammatory and chemopreventive properties [47]. Importantly, this natural substance, as a possible enhanced component of nutrition may become a significant element supporting the treatment of many diseases [85]. The available information on the possible effects of Xanthohumol in brain injury is limited but it was found to have neuroprotective effect in cerebral infarction studies in the rat, presumably related to its anti-inflammatory activities. The focal cerebral ischemia in the rat model was associated with increases in hypoxia-inducible factor (HIF)-1α, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), and active caspase-3 protein expressions in ischemic regions [47]. Yen et al. showed that expression of these genes was inhibited by treatment with Xanthohumol [86]. The neuroinflammatory response in Parkinson's disease is mediated by the presence of activated microglial cells in the substantia nigra, with increased levels of proinflammatory cytokines in the striatum and the substantia nigra, and activation of the NF-κB pro-inflammatory pathway, which regulates target genes encoding proinflammatory cytokines, chemokines, growth factors and inducible enzymes [87]. In experimental models, Xanthohumol significantly reduced the production of pro-inflammatory cytokines IL-1β and TNF-α, and decreased the imbalance between pro- and anti-apoptotic factors observed in the brain [88]. Moreover, Xanthohumol increased the expression of the neurotrophic factor BDNF what suggests a capacity to modulate inflammatory responses in the brain [89]. Xanthohumol inhibits iNOS and activation of Ito cells, central mediators of hepatic fibrogenesis [90]. It induces apoptotic processes and prevents DNA damage secondary to carcinogens in liver and colon [91]. In addition, Zhang and al. have demonstrated that Xanthohumol administration decreases the diversity of intestinal microbiota and reduces counts of Tenericutes sp. [92]. Xanthohumol alters bile acid metabolism and specifically reduces inflammation in metabolic syndrome and in obesity.
3.3. Baicalin
Baicalin is a 7-D-Glucuronic acid-5,6-dihydroxyflavone, an active flavonoid isolated from the radix of Scutellaria baicalensis, with antitumor, antiviral, antimicrobial anti-inflammatory antioxidative and neuroprotective effects [93]. In recent studies, Baicalin administration reduced brain edema, apoptotic cell death and activated antioxidative enzymes [94]. Baicalin demonstrates neuroprotective effect via activating the Akt/Nrf2 pathway and through the inhibition of TLR4 expression via the PI3K/AKT/FoxO1 pathway [93, 94]. Application of baicalin significantly decreased levels of IL-1, IL-6 and TNF-α in the hippocampus.
Baicalin-aluminium complexes altered the composition of the gut microbiome in piglets [95]. In animals treated with Baicalin-aluminium complexes, the counts of Viruses noname, Proteobacteria sp., Euryarchaeota sp., and Fusobacteria sp., were increased, while the counts of Firmicutes sp., Actinobacteria sp., and Bacteroidetes sp., were reduced [95]. In addition, Kang et al. demonstrated that intestinal microbiota may play a crucial role in the pharmacokinetics of Baicalin administered orally [96].
3.4. Metformin
Metformin is an oral hypoglycaemic drug with anti-inflammatory, antioxidative, and anti-ischemic activity [97]. In an animal model of TBI, metformin reduced cerebral oedema and neuronal apoptosis, and significantly improved neurological deficits presumably related to a neuroprotective effect. Metformin reduced levels of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, inhibited microglial activation and the translocation into the cellular nucleus of NF-κB p65 and the phosphorylation of ERK1/2 and p38 MAPK [98]. Tao et al. have shown neuroprotective effect of metformin in TBI associated with its anti-inflammatory effect involving specific intracellular pathways indicated above [98]. In a randomized study, metformin had a strong effect on the gut microbiome by reducing abundance in microbiome, of γ Proteobacteria including Escherichia coli and Firmicutes sp [99]. These authors also observed decreased counts of Intestinibacter sp. and increased counts of Bifidobacterium sp. In addition, recent data demonstrated connection between the administration of metformin and the abundance of Akkermansia muciniphila and potentially related metabolic effects [100-103]. A. muciniphila contributed to metformin antidiabetic effect via increased mucin degradation and enrichment of SCFA secretion [104]. Sun et al. have shown that individuals treated with metformin had decreased Bacteroides fragilis counts in the gut and increased levels of glycoursodeoxycholic acid (GUDCA) in the bile related to inhibition of intestinal farnesoid X receptor (FXR) signaling [105].
3.5. Melatonin
Melatonin is another agent with a neuroprotective activity whose administration attenuates early brain damage and behavioral deficits after TBI [106]. In addition, exogenous melatonin reduces inflammation; attenuates levels of IL-1β, TNF-α, IL-10, IL-4, superoxide dismutase, glutathione, glutathione peroxidase, and inhibits mitochondrial apoptotic pathways [106, 107]. In recent studies, melatonin attenuated brain edema and increased permeability of the BBB [108, 109]. Furthermore, Jing et al. have shown that administration of melatonin reduced gut dysbiosis in mice after SCI [110]. Gut microbiome in animals treated with melatonin had decreased relative counts of Clostridia sp. [110]. In addition, reduction of monocyte chemotactic protein 1 expression and of gut barrier permeability were correlated with increase in counts of Lactobacillales sp. and Lactobacillus sp. [110]. Animal studies have shown that administration of melatonin improved locomotor recovery and intestinal integrity [111]. https://www.nature.com/articles/s41598-020-59314-7 In addition, melatonin significantly elevated goblet cells, Reg3β, and Firmicutes sp. to Bacteriodetes sp. ratio by inhibiting Gram-negative bacteria through TLR4 signaling [112]. Herein, it is showed that melatonin administration suppresses proinflammatory mediators in colitis and has a regulatory effect on microbiota in the intestine [113].
3.6. Ketamine
Ketamine has anti-inflammatory activity and inhibits neuronal apoptosis [114, 115]. In addition, ketamine interacted with gut microbiota as it reduced counts of Deferrribacteres sp. and Mollicutes sp. and increased the abundance of Turicibacterales sp. [116]. Furthermore, low Sarcia sp. levels were associated with inflammatory actions due to changes in degradation of polysaccharides complex to short chain fatty acids including butyrate, and ketamine significantly increased counts of these bacteria [117].
