Abstract
Background
Nonylphenol (NP), a chemical compound widely used in industry, is the result of the nonylphenol ethoxylate decomposition and it is known as an estrogen-like compound. Numerous studies and researches have shown that it has many destructive functions of various organs such as the brain. This toxicant causes oxidative stress in the cortex and hippocampus cells, which are two essential regions to preserve memory and learning in the brain.
Methods
This review examines recent findings to better understanding the mechanisms of NP neurotoxicity. We used Scopus, Google Scholar, and PubMed databases to find articles focused on the destructive effects of NP on the oxidative stress pathway and its defense mechanisms.
Results
NP has potential human health hazards associated with gestational, peri- and postnatal exposure. NP can disrupt brain homeostasis in different ways, such as activation of inflammatory factors in brain especially in hippocampus and cortex, disruption of the cell cycle, changes in neuron, dendrites and synapses morphology, disruption of extra and intracellular calcium ion balance and also memory and learning disorders
Keywords: Nonylphenol, neurotoxicity, hippocampus, oxidative stress, neuro inflammation, apoptosis
1. INTRODUCTION
In today's industrialized world, there are growing concerns about the damages caused by EDCs to the human body, animals, and microorganisms [1, 2]. EDCs are estrogen recipient agonists that interfere with metabolism, synthesis, storage, the release of hormones, and alter their normal function [3]. Alkylphenol ethoxylates (APEOs) are a class of non-ionic surfactants that are potentially a dangerous class of EDCs. These compounds are widely used in the production of various detergents, cleaners, and emulsifiers [4]. NP is the final product of the APEOs decomposition in an environment that makes up about 80% of the APEOs [4, 5]. This chemical compound is stable in the environment and is present in domestic and industrial wastewater. NP is commonly used in the manufacture of household detergents such as pesticides, cosmetics, additives, plastics, polyvinyl chloride pipes, food processing industries, packaging, paint, and other agricultural products are used [6, 7]. NP is also widely used in a variety of fields, including rubber antioxidants, gasoline additives, and phenolic resin modifiers [8]. The presence of NP residues is often identified in various environmental sectors such as air, soil, sediments, and water [9]. Specifically, in the river and lake water of China, 28.6μg/L concentration of NP was identified, and a much higher level of NP with a concentration of 644μg/L was detected in Spanish surface water [10]. Humans are in extensive contact with the NP by espousing contaminated water and food [11]. Recent reports indicate that 100% of non-employed women in southern Spain were exposed to NP [12]. In addition, the highest level of NP in the breast milk of Italian women was 32 ng / ml [13]. The average estimated NP per capita food intake was 27 micrograms per day (average) in the general Swedish population [14].
Studies have shown that NP can pass NP by espousing contaminated water through the placenta [15] and BBB [16]. After ingestion, NP is circulated throughout the CNS by the bloodstream and finally penetrates the BBB [16]. Due to its lipophilic properties, it can accumulate in various tissues rich in fat, such as the brain [17]. It is also found in many biological fluids in the body, including human milk and urine [18-20]. This toxicant has different toxic effects on various organs such as liver [21, 22] and reproductive system [23, 24]. Numerous studies have shown that exposure to certain chemicals, such as minerals, solvents, and some compounds in the POP category, can disrupt certain regions of the brain and impair memory and learning. These facts provided the basis for further studies on neurotoxicity and changes in brain homeostasis [25, 26]. NP, a toxicant that is resistant to decomposition, can affect brain functions in different ways [27]. The effects of NP, like other EDCs on the neuroendocrine system, have been demonstrated by researchers [28]. Studies report that exposure to NP can cause neurotoxicity, behavioral changes, and adverse effects on memory and learning [29-31]. According to a report, mother rats were exposed to chronic NP orally, which caused cognitive impairment in young mice [32]. According to Yokosuka et al., NP causes a change in the dendrites and synaptic growth of the mouse fetal hypothalamus [31]. Zhen Mao et al. also found that NP increased CAS-3 expression in the hippocampus and cortex cells and induced apoptosis in these cells [33]. It can interfere with the metabolism and development of neurocytes and as well as synthesizing and releasing neurotransmitters [27]. Also, NP activates inflammatory cell signaling, especially in the hippocampus and cortex, such as increasing the secretion of inflammatory cytokines [34]. Based on the results of previous studies about NP effect on neurotoxicity, this review aimed to summarize the mechanisms involved in NP-induced neural toxicity.
2. EFFECTS OF NP ON DIFFERENT NEURAL CELLS
Glial cells are a group of non-nerve cells include MG, astrocytes, and oligodendrocyte stem cells that make up the bulk of mammalian brain tissue. In a simple word, MGs are immune and phagocytic cells of the nervous system that are mostly distributed in the hippocampus [35]. These cells perform essential functions such as neuronal metabolism, signal conduction, neurogenesis, and synaptic flexibility in the brain. All types of glial cells are necessary for maintaining in the CNS homeostasis. MGs are the basic source of inflammatory cytokines in CNS [36].
CNS changes, such as infection or nerve damage, trigger MG and astrocytes [35]. Depending on the injury, the first response of these cells may be the production and release of inflammatory factors or cytokines [37]. Severe and long-term damage to the nervous system can lead to the release of inflammatory cytokines by astrocytes and MG, which ultimately leads to inflammation. In addition, abnormal activation of MG by excessive secretion of cytokines may cause severe CNS damage [38]. Complex signaling cascades play a role in activating MG. TNF, which is released from active MG and astrocytes, can exacerbate inflammation and release ROS from the microglia, thereby destroying the nerve [37].
