Abstract
Introduction
Oculomotor function has not been systematically studied in frontotemporal dementia (FTD) and yet may offer a simple target to monitor disease activity.
Methods
We assessed fixation stability, smooth pursuit, pro‐saccades, and anti‐saccades using the Eyelink 1000‐plus eye‐tracker in 19 individuals with behavioral variant FTD (bvFTD) and 22 controls. Neuroanatomical correlates were assessed using a region of interest magnetic resonance imaging (MRI) analysis.
Results
Measures of fixation stability were impaired in the bvFTD group compared with controls. However, performance did not differ from controls in the pro‐saccade tasks except in the vertical overlap condition. The bvFTD group performed worse in the anti‐saccade task, which correlated strongly with executive function. Neural correlates included the orbitofrontal and ventromedial prefrontal cortices and striatum for fixation stability, and the dorsolateral prefrontal and parietal cortices and striatum for anti‐saccades.
Discussion
Overall, oculomotor function is abnormal in bvFTD, with performance likely related to impairment of inhibitory control and executive dysfunction.
Keywords: anti‐saccades, behavioral variant frontotemporal dementia, eye‐tracking, fixations, oculomotor function, pursuit, saccades
1. INTRODUCTION
Eye movements are easily observed and measured. They are classified by the way they serve vision, with evaluation of how these different types of movements are affected having been shown to be helpful in facilitating the diagnosis of neurodegenerative disorders. 1 , 2 The measurement of eye movements can also act as a powerful tool to study cognition including memory, language, and spatial learning. 3 Despite this, oculomotor function has never been systematically assessed in behavioral variant frontotemporal dementia (bvFTD), a neurodegenerative disease associated with progressive changes in personality, impaired social cognition, and executive dysfunction.
The neuroanatomical regions involved in oculomotor function vary by the type of eye movements (Figure 1A). Although initial generation of saccades is associated with projections to the superior colliculus and onto brainstem structures that directly innervate the eye muscles, saccadic function is ultimately under cortical control, with the frontal eye field (FEF), the supplementary eye field (SEF), parietal eye fields (PEFs), and the dorsolateral prefrontal cortex (DLPFC) being critical regions. 4 , 5 Voluntary pro‐saccades (toward a target: Figure 1A) are primarily under FEF control, whereas reflexive saccades are largely triggered via PEF neurons. Some saccadic tasks, such as anti‐saccades (looking in the opposite direction to a suddenly appearing target; Figure 1A) add cognitive layers to the final oculomotor execution and more directly involve the DLPFC and PEF. 5 In contrast, smooth pursuit and fixation eye movements are initially processed by the extrastriatal cortical regions including V5 and the medial superior temporal visual area, connecting to the posterior parietal cortex, FEF, and SEF before being projected down to the pontine nuclei and cerebellum. 5 Given the cortical changes associated with bvFTD, typically in the frontal and temporal regions, 6 , 7 one might expect a range of oculomotor disturbances.
FIGURE 1.
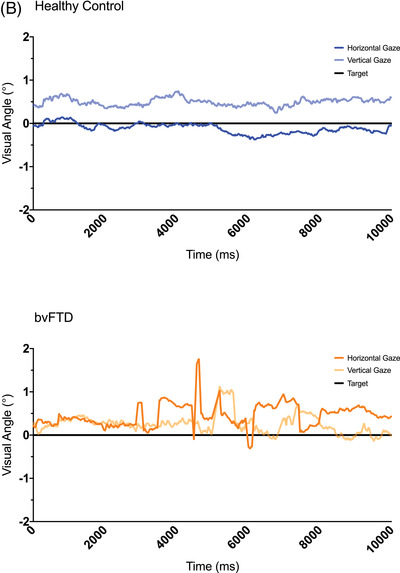
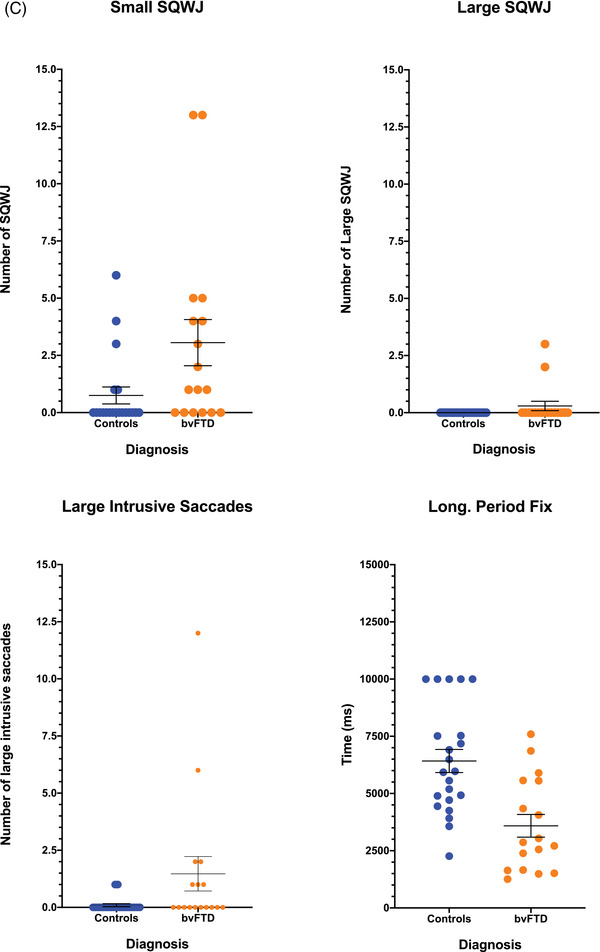
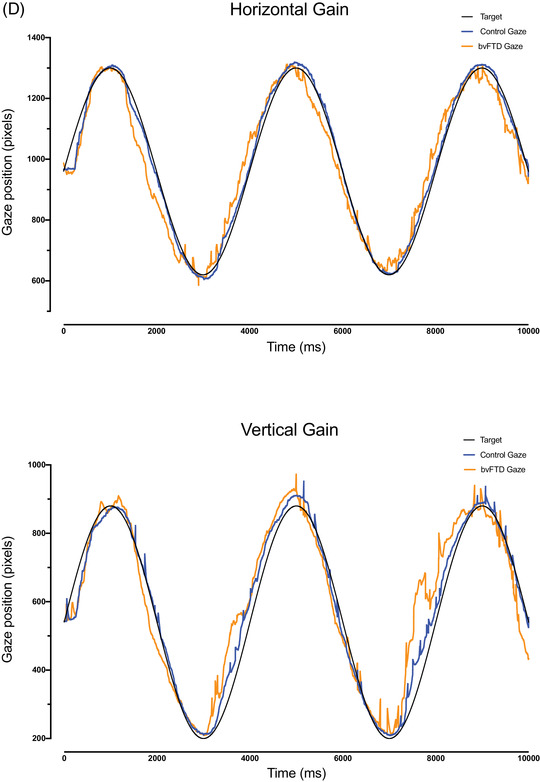
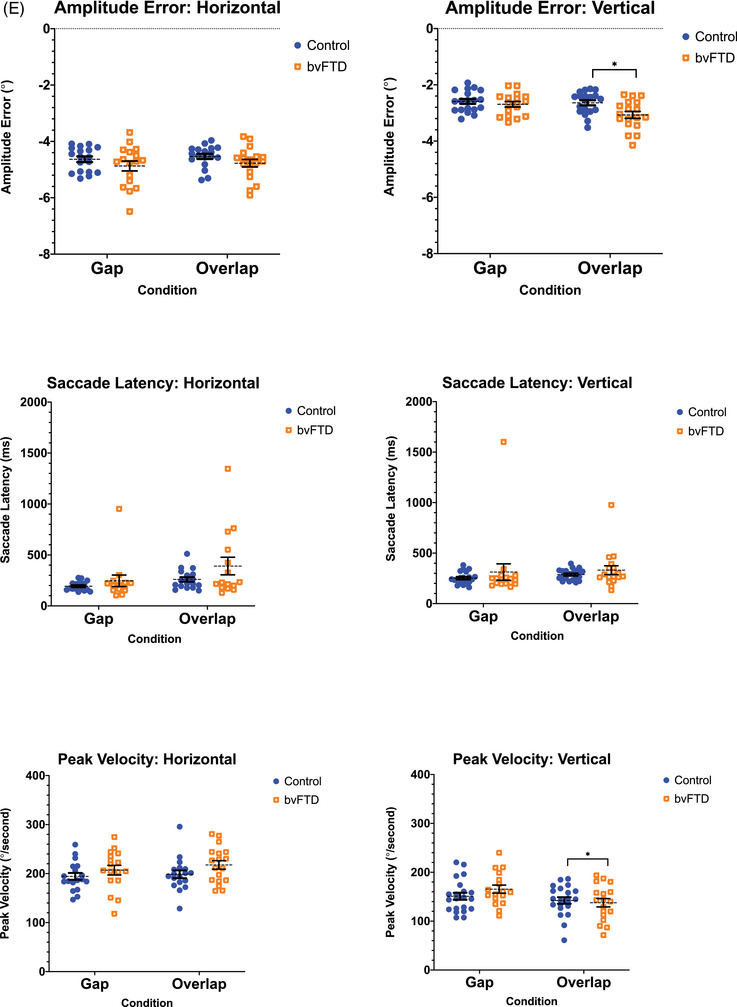
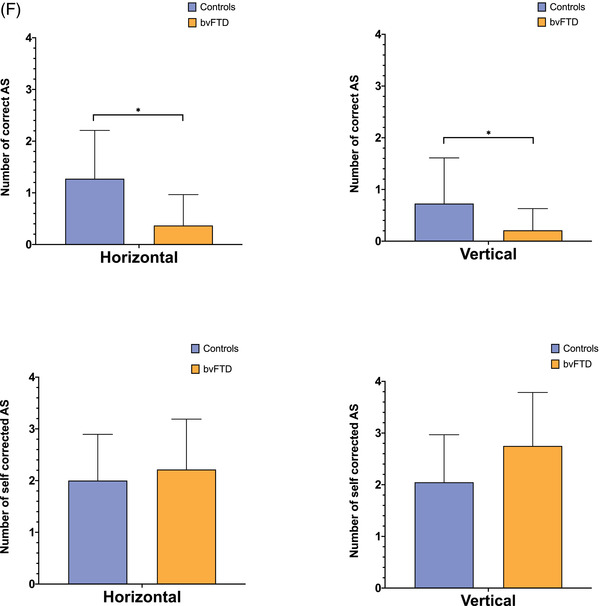