3.7. Statins
Recent studies demonstrated immunomodulatory and anti-inflammatory effects of statins. In animal models of TBI statins reduced apoptosis of microglia and downregulated expression of TNF-α [118, 119]. Atorvastatin reduced neuroinflammation by increasing the proportion of regulatory T cells (Tregs), IL-10 and transforming growth factor (TGF)-β1 [120]. In vitro research showed gut microbiota composition changes during statins treatment. Administration of fluvastatin increased the abundance of Escherichia/Shigella sp., Ruminococcaceae UCG 014, and Sutterella sp. [121] with concurrent reduction in production of short chain fatty acids. However, gut composition after rosuvastatin, simvastatin and atorvastatin treatment were almost unchanged. Kummen et al. have shown that patients treated with resuvastatin demonstrated a decreased genetic potential to transport and metabolize TMAO (pro-atherogenic metabolite trimethylamine-N-oxide) [122]. Furthermore, authors observed elevated metabolites of betaine and γ-butyrobetaine in plasma. Atorvastatin and rosuvastatin markedly elevated the counts of Bacteroides sp., Butyricimonas sp., and Mucispirillum sp. in the gut [122]. Changes in the content of gut microbiota in the ileum corresponded to the levels of IL-1β and TGFβ1 [123].
3.8. Mesenchymal Stem/Stromal Cells
The ribosome, glycolysis, amino acid biosynthesis, carbon metabolism, and oxidative phosphorylation are involved in regulation of mesenchymal stem/stromal cell (MSC) proliferation and differentiation. The HIF-1 and several infection/inflammation signaling pathways are connected with chemokine and cytokine up-regulation by MSC [124]. These immunomodulatory effects have been assessed after TBI. Normal microbiota is involved in immunomodulatory properties of bone marrow stromal cells (MSCs). Microbiome induced immune-regulatory mediator secretions, cytokine gene transcription and surface protein expressions in MSCs [125]. These MSC-microbiome interactions markedly augmented transcription of immunomodulatory genes including COX2, IL-6, and IL-8 and upregulated secretion of prostaglandin E2 (PGE2), IL-6, and IL-8 [126]. Gut microbiome markedly activated T-cell apoptosis and cytokine secretion [125]. Experimental data indicate that MSCs influenced by microbiota had enhanced expression of chemokines and IL-10 [125, 127]. This MSC-gut microbiome axis may lead to novel discoveries in therapeutics helping regulate pathologic inflammatory processes. In animal models of bowel inflammatory disease, administration through systemic infusion or local inoculation of MSC restored the physiologic composition of gut microbiome and enhanced suppression of pathogenic bacteria [128]. Furthermore, subepithelial MSC induced diversity of gut microbiome and regulated IgA production [127]. MSC interacts directly with the gut epithelium to control expression of CCL20 and microfold (M) cell differentiation [127]. These combined influences of microbiome and MSC therapy inhibited inflammation and restored gut function [128]. The cytosolic bacterial peptidoglycan Nod2 triggered MSC protective effect against oxidative stress-mediated apoptosis [129]. In addition pluripotent stem cells therapy increased numbers of Lgr5+ intestinal stem cells, increased proliferation of intestinal epithelial cells, promoted angiogenesis and restored changes of gut microbiome composition in a mouse model [130]. Thus interactions between MSC and gut microbiome could be used in addressing therapies for inflammatory diseases including the TBI. Recent data demonstrated that MSC administrated by systematically infusion or intracerebral injection migrates preferentially to the ischemic area [131, 132]. MSC therapy and migration in TBI depends on specific signals crosstalk with the brain tissue involving the stromal-derived factor-1 (SDF1), a chemokine expressed in astrocytes, neurons and endothelial cells and CXCR4 (receptor of SDF-1) expressed in MSCs [133]. MSC can reduce apoptosis of astrocytes in the ischemic boundary zone of the TBI and augment astrocyte proliferation post-ischemia. Gao et al. demonstrated that these processes may specifically involve activation of mitogen-activated protein kinase/extracellular signal-regulated kinase and phosphoinositide 3-kinase/threonine protein kinase pathways [134]. Transplantation of MSC elicited up-regulation of brain-derived neurotrophic factor (BDNF), epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), insulin-like growth factor 1 (IGF1) and vascular endothelial growth factor (VEGF). The increase in the production of growth factors stimulated regeneration of resident brain cells [135]. Promising data have shown that MSCs can reduce inflammation and edema [131]. MSC with purified immune cells including NK, dendritic cells, naive and effector T cells, upsurge the production of interleukins IL-4 and IL-10 and inhibit TNF-α and IFN-γ expression [136]. In rat model of hemorrhagic stroke intraventricular infusion BM-MSCs suppressed pro-inflammatory cytokine expression including IL‐6, IL‐1α, and IFN‐γ [137]. It is important to note that increased IL-4 levels opposite to decreased INF-γ reverse the helper T cell subsets, from cytotoxic Th1 cells to Th2 cells [131]. Furthermore, MSC therapy inhibits T cells proliferation and finally, these may correlate with alterations of cell damage [136]. In recent years BM-MNCs therapy is a subject of clinical trials for TBI. Cox et al. have shown in twenty-five patients that intravenous delivery of BM-MNCs is safe and had no severe adverse effects [138].
3.9. The Stable Gastric Pentadecapeptide (BPC 157)
The stable gastric pentadecapeptide is a small anti-ulcer and anti-inflammatory peptide founding in the human gastric juice [139]. It is freely soluble in water at pH 7.0 and in saline [140]. BPC 157 presents multiorgan activity. It was successfully used for the treatment of esophagus, stomach, duodenum, intestine, liver and pancreas lesions [141, 142]. Interestingly, BPC 157 also presents neuroprotective properties [143, 144]. Experimental study has confirmed the BPC 157 counteracts the immediate consequences of severe head injury and reduces post-traumatic brain oedema, number and size of haemorrhagic post-traumatic lacerations, intensity of subarachnoidal bleeding and intraventricular haemorrhage [144]. Additionally, BPC 157 significantly reduces the cuprizone-induced demyelination and then risk of severe encephalopathy [141, 145]. A single dose of pentadecapeptide BPC 157 improved motor function and decreased limb spasticity induced by experimental spinal cord injury [146]. The administration of BPC 157 30 sec after 20 minutes experimental-induced cerebral ischemia significantly reduced neuronal damage and attenuated or even completely prevented ischemia/reperfusion-induced behavioural deficits and motor dysfunction [147]. The neuroprotective effect of BPC 157 may result from its interaction with dopaminergic and serotonergic system, however BPC 157 also affects GABAergic system, upregulates the GABAergic-dependent neurotransmission and interacts with opioid system [148-150]. Ultimately, neuro-beneficial activity of peripheral administration of the stable gastric pentadecapeptide BPC 157 can document the link between intestinal and brain, however, this relationship should be confirmed in further clinical studies.
