Abstract
The growth factors BDNF and GDNF are gaining more and more attention as modulators of synaptic transmission in the mature central nervous system (CNS). The two molecules undergo a regulated secretion in neurons and may be anterogradely transported to terminals where they can positively or negatively modulate fast synaptic transmission. There is today a wide consensus on the role of BDNF as a pro-nociceptive modulator, as the neurotrophin has an important part in the initiation and maintenance of inflammatory, chronic, and/or neuropathic pain at the peripheral and central level. At the spinal level, BDNF intervenes in the regulation of chloride equilibrium potential, decreases the excitatory synaptic drive to inhibitory neurons, with complex changes in GABAergic/glycinergic synaptic transmission, and increases excitatory transmission in the superficial dorsal horn. Differently from BDNF, the role of GDNF still remains to be unraveled in full. This review resumes the current literature on the interplay between BDNF and GDNF in the regulation of nociceptive neurotransmission in the superficial dorsal horn of the spinal cord. We will first discuss the circuitries involved in such a regulation, as well as the reciprocal interactions between the two factors in nociceptive pathways. The development of small molecules specifically targeting BDNF, GDNF and/or downstream effectors is opening new perspectives for investigating these neurotrophic factors as modulators of nociceptive transmission and chronic pain. Therefore, we will finally consider the molecules of (potential) pharmacological relevance for tackling normal and pathological pain.
Keywords: BDNF, GDNF, nociception, spinal dorsal horn, chronic pain, neuropathic pain
1. INTRODUCTION
Neurotrophic factors (NFs) are a large and heterogeneous group of molecules supporting the growth, survival, and differentiation of developing and mature neurons. However, besides their “classical” trophic activity, NFs also act as neuronal modulators, affecting synaptic plasticity and neuronal endeavor [1]. Several NFs have received particular attention as modulators of nociception and players in the maladaptive changes leading to chronic pain under pathological conditions [2, 3]. Initial studies on the intervention of NFs in the modulation of the nerve pathways that convey pain-related information to the brain have mainly focused on the role of nerve growth factor (NGF) in nociception and led to the development of novel pharmacological approaches to treat certain altered pain conditions [4]. More recently, the brain-derived neurotrophic factor (BDNF) [5] and the glial cell line-derived neurotrophic factor (GDNF) [6] have gained increasing attention as pain modulators. The reasons for such an interest are that the two factors and their receptors are expressed by the nociceptors, and the former likely play a role in the sensitization processes leading to chronic pain. However, the anatomical localization and the effects on neuronal activity induced by BDNF and GDNF are different, thus suggesting a dissimilar contribution to the processing of nociceptive stimuli.
In this review, we will reconsider the state of art roles played by BDNF and GDNF in the control of nociceptive transmission and in the maladaptive changes that occur in pain pathological conditions. We will pay particular attention to the putative interactions and complementarities between the two molecules and, eventually, to the pharmacological strategies that may derive from this knowledge.
2. NEUROTROPHIC FACTOR FAMILIES AND THEIR RECEPTORS: AN OVERVIEW
NFs belong to three main families: the neurotrophin (NT) family, the GDNF ligand (GFL) family and the ciliary neurotrophic factor (CNTF) family. Here, we will focus on the NT and GFL families, which include BDNF and GDNF, respectively. There are two reasons for this choice. First, during development, NTs and GFLs promote the differentiation and survival of the primary sensory neurons [7-11]. Second, in adulthood, they intervene in the regulation of the functional properties of these neurons, particularly in the modulation of their synaptic activity [5, 6, 12, 13].
2.1. The Neurotrophin Family
NGF, BDNF, neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5) compose the NT family. NTs share numerous commonalities: first, they all express extensive structural homologies in both genes and proteins [14]; second, the encoding genes contain a signal sequence and a prodomain; hence NTs are synthesized as pro-NTs, which need to be subsequently proteolyzed to obtain the functionally active mature molecules; third, NT molecules are typically released as homodimers, which is the required configuration for binding to their respective receptors.
NTs activate two different families of membrane receptors: a pan-NT receptor, known as p75NTR, and a group of tyrosine kinase receptors, collectively known as Trk receptors [15, 16].
p75NTR is a member of the tumor necrosis factor superfamily. It consists of an extracellular domain with four cysteine-rich motifs and an intracellular domain (including a “death domain”) acting on a pool of cytoplasmatic proteins. The receptor expression level in the peripheral and central nervous system decreases with the progression of development, but typically increases following injury [17]. p75NTR lacks an intrinsic catalytic activity, however, it can interact with other NT receptors (i.e., the Trk receptors), as well as with sortilin and Nogo, to produce a broad spectrum of downstream effects [17].
Both NT and pro-NT dimers activate p75NTR, although with opposing effects [18]. Specifically, pro-NTs bind p75NTR with high affinity and, in combination with the co-receptor sortilin, induce apoptosis or inhibit axonal growth [19]. This “death pathway” is intracellularly mediated by JNK kinases, which, in turn, cause cell death via the p53 and Bax pro-apoptotic pathways [18]. Unlike pro-NTs, mature NTs exhibit a low affinity for p75NTR and promote neuronal survival and differentiation [14, 20]. This “life pathway” seems largely mediated via the interaction between p75NTR and the Trk receptors, i.e. by increasing the affinity of the mature NTs for the Trks [21].
Each mature member of the NT family binds with high affinity to a specific Trk receptor: NGF preferentially activates TrkA, BDNF and NT4/5 bind TrkB, and NT3 is associated with TrkC, but it is also a low-affinity agonist for TrkA and TrkB [22]. Trk receptors have an extracellular domain consisting of a cysteine-rich cluster, three leucine-rich repeats, another cysteine-rich cluster, two immunoglobulin- like domains, and an intracellular domain that possesses tyrosine kinase activity [14]. NTs binding induces Trk receptor dimerization, followed by phosphorylation of the cytoplasmic tyrosine residues, which, in turn, initiates the activation of downstream effectors [22]. Interestingly, Trk receptors are also active in the absence of NTs. Namely, they can be trans-activated via a signaling pathway triggered by several G protein-coupled receptors (GPCRs [14, 23]). The adenosine receptor A2A was firstly proposed to trans-activate TrkB receptors via G protein-dependent activation of Src kinases [24]. Subsequently, other GPCR neuromodulators were shown to induce Trk transactivation, such as the pituitary adenylate cyclase-activating polypeptide receptor (PACAP [23],), the angiotensin receptor type 2 [25], and the low-density lipoprotein receptor-related protein-1 [26]. Tyrosine kinase activity can also be induced by mechanisms other than those mediated by GPCRs, for instance, by the epidermal growth factor receptor [27], or zinc, which is co-released with glutamate at excitatory synapses [28].
Trk receptor activation is associated with three major signaling pathways: the Ras/mitogen-activated protein kinase (MAPK) pathway; the phosphatidylinositol-3-kinase (PI3K)/Akt pathway, and the phospholipase C-γ (PLCγ)/diacylglycerol (DAG)/protein kinase C (PKC) pathway [14, 29]. All these pathways are involved in cell survival and differentiation, but also in the modulation of neuronal activity (i.e., acting on ion channels and ion transporters) and in the regulation of neuronal plasticity [14, 16, 22]. PLCγ is then thought to play a major role in synaptic plasticity through inositol-3-phosphate release and Ca2+ mobilization from intracellular stores.
To add another level of complexity, different splicing variants exist in each group of Trk receptors. Besides the “classical” full-length forms, splicing generates TrkB and TrkC isoforms that miss the tyrosine kinase domain and are often referred to as truncated receptor variants [30]. Although the role of these isoforms is still poorly understood, yet different studies have demonstrated that they are not inactive, as originally thought, but can evoke Ca2+ transients and trigger intracellular signals. Interestingly, a growing number of evidence indicates that signals from truncated Trk isoforms typically counteract the function of the respective full-length receptors, for instance, by inhibiting dimerization or by disrupting intracellular responses [31-35].
2.2. The GDNF Ligand Family
The members of the GFL family, GDNF, neurturin, artemin and persephin, are structurally related to the transforming growth factor β (TGF-β) superfamily [36, 37]. Each member of the family is translated as a pre-pro-precursor protein, which is subsequently cleaved to generate an active form [38, 39]. GFLs signal through a multi-competent receptor complex comprising the tyrosine kinase transmembrane receptor Ret and a high-affinity ligand-binding glycosylphosphatidylinositol (GPI)-anchored co-receptor referred to as GFRα [37]. Ret is a tyrosine kinase receptor constituted by an extracellular domain containing several cadherin-like repeats, a hydrophobic transmembrane domain, and an intracellular tyrosine kinase domain [40]. For the ligand to activate RET, it must bind first to the appropriate GFRα co-receptor. Each member of the GFL family matches with a specific GFRα isoform characterized by a distinct structure of the cysteine-rich repeats in the ligand-binding domains [37]. Namely, GDNF preferentially binds to GFRα1, neurturin to GFRα2, artemin to GFRα3 and persephin to GFRα4 [36]. Like NTs, also GFLs are released as homodimers, each binding two GFRα receptors that, in turn, induce Ret dimerization, thus conserving a 2:2:2 stoichiometry [41]. Ret dimers activate downstream intracellular pathways through their cytosolic tyrosine kinase domain. The interaction between GFLs, GFRα and Ret is favored by glycosaminoglycans, such as heparan sulphate, as the lack of these molecules is sufficient to abolish the survival and axonal growth effects of GDNF [42, 43]. Moreover, receptor/co-receptor interaction mainly occurs within sphingolipid- and cholesterol-rich membrane microdomains, known as lipid rafts, in which GFRsα are confined [44]. Therefore, the disruption of Ret localization in lipid rafts significantly weakens the associated intracellular signals [44]. GFRα-Ret activation mainly signals through Ras/MAPK, phosphatidylinositol-3 kinase (PI3K)/Akt and PLCγ, which play a key role in neurite outgrowth and cell survival in the nervous system [45-47].
Consistently with a broader expression of GFRα than Ret in neurons, GFLs can also transduce through GFRα in a Ret-independent pathway [47, 48]. This was clearly demonstrated in Ret-deficient cell lines, in which GFRα-mediated signals activate the Src-like kinase that phosphorylates the cAMP response element-binding protein (CREB) and induces c-fos expression and cell survival [48]. A special role in the activation of the Ret-independent pathway is played by the neural cell adhesion molecule (NCAM). Actually, NCAM acts as an alternative signaling pathway for GFLs, at the point that GFRα-NCAM, GDNF-NCAM and GDNF-GFRα-NCAM may differentially transduce various intracellular pathways (vertical signaling) or engage other molecules at the cell membrane (horizontal signaling) [46, 47, 49].
3. LOCALIZATION OF BDNF AND GDNF AND THEIR RECEPTORS IN NOCICEPTIVE PATHWAYS
Spinal nociceptive pathways, in their most simple configuration, consist of a chain of three neurons: a primary sensory neuron, or nociceptor, located in dorsal root ganglia (DRGs), a secondary neuron in the spinal cord dorsal horn, and a third neuron in the ventroposterolateral nucleus of the thalamus, which eventually transmits the nociceptive information to the somatosensory cortex. An analogue chain of neurons conveys nociceptive stimuli from the head and the initial part of the neck along the trigeminal pathway.
BDNF, GDNF and their receptors have been consistently described in these pathways, and particularly in DRGs and in spinal dorsal horn neurons (see for review [5, 6] and Fig. 1). Understanding the anatomical distribution of these molecules into labeled nociceptive lines is the necessary premise to get insight into their role in pain processing.
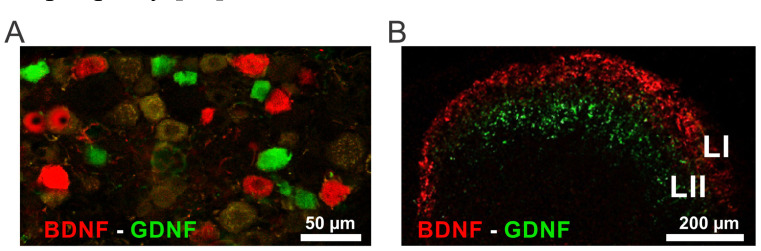
Fig. (1).
Localization of BDNF and GDNF in the mouse DRGs and spinal dorsal horn. (A) Distribution of the two NFs in DRGs. Both BDNF (red) and GDNF (green) are expressed in small- to-medium primary sensory neurons, yet in distinct populations. (B) Distribution of BDNF (red) and GDNF (green) in the superficial dorsal horn. BDNF immunoreactive fibers are mainly localized in laminae I-II outer, while GDNF immunoreactive fibers mainly end in lamina II. Abbreviations: LI = lamina I, LII = lamina II. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Localization of BDNF and its Preferred Receptor TrkB in DRGs and Dorsal Horn of the Spinal Cord
Early in situ hybridisation and immunohistochemical studies demonstrated the expression of BDNF mRNA and protein by a subpopulation of sensory neurons in DRGs, both in rodent and human tissues [50-52]. In mice and rats, BDNF positive DRG neurons are mainly small-to-medium- sized [53-55]. BDNF is localized in a population of peptidergic neurons expressing the calcitonin gene-related peptide (CGRP), which typically characterizes the nociceptors encoding thermal pain [56, 57]. Conversely, the neurotrophin is not expressed by non-peptidergic sensory neurons, that are identified by isolectin B4 (IB4), and represent a group of nociceptors principally involved in the transduction of mechanical pain [56, 57]. Accordingly, BDNF-positive fibres in the superficial dorsal horn overlap the localization of CGRP-expressing fibres in lamina I and lamina II outer [55, 58]. Interestingly, BDNF has also been observed in sensory neurons that express neither CGRP nor IB4 [55]. Consistently, by using a BDNF-LAcZ reporter transgenic mouse, Basbaum’s group has recently demonstrated a broader expression of BDNF than previously described [59]. In particular, these authors observed expression in large myelinated primary afferents, suggesting a still poorly understood role in the processing of non-nociceptive sensory information from the periphery [59].
The TrkB protein and mRNA are also expressed by DRG neurons of variable size in both rodents and humans [51, 52, 54, 60], with a majority of medium-to-large-sized neurons [60]. By using transgenic TrkBtauEGFP, Li and colleagues [61] demonstrated a foremost expression of the BDNF receptor in Aδ low threshold mechanoreceptors, which heavily terminate in lamina III of the dorsal horn. On the other hand, we have previously shown that a subset of CGRP-positive nociceptors also co-express BDNF and TrkB, which suggests the existence of an autocrine activation loop at their peptidergic terminals [54].
BDNF and TrkB are also expressed by sparse propriospinal neurons. In situ hybridization studies showed the expression of the BDNF mRNA in the deep dorsal horn of adult rats [52, 58]. Conversely, the receptor is expressed by the majority of neurons in the spinal cord [52, 62], including most ascending projection neurons in lamina I and in the deep dorsal horn laminae [62].
