Abstract
The spinal cord injury (SCI) initiates an extraordinarily protracted disease with 3 phases; acute, inflammatory, and resolution that are restricted to the cavity of injury (COI) or arachnoiditis by a unique CNS reaction against the severity of destructive inflammation. While the severity of inflammation involving the white matter is fueled by a potently immunogenic activity of damaged myelin, its sequestration in the COI and its continuity with the cerebrospinal fluid of the subdural space allow anti-inflammatory therapeutics infused subdurally to inhibit phagocytic macrophage infiltration and thus provide neuroprotection. The role of astrogliosis in containing and ultimately in eliminating severe destructive inflammation post-trauma appears obvious but is not yet sufficiently understood to use in therapeutic neuroprotective and neuroregenerative strategies. An apparent anti-inflammatory activity of reactive astrocytes is paralleled by their active role in removing excess edema fluid in blood-brain barrier damaged by inflammation. Recently elucidated pathogenesis of neurotrauma, including SCI, traumatic brain injury (TBI), and stroke, calls for the following principal therapeutic steps in its treatment leading to the recovery of neurologic function: (1) inhibition and elimination of destructive inflammation from the COI with accompanying reduction of vasogenic edema, (2) insertion into the COI of a functional bridge supporting the crossing of regenerating axons, (3) enabling regeneration of axons to their original synaptic targets by temporary safe removal of myelin in targeted areas of white matter, (4) in vivo, systematic monitoring of the consecutive therapeutic steps. The focus of this paper is on therapeutic step 1.
Keywords: Neurotrauma, inflammatory phase of SCI, cavity of injury, arachnoiditis, astrogliosis, subdural infusion, neuroprotective therapy, vasogenic edema
1. INTRODUCTION
Major trauma to the central nervous system (CNS) and stroke are serious medical consequences in surviving individuals that have no effective treatment, thus posing the greatest challenge to intensive care and beyond. This frustrating status quo has persisted despite the realization that damage, secondary to the initial trauma, plays an important role, but the pathogenesis of the disease indicated as the “secondary damage” has not been studied systematically for its elucidation until recently [1]. In the rat model of the spinal cord injury (SCI), a localized, massive injury involving the white matter that destroys all cellular elements and blood vessels initiates a severe, destructive inflammation characterized by infiltration of the site of injury by phagocytic, pro-inflammatory, CD68+/CD163- macrophages persisting for >16 weeks post-SCI [1]. This paper addresses and discusses the pathogenesis of neurotrauma in the model of SCI in an attempt to provide direction for studies on novel therapies addressing neuroprotection. Implications for treatments of cerebral edema and treatments addressing neuroregeneration are also discussed in order to introduce a strategic view for multiple therapeutic steps potentially leading to the cure for medical specialists and biomedical investigators involved in neurotrauma and stroke.
1.1. Pathogenesis of Spinal Cord Injury
Localized massive trauma involving the white matter in the SCI results in hemorrhages, cellular necrosis, and edema constituting pathology designated as the Acute Phase that lasts 2 days in the rat model [1]. Infiltration of the area of injury by macrophages on day 3 initiates the Inflammatory Phase with phagocytic macrophages persisting beyond 16 weeks post-SCI. The Inflammatory Phase is the main subject of this review. It is supervened and overlapped by a Resolution Phase resulting in marked lowering of numbers of infiltrating macrophages after 2 months post-SCI likely regulated by a CNS tissue response involving astrogliosis [1].
1.1.1. Inflammatory Phase
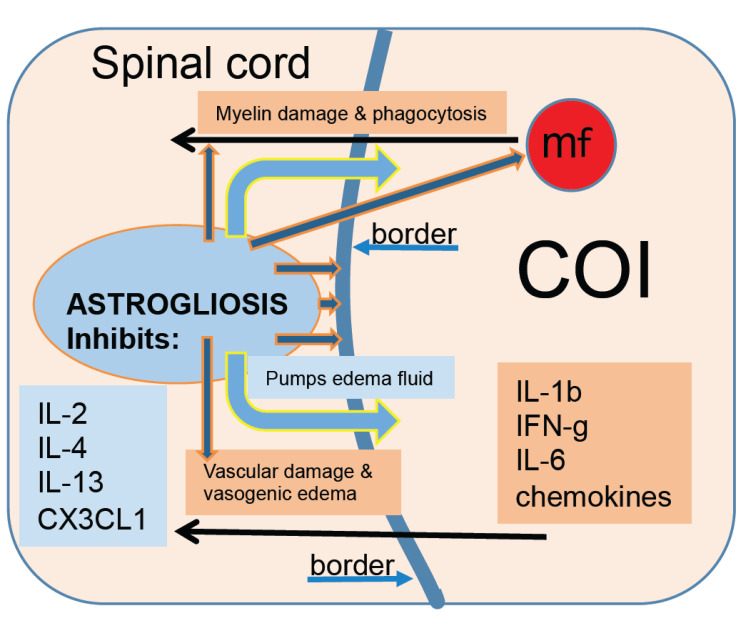
Mechanisms involved in the inflammatory phase of the SCI are presented in Fig. 1. Neurotrauma involving the white matter damage initiates inflammatory response with destructive severity and extraordinarily protracted course unprecedented in any extraneural injury. Destructive inflammation involves infiltration of the area of injury by numerous macrophages actively phagocytizing myelin-rich debris and
Fig. (1).
