Abstract
Background
Allergic rhinitis and asthma are mediated by similar allergic mechanisms. They may represent two manifestations of the same united airway disease and therefore intranasal corticosteroids (INCS) could improve asthma. Nevertheless none of the asthma guidelines have advocated intranasal corticosteroids for asthma.
Objectives
To assess the efficacy of intranasal corticosteroids on asthma outcomes in people with rhinitis and asthma.
Search methods
We searched the Cochrane Airways Group trials register, the Cochrane Central Register of Controlled Trials (Issue 1, 2003), MEDLINE and reference lists of articles. We also contacted researchers in the field. The last search was conducted in March 2004.
Selection criteria
Randomised controlled trials comparing intranasal corticosteroids to intranasal placebo or to other traditional asthma treatments were included. Intrabronchial corticosteroids were not allowed but a device combining intranasal and intrabronchial corticosteroid was considered as being a primary INCS technique and was therefore also compared to placebo.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data. Study authors were contacted for additional information. Quality assessment for the 14 eligible studies was performed using the Jadad score and by ranking allocation concealment. Statistical analysis for continuous data was done by weighted mean difference or standardised mean difference.
Main results
Fourteen trials involving 477 people were included. Meta‐analysis for asthma outcomes failed to show a statistically significant benefit of INCS in asthma. However, for symptom scores and forced expiratory volume in one second, the trend favoured a beneficial effect of INCS. For asthma symptom scores (two parallel studies), the standardised mean difference was 0.61 (95% confidence interval (CI) ‐0.04 to 1.26). Meta‐analysis for forced expiratory volume in one second (five parallel studies) gave a standardised mean difference of 0.31 (95% CI ‐0.04 to 0.65). In the parallel studies, meta‐analysis of peak expiratory flow gave a standardised mean difference of ‐0.10 Litres/min (95% CI ‐0.55 to 0.35) for mean peak flow (three studies). Meta‐analysis for methacholine airway responsiveness (three parallel studies) showed a standardised mean difference of ‐0.20 (‐95% CI 0.64 to 0.24).
Authors' conclusions
Intranasal corticosteroids were well tolerated. While INCS tended to improve asthma symptoms and forced expiratory volume in one second, the results did not reach significance. The combination of intranasal plus intrabronchial corticosteroids should remain the current clinical practice until more research is done.
Plain language summary
Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis
It has been suggested for nearly twenty years that nasal sprays containing corticosteroids might improve asthma outcomes in people suffering from both asthma and rhinitis. Intranasal corticosteroids had few side effects in people with mild asthma, but the improvements in symptoms scores and lung function could have arisen by chance. Intranasal corticosteroids may be a promising alternative treatment for patients with rhinitis and mild asthma. More research is needed before considering changing the current practice of prescribing corticosteroids delivered by oral inhalers for asthma, and by nasal sprays for rhinitis.
Background
Allergic rhinitis and asthma are high prevalence and high cost diseases in both developed and undeveloped countries (Weiss 2001). Numerous studies have reported that the prevalence of these two conditions has increased over the last 30 to 40 years. It is estimated that worldwide between 1% and 20% of children and young adults have asthma and around 20% of individuals of all ages have allergic rhinitis (Lundback 1998). These two diseases commonly coexist and rhinitis is recognised as a risk factor for subsequent asthma (Greisner 2000).
The mechanisms connecting upper and lower airway dysfunction are still under investigation but might include nasal‐bronchial reflex, mouth breathing caused by nasal obstruction, and pulmonary aspiration of nasal contents. Allergic rhinitis and asthma are both mediated by similar allergic inflammatory mechanisms and may represent two manifestations of the same disease (Togias 2003).
Epidemiological data indicate that nasal symptoms are experienced by as many as 30 to 99% of patients with allergic asthma (Simons 1999). Likewise asthma is experienced by 15 to 40% of patients with rhinitis (Leynaert 2000). Moreover, up to 30% of the people with allergic rhinitis, with no past history of asthma, will show bronchial hyperreactivity after provocative bronchial challenge with metacholine (Kapsali 1997; Fireman 2000). Both nose and bronchi biopsy results show eosinophilic inflammation, reticular basement membrane thickening and epithelium shedding but in a greater extent in the bronchial than in the nasal mucosa (Chanez 1999). Recent studies identify that nasal allergen challenge induces bronchial eosinophilic inflammation giving additional weight to a common immunopathogenesis in rhinitis and asthma.
To emphasise the strong link between upper and lower airways, the terms united airways disease (Passalacqua 2001) or combined allergic rhinitis and asthma syndrome have recently been proposed by the World Asthma Organization (Bousquet 2001) with no definite consensus.
Treatment of rhinitis with intranasal corticosteroids (INCS) is both safe and effective on most nasal symptoms. Since there is a link between nasal and bronchial disease in asthma and rhinitis, it has been postulated that INCS may improve asthma.
Rhinitis and particularly asthma are also associated with an enormous social, psychological and economic burden. Optimisation of asthma control is therefore of clinical and financial importance.
Some studies have shown that INCS have some additional benefit on controlling allergic seasonal asthma in adults (Watson 1993), allergic seasonal asthma in children (Allen 1997; Pedersen 1998), allergic perennial and seasonal asthma in children (Henriksen 1984) and allergic asthma after cat exposure in children (Wood 1995). Others however have shown that INCS do not influence bronchial hyper‐responsiveness in children and young adults with allergic seasonal rhinitis and asthma (Thio A 2000).
Most available narrative reviews support a positive effect of INCS on various asthma outcomes (Corren 1992; Spector 1997; Pauwels 1998; Mygind 1998; Fireman 2000; Gawchick 2000; Scadding 2000). However, none of the major asthma guidelines recommend intranasal glucocorticoids to control asthma (WHO/NHLBI 1995; BTS 1997; NHLBI 1997; NAC 1998). In contrast a panel of experts has recently published in collaboration with the WHO a global guideline on rhinitis and asthma named Allergic Rhinitis and its Impact on Asthma initiative (Bousquet 2001). This guideline states that optimal management of rhinitis may improve coexisting asthma.
In the face of recent variations between guidelines, this systematic review aims to determine whether treatment with intranasal corticosteroids is effective on asthma outcomes, in patients suffering from both allergic and non allergic rhinitis and asthma.
Objectives
To assess the efficacy of INCS on asthma outcomes in people with allergic or non allergic rhinitis with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that report at least one asthma outcome and that compared the efficacy of INCS to placebo, or to other conventional treatments for asthma or in addition to other conventional asthma medications. Some trials may be only single blind.
Types of participants
We included studies recruiting children and adults. The diagnosis of rhinitis was made on clinical criteria such as sneezing, rhinorrhoea, nasal obstruction and/or nasal itching. Intermittent/seasonal and persistent/perennial rhinitis were considered for inclusion with severity ranging from mild to moderate‐severe. Asthma was defined by clinical criteria, typical physiological features, or American Thoracic Society (ATS) criteria. Both allergic and non‐allergic rhinitis and asthma cases were included. The definition of allergic asthma and rhinitis was established by the presence of the previously described criteria in addition with one or more positive atopic markers relevant to the site area. These tests included the presence of at least one positive skin prick test (SPT) to a common inhalant allergen (wheal greater than three mm compared with the diluent within 15 min of the SPT) or the presence of at least one positive in serum specific IgE to a common inhalant allergen. We considered studies conducted in primary care, hospital outpatients and an institutional care setting.
Types of interventions
Three types of intervention were considered: (1) RCTs comparing INCS to placebo; (2) RCTs comparing INCS to other conventional treatments for asthma; and (3) RCTs comparing INCS in addition with other conventional asthma medications to conventional treatment for asthma.
Conventional treatment for asthma could include: inhaled, oral and parenteral corticosteroids, short and long acting ß2 agonists, ipratropium bromide, sodium cromoglycate, theophylline, leukotriene receptor antagonists, 5‐lipoxygenase inhibitors and specific immunotherapy. Intrabronchial corticosteroids were not allowed but a device combining intranasal and intrabronchial corticosteroid was considered as being a primary INCS technique and was therefore also compared to placebo.
Types of outcome measures
We considered all outcomes. Some parameters of particular interest considered to be important a priori were: (1) Outcomes reflecting symptoms: nocturnal/day time symptoms scores and nocturnal/day time, rescue ß2 agonist use (2) Outcomes reflecting asthma exacerbations: hospital admission rates and length of stay, unscheduled doctor visits, emergency room visits and days off school/work (3) Outcomes reflecting well‐being: quality of life assessments (4) Outcomes reflecting airway calibre: forced expiratory volume at one second (FEV1) (Litres), peak expiratory flow (PEF) (Litres/min) and methacholine challenge test (PC20 FEV1, PD20 FEV1). (5) Outcomes reflecting inflammation: eosinophils in induced sputum (x 10E6/ml and/or percentage), eosinophil cationic protein (ECP) (mg/l) and exhaled gases.
Search methods for identification of studies
We searched the Cochrane Airways Group trials register (March 2004), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2004), MEDLINE (January 1966 to March 2004) using the terms:
(( rhinitis or hay fever) AND (asthma OR wheez* OR hyper‐responsiveness)) AND ((intranasal OR nasal) AND (steroid OR corticosteroid OR glucocorticoid OR beclomethasone OR fluticasone OR triamcinolone OR budesonide OR mometasone)).
We considered all articles regardless of language.
We also examined reference lists from the articles obtained and approached the authors of published guidelines and recognised experts in rhinitis and asthma management for additional studies.
Data collection and analysis
Study selection One reviewer screened titles and abstracts of the electronic search. From the results of the screened electronic search, bibliographic searches and other contacts, two reviewers independently selected trials which met previously defined inclusion criteria.
Quality Assessment Two reviewers independently abstracted study characteristics and outcome measures. The trials were scored using the Cochrane approach: Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
The studies were also assessed using a five point scoring instrument (Jadad 1996) (1) Was the study described as randomised? (yes = 1 no = 0) (2) Was the study described as double blind? (yes = 1 no = 0) (3) Was there a description of withdrawals and dropouts? (yes = 1 no = 0) (4) Was the method of randomisation well described and appropriate? (yes = 1 no = 0) (5) Was the method of double blinding well described and appropriate? (yes = 1 no = 0) (6) Deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. There was a 92.50% agreement between reviewers, with a kappa of 0.82. Disagreement was resolved by consensus.