(A) The oculomotor task stimuli and the associated eye movements: fixation (with small wave jerk [SWJ] intrusions), smooth pursuit, pro‐saccades, and anti‐saccades (showing a correct anti‐saccade and an anti‐saccadic error). Deg = degrees. (B) The mean fixation traces across all trials for control and behavioral variant frontotemporal dementia (bvFTD) groups. The darker line represents the horizontal gaze position on the screen, whereas the lighter line represents the vertical gaze position. The solid black line represents the position of the target throughout the trials. (C) Performance on fixation tasks in the control and bvFTD groups. (D) The pursuit traces are shown for the control and bvFTD groups in each condition. The lines represent the mean eye position across the trials for each group while pursuing the target as it moves across the screen. Controls are represented by the blue line, the bvFTD by the orange line, and the target by the black line. The top image represents the horizontal condition and the bottom image represents the vertical condition. (E)€ Performance on the pro‐saccade tasks in the control and bvFTD groups. (F) Mean number of correct and self‐corrected anti‐saccades (AS) in the control and bvFTD groups
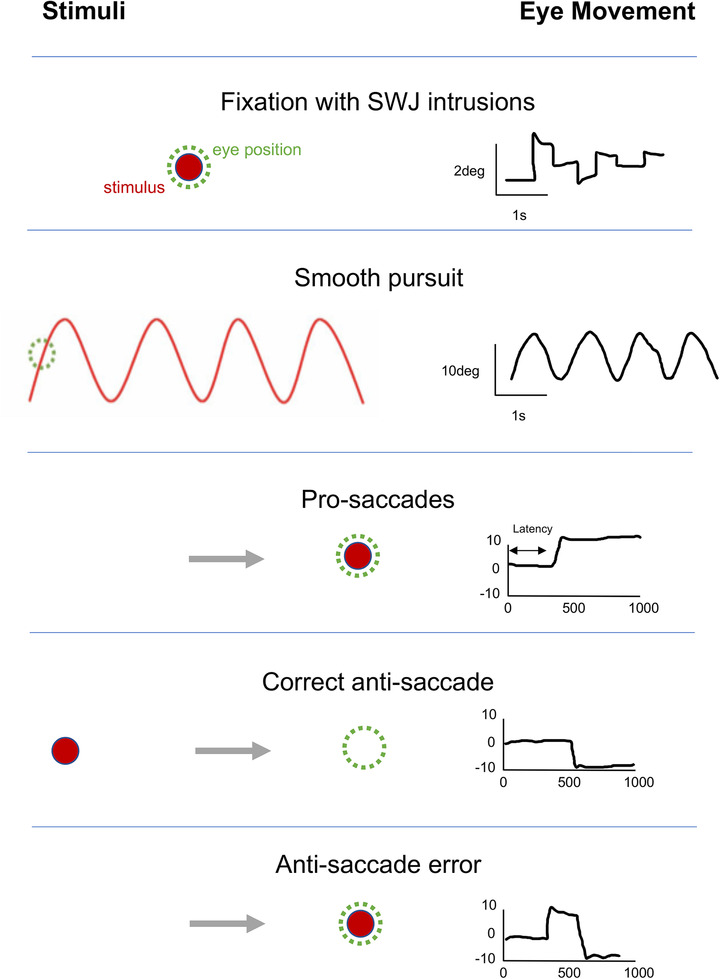
Previous investigation of oculomotor function in bvFTD has produced conflicting findings. For example, although some studies of pro‐saccades in bvFTD suggest that the time taken to generate a saccade is longer than in healthy controls, 8 , 9 , 10 and that saccades are slower and less accurate when looking at a specific target, 10 , 11 others have shown that people with bvFTD have normal saccadic speed and accuracy. 9 , 12 Similarly, although some studies have shown that individuals with bvFTD can have difficulty following a moving target across a screen compared to healthy controls when a step‐wise smooth pursuit task is performed, 11 , 12 others have shown normal pursuit movements. In contrast to these conflicting results for pro‐saccades and pursuit eye movements, the majority of studies have shown that performance on anti‐saccade tasks in individuals with bvFTD is impaired, with difficulties inhibiting their saccades toward the new target. 8 , 9 , 10 , 11 , 12 , 13
The aim of this study was therefore to systematically assess oculomotor function in individuals with bvFTD and correlate this with neuropsychometric abilities and neuroanatomical changes.
2. METHODS
2.1. Participants
Participants were recruited from the longitudinal FTD studies at the University College London (UCL) Dementia Research Centre (DRC). A total of 19 consecutively recruited people fulfilling current diagnostic criteria for bvFTD 7 were included in the study, of whom 10 were genetically confirmed (carrying mutations in chromosome 9 open reading frame 72 [C9orf72] = 5, progranulin [GRN] = 3 and microtubule‐associated protein tau [MAPT] = 2). Twenty‐two healthy controls also took part in the study. All participants gave fully informed consent in line with the Declaration of Helsinki.
RESEARCH IN CONTEXT
Systematic review: Previous reports of oculomotor function in individuals with behavioral variant frontotemporal dementia (bvFTD) suggest the presence of abnormalities but this has not been studied comprehensively previously.
Interpretation: This study shows that oculomotor function is impaired in bvFTD across multiple types of eye movements, but particularly in fixation stability and the ability to perform anti‐saccades. Impaired performance in bvFTD is likely related to impairment of inhibitory control and executive dysfunction.
