Abstract
Taxonomies of human memory, influenced heavily by Endel Tulving, make a fundamental distinction between explicit and implicit memory. Humans are aware of explicit memories, whereas implicit memories control behavior even though we are not aware of them. Efforts to understand the evolution of memory, and to use nonhuman animals to model human memory, will be facilitated by better understanding the extent to which this critical distinction exists in nonhuman animals. Work with metacognition paradigms in the past 20 years has produced a strong case for the existence of explicit memory in nonhuman primates and possibly other nonhuman animals. Clear dissociations of explicit and implicit memory by metacognition have yet to be demonstrated in nonhumans, although dissociations between memory systems by other behavioral techniques, and by brain manipulations, suggest that the explicit-implicit distinction applies to nonhumans. Neurobehavioral studies of metamemory are beginning to identify neural substrates for memory monitoring in the frontal cortex of monkeys. We have strong evidence that at least some memory systems are explicit in rhesus monkeys, but we need to learn more about the distribution of explicit processes across cognitive systems within monkeys, and across species.
Keywords: Memory systems, Implicit, Metacognition, Memory monitoring, Monkey, Primate, Tulving
It is widely recognized that both human and nonhuman brains consist of distinct memory systems, each specialized for different cognitive demands (Cohen and Eichenbaum, 1994; Eichenbaum and Cohen, 2001; Kim and Baxter, 2001; Sherry, 2006; Sherry and Schacter, 1987; Squire, 2004; Squire et al., 1993). Influential taxonomies of human memory, including that developed by Tulving, make a primary distinction between memories that are consciously accessible to monitoring (explicit or declarative) and those that are unconscious (Fig. 1; implicit or nondeclarative, e.g., Cohen and Eichenbaum, 1994; Squire et al., 1993; Squire and Zola-Morgan, 1991; Tulving, 1985; Tulving and Schacter, 1990).
Fig. 1. Taxonomy of human memory systems (Squire and Zola-Morgan, 1991).
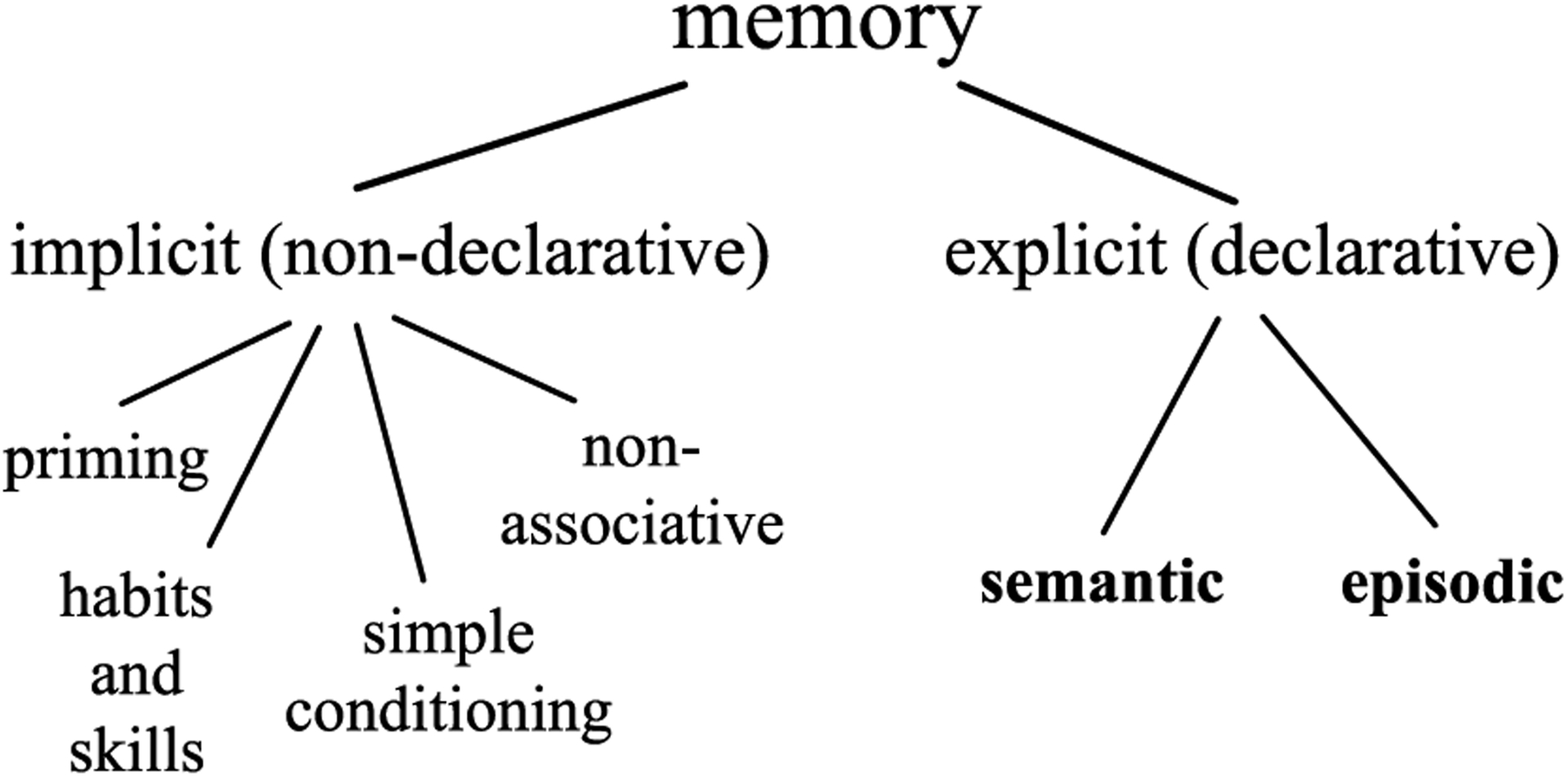
Implicit memory affects behavior without awareness. In contrast, humans are consciously aware of explicit memories. In other words, explicit memories and accessible to cognitive monitoring, but implicit memories are not. We use this difference in accessibility to metacognitive monitoring to classify nonhuman primate cognitive systems.
While it is clear that distinct memory systems also exist in many nonhuman animals, without the benefit of language-based assessments of memory, it has been difficult to make measurements relevant to the explicit-implicit distinction in species other than humans. The systems we observe in other species may parallel those found in humans in dependence on specific neural substrates, such as the hippocampus or striatum, and may show functional similarities, for instance in speed of learning, resistance to interference, or duration of retention. Both anatomical and functional parallels are important kinds of converging evidence required in the comparison of memory systems across species. Because the explicit-implicit distinction is such a conspicuous and important characteristic of human memory systems, measures of whether nonhuman memory systems are explicit are another critical type of evidence.
Awareness of memory permits humans to comment on memory, for example by reporting that they have forgotten, or are uncertain. Because animals do not verbally report their experience of memory, it has sometimes been argued either that nonhuman species do not possess explicit memory (e.g., Tulving and Markowitsch, 1994), or that it is impossible to determine whether or not they do (e.g., Shettleworth, 1998). But awareness of memory also permits overt behavior other than verbal commentary responses, and these behaviors can be studied in nonverbal animals (Weiskrantz, 2001). Monitoring memory allows adaptive behavioral choices such as information seeking or avoidance of situations where specific knowledge is required for success. These functions of explicit memory have been studied using metacognition paradigms in nonhuman animals, particularly rhesus monkeys, and we have found that some species are capable of introspecting about, and controlling, their own cognition, indicating the presence of explicit cognitive processes.
Given the accumulation of evidence for the existence of explicit memory in rhesus monkeys (hereafter “monkeys”), we can now begin to answer more refined questions about the distribution, mechanisms, and function of explicit cognition in monkeys. Among these questions are the following. How taxonomically limited or widespread is explicit representation? Which cognitive systems are explicit and which implicit, and how does metacognitive monitoring and control modulate the contributions of these systems to behavior? What neurobiological mechanisms enable cognitive monitoring in nonhumans, and how are they organized in the brain? For example, are the substrates of cognitive monitoring co-located with substrates for the processes being monitored, or are metacognitive processes instantiated in distinct systems? What are the contributions of cognitive monitoring to cognitive control? What ecological or social demands selected for the evolution of explicit representation? Below we review some initial progress addressing these questions. Much additional work will be required to provide satisfying answers.
1. Metacognition and memory monitoring paradigms provide psychologically valid measures of explicit memory for drawing parallels in cognition among species
In humans, memory monitoring is associated with consciousness and is often identified on the basis of verbal reports of private experience (e. g., “I knew” versus “I guessed”). Because nonhuman species cannot provide verbal reports on their experience of memory, to determine whether nonhumans have explicit cognition, we need to establish other behavioral criteria that discriminate between explicit and implicit memories. Given that even complex cognitions, such as correct use of grammar (Knowlton et al., 1992), classical conditioning (Clark and Squire, 1998), and skill learning (Cohen et al., 1985; Knowlton and Squire, 1993), can proceed without conscious awareness in humans, we cannot identify explicit cognition in nonhumans simply on the basis of the apparent complexity of the behavior involved. This has led theorists to propose kinds of behavior that require explicit processing. One influential proposal was that relational memories are uniquely associated with explicit cognition (Eichenbaum and Cohen, 2001). Relational memories are those in which the relations among memoranda are critical, such as when determining which of a sequence of events occurred first, or in determining how items are ordered through transitive inference. However, whether or not memories are consciously accessible in humans does not reliably predict type of cognitive processing, such as relational versus nonrelational coding, nor does it predict whether particular neural substrates such as the hippocampus are involved (e.g. Greene et al., 2007; Hannula et al., 2006; Ryan et al., 2000). The fact that we cannot use neural substrates or relational coding as reliable indicators of whether cognition is explicit highlights the need for direct measures of memory access in nonverbal species. It is also insufficient to state that a particular behavior is “complex” and therefore must be explicit (e.g. Griffin, 1976, 2001). To progress, we must use replicable paradigms that capture the accessibility of memory to cognitive monitoring.