Data extraction Two reviewers extracted data from each outcome for the published results of included trials. All the outcomes listed above were assessed and the results were reported as both absolute values and changes compared to baseline. When outcomes were measured at several time‐points, only data from the last time‐point that could be ascertained was used. When data were extracted from graphical plots, we made an attempt to verify such data by contacting authors. If an intention to treat analysis (ITT) was not used by the investigators, and it was not explicit in the presentation of how many participants (N) were in each group at the time of last evaluation of that outcome, the appropriate N value for each intervention group was calculated by subtracting the number of participants who withdrew in each intervention group from those randomised to each intervention group. We contacted authors to clarify details of randomisation and/or to request missing outcome data. Data for reported outcomes which could not be calculated in the meta‐analysis have been listed in Table 01. Data analysis Outcomes were analysed as continuous or dichotomous variables, using standard statistical techniques available in Review Manager:
(1) For continuous outcomes, the weighted mean difference (WMD) and 95% confidence intervals were calculated. (2) For dichotomous outcomes, the relative risk (RR) was calculated with 95% confidence intervals
We conducted a subgroup analysis based on study design: Crossover versus parallel group design.
Results
Description of studies
Results of the search
The literature search identified a total of 19 possible publications reporting 18 distinct trials. Data was in a form adequate for analysis in 14 of the 18 trials. Attempts to contact the author of one trial for more detailed information have been unsuccessful (Gupta 1996). Another author was unable to provide the required additional information to allow assessment of the study (Wilson 2000). This study was therefore excluded from the systematic review. One study compared two different intrabronchial corticosteroids and did not match the type of comparison we had predefined (Gale 1981). A fourth study was neither blinded or controlled and had therefore to be excluded (Bilancia 1992). Study design, generic name and dose of intranasal corticosteroids, technique of intranasal inhalation and reported outcomes varied widely. These are reported in the Table of Included Studies. An update search conducted in March 2004 did not yield any new studies.
Included studies
The 14 studies that were analysed were comprised of nine parallel and five cross‐over randomised placebo‐controlled trials. One of the cross‐over studies was also able to be analysed as a parallel study by extracting the outcomes for the first treatment period only (Pedersen 1998).
There were a total of 477 participants enrolled in the 14 included trials. Of these 453 completed the various studies, with 44 withdrawals (9.2%). Seventy‐two participants came from one study (Thio B 2000). The other trials enrolled a smaller number of participants with a range from 11 to 60.
Three studies were assessing a pediatric population, 10 were studying adults and one study included a heterogeneous group with a mean age of 17 years.
Most trials were of short or intermediate duration with six studies lasting from one to four weeks and seven trials from six to eight weeks. One additional trial extended to 12 weeks (Armitage 1992).
Five studies were appraising a population with perennial rhinitis (Henriksen 1984; Aubier 1992; Watson 1993; Wood 1995; Pedersen 1998). The latest of these trials was focusing on patients with rhinitis to cat exposure who underwent several cat challenges. Nine studies were assessing a population with seasonal allergic rhinitis during the pollen season (Reed 1988; Pedersen 1990; Armitage 1992; Corren 1992; Pelucchi 1995; Foresi 1996; Thio A 2000; Thio B 2000; Rak 2001).
All studies but Pedersen 1998 included patients with allergic rhinitis defined by typical clinical symptoms in association with positive atopy markers such as skin prick tests and/or specific IgE. Pedersen 1998 focused on a mixed population with children featuring allergic and non allergic rhinitis.
Seven out of 14 studies were including patients with asthma. Four additional trials had an heterogeneous population where some had asthma and others not (Reed 1988; Armitage 1992; Wood 1995; Rak 2001). Three studies excluded asthmatics but measured asthma outcomes (Aubier 1992; Pelucchi 1995; Foresi 1996).
Severity of asthma was overall poorly described. Six studies classified asthma as mild or intermittent (Reed 1988; Pedersen 1990; Bilancia 1992; Corren 1992; Watson 1993;Thio A 2000; Thio B 2000). One other trial did not specify the degree of severity but stated that the participants were requiring inhaled corticosteroids (Pedersen 1998). Another four papers did not clarify severity at all (Henriksen 1984; Armitage 1992; Wood 1995; Rak 2001). Overall asthma severity of included participants can therefore be extrapolated as mild.
Intervention
Twelve studies compared intranasal corticosteroids to intranasal placebo (Henriksen 1984; Reed 1988; Pedersen 1990; Armitage 1992; Corren 1992; Watson 1993; Pelucchi 1995; Wood 1995; Foresi 1996; Pedersen 1998; Thio A 2000; Thio B 2000). One of the two active treatment arms of Pelucchi 1995 compared intranasal corticosteroids to intranasal antihistamines. Aubier 1992 compared intranasal to intrabronchial corticosteroids. One study compared INCS to specific immunotherapy (Rak 2001).
Seven out of 14 studies used intranasal beclomethasone at a dose ranging from 200 to 400 ug/day (Reed 1988; Armitage 1992; Aubier 1992; Corren 1992; Watson 1993; Pelucchi 1995; Thio B 2000). Four out of 14 studies used intranasal budesonide with a dose ranging from 400 to 1292 ug/day (Henriksen 1984; Pedersen 1990; Pedersen 1998; Rak 2001. Three trials used fluticasone 200 ug/d (Foresi 1996; Thio A 2000; Thio B 2000) and another study used triamcinolone 440 ug/day (Wood 1995).
The intranasal inhalation technique was not specified except in two studies (Pedersen 1990; Pedersen 1998). A pear shaped, valved holding device equipped with a nozzle was added to the metered dose inhaler for drug delivery in these two cases. The inhalation technique was performed as follows: after a deep expiration to residual volume, drug actuation was synchronised at commencement of a deep inspiration through each nostril and then a breath hold for 10 seconds. The aim of this technique was to achieve a combined intranasal and intrabronchial corticosteroid deposition.
Outcomes
All asthma outcomes were considered and in particular asthma symptom scores, preference for treatment period, rescue ß2 agonist use, rescue terfenadine use, quality of life assessments, forced expiratory volume at one second (FEV1) (Litres and percentage predicted), Tiffenau ratio (FEV1/FVC) (percentage predicted), maximal mid‐expiratory flow rate (FEF 25‐75) (percentage predicted), peak expiratory flow (PEF) (Litres/min and percentage predicted), bronchial challenge test (PC20 FEV1 mg/ml, PD20 FEV1 mg/ml, Log PD20 umol), global side effects, specific side effects, drop‐out due to side‐effects, drop‐out due to ineffective treatment.
Objective lung function measures such as FEV1 and PEF, various asthma symptom scores and bronchial hyperreactivity challenges were the main outcomes that could be compared for meta‐analysis. Inconsistent reporting of the other above mentioned outcomes precluded meta‐analysis.
Risk of bias in included studies
All studies stated that the treatment allocation was random. None of the papers nevertheless described the method and the concealment of randomisation. All the studies had therefore an unclear allocation concealment corresponding to a Cochrane B grade.
Jadad scoring varied between two and three. The rating of the 14 studies was (Armitage 1992: B3), (Aubier 1992: B2), (Corren 1992: B3), (Foresi 1996: B3), (Henriksen 1984: B3), (Pedersen 1990: B3), (Pedersen 1998: B3), (Pelucchi 1995: B3), (Rak 2001: B3), (Reed 1988: B3), (Thio A 2000: B3), (Thio B 2000 and Thio C 2000: B3), (Watson 1993: B3), (Wood 1995: B2).
The 14 studies were said to be double‐blind. Blinding of patients was therefore assumed in all studies but was only specified in two (Aubier 1992; Wood 1995). Blinding of the care providers was again assumed in all trials but was only specified in one study (Aubier 1992). None of the 14 studies mentioned blinding of outcome assessors.
Withdrawals and drop‐outs were accounted in 12 papers and could be deducted in the three additional studies (Aubier 1992; Wood 1995; Foresi 1996).
Effects of interventions
FEV1
Eight out of 14 studies described FEV1 outcomes. Seven studies assessed FEV1 for the comparison of INCS versus intranasal placebo: (Henriksen 1984; Corren 1992; Pelucchi 1995; Wood 1995; Foresi 1996; Thio A 2000; Thio B 2000). The meta‐analysis found a non significant improvement in FEV1 of 0.31 standardised mean difference (95% CI ‐0.04 to 0.65, Analysis 1.1) favouring treatment. There was no significant heterogeneity (p = 0.36) in this result.
1.1. Analysis.
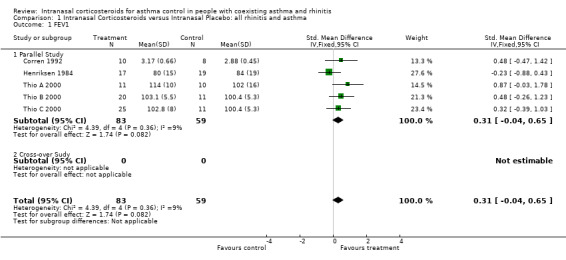
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 1 FEV1.
FEV1 was expressed as percentage predicted in four studies (Henriksen 1984; Pelucchi 1995; Thio A 2000; Thio B 2000), as percentage of change in one study (Wood 1995) and in litres in two studies (Corren 1992; Foresi 1996). Six studies found no significant change in FEV1 between groups at baseline and at completion of the studies. Wood 1995 focused on the effect of intranasal steroids after cat challenge. He found that the maximum change in FEV1 for challenges on placebo ranged from 4 to 53% (median 17.5%) and from 1 to 59% (median 15%) (p = 0.07) for challenges on the active drug. The difference in FEV1 was more obvious during the first half of the challenge and only reached statistical significance at the 30 minute mark (p = 0.05). Data for FEV1 from four studies could be pooled together for meta‐analysis. These studies compared INCS versus intranasal placebo, in a population of asthmatics with rhinitis (Henriksen 1984; Corren 1992; Thio A 2000; Thio B 2000; Thio C 2000). As mentioned above under description of studies, Thio B 2000 had two treatment arms and one control group, namely: intranasal beclomethasone, intranasal fluticasone and intranasal placebo. By convenience, Thio B 2000 refers to the intranasal beclomethasone group when compared to intranasal placebo and Thio C 2000 refers to the intranasal fluticasone group when compared to the intranasal placebo group. Although these two distinct results for FEV1 come from the same study, they are expressed as if they were extracted from two different studies when pooled into a meta‐analysis. One study assessed FEV1 for the comparison of intranasal versus intrabronchial corticosteroids. Aubier 1992 found no statistical differences in FEV1 (percentage predicted) between or within groups at the end of the study.