4. QUERCETIN AND ANTI-QUORUM SENSING ACTIVITY
Quorum sensing (QS) plays a crucial role in the activity of important microbial physiology processes, including production of biofilm [151]. Populations of bacteria synchronize their gene expression by range of intra and intercellular signals, and production of different compounds including autoinducer-1, autoinducer-2, autoinducer-3, Pseudomonas quinolone signal (PQS) and diffusible signal factors (DSFs). Because these processes are connected with virulence, there is a theory that anti-quorum activity of some agents reduces pathogenicity and may present smaller effect on the host of commensal microbes and modulation of gut microbiome [152]. Recent studies share an interest in the use of new anti quaorum therapies to limit collateral damage to microbiota by antibiotics [153, 154]. Experimental study has confirmed the anti-quorum sensing activity of the quercetin and reduction the formation of Pseudomonas aeruginosa PAO1 biofilm [155]. Quercetin is natural aglycone flavonoid occurring in fruits and vegetables, with anti-inflammatory, anti‐proliferative and antioxidative effects [156]. In addition these commands alleviate cerebral edema, decrease neuronal degeneration, reduce oxidative stress in the mitochondria. Additionaly quercetin attenuate neuronal apoptosis via inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and activated Akt serine/threonine protein kinase phosphorylation [157, 158]. The administration of quercetin resulted in the modulation of the Nrf2 pathway and presented antioxidant enzyme activity on mitochondrial biogenesis after TBI [159]. In mice model of atherosclerosis quercetin significantly effects on gut microbiom and Firmicutes phyla were the most sensitive commensals [160]. However, the relationship between quercetin and host of commensals needs more further studies.
CONCLUSION
Influences of microbiome on the CNS constitute a potential target for therapeutic interventions in neurotrauma. Further detailed investigations are needed to better understand complex brain-gut interactions in respect to regulation of destructive inflammation initiated by neurotrauma.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ATP
Adenosin-triphosphate
- Bax/Bcl
Pro-apoptotic /anti-apoptotic proteins
- BBB
Blood-brain barrier
- BDNF
Brain-derived neurotrophic factor
- BPC 157
Stable gastric pentadecapeptide
- CNS
Central nervous system
- COI
Cavities of injury
- DAMPS
Danger associated molecular patterns
- EGF
Epidermal growth factor
- FGF2
Fibroblast growth factor 2
- GLP-1
Glucagon-like peptide-1
- GUDCA
Glycoursodeoxycholic acid
- HDACs
Inhibitor of histone deacetylases
- HIF
Hypoxia-inducible factor
- HMGB 1
High mobility group box 1 protein
- HPA axis
Hypothalamus-pituitary-adrenal axis
- ICU
Intensive Care Unit
- IGF1
Insulin-like growth factor 1
- MSC
Bone marrow stromal cells
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NOS
Nitric oxide synthase
- NOX
NOXs, NADPH oxidase
- PGE2
Prostaglandin E2
- PRRs
Pattern Recognition Receptors
- ROS
Reactive products of oxygen
- SCFA
Short-chain fatty acids
- SCI
Spinal cord injury
- TBI
Trauma brain injury
- TJs
Tight junctions
- TLR
Toll like receptors
- TMAO
Trimethylamine-N-oxide
- TNFα
Tumor necrosis factor
- Treg
Regulatory T cells
- VEGF
Vascular endothelial growth factor
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Capizzi A., Woo J., Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med. Clin. North Am. 2020;104:213–238. doi: 10.1016/j.mcna.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Kwiecien J.M., Dabrowski W., Dąbrowska-Bouta B., Sulkowski G., Oakden W., Kwiecien-Delaney C.J., Yaron J.R., Zhang L., Schutz L., Marzec-Kotarska B., Stanisz G.J., Karis J.P., Struzynska L., Lucas A.R. Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS One. 2020;15(3):e0226584. doi: 10.1371/journal.pone.0226584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Freitas Cardoso M.G., Faleiro R.M., de Paula J.J., Kummer A., Caramelli P., Teixeira A.L., de Souza L.C., Miranda A.S. Cognitive impairment following acute mild traumatic brain injury. Front. Neurol. 2019;10:198. doi: 10.3389/fneur.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazaridis C., Rusin C.G., Robertson C.S. Secondary brain injury: predicting and preventing insults. Neuropharmacology. 2019:145–152. doi: 10.1016/j.neuropharm.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Siwicka-Gieroba D., Malodobry K., Biernawska J., Robba C., Bohatyrewicz R., Rola R., Dabrowski W. The neutrophil/lymphocyte count ratio predicts mortality in severe traumatic brain injury patients. J. Clin. Med. 2019;8(9):1453. doi: 10.3390/jcm8091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mu J., Li M., Wang T., Li X., Bai M., Zhang G., Kong J. Myelin damage in diffuse axonal injury. Front. Neurosci. 2019;13:217. doi: 10.3389/fnins.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamoun R., Suki D., Gopinath S.P., Goodman J.C., Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010;113(3):564–570. doi: 10.3171/2009.12.JNS09689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thelin E.P., Hall C.E., Gupta K., Carpenter K.L.H., Chandran S., Hutchinson P.J., Patani R., Helmy A. Elucidating Pro-inflammatory cytokine responses after traumatic brain injury in a human stem cell model. J. Neurotrauma. 2018;35(2):341–352. doi: 10.1089/neu.2017.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price L., Wilson C., Grant G. Blood-brain barrier pathophysiology following traumatic brain injury. Translational Research in Traumatic Brain Injury. CRC Press; 2016. pp. 85–96. [PubMed] [Google Scholar]
- 11.Kwiecien J.M., Dabrowski W., Marzec-Kotarska B., Kwiecien-Delaney C.J., Yaron J.R., Zhang L., Schutz L., Lucas A.R. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019;57(1):41–50. doi: 10.5114/fn.2019.83830. [DOI] [PubMed] [Google Scholar]