Mechanisms involved in inflammation induced by the spinal cord injury. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
red blood cells that are completely removed by the week 2 post-SCI in the rat model [1]. Administration of anti-inflammatory treatments such as dexamethasone, MT-7, or Serp-1, both immunomodulatory proteins derived from Myxomavirus [2-19], lowers the numbers of infiltrating macrophages and slows down the removal of necrotic debris [20-23] beyond 2 weeks post-SCI. This observation indicates that for an anti-inflammatory treatment to be an effective neuroprotectant, it needs to be administered for a period of time considerably exceeding 2 weeks post-trauma to allow for slower removal of immunogenic myelin-rich debris by lowered numbers of phagocytic macrophages. During the first week post-SCI, numbers of infiltrating, myelin-phagocytizing macrophages rise rapidly and remain so for the 4 weeks before declining thereafter in a gradual fashion but persist in low numbers beyond week 16, indicating an active status of inflammation [1]. Since the myelin-rich necrotic debris is removed by the second-week post-SCI, the source of myelin for phagocytic macrophages appears to be in the spinal cord surrounding the area of inflammation. This indicates a mechanism of a vicious cycle that supports the destructive inflammation for a long period of time where infiltrating blood-borne macrophages are pro-inflammatory, CD68+/CD163-, and via numerous released inflammatory factors [1] damage the spinal cord around the lesion leading to the death of neural cells including oligodendrocytes resulting in ongoing myelin damage [24-26] that sustains chemotaxis of further macrophages [1]. In a systematic proteomic study, the tissue of the spinal cord post-SCI was collected from rats perfused with chilled lactated Ringer’s solution to obtain it blood-free and to minimize uncontrolled endogenous proteolysis [1]. Levels of inflammatory cytokines including IL-1β, IL-6, IFN-γ, and chemokines were markedly elevated during the first 4 weeks post-SCI, coinciding with the severity of macrophage-rich inflammation, supporting the notion of the severity of destructive inflammation [1]. Persistence of inflammatory type of infiltrating macrophages, CD68+/CD163-, throughout the studied course of 16 weeks indicates that lowering of their numbers 8 weeks after the SCI combined with the reduction of levels of inflammatory cytokines in parallel may not involve the conversion of inflammatory (CD68+) into anti-inflammatory (CD163+) macrophages observed in trauma-induced inflammation in extraneural tissues [27]. Rather, the inhibition and elimination of the most severe and destructive inflammation in the body may be caused by a CNS tissue reaction, specifically astrogliosis [1, 28]. In a recently completed study, subdural infusion of 0.2 mg of Serp-1 per week, sustained for 8 weeks, elimination of CD68+ phagocytic macrophages was accelerated with few macrophages remaining in the COI similar in numbers to those in the COI of untreated rats at 16 weeks post-SCI [1, 23]. Importantly, un-phagocytized myelin-rich necrotic debris persisted in the COI at 4 weeks in Serp-1 infused rats, indicating a delay of its removal by lowered numbers of macrophages. The observations made in this study testing Serp-1 anti-inflammatory agent of (1) lowered numbers of macrophages, (2) their accelerated removal from the COI, and (3) extended persistence of myelin-rich necrotic debris, indicating that this treatment was neuroprotective [23]. It needs to be also pointed out that the duration of this treatment needs to be at least 8 weeks to eliminate inflammation and accelerate the formation of the syrinx [23]. This therapeutic strategy stands out against numerous attempts at short-duration anti-inflammatory treatments of SCI studied previously and introduces a new fundamental parameter, that of a duration of treatment (8 weeks in case of Serp-1 constituting the current standard) sufficient to eliminate a destructive inflammation. Disregarding this parameter may lead to failure to eliminate inflammation since myelin-rich necrotic debris persisting in the COI may reignite the severity of macrophage-rich, destructive inflammation [20-24].
1.1.2. Vasogenic Edema
Post-traumatic cerebral edema remains a serious and not well-addressed challenge to intensive therapists. Edema caused by the initial traumatic event appears to be sustained by the supervening inflammation with its damaging effects to surrounding blood vessels mediated by elevated inflammatory cytokines and chemokines and by the presence of numerous inflammatory macrophages [1]. In a T2-weighed MRI study of the formalin-fixed spinal cords from SCI rats, edema persisted for 16 weeks, parallel with the persistence of inflammatory CD68+ macrophages [1]. Severe inflammation elucidated in the rat SCI can explain vascular pathology with damage to the blood-brain barrier (BBB) or blood spinal cord barrier (BSCB) related to a number of specific molecular changes leading to extravascular leakage and vasogenic edema [29, 30]. While vasogenic edema appears to be sustained by post-traumatic inflammation for a long time, tissue reaction counteracting the accumulation of intercellular water in the CNS comes to importance. Astrocytes use bi-directional aquaporin (AQP) water channels, particularly AQP-4, to direct excess water to; (1) the subarachnoid space via the glia limitans externa, (2) the cerebral ventricles and central canal via the glial limitans interna underlying ependymal cells and, (3) blood vessels via the BBB/BSCB [31-35]. An additional 4th path of elimination of excess edema water into the site of injury converted into the COI has been recently postulated in the SCI [36]. As the astrocytic reaction and corresponding remarkable increase in expression of AQP-4 may indicate an increased transfer of excess edema fluid out of the brain or spinal cord, the persistence of inflammation along with edema [1] supports the notion of persistent damage to BBB/BSCB with resulting protracted course of vasogenic edema counteracted by progressively severe reactive astrogliosis. The importance of an apparent dynamic balancing of BBB-damaging inflammation on the one hand and reactive astrogliosis attempting to counteract the damage in the BBB appears obvious and of critical importance to neuropharmacology. A systematic administration route, such as oral or intravenous, of an anti-inflammatory treatment of neurotrauma needs to be likely determined empirically in pre-clinical studies to address CNS tissue availability via damaged BBB. In the case of
anti-inflammatory proteins, Serp-1 and M-T7, intraperitoneal infusion, corresponding to an intravenous administration, did not result in lower numbers of macrophages in the COI, whereas a subdural infusion allowing for the direct access of either protein to the COI and circumventing the BSCB altogether had significant macrophage-lowering, therefore anti-inflammatory effect [22].
Currently used treatment of cerebral edema that involves the intravenous administration of hyperosmotic solutes such as 20% mannitol and 1.8-3% NaCl and diuretics [37-38] was essentially established at the end of the First World War, >100 years ago. It needs to be emphasized that this treatment addresses only a minor mechanism serving to remove excess water from the CNS, via the BBB/BSCB into the bloodstream. In a cat model of cerebral trauma, this mechanism accounted only for 11% of the total removal of excess cerebral water [35]. The remaining paths moved 87% of water to the subarachnoid space and the cerebral ventricles [35] and perhaps to the COI [36] but are not addressed in therapies alleviating cerebral edema currently in use. Two obvious therapeutic approaches; (i) inhibition of inflammation to suppress vasogenic edema and, (ii) promotion of astrogliosis in areas targeted for removal of excess edema fluid, should be considered but have not yet been addressed in pre-clinical studies. It needs to be emphasized that the CNS tissue reaction to an injury known as astrogliosis appears to act to suppress inflammation and vasogenic edema, which are beneficial mechanisms. Therefore, anti-inflammatory and anti-edema therapies must not inhibit astrogliosis. Furthermore, astrogliosis-promoting treatments may prove beneficial, which flies against common beliefs presently.
