Abstract
Introduction
We examined relationships of body mass index (BMI) with cognition in middle‐aged adults at Alzheimer's disease (AD) risk due to parental family history.
Methods
Participants are offspring of AD patients from the Israel Registry of Alzheimer's Prevention (N = 271). Linear regressions assessed associations of BMI and cognition, and whether associations differed by maternal/paternal history. Analyses of covariance examined associations of long‐term trajectories of BMI with cognition.
Results
Higher BMI was associated with worse language (P = .045). Interactions of BMI with parental history were significant for episodic memory (P = .023), language (p = .027), working memory (P = .006), global cognition (P = .008); associations were stronger among participants with maternal history. Interactions of BMI trajectories with parental history were significant for episodic memory (P = .017), language (P = .013), working memory (P = .001), global cognition (P = .005), with stronger associations for maternal history.
Discussion
Higher BMI and overweight/obese trajectories were associated with poorer cognition in adults with maternal history of AD, but not those with paternal history.
Keywords: adiposity, Alzheimer's disease, cognition, cognitive decline, obesity, parental history of Alzheimer's disease, risk factors
1. INTRODUCTION
Adiposity, often represented by body mass index (BMI), is associated with Alzheimer's disease (AD) and cognitive decline, as well as with risk factors for cognitive decline, including cardiovascular disease and type 2 diabetes mellitus (T2DM). 1 , 2 , 3 As AD neuropathology begins accumulating years to decades prior to the appearance of overt clinical symptoms, it is crucial to identify risk factors in middle‐aged adults to understand the pathways underlying the earliest stages of AD and cognitive decline.
Family history of AD is the second most significant risk factor for AD, after age. 4 Growing evidence suggests that compared to individuals with a paternal risk of AD, 5 individuals with a maternal history of AD are at significantly greater risk of AD themselves, both clinically 5 and neuropathologically. 4 , 6 , 7 , 8 Individuals with a maternal history of obesity have a higher risk of developing cardiovascular disease. In an AD‐like mouse model, offspring of mothers who were obese during pregnancy had more memory impairment, and more tau‐positive neurons in the hippocampus, than offspring of lean mothers. Individuals with a maternal history of AD have more amyloid, as seen by Pittsburgh compound B positron emission tomography, 6 reduced gray matter, 6 and other AD‐related brain changes. 7 , 8 , 9 Further, they have a reduced cerebral metabolic rate of glucose 9 compared to individuals with no parental or paternal history of AD. However, little is known about the inter‐relationships of adiposity and AD family history on cognition.
In this study, we examined the relationship of BMI with cognitive functioning in 271 middle‐aged adults at high AD risk due to a parental family history of AD, all participants of the Israel Registry for Alzheimer's Prevention (IRAP). 10 All participants are from the Maccabi Healthcare Services (MHS), which provides approximately 20 years of BMI data, permitting the examination of associations of long‐term trajectories of BMI with cognition in addition to BMI at the time of cognitive assessment. We further investigated whether these associations differed by a maternal and paternal family history of AD.