The study of explicit memory and metacognition in nonhuman animals is possible if we focus on the functional rather than the experiential properties of the accessibility of memory (Basile and Hampton, 2014; Hampton, 2001, 2003; Hampton and Schwartz, 2004; Hampton et al., 2004). A functional approach begins by posing the question, “what can an organism with memory access do that one without it cannot do?” In formulating this question we can arrive at operational definitions of memory access that capture important functional capacities while avoiding the pitfalls associated with attempts to study phenomenology in animals. One thing memory monitoring allows humans to do is to introspectively discriminate between knowing and not knowing. For example, when considering greeting an acquaintance at a party, humans are often able to determine whether or not they know the person’s name before speaking. We can then choose adaptively to state the name if we know it, or select a different course of action when we do not know it. These alternative actions might include asking a friend for the name, thus correcting our ignorance, or avoiding the person altogether. We are able to offer analogous alternatives in experiments with nonhuman animals.
2. Rhesus monkeys monitor memory
In traditional tests of memory in nonhuman animals, subjects are given “forced-choice” tests in which they simply do the best they can with what information they have. There are no behavioral options analogous to asking a friend for the name of the acquaintance. But paradigms have been developed in several laboratories that do provide animals with such alternatives, thus more accurately modeling situations in which humans make adaptive choices based on memory monitoring. Most of this work has been conducted with rhesus monkeys (Macaca mulatta). Fig. 2 outlines one such paradigm and illustrates the logic employed in related paradigms. In these experiments monkeys were given a choice between taking a memory test and declining the test, which is analogous to a human saying “I remember” or “I forget” respectively. Monkeys show that they accurately monitor memory in these paradigms by selectively declining to take tests when their memory is poor, while taking tests and performing accurately when their memory is good (Brown et al., 2017; Hampton, 2001; Templer et al., 2018; Templer and Hampton, 2012). Studies using this “decline test” paradigm have tested the robustness of the initial findings with generalization tests in which it was found that monkeys are more likely to decline tests after long memory intervals, and on trials where they were not shown a sample to remember, bolstering the interpretation that monkeys monitor whether or not memory is present.
Fig. 2. A memory monitoring paradigm for detecting explicit memory in monkeys.
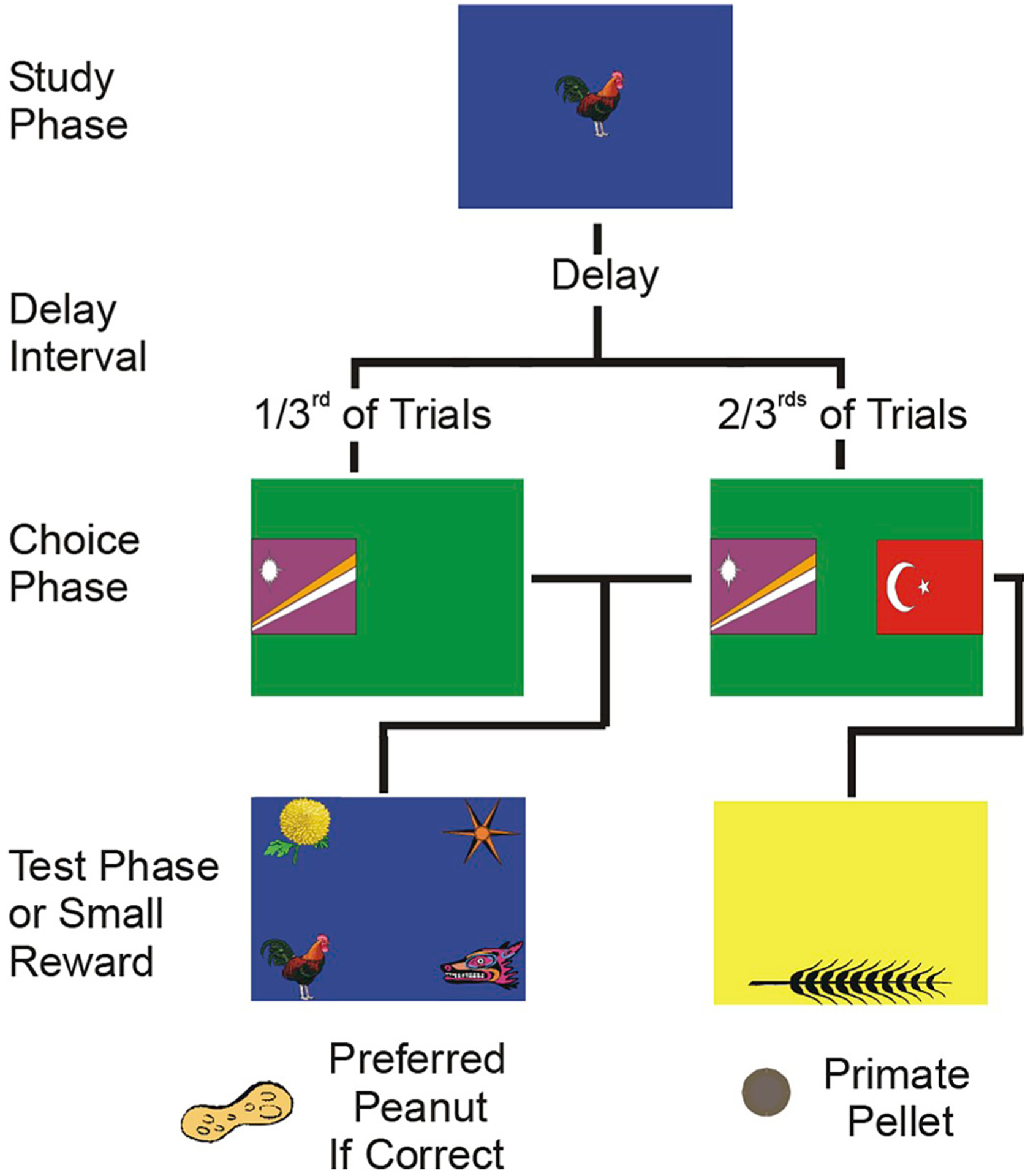
Each panel depicts what the monkey saw on a touch-sensitive computer monitor at different stages in a trial.
Many other studies have assessed memory monitoring based on similar reasoning that if monkeys have explicit memory, or “know when they know,” they should approach memory tests differently when they know the answer than when they do not. In addition to avoiding memory tests when they have forgotten, monkeys seek information when ignorant. For example, monkeys, apes, and children that do not know in which of several tubes food is hidden will bend down to look before choosing. In contrast, when they do know the location of the food, they choose without checking first (Call and Carpenter, 2001; Hampton et al., 2004). A large number of explanations for this pattern of behavior, other than memory monitoring, were evaluated and rejected in a computerized version of this paradigm (Basile et al., 2015). Similarly, monkeys made the effort to “reveal” a hidden sample image on a computer screen before proceeding to a memory test (Beran and Smith, 2011). Monkeys also appear to show spontaneous memory monitoring, without training. One monkey was found to express apparent frustration in advance of getting feedback on memory tests he was about to get wrong (Hampton and Hampstead, 2006), and free-ranging monkeys on Cayo Santiago made information seeking responses with little or no training in a search task (Rosati and Santos, 2016). In addition to accurately monitoring whether or not a memory was present, as shown above, monkeys accurately monitored their ability to report the order in which events occurred, more frequently accepting easy memory tests for events that were relatively widely separated in time (Templer et al., 2018). Of course we do not expect introspection to be faultless, and monkeys are subject to metacognitive illusions as are humans (Ferrigno et al., 2017).
In contrast to the strong evidence of memory monitoring in rhesus monkeys, the current pattern of results from other species is puzzling. First, it appears that memory monitoring may not be universal among primates, or at least comes much more easily to some species than others. Strikingly, New World brown capuchin monkeys (Cebus apella) are much less likely than are rhesus monkeys to behave in ways indicative of memory monitoring or metacognition generally (Basile et al., 2009; Beran et al., 2009; Fujita, 2009; Paukner et al., 2006; Smith et al., 2018). This is true even when tested with the same procedures used with rhesus monkeys (Basile et al., 2009; Smith et al., 2018). Second, evidence for memory monitoring in pigeons and dogs has been weak (Belger and Bräuer, 2018; Brauer et al., 2004; McMahon et al., 2010; Roberts et al., 2009; Sutton and Shettleworth, 2008), while there seems to be comparatively strong, but limited, evidence for metacognition in rats (Foote and Crystal, 2007; Templer et al., 2017). It is probably still early to state whether this pattern reflects true species differences, or is a result of differences in techniques or research effort. Better addressing this question of species differences is an exciting part of current comparative work. Getting answers will inform us about the evolution of cognitive monitoring generally, and may specifically tell us whether memory monitoring evolved in response to specific ecological or social selection pressures, or represents a general cognitive capacity shared by most species.
Understanding the evolution of cognitive monitoring will require comparative studies across species, but will also be informed by comparisons of cognitive monitoring within species. It is likely that cognitive systems differ in accessibility to cognitive monitoring. To address this issue, we need to both determine which cognitive systems can be identified in a given species, and then conduct additional studies that assess cognitive monitoring in those systems.