Future directions: It may be possible that eye‐tracking assessments of oculomotor function, particularly anti‐saccade tasks, could be used as outcome measures in upcoming clinical trials for bvFTD.
All participants underwent a standardized history, neurological examination (with normal or corrected normal visual acuity noted), and neuropsychological battery. Participants also underwent a volumetric 3T T1‐weighted magnetic resonance imaging (MRI) on a Siemens Prisma scanner within a week of the oculomotor testing, with scans included in the final study following a quality check for movement, artifacts, and the presence of moderate to severe vascular disease or other non‐degenerative brain pathology.
Participants underwent a set of tests of oculomotor function lasting ≈10 to 15 min in total. The stimuli were presented on an 18‐inch Dell Latitude E6540 Laptop (resolution of 1920 × 1080 pixels) from a fixed viewing distance of 70 cm, linked to the SR Research Eyelink 1000 Plus table‐mounted eye‐tracker. See Figure 1A adapted from Klarendic, Kaski 5 for examples of stimuli and the associated eye movements. Viewing was binocular but only the right eye was tracked. Individuals’ heads were stabilized with the use of a chin rest. Before starting the experiment, a nine‐point calibration procedure was carried out. This was repeated throughout the experiment if the individual needed a break or moved their heads away from the chin rest for any reason.
2.1.1. Fixation task
A red cross (Color in Red Green Blue (RGB) = 128, 128, 128; Size = 0.5 degrees of visual angle [° VA]) was presented in the middle of the screen for 10 s and individuals were instructed to look at the red cross without blinking. 14 , 15 There was a total of four trials.
2.1.2. Smooth pursuit task
Participants were asked to follow a red dot as it moved across the screen. The red target (RGB = 255, 0, 0; Size = 0.5° VA) appeared in the center of the screen and moved 10° to either side of the center (20° total amplitude) in both horizontal and vertical directions. Each trial lasted 10 s with the sinusoidal target frequency set at 0.25 Hz. There were two practice trials and two active trials, one in each direction.
2.1.3. Pro‐saccade task
A red cross (RGB = 255, 0, 0; Size = 0.5° VA) appeared in the middle of the screen, and then once the participants had fixated on the cross, a green dot (RGB = 0, 200, 0; Diameter = 0.5° VA) appeared at 8° VA in the horizontal direction and 5° VA in the vertical directions on either side of the target fixation cross. The difference in visual angle was chosen to reflect a naturally wider horizontal viewing plane. Participants were asked to look as quickly and as accurately as possible to the green dot when it appeared. There were two conditions, an overlap and a gap condition. In the overlap condition, both the green dot and the fixation cross were on the screen for 500 ms before the cross disappeared. In the gap condition, the cross had disappeared from the screen for 200 ms before the dot appeared. There were 16 trials in total (8 overlap, 8 gap). 11 , 14
2.1.4. Anti‐saccade task
This test was similar in structure to the pro‐saccade test; however, the dot was red (RGB = 255, 0, 0; Size = 0.5° VA), and participants were told to look in the opposite direction to the dot when it appeared, that is, if the dot appeared on the right, they should look to the left, and if it appeared at the top, they should look at the bottom. The test included the same number of trials and locations as the pro‐saccade test; however, only the gap condition was administered.