2. METHODS
2.1. Study population
Participants were from the IRAP study, a longitudinal study of risk factors for cognitive decline in initially cognitively normal middle‐aged individuals at risk for AD due to parental family history. The study is a collaboration between the Sheba Medical Center, Israel, and the MHS, Israel. Both institutional review boards approved the study and all participants signed informed consent. All participants are members of the MHS, the second‐largest Israeli health maintenance organization, which provides care for ≈25% of the Israeli population and contains extensive medical information since 1998, including diagnoses, medication purchases, and laboratory results. Details on the methods of IRAP were described previously by Ravona‐Springer et al. 10 Briefly, the study collects detailed cognitive, health‐related, genetic, lifestyle, and brain imaging data, with follow‐up visits every 3 years. The main source of participant recruitment is through advertisements on the MHS website and participants’ word of mouth. Eligibility criteria are (1) age between 40 and 65, (2) member of MHS, (3) a parental family history of AD, and (4) fluency in Hebrew. After obtaining informed consent, each IRAP participant completes an entry assessment that includes anthropometric measurements, neuropsychological testing by trained neuropsychologists, laboratory testing, and a detailed health and lifestyle history. Offspring of probands with partial information about dementia type or with dementia other than AD are excluded from the study. Parents with an AD onset before the age of 55 are assumed to have familial early onset AD and their offspring are excluded from the study. Siblings are excluded from the study as their data may substantively confound results, especially of analyses including genetic components. Thus, the first offspring who volunteers and is eligible is included in the study. Assessments are performed at the Sheba Medical Center.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (PubMed and other) sources. The association between adiposity (represented by body mass index [BMI]) and cognitive decline and Alzheimer's disease (AD) has been frequently reported. Because the accumulation of AD neuropathology begins well before symptoms, it is important to examine the relationship between BMI and cognition in middle‐aged adults. Family history of AD is a significant risk factor for AD, but the interaction of BMI with family history is not well described. Relevant citations regarding the relationships among BMI, AD, and family history are appropriately cited.
Interpretation: Our findings suggest that adiposity in midlife is associated with worse cognition, particularly in those with a maternal family history of AD. This finding expands upon the current knowledge regarding risk factors for AD.
Future directions: The article recommends further investigations of genetic and environmental contributions of maternal history of AD.
At the time of the present data analysis, 271 participants (179 with maternal, 85 with a paternal, and 7 with maternal and paternal family history) had BMI, cognitive, and covariate data. We present baseline results, as the first follow‐up of the IRAP cohort has just begun.
2.1.1. Determination of parental AD status
Individuals who approach the study team undergo questioning about their age, MHS membership, and parental dementia. Then, medical records of proband parents of potential participants are made available to the study team and a dementia questionnaire (DQ) is administered by telephone prior to the invitation of potential participants to the study site. The DQ is a validated, informant‐based instrument, to determine the likely presence of AD in parents of potential study volunteers. 11 The DQ obtains information similar to that obtained from a medical records review using Diagnostic and Statistical Manual of Mental Disorders, 4th revision criteria. During DQ administration, offspring are asked about the type, course, and progression of dementia‐related symptoms and about the presence or absence of comorbid conditions that could explain or contribute to the symptoms in their parents. When administered to close family members, diagnostic classifications based on the DQ show high sensitivity and specificity as well as a high degree of concordance with the outcomes of clinical and neuropathological diagnostic evaluations. 11 All the medical history and diagnostic workup available are reviewed together with the DQ by the study team in order to reach a probable AD diagnosis (according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria 12 ).
2.2. Body mass index
Height and weight were measured (barefoot, wearing light clothing) by nurses, using a beam balance and stadiometer to the nearest centimeter and 0.1 kg, respectively. BMI was calculated (weight [in kilograms] divided by squared height [in meters]). Characterization of BMI subgroups was based on World Health Organization criteria as follows: “normal” (between 18.5 and 24.99 kg/m2), overweight (between 25 and 29.99 kg/m2), and obese (> 30 kg/m2). 13
2.3. BMI trajectories
The MHS electronic registry has BMI registered since 1998 for patients who come for their annual visits. Participants in this study had an average of 13.21 (14.29) BMI measurements in the registry. We thus evaluated trajectories of BMI as predictors of cognition. Trajectories were identified using an SAS macro called PROC TRAJ, which applies a multinomial modeling strategy to identify relatively homogenous clusters of developmental trajectories within a sample population. Trajectory parameters are derived by latent class analysis using maximum likelihood estimation. In particular, the distinctive trajectories of BMI were derived by modeling BMI as a function of the number of follow‐up years in the Diabetes Registry prior to the start date of IRAP (defined as the intercept) with the adjustment of IRAP baseline age and sex. Distinct time points were created for each follow‐up visit observed. The number of trajectories and degree of curvature were determined using the guidelines suggested by Jones et al. 14 The output of PROC TRAJ includes the equations for the different trajectories along with the assignment of each patient to one of the trajectory groups. Three trajectories were identified with linear, quadratic, and cubic curves corresponding to “normal” (between 18.5 and 24.99; 46%), overweight (between 25 and 29.99; 43.6%), or obese (> 30; 10.3%). 15 Because the number of participants in the obese trajectory was small (N = 15), we collapsed the obese and overweight trajectory into one trajectory, so analyses compared normal to overweight/obese trajectories.