1.1.3. Astrogliosis
1.1.3.1. The Cavity of Injury (COI)
The CNS tissue reaction to trauma and severe supervening inflammation is rapid and formidable [1]. An area of injury deep in the spinal cord, targeted for infiltration of phagocytic macrophages, is delineated by hypertrophied astrocytic processes and accumulates fluid within the first week post-SCI [1]. Thus, the initial site of the SCI is converted to a COI that serves to sequester the severity of inflammation in an early attempt to preserve the surrounding spinal cord and restore homeostasis therein [1, 36, 39, 40]. The formation of the COI in response to injury and supervening inflammation is unique to the CNS and does not occur in response to trauma in extraneural tissues. Systematic histologic analysis of the COI involving the GFAP stain demonstrates rapid reorganization of astrocytic processes from random to parallel to the margin of the necrotic lesion and formation of progressively thickening, a continuous wall of GFAP-positive hypertrophied processes sharply delineating the COI [1] and then a maturing syrinx [1, 36, 39, 40]. The progression of astrogliosis delineating the COI coincides with the reduction of numbers of macrophages therein after 4 weeks and with declining levels of inflammatory cytokines and chemokines while anti-inflammatory cytokines including IL-2, IL-4, and IL-13 and also the chemokine CX3CL1 (fractalkine) become elevated [1]. A gradually increasing and evidently powerful anti-inflammatory effect of astrogliosis is supported by an independent observation from SCI studies in the Long Evans Shaker (LES) rat, a severely dysmyelinated mutant of myelin basic protein gene [41,42] where inflammatory response involving infiltration by macrophages is entirely eliminated from the COI by the day 7 post-SCI [28] which is extraordinarily fast even in extraneural soft tissue trauma where inflammation tends to be eliminated in 2-3 weeks. An anti-inflammatory activity appeared to be water-soluble [28]. Severe, diffuse astrogliosis develops in the LES rat CNS in response to the lack of myelin [43], resulting in a lack of nodes of Ranvier [44] and the need to restore and maintain homeostasis [40] rather than due to an inflammatory pathology indicating that this CNS tissue response may include multifactorial activities including; (1) anti-inflammatory and (2) anti-vasogenic edema.
1.1.3.2. Arachnoiditis
A superficial area of the SCI adjacent to a widely disrupted pia terminalis becomes invaded by granulomatous cells from the subarachnoid space, including macrophages, fibroblasts, and capillary blood vessels resulting in obliteration of the infiltrated spinal cord [1]. This type of inflammatory infiltration called arachnoiditis [1, 45, 46] is devoid of glial cells [1]; therefore, it is not a ‘glial scar’ but rather a granulomatous inflammatory tissue that ultimately becomes a scar excluded from the adjacent CNS by a thick wall of reactive astrogliosis [1]. A detailed systematic histologic study indicates that while in initial stages, arachnoiditis is an aggressive pathology involving numerous phagocytic macrophages, expanding at the cost of the destroyed adjacent spinal cord, progressing to severe astrogliosis and the leading edge of arachnoiditis, eventually containing it [1], which indicates an anti-inflammatory activity of this CNS tissue response.
Anti-inflammatory activity mounted by progressive astrogliosis in response to neurotrauma has profound implications for future effective neuroprotective therapies.
There is a dramatic difference between two types of inflammatory pathologies initiated by the SCI; the COI and arachnoiditis; the former connects with the subarachnoid space and is accessible by simple diffusion to anti-inflammatory drugs administered subdurally [20-23], resulting in the reduction of levels of phagocytic macrophages, considered a neuroprotective effect. The COI is surrounded by the CNS with damaged BSCB that can still be selective to large molecular weight anti-inflammatory agents [22]. Arachnoiditis is a solid vascularized extraneural tissue without a BSCB, and its anti-inflammatory treatment by parenteral or oral administration can be considered. While the COI, after elimination of inflammation, may be implanted by a synthetic [47] or a cellular [28] bridge to allow regrowth of axons across its aqueous milieu, a scar resulting from arachnoiditis does not contain neural cells, is excluded from the rest of the CNS, and constitutes a potently inhibitory barrier to axonal regeneration. Therefore, to consider axonal regeneration, such scar would have to be resected, following inflammation inhibited and eliminated before a bridging implant could be inserted.
A curve of the reduction in numbers of phagocytic macrophages in the COI of untreated subjects in pre-clinical trials needs to be applied against a flatter curve produced by the anti-inflammatory effect of a candidate drug, also leading to considerably faster elimination of phagocytic macrophages than >16 weeks post-SCI [1, 23].
Direct access to the aqueous content of the COI from the subarachnoid space allows for simple diffusion of anti-inflammatory drugs with resulting inhibition of macrophage-rich inflammation. This invasive therapeutic administration is of importance to drugs of a large molecular size, such as immunomodulatory proteins, Serp-1 and M-T7 [19], that have been shown to have a strong macrophage-inhibitory effect in a recent rat SCI study following the subdural infusion but not the intraperitoneal infusion [22]. This study clearly demonstrated that although damage to the BSCB around the COI due to severe inflammation therein may be obvious, selective exclusion of some compounds administered parenterally may persist, requiring empirical testing in pre-clinical studies of each candidate anti-inflammatory agent for an optimal route of administration.
The excess edema water removal effect by astrocytes via the brain and spinal cord external and internal surfaces needs to be considered in respect to progressive astrogliosis. Although treatments leading to an acceleration of astroglial reaction with its theoretical potential to remove excess edema fluid faster are currently not obvious, it needs to be emphasized that pharmacologic inhibition of vasogenic edema by anti-inflammatory drugs active in the COI indicate a promising therapeutic strategy. The potential astrogliosis-inhibiting activity of any candidate anti-inflammatory compounds needs to be considered counterproductive after it was shown on histologic analysis that dexamethasone infused subdurally was associated with reduced size of hypertrophic astrocytes around the COI [20].
2.1. Anti-inflammatory Treatments for Neuroprotection
2.1.1. Methylprednisolone Succinate
Methylprednisolone succinate administered to the SCI patients shortly after the accident in an intravenous bolus of 30 mg/kg body weight over 1 hour followed by the intravenous dose of 5.4 mg/kg for 23-47 hours has been recommended by the North American Spinal Cord Injury Study (NASCIS) [48] and used by others [49-51]. But this treatment does not address the pathogenesis of the SCI, specifically the onset, at day 3 post-SCI and the long duration of the Inflammatory Phase [1]. This treatment is considered ineffective and no longer recommended [52] due to the high risk of adverse side effects and no improved neurologic deficits [22, 48, 53-55]. However, much of the published literature reported on the examination of steroids used as potent acute inhibitors of inflammation. Since methylprednisolone is unstable beyond 24 hours, dexamethasone, a more powerful and stable synthetic corticosteroid, was used in studies requiring longer periods of continuous administration. In studies using the rat model of SCI, the constant subdural infusion of dexamethasone for 1-2 weeks [20, 21] allowed for a remarkable inhibition of macrophage infiltration in the COI considered a neuroprotective effect. This duration, however, was too short of eliminating the myelin-rich debris and inflammation from the COI [21]. The persistence of myelin-rich necrotic debris in the COI after a premature termination of dexamethasone treatment can reignite the severity of destructive inflammation [20,21]; therefore, it is not effective. Longer treatment cannot be considered since it is unduly toxic to rats developing Cushingoid syndrome and hypovolemic shock [20, 22]. This consideration indicates that for a continuous anti-inflammatory treatment beyond 2 weeks, novel, powerful anti-inflammatory agents of low or no toxicity are required for an effective neuroprotective outcome. A potentially inhibitory effect of dexamethasone on astroglial hypertrophy [20] with its putative anti-inflammatory and edema-resolving activity in the SCI studies [1, 28, 36] further precludes the use of steroids in the management of neurotrauma and cerebral edema.