PEF
Seven out of 14 studies described PEF outcomes. Six studies assessed PEF for the comparison of INCS versus intranasal placebo: ( Henriksen 1984; Pedersen 1990; Armitage 1992; Corren 1992; Watson 1993; Pedersen 1998). Data for morning PEF from two parallel studies (Henriksen 1984; Pedersen 1998) comparing intranasal corticosteroids versus intranasal placebo in "united airway disease" could be pooled together for meta‐analysis. Morning PEF declined by ‐25.33 (WMD 95% CI ‐62.42 to 11.76, Analysis 1.2) (Litres/min) after active treatment. No significant heterogeneity was detected between these two studies. Data for morning PEF from two cross‐over studies (Pedersen 1990; Watson 1993) comparing INCS versus intranasal placebo in a population with asthma and rhinitis, could also be aggregated. It found an improvement in morning PEF of 36.51 (WMD 95% CI ‐1.80 to 74.82) (Litres/min). No significant heterogeneity was detected between these two studies.
1.2. Analysis.
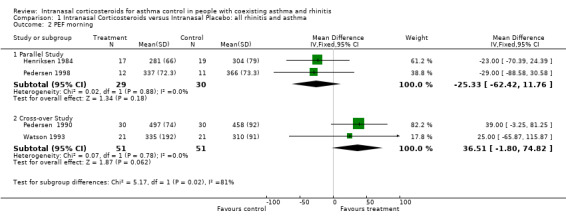
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 2 PEF morning.
Five trials provided the results of morning and evening PEF in L/min. Corren 1992 presented only morning average PEF in L/min. No differences were noted in morning and evening PEF between active and placebo treatments in two studies ( Henriksen 1984; Watson 1993). Similarly (Corren 1992) found no differences in daily morning PEF between the two treatment groups. There was a small but statistically significant increase in morning PEF (p = 0.02) in favour of the intranasal budesonide group in Pedersen 1990 and a borderline statistically significant elevation in evening PEF (p = 0.05) in favour of the beclomethasone group in Armitage 1992. Pedersen 1998 noted a change in the in office PEF favouring budesonide (p = 0.01), an improvement in the mean morning PEF favouring budesonide (p < 0.05) and an increase in the mean evening PEF favouring budesonide (p = 0.05).
Data for evening PEF from two parallel studies (Henriksen 1984; Pedersen 1998) comparing intranasal corticosteroids versus intranasal placebo in "united airway disease" could be pooled together for meta‐analysis. There was a decline in evening PEF of ‐27.37 (WMD 95% CI ‐65.43 to 10.68, Analysis 1.3) after active treatment. No significant heterogeneity was detected between these two studies. Data for evening PEF from two cross‐over studies (Pedersen 1990; Watson 1993) comparing intranasal corticosteroids versus intranasal placebo in rhinitis and asthma patients, could also be aggregated. It found a WMD in evening PEF of 17.11 (95% CI ‐19.07 to 53.29) (l/min) indicating a non significant trend favouring the active treatment group. No significant heterogeneity was detected between these two studies.
1.3. Analysis.
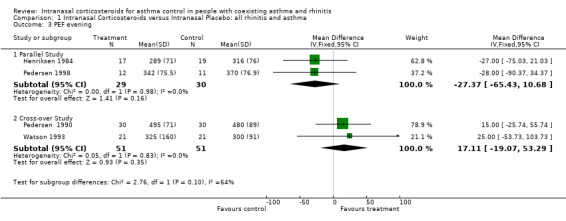
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 3 PEF evening.
Data for mean PEF from three parallel studies (Henriksen 1984; Corren 1992; Pedersen 1998) comparing intranasal corticosteroids versus intranasal placebo in "allergic rhino‐bronchitis" could be pooled together for meta‐analysis. It found a SMD in mean PEF of ‐0.10 (95% CI ‐0.55 to 0.35, Analysis 1.3) (Litres/min and percentage predicted) that favoured placebo in a non significant manner. Heterogeneity was not detected.
Overall there was no consistent benefit of INCS on PEF in asthma.
One study assessed PEF for the comparison of INCS versus other asthma conventional treatment such as specific immunotherapy in this case (Rak 2001). The authors expressed the mean weekly PEF in percentage predicted. Rak 2001 found that specific immunotherapy to birch pollen did not alter the PEF throughout the study. Surprisingly, the other group who was treated by intranasal budesonide and specific immunotherapy to placebo, experienced a significant decrease in PEF during week three (p = 0.01) and week four (p = 0.02), nevertheless these differences were not recorded at week five and week six at the end of the trial.
Asthma Symptom Scores
Ten trials reported various asthma symptom scores measured using different scales for the comparison of INCS versus intranasal placebo: (Henriksen 1984; Reed 1988; Armitage 1992; Corren 1992; Watson 1993; Pelucchi 1995; Wood 1995; Pedersen 1998; Thio A 2000; Thio B 2000). The following four studies showed meaningful differences in asthma symptom scores between the two treatment groups. Pedersen 1998, reported that the asthma symptom scores improved significantly during the last week of the budesonide treatment group (p < 0.05). Reed 1988, demonstrated that the placebo treated participants reported a 10‐fold increase in symptoms of asthma in comparison with the glucocorticoid treated participants, during the ragweed pollen season (p < 0.001). Moreover the expected increase in seasonal asthma did not occur in the glucocorticoid treated group. Wood 1995, showed that there was a trend towards reduced asthma symptoms score in the treatment group when compared to placebo, but only 45 to 60 minutes after a cat challenge (p < 0.05). Last, Watson 1993 showed no differences in asthma symptom scores at the end of his cross‐over study. Nevertheless evening asthma scores were significantly lower at week 2 and 3 in the active treatment group when the first treatment period was analysed as a parallel study design (p = 0.001 and p = 0.02, respectively).
Data for asthma symptom scores from two parallel studies (Corren 1992; Pedersen 1998) comparing INCS versus intranasal placebo could be pooled together for meta‐analysis. There was a non significant improvement in symptoms with a SMD of 0.61 (95% CI ‐0.04 to 1.26) (p = 0.07) favouring intranasal corticosteroids. There was nevertheless a mild degree of heterogeneity (p < 0.05) that was mainly related to the different inhalation techniques, the different budesonide doses and the different symptom scales used. Pedersen 1998 used a nozzle allowing the inhalation to go through the nose into the bronchi, achieving therefore a combined intranasal and intrabronchial corticosteroid deposition. Moreover the Pedersen 1998 study used a very high budesonide dose, that was 2.4‐fold greater than the average dose of the three other studies implementing budesonide. Pedersen 1998 was studying children and Corren 1992 studied adults.
Airway Responsiveness
Nine out of 14 studies described bronchial hyper‐responsiveness outcomes. Seven studies described airway reactivity tests in various ways for the comparison of INCS versus intranasal placebo: (Armitage 1992; Corren 1992; Watson 1993; Pelucchi 1995; Foresi 1996; Thio A 2000; Thio B 2000).
Data for methacholine challenges from three parallel studies, comparing INCS versus intranasal placebo in "allergic rhino‐bronchitis" could be pooled together for meta‐analysis (Thio A 2000; Thio B 2000; Thio C 2000). There was no overall effect of INCS on bronchial hyper‐responsiveness with a SMD of ‐0.20 (95% CI ‐0.64 to 0.24, Analysis 1.6) (log PD20 methacholine) (p = 0.4). Heterogeneity was not detected.
1.6. Analysis.
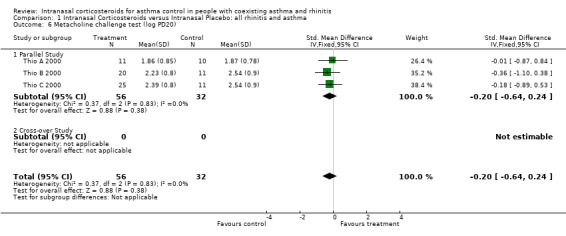
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 6 Metacholine challenge test (log PD20).
Armitage 1992 showed no differences in bronchial responsiveness when comparing the two treatment groups. Corren 1992 showed that participants treated with beclomethasone were significantly protected from the seasonal increase, at week six, in bronchial reactivity seen in the placebo group (p = 0.022). In Foresi 1996, after four weeks of treatment during the pollen season, the change in PD20 methacholine in the fluticasone group was not significant, whereas decrease in PD20 methacholine in the placebo group was highly significant (p < 0.01). Neither beclomethasone nor azelastine significantly attenuated the increase in bronchial responsiveness to methacholine, occurring during the pollen season in Pelucchi 1995. Thio A 2000 demonstrated that the log PD20 methacholine decreased significantly both in the fluticasone (p = 0.002) and in the placebo group (p = 0.01), but without intergroup differences (p = 0.2). Thio B 2000 showed that six weeks of fluticasone, beclomethasone or placebo tended to increase the log PD20 methacholine in all three groups but the changes were not significant within or between groups (p = 0.97). Watson 1993 demonstrated that there was a significant improvement in geometric mean PC 20 methacholine after four weeks of intranasal beclomethasone (p = 0.04) but not after placebo.
One trial assessed bronchial hyper‐responsiveness for the comparison of intranasal versus intrabronchial corticosteroids (Aubier 1992). The authors described that two weeks of intranasal beclomethasone markedly decreased bronchial hyper‐responsiveness (p < 0.001) whereas no change was noted after two weeks of intrabronchial beclomethasone.
One study assessed bronchial hyper‐responsiveness for the comparison of INCS versus other asthma conventional treatment such as specific immunotherapy in this case. In Rak 2001, patients were analysed in two subgroups: rhinitis only and rhinitis plus asthma. They underwent a six weeks treatment of intranasal budesonide or benefited from specific immunotherapy to birch. Methacholine sensitivity increased significantly (p = 0.007) only in rhinitics. In contrast, methacholine sensitivity did not increase in the asthmatic subgroup. Data were not available to compare both treatment arms at the end of the trial.
Side Effects
Side effects were rare and mild and did not cause any patient to withdraw from the 14 included trials. Side effects were reported as specific outcomes in three studies in a number of different ways (Reed 1988; Pedersen 1990; Pedersen 1998), making direct comparisons impossible. None of the side effects outcomes were statistically significant when compared to the control group within any of these three studies. Ten participants treated by intranasal placebo and one participant treated with intranasal budesonide complained from sneezing immediately after the use of the spray (Henriksen 1984). In the same study one participant in the intranasal budesonide group suffered from transient haemorrhagic nasal secretions.
Withdrawals and Drop‐outs
There were 44 withdrawals out of 477 participants initially enrolled in the 14 trials reviewed. Nine out of 14 trials described withdrawals. There were various reasons for withdrawals but none due to side effects of intranasal glucocorticoids. Nine studies reported withdrawals and drop‐out all for the comparison of INCS versus intranasal placebo: (Henriksen 1984; Reed 1988; Pedersen 1990; Armitage 1992; Corren 1992; Watson 1993; Pelucchi 1995; Thio A 2000; Thio B 2000). Thio B 2000 was the biggest trial that included 72 participants. There were five withdrawals all because of non‐compliance with the protocol namely two from the beclomethasone group and three from the placebo group. Reed 1988 included 60 participants but 12 withdrew. Eight withdrawals came from the placebo group: five for inefficacy, two for non compliance and one for moving away. Four withdrawals came from the beclomethasone group: two for inefficacy, one for non compliance and one for unrelated disease.
