Abstract
Objectives:
Adverse childhood experiences (ACEs; ie, exposure to abuse, neglect, household dysfunction in childhood) are associated with poor mental and physical health outcomes across the lifespan. Emerging research suggests parent ACEs also confer risk for poor child outcomes. The relation between parent ACEs and child pain in youth with chronic pain has not yet been examined. The aim of the current longitudinal study was to examine the associations among parent ACEs, parent health, and child pain, in a clinical sample of youth with chronic pain.
Methods:
In total, 192 youth (75.5% female, 10 to 18 y old) and one of their parents (92.2% female) were recruited from tertiary pediatric chronic pain clinics in Canada. At baseline, parents completed self-report measures of ACEs, chronic pain status, anxiety and depressive symptoms, and posttraumatic stress disorder symptoms. At a 3-month follow-up, youth completed self-report measures of pain intensity and pain interference.
Results:
Regression and mediation analyses revealed that parent ACEs significantly predicted parent chronic pain status and depressive symptoms, but not parent anxiety or posttraumatic stress disorder symptoms. Moreover, parent ACEs were not significantly related to youth pain, either directly or indirectly through parent health variables.
Discussion:
Findings suggest that an intergenerational cascade from parent ACEs to parent health to child pain was not present in the current sample. Further research that examines the role of parent ACEs in the development of child chronic pain, as well as other risk and resiliency factors that may mediate or moderate the association between parent ACEs and child chronic pain, is needed.
Key Words: maltreatment, trauma, chronic pain, mental health, children
Research is increasingly demonstrating the critical role of parent health in pediatric chronic pain. Studies have found high rates of physical and mental health problems among parents of youth with chronic pain, with 50% to 75% endorsing their own chronic pain,1,2 50% reporting moderate to severe pain-related disability,3 40% and 62% reporting clinically-elevated depressive and anxiety symptoms, respectively,4 and 20% meeting the clinical cut-off for posttraumatic stress disorder (PTSD).5 Importantly, these health complaints have been linked to worse physical and mental health outcomes for youth with chronic pain, including poorer response to psychological treatments for their chronic pain.6,7
Despite recent attention to parent health in pediatric chronic pain, minimal research has examined adverse childhood experiences (ACEs) among parents of youth with chronic pain. ACEs typically encompass 10 stressful and/or traumatic events (ie, 5 types of maltreatment, 5 types of household dysfunction) that an individual may experience in childhood.8 ACEs have been widely studied and tied to a host of negative physical and mental health outcomes in adulthood,8–11 including those identified as parent factors contributing to poor outcomes in pediatric chronic pain (eg, chronic pain, elevated depressive, anxiety, and PTSD symptoms1,2,5,12–18). Thus, parent ACEs may be a distal risk factor for the development and maintenance of chronic pain in youth, which by increasing parent risk for poor health outcomes may in turn increase child risk for chronic pain and disability. Indeed, emerging research has shown that parent ACEs are related to poorer developmental, health, and psychosocial outcomes in children, with biological (eg, self-rated health, cortisol response) and psychosocial (eg, anxiety and depressive symptoms) parent factors mediating these relations.19–29
Recently, we demonstrated that ACEs, especially physical neglect, are prevalent in parents of youth with chronic pain;30 however, the relation between parent ACEs and pain in youth with chronic pain has not yet been examined. Conceptual and empirical literature suggest that exposure to ACEs can lead to a toxic stress response, wherein prolonged activation of the hypothalamic-pituitary-adrenal (HPA) axis during critical and sensitive periods of development can cause lasting changes in neurobiological (eg, alterations in brain architecture, regional neural connectivity, and gene expression) and psychological (eg, alterations in cognitive and interpersonal styles) processes, which in turn increase risk for poor health outcomes in mothers as well as their offspring.9,31–35 Indeed, parent ACEs have been shown to relate to child outcomes through both direct (eg, epigenetics, elevated stress hormones during pregnancy) and indirect (eg, parenting behaviors) mechanisms.22,24,29,31,36 In this way, a parent’s ACEs may be directly and/or indirectly related to their child’s chronic pain.
