Abstract
Background
The cervical cap and the diaphragm are vaginal barrier contraceptive methods that prevent pregnancy by covering the cervix. The two devices also act as a reservoir for spermicide. The cervical cap is smaller and can remain in place longer than the diaphragm. The Prentif cap and the FemCap have been compared to the diaphragm in randomized controlled trials.
Objectives
To compare the contraceptive efficacy, safety, discontinuation, and acceptability of the cervical cap with that of the diaphragm.
Search methods
In February 2012, we searched MEDLINE, POPLINE, CENTRAL, and LILACS for randomized controlled trials of cervical caps. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). Previous searches also included EMBASE. For the initial review, we wrote to manufacturers and investigators for information about other published or unpublished trials.
Selection criteria
All randomized controlled trials in any language comparing a cervical cap with a diaphragm were eligible for inclusion.
Data collection and analysis
Articles identified for inclusion were independently abstracted by two reviewers. Data were entered into RevMan, and a second reviewer verified the data entered. Outcome measures include contraceptive efficacy, safety, discontinuation, and acceptability. Outcomes were calculated as Peto odds ratios (OR) with 95% confidence intervals (CI). Life‐table and Kaplan‐Meier cumulative rate ratios for selected measures are presented.
Main results
The curves for the life‐table cumulative pregnancy rates for the Prentif cap and the diaphragm did not differ. However, the Kaplan‐Meier six‐month cumulative pregnancy rates for the FemCap and the diaphragm were not clinically equivalent. The Prentif cap had more Class I to Class III cervical cytologic conversions than the diaphragm (OR 2.31; 95% CI 1.04 to 5.11). The FemCap trial did not find differences in Papanicolaou smear results between the groups. Fewer Prentif cap users had vaginal ulcerations or lacerations (OR 0.31; 95% CI 0.14 to 0.71) than diaphragm users. Fewer FemCap users had blood in the device (OR 2.29; 95% CI 1.27 to 4.14), but more had urinary tract infections (OR 0.59; 95% CI 0.39 to 0.95). In the FemCap trial, similar proportions of women reported liking their device. However, FemCap users were less likely to use the device alone after the trial (OR 0.47; 95% CI 0.31 to 0.71) or recommend it to a friend (OR 0.48; 95% CI 0.29 to 0.81).
Authors' conclusions
The Prentif cap was as effective as its comparison diaphragm in preventing pregnancy, but the FemCap was not. Both cervical caps appear to be medically safe.
Keywords: Adult, Female, Humans, Intrauterine Devices, Contraception, Contraception/instrumentation, Contraception/methods, Randomized Controlled Trials as Topic
Plain language summary
Cervical cap versus diaphragm for birth control
The cervical cap and the diaphragm are small, rubber devices that women put in their vagina (birth canal) and place over their cervix. Both devices block sperm and help prevent pregnancy. Also, both hold a chemical that kills sperm. Birth control with these methods can be stopped at any time and can be used without involving the partner. The cervical cap is smaller than the diaphragm and can be left in place longer. The cervical cap can be worn up to 72 hours, and the diaphragm can be used up to 30 hours. In this review, we compared the cervical cap with the diaphragm for how well it worked for birth control. We also looked at its safety and whether women stopped using it early.
In February 2012, we did a computer search for studies of cervical caps. We wrote to manufacturers and researchers for information about other trials. We included randomized controlled trials that compared a cervical cap with a diaphragm.
We found two trials that compared the cervical cap with the diaphragm. Two types of cervical caps were studied: the Prentif cap and the FemCap. The Prentif cap prevented pregnancy as well as the diaphragm, but the FemCap did not. Women who used the Prentif cap had more abnormal changes in the cervix than diaphragm users. The FemCap users did not have more abnormal changes than the diaphragm users. Many women from both groups dropped out early from the two trials. Similar numbers of FemCap users and diaphragm users reported liking their assigned method.
The Prentif cap worked as well as the diaphragm to prevent pregnancy. The FemCap did not prevent pregnancy as well as the diaphragm. Both cervical caps appear to be medically safe.
Background
The cervical cap and the diaphragm are vaginal contraceptive methods that prevent pregnancy by blocking the cervix as well as by providing a reservoir for spermicide. The cervical cap is a soft, small, cup‐like device that completely covers the cervix with a dome designed to be deep enough to prevent contact with the cervical os (Shihata 1991; Stewart 1998). Some types of cervical caps are designed to also cover the vaginal fornices. The cap's position is maintained either by suction with, or adherence to, the surface of the cervix. Currently, five types of cervical caps are manufactured. The Prentif Cavity Rim Cervical Cap, the Dumas or vault cap, and the Vimule Cap are made of latex and have the same manufacturer (Lamberts LTD, Oxford, England). The FemCap is made of silicone rubber (rubber manufactured by Hi‐Tech Rubber, Anaheim, CA) and the Oves Cap (Veos UK Limited, London, England) is constructed of silicone elastomer. Cervical caps are reusable except for the Oves Cap, which is a single‐use device. Caps are to be worn for a minimum of 6 to 8 hours after intercourse and a maximum of 30 to 72 hours after insertion (Shihata 1991; Stewart 1998).
The diaphragm is designed to be placed in the vagina so that the dome of the device completely covers the cervix with the anterior rim fit behind the pubic bone and the posterior rim fit in the posterior fornix (Stewart 1998). Diaphragm types vary according to their spring and rim designs. The flexible rim of the diaphragm contains one of three spring designs: arcing, coil or flat spring. The arcing and coil spring types are also available in a model with a wide‐seal rim. This design is thought to provide a better seal between the diaphragm and the vaginal wall. No type of diaphragm, though, forms a complete seal with the vaginal wall. Diaphragms are constructed out of natural rubber, rubber latex, or silicone rubber. They range from 50 mm to 105 mm in diameter size and are to be worn for a minimum of 6 hours after intercourse and a maximum of 24 to 30 hours after insertion. The recommended duration of cap and diaphragm use varies by device type, health care provider and country guidelines.