2.4. Cognitive evaluation and diagnostic consensus conference
IRAP participants complete a comprehensive neuropsychological battery at baseline and at follow‐up assessments. Factor analysis summarized the neuropsychological measures into four domains: executive‐motor functions (Trail Making Test [TMT] A and B time, Block design, Digit Symbol), working memory (digit span forward, digit span backward, letter‐number sequencing), language (verbal IQ similarities, verbal IQ vocabulary, and the average of phonemic and animal fluency), and episodic memory (Rey Auditory Verbal Learning Test immediate recall [sum of trials 1–5], delayed recall, recognition). The neuropsychological tests scores were transformed into Z scores (reversing scores representing time—TMT A and B—so high scores represented good cognition) and averaged for each domain. A global cognition measure averaged the scores of all four domains. We examined the associations of BMI with global cognition and with each of the cognitive domains.
2.5. Measurement of covariates
Sociodemographic covariates were age, years of education, and sex. Cardiovascular risk factors included total cholesterol, systolic and diastolic blood pressure, creatinine (measured as the mean of all measures from the MHS electronic medical records), and the diagnosis of T2DM, also received through MHS and confirmed by the participants at the study physician's assessment. Self‐reported physical activity (never, < 1x/month, 1–4x/month, or < 1x/week), and apolipoprotein E (APOE) ε4 status were evaluated by the IRAP study. 16 , 17
2.6. Statistical analysis
Primary analyses were linear regressions performed to examine the association between baseline BMI with cognition. Global cognition is presented in the main article, and results on the specific cognitive domains are available in supporting information. In model 1, we adjusted for sociodemographic variables (age, years of education, and sex). In model 2, we added the cardiovascular and other health covariates (total cholesterol, systolic and diastolic blood pressure, self‐reported physical activity, creatinine, diabetes, and APOE ε4 status). Additionally, we examined the interaction of parental history of AD and BMI on cognition, adding an interaction term of BMI x paternal/maternal AD, using the same two‐model approach. To identify the source of significant interactions, we repeated the linear regressions separately for those with a maternal and paternal family history of AD. We then examined the relationship between long‐term BMI with cognition. BMI group assignments (based on long‐term trajectories of BMI) were analyzed using analysis of covariance (ANCOVA) to compare cognitive functioning of the two trajectory groups (normal and overweight/obese), adjusting for the same covariates as in the linear regressions. Additionally, we examined the interaction of parental history of AD and BMI trajectory on cognition, adding an interaction term of BMI trajectory x parental/maternal AD, using the same two‐model regression approach described above. To identify the source of significant interactions, we repeated the ANCOVA for those with a maternal and paternal family history of AD separately.
3. RESULTS
Participant characteristics are presented in Table 1. Participants had a mean age of 54.1 years, and approximately 60.5% were women. Their baseline BMI averaged 26.2 (4.3) kg/m2, reflecting a slightly overweight sample; 151 (55.7%) of them were either overweight or obese. Participants with a maternal history of AD did not differ from participants with a paternal history of AD on any of the covariates, or on BMI. Participants with an overweight or obese trajectory of BMI had higher systolic and diastolic blood pressure, compared to participants with a normal trajectory of BMI, but did not differ in any other covariate.
TABLE 1.