- 12.Büki A., Povlishock J.T. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir. (Wien) 2006;148(2):181–193. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- 13.Kwiecien J.M. Cellular mechanisms of white matter regeneration in an adult dysmyelinated rat model. Folia Neuropathol. 2013;51(3):189–202. doi: 10.5114/fn.2013.37703. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Taylor-Nguyen N.N., Riley A.M., Herring B.P., White F.A., Obukhov A.G. The TRPC6 inhibitor, larixyl acetate, is effective in protecting against traumatic brain injury-induced systemic endothelial dysfunction. J. Neuroinflammation. 2019;16(1):21. doi: 10.1186/s12974-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X.J., Kong K.M., Qi W.L., Ye W.L., Song P.S. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol. Sin. 2005;26(8):934–942. doi: 10.1111/j.1745-7254.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 16.Barkho B.Z., Song H., Aimone J.B., Smrt R.D., Kuwabara T., Nakashima K., Gage F.H., Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Network. Korean Association of Immunologists. 2018;18(4):e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortezaee K., Khanlarkhani N., Beyer C., Zendedel A. Inflammasome: Its Role in Traumatic Brain and Spinal Cord Injury. J. Cell. Physiol. 2018:5160–5169. doi: 10.1002/jcp.26287. [DOI] [PubMed] [Google Scholar]
- 19.Kimbler D.E., Shields J., Yanasak N., Vender J.R., Dhandapani K.M. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7(7):e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma M.W., Wang J., Dhandapani K.M., Brann D.W. NADPH Oxidase 2 Regulates NLRP3 inflammasome activation in the brain after traumatic brain injury. Oxid. Med. Cell. Longev. 2017;2017:6057609. doi: 10.1155/2017/6057609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peron J.P.S., Oliveira D. Central Nervous system resident cells in neuroinflammation: a brave new world. Autoimmune Diseases-Contributing Factors, Specific Cases of Autoimmune Diseases, and Stem Cell and Other Therapies. InTech; 2012. [Google Scholar]
- 22.Kwiecien J.M., Dabrowski W., Kwiecien-Delaney B.J., Kwiecien-Delaney C.J., Siwicka-Gieroba D., Yaron J.R., Zhang L., Delaney K.H., Lucas A.R. Neuroprotective effect of subdural infusion of serp-1 in spinal cord trauma. Biomedicines. 2020;8(10):E372. doi: 10.3390/biomedicines8100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villalba N., Sackheim A.M., Nunez I.A., Hill-Eubanks D.C., Nelson M.T., Wellman G.C., Freeman K. traumatic brain injury causes endothelial dysfunction in the systemic microcirculation through arginase-1-dependent uncoupling of endothelial nitric oxide synthase. J. Neurotrauma. 2017;34(1):192–203. doi: 10.1089/neu.2015.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. Host-bacterial mutualism in the human intestine. . Science (80-.), . 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson S.E., Watts L.T., Burmeister D.M., Merrill D., Scroggins S., Zou Y., Lai Z., Grandhi R., Lewis A.M., Newton L.M., Eastridge B.J., Schwacha M.G. Moderate traumatic brain injury alters the gastrointestinal microbiome in a time-dependent manner. Shock. 2019;52(2):240–248. doi: 10.1097/SHK.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., Gulyás B., Halldin C., Hultenby K., Nilsson H., Hebert H., Volpe B.T., Diamond B., Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houser M.C., Tansey M.G. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3(1):3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang H.X., Wang Y.P. Gut Microbiota-brain axis. Chin. Med. J. (Engl.) 2016:2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauma S., Casaccia P. Gut-brain communication in demyelinating disorders. Curr. Opin. Neurobiol. 2020:92–101. doi: 10.1016/j.conb.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A., Kanner R., Bencosme Y., Lee Y.K., Hauser S.L., Crabtree-Hartman E., Sand I.K., Gacias M., Zhu Y., Casaccia P., Cree B.A.C., Knight R., Mazmanian S.K., Baranzini S.E. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson S.E., Burmeister D.M., Johnson T.R., Zou Y., Lai Z., Scroggins S., Derosa M., Jonas R.B., Merrill D.R., Zhu C., Newton L.M., Stewart R.M., Schwacha M.G., Jenkins D.H., Eastridge B.J. A Prospective Study in Severely Injured Patients Reveals an altered gut microbiome is associated with transfusion volume. . J. Trauma and Acute Care Surgery; Lippincott Williams and Wilkins, . 2019; 86 doi: 10.1097/TA.0000000000002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice M.W., Pandya J.D., Shear D.A. Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front. Neurol. 2019;10:875. doi: 10.3389/fneur.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson T.T., Nicholson S., Wallace D., Hawryluk G.W.J., Grandhi R. Complex Feed-forward and feedback mechanisms underlie the relationship between traumatic brain injury and the gut-microbiota-brain axis. Shock. 2019;52(3):318–325. doi: 10.1097/SHK.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 39.Hang C.H., Shi J.X., Li J.S., Wu W., Yin H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003;9(12):2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bansal V., Costantini T., Ryu S.Y., Peterson C., Loomis W., Putnam J., Elicieri B., Baird A., Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J. Trauma Inj. Infect. Crit. Care. 2010;68(5):1059–1063. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houlden A., Goldrick M., Brough D., Vizi E.S., Lénárt N., Martinecz B., Roberts I.S., Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bansal V., Costantini T., Kroll L., Peterson C., Loomis W., Eliceiri B., Baird A., Wolf P., Coimbra R. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J. Neurotrauma. 2009;26(8):1353–1359. doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundman M.H., Chen N. The Bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav. Immun. 2017;66:31–44. doi: 10.1016/j.bbi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Winek K., Dirnagl U., Meisel A. The gut microbiome as therapeutic target in central nervous system diseases: implications for stroke. Neurotherapeutics. 2016;13(4):762–774. doi: 10.1007/s13311-016-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winek K., Meisel A., Dirnagl U. Gut microbiota impact on stroke outcome: Fad or Fact? J. Cereb. Blood Flow Metab. 2015:891–898. doi: 10.1177/0271678X16636890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh V., Roth S., Llovera G., Sadler R., Garzetti D., Stecher B., Dichgans M., Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kigerl K.A., Mostacada K., Popovich P.G. Gut microbiota are disease-modifying factors after traumatic spinal cord injury. Neurotherapeutics. 2018;15(1):60–67. doi: 10.1007/s13311-017-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepage P., Leclerc M.C., Joossens M., Mondot S., Blottière H.M., Raes J., Ehrlich D., Doré J. A metagenomic insight into our gut’s microbiome. Gut. 2013;62(1):146–158. doi: 10.1136/gutjnl-2011-301805. [DOI] [PubMed] [Google Scholar]
