Abstract
Evidence suggests severe coronavirus disease-19 (COVID-19) infection is characterised by pulmonary and systemic microvasculature dysfunction, specifically, acute endothelial injury, hypercoagulation and increased capillary permeability. Diabetes, which is also characterised by vascular injury in itself, confers an increased risk of adverse COVID-19 outcomes. It has been suggested that pre-existing endothelial dysfunction and microvascular disease in diabetes will exacerbate the vascular insults associated with COVID-19 and thus lead to increased severity of COVID-19 infection. In this article, we evaluate the current evidence exploring the impact of microvascular complications, in the form of diabetic retinopathy and nephropathy, in individuals with COVID-19 and diabetes. Future insights gained from exploring the microvascular injury patterns and clinical outcomes may come to influence care delivery algorithms for either of these conditions.
Keywords: COVID-19, Diabetes, Microvascular disease, Endothelial dysfunction, Retinopathy, Albuminuria, Diabetic nephropathy
1. Introduction
Coronavirus disease 2019 (COVID-19), initially identified in Wuhan, China, is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is a single-stranded RNA virus and is believed to be of zoonotic origin (Asselah et al., 2021). Since COVID-19 was declared by the World Health Organization as a global pandemic in March 2020 (WHO, 2020), the total number of reported cases has surpassed 102 million and with a death toll in excess of 2 million individuals (WHO, 2021). Individuals with COVID-19 may present in a wide-spectrum of severity, requiring individualised management. The majority exhibit mild COVID-19 infection and present with fever, cough and dyspnoea (Huang et al., 2020a). However, approximately 5% of people develop severe forms of the disease requiring hospitalisation (Beltrán-García et al., 2020). The respiratory tract is predominantly affected, and respiratory failure is the leading cause of death in COVID-19 (Torres Acosta and Singer, 2020). However, extrapulmonary organ involvement has also been observed which may relate to the extensive distribution of angiotensin-converting enzyme 2 receptor, the receptor responsible for SARS-CoV-2 host cell invasion across body systems (Shang et al., 2020).
In its severe form, COVID-19 is associated with an excessive host inflammatory response, endothelial injury, increased pulmonary capillary permeability and venous thrombosis formation. These acute micro- and macro-circulatory insults likely contribute to the development of adverse complications, such as acute respiratory distress syndrome (ARDS) (Huertas et al., 2020; Whyte et al., 2020).
2. Vascular Injury in COVID-19
Pulmonary microvascular dysfunction is associated with severe COVID-19 infection (Teuwen et al., 2020). Pulmonary interstitial oedema, a result of increased capillary permeability, has been observed in COVID-19 patient autopsies (Carsana et al., 2020). Pulmonary oedema is a component of ARDS, a life-threatening condition occurring in approximately one third of hospitalised COVID-19 patients (Endeman et al., 2020; Tzotzos et al., 2020). Additionally, hypercoagulability is a consistent characteristic of patients hospitalised with COVID-19. Pulmonary emboli have been observed in approximately 15% of COVID-19 intensive care unit (ICU) patients (Nopp et al., 2020) and COVID-19 autopsies have consistently revealed pulmonary microthrombosis with microangiopathy (Ackermann et al., 2020; Lax et al., 2020).
Endothelial inflammation and apoptosis have been identified in the lungs of COVID-19 patient autopsies and likely contribute to the microcirculatory impairments observed in severe disease (Varga et al., 2020). This acute endothelial injury may be the result of direct viral endothelial cell entry or the result of indirect host inflammatory cytokines. An excessive host inflammatory response, known as a ‘cytokine storm’, is a typical presentation of patients with severe COVID-19 (Siddiqi and Mehra, 2020). Perivascular T-cell infiltration, in addition to high plasma concentrations of tissue necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) are observed in COVID-19 ICU patients (Huang et al., 2020b). These cytokines can disrupt endothelial cell tight junctions and dysregulate adhesive and pro-coagulative molecules (Pober and Sessa, 2007).
Landecho et al. (2021) investigated the impact on new incident retinal changes in patients discharged from hospital due to COVID-19 associated pneumonia. When assessed with fundoscopy, optical coherence tomography (OCT) and OCT angiography fourteen days following discharge, new cotton wool exudates were observed in 22% of patients, irrespective of whether they had concomitant diabetes or not. These results suggest COVID-19 may be associated with systemic microangiopathy with indolent progression in the immediate recovery period – whether this is directly related to the virus itself or secondary to the hypoxia and the interventions offered to correct this remains unclear.
One of the most commonly reported cutaneous feature of COVID-19 is the chilblain-alike ‘perniosis’ particularly notable in the feet – sometimes referred to has COVID-toes (Kanitakis et al., 2020; Vas et al., 2021). Histological examination of these have revealed dermal perivascular inflammation, endothelial cell swelling and immune deposition within the microvasculature. Post-mortem histopathological examination of the individuals with COVID-19 has observed thinning of the endothelial cell basal lamina, congested blood vessels and relatively intact vasculature even in those without antemortem neurological symptoms (Lee et al., 2021).
3. Diabetes and COVID-19 outcomes
Diabetes increases the risk of hospitalisation, admission to critical care and mortality in COVID-19. Studies indicate diabetes is present in approximately 8 to 33% of hospitalised COVID-19 patients (Vas et al., 2020). The concentrations of interleukin-6 (IL-6), C-reactive protein (CRP), and D-dimer were significantly greater in hospitalised COVID-19 patients with diabetes compared to patients without, implying those with diabetes are more vulnerable to developing a greater degree of systemic inflammatory response to infection (Guo et al., 2020). A meta-analysis of COVID-19 patients determined diabetes was associated with over two-fold increased risk of greater COVID-19 severity and mortality (Kumar et al., 2020). However, this study did not adjust for additional COVID-19 risk factors such as obesity and old age. Among those with diabetes certain individuals are at even greater COVID-19 risk than others - for example, Holman et al. (2020) observed independent associations between HbA1c and body mass index (BMI) with mortality in individuals with COVID-19 and diabetes.
The COVID-19 pandemic has interrupted the normal monitoring and treatment of diabetic complications. Patients with diabetic retinopathy have been subject to reduced clinics and treatment with anti-VEGF interventions (Ahmed and Liu, 2021). This may lead to long-term impacts on visual acuity. Reports of suboptimal outcomes and a higher risk of amputation during the pandemic have now started to emerge from many parts of the world. On study from Italy, reported a threefold higher amputation rate in 2020 compared to the year 2019 (Caruso et al., 2020) and another from China also reported significant disruption of routine foot care provision (Liu et al., 2020). One study reported that there was a precipitous decrease in hospitalisation rates for diabetic foot ulcers during the first French lockdown and fewer revascularisations were undertaken (Mariet et al., 2021). Monitoring for diabetic kidney disease has also been reported to decline during the pandemic due to redirection of scare healthcare resources (Gregg et al., 2021). These studies highlight the disruptive impact of the COVID-19 pandemic at a population level and temporal data to assess the impact of microvascular (and by extension, future macrovascular) outcomes is awaited.
3.1. Microvascular complications of diabetes
The endothelium is the innermost lining of blood vessels and acts as a paracrine organ, via the secretion of factors, to control vascular homeostasis while simultaneously influencing vascular tone, platelet aggregation local responses to inflammation and angiogenesis (Madonna and De Caterina, 2011). Endothelial dysfunction is a common complication in diabetes. Endothelial dysfunction is associated with altered vasomotor tone, promotion of a procoagulant state, increased vessel permeability and abnormal expression of inflammatory cytokines (Stehouwer et al., 1997).
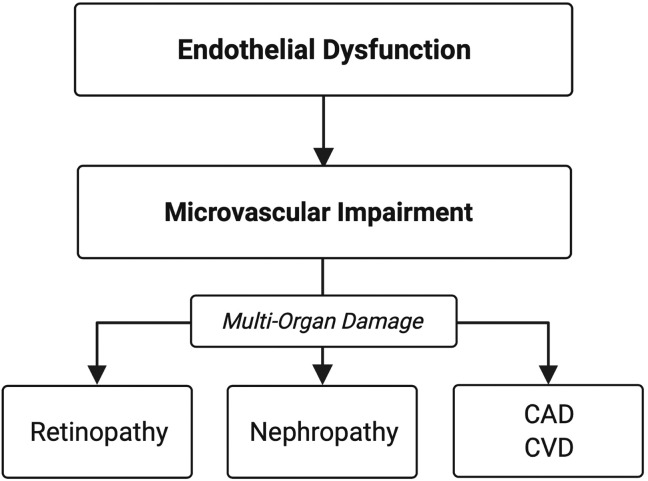
Microvascular complications observed in one end-organ is suggestive of systemic vascular damage (Ikram et al., 2008; Nowroozpoor et al., 2021). This is likely due to a common aetiology of endothelial dysfunction. Endothelial dysfunction is closely associated with the initiation and perpetuation of chronic macrovascular and microvascular disease (Avogaro et al., 2011; Farrah et al., 2020) (Fig. 1 ).
Fig. 1.
- Chronic diabetic complications are a manifestation of endothelial and microvascular dysfunction Abbreviations: CAD = coronary artery disease, CVD = cerebrovascular disease.
Hyperglycaemia contributes to the development of endothelial dysfunction. Endothelial dysfunction disrupts vascular homeostasis and is closely associated with the development of microvascular and macrovascular disease; including diabetic nephropathy (persistent albuminuria and decreased glomerular filtration rate), retinopathy and cardiovascular and cerebrovascular disease. Therefore, microvascular damage in one end organ is indicative of systemic microvascular damage.
3.2. Diabetic retinopathy
Diabetic retinopathy is a common complication of diabetes. In 2015, the prevalence of diabetic retinopathy was 56% in patients with type 1 diabetes and 30% in patients with type 2 (Thomas et al., 2015). In the SOULD-D study, a similar incidence of diabetic retinopathy and nephropathy was observed after two years in a cohort of patients with newly diagnosed type 2 diabetes (16% and 18% respectively) (Ismail et al., 2017). Diabetic retinopathy is an independent risk for the development of diabetic nephropathy and end-stage renal disease (Gall et al., 1997; Looker et al., 2003; Zhang et al., 2018). Therefore, it is unsurprising diabetic retinopathy frequently coexists with extra-retinal microvascular complications (Gross et al., 2005). Furthermore, diabetic retinopathy, in type 1 and 2 diabetes is associated with increased risk of cardiovascular disease and mortality (Targher et al., 2006), suggesting that retinopathy is one manifestation of generalised microvascular damage and endothelial dysfunction (Fig. 1).
3.3. Diabetic nephropathy and albuminuria
Albuminuria (urinary albumin creatinine ratio ≥3 mg/mmol) is an early marker for diabetic nephropathy, a common complication of diabetes, as well as a marker for cardiovascular disease (Dinneen and Gerstein, 1997; Hillege et al., 2002). Microalbuminuria is present in approximately 40% of individuals with diabetes (Parving et al., 2006). The Steno hypothesis (Deckert et al., 1989) suggested albuminuria is a manifestation of systemic microvascular damage and evidence continues to support this (Goligorsky, 2017; Martens et al., 2016). Thus, albuminuria is a useful clinical marker of generalised endothelial dysfunction and microvascular damage in patients with diabetes (Jensen et al., 2000; Toto, 2004).
3.4. Diabetic neuropathy
Diabetic somatic neuropathy is associated with microvascular complications elsewhere as well as macrovascular complications such as cardiovascular and cerebrovascular events (Pop-Busui et al., 2017). Presence of diabetic autonomic neuropathy (DAN) can lead to direct microvascular and sudomotor dysfunction and may also affect the lungs. Autonomic neuropathy in patients with type 1 diabetes has been associated with dysfunctional bronchial neuro-adrenergic innervation (Antonelli Incalzi et al., 2002). Additionally, cardiac autonomic neuropathy is significantly associated with decreased diffusing capacity of carbon monoxide (DLCO) (Pitocco et al., 2008). The autonomic nervous system also provides negative feedback during an inflammatory response to decrease macrophage production of inflammatory cytokines (Pitocco et al., 2021).
4. Microvascular complications of diabetes as a risk factor for severe COVID-19
Several studies of COVID-19 patients with diabetes demonstrate increased risk of adverse outcomes in the presence of diabetic microvascular complications. The CORONADO study investigated the characteristics and outcomes of 1317 hospitalised COVID-19 patients with diabetes from 53 different hospitals in France (Cariou et al., 2020). The study identified underlying microvascular disease, defined as severe diabetic retinopathy and/or diabetic kidney disease and/or diabetic foot ulcer, was independently associated with the risk of mortality on day 7 of hospitalisation, when adjusting for age and gender (odds ratio (OR) 2.14, 95% confidence interval (CI) 1.16–3.94).
Only a few studies have investigated the effect of individual diabetic microvascular complications on COVID-19 outcomes. A single centre study of 189 hospitalised COVID-19 patients with diabetes (Corcillo et al., 2020), identified that the presence of diabetic retinopathy was associated with over five-fold increased odds of intubation (OR 5.81, 95% CI 1.37–24.66 [P < 0.001]), independent of other well-established COVID-19 risk factors. In the study, 80% of patients with retinopathy had background retinopathy. Apart from the study by Landecho et al. (2021), there are no published studies exploring the relationship between past COVID-19 positivity and changes to current diabetic retinopathy status independent of glycaemic status.
McGurnaghan et al. (2021) investigated the outcomes and associated risk factors of 319,349 individuals, in a large Scottish database, with COVID-19 and diabetes. There was a greater risk of death and/or requirement for critical care in COVID-19 individuals with referable retinopathy (OR 1.67, 95% CI 1.38–2.03 [P < 0.0001]). Additionally, these authors determined that the prevalence of albuminuria was significantly greater in COVID-19 individuals who died and/or required critical care-unit treatment. However, neither retinopathy nor albuminuria were significant after carrying out a stepwise logistic regression which included age, sex, type of diabetes and duration of diabetes at baseline. Therefore, retinopathy and albuminuria were not included in the final risk prediction model for fatal or critical care unit-treated COVID-19. McGurnaghan et al. (2021) also observed that the estimated glomerular filtration rate (eGFR) was significantly lower in patients with fatal or critical care unit-treated COVID-19 and eGFR remained significant in the final prediction risk model (OR 0.992, 95% CI 0·989–0·995 [P < 0.0001]). Thus, it is possible albuminuria was not selected in the final risk prediction model due to the close association between eGFR and albuminuria in diabetes. Furthermore, 43% of patients who died or required critical care unit-treated COVID-19 had missing albuminuria status.
In an unpublished study, we investigated the effects of albuminuria on adverse outcomes in a small cohort of 80 hospitalised COVID-19 patients with diabetes, at a single centre. The prevalence of albuminuria (defined as an albumin-to-creatinine ratio of ≥3 mg/mmol or a protein-to-creatinine ratio ≥15 mg/mmol) was greater in patients who died (n = 8, 100%) versus those who survived (n = 30, 42%) [P = 0.002].
In individuals with and without diabetes, chronic kidney disease (CKD) is an independent risk factor for mortality in COVID-19 (ERA-EDTA, 2021; Williamson et al., 2020). However, compared to non-diabetic individuals with CKD, individuals with diabetic nephropathy have a significantly greater probability of developing adverse outcomes in COVID-19 (Leon-Abarca et al., 2020). Holman et al. (2020) showed a negative linear relationship between eGFR and adverse COVID-19 outcomes in individuals with diabetes. Compared to eGFR≥90 mL/min/1.73 m2, an eGFR<15 mL/min/1.73 m2 was associated with an increased risk of mortality in COVID-19 patients in both type 1 diabetes mellitus (adjusted hazard ratio (aHR) 8.35, 95% CI 5.50–12.70) and type 2 diabetes mellitus (aHR 4.91, 95% CI 4.34–5.56) (Holman et al., 2020).
Development of severe COVID-19 infection with hypoxemia requiring non-invasive ventilation has been shown to be associated with the development of neuropathic symptoms and widespread sensory dysfunction in diabetes patients without previous evidence of clinical neuropathic abnormalities (Odriozola et al., 2021). Studies exploring the presence of diabetic neuropathy on COVID-19 outcomes are unavailable presently, partly due to the challenging nature of diagnostic certainty as well as the lack of routine characterisation of neuropathic deficits, apart from foot ulcer risk, in people with diabetes. As previously mentioned, the pro-inflammatory ‘cytokine storm’ is a hallmark of severe COVID-19. Pitocco et al. (2021) suggest diabetic autonomic neuropathy may lead to impaired negative feedback of the inflammatory response. This may, in part, contribute to the excessive inflammation in COVID-19 infection leading to more sever forms of the disease (Pitocco et al., 2021). Studies investigating the association between diabetic neuropathy and COVID-19 severity are required to confirm this hypothesis.
5. Proposed mechanism – ‘the clash of two injuries’
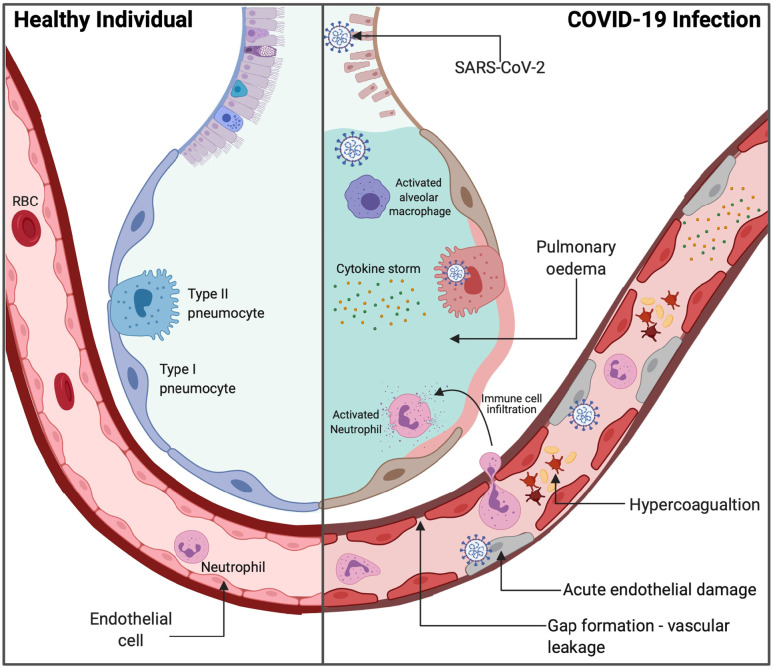
Pulmonary endothelial cells have a pivotal role in optimising gas exchange and regulating pulmonary vascular tone through the nitric oxide (NO), prostacyclin (PGI2), endothelin (ET) and serotonin (5-HT) pathways (Huertas et al., 2018). In COVID-19, acute endothelial injury, in addition to excessive host inflammation, likely contributes to microcirculatory impairments responsible for the formation of adverse complications in COVID-19 (Ackermann et al., 2020; Huertas et al., 2020) (Fig. 2 ). As well as COVID-19, diabetes is a well-established risk factor for additional viral respiratory illnesses. A study by Badawi and Ryoo, 2016 observed a high prevalence of diabetes in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) (54.4%) and influenza A H1N1 (14.6%). Pre-existing pulmonary vascular injury from diabetic microvascular disease may be responsible for the increased disease severity observed in patients with MERS-CoV and H1N1.
Fig. 2.
Healthy alveolar cross section versus the acute pulmonary and vascular injuries in COVID-19. Adapted from “Acute Respiratory Distress Syndrome (ARDS)” by BioRender.com (2020).
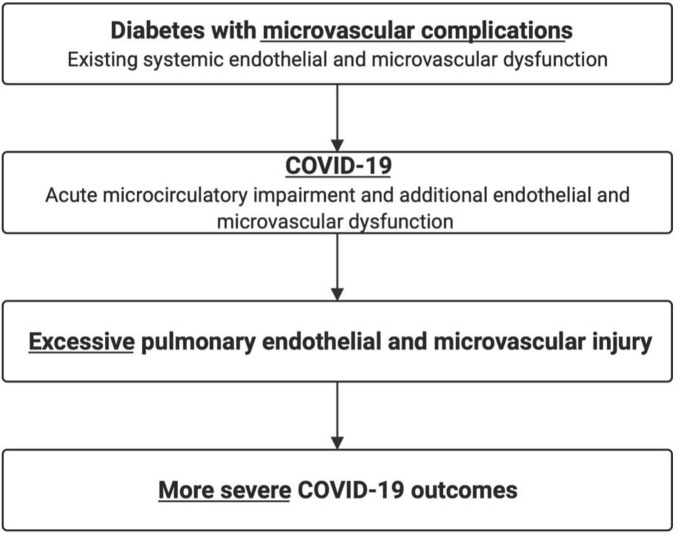
Evidence suggests the pulmonary vasculature is a site that manifests diabetic microvascular damage (Khateeb et al., 2019). Individuals with diabetes are observed to have impaired lung function, as indicated by a significant reduction in the diffusing capacity of carbon monoxide (DLCO). Additionally, individuals with diabetes are at increased risk of developing adverse respiratory conditions such as asthma, fibrosis and chronic obstructive pulmonary disease (COPD) (Ehrlich et al., 2010; Fontaine-Delaruelle et al., 2016). Pre-existing pulmonary microvascular dysfunction would mean less physiological reserve to facilitate pulmonary vascular shunting to maintain oxygenation with COVID-19, which is characterised by pulmonary capillary leakage and microthrombi formation (Fig. 3 ).
Fig. 3.
Diabetic microvascular disease increases the risk of severe COVID-19 infection.
In diabetes, microvascular complications in the form of diabetic retinopathy and diabetic nephropathy, may exacerbate the acute pulmonary endothelial and microvascular injuries associated with COVID-19. This may lead to increased alveolar infiltration, thrombosis and inflammation and thus increased risk of adverse COVID-19 outcomes.
6. Interplay between diabetes and obesity
Obesity, hypertension and advanced age are each independently associated with increased risk of adverse COVID-19 outcomes (Grasselli et al., 2020; Simonnet et al., 2020; Zhou et al., 2020). These conditions are additionally associated with systemic vascular endothelial dysfunction (Taddei et al., 2001; Ungvari et al., 2018; Virdis, 2016). Like in diabetes, underlying endothelial damage in obesity, hypertension and advanced age may predispose patients to excessive pulmonary vascular injury in COVID-19 complicated by obesity (Vas et al., 2020).
7. Conclusion
In individuals with diabetes, underlying microvascular disease is associated with higher risk of adverse outcomes in COVID-19. This may be due to an exacerbation of underlying pulmonary microcirculatory impairments. Further studies are required which directly investigate the pathophysiological mechanisms responsible for severe COVID-19 outcomes in patients with diabetes and pre-existing endothelial and microvascular dysfunction. Future insights gained from exploring the microvascular injury patterns and clinical outcomes are likely to play a significant part in influencing optimal care delivery algorithms for both of these conditions.
Funding
No external funding was received for the preparation of this manuscript.
CRediT authorship contribution statement
All authors contributed to the approach, literature search, review of supplements and editing the manuscript. RB and JK wrote the initial draft. All authors approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I., Liu T. The impact of COVID-19 on diabetic retinopathy monitoring and treatment. Curr. Diabetes Rep. 2021;21(10):40. doi: 10.1007/s11892-021-01411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli Incalzi R., Fuso L., Giordano A., Pitocco D., Maiolo C., Calcagni M.L., et al. Neuroadrenergic denervation of the lung in type I diabetes mellitus complicated by autonomic neuropathy. Chest. 2002;121(2):443–451. doi: 10.1378/chest.121.2.443. [DOI] [PubMed] [Google Scholar]
- Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R.F. COVID-19: discovery, diagnostics and drug development. J. Hepatol. 2021;74(1):168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avogaro A., Albiero M., Menegazzo L., de Kreutzenberg S., Fadini G.P. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(2):S285–S290. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A., Ryoo S.G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the Middle East respiratory syndrome coronavirus: a systematic review and meta-analysis. J. Public Health Res. 2016;5(3):733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., et al. Sepsis and coronavirus disease 2019: common features and anti-inflammatory therapeutic approaches. Crit. Care Med. 2020;48(12):1841–1844. doi: 10.1097/CCM.0000000000004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso P., Longo M., Signoriello S., Gicchino M., Maiorino M.I., Bellastella G., Chiodini P., Giugliano D., Esposito K. Diabetic foot problems during the COVID-19 pandemic in a tertiary care center: the emergency among the emergencies. Diabetes Care. 2020;43(10) doi: 10.2337/dc20-1347. e123-e12. [DOI] [PubMed] [Google Scholar]
- Corcillo A., Cohen S., Li A., Crane J., Kariyawasam D., Karalliedde J. Diabetic retinopathy is independently associated with increased risk of intubation: a single centre cohort study of patients with diabetes hospitalised with COVID-19. Diabetes Res. Clin. Pract. 2020;171 doi: 10.1016/j.diabres.2020.108529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert T., Feldt-Rasmussen B., Borch-Johnsen K., Jensen T., Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage.The Steno hypothesis. Diabetologia. 1989;32(4):219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- Dinneen S.F., Gerstein H.C. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch. Intern. Med. 1997;157(13):1413–1418. doi: 10.1001/archinte.1997.00440340025002. [DOI] [PubMed] [Google Scholar]
- Ehrlich S.F., Quesenberry C.P., Jr., Van Den Eeden S.K., Shan J., Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeman H., van der Zee P., van Genderen M.E., van den Akker J., Gommers D. Progressive respiratory failure in COVID-19: a hypothesis. Lancet Infect. Dis. 2020;20(12):1365. doi: 10.1016/S1473-3099(20)30366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERA-EDTA Council. ERACODA Working Group Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021;36(1):87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah T.E., Dhillon B., Keane P.A., Webb D.J., Dhaun N. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int. 2020;98(2):323–342. doi: 10.1016/j.kint.2020.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine-Delaruelle C., Viart-Ferber C., Luyton C., Couraud S. Fonction pulmonaire du patient diabétique [Lung function in patients with diabetes mellitus] Rev. Pneumol. Clin. 2016;72(1):10–16. doi: 10.1016/j.pneumo.2015.03.010. French. [DOI] [PubMed] [Google Scholar]
- Gall M.A., Hougaard P., Borch-Johnsen K., Parving H.H. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314(7083):783–788. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky M.S. Vascular endothelium in diabetes. Am. J. Physiol. Renal Physiol. 2017;312(2):F266–F275. doi: 10.1152/ajprenal.00473.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy,Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg E.W., Sophiea M.K., Weldegiorgis M. Diabetes and COVID-19: population impact 18 months into the pandemic. Diabetes Care. 2021;44(9):1916–1923. doi: 10.2337/dci21-0001. [DOI] [PubMed] [Google Scholar]
- Gross J.L., de Azevedo M.J., Silveiro S.P., Canani L.H., Caramori M.L., Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillege H.L., Fidler V., Diercks G.F., van Gilst W.H., de Zeeuw D., van Veldhuisen D.J., Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan,China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Wei F., Hu L., Wen L., Chen K. Epidemiology and clinical characteristics of COVID-19. Arch. Iran. Med. 2020;23(4):268–271. doi: 10.34172/aim.2020.09. [DOI] [PubMed] [Google Scholar]
- Huertas A., Guignabert C., Barberà J.A., Bärtsch P., Bhattacharya J., Bhattacharya S., et al. Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases: highlights from basic research to therapy. Eur. Respir. J. 2018;51(4):1700745. doi: 10.1183/13993003.00745-2017. [DOI] [PubMed] [Google Scholar]
- Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram M.A., Vernooij M.W., Hofman A., Niessen W.J., van der Lugt A., Breteler M.M. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39(1):55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- Ismail K., Moulton C.D., Winkley K., Pickup J.C., Thomas S.M., Sherwood R.A., Stahl D., Amiel S.A. The association of depressive symptoms and diabetes distress with glycaemic control and diabetes complications over 2 years in newly diagnosed type 2 diabetes: a prospective cohort study. Diabetologia. 2017;60(10):2092–2102. doi: 10.1007/s00125-017-4367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.S., Feldt-Rasmussen B., Strandgaard S., Schroll M., Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35(4):898–903. doi: 10.1161/01.hyp.35.4.898. [DOI] [PubMed] [Google Scholar]
- Kanitakis J., Lesort C., Danset M., Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic ("COVID toes"): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J. Am. Acad. Dermatol. 2020;83(3):870–875. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateeb J., Fuchs E., Khamaisi M. Diabetes and lung disease: a neglected relationship. Rev. Diabet. Stud. 2019;15:1–15. doi: 10.1900/RDS.2019.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19?A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landecho M.F., Yuste J.R., Gándara E., Sunsundegui P., Quiroga J., Alcaide A.B., et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J. Intern. Med. 2021;289(1):116–120. doi: 10.1111/joim.13156. [DOI] [PubMed] [Google Scholar]
- Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center,clinicopathologic case series. Ann. Intern. Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., et al. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Abarca J.A., Memon R.S., Rehan B., Iftikhar M., Chatterjee A. The impact of COVID-19 in diabetic kidney disease and chronic kidney disease: a population-based study. Acta Bio-med. Atenei Parmensis. 2020;91(4) doi: 10.23750/abm.v91i4.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., You J., Zhu W., Chen Y., Li S., Zhu Y., Ji S., Wang Y., Li H., Li L., Fan S. The COVID-19 outbreak negatively affects the delivery of care for patients with diabetic foot ulcers. Diabetes Care. 2020;43(10):e125–e126. doi: 10.2337/dc20-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker H.C., Krakoff J., Knowler W.C., Bennett P.H., Klein R., Hanson R.L. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima Indians. Diabetes Care. 2003;26(2):320–326. doi: 10.2337/diacare.26.2.320. [DOI] [PubMed] [Google Scholar]
- Madonna R., De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part II: cellular mechanisms and therapeutic targets. Vasc. Pharmacol. 2011;54(3–6):75–79. doi: 10.1016/j.vph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Mariet A.S., Benzenine E., Bouillet B., Vergès B., Quantin C., Petit J.M. Impact of the COVID-19 epidemic on hospitalization for diabetic foot ulcers during lockdown: a French nationwide population-based study. Diabet. Med. 2021;38(7) doi: 10.1111/dme.14577. [DOI] [PubMed] [Google Scholar]
- Martens R.J., Henry R.M., Houben A.J., van der Kallen C.J., Kroon A.A., Schalkwijk C.G., et al. Capillary rarefaction associates with albuminuria: the Maastricht study. J. Am. Soc. Nephrol. 2016;27(12):3748–3757. doi: 10.1681/ASN.2015111219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurnaghan S.J., Weir A., Bishop J., Kennedy S., Blackbourn L., McAllister D.A., Scottish Diabetes Research Network Epidemiology Group Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 2020;4(7):1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowroozpoor A., Gutterman D., Safdar B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys? - a scoping review. Microvasc. Res. 2021;134 doi: 10.1016/j.mvr.2020.104123. [DOI] [PubMed] [Google Scholar]
- Parving H.H., Lewis J.B., Ravid M., Remuzzi G., Hunsicker L.G., DEMAND investigators Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- Odriozola A., Ortega L., Martinez L., Odriozola S., Torrens A., Corroleu D., Martínez S., Ponce M., Meije Y., Presas M., Duarte A., Belén Odriozola M., Malik R.A. Widespread sensory neuropathy in diabetic patients hospitalized with severe COVID-19 infection. Diabetes Res Clin Pract. 2021;172 doi: 10.1016/j.diabres.2020.108631. 2021 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitocco D., Viti L., Santoliquido A., Tartaglione L., Di Leo M., Bianchi A., Caputo S., Pontecorvi A. Diabetic neuropathy: a risk factor for severe COVID-19? Acta Diabetol. 2021;58(5):669–670. doi: 10.1007/s00592-020-01658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitocco D., Santangeli P., Fuso L., Zaccardi F., Longobardi A., Infusino F., et al. Association between reduced pulmonary diffusing capacity and cardiac autonomic dysfunction in type 1 diabetes. Diabet. Med. 2008;25(11):1366–1369. doi: 10.1111/j.1464-5491.2008.02571.x. [DOI] [PubMed] [Google Scholar]
- Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R., Boulton A.J., Feldman E.L., Bril V., Freeman R., Malik R.A., Sosenko J.M., Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer C.D., Lambert J., Donker A.J., van Hinsbergh V.W. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc. Res. 1997;34(1):55–68. doi: 10.1016/s0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- Targher G., Bertolini L., Tessari R., Zenari L., Arcaro G. Retinopathy predicts future cardiovascular events among type 2 diabetic patients: the valpolicella heart diabetes study. Diabetes Care. 2006;29(5):1178. doi: 10.2337/diacare.2951178. [DOI] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.L., Dunstan F.D., Luzio S.D., Chowdhury S.R., North R.V., Hale S.L., et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br. J. Ophthalmol. 2015;99(1):64–68. doi: 10.1136/bjophthalmol-2013-304017. [DOI] [PubMed] [Google Scholar]
- Torres Acosta M.A., Singer B.D. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur. Respir. J. 2020;56(3) doi: 10.1183/13993003.02049-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto R.D. Microalbuminuria: definition, detection, and clinical significance. J. Clin. Hypertens. (Greenwich) 2004;6(11 Suppl 3):2–7. doi: 10.1111/j.1524-6175.2004.4064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S., Virdis A., Ghiadoni L., Sudano I., Salvetti A. Endothelial dysfunction in hypertension. J. Cardiovasc. Pharmacol. 2001;38(2):S11–S14. doi: 10.1097/00005344-200111002-00004. [DOI] [PubMed] [Google Scholar]
- Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit. Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Kiss T., Wren J.D., Giles C.B., Griffin C.T., et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15(9):555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas PRJ, Georgiadis GS, Papanas N. COVID-19 Toes and Other Skin Lesions During the Pandemic: Emerging Entities? Int J Low Extrem Wounds. 2021 Apr;23 doi: 10.1177/15347346211011843. 15347346211011844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas P., Hopkins D., Feher M., Rubino F., B Whyte M. Diabetes, obesity and COVID-19: a complex interplay. Diabetes Obes Metab. 2020;22(10):1892–1896. doi: 10.1111/dom.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A. Endothelial dysfunction in obesity: role of inflammation. High Blood Press Cardiovasc. Prev. 2016;23(2):83–85. doi: 10.1007/s40292-016-0133-8. [DOI] [PubMed] [Google Scholar]
- Whyte M.B., Vas P., Heiss C., Feher M.D. The contribution of diabetic micro-angiopathy to adverse outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020;164 doi: 10.1016/j.diabres.2020.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO Director-General's Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Accessed 8th February 2021]
- World Health Organization Weekly epidemiological update - 2 February 2021. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---2-february-2021 [Accessed 8th February 2021]
- Zhang J., Wang Y., Li L., Zhang R., Guo R., Li H., Han Q., Teng G., Liu F. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Ren. Fail. 2018;40(1):243–251. doi: 10.1080/0886022X.2018.1456453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]