The aim of the current longitudinal study was to examine the associations between parent ACEs, parent health at baseline (chronic pain status and mental health symptoms), and child pain variables at follow-up (pain intensity and interference) in a clinical sample of youth with chronic pain. We hypothesized that (1) higher parent ACEs would be related to the presence of chronic pain and greater depressive, anxiety, and PTSD symptoms in parents as well as greater pain intensity and pain interference in youth and that (2) parent health would mediate the associations between parent ACEs and youth pain.
METHODS
This study is part of a broader program of research, entitled the Pain and Mental Health in Youth (PATH) Study, that examined cognitive, behavioral, neurobiological, and social factors in the co-occurrence of chronic pain and internalizing mental health disorders in youth. The data for this research program was collected across multiple sites in Canada, including Alberta Children’s Hospital (ACH) in Calgary, Alberta and the IWK Health Centre in Halifax, Nova Scotia. The aims of the current study were distinct from previously published articles that have used data from the PATH Study.30,37–43
Participants
Youth with chronic pain and one of their parents were recruited from tertiary-level, outpatient chronic pain clinics at pediatric hospitals in Canada. Youth were eligible to participate if they were 10 to 18 years of age and had chronic pain (ie, pain for ≥3 mo) at the time of recruitment that was not associated with an underlying disease (eg, juvenile idiopathic arthritis, cancer). Youth were not eligible if they were unable to read/speak English, did not have access to the internet, or had any of the following: severe cognitive impairment or a developmental disorder, schizophrenia spectrum or other psychotic disorder, or presence of a serious chronic health or life-threatening condition (eg, cancer). Parents were eligible to participate if they were the legal guardian of the youth, could read/speak English, and had access to the internet.
At ACH, 360 families were contacted about the study. Of these, 63 were not eligible and 107 either did not want to participate or could not be reached after initial contact to be enrolled. At the IWK, 76 families were contacted about the study. Of these, 5 were not eligible, 39 either declined participation or could not be reached after initial contact to be enrolled, and 2 did not consent. Of the 220 families enrolled, six could not be reached after enrollment, 6 withdrew before baseline, 1 was not able to participate due to the COVID-19 pandemic, 10 parents either did not complete the baseline survey or the ACE Questionnaire, 2 parents enrolled twice (with a different child), and 3 parent-child dyads were excluded because they were not biologically related. The sample for the current study included 192 parent-child dyads.
Procedure
Study procedures were approved by the institutional research ethics board at each site and have been described in detail elsewhere.38,41,43 In brief, clinic staff provided the research team with the contact information of families who had recently been referred or treated in the chronic pain clinics. The research team also had access to lists of families who were potentially eligible (eg, families who had participated in a clinical outcomes study, families who had been treated in the hospital and were interested in being contacted about research studies). Potential participants were contacted via email, telephone, or standard mail with information about the study. Interested parent-child dyads were screened for eligibility over the phone and an oral informed consent procedure was conducted with eligible dyads. Online versions of the consent and/or assent forms were also emailed to obtain written consent.
At the baseline and 3-month follow-up, parents and youth were each sent a battery of self-report measures. Parents provided sociodemographic information and completed measures of exposure to ACEs, chronic pain, depressive and anxiety symptoms, and PTSD symptoms. Youth completed measures of pain. This study used baseline data for the sample characteristics (ie, sociodemographic variables, youth pain characteristics) and the parent variables of interest (ie, ACEs, chronic pain, mental health symptoms), and follow-up data for the youth variables of interest (ie, pain intensity and pain interference). All forms and measures were administered and completed through Research Electronic Data Capture (REDCap), a secure web-based data collection site.44,45 Parents and youth each received an honorarium (ie, $10 or $20 CAD gift cards) for their participation at each timepoint. Data were collected between February 2017 and September 2020. On average, follow-up was completed 109 days after baseline (SD=17 d, range: 58 to 184 d).
Measures
Sociodemographic Information
Parents completed a sociodemographic questionnaire that asked about their own age, gender, race/ethnicity, marital status, education, employment status, and annual household income, as well as their child’s age, gender, and race/ethnicity.