Nine patients dropped‐out from Pelucchi 1995. Two participants withdrew from the beclomethasone group for unrelated reasons and for asthma and two patients withdrew from the placebo group for uncontrolled rhinitis and asthma. Five remaining participants dropped‐out from the azelastine group for various reasons.
Seven participants dropped‐out in the first arm of a cross‐over study while treated by intranasal beclomethasone: five for protocol failure and two for upper respiratory tract infection (Watson 1993).
In Thio A 2000, four participants dropped‐out: two from the placebo group and two from the treatment group. One participant dropped‐out in the placebo group because of uncontrollable symptoms and the three remaining all because of non‐compliance with the protocol. Four participants dropped‐out from another study: one for an upper respiratory tract infection and one for being unable to be contacted from the treatment group; one for an upper respiratory tract infection and one for rhinitis symptoms in the placebo group (Corren 1992).
One patient dropped‐out because of viral meningitis (Armitage 1992). In Henriksen 1984, one patient dropped‐out for inadequate symptom recording. Another patient withdrew for personal reasons from Pedersen 1990.
Discussion
The recent WHO/ARIA guidelines on rhinitis suggested that optimal treatment of rhinitis may improve coexisting asthma (Bousquet 2001). Various randomised controlled trials on INCS in united airway disease were listed and described, however, no clear therapeutical proposition could be advised.
We have therefore performed a systematic review on this topic and have analysed 14 randomised controlled trials which addressed this issue in order to provide the best possible evidence for the efficacy of intranasal corticosteroids in patients with coexisting asthma and rhinitis.
All studies included patients with a documented seasonal or perennial allergic condition apart from Pedersen 1998 who enrolled a population suffering from allergic and nonallergic rhinitis
All the asthma outcomes were initially considered but only FEV1, FEF 25‐75, different PEF measurements, bronchial hyperreactivity challenges, various asthma symptom scores, rescue medication use and side effects were repeated through studies with some consistency. Data analysing outcomes related to inflammation, quality of life and asthma exacerbation were not available in the included studies.
All the above listed outcomes were first analysed separately and, in a second stage, eight meta‐analyses all focusing on a population suffering from rhinitis and mild asthma could be aggregated and interpreted. The outcomes that could be pooled in parallel studies were Asthma Symptom Scores, FEV1, PEF morning, PEF evening, mean PEF and methacholine challenge and the outcomes that could be aggregated together in cross‐over studies were PEF morning and PEF evening. The quality of the studies were classified as intermediate and all of them had an unclear allocation concealment rated B.
FEV1
A total of eight studies described FEV1 outcomes. There was a non significant improvement in FEV1 of 0.31 (SMD 95% CI ‐0.04 to 0.65). These findings suggest that intranasal corticosteroids improve objective lung function such as FEV1 but only in a non statistically significant matter. Despite the fact that this combined result is not statistically significant, there is nevertheless a strong trend towards efficacy with four out of five outcomes in this meta‐analysis favouring treatment and this result has only very mild heterogeneity (p = 0.36). Reasons for homogeneity in this meta‐analysis can be related to the fact that the studied population had asthma of similar severity classified as mild and that the length of all studies was identical with six week duration. It appears clearly that there is a need for new studies to increase the statistical power to assess more adequately if FEV1 can be significantly improved by intranasal glucocorticoids.
PEF
A total of seven studies described PEF outcomes. Three meta‐analyses were performed in the parallel study subgroup. The first meta‐analysis was aggregating three studies assessing mean PEF (Henriksen 1984; Corren 1992; Pedersen 1998). The second and the third meta‐analyses were pooling together the same two studies to analyse respectively morning and evening PEF (Henriksen 1984; Pedersen 1998). Another two meta‐analyses were performed in the cross‐over subgroup, for morning and evening PEF including the same two studies (Pedersen 1990; Watson 1993).
These five separate meta‐analyses for various PEF measurements all failed to show a positive impact of INCS. This result is disconcerting in the light of the previous data showing a clear trend towards improvement of FEV1 after INCS. These findings seem nevertheless to be valid because there was no heterogeneity in all five meta‐analysis and because all five meta‐analyses expressed the same result. Part of the explanation may be that PEF measurements are less sensitive than spirometry and that they rely on compliance. The lower sensitivity of PEF measurements is probably too subtle to be of significance in moderate to severe asthmatics but might be sufficient to prevent detection of airway caliber modifications in mild or intermittent asthma. A limitation of our PEF assessment is that the average of studies included in each meta‐analyses was only 2.2, thus lowering the statistical power of the global result. It is interesting to underline that Pedersen 1990 and Armitage 1992 were the only studies to demonstrate an increase in PEF after intranasal corticosteroids. The potential positive impact of the nozzle technique, used in Pedersen 1990 has already been raised above. Armitage 1992 is one of the two longest trials of three months duration. It is therefore possible that a sufficient duration as well as a combined intranasal‐intrabronchial corticosteroid inhalation techniques are prerequisite to properly evaluate PEF in mild asthmatics.
Asthma Symptom Scores
Ten out of 14 studies reported various asthma symptoms scores but only four showed a positive impact of INCS when compared to intranasal placebo. A meta‐analysis that pooled results for asthma symptom score from two parallel studies (Corren 1992; Pedersen 1998) demonstrated a non significant improvement in asthma symptom score of 0 .61 (SMD 95% CI ‐0.04 to 1.26).
Although this combined result is not statistically significant, it suggests nevertheless that intranasal steroids could have some impact in reducing asthma symptoms and it highlights in particular that the combined intranasal and intrabronchial corticosteroid deposition technique implemented by Pedersen 1998 might be of potential benefits. These findings are however weakened by mild heterogeneity (p < 0.05) possibly related to the different inhalation techniques, the variation of budesonide doses and the use of different symptom scales. Pedersen 1998 used a specific nozzle device allowing a combined topical glucocorticoid deposition in the nose and in the bronchi. Moreover, the Pedersen 1998 study used a 2.4‐fold higher dose of budesonide than the average dose used in the three other studies implementing budesonide. This specific corticosteroid deposition in two locations of the naso‐bronchial airway is possibly of great importance and has not been sufficiently studied. The potential advantages include a greater corticosteroid deposition in the airways without an increase of total corticosteroid dose, a potential reduction in oral candidosis and an improvement in compliance. The optimal corticosteroid dose for this combined naso‐bronchial deposition is not defined yet.
Bronchial reactivity
A total of nine studies described bronchial reactivity outcomes. A meta‐analysis could be done with three results of methacholine challenges extracted from two separate parallel studies (Thio A 2000; Thio B 2000; Thio C 2000). The global result showed no effect of intranasal steroids on bronchial reactivity and there was no heterogeneity in these results. This finding is surprising mainly because the methacholine challenge test is more sensitive than spirometry and also because nearly half of the studies (44%) that described this outcome, demonstrated a reduction in bronchial reactivity after active treatment. A possible explanation is again the fact that, the statistical power of this meta‐analysis, done with only three outcomes, is too low.
One study assessed PC20 methacholine for the comparison of INCS versus specific immunotherapy but did not demonstrate a difference between the two treatment groups. One study assessed bronchial responsiveness for the comparison of intranasal versus intrabronchial corticosteroids. After two weeks of INCS PD35 for SGaw increased significantly, but surprisingly not after two weeks of intrabronchial corticosteroids at equivalent dose. Seven out of 14 studies assessed airway responsiveness with methacholine challenges for the comparison of intranasal corticosteroids versus intranasal placebo. A reduction in airway reactivity or the avoidance of seasonal increase seen in the placebo group was demonstrated in three trials (43%), after treatment with intranasal corticosteroids (Corren 1992; Watson 1993; Foresi 1996).
Side effects
INCS were well tolerated and caused only mild side effects. No withdrawals were related to side effects even in Pedersen 1998, who used the highest dose of intranasal budesonide (1292 ug/d). The two longest studies of three months duration each, did not mention any side‐effects.
Authors' conclusions
Implications for practice.
INCS are well tolerated but their overall efficacy in "united airway disease" still remains unclear. In the small number of studies reported, INCS improve did not lead to significant improvement of asthma symptom scores, FEV1, morning and evening PEF and bronchial reactivity in mild asthma. There were trends favouring INCS which indicate that INCS as monotherapy may be a promising alternative in coexisting rhinitis and mild asthma. The classical combination of an INCS in addition to an intrabronchial corticosteroid should nevertheless remain the current treatment practice in mild, moderate and severe asthma until more research is done on this topic.
Implications for research.
There is a need for further research on the role of INCS in co‐existing asthma and rhinitis. The most interesting issue is the efficacy of the nozzle device allowing the inhalation to go through the nose and into the bronchi, achieving therefore a combined intranasal and intrabronchial corticosteroid deposition. There is also an urgent need to focus on patients presenting with rhinitis and moderate to severe forms of asthma in the absence of existing data. Optimal corticosteroid dosage should also be defined mainly for the nozzle inhalation technique and in the more severe asthmatic population. The duration of a future study should extend to at least three months to assess the efficacy of intranasal glucocorticoids on various asthma outcome and to explore potential side effects. Further studies comparing monotherapy with INCS versus intrabronchial corticosteroids are also required.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 19 March 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to acknowledge the Australian Cochrane Airways Group Network for financial assistance towards the completion of this systematic review, by granting the Scholarship Award 2002 to Dr. Philip Taramarcaz. Also thanks to Claire Allen for her helpful comments on the synopsis.
Data and analyses
Comparison 1. Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 5 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.04, 0.65] |
| 1.1 Parallel Study | 5 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.04, 0.65] |
| 1.2 Cross‐over Sudy | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PEF morning | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Parallel Study | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐25.33 [‐62.42, 11.76] |
| 2.2 Cross‐over Study | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | 36.51 [‐1.80, 74.82] |
| 3 PEF evening | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Parallel Study | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐27.37 [‐65.43, 10.68] |
| 3.2 Cross‐over Study | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | 17.11 [‐19.07, 53.29] |
| 4 Mean PEF | 3 | 77 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.55, 0.35] |
| 4.1 Parallel Study | 3 | 77 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.55, 0.35] |
| 4.2 Cross‐over Study | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Asthma Symptom Score | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.04, 1.26] |
| 5.1 Parallel Study | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.04, 1.26] |
| 5.2 Cross‐over Study | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Metacholine challenge test (log PD20) | 3 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 6.1 Parallel Study | 3 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 6.2 Cross‐over Study | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.4. Analysis.