Both the cervical cap and the diaphragm offer women a reversible, user‐controlled contraceptive method that can be used without the involvement of her partner. Since they are only needed at the time of coitus, both methods are ideal for women who require contraception only intermittently. They may also be appropriate for women with contraindications to hormonal contraception. Some guidelines suggest that the cervical cap, unlike the diaphragm, has the ability to protect against multiple coital acts without requiring the application of additional spermicide; however, this practice is not unanimously accepted. The diaphragm has the advantage over the cap of being offered in a wider range of sizes; the restricted number of available sizes prevents the cap from being an option for all women. For example, a large U.S. study found that 6% to 10% of women could not be fitted properly with the Prentif Cavity Rim cervical cap (Bernstein 1986). Fit is important because caps that are fitted too tightly could possibly result in cervical trauma, and those without a secure placement could be more likely to dislodge from the cervix during use. Concerns related to both cervical cap and diaphragm use have been hypothesized: inadequate contraceptive efficacy, increased risk of abnormalities of the cervix and urinary tract infections; difficulties or inconveniences related to insertion and removal; user or partner discomfort; latex reactions; and unpleasant odor, especially when left in place longer than the recommended amount of time (Powell 1986; Trussell 1993).
Objectives
To compare the contraceptive efficacy, safety, discontinuation, and acceptability of the cervical cap with that of the diaphragm.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials in any language comparing a cervical cap with a diaphragm.
Types of participants
Women of reproductive age who do not have contraindications to either the cervical cap or the diaphragm.
Types of interventions
Any cervical cap was eligible for study inclusion. However, to date, only two types of cervical caps have been evaluated against a diaphragm in a randomized controlled trial. The Prentif Cavity Rim Cervical Cap® (Lamberts LTD, Oxford, England) is a latex cup with a firm, round rim that uses suction to fit snugly around the base of the cervix. The suction between the rim and the surface of the cervix is facilitated by a groove located along the inside of the rim of the cap. The Prentif Cap comes in four internal rim diameter sizes: 22, 25, 28 and 31 mm.
The FemCap® (rubber manufactured by Hi‐Tech Rubber, Anaheim, CA) is a silicone rubber cervical cap with a sailor‐hat shape. The dome completely covers the cervix with the rim securely fit into the vaginal fornices and a brim that adheres and conforms to the vaginal walls around the cervix. The FemCap is available in three sizes: 22 mm for nulliparous women, 26 mm for women who have been pregnant but who have never vaginally delivered, and 30 mm for women who have vaginally delivered. The FemCap is formulated of medical‐grade silicone rubber, which has the advantage of being less allergenic and less susceptible to oxidative forces than the latex‐containing Prentif Cap. Accordingly, the FemCap has potential for being easier to clean and more durable. Any diaphragm could be the comparison method.
Types of outcome measures
Outcome measures included contraceptive efficacy, method‐related and non‐method‐related discontinuation, safety, and acceptability.
Search methods for identification of studies
Electronic searches
In February 2012, we searched the computerized databases MEDLINE, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), and LILACS. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The search strategy is given in Appendix 1. The previous search strategy, which also included EMBASE, can be found in Appendix 2.
Searching other resources
For the initial review, we reviewed the references of publications identified for inclusion. We also attempted to contact the manufacturers and known investigators to request information about any other published or unpublished trials not discovered in our search.
Data collection and analysis
All titles and abstracts located in the literature searches were assessed for inclusion. Two reviewers independently abstracted data from the studies identified for inclusion to increase accuracy. Data were entered and analyzed with RevMan 4.1, and a second reviewer verified the data entered. Peto odds ratios with 95% confidence intervals were used for the dichotomous outcomes. Life‐table and Kaplan‐Meier cumulative rate ratios for contraceptive efficacy, colposcopy findings, and discontinuation were presented in 'Additional tables.' Rates per women‐years for gynecological infection were also included in a table. We examined the outcomes from the two trials separately because the trials differed in terms of cervical cap and diaphragm types. The trials were critically appraised for potential biases by qualitatively assessing the study design, blinding, randomization method, group allocation concealment, and loss to follow‐up and early discontinuation rates.
Results
Description of studies
Two randomized controlled trials comparing the cervical cap to the diaphragm were identified. Both trials recruited sexually active women aged 18 to 40 years. The first trial (Bernstein 1986) evaluated the Prentif Cavity Rim Cervical Cap® in comparison to the Ortho® diaphragm (Ortho Pharmaceutical Corporation, Raritan, NJ). The standard study diaphragm was a coil‐spring device, but an arcing or a flat diaphragm was assigned if it provided a better fit or was easier for the woman to insert. Both intervention groups received the spermicides Ortho‐Gynol®, which contains 1.00% nonoxynol‐9, and Gynol II®, which has 2% nonoxynol‐9 (Advanced Care Products, Ortho Pharmaceutical Corporation, Raritan, NJ). The Prentif Cap users were to fill one‐third of the cap with the spermicidal jelly prior to insertion, and the diaphragm users were to follow the manufacturer's instructions regarding spermicide application. Women in both intervention groups were instructed to leave the device in place for a minimum of eight hours following intercourse. The Prentif cap had a longer maximum length of use than the diaphragm (72 hours versus 24 hours, respectively). The groups also differed in that additional spermicide was optional for the cap users, but the diaphragm users were instructed to insert more spermicide before any additional coital acts or if more than six hours had elapsed since insertion. The length of follow up for participants varied from 6 to 42 months depending on the time of study entry. Participants were to return for follow‐up visits at one week after randomization and then every subsequent three months. Cervical cytology was to be assessed with gynecologic exams and Papanicolaou smears at the initial visit and the 3‐month and 12‐month follow‐up visits.
The second trial (Mauck 1999) compared the FemCap® cervical cap to the latex, arcing spring All‐Flex® diaphragm (Ortho Pharmaceutical Corporation, Raritan, NJ). One‐half teaspoon of spermicide was to be used with the FemCap and one teaspoon with the diaphragm; the spermicide Gynol II® (Advanced Care Products, Ortho Pharmaceutical Corporation, Raritan, NJ) containing 2% nonoxynol‐9 was used with both groups. Women in 10 centers in the U.S. had to be willing not to become pregnant during the 28‐week trial while also accepting an unknown risk of pregnancy to be eligible for trial participation. FemCap users were instructed to use the device for a minimum of 6 hours after intercourse and a maximum of 48 hours after insertion. They were to either refrain from intercourse or use condoms instead of the cap during menses due to a lack of evidence on toxic shock syndrome. While FemCap users were not required to use more spermicide than the initial amount, women using the diaphragm were instructed to insert additional spermicide in the case of more than one coital act. Diaphragm users were instructed to remove the device immediately after six hours after the last act of intercourse had elapsed. Follow‐up visits for both groups were scheduled for 2, 6, 12, and 28 weeks after randomization, and participants were contacted by telephone at 20 weeks following randomization. A small subset of women (21 cap users and 21 diaphragm users) was enrolled in a nested colposcopic study of cervical changes.