Parent ACEs
The ACE Questionnaire retrospectively assessed parent exposure to 10 types of ACEs (ie, emotional, physical, and sexual abuse, emotional and physical neglect, lived with someone with substance use problems, mental illness, or who went to jail, witnessed physical violence between parents, parents separated or divorced) in the first 18 years of life. This 28-item measure was developed for the original ACE Study8 by adapting items from existing measures of childhood abuse, neglect, and household dysfunction.46–48 On this measure, ACE types are assessed with one or more items, which are rated on dichotomous (yes/no) or 5-point Likert-type (0=“never true” or “never” to 4=“very often true” or “very often”) scales. If at least one item of the ACE type is endorsed, the ACE is coded as present. Total ACE scores were obtained by summing responses for the 10 types (range: 0 to 10), with higher scores indicating exposure to more types of ACEs. The ACE Questionnaire has demonstrated good psychometric properties in community and high-risk populations (eg, low income women, individuals with major depression49–52) and demonstrated excellent internal consistency (a=0.92−0.94) in the current study.
Parent Chronic Pain
Similar to previous research,1,53 and consistent with the current definition of chronic pain,54 parent chronic pain status was assessed with a dichotomous item (yes/no) that asked about the presence of pain for at least 3 months in a row. If parents selected yes, a follow-up item assessed the duration of their pain in months and years.
Parent Depressive and Anxiety Symptoms
The Hospital Anxiety and Depression Scale (HADS) was administered to assess parent depressive and anxiety symptoms.55 The HADS consists of 14 items that ask about symptoms of depression and anxiety experienced in the past week. Items are rated on a 4-point Likert-type scale, with each item having different anchors (range: 0 to 3). Total scores for the subscales of depression (HADS-D) and anxiety (HADS-A) are obtained by summing the ratings of the relevant items for each subscale (ranges: 0 to 21). Higher scores indicate greater depressive or anxiety symptoms, with a score of 8 on each subscale suggested as the clinical cut-off.56 In the current study, we used the clinical cut-off for descriptive purposes, to identify the percentage of parents reporting clinically elevated depressive or anxiety symptoms, and the continuous score of each subscale for the main analyses. The HADS has demonstrated good reliability and good to very good concurrent validity in patient (eg, primary care, cancer) and community populations.56 In the current study, both the HADS-D and HADS-A showed good internal consistency (as=0.85 and 0.84, respectively).
Parent PTSD Symptoms
Parent PTSD symptoms were assessed with the PTSD Checklist for DSM-5 (PCL-5) with Criterion A.57 Respondents are first asked to describe the worst event they have experienced that continues to bother them in a textbox and answer questions about that event, including how long ago it happened. The 20-item PCL-5 then asks respondents to think about this event and rate how much PTSD-specific symptoms have bothered them in the past month on a 5-point Likert-type scale (0=“not at all” to 4=“extremely”). A total symptom severity score is obtained by summing the ratings for each item (range: 0 to 80). Higher scores indicate greater PTSD symptoms, with a score of 33 suggested as the clinical cut-off.58 Similar to previous research,5,15 we used the clinical cut-off for descriptive purposes, to identify the percentage of parents reporting clinically elevated PTSD symptoms, and the continuous total score for the main analyses. The PCL-5 has excellent reliability and validity59 and has been used in previous research with parents of youth with chronic pain.1,5 It demonstrated excellent internal consistency (a=0.94) in this study.
Youth Pain
Youth pain characteristics were assessed with single items from the widely-used Pain Questionnaire.60 Specifically, pain duration was assessed with an item that asks respondents to indicate how long they have had pain in years and months. Pain locations were assessed with an item that asks respondents to select the parts of their body where they experienced the most aches or pains in the past week from a checklist of 6 options (eg, stomach, head). Pain frequency was assessed with an item that asks respondents to indicate how often they had pain in the past week on a 5-point Likert-type scale (0=“not at all” to 4=“daily”). These items were used to characterize the pain experience of youth in the current study, and have been used in previous research with youth with chronic pain.1,5,15 Youth pain intensity was measured using a validated and reliable 11-point Numerical Rating Scale (0=“no pain” to 10=“worst pain possible”).61 Youth pain interference was assessed with the Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Interference—Short Form. This 4-item measure asks youth to rate the extent to which pain interfered with daily activities such as sleeping in the past week on a 5-point Likert-type scale (1=“never” to 5=“almost always”). A total score is obtained by summing the ratings for each item and then translating the raw score into a standardized T score (range: 36.7 to 74). Higher scores indicate greater pain interference. This measure was developed by the National Institutes of Health and has been validated in youth with chronic pain.62 It demonstrated good internal consistency (a=0.84) in this study.