The expression of BDNF and TrkB in pain-related supraspinal centres has received less attention. However, few studies have demonstrated the involvement of the neurotrophin in different key areas for pain encoding, including the thalamus [63] and the parabrachial-amygdaloid pathways [64]. Previously, our group, in collaboration with Priestley’s group, demonstrated that BDNF is costored in individual dense-core vesicles (DCVs) with CGRP in the parabrachial projection to the amygdala [65].
3.2. Localization of GDNF and Its Preferred Receptor GFRα1/Ret in DRGs and the Dorsal Horn of the Spinal Cord
As BDNF, GDNF is mainly expressed by small-to-medium-sized neurons in DRGs [55, 66, 67], although in a distinct and smaller population [55]. In mouse DRGs, GDNF is localized in a specific subgroup of peptidergic neurons containing CGRP and somatostatin [55]. The neurotrophic factor is anterogradely transported to the spinal dorsal horn, as suggested by immunohistochemical studies that localized the protein in the peptidergic afferent fibres within lamina I and lamina II outer [55, 68-70].
The mRNA of the GFL family receptor Ret is expressed by more than half of the lumbar DRG neurons in both the rat [66, 71-73] and mouse [74]. Most of the RET-expressing neurons also express the GDNF specific co-receptor GFRα1: 66% in adult rats [73] and 89% in adult mice [75]. A similar expression pattern is also detected in the thoracic DRGs from adult humans, albeit in a sensibly lower percentage [51]. Interestingly, while GDNF is mainly expressed by the peptidergic neurons, the GFRα1/Ret receptor complex is typically found in the non-peptidergic IB4-positive neurons, as well as in a small subset of non-peptidergic IB4-negative neurons [75, 76]. During development, Ret is up-regulated in non-peptidergic neurons, while TrkA is depleted; thus these neurons pass from NGF- to GDNF-dependence [75]. Ret expression in these sensory neurons is critical for their differentiation and for the expression of several important molecules that are necessary for the detection of noxious stimuli [76, 77].
Consistently with the expression of GFRα1/Ret in non-peptidergic primary sensory neurons, GDNF receptors have been detected in primary afferent fibres in the dorsal horn, and, particularly, in the inner aspect of lamina II [70, 78]. Moreover, at the ultrastructural level, GFRα1 receptors are specifically expressed by IB4-positive terminals in the mouse spinal dorsal horn and are mainly localized ventrally to GDNF-expressing peptidergic terminals in lamina II [55, 70].
There is not compelling evidence of a propriospinal source of GDNF. Conversely, GFRα1/Ret are also expressed by some neurons in the dorsal horn, including putative projection neurons in lamina I that also express the substance P preferred receptor NK1 [78].
4. ROLE OF BDNF AND GDNF IN NOCICEPTION
Although the localization of BDNF and GDNF and their receptors in primary sensory neurons strongly suggests an intervention in nociception, yet their significance in pain physiology remains elusive. The lack of specific pharmacological tools targeting the two molecules together with the difficulty to generate classical knockout animals due to the requirement of both factors for neuronal survival have for long hampered the investigation of the role of BDNF and GDNF in nociception. In recent times, however, the development and availability of conditional mutant mice in which the expression of specific molecules can be selectively depleted in mature neurons has opened new avenues to interrogate the contribution of the two NFs to pain transmission.
Initial studies on the conditional blockade of a mutant TrkB receptor in mice led to conclude that BDNF does not contribute to acute heat, mechanical or chemical pain sensitivity [79]. More recently, Dembo and colleagues [59] have investigated the contribution of BDNF to nociceptive transmission by using a conditional transgenic mouse model in which BDNF can be selectively deleted in virtually all primary sensory neurons by tamoxifen injection. The ablation of BDNF produced only minor effects, including a slight increase in the heat threshold of male mice, but not females, to the tail immersion test and no effects on mechanical or cold sensitivity [59]. Consistently, it seems reasonable to infer that ongoing TrkB signaling facilitates the development of synaptic plasticity in the outer aspect of the superficial dorsal horn, where thermal input is mainly processed, while it has a minor impact deeper, where mechanical input is conveyed, thus making the system more prone to thermal sensitization [80].
A different scenario has been described when the GDNF receptor Ret was ablated from sensory neurons. By the conditional deletion of Ret in Nav1.8 expressing nociceptors in mice, Golden and colleagues [77] have demonstrated that the mutants express increased sensitivity to cold and increased formalin-induced pain, thus demonstrating a constitutive inhibitory role of Ret signaling in modulating nociception. These data are consistent with our findings ex vivo [70], demonstrating that GDNF, acting on the GFRα1 receptor complex, constitutively constrains the excitatory drive induced by afferent fiber activation upon spinal dorsal horn neurons (Fig. 2).
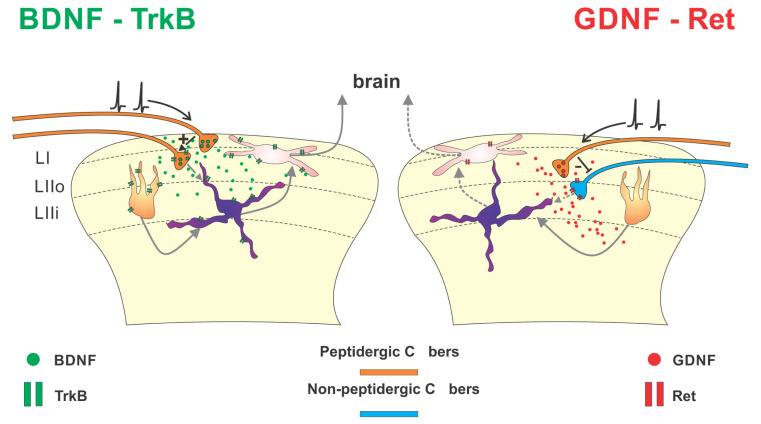
Fig. (2).
Effects of BDNF and GDNF on nociceptive transmission. The cartoon schematically illustrates the localization of BDNF (left) and GDNF (right) in spinal nociceptive pathways and their putative role in modulating nociceptive transmission. Left: BDNF (green dots) is synthesized by peptidergic primary sensory neurons in dorsal root ganglia and anterogradely transported to lamina I (LI) and outer lamina II (LIIo) of the dorsal horn via primary afferent C fibers (orange). BDNF acts both pre- and post-synaptically through its preferred receptor TrkB. Notably, BDNF+ peptidergic afferent fibers express pre-synaptic TrkB receptors, suggesting an autocrine function. BDNF/TrkB activation in superficial dorsal horn plays a major pro-nociceptive role by increasing local network excitability. Right: Another sub-population of peptidergic fibers (orange), distinct from that expressing BDNF, ends in lamina IIo and contains GDNF (red dots). Once released, GDNF activates the Ret/GFRα1 complex (in the diagram, only Ret is shown for simplicity), which is mainly expressed by non-peptidergic afferent terminals (blue) ending in inner lamina II (LIIi). GDNF, acting on pre-synaptic Ret/GFRα1, reduces the glutamate release associated with the activation of non-peptidergic fibers, thus limiting their associated excitatory drive. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
While the role of BDNF and GNDF in the physiology of pain transmission is still debated, it is, on the other hand, well established that both NFs significantly contribute to the maladaptive changes occurring in pathological conditions, as it will be described in the following paragraphs.
5. ROLE OF BDNF IN PATHOLOGICAL PAIN
Several lines of evidence converge to indicate that BDNF principally acts as a pro-nociceptive modulator in sensory pathways, with a central role in the initiation and maintenance of inflammatory, chronic and/or neuropathic pain [81]. Nevertheless, a BDNF anti-nociceptive modulation has also been occasionally described, suggesting heterogeneity of BDNF actions that depend on the molecular pathways involved, the cells affected and the type of pathological conditions (for review [5, 82-85]). In this section, we review the role of BDNF as a pain modulator emerging from different preclinical models of pathological pain.
5.1. BDNF as Pro-nociceptive Neuromodulator in Inflammatory Pain
BDNF expressed by the sensory neurons and released by their primary afferent fibers is necessary for the development of both formalin and carrageenan-induced inflammatory pain [86]. The pro-nociceptive effect of BDNF in inflammatory pain has been typically associated with a pre- and post-synaptic potentiation of the glutamatergic neurotransmission in the spinal dorsal horn via NMDA receptor plasticity. In carrageenan-treated rats, BDNF potentiates nociceptive spinal reflex activity and induces c-fos expression in dorsal horn neurons in NMDA receptor-dependent manner [87]. The BDNF-sequestering molecule trkB-IgG has also been observed to rescue hyperalgesic behavior and functional alterations [87]. Similarly, the co-administration of BDNF with the NMDA receptor antagonist D(-)-2-amino-5-phosphonovaleric acid (D-APV) prevents the BDNF-induced hyperalgesia in mice [88]. NMDA potentiation is mediated through the activation of PKC and PLC-dependent pathways [89, 90].
BDNF may act pre-synaptically to induce hyperalgesic behavior. A pre-synaptic mechanism has been described in a rat model of inflammation based on subcutaneous injections of complete Freund’s adjuvant (CFA) [91]. The authors showed that BDNF increases the presynaptic release of glutamate upon lamina II neurons. The effect was associated with an increased synaptic input from large myelinated Aβ afferent fibers in lamina II, which typically encode light touch stimuli and thus may underlie allodynia in CFA rats [91].
More recently, the contribution of peripheral BDNF to inflammatory pain has been reconsidered in a conditional Advilin-CreERT2 knock-out mouse model, which lacks BDNF specifically in sensory neurons [59, 81]. Both studies observed a reduced nocifensive response in the second phase of the formalin test, which indicates a reduced central sensitization [59, 81], although the effect was observed only in males [59]. Surprisingly, however, Dembo and colleagues [59] did not detect any difference in the development of pain hypersensitivity in the CFA model of chronic inflammatory pain. On the other hand, Sikandar and colleagues [81] observed that BDNF deletion critically affects hyperalgesic priming, i.e. a neuroplastic mechanism producing a latent state of hyperresponsiveness in sensory neurons, which is thought to favor the transition from acute to chronic pain [81]. Hyperalgesic priming was induced by intraplantar injection of carrageenan and the effect was uncovered by prostaglandin E2 (PGE2) injection after six days. While primed control mice expressed long-lasting hyperalgesia following PGE2 injection, mice lacking BDNF in sensory neurons only exhibit a transient decrease of nociceptive thresholds [81].
5.2. BDNF as Pro-nociceptive Neuromodulator in Neuropathic Pain
BDNF also underlies the maladaptive plasticity occurring in neuropathic pain. Early studies in rats and mice with partial sciatic nerve lesion demonstrated that spinal BDNF contributes to the alterations resulting from the nerve damage in nociceptive pathways [92-95]. Systemic administration of either anti-BDNF or anti-TrkB neutralizing antibodies, Trk inhibitors or the BDNF scavenger TrkB/Fc abolishes behavioral pain hypersensitivity [92-94]. Up-regulation of BDNF in sensory neurons and its central release upon spinal dorsal horn neurons have been observed in different nerve-injury models, including sciatic nerve transection, chronic constriction injury and nerve ligation [96-102].
A significant leap forward in understanding the role of BDNF in neuropathic pain was achieved with the study by Coull and colleagues [103]. The authors identified the spinal microglia as the main source of BDNF in nerve-injured rats. In this model, the purinergic P2X4 receptors activate spinal microglia to trigger the release of BDNF, which, in turn, decreases KCC2 function, the main Cl- extruder in lamina I projection neurons. The subsequent accumulation of Cl- in these neurons reduces the strength of GABA/glycine ionotropic transmission, hence leading to hyperexcitability and pain hypersensitivity. These effects are recapitulated by intrathecal transfer of ATP-stimulated microglia or by BDNF administration [103, 104], and are prevented by either depleting BDNF in microglia [103, 105], blocking TrkB [103, 105], or knocking out P2X4 expression [105, 106].
BDNF also causes hyperexcitability of spinal neurons by increasing the NMDA receptor function, as reported in inflammatory pain. Indeed, in rats with spinal nerve ligation, intrathecal injection of BDNF induces mechanical allodynia and long-term potentiation (LTP) via a mechanism involving the activation of NR2B-containing NMDA receptors [96, 107].
The concomitant alterations of inhibitory and excitatory transmission likely interact to sensitize central sensory neurons. Recent studies have specifically addressed this question. In particular, Hildebrand and colleagues [108] have reported that the downregulation of KCC2 induced by BDNF is both sufficient and necessary to promote NR2B-NMDA phosphorylation and LTP induction in lamina I neurons of nerve-injured rats. A key role in the signaling between BDNF-dependent Cl- dysregulation and NMDA phosphorylation is played by STEP61 phosphatase, whose down-regulation is coupled with KCC2 depletion and is sufficient to drive the potentiation of excitation [109]. Importantly, these mechanisms were also recapitulated in an ex-vivo spinal cord preparation obtained from human tissue, thus shortening the distance to clinical interventions [109].
Although the above-described mechanisms are based on post-synaptic alterations, pre-synaptic effects have also been reported. In mice with chronic constriction injury, BDNF increased the expression of the Cl- transporter NKCC1 in DRGs, thus causing an increase of intracellular Cl-, leading to transient presynaptic disinhibition and hypersensitivity [110]. The presynaptic effects are also likely mediated by microglial BDNF, which has been shown to act on afferent terminals for potentiating the NMDA-mediated responses [111]. Altogether, these data confirm the existence of coupling mechanisms involving Cl- dysregulation and NMDA potentiation at both pre- and post-synaptic levels, which may be affected by BDNF.
5.3. BDNF Involvement in other Forms of Chronic Pain
Besides inflammatory and neuropathic pain, BDNF is involved in several other forms of chronic pain, including pain following spinal cord injury [34], trigeminal pain and migraine [112-114], opioid-and nicotine-induced hyperalgesia [105, 115], painful diabetic neuropathy [116, 117] and cancer pain [118-122]. Although these different models of pathological pain are based on distinct etiological grounds, yet pain hypersensitivity gated by BDNF is associated with the recurrent involvement of a restricted number of molecular actors, including P2X4 receptors, KCC2/NKCC1 transporters and NMDA receptors. Such commonality of mechanisms has important implications in conceiving new treatments for future therapeutic approaches, as later discussed in this review.
5.4. BDNF as an Anti-nociceptive Neuromodulator
While most published data indicate BDNF as a pain amplifier, yet other lines of evidence suggest that it may also exert anti-nociceptive effects. Early studies in the field showed that the central administration of BDNF may produce analgesic behavior. For instance, injections of BDNF into the rat midbrain caused thermal analgesia, likely acting on endogenous opioidergic/serotoninergic system [123]. Similarly, intracerebroventricular injections of BDNF in rats increased the latency of hind paw licking in the hot-plate test [124].