Risk of bias in included studies
The blinding of the participants and the investigators to group assignment was not feasible in either trial as the cervical cap and the diaphragm differ in appearance. In the Prentif cap trial (Bernstein 1986), the staff members who interpreted the cytology results were blinded to the assigned contraceptive method. The method of randomization, allocation concealment, a priori hypotheses, and sample size calculations were not described for the Prentif cap trial. About 25% of the women (185 women assigned to the Prentif cap and 191 to the diaphragm) were inappropriately excluded after randomization because of eventual discovery of ineligibility. Excluding from the analysis participants who have been randomized is not consistent with intent to treat analysis. Failure to use intent to treat analysis can lead to biased results (Weiss 1998). Reasons for disqualification from the Prentif or diaphragm group included pregnancy at entry or the one‐week visit (10 cap users and 14 diaphragm users); inability to be fit with the device (43, 26); medical contraindication (26, 16); personal reason related to the method (39, 43); personal reason not method‐related (5, 12); and investigator's choice (62, 80). Loss to follow up during the 42‐month trial was about 19% for the Prentif cap users and 21% for the diaphragm users.
Although the participants and the investigators in the FemCap trial were unblinded regarding group assignment, the staff members who analyzed adverse events were blinded and were not connected to the trial in any other manner. Randomization was based on a computer‐generated scheme with a fixed block size of four, stratified by center and prior female barrier method use. The concealment of group allocation was not described. The authors included a priori hypotheses and sample size calculations. About 7% of the women (53 women assigned to the FemCap and 6 to the diaphragm) were inappropriately excluded after randomization. Most exclusions at baseline from the FemCap or diaphragm group were due to user‐inability to insert or remove the device (40 cap users, 3 diaphragm users). Other reasons included inability to fit the device (5 cap users, 0 diaphragm users); a non‐safety reason related to the device (1, 0); a non‐safety reason not related to the device (4, 1); and protocol violation (3, 2). Loss to follow up in this shorter trial was low (6.4%).
Effects of interventions
While the Prentif cap was comparable to the diaphragm in preventing pregnancy, the FemCap was not as effective as its comparison diaphragm. The cumulative life‐table rate ratios of pregnancy for the Prentif cap in comparison to the diaphragm were 1.3, 1.0, 1.1, and 1.1 at 6, 12, 18, and 24 months, respectively (Table 1). The curves for the cumulative life‐table pregnancy rates through 24 months for the Prentif cap and the diaphragm were not significantly different (P value of 0.4). The Kaplan‐Meier six‐month cumulative pregnancy rate ratio was 1.7 for the FemCap in comparison to the diaphragm. Mauck 1999 concluded that the FemCap did not meet the a priori definition of clinical equivalence with the diaphragm because a difference of six percentage points was possible in the six‐month rates of pregnancy with the two devices.
1. Cap versus diaphragm: Cumulative pregnancy rate ratio.
| Outcome | Bernstein‐life table | Mauck ‐ Kaplan‐Meier |
| 6‐month rate ratio | 1.3 | 1.7 |
| 12‐month rate ratio | 1.0 | |
| 18‐month rate ratio | 1.1 | |
| 24‐month rate ratio | 1.1 |
In the Prentif cap trial, Papanicolaou examinations were performed at the initial, 3‐month, and 12‐month visits to assess changes in cervical intraepithelial neoplasia and inflammation (Bernstein 1986). The odds ratio of having a Class I to a Class III cytologic conversion at the three‐month visit for the Prentif cap versus the diaphragm was 2.31 (95% CI 1.04 to 5.11). In the FemCap trial, Papanicolaou smears were performed at the initial, 12‐week, and discontinuation visits (Mauck 1999). No statistically significant differences in abnormal Papanicolaou smears between the FemCap and diaphragm users were found. Also, the nested colposcopy analysis revealed similar rates of lesions for women in the two methods; the Kaplan‐Meier six‐month cumulative rate ratio of developing a detectable colposcopy finding was 1.2 for the FemCap versus the diaphragm users (Table 2).
2. Cap versus diaphragm: Kaplan‐Meier cumulative 6‐month rate ratio of colposcopy find.
| Outcome | Bernstein |
| Colposcopy finding | 1.2 |
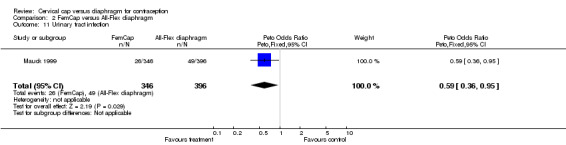
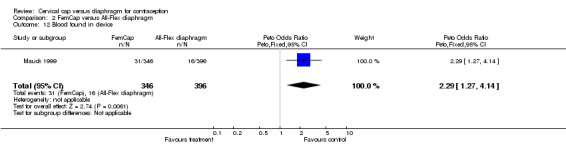
Commonly reported infections in the Prentif cap trial included etiology‐unspecified vaginitis, bacterial vaginosis, candidiasis, and urinary tract infection (Bernstein 1986). The total episodes per 100 women‐years for these gynecological infections were similar for the cap and diaphragm users (Table 3). The data available do not allow the rates for the two methods to be tested for statistical significance. Five Prentif cap and 18 diaphragm users developed vaginal ulcerations or lacerations. The odds ratio of this adverse event was 0.31 (95% CI 0.14 to 0.71) for women in the Prentif group in comparison to those in the diaphragm group. In Mauck 1999, FemCap users reported blood in the device on removal more often than diaphragm users with an odds ratio of 2.29 (95% CI 1.27 to 4.14). Women in the FemCap group were less likely to have urinary tract infections than those in the diaphragm group (OR 0.59; 95% CI 0.36 to 0.95). Other adverse events included vaginal candidiasis, etiology‐unspecified vaginitis, bacterial vaginosis, leukorrhea, genital irritation and dysmenorrhea. However, the rates of these adverse events were similar between the FemCap and diaphragm users.