Data Preparation and Analysis
Data preparation included removing participants who did not complete the ACE Questionnaire, were enrolled twice (same parent with a different child), or were not biologically related. In total, 192 parent-child dyads were included in analyses that used baseline data and 169 parent-child dyads were included in analyses that used follow-up data (88% retention). However, the number of participants in each analysis vary slightly due to missing data. The lower sample size at follow-up was due to attrition (n=2 families withdrew from the study before follow-up, n=4 families could not be reached for follow-up) and incomplete data on the follow-up survey (n=17). The proportion of missing data for baseline variables was 0% for parent ACEs, parent chronic pain status, and parent depressive symptoms, 0.5% for parent anxiety symptoms, and 7.3% for parent PTSD symptoms. The proportion of missing data for follow-up variables was 3.6% for youth pain intensity and 5.9% for youth pain interference. Little’s Missing Completely at Random (MCAR) test,63 which included the continuous study variables, was nonsignificant, χ2 (21)=22.66, P=0.362, indicating that the pattern of missing data did not differ significantly from data missing completely at random. Within-person mean imputation was used for item-level missing data on the measures of parent anxiety and PTSD symptoms, but not youth pain intensity or interference given the short length of these measures. Given the low percentage of missing data, and results of Little’s MCAR test, pairwise deletion was used for all analyses.
Statistical analyses were performed using IBM SPSS Statistics (version 24). Statistical significance was set at P<0.05 for all analyses. Descriptive statistics were used to (1) characterize the sample on sociodemographic characteristics, youth pain characteristics, parent exposure to ACEs, and parent health characteristics, (2) identify the percentage of parents who reported an ACE as their worst event on the measure of parent PTSD symptoms, and (3) calculate mean scores for the study variables. Independent samples t tests, χ2 tests, or Fischer exact tests were used to compare participants at each site on sociodemographic and pain characteristics. Pearson correlations were used to examine relations between the study variables.
Binary logistic regression was used to examine the association between parent ACEs and parent chronic pain status whereas linear regression was used to examine the associations between parent ACEs and parent mental health symptoms and youth pain. For each analysis, omnibus test statistics and regression coefficients of the predictor variables are reported. In addition, for the logistic regression analyses, odds ratios (OR) and 95% confidence intervals (CI) are reported. Both unadjusted and adjusted models were conducted for each outcome, with covariates entered in a separate, first step in the adjusted models. Results of the unadjusted models are reported in the relevant tables and figure but are not described in the text.
To examine the indirect effect of parent ACEs on youth pain through parent health, mediation analyses were conducted when significant correlations were found between key variables (ie, predictor variable correlated with mediator variable and mediator variable correlated with outcome variable). For simple mediation analyses with a continuous mediator variable, PROCESS Macro for SPSS (Version 3.5) was used.64 This analysis uses a bootstrap estimation approach to test for the indirect (ie, mediation) effect. Bootstrapped CI that do not cross zero are indicative of a statistically significant indirect effect. A bootstrapping sample of 5000 was used in the current analyses. For simple mediation analyses with a dichotomous mediator variable, Valeri and VanderWeele’s SPSS Macro for nonlinear mediation models was planned.65 This technique is based on the counterfactual approach and uses a logistic regression model to allow for binary mediators. The macro generates estimates and CI for direct and indirect effects as well as bootstrap CI to test for the significance of the indirect effect.
Covariates were selected a priori. Based on previous ACEs research,8,17,21,66,67 parent age, gender, race/ethnicity, and annual household income were included as covariates in analyses that examined parent variables and youth age and gender were included as covariates in analyses that examined youth outcomes. Site of data collection was included as a covariate in all analyses. With the exception of the continuous variable of age, covariates were dichotomized as follows: gender (female=focus group, male=reference group), race/ethnicity (other categories=focus group, White/Caucasian=reference group), annual household income (<$90,000=focus group, >$90,000=reference group), and site (IWK=focus group, ACH=reference group).
