Abstract
Background
Characteristics of children with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Taiwanese households is nascent. We sought to characterize SARS-CoV-2 infection, and estimate the relative risk of infection among children within households during school closures in Taipei and New Taipei City.
Methods
We reviewed consecutive children below 18 years presenting to our emergency department from May 18, 2021 to July 12, 2021 who underwent real-time reverse-transcription polymerase chain reaction (rRT-PCR) for SARS-CoV-2 from respiratory swabs. Demographics, symptoms, and contacts were captured from medical records. Household contact was defined as an individual with confirmed COVID-19 living in the same residence as the child.
Results
Among 56 children with SARS-CoV-2, twenty-five (45%) were male with mean age of 7.9 years. Symptoms were nonspecific, with 29% having fever, 32% having cough, and 48% were asymptomatic. The median cycle threshold (Ct) value of SARS-CoV-2 rRT-PCR was 25 (range 11–38). All 56 children reported 94 contacts with a COVID-19 patient, of which 99% were household contacts. The relative risk of infection was 8.5 (95% CI 5.0–14.7) for children whose parent(s) were COVID-19 patients, and 7.3 (95% CI 4.9–11.0) for children whose household grandparent(s) were patients, as compared to children without respective contacts. Children without COVID-19 contacts were all tested negative.
Conclusions
During school closures in Taipei and New Taipei City, children with SARS-CoV-2 infection in our cohort had one or more COVID-19 contacts, mostly within their households. While diagnosing pediatric COVID-19 is challenging as children were often asymptomatic, those without contacts were likely uninfected.
Keywords: SARS-CoV-2, COVID-19, Household, Children, School closure, Taiwan
Introduction
School closure is one of the most effective nonpharmaceutical intervention employed by governments to curb the community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 While reports have indicated children are less likely than adults to be infected,2 , 3 and infected children is often asymptomatic or results in only mild disease,4 household secondary infection rate of up to 42% has been reported in children living with a primary patient with COVID-19.5 As school closures and stay-at-home orders continue due to SARS-CoV-2 variants, the burden of disease will eventually shift to unvaccinated children who acquire the virus from household contacts.6
Following a surge of locally transmitted cases in Taiwan, the Central Epidemic Command Center (CECC) raised the epidemic alert level to three out of a four-tier system on May 15, 2021. Education institutions in Taipei and New Taipei City were closed from May 18, 2021 until the end of summer holidays. Entertainment and sports venues nationwide were closed while workers were encouraged to work from home during the level three alert initially until July 12, 2021. By early July, the 7-day moving average of new COVID-19 cases dropped to below 50 from a peak of 500 in late May, prompting the CECC to ease some restrictions such as allowing museums and libraries to reopen, although the level three alert was to remain until July 26, 2021. As interaction outside the home decreased during the period, we hypothesized that households will become a major setting of SARS-CoV-2 transmission for children during school closures, and sought to estimate the relative risk of secondary infection among children whose family members were infected.
Materials and methods
Study subjects
This retrospective study was undertaken in Taipei Tzu Chi Hospital located in New Taipei City, one of the epicenters of COVID-19 epidemic in Taiwan. As of July 12, 2021, cumulative cases in New Taipei City was 6659 or 43.6% of all cases nationwide, while total inpatient COVID-19 cases for Taipei Tzu Chi Hospital were 631 from May 16 to July 12, 2021. Children aged 18 years or younger seen at the emergency department of Taipei Tzu Chi Hospital from May 18 to July 12, 2021 were enrolled. Children received SARS-CoV-2 real-time reverse-transcription polymerase chain reaction (rRT-PCR) because they were symptomatic, were close contacts with an individual with a confirmed infection, or were returning international travelers. Diagnosis of COVID-19 was based on a detection of SARS-CoV-2 by rRT-PCR in samples obtained from the nasopharynx or oropharynx. Children were excluded if they did not receive rRT-PCR for SARS-CoV-2 at the emergency department. Demographic, clinical, radiologic data, household contacts, and travel histories of children who received rRT-PCR for SARS-CoV-2 were collected and analyzed.