BDNF has also been reported to reverse established allodynic/hyperalgesic behaviors in animals with neuropathic pain clinical signs. Indeed, the administration of BDNF intrathecally [125] or through viral vectors [126] reverses thermal and/or mechanical hypersensitivity following nerve injury. These anti-nociceptive effects of BDNF consistently rely on specific interactions between the trophic factor and synaptic inhibition mechanisms at both pre-and post-synaptic level. Pre-synaptically, TrkB activation stimulates the release of GABA from dorsal horn neurons upon primary afferent terminals [125, 127], while post-synaptically, it enhances the spontaneous release of GABA and glycine upon lamina II neurons [128]. However, it should be stressed out that the comprehension of the mechanisms of action of BDNF (and other modulators) in the superficial dorsal horn is made difficult by the still disguised circuitry of this area of CNS. As an example, the observations by Bardoni and colleagues [128] regarding the enhancement of inhibitory neurotransmission in lamina II have been recently correlated with a polysynaptic chain consisting of a C peptidergic nociceptor, two islet cells in sequence, a central cell, a large vertical cell, and, eventually, a lamina I excitatory projection neuron [129]. As the first islet cell inhibits the second islet cell that, in sequence, is inhibitory to the central cell, the circuit is excitatory onto the large vertical cell. Thus, this type of configuration can explain why BDNF was shown to produce ex vivo a depression of the evoked IPSCs as well as an increase of mIPSCs at synapses in the superficial dorsal horn [128].
Yet, the apparently contradictory co-existence of pro-nociceptive and anti-nociceptive effects is not surprising, as it belongs to the pleomorphic nature of BDNF. Recently, in fact, Huang and colleagues demonstrated that while the intrathecal administration of BDNF to the spinal cord of uninjured rats decreases KCC2, thus promoting disinhibition and hypersensitivity, nonetheless after spinal cord injury, the same BDNF treatment increases KCC2 and restores synaptic inhibition [130]. Such dual behavior likely depends on the activation of specific intracellular pathways, as well as on the involvement of different cell types, thus highlighting that it is not possible to achieve efficacious BDNF-targeted pharmacological solutions without an accurate understanding of the cellular and molecular actors and circuitries involved.
6. ROLE OF GDNF IN PATHOLOGICAL PAIN
GDNF displays both pro-nociceptive and anti-nociceptive functions in the sensory system, depending on the site of action and on the type of pain. While GDNF principally acts as an anti-nociceptive modulator in neuropathic pain, pro-nociceptive effects are often reported at the peripheral level and in experimental models of inflammatory pain (see for review [6, 12]).
6.1. GDNF as an Anti-nociceptive Neuromodulator
GDNF anti-nociceptive effects have been well established in different models of neuropathic pain, including nerve-injury and diabetic neuropathy [131, 132].
In rats with sciatic nerve partial ligation or L5 spinal nerve ligation, intrathecal infusion of GDNF reverses mechanical and thermal hyperalgesia and GDNF pre-treatment avoids the insurgence of these neuropathic pain signs by preventing the alterations induced by nerve-injury on peripheral sodium channel subunits [12, 131]. Similarly, injections of viral vectors inducing the expression of GNDF [133-135] or of mesenchymal stem cells over-expressing the trophic factor [136] alleviate the allodynic and hyperalgesic signs associated with a nerve injury in rodents. Specifically, intraspinal delivery of GDNF-expressing lentiviral vectors in rats prevented the up-regulation of the activating transcription factor 3 (ATF-3), a marker of nerve injury, as well as the down-regulation of the phenotypic marker of non-peptidergic nociceptors IB4 [134]. Injection of adenovirus vectors encoding GDNF attenuated allodynia and thermal hyperalgesia, reduced chronic constriction injury-induced neuronal apoptosis, inhibited microglia activation and cytokine production, indicating a neuroprotective action of the NF [133]. Similar effects have also been reported in mice [135]. Importantly, also human patients with neuropathic pain symptoms exhibit decreased levels of GDNF in their cerebrospinal fluid, thus reinforcing the clinical significance of the observations in animal models [137].
Different mechanisms have been proposed to underlie the anti-hyperalgesic effects of GDNF. GDNF released from peptidergic nociceptors may exert an inhibitory control on the glutamate excitatory drive from non-peptidergic fibres, with a subsequent reduction in neuronal activity [70]. Alternatively, GDNF may act on other targets, such as NCAM and other adhesion molecules, which concur to GDNF analgesic effects at the spinal level in a Ret-independent manner [138-140]. In addition, GDNF and its receptors may alter pre-synaptically the neuronal excitability by directly acting on voltage-dependent channels. Indeed, a potentiation of the Kv4.1 potassium channel in the sensory neurons of nerve-injured animals has been recently described [141]. These interactions may also require the modulation of other transmitters activating GPCRs that, in turn, reduce neuronal excitability and promote analgesia. In particular, intrathecal application of GDNF enhances the expression and the central release of the analgesic peptide somatostatin, suggesting a GDNF anti-allodynic effect via somatostatinergic mechanisms [142, 143].
Monoamines are another group of analgesic transmitters that have been associated with the GDNF analgesic effects. Indeed, injection of GDNF into the locus coeruleus of nerve-injured rats exerted prolonged analgesia by enhancing the descending noradrenergic inhibition to spinal dorsal horn neurons via α2-adrenoceptor [144].
Little is known, however, regarding the signaling pathways through which GDNF and its receptors produce analgesia. In spinal dorsal horn neurons, GDNF administration to nerve-injured rodents leads to the down-regulation of different intracellular pathways associated with pain hypersensitivity, such as nectin-1/c-Src [138] and integrin β1/FAK [145], or the up-regulation of anti-nociceptive pathways, such as E- cadherin/p120 catenin [146].
6.2. GDNF as a Pro-nociceptive Neuromodulator
While GDNF is frankly anti-nociceptive in neuropathic pain, it has more prominent pro-nociceptive functions in inflammatory pain. Ogun-Muyiwa and colleagues [147] observed that exogenous GDNF up-regulates pro-nociceptive mediators, such as substance P, in adult rat DRG cultures. The pronociceptive role was subsequently confirmed by direct intraplantar injection of GDNF, which enhances both thermal sensitivity mediated by the thermal transducer TRPV1 [148] and mechanical sensitivity associated with IB4- positive nociceptors [149, 150]. As IB4-positive neurons are known to express GFRα/Ret receptor complex, these sensory neurons are the more obvious target for GDNF-dependent sensitization. Indeed, ablating IB4-positive nociceptors prevent GDNF-induced mechanical hyperalgesia [149]. In the same study, Bogen and colleagues were able to reverse the GDNF-dependent hyperalgesia by interfering with several kinases downstream to GFRα/Ret, including PLCγ, MAPK/ERK, PI3K, CDK5 and Src family kinase.
The effect of GDNF on IB4-positive neurons is not only limited to nociceptor sensitization but also extends to hyperalgesic priming [151, 152]. GDNF induces priming in IB4- positive neurons via PKCε signaling pathway [151] and priming is directly associated with a reduction in the levels of the GPCR kinase 2 (GRK2) [152].
The involvement of GDNF in the development of inflammatory pain has been described in different chronic pain models, such as CFA-induced inflammation [153, 154]. In rats with CFA-induced arthritis, GDNF stored in DRGs is depleted and the intrathecal injection of a function-blocking antibody against GDNF decreases the inflammatory hyperalgesia [153]. GDNF, as well as other related GFLs (in particular artemin), have also been involved in several other models of inflammatory pain, including bone inflammation [155] and cystitis [156, 157].
7. INTERPLAY BETWEEN GDNF AND BDNF IN NOCICEPTIVE PATHWAYS
In the previous sections, we have explored in-depth the current knowledge on the role of BDNF and GDNF in the control of nociceptive neurotransmission under normal and pathological conditions. Interestingly, several distinctive and somehow complementary features characterized the localization of these two NFs in nociceptive pathways (Fig. 2). Specifically: 1- BDNF and GDNF are basically expressed by two distinct populations of peptidergic nociceptors [55]; 2- in DRGs, BDNF is mainly expressed by the same neurons expressing its cognate receptor TrkB, suggesting an autocrine function [5, 54], while the GDNF receptor complex (GFRα-Ret) is expressed by the non-peptidergic sensory neurons [66, 70]; 3- while clearly, pro-nociceptive and anti-nociceptive effects cannot be univocally associated to one trophic factor or the other, yet a general consensus supports the concept that in neuropathic pain, BDNF is pro-nociceptive, while GDNF is anti-nociceptive [5, 6, 129].
Several works in the last years have explored how pathological conditions affect BDNF and GDNF expression in CNS, thus demonstrating the implications of both factors in the shift from normality to an altered neurological situation Table (1). In several cases, the levels of the two NFs are regulated in an opposing direction, reinforcing the hypothesis that they play alternative/complementary roles.
Table 1.
BDNF and GDNF levels in neurological disorders.
| Disease | Model | BDNF | GDNF |
|---|---|---|---|
| Spinal cord ischemia | Rat model of transient ischemia [158] | ↑ in spinal neurons | ↑in spinal neurons and astrocytes |
| Parkinson’s disease | Lesion of the rat nigrostriatal pathway by 6-hydroxydopamine [159] | ↑ in striatum and midbrain of young rats ↓ in striatum and ↑ in midbrain of old rats |
↑ in striatum and ↔ in midbrain of young rats ↔ in striatum and midbrain of old rats |
| Alzheimer’s disease | B6C3-Tg transgenic mouse [160] | ↓in cortex | ↑in cortex |
| Learning and memory impairment | Pneumococcal meningitis in rat [161] | ↓in hippocampus | ↓in hippocampus |
| Alcohol spectrum disorder | Ethanol administration to postnatal rats [162] | ↑ in amygdala and ↔ in pyriform cortex in P7 rats ↓ in amygdala and ↓in pyriform cortex in P15 rats |
↑in amygdala and ↑ in pyriform cortex in P7 rats ↑ amygdala and ↓pyriform cortex in P15 rats |
| Hyperoxia | Exposure of P7-12 rats to the hyperoxic condition [163] | ↓mRNA in prefrontal cortex and ↑ in isocortex | ↑ mRNA in prefrontal cortex and ↑ in isocortex |
However, only a few studies have properly addressed whether BDNF and GDNF directly interact in modulating the function of central neurons. For instance, Giehl and colleagues [164] showed that GDNF prevents the degeneration of lesioned corticospinal neurons via BDNF signaling. Similarly, combined administration of GDNF and BDNF, but not each factor individually, favors motor neuron differentiation in vitro [165] and improves motor dysfunction in a rat model of spinal cord injury [166]. An interaction between GDNF and BDNF was also observed in the hippocampus in 3xTg-AD mice that modeled Alzheimer’s disease [167]. Here, the over-expression of GDNF improved cognition performances and increased BDNF expression.
Importantly, imbalances in the expression of BDNF and GDNF are associated with several neurological alterations in humans. Aged subjects exhibiting cognitive decline or with mild Alzheimer’s disease display decreased BDNF and GDNF serum levels when compared with healthy subjects [168]. A significant decrease in both NFs has also been reported in patients with schizophrenia [169]. Conversely, in patients diagnosed with bipolar disorder where only BDNF was decreased, lithium-based therapy inverted the ratio of circulating BDNF/GDNF, suggesting that the reciprocal levels determine the overall effect on the nervous system [170].
8. THERAPEUTIC PERSPECTIVES
Given the part played by BDNF and GDNF in pain modulation preclinically, the two molecules certainly represent an attractive target for the development of novel therapeutic strategies. Several approaches have been proposed in animal studies, particularly for the treatment of neuropathic pain in which, as highlighted above, BDNF and GDNF have opposite effects.
8.1. Targeting BDNF/TrkB
The main approaches derived from preclinical studies to treat pain by blocking BDNF/TrkB signaling can be distinct in: i) scavenging BDNF, ii) targeting the extracellular TrkB domain, iii) interfering with the intracellular kinase domain.
8.1.1. Scavenging BDNF
Scavenging BDNF can be achieved in vivo by delivering a TrkB-Fc fusion protein, obtained from the extracellular domain of TrkB and the Fc domain of human IgGs [171]. This approach has been extensively used to probe the role of endogenous BDNF in pain models, including nerve injury [103], diabetic neuropathy [172], and pain incision model [173]. Although highly specific, the method is poorly translatable in clinical settings since the scavenger needs to be applied intrathecally or locally at the central sites where BDNF is released.
8.1.2. Targeting the Extracellular TrkB Domain
Targeting the extracellular domain, thus reducing the probability of ligand binding, can be achieved either through TrkB blocking antibodies [174] or by developing new receptor antagonists [175]. Monoclonal function-blocking antibodies against TrkB receptors have been successfully employed in preclinical investigations (i.e., mouse clone 47 [176]), and proved to be effective in blocking the effects of BDNF on neuronal activity in acute spinal cord slices [177], as well as in reversing neuropathic [103] and other forms of pathological [105] pain in rodents. Similarly to TrkB-Fc, systemically administered anti-trkB blocking antibodies do not reach the CNS, which, together with the relatively low sensitivity [176], makes them poor candidates for clinical treatments. In the last decade, the identification of novel small molecules acting as negative allosteric modulators of TrkB receptors has brought important advances in pharmacokinetics and drug bioavailability. By using a peptidomimetic approach, Cazorla and colleagues identified a small BDNF fragment, cyclotraxin-B, which allosterically alters the conformation of TrkB [178]. Cyclotraxin-B was thereafter proved to have clear antinociceptive effects in different pain models, including post-stroke pain [63], nerve ligation injury [113], and inflammatory pain [179]. On the other hand, cyclotraxin-B does not only act on the BDNF dependent mechanisms but also ont the BDNF independent forms of TrkB activation, which raises the issue of the specificity of the effect. Moreover, the peptide is effective if delivered intravenously, but not orally. These limitations have been overcome by the same group through the identification of ANA-12, a non-peptidic small molecule [175]. ANA-12 allosterically prevents BDNF from binding TrkB with nanomolar potency [175] and, unlike cyclotraxin-B, can be delivered orally, thus representing a promising and specific approach for clinical trials. Very promising, an increasing number of preclinical studies have confirmed the antinociceptive efficacy of ANA-12 in different pain models, including nerve injury [111], inflammatory pain [180], trigeminal pain [114] and migraine [112, 181]. Recently, ANA-12 has also been documented to counteract chronic hypersensitivity due to visceral pain in cyclophosphamide-induced cystitis [182] and in a model of irritable bowel syndrome induced by maternal separation in rats [183].