RESULTS
Participant Characteristics
Sociodemographic and pain characteristics of the sample are reported in Table 1. Parents ranged in age from 32 to 63 years old (M=44.89 y, SD=5.15 y) and were predominately female, White, and married or common-law. The majority of parents had a college or university degree, were employed full-time, and had an annual household income >$90,000 CAD. Parents from the 2 sites did not significantly differ on any sociodemographic variable. Youth ranged in age from 10 to 18 years old (M=14.38 y, SD=2.20 y) and were also predominately female and White. Youth from the 2 sites did not significantly differ by age or race/ethnicity but did significantly differ by gender, with more males in the ACH sample than the IWK sample.
TABLE 1.
Sociodemographic and Pain Characteristics of the Sample
| n (%) | Significance Test | ||||
|---|---|---|---|---|---|
| Variable | Total Sample | ACH (n=168) | IWK (n=24) | Statistic | P |
| Parent age, M (SD) (y) | 44.89 (5.15) | 44.94 (5.14) | 44.54 (5.32) | t=0.36 | 0.721 |
| Parent gender | FET | 0.223 | |||
| Female | 177 (92.2) | 153 (91.1) | 24 (100) | ||
| Male | 14 (7.3) | 14 (8.3) | 0 (0.0) | ||
| Other | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Parent race/ethnicity | FET | 0.209 | |||
| White/Caucasian | 165 (85.9) | 142 (84.5) | 23 (95.8) | ||
| Biracial or multiracial | 12 (6.3) | 11 (6.5) | 1 (4.2) | ||
| Latin American | 4 (2.1) | 4 (2.4) | 0 (0.0) | ||
| Arab/West Asian | 3 (1.6) | 3 (1.8) | 0 (0.0) | ||
| South Asian | 2 (1.0) | 2 (1.2) | 0 (0.0) | ||
| Indigenous | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Black/African American | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Chinese | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Filipino | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Other | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Did not answer | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Parent marital status | FET | 1.00 | |||
| Married or common-law | 155 (80.7) | 135 (80.4) | 20 (83.3) | ||
| Divorced or separated | 27 (14.1) | 24 (14.3) | 3 (12.5) | ||
| Single | 7 (3.6) | 6 (3.6) | 1 (4.2) | ||
| Widowed | 3 (1.6) | 3 (1.8) | 0 (0.0) | ||
| Parent education | χ2=2.55 | 0.110 | |||
| High school or less | 18 (9.4) | 17 (10.1) | 1 (4.2) | ||
| Vocational school or some college (no degree) | 41 (21.4) | 38 (22.6) | 3 (12.5) | ||
| College or Bachelor’s degree | 106 (55.2) | 93 (55.4) | 13 (54.2) | ||
| Graduate/professional school (Master’s degree, PhD) | 27 (14.1) | 20 (11.9) | 7 (29.2) | ||
| Parent employment status | FET | 1.00 | |||
| Full-time | 105 (54.7) | 88 (52.4) | 17 (70.8) | ||
| Part-time | 50 (26.0) | 49 (29.2) | 1 (4.2) | ||
| Not working | 35 (18.2) | 31 (18.5) | 4 (16.7) | ||
| Did not answer | 2 (1.0) | 0 (0.0) | 2 (8.3) | ||
| Annual household income, CAD | χ2=0.32 | 0.570 | |||
| 0-29,999 | 11 (5.7) | 9 (5.4) | 2 (8.3) | ||
| 30,000-59,999 | 18 (9.4) | 15 (8.9) | 3 (12.5) | ||
| 60,000-89,999 | 23 (12.0) | 20 (11.9) | 3 (12.5) | ||
| >90,000 | 115 (59.9) | 101 (60.1) | 14 (58.3) | ||
| Did not answer | 25 (13.1) | 23 (13.7) | 2 (8.3) | ||
| Youth age, y | 14.38 (2.20) | 14.30 (2.18) | 14.92 (2.26) | t=−1.29 | 0.198 |
| Youth gender | χ2=5.62 | 0.018 | |||