Contact tracing, quarantine of close contacts, and epidemiological investigation were conducted by the Taiwan Centers for Disease Control as described previously.7 Briefly, besides a 14-day home quarantine since last exposure, household contacts of a patient with COVID-19 would be referred to hospitals to undergo rRT-PCR for SARS-CoV-2 regardless of symptoms when first identified, as well as when they develop any new symptoms.
Data collection
Date of visit, age, gender, residence, symptoms, travel histories, attendance of daycare or educational institutions, contacts with another individual with COVID-19, relationship of contacts, disposition at the emergency department, laboratory findings, and clinical course were collected from medical records. Radiological evaluation was performed by an experienced radiologist with expertise in pediatric chest imaging using Picture Archiving and Communication Systems (PACS). Parameters used in evaluation of chest X-ray were: (1) whether it was normal or pathological, (2) affected lung was unilateral or bilateral, (3) lesions were scattered or consolidated. This study was approved by the Institutional Review Board of Taipei Tzu Chi Hospital (IRB No. 10-X-100).
Definitions
Children were determined to be asymptomatic if medical records did not document any clinical signs and symptoms, or the patient was documented to be asymptomatic. Household contact was defined as an individual with confirmed COVID-19 who was living in the same residence as the child. This may include the child's extended family members as long as medical records documented them to be living together. When no documentation of residence was found, the child's parents were assumed to be living together, while other relations to be living separately.
Symptom onset dates of children with household or non-household COVID-19 contacts were documented to construct a case distribution timeline in relation to school closure. For asymptomatic patients, onset was defined as the date of specimen collection for the first positive rRT-PCR.
Statistical analyses
All analyses were performed with commercially available statistical software (SPSS v25, IBM Corp., Armonk, NY, USA). Descriptive statistics were performed and reported by percentages for qualitative data, and by median with ranges or mean with standard deviation for quantitative data, where appropriate. Continuous variables were analyzed with t-test. Categorical variables were analyzed with the Fisher's exact and Mann–Whitney U test. Relative risk of infection among contacts was calculated by standard statistical methods. All tests were two-tailed. Statistical significance was defined as p < 0.05.
Results
Demographics
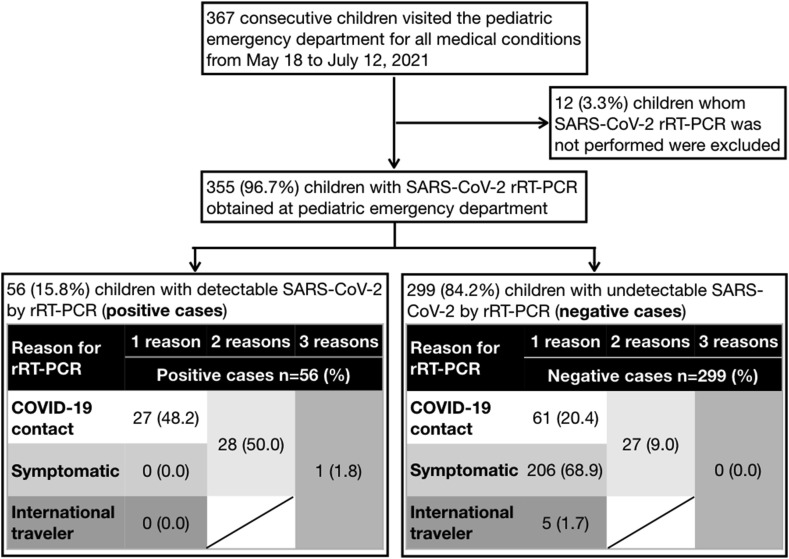
From May 18 to July 12, 2021, a total of 367 children visited the pediatric emergency department of our hospital, which was open for all medical conditions. All consecutive emergency records were reviewed by study investigators, who excluded 12 (3.3% out of 367) children whom SARS-CoV-2 rRT-PCR were not performed. Of 355 children with respiratory swabs obtained, 56 (15.8%) children had SARS-CoV-2 detected by rRT-PCR (positive cases), while 299 (84.2%) children had no detectable SARS-CoV-2 (negative cases). Children without COVID-19 contacts were all tested negative (Fig. 1 ).