8.1.3. Interfering with the Itracellular Kinase Domain
The main approach to intracellularly block tyrosine kinases activity is to interfere with the ATP binding site of the enzymes [184, 185]. The indolocarbazole K252a is one of the first used compounds acting as a competitive inhibitor of the ATP site, thus blocking Trk catalytic activity [186, 187]. K252a administration effectively blocks the rise of intracellular Ca2+ promoted by BDNF in acute slices of the rat superficial dorsal horn [177] and, in vivo, it reduces hypersensitivity in visceral [188, 189], neuropathic [103, 190], inflammatory [191], and skeletal pain [192].
Encouraged by preclinical studies, different pharmaceutical companies have invested considerable resources in improving kinases inhibitors, some of which are currently under clinical trials or investigations for a broad range of diseases, including oncological disorders and pain (for review [184, 193-196]). Although most of them exhibit high potency in vitro, yet the development of a TrkB inhibitor as a marketed product has not been successful at present. Unfortunately, developing tyrosine kinase antagonists for specific Trk receptors is challenging since ATP competitive Trk inhibitors are at best Trk specific (pan-Trk inhibitors) but can hardly discriminate between Trk subtypes [184]. Therefore, a major limitation concerning the use of these molecules is the risk to develop central side effects. To limit adverse effects, several attempts have been made to develop molecules that do not cross the blood-brain barrier (BBB) but mainly act at the periphery. Some of these molecules, e.g., ARRY-470 [197] or PF-06273340A [198], exhibit antinociceptive effects in animal models of chronic pain. In a recent randomized, double-blinded clinical trial, the pan-Trk inhibitor ONO-4474 was proved to be analgesic in patients with moderate to severe osteoarthritis [199]. Yet, it is likely that all these molecules also target peripheral Trk receptors other than TrkB (e.g., TrkA).
8.2. Alternative Targets to Counteract BDNF Pronociceptive Effects
Due to the above-described limitations, to date, there are no effective pain treatments based on BDNF/TrkB. The critical role played by BDNF and TrkB receptors in the survival of central neurons further limits the use of TrkB antagonists as a safe therapeutic approach. Moreover, the complexity and plurality of the intracellular signaling pathways activated by tyrosine kinase receptors make it difficult to develop selective pharmacological treatments. Targeting BDNF/TrkB can lead to both on-target side effects, due to the inactivation of BDNF-dependent pathways important for the normal functioning of the healthy nervous system, and off- target side effects, due to the lack of specificity and the interaction with relevant non-BDNF pathways [200].
Other viable therapeutic options to counteract TrkB-dependent pathological alterations can be achieved by targeting upstream or downstream effectors.
Upstream targeting of microglia can effectively reduce the BDNF-dependent alterations in neuropathic pain [201]. In this respect, microglial P2X4 receptors, whose activation is necessary for the subsequent release of BDNF, are viable targets for clinical interventions [202]. Alternatively, several GPCR receptors, such as dopamine receptor 1 (D1R [203]), PACAP receptor [23], adenosine A2 receptor [204] and the cannabinoid receptor 1 [205], can transactivate TrkB, thus representing alternative targets to reduce TrkB signaling.
Among downstream signaling pathways, a key mechanism underlying TrkB-dependent pain hypersensitivity, acts through the downregulation of KCC2, which causes an imbalance between excitatory and inhibitory neurotransmission [103]. KCC2 itself represents a putative pharmacological target [206]. Therefore, the development of KCC2 enhancers is a promising therapeutic avenue to restore inhibition and counteract the symptoms associated with the BDNF-TrkB-KCC2 cascade [207].
8.3. Targeting GDNF/GFRα1/Ret
Although GDNF and its receptor, as well as other GFLs, have been shown to either increase or decrease sensitivity in different preclinical models of pain, the antinociceptive role of GDNF in neuropathic pain has been consistently reported (for review, see [6]). Thus, potentiating the GDNF system is, theoretically, a viable strategy to counteract neuropathic pain symptoms. To achieve this goal, the following strategies have been explored: i) direct administration of GDNF; ii) synthesis and delivery of small molecule agonists; iii) alternative druggable targets to potentiate GDNF/GFRα1/Ret pathways.
8.3.1. Administration of GDNF
In different experimental neuropathic pain models, the antinociceptive effects of GDNF have been demonstrated by the direct administration of GDNF, mostly intrathecally [131, 142, 143, 208]. In terms of translatability into clinical settings, however, intrathecal administration of GDNF is hampered by the invasiveness of the procedure and potential side effects [209]. On the other hand, the poor CNS bioavailability of proteinaceous molecules with high molecular weight, such as the NFs, strongly limits the use of systemic administration in clinical interventions [210, 211]. Many efforts have been made to overcome the BBB obstacle and improve GDNF availability to the nervous tissue, for instance, by intranasal administration using cationic liposomes [212, 213] or by encapsulating GDNF in microspheres, allowing a slower release when centrally injected [214-216].
Gene-transfer approaches represent a more effective way to deliver GDNF in the CNS. These approaches can be either implemented by trojan horses, such as liposomes, that carry GDNF-encoding plasmids across the BBB to the central neurons [217], or by gene transfer with recombinant viral vectors locally infecting the target neurons [210]. A number of viral vectors carrying the gdnf gene have been developed, in particular, for rescuing motor performance in animal models of Parkinson’s disease [210]. These include the herpes simplex virus (HSV [218]), lentiviruses [219], adenoviruses [220] and adeno-associated viruses (AVV [221-223]). Vector-mediated GDNF delivery has also been proposed to reverse neuropathic pain symptoms after nerve injury, either by spinal injection of lentiviral vectors [134, 135], intracutaneous inoculation of engineered herpes simplex virus [224] or intramuscular injection of recombinant adenovirus [133, 225].
Eventually, cell therapy represents another putative strategy to deliver GDNF to central neurons. Administration of astrocytes, macrophages and neural stem cells engineered to express GDNF, or carotid bulb cells, an endogenous source of GDNF, have been successfully employed to prevent cell loss in certain neurodegenerative diseases [226-228]. The use of GDNF-based cell therapy has also been successfully adopted for the treatment of pain hypersensitivity in nerve-injured rats [229].
Clinical trials to test the effectiveness of GDNF-based therapies in humans have so far largely failed, and more importantly, have unveiled the main limitations of this approach: first, the onset of unwanted side effects, such as nausea, weight loss, anorexia; second, the inadequate concentration reached by GDNF in target tissues; and third, the production of neutralizing anti-GDNF antibodies in a number of patients [230, 231]. Gene therapy may represent an improvement as compared to the direct administration of the NF, as demonstrated in preclinical investigations. A large clinical trial is currently ongoing to address the effectiveness of AAV-gdnf administration in the putamen of patients with Parkinson’s disease [232], which may subsequently open the avenue for similar approaches for the treatment of neuropathic pain. Indeed, GDNF has not yet been employed to treat neuropathic pain in humans. Artemin, instead, has already been tested in two clinical studies in patients with sciatica, with the main purpose to verify the safety, tolerability, and pharmacokinetics of the NF [233, 234].
8.3.2. Small Molecule Agonists
To overcome the BBB, another viable strategy is the synthesis of non-peptidyl small molecule agonists activating GDNF receptors complex GFRα1/Ret. The first described agonist of GFRα1 was XIB4035 [235]. Local XIB4035 administration was shown to alleviate the symptoms of diabetic neuropathy [132]. More recently, Sidorova and colleagues [236] identified by high-throughput screening, a novel Ret agonist, named BT13. This molecule directly modulates RET and its downstream intracellular signaling cascades. BT13 systemic administration reverses nerve injury-induced alterations in sensory neurons [236].
8.3.3. Alternative Druggable Targets to Potentiate GDNF/GFRα1/Ret Pathways
Emerging evidence suggests that the activation of the intracellular Ret signaling cascade is the endpoint of a complex and multifaceted ensemble of interacting factors, among which GFLs and GFRs likely represent a necessary but not sufficient component. Understanding the specific relevance and involvement of these interacting factors may provide novel approaches to restore pharmacologically Ret signaling in pathological settings.
The molecular composition of the membrane microdomains (lipid rafts) in which the GFRs are anchored represents a key factor influencing intracellular Ret signaling pathways [44]. Indeed, GDNF-mediated activation of GFRα1 fosters the recruitment of Ret to lipid rafts, thus favoring Ret/Src kinases coupling [44]. The interaction of GFRα1 and Ret outside lipid rafts microdomains does not preclude Ret activation but attenuates downstream functional effects [237]. GM1 ganglioside is an important component of lipid rafts and displays neurotrophic actions in vitro and in vivo [238, 239]. A recent study showed that the administration of GM1 promotes Ret phosphorylation by enhancing the interaction between GDNF and GFRα1 [239]. Interestingly, the administration of GM1 to patients with post-herpetic neuralgia or spinal cord injury was found to sensibly reduce pain severity [240].
Transcription factors represent other alternative targets for indirectly manipulating the Ret function. Specifically, the transcription factor nurr1 has been shown to stimulate Ret tyrosine kinase activity in animal models of Parkinson’s disease [241, 242]. Pharmacologically, nurr1 can be targeted by delivering bexarotene, an agonist of retinoic X receptors, which in turn forms heterodimers with the transcription factor [242]. Interestingly, bexarotene also exerts antinociceptive effects in neuropathic pain by counteracting certain spinal neuroinflammatory processes [243], thus leaving open the possibility that nurr1-Ret pathways may also be targeted in the spinal cord.
Eventually, as reported earlier, GDNF may exert antinociceptive effects, also acting upon receptors other than Ret. In particular, adhesion molecules such as NCAM, E-cadherin and nectin-1 have been implicated in GDNF antinociceptive signaling pathways [49, 138, 140]. Importantly, C3D, a NCAM mimetic peptide, recapitulates the analgesic effects of GDNF in the model of nerve injury [49], thus representing another alternative strategy for pain treatment.
CONCLUSION
While pre-clinical studies in the last 20 years have consistently reported that BDNF and GDNF play a significant role in the development of pain hypersensitivity, no novel pharmacological strategies have been successfully developed based on this knowledge. The reasons are multiple (see for review [244, 245]), and include the difficulty to correctly dose and deliver the treatments, as well as the necessity to deal with advanced stages of the pathology in humans. On the mechanistic side, however, one major obstacle is the tremendous fluidity of NFs activity. As discussed in this review, the same NFs can generate opposite effects by activating different intracellular pathways or different receptors; not to say that the same molecules have short- and medium-term effects on neuronal function, but also long term trophic effects on neuronal survival and differentiation. Therefore, the identification of specific downstream targets in Ret- and TrkB- dependent pathways involved in the onset of pain symptoms (or in their reduction) is probably a more viable and safer strategy rather than directly tackling NFs receptors. In this respect, defining the mechanistic ground through which NFs promote sensitization in pathological pain is necessary to refine pharmacological interventions, reduce side effects and increase effectiveness. Additionally, more efforts are needed to be made not only in the analysis of the effects of individual relevant factors but also in their reciprocal interactions. In this review, we showed that BDNF and GDNF exert distinct complementary effects in pain processing. Their reciprocal contribution appears particularly evident in neuropathic pain, where BDNF/TrkB signaling plays a dominant pro-nociceptive role, while GDNF/GFRα1/Ret signaling mainly exerts anti-nociceptive effects. Any imbalance in their contribution to the modulation of pain processing may concur with the development of pathological conditions. Although we are still far from understanding properly the circuitry that triggers the opposing effects of BDNF and GDNF in the initial process of nociceptive stimuli [129], this dual mechanism of action provides, at least theoretically, the possibility to pharmacologically modulate its final outcome by the combinatory use of agonist (mimetic) and antagonist (lytic) drugs to each NF in a fashion similar to that currently in use in the pharmacological control of sympathetic and parasympathetic visceral neurotransmission. It is at present difficult to foresee which could be the advantages or disadvantages of such an approach. If indeed, it will be possible to develop adequate pharmacological tools that overcome the limitations described in the previous section of this paper, however, one has to keep in mind that any drug acting on either of the two NFs will influence their relative functional balance. Thus, it might be speculated that, for e.g., the development of a GDNF agonist would be equally effective than that of a BDNF antagonist in the control of pathological pain. In the transition from preclinical studies to therapeutic intervention, the interactions between the two NFs should be taken into consideration. Most of the clinical trials in the past were focused on single targets, with the idea that a single molecule or receptor can effectively restore a pathological condition. The interactions of BDNF and GDNF in nociceptive pathways impose a change of approach toward combinational therapies [246], including the delivery of both trophic factors and/or small molecules acting on downstream targets, aiming to restore an altered balance. On the other hand, the successful development of combinational therapies based on a homeostatic approach implies the availability of diagnostic tools to non-invasively explore the neurotrophic content at both central and peripheral levels in different types of painful neuropathies [247-249].