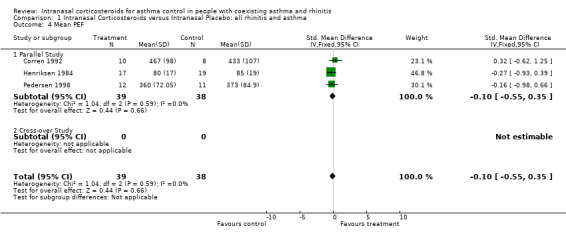
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 4 Mean PEF.
1.5. Analysis.
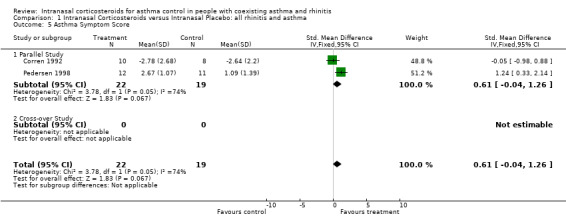
Comparison 1 Intranasal Corticosteroids versus Intranasal Placebo: all rhinitis and asthma, Outcome 5 Asthma Symptom Score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armitage 1992.
| Methods | DESIGN: double‐blind, parallel, placebo‐ controlled study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT BLINDING: said to be double‐blind PROVIDER BLINDING: said to be double‐blind OUTCOME ASSESSOR BLINDING: not specified WITHDRAWALS: 0 DROP‐OUTS: 1 (from the treatment group because of viral meningitis) | |
| Participants | ELIGIBLE: 23 ENROLLED: 20 COMPLETED: 19 AGE (MEAN): 35 SEX (male/female): not specified NUMBER IN THE TREATMENT GROUP: 9 NUMBER IN THE CONTROL GROUP: 11 RECRUITMENT: local GP and the Allergy and Chest Clinic of the Churchill Hospital in Oxford, UK ASTHMA DIAGNOSIS: questionnaire (clinical criteria) ASTHMA SEVERITY: not homogeneous (some with asthma = FEV1 < 76% and some without asthma FEV1 > 85%) RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT > 3 mm for grass mix and negative for house dust mites, specific IgE for grass pollen mix, tree pollen mix and rape pollen) INCLUSION: unselected hay fever sufferers, half with a history of previous seasonal wheezing EXCLUSION: not specified | |
| Interventions | SETTING: not specified TREATMENT GROUP: intranasal beclomethasone 50 ug 2 puffs in each nostril 2/d (concomitant treatment: cetrizine 10 mg prn, inhaled salbutamol 0.1 ug prn, topical eye drops prn) PLACEBO GROUP: intranasal placebo 2 puffs each nostril 2/d (concomitant treatment: cetirizine 10 mg prn, inhaled salbutamol 0.1 ug prn, topical eye drops prn) DURATION: 3 months | |
| Outcomes | ‐asthma score (cough, wheeze and number of puffs of salbutamol) from julian days D160‐175 ‐wheeze score from D160‐175 ‐cough score from D160‐175 ‐log PD20 methacholine (umol) from D160‐175 ‐number of puffs of Ventolin from D160‐175 ‐morning PEF (l/m) from D160‐175 ‐evening PEF (l/m) from D160‐175 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Aubier 1992.
| Methods | DESIGN: double‐blind, randomised, cross‐over study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT BLINDING: stated, PROVIDER BLINDING: stated OUTCOME ASSESSOR BLINDING: not specified WITHDRAWALS/DROP‐OUTS: not specified (probably 0) | |
| Participants | ELIGIBLE: not stated ENROLLED: 11 COMPLETED: probably 11 AGE (MEAN): 33+‐9 SEX (male/female): stated 5/9 =14 with an error (total should be 11) NUMBER IN THE TREATMENT GROUP: not stated NUMBER IN THE CONTROL GROUP: not stated RECRUITMENT: Chest and Allergy Unit, Hopital Bichat, Paris, France ASTHMA DIAGNOSIS: no asthma (no history of asthma, normal physical examination and spirometry) ASTHMA SEVERITY: 0 RHINITIS DIAGNOSIS: perennial symptoms RHINITIS CLASSIFICATION: perennial allergic rhinitis ATOPY MARKERS: SPT for common aeroallergens INCLUSION: no asthma, bronchial hyperresponsiveness, positive SPT EXCLUSION: sinusitis, smoker, on intranasal or inhaled corticosteroids, acute flare‐up of rhinitis or upper respiratory tract infection < 2 months | |
| Interventions | SETTING: not specified RUN IN PHASE: 1 week TREATMENT GROUP: intranasal beclomethasone 50 ug 4/d + inhaled placebo 4/d CONTROL GROUP: inhaled beclomethasone 50 ug 4/d + intranasal placebo 4/d WASH‐OUT: 0 then cross‐over DURATION: 2 weeks | |
| Outcomes | ‐FEV1 (% pred) at the end of treatment period ‐FEF 25‐75 (% pred) at the end of treatment period ‐SGaw (L/S/cm H2O/L) at the end of treatment period ‐PD35 for SGaw (ug carbacol) at the end of the treatment period ‐change in PD35 SGaw at the end of the treatment period | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Corren 1992.
| Methods | DESIGN: randomised, double‐blinded, placebo‐ controlled, parallel study METHOD OF RANDOMISATION: not stated CONCEALMENT OF RANDOMISATION: not stated PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: not described in detail but double‐blinded stated WITHDRAWALS/ DROP‐OUTS: 2 drop‐outs from the treatment group (1 upper respiratory tract infection, 1 unreachable), 2 from the placebo group (1 upper respiratory tract infection, 1 rhinitis symptoms) | |
| Participants | ELIGIBLE: not stated ENROLLED: 22 COMPLETED: 18 AGE (MEAN): 25.6 SEX (male/female): 5/13 NUMBER IN THE TREATMENT GROUP: 12 NUMBER IN THE CONTROL GROUP: 10 RECRUITMENT: National Jewish Center for Immunology and Respiratory Medicine, Denver, USA ASTHMA DIAGNOSIS: clinical criteria and PC20 methacholine < 8 mg/ml ASTHMA SEVERITY: mild RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: allergic seasonal rhinitis ATOPY MARKERS: SPT for ragweed INCLUSION: SPT for ragweed, history of seasonal rhinitis and asthma during August and September, PC20 methacholine < 8 mg/ml EXCLUSION: intranasal or inhaled or oral glucocorticosteroids or cromolyn sodium < 3 months, upper respiratory tract infection < 6 weeks, specific immunotherapy < 3 years, quit smoking < 1 year, pet allergy | |
| Interventions | SETTING: National Jewish Center for Immunology and Respiratory Medicine, Denver, USA TREATMENT GROUP: intranasal beclomethasone 42 ug 2 puffs in every nostril bd = 336 ug/d CONTROL GROUP: intranasal placebo 2 puffs each nostril bd (rescue medication for both groups: albuterol 2 puffs every 4 hours, dexbrompheniramine with pseudoephedrine 1 bd) DURATION: 6 weeks | |
| Outcomes | ‐chest symptoms at the end of trial ‐daily average PEF (L/min) ‐FEV1 (L) at the end of the trial ‐PC20 methacholine (ug/ml) at the end of the trial | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Foresi 1996.
| Methods | DESIGN: randomised, double‐blind, placebo‐ controlled, parallel study METHOD OF RANDOMISATION: not stated CONCEALMENT OF RANDOMISATION: not stated PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: not described in detail but double‐blind stated WITHDRAWALS/ DROP‐OUTS: not stated | |
| Participants | ELIGIBLE: not stated ENROLLED: 50 COMPLETED: 50 AGE (MEAN): 27.8 SEX (male/female): stated but with an error (33/21) NUMBER IN THE TREATMENT GROUP: 24 NUMBER IN THE CONTROL GROUP: 26 RECRUITMENT: 2 outpatient clinics from northern Italy ASTHMA DIAGNOSIS: no asthma ASTHMA SEVERITY: 0 RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: allergic seasonal rhinitis ATOPY MARKERS: SPT for a mixture of grasses or parieteria INCLUSION: history of seasonal allergic rhinitis of at least 3 years, no asthma, no previous specific immunotherapy EXCLUSION: nasal polyps and sinusitis, esthablished asthma or wheeze, antihistamines in the previous 6 weeks, nasal surgery in the previous 6 months | |
| Interventions | SETTING: Not certain but in northen Italy TREATMENT GROUP: intranasal fluticasone 50 ug/ in 2 puffs in each nostril once a day = 200 ug/d CONTROL GROUP: intranasal placebo 2 puffs in each nostril 1 day (rescue medication for both groups: salbutamol spray, terfenadine 60 mg, Na cromoglycate eye drops (dose not specified) DURATION: 6 weeks | |
| Outcomes | ‐chest symptoms at the end of trial (visit 4) ‐FEV1 (L) at the end of the trial at week 6 ‐PC20 methacholine (mg) at the end of the trial | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Henriksen 1984.
| Methods | DESIGN: double‐blind, parallel study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: not detailed but blinding stated WITHDRAWALS/DROP‐OUTS: 1 for inadequate symptom recording but group not stated | |
| Participants | ELIGIBLE: not stated ENROLLED: 37 COMPLETED: 36 AGE (MEAN): 11.6 SEX (male/female): 26/11 NUMBER IN THE TREATMENT GROUP: 18 NUMBER IN THE CONTROL GROUP: 19 RECRUITMENT: Pediatric Department, Aarhus, Denmark ASTHMA DIAGNOSIS: ATS criteria ASTHMA SEVERITY: not specified RHINITIS DIAGNOSIS: history of nasal obstruction > 3 months RHINITIS CLASSIFICATION: perennial allergic rhinitis ATOPY MARKERS: SPT and RAST to either/or HDM, animal dander grass INCLUSION: ATS criteria, nasal obstruction for > 3 months, atopy markers, no use of topical or systemic steroids < 5 months EXCLUSION: septal deviation, nasal polyps, enlarged adenoids | |
| Interventions | SETTING: Pediatric Department, Aarhus, Denmark TREATMENT GROUP: intranasal budesonide 50 ug 2 puffs every nostril bd = 400 ug/d PLACEBO GROUP: intranasal placebo 2 puffs every nostril bd DURATION: 4 weeks + 2 weeks of run‐in | |
| Outcomes | ‐PEF morning (% pred) D15‐42 ‐PEF evening (% pred) D15‐42 ‐FEV1 (% pred) at week 4 ‐FEF 25‐75 (% pred) at week 4 ‐mean daily asthma symptom score | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Pedersen 1990.