| Female | 145 (75.5) | 122 (72.6) | 23 (95.8) | ||
| Male | 44 (22.9) | 43 (25.6) | 1 (4.2) | ||
| Other | 3 (1.6) | 3 (1.8) | 0 (0.0) | ||
| Youth race/ethnicity | FET | 0.578 | |||
| White/Caucasian | 157 (81.8) | 136 (81.0) | 21 (87.5) | ||
| Biracial or multiracial | 15 (7.8) | 13 (7.7) | 2 (8.3) | ||
| Arab/West Asian | 3 (1.6) | 3 (1.8) | 0 (0.0) | ||
| South Asian | 3 (1.6) | 3 (1.8) | 0 (0.0) | ||
| Indigenous | 2 (1.0) | 2 (1.2) | 0 (0.0) | ||
| Latin American | 2 (1.0) | 2 (1.2) | 0 (0.0) | ||
| Black/African American | 2 (1.0) | 1 (0.6) | 1 (4.2) | ||
| Filipino | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Other | 6 (3.1) | 6 (3.6) | 0 (0.0) | ||
| Did not answer | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
| Youth pain duration | 3.42 (3.16) | 3.23 (3.10) | 4.65 (3.33) | t=−1.93 | 0.055 |
| Youth pain locations | |||||
| Head | 131 (68.2) | 123 (73.2) | 8 (33.3) | χ2=15.41 | <0.001 |
| Muscle and joints | 49 (25.5) | 40 (23.8) | 9 (37.5) | χ2=2.07 | 0.150 |
| Stomach | 38 (19.8) | 33 (19.6) | 5 (20.8) | FET | 1.00 |
| Legs | 34 (17.7) | 23 (13.7) | 11 (45.8) | FET | 0.001 |
| Chest | 22 (11.5) | 20 (11.9) | 2 (8.3) | FET | 1.00 |
| Other | 54 (28.1) | 42 (25.0) | 12 (50.0) | χ2=6.49 | 0.011 |
| >1 location | 85 (44.3) | 73 (43.5) | 12 (50.0) | χ2=0.37 | 0.546 |
| Youth pain frequency | χ2=4.89 | 0.027 | |||
| Not at all | 4 (2.1) | 4 (2.4) | 0 (0.0) | ||
| Once per week | 14 (7.3) | 13 (7.7) | 1 (4.2) | ||
| 2-3 times per week | 52 (27.1) | 48 (28.6) | 4 (16.7) | ||
| 4-6 times per week | 26 (13.5) | 24 (14.3) | 2 (8.3) | ||
| Daily | 95 (49.5) | 78 (46.4) | 17 (70.8) | ||
| Did not answer | 1 (0.5) | 1 (0.6) | 0 (0.0) | ||
For significance testing, independent samples t tests were used when variables were continuous, χ2 tests were used when variables were categorical and met the minimum expected cell count, and the Fischer exact tests were used when variables were categorical and the expected cell count was below 5. All multicategorical variables were dichotomized for significance testing as follows: gender (female, male), race/ethnicity (White, other categories), marital status (married or common law, other categories), education (high school or less or some postsecondary training, college or University degree), employment status (working full-time or part-time, not working), annual household income (<$90,000, >$90,000), and youth pain frequency (daily, other categories). The youth pain location variable was naturally dichotomous (yes/no to having pain in each location) and thus the prevalence of each pain location was compared across samples.
ACH indicates Alberta Children’s Hospital; FET, Fischer exact test.
Youth reported an average pain duration of 3.42 years (SD=3.16 y, range: 3 mo to 17 y and 1 mo). Youth from the 2 sites did not significantly differ in average pain duration but did significantly differ in the most frequently reported pain locations as well as pain frequency. Specifically, significantly more youth at ACH than IWK reported head pain whereas significantly more youth at IWK than ACH reported leg pain and pain in “other” locations. Approximately half of the youth at each site reported more than 1 pain location. Significantly more youth at IWK reported pain on a daily basis than youth at ACH.