Various studies have shown that exposure to different doses of NP can affect the number and function of glial cells [39]. NP can induce some inflammatory factors in glial cells, subsequent activation of these factors and provide the basis for inflammation in the CNS. According to Weijia GU et al., Maternal exposure to NP induces MG activation in the offspring hippocampus. NP exposure in a dosage of 10 and 50mg/kg increased the production of IL-1β, IL-6, and TNF-α in the offspring hippocampus [40]. Previous studies have provided evidence that the MAPK signaling can begin by phosphorylated Akt and can balance inflammatory responses in MG. The Akt/MAPK/AP-1 signaling may be involved in the activation of MG and their production of pro-inflammatory cytokines. Activating the Akt / MAPK / AP-1 signaling by NP, can increase the secretion of inflammatory cytokines at the MG level and can initiate the CNS inflammation [39]. Neuroinflammation, caused by excessive MG activity is one of the most common causes of neurodegenerative diseases such as Alzheimer's, Parkinson's, and Huntington's disease [41, 42].
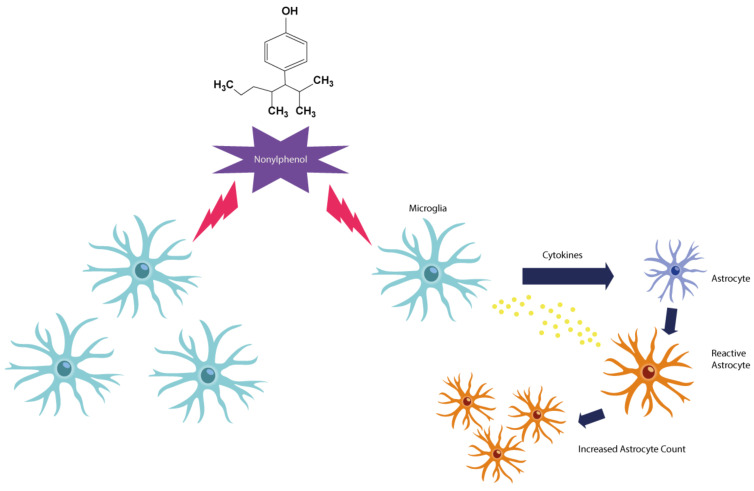
Based on Zhenmin Qiu et al. both in vitro and in vivo findings, prenatal contact with NP activates the MG and induces NO production in F1. In this study, pregnant rats were randomly assigned to each group. Gavage of 10, 50, and 100 mg/kg doses were administered daily for 21 days [43]. In general, increased MG activity induces NO production in MG. During a process of neural inflammation, excess NO and PGE2 are mainly produced by activated MG [44]. NO is mainly produced by iNOS in MG. NO is an essential biological messenger, but if overproduced in the CNS, it can cause inflammation and death of nerve cells in the brain. Other studies also show that NP significantly increases NO levels in the rat brain. Appropriate amounts of NO can dilate blood vessels, inhibit apoptosis, and play an essential role in memory processes, but can be harmful if too much NO is produced (Fig. 1).
Fig. (1).
Exposure to NP can increase the number of MG and astrocytes, as well as release inflammatory cytokines, and induce NO production in MG. When there is damage to the brain, astrocytes Are subjected to a series of morphological changes and the term astrogliosis is attributed to it. Activation of the inflammatory state of astrocytes is associated with the regulation of GFAP. In addition to inflammation, astrogliosis is associated with an increase in the number of astrocytes, which can lead to brain damage and neurodegenerative diseases following inflammation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In general, in conditions of oxidative stress, NO is one of the reactive species of nitrogen. It can easily change to anion proxy nitrite and cause cytotoxicity [45].
One of the most common glia in the adult brain is astrocyte. Astrocytes play key and regulatory roles in brain function, including neurogenesis, synaptogenesis, controlling the permeability of the BBB, and maintaining extracellular homeostasis. Activation of the inflammatory state of astrocytes is usually associated with GFAP that is a protein that is expressed by a variety of nerve cells, especially astrocytes during development and evolution. GFAP plays an essential role in synaptic function, and changes in GFAP levels can reflect impaired synaptogenesis as well as pathological regulation of neural function [38]. According to studies, Nonylphenol can increase the production of GFAP. In a study by Jie Y, et al., rats were gavaged with NP since gestational day 6 to postnatal day (PND) 21 at dose levels of 25 mg/kg/day as a low dose, 50 mg/kg/day as a middle dose and 100 mg/kg/day as a high dose. Results showed a positive correlation between the number of GFAP positive astrocytes and NP levels as an increased GFAP protein expression with a high dose of NP exposure [46]. Astrocytes can exacerbate the disease through Ca2+ signals and induction of inflammation progression. Therefore, any dysfunction of these cells can interfere with cerebral hemostasis. Astrocytes have different functions such as Protection against glutamate toxicity by absorbing extracellular glutamate. When the normal function of the astrocyte is disrupted by NP, the concentration of extracellular glutamate increases, causing calcium ion influx and increasing the permeability of the mitochondrial membrane. As a result, it damages the mitochondria and induces apoptosis [47]. It has been investigated that NP exposure during pregnancy could interfere with the links that are necessary for neuronal growth and development through inhibiting GAP-43 (a nervous tissue-specific cytoplasmic protein) expression [48]. GAP-43 is associated with learning and memory functions [47]. It is also involved in signal transmission, synaptic flexibility, neural growth, neurotransmitter emission as well as the release of monoamine transmitters [46]. Thus NP exposure can lead to learning and memory impairment in male rats.