| Methods | DESIGN: placebo‐controlled, double‐blind, cross‐over study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: only stated WITHDRAWALS/DROP‐OUTS: 0 | |
| Participants | ELIGIBLE: not specified ENROLLED: 30 COMPLETED: 30 AGE (MEAN): 25.5 SEX (male/female): 16/14 NUMBER IN THE TREATMENT GROUP: not specified NUMBER IN THE CONTROL GROUP: not specified RECRUITMENT: Respiratory Department, Aarhus, Denmark ASTHMA DIAGNOSIS: clinical criteria (wheeze, cough, dyspnoea) ASTHMA SEVERITY: intermittent to mild persistent (didn't require inhaled or intranasal corticosteroids) RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT and RAST to grass pollen INCLUSION: positive SPT and RAST to grass pollens, rhinitis and asthma during the pollen season during at least 1 of the last 5 seasons EXCLUSION: perennial rhinitis, asthma requiring corticosteroids, severe nasal blockage, women likely to become pregnant | |
| Interventions | SETTING: Respiratory Department, Aarhus, Denmark RUN IN PHASE: 10 days TREATMENT GROUP: intranasal budesonide 100 ug 2 puffs in every nostril bd = 800 ug/d CONTROL GROUP: intranasal placebo 2 puffs in every nostril bd WASH OUT: 0 then cross‐over DURATION: 14 days | |
| Outcomes | ‐preference for specific bronchial symptoms (wheezing) in week 2 ‐preference for specific bronchial symptoms (breathlessness) in week 2 ‐preference for specific bronchial symptoms (cough) in week 2 ‐morning PEF (l/m probably) for trial period ‐evening PEF (l/m probably) for trial period ‐use of rescue terbutaline for the trial period ‐dry nose for the trial period ‐bleeding nose for the trial period ‐hoarseness for the trial period ‐nose irritation from spray for the trial period ‐mouth irritation from spray for the trial period ‐throat irritation from spray for the trial period ‐lung irritation from spray for the trial period ‐other adverse effects for the trial period | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Pedersen 1998.
| Methods | DESIGN: double‐blind, placebo‐controlled, cross‐over study METHOD OF RANDOMISATION: not stated CONCEALMENT OF RANDOMISATION: not stated PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: not specified WITHDRAWALS/DROP‐OUTS: 1 withdrawal for personal reasons from (placebo‐budesonide group) | |
| Participants | ELIGIBLE: not specified ENROLLED: 24 COMPLETED:23 AGE (MEAN): 11.3 SEX (male/female): 13/9 NUMBER IN THE TREATMENT GROUP: not stated NUMBER IN THE CONTROL GROUP: not stated RECRUITMENT: Department of Pediatrics, Rigs Hospital, Copenhagen, Denmark ASTHMA DIAGNOSIS: lung function with increase of > 20% after bronchodilation ASTHMA SEVERITY: not specified but were requiring inhaled corticosteroids in average 646 ug/d RHINITIS DIAGNOSIS: not specified RHINITIS CLASSIFICATION: perennial allergic and non allergic rhinitis ATOPY MARKERS: not specified INCLUSION: perennial allergic and non allergic rhinitis and asthma. Asthma was defined by FEV1 increase of more than 20% after inhalation of a B2 agonist EXCLUSION: not specified | |
| Interventions | SETTING: Department of Pediatrics, Rigs Hospital, Copenhagen, Denmark RUN IN: 2 weeks TREATMENT GROUP: intranasal budesonide (double dose of usual inhaled budesonide given bd): mean 1292 ug/d CONTROL GROUP: intranasal placebo bd WASH‐OUT: 2 weeks then cross‐over DURATION: 3 weeks | |
| Outcomes | ‐morning home PEF (l/min) for week 3 ‐evening home PEF (l/min) for week 3 ‐asthma symptom score for week 3 ‐number of puffs from B2 agonist during week 3 ‐side effect score for nose bleed during week 3 ‐side effect score for dry nose during week 3 ‐side effect score for hoarseness during week 3 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Pelucchi 1995.
| Methods | DESIGN: randomised, double‐blind, placebo‐ controlled, parallel study METHOD OF RANDOMISATION: not stated CONCEALMENT OF RANDOMISATION: not stated PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: just stated WITHDRAWALS/DROP‐OUTS: 5 withdrawals from the intranasal azelastine treatment group: 2 for personal reasons, 1 for asthma, 2 for pregnancy . Two others withdrew from the intranasal beclomethasone group for unrelated disease and for asthma. Two withdrew from the placebo group for uncontrolled rhinitis and asthma. | |
| Participants | ELIGIBLE: not specified ENROLLED: 45 COMPLETED: 36 AGE (MEAN): 25.6 SEX (male/female) for those completing the study: 21/15 NUMBER IN THE AZELASTINE TREATMENT GROUP: 10 NUMBER IN THE BECLOMETHASONE TREATMENT GROUP: 13 NUMBER IN THE CONTROL GROUP: 13 RECRUITMENT: Servizio of respiratory physiologia, Opedale di Sesto San Giovanni, Italy ASTHMA DIAGNOSIS: no asthma ASTHMA SEVERITY: 0 RHINITIS DIAGNOSIS: clinical seasonal symptoms for 3 consecutive years RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT only to grasses INCLUSION: history of allergic rhinoconjonctivitis for at least 3 consecutive years, SPT to grasses, no asthma EXCLUSION: positive SPT to other aeroallergens than grasses, previous specific immunotherapy, asthma, deviated nasal septum, polyps | |
| Interventions | SETTING: Servizio of respiratory physiologia, Opedale di Sesto San Giovanni, Italy TREATMENT GROUP A: intranasal beclomethasone 50 ug 2 puffs per nostril bd = 200 ug/d TREATMENT GROUP B: Intranasal azelastine 0.14 ug 1 puff in each nostril bd CONTROL GROUP: intranasal placebo 1 day DURATION: 6 weeks | |
| Outcomes | ‐FEV1 (% pred) during the pollen season (week 4) when comparing beclomethasone and placebo ‐FEV1 (% pred) during week 4 when comparing beclomethasone and azelastine ‐PD20 methacholine (umol) at week 4 when comparing beclomethasone to placebo ‐PD20 methacholine (umol) at week 4 when comparing beclomethasone to azelastine. ‐Chest symptoms at week 4 when comparing beclomethasone to placebo ‐Chest symptoms at week 4 when comparing beclomethasone to azelastine ‐medication requirement at week 4 when comparing beclomethasone to placebo ‐medication requirement at week 4 when comparing beclomethasone to azelastine | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Rak 2001.
| Methods | DESIGN: double‐blind, randomised, comparative, parallel study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: stated double‐blinded WITHDRAWALS/DROP‐OUTS: 0 | |
| Participants | ELIGIBLE: not specified ENROLLED: 41 COMPLETED: 41 AGE (MEAN): 29 SEX (male/female): 22/19 NUMBER IN THE TREATMENT GROUP: 20 NUMBER IN THE CONTROL GROUP: 21 RECRUITMENT: Ear, nose and throat department, Uppsala, Sweden ASTHMA DIAGNOSIS: history, need for B2 agonists, PC20 methacholine < 8 mg/ ml outside the pollen season ASTHMA SEVERITY: not sated: but 20 had no asthma and 21 had asthma RHINITIS DIAGNOSIS: history RHINITIS CLASSIFICATION: seasonal allergic rhinoconjonctivitis ATOPY MARKERS: SPT and IgE for birch INCLUSION: birch pollen allergic patients with some having only rhinoconjonctivitis and other having rhinoconjonctivitis and asthma. EXCLUSION: perennial rhinitis symptoms, SPT to house dust mites or moulds | |
| Interventions | SETTING: Ear, nose and throat department, Uppsala, Sweden TREATMENT GROUP: intranasal budesonide 200 per nostril daily = 400 ug ug/d + specific immunotherapy to placebo (concomitant drugs: local antihistamine, decongestant drops, antihistamine tablets) PLACEBO GROUP: intranasal placebo 2 puffs every nostril daily + specific immunotherapy to birch (concomitant drugs: local antihistamine, decongestant drops, antihistamine tablets) DURATION: 6 weeks | |
| Outcomes | ‐mean weekly PEF (% pred) at week 6 ‐B2 agonist use (number of puffs) at week 6 ‐methacholine challenge (mg/ml) at week 6 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Reed 1988.
| Methods | DESIGN: double‐blind, parallel, randomised study for beclomethasone and placebo. Single‐blinded for flunisolide and cromolyn but data not used. METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: stated double‐blinded WITHDRAWALS/DROP‐OUTS: 8 withdrawals from placebo group and 4 withdrawals from beclomethasone group | |
| Participants | ELIGIBLE: not specified ENROLLED: 60 (for beclomethasone and placebo) COMPLETED: 48 AGE (MEAN): not clear SEX (male/female): not clear NUMBER IN THE TREATMENT GROUP: 30 NUMBER IN THE CONTROL GROUP: 30 RECRUITMENT: Department of Allergic Diseases and Internal Medicine, Mayo clinic, Minesota, USA ASTHMA DIAGNOSIS: clinical criteria: asthma score, but only 48% had asthma ASTHMA SEVERITY: not stated for the 48 % with asthma RHINITIS DIAGNOSIS: hay fever score RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT and IgE for ragweed INCLUSION: history of seasonal rhinitis in August and September, no ragweed hyposensitization therapy for the last 2 years, SPT to ragweed, elevated pre‐seasonal ragweed IgE, patent nasal airways, good general health EXCLUSION: pregnant, nasal polyps | |
| Interventions | SETTING: Allergic Diseases and Internal Medicine, Mayo clinic, Minnesota, USA TREATMENT GROUP: intranasal beclomethasone 2 puffs in every nostril bd = 336 ug/d (concomitant drugs: antihistamine, bronchodilators decongestant tablets) PLACEBO GROUP: intranasal placebo 2 puffs every nostril bd (concomitant drugs: antihistamine, bronchodilators decongestant tablets) DURATION: 8 weeks | |
| Outcomes | ‐mean weekly asthma symptom score ‐drop‐out due to ineffective treatment for all the treatment period ‐drop‐out due to side‐effects for all the treatment period | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Thio A 2000.