2.2. Statistical analysis
All data were analyzed using Stata version 14.2 (Stata‐Corp, College Station, TX). Demographic and psychometric data were analyzed using independent t‐tests on normally distributed data, or Mann‐Whitney U tests for data that were not normally distributed to compare between the two groups (Table 1).
TABLE 1.
Demographic and neuropsychometric data for the control and bvFTD participants
| Controls (N = 22) | bvFTD (N = 19) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P value | |
| Sex (F : M) | 9 : 13 | 5 : 14 | 0.326 | ||
| Age | 64.2 | 5.7 | 63.7 | 6.2 | 0.821 |
| MMSE (/30) | 29.5 | 0.7 | 24.8 | 4.0 | < 0.001 |
| CDR® plus NACC FTLD sum of boxes | 0.80 | 0.8 | 10.3 | 3.7 | < 0.001 |
| WMS‐R Digit Span Forwards (/12) | 9.0 | 2.2 | 6.8 | 0.5 | 0.005 |
| WMS‐R Digit Span Backwards (/12) | 8.3 | 2.6 | 4.8 | 1.9 | < 0.001 |
| Phonemic Fluency (1 min) | 15.1 | 5.7 | 8.2 | 4.9 | < 0.001 |
| D‐KEFS Color‐Word Interference Test (seconds) | 56.5 | 17.3 | 93.3 | 36.4 | < 0.001 |
| Trail Making Test Part A (seconds) | 30.3 | 11.2 | 52.0 | 29.1 | 0.001 |
| Trail Making Test Part B (seconds) | 69.2 | 24.7 | 171.5 | 90.9 | < 0.001 |
| British Picture Vocabulary Scale (/150) | 147.9 | 1.3 | 124.9 | 23.2 | < 0.001 |
Abbreviation: SD, standard deviation; F, Female; M, Male; MMSE, Mini Mental State Examination; CDR® plus NACC FTLD, CDR® Dementia Staging Instrument with the National Alzheimer Coordinating Centre Frontotemporal Lobar Degeneration component; WMS‐R, Wechsler Memory Scale Revised; D‐KEFS, Delis Kaplan Executive System.
All gaze data were loaded into the Data Viewer Program provided by SR Research for pre‐processing of the eye‐tracking data. The Reaction Time Manager tool in Data Viewer was used to zero all values to the onset of the cross for the fixation task and to the onset of the target in both the pro‐saccade and anti‐saccade tasks. Two individuals with bvFTD did not have sufficient data to be included in the fixation analysis, and one individual did not have enough data to be included in the smooth pursuit and pro‐saccade analysis. The following oculomotor function measures were calculated from the output reports generated by the Data Viewer software:
2.2.1. Fixation
Small square wave jerk frequency: A small square wave jerk was counted in a predefined algorithm for each individual on each trial. It was defined as a saccade that moved away from the central fixation cross, and was followed by another saccade, which moved back toward the fixation cross in the direction from which it had come. The first saccade had to be < 2° in amplitude, whereas the second saccade had to be less than 300 ms later with a similar amplitude (< 0.75° difference) to the first. 16
Large square wave jerk frequency: The large square wave jerks followed the same algorithm as small square wave jerks; however, the first saccade had to be between 2° and 6° in amplitude. The numbers of large square wave jerks were then counted for each individual on each trial.
Number of large intrusive saccades: A saccade was classed as a large intrusive saccade if the amplitude was greater than 2°, and it did not contain a blink. 17 These were then counted for each individual on each trial.
Longest period of fixation: The maximum time period spent looking at the fixation cross (time between saccades) without blinking was classed as the longest period of fixation for each individual across the trials. 17
2.2.2. Smooth pursuit
Pursuit gain: Potential pursuit segments were classed as any consecutive samples that were not parsed by the Eyelink software as being in a blink or a saccade. For each potential pursuit segment, velocity gain was calculated by dividing the average eye velocity by the average target velocity in that segment. The number of samples within each pursuit segment was also counted to determine the length of the pursuit segment (the eye tracker sampled at 1000 Hz, so 20 samples equates to 20 ms). The duration of the pursuit segments was taken into consideration when averaging the overall pursuit gain for each trial by calculating a weighted mean, so that the gain values for longer periods of pursuit contribute more to the mean than the gain values from shorter periods. The weighted average velocity gain for each individual on each trial was used in the group analysis.
2.2.3. Pro‐saccades
The first saccade that met the following criteria was used for the analysis: the first saccade that did not contain a blink, did not start before the onset of the target, went in the same direction as the target and started at the fixation cross. If this first saccade happened to be greater than the sixth saccade in the trial, it was not included in the analysis.
Amplitude error: This was a measure of how close to the target the initial saccade amplitude was. It was calculated by taking the visual angle of the target away from the amplitude of the saccade. It is measured in degrees of visual angle.
Saccade latency: This was a measure of the time taken (in milliseconds) for the individual to generate the first saccade after the target has appeared.
Peak velocity: This was calculated in degrees per second and was the maximum velocity reached for the saccade of interest.
2.2.4. Anti‐saccades
Correct anti‐saccades: An anti‐saccade was defined in the same way as a pro‐saccade (above) except being one that went in the opposite direction to the target. The total number of correct anti‐saccades was measured.
Self‐corrected anti‐saccades: Self‐corrected anti‐saccades occur when an individual makes a small eye movement toward the target but then realizes that they should look in the other direction. To calculate this, those trials that contained correct anti‐saccades were removed from the data. The remaining trials then contained all data that were not a correct anti‐saccade, and a self‐corrected anti‐saccade was counted as one where there was a pro‐saccade (the first saccade that did not contain a blink, did not start before the target onset, was greater than 2° in amplitude and went toward the target) followed by an anti‐saccade (a following saccade that went back in the direction it had come from, away from the target, and was within 500 ms of the first saccade) as long as it was less than the sixth saccade in the trial. The total number of self‐corrected anti‐saccades was measured.
Multiple linear regression models were run for each of the oculomotor function measures, comparing the measure of interest between groups, with age as a covariate. For the peak velocity, saccade amplitude was also included as a covariate, as this was highly correlated with the peak velocity (r = 0.837, P < 0.001). As the distance of the eye movement increases, so too does the speed with which the eye moves. As we had differences in the distance the individual had to look in the horizontal and vertical conditions, it was important to include this as a covariate in the analysis. Bootstrapping with 1000 replicates was carried out for saccade latency and peak velocity metrics on the pro‐saccade tests, and on the correct and self‐corrected metrics on the anti‐saccade measures, as the data were not normally distributed.