Characteristics of the study sample
| Characteristic and measure | Entire sample N = 271 | Maternal N = 179 | Paternal N = 85 | Normal BMI trajectory N = 154 | Overweight or obese BMI trajectory N = 86 |
|---|---|---|---|---|---|
| Demographic | |||||
| Age | 54.1 (6.8), 39–66 | 54.0 (7.2), 39–66 | 53.9 (6.0), 43–65 | 54.2 (6.7), 39–65 | 54.7 (6.6), 41–66 |
| Sex (% female) | 60.5% | 63.1% | 54.1% | 59.1% | 62.8% |
| Education years | 16.6 (3.1), 10–29 | 16.4 (2.9), 11–24 | 16.9 (3.2), 10–27 | 16.5 (3.2), 10–29 | 16.5 (3.0), 11–24 |
| APOE ε4 carriers (%) | 33.2% | 34.4% | 29.7% | 33.3% | 39.7% |
| Health history | |||||
| Diabetes, (%) | 13.0% | 13.5% | 11.8% | 11.6% | 14.6% |
| Laboratory values/vitals (fasting) | |||||
| Creatinine (mg/dL) | 0.8 (0.2), 0–1.6 | 0.9 (0.2), 0.0–1.6 | 0.9 (0.1),0 .6–1.3 | 0.9(0.1), 0.6–1.3 | 0.9 (0.2), 0.0–1.6 |
| Cholesterol (mg/dL) | 198.1 (28.2), 117–318 | 198.8 (28.0), 138–260 | 197.20 (29.9), 117–318 | 197.2 (27.8), 117–254 | 200.1 (28.3), 141–318 |
| BMI | 26.2 (4.3), 17.0–41.8 | 26.0 (4.2), 17.0–40.5 | 26.8 (4.5), 17.4–41.8 | 23.9 (2.5), 16.9–31.7 | 30.6 (3.6), 20.3–41.8 |
| Systolic blood pressure | 120.7 (10.6), 95.2–156.2 | 120.3 (11.0), 95.2–156.2 | 121.3 (9.4), 97.3–141.3 | 118.9 (10.5), 95.2–152.9 | 124.9 (9.4), 105–2‐156.2 |
| Diastolic blood pressure | 75.5 (6.0), 59.2–95.1 | 75.2 (6.3), 59.2–95.1 | 75.9 (5.3), 62.7–88.4 | 74.7 (6.1), 59.2–92.4 | 77.4 (5.3), 65.7–95.1 |
| Lifestyle variables | |||||
| Exercise frequency | |||||
| Never | 29.6% | 29.1% | 30.9% | 25.0% | 36.4% |
| 1x/month | 13.6% | 14.9% | 11.8% | 10.3% | 16.7% |
| 1–4x/month | 24.8% | 23.1% | 27.9% | 27.6% | 15.2% |
| >1x/week | 32.0% | 32.8% | 29.4% | 37.1% | 31.8% |
Values represent mean (SD), range unless otherwise specified.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; SD, standard deviation.
3.1. Associations of BMI (measured at the time of cognitive assessment) and of long‐term trajectories of BMI with global cognition—moderation by parental history
Table 2 presents the results for global cognitive functioning; results for the specific cognitive domains (executive functions, episodic memory, language, and working memory) are presented Tables S1 and S2 in supporting information. In model 1, there was an association between BMI and global cognition for the entire sample (P = .004), which did not withstand adjustment for additional clinical covariates (model 2; P = .160). An interaction between BMI and parental history of AD (maternal/paternal) on global cognition (see Figure 1) approached significance for model 1 (P = .074) and was significant for model 2 (P = .014). Maternal history of AD drove this association such that the associations of BMI with global cognition were significant in this group for model 1 (P < .001) and model 2 (P = .019) but were not significant, in either model (model 1, P = .952; model 2, P = .249), for those with a paternal history of AD.
TABLE 2.
Association of BMI with global cognition
| Model 1 | Model 2 | |
|---|---|---|
| Entire sample | r = –.174, β = –.165, P = .004* | r = –.106, β = –.117, P = .160 |
| Maternal AD | r = –.264, β = –.253, P < .001* | r = –.216, β = –.236, P = .019* |
| Paternal AD | r = .007, β = .006, P = .952 | r = .182, β = .242, P = .249 |
| Interactions a | r = .110, β = .745, P = .074 | r = .185, β = 1.32, P = .014* |
Notes: Model 1 covariate: age, sex, education. Model 2 covariates: age, sex, education, APOE ε4, physical activity, diastolic blood pressure, systolic blood pressure, total cholesterol, diabetes status, creatinine.