3. Nonhumans have dissociable memory systems
Understanding how different memory systems act together or independently to control behavior is a major challenge in the study of the brain’s multiple memory systems (Cohen and Eichenbaum, 1994; Eichenbaum et al., 2007; Fernandez-Ruiz et al., 2001; Kim and Baxter, 2001; McDonald and White, 1993; Packard, 1999; Packard and McGaugh, 1996; Poldrack and Packard, 2003; Schroeder et al., 2002; Sherry, 2006). Often more than one memory system participates even in “simple” memory tests. In a particularly clear example, rats were trained in a plus-shaped maze to start from the same location each trial and travel to a consistently baited arm of the maze (Packard and McGaugh, 1996). Because the same start and goal arms were used across training trials, rats could learn either to navigate to a particular place in the room as defined by landmarks, or learn to turn in a particular direction (e.g. turn right – a so-called response strategy). They did both. On probe trials the rats started from the arm directly opposite the start location used on training trials. These probe trials tested whether the rats were using the place or response strategy because the two strategies resulted in entry into opposite arms of the maze. Early in training rats used a place strategy, but after extensive training they followed the response rule. Furthermore, by inactivating the dorsal striatum or hippocampus on probe trials it was found that the place strategy required the hippocampus while the response strategy required the dorsal striatum. Most interesting was the finding that inactivation of the striatum after extensive training resulted in clear expression of the place strategy again, demonstrating that both the place and response strategies were available late in training, but that under normal conditions it was the response strategy that controlled behavior after extensive training.
Other animal work strongly suggests that many tasks recruit multiple simultaneously active memory systems (Cohen and Eichenbaum, 1994; DeCoteau and Kesner, 2000; Fernandez-Ruiz et al., 2001; Kesner et al., 1993; Kim and Baxter, 2001; McDonald and White, 1993; Poldrack and Packard, 2003; Schroeder et al., 2002; White and McDonald, 2002). To date, no published work with nonhuman animals has addressed whether these interacting memory systems are differentially accessible to monitoring in nonhuman species.
We describe Process Dissociation Paradigm (PDP) below, a technique adapted from humans for work with monkeys. This behavioral technique has the potential to measure both explicit and implicit cognition in monkeys in a manner that parallels work done in humans. We also provide evidence that the distinctions found in monkeys using this procedure map to neurobiological interventions.
4. Some dissociations of cognitive systems are suggestive of the explicit-implicit distinction in monkeys, but are not conclusive
Because both implicit and explicit memory may contribute to performance in a given cognitive task, it will be rare for there to be a one-to-one correspondence between specific memory tests and these types of memory. One approach that may distinguish the relative contributions of explicit and implicit memory systems is Process Dissociation Paradigm (PDP), which was specifically designed to quantify the contributions of multiple memory systems within a single cognitive test (Hay and Jacoby, 1996; Jacoby, 1991). In PDP two memory systems cooperate in one test condition, by providing the same answer to a memory test, and conflict in another, by providing different answers. One memory system is typically called automatic, reflecting the fact that influence on behavior by this memory process proceeds without awareness or cognitive control, and the other is controlled, reflecting the fact that subjects are aware of these memories and able to regulate how they contribute to behavior.
A person who has for years driven a stick shift and borrows a friend’s automatic transmission car may learn about the distinction between automatic habits and controlled memory. Their friend reminds them that the car has no stick shift, but the person nonetheless reaches repeatedly for the stick while driving. The implicit habit of shifting gears sometimes achieves expression in behavior despite the explicit knowledge that the car has no stick shift. In contrast, when this person is driving their own car, the explicit knowledge that they are driving a stick shift, and their implicit habit to shift, yield the same appropriate behavior – shifting gears. PDP uses the pattern of errors made by subjects to quantify the expression of these two types of memory. It is important to appreciate that habits, like shifting gears, which are “stamped in” by repeated experience, and knowledge about why and when one should shift gears, are both kinds of memory. Both habits and explicit knowledge are records of past experience, and both can control behavior.
PDP is particularly useful in work spanning humans and nonhumans because it does not depend on verbal reports of private experience to distinguish between memory systems. Criterion validity for PDP as a measure of explicit and implicit memory is found in experiments with humans demonstrating that measures of explicit and implicit memory correlate strongly with measures of memory derived from PDP. The type of memory labelled “controlled” in PDP appears to be the same type of memory labelled “explicit” in other paradigms, while the same is true for “automatic” influences and implicit memory (Hay and Jacoby, 1996; Jacoby et al., 1993; Reingold and GoshenGottstein, 1996; Toth et al., 1994). The correspondence of the explicit-implicit distinction with PDP measures in humans suggests that parallel dissociations found in non-humans using PDP may capture the explicit-implicit distinction too. While such dissociations are interesting and suggestive, it will still be important to assess this with more direct tests, and potentially converging evidence, provided by metacognition measures.
PDP has been implemented in behavioral studies with nonhuman animals and the resulting dissociations are consistent with the existence of both an explicit, and implicit, memory process (Roberts et al., 2015; Tu and Hampton, 2013). In contrast to the procedure used in standard recognition memory tests, in the implementation of PDP (Tu and Hampton, 2013; Tu et al., 2011), “high frequency” images were created by using some images as the to-be-remembered sample image much more often than others (Fig. 3). All the stimuli used in these matching-to-sample tests were highly familiar because they were all used in every day of testing. However, the high frequency manipulation induced a “habit” of selecting particular stimuli because they have been correct, and reinforced, much more often than other equally familiar stimuli. These manipulations parallel the logic of tests used in humans by Larry Jacoby and colleagues, such as in the “false fame” and other “ironic” memory influence paradigms (Hay and Jacoby, 1996). Although this implementation may not measure identical memory processes to those measured in the Jacoby studies, these procedures do appear to capture the distinction between automatic implicit, and controlled explicit, memory processes considered broadly.
Fig. 3. Process Dissociation Paradigm for monkeys.
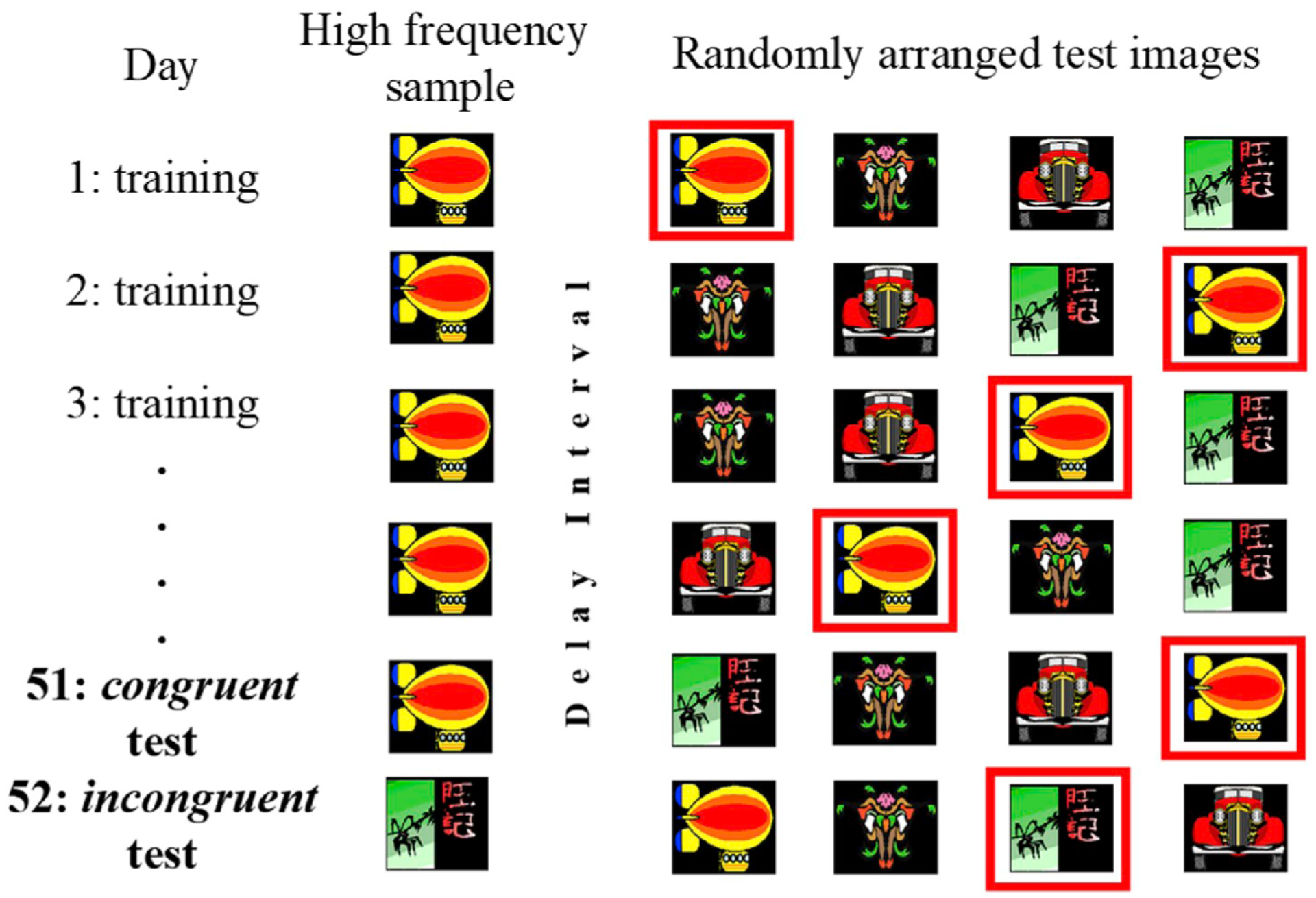
Each row represents one trial with one of the image quads that was shown to monkeys each day. The leftmost images represent the to-be-remembered sample image monkeys saw at the beginning of a trial. The four images to the right represent the choice images displayed at test (these images were randomly assigned to the four corners of the touch screen; the box indicates which image was correct and was not shown to the monkeys). Unlike in a normal recognition memory test procedure, here the selection of the sample image was parametrically biased toward the high frequency image (the blimp in this one case). After many days of training, monkeys were given probe trials of two types. On congruent probes, a high frequency image was the to-be-remembered sample, just as in training. On incongruent probes, a different image was selected as the sample, and the same choice stimuli appeared at test. Monkeys tended to make errors by selecting the high frequency image on incongruent trials (the blimp in this case). A double dissociation was revealed both by manipulating the strength of habits by varying the bias used with samples, and by manipulating memory by varying the memory interval between study and test. Habit and memory varied independently.