Pearson's correlation coefficients were calculated to assess the association of measures with disease severity on task performance in the bvFTD group, as well as determining the relationship between the anti‐saccade task and the neuropsychological measures of executive function and language comprehension. Correlation coefficients were also calculated to assess the association of oculomotor function measures with neuroanatomical regions of interest (cortical and subcortical areas known to be implicated in eye movements), specifically the orbitofrontal cortex, dorsolateral prefrontal cortex (DLPFC), ventromedial prefrontal cortex (VMPFC), parietal cortex, and striatum, calculated as described previously using an automated atlas segmentation propagation and label fusion strategy called Geodesic Information Flow or GIF, 18 and expressed as a percentage of total intracranial volume, computed with SPM12 (Statistical Parametric Mapping, Welcome Trust Centre for Neuroimaging, London, UK) running under Matlab R20014b (MathWorks, USA). 19
3. RESULTS
Demographic and neuropsychometric data can be found in Table 1. No significant differences were found between groups in age or sex. Mini Mental State Examination (MMSE) scores were lower and CDR® Dementia Staging Instrument with the National Alzheimer Coordinating Centre Frontotemporal Lobar Degeneration component (CDR® plus NACC FTLD sum of boxes were higher in the bvFTD group (both P < 0.001). For the neuropsychometric battery, the bvFTD group performed significantly worse on all tests compared to controls (Table 1). Oculomotor function measures are summarized in Table 2:
TABLE 2.
Fixation, pursuit, pro‐saccade, and anti‐saccade metrics for the control and bvFTD groups
| Controls | bvFTD | |||||||
|---|---|---|---|---|---|---|---|---|
| Test | Analysis | Direction | Condition | Mean | SD | Mean | SD | P value |
| Fixation | Small square wave jerks (n) | – | – | 0.75 | 1.65 | 3.06 | 4.16 | 0.028 |
| Large square wave jerks (n) | – | – | 0.00 | 0.00 | 0.44 | 0.97 | 0.129 | |
| Large intrusive saccades (n) | – | – | 0.10 | 0.30 | 1.47 | 3.11 | 0.055 | |
| Longest period of fixation (ms) | – | – | 6140.8 | 2527.0 | 3322.6 | 2135.8 | 0.001 | |
| Smooth pursuit | Pursuit gain | Horizontal | – | 0.81 | 0.15 | 0.71 | 0.17 | 0.063 |
| Vertical | – | 0.70 | 0.19 | 0.61 | 0.17 | 0.167 | ||
| Pro‐saccades | Amplitude error (degrees) | Horizontal | Gap | −4.70 | 0.45 | −4.87 | 0.72 | 0.440 |
| Overlap | −4.59 | 0.45 | −4.77 | 0.57 | 0.297 | |||
| Vertical | Gap | −2.61 | 0.38 | −2.69 | 0.40 | 0.529 | ||
| Overlap | −2.65 | 0.38 | −3.07 | 0.52 | 0.008 | |||
| Saccade latency (ms) | Horizontal | Gap | 201.56 | 49.19 | 249.55 | 196.19 | 0.181 | |
| Overlap | 258.67 | 89.26 | 380.72 | 307.69 | 0.123 | |||
| Vertical | Gap | 255.63 | 59.50 | 312.81 | 334.69 | 0.483 | ||
| Overlap | 287.09 | 53.29 | 331.33 | 183.18 | 0.235 | |||
| Peak velocity (degrees/s) | Horizontal | Gap | 199.44 | 29.44 | 207.02 | 40.71 | 0.187 | |
| Overlap | 176.19 | 23.14 | 180.74 | 30.25 | 0.086 | |||
| Vertical | Gap | 150.79 | 32.35 | 165.49 | 34.10 | 0.125 | ||
| Overlap | 144.00 | 26.51 | 137.62 | 36.35 | 0.047 | |||
| Anti‐saccades | Correct anti‐saccades (n) | Horizontal | Gap | 1.27 | 0.94 | 0.37 | 0.60 | <0.001 |
| Vertical | Gap | 0.73 | 0.88 | 0.21 | 0.42 | 0.016 | ||
| Self‐corrected anti‐saccades (n) | Horizontal | Gap | 2.00 | 0.89 | 2.21 | 0.97 | 0.517 | |
| Vertical | Gap | 2.05 | 0.92 | 2.75 | 1.04 | 0.111 | ||
Significant results are shown in bold. Abbreviations: ms, milliseconds; n, number; S, seconds.
3.1. Fixation
The mean eye position for each group is shown in Figure 1B, with individual performance on each of the metrics displayed in Figure 1C. Differences were observed between the bvFTD and control groups on the number of square wave jerks with significantly higher numbers of small square wave jerks (P = 0.028) in the bvFTD group, although no differences in number of large square wave jerks (Table 2). There was also a trend toward a higher number of large intrusive saccades (P = 0.055), as well as a significantly shorter longest period of fixation (P = 0.001) in the bvFTD group (Table 2).
3.2. Smooth pursuit
There were no significant differences observed between the two groups in the ability to pursue a target in either the horizontal or vertical direction. However, there was a trend toward the bvFTD group being less accurate than controls in the horizontal condition (P = 0.063) (Table 2; Figure 1D).
3.3. Pro‐saccades
Few differences were observed between groups on the pro‐saccade tasks (Table 2; Figure 1E). However, on the most challenging pro‐saccade task, the vertical overlap condition, the bvFTD group had a greater amplitude error and a slower peak velocity relative to the controls (amplitude error: P = 0.008; peak velocity, P = 0.047).
3.4. Anti‐saccades
The bvFTD group were significantly impaired at performing correct anti‐saccades relative to controls on both the horizontal (P < 0.001) and vertical (P = 0.016) conditions (Table 2; Figure 1F). No differences were observed between the two groups on the self‐corrected anti‐saccades measure.
3.5. Correlational analysis
Worse performance on the anti‐saccade task correlated with executive dysfunction as measured in the neuropsychometric battery, although more strongly for the horizontal than the vertical condition (Table 3). There was no correlation with language comprehension in either condition (Table 3).
TABLE 3.