P < .05.
Interactions between BMI and maternal/paternal family history on cognitive function.
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; BMI, body mass index.
FIGURE 1.
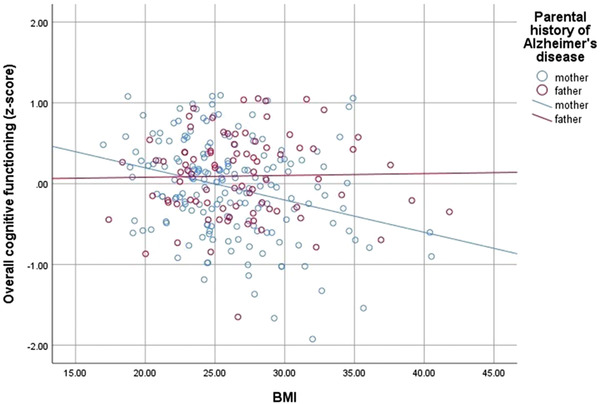
Interaction of global cognitive functioning with body mass index (BMI) by parental history of Alzheimer's disease
Using ANCOVA to examine the differences between the two groups of long‐term trajectories of BMI in global cognition and applying the same two‐model approach as previously described, we found significantly lower global cognition (model 1, P = .040) in the BMI trajectories of overweight/obese compared to the trajectory of normal weight (Table 3). After adjustment for additional clinical covariates, the association was not significant (P = .341). However, the interaction between BMI trajectory and maternal/paternal history was significant in both models (P = .006, model 1; P = .005, model 2). To investigate the source of the significant interaction, we repeated the analyses stratifying the sample by a maternal and paternal family history of AD, and found that among participants with a maternal history of AD, those in the overweight/obese trajectory demonstrated significantly poorer global cognition ( P = .001, model 1; P = .036, model 2). No associations between BMI trajectories and global cognition were found among participants with a paternal family history (model 1, P = .310; model 2, P = .325).
TABLE 3.
Association of BMI trajectories with cognition
| Model 1 | Model 2 | |
|---|---|---|
| Entire sample | F(1,235) = 4.29, P = .040* | F(1,183) = 0.912, P = .341 |
| Maternal AD | F(1,154) = 12.483, P = .001* | F(1,120) = 4.503, P = .036* |
| Paternal AD | F(1,70) = 1.047, P = .310 | F(1,47) = .990, P = .325 |
| Interactions a | r = .179 β = .704, P = .006* | r = .206, β = .817, P = .005* |
Notes: Model 1 covariates: age, sex, education. Model 2 covariates: age, sex, education, APOE ε4, physical activity, diastolic blood pressure, systolic blood pressure, total cholesterol, diabetes status, creatinine.
P < .05.
Interactions between BMI and maternal/paternal family history on cognitive function.
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; BMI, body mass index.
3.2. Associations of BMI (measured at the time of cognitive assessment) and of long‐term trajectories of BMI with specific cognitive domains—moderation by parental history
Table S1 presents the results for the associations between BMI and specific cognitive domains (executive functions, episodic memory, language, and working memory). In model 1, there was an association between BMI and executive function (P = .004) and language (P = .002) for the entire sample; there were no significant associations for episodic memory (P = .069) or working memory (P = .198). In model 2, only the language association with BMI was significant (P = .045); associations of BMI with executive function (P = .089), episodic memory (P = .191), and working memory (P = .843) were not significant. The interaction between BMI and parental history of AD (maternal/paternal), shown in Table S2, was significant for model 1 (P = .019) for working memory, but not for the other functions (P‐values above .10). In model 2, the interaction of BMI with maternal/paternal AD status was significant for episodic memory (P = .023), language (P = .027), and working memory (P = .006), but not for executive function (P = .299). As seen in Table S3 in supporting information, maternal history of AD drove this association as the regressions of BMI with cognition were significant in this group for model 1 for all cognitive domains (executive functioning, P = .017; episodic memory, P = .029; language, P < .001; working memory, P = .001). There were no significant associations between BMI and any of the cognitive domains, in either model, for those with a paternal history of AD. The interactions (see Table S4 in supporting information) for episodic memory (P = .017), working memory (P = .001), and language (P = .013) withstood further adjustment for clinical covariates (model 2), but not for executive functions (P = .378).