Habits biased monkeys to select high frequency images at test, regardless of which image they saw during the study phase of that trial. Thus, two types of memory could control monkeys’ choice behavior at test: 1) memory for the image presented as the sample on that particular trial, and 2) habit memory of a high frequency image. On a majority of trials, the two memory types acted in concert (congruent trials). On these trials the sample image was a high frequency image. On the remaining incongruent trials, the two memory types were in conflict because the sample image presented at study was not a high frequency image. Instead one of the distracter images was a high frequency image. Habits enhanced performance on congruent trials; habits impaired performance on incongruent trials. The strength of habits and memories could be manipulated entirely independently, demonstrating a behavioral double dissociation (Tu and Hampton, 2013). Direct tests using metacognition paradigms are needed to evaluate whether these systems differ in the extent to which they are explicit. Such tests would provide monkeys with the opportunity to avoid tests they subjectively perceive as difficult. Selectively avoiding test trials on which accuracy is low would be indicative of explicit cognition.
5. Behavioral dissociation of putative explicit and implicit memory systems by PDP is consistent with neurobiological evidence in nonhuman animals
Evidence from neurobiological studies in primates has identified the temporal lobe and striatum as distinct recipients of visual information. The primate ventral visual processing stream conveys highly processed visual information to both the medial temporal lobes (Suzuki, 1996) and tail of caudate and ventral putamen (Saintcyr et al., 1990; Webster et al., 1993). This is consistent with the idea that the temporal lobe and striatum support parallel visual memory systems, the former associated with explicit memory and the latter with implicit habits (Packard and Knowlton, 2002; Seger, 2006). Evidence from rodents implicates the neostriatum in formation of some habits (Kesner et al., 1993; Packard, 1999; Packard and McGaugh, 1996), and the little evidence there is from monkeys suggests the same (Fernandez-Ruiz et al., 2001; Teng et al., 2000). However, again, the extent to which these brain areas in non-humans are distinct in terms of explicit processing has yet to be directly tested using metacognition paradigms.
In contrast to the habits supported by the striatum, recognition memory for recently seen images is critically dependent on the primate perirhinal cortex (Buffalo et al., 1999; Malkova et al., 2001; Meunier et al., 1993; Tu et al., 2011; Turchi et al., 2005). The perirhinal cortex receives strong input from the ventral visual pathway, projects heavily to the hippocampus via the entorhinal cortex (Suzuki, 1996), and neurons here have large receptive fields (Jagadeesh et al., 2001) and respond selectively to complex visual stimuli (Logothetis, 1998). Thus, it has a variety of properties that would well serve recognition memory.
Using the logic of PDP we quantified the influence of habits and memory in the control of behavior in intact monkeys and monkeys lacking perirhinal cortex (Tu et al., 2011). Memory was significantly attenuated in monkeys lacking perirhinal cortex, but habits were entirely intact (Fig. 4). Deficits in perception could not explain these results, as perception was equally important for successful habits and successful memory. These results dissociate memory and habit within a single cognitive test and emphasize the importance of perirhinal cortex for memory. Because of the close association between PDP scores and the explicit-implicit distinction, this dissociation may be one between explicit and implicit memory, although this should be evaluated with direct tests using metamemory paradigms.
Fig. 4. Memory, but not habit, was impaired by perirhinal cortex lesions (Tu et al., 2011).
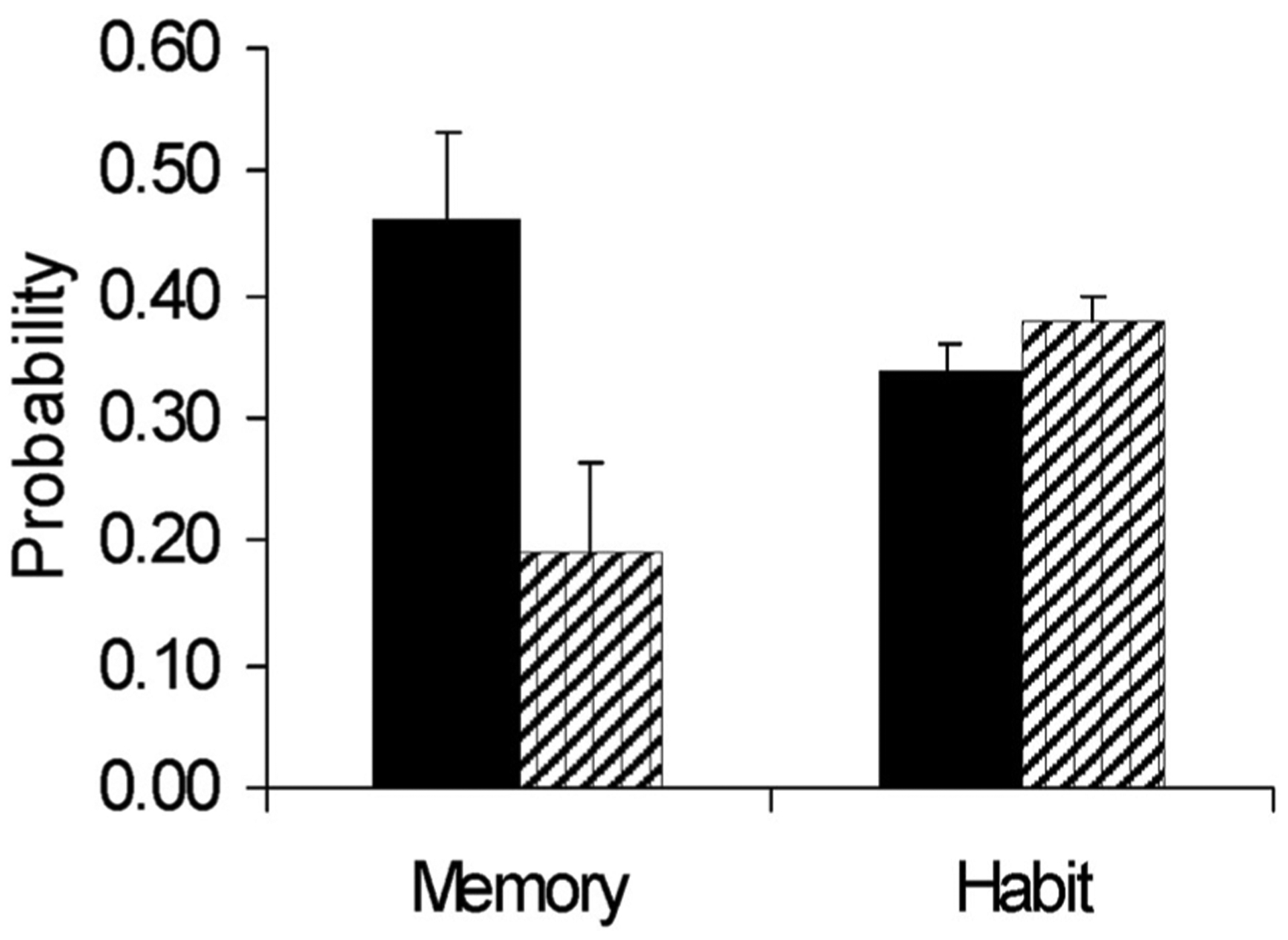
Lesions impaired intra-trial memory (“memory”) but left extra-trial memory (“habits”) intact. Bars represent the scores resulting from the analysis of congruent and incongruent trials in the PDP paradigm.
6. Frontal brain regions are involved in metamemory in monkeys
While temporal lobe and basal ganglia structures have been identified as critical for memory, adaptive behavior results from activation of a larger network of brain areas, often including the frontal cortex (e.g. Burgess, Maguire, Spiers and O’Keefe, 2001). There are strong reciprocal connections between the temporal lobes and frontal cortices (e.g. Goldman-Rakic et al., 1984; Lavenex and Amaral, 2000; Rempel-Clower and Barbas, 2000), such that frontal cortices are positioned to monitor and control temporal lobe activity. Frontal cortices play critical roles in directing memory search and validating retrieved information (Dellarocchetta and Milner, 1993; Dobbins et al., 2002; Rugg et al., 1999). In studies in which temporal lobe and frontal lobe structures were disconnected, learning and memory impairments were observed (Baxter et al., 2000; Gaffan and Harrison, 1988). The frontal lobes in humans are critical for memory monitoring (e.g. Budson et al., 2005; Fernandez--Duque et al., 2000; Shimamura, 2000). Metacognition is often conceived of as involving monitoring and control processes in the frontal lobes and object-level processes such as memory elsewhere in the brain (e.g. Nelson, 1996). Thus, memory monitoring might involve the frontal lobes monitoring the reliability of memories in the temporal lobe as a person studies a list of terms for an upcoming test. Signals resulting from memory monitoring could drive the decision to terminate study at the appropriate time.