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Gibon J., Barker P.A. Neurotrophins and proneurotrophins: focus on synaptic activity and plasticity in the brain. Neuroscientist. 2017;23(6):587–604. doi: 10.1177/1073858417697037. [DOI] [PubMed] [Google Scholar]
- 2.Khan N., Smith M.T. Neurotrophins and neuropathic pain: role in pathobiology. Molecules. 2015;20(6):10657–10688. doi: 10.3390/molecules200610657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossipov M.H. Growth factors and neuropathic pain. Curr. Pain Headache Rep. 2011;15(3):185–192. doi: 10.1007/s11916-011-0183-5. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher J.H., Tewari D., McMahon S.B. Neurotrophic factors and their inhibitors in chronic pain treatment. Neurobiol. Dis. 2017;97(Pt B):127–138. doi: 10.1016/j.nbd.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Merighi A., Salio C., Ghirri A., Lossi L., Ferrini F., Betelli C., Bardoni R. BDNF as a pain modulator. Prog. Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Merighi A. Targeting the glial-derived neurotrophic factor and related molecules for controlling normal and pathologic pain. Expert Opin. Ther. Targets. 2016;20(2):193–208. doi: 10.1517/14728222.2016.1085972. [DOI] [PubMed] [Google Scholar]
- 7.Acheson A., Conover J.C., Fandl J.P., DeChiara T.M., Russell M., Thadani A., Squinto S.P., Yancopoulos G.D., Lindsay R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 8.Buj-Bello A., Buchman V.L., Horton A., Rosenthal A., Davies A.M. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15(4):821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 9.Luo W., Wickramasinghe S.R., Savitt J.M., Griffin J.W., Dawson T.M., Ginty D.D. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54(5):739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Marmigère F., Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 2007;8(2):114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 11.Valdés-Sánchez T., Kirstein M., Pérez-Villalba A., Vega J.A., Fariñas I. BDNF is essentially required for the early postnatal survival of nociceptors. Dev. Biol. 2010;339(2):465–476. doi: 10.1016/j.ydbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Boucher T.J., McMahon S.B. Neurotrophic factors and neuropathic pain. Curr. Opin. Pharmacol. 2001;1(1):66–72. doi: 10.1016/S1471-4892(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 13.Denk F., Bennett D.L., McMahon S.B. Nerve growth factor and pain mechanisms. Annu. Rev. Neurosci. 2017;40:307–325. doi: 10.1146/annurev-neuro-072116-031121. [DOI] [PubMed] [Google Scholar]
- 14.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao M.V., Hempstead B.L. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18(7):321–326. doi: 10.1016/0166-2236(95)93922-K. [DOI] [PubMed] [Google Scholar]
- 16.Mitre M., Mariga A., Chao M.V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2017;131(1):13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeker R., Williams K. Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J. Neuroimmune Pharmacol. 2014;9(5):615–628. doi: 10.1007/s11481-014-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underwood C.K., Coulson E.J. The p75 neurotrophin receptor. Int. J. Biochem. Cell Biol. 2008;40(9):1664–1668. doi: 10.1016/j.biocel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Barker P.A. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42(4):529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ceni C., Unsain N., Zeinieh M.P., Barker P.A. Neurotrophins in the regulation of cellular survival and death. Handb. Exp. Pharmacol. 2014;220:193–221. doi: 10.1007/978-3-642-45106-5_8. [DOI] [PubMed] [Google Scholar]
- 21.Meeker R.B., Williams K.S. The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen. Res. 2015;10(5):721–725. doi: 10.4103/1673-5374.156967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bothwell M. Recent advances in understanding neurotrophin signaling. F1000 Res. 2016;5:5. doi: 10.12688/f1000research.8434.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee F.S., Rajagopal R., Kim A.H., Chang P.C., Chao M.V. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 2002;277(11):9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- 24.Lee F.S., Chao M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA. 2001;98(6):3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diniz C.R.A.F., Casarotto P.C., Fred S.M., Biojone C., Castrén E., Joca S.R.L. Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology. 2018;135:163–171. doi: 10.1016/j.neuropharm.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi K., Yamauchi T., Mantuano E., Murakami K., Henry K., Takahashi K., Campana W.M. Low-density lipoprotein receptor related protein-1 (LRP1)-dependent cell signaling promotes neurotrophic activity in embryonic sensory neurons. PLoS One. 2013;8(9):e75497. doi: 10.1371/journal.pone.0075497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puehringer D., Orel N., Lüningschrör P., Subramanian N., Herrmann T., Chao M.V., Sendtner M. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat. Neurosci. 2013;16(4):407–415. doi: 10.1038/nn.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y.Z., Pan E., Xiong Z.Q., McNamara J.O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57(4):546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Richner M., Ulrichsen M., Elmegaard S.L., Dieu R., Pallesen L.T., Vaegter C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014;50(3):945–970. doi: 10.1007/s12035-014-8706-9. [DOI] [PubMed] [Google Scholar]
- 30.Blum R., Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- 31.Dedoni S., Olianas M.C., Ingianni A., Onali P. Interferon-β Inhibits Neurotrophin 3 Signalling and Pro-survival activity by upregulating the expression of truncated TrkC-T1 Receptor. Mol. Neurobiol. 2017;54(3):1825–1843. doi: 10.1007/s12035-016-9789-2. [DOI] [PubMed] [Google Scholar]
- 32.Esteban P.F., Yoon H.Y., Becker J., Dorsey S.G., Caprari P., Palko M.E., Coppola V., Saragovi H.U., Randazzo P.A., Tessarollo L. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J. Cell Biol. 2006;173(2):291–299. doi: 10.1083/jcb.200512013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulgenzi G., Tomassoni-Ardori F., Babini L., Becker J., Barrick C., Puverel S., Tessarollo L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015;210(6):1003–1012. doi: 10.1083/jcb.201502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matyas J.J., O’Driscoll C.M., Yu L., Coll-Miro M., Daugherty S., Renn C.L., Faden A.I., Dorsey S.G., Wu J. Truncated TrkB.T1-mediated astrocyte dysfunction contributes to impaired motor function and neuropathic pain after spinal cord injury. J. Neurosci. 2017;37(14):3956–3971. doi: 10.1523/JNEUROSCI.3353-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose C.R., Blum R., Pichler B., Lepier A., Kafitz K.W., Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426(6962):74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 36.Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 37.Wang X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim. Biophys. Acta. 2013;1834(10):2205–2212. doi: 10.1016/j.bbapap.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 39.Penttinen A.M., Parkkinen I., Voutilainen M.H., Koskela M., Bäck S., Their A., Richie C.T., Domanskyi A., Harvey B.K., Tuominen R.K., Nevalaita L., Saarma M., Airavaara M. Pre-α-pro-GDNF and Pre-β-pro-GDNF isoforms are neuroprotective in the 6-hydroxydopamine rat model of Parkinson’s Disease. Front. Neurol. 2018;9:457. doi: 10.3389/fneur.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibáñez C.F. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 2013;5(2):5. doi: 10.1101/cshperspect.a009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlee S., Carmillo P., Whitty A. Quantitative analysis of the activation mechanism of the multicomponent growth-factor receptor Ret. Nat. Chem. Biol. 2006;2(11):636–644. doi: 10.1038/nchembio823. [DOI] [PubMed] [Google Scholar]
- 42.Barnett M.W., Fisher C.E., Perona-Wright G., Davies J.A. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J. Cell Sci. 2002;115(Pt 23):4495–4503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M., Xiao H., Kiuchi K. Heparin facilitates glial cell line-derived neurotrophic factor signal transduction. Neuroreport. 2002;13(15):1913–1916. doi: 10.1097/00001756-200210280-00016. [DOI] [PubMed] [Google Scholar]
- 44.Tansey M.G., Baloh R.H., Milbrandt J., Johnson E.M., Jr GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25(3):611–623. doi: 10.1016/S0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 45.Creedon D.J., Tansey M.G., Baloh R.H., Osborne P.A., Lampe P.A., Fahrner T.J., Heuckeroth R.O., Milbrandt J., Johnson E.M., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc. Natl. Acad. Sci. USA. 1997;94(13):7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibáñez C.F. Beyond the cell surface: new mechanisms of receptor function. Biochem. Biophys. Res. Commun. 2010;396(1):24–27. doi: 10.1016/j.bbrc.2010.01.136. [DOI] [PubMed] [Google Scholar]
- 47.Sariola H., Saarma M. Novel functions and signalling pathways for GDNF. J. Cell Sci. 2003;116(Pt 19):3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 48.Trupp M., Scott R., Whittemore S.R., Ibáñez C.F. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J. Biol. Chem. 1999;274(30):20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 49.Paratcha G., Ledda F., Ibáñez C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113(7):867–879. doi: 10.1016/S0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 50.Ernfors P., Merlio J.P., Persson H. Cells Expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur. J. Neurosci. 1992;4(11):1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 51.Josephson A., Widenfalk J., Trifunovski A., Widmer H.R., Olson L., Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J. Comp. Neurol. 2001;440(2):204–217. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- 52.Widenfalk J., Lundströmer K., Jubran M., Brene S., Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001;21(10):3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michael G.J., Averill S., Nitkunan A., Rattray M., Bennett D.L., Yan Q., Priestley J.V. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salio C., Lossi L., Ferrini F., Merighi A. Ultrastructural evidence for a pre- and postsynaptic localization of full-length trkB receptors in substantia gelatinosa (lamina II) of rat and mouse spinal cord. Eur. J. Neurosci. 2005;22(8):1951–1966. doi: 10.1111/j.1460-9568.2005.04392.x. [DOI] [PubMed] [Google Scholar]
- 55.Salio C., Ferrini F. BDNF and GDNF expression in discrete populations of nociceptors. Ann. Anat. 2016;207:55–61. doi: 10.1016/j.aanat.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Cavanaugh D.J., Lee H., Lo L., Shields S.D., Zylka M.J., Basbaum A.I., Anderson D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA. 2009;106(22):9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rau K.K., McIlwrath S.L., Wang H., Lawson J.J., Jankowski M.P., Zylka M.J., Anderson D.J., Koerber H.R. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J. Neurosci. 2009;29(26):8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conner J.M., Lauterborn J.C., Yan Q., Gall C.M., Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 1997;17(7):2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dembo T., Braz J.M., Hamel K.A., Kuhn J.A., Basbaum A.I. Primary afferent-derived BDNF contributes minimally to the processing of pain and itch. eNeuro. 2018;5(6):5. doi: 10.1523/ENEURO.0402-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karchewski L.A., Kim F.A., Johnston J., McKnight R.M., Verge V.M. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J. Comp. Neurol. 1999;413(2):327–341. doi: 10.1002/(SICI)1096-9861(19991018)413:2<327::AID-CNE11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Li L., Rutlin M., Abraira V.E., Cassidy C., Kus L., Gong S., Jankowski M.P., Luo W., Heintz N., Koerber H.R., Woodbury C.J., Ginty D.D. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradbury E.J., King V.R., Simmons L.J., Priestley J.V., McMahon S.B. NT-3, but not BDNF, prevents atrophy and death of axotomized spinal cord projection neurons. Eur. J. Neurosci. 1998;10(10):3058–3068. doi: 10.1046/j.1460-9568.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 63.Shih H.C., Kuan Y.H., Shyu B.C. Targeting brain-derived neurotrophic factor in the medial thalamus for the treatment of central poststroke pain in a rodent model. Pain. 2017;158(7):1302–1313. doi: 10.1097/j.pain.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarhan M., Pawlowski S.A., Barthas F., Yalcin I., Kaufling J., Dardente H., Zachariou V., Dileone R.J., Barrot M., Veinante P. BDNF parabrachio-amygdaloid pathway in morphine-induced analgesia. Int. J. Neuropsychopharmacol. 2013;16(7):1649–1660. doi: 10.1017/S146114571200168X. [DOI] [PubMed] [Google Scholar]
- 65.Salio C., Averill S., Priestley J.V., Merighi A. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev. Neurobiol. 2007;67(3):326–338. doi: 10.1002/dneu.20358. [DOI] [PubMed] [Google Scholar]
- 66.Bennett D.L., Michael G.J., Ramachandran N., Munson J.B., Averill S., Yan Q., McMahon S.B., Priestley J.V. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998;18(8):3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honda T., Takahashi M., Sugiura Y. Co-localization of the glial cell-line derived neurotrophic factor and its functional receptor c-RET in a subpopulation of rat dorsal root ganglion neurons. Neurosci. Lett. 1999;275(1):45–48. doi: 10.1016/S0304-3940(99)00729-6. [DOI] [PubMed] [Google Scholar]
- 68.Holstege J.C., Jongen J.L., Kennis J.H., van Rooyen-Boot A.A., Vecht C.J. Immunocytochemical localization of GDNF in primary afferents of the lumbar dorsal horn. Neuroreport. 1998;9(12):2893–2897. doi: 10.1097/00001756-199808240-00039. [DOI] [PubMed] [Google Scholar]
- 69.Ohta K., Inokuchi T., Gen E., Chang J. Ultrastructural study of anterograde transport of glial cell line-derived neurotrophic factor from dorsal root ganglion neurons of rats towards the nerve terminal. Cells Tissues Organs. 2001;169(4):410–421. doi: 10.1159/000047909. [DOI] [PubMed] [Google Scholar]
- 70.Salio C., Ferrini F., Muthuraju S., Merighi A. Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (GDNF). J. Neurosci. 2014;34(41):13819–13833. doi: 10.1523/JNEUROSCI.0808-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennett D.L., Boucher T.J., Armanini M.P., Poulsen K.T., Michael G.J., Priestley J.V., Phillips H.S., McMahon S.B., Shelton D.L. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J. Neurosci. 2000;20(1):427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashiba H., Hyon B., Senba E. Glial cell line-derived neurotrophic factor and nerve growth factor receptor mRNAs are expressed in distinct subgroups of dorsal root ganglion neurons and are differentially regulated by peripheral axotomy in the rat. Neurosci. Lett. 1998;252(2):107–110. doi: 10.1016/S0304-3940(98)00558-8. [DOI] [PubMed] [Google Scholar]
- 73.Kashiba H., Uchida Y., Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res. Mol. Brain Res. 2003;110(1):52–62. doi: 10.1016/S0169-328X(02)00584-3. [DOI] [PubMed] [Google Scholar]