| Methods | DESIGN: double‐blind, placebo controlled, parallel study study. METHOD OF RANDOMISATION: not specified but stated CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: stated double‐blinded WITHDRAWALS/DROP‐OUTS: 3 withdrawals from the treatment group for non compliance and 1 drop‐out for uncontrolled symptoms in the control group | |
| Participants | ELIGIBLE: not specified ENROLLED: 25 COMPLETED: 21 AGE (MEAN): 17.3 SEX (male/female): 8/13 NUMBER IN THE TREATMENT GROUP: 11 NUMBER IN THE CONTROL GROUP: 10 RECRUITMENT: University Hospital of Leiden, Netherlands ASTHMA DIAGNOSIS: ATS criteria: ASTHMA SEVERITY: mild RHINITIS DIAGNOSIS: clinical history RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT or IgE for grasses INCLUSION: steady state EXCLUSION: use of intranasal, intrabronchial or oral corticosteroids in the last 3 months, concurrent treatment with theophylline, anticholinergic agents, long acting B2 agonists, cromones, specific immunotherapy, upper or lower respiratory tract infection in the last 3 weeks | |
| Interventions | SETTING: University Hospital of Leiden, Netherlands TREATMENT GROUP: intranasal fluticasone 50 ug 2 puffs in each nostril daily = 200 ug/d (concomitant drugs: salbutamol 200 ug prn, levocabastine eye drops prn) PLACEBO GROUP: intranasal placebo (concomitant drugs: salbutamol 200 ug prn, levocabastine eye drops prn) DURATION: 6 weeks | |
| Outcomes | ‐ asthma symptom score at week 6 ‐ FEV1 at week 6 (% pred) ‐log PD20 methacholine (ug) at week 6 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Thio B 2000.
| Methods | DESIGN: double‐blind, placebo‐ controlled, parallel study METHOD OF RANDOMISATION: not specified but stated CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: stated double‐blinded WITHDRAWALS/DROP‐OUTS: 3 withdrawals from treatment group and 3 withdrawals from the placebo group | |
| Participants | ELIGIBLE: not specified ENROLLED: 72 COMPLETED: 67 AGE (MEAN): 19.1 SEX (male/female): 27/45 NUMBER IN THE TREATMENT GROUP with beclomethasone: 23 NUMBER IN THE CONTROL GROUP: 24 RECRUITMENT: University Hospital of Leiden, Netherlands ASTHMA DIAGNOSIS: ATS criteria ASTHMA SEVERITY: mild RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT to grasses or specific IgE to grasses > 0.7kU/l INCLUSION: steady state EXCLUSION: use of intranasal, intrabronchial or oral corticosteroids in the last 3 months, concurrent treatment with theophylline, anticholinergic agents, long acting B2 agonists, cromones, specific immunotherapy, upper or lower respiratory tract infection in the last 3 weeks | |
| Interventions | SETTING: University Hospital of Leiden, Netherlands TREATMENT GROUP: intranasal beclomethasone 50 ug 2 puffs in each nostril bd = 400 ug/d (concomitant drugs: salbutamol 200 ug prn, levocabastine eye drops prn) PLACEBO GROUP: intranasal placebo (concomitant treatment: salbutamol 200 ug prn, levocabastine eye drops prn) DURATION: 6 weeks | |
| Outcomes | ‐asthma symptom score at the end of trial at week 6 ‐FEV1 (% pred) at week 6 ‐logPD 20 methacholine (ug) at week 6 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Thio C 2000.
| Methods | DESIGN: double‐blind, placebo‐controlled, parallel study METHOD OF RANDOMISATION: not specified but stated CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: stated double‐blinded WITHDRAWALS/DROP‐OUTS: 0 withdrawals from treatment group and 3 withdrawals from the placebo group | |
| Participants | ELIGIBLE: not specified ENROLLED: 72 COMPLETED: 67 AGE (MEAN): 19.1 SEX (male/female): 27/45 NUMBER IN THE TREATMENT GROUP with fluticasone: 25 NUMBER IN THE CONTROL GROUP: 24 RECRUITMENT: University Hospital of Leiden, Netherlands ASTHMA DIAGNOSIS: ATS criteria ASTHMA SEVERITY: mild RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: seasonal allergic rhinitis ATOPY MARKERS: SPT to grasses or specific IgE to grasses > 0.7kU/l INCLUSION: steady state EXCLUSION: use of intranasal, intrabronchial or oral corticosteroids in the last 3 months, concurrent treatment with theophylline, anticholinergic agents, long acting B2 agonists, cromones, specific immunotherapy, upper or lower respiratory tract infection in the last 3 weeks | |
| Interventions | SETTING: University Hospital of Leiden, Netherlands TREATMENT GROUP: intranasal fluticasone 50 ug 2 puffs in every nostril daily = 200 ug/d (concomitant drugs: salbutamol 200 ug prn, levocabastine eye drops prn) PLACEBO GROUP: intranasal placebo (concomitant treatment: salbutamol 200 ug prn, levocabastine eye drops prn) DURATION: 6 weeks | |
| Outcomes | ‐asthma symptom score at the end of trial at week 6 ‐FEV1 (%pred) at week 6 ‐logPD 20 methacholine (ug) at week 6 | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Watson 1993.
| Methods | DESIGN: randomised, double‐blind, placebo‐ controlled, cross‐over study METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT, PROVIDER AND OUTCOME ASSESSOR BLINDING: not specified but said to be double‐blinded WITHDRAWALS/DROP‐OUTS: 5 drop‐outs in the first arm of treatment group because of failure of protocol and 2 drop‐outs for an upper respiratory tract infection | |
| Participants | ELIGIBLE: not specified ENROLLED: 28 COMPLETED: 21 AGE (MEAN): 10.0 SEX (male/female): not specified NUMBER IN THE TREATMENT GROUP: 10 for 1st period before cross‐over NUMBER IN THE CONTROL GROUP: 11 for 1st period before cross‐over RECRUITMENT: Department of Pediatrics, Allergic and Clinical Immunology Section, Children Hospital of Winnipeg, Manitoba, Canada ASTHMA DIAGNOSIS: methacholine challenge (PC20 < 8 mg/ml) ASTHMA SEVERITY: mild RHINITIS DIAGNOSIS: clinical criteria RHINITIS CLASSIFICATION: perennial allergic rhinitis ATOPY MARKERS: SPT and or IgE to non specified perennial antigens INCLUSION: perennial documented allergic rhinitis, mild asthma (level 1 of Canadian guidelines), IgE or SPT for perennial antigens, airway responsiveness (PC 20 < 8 mg/ml) EXCLUSION: other causes of nasal obstruction, use of intranasal corticosteroids for the last 30 days before the beginning of the trial | |
| Interventions | SETTING: Department of Pediatrics, Allergic and Clinical Immunology Section, Children Hospital of Winnipeg, Manitoba, Canada RUN‐IN: 0 but no steroids for 30 days prior trial initiation TREATMENT GROUP: intranasal beclomethasone 100 ug each nostril bd = 400 ug/d CONTROL GROUP: intranasal placebo each nostril bd WASH‐OUT: 0 then cross‐over DURATION: 4 weeks | |
| Outcomes | ‐PEF morning (l/min) for last week of trial ‐PEF evening (l/min) for last week of trial PC20 metacholine (mg/ml) at the end of the trial ‐asthma symptom score at the end of the trial | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Wood 1995.
| Methods | DESIGN: double‐blind crossover trial METHOD OF RANDOMISATION: not specified CONCEALMENT OF RANDOMISATION: not specified PATIENT BLINDING: yes patient was unaware PROVIDER BLINDING: was stated double‐blinded OUTCOME ASSESSOR BLINDING not stated WITHDRAWALS/DROP‐OUTS: not described | |
| Participants | ELIGIBLE: not specified ENROLLED: 12 COMPLETED: 12 AGE (MEAN): 21 to 44 years SEX (male/female): 5/7 NUMBER IN THE TREATMENT GROUP: 6 probably NUMBER IN THE CONTROL GROUP: 6 probably RECRUITMENT: Asthma and Allergy Center, John Hopkins Hospital, Baltimore, USA ASTHMA DIAGNOSIS: not all had asthma. 11 had asthma with 6 diagnosed on history and 5 diagnosed on methacholine test ASTHMA SEVERITY: not specified for those with asthma RHINITIS DIAGNOSIS: history RHINITIS CLASSIFICATION: allergic perennial rhinitis with sensitisation to cat ATOPY MARKERS: SPT and IgE to cat and various common aeroallergens INCLUSION: SPT to cat > 2 mm, rest unclear EXCLUSION: not specified | |
| Interventions | SETTING: Asthma and Allergy Center, John Hopkins Hospital, Baltimore, USA RUN‐IN: 72 hours for intranasal and inhaled corticosteroids TREATMENT GROUP: triamcinolone 55 ug 2 sprays in each nostril bd = 440 ug/d CONTROL GROUP: intranasal placebo 2 sprays in every nostril bd WASH‐OUT: 2 weeks then cross‐over DURATION: 1 week | |
| Outcomes | ‐lower respiratory symptoms during cat challenge ‐total coughs during cat challenge ‐FEV1 (% change) during cat challenge ‐late phase response (PEF monitoring for 12 hours after the challenge) | |
| Notes | Jadad score 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bilancia 1992 | This study was randomised but not blinded and there was no control group. |
| Gale 1981 | This study was comparing 2 different intrabronchial corticosteroids in addition to the same intranasal corticosteroid. Therefore, it did not conform to the type of intervention we had predefined. |
| Wilson 2000 | This study was probably not a RCT. The authors couldn't provide the additional requested information. |
Contributions of authors
Taramarcaz P (PT01) ‐ inclusion/exclusion, quality assessment, data extraction, analysis and interpretation, drafting/writing/editing the protocol and the review
Gibson PG (MDPG) ‐ instigation of the review, intellectual direction and supervision, inclusion/exclusion, quality assessment, data extraction, analysis and interpretation.
Sources of support
Internal sources
Airway Inflammation Research Group and Hunter Medical Research Institute, Australia.
External sources
Geneva University Hospital, Switzerland.