The first neurobiological work published about metacognition in nonhuman primates did not implicate frontal cortex. This work used a perceptual task requiring monkeys to report the direction of movement of arrays of dots, with an option to decline difficult tests. This work has many similarities to the metamemory paradigms described above (e.g. Fig. 2), but involves monitoring of a perceptual decision process rather than memory. The authors reported that the same parietal cortex neurons that encoded the decision about which way the dots were moving also represented the confidence of the monkeys’ decision (Kiani and Shadlen, 2009). This work raises the interesting possibility that “metacognitive” signals could be one and the same as decisional signals. However, it is difficult to discriminate this possibility from the alternative that difficult decisions correlate with characteristic activity of neurons responsible for the decision, as well as “metacognitive” neurons elsewhere in the brain.
The neurobiology of metamemory was first studied in a spatial memory task that required monkeys to saccade to a cued location after a brief memory interval. Signals that correlated with metamemory judgments were found in supplementary eye fields, but not frontal eye fields or dorsolateral prefrontal cortex (Middlebrooks and Sommer, 2012). In contrast to the interpretation for metacognition of perceptual judgments provided by Kiani and Shadlen (2009), recent work on metamemory has provided clear evidence of dissociable memory and metamemory processes in monkeys. Reversible inactivation of areas of monkey frontal cortex impaired retrospective metamemory judgments while leaving intact accuracy in the primary memory tests about which monkeys made metamemory judgements (Miyamoto et al., 2017; Miyamoto et al., 2018). A third study was recently published that claims to address the neurobiology of metamemory in monkeys (Buckley, 2019), but unfortunately, this study, in which metacognition is inferred solely from response latency, does not meet even modest criteria for introspective metacognition (Hampton, 2009).
The studies of the neurobiology of metamemory reviewed above have used only retrospective metacognitive paradigms, where monkeys judge the quality of memory after completing memory tests. These tests are sometimes called retrospective betting paradigms. Combining neurophysiology with metamemory paradigms is extremely challenging, and this relatively new work is exciting and commendable. While prospective metacognition paradigms may provide stronger evidence for memory monitoring (Hampton, 2009), the cited studies provide the best evidence we currently have regarding the neurobiology of metamemory in primates, and encourage additional work using prospective metamemory paradigms.
It appears that the study of metamemory in monkeys is entering an exciting new phase in which neural recordings and causal neural interventions are adding to the substantial behavioral evidence collected in the last decades. We can expect to learn more both about the neurobiology of memory and metacognition from this work, and also to acquire new evidence on which to evaluate broad questions in metacognition research, such as the extent to which metacognitive judgments, and the cognitive processes that are supposed to be the target of such judgements, are dissociable.
7. Evidence from perceptual tasks is consistent with a distinction between implicit and explicit cognition in monkeys
Much of this review has focused on work in metamemory, and the question of whether monkeys metacognitively monitor at least some of their memory processes. Memory is just one of many possible target cognitive processes for metacognition. Humans also have introspective access to some perceptual processes, evident in our ability to predict the accuracy of perceptual and other judgements (Shields et al., 2005; Shields et al., 1997). At the same time, it is well documented that vast portions of perceptual processing are almost entirely inaccessible to introspection (e.g. Milner, 2012; Milner and Goodale, 2008). The phenomenon of “blindsight” in which humans with visual cortex damage show impairments in visual experience, but residual capacity to make perceptual judgments, is one area in which the differences between explicit and implicit perception are evident.
Humans with primary visual cortex damage have “scotomas,” or areas in the visual field where visual perception is abnormal. While it seemed obvious initially that subjects were blind in the scotoma, later work showed that at least sometimes people had some residual function in that area, despite reporting a lack of visual experience. In experimental settings subjects were well above chance localizing stimuli that they reported they had not seen (Kentridge et al., 1999; Sanders et al., 1974). This apparent dissociation of visual awareness from visual processing was replicated in monkeys with primary visual cortex lesions (Cowey and Stoerig, 1995, 1997; Moore et al., 1998). Intact human and nonhuman primates show a parallel to the blindsight that results from primary visual cortex damage in paradigms in which stimuli are presented very briefly and followed by a visual mask. Under appropriate conditions both humans (Klotz and Neumann, 1999) and monkeys (Andersen et al., 2014) report that they did not detect a stimulus, and yet can report the location where the stimulus occurred when forced to guess. This dissociation of perception and action, when it occurs in humans, is one between explicit awareness of a stimulus and an implicit capacity to localize it.
While the blindsight paradigms have not been combined with metacognition procedures in monkeys, a number of other perceptual tasks have been, and these experiments appear to show that some aspects of perceptual processes in monkeys are explicit. Monkeys avoid difficult visual discriminations in favor of easier ones (Brown et al., 2017; Shields et al., 1997). They make adaptive retrospective judgments about their accuracy on perceptual tasks (Kornell et al., 2007). Competing cognitive load impairs metacognition to a greater extent than it does the perceptual judgments about which monkeys metacognize (Smith et al., 2013). This last study very neatly shows both that monkeys metacognize about perception, and also that the metacognitive judgments are distinct from the primary perceptual process they monitor. Because these perceptual processes can be monitored, they appear to be explicit.
8. Cognitive control is likely limited to explicit cognition and likely depends on metacognition
It is likely that much of the adaptive function of metacognitive monitoring manifests in the role monitoring fills– providing feedback to regulate cognitive control. Knowing you don’t know is not much use if there is nothing you can do about it. The establishment of metacognition in monkeys positions us to shift the focus of our studies from whether metacognition occurs at all to identification of the properties of the interplay between monitoring processes and the control of cognitive states. In one such approach using a delayed matching-to-sample task, the sample and the test were both occluded at the beginning of each trial (Beran and Smith, 2011; Roberts et al., 2009). Monkeys and pigeons were trained to contact one icon to reveal the sample, correcting their state of ignorance, and contact another icon to reveal the comparison stimuli for matching tests. If subjects monitored their own knowledge and responded adaptively, they should uncover the sample before proceeding to the test. On some trials the sample was already uncovered at the beginning of the trial, and efficient subjects would respond immediately to the icon that revealed the matching test. Monkeys, but not pigeons, flexibly changed their use of the “reveal” option, reflecting sensitivity to their ignorance of the sample and the “need to know before you go” (Beran and Smith, 2011; Roberts et al., 2009). These results are consistent with metacognition serving cognitive control in monkeys, and also reinforces other findings suggesting that pigeons may not be metacognitive.
Unlike natural circumstances in which information may be acquired gradually and the amount of information needed to behave adaptively varies, most metacognition experiments have implemented “information” in an all-or-none fashion. The location of hidden food is either seen or not seen (Call and Carpenter, 2001; Hampton et al., 2004); the next correct choice is either provided or not provided (Kornell et al., 2007); the sample is either presented or not presented (Beran and Smith, 2011). This dichotomous approach limits the investigation of dynamic interactions between monitoring and seeking of additional information in the development of behavioral decisions. To better understand the extent to which monitoring of gradually changing cognitive states controls information seeking, we developed an information-seeking paradigm that allowed us to manipulate the amount of information available in a classification task and examine information seeking and accuracy of classification decisions (Fig. 5; Tu et al., 2015). Monkeys that monitor and respond adaptively to accumulating information should make many “revelation” responses when information is poor and few such responses when information is rich. We found that monkeys indeed adjusted information seeking effort in response to the difference between information accumulated and information needed. A dynamic interaction of memory monitoring and memory control is also suggested by evidence that monkeys actively hold memories in mind, perhaps refreshing them as they begin to fade (Basile and Hampton, 2013; Tu and Hampton, 2014). Monkeys also selectively enhanced processing of cued items in working memory, a so-called retro-cue effect (Brady and Hampton, 2018).
Fig. 5. Test of dynamic cognitive monitoring of decision-making.