In total, 68.2% (n=131) of parents reported 1 or more ACEs and 22.4% (n=43) reported 4 or more ACEs. Approximately half of the parent sample (51.0%; n=98) reported chronic pain. These parents reported an average pain duration of 12.42 years (SD=12.15 y, range: 4 mo to 48 y), with 15.3% (n=15) indicating that their chronic pain began in childhood and 30.6% (n=30) indicating it was present before their child was born. Overall, 16.1% (n=31) of parents reported clinically elevated depressive symptoms, 38.5% (n=74) reported clinically elevated anxiety symptoms, and 5.2% (n=10) reported clinically elevated PTSD symptoms. When asked to describe their “worst” traumatic event, 6.8% of parents reported an ACE (eg, sexual abuse), 8.3% reported an event from their childhood that is not captured by the traditional ACE categories (eg, death of family or friends, being bullied or socially excluded), 75.5% reported an event from their adulthood, and 9.4% did not report an event (Table 2). Descriptives and correlations for the study variables are reported in Table 3.
TABLE 2.
Traumatic Events Reported by Parents of Youth With Chronic Pain
| Traumatic Event | n (%) |
|---|---|
| Traditional ACE category | 13 (6.8) |
| Sexual abuse | 9 (4.7) |
| Physical abuse | 2 (1.0) |
| Physical violence between parents | 1 (0.5) |
| Parent separation/divorce | 1 (0.5) |
| Other childhood event | 16 (8.3) |
| Death of family or friend | 6 (3.1) |
| Witnessed fatal accident | 2 (1.0) |
| Parent physical illness/medical emergency | 2 (1.0) |
| Bullied or socially excluded | 2 (1.0) |
| Involved in serious accident | 1 (0.5) |
| Physical violence against sibling | 1 (0.5) |
| Natural disaster | 1 (0.5) |
| Fire | 1 (0.5) |
| Adulthood event | 145 (75.5) |
| No event reported | 18 (9.4) |
ACEs indicates adverse childhood experiences.
TABLE 3.
Descriptive Statistics and Correlation Coefficients for Study Variables
| Variables | 2 | 3 | 4 | 5 | 6 | 7 | M (SD) | Range | n |
|---|---|---|---|---|---|---|---|---|---|
| 1. Parent ACE score | 0.19** | 0.25** | 0.11 | 0.20** | −0.07 | 0.09 | 2.10 (2.35) | 0-10 | 192 |
| 2. Parent chronic pain status | — | 0.31*** | 0.22** | 0.26*** | −0.10 | 0.02 | — | Yes/no | 192 |
| 3. Parent depressive symptoms | — | 0.68*** | 0.69*** | 0.03 | 0.20* | 3.76 (3.77) | 0-21 | 192 | |
| 4. Parent anxiety symptoms | — | 0.58*** | −0.06 | 0.10 | 6.46 (3.95) | 0-21 | 192 | ||
| 5. Parent PTSD symptoms | — | 0.05 | 0.19* | 10.63 (12.25) | 0-80 | 188 | |||
| 6. Youth pain intensity | — | 0.47*** | 5.33 (2.04) | 0-10 | 163 | ||||
| 7. Youth pain interference | — | 53.30 (9.57) | 36.7-74 | 159 |
ACE indicates adverse childhood experiences; M, mean; PTSD, posttraumatic stress disorder.
P<0.05.
P<0.01.
P<0.001.
Parent ACEs to Parent Health
Parent ACEs Predicting Parent Chronic Pain Status
To examine the association between parent ACEs and parent chronic pain status, binary logistic regression analyses were conducted. Results of the unadjusted and adjusted analyses are reported in Table 4. The omnibus test for the adjusted model was significant, with parent ACE score and covariates accounting for 16.9% of the total variance in parent chronic pain status (Nagelkerke R2=0.169). Parent ACE score was a significant independent predictor of parent chronic pain status while controlling for covariates (ΔNagelkerke R2=0.035, P=0.034), such that for every additional ACE reported, the odds of parent chronic pain increased (ORa=1.19; 95% CI=1.01, 1.40). Household income was the only covariate that significantly predicted parent chronic pain status. Parents with a household income less (vs. more) than $90,000 showed higher odds of reporting chronic pain (ORa=3.74; 95% CI=1.78, 7.83).