3. Cap versus diaphragm: Total episodes of infection per 100 women‐years.
| Infection | Prentif cap | Diaphragm | Ratio (Bernstein) |
| Etiology‐unspecified vaginitis | 29.7 | 28.2 | 1.1 |
| Bacterial vaginosis | 39.7 | 29.5 | 1.3 |
| Candidiasis | 39.5 | 34.1 | 1.2 |
| Urinary tract infection | 14.8 | 16.5 | 0.9 |
The most commonly reported problem among the women in the FemCap trial was dislodgement of the device (Mauck 1999). About 31% of the FemCap users reported that the device dislodged or moved in comparison to only 6% of diaphragm users reporting this problem (OR 5.46; 95% CI 3.74 to 7.96). Women assigned to the FemCap were also more likely than those in the diaphragm group to report at least one occurrence of difficulty removing the device (OR 3.14; 95% CI 1.82 to 5.42) and that their partner could feel the device (OR 2.82; 95% CI 1.43 to 5.56). Although FemCap users were less likely than diaphragm users to report coital pain (OR 0.28; 95% CI 0.09 to 0.83), very few women in either group reported ever experiencing this problem. Women reported other problems, such as breakthrough bleeding or spotting, difficulty inserting the device, vaginal symptoms, and device feeling uncomfortable. However, the reporting of these problems did not differ by group assignment.
Only 34% of Prentif cap users and 28% of diaphragm users completed the trial (Bernstein 1986). Prentif cap users discontinued more often than diaphragm users, because of cervical changes (six‐month life‐table cumulative rate ratio of 1.8) and less often for other medical reasons (six‐month life‐table cumulative rate ratio of 0.5), but these differences were not significant (Table 4). Women using the Prentif cap were significantly more likely to exit the trial due to concern about the effectiveness of the method than those using the diaphragm (six‐month life‐table cumulative rate ratio of 3.0), but were less likely than diaphragm users to discontinue as a result of disliking the method (six‐month life‐table cumulative rate ratio of 0.3). Discontinuation rates for non‐device related study reasons or personal reasons were significantly lower for Prentif cap users than diaphragm users (six‐month life‐table cumulative rate ratios of 0.1 and 0.6, respectively). Thirty‐seven percent of FemCap users and 29% of diaphragm users discontinued early from the second trial (Mauck 1999). The overall six‐month Kaplan‐Meier rate ratio of discontinuation for cap versus diaphragm users of 1.2 (Table 4) was not statistically significant (P = 0.08).
4. Cap versus diaphragm: 6‐month cumulative discontinuation rate ratio.
| Outcome | Bernstein‐life table | Mauck‐ Kaplan‐Meier |
| Overall | 1.2 | |
| Cervical change | 1.8 | |
| Medical/safety reason | 0.5 | |
| Concern about effectiveness | 3.0 | |
| Disliked method | 0.3 | |
| Partner disliked method | 1.7 | |
| Study reason: related to device | 0.1 | |
| Personal reason: unrelated to device | 0.6 |
Women in the Prentif cap trial (Bernstein 1986) were interviewed regarding the acceptability of the devices without the use of a questionnaire and, therefore, their comments are difficult to compare. In the FemCap trial (Mauck 1999), similar proportions of women reported liking their assigned device 'somewhat' or 'a lot' at the two‐week interview. However, FemCap users were less likely than the diaphragm users to state that they were 'probably' or 'definitely' likely to use the device alone after completing the trial (OR 0.47; 95% CI 0.31 to 0.71) or that they would recommend it to a friend (OR 0.48; 95% CI 0.29 to 0.81).
Discussion
Although the contraceptive efficacy of the Prentif cap was similar to the comparison diaphragm, the FemCap was not as effective in preventing pregnancy as the diaphragm.
The possible increased risk of cytologic changes associated with the cervical cap remains unclear. Prentif cap users had a higher rate of Class I to Class III changes in cervical cytology after three months of use than diaphragm users. As a result, the U.S. Food and Drug Administration's approval of the Prentif cap included the requirement that women have a Papanicolaou smear at baseline and after three months of cap use. Gollub 1989 critiqued this interpretation of the data on the grounds that the increased risk of cytologic conversions was based on an analysis using multiple testing with selected subsets of women (Gollub 1989). The authors argued that if the 134 women with Class II smears at the initial or three‐month visit were included in the analysis, the apparent difference in cytologic conversions between the cap and diaphragm groups would not remain. In Mauck 1999, the nested colposcopy analysis did not detect differences between FemCap and diaphragm users. This analysis, though, was based on a small subset (41 women) and had sufficient power to detect only large differences in lesions rates (i.e., probability difference of 30 percentage points).
Some proponents of the cervical cap have hypothesized that the device would result in fewer urinary tract infections (UTI) than the diaphragm, because the cap, unlike the diaphragm, does not put pressure on the urethra. Others have argued the diaphragm and the cap pose similar UTI risk as both methods require the application of spermicide. Spermicide alone, the spermicide‐coated condom, the diaphragm with spermicide and the cap have been shown to be risk factors for UTI (Hooton 2000; Fihn 1996). The data from the Prentif and FemCap trials regarding this outcome suggest conflicting interpretations. While FemCap users were significantly less likely to have UTI than diaphragm users (Mauck 1999), the UTI rates for women using the Prentif cap and the diaphragm were similar (Bernstein 1986).
Despite instructions that the Prentif cap could be worn for up to 72 hours after insertion, the mean length of wear was 31.5 hours (Bernstein 1986). Based on a lack of data regarding longer periods of use, the U.S. Food and Drug Administration's guidelines set a maximum length of wear for 48 hours following insertion of the Prentif cap.
Many women could not be fitted properly with the cervical cap. The limited number of cap sizes prevented the device from being an option for some because the fit of the cap varies according to the size, shape, length, position and location of the cervix. The eligibility requirement in the Bernstein trial (requiring an adequate fit with the study devices) prevented the enrollment of 6% of potential participants who could not be fitted with the cap and 0.1% who could not be fitted with the diaphragm. Despite the enrollment criteria, an additional 5% of the women assigned to the cap group and 3% of those assigned to the diaphragm group were disqualified after randomization because of a lack of proper fit. In Mauck 1999, about 1% of the women assigned to use the FemCap and none of those assigned to use the diaphragm were disqualified at baseline after randomization for an inability to be fit. An additional 4% of FemCap users and 1% of diaphragm users discontinued during follow up for this reason. Bernstein recommended the manufacturing of the cap in additional small sizes to improve the proportion of women who could be fit (Bernstein 1986).