- 74.Zwick M., Davis B.M., Woodbury C.J., Burkett J.N., Koerber H.R., Simpson J.F., Albers K.M. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J. Neurosci. 2002;22(10):4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molliver D.C., Wright D.E., Leitner M.L., Parsadanian A.S., Doster K., Wen D., Yan Q., Snider W.D. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19(4):849–861. doi: 10.1016/S0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 76.Franck M.C., Stenqvist A., Li L., Hao J., Usoskin D., Xu X., Wiesenfeld-Hallin Z., Ernfors P. Essential role of Ret for defining non-peptidergic nociceptor phenotypes and functions in the adult mouse. Eur. J. Neurosci. 2011;33(8):1385–1400. doi: 10.1111/j.1460-9568.2011.07634.x. [DOI] [PubMed] [Google Scholar]
- 77.Golden J.P., Hoshi M., Nassar M.A., Enomoto H., Wood J.N., Milbrandt J., Gereau R.W., IV, Johnson E.M., Jr, Jain S. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J. Neurosci. 2010;30(11):3983–3994. doi: 10.1523/JNEUROSCI.5930-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jongen J.L., Jaarsma D., Hossaini M., Natarajan D., Haasdijk E.D., Holstege J.C. Distribution of RET immunoreactivity in the rodent spinal cord and changes after nerve injury. J. Comp. Neurol. 2007;500(6):1136–1153. doi: 10.1002/cne.21234. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Ratnam J., Zou B., England P.M., Basbaum A.I. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J. Neurosci. 2009;29(17):5508–5515. doi: 10.1523/JNEUROSCI.4288-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrini F., Perez-Sanchez J., Ferland S., Lorenzo L.E., Godin A.G., Plasencia-Fernandez I., Cottet M., Castonguay A., Wang F., Salio C., Doyon N., Merighi A., De Koninck Y. Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization. Nat. Commun. 2020;11(1):3935. doi: 10.1038/s41467-020-17824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sikandar S., Minett M.S., Millet Q., Santana-Varela S., Lau J., Wood J.N., Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141(4):1028–1039. doi: 10.1093/brain/awy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garraway S.M., Huie J.R. Spinal plasticity and behavior: BDNF-induced neuromodulation in uninjured and injured spinal cord. Neural Plast. 2016;2016:9857201. doi: 10.1155/2016/9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merighi A., Carmignoto G., Gobbo S., Lossi L., Salio C., Vergnano A.M., Zonta M. Neurotrophins in spinal cord nociceptive pathways. Prog. Brain Res. 2004;146:291–321. doi: 10.1016/S0079-6123(03)46019-6. [DOI] [PubMed] [Google Scholar]
- 84.Pezet S., Malcangio M., McMahon S.B. BDNF: a neuromodulator in nociceptive pathways? Brain Res. Brain Res. Rev. 2002;40(1-3):240–249. doi: 10.1016/S0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 85.Pezet S., McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 86.Zhao J., Seereeram A., Nassar M.A., Levato A., Pezet S., Hathaway G., Morenilla-Palao C., Stirling C., Fitzgerald M., McMahon S.B., Rios M., Wood J.N. London Pain Consortium. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol. Cell. Neurosci. 2006;31(3):539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Kerr B.J., Bradbury E.J., Bennett D.L., Trivedi P.M., Dassan P., French J., Shelton D.B., McMahon S.B., Thompson S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999;19(12):5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Groth R., Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100(1-2):171–181. doi: 10.1016/S0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 89.Garraway S.M., Petruska J.C., Mendell L.M. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur. J. Neurosci. 2003;18(9):2467–2476. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- 90.Slack S.E., Thompson S.W. Brain-derived neurotrophic factor induces NMDA receptor 1 phosphorylation in rat spinal cord. Neuroreport. 2002;13(15):1967–1970. doi: 10.1097/00001756-200210280-00027. [DOI] [PubMed] [Google Scholar]
- 91.Matayoshi S., Jiang N., Katafuchi T., Koga K., Furue H., Yasaka T., Nakatsuka T., Zhou X.F., Kawasaki Y., Tanaka N., Yoshimura M. Actions of brain-derived neurotrophic factor on spinal nociceptive transmission during inflammation in the rat. J. Physiol. 2005;569(Pt 2):685–695. doi: 10.1113/jphysiol.2005.095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Theodosiou M., Rush R.A., Zhou X.F., Hu D., Walker J.S., Tracey D.J. Hyperalgesia due to nerve damage: role of nerve growth factor. Pain. 1999;81(3):245–255. doi: 10.1016/S0304-3959(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 93.Yajima Y., Narita M., Narita M., Matsumoto N., Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002;958(2):338–346. doi: 10.1016/S0006-8993(02)03666-1. [DOI] [PubMed] [Google Scholar]
- 94.Yajima Y., Narita M., Usui A., Kaneko C., Miyatake M., Narita M., Yamaguchi T., Tamaki H., Wachi H., Seyama Y., Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 2005;93(3):584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhou X.F., Deng Y.S., Xian C.J., Zhong J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 2000;12(1):100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 96.Geng S.J., Liao F.F., Dang W.H., Ding X., Liu X.D., Cai J., Han J.S., Wan Y., Xing G.G. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp. Neurol. 2010;222(2):256–266. doi: 10.1016/j.expneurol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Miletic G., Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci. Lett. 2002;319(3):137–140. doi: 10.1016/S0304-3940(01)02576-9. [DOI] [PubMed] [Google Scholar]
- 98.Obata K., Yamanaka H., Kobayashi K., Dai Y., Mizushima T., Katsura H., Fukuoka T., Tokunaga A., Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J. Neurosci. 2004;24(45):10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obata K., Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci. Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Obata K., Yamanaka H., Kobayashi K., Dai Y., Mizushima T., Katsura H., Fukuoka T., Tokunaga A., Noguchi K. The effect of site and type of nerve injury on the expression of brain-derived neurotrophic factor in the dorsal root ganglion and on neuropathic pain behavior. Neuroscience. 2006;137(3):961–970. doi: 10.1016/j.neuroscience.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 101.Vanelderen P., Rouwette T., Kozicz T., Roubos E., Van Zundert J., Heylen R., Vissers K. The role of brain-derived neurotrophic factor in different animal models of neuropathic pain. Eur. J. Pain. 2010;14(5):473.e1–473.e9. doi: 10.1016/j.ejpain.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 102.Walker S.M., Mitchell V.A., White D.M., Rush R.A., Duggan A.W. Release of immunoreactive brain-derived neurotrophic factor in the spinal cord of the rat following sciatic nerve transection. Brain Res. 2001;899(1-2):240–247. doi: 10.1016/S0006-8993(01)02259-4. [DOI] [PubMed] [Google Scholar]
- 103.Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 104.Constandil L., Aguilera R., Goich M., Hernández A., Alvarez P., Infante C., Pelissier T. Involvement of spinal cord BDNF in the generation and maintenance of chronic neuropathic pain in rats. Brain Res. Bull. 2011;86(5-6):454–459. doi: 10.1016/j.brainresbull.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Ferrini F., Trang T., Mattioli T.A., Laffray S., Del’Guidice T., Lorenzo L.E., Castonguay A., Doyon N., Zhang W., Godin A.G., Mohr D., Beggs S., Vandal K., Beaulieu J.M., Cahill C.M., Salter M.W., De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl⁻ homeostasis. Nat. Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulmann L., Hatcher J.P., Hughes J.P., Chaumont S., Green P.J., Conquet F., Buell G.N., Reeve A.J., Chessell I.P., Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ding X., Cai J., Li S., Liu X.D., Wan Y., Xing G.G. BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol. Dis. 2015;73:428–451. doi: 10.1016/j.nbd.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 108.Hildebrand M.E., Xu J., Dedek A., Li Y., Sengar A.S., Beggs S., Lombroso P.J., Salter M.W. Potentiation of synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 2016;17(10):2753–2765. doi: 10.1016/j.celrep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 109.Dedek A., Xu J., Kandegedara C.M., Lorenzo L.É., Godin A.G., De Koninck Y., Lombroso P.J., Tsai E.C., Hildebrand M.E. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain. 2019;142(6):1535–1546. doi: 10.1093/brain/awz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J.T., Guo D., Campanelli D., Frattini F., Mayer F., Zhou L., Kuner R., Heppenstall P.A., Knipper M., Hu J. Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction. Nat. Commun. 2014;5:5331. doi: 10.1038/ncomms6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen W., Walwyn W., Ennes H.S., Kim H., McRoberts J.A., Marvizón J.C. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci. 2014;39(9):1439–1454. doi: 10.1111/ejn.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burgos-Vega C.C., Quigley L.D., Avona A., Price T., Dussor G. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain. 2016;157(12):2722–2730. doi: 10.1097/j.pain.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Constandil L., Goich M., Hernández A., Bourgeais L., Cazorla M., Hamon M., Villanueva L., Pelissier T. Cyclotraxin-B, a new TrkB antagonist, and glial blockade by propentofylline, equally prevent and reverse cold allodynia induced by BDNF or partial infraorbital nerve constriction in mice. J. Pain. 2012;13(6):579–589. doi: 10.1016/j.jpain.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 114.Liu C., Zhang Y., Liu Q., Jiang L., Li M., Wang S., Long T., He W., Kong X., Qin G., Chen L., Zhang Y., Zhou J. P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia. Mol. Pain. 2018;14:1744806918795930. doi: 10.1177/1744806918795930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X., Xu P., Li C., Zhu W., Wu S., Yu A., Ding Y., Wang Q., Zhang Z. Spinal microglial P2X4 receptor-brain-derived neurotrophic factor signaling regulates nicotine withdrawal-induced hyperalgesia. Neuroreport. 2017;28(6):339–347. doi: 10.1097/WNR.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 116.Ismail C.A.N., Suppian R., Aziz C.B.A., Long I. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J. Diabetes Metab. Disord. 2019;18(1):181–190. doi: 10.1007/s40200-019-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee-Kubli C.A., Calcutt N.A. Altered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic pain. Pain. 2014;155(2):250–260. doi: 10.1016/j.pain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bao Y., Hou W., Liu R., Gao Y., Kong X., Yang L., Shi Z., Li W., Zheng H., Jiang S., Li C., Qin Y., Hua B. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Mol. Pain. 2014;10:28. doi: 10.1186/1744-8069-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin X.H., Wang L.N., Zuo J.L., Yang J.P., Liu S.L. P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J. Neurosci. Res. 2014;92(12):1690–1702. doi: 10.1002/jnr.23443. [DOI] [PubMed] [Google Scholar]
- 120.Meng X.W., Gao J.L., Zuo J.L., Wang L.N., Liu S.L., Jin X.H., Yao M., Namaka M. Toll-like receptor-4/p38 MAPK signaling in the dorsal horn contributes to P2X4 receptor activation and BDNF over-secretion in cancer induced bone pain. Neurosci. Res. 2017;125:37–45. doi: 10.1016/j.neures.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Wang L.N., Yang J.P., Ji F.H., Zhan Y., Jin X.H., Xu Q.N., Wang X.Y., Zuo J.L. Brain-derived neurotrophic factor modulates N-methyl-D-aspartate receptor activation in a rat model of cancer-induced bone pain. J. Neurosci. Res. 2012;90(6):1249–1260. doi: 10.1002/jnr.22815. [DOI] [PubMed] [Google Scholar]
- 122.Wang L.N., Yang J.P., Zhan Y., Ji F.H., Wang X.Y., Zuo J.L., Xu Q.N. Minocycline-induced reduction of brain-derived neurotrophic factor expression in relation to cancer-induced bone pain in rats. J. Neurosci. Res. 2012;90(3):672–681. doi: 10.1002/jnr.22788. [DOI] [PubMed] [Google Scholar]
- 123.Siuciak J.A., Altar C.A., Wiegand S.J., Lindsay R.M. Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994;633(1-2):326–330. doi: 10.1016/0006-8993(94)91556-3. [DOI] [PubMed] [Google Scholar]
- 124.Cirulli F., Berry A., Alleva E. Intracerebroventricular administration of brain-derived neurotrophic factor in adult rats affects analgesia and spontaneous behaviour but not memory retention in a Morris Water Maze task. Neurosci. Lett. 2000;287(3):207–210. doi: 10.1016/S0304-3940(00)01173-3. [DOI] [PubMed] [Google Scholar]
- 125.Lever I., Cunningham J., Grist J., Yip P.K., Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 2003;18(5):1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- 126.Eaton M.J., Blits B., Ruitenberg M.J., Verhaagen J., Oudega M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002;9(20):1387–1395. doi: 10.1038/sj.gt.3301814. [DOI] [PubMed] [Google Scholar]
- 127.Pezet S., Cunningham J., Patel J., Grist J., Gavazzi I., Lever I.J., Malcangio M. BDNF modulates sensory neuron synaptic activity by a facilitation of GABA transmission in the dorsal horn. Mol. Cell. Neurosci. 2002;21(1):51–62. doi: 10.1006/mcne.2002.1166. [DOI] [PubMed] [Google Scholar]
- 128.Bardoni R., Ghirri A., Salio C., Prandini M., Merighi A. BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Dev. Neurobiol. 2007;67(7):960–975. doi: 10.1002/dneu.20401. [DOI] [PubMed] [Google Scholar]
- 129.Merighi A. The histology, physiology, neurochemistry and circuitry of the substantia gelatinosa Rolandi (lamina II) in mammalian spinal cord. Prog. Neurobiol. 2018;169:91–134. doi: 10.1016/j.pneurobio.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 130.Huang Y.J., Lee K.H., Grau J.W. Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Exp. Neurol. 2017;288:38–50. doi: 10.1016/j.expneurol.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Boucher T.J., Okuse K., Bennett D.L., Munson J.B., Wood J.N., McMahon S.B. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 132.Hedstrom K.L., Murtie J.C., Albers K., Calcutt N.A., Corfas G. Treating small fiber neuropathy by topical application of a small molecule modulator of ligand-induced GFRα/RET receptor signaling. Proc. Natl. Acad. Sci. USA. 2014;111(6):2325–2330. doi: 10.1073/pnas.1308889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chou A.K., Yang M.C., Tsai H.P., Chai C.Y., Tai M.H., Kwan A.L., Hong Y.R. Adenoviral-mediated glial cell line-derived neurotrophic factor gene transfer has a protective effect on sciatic nerve following constriction-induced spinal cord injury. PLoS One. 2014;9(3):e92264. doi: 10.1371/journal.pone.0092264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pezet S., Krzyzanowska A., Wong L.F., Grist J., Mazarakis N.D., Georgievska B., McMahon S.B. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol. Ther. 2006;13(6):1101–1109. doi: 10.1016/j.ymthe.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 135.Takasu K., Sakai A., Hanawa H., Shimada T., Suzuki H. Overexpression of GDNF in the uninjured DRG exerts analgesic effects on neuropathic pain following segmental spinal nerve ligation in mice. J. Pain. 2011;12(11):1130–1139. doi: 10.1016/j.jpain.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Yu H., Fischer G., Ebert A.D., Wu H.E., Bai X., Hogan Q.H. Analgesia for neuropathic pain by dorsal root ganglion transplantation of genetically engineered mesenchymal stem cells: initial results. Mol. Pain. 2015;11:5. doi: 10.1186/s12990-015-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Capelle H.H., Weigel R., Schmelz M., Krauss J.K. Neurotrophins in the cerebrospinal fluid of patient cohorts with neuropathic pain, nociceptive pain, or normal pressure hydrocephalus. Clin. J. Pain. 2009;25(8):729–733. doi: 10.1097/AJP.0b013e3181a776e4. [DOI] [PubMed] [Google Scholar]