Correlations of executive function tasks with the correct number of anti‐saccades in individual with bvFTD
| Correct anti‐saccades | ||
|---|---|---|
| Horizontal | Vertical | |
| WMS‐R Digit Span Backwards | 0.54 | 0.45 |
| <0.001 | 0.003 | |
| Phonemic Fluency | 0.44 | 0.27 |
| 0.005 | 0.094 | |
| D‐KEFS Color‐Word Interference Test | −0.45 | −0.37 |
| 0.006 | 0.024 | |
| Trail Making Test Part A | −0.44 | −0.35 |
| 0.005 | 0.025 | |
| Trail Making Test Part B | −0.41 | −0.27 |
| 0.016 | 0.113 | |
| British Picture Vocabulary Scale | 0.28 | 0.16 |
| 0.084 | 0.318 | |
Abbreviation: WMS‐R, Wechsler Memory Scale Revised; D‐KEFS, Delis Kaplan Executive System.
Only performance on the longest period of fixation (r = ‐0.47, P = 0.003), and the number of correct anti‐saccades made (r = ‐0.52, P < 0.001) in the horizontal condition correlated with disease severity (as measured by CDR® plus NACC FTLD sum of boxes).
Table 4 displays the correlations between the oculomotor function tasks and the regions of interest. For the fixation task, small square wave jerks negatively correlated with the orbitofrontal cortex (r = ‐0.39, P = 0.015), the ventromedial prefrontal cortex (VMPFC: r = ‐0.48, P = 0.002), and the striatal volume (r = ‐0.39, P = 0.014). The longest period of fixation positively correlated with the orbitofrontal cortex volume as well (r = 0.42, P = 0.009). The VMPFC volume (r = 0.36, P = 0.026) was also found to correlate with performance on the vertical gain condition of the smooth pursuit task. There were no significant correlations found with any of the pro‐saccade tasks. However, the number of correct anti‐saccades made on both the horizontal and vertical conditions, correlated positively with the volume of the DLPFC (Horizontal: r = 0.35, P = 0.026; Vertical: r = 0.38, P = 0.015), and parietal cortex (Horizontal: r = 0.34, P = 0.029; Vertical: r = 0.36, P = 0.021). The striatal volume also positively correlated with the horizontal condition (r = 0.47, P = 0.002). Negative correlations were found between the number of self‐corrected anti‐saccades (horizontal) and the parietal cortex volume (r = ‐0.43, P = 0.011).
TABLE 4.
Correlations between oculomotor function tasks and neuroanatomical regions of interest within the bvFTD group
| Fixation | Pursuit | Pro‐saccade: Amplitude Error | Pro‐saccade: Saccade latency | Pro‐saccade: Peak Velocity | Anti‐saccade: Correct | Anti‐saccade: Self‐Corrected | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small SQWJ | Large SQWJ | Large intrusive saccades | Longest period fixation | Horizontal | Vertical | Horizontal Gap | Horizontal Overlap | Vertical Gap | Vertical Overlap | Horizontal Gap | Horizontal Overlap | Vertical Gap | Vertical Overlap | Horizontal Gap | Horizontal Overlap | Vertical Gap | Vertical Overlap | Horizontal Gap | Vertical Gap | Horizontal Gap | Vertical Gap | |
| Orbitofrontal cortex | −0.39 | −0.23 | −0.29 | 0.42 | 0.08 | 0.29 | 0.13 | 0.14 | −0.07 | 0.18 | −0.10 | −0.03 | −0.13 | −0.06 | 0.08 | 0.12 | 0.10 | 0.24 | −0.06 | −0.25 | 0.28 | 0.22 |
| 0.015 | 0.154 | 0.075 | 0.009 | 0.640 | 0.076 | 0.418 | 0.399 | 0.679 | 0.258 | 0.534 | 0.852 | 0.448 | 0.693 | 0.623 | 0.443 | 0.538 | 0.129 | 0.717 | 0.120 | 0.102 | 0.247 | |
| Dorsolateral prefrontal cortex | 0.03 | 0.09 | 0.05 | 0.00 | 0.08 | −0.08 | −0.03 | −0.04 | 0.04 | −0.08 | 0.15 | −0.02 | 0.12 | 0.28 | −0.03 | −0.23 | −0.19 | −0.23 | 0.35 | 0.38 | −0.18 | −0.28 |
| 0.864 | 0.598 | 0.758 | 0.999 | 0.643 | 0.617 | 0.850 | 0.790 | 0.810 | 0.633 | 0.360 | 0.885 | 0.483 | 0.085 | 0.857 | 0.161 | 0.251 | 0.145 | 0.026 | 0.015 | 0.288 | 0.144 | |
| Ventromedial prefrontal cortex | −0.48 | −0.22 | −0.30 | 0.30 | 0.09 | 0.36 | 0.09 | 0.14 | −0.02 | 0.14 | −0.15 | 0.02 | −0.10 | −0.16 | 0.09 | 0.23 | 0.20 | 0.22 | −0.10 | −0.18 | 0.31 | 0.31 |
| 0.002 | 0.178 | 0.068 | 0.061 | 0.584 | 0.026 | 0.600 | 0.375 | 0.902 | 0.406 | 0.373 | 0.919 | 0.544 | 0.310 | 0.606 | 0.157 | 0.218 | 0.166 | 0.521 | 0.251 | 0.074 | 0.100 | |
| Parietal cortex | −0.12 | 0.14 | 0.14 | −0.05 | −0.16 | 0.24 | −0.12 | −0.02 | −0.08 | 0.08 | 0.02 | 0.06 | −0.02 | 0.14 | −0.13 | −0.16 | −0.10 | −0.04 | 0.34 | 0.36 | −0.43 | −0.37 |
| 0.468 | 0.385 | 0.403 | 0.759 | 0.311 | 0.139 | 0.450 | 0.906 | 0.613 | 0.65 | 0.904 | 0.735 | 0.901 | 0.376 | 0.419 | 0.310 | 0.554 | 0.792 | 0.029 | 0.021 | 0.011 | 0.051 | |
| Striatum | −0.39 | 0.14 | −0.02 | 0.22 | 0.20 | 0.08 | −0.03 | 0.06 | −0.05 | −0.05 | 0.07 | −0.02 | 0.09 | 0.22 | −0.08 | −0.14 | −0.10 | −0.17 | 0.47 | 0.17 | −0.14 | −0.17 |
| 0.014 | 0.384 | 0.902 | 0.180 | 0.209 | 0.625 | 0.871 | 0.697 | 0.758 | 0.747 | 0.657 | 0.925 | 0.583 | 0.179 | 0.624 | 0.406 | 0.540 | 0.304 | 0.002 | 0.283 | 0.436 | 0.369 | |
The r value is presented at the top, followed by the P‐value below.
4. DISCUSSION
We show that people with bvFTD have oculomotor dysfunction with specific impairments of fixation stability, pro‐saccades and, in particular, anti‐saccades. On the fixation tasks, people with bvFTD have more small square wave jerks and a shorter longest period of fixation. No significant differences were observed on the pursuit test but there was a trend to impairment in the bvFTD group on the horizontal condition. On pro‐saccade tasks, there was a greater amplitude error and slower peak velocity in the vertical overlap condition. Finally, performance on the anti‐saccade task demonstrated significant impairment in the bvFTD group and correlated with executive dysfunction.
In order to see continuously, our visual system consistently makes microsaccades to prevent foveal fixation on a particular point. 20 Small square wave jerks are a malfunction of this process in which the microsaccades are exaggerated. This is because saccadic intrusions occur that take the eye away from the target and then back again towards it in a corrective manner. 21 Although small square wave jerks do occur in the healthy population, they are more common in brainstem and cerebellar disorders, such as progressive supranuclear palsy (PSP) 22 , 23 , 24 as well as cortical disorders such as Alzheimer's disease (AD). 14 , 25 Our findings suggest an association between orbitofrontal cortex and VMPFC atrophy and the number of square wave jerks produced, which is consistent with previous findings that demonstrate a link between the thickness of the frontal lobe in people with AD and the number of small square wave jerks. 14 These results also suggest the involvement of the striatum, which is also supported by findings in PSP and Parkinson disease. 26
People with bvFTD fixated on the target for a shorter period than the control group, indicating that individuals with bvFTD are struggling to maintain fixation for very long. It is possible that this is due to a problem with saccadic inhibition, especially given the correlation with the orbitofrontal cortex. 27 , 28 This is further supported by a trend toward an increased number of large intrusive saccades in the bvFTD group relative to controls, and its trend in correlation with the orbitofrontal cortex.
Throughout the remaining oculomotor tests, participants were presented with two conditions, horizontal versus vertical, and both bvFTD and controls had worse performance on the vertical than the horizontal condition. It is possible that this is because much of the visual information we see on a daily basis, is processed in the horizontal plane, for example, when reading, it is much easier to read when it is displayed horizontally than it is vertically for individuals in the western world. 29 , 30 As a result, we have much less need, and therefore less practice, at moving our eyes in the vertical plane, and this is reflected in the results shown here across the tests.
Overall, participants with bvFTD did not show any difficulties pursuing a moving target when compared to controls, although there was a trend toward a deficit in the horizontal condition. This is interesting given that previous literature indicates a significant deficit. 11 , 12 These previous studies used a ramp‐step pursuit test, which is likely to be a much harder task than the one used in this study because the individual is required to make an initial saccade to identify the location of the target, and then track the movement, 31 perhaps accounting for this discrepancy.
On the whole, performance on the pro‐saccade task remained relatively intact. Differences emerged only on the vertical overlap condition for the amplitude error and peak velocity, in which the patients were less accurate and had slower saccades. In addition to differences being seen on the vertical condition, the overlap condition represents a greater challenge because it requires an attentional shift away from the current target, which nevertheless remains temporarily on the screen (together with the new target). Thus this task more deeply probes executive function abilities that are impaired in bvFTD.
The results found in this study are in line with previous literature, which found that individuals with bvFTD did not display a delay in being able to generate saccades. 12 One study, however, found an impairment in saccade latency in the vertical condition that we did not find here, 11 whereas others suggest that there is an impairment in both the horizontal and vertical conditions. 9 , 10 Again, in line with our findings, some studies found no deficits in peak velocity or the amplitude error of the bvFTD individuals 9 , 12 ; however, others did. 10 , 11 It is possible that the different results seen in the literature are due to differences in the equipment used (only three of the six studies used the same eye‐tracking equipment) or differences in the design and set up of the trials.
As part of the bvFTD diagnostic criteria, individuals commonly present with difficulties with executive control. Our findings are in line with this, as fewer anti‐saccades were made in the bvFTD group across all conditions when compared with the number made by the control group. The anti‐saccade test is an extremely difficult test for both the bvFTD and control group, as it requires individuals to go against their instincts to look at new stimuli. 32 Our findings are in line with prior studies of anti‐saccades in individuals with bvFTD, 9 , 12 , 13 and reflected in the psychometric correlations, as well as in the region of interest (ROI) analysis where there was correlation with the DLPFC, the key area associated with executive function. 33 Executive function tests have also been known to correlate with the parietal lobes, 34 even in FTD, 35 , 36 and this is also found in these results.
Despite using reliable and accurate equipment and software, there were a limited number of trials in each condition. This was to ensure that the combination of these measures did not take too long to administer. Furthermore, although the sample size was sufficient for a cross‐sectional study in bvFTD, larger sample sizes with increased stratification and more longitudinal follow‐up would provide further informative data. Another limitation to this work is the use of the Eyelink eye tracker. Although it is highly accurate and reliable, it comes at a high cost. Caution should be applied when using different types of eye trackers for the same task as it could heavily impact performance. If this approach is to be taken and multiple different eye trackers are to be used, a validation of the task across the different equipment should be carried out.
This work gives a comprehensive overview of oculomotor functioning in bvFTD with deficits of fixation, pro‐saccades, and anti‐saccades. This latter task provides a particularly simple way of measuring executive function, and given selective abnormalities in patients with bvFTD compared to controls, it is possible that this type of test along with the fixation tasks could be used as an outcome measure in upcoming clinical trials for FTD. Further work in pre‐symptomatic cohorts and longitudinal follow‐up will be helpful.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
We thank the research participants for their contribution to the study. The Dementia Research Centre is supported by Alzheimer's Research UK, Alzheimer's Society, Brain Research UK, and The Wolfson Foundation. This work was supported by the NIHR UCL/H Biomedical Research Centre, the Leonard Wolfson Experimental Neurology Centre (LWENC) Clinical Research Facility, and the UK Dementia Research Institute, which receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society, and Alzheimer's Research UK. JDR is supported by an MRC Clinician Scientist Fellowship (MR/M008525/1) and has received funding from the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH). This work was also supported by the MRC UK GENFI grant (MR/M023664/1), the Bluefield Project, and the JPND GENFI‐PROX grant (2019‐02248). MB is supported by a Fellowship award from the Alzheimer's Society, UK (AS‐JF‐19a‐004‐517). MB's work is also supported by the UK Dementia Research Institute, which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society, and Alzheimer's Research UK. RC/CG are supported by a Frontotemporal Dementia Research Studentships in Memory of David Blechner funded through The National Brain Appeal (RCN 290173). JDW is supported by the Alzheimer's Society, Alzheimer's Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. The GIF template database includes volumetric MRI scans from the University College London Genetic FTD Initiative (GENFI) study (www.genfi.org.uk), which is funded by the Medical Research Council UK GENFI grant (MR/M023664/1).