Parity has been suggested to play a role in the effectiveness of barrier methods. Parous women had a higher pregnancy rate than nulliparous women in both the Prentif cap (30% versus 15%) and diaphragm (29% versus 15%) arms (Bernstein 1986). Multivariate analysis showed a significant difference in pregnancy rates by parity among cap users. However, the pregnancy rate among diaphragm users did not differ by parity. The pregnancy rate among FemCap users was not statistically different for women who had given birth versus those who had not (Mauck 1999). These analyses of the role of parity should be interpreted with caution because subgroup analyses are appropriate only for hypothesis‐generating studies (Lau 1998).
Female barrier contraceptives are not widely used. For example, a 1995 national U.S. survey found that only 2% of reproductive‐age women used the diaphragm, and the use of other female barrier methods was even less prevalent (Piccinino 1998). From 2001 to 2005 in New South Wales (Australia), 5% of women attending family planning centers were fitted with a diaphragm or cervical cap (Bateson 2007). These women were likely to be older, more educated, and have health insurance than the women prescribed oral contraceptives. The cervical cap is not an appropriate contraceptive method for some women, including those who cannot be properly fit with the device. Suitable candidates for the cap should be able to correctly and consistently insert and remove the device and be comfortable with this process. Although not appropriate for all women, the cervical cap offers a safe, user‐controlled option for women seeking a non‐hormonal, reversible method of contraception. The Prentif cap may be more effective in preventing pregnancy than the FemCap, but no direct comparisons of these caps has been done.
Authors' conclusions
Implications for practice.
The Prentif cap was as effective as its comparison diaphragm in preventing pregnancy, but the FemCap was not. Both cervical caps offer a medically safe method of contraception.
Implications for research.
The degree and duration of spermicide effectiveness in preventing pregnancy when used with the cervical cap is unclear. Because the safety of spermicide used with the cervical cap has been questioned, its role should be evaluated. In addition, the FemCap design has been modified to include a strap to facilitate removal (CONRAD 2000), and this new design should be compared in a randomized controlled trial with the diaphragm.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2012 | New search has been performed | Searches updated; no new trials included. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 20 August 2009 | New search has been performed | Searches updated; no new trials found. |
| 14 April 2008 | Amended | Converted to new review format. |
| 4 June 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI 360 assisted with the literature search.
Appendices
Appendix 1. 2012 search strategies
PubMed search of MEDLINE (01 Jun 2009 to 27 Feb 2012)
cervical[Title/Abstract] AND cap[Title/Abstract] AND ((Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp])
POPLINE (2009 to 27 Feb 2012)
cervical cap
Cochrane Central Register of Controlled Trials (CENTRAL) (2009 to 23 Feb 2012)
(cervical cap AND diaphragm) in Title, Abstract or Keywords
LILACS (29 Feb 2012)
cervical AND cap
ClinicalTrials.gov (27 Feb 2012)
Interventions: cervical cap
ICTRP (27 Feb 2012)
Search terms: cervical cap
Appendix 2. 2009 Search strategies
PubMed search of MEDLINE (6 Feb 2007 to 21 Aug 2009)
(((((((((((((((((((((((("randomized controlled trials"[MESH:noexp] OR "random allocation"[MESH:noexp]) OR "double‐blind method" [MESH:noexp]) OR "single‐blind method" [MESH:noexp]) OR "clinical trials"[MESH]) OR "placebos"[MESH:noexp]) OR "research design"[MESH:noexp]) OR "comparative study"[MESH:noexp]) OR "evaluation studies"[MESH]) OR "follow‐up studies" [MESH:noexp]) OR "prospective studies"[MESH:noexp]) OR "cross‐over studies"[MESH:noexp]) OR "intervention studies"[MESH:noexp]) OR "randomized controlled trial" [pt]) OR "controlled clinical trial"[pt]) OR "clinical trial"[pt]) OR "clinic* trial*" [title/abstract word]) OR (((("singl*"[title/abstract word] OR "doubl*"[title/abstract word]) OR "trip*"[title/abstract word]) OR "trebl*"[title/abstract word]) AND ("blind*"[title/abstract word] OR "mask*"[title/abstract word])) OR "placebo*" [title/abstract word]) OR "random*"[title/abstract word]) OR "latin square"[title/abstract word]) OR "control*"[title/abstract word]) OR "prospectiv*"[title/abstract word]) OR "volunteer*"[title/abstract word]) NOT ("animal"[MESH] NOT "human"[MESH])) AND ("cervical"[title/abstract word]) AND ("cap"[title/abstract word])
POPLINE (6 Feb 2007 to 20 Aug 2009)
cervical cap
AND (compar* OR clinical trials OR comparative studies OR random OR double‐blind studies)
Cochrane Central Register of Controlled Trials (CENTRAL) (20 Aug 2009)
(cervical cap AND diaphragm) in Title, Abstract or Keywords
EMBASE (6 Feb 2007 to 28 Aug 2009)
cervical(w)cap AND diaphragm
LILACS (14 Feb 2007 to 21 Aug 2009)
cervical AND cap
Data and analyses
Comparison 1. Prentif cap versus Ortho diaphragm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy | 1 | 1153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.89, 1.74] |
| 2 Disqualification at baseline | 1 | 1529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.76, 1.20] |
| 3 Completed trial | 1 | 1153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [1.00, 1.65] |
| 4 Vaginal ulcerations or lacerations | 1 | 1201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.14, 0.71] |
| 5 CIN Class I to Class III conversion (at three months) | 1 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.04, 5.11] |
| 6 Device dislodge | 1 | 1152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.10 [3.18, 5.30] |
1.1. Analysis.
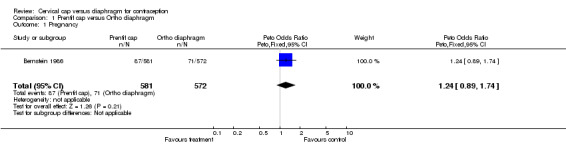
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 1 Pregnancy.
1.2. Analysis.
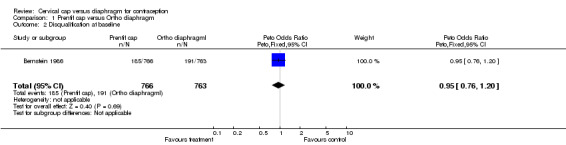
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 2 Disqualification at baseline.