- 138.Gao Y.Y., Hong X.Y., Wang H.J. Role of Nectin-1/c-Src Signaling in the analgesic effect of GDNF on a rat model of chronic constrictive injury. J. Mol. Neurosci. 2016;60(2):258–266. doi: 10.1007/s12031-016-0792-x. [DOI] [PubMed] [Google Scholar]
- 139.Patil S.B., Brock J.H., Colman D.R., Huntley G.W. Neuropathic pain- and glial derived neurotrophic factor-associated regulation of cadherins in spinal circuits of the dorsal horn. Pain. 2011;152(4):924–935. doi: 10.1016/j.pain.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 140.Sakai A., Asada M., Seno N., Suzuki H. Involvement of neural cell adhesion molecule signaling in glial cell line-derived neurotrophic factor-induced analgesia in a rat model of neuropathic pain. Pain. 2008;137(2):378–388. doi: 10.1016/j.pain.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 141.Shinoda M., Fukuoka T., Takeda M., Iwata K., Noguchi K. Spinal glial cell line-derived neurotrophic factor infusion reverses reduction of Kv4.1-mediated A-type potassium currents of injured myelinated primary afferent neurons in a neuropathic pain model. Mol. Pain. 2019;15:1744806919841196. doi: 10.1177/1744806919841196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Adler J.E., Nico L., VandeVord P., Skoff A.M. Modulation of neuropathic pain by a glial-derived factor. Pain Med. 2009;10(7):1229–1236. doi: 10.1111/j.1526-4637.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 143.Malcangio M., Getting S.J., Grist J., Cunningham J.R., Bradbury E.J., Charbel Issa P., Lever I.J., Pezet S., Perretti M. A novel control mechanism based on GDNF modulation of somatostatin release from sensory neurones. FASEB J. 2002;16(7):730–732. doi: 10.1096/fj.01-0971fje. [DOI] [PubMed] [Google Scholar]
- 144.Kimura M., Sakai A., Sakamoto A., Suzuki H. Glial cell line-derived neurotrophic factor-mediated enhancement of noradrenergic descending inhibition in the locus coeruleus exerts prolonged analgesia in neuropathic pain. Br. J. Pharmacol. 2015;172(10):2469–2478. doi: 10.1111/bph.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang H.J., Song G., Liang J., Gao Y.Y., Wang C.J. Involvement of integrin β1/FAK signaling in the analgesic effects induced by glial cell line-derived neurotrophic factor in neuropathic pain. Brain Res. Bull. 2017;135:149–156. doi: 10.1016/j.brainresbull.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 146.Wang C., Wang H., Pang J., Li L., Zhang S., Song G., Li N., Cao J., Zhang L. Glial cell-derived neurotrophic factor attenuates neuropathic pain in a mouse model of chronic constriction injury: possible involvement of E-cadherin/p120ctn signaling. J. Mol. Neurosci. 2014;54(2):156–163. doi: 10.1007/s12031-014-0266-y. [DOI] [PubMed] [Google Scholar]
- 147.Ogun-Muyiwa P., Helliwell R., McIntyre P., Winter J. Glial cell line derived neurotrophic factor (GDNF) regulates VR1 and substance P in cultured sensory neurons. Neuroreport. 1999;10(10):2107–2111. doi: 10.1097/00001756-199907130-00021. [DOI] [PubMed] [Google Scholar]
- 148.Malin S.A., Molliver D.C., Koerber H.R., Cornuet P., Frye R., Albers K.M., Davis B.M. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J. Neurosci. 2006;26(33):8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bogen O., Joseph E.K., Chen X., Levine J.D. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur. J. Neurosci. 2008;28(1):12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferrari L.F., Bogen O., Levine J.D. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165(3):896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Joseph E.K., Levine J.D. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169(1):431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang H.J., Gu H.X., Eijkelkamp N., Heijnen C.J., Kavelaars A. Low GRK2 underlies hyperalgesic priming by glial cell-derived neurotrophic factor. Front. Pharmacol. 2018;9:592. doi: 10.3389/fphar.2018.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fang M., Wang Y., He Q.H., Sun Y.X., Deng L.B., Wang X.M., Han J.S. Glial cell line-derived neurotrophic factor contributes to delayed inflammatory hyperalgesia in adjuvant rat pain model. Neuroscience. 2003;117(3):503–512. doi: 10.1016/S0306-4522(02)00958-2. [DOI] [PubMed] [Google Scholar]
- 154.Amaya F., Shimosato G., Nagano M., Ueda M., Hashimoto S., Tanaka Y., Suzuki H., Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur. J. Neurosci. 2004;20(9):2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- 155.Nencini S., Ringuet M., Kim D.H., Greenhill C., Ivanusic J.J. GDNF, Neurturin, and artemin activate and sensitize bone afferent neurons and contribute to Inflammatory Bone Pain. J. Neurosci. 2018;38(21):4899–4911. doi: 10.1523/JNEUROSCI.0421-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DeBerry J.J., Saloman J.L., Dragoo B.K., Albers K.M., Davis B.M. Artemin immunotherapy is effective in preventing and reversing cystitis-induced bladder hyperalgesia via TRPA1 Regulation. J. Pain. 2015;16(7):628–636. doi: 10.1016/j.jpain.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Forrest S.L., Osborne P.B., Keast J.R. Characterization of bladder sensory neurons in the context of myelination, receptors for pain modulators, and acute responses to bladder inflammation. Front. Neurosci. 2013;7:206. doi: 10.3389/fnins.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tokumine J., Kakinohana O., Cizkova D., Smith D.W., Marsala M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J. Neurosci. Res. 2003;74(4):552–561. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- 159.Yurek D.M., Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891(1-2):228–235. doi: 10.1016/S0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- 160.Li B., Gao Y., Zhang W., Xu J.R. Regulation and effects of neurotrophic factors after neural stem cell transplantation in a transgenic mouse model of Alzheimer disease. J. Neurosci. Res. 2018;96(5):828–840. doi: 10.1002/jnr.24187. [DOI] [PubMed] [Google Scholar]
- 161.Simões L.R., Abreu R.R.E.S., Generoso J.S., Goularte J.A., Collodel A., Giridharan V.V., Arumanayagam A.C.S., Valvassori S.S., Quevedo J., Barichello T. Prevention of Memory Impairment and Neurotrophic Factors Increased by Lithium in Wistar Rats Submitted to Pneumococcal Meningitis Model. Mediators Inflamm. 2017;2017:6490652. doi: 10.1155/2017/6490652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Balaszczuk V., Bender C., Pereno G., Beltramino C.A. Binge alcohol-induced alterations in BDNF and GDNF expression in central extended amygdala and pyriform cortex on infant rats. Int. J. Dev. Neurosci. 2013;31(5):287–296. doi: 10.1016/j.ijdevneu.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Sengoku T., Murray K.M., Wilson M.E. Neonatal hyperoxia induces alterations in neurotrophin gene expression. Int. J. Dev. Neurosci. 2016;48:31–37. doi: 10.1016/j.ijdevneu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 164.Giehl K.M., Schütte A., Mestres P., Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J. Neurosci. 1998;18(18):7351–7360. doi: 10.1523/JNEUROSCI.18-18-07351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zurn A.D., Winkel L., Menoud A., Djabali K., Aebischer P. Combined effects of GDNF, BDNF, and CNTF on motoneuron differentiation in vitro. J. Neurosci. Res. 1996;44(2):133–141. doi: 10.1002/(SICI)1097-4547(19960415)44:2<133::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 166.Sharma H.S. Post-traumatic application of brain-derived neurotrophic factor and glia-derived neurotrophic factor on the rat spinal cord enhances neuroprotection and improves motor function. Acta Neurochir. Suppl. (Wien) 2006;96:329–334. doi: 10.1007/3-211-30714-1_69. [DOI] [PubMed] [Google Scholar]
- 167.Revilla S., Suñol C., García-Mesa Y., Giménez-Llort L., Sanfeliu C., Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 168.Forlenza O.V., Miranda A.S., Guimar I., Talib L.L., Diniz B.S., Gattaz W.F., Teixeira A.L. Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J. Alzheimers Dis. 2015;46(2):423–429. doi: 10.3233/JAD-150172. [DOI] [PubMed] [Google Scholar]
- 169.Xiao W., Ye F., Liu C., Tang X., Li J., Dong H., Sha W., Zhang X. Cognitive impairment in first-episode drug-naïve patients with schizophrenia: Relationships with serum concentrations of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;76:163–168. doi: 10.1016/j.pnpbp.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 170.Tunca Z., Ozerdem A., Ceylan D., Yalçın Y., Can G., Resmi H., Akan P., Ergör G., Aydemir O., Cengisiz C., Kerim D. Alterations in BDNF (brain derived neurotrophic factor) and GDNF (glial cell line-derived neurotrophic factor) serum levels in bipolar disorder: The role of lithium. J. Affect. Disord. 2014;166:193–200. doi: 10.1016/j.jad.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 171.Croll S.D., Chesnutt C.R., Rudge J.S., Acheson A., Ryan T.E., Siuciak J.A., DiStefano P.S., Wiegand S.J., Lindsay R.M. Co-infusion with a TrkB-Fc receptor body carrier enhances BDNF distribution in the adult rat brain. Exp. Neurol. 1998;152(1):20–33. doi: 10.1006/exnr.1998.6836. [DOI] [PubMed] [Google Scholar]
- 172.Morgado C., Pereira-Terra P., Cruz C.D., Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes. Metab. 2011;13(2):150–159. doi: 10.1111/j.1463-1326.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 173.Moy J.K., Szabo-Pardi T., Tillu D.V., Megat S., Pradhan G., Kume M., Asiedu M.N., Burton M.D., Dussor G., Price T.J. Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats. Neurobiol. Pain. 2018;5:100024. doi: 10.1016/j.ynpai.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rosenthal A., Lin J.C. Modulation of neurotrophin signaling by monoclonal antibodies. Handb. Exp. Pharmacol. 2014;220:497–512. doi: 10.1007/978-3-642-45106-5_19. [DOI] [PubMed] [Google Scholar]
- 175.Cazorla M., Prémont J., Mann A., Girard N., Kellendonk C., Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 2011;121(5):1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cazorla M., Arrang J.M., Prémont J. Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor. Br. J. Pharmacol. 2011;162(4):947–960. doi: 10.1111/j.1476-5381.2010.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Merighi A., Bardoni R., Salio C., Lossi L., Ferrini F., Prandini M., Zonta M., Gustincich S., Carmignoto G. Presynaptic functional trkB receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina II (substantia gelatinosa) of postnatal rat spinal cord. Dev. Neurobiol. 2008;68(4):457–475. doi: 10.1002/dneu.20605. [DOI] [PubMed] [Google Scholar]
- 178.Cazorla M., Jouvenceau A., Rose C., Guilloux J.P., Pilon C., Dranovsky A., Prémont J. Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice. PLoS One. 2010;5(3):e9777. doi: 10.1371/journal.pone.0009777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thibault K., Lin W.K., Rancillac A., Fan M., Snollaerts T., Sordoillet V., Hamon M., Smith G.M., Lenkei Z., Pezet S. BDNF-dependent plasticity induced by peripheral inflammation in the primary sensory and the cingulate cortex triggers cold allodynia and reveals a major role for endogenous BDNF as a tuner of the affective aspect of pain. J. Neurosci. 2014;34(44):14739–14751. doi: 10.1523/JNEUROSCI.0860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tillu D.V., Hassler S.N., Burgos-Vega C.C., Quinn T.L., Sorge R.E., Dussor G., Boitano S., Vagner J., Price T.J. Protease-activated receptor 2 activation is sufficient to induce the transition to a chronic pain state. Pain. 2015;156(5):859–867. doi: 10.1097/j.pain.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Long T., He W., Pan Q., Zhang S., Zhang D., Qin G., Chen L., Zhou J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain. 2020;21(1):4. doi: 10.1186/s10194-019-1070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ding H., Chen J., Su M., Lin Z., Zhan H., Yang F., Li W., Xie J., Huang Y., Liu X., Liu B., Zhou X. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflammation. 2020;17(1):19. doi: 10.1186/s12974-020-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fan F., Tang Y., Dai H., Cao Y., Sun P., Chen Y., Chen A., Lin C. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. Eur. J. Pain. 2020;24(4):839–850. doi: 10.1002/ejp.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yan W., Lakkaniga N.R., Carlomagno F., Santoro M., McDonald N.Q., Lv F., Gunaganti N., Frett B., Li H.Y. Insights into current tropomyosin receptor kinase (TRK) inhibitors: development and clinical application. J. Med. Chem. 2019;62(4):1731–1760. doi: 10.1021/acs.jmedchem.8b01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stachel S.J., Sanders J.M., Henze D.A., Rudd M.T., Su H.P., Li Y., Nanda K.K., Egbertson M.S., Manley P.J., Jones K.L., Brnardic E.J., Green A., Grobler J.A., Hanney B., Leitl M., Lai M.T., Munshi V., Murphy D., Rickert K., Riley D., Krasowska-Zoladek A., Daley C., Zuck P., Kane S.A., Bilodeau M.T. Maximizing diversity from a kinase screen: identification of novel and selective pan-Trk inhibitors for chronic pain. J. Med. Chem. 2014;57(13):5800–5816. doi: 10.1021/jm5006429. [DOI] [PubMed] [Google Scholar]
- 186.Angeles T.S., Yang S.X., Steffler C., Dionne C.A. Kinetics of trkA tyrosine kinase activity and inhibition by K-252a. Arch. Biochem. Biophys. 1998;349(2):267–274. doi: 10.1006/abbi.1997.0490. [DOI] [PubMed] [Google Scholar]
- 187.Tapley P., Lamballe F., Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7(2):371–381. [PubMed] [Google Scholar]
- 188.Winston J.H., Toma H., Shenoy M., He Z.J., Zou L., Xiao S.Y., Micci M.A., Pasricha P.J. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J. Pain. 2003;4(6):329–337. doi: 10.1016/S1526-5900(03)00636-9. [DOI] [PubMed] [Google Scholar]
- 189.Frias B., Allen S., Dawbarn D., Charrua A., Cruz F., Cruz C.D. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience. 2013;234:88–102. doi: 10.1016/j.neuroscience.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 190.Miletic G., Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137(3):532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang H.H., Zhang X.Q., Xue Q.S., Yan-Luo, Huang J.L., Zhang S., Shao H.J., Lu H., Wang W.Y., Yu B.W. The BDNF/TrkB signaling pathway is involved in heat hyperalgesia mediated by Cdk5 in rats. PLoS One. 2014;9(1):e85536. doi: 10.1371/journal.pone.0085536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yasui M., Shiraishi Y., Ozaki N., Hayashi K., Hori K., Ichiyanagi M., Sugiura Y. Nerve growth factor and associated nerve sprouting contribute to local mechanical hyperalgesia in a rat model of bone injury. Eur. J. Pain. 2012;16(7):953–965. doi: 10.1002/j.1532-2149.2011.00094.x. [DOI] [PubMed] [Google Scholar]