Table S3 presents the detailed results for the differences between BMI trajectories and specific cognitive domains. In model 1, the overweight/obese trajectory group had significantly lower functioning in executive function (P = .032) and language (P = .018) for the entire sample; there were no significant differences between BMI trajectory groups for episodic memory (P = .344) or working memory (P = .215). In model 2, there were no significant differences between the BMI trajectory groups for any of the cognitive domains (language, P = .165; executive functions, P = .516; episodic memory, P = .376; or working memory, P = .868). In contrast, an interaction between BMI trajectory and parental history of AD (maternal/paternal), shown in Table S4, was significant for model 1 for language (P = .014), episodic memory (P = .010), and working memory (P < .001). These interactions withstood adjustment for covariates in model 2 (episodic memory, P = .017, language, P = .013, and working memory, P = .001). The interaction of parental family history with BMI trajectories was not significant for executive function in both models (P > .378). As seen in Table S3, the significant associations were driven by a maternal family history of AD as among offspring of maternal family history of AD, the overweight/obese group had significantly lower functioning for all cognitive domains (executive function, P = .017; episodic memory, P = .029; language, P < .001; working memory, P = .001). These differences withstood additional adjustment for clinical variables for episodic memory (P = .046), language (P = .020), and working memory (P = .046), but not for executive function (P = .544). There were no significant differences in the cognitive domains between BMI trajectory groups for those with a paternal history of AD (model 2, executive function, P = .976; episodic memory, P = .316; language, P = .701; working memory, P = .089).
4. DISCUSSION
Higher BMI was significantly associated with worse cognitive functioning in cognitively normal middle‐aged adults, after adjustment for sociodemographic and cardiovascular risk factors, including the APOE ε4 genotype. The source of parental history of AD moderated these associations such that significant associations of higher BMI with lower cognitive function were found in those with maternal AD, but not in those with paternal AD. Our results were reinforced by the finding that long‐term trajectories of BMI—based on ≈20 years of BMI measurements, starting when the sample was on average 34 years of age—were also associated with cognition, and more strongly among participants with a maternal history of AD. In these participants, but not in those with a paternal history of AD, long‐term normal BMI was associated with better cognitive functioning compared to those with long‐term overweight/obesity trajectories. Associations were more prominent for the cognitive domains of episodic memory, working memory, and language and less so for executive function, suggesting that BMI affects cognitive abilities differently. Our results suggest that in midlife, adiposity may already have a detrimental impact on the brain, and this effect is most prominent in offspring of mothers with AD.
The association between midlife BMI with cognition and risk of dementia is well established, 1 and evidence is emerging that higher BMI during early adulthood can increase the risk for dementia. 18 Several mechanisms may underlie the BMI–cognition link. Obesity is associated with hypertension and high cholesterol; 19 however, adjustment for these vascular risk factors did not alter substantively the results, suggesting other pathways. Obesity causes chronic inflammation, 19 including alterations in adipokines that affect the brain 3 and which may contribute to the initiation and progress of the dementia process. 3 Yet, adjustment for C‐reactive protein, an acute phase reactant, did not alter the associations (data not shown). It is possible that adipokines, secreted from adipose tissue, may be more directly relevant to adiposity‐related cognitive impairment. Finally, in addition to the fat and obesity (FTO) gene, which has been implicated in AD risk, 20 a total of 31 single nucleotide polymorphisms, linked to seven genes, were found to be shared by AD and obesity. 21 This suggests a genetic involvement in poor cognitive function in the context of overweight/obesity.