1.3. Analysis.
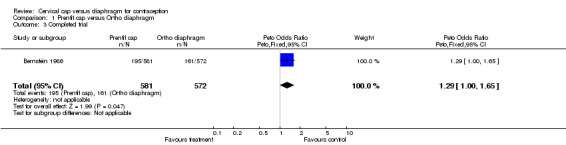
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 3 Completed trial.
1.4. Analysis.
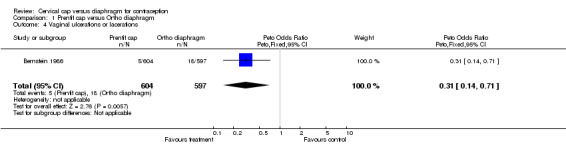
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 4 Vaginal ulcerations or lacerations.
1.5. Analysis.
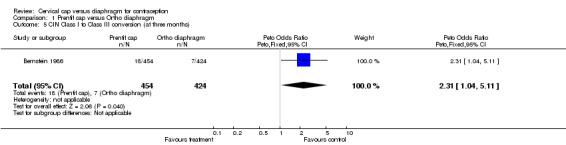
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 5 CIN Class I to Class III conversion (at three months).
1.6. Analysis.
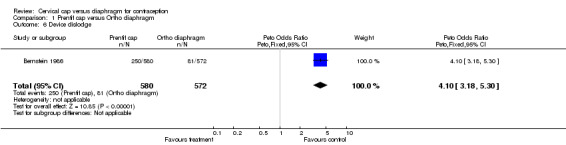
Comparison 1 Prentif cap versus Ortho diaphragm, Outcome 6 Device dislodge.
Comparison 2. FemCap versus All‐Flex diaphragm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [1.02, 3.07] |
| 2 Disqualification at baseline | 1 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.58 [3.29, 9.47] |
| 3 Disqualification during follow‐up | 1 | 841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [1.10, 2.00] |
| 4 Atypical squamous cell of undetermined significance | 1 | 657 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 5 Atypical glandular cell of undetermined significance | 1 | 657 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.17, 8.56] |
| 6 Low‐grade squamous intraepithelial lesion | 1 | 657 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.25, 3.56] |
| 7 Vaginal candidasis | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.71, 1.48] |
| 8 Etiology unspecified vaginitis | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.50, 1.25] |
| 9 Bacterial vaginosis | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.40, 1.32] |
| 10 Leukorrhea | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.34, 1.14] |
| 11 Urinary tract infection | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.36, 0.95] |
| 12 Blood found in device | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.29 [1.27, 4.14] |
| 13 Genital irritation | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.38, 1.42] |
| 14 Dysmenorrhea | 1 | 742 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.74, 2.85] |
| 15 Breakthrough bleeding and/or spotting | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.60, 3.07] |
| 16 Device dislodge/move | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.46 [3.74, 7.96] |
| 17 Difficulty removing | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.14 [1.82, 5.42] |
| 18 Difficulty inserting | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.97, 2.76] |
| 19 Vaginal symptoms | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.49, 1.45] |
| 20 Device uncomfortable | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.25, 1.50] |
| 21 Coital pain | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.09, 0.83] |
| 22 Partner could feel device | 1 | 748 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.82 [1.43, 5.56] |
| 23 Liked device | 1 | 705 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.78, 1.56] |
| 24 Future use | 1 | 705 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.31, 0.71] |
| 25 Recommend to friend | 1 | 687 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.29, 0.81] |
2.1. Analysis.
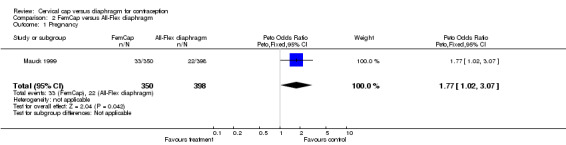
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 1 Pregnancy.
2.2. Analysis.
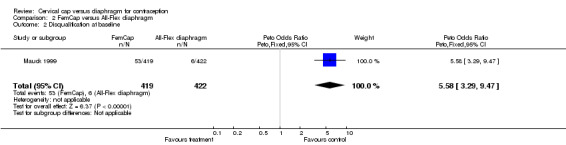
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 2 Disqualification at baseline.
2.3. Analysis.
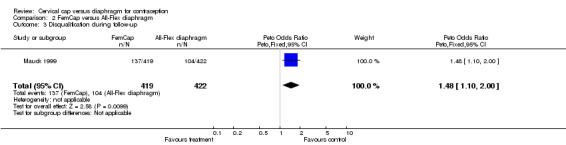
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 3 Disqualification during follow‐up.
2.4. Analysis.
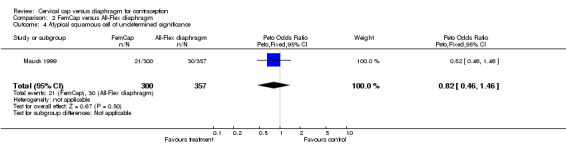
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 4 Atypical squamous cell of undetermined significance.
2.5. Analysis.
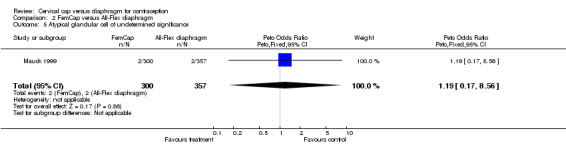
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 5 Atypical glandular cell of undetermined significance.
2.6. Analysis.
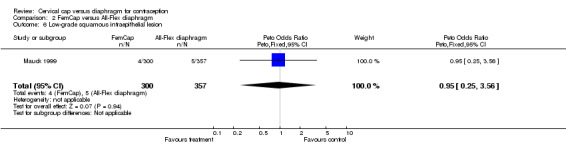
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 6 Low‐grade squamous intraepithelial lesion.
2.7. Analysis.
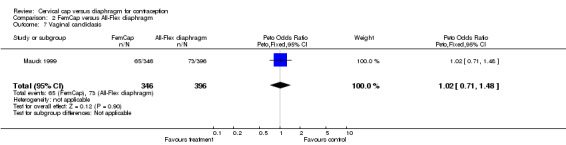
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 7 Vaginal candidasis.
2.8. Analysis.
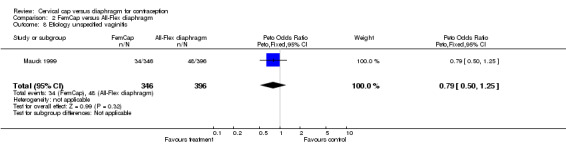
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 8 Etiology unspecified vaginitis.