- 193.Bailey J.J., Jaworski C., Tung D., Wängler C., Wängler B., Schirrmacher R. Tropomyosin receptor kinase inhibitors: an updated patent review for 2016-2019. Expert Opin. Ther. Pat. 2020;30(5):325–339. doi: 10.1080/13543776.2020.1737011. [DOI] [PubMed] [Google Scholar]
- 194.Bailey J.J., Schirrmacher R., Farrell K., Bernard-Gauthier V. Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part II. Expert Opin. Ther. Pat. 2017;27(7):831–849. doi: 10.1080/13543776.2017.1297797. [DOI] [PubMed] [Google Scholar]
- 195.Bailey J.J., Schirrmacher R., Farrell K., Bernard-Gauthier V. Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part I. Expert Opin. Ther. Pat. 2017;27(6):733–751. doi: 10.1080/13543776.2017.1297796. [DOI] [PubMed] [Google Scholar]
- 196.Wang T., Yu D., Lamb M.L. Trk kinase inhibitors as new treatments for cancer and pain. Expert Opin. Ther. Pat. 2009;19(3):305–319. doi: 10.1517/13543770902721261. [DOI] [PubMed] [Google Scholar]
- 197.Ghilardi J.R., Freeman K.T., Jimenez-Andrade J.M., Mantyh W.G., Bloom A.P., Bouhana K.S., Trollinger D., Winkler J., Lee P., Andrews S.W., Kuskowski M.A., Mantyh P.W. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone. 2011;48(2):389–398. doi: 10.1016/j.bone.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Skerratt S.E., Andrews M., Bagal S.K., Bilsland J., Brown D., Bungay P.J., Cole S., Gibson K.R., Jones R., Morao I., Nedderman A., Omoto K., Robinson C., Ryckmans T., Skinner K., Stupple P., Waldron G. The discovery of a potent, selective, and peripherally restricted pan-Trk Inhibitor (PF-06273340) for the treatment of pain. J. Med. Chem. 2016;59(22):10084–10099. doi: 10.1021/acs.jmedchem.6b00850. [DOI] [PubMed] [Google Scholar]
- 199.Ishiguro N., Oyama S., Higashi R., Yanagida K. Efficacy, safety, and tolerability of ONO-4474, an orally available pan-tropomyosin receptor kinase inhibitor, in japanese patients with moderate to severe osteoarthritis of the knee: a randomized, placebo-controlled, double-blind, parallel-group comparative study. J. Clin. Pharmacol. 2020;60(1):28–36. doi: 10.1002/jcph.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Boulle F., Kenis G., Cazorla M., Hamon M., Steinbusch H.W., Lanfumey L., van den Hove D.L. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog. Neurobiol. 2012;98(2):197–206. doi: 10.1016/j.pneurobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 201.Ferrini F., De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Beggs S., Trang T., Salter M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 2012;15(8):1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iwakura Y., Nawa H., Sora I., Chao M.V. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J. Biol. Chem. 2008;283(23):15799–15806. doi: 10.1074/jbc.M801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wiese S., Jablonka S., Holtmann B., Orel N., Rajagopal R., Chao M.V., Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc. Natl. Acad. Sci. USA. 2007;104(43):17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Berghuis P., Dobszay M.B., Wang X., Spano S., Ledda F., Sousa K.M., Schulte G., Ernfors P., Mackie K., Paratcha G., Hurd Y.L., Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by trans activating the TrkB receptor. Proc. Natl. Acad. Sci. USA. 2005;102(52):19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Doyon N., Ferrini F., Gagnon M., De Koninck Y. Treating pathological pain: is KCC2 the key to the gate? Expert Rev. Neurother. 2013;13(5):469–471. doi: 10.1586/ern.13.40. [DOI] [PubMed] [Google Scholar]
- 207.Gagnon M., Bergeron M.J., Lavertu G., Castonguay A., Tripathy S., Bonin R.P., Perez-Sanchez J., Boudreau D., Wang B., Dumas L., Valade I., Bachand K., Jacob-Wagner M., Tardif C., Kianicka I., Isenring P., Attardo G., Coull J.A., De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013;19(11):1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Charbel Issa P., Lever I.J., Michael G.J., Bradbury E.J., Malcangio M. Intrathecally delivered glial cell line-derived neurotrophic factor produces electrically evoked release of somatostatin in the dorsal horn of the spinal cord. J. Neurochem. 2001;78(2):221–229. doi: 10.1046/j.1471-4159.2001.00430.x. [DOI] [PubMed] [Google Scholar]
- 209.Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr, Lozano A.M., Penn R.D., Simpson R.K., Jr, Stacy M., Wooten G.F. ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/WNL.60.1.69. [DOI] [PubMed] [Google Scholar]
- 210.d’Anglemont de Tassigny X., Pascual A., López-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boado R.J., Pardridge W.M. Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Diabetes Obes. Metab. 2009;37(12):2299–2304. doi: 10.1124/dmd.109.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gartziandia O., Herrán E., Ruiz-Ortega J.A., Miguelez C., Igartua M., Lafuente J.V., Pedraz J.L., Ugedo L., Hernández R.M. Intranasal administration of chitosan-coated nanostructured lipid carriers Loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J. Biomed. Nanotechnol. 2016;12(12):2220–2230. doi: 10.1166/jbn.2016.2313. [DOI] [PubMed] [Google Scholar]
- 213.Hernando S., Herran E., Figueiro-Silva J., Pedraz J.L., Igartua M., Carro E., Hernandez R.M. Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol. Neurobiol. 2018;55(1):145–155. doi: 10.1007/s12035-017-0728-7. [DOI] [PubMed] [Google Scholar]
- 214.Garbayo E., Ansorena E., Lana H., Carmona-Abellan M.D., Marcilla I., Lanciego J.L., Luquin M.R., Blanco-Prieto M.J. Brain delivery of microencapsulated GDNF induces functional and structural recovery in parkinsonian monkeys. Biomaterials. 2016;110:11–23. doi: 10.1016/j.biomaterials.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 215.Garbayo E., Montero-Menei C.N., Ansorena E., Lanciego J.L., Aymerich M.S., Blanco-Prieto M.J. Effective GDNF brain delivery using microspheres--a promising strategy for Parkinson’s disease. J. Control. Release. 2009;135(2):119–126. doi: 10.1016/j.jconrel.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 216.Herrán E., Ruiz-Ortega J.Ã., Aristieta A., Igartua M., Requejo C., Lafuente J.V., Ugedo L., Pedraz J.L., Hernández R.M. In vivo administration of VEGF- and GDNF-releasing biodegradable polymeric microspheres in a severe lesion model of Parkinson’s disease. Eur. J. Pharm. Biopharm. 2013;85(3 Pt B):1183–1190. doi: 10.1016/j.ejpb.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 217.Xia C.F., Boado R.J., Zhang Y., Chu C., Pardridge W.M. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson’s disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. J. Gene Med. 2008;10(3):306–315. doi: 10.1002/jgm.1152. [DOI] [PubMed] [Google Scholar]
- 218.Monville C., Torres E., Thomas E., Scarpini C.G., Muhith J., Lewis J., Finn J., Smith C., Cai S., Efstathiou S., Howard K., Dunnett S.B. HSV vector-delivery of GDNF in a rat model of PD: partial efficacy obscured by vector toxicity. Brain Res. 2004;1024(1-2):1–15. doi: 10.1016/j.brainres.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 219.Palfi S., Leventhal L., Chu Y., Ma S.Y., Emborg M., Bakay R., Déglon N., Hantraye P., Aebischer P., Kordower J.H. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J. Neurosci. 2002;22(12):4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Choi-Lundberg D.L., Lin Q., Chang Y.N., Chiang Y.L., Hay C.M., Mohajeri H., Davidson B.L., Bohn M.C. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 221.Kirik D., Rosenblad C., Bjorklund A., Mandel R.J. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000;20(12):4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang L.J., Lu Y.Y., Muramatsu S., Ikeguchi K., Fujimoto K., Okada T., Mizukami H., Matsushita T., Hanazono Y., Kume A., Nagatsu T., Ozawa K., Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurosci. 2002;22(16):6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Heiss J.D., Lungu C., Hammoud D.A., Herscovitch P., Ehrlich D.J., Argersinger D.P., Sinharay S., Scott G., Wu T., Federoff H.J., Zaghloul K.A., Hallett M., Lonser R.R., Bankiewicz K.S. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov. Disord. 2019;34(7):1073–1078. doi: 10.1002/mds.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hao S., Mata M., Wolfe D., Huang S., Glorioso J.C., Fink D.J. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol. Ther. 2003;8(3):367–375. doi: 10.1016/S1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 225.Shi J.Y., Liu G.S., Liu L.F., Kuo S.M., Ton C.H., Wen Z.H., Tee R., Chen C.H., Huang H.T., Chen C.L., Chao D., Tai M.H. Glial cell line-derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum. Gene Ther. 2011;22(6):721–731. doi: 10.1089/hum.2010.036. [DOI] [PubMed] [Google Scholar]
- 226.Chen C., Li X., Ge G., Liu J., Biju K.C., Laing S.D., Qian Y., Ballard C., He Z., Masliah E., Clark R.A., O’Connor J.C., Li S. GDNF-expressing macrophages mitigate loss of dopamine neurons and improve Parkinsonian symptoms in MitoPark mice. Sci. Rep. 2018;8(1):5460. doi: 10.1038/s41598-018-23795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Akerud P., Canals J.M., Snyder E.Y., Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 2001;21(20):8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cunningham L.A., Su C. Astrocyte delivery of glial cell line-derived neurotrophic factor in a mouse model of Parkinson’s disease. Exp. Neurol. 2002;174(2):230–242. doi: 10.1006/exnr.2002.7877. [DOI] [PubMed] [Google Scholar]
- 229.Xu Q., Zhang M., Liu J., Li W. Intrathecal transplantation of neural stem cells appears to alleviate neuropathic pain in rats through release of GDNF. Ann. Clin. Lab. Sci. 2013;43(2):154–162. [PubMed] [Google Scholar]
- 230.Salvatore M.F., Ai Y., Fischer B., Zhang A.M., Grondin R.C., Zhang Z., Gerhardt G.A., Gash D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 231.Lang A.E., Gill S., Patel N.K., Lozano A., Nutt J.G., Penn R., Brooks D.J., Hotton G., Moro E., Heywood P., Brodsky M.A., Burchiel K., Kelly P., Dalvi A., Scott B., Stacy M., Turner D., Wooten V.G., Elias W.J., Laws E.R., Dhawan V., Stoessl A.J., Matcham J., Coffey R.J., Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59(3):459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 232.McFarthing K., Prakash N., Simuni T. Clinical trial highlights: 1. gene therapy for parkinson’s, 2. phase 3 study in focus - Intec Pharma’s accordion pill, 3. clinical trials resources. J. Parkinsons Dis. 2019;9(2):251–264. doi: 10.3233/JPD-199001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rolan P.E., O’Neill G., Versage E., Rana J., Tang Y., Galluppi G., Aycardi E. First-In-Human, Double-Blind, Placebo-Controlled, Randomized, Dose-Escalation Study of BG00010, a glial cell line-derived neurotrophic factor family member, in subjects with unilateral sciatica. PLoS One. 2015;10(5):e0125034. doi: 10.1371/journal.pone.0125034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Okkerse P., Hay J.L., Versage E., Tang Y., Galluppi G., Ravina B., Verma A., Williams L., Aycardi E., Groeneveld G.J. Pharmacokinetics and pharmacodynamics of multiple doses of BG00010, a neurotrophic factor with anti-hyperalgesic effects, in patients with sciatica. Br. J. Clin. Pharmacol. 2016;82(1):108–117. doi: 10.1111/bcp.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tokugawa K., Yamamoto K., Nishiguchi M., Sekine T., Sakai M., Ueki T., Chaki S., Okuyama S. XIB4035, a novel nonpeptidyl small molecule agonist for GFRalpha-1. Neurochem. Int. 2003;42(1):81–86. doi: 10.1016/S0197-0186(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 236.Sidorova Y.A., Bespalov M.M., Wong A.W., Kambur O., Jokinen V., Lilius T.O., Suleymanova I., Karelson G., Rauhala P.V., Karelson M., Osborne P.B., Keast J.R., Kalso E.A., Saarma M. A Novel small molecule GDNF Receptor RET Agonist, BT13, promotes neurite growth from sensory neurons in Vitro and Attenuates Experimental Neuropathy in the Rat. Front. Pharmacol. 2017;8:365. doi: 10.3389/fphar.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tsui C.C., Gabreski N.A., Hein S.J., Pierchala B.A. Lipid rafts are physiologic membrane microdomains necessary for the morphogenic and developmental functions of glial cell line-derived neurotrophic factor in vivo. J. Neurosci. 2015;35(38):13233–13243. doi: 10.1523/JNEUROSCI.2935-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hadjiconstantinou M., Neff N.H. GM1 ganglioside: in vivo and in vitro trophic actions on central neurotransmitter systems. J. Neurochem. 1998;70(4):1335–1345. doi: 10.1046/j.1471-4159.1998.70041335.x. [DOI] [PubMed] [Google Scholar]
- 239.Newburn E.N., Duchemin A.M., Neff N.H., Hadjiconstantinou M. GM1 ganglioside enhances Ret signaling in striatum. J. Neurochem. 2014;130(4):541–554. doi: 10.1111/jnc.12760. [DOI] [PubMed] [Google Scholar]
- 240.Koju R.P., Lei T.C. Efficacy of Ganglioside GM1 in the treatment of postherpetic neuralgia. J. Coll. Physicians Surg. Pak. 2016;26(7):633–634. [PubMed] [Google Scholar]
- 241.Dong J., Li S., Mo J.L., Cai H.B., Le W.D. Nurr1-based therapies for Parkinson’s Disease. CNS Neurosci. Ther. 2016;22(5):351–359. doi: 10.1111/cns.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Volakakis N., Tiklova K., Decressac M., Papathanou M., Mattsson B., Gillberg L., Nobre A., Björklund A., Perlmann T. Nurr1 and retinoid X receptor ligands stimulate ret signaling in dopamine neurons and can alleviate α-synuclein disrupted gene expression. J. Neurosci. 2015;35(42):14370–14385. doi: 10.1523/JNEUROSCI.1155-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gui Y., Duan S., Xiao L., Tang J., Li A. Bexarotent attenuated CCI-induced spinal neuroinflammation and neuropathic pain by targeting MKP-1. J. Pain. 2019:S1526-5900(18)30607-2. doi: 10.1016/j.jpain.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 244.Bartus R.T., Johnson E.M., Jr Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: Where have we been and what have we learned? Neurobiol. Dis. 2017;97(Pt B):156–168. doi: 10.1016/j.nbd.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 245.Bartus R.T., Johnson E.M., Jr Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 2: Where do we stand and where must we go next? Neurobiol. Dis. 2017;97(Pt B):169–178. doi: 10.1016/j.nbd.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 246.Weissmiller A.M., Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012;1(1):14. doi: 10.1186/2047-9158-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Anand P. In: NGF and Related Molecules in Health and Disease. Aloe L., Calza L., editors. Vol. 146. Amsterdam: Elsevier; 2004. Neurotrophic factors and their receptors in human sensory neuropathies. pp. 477–492. [DOI] [PubMed] [Google Scholar]
- 248.Saudek F., Cahova M., Havrdova T., Zacharovova K., Dankova H., Voska L., Lanska V., Uceyler N., Sommer C. Preserved expression of skin neurotrophic factors in advanced diabetic neuropathy does not lead to neural regeneration despite pancreas and kidney transplantation. J. Diabetes. Res. 2018;2018:2309108. doi: 10.1155/2018/2309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sun Q., Tang D.D., Yin E.G., Wei L.L., Chen P., Deng S.P., Tu L.L. Diagnostic significance of serum levels of nerve growth factor and brain derived neurotrophic factor in diabetic peripheral neuropathy. Med. Sci. Monit. 2018;24:5943–5950. doi: 10.12659/MSM.909449. [DOI] [PMC free article] [PubMed] [Google Scholar]