2.1.2. Other Candidate Treatments for Neurotrauma
Other candidate treatments for neurotrauma include but are not limited to; riluzole, glibenclamide and cethrin [56], and also fumaric acid esters [57], estrogen [58], edaravone [59], mithramycine A [60], and N-Palmytiolethalonamine-oxazoline [61] have recently been studied in SCI animal models and clinical trials are discussed in detail in the respective citations. However, these candidate treatments were used only in short-term duration, corresponding to initial stages of SCI, therefore not addressing the duration of the destructive inflammation whose inhibition and accelerated total elimination are required from effective neuroprotective therapies of sufficient duration. It is possible that some of the above treatments may prove to be effective neuroprotectants once tested in properly designed preliminary pre-clinical studies addressing the pathogenesis of neurotrauma [1, 23].
2.1.3. Subdural Infusions of Anti-inflammatory Compounds
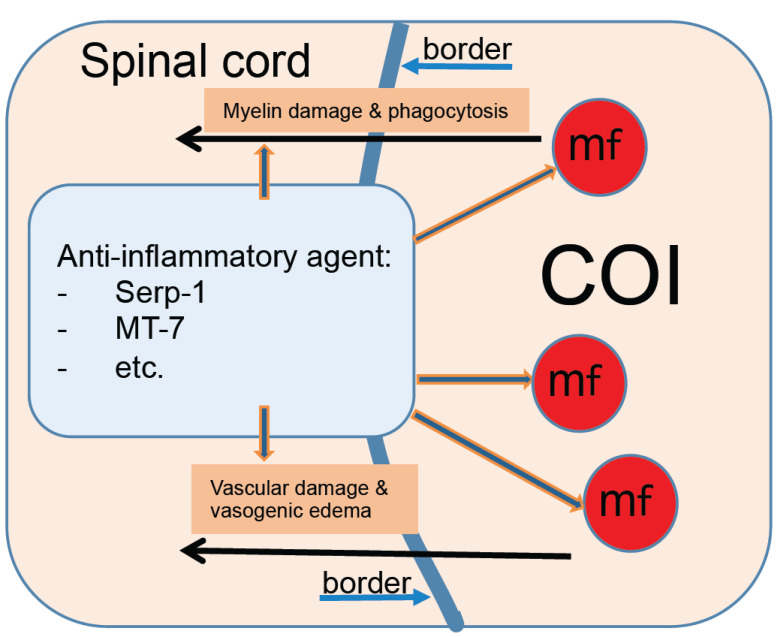
Subdural infusions of anti-inflammatory compounds have been recently attempted in the rat model where the SCI was caused by an epidural balloon crush leaving the dura mater and the subarachnoid space intact [62]. Mechanisms involved in neuroprotection and inhibition of vasogenic edema are summarized in Fig. 2. Two immunomodulatory proteins derived from Myxoma virus; Serp-1, a serpin inhibitor of thrombosis and thrombolysis [15, 19] and M-T7 a chemokine inhibitor [2, 6, 9, 14] were administered subdurally by a continuous infusion from an osmotic pump. A dose of either protein at 0.2 mg/week resulted in improved neurological signs and in lowering of numbers of phagocytic macrophages in the COI by 80% for M-T7 and by 50% for Serp-1 [22]. Intraperitoneal administration of the same dose of either protein resulted in little or no effect, correspondingly indicating that despite apparent damage to the BSCB around the COI [1], it likely remained impermeable or poorly permeable to Serp-1 and to M-T7. Serp-1 has been shown to reduce inflammatory damage in the myocardium of patients with a mild heart infarction or unstable angina in a Phase IIa trial [16]. In a recently completed study performed in an attempt to not only inhibit but also to eliminate the post-SCI inflammation in the COI, administration of the same weekly dose of Serp-1 for 8 weeks (max. total 1.6 mg/rat) resulted in a consistent reduction of macrophages at 2-8 weeks post-SCI and a COI with few macrophages similar to the COI of untreated rats at 16 weeks post-SCI [1, 23] indicating accelerated inhibition and elimination of macrophage-rich inflammation by continuous infusion of Serp-1. It needs to be noted that in Serp-1 infused rats, the COI with reduced numbers of macrophages still contained myelin-rich necrotic debris at 4 weeks post-SCI [23]. Whereas in untreated rats, necrotic debris and red blood cells are removed before 2 weeks post-SCI [1], indicating that; (a) Serp-1 administered subdurally did inhibit destructive inflammation by lowering numbers of phagocytic macrophages in the COI, (b) administration of Serp-1 or any other effective anti-inflammatory drug studied, should be continued beyond the complete removal of necrotic debris to avoid a re-ignition of macrophage-rich inflammation and preferably until all macrophages are absent from the COI, (c) since the macrophage count in the COI test [1, 22, 23] is based on histologic analysis of the injured spinal cord it is not suitable for clinical studies; therefore novel in vivo assays need to be developed to systematically monitor the progress of an anti-inflammatory treatment in individual patients with neurotrauma or stroke enabling optimization of treatment along its course in individual patients.
Fig. (2).
Anti-inflammatory treatment is neuroprotective in spinal cord injury. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1.4. Neurological Tests Assessing Anti-inflammatory Effects of Neuroprotective Drugs in Pre-clinical Studies on the SCI
In the experiments using the subdural infusion of dexamethasone, M-T7 and Serp-1, the Basso Beattie Bresnahan (BBB) locomotor test used to evaluate SCI rats [63] by others was substituted by a simpler hind end locomotor test and supplemented by a hind end pinch withdrawal test developed by the author [22, 23, 64] to allow for a more detailed analysis of the neurologic function of SCI rats every day after the SCI. While both tests detected neurological improvements in rats treated with anti-inflammatory infusions in a 7 days long study [22], this was not the case in the follow-up study involving 56 days of treatment with Serp-1 despite the lowering effect of this treatment on the numbers of phagocytic macrophages in the COI [23]. This observation suggests that scores in both neurological tests and perhaps the BBB test do not adequately represent the pathogenesis of the SCI where severe inflammation likely contributes to further destruction of the spinal cord [1] while the scores improve in untreated rats during the first 4 weeks post-SCI [23, 64, 65]. In another contradiction, while the phagocytic macrophage-rich inflammation persists beyond 4 weeks in untreated rats and in Serp-1 infused rats [1, 23], the scores of both neurological tests stabilized during the week 4 post-SCI [23], an observation also made with the BBB test in treatment experiments lasting beyond 4 weeks [66-68]. Thus the usefulness of the neurological test in pre-clinical studies on candidate neuroprotective compounds appears quite limited as it may not correspond to the pathogenesis of the SCI and cannot be used beyond week 4 while the effective treatments are expected to inhibit inflammation by lowering numbers of macrophages and the duration of treatment needs to last well beyond 4 weeks [23, 64].