According to research on the toxic effects of NP on the nervous system, it can affect different parts of the cell and disrupt various cellular signals. The exact mechanism of toxicity by this toxicant is yet to be known. In this regard, we have summarized and compiled to provide an introduction of new information about NP cell toxicity.
3. OXIDATIVE STRESS AND INFLAMMATION
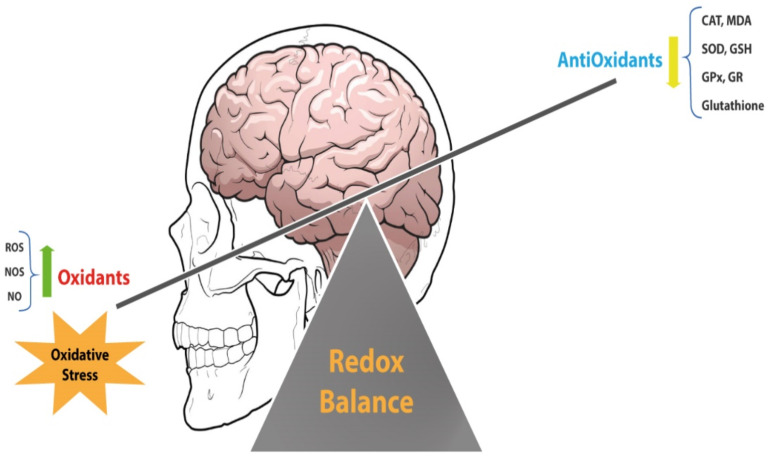
Oxidative stress is an occurrence caused by an imbalance between products such as ROS and RNS at the cellular and tissue levels and the biological efficiency of the body's antioxidant systems such as antioxidant enzymes to reduce and eliminate the factors that cause oxidative stress [48]. Atomic free radicals or molecules are highly reactive that can react with other important cellular structures [49]. Free radicals can damage parts of cells, including proteins, DNA, and cell membranes, by stealing their electrons through a process called oxidation [32, 50]. NP causes ROS accumulation in the brain and may cause lipid peroxidation. The brain is sensitive to lipid peroxidation why is enriched in polyunsaturated fatty acids (PUFAs). By measuring the level of MDA enzyme as a marker of lipid peroxidation and oxidative stress, the rate of lipid peroxidation of the brain can be determined. The accumulation of ROS, and the resulting cellular redox, can be one of the triggers for the apoptosis process [32].
Production of ROS at low and appropriate levels may be necessary because it initiates some processes such as protein phosphorylation, activation of several transcriptional factors, apoptosis, immunity, and differentiation. When ROS production increases, it can damage important cellular structures, such as lipids, nucleic acids, and proteins. Various evidence suggests that excessive ROS can lead to the onset and progression of diseases such as metabolic disorders, diabetes, cancer, cardiovascular diseases, and atherosclerosis [51]. There are two types of protective mechanisms to counteract the threat posed by ROS in the brain, which acts as the antioxidant enzyme system and the low-molecular-weight antioxidants. The antioxidant enzyme system includes SOD, glyoxalase, GR, GPx, and CAT [50, 52]. Low molecular weight antioxidants Consist of melatonin, ascorbic acid, glutathione, and uric acid [53]. Cells increase their antioxidant defenses in response to nitrosative and oxidative stress by activation of Nrf2, an important transcription factor. Some biological and industrial toxins can cause oxidative stress in various cells and tissues of the body. According to a study of NP neurotoxicity, behavioral impairments, including movement and cognitive function, are caused by NP with the loss of antioxidant enzymes [29]. Oxidative stress can be responsible for the toxicity of NP exposure. Based on recent findings, NP can cause cell damage by creating oxidative stress and increasing the ROS and RNS, as well as inhibiting the antioxidant defense mechanisms in cells [54]. ROS may react with biological molecules and directly damage proteins, amino acids, porphyrins, nucleic acids, and phenolic substances. According to a study by Tabassum et al., Nonylphenol can increase oxygen free radicals by reducing the concentration of glutathione, resulting in lipid peroxidation in the brain. Gavage doses of 20, 50, 100 mg/kg of NP in the same study showed a significant reduction in SOD, CAT, and GPx, GR enzymes (Fig. 2) [52]. NP caused excessive ROS in the head of nematodes and by overcoming the antioxidant system [9]. Mufide et al. examined the effect of ascorbic acid on the neurotoxicity of BPA, NP, and actylphenol. They found that in the NP group, there was a significant decrease in the levels of MDA and GSH enzymes [55]. In addition to oxidative stress, NP can cause inflammation in the brain by stimulating inflammatory factors. Proinflammatory cytokine production is increased under some pathological conditions and leads to CNS damage According to Weijia GU et al., finding NP exposure during lactation and pregnancy on offspring caused MG activation that produced excessive pro-inflammatory cytokines in offspring. NP can significantly increase TNF -α levels at doses of 10 and 50 mg/kg [56].