- 50.Treangen T.J., Wagner J., Burns M.P., Villapol S. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front. Immunol. 2018;9(NOV):2757. doi: 10.3389/fimmu.2018.02757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard B.M., Kornblith L.Z., Christie S.A., Conroy A.S., Nelson M.F., Campion E.M., Callcut R.A., Calfee C.S., Lamere B.J., Fadrosh D.W., Lynch S., Cohen M.J. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg. Acute Care Open. 2017;2(1):e000108. doi: 10.1136/tsaco-2017-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chodobski A., Zink B.J., Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoban A.E., Stilling R.M., Ryan F.J., Shanahan F., Dinan T.G., Claesson M.J., Clarke G., Cryan J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 2016;6(4):e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E., Ben-Amram H., Koren O., Forsythe P., Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017;8:15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Zeng G., Zheng X., Wang W., Ling Y., Tang H., Zhang J. Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed. Pharmacother. 2018;106:349–354. doi: 10.1016/j.biopha.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 56.Sun J., Wang F., Ling Z., Yu X., Chen W., Li H., Jin J., Pang M., Zhang H., Yu J., Liu J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Desbonnet L., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. 2011. [DOI] [PubMed]
- 59.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao H.W., Ge C., Feng G.X., Li Y., Luo D., Dong J.L., Li H., Wang H., Cui M., Fan S.J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018;287(1):23–30. doi: 10.1016/j.toxlet.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandya J.D., Pauly J.R., Sullivan P.G. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 2009;218(2):381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., Sullivan P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014;257:106–113. doi: 10.1016/j.expneurol.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajpai P., Darra A., Agrawal A. Microbe-mitochondrion crosstalk and health: an emerging paradigm. Mitochondrion. 2018:20–25. doi: 10.1016/j.mito.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Frye R.E., Rose S., Chacko J., Wynne R., Bennuri S.C., Slattery J.C., Tippett M., Delhey L., Melnyk S., Kahler S.G., MacFabe D.F. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl. Psychiatry. 2016;6(10):e927. doi: 10.1038/tp.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie R., Jiang P., Lin L., Jiang J., Yu B., Rao J., Liu H., Wei W., Qiao Y. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J. Psychiatr. Res. 2020;122:70–78. doi: 10.1016/j.jpsychires.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Davari S., Talaei S.A., Alaei H., Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 67.Han B., Sivaramakrishnan P., Lin C.J., Neve I.A.A., He J., Tay L.W.R., Sowa J.N., Sizovs A., Du G., Wang J., Herman C., Wang M.C. Microbial genetic composition tunes host longevity. Cell. 2017;169(7):1249–1262.e13. doi: 10.1016/j.cell.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasubuchi M., Hasegawa S., Hiramatsu T., Ichimura A., Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang T.W.H., Chen H.C., Chen C.Y., Yen C.Y.T., Lin C.J., Prajnamitra R.P., Chen L.L., Ruan S.C., Lin J.H., Lin P.J., Lu H.H., Kuo C.W., Chang C.M., Hall A.D., Vivas E.I., Shui J.W., Chen P., Hacker T.A., Rey F.E., Kamp T.J., Hsieh P.C.H. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139(5):647–659. doi: 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 70.https://www.ncbi.nlm.nih.gov/pubmed/29805804/
- 71.Stilling R.M., van de Wouw M., Clarke G., Stanton C., Dinan T.G., Cryan J.F. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Volmar C.H., Wahlestedt C. Histone Deacetylases (HDACs) and Brain Function. Neuroepigenetics. 2015:20–27. [Google Scholar]
- 73.Lu J., Frerich J.M., Turtzo L.C., Li S., Chiang J., Yang C., Wang X., Zhang C., Wu C., Sun Z., Niu G., Zhuang Z., Brady R.O., Chen X. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc. Natl. Acad. Sci. USA. 2013;110(26):10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H., Sun J., Du J., Wang F., Fang R., Yu C., Xiong J., Chen W., Lu Z., Liu J. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol. Motil. 2018;30(5):e13260. doi: 10.1111/nmo.13260. [DOI] [PubMed] [Google Scholar]
- 75.D’Mello C., Ronaghan N., Zaheer R., Dicay M., Le T., MacNaughton W.K., Surrette M.G., Swain M.G. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 2015;35(30):10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Möhle L., Mattei D., Heimesaat M.M., Bereswill S., Fischer A., Alutis M., French T., Hambardzumyan D., Matzinger P., Dunay I.R., Wolf S.A. Ly6C(hi) Monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15(9):1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 77.Tan M., Zhu J.C., Du J., Zhang L.M., Yin H.H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit. Care. 2011;15(6):R290. doi: 10.1186/cc10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavelescu D., Mirea L., Grintescu I. Could selected probiotics have beneficial effects on clinical outcome of severe traumatic brain injury patients? Crit. Care. 2014;18(Suppl. 1):472. doi: 10.1186/cc13662. [DOI] [Google Scholar]
- 79.Wang J., Liu K.X., Ariani F., Tao L.L., Zhang J., Qu J-M. Probiotics for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of high-quality randomized controlled trials. PLoS One. 2013;8(12):e83934. doi: 10.1371/journal.pone.0083934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenner L.A., Stearns-Yoder K.A., Hoffberg A.S., Penzenik M.E., Starosta A.J., Hernández T.D., Hadidi D.A., Lowry C.A. Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav. Immun. 2017;65:57–67. doi: 10.1016/j.bbi.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt E.K.A., Torres-Espin A., Raposo P.J.F., Madsen K.L., Kigerl K.A., Popovich P.G., Fenrich K.K., Fouad K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One. 2020;15(1):e0226128. doi: 10.1371/journal.pone.0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammond F.M., Grattan K.D., Sasser H., Corrigan J.D., Rosenthal M., Bushnik T., Shull W. Five years after traumatic brain injury: a study of individual outcomes and predictors of change in function. NeuroRehabilitation. 2004;19(1):25–35. doi: 10.3233/NRE-2004-19104. [DOI] [PubMed] [Google Scholar]
- 83.Washington P. M., Villapol S., Burns M. P. Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? 2016. [DOI] [PMC free article] [PubMed]