Individuals with a maternal history of AD are at significantly greater risk of AD themselves, compared to individuals with a paternal history. 9 There is consistent literature suggesting that individuals with a maternal family history who are currently unaffected themselves, have poorer brain outcomes compared to those with a paternal history or no parental history. These outcomes include lower gray matter volume, 6 , 22 reduced cerebral metabolic rate of glucose (CMRglc), 4 hypoperfusion in the hippocampus and parieto‐frontal regions (brain regions impacted by AD), 7 and hippocampal atrophy. 23 Brain atrophy and hypoperfusion have been linked to obesity suggesting that these brain pathologies may underlie associations between excess adiposity and cognition in offspring with a maternal history of AD.
The greater impact of a maternal family history of AD may be due to the role of mitochondrial and X chromosome effects on AD. 5 For example, a mutation located on the PCDH11 gene on chromosome X has been linked to AD. 9 Mutations in mitochondrial DNA (inherited maternally) may cause mitochondrial dysfunction and oxidation that may be involved in the development of AD. 4 Mitochondrial dysfunction has emerged as a feature common to chronic conditions including AD and obesity, and may serve as a common cause for cognitive impairment. 24
Maternal weight is a significantly stronger predictor of offspring weight than paternal weight, 25 and obesity is a significant risk factor for AD. 1 This may suggest a multiplied risk of AD in offspring of mothers with AD if those mothers were also overweight. Alternatively, these results may simply reflect an additive effect of several risk factors; participants who have a mother with AD and who are overweight/obese are at greater risk for cognitive impairment compared to participants with only one risk factor. The mechanisms underlying the greater impact of maternal family history combined with obesity on cognition are yet to be elucidated.
This study was limited by its cross‐sectional outcomes. Longitudinal assessments of the IRAP study are ongoing; and we expect to integrate additional cognitive, functional, and health measures into future data analysis. Strengths of the study include a broad assessment of multiple, directly measured anthropometric indices and cardiovascular risk factors (rather than self‐reported) and the neuropsychological battery that allowed assessment of different cognitive domains. Also, the trajectories of BMI included data from 20 years, providing evidence for the possibility that our results are not due to reverse causality. The study innovates by demonstrating that this BMI–cognition relationship may be especially impactful for individuals with a maternal history of AD, a finding that to our knowledge has not yet been reported. Importantly, the lack of significant results for the paternal group was not due to a small sample size but rather due to a small effect in contrast to the associations among maternal AD participants.
Our results suggest that already in midlife, greater adiposity is associated with worse cognition. Future studies are needed to determine whether interventions to reduce adiposity may protect against cognitive decline and ultimately, from dementia, particularly in those carrying a maternal family history of AD. Additionally, future studies are needed to better clarify the relationship between BMI and parental AD and its effect on offspring cognition; few studies have interpreted the impact of BMI on AD risk within the context of maternal versus paternal AD.
CONFLICTS OF INTEREST
Rebecca K. West reports that in addition to her work at the Icahn School of Medicine at Mount Sinai, she is paid as an adjunct assistant professor and a researcher at the City University of New York. Ramit Ravona‐Springer received funding from Tel Aviv University internal grants—the Brecher foundation and the Ofer Mordechai Foundation, the ARC (Accelerate, Redesign, Collaborate) Innovation Center at the Sheba Medical Center, the ADDF (Alzheimer's Drug Discovery Foundation). Inbal Sharvit Ginon received travel support for attending AAIC 2018 and AAIC 2019, as PhD student at the Joseph Sagol Neuroscience Center at Sheba Medical Center, Israel. Ithamar Ganmore reports no disclosures. Sigalit Manzali reports no disclosures. Sapir Golan reports no disclosures. Ethel Boccara reports no disclosures.Anthony Heymann received consulting fees from pharmaceutical companies Boehringer Ingelheim Israel, Rafa Israel, and Kamada, and payment for expert testimony in medical negligence cases in Israel courts. Michal Schnaider Beeri reports no disclosures.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
We thank the LeRoy Schecter Foundation and Dr. Marina Nissim for their kind financial gifts that supported this study.