2.1.5. Histologic Analysis of the SCI, the Macrophage Count in the COI Test
Detailed histologic and immunohistochemical analysis of the SCI along with its progression over a period of 16 weeks allowed to distinguish particular pre-eminence of phagocytic macrophages infiltrating the COI and also arachnoiditis [1]. A rapid increase in numbers of macrophages in the COI starting at day 3 and their numbers peaking at day 7 allows for reliable determination of reduction of infiltrating macrophages in rats treated with anti-inflammatory agents [20-22]. Therefore, the macrophage count in the COI test addresses the severity of the inflammatory process in the COI, and previous studies have shown that anti-inflammatory treatments can reduce this severity [64].
In a study addressing the determination of the duration of continuous treatment required to eliminate inflammation from the COI by subdural infusion of Serp-1 at 0.2 mg/week for 8 weeks, the numbers of macrophages were consistently lower at 2, 4, and 8 weeks than in untreated SCI rats [23]. Very low numbers of macrophages at 8 weeks in Serp-1 treated rats were comparable to those in untreated rats at 16 weeks, indicating acceleration of elimination of destructive inflammation from the COI [1, 23]. Reliable recognition of phagocytic macrophages containing blue granules of myelin and/or red blood cells on the luxol fast blue stain counterstained with hematoxylin and eosin (LFB+H&E) in the rat spinal cord is not challenging for a trained veterinary or medical pathologist, but the macrophage count in the COI test is not applicable to clinical trials since the segments of the spinal cord are required. A volumetric test addressing the area of the inflammatory obliteration in sections of the spinal cord containing the SCI from segments of a standardized thickness (3mm) is currently being developed by the author to directly measure the neuroprotective effect of tested anti-inflammatory agents. Given its dependence on histology, this test however, will be restricted to pre-clinical studies and will not be applicable to clinical trials.
Histologic studies of the SCI brought to focus an issue that has eluded the field devoted to studies on TBI. Since TBI models in the mouse and rat brain have been widely used for basic research and pre-clinical drug testing to address brain injury and stroke, it needs to be emphasized that the small content of the white matter in the rodent brain makes it a convenient model of gray matter injury. This is dramatically different from the white matter injury commonly encountered in human TBI and stroke. While the white matter injury initiates a severe, destructive inflammation fuelled by a mechanism of the vicious cycle due to the powerful immunogenic effect of a large quantity of damaged myelin and sustained by it for a very long time [1], gray matter injury is expected to be self-limiting with little inflammation involved [28]. Therefore, while the rodent brain injury may not be a suitable model of TBI, due to the paucity of the white matter, the rodent models of SCI may serve as adequate models of TBI due to the rich content of the white matter and its involvement in the pathogenesis of neurotrauma as in human TBI. Given the large size of the complex brain with rich content of the white matter in the hemispheres in the pig, this animal may serve as a better model of TBI versus rodent models.
2.1.6. Imaging of Neurotrauma
Imaging of neurotrauma is an excellent diagnostic tool given its ability to localize the lesion, measure its size and also characterize the edema in the tissue surrounding the lesion itself. Since the inflammatory disease initiated by neurotrauma is very prolonged and destructive, good quality imaging such as T2-weighed MRI should be performed repeatedly in a systematic fashion in a patient treated with an anti-inflammatory agent. Volumetric measurements of the lesion and then of the inflammation (COI and arachnoiditis) may be able to determine a neuroprotective effect; smaller progressive increases in the size of the inflammatory lesion when compared to untreated controls. The use of systematic imaging of neurotrauma patients treated with anti-inflammatory agents is anticipated. New detailed information on the development of histologic changes in inflammation involving the white matter should be helpful with an interpretation of MRI images. Specifically, the cellular content in the COI corresponding to inflammatory macrophages and indicating destruction of the surrounding tissue should be considered [1]. An inhibitory effect of effective anti-inflammatory treatment on peri-lesional vasogenic edema may also be detected by T2-MRI [1], contributing to an important aspect of the in vivo assessment of a candidate therapy in clinical trials.
The usefulness of in vivo MR imaging in the rodent models of neurotrauma has been demonstrated in a number of studies on the SCI [69-71] and TBI [72-74]. It needs to be, however, pointed out that the breathing movements of the thorax in the rat in the supine recumbency affect the position of the cervical and thoracic spinal cord, therefore the quality of imaging of the spinal cord.
Ultimately limited resolution of the MR imaging will require additional analytic methods to measure CNS tissue protective and anti-inflammatory effects of candidate agents in clinical trials. Specific biomarkers of the CNS destruction and the severity of neuroinflammation fulfilling this requirement will need to be developed. This need is further underscored by an inability to use histologic analyses, including the macrophage count in the COI test in human patients.
CONCLUSION