Figure 1.
A diagram showing the study flow. Abbreviations: SARS-CoV-2 denotes severe acute respiratory syndrome coronavirus-2; rRT-PCR denotes real-time reverse-transcription polymerase chain reaction. There were no children who were both symptomatic and international travelers.
The demographics of 355 children were presented on Table 1 . The proportion of positive cases with residence unrecorded was higher than negative cases. There were no differences in gender, age, residence, chest film obtained, or disposition between positive and negative cases.
Table 1.
Demographics of children with positive and negative real-time reverse-transcription polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 from respiratory swabs obtained at our emergency department from May 18 to July 12, 2021.
| Positive cases, n = 56 (%) | Negative cases, n = 299 (%) | p-value | |
|---|---|---|---|
| Gender | |||
| Male | 25 (44.6) | 155 (51.8) | 0.383 |
| Age, years, mean ± SD | 7.9 ± 5.1 | 6.9 ± 5.6 | 0.203 |
| Residence | 0.438a | ||
| New Taipei City | 41 (73.2) | 257 (86.0) | |
| Xindian District | 9 (16.1) | 126 (42.1) | |
| Zhonghe District | 12 (21.4) | 69 (23.1) | |
| Yonghe District | 5 (8.9) | 32 (10.7) | |
| Sanchong District | 7 (12.5) | 3 (1.0) | |
| Other districts | 8 (14.3) | 27 (9.0) | |
| Taipei City | 5 (8.9) | 22 (7.4) | |
| Wenshan District | 5 (8.9) | 16 (5.4) | |
| Other districts | 0 (0.0) | 6 (2.0) | |
| Taoyuan City | 1 (1.8) | 3 (1.0) | |
| Not recorded | 9 (16.1) | 17 (5.7) | 0.011 |
| Chest film obtained | 39 (69.6) | 175 (58.5) | 0.137 |
| Disposition | 0.175 | ||
| Discharged home | 38 (67.9) | 226 (75.6) | |
| Admitted to ward | 18 (32.1) | 69 (23.1) | |
| Admitted to ICU | 0 (0.0) | 4 (1.3) | |
Abbreviations: ICU denotes intensive care unit; SD denotes standard deviation.
Between New Taipei City, Taipei City, and Taoyuan City.
Symptoms and contacts of positive and negative cases
Major symptoms, travel, and school attendance among positive and negative cases were presented on Table 2 . Compared with negative cases, a significantly higher proportion of positive cases reported changes in taste and smell, reported no symptoms, and reported contact with another COVID-19 patient. There were no differences between positive and negative cases who reported overseas travel, attendance of daycare or educational institution 14 days prior to PCR, nor non-household contacts with COVID-19. Among different types of household contacts, a higher proportion of positive cases reported one or both parents, and one or more grandparents being COVID-19 patients, compared to negative cases. The relative risk of infection was 8.5 (95% CI 5.0–14.7) for children whose parent(s) were diagnosed with COVID-19, and 7.3 (95% CI 4.9–11.0) for children whose household grandparent(s) were diagnosed with COVID-19, as compared to children without respective COVID-19 household contacts (Table 3 ).
Table 2.
Characteristics of children with positive and negative real-time reverse-transcription polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 from respiratory swabs obtained at our emergency department from May 18 to July 12, 2021.