TABLE 4.
Results From Binary Logistic Regression Analyses of Parent ACEs Predicting Parent Chronic Pain Status
| Predictor Variables | b | SE−b | P | OR [95% CI] | ΔR2 | χ2 | P |
|---|---|---|---|---|---|---|---|
| Unadjusted model | |||||||
| Step 1: parent ACE score | 0.18 | 0.07 | 0.009 | 1.19 [1.05, 1.36] | 0.051 | 7.48 | 0.006 |
| Adjusted model | — | — | — | — | 0.169 | 22.12 | 0.001 |
| Step 1: covariates | — | — | — | — | 0.134 | 17.32 | 0.004 |
| Site | 0.28 | 0.49 | 0.576 | 1.32 [0.50, 3.44] | — | — | — |
| Parent age | 0.02 | 0.03 | 0.666 | 1.02 [0.95, 1.08] | — | — | — |
| Parent gender | 0.99 | 0.66 | 0.132 | 2.70 [0.74, 9.78] | — | — | — |
| Parent race/ethnicity | 0.58 | 0.50 | 0.246 | 1.79 [0.67, 4.76] | — | — | — |
| Household income | 1.32 | 0.38 | <0.001 | 3.74 [1.78, 7.83] | — | — | — |
| Step 2: parent ACE score | 0.18 | 0.08 | 0.034 | 1.19 [1.01, 1.40] | 0.035 | 4.81 | 0.028 |
n=192 for unadjusted models, n=163 for adjusted models. The target category for the dependent variable was presence of chronic pain and the reference category was absence of chronic pain; focus and reference groups of covariates are listed under “Data Preparation and Analysis.”
ACEs indicates adverse childhood experiences; b, unstandardized b-weight; CI, confidence interval; OR, odds ratio; R2, Nagelkerke pseudo R2; χ2, chi-square omnibus test for logistic regression.
Parent ACEs Predicting Parent Mental Health Symptoms
To examine the associations between parent ACEs and parent depressive, anxiety, and PTSD symptoms, linear regression analyses were conducted. Results of the unadjusted and adjusted analyses are reported in Table 5.
TABLE 5.
Results From Linear Regression Analyses of Parent ACEs Predicting Parent Mental Health Symptoms
| Predictor Variables | b | SE−b | β | ΔF | P | ΔR2 (sr2) |
|---|---|---|---|---|---|---|
| Predicting parent depressive symptoms | ||||||
| Unadjusted model | ||||||
| Step 1: parent ACE score | 0.40 | 0.11 | 0.25 | 12.37 | 0.001 | 0.061 |
| Adjusted model | — | — | — | 7.60 | <0.001 | 0.226 |
| Step 1: covariates | — | — | — | 7.59 | <0.001 | 0.195 |
| Site | 3.36 | 0.78 | 0.31 | — | <0.001 | (0.094) |
| Parent age | 0.03 | 0.05 | 0.04 | — | 0.601 | (0.001) |
| Parent gender | −1.68 | 1.03 | −0.12 | — | 0.104 | (0.014) |
| Parent race/ethnicity | −0.14 | 0.76 | −0.01 | — | 0.857 | (<0.001) |
| Household income | 2.19 | 0.58 | 0.28 | — | <0.001 | (0.074) |
| Step 2: parent ACE score | 0.31 | 0.12 | 0.20 | 6.37 | 0.013 | 0.032 |
| Predicting parent anxiety symptoms | ||||||
| Unadjusted model | ||||||
| Step 1: parent ACE score | 0.19 | 0.12 | 0.11 | 2.41 | 0.122 | 0.013 |
| Adjusted model | — | — | — | 2.47 | 0.026 | 0.087 |
| Step 1: covariates | — | — | — | 2.81 | 0.018 | 0.082 |
| Site | 2.32 | 0.88 | 0.20 | — | 0.009 | (0.041) |
| Parent age | 0.05 | 0.06 | 0.07 | — | 0.397 | (0.004) |
| Parent gender | 0.24 | 1.15 | 0.02 | — | 0.838 | (<0.001) |
| Parent race/ethnicity | −1.59 | 0.86 | −0.15 | — