Abstract
An ongoing global pandemic, the coronavirus disease 2019 is posing threat to people all over the world. The association between COVID-19 and the risk of ischemic stroke remains unclear. This study systematically reviewed published studies and conducted meta-analysis to evaluate the association between the risk of ischemic stroke and COVID-19. This study was conducted according to guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The protocol used in this study had been registered in the International Prospective Register of Systematic Reviews. EMBASE, PubMed, Cochrane Library and Web of Science were searched from 1st December 2019–19th February 2021. This systematic review and meta-analysis analysed the combined effect estimations based on odds ratios (OR) with the random-effects model. Four studies were screened from 31,634 participants including 171 COVID-19 positive patients with ischemic stroke were included. The mean age of COVID-19 positive patients with ischemic stroke was 69.45 years (Range: 63–77 years) and the male patients were 56%. Countries covered by these articles were USA, Italy and France. Three of the articles were retrospective cohort studies and one was prospective cohort study. Our analysis revealed that the risk of ischemic stroke (combined OR: 2.41; 95% CI: 1.08–5.38) was significantly increased. Four included studies were significantly heterogeneous (I2 = 75.2%, P = 0.007). Significant association between the risk of ischemic stroke and COVID-19 was observed in the North America group (combined OR: 2.90; 95% CI: 0.45–18.80, I2 = 89.60%, P = 0.002). This study found that the risk for ischemic stroke was increased in COVID-19 patients, especially in patients from North America. Further studies with larger sample sizes that include different ethnic populations are required to confirm our analysis.
Keywords: Ischemic stroke, COVID-19, Risk, Meta-analysis, Systematic review
1. Introduction
A novel corona virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China in December 2019 (Mao et al., 2020, Zhu et al., 2020). It is now well-known that SARS-CoV-2 can cause a series of acute respiratory syndromes including pneumonia and these syndromes are collectively termed corona virus disease 2019 (COVID-19) according to the World Health Organization. Although COVID-19 affects the respiratory system, it can also cause damage to other systems, such as the immune system and the nervous system (Siow et al., 2021). The COVID-19 has been associated with many neurological complications including strokes (Montalvan et al., 2020). Indeed, previous studies found that 78 of 214 COVID-19 patients from Wuhan, China experienced neurological complications (Mao et al., 2020, Wang et al., 2020). As the most serious form of neurological complications, strokes can be mainly categorized into hemorrhagic strokes and ischemic strokes (Nannoni et al., 2020). To the best of our knowledge, the association between the risk of ischemic stroke and COVID-19 remains inconclusive. While studies pointed out that COVID-19 was a risk factor for the ischemic stroke (Dhamoon et al., 2021, Perry et al., 2020), other studies suggested that COVID-19 posed an independent risk to the acute ischemic stroke (Bekelis et al., 2020, Belani et al., 2020, Katz et al., 2020, Nogueira et al., 2021). To resolve this contradiction, we systematically reviewed published studies and conducted meta-analysis to evaluate the association between the risk of ischemic stroke and COVID-19.
2. Methods
This study was conducted according to guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The protocol used in this study had been registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021244691).
2.1. Search strategy
Databases used in this study included the EMBASE, PubMed, Cochrane Library and Web of Science. MeSH terms and search keywords used were: (COVID-19) OR (COVID 19) OR (COVID-19 Virus Disease) OR (COVID 19 Virus Disease) OR (COVID-19 Virus Diseases) OR (Disease, COVID-19 Virus) AND ((Ischemic Stroke) OR (Ischemic Strokes) OR (Stroke, Ischemic) OR (Ischemic Stroke) OR (Ischemic Strokes) OR (Stroke, Ischemic) OR (Acute Ischemic Stroke) OR (Acute Ischemic Strokes) OR (Ischemic Stroke, Acute) OR (Stroke, Acute Ischemic)). Two authors Yanhua Cui (YHC) and Tianbai Li (TBL) screened all titles and abstracts independently. Any disagreements were discussed with a third evaluator Zhao-fei Yang (ZFY) or resolved through consensus.
2.2. Eligibility criteria
Identified articles were further selected based on the following criteria: observational cohort studies, studies reporting on a minimum of ten patients aged ≥ 18 years; the studies on COVID-19 patients with and ischemic stroke, studies published from 1st December 2019–19th February 2021.
2.3. Excluded criteria
We excluded all studies conducted on animals and reported only hemorrhagic stroke, cerebral venous thrombosis. Other exclusion criteria included publications on patient cohorts previously reported, non-English articles, full-texts unavailable, non-original research papers (systematic reviews, editorials commentaries, opinion papers, letters, protocols, reports, book chapters), non-clinical characteristics reported, as well as COVID-19 diagnosis but no reporting of ischemic stroke as a complication.
2.4. Data extraction
Two authors YHC and TBL independently extracted data from each study based on eligibility criteria and excluded criteria. Any disagreements between these two authors (YHC and TBL) were discussed with a third evaluator ZFY or by consensus. We extracted data about the name of authors, year, study design, country, continent, age, sex, sample size, the number of COVID-19 positive patients with or without ischemic stroke, the number of COVID-19 negative patients with or without ischemic stroke.
2.5. Assessment of quality
Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of all included observational cohort studies. All studies were evaluated based on their outcome, selection and comparability. Two authors YHC and TBL evaluated all included studies independently and discussed discrepancies by consensus.
2.6. Data analysis
To determine the correlation between ischemic stroke and COVID-19, odds ratios (OR) and confidence intervals (CI) were assessed. To assess the heterogeneity between studies, the Cochran’s Q test was performed. The heterogeneity was estimated by calculating the I² with levels of 0% (absent), 25% (low), 50% (moderate) and 75% (high). The random effect model was used when the severe heterogeneity had I² > 50%, otherwise the fixed effect model was applied. A P-value less than 0.05 was defined as statistic significant. Subgroup analyses were performed according to the type of continent, number of sample size, study design and NOS score. Moreover, sensitivity analysis was conducted by deleting each study individually and thus the quality and consistency of results were evaluated. Begg’s test was used to analysis the publication bias. In this study, statistical analyses were performed using the Stata software package (version 15.1).
3. Results
3.1. Study selection
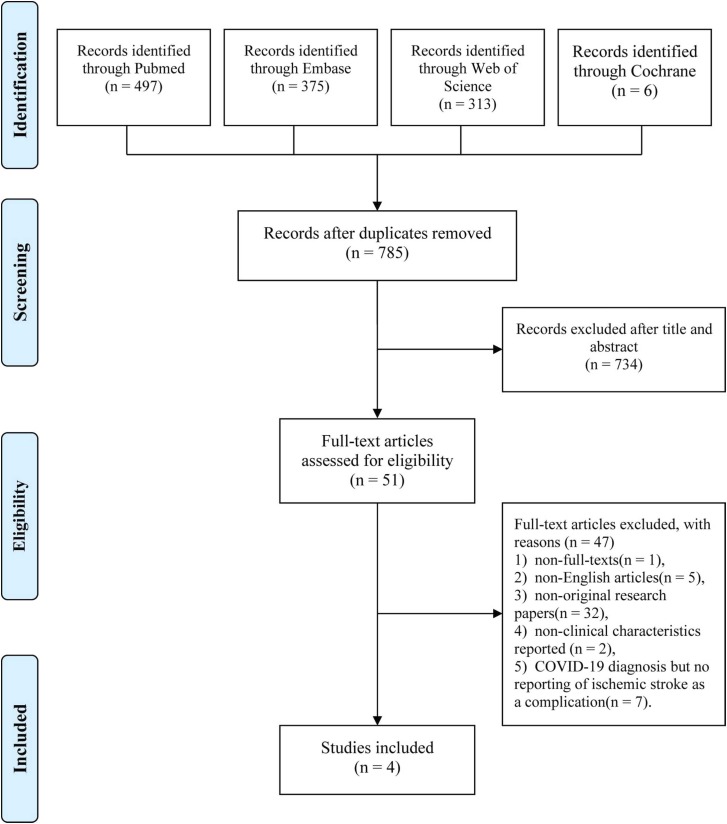
A total of 1191 publications was identified in the initial literature search. Among these studies, 497 articles were from PubMed, 375 articles were from EMBASE, 313 articles were from Web of Science and 6 articles were from Cochrane Library, and 406 duplicated articles were removed. Thus, we identified 785 unique papers and selected 51 papers for full-text review after reviewing the title and abstract. We excluded 47 articles based on the exclusion criteria. Finally, we included 4 eligible studies in our systematic review and meta-analysis. The flowchart showing the systematic screening and process of selection is reflected in Fig. 1.
Fig. 1.
The process of study selection shown in the PRISMA flowchart.
3.2. Quality assessment and baseline characteristics
We summarized the main characteristics of included articles in Table 1 The mean age of COVID-19 positive patients with ischemic stroke was 69.45 years (Range: 63–77 years) and the male patients were 56%. Countries covered by these articles were USA, Italy and France. Three of the articles were retrospective cohort studies and one was prospective cohort study. The study quality indicated by the score of NOS was ranged from 7 to 8 (Table S1).
Table 1.
Baseline features of COVID-19 patients with ischemic stroke.
| Study | Year | Study Design | Country | Continent | Age | Sex (Male%) | Sample size (n) | IS in COVID-19 (+) | COVID-19 (+) (n) | IS in COVID − 19 (-) | COVID-19 (-) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qureshi | 2021 | retrospective cohort | USA | North America | 68.8 | 44.7 | 27676 | 103 | 8163 | 199 | 19513 |
| Benussi | 2020 | retrospective cohort | Italy | Europe | 77 | 50 | 173 | 35 | 56 | 50 | 117 |
| Merkler | 2020 | retrospective cohort | USA | North America | 69 | 58 | 3402 | 31 | 1916 | 3 | 1486 |
| Helms | 2020 | prospective cohort | France | Europe | 63 | 81.3 | 383 | 2 | 150 | 1 | 233 |
Abbreviations: IS, Ischemic Stroke, COVID-19,Coronavirus Disease 2019
3.3. Overall analysis of the correlation between the risk of ischemic stroke and COVID-19
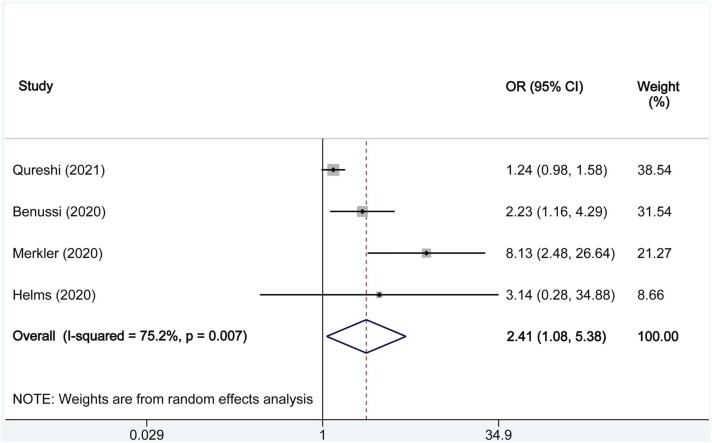
We analysed the combined effect estimations based on OR with the random-effects model ( Fig. 2). We found that the risk of ischemic stroke (combined OR: 2.41; 95% CI: 1.08–5.38) was significantly increased. Four included studies were significantly heterogeneous (I2 = 75.2%, P = 0.007).
Fig. 2.
Forest plots indicating COVID-19 and the risk of ischemic stroke. The X axis represents odds ratios (OR) for each individual study. Square size represents individual study weight. Bar represents 95% confidence interval (CI); diamond represents combined OR. Abbreviations: OR, odds ratios; CI, confidence intervals.
3.4. Subgroup analyses of correlation between the risk of ischemic stroke and COVID-19
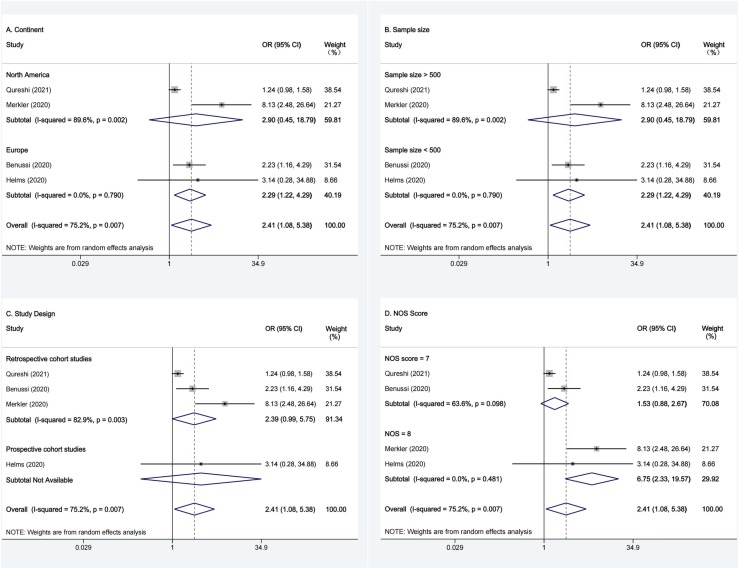
We conducted subgroup analyses based on the continent (North America or Europe), sample size (>500 or <500), study design (prospective, retrospective) and NOS score (7, 8) in Fig. 3. Significant association between the risk of ischemic stroke and COVID-19 was observed in the North America group (combined OR: 2.90; 95% CI: 0.45–18.80, I2 = 89.60%, P = 0.002), but not in the Europe group (combined OR: 2.29; 95% CI: 1.22–4.29, I2 = 0.00%, P = 0.79); Significant association between the risk of ischemic stroke and COVID-19 was also observed in studies with sample size > 500 (combined OR: 2.90; 95% CI: 0.45–18.80, I2 = 89.60%, P = 0.002), but not in studies with sample size < 500 (combined OR: 2.29; 95% CI: 1.22–4.29, I2 = 0.00%, P = 0.79); Moreover, significant association between the risk of ischemic stroke and COVID-19 was further observed in retrospective studies (combined OR: 2.39; 95% CI: 0.99–5.45, I2 = 82.90%, P = 0.003). In addition, we also assessed the quality of studies based on the NOS score. We found that there was no significant association between the risk of ischemic stroke and COVID-19 studies with a score of 7 (combined OR: 1.53; 95% CI: 0.88–2.67, I2 = 63.60%, P = 0.10) or 8 (combined OR: 6.75; 95% CI: 2.33–19.57, I2 = 0.0%, P = 0.48).
Fig. 3.
Subgroup Analyses Forest plots of the risk of ischemic stroke and COVID-19. (A) Subgroup Analyses Forest plots of the risk of ischemic stroke and COVID-19 based on continent; (B) Subgroup Analyses Forest plots of the risk of ischemic stroke and COVID-19 based on sample size; (C) Subgroup Analyses Forest plots of COVID-19 and risk of ischemic stroke for study design; (D) Subgroup Analyses Forest plots of the risk of ischemic stroke and COVID-19 based on NOS Score; The X axis represents odds ratios (OR) for each individual study; Square size represents individual study weight. Bar represents 95% confidence interval (CI); diamond represents combined OR. Abbreviations: OR, odds ratios; CI, confidence intervals; NOS, Newcastle–Ottawa Scale.
3.5. Sensitivity analysis
We next confirmed whether modification of the inclusion criteria had affected the final results using the sensitivity analysis. By excluded single study sequentially, we analysed the effect of each study on the pooled results and found no significant changes in the final results (Fig. S1). Thus, our meta-analysis had validated rationality and reliability.
3.6. Publication bias
To assess publication bias, we performed Begg’s test and examined the funnel plot symmetry (Fig. S2). We observed no publication bias with the Begg’s test (Z = 0.73, P = 0.67). In addition, all studies had CIs within 95% and were evenly distributed around the vertical.
4. Discussion
This study analyzed the associations between the risk of ischemic stroke and COVID-19 with a systematic review and meta-analysis. This is the first study comprehensively analysed the relationship between the risk of ischemic stroke and COVID-19 when previous studies failed to draw consistent conclusions. This meta-analysis showed that the risk of ischemic stroke was positively associated with COVID-19. Specifically, COVID-19 might have increased the risk of ischemic stroke by 1.4-fold.