| Positive cases, n = 56 (%) | Negative cases, n = 299 (%) | p-value | |
|---|---|---|---|
| Symptomsa | |||
| Fever | 16 (28.6) | 177 (59.2) | <0.001 |
| Cough | 18 (32.1) | 71 (23.7) | 0.183 |
| Rhinorrhea | 4 (7.1) | 40 (13.3) | 0.269 |
| Sore throat | 1 (1.8) | 26 (8.7) | 0.100 |
| Change in taste and smell | 3 (5.4) | 1 (0.3) | 0.013 |
| Abdominal pain | 1 (1.8) | 32 (10.7) | 0.041 |
| Vomiting | 2 (3.6) | 25 (8.4) | 0.280 |
| Diarrhea | 4 (7.1) | 55 (18.4) | 0.049 |
| Other symptomsb | 0 (0.0) | 4 (1.3) | |
| Asymptomatic | 27 (48.2) | 66 (22.1) | <0.001 |
| Overseas travel history 14 days prior to rRT-PCR | 1 (1.8) | 5 (1.7) | 1.000 |
| Attending daycare or educational institution 14 days prior to rRT-PCR | 6 (10.7) | 41 (13.7) | 0.670 |
| Contact with another COVID-19 patient | 56 (100.0) | 88 (29.4) | <0.001 |
| Amount of contacts, median (range) | 1.0 (1.0–4.0) | 0.0 (0.0–4.0) | <0.001 |
| COVID-19 household contactsa | |||
| One or both parents | 41 (73.2) | 45 (15.1) | <0.001 |
| One or more grandparents | 27 (48.2) | 13 (4.3) | <0.001 |
| Uncle and/or aunt | 6 (10.7) | 5 (1.7) | 0.052 |
| Sibling | 0 (0.0) | 5 (1.7) | 1.000 |
| Domestic helper | 1 (1.8) | 0 (0.0) | 1.000 |
| COVID-19 non-household contactc | 1 (1.8) | 27 (9.0) | 0.100 |
Abbreviations: rRT-PCR denotes real-time reverse-transcription polymerase chain reaction.
Some patients report more than 1 symptom or had more than 1 household contact, and therefore results sum up to more than 100%.
Include 4 patients, 1 each for chest pain, choking, feeling lethargic, and poor appetite, all in the cohort of negative cases.
Include 1 grandfather (not living together) in the cohort with positive SARS-CoV-2 rRT-PCR; 7 grandparents (not living together) 5 classmates, 5 teachers, 5 parent's colleagues, 3 uncles and/or aunts (not living together), 1 cousin, and 1 colleague in the cohort with negative SARS-CoV-2 rRT-PCR.
Table 3.
Relative risks of testing positive for severe acute respiratory syndrome coronavirus-2 by real-time reverse-transcription polymerase chain reaction when different household contacts were COVID-19 patients, as compared to not having respective contacts with COVID-19.
| COVID-19 contacts | Positive cases |
Negative cases |
Relative risk (95% CI) |
|---|---|---|---|
| No. of children/total no. | |||
| One or both parents were COVID-19 patients | 41/56 | 45/299 | 8.5 (5.0–14.7) |
| One or more household grandparents were COVID-19 patients | 27/56 | 13/299 | 7.3 (4.9–11.0) |
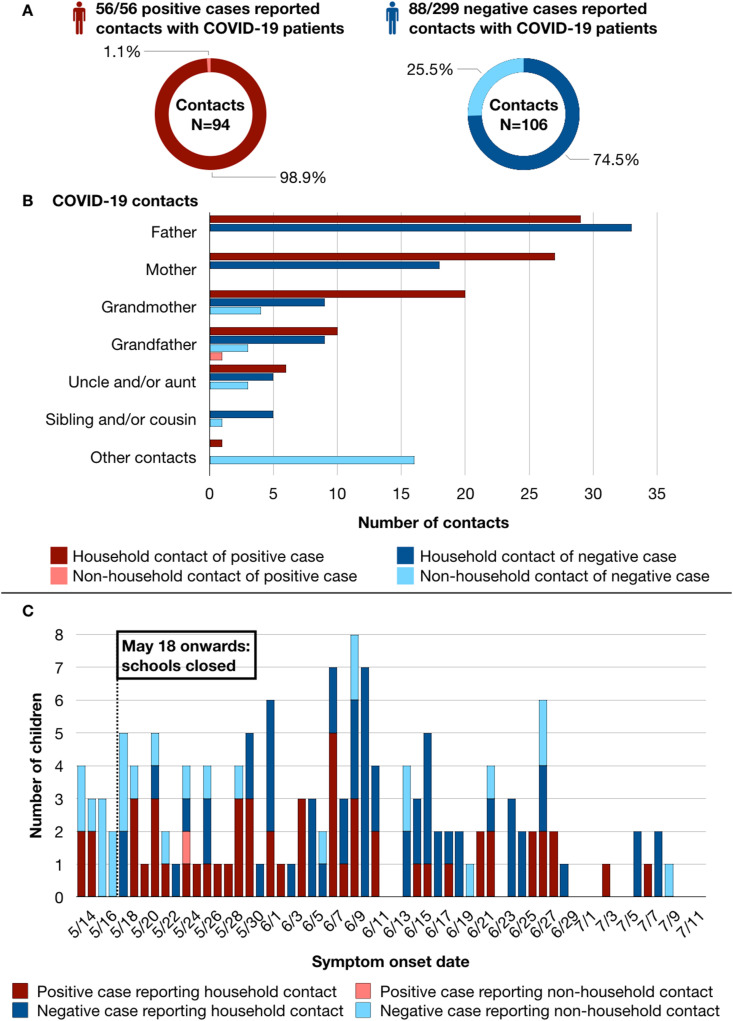
As children may be living with more than 1 individual with COVID-19, there were 144 children who reported a total of 200 contacts: all 56 positive cases reported 93 household and 1 non-household contacts, while 88 negative cases reported 79 household and 27 non-household contacts (Fig. 2 A).
Figure 2.
Interpersonal and temporal relationships of children with household and non-household COVID-19 contacts. (A) The proportion of contacts among positive and negative cases were presented as donut charts. All 56 positive cases reported 94 contacts: 93 household (dark red) and 1 non-household (light red) contacts; while 88 out of 299 negative cases reported 106 contacts: 79 household (dark blue) and 27 non-household (light blue) contacts. (B) A breakdown of all 200 COVID-19 contacts by interpersonal relationship to positive and negative cases presented as a horizontal bar chart with a similar household/non-household legend to (A). As children may report more than 1 COVID-19 contact, the amount of contacts was greater than the amount of children. (C) The distribution of positive and negative cases (red or blue in color) who had household or non-household contacts (dark or light in shade) presented as a timeline by symptom onset date. Even though schools were closed in Taipei and New Taipei City on May 18, 2021, the earliest symptom onset was on May 14. Three-quarters of positive cases started to have symptoms 1 week after school closure (May 25, 2021 onwards). For asymptomatic positive cases, onset date was the date of specimen collection.
Interpersonal and temporal relationships of children with COVID-19 contacts
A breakdown of 200 COVID-19 contacts was shown in Fig. 2B. A child's father (n = 62), mother (n = 45), and grandmother (n = 33) ranked among the 3 most frequently reported contacts. Only 1 positive case reported a non-household contact: a grandfather living separately with the child; while another positive case reported simultaneous contacts with the father, and live-in domestic helper (classified under other contacts).
Symptom onset for 56 positive cases and 88 negative cases, further differentiated by household or non-household contact was shown in Fig. 2C. More than half (57.1%, or 16) of 28 children with non-household contacts had symptom onset 4 days before and 1 week after school closure. Out of these 16 children, only 1 tested positive for SARS-CoV-2 by rRT-PCR. In contrary, most children with household COVID-19 contacts started to have symptoms 1 week after school closure, with 84.5% (98/116) of them reporting symptoms from May 25 to July 12, 2021. Similarly, the majority of positive cases (42 out of 56 children, 75.0%) had symptom onset 1 week after school closure.
Laboratory findings of positive cases
The median cycle threshold (Ct) value of SARS-CoV-2 rRT-PCR obtained at the emergency department for all positive cases was 25 (range 11–38). Of 11 children with hematological data, the median white cell, neutrophil, and lymphocyte counts were 5460 per μL (range 2370–10,540), 2289 per μL (range 841–8580), and 2139 per μL (range 928–4277) respectively. Only 1 child had mild elevation of C-reactive protein at 1.45 mg/dL, out of 11 whose blood was drawn.
Radiographic findings and clinical course of positive cases
Of 39 positive cases who obtained a chest film, there were 11 (28.2%) cases with bilateral ground glass opacities, 10 (25.6%) cases with bilateral scattered consolidations, and 18 (46.2%) with no pathology observed. There were 18 cases who were admitted for a median of 9 days (range 1–12 days). Only 1 adolescent required supplemental oxygen while none required intensive care. There were no deaths among positive cases.
Discussion
In this retrospective study of consecutive children who visited the emergency department in a hospital located at one of the epicenters of COVID-19 epidemic in Taiwan, we found SARS-CoV-2 infection among children to be predominantly household contacts during school closures. During this period of reduced mobility, almost all pediatric patients with COVID-19 reported household contacts, with a relative risk of infection of 8.5 when one or both parents were diagnosed, and 7.3 when one or more household grandparents were diagnosed. These findings highlighted the importance of obtaining accurate household contact and cluster histories to differentiate children at high risk of infection visiting the emergency department during school closures.
Similar to our observation, most children with SARS-CoV-2 in previous studies were identified through contact tracing of family clusters.2 , 5 , 8 In our study, more than half of all children with household COVID-19 contacts (93 out of 172, 54.1%) tested positive; nearly all children with non-household contacts (27 out of 28, 96.4%) tested negative; and all children without a COVID-19 contact tested negative. During school closures, children were predominantly infected by their household contacts, and were usually not the index case in a household.2 While implementation of social distancing interventions significantly reduced risk of transmission from social and community contacts, risk of transmission in households increased during the lockdown period, especially so among children.9 The level three epidemic alert in Taiwan was not essentially a lockdown, but most parents stayed home to care for their offspring as daycare and schools were closed, potentially creating spread within the household.10 Households are favorable environments for transmission.11 Like other urban dwellings, most residences in Taipei and New Taipei City are confined spaces where family members may crowd and be in close contact. Personal protective equipment such as masks was also less likely to be worn at home. Among family members, children of a primary patient suffered the highest secondary infection rate compared to spouses or partners, possibly due to intimate and sustained childcare needs.5 Data on secondary attack rates varied, potentially confounded by underreporting due to minimal symptoms among children and ascertainment bias.12 , 13 Regardless, as we continue to struggle with limited vaccine supplies and ongoing lockdowns due to novel variants, the burden of COVID-19 will inevitably shift to younger children and adolescents who are unvaccinated, as was seen in Israel.6
Our study adds to the wealth of literature confirming a high proportion of children infected with SARS-CoV-2 being asymptomatic, and a vast majority having a favorable prognosis.4 , 14 Nevertheless, diagnosing pediatric COVID-19 remains a huge challenge as children may report nonspecific, subtle, transient, or even no symptoms at all.4 Given the high transmissibility of presymptomatic patients,7 prolonged viral shedding in stools15 and respiratory secretions of children,16 timely identification of infected children would be mainly epidemiological in order to break the chain of transmission.
The CECC's decision to close schools likely mitigated in-school transmissions, decreased total COVID-19 cases, and mortalities in Taiwan.17 In our study conducted during school closure, only 5% of all contacts were related to school attendance, and none were positive. While a review,18 studies from the UK19 and Hong Kong20 found an overall low risk of SARS-CoV-2 outbreak among staff and students in educational settings, numerous reports had shown the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members, some even leading to the hospitalization of adults who were secondary cases.21, 22, 23 Despite lower susceptibility for SARS-CoV-2 among children, data on the contagiousness of infected children remains limited and controversial.2 , 3 , 24 While not a direct comparison, a study conducted at a public kindergarten in Taipei City estimated the attack rate of seasonal influenza to be 27% among classmates.25 The attack rate could potentially be higher for SARS-CoV-2 as young children often have difficulty adhering to hand hygiene and mask wearing,21 and unlike high yearly uptake of influenza vaccines,26 there is currently no SARS-CoV-2 vaccines available for children below 12 years old. Further, Taiwanese schoolchildren typically spend long hours at school with class sizes of up to 35 classmates,25 while most households consist of both working parents who often require additional help such as their extended family or babysitter to assist in childcare. Taken together, these factors may potentially create superspreading events of SARS-CoV-2 given the frequent contacts with multiple individuals,27 especially at a time when national vaccination rates were low.28
Although school closures are effective in virus mitigation, education and health officials must balance pandemic response with academic, health, and economic consequences.29 Analyses estimated that school disruptions due to war and teacher strikes were associated with projected annual income losses of 2–3% over the course of students’ lifetimes.30 , 31 Other consequences of school closures include loss of income and productivity, gaps in childcare, deteriorating child-parental psychological health, and missed developmental opportunities.29 , 32 , 33
This study has several limitations. First, being retrospective in nature meant that symptoms and contacts may have been underestimated due to missing or inconsistent reporting, or patients being tested whilst presymptomatic or contact tracing has yet to conclude. As the Taiwan Centers for Disease Control may refer patients to different hospitals for rRT-PCR, a proportion of negative cases may had been tested prior to threshold of viral detection. Hence, positive cases may be underreported, and negative cases may be overrepresented. Second, we were unable to ascertain complete household members and index patients in most households as such information were not routinely recorded in emergency medical records. Therefore, we could not estimate household transmission including secondary attack rate and serial interval of SARS-CoV-2 infection. Our analyses reflected that children are almost always not an index case during school closure, and the common family dynamics in Taiwan, where a child may receive frequent care from multiple extended family members even if not living together. Third, our study was conducted during school closure and a period of heightened alert, and hence may not reflect infections and transmissions when social activity normalizes. Finally, the amount of positive cases who received blood sampling was small and hematological data should be interpreted with caution.
In conclusion, diagnosing pediatric COVID-19 by symptoms alone is challenging as children were often asymptomatic or pauci-symptomatic. During school closures in Taipei and New Taipei City, children with SARS-CoV-2 infection in our cohort had one or more COVID-19 contacts, mostly within their households. They suffer an increased risk of infection when their parents or household grandparents were COVID-19 patients, while those without COVID-19 contacts were likely uninfected.
Declaration of competing interests
All authors declare no conflict of interests.
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan [MOST 108-2314-B-303 -019 -MY2(CHY)], and Taipei Tzu Chi Hospital, Taiwan [TCRD - TPE -108-RT-2; TCRD-TPE-110-19 (CHY)].
Acknowledgments
The authors would like to thank all patients for participation, and all hospital staff for their unwavering dedication and determination during a period of hardship.
References
- 1.Brauner J.M., Mindermann S., Sharma M., Johnston D., Salvatier J., Gavenciak T., et al. Inferring the effectiveness of government interventions against COVID-19. Science. 2021;371 doi: 10.1126/science.abd9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spielberger B.D., Goerne T., Geweniger A., Henneke P., Elling R. Intra-household and close-contact SARS-CoV-2 transmission among children - a systematic review. Front Pediatr. 2021;9:613292. doi: 10.3389/fped.2021.613292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y., Bloxham C.J., Hulme K.D., Sinclair J.E., Tong Z.W.M., Steele L.E., et al. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis. 2021;72:e1146–e1153. doi: 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang T.H., Wu J.L., Chang L.Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. 2020;119:982–989. doi: 10.1016/j.jfma.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis N.M., Chu V.T., Ye D., Conners E.E., Gharpure R., Laws R.L., et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1166. Epub 2020 Aug 16. [DOI] [Google Scholar]
- 6.Will S.M. COVID become a disease of the young? Nature. 2021;595:343–344. doi: 10.1038/d41586-021-01862-7. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H., et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Q., Chen Y.C., Chen C.L., Chiu C.H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K., Wang W., Gao L., Wang Y., Luo K., Ren L., et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science. 2021;371 doi: 10.1126/science.abe2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park Y.J., Choe Y.J., Park O., Park S.Y., Kim Y.M., Kim J., et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta N.S., Mytton O.T., Mullins E.W.S., Fowler T.A., Falconer C.L., Murphy O.B., et al. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza T.H., Nadal J.A., Nogueira R.J.N., Pereira R.M., Brandao M.B. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020;55:1892–1899. doi: 10.1002/ppul.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D., et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiehao C., Jin X., Daojiong L., Zhi Y., Lei X., Zhenghai Q., et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auger K.A., Shah S.S., Richardson T., Hartley D., Hall M., Warniment A., et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324:859–870. doi: 10.1001/jama.2020.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein E., Lipsitch M., Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223:362–369. doi: 10.1093/infdis/jiaa691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail S.A., Saliba V., Lopez Bernal J., Ramsay M.E., Ladhani S.N. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344–353. doi: 10.1016/S1473-3099(20)30882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua G.T., Wong J.S.C., Lam I., Ho P.P.K., Chan W.H., Yau F.Y.S., et al. Clinical characteristics and transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez A.S.H.M., Antezano J., Vilven D., Rutner T., Bogdanow L., Claflin C., et al. Transmission dynamics of COVID-19 outbreaks associated with child care facilities - Salt lake city, Utah, April-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1319–1323. doi: 10.15585/mmwr.mm6937e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu V.T.Y.A., Chang K., Schwartz N.G., McDaniel C.J., Lee S.H., Szablewski C.M., et al. Georgia camp investigation team. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med. 2021 doi: 10.1056/NEJMc2031915. Epub 2021 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okarska-Napierała M.M.J., Kuchar E. SARS-CoV-2 cluster in nursery, Poland. Emerg Infect Dis. 2021;27:317–319. doi: 10.3201/eid2701.203849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C.Y., Huang L.M., Fan T.Y., Cheng A.L., Chang L.Y. Incidence of respiratory viral infections and associated factors among children attending a public kindergarten in Taipei City. J Formos Med Assoc. 2018;117:132–140. doi: 10.1016/j.jfma.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Meyer D., Shearer M.P., Chih Y.C., Hsu Y.C., Lin Y.C., Nuzzo J.B. Taiwan's annual seasonal influenza mass vaccination program-lessons for pandemic planning. Am J Publ Health. 2018;108:S188–S193. doi: 10.2105/AJPH.2018.304527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P.Y., Wu T.S., Cheng C.W., Chen C.J., Huang C.G., Tsao K.C., et al. A hospital cluster of COVID-19 associated with a SARS-CoV-2 superspreading event. J Microbiol Immunol Infect. 2021 doi: 10.1016/j.jmii.2021.07.006. Epub 2021 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie H., Ortiz-Ospina E., Beltekian D., Mathieu E., Hasell J., Macdonald B., et al. Statistics and research coronavirus pandemic (COVID-19) https://ourworldindata.org/coronavirus Published online at OurWorldInData.org. Available at:
- 29.Donohue J.M., Miller E. COVID-19 and school closures. JAMA. 2020;324:845–847. doi: 10.1001/jama.2020.13092. [DOI] [PubMed] [Google Scholar]
- 30.Ichino A.W.-E.R. The long-run educational cost of World War II. J Labor Econ. 2004;22:57–86. [Google Scholar]
- 31.Jaume D., Willén A. The long-run effects of teacher strikes: evidence from Argentina. J Labor Econ. 2019;37:1097–1139. [Google Scholar]
- 32.Patrick S.W.H.L., Zickafoose J.S., Lovell K., Halvorson A., Loch S., Letterie M., et al. Well-being of parents and children during the COVID-19 pandemic: a national Survey. Pediatrics. 2020;146 doi: 10.1542/peds.2020-016824. [DOI] [PubMed] [Google Scholar]
- 33.Kamidani S., Rostad C.A., Anderson E.J. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. 2021;33:144–151. doi: 10.1097/MOP.0000000000000978. [DOI] [PubMed] [Google Scholar]