While the pathogenesis of neurotrauma has been largely elucidated in a recent systematic study of the rat SCI, anti-inflammatory treatments to limit destructive effects of the supervening inflammation and provide neuroprotection remain unavailable. The rat model of the SCI with a massive injury to the white matter is appropriate to study candidate anti-inflammatory agents for clinical trials involving SCI, TBI, and stroke patients, but analytic methods to systematically measure neuroprotective effects in vivo remain to be developed. It is anticipated that in a large proportion of SCI, TBI, and stroke cases, effective inhibition and accelerated elimination of inflammatory infiltration in the COI will result in neuroprotection and inhibition of vasogenic edema leading to improved neurologic outcomes. In cases of initial extensive damage of the CNS, the elimination of the inflammation will need to be followed by therapies targeting neuroregeneration leading to improved neurologic deficits.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Partial funding was provided by VPC NeuroPath CONSULTING, Inc.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kwiecien J.M., Dabrowski W., Dąbrowska-Bouta B., Sulkowski G., Oakden W., Kwiecien-Delaney C.J., Yaron J.R., Zhang L., Schutz L., Marzec-Kotarska B., Stanisz G.J., Karis J.P., Struzynska L., Lucas A.R. Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS One. 2020;15(3):e0226584. doi: 10.1371/journal.pone.0226584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartee M.Y., Chen H., Dai E., Liu L.Y., Davids J.A., Lucas A. Defining the anti-inflammatory activity of a potent myxomaviral chemokine modulating protein, M-T7, through site directed mutagenesis. Cytokine. 2014;65(1):79–87. doi: 10.1016/j.cyto.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Bédard E.L., Jiang J., Arp J., Qian H., Wang H., Guan H., Liu L., Parry N., Kim P., Garcia B., Li X., Macaulay C., McFadden G., Lucas A., Zhong R. Prevention of chronic renal allograft rejection by SERP-1 protein. Transplantation. 2006;81(6):908–914. doi: 10.1097/01.tp.0000203141.02725.8a. [DOI] [PubMed] [Google Scholar]
- 4.Bédard E.L.R., Kim P., Jiang J., Parry N., Liu L., Wang H., Garcia B., Li X., McFadden G., Lucas A., Zhong R. Chemokine-binding viral protein M-T7 prevents chronic rejection in rat renal allografts. Transplantation. 2003;76(1):249–252. doi: 10.1097/01.TP.0000061604.57432.E3. [DOI] [PubMed] [Google Scholar]
- 5.Bot I., von der Thüsen J.H., Donners M.M.P.C., Lucas A., Fekkes M.L., de Jager S.C.A., Kuiper J., Daemen M.J.A.P., van Berkel T.J.C., Heeneman S., Biessen E.A.L. Serine protease inhibitor Serp-1 strongly impairs atherosclerotic lesion formation and induces a stable plaque phenotype in ApoE-/-mice. Circ. Res. 2003;93(5):464–471. doi: 10.1161/01.RES.0000090993.01633.D4. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., Ambadapadi S., Wakefield D., Bartee M., Yaron J.R., Zhang L., Archer-Hartmann S.A., Azadi P., Burgin M., Borges C., Zheng D., Ergle K., Muppala V., Morshed S., Rand K., Clapp W., Proudfoot A., Lucas A. Selective deletion of heparan sulfotransferase enzyme, Ndst1, in donor endothelial and myeloid precursor cells significantly decreases acute allograft rejection. Sci. Rep. 2018;8(1):13433. doi: 10.1038/s41598-018-31779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Zheng D., Abbott J., Liu L., Bartee M.Y., Long M., Davids J., Williams J., Feldmann H., Strong J., Grau K.R., Tibbetts S., Macaulay C., McFadden G., Thoburn R., Lomas D.A., Spinale F.G., Virgin H.W., Lucas A. Myxomavirus-derived serpin prolongs survival and reduces inflammation and hemorrhage in an unrelated lethal mouse viral infection. Antimicrob. Agents Chemother. 2013;57(9):4114–4127. doi: 10.1128/AAC.02594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai E., Guan H., Liu L., Little S., McFadden G., Vaziri S., Cao H., Ivanova I.A., Bocksch L., Lucas A. Serp-1, a viral anti-inflammatory serpin, regulates cellular serine proteinase and serpin responses to vascular injury. J. Biol. Chem. 2003;278(20):18563–18572. doi: 10.1074/jbc.M209683200. [DOI] [PubMed] [Google Scholar]
- 9.Dai E., Liu L.Y., Wang H., McIvor D., Sun Y.M., Macaulay C., King E., Munuswamy-Ramanujam G., Bartee M.Y., Williams J., Davids J., Charo I., McFadden G., Esko J.D., Lucas A.R. Inhibition of chemokine-glycosaminoglycan interactions in donor tissue reduces mouse allograft vasculopathy and transplant rejection. PLoS One. 2010;5(5):e10510. doi: 10.1371/journal.pone.0010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalani A.S., Graham K., Mossman K., Rajarathnam K., Clark-Lewis I., Kelvin D., McFadden G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 1997;71(6):4356–4363. doi: 10.1128/JVI.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas A., Liu L., Macen J., Nash P., Dai E., Stewart M., Graham K., Etches W., Boshkov L., Nation P.N., Humen D., Hobman M.L., McFadden G. Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation. 1996;94(11):2890–2900. doi: 10.1161/01.CIR.94.11.2890. [DOI] [PubMed] [Google Scholar]
- 12.Lucas A.R., Verma R.K., Dai E., Liu L., Chen H., Kesavalu S., Rivera M., Velsko I., Ambadapadi S., Chukkapalli S., Kesavalu L. Myxomavirus anti-inflammatory chemokine binding protein reduces the increased plaque growth induced by chronic Porphyromonas gingivalis oral infection after balloon angioplasty aortic injury in mice. PLoS One. 2014;9(10):e111353. doi: 10.1371/journal.pone.0111353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahon B.P., Ambadapadi S., Yaron J.R., Lomelino C.L., Pinard M.A., Keinan S., Kurnikov I., Macaulay C., Zhang L., Reeves W., McFadden G., Tibbetts S., McKenna R., Lucas A.R. Crystal structure of cleaved Serp-1, a myxomavirus-derived immune modulating serpin: structural design of serpin reactive center loop peptides with improved therapeutic function. Biochemistry. 2018;57(7):1096–1107. doi: 10.1021/acs.biochem.7b01171. [DOI] [PubMed] [Google Scholar]
- 14.Mossman K., Upton C., McFadden G. The myxoma virus-soluble interferon-gamma receptor homolog, M-T7, inhibits interferon-gamma in a species-specific manner. J. Biol. Chem. 1995;270(7):3031–3038. doi: 10.1074/jbc.270.7.3031. [DOI] [PubMed] [Google Scholar]
- 15.Nash P., Whitty A., Handwerker J., Macen J., McFadden G. Inhibitory specificity of the anti-inflammatory myxoma virus serpin, SERP-1. J. Biol. Chem. 1998;273(33):20982–20991. doi: 10.1074/jbc.273.33.20982. [DOI] [PubMed] [Google Scholar]
- 16.Tardif J-C., L’Allier P.L., Grégoire J., Ibrahim R., McFadden G., Kostuk W., Knudtson M., Labinaz M., Waksman R., Pepine C.J., Macaulay C., Guertin M.C., Lucas A. A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2010;3(6):543–548. doi: 10.1161/CIRCINTERVENTIONS.110.953885. [DOI] [PubMed] [Google Scholar]
- 17.Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258(5086):1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 18.Viswanathan K., Liu L., Vaziri S., Dai E., Richardson J., Togonu-Bickersteth B., Vatsya P., Christov A., Lucas A.R. Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 2006;95(3):499–510. doi: 10.1160/TH05-07-0492. [DOI] [PubMed] [Google Scholar]
- 19.Yaron J.R., Zhang L., Guo Q., Burgin M., Schutz L.N., Awo E., Wise L., Krause K.L., Ildefonso C.J., Kwiecien J.M., Juby M., Rahman M.M., Chen H., Moyer R.W., Alcami A., McFadden G., Lucas A.R. Deriving immune modulating drugs from viruses - a new class of biologics. J. Clin. Med. 2020;9(4):E972. doi: 10.3390/jcm9040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwiecien J., Jarosz B., Urdzikova L.M., Rola R., Dabrowski W. Subdural infusion of dexamethasone inhibits leukomyelitis after acute spinal cord injury in a rat model. Folia Neuropathol. 2015;53(1):41–51. doi: 10.5114/fn.2015.49973. [DOI] [PubMed] [Google Scholar]