Fig. (2).
The brain, as an organ that contains a lot of unsaturated fats, is very sensitive to oxidative stress. According to various findings, NPs can reduce the level of antioxidants in the cell and cause oxidative stress in the cell by reducing the antioxidant power of the organ. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. CELL CYCLE REGULATION
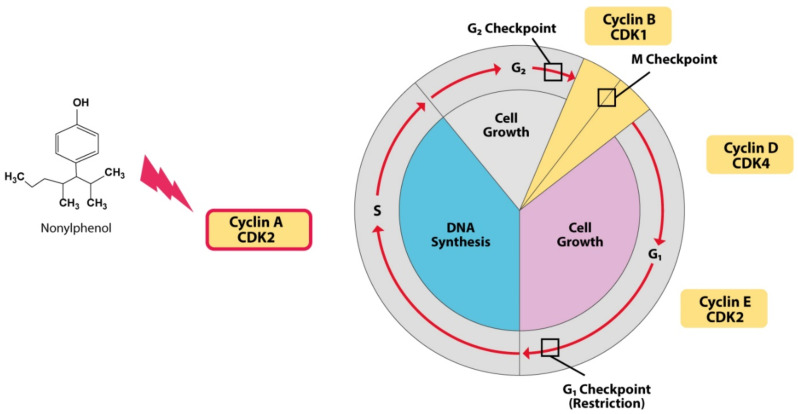
The cell cycle is an essential event in the cell that begins with cell growth and ends with cell division. The cell spends the most time in the interphase stage. During this time, with the growth and replication of its chromosomes, the cell prepares for cell division. After this stage, the cell can perform mitosis and complete its division [56]. The resulting cells (daughter cells), each enter their own time intervals and initiate a new cycle of the cell cycle. Cell division is a process that requires the precise regulation of the proteins responsible for regulating cell division. However, any inconsistency in this cycle can lead to damage and destruction of the organ or even cause diseases such as cancer. Chiho Kudo et al.; studied the destructive effects of five chemical compounds on neural stem cells (NSCs) from the EDCs category in a study. A study has shown that exposure to 4-nonylphenol leads to the accumulation of cells in the G2 / M phase decreases the regulation of protein cycling A and B1, which are the main control proteins in the G2 to M cell transfer cycle [57]. Understanding the exact effects of NP on the cell cycle and regulating it is not entirely clear and requires further study (Fig. 3).
Fig. (3).
NP stops the cell in the G2 / M phase by reducing the levels of Cyclin A and B1 proteins, which are the two major regulatory proteins in cell transport from phase G2 to M in the cell cycle. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
5. APOPTOSIS SIGNALING
Apoptosis is a highly regulated phenomenon that is triggered by the activation of a series of molecules and proteins, like cysteine proteases called caspases. This is needed for the normal performing and survival of multicellular organisms. It happens under physiological and pathological conditions [58] and Recognizing the underlying mechanism of apoptosis is necessary because it plays a key role in the pathogenesis of many diseases. Signaling pathways that begin apoptosis are divided into two main categories: intrinsic and non-intrinsic pathways. Intrinsic pathways are initiated by mitochondrial events and extrinsic pathways begin by death receptors such as those of TRAIL, TNF-α and FAS-L [59]. In general, in the apoptosis process, when Caspase 3 is activated, the signal is sent to the cell, and apoptosis is induced. This leads to morphological changes in the cell, containing chromatin condensation and DNA fragmentation [60]. According to the evidence, NP can play a role in inducing nerve cells apoptosis, so that NP increases CAS-3 expression in the hippocampus and cortex cells and induces apoptosis in these cells. Also NP at concentrations of 5 and 10 μM caused the loss of mitochondrial membrane potential, apoptotic nuclei fragmentation, and neuronal cell death [33].
NP treatment induced apoptosis in mouse hippocampal neuronal cells in early developmental stages. NP can activate the caspase cascade leading to apoptosis of NSCs [57]. Treatment with NP induced apoptosis in mouse hippocampal neuronal cells in early developmental stages. Based on the results of Chiho Kudo et al. findings the destructive effects of five chemical compounds on NSCs from the EDCs category, Chronic exposure of NP by oral gavage in a dosage of (100 and 200 mg/kg/day) activated abnormal apoptosis in the young mouse brain. In a study, the tendency of the increase in caspase activity was discovered on NSCs treated with 4-NP [57].
Similarly, two separate studies found that NP induces apoptosis in murine neural stem cells, neuronally differentiated PC12 cells, and the mouse brain due to the activation/up-regulation of caspases, the suppression of BCL-2 transcription, and an increased intracellular Ca2+ concentration [57, 61]. Apparently, another way to induce apoptosis by NP is to increase the expression of the JNK gene [62]. JNK belongs to the superfamily of MAP kinases and plays an important role in regulating cell proliferation, differentiation, and intrinsic mitochondrial apoptotic pathways. Members of the MAPK family play essential roles in inflammation. Many stimulating factors, such as growth factors, cytokines, stress factors, and genotoxic, and cytotoxic toxins, can trigger JNK activation. As explained above, the process of apoptosis begins in two ways, intrinsic and non-intrinsic. It seems that JNK has a central role in both pathways mentioned [63]. Based on the results of various studies, it can be concluded that NP activates the cell's inflammatory signaling, especially in the hippocampus and cortex, and increases the secretion of inflammatory cytokines, including IL-6, IL-1β, and TNF-α [41] (81). This means that NP can induce apoptosis not only by increasing the activity of inflammatory cytokines, including TNF-α, but also by stimulating JNK signaling. Bcl-2 family proteins play an important role in modulating and controlling the intrinsic apoptotic pathway, which blocks apoptotic demise by refraining the efflux of cyt-c from the mitochondria and the subsequent caspase cascade activation. Based on the study of Zhen Mao et al., daily oral gavage of mice with NP at dose levels of 50, 100, 200 mg/ (kg day) for 90 days significantly reduced the expression of Bcl2 gene mRNA expression in the hippocampus and cerebral cortex at doses of 100 and 200 mg/kg/day [33].