Declarations of interest
Nil
Edited (no change to conclusions)
References
References to studies included in this review
Armitage 1992 {published data only}
- Armitage JM, Sin Fai Lam K, Wilkinson I, Faux JA, Hopkin JM. Investigation of the tendency to wheeze in pollen sensitive patients. Clinical and Experimental Allergy 1992;22:916‐22. [DOI] [PubMed] [Google Scholar]
Aubier 1992 {published data only}
- Aubier M, Levy J, Clerici C, Neukirch F, Herman D. Different effects of nasal and bronchial glucocorticosteroid administration on bronchial hyperresponsiveness in patients with allergic rhinitis. American Review of Respiratory Disease 1992;146:122‐6. [DOI] [PubMed] [Google Scholar]
Corren 1992 {published data only}
- Corren J, Adinoff AD, Buchmeier AD, Irvin CG. Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. Journal of Allergy and Clinical Immunology 1992;90:250‐6. [DOI] [PubMed] [Google Scholar]
Foresi 1996 {published data only}
- Foresi A, Pelucchi A, Gherson G, Mastropasqua B, Chiapparino A, Testi R. Once daily intranasal fluticasone propionate (200 ug) reduces nasal symptoms and inflammation but also attenuates the increase in bronchial responsiveness during the pollen season in allergic rhinitis. Journal of Allergy and Clinical Immunology 1996;98:274‐82. [DOI] [PubMed] [Google Scholar]
Henriksen 1984 {published data only}
- Henriksen JM, Wenzel A. Effect of an intranasally administered corticosteroid (budesonide) on nasal obstruction, mouth breathing and asthma. American Review of Respiratory Disease 1984;130:1014‐8. [DOI] [PubMed] [Google Scholar]
Pedersen 1990 {published data only}
- Mygind N, Bisgaard H, Dahl R. Simultaneous treatment of rhinitis and asthma by nasal inhalation of corticosteroid from a spacer. Allergy 1999;54 Suppl 57:132‐53. [DOI] [PubMed] [Google Scholar]
- Pedersen B, Dahl R, Lindqvist N, Mygind N. Nasal inhalation of the glucocorticoid budesonide from a spacer for treatment of patients with pollen rhinitis and asthma. Allergy 1990;45:451‐6. [DOI] [PubMed] [Google Scholar]
Pedersen 1998 {published data only}
- Pedersen W, Hjuler I, Bisgaard H, Mygind N. Nasal inhalation of budesonide from a spacer in children with perennial rhinitis and asthma. Allergy 1998;53:383‐7. [DOI] [PubMed] [Google Scholar]
Pelucchi 1995 {published data only}
- Pelucchi A, Chiapparino A, Mastropasqua B, Marazzini L, Hernandez A, Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to metacholine in allergic rhinitis in response to grass pollens. Journal of Allergy and Clinical Immunology 1995;95:515‐23. [DOI] [PubMed] [Google Scholar]
Rak 2001 {published data only}
- Rak S, Heinrich C, Jacobsen L, Scheynius A, Venge P. A double‐blinded, comparative study of the effects of short preseason specific immunotherapy and topical steroids in patients with allergic rhinoconjonctivitis and asthma. Journal of Allergy and Clinical Immunology 2001;108:921‐8. [DOI] [PubMed] [Google Scholar]
Reed 1988 {published data only}
- Reed CE, Marcoux JP, Welsh PW. Effects of topical nasal treatment on asthma symptoms. Journal of Allergy and Clinical Immunology 1988;81(5):1042‐7. [DOI] [PubMed] [Google Scholar]
- Welch PW, Stricker WE, Chu C‐P, Naessens JM, Reese ME, Reed CE, et al. Efficacy of Beclomethasone nasal solution, Flunisolide, and Cromolyn in relieving symptoms of ragweed allergy. Mayo Clin Proc 1987;62:125‐34. [DOI] [PubMed] [Google Scholar]
Thio A 2000 {published data only}
- Thio BJ, Slingerland GLM, Fredriks AM, Nagelkerke AF, Scheeren RA, Neijens HJ, et al. Influence of intranasal steroids during grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax 2000;55:826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thio B 2000 {published data only}
- Thio BJ, Slingerland GLM, Fredriks AM, Nagelkerke AF, Scheeren RA, Neijens HJ, et al. Influence of intranasal steroids during grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax 2000;55:826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thio C 2000 {published data only}
- Thio BJ, Slingerland GLM, Fredriks AM, Nagelkerke AF, Scheeren RA, Neijens HJ, et al. Influence of intranasal steroids during grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax 2000;55:826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1993 {published data only}
- Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: Effect on lower airways responsiveness. Journal of Allergy and Clinical Immunology 1993;91(1):97‐101. [DOI] [PubMed] [Google Scholar]
Wood 1995 {published data only}
- Wood RA, Eggleston PA. The effects of intranasal steroids on nasal and pulmonary responses to cat exposure. American Journal of Respiratory and Critical Care Medicine 1995;151:315‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bilancia 1992 {published data only}
- Bilancia R, Federici F, Ricatti DG, Buonpensiere L, Ferrannini A, Gramiccioni E. Spirometric value modifications caused by beclamethasone dipropionate nasal spray in patients with persistent allergic rhinitis [Modificazione dei valori spirometrici indotti dal beclomethasone dipropionato per via nasale in pazienti con rhinite allergica perenne]. Archivo Monaldi 1992;47(6):47‐53. [PubMed] [Google Scholar]
Gale 1981 {published data only}
- Gale AE, Harding P, Solomon E. Flunisolide intranasal solution combined with intrabronchial steroids in adults with both bronchial asthma and perennial rhinitis. Annals of Allergy 1981;46:268‐72. [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Subjective and objective markers of treatment response in patients with seasonal allergic rhinitis. Annals of Allergy Asthma and Immunology 2000;85:111‐4. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gupta 1996 {published data only}
- Gupta SK. Reversion and antihistamines. Journal of Association of Physicians of India 1996;44(1):72‐3. [PubMed] [Google Scholar]
Additional references
Allen 1997
- Allen ED, Whitaker ER, Ryu G. [Six‐week trial of nebulised flunisolide nasal spray: efficacy in young children with moderately severe asthma]. Pediatric Pulmonology 1997;24:397‐405. [DOI] [PubMed] [Google Scholar]
Bousquet 2001
- Bousquet J, Cauwenberge P, Khaltaev N. [Allergic Rhinitis and its Impact on Asthma (ARIA)]. Journal of Allergy and Clinical Immunology 2001;108(5 Suppl):S147‐334. [DOI] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. [The british guidelines on asthma management 1995, review and position statement]. Thorax 1997;52(S51). [Google Scholar]
Chanez 1999
- Chanez P, Vignola AM, Vic P, Guddo F, Buonsignore G, Godard P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. American Journal of Respiratory and Critical Care Medicine 1999;159:588‐95. [DOI] [PubMed] [Google Scholar]
Fireman 2000
- Fireman P. [Rhinitis and asthma connection: management of coexisting upper airway allergic disease and asthma]. Allergy and Asthma Proceedings 2000;21(1):45‐54. [DOI] [PubMed] [Google Scholar]
Gawchick 2000
- Gawchik SM, Saccar CL. [A risk‐benefit assessment of intranasal triamcinolone acetonide in allergic rhinitis]. Drug Safety 2000;23(4):309‐22. [DOI] [PubMed] [Google Scholar]
Greisner 2000
- Greisner WA, Settipane RJ, Settipane GA. [Allergy and Asthma Proceedings]. 2000 21;6(371‐6). [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jadad 2000
- Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. [Systematic reviews and meta‐analysis on treatment of asthma: critical evaluation]. British Medical Journal 2000;320:537‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapsali 1997
- Kapsali T, Horowitz E, Diemer F, Togias A. [Rhinitis is ubiquitous in allergic asthmatics]. Journal of Allergy and Clinical Immunology 1997;99(S138). [Google Scholar]
Leynaert 2000
- Leynaert B, Neukirch F, Demoly P, Bousquet J. [Epidemiologic evidence for asthma and rhinitis comorbidity]. Journal of Allergy and Clinical Immunology 2000;106(5):S201‐5. [DOI] [PubMed] [Google Scholar]
Lundback 1998
- Lundback B. [Epidemiology of rhinitis and asthma]. Clinical and Experimental Allergy 1998;28(2):S3‐10. [PubMed] [Google Scholar]
Mygind 1998
- Mygind N, Dahl R, Nielsen LP. [Effect of nasal inflammation and of intranasal anti‐inflammatory treatment on bronchial asthma]. Respiratory Medicine 1998;92(547‐9). [DOI] [PubMed] [Google Scholar]
NAC 1998
- NAC. [Asthma Management Plan]. Asthma Management Handbook 1998. [http://www.NationalAsthma.org.au]
NHLBI 1997
- National Heart, Lung and Blood Institute. [National asthma education and prevention program expert panel report 2. Guidelines for the diagnosis and management of asthma]. NIH Publication 1997, (97‐4051). [Google Scholar]
Passalacqua 2001
- Passalacqua G, Ciprandi G, Canonica GW. The nose‐lung interaction in allergic rhinitis and asthma: united airways disease. Current Opinion in Allergy and Clinical Immunology 2001;1:7‐13. [DOI] [PubMed] [Google Scholar]
Pauwels 1998
- Pauwels R. [Influence of treatment on the nose and/or the lungs]. Clinical Experimental Allergy 1998;28:S37‐40. [PubMed] [Google Scholar]
Pedersen 1998
- Pedersen W, Hjuler I, Bisgaard H, Mygind N. [Nasal inhalation of budesonide from a spacer in children with perennial rhinitis and asthma]. Allergy 1998;53(4):383‐7. [DOI] [PubMed] [Google Scholar]
Scadding 2000
- Scadding GK. [Other anti‐inflammatory uses of intranasal corticosteroids in upper respiratory inflammatory diseases]. Allergy 2000;55(62):S19‐23. [DOI] [PubMed] [Google Scholar]
Simons 1999
- Simons FE. [Allergic rhinobronchitis: The asthma‐allergic rhinitis link]. Journal of Allergy and Clinical Immunology 1999;104:534‐40. [DOI] [PubMed] [Google Scholar]
Spector 1997
- Spector SL. [Overview of comorbid associations of allergic rhinitis]. Journal of Allergy and Clinical Immunology 1997;99:S773‐80. [DOI] [PubMed] [Google Scholar]
Thio 2000
- Thio BJ, Slingerland GL, Fredriks AM, Nagelkerke AF, Scheeren RA, Neijens HJ, et al. [Influence of intranasal steroids during the grass pollen season on bronchial responsiveness in children with asthma and hay fever]. Thorax 2000;55:826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Togias 2003
- Togias A. Rhinitis and asthma: evidence for respiratory system integration. Journal of Allergy and Clinical Immunology 2003;111:1171‐83. [DOI] [PubMed] [Google Scholar]
Weiss 2001
- Weiss KB, Sullivan SD. [The health economics of asthma and rhinitis.I. Assessing the economic impact]. Journal of Allergy and Clinical Immunology 2001;107:3‐8. [DOI] [PubMed] [Google Scholar]
WHO/NHLBI 1995
- WHO and National Heart, Lung and Blood Institute. [Global strategy for asthma management and prevention workshop report. Global Initiative for Asthma (GINA)]. NIH Publication No 96‐3659A 1995. [Google Scholar]
Wood 1995
- Wood RA, Eggleston PA. [The effects of intranasal steroids on nasal and pulmonary responses to cat exposure]. American Journal or Respiratory and Critical Care Medicine 1995;151:315‐20. [DOI] [PubMed] [Google Scholar]