2.9. Analysis.
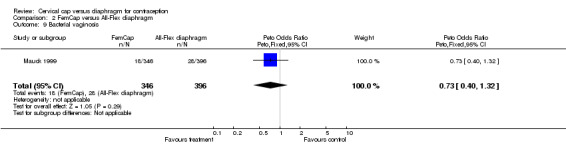
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 9 Bacterial vaginosis.
2.10. Analysis.
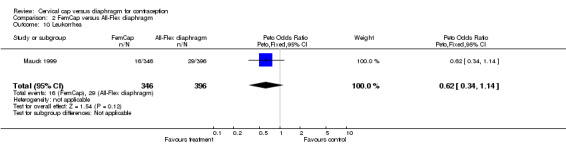
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 10 Leukorrhea.
2.11. Analysis.
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 11 Urinary tract infection.
2.12. Analysis.
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 12 Blood found in device.
2.13. Analysis.
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 13 Genital irritation.
2.14. Analysis.
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 14 Dysmenorrhea.
2.15. Analysis.
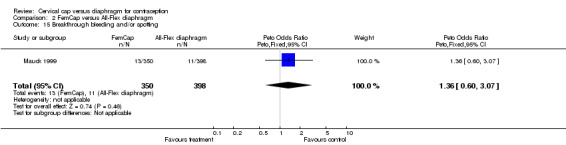
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 15 Breakthrough bleeding and/or spotting.
2.16. Analysis.
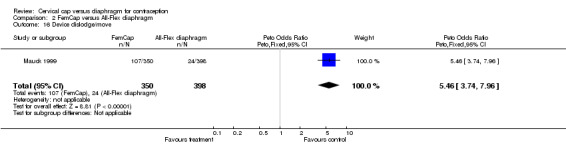
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 16 Device dislodge/move.
2.17. Analysis.
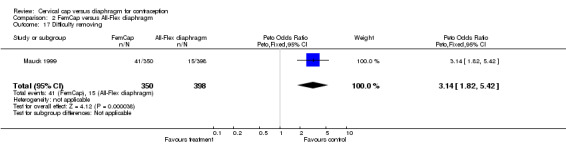
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 17 Difficulty removing.
2.18. Analysis.
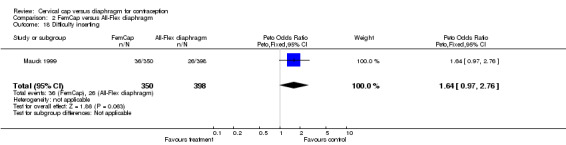
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 18 Difficulty inserting.
2.19. Analysis.
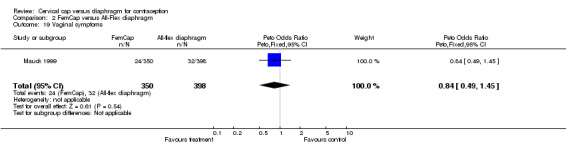
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 19 Vaginal symptoms.
2.20. Analysis.
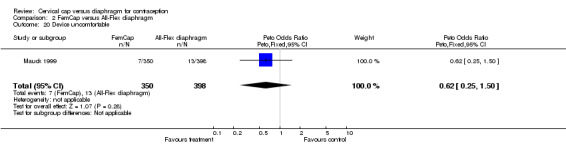
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 20 Device uncomfortable.
2.21. Analysis.
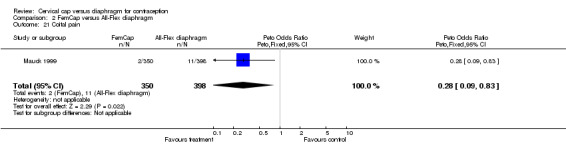
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 21 Coital pain.
2.22. Analysis.
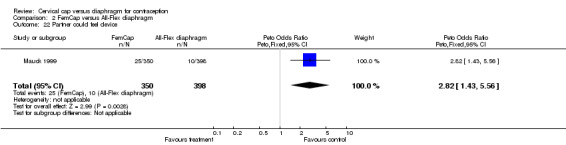
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 22 Partner could feel device.
2.23. Analysis.
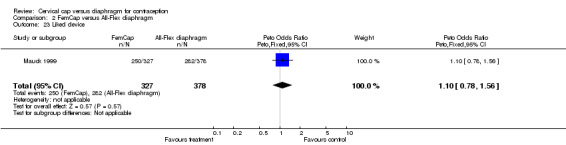
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 23 Liked device.
2.24. Analysis.
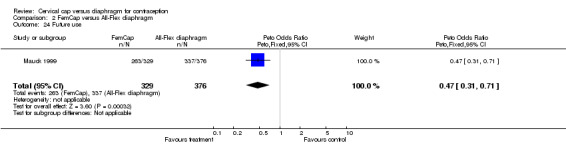
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 24 Future use.
2.25. Analysis.
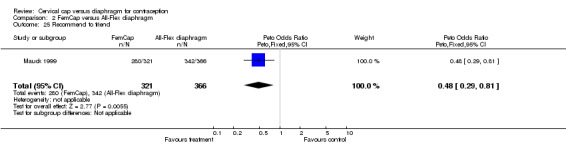
Comparison 2 FemCap versus All‐Flex diaphragm, Outcome 25 Recommend to friend.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bernstein 1986.
| Methods | Randomized parallel group trial. 8 family planning clinics in the USA. Unblinded. Follow up for 6 to 24 months. | |
| Participants | 1529 sexually active women aged 18 to 40 years. Exclusion criteria: irregular menses; health conditions associated with high maternal risk; inability to be fitted with either cap or diaphragm. | |
| Interventions | Prentif Cavity Rim Cervical Cap® (Lamberts LTD, Oxford, England) in comparison with a coil‐spring, arcing, or flat diaphragm. Both groups received the spermicides Ortho‐Gynol®, which contains 1.00% nonoxynol‐9, and Gynol II®, which has 2% nonoxynol‐9 (Advanced Care Products, Ortho Pharmaceutical Corporation, Raritan, NJ). | |
| Outcomes | Contraceptive efficacy; acceptability; method‐related problems; changes in cervical cytology; dislodgment; vaginal lesions; early discontinuation. | |
| Notes | Randomization and allocation concealment processes not described. Lost to follow‐up or refused follow‐up rate was 20%. | |
Mauck 1999.