6. CALCIUM SIGNALING
Ca2+ is one of the main regulators of cell survival. It can also cause apoptosis in response to a variety of pathological conditions —the Precise regulation of intracellular Ca2+ homeostasis by anti- and pro-apoptotic proteins forms. The Ca2+ signal to which mitochondria and other cellular effects are exposed has various efficiencies in inducing cell death. There is a growing concern about different forms of cell death (necrosis, apoptosis, and autophagy) [64]. The molecular effects and signaling pathways of these three types are not independent of each other. In addition to being a secondary messenger, Ca2+ plays an essential role in controlling a variety of physiological events [65]. According to the findings of various articles on the effects of NP on calcium signaling, it can be concluded that this toxicant can, directly and indirectly, alter the function of intracellular and extracellular calcium ions. Based on Jue-Long et al. findings, which examined the effects of NP on bone cells, NP can induce the release of Ca2+ and can cause cell death by increasing Ca2+ in MG63 osteoporosis cells [66]. Because increasing in Ca2+ can interfere with many cellular processes, it should be used for other laboratory studies using low concentrations of NP. NP causes Lack of Ca2+ modulation and extracellular Ca2+ ion by inhibiting the Ca2+ ATPase as well as inhibiting the NAchR [67]. However, the understanding of the exact relationship between the toxic effects of NP on calcium signaling is not yet clear. A closer look at these relationships requires further investigation and testing in future studies.
CONCLUSION
The potential effects of EDCs—NP on CNS have created concern in recent years. The disruption of cognitive function and various behavioral problems in fish, reptiles, and birds due to NP exposure has been suspected because the development of the CNS is highly regulated by endogenous hormones (e.g., gonadal hormones) directly that occur early in development. Due to the structural similarity with endogenous hormones, NP has the potential to mimic, or in some cases, block the effects of the endogenous hormone and disrupt the normal function of the body. It is now clear that mitochondria play a Fundamental role in the apoptosis. Along with mitochondrial defects, some major and important participants in the apoptosis, consist of cytochrome C, pro-caspases, APAF-1 and AIF are secreted into the cytosol. Another factor that NP increases secretion is nitric oxide. In addition to being an active species of nitrogen and causing oxidative stress, nitric oxide can trigger the onset of the apoptosis process. This pathway can be one of apoptosis pathways induced by NP. As mentioned earlier in this study, NP can cause inflammation by stimulating the number and function of two important cell groups if activated in brain tissue. As mentioned earlier, NP can cause Lack of calcium modulation and extracellular calcium ion by inhibiting the Ca2+- ATPase as well as inhibiting the nicotinic acetylcholine receptor (NAchR). This mechanism also increases extracellular calcium ions. Calcium signaling is one of the triggers for the onset of apoptosis, so NP This mechanism also increases extracellular calcium ions. Calcium signaling is one of the triggers for the onset of apoptosis, so NP can induce apoptosis by stimulating multiple pathways. The fact that molecular pathways are completely interdependent is unavoidable. The pathways of toxicity can reinforce or weaken each other's effect. The pathways of NP toxicity are still unclear and the relationship between NP toxicity requires further study. In this review, we tried to gain a deeper understanding of the toxicity of NP. It is hoped that we have been able to provide a context for future studies.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AIF
Apoptosis-inducing factor
- APAF-1
Apoptotic protease-activating factor-1
- BBB
Blood-brain barrier
- BCL-2
B-cell lymphoma 2
- BPA
Bisphenol A
- CAS-3
Caspase 3
- CAT
Catalase
- CNS
Central nervous system
- EDCs
Endocrine disrupter chemical
- GFAP
Glial fibrillary acidic protein
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- iNOS
Nitric oxide synthase
- JNKs
c-Jun N-terminal Kinases
- MDA
Malondialdehyde
- MG
Microglia
- NAchR
Nicotinic acetylcholine receptor
- NO
Nitric oxide
- NPnEO
Nonyphenol ethoxylates
- Nrf2
Nuclear factor erythroid 2–related factor
- NSCs
Neural Stem Cells
- PGE2
Prostaglandin 2
- POP
Persistent organic pollutant
- PUFAs
Polyunsaturated fatty acids
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TNF
Tumor necrosis factor
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Aly H.A., Domènech O., Banjar Z.M. Effect of nonylphenol on male reproduction: Analysis of rat epididymal biochemical markers and antioxidant defense enzymes. Toxicol. Appl. Pharmacol. 2012;261(2):134–141. doi: 10.1016/j.taap.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Lagos-Cabré R., Moreno R.D. Contribution of environmental pollutants to male infertily: A working model of germ cell apoptosis induced by plasticizers. Biol. Res. 2012;45(1):5–14. doi: 10.4067/S0716-97602012000100001. [DOI] [PubMed] [Google Scholar]