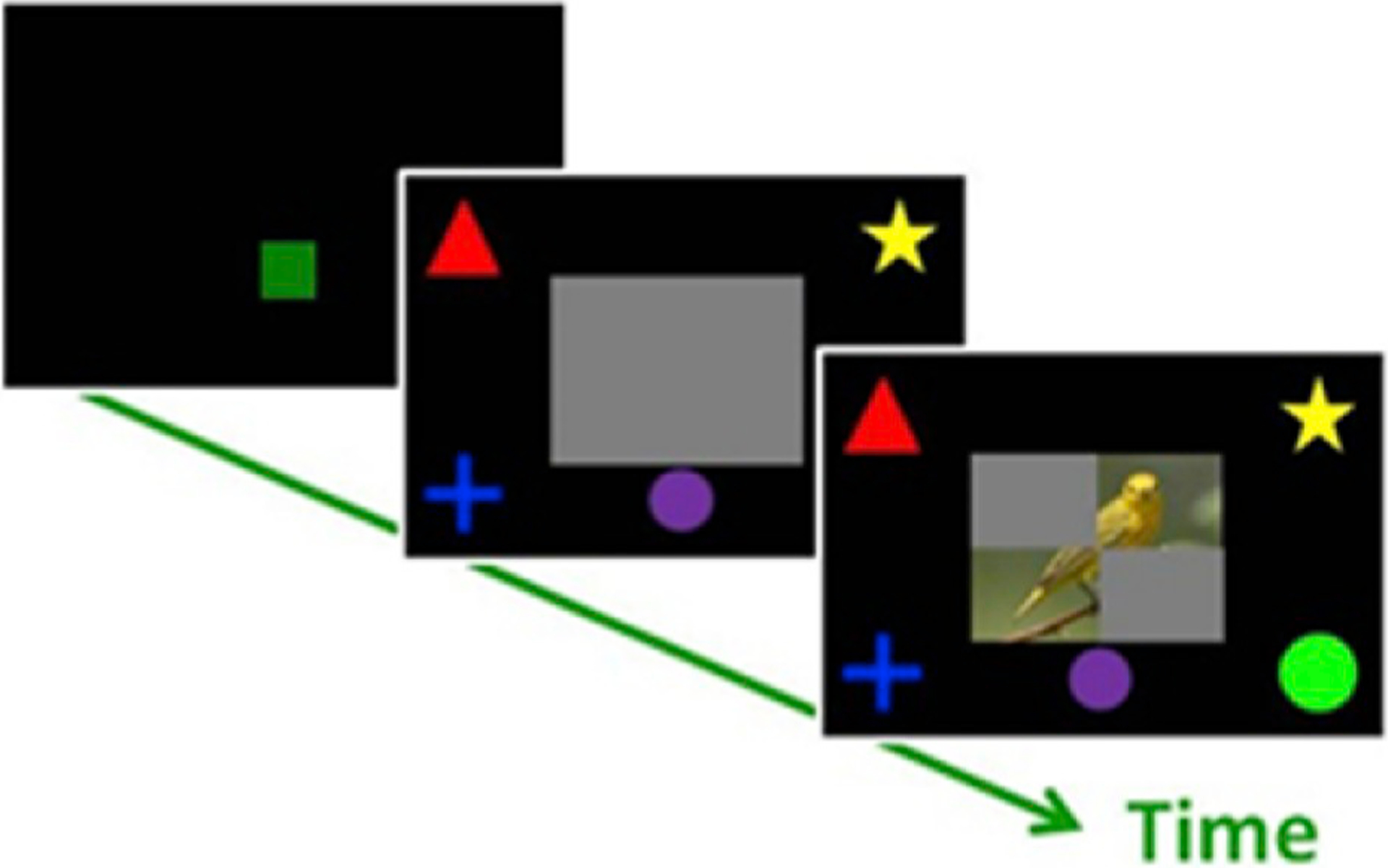
Monkeys touched the green square to start the trial. When the grey plaque appeared, monkeys could touch the purple button to gradually reveal the image. They were free to identify the image as a bird, fish, person, or flower at any time by contacting the choice stimuli in the corners of the screen. Monkeys regulated how much of the image they revealed, pressing the button more times when each button press revealed only a small part of the image, and pressing fewer times when each press revealed a large part of the image (Tu et al., 2015).
9. Some speculations on how working memory and explicit cognition are intertwined
To a large extent, material processed in working memory is the material of which we are conscious. Working memory actively maintains information in a heightened state of accessibility using extremely limited cognitive resources (Baddeley, 2003; Baddeley and Hitch, 1974; Basile and Hampton, 2013; Cowan, 2008; Unsworth and Engle, 2007). In contrast to long term memory, which is capacious and apparently passive once established, when we fail to “attend” to working memory, information is rapidly lost (Awh and Jonides, 2001; Cowan, 1998; Pertzov et al., 2013). The information held active by working memory comes from at least two sources. Perceptual information that is attended to provides new material for working memory. Some of this information may eventually be stored in long-term memory; most is rapidly forgotten. In humans, information that does get stored in LTM may remain outside of awareness for long periods of time, even decades, before again becoming the object of awareness when “activated” by working memory (Larocque et al., 2014). Although we label some long-term memories explicit, it is the case that they are only explicit during the relatively brief periods of time during which they are made “active” by working memory. The rest of the time we are as unaware of them as we are of the control of the release of hormones by the hypothalamus. Rather than calling some memories explicit and others implicit, it might be more correct, if awkward, to label some memories capable of becoming explicit and others not.
Given the correspondence between working memory and explicit awareness, metacognition is likely highly dependent on working memory. We can only be metacognitive about material activated by working memory. The reason priming is implicit is that it occurs without working memory. The reason habits are implicit is because once established they control behavior without working memory, freeing working memory for other tasks.
All of the evidence for metacognition in monkeys presented here may reflect the operation of working memory. While the material active in working memory is normally conceived of as having come from either recent perception or from long-term memory, probably all of the existing evidence for explicit memory in monkeys reflects working memory for recently perceived material, not material that was activated in, or from, long-term memory. The evidence we have presented comes from tests of memory for recently seen images or locations, or decisions about currently or recently visible disriminanda. Evidence for explicit long term memories is monkeys appears lacking. It is possible that working memory in monkeys operates on material “retrieved” from long-term memory only under highly restricted conditions, but it is more likely that research effort in studying metacognition has focused selectively on short term memory and decisional processes rather than longer-term memory. Expanding this focus should be a priority for future research. We recently described the interaction of working memory and long-term memory in the execution of “simultaneous chains” in monkeys, and this might be one paradigm that would allow tests of whether long-term memories can become explicit in monkeys (Templer et al., 2019).
10. Summary and prospects for the future
The studies reviewed demonstrate that monkeys monitor some of their memories. They know when they remember. They avoid tests when they do not know the answer. They seek information when ignorant. Their behavior captures core functional properties of explicit memory, as conveyed to us by Tulving. Monkeys clearly have multiple memory systems, and these systems dissociate in patterns that parallel findings in humans, such as with process dissociation paradigm. It is reasonable to state that monkeys have explicit memory.
Adopting the working hypothesis that monkeys have some explicit memories is not an end in itself, but a beginning, because it raises many fascinating questions about the evolution and neurobiology of memory. We have some ideas about functions that might be served by metacognition and explicit cognitive processes, but there are no convincing arguments, much less evidence, indicating the conditions under which explicit cognition and metacognition should evolve. Our understanding of why these processes are conscious in humans, and whether there might be similar phenomenology in nonhumans remains shockingly weak.
Endel Tulving has identified many exciting questions in the cognitive science of memory, and provided many stimulating answers. He has helped convince us all of the importance of distinctions between explicit and implicit memory. As a leader in our field Tulving does not just provide us with answers, and elegant experimental techniques – although he certainly does these things – he has pushed us toward new discoveries. He has often done this through what we might call “strategic provocation,” where he sets a bar for the demonstration of episodic memory in nonhumans (Tulving, 2002), or states that nonhumans do not have explicit memory (Tulving and Markowitsch, 1994). Tulving’s strategic provocations are not dogmatic. Having thrown down the gauntlet, he is eager to good naturedly “fence” in a way that supports the development of the next generation of scientists and scientific ideas. In addition to all the explicit knowledge Tulving has provided us, he has also taught us procedures for finding new knowledge and encouraging young scientists. Thank you again, Endel, for the incitement!
Acknowledgment
Preparing this manuscript was support by National Science Foundation grant BCS-1632477 and by the National Institutes of Health’s Office of the Director, Office of Research Infrastructure Programs, P51OD011132.