| Methods | Randomized parallel group trial. 10 centers in the USA. Unblinded. 28 week follow‐up. Nested study of colposcopic changes (n=42). | |
| Participants | 841 sexually active women aged 18 to 40 years in a monogamous, heterosexual relationship and at risk for pregnancy. Women had to be willing not to become pregnant for the study length while also accepting an unknown risk of pregnancy. Exclusion criteria: pregnancy; lactation; irregular menses; suspected infertility; sensitivity to latex, silicone or spermicide; history of certain medical conditions; high risk for sexually transmitted infections. | |
| Interventions | FemCap® cervical cap (silicone rubber for cap manufactured by Hi‐Tech Rubber, Anaheim, CA; n=419) versus All‐Flex® contraceptive diaphragm (Ortho Pharmaceutical Corporation, Raritan, New Jersey; n=422). The spermicide Gynol II® (Advanced Care Products, Ortho Pharmaceutical Corporation, Raritan, NJ) containing 2% nonoxynol‐9 was used with both study groups. | |
| Outcomes | Contraceptive efficacy; early discontinuation (at baseline and during trial); acceptability; safety. Safety was evaluated in three areas: discontinuations for medical reasons; adverse events; and changes in pelvic examination, Papanicolaou smears and colposcopy. | |
| Notes | Authors included a priori hypotheses and sample size calculations. Computer‐generated randomization scheme with stratification by center and prior female barrier method use. Method of allocation concealment not described. Low (6.4%) loss to follow up. | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Creatsas 2002 | No diaphragm comparison group. |
| Mauck 1997 | Outcomes not relevant. |
| van der Straten 2010 | Outcomes not relevant. Study examined 'potential' acceptability; participants inserted and removed the barrier contraceptives in a clinical setting. They did not use them during intercourse. |
Contributions of authors
Maria Gallo performed the initial literature search, abstracted data, and drafted the protocol and review. David Grimes abstracted data, edited and advised on the protocol and review drafts, and provided clinical expertise. Ken Schulz reviewed the protocol and review drafts and provided statistical expertise. From 2007 to 2012: Laureen Lopez reviewed the search results, wrote the Plain Language Summary, and edited the manuscript for current style issues.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
U.S. Agency for International Development, USA.
Declarations of interest
Dr. Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bernstein 1986 {unpublished data only}
- Bernstein GS. Final Report: Use‐effectiveness study of cervical caps. Contract No. 1‐HD‐1‐2804, NICCHD; 1986.
Mauck 1999 {published data only}
- Anonymous. Safety and efficacy study of FemCap used with spermicide vs the Ortho All‐Flex Diaphragm used with spermicide: final report. Contraceptive Research and Development Program (CONRAD), Family Health International 1998.
- Mauck C, Callahan M, Weiner DH, Dominik R. A comparative study of the safety and efficacy of FemCap, a new vaginal barrier contraceptive, and the Ortho All‐Flex diaphragm. The FemCap Investigators' Group. Contraception 1999;60:71‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Creatsas 2002 {published data only}
- Creatsas G, Elsheikh A, Colin P. Safety and tolerability of the new contraceptive sponge Protectaid. The European Journal of Contraception & Reproductive Health Care 2002;7:91‐5. [PubMed] [Google Scholar]
Mauck 1997 {published data only}
- Mauck CK, Baker JM, Barr SP, Johanson W, Archer DF. A phase I study of Femcap used with and without spermicide. Postcoital testing. Contraception 1997;56:111‐5. [DOI] [PubMed] [Google Scholar]
van der Straten 2010 {published data only}
Additional references
Bateson 2007
- Bateson D, Weisberg E. Comparison of diaphragm and combined oral contraceptive pill users in the Australian family planning setting. European Journal of Contraception and Reproductive Health Care 2007;12:24‐9. [DOI] [PubMed] [Google Scholar]
CONRAD 2000
- CONRAD. Mechanical and Chemical Barriers for Women [Bienniel report]. http://www.conrad.org/f‐2.html#mechanicalbarriers (accessed 13 Apr 2012).
Fihn 1996
- Fihn SD, Boyko EJ, Normand EH, Chen CL, Grafton JR, Hunt M, et al. Association between use of spermicide‐coated condoms and Escherichia coli urinary tract infection in young women. American Journal of Epidemiology 1996;144:512‐20. [DOI] [PubMed] [Google Scholar]
Gollub 1989
- Gollub EL, Sivin I. The Prentif cervical cap and pap smear results: a critical appraisal. Contraception 1989;40:343‐9. [DOI] [PubMed] [Google Scholar]
Hooton 2000
- Hooton TM, Scholes D, Stapleton AE, Roberts PL, Winter C, Gupta K, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. New England Journal of Medicine 2000;343:992‐7. [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D editor(s). Systematic Reviews: Synthesis of Best Evidence for Health Care Decisions. Philadelphia, PA: American College of Physicians, 1998:91‐101. [Google Scholar]
Piccinino 1998
- Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982‐1995. Family Planning Perspectives 1998;30:4‐10, 46. [PubMed] [Google Scholar]
Powell 1986
- Powell MG, Mears BJ, Deber RB, Ferguson D. Contraception with the cervical cap: effectiveness, safety, continuity of use, and user satisfaction. Contraception 1986;33:215‐32. [DOI] [PubMed] [Google Scholar]
Shihata 1991
- Shihata AA, Trussell J. New female intravaginal barrier contraceptive device. Preliminary clinical trial. Contraception 1991;44:11‐9. [DOI] [PubMed] [Google Scholar]
Stewart 1998
- Stewart F. Vaginal barriers: the diaphragm, contraceptive sponge, cervical cap, and female condom. In: Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Guest F, et al. editor(s). Contraceptive Technology. 17th Edition. New York: Ardent Media, Inc., 1998:371‐404. [Google Scholar]
Trussell 1993
- Trussell J, Strickler J, Vaughan B. Contraceptive efficacy of the diaphragm, the sponge and the cervical cap. Family Planning Perspectives 1993;25:100‐5,35. [PubMed] [Google Scholar]
Weiss 1998
- Weiss NS. Clinical epidemiology. In: RJ Rothman, S Greenland editor(s). Modern Epidemiology. 2nd Edition. New York: Lippincott Williams and Wilkins, 1998:519‐28. [Google Scholar]