- 3.Dobrzyńska M.M. DNA damage in organs of female and male mice exposed to nonylphenol, as a single agent or in combination with ionizing irradiation: A comet assay study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;772:14–19. doi: 10.1016/j.mrgentox.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y., Wu J., Huang Y., Shen S., Han X. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol. Lett. 2009;186(2):84–95. doi: 10.1016/j.toxlet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y., Han X.D. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprod. Toxicol. 2006;22(4):623–630. doi: 10.1016/j.reprotox.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X., Yang G., Toyooka T., Ibuki Y. New mechanism of γ-H2AX generation: Surfactant-induced actin disruption causes deoxyribonuclease I translocation to the nucleus and forms DNA double-strand breaks. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;794:1–7. doi: 10.1016/j.mrgentox.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Yao G., Hu Y., Liang J., Hou Y. Nonylphenol-induced thymocyte apoptosis is related to Fas/FasL pathway. Life Sci. 2005;77(26):3306–3320. doi: 10.1016/j.lfs.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Soares A., Guieysse B., Jefferson B., Cartmell E., Lester J.N. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008;34(7):1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Cao X., Wang X., Chen H., Li H., Tariq M., Wang C., Zhou Y., Liu Y. Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ. Pollut. 2019;244:947–957. doi: 10.1016/j.envpol.2018.09.140. [DOI] [PubMed] [Google Scholar]
- 10.Fu M., Li Z., Gao H. Distribution characteristics of nonylphenol in Jiaozhou Bay of Qingdao and its adjacent rivers. Chemosphere. 2007;69(7):1009–1016. doi: 10.1016/j.chemosphere.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Uguz C., Iscan M., Ergüven A., Isgor B., Togan I. The bioaccumulation of nonyphenol and its adverse effect on the liver of rainbow trout (Onchorynchus mykiss). Environ. Res. 2003;92(3):262–270. doi: 10.1016/S0013-9351(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Espinosa M.J., Freire C., Arrebola J.P., Navea N., Taoufiki J., Fernandez M.F., Ballesteros O., Prada R., Olea N. Nonylphenol and octylphenol in adipose tissue of women in Southern Spain. Chemosphere. 2009;76(6):847–852. doi: 10.1016/j.chemosphere.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Ademollo N., Ferrara F., Delise M., Fabietti F., Funari E. Nonylphenol and octylphenol in human breast milk. Environ. Int. 2008;34(7):984–987. doi: 10.1016/j.envint.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Gyllenhammar I., Glynn A., Darnerud P.O., Lignell S., van Delft R., Aune M. 4-Nonylphenol and bisphenol A in Swedish food and exposure in Swedish nursing women. Environ. Int. 2012;43:21–28. doi: 10.1016/j.envint.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Bechi N., Ietta F., Romagnoli R., Jantra S., Cencini M., Galassi G., Serchi T., Corsi I., Focardi S., Paulesu L. Environmental levels of para-nonylphenol are able to affect cytokine secretion in human placenta. Environ. Health Perspect. 2010;118(3):427–431. doi: 10.1289/ehp.0900882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arukwe A., Thibaut R., Ingebrigtsen K., Celius T., Goksøyr A., Cravedi J. In vivo and in vitro metabolism and organ distribution of nonylphenol in Atlantic salmon (Salmo salar). Aquat. Toxicol. 2000;49(4):289–304. doi: 10.1016/S0166-445X(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 17.Geens T., Neels H., Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere. 2012;87(7):796–802. doi: 10.1016/j.chemosphere.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Azzouz A., Rascón A.J., Ballesteros E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016;119:16–26. doi: 10.1016/j.jpba.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Jing X., Bing S., Xiaoyan W., Xiaojie S., Yongning W. A study on bisphenol A, nonylphenol, and octylphenol in human urine amples detected by SPE-UPLC-MS. Biomed. Environ. Sci. 2011;24(1):40–46. doi: 10.3967/0895-3988.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Calafat A.M., Kuklenyik Z., Reidy J.A., Caudill S.P., Ekong J., Needham L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazemi S., Mousavi Kani S.N., Ghasemi-Kasman M., Aghapour F., Khorasani H., Moghadamnia A.A. Nonylphenol induces liver toxicity and oxidative stress in rat. Biochem. Biophys. Res. Commun. 2016;479(1):17–21. doi: 10.1016/j.bbrc.2016.08.164. [DOI] [PubMed] [Google Scholar]
- 22.Sayed A.E.H., Soliman H.A.M. Modulatory effects of green tea extract against the hepatotoxic effects of 4-nonylphenol in catfish (Clarias gariepinus). Ecotoxicol. Environ. Saf. 2018;149:159–165. doi: 10.1016/j.ecoenv.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Kazemi S., Feizi F., Aghapour F., Joorsaraee G.A., Moghadamnia A.A. Histopathology and histomorphometric investigation of bisphenol A and nonylphenol on the male rat reproductive system. N. Am. J. Med. Sci. 2016;8(5):215–221. doi: 10.4103/1947-2714.183012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Dakdoky M.H., Helal M.A. Reproductive toxicity of male mice after exposure to nonylphenol. Bull. Environ. Contam. Toxicol. 2007;79(2):188–191. doi: 10.1007/s00128-007-9158-y. [DOI] [PubMed] [Google Scholar]