Russell LL, Greaves CV, Convery RS, et al. Eye movements in frontotemporal dementia: Abnormalities of fixation, saccades and anti‐saccades. Alzheimer's Dement. 2021;7:e12218. 10.1002/trc2.12218
Diego Kaski and Jonathan D. Rohrer are joint senior authors.
REFERENCES
- 1. Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013;9(2): 4–85. [DOI] [PubMed] [Google Scholar]
- 2. Antoniades C, Kennard C. Ocular motor abnormalities in neurodegenerative disorders. Eye. 2015;29(2):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bueno A, Sato J, Hornberger M. Eye tracking–The overlooked method to measure cognition in neurodegeneration?. Neuropsychologia. 2019;133:107191. [DOI] [PubMed] [Google Scholar]
- 4. Pierrot‐Deseilligny C, Milea D, Müri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. [DOI] [PubMed] [Google Scholar]
- 5. Klarendic M, Kaski D. Deep brain stimulation and eye movements. Eur J Neurosci. 2021;53(7):2344–2361. [DOI] [PubMed] [Google Scholar]
- 6. Gordon E, Rohrer JD, Fox NC. Advances in neuroimaging in frontotemporal dementia. J Neurochem. 2016; 138: 193–210. [DOI] [PubMed] [Google Scholar]
- 7. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyniel C, Rivaud‐Pechoux S, Damier P, Gaymard B. Saccade impairments in patients with fronto‐temporal dementia. J Neurol Neurosurg Psychiatry. 2005;76(11):1581–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burrell JR, Hornberger M, Carpenter RH, Kiernan MC, Hodges JR. Saccadic abnormalities in frontotemporal dementia. Neurology. 2012;78(23):1816–1823. [DOI] [PubMed] [Google Scholar]
- 10. Douglass A, Walterfang M, Velakoulis D, Abel L. Behavioral variant frontotemporal dementia performance on a range of saccadic tasks. J Alzheimers Dis. 2018;65(1):231–242. [DOI] [PubMed] [Google Scholar]
- 11. Garbutt S, Matlin A, Hellmuth J, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131(5):1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boxer AL, Garbutt S, Rankin KP, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boxer AL, Garbutt S, Seeley WW, et al. Saccade abnormalities in autopsy‐confirmed frontotemporal lobar degeneration and Alzheimer disease. Arch Neurol. 2012;69(4):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shakespeare TJ, Kaski D, Yong KX, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015;138(7):1976–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crossland MD, Rubin Gary S. The use of an infrared eyetracker to measure fixation stability. Optom Vis Sci. 2002;79(11):735–739. [DOI] [PubMed] [Google Scholar]
- 16. Leigh R, Zee D. The neurology of eye movements fourth edition. Contemp Neurol Ser. 2006;70(1): –. [Google Scholar]
- 17. Bylsma FW, Rasmusson DX, Rebok GW, Keyl PM, Tune L, Brandt J. Changes in visual fixation and saccadic eye movements in Alzheimer's disease. Int J Psychophysiol. 1995;19(1):33–40. [DOI] [PubMed] [Google Scholar]
- 18. Cardoso MJ, Modat M, Wolz R, et al. Geodesic information flows: spatially‐variant graphs and their application to segmentation and fusion. IEEE Trans Med Imaging. 2015;34(9):1976–1988. [DOI] [PubMed] [Google Scholar]
- 19. Malone IB, Leung KK, Clegg S, et al. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez‐Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006;49(2):297–305. [DOI] [PubMed] [Google Scholar]
- 21. Otero‐Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez‐Conde S. Saccades during attempted fixation in Parkinsonian disorders and recessive ataxia: from microsaccades to square‐wave jerks. PLoS One. 2013;8(3):e58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phokaewvarangkul O, Bhidayasiri R. How to spot ocular abnormalities in progressive supranuclear palsy? A practical review. Transl Neurodegener. 2019;8(1): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinnock RA, McGivern RC, Forbes R, Gibson JM. An exploration of ocular fixation in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy. J Neurol. 2010;257(4):533–539. [DOI] [PubMed] [Google Scholar]
- 24. Rascol O, Sabatini U, Simonetta‐Moreau M, Montastruc J, Rascol A, Clanet M. Square wave jerks in Parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 1991;54(7):599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamagoe K, Yamada S, Kawakami R, Koganezawa T, Tamaoka A. Abnormal saccadic intrusions with Alzheimer's disease in darkness. Curr Alzheimer Res. 2019;16(4):293–301. [DOI] [PubMed] [Google Scholar]
- 26. Shaikh AG, Xu‐Wilson M, Grill S, Zee DS. ‘Staircase'square‐wave jerks in early Parkinson's disease. Br J Ophthalmol. 2011;95(5):705–709. [DOI] [PubMed] [Google Scholar]
- 27. Peters F, Perani D, Herholz K, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21(5‐6):373–379. [DOI] [PubMed] [Google Scholar]
- 28. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(9):2502–2512. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt D, Ullrich D, Rossner R. Horizontal and vertical reading: a comparative investigation of eye movements. Ger J Ophthalmol. 1993;2(4‐5):251–255. [PubMed] [Google Scholar]
- 30. Yu D, Park H, Gerold D, Legge GE. Comparing reading speed for horizontal and vertical English text. J Vis. 2010;10(2):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159(2):326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munoz DP, Everling S. Look away: the anti‐saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218. [DOI] [PubMed] [Google Scholar]
- 33. Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17(3):213–233. [DOI] [PubMed] [Google Scholar]
- 34. Lynch J, Mountcastle V, Talbot W, Yin T. Parietal lobe mechanisms for directed visual attention. J Neurophysiol. 1977;40(2):362–389. [DOI] [PubMed] [Google Scholar]
- 35. Rohrer JD, Warren JD, Omar R, et al. Parietal lobe deficits in frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch Neurol. 2008;65(4):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roca M, Manes F, Gleichgerrcht E, et al. Intelligence and executive functions in frontotemporal dementia. Neuropsychologia. 2013;51(4):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]