- 21.Kwiecien J.M., Jarosz B., Oakden W., Klapec M., Stanisz G.J., Delaney K.H., Kotlinska-Hasiec E., Janik R., Rola R., Dabrowski W. An in vivo model of anti-inflammatory activity of subdural dexamethasone following the spinal cord injury. Neurol. Neurochir. Pol. 2016;50(1):7–15. doi: 10.1016/j.pjnns.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Kwiecien J.M., Dabrowski W., Marzec-Kotarska B., Kwiecien-Delaney C.J., Yaron J.R., Zhang L., Schutz L., Lucas A.R. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019;57(1):41–50. doi: 10.5114/fn.2019.83830. [DOI] [PubMed] [Google Scholar]
- 23.Kwiecien J.M., Dabrowski W., Kwiecien-Delaney B.J., Kwiecien-Delaney C.J., Siwicka-Gieroba D., Yaron J.R., Zhang L., Delaney K.H., Lucas A.R. Neuroprotective effect of subdural infusion of Serp-1 in spinal cord trauma. Biomedicines. 2020;8(10):372. doi: 10.3390/biomedicines8100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blomster L.V., Brennan F.H., Lao H.W., Harle D.W., Harvey A.R., Ruitenberg M.J. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 2013;247:226–240. doi: 10.1016/j.expneurol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Ramer L.M., Au E., Richter M.W., Liu J., Tetzlaff W., Roskams A.J. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J. Comp. Neurol. 2004;473(1):1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- 26.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero A.R., Uchida K., Nakajima H., Watanabe S., Nakamura M., Johnson W.E., Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwiecien J.M. Cellular mechanisms of white matter regeneration in an adult dysmyelinated rat model. Folia Neuropathol. 2013;51(3):189–202. doi: 10.5114/fn.2013.37703. [DOI] [PubMed] [Google Scholar]
- 29.Klatzo I. Evolution of brain edema concepts. Acta Neurochir. Suppl. (Wien) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- 30.Bloch O., Papadopoulos M.C., Manley G.T., Verkman A.S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005;95(1):254–262. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- 31.Amiry-Moghaddam M., Otsuka T., Hurn P.D., Traystman R.J., Haug F.M., Froehner S.C., Adams M.E., Neely J.D., Agre P., Ottersen O.P., Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. USA. 2003;100(4):2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemley S.J., Bilston L.E., Cheng S., Chan J.N., Stoodley M.A. Aquaporin-4 expression in post-traumatic syringomyelia. J. Neurotrauma. 2013;30(16):1457–1467. doi: 10.1089/neu.2012.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen S., Nagelhus E.A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O.P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rash J.E., Yasumura T., Hudson C.S., Agre P., Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marmarou A., Hochwald G., Nakamura T., Tanaka K., Weaver J., Dunbar J. Brain edema resolution by CSF pathways and brain vasculature in cats. Am. J. Physiol. 1994;267(2 Pt 2):H514–H520. doi: 10.1152/ajpheart.1994.267.2.H514. [DOI] [PubMed] [Google Scholar]
- 36.Kwiecien J.M., Dabrowski W., Yaron R.J., Zhang L., Delaney K.H., Lucas A.R. The role of astrogliosis in formation of the syrinx in spinal cord injury. Curr. Neuropharmacol. 2020;18:1. doi: 10.2174/1570159X18666200720225222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw K., Smith M. Disorders of sodium balance after brain injury. Contin. Educ. Anaesth. Crit. Care Pain. 2008;8:129. doi: 10.1093/bjaceaccp/mkn019. [DOI] [Google Scholar]
- 38.Human T., Cook A.M., Anger B., Bledsoe K., Castle A., Deen D., Gibbs H., Lesch C., Liang N., McAllen K., Morrison C., Parker D., Jr, Rowe A.S., Rhoney D., Sangha K., Santayana E., Taylor S., Tesoro E., Brophy G. Treatment of hyponatremia in patients with acute neurological injury. Neurocrit. Care. 2017;27(2):242–248. doi: 10.1007/s12028-016-0343-x. [DOI] [PubMed] [Google Scholar]
- 39.Escartin C., Bonvento G. Targeted activation of astrocytes: a potential neuroprotective strategy. Mol. Neurobiol. 2008;38(3):231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- 40.Karimi-Abdolrezaee S., Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol. Neurobiol. 2012;46(2):251–264. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 41.Kwiecien J.M., O’Connor L.T., Goetz B.D., Delaney K.H., Fletch A.L., Duncan I.D. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J. Neurocytol. 1998;27(8):581–591. doi: 10.1023/A:1006922227791. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor L., Goetz B., Kwiecien J.M., Delaney K., Fletch A., Duncan I.D. Insertion of a retrotransposon into the myelin basic protein gene causes CNS dysmyelination in the Long Evans shaker (LES) rat. J. Neurosci. 1999;19:3404–3413. doi: 10.1523/JNEUROSCI.19-09-03404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwiecien J.M. Cellular compensatory mechanisms in the CNS of dysmyelinated rats. Comp. Med. 2010;60(3):205–217. [PMC free article] [PubMed] [Google Scholar]
- 44.Eftekharpour E., Karimi-Abdolrezaee S., Sinha K., Velumian A.A., Kwiecien J.M., Fehlings M.G. Structural and functional alterations of spinal cord axons in adult Long Evans Shaker (LES) dysmyelinated rats. Exp. Neurol. 2005;193(2):334–349. doi: 10.1016/j.expneurol.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Seki T., Fehlings M.G. Mechanistic insights into posttraumatic syringomyelia based on a novel in vivo animal model. Laboratory investigation. J. Neurosurg. Spine. 2008;8(4):365–375. doi: 10.3171/SPI/2008/8/4/365. [DOI] [PubMed] [Google Scholar]
- 46.Austin J., Afshar M., Fehlings M.G. The relationship between localized subarachnoid inflammation and parenchymal pathophysiology after spinal cord injury. J. Neurotrauma. 2012;29:1838–1849. doi: 10.1089/neu.2012.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwiecien J.M. 2016. [Google Scholar]
- 48.Bracken M.B., Shepard M.J., Collins W.F., Holford T.R., Young W., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J., Marshall L.F., Perot P.L., Jr, Piepneier J., Sonntag V.K.H., Wagner F.C., Wilberger J.E., Winn H.R. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 49.Bowes A.L., Yip P.K. Modulating inflammatory cell responses to spinal cord injury: all in good time. J. Neurotrauma. 2014;31(21):1753–1766. doi: 10.1089/neu.2014.3429. [DOI] [PubMed] [Google Scholar]