Study funded by NIH (AG02219/AG/NIA NIH HHS, P01‐AG02219/AG/NIA NIH HHS, R01 AG034087/AG/NIA NIH HHS, AG051545/AG/NIA NIH HHS, AG053446/AG/NIA NIH HHS, AG061093/AG/NIA NIH HHS), provided to the institutions listed in this manuscript.
West RK, Ravona‐Springer R, Sharvit‐Ginon I, et al. Long‐term trajectories and current BMI are associated with poorer cognitive functioning in middle‐aged adults at high Alzheimer's disease risk. Alzheimer's Dement. 2021;13:e12247. 10.1002/dad2.12247
REFERENCES
- 1. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimer's Dement. 2015;11(6):718‐726. [DOI] [PubMed] [Google Scholar]
- 2. Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369‐381. [DOI] [PubMed] [Google Scholar]
- 3. José A, Luchsinger DRG. Adiposity, type 2 diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16(4):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104(48):19067‐19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon‐Albright LA, Foster NL, Schliep K, et al. Relative risk for Alzheimer disease based on complete family history. Neurology. 2019;92(15):e1745‐e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Burns JM. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74(2):113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okonkwo OC, Xu G, Oh JM, et al. Cerebral blood flow is diminished in asymptomatic middle‐aged adults with maternal history of alzheimer's disease. Cereb Cortex. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okonkwo OC, Oh JM, Koscik R, et al. Amyloid burden, neuronal function, and cognitive decline in middle‐aged adults at risk for Alzheimer's disease. J Int Neuropsychol Soc. 2014;20(4):422‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010;4(3):170‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ravona‐Springer R, Sharvit‐Ginon I, Ganmore I, et al. The Israel Registry for Alzheimer's Prevention (IRAP) Study: design and Baseline Characteristics. J Alzheimer's Dis. 2020;78(2):777‐788. [DOI] [PubMed] [Google Scholar]
- 11. Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A Validation Study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901‐906. [DOI] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group uder the auspices of Department of Health and Human Servies Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939. [DOI] [PubMed] [Google Scholar]
- 13. (WHO) WHO . Mean Body Mass Index (BMI). https://www.who.int/gho/ncd/risk_factors/bmi_text/en/
- 14. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374‐393. [Google Scholar]
- 15. (WHO) WHO . Obesity and Overweight. Fact Sheet.; 2017. 10.1007/s12630-015-0458-0. [DOI]
- 16. Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi‐dominant inheritance. Mol Psychiatry. 2011;16(9):903‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y, Epidemiologic studies of modifiable factors associated with cognition and dementia : systematic review and meta‐analysis. 2014;14(1):1‐33. 10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Hazzouri Z, Vittinghoff E, Hoang T, et al. Body mass index in early adulthood and dementia in late life: findings from a pooled cohort. Alzheimer's Dement. 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tchernof A, Després J‐P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359‐404. [DOI] [PubMed] [Google Scholar]
- 20. Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer's disease risk: a prospective cohort study. J Alzheimer's Dis. 2011;23(3):461‐469. [DOI] [PubMed] [Google Scholar]
- 21. Zhuang QS, Zheng H, Gu XD, Shen L, Ji HF. Detecting the genetic link between Alzheimer's disease and obesity using bioinformatics analysis of GWAS data. Oncotarget. 2017;8(34):55915‐55919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berti V, Mosconi L, Glodzik L, et al. Structural brain changes in normal individuals with a maternal history of Alzheimer's. Neurobiol Aging. 2011;32(12):2325.e17‐2325.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrawis JP, Hwang KS, Green AE, et al. Thompson and LGA. Effects of ApoE4 and maternal history of dementia on hippocampal atrophy John. Neurobiol Aging. 2012;33(5):856‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rigotto G, Basso E. Mitochondrial dysfunctions: a thread sewing together Alzheimer's disease, diabetes, and obesity. Oxid Med Cell Longev. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population‐based sample. Am J Clin Nutr. 2010;91(6):1560‐1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