- 25.Gillette R., Reilly M.P., Topper V.Y., Thompson L.M., Crews D., Gore A.C. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm. Behav. 2017;87:8–15. doi: 10.1016/j.yhbeh.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandjean P., Landrigan P.J. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 27.Lü B., Zhan P. Effects of nonylphenol on the brain development-associated gene expression profiles of F1 generation rats. Toxicol. Environ. Chem. 2008;90(1):127–134. doi: 10.1080/02772240701340642. [DOI] [PubMed] [Google Scholar]
- 28.Gore A.C. Neuroendocrine systems as targets for environmental endocrine-disrupting chemicals. Fertil. Steril. 2008;89(2) Suppl.:e101–e102. doi: 10.1016/j.fertnstert.2007.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazemi S., Khalili-Fomeshi M., Akbari A., Kani S.N.M., Ahmadian S.R., Ghasemi-Kasman M. The correlation between nonylphenol concentration in brain regions and resulting behavioral impairments. Brain Res. Bull. 2018;139:190–196. doi: 10.1016/j.brainresbull.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Jie Y., Pan W., Wenxia Y., Feng G., Liting H., Wenmei L., Jie X. The effects of gestational and lactational exposure to Nonylphenol on c-jun, and c-fos expression and learning and memory in hippocampus of male F1 rat. Iran. J. Basic Med. Sci. 2017;20(4):386–391. doi: 10.22038/IJBMS.2017.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokosuka M., Ohtani-Kaneko R., Yamashita K., Muraoka D., Kuroda Y., Watanabe C. Estrogen and environmental estrogenic chemicals exert developmental effects on rat hypothalamic neurons and glias. Toxicol. In Vitro. 2008;22(1):1–9. doi: 10.1016/j.tiv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Mao Z., Zheng Y-L., Zhang Y-Q. Behavioral impairment and oxidative damage induced by chronic application of nonylphenol. Int. J. Mol. Sci. 2010;12(1):114–127. doi: 10.3390/ijms12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao Z., Zheng Y.L., Zhang Y.Q., Han B.P., Chen L.T., Li J., Li F., Shan Q. Chronic application of nonylphenol-induced apoptosis via suppression of bcl-2 transcription and up-regulation of active caspase-3 in mouse brain. Neurosci. Lett. 2008;439(2):147–152. doi: 10.1016/j.neulet.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Che X., Fang Y., You M., Xu Y., Wang Y. Exposure to nonylphenol in early life increases pro-inflammatory cytokines in the prefrontal cortex: Involvement of gut-brain communication. Chem. Biol. Interact. 2020;323:109076. doi: 10.1016/j.cbi.2020.109076. [DOI] [PubMed] [Google Scholar]
- 35.Jäkel S., Dimou L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro A., Spinelli C.C., Martucciello S., Nori S.L., Capunzo M., Puca A.A., Ciaglia E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018;103(3):509–524. doi: 10.1002/JLB.3MR0118-003R. [DOI] [PubMed] [Google Scholar]
- 37.Chan C-K., Tan L.T., Andy S.N., Kamarudin M.N.A., Goh B.H., Kadir H.A. Anti-neuroinflammatory activity of Elephantopus scaber L. via activation of Nrf2/HO-1 signaling and inhibition of p38 MAPK pathway in LPS-induced microglia BV-2 cells. Front. Pharmacol. 2017;8:397. doi: 10.3389/fphar.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siracusa R., Fusco R., Cuzzocrea S. Astrocytes: Role and functions in brain pathologies. Front. Pharmacol. 2019;10:1114. doi: 10.3389/fphar.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelmsson U., Li L., Pekna M., Berthold C.H., Blom S., Eliasson C., Renner O., Bushong E., Ellisman M., Morgan T.E., Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 2004;24(21):5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafit-Zagardo B., Sharma N., Berman J.W., Bornstein M.B., Brosnan C.F. CSF-1 expression is upregulated in astrocyte cultures by IL-1 and TNF and affects microglial proliferation and morphology in organotypic cultures. Int. J. Dev. Neurosci. 1993;11(2):189–198. doi: 10.1016/0736-5748(93)90078-R. [DOI] [PubMed] [Google Scholar]
- 41.Gu W., Wang Y., Qiu Z., Dong J., Wang Y., Chen J. Maternal exposure to nonylphenol during pregnancy and lactation induces microglial cell activation and pro-inflammatory cytokine production in offspring hippocampus. Sci. Total Environ. 2018;634:525–533. doi: 10.1016/j.scitotenv.2018.03.329. [DOI] [PubMed] [Google Scholar]