References
- Andersen, Basile, Hampton, 2014. Dissociation of visual localization and visual detection in rhesus monkeys (Macaca mulatta). Anim. Cognit 17 (3), 681–687. 10.1007/s10071-013-0699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh, Jonides, 2001. Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci 5 (3), 119–126. 10.1016/S1364-6613(00)01593-X. [DOI] [PubMed] [Google Scholar]
- Baddeley, 2003. Working memory: looking back and looking forward. Nat. Rev. Neurosci 4 (10), 829–839. [DOI] [PubMed] [Google Scholar]
- Baddeley, Hitch, 1974. Working memory. In: Bower GH (Ed.), Psychology of Learning and Motivation, vol. 8. Academic Press, pp. 47–89. [Google Scholar]
- Basile, Hampton, 2013. Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition 126 (3), 391–396. 10.1016/j.cognition.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile, Hampton, 2014. Metacognition as Discrimination: commentary on Smith et al. (2014). J. Comp. Psychol 128 (2), 135–137. 10.1037/a0034412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile, Hampton, Suomi, Murray, 2009. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella). Anim. Cognit 12 (1), 169–180. 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile, Schroeder, Brown, Templer, Hampton, 2015. Evaluation of seven hypotheses for metamemory performance in rhesus monkeys. J. Exp. Psychol. Gen 144 (1), 85–102. 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, Parker, Lindner, Izquierdo, Murray, 2000. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J. Neurosci 20 (11), 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belger, Bräuer, 2018. Metacognition in dogs: do dogs know they could be wrong? Learn. Behav 46 (4), 398–413. 10.3758/s13420-018-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran, Smith, 2011. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella). Cognition 120 (1), 90–105. 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran, Smith, Coutinho, Couchman, Boomer, 2009. The psychological organization of “uncertainty” responses and “middle” responses: a dissociation in capuchin monkeys (Cebus apella). J. Exp. Psychol. Anim. Behav. Process 35 (3), 371–381. 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, Hampton, 2018. Post-encoding control of working memory enhances processing of relevant information in rhesus monkeys (Macaca mulatta). Cognition 175, 26–35. 10.1016/j.cognition.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer, Call, Tomasello, 2004. Visual perspective taking in dogs (Canis familiaris) in the presence of barriers. Appl. Anim. Behav. Sci 88 (3–4), 299–317. [Google Scholar]
- Brown, Templer, Hampton, 2017. An assessment of domain-general metacognitive responding in rhesus monkeys. Behav. Process 135, 132–144. 10.1016/j.beproc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, 2019. Mnemonic introspection in macaques is dependent on superior dorsolateral prefrontal cortex but not orbitofrontal cortex. J. Neurosci 39 (30), 5922–5934. 10.1523/jneurosci.0330-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson, Dodson, Vatner, Daffner, Black, Schacter, 2005. Metacognition and false recognition in patients with frontal lobe lesions: the distinctiveness heuristic. Neuropsychologia 43 (6), 860–871. [DOI] [PubMed] [Google Scholar]
- Buffalo, Ramus, Clark, Teng, Squire, Zola, 1999. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn. Mem 6, 572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, Maguire, Spiers, O’Keefe, 2001. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14 (2), 439–453. [DOI] [PubMed] [Google Scholar]
- Call, Carpenter, 2001. Do apes and children know what they have seen? Anim. Cognit 4, 207–220. [Google Scholar]
- Clark, Squire, 1998. Classical conditioning and brain systems: the role of awareness. Science 280 (5360), 77–81. [DOI] [PubMed] [Google Scholar]
- Cohen, Eichenbaum, 1994. Memory, Amnesia, and the Hippocampal System MIT Press, Cambridge, M.A. [Google Scholar]
- Cohen, Eichenbaum, Deacedo Corkin, 1985. Different memory systems underlying acquisition of procedural and declarative knowledge. Ann. N. Y. Acad. Sci 444, 54–71. [DOI] [PubMed] [Google Scholar]
- Cowan, 1998. Attention and Memory: An Integrated Framework Oxford University Press, Oxford, UK. [Google Scholar]
- Cowan, 2008. What are the differences between long-term, short-term, and working memory?. In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S (Eds.), Essence of Memory, vol. 169. Elsevier Science Bv, Amsterdam, pp. 323–338. [Google Scholar]
- Cowey, Stoerig, 1995. Blindsight in monkeys. Nature 373 (6511), 247–249. [DOI] [PubMed] [Google Scholar]
- Cowey, Stoerig, 1997. Visual detection in monkeys with blindsight. Neuropsychologia 35 (7), 929–939. [DOI] [PubMed] [Google Scholar]
- DeCoteau, Kesner, 2000. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav. Neurosci 114, 1096–1108. [DOI] [PubMed] [Google Scholar]
- Dellarocchetta, Milner, 1993. Strategic search and retrieval inhibition - the role of the frontal lobes. Neuropsychologia 31 (6), 503–524. [DOI] [PubMed] [Google Scholar]
- Dobbins, Foley, Schacter, Wagner, 2002. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron 35 (5), 989–996. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, Cohen, 2001. From Conditioning to Conscious Recollection: Memory Systems of the Brain Oxford University Press, Oxford. [Google Scholar]
- Eichenbaum, Yonelinas, Ranganath, 2007. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque, Baird, Posner, 2000. Executive attention and metacognitive regulation. Conscious. Cognit 9 (2), 288–307. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz, Wang, Aigner, Mishkin, 2001. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proc. Natl. Acad. Sci. U.S.A 98 (7), 4196–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno, Kornell, Cantlon, 2017. A metacognitive illusion in monkeys. Proc. R. Soc. Biol. Sci 284, 6. 10.1098/rspb.2017.1541, 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote, Crystal, 2007. Metacognition in the rat. Curr. Biol 17 (6), 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, 2009. Metamemory in tufted capuchin monkeys (Cebus apella). Anim. Cognit 12 (4), 575–585. 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Gaffan, Harrison, 1988. Inferotemporal-frontal disconnection and fornix transection in visuomotor conditional learning by monkeys. Behav. Brain Res 31 (2), 149–163. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic, Selemon, Schwartz, 1984. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal-formation and parahippocampal cortex in the rhesus-monkey. Neuroscience 12 (3), 719–743. [DOI] [PubMed] [Google Scholar]
- Greene, Gross, Elsinger, Rao, 2007. Hippocampal differentiation without recognition: an fMRI analysis of the contextual cueing task. Learn. Mem 14 (8), 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, 1976. The Question of Animal Awareness : Evolutionary Continuity of Mental Experience Rockefeller University Press, New York. [Google Scholar]
- Griffin, 2001. Animal Minds : beyond Cognition to Consciousness University of Chicago Press, Chicago. [Google Scholar]
- Hampton, 2001. Rhesus monkeys know when they remember. Proc. Natl. Acad. Sci. U.S. A 98 (9), 5359–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton, 2003. Metacognition as evidence for explicit representation in nonhumans. Behav. Brain Sci 26, 346–347. [DOI] [PubMed] [Google Scholar]
- Hampton, 2009. Multiple demonstrations of metacognition in nonhumans: converging evidence or multiple mechanisms? Comp Cogn Behav Rev 4, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton, Hampstead, 2006. Spontaneous behavior of a rhesus monkey (Macaca mulatta) during memory tests suggests memory awareness. Behav. Process 72, 184–189. [DOI] [PubMed] [Google Scholar]
- Hampton, Schwartz, 2004. Episodic memory in nonhumans: what, and where, is when? Curr. Opin. Neurobiol 14, 192–197. [DOI] [PubMed] [Google Scholar]
- Hampton, Zivin, Murray, 2004. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim. Cognit 7, 239–254. [DOI] [PubMed] [Google Scholar]
- Hannula, Tranel, Cohen, 2006. The long and the short of it: relational memory impairments in amnesia, even at short lags. J. Neurosci 26 (32), 8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, Jacoby, 1996. Separating habit and recollection: memory slips, process dissociations, and probability matching. J. Exp. Psychol. Learn. Mem. Cogn 22 (6), 1323–1335. [DOI] [PubMed] [Google Scholar]
- Jacoby, 1991. A process dissociation framework - separating automatic from intentional uses of memory. J. Mem. Lang 30 (5), 513–541. [Google Scholar]
- Jacoby, Toth, Yonelinas, 1993. Separating conscious and unconscious influences of memory - measuring recollection. J. Exp. Psychol. Gen 122 (2), 139–154. [Google Scholar]
- Jagadeesh, Chelazzi, Mishkin, Desimone, 2001. Learning increases stimulus salience in anterior inferior temporal cortex of the macaque. J. Neurophysiol 86, 290–303. [DOI] [PubMed] [Google Scholar]
- Kentridge, Heywood, Weiskrantz, 1999. Attention without awareness in blindsight. Proc. R. Soc. Lond. Ser. B Biol. Sci 266 (1430), 1805–1811. 10.1098/rspb.1999.0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner, Bolland, Dakis, 1993. Memory for spatial locations, motor-responses, and objects - triple dissociation among the Hippocampus, caudate-nucleus, and extrastriate visual-cortex. Exp. Brain Res 93, 462–470. [DOI] [PubMed] [Google Scholar]
- Kiani, Shadlen, 2009. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324 (5928), 759–764. 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Baxter, 2001. Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends Neurosci 24 (6), 324–330. [DOI] [PubMed] [Google Scholar]
- Klotz, Neumann, 1999. Motor activation without conscious discrimination in metacontrast masking. J. Exp. Psychol. Hum. Percept. Perform 25 (4), 976–992. 10.1037/0096-1523.25.4.976. [DOI] [Google Scholar]
- Knowlton, Ramus, Squire, 1992. Intact artificial grammar learning in amnesia - dissociation of classification learning and explicit memory for specific instances. Psychol. Sci 3 (3), 172–179. [Google Scholar]
- Knowlton, Squire, 1993. The learning of categories - parallel brain systems for item memory and category knowledge. Science 262 (5140), 1747–1749. [DOI] [PubMed] [Google Scholar]
- Kornell, Son, Terrace, 2007. Transfer of metacognitive skills and hint seeking in monkeys. Psychol. Sci 18 (1), 64–71. [DOI] [PubMed] [Google Scholar]