Moreover, we found that the increased risk of ischemic stroke was associated with COVID-19 in retrospective cohort studies or studies with COVID-19 participants in North America but not elsewhere. This might be explained by the high risk of ischemic stroke among COVID-19 participants from USA and a low risk of ischemic stroke among COVID-19 participants from Italy and France. As we know that blacks have almost twice the risk of having a stroke for the first time as whites (Virani et al., 2020). Blacks have the highest death rate from stroke (Mozaffarian et al., 2016). Furthermore, a high percentage of COVID-19 patients are black people in the USA (Tai et al., 2021). These reasons may be the high risk of ischemic stroke among COVID-19 participants from USA. Studies with smaller sample size also failed to demonstrate an association between the risk of ischemic stroke and COVID-19 probably due to insufficient data for statistical analysis.
Several mechanisms had been proposed to explain the association between the risk of ischemic stroke and COVID-19. The first possible mechanism is the cytokine storm occurred in response to COVID-19 (Chen et al., 2020, Guzik et al., 2020, Zakeri et al., 2020). Indeed, increased levels of proinflammatory cytokines such as interleukine-1β (IL-1β), IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, granulocyte-macrophage colony-stimulating factor, interferon-γ, and monocyte chemotactic protein 1 were associated with the risk of multiple organ dysfunction in patients with COVID-19 (Huang et al., 2020, Mehta et al., 2020, Ojo et al., 2020). In addition, the increased levels of C-reactive protein and IL-6 might also contribute to the increased risk of acute ischemic stroke (Tzoulaki et al., 2007, Zakeri et al., 2020). Another possible mechanism for COVID-19-induced ischemic stroke is the activated innate immune system and the coagulation induced by infection with SARS-CoV-2 (Beyrouti et al., 2020, Campbell and Kahwash, 2020, Majidi et al., 2020, Sweid et al., 2020, Yaghi et al., 2020). In fact, the level of D-dimer was increased in COVID-19 patients with acute ischemic stroke, suggesting that COVID-19 may cause an acute systemic inflammatory response and lead to a procoagulant state (Grau et al., 2010, Merad and Martin, 2020, Wang et al., 2020). Inflammatory cytokines can cause endothelial activation which refers to the procoagulant state of the endothelial cells of blood vessels (Ojo et al., 2020). Thrombin, the serine protease which regulates blood coagulation, can be released by activated endothelial cells and is normally controlled by natural anticoagulants. Uncontrolled thrombin in the blood stream can activate platelets and lead to thrombosis which increases the risk of stroke (Beyrouti et al., 2020, Tang et al., 2020). Moreover, the antiphospholipid antibody syndrome (APS), an autoimmune disorder featured by excess formation of blood clot, might be another mechanism that associated COVID-19 with the ischemic stroke (Beyrouti et al., 2020, Zhang et al., 2020). The APS is mainly classified by an increased level of primary antiphospholipid antibodies including the lupus anticoagulant and aβ2GPI immunoglobulin M or immunoglobulin G antibodies. One study reported that 14 patients were tested positive for lupus anticoagulant and 5 patients were positive for β2GPI immunoglobulin M or immunoglobulin G antibodies among 56 COVID-19 patients (Harzallah et al., 2020, Ojo et al., 2020). Therefore, the level of antiphospholipid antibodies might be a good indicator for COVID-19 patients in predicting the risk of ischemic stroke. However, more studies are required to clarify the contribution of antiphospholipid antibodies to increase the risk of ischemic stroke in COVID-19 patients.
Our study has some strengthens. First, we analysed cohort studies instead of case report or case series. Second, we also considered the generalizability by including studies from different countries. Third, multiple analyses including the publication bias analysis, sensitivity analysis and subgroup analysis, were conducted to evaluate the combined effect size estimation.
Our study has some limitations. First, although we analysed four studies, the sample size remained small. Second, the language bias that favor English exists in this study. Therefore, further studies with larger sample sizes that include different ethnic populations are required to confirm our analysis.
5. Conclusions
Our meta-analysis showed a strong correlation between the increased incidence rate of ischemic stroke and COVID-19, especially in North America COVID-19 patients. Further study is required to develop effective treatments to decrease the ischemic stroke risk in COVID-19 patients.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC 81771521).