- 84.Lynch S.V., Ng S.C., Shanahan F., Tilg H. Translating the gut microbiome: ready for the clinic? Nat. Rev. Gastroenterol. Hepatol. 2019;16(11):656–661. doi: 10.1038/s41575-019-0204-0. [DOI] [PubMed] [Google Scholar]
- 85.Marín C., Yubero-Serrano E.M., López-Miranda J., Pérez-Jiménez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. Int. J. Mol. Sci. 2013;14(5):8869–8889. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yen T-L., Hsu C-K., Lu W-J., Hsieh C-Y., Hsiao G., Chou D-S., Wu G-J., Sheu J-R. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J. Agric. Food Chem. 2012;60(8):1937–1944. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 87.Wang Q., Liu Y., Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho J.M., Yun S.M., Choi Y.H., Heo J., Kim N.J., Kim S.H., Kim E.H. Xanthohumol prevents dextran sulfate sodium-induced colitis via inhibition of IKKβ/NF-κB signaling in mice. Oncotarget. 2017;9(1):866–880. doi: 10.18632/oncotarget.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rancán L., Paredes S.D., García I., Muñoz P., García C., López de Hontanar G., de la Fuente M., Vara E., Tresguerres J.A.F. Protective effect of xanthohumol against age-related brain damage. J. Nutr. Biochem. 2017;49:133–140. doi: 10.1016/j.jnutbio.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Dorn C., Kraus B., Motyl M., Weiss T.S., Gehrig M., Schölmerich J., Heilmann J., Hellerbrand C. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol. Nutr. Food Res. 2010;54(S2) Suppl. 2:S205–S213. doi: 10.1002/mnfr.200900314. [DOI] [PubMed] [Google Scholar]
- 91.Scagliarini A., Mathey A., Aires V., Delmas D. Xanthohumol, a prenylated flavonoid from hops, induces dna damages in colorectal cancer cells and sensitizes SW480 Cells to the SN38 Chemotherapeutic Agent. Cells. 2020;9(4):E932. doi: 10.3390/cells9040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Bobe G., Revel J.S., Rodrigues R.R., Sharpton T.J., Fantacone M.L., Raslan K., Miranda C.L., Lowry M.B., Blakemore P.R., Morgun A., Shulzhenko N., Maier C.S., Stevens J.F., Gombart A.F. Improvements in metabolic syndrome by xanthohumol derivatives are linked to altered gut microbiota and bile acid metabolism. Mol. Nutr. Food Res. 2020;64(1):e1900789. doi: 10.1002/mnfr.201900789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo L.T., Wang S.Q., Su J., Xu L.X., Ji Z.Y., Zhang R.Y., Zhao Q.W., Ma Z.Q., Deng X.Y., Ma S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation. 2019;16(1):95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fang J., Wang H., Zhou J., Dai W., Zhu Y., Zhou Y., Wang X., Zhou M. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des. Devel. Ther. 2018;12:2497–2508. doi: 10.2147/DDDT.S163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu S., Zhuang F., Guo L., Qiu Y., Xiong J., Ye C., Liu Y., Wu Z., Hou Y., Hu C.A.A. Effect of baicalin-aluminum complexes on fecal microbiome in piglets. Int. J. Mol. Sci. 2019;20(10):E2390. doi: 10.3390/ijms20102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang M.J., Ko G.S., Oh D.G., Kim J.S., Noh K., Kang W., Yoon W.K., Kim H.C., Jeong H.G., Jeong T.C. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch. Pharm. Res. 2014;37(3):371–378. doi: 10.1007/s12272-013-0179-2. [DOI] [PubMed] [Google Scholar]
- 97.Taheri A., Emami M., Asadipour E., Kasirzadeh S., Rouini M.R., Najafi A., Heshmat R., Abdollahi M., Mojtahedzadeh M. A randomized controlled trial on the efficacy, safety, and pharmacokinetics of metformin in severe traumatic brain injury. J. Neurol. 2019;266(8):1988–1997. doi: 10.1007/s00415-019-09366-1. [DOI] [PubMed] [Google Scholar]
- 98.Tao L., Li D., Liu H., Jiang F., Xu Y., Cao Y., Gao R., Chen G. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res. Bull. 2018;140:154–161. doi: 10.1016/j.brainresbull.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 99.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L.M., Serino M., Planas-Fèlix M., Xifra G., Mercader J.M., Torrents D., Burcelin R., Ricart W., Perkins R., Fernàndez-Real J.M., Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X., Zhang X., Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015;5(1):14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H., Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014;80(19):5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de la Cuesta-Zuluaga J., Mueller N.T., Corrales-Agudelo V., Velásquez-Mejía E.P., Carmona J.A., Abad J.M., Escobar J.S. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 103.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 104.Xu Y., Wang N., Tan H.Y., Li S., Zhang C., Feng Y. Function of akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front. Microbiol. 2020;11:219. doi: 10.3389/fmicb.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun L., Xie C., Wang G., Wu Y., Wu Q., Wang X., Liu J., Deng Y., Xia J., Chen B., Zhang S., Yun C., Lian G., Zhang X., Zhang H., Bisson W.H., Shi J., Gao X., Ge P., Liu C., Krausz K.W., Nichols R.G., Cai J., Rimal B., Patterson A.D., Wang X., Gonzalez F.J., Jiang C. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018;24(12):1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J., Jiang C., Zhang K., Lan X., Chen X., Zang W., Wang Z., Guan F., Zhu C., Yang X., Lu H., Wang J. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic. Biol. Med. 2019;131:345–355. doi: 10.1016/j.freeradbiomed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 107.Ding K., Xu J., Wang H., Zhang L., Wu Y., Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem. Int. 2015;91:46–54. doi: 10.1016/j.neuint.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 108.Alluri H., Wilson R.L., Anasooya Shaji C., Wiggins-Dohlvik K., Patel S., Liu Y., Peng X., Beeram M.R., Davis M.L., Huang J.H., Tharakan B. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS One. 2016;11(5):e0154427. doi: 10.1371/journal.pone.0154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seifman M.A., Adamides A.A., Nguyen P.N., Vallance S.A., Cooper D.J., Kossmann T., Rosenfeld J.V., Morganti-Kossmann M.C. Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J. Cereb. Blood Flow Metab. 2008;28(4):684–696. doi: 10.1038/sj.jcbfm.9600603. [DOI] [PubMed] [Google Scholar]
- 110.Jing Y., Yang D., Bai F., Zhang C., Qin C., Li D., Wang L., Yang M., Chen Z., Li J. Melatonin Treatment Alleviates Spinal Cord Injury-Induced Gut Dysbiosis in Mice. J. Neurotrauma. 2019;36(18):2646–2664. doi: 10.1089/neu.2018.6012. [DOI] [PubMed] [Google Scholar]