- Larocque, Lewis-Peacock, Postle, 2014. Multiple neural states of representation in short-term memory? It’s a matter of attention. Front. Hum. Neurosci 8, 5. 10.3389/fnhum.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex, Amaral, 2000. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10 (4), 420–430. [DOI] [PubMed] [Google Scholar]
- Logothetis, 1998. Object vision and visual awareness. Curr. Opin. Neurobiol 8, 536–544. [DOI] [PubMed] [Google Scholar]
- Malkova, Bachevalier, Mishkin, Saunders, 2001. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport 12 (9), 1913–1917. [DOI] [PubMed] [Google Scholar]
- McDonald, White, 1993. A triple dissociation of memory-systems - Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci 107 (1), 3–22. [DOI] [PubMed] [Google Scholar]
- McMahon, Macpherson, Roberts, 2010. Dogs choose a human informant: metacognition in canines. Behav. Process 85 (3), 293–298. 10.1016/j.beproc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Meunier, Bachevalier, Mishkin, Murray, 1993. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus-monkeys. J. Neurosci 13 (12), 5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks, Sommer, 2012. Neuronal correlates of metacognition in primate frontal cortex. Neuron 75 (3), 517–530. 10.1016/j.neuron.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, 2012. Is visual processing in the dorsal stream accessible to consciousness? Proc. R. Soc. Biol. Sci 279 (1737), 2289–2298. 10.1098/rspb.2011.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, Goodale, 2008. Two visual systems re-viewed. Neuropsychologia 46 (3), 774–785. 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Miyamoto, Osada, Setsuie, Takeda, Tamura, Adachi, Miyashita, 2017. Causal neural network of metamemory for retrospection in primates. Science 355 (6321), 188–193. 10.1126/science.aal0162. [DOI] [PubMed] [Google Scholar]
- Miyamoto, Setsuie, Osada, Miyashita, 2018. Reversible silencing of the frontopolar cortex selectively impairs metacognitive judgment on non-experience in primates. Neuron 97 (4), 980–989. 10.1016/j.neuron.2017.12.040e986. [DOI] [PubMed] [Google Scholar]
- Moore, Rodman, Gross, 1998. Man, monkey, and blindsight. The Neuroscientist 4 (4), 227–230. 10.1177/107385849800400410. [DOI] [Google Scholar]
- Nelson, 1996. Consciousness and metacognition. Am. Psychol 51 (2), 102–116. [Google Scholar]
- Packard, 1999. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc. Natl. Acad. Sci. U.S.A 96 (22), 12881–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard, Knowlton, 2002. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci 25, 563–593. [DOI] [PubMed] [Google Scholar]
- Packard, McGaugh, 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem 65, 65–72. [DOI] [PubMed] [Google Scholar]
- Paukner, Anderson, Fujita, 2006. Redundant food searches by capuchin monkeys (Cebus apella): a failure of metacognition? Anim. Cognit 9 (2), 110–117. [DOI] [PubMed] [Google Scholar]
- Pertzov, Bays, Joseph, Husain, 2013. Rapid forgetting prevented by retrospective attention cues. J. Exp. Psychol. Hum. Percept. Perform 39 (5), 1224–1231. 10.1037/a0030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack, Packard, 2003. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia 41, 245–251. [DOI] [PubMed] [Google Scholar]
- Reingold, GoshenGottstein, 1996. Separating consciously controlled and automatic influences in memory for new associations. J. Exp. Psychol. Learn. Mem. Cogn 22 (2), 397–406. [Google Scholar]
- Rempel-Clower, Barbas, 2000. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cerebr. Cortex 10 (9), 851–865. [DOI] [PubMed] [Google Scholar]
- Roberts, Feeney, McMillan, MacPherson, Musolino, Petter, 2009. Do pigeons (Columba livia) study for a test? J. Exp. Psychol. Anim. Behav. Process 35 (2), 129–142. 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Roberts, Strang, Macpherson, 2015. Memory systems interaction in the pigeon: working and reference memory. Journal of Experimental Psychology-Animal Learning and Cognition 41 (2), 152–162. 10.1037/xan0000053. [DOI] [PubMed] [Google Scholar]
- Rosati, Santos, 2016. Spontaneous metacognition in rhesus monkeys. Psychol. Sci 27 (9), 1181–1191. 10.1177/0956797616653737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg, Fletcher, Chua, Dolan, 1999. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage 10 (5), 520–529. [DOI] [PubMed] [Google Scholar]
- Ryan, Althoff, Whitlow Cohen, 2000. Amnesia is a deficit in relational memory. Psychol. Sci 11 (6), 454–461. [DOI] [PubMed] [Google Scholar]
- Saintcyr, Ungerleider, Desimone, 1990. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallidonigral complex in the monkey. J. Comp. Neurol 298 (2), 129–156. [DOI] [PubMed] [Google Scholar]
- Sanders, Warrington, Marshall, Weiskrantz, 1974. BLINDSIGHT”: VISION IN a field defect. The Lancet 303 (7860), 707–708. 10.1016/S0140-6736(74)92907-9. [DOI] [PubMed] [Google Scholar]
- Schroeder, Wingard, Packard, 2002. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus 12, 280–284. [DOI] [PubMed] [Google Scholar]
- Seger, 2006. The basal ganglia in human learning. The Neuroscientist 12 (4), 285–290. [DOI] [PubMed] [Google Scholar]
- Sherry, 2006. Neuroecology. Annu. Rev. Psychol 57, 167–197. [DOI] [PubMed] [Google Scholar]
- Sherry, Schacter, 1987. The evolution of multiple memory-systems. Psychol. Rev 94 (4), 439–454. [Google Scholar]
- Shettleworth, 1998. Cognition, Evolution, and Behavior Oxford University Press, New York. [Google Scholar]
- Shields, Smith, Guttmannova, Washburn, 2005. Confidence judgments by humans and rhesus monkeys. J. Gen. Psychol 132 (2), 165–186. [PMC free article] [PubMed] [Google Scholar]
- Shields, Smith, Washburn, 1997. Uncertain responses by humans and rhesus monkeys (Macaca mulatta) in a psychophysical same-different task. J. Exp. Psychol. Gen 126 (2), 147–164. [DOI] [PubMed] [Google Scholar]
- Shimamura, 2000. Toward a cognitive neuroscience of metacognition. Conscious. Cognit 9 (2), 313–323. [DOI] [PubMed] [Google Scholar]
- Smith, Coutinho, Church, Beran, 2013. Executive-attentional uncertainty responses by rhesus macaques (Macaca mulatta). J. Exp. Psychol. Gen 142 (2), 458–475. 10.1037/a0029601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, Smith, David, Beran, 2018. Not Knowing what One Knows: A Meaningful Failure of Metacognition in Capuchin Monkeys, vol. 5. [Google Scholar]
- Squire, 2004. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem 82, 171–177. [DOI] [PubMed] [Google Scholar]
- Squire, Knowlton, Musen, 1993. The structure and organization of memory. Annu. Rev. Psychol 44, 453–495. [DOI] [PubMed] [Google Scholar]
- Squire, Zola-Morgan, 1991. The medial temporal-lobe memory system. Science 253 (5026), 1380–1386. [DOI] [PubMed] [Google Scholar]
- Sutton, Shettleworth, 2008. Memory without awareness: pigeons do not show metamemory in delayed matching to sample. J. Exp. Psychol. Anim. Behav. Process 34 (2), 266–282. [DOI] [PubMed] [Google Scholar]
- Suzuki, 1996. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin. Neurosci 8, 3–12. [Google Scholar]
- Templer, Brown, Hampton, 2018. Rhesus monkeys metacognitively monitor memories of the order of events. Sci. Rep 8 (1), 11541. 10.1038/s41598-018-30001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer, Gazes, Hampton, 2019. Co-operation of long-term and working memory representations in simultaneous chaining by rhesus monkeys (Macaca mulatta). Q. J. Exp. Psychol 1747021819838432 10.1177/1747021819838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer, Hampton, 2012. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Anim. Cognit 15 (3), 409–419. 10.1007/s10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer, Lee, Preston, 2017. Rats know when they remember: transfer of metacognitive responding across odor-based delayed match-to-sample tests. Anim. Cognit 20 (5), 891–906. 10.1007/s10071-017-1109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, Stefanacci, Squire, Zola, 2000. Contrasting effects on discrimination learning after hippocampal lesions and conjoint hippocampal-caudate lesions in monkeys. J. Neurosci 20, 3853–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, Reingold, Jacoby, 1994. Toward a redefinition of implicit memory - process dissociations following elaborative processing and self-generation. J. Exp. Psychol. Learn. Mem. Cogn 20 (2), 290–303. [DOI] [PubMed] [Google Scholar]
- Tu, Hampton, 2013. One-trial memory and habit contribute independently to matching-to-sample performance in rhesus monkeys (Macaca mulatta). J. Comp. Psychol 127 (3), 319–328. 10.1037/a0030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Hampton, 2014. Control of working memory in rhesus monkeys (Macaca mulatta). J. Exp. Psychol. Anim. Behav. Process 40 (4), 467–476. 10.1037/xan0000030. [DOI] [PubMed] [Google Scholar]
- Tu, Hampton, Murray, 2011. Perirhinal cortex removal dissociates two memory systems in matching-to-sample performance in rhesus monkeys. J. Neurosci 31 (45), 16336–16343. 10.1523/jneurosci.2338-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, Pani, Hampton, 2015. Rhesus monkeys (Macaca mulatta) adaptively adjust information seeking in response to information accumulated. J. Comp. Psychol 129, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving, 1985. Memory and consciousness. Canadian Psychology-Psychologie Canadienne 26 (1), 1–12. [Google Scholar]
- Tulving, 2002. Episodic memory and common sense: how far apart? In: Baddeley AD, Conway M, Aggleton J (Eds.), Episodic Memory: New Directions in Research Oxford University Press, New York, pp. 269–288. [Google Scholar]
- Tulving, Markowitsch, 1994. What do animal-models of memory model? Behav. Brain Sci 17 (3), 498–499. [Google Scholar]
- Tulving, Schacter, 1990. Priming and human-memory systems. Science 247 (4940), 301–306. [DOI] [PubMed] [Google Scholar]
- Turchi, Saunders, Mishkin, 2005. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc. Natl. Acad. Sci. U.S.A 102 (6), 2158–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth, Engle, 2007. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychol. Rev 114 (1), 104–132. 10.1037/0033-295x.114.1.104. [DOI] [PubMed] [Google Scholar]
- Webster, Bachevalier, Ungerleider, 1993. Subcortical connections of inferior temporal areas Te and teo in macaque monkeys. J. Comp. Neurol 335 (1), 73–91. [DOI] [PubMed] [Google Scholar]
- Weiskrantz, 2001. Commentary responses and conscious awareness in humans: the implications for awareness in non-human animals. Anim. Welf 10, S41–S46. [Google Scholar]
- White, McDonald, 2002. Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem 77 (2), 125–184. [DOI] [PubMed] [Google Scholar]