CRediT authorship contribution statement
Yanhua Cui, Taibai Li and Zhaofei Yang searched literatures and performed data extraction. Yanhua Cui and Bing Zhao analyzed the data, drafted and finalized the manuscript. Song Li revised the manuscript. Wei-dong Le designed the study concept and revised the manuscript. All authors have approved the final article.
Acknowledgments
Not applicable.
Declaration of Conflicts of interest
All authors declared no financial or other conflicts of interest involved.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.brainresbull.2021.12.011.
Appendix A. Supplementary material
Supplementary material
.
References
- Bekelis K., Missios S., Ahmad J., Labropoulos N., Schirmer C.M., Calnan D.R., Skinner J., MacKenzie T.A. Ischemic stroke occurs less frequently in patients with COVID-19 a multicenter cross-sectional study. Stroke. 2020;51(12):3570–3576. doi: 10.1161/strokeaha.120.031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K., Mocco J., Majidi S., Yeckley J., Aggarwal A., Lefton D., Doshi A.H. COVID-19 is an independent risk factor for acute ischemic stroke. Am. J. Neuroradiol. 2020;41(8):1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/circulationaha.120.047419. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130(5):2620–2629. doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamoon M.S., Thaler A., Gururangan K., Kohli A., Sisniega D., Wheelwright D., Mensching C., Fifi J.T., Fara M.G., Jette N., Cohen E., Dave P., DiRisio A.C., Goldstein J., Loebel E.M., Mayman N.A., Sharma A., Thomas D.S., Vega Perez R.D., Weingarten M.R., Wen H.H., Tuhrim S., Stein L.K., Mount Sinai Stroke I. Acute cerebrovascular events with COVID-19 infection. Stroke. 2021;52(1):48–56. doi: 10.1161/strokeaha.120.031668. [DOI] [PubMed] [Google Scholar]
- Grau A.J., Urbanek C., Palm F. Common infections and the risk of stroke. Nat. Rev. Neurol. 2010;6(12):681–694. doi: 10.1038/nrneurol.2010.163. [DOI] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020;18(8):2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 395(10223), 497–506. 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed]
- Katz J.M., Libman R.B., Wang J.J., Sanelli P., Filippi C.G., Gribko M., Pacia S.V., Kuzniecky R.I., Najjar S., Azhar S. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):E227–E231. doi: 10.1161/strokeaha.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi S., Fifi J.T., Ladner T.R., Lara-Reyna J., Yaeger K.A., Yim B., Dangayach N., Oxley T.J., Shigematsu T., Kummer B.R., Stein L.K., Weinberger J., Fara M.G., De Leacy R., Dhamoon M.S., Tuhrim S., Mocco J. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak clinical characteristics and paraclinical findings. Stroke. 2020;51(9):2656–2663. doi: 10.1161/strokeaha.120.030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., Chang, J., Hong, C., Zhou, Y., Wang, D., et al. 2020. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA neurology 77(6), 683–690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- Mehta, P., McAuley, D.F., Brown, M., Sanchez, E., Tattersall, R.S., and Manson, J.J.2020. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 395(10229), 1033–1034. 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Després J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jiménez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner M.B. Heart disease and stroke statistics-2016 update: a report from the American Heart association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int. J. Stroke. 2020 doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo A.S., Balogun S.A., Idowu A.O. Acute ischemic stroke in COVID-19: putative mechanisms, clinical characteristics, and management. Neurol. Res. Int. 2020;2020 doi: 10.1155/2020/7397480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Smith C.J., Roffe C., Simister R.J., Narayanamoorthi S., Marigold R., Willmot M., Dixit A., Hassan A., Quinn T., et al. Characteristics and outcomes of COVID-19-associated stroke: a UK multicentre case-control study. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-324927. [DOI] [PubMed] [Google Scholar]