- 111.Kim S.W., Kim S., Son M., Cheon J.H., Park Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020;10(1):2232. doi: 10.1038/s41598-020-59314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao T., Wang Z., Dong Y., Cao J., Lin R., Wang X., Yu Z., Chen Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019;67(1):e12574. doi: 10.1111/jpi.12574. [DOI] [PubMed] [Google Scholar]
- 113.Effect of Melatonin on Renewal of Chicken Small Intestinal Mucosa https://pubmed.ncbi.nlm.nih.gov/28431176/ [DOI] [PubMed]
- 114.Liang J., Wu S., Xie W., He H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des. Devel. Ther. 2018;12:845–853. doi: 10.2147/DDDT.S160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.The Effect of Ketamine on Immune Function and Prognosis in Patients Undergoing Colorectal Cancer Resection https://clinicaltrials.gov/ct2/show/NCT03273231
- 116.Getachew B., Aubee J.I., Schottenfeld R.S., Csoka A.B., Thompson K.M., Tizabi Y. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018;18(1):222. doi: 10.1186/s12866-018-1373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rossi G., Pengo G., Caldin M., Palumbo Piccionello A., Steiner J.M., Cohen N.D., Jergens A.E., Suchodolski J.S. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One. 2014;9(4):e94699. doi: 10.1371/journal.pone.0094699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim S.W., Shiue Y.L., Liao J.C., Wee H.Y., Wang C.C., Chio C.C., Chang C.H., Hu C.Y., Kuo J.R. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behavior in rats by reducing neuroinflammation in the hippocampus. Neurocrit. Care. 2017;26(1):122–132. doi: 10.1007/s12028-016-0290-6. [DOI] [PubMed] [Google Scholar]
- 119.Mountney A., Boutté A.M., Gilsdorf J., Lu X.C., Tortella F.C., Shear D.A. Intravenous administration of simvastatin improves cognitive outcome following severe traumatic brain injury in rats. J. Neurotrauma. 2016;33(16):1492–1500. doi: 10.1089/neu.2015.4139. [DOI] [PubMed] [Google Scholar]
- 120.Xu X., Gao W., Cheng S., Yin D., Li F., Wu Y., Sun D., Zhou S., Wang D., Zhang Y., Jiang R., Zhang J. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J. Neuroinflammation. 2017;14(1):167. doi: 10.1186/s12974-017-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao C., Hu Y., Chen H., Li B., Cao L., Xia J., Yin Y. An in vitro evaluation of the effects of different statins on the structure and function of human gut bacterial community. PLoS One. 2020;15(3):e0230200. doi: 10.1371/journal.pone.0230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kummen M., Solberg O.G., Storm-Larsen C., Holm K., Ragnarsson A., Trøseid M., Vestad B., Skårdal R., Yndestad A., Ueland T., Svardal A., Berge R.K., Seljeflot I., Gullestad L., Karlsen T.H., Aaberge L., Aukrust P., Hov J.R. Rosuvastatin alters the genetic composition of the human gut microbiome. Sci. Rep. 2020;10(1):5397. doi: 10.1038/s41598-020-62261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J., Lee H., An J., Song Y., Lee C.K., Kim K., Kong H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front. Microbiol. 2019;10:1947. doi: 10.3389/fmicb.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pattappa G., Heywood H.K., de Bruijn J.D., Lee D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011;226(10):2562–2570. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 125.Xiao E., He L., Wu Q., Li J., He Y., Zhao L., Chen S., An J., Liu Y., Chen C., Zhang Y. Microbiota regulates bone marrow mesenchymal stem cell lineage differentiation and immunomodulation. Stem Cell Res. Ther. 2017;8(1):213. doi: 10.1186/s13287-017-0670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kol A., Foutouhi S., Walker N.J., Kong N.T., Weimer B.C., Borjesson D.L. Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions. Stem Cells Dev. 2014;23(16):1831–1843. doi: 10.1089/scd.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagashima K., Sawa S., Nitta T., Tsutsumi M., Okamura T., Penninger J.M., Nakashima T., Takayanagi H. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 2017;18(6):675–682. doi: 10.1038/ni.3732. [DOI] [PubMed] [Google Scholar]
- 128.Ocansey D.K.W., Wang L., Wang J., Yan Y., Qian H., Zhang X., Xu W., Mao F. Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: an enhanced therapeutic effect. Clin. Transl. Med. 2019;8(1):31. doi: 10.1186/s40169-019-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nigro G., Rossi R., Commere P.H., Jay P., Sansonetti P.J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15(6):792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 130.Soontararak S., Chow L., Johnson V., Coy J., Wheat W., Regan D., Dow S. Mesenchymal Stem Cells (MSC) Derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl. Med. 2018;7(6):456–467. doi: 10.1002/sctm.17-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bonsack B., Corey S., Shear A., Heyck M., Cozene B., Sadanandan N., Zhang H., Gonzales-Portillo B., Sheyner M., Borlongan C.V. Mesenchymal stem cell therapy alleviates the neuroinflammation associated with acquired brain injury. CNS Neurosci. Ther. 2020;26(6):603–615. doi: 10.1111/cns.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen J., Li Y., Katakowski M., Chen X., Wang L., Lu D., Lu M., Gautam S.C., Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 133.Cui X., Chen J., Zacharek A., Li Y., Roberts C., Kapke A., Savant-Bhonsale S., Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25(11):2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao Q., Li Y., Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136(1):123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 135.Qu R., Li Y., Gao Q., Shen L., Zhang J., Liu Z., Chen X., Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27(4):355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walker P.A., Shah S.K., Harting M.T., Cox C.S. Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis. Model. Mech. 2009;2(1-2):23–28. doi: 10.1242/dmm.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang P., Freeman W.D., Edenfield B.H., Brott T.G., Meschia J.F., Zubair A.C. Safety and Efficacy of Intraventricular Delivery of Bone Marrow-Derived Mesenchymal Stem Cells in Hemorrhagic Stroke Model. Sci. Rep. 2019;9(1):5674–5674. doi: 10.1038/s41598-019-42182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cox C.S., Jr, Hetz R.A., Liao G.P., Aertker B.M., Ewing-Cobbs L., Juranek J., Savitz S.I., Jackson M.L., Romanowska-Pawliczek A.M., Triolo F., Dash P.K., Pedroza C., Lee D.A., Worth L., Aisiku I.P., Choi H.A., Holcomb J.B., Kitagawa R.S. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079. doi: 10.1002/stem.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sikiric P., Seiwerth S., Brcic L., Blagaic A.B., Zoricic I., Sever M., Klicek R., Radic B., Keller N., Sipos K., Jakir A., Udovicic M., Tonkic A., Kokic N., Turkovic B., Mise S., Anic T. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006;14(5-6):214–221. doi: 10.1007/s10787-006-1531-7. [DOI] [PubMed] [Google Scholar]