- 50.Schoenfeld A.J., Laughlin M.D., McCriskin B.J., Bader J.O., Waterman B.R., Belmont P.J. Jr Spinal injuries in United States military personnel deployed to Iraq and Afghanistan: an epidemiological investigation involving 7877 combat casualties from 2005 to 2009. Spine. 2013;38(20):1770–1778. doi: 10.1097/BRS.0b013e31829ef226. [DOI] [PubMed] [Google Scholar]
- 51.Shank C.D., Walters B.C., Hadley M.N. Current topics in the management of acute traumatic spinal cord injury. Neurocrit. Care. 2019;30(2):261–271. doi: 10.1007/s12028-018-0537-5. [DOI] [PubMed] [Google Scholar]
- 52.Hurlbert R.J., Hadley M.N., Walters B.C., Aarabi B., Dhall S.S., Gelb D.E., Rozzelle C.J., Ryken T.C., Theodore N. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(Suppl. 2):93–105. doi: 10.1227/NEU.0b013e31827765c6. [DOI] [PubMed] [Google Scholar]
- 53.Pettiford J.N., Bikhchandani J., Ostlie D.J., St Peter S.D., Sharp R.J., Juang D. A review: the role of high dose methylprednisolone in spinal cord trauma in children. Pediatr. Surg. Int. 2012;28(3):287–294. doi: 10.1007/s00383-011-3012-3. [DOI] [PubMed] [Google Scholar]
- 54.Ahuja C.S., Martin A.R., Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000 Res. 2016;5:1017. doi: 10.12688/f1000research.7586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brodbelt A.R., Stoodley M.A., Watling A.M., Tu J., Jones N.R. Fluid flow in an animal model of post-traumatic syringomyelia. Eur. Spine J. 2003;12(3):300–306. doi: 10.1007/s00586-002-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulndreaj A., Badner A., Fehlings M.G. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000 Res. 2017;6:1907. doi: 10.12688/f1000research.11633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordaro M., Casili G., Paterniti I., Cuzzocrea S., Esposito E. Fumaric acid esters attenuate secondary degeneration after spinal cord injury. J. Neurotrauma. 2017;34(21):3027–3040. doi: 10.1089/neu.2016.4678. [DOI] [PubMed] [Google Scholar]
- 58.Cox A., Varma A., Barry J., Vertegel A., Banik N. Nanoparticle estrogen in rat spinal cord injury elicits rapid anti-inflammatory effects in plasma, cerebrospinal fluid, and tissue. J. Neurotrauma. 2015;32(18):1413–1421. doi: 10.1089/neu.2014.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta S., Iwashita Y., Kakinoki R., Noguchi T., Nakamura T. Effects of continuous intravenous infusion of MCI-186 on functional recovery after spinal cord injury in rats. J. Neurotrauma. 2011;28(2):289–298. doi: 10.1089/neu.2010.1477. [DOI] [PubMed] [Google Scholar]
- 60.Lee J.Y., Choi H.Y., Park C.S., Ju B.G., Yune T.Y. Mithramycin A improves functional recovery by inhibiting BSCB disruption and hemorrhage after spinal cord injury. J. Neurotrauma. 2018;35(3):508–520. doi: 10.1089/neu.2017.5235. [DOI] [PubMed] [Google Scholar]
- 61.Impellizzeri D., Cordaro M., Bruschetta G., Siracusa R., Crupi R., Esposito E., Cuzzocrea S. N-palmitoylethanolamine-oxazoline as a new therapeutic strategy to control neuroinflammation: neuroprotective effects in experimental models of spinal cord and brain injury. J. Neurotrauma. 2017;34(18):2609–2623. doi: 10.1089/neu.2016.4808. [DOI] [PubMed] [Google Scholar]
- 62.Kwiecien J.M. Methods for Assessing Serpins as Neuroprotective Therapeutics. Methods Mol. Biol. 2018;1826:223–235. doi: 10.1007/978-1-4939-8645-3_15. [DOI] [PubMed] [Google Scholar]
- 63.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 64.Kwiecien J.M., Yaron J.R., Zhang L., Delaney K.H., Lucas A.R. Neurologic and histologic tests used to measure neuroprotective effectiveness of serpins. Methods Mol. Biol. 2021;222:227–239. doi: 10.1007/978-1-0716-1012-1_13. [DOI] [PubMed] [Google Scholar]
- 65.Kwiecien J.M., Zhang L., Yaron J.R., Schutz L.N., Kwiecien‐Delaney C.J., Enkidia A., Awo E.A., Burgin M., Dabrowski W., Lucas A.R. Local chitosan‐serpin injection after spinal cord injury reduces inflammatory damage and improves neurologic function. J. Clin. Med. 2020;9:E1221. doi: 10.3390/jcm9041221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao K., Shen Z., Yuan Y., Han D., Song C., Guo Y., Mei X. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/β-catenin signaling pathway after spinal cord injury. J. Neurochem. 2016;138(1):139–149. doi: 10.1111/jnc.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia G., Zhang Y., Li W., Dai H. Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, anti-neuroinflammatory and anti-apoptotic properties. Mol. Med. Rep. 2019;20(4):3433–3439. doi: 10.3892/mmr.2019.10537. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L.Y., Tian Z.R., Yao M., Chen X.Q., Song Y.J., Ye J., Yi N.X., Cui X.J., Wang Y.J. Riluzole promotes neurological function recovery and inhibits damage extension in rats following spinal cord injury: a meta-analysis and systematic review. J. Neurochem. 2019;150(1):6–27. doi: 10.1111/jnc.14686. [DOI] [PubMed] [Google Scholar]
- 69.Oakden W., Kwiecien J.M., O’Reilly M.A., Lake E., Akens M.K., Aubert I., Whyne C., Hynynen K., Stanisz G.J. A non-invasive model of cervical spinal cord injury induced with focused ultrasound. J. Neurosci. Methods. 2014;235:92–100. doi: 10.1016/j.jneumeth.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 70.Oakden W., Kwiecien J.M., O’Reilly M.A., Dabrowski W., Whyne C., Finkelstein J., Hynynen K., Stanisz G.J. Quantitative MRI in a non-surgical model of cervical spinal cord injury. NMR Biomed. 2015;28(8):925–936. doi: 10.1002/nbm.3326. [DOI] [PubMed] [Google Scholar]
- 71.Zhang C., Morozova A.Y., Abakumov M.A., Gubsky I.L., Douglas P., Feng S., Bryukhovetskiy A.S., Chekhonin V.P. Precise delivery into chronic spinal cord injury syringomyelic cysts with magnetic nanoparticles MRI visualization. Med. Sci. Monit. 2015;21:3179–3185. doi: 10.12659/MSM.895624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider G., Fries P., Wagner-Jochem D., Thome D., Laurer H., Kramann B., Mautes A., Hagen T. Pathophysiological changes after traumatic brain injury: comparison of two experimental animal models by means of MRI. MAGMA. 2002;14(3):233–241. doi: 10.1007/BF02668217. [DOI] [PubMed] [Google Scholar]
- 73.Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., Diaz-Arrastia R. Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 2016;275(Pt 3):353–366. doi: 10.1016/j.expneurol.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Yu F., Shukla D.K., Armstrong R.C., Marion C.M., Radomski K.L., Selwyn R.G., Dardzinsky B.J. Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J. Neurotrauma. 2017;34:1364–1381. doi: 10.1089/neu.2016.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]