- Nogueira R.G., Abdalkader M., Qureshi M.M., Frankel M.R., Mansour O.Y., Yamagami H., Qiu Z., Farhoudi M., Siegler J.E., Yaghi S., Raz E., Sakai N., Ohara N., Piotin M., Mechtouff L., Eker O., Chalumeau V., Kleinig T.J., Pop R., Liu J., Winters H.S., Shang X., Vasquez A.R., Blasco J., Arenillas J.F., Martinez-Galdamez M., Brehm A., Psychogios M.N., Lylyk P., Haussen D.C., Al-Bayati A.R., Mohammaden M.H., Fonseca L., Luís Silva M., Montalverne F., Renieri L., Mangiafico S., Fischer U., Gralla J., Frei D., Chugh C., Mehta B.P., Nagel S., Mohlenbruch M., Ortega-Gutierrez S., Farooqui M., Hassan A.E., Taylor A., Lapergue B., Consoli A., Campbell B.C., Sharma M., Walker M., Van Horn N., Fiehler J., Nguyen H.T., Nguyen Q.T., Watanabe D., Zhang H., Le H.V., Nguyen V.Q., Shah R., Devlin T., Khandelwal P., Linfante I., Izzath W., Lavados P.M., Olavarría V.V., Sampaio Silva G., de Carvalho Sousa A.V., Kirmani J., Bendszus M., Amano T., Yamamoto R., Doijiri R., Tokuda N., Yamada T., Terasaki T., Yazawa Y., Morris J.G., Griffin E., Thornton J., Lavoie P., Matouk C., Hill M.D., Demchuk A.M., Killer-Oberpfalzer M., Nahab F., Altschul D., Ramos-Pachón A., Pérez de la Ossa N., Kikano R., Boisseau W., Walker G., Cordina S.M., Puri A., Luisa Kuhn A., Gandhi D., Ramakrishnan P., Novakovic-White R., Chebl A., Kargiotis O., Czap A., Zha A., Masoud H.E., Lopez C., Ozretic D., Al-Mufti F., Zie W., Duan Z., Yuan Z., Huang W., Hao Y., Luo J., Kalousek V., Bourcier R., Guile R., Hetts S., Al-Jehani H.M., AlHazzani A., Sadeghi-Hokmabadi E., Teleb M., Payne J., Lee J.S., Hong J.M., Sohn S.I., Hwang Y.H., Shin D.H., Roh H.G., Edgell R., Khatri R., Smith A., Malik A., Liebeskind D., Herial N., Jabbour P., Magalhaes P., Ozdemir A.O., Aykac O., Uwatoko T., Dembo T., Shimizu H., Sugiura Y., Miyashita F., Fukuda H., Miyake K., Shimbo J., Sugimura Y., Beer-Furlan A., Joshi K. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;16:573–584. doi: 10.1161/strokeaha.120.031786. [DOI] [Google Scholar]
- Siow I., Lee K.S., Zhang J.J.Y., Saffari S.E., Ng A., Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J. Stroke Cerebrovasc. Dis.: Off. J. Natl. Stroke Assoc. 2021;30(3) doi: 10.1016/j.jstrokecerebrovasdis.2020.105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweid A., Hammoud B., Weinberg J.H., Oneissi M., Raz E., Shapiro M., DePrince M., Tjoumakaris S., Gooch M.R., Herial N.A., Zarzour H., Romo V., Rosenwasser R.H., Jabbour P. Letter: thrombotic neurovascular disease in COVID-19 patients. Neurosurgery. 2020;87(3):E400–e406. doi: 10.1093/neuros/nyaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai D.B.G., Shah A., Doubeni C.A., Sia I.G., Wieland M.L. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin. Infect. Dis. 2021;72(4):703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulaki I., Murray G.D., Lee A.J., Rumley A., Lowe G.D., Fowkes F.G. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh artery study. Circulation. 2007;115(16):2119–2127. doi: 10.1161/circulationaha.106.635029. [DOI] [PubMed] [Google Scholar]
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics S. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/cir.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Wang Z., Yang Y., Liang X., Gao B., Liu M., Li W., Chen Z., Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism and management. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., Henninger N., Trivedi T., Lillemoe K., Alam S., Sanger M., Kim S., Scher E., Dehkharghani S., Wachs M., Tanweer O., Volpicelli F., Bosworth B., Lord A., Frontera J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri A., Jadhav A.P., Sullenger B.A., Nimjee S.M. Ischemic stroke in COVID-19-positive patients: an overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J. Neurointerv. Surg. 2020 doi: 10.1136/neurintsurg-2020-016794. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. New Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research T A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material