- 42.Park H.Y., Han M.H., Park C., Jin C.Y., Kim G.Y., Choi I.W., Kim N.D., Nam T.J., Kwon T.K., Choi Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011;49(8):1745–1752. doi: 10.1016/j.fct.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Qiu Z., Wang Y., Chen J. Perinatal exposure to nonylphenol induces microglia-mediated nitric oxide and prostaglandin E2 production in offspring hippocampus. Toxicol. Lett. 2019;301:114–124. doi: 10.1016/j.toxlet.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Kwon O.W., Moon E., Chari M.A., Kim T.W., Kim A.J., Lee P., Ahn K.H., Kim S.Y. A substituted 3,4-dihydropyrimidinone derivative (compound D22) prevents inflammation mediated neurotoxicity; role in microglial activation in BV-2 cells. Bioorg. Med. Chem. Lett. 2012;22(16):5199–5203. doi: 10.1016/j.bmcl.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y-Q., Mao Z., Zheng Y.L., Han B.P., Chen L.T., Li J., Li F. Elevation of inducible nitric oxide synthase and cyclooxygenase-2 expression in the mouse brain after chronic nonylphenol exposure. Int. J. Mol. Sci. 2008;9(10):1977–1988. doi: 10.3390/ijms9101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jie Y., Xuefeng Y., Mengxue Y., Xuesong Y., Jing Y., Yin T., Jie X. Mechanism of nonylphenol-induced neurotoxicity in F1 rats during sexual maturity. Wien. Klin. Wochenschr. 2016;128(11-12):426–434. doi: 10.1007/s00508-016-0960-6. [DOI] [PubMed] [Google Scholar]
- 47.Murata Y., Higo N., Oishi T., Yamashita A., Matsuda K., Hayashi M. Developmental changes in the expression of growth-associated protein-43 mRNA in the monkey thalamus: northern blot and in situ hybridization studies. Neuroscience. 2005;136(2):497–507. doi: 10.1016/j.neuroscience.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Pizzino G. Oxidative stress: Harms and benefits for human health. Neurobiol. Dis. 2017;84:4–21. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobb C.A., Cole M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015;84:4–21. doi: 10.1016/j.nbd.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griendling K.K., Sorescu D., Lassègue B., Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2000;20(10):2175–2183. doi: 10.1161/01.ATV.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 51.Karageorgos N., Patsoukis N., Chroni E., Konstantinou D., Assimakopoulos S.F., Georgiou C. Effect of N-acetylcysteine, allopurinol and vitamin E on jaundice-induced brain oxidative stress in rats. Brain Res. 2006;1111(1):203–212. doi: 10.1016/j.brainres.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 52.Tabassum H., Ashafaq M., Parvez S., Raisuddin S. Role of melatonin in mitigating nonylphenol-induced toxicity in frontal cortex and hippocampus of rat brain. Neurochem. Int. 2017;104:11–26. doi: 10.1016/j.neuint.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Chance B., Schoener B., Oshino R., Itshak F., Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J. Biol. Chem. 1979;254(11):4764–4771. doi: 10.1016/S0021-9258(17)30079-0. [DOI] [PubMed] [Google Scholar]
- 54.Magnifico M.C. Nonylphenol and octylphenol differently affect cell redox balance by modulating the nitric oxide signaling. Oxid. Med. Cell. Longev. 2018;2018:1684827. doi: 10.1155/2018/1684827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aydoğan M., Korkmaz A., Barlas N., Kolankaya D. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology. 2008;249(1):35–39. doi: 10.1016/j.tox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Baserga R. The relationship of the cell cycle to tumor growth and control of cell division: A review. Cancer Res. 1965;25(5 Part 1):581–595. [PubMed] [Google Scholar]
- 57.Kudo C., Wada K., Masuda T., Yonemura T., Shibuya A., Fujimoto Y., Nakajima A., Niwa H., Kamisaki Y. Nonylphenol induces the death of neural stem cells due to activation of the caspase cascade and regulation of the cell cycle. J. Neurochem. 2004;88(6):1416–1423. doi: 10.1046/j.1471-4159.2003.02270.x. [DOI] [PubMed] [Google Scholar]
- 58.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30(1):87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Zou H., Slaughter C., Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89(2):175–184. doi: 10.1016/S0092-8674(00)80197-X. [DOI] [PubMed] [Google Scholar]
- 61.Kusunoki T., Shimoke K., Komatsubara S., Kishi S., Ikeuchi T. p-Nonylphenol induces endoplasmic reticulum stress-mediated apoptosis in neuronally differentiated PC12 cells. Neurosci. Lett. 2008;431(3):256–261. doi: 10.1016/j.neulet.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 62.Gu W., Wang Y., Qiu Z., Dong J., Wang Y., Chen J. Mitogen-activated protein kinase signaling is involved in nonylphenol-induced proinflammatory cytokines secretion by BV2 microglia. J. Appl. Toxicol. 2018;38(7):958–967. doi: 10.1002/jat.3602. [DOI] [PubMed] [Google Scholar]
- 63.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajnóczky G., Davies E., Madesh M. Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 2003;304(3):445–454. doi: 10.1016/S0006-291X(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 65.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J-L., Liu C.S., Lin K.L., Chou C.T., Hsieh C.H., Chang C.H., Chen W.C., Liu S.I., Hsu S.S., Chang H.T., Jan C.R. Nonylphenol-induced Ca2+ elevation and Ca2+ independent cell death in human osteosarcoma cells. Toxicol. Lett. 2005;160(1):76–83. doi: 10.1016/j.toxlet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Liu P-S., Liu G-H., Chao W-L. Effects of nonylphenol on the calcium signal and catecholamine secretion coupled with nicotinic acetylcholine receptors in bovine adrenal chromaffin cells. Toxicology. 2008;244(1):77–85. doi: 10.1016/j.tox.2007.11.005. [DOI] [PubMed] [Google Scholar]