- 140.Sikiric P. The pharmacological properties of the novel peptide BPC 157 (PL-10). Inflammopharmacology. 1999;7(1):1–14. doi: 10.1007/s10787-999-0022-z. [DOI] [PubMed] [Google Scholar]
- 141.Sikiric P., Seiwerth S., Rucmsn R., Kolenc D., Vuletic B.L., Drmic D., Grgic T., Strbe S., Zukanovic G., Crvenkovic D., Madzarac G., Rukavina I., Sucic M., Baric M., Starcevic N., Krstonijevic Z., Bencic M.L., Filipcic I., Rokotov D.S., Vlainic J. Brain-gut axis and pentadecapeptide bpc 157: theoretical and practical implications. Curr. Neuropharmacol. 2016;14:857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sikiric P., Seiwerth S., Rucman R., Turkovic B., Rokotov D.S., Brcic L., Sever M., Klicek R., Radic B., Drmic D., Ilic S., Kolenc D., Vrcic H., Sebecic B. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr. Pharm. Des. 2011;17(16):1612–1632. doi: 10.2174/138161211796196954. [DOI] [PubMed] [Google Scholar]
- 143.Sikirić P., Seiwerth S., Grabarević Z., Rucman R., Petek M., Jagić V., Turković B., Rotkvić I., Mise S., Zoricić I., Gjurasin M., Konjevoda P., Separović J., Ljubanović D., Artuković B., Bratulić M., Tisljar M., Jurina L., Buljat G., Miklić P., Marović A. Beneficial effect of a novel pentadecapeptide BPC 157 on gastric lesions induced by restraint stress, ethanol, indomethacin, and capsaicin neurotoxicity. Dig. Dis. Sci. 1996;41(8):1604–1614. doi: 10.1007/BF02087908. [DOI] [PubMed] [Google Scholar]
- 144.Tudor M., Jandric I., Marovic A., Gjurasin M., Perovic D., Radic B., Blagaic A.B., Kolenc D., Brcic L., Zarkovic K., Seiwerth S., Sikiric P. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul. Pept. 2010;160(1-3):26–32. doi: 10.1016/j.regpep.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 145.Klicek R., Kolenc D., Suran J., Drmic D., Brcic L., Aralica G., Sever M., Holjevac J., Radic B., Turudic T., Kokot A., Patrlj L., Rucman R., Seiwerth S., Sikiric P. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013;64(5):597–612. [PubMed] [Google Scholar]
- 146.Perovic D., Kolenc D., Bilic V., Somun N., Drmic D., Elabjer E., Buljat G., Seiwerth S., Sikiric P. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J. Orthop. Surg. Res. 2019;14(1):199. doi: 10.1186/s13018-019-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vukojević J., Vrdoljak B., Malekinušić D., Siroglavić M., Milavić M., Kolenc D., Boban Blagaić A., Batelja L., Drmić D., Seiverth S., Sikirić P. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020;10(8):e01726. doi: 10.1002/brb3.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boban Blagaic A., Blagaic V., Mirt M., Jelovac N., Dodig G., Rucman R., Petek M., Turkovic B., Anic T., Dubovecak M., Staresinic M., Seiwerth S., Sikiric P. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur. J. Pharmacol. 2005;512(2-3):173–179. doi: 10.1016/j.ejphar.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 149.Sikiric P., Jelovac N., Jelovac-Gjeldum A., Dodig G., Staresinic M., Anic T., Zoricic I., Ferovic D., Aralica G., Buljat G., Prkacin I., Lovric-Bencic M., Separovic J., Seiwerth S., Rucman R., Petek M., Turkovic B., Ziger T. Anxiolytic effect of BPC-157, a gastric pentadecapeptide: shock probe/burying test and light/dark test. Acta Pharmacol. Sin. 2001;22(3):225–230. [PubMed] [Google Scholar]
- 150.Sikiric P., Marovic A., Matoz W., Anic T., Buljat G., Mikus D., Stancic-Rokotov D., Separovic J., Seiwerth S., Grabarevic Z., Rucman R., Petek M., Ziger T., Sebecic B., Zoricic I., Turkovic B., Aralica G., Perovic D., Duplancic B., Lovric-Bencic M., Rotkvic I., Mise S., Jagic V., Hahn V. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J. Physiol. Paris. 1999;93(6):505–512. doi: 10.1016/S0928-4257(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 151.Krzyżek P. Challenges and limitations of anti-quorum sensing therapies. Front. Microbiol. 2019;10:2473. doi: 10.3389/fmicb.2019.02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pejin B., Ciric A., Glamoclija J., Nikolic M., Sokovic M. In vitro anti-quorum sensing activity of phytol. Nat. Prod. Res. 2015;29(4):374–377. doi: 10.1080/14786419.2014.945088. [DOI] [PubMed] [Google Scholar]
- 154.Pejin B., Iodice C., Tommonaro G., Stanimirovic B., Ciric A., Glamoclija J., Nikolic M., De Rosa S., Sokovic M. Further in vitro evaluation of antimicrobial activity of the marine sesquiterpene hydroquinone avarol. Curr. Pharm. Biotechnol. 2014;15(6):583–588. doi: 10.2174/138920101506140910152253. [DOI] [PubMed] [Google Scholar]
- 155.Pejin B., Ciric A., Markovic J.D., Glamoclija J., Nikolic M., Stanimirovic B., Sokovic M. Quercetin potently reduces biofilm formation of the strain Pseudomonas aeruginosa PAO1 in vitro. Curr. Pharm. Biotechnol. 2015;16(8):733–737. doi: 10.2174/1389201016666150505121951. [DOI] [PubMed] [Google Scholar]
- 156.Li X., Wang H., Wen G., Li L., Gao Y., Zhuang Z., Zhou M., Mao L., Fan Y. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1α pathway as a potential mechanism. J. Cell. Mol. Med. 2018;22(2):883–891. doi: 10.1111/jcmm.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu P., Zou D., Yi L., Chen M., Gao Y., Zhou R., Zhang Q., Zhou Y., Zhu J., Chen K., Mi M. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor. Neurol. Neurosci. 2015;33(2):143–157. doi: 10.3233/RNN-140446. [DOI] [PubMed] [Google Scholar]
- 158.Du G., Zhao Z., Chen Y., Li Z., Tian Y., Liu Z., Liu B., Song J. Quercetin protects rat cortical neurons against traumatic brain injury. Mol. Med. Rep. 2018;17(6):7859–7865. doi: 10.3892/mmr.2018.8801. [DOI] [PubMed] [Google Scholar]
- 159.Li X., Wang H., Gao Y., Li L., Tang C., Wen G., Zhou Y., Zhou M., Mao L., Fan Y. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway. PLoS One. 2016;11(10):e0164237. doi: 10.1371/journal.pone.0164237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu D.N., Guan L., Jiang Y.X., Ma S.H., Sun Y.N., Lei H.T., Yang W.F., Wang Q.F. Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc. Diagn. Ther. 2019;9(6):545–560. doi: 10.21037/cdt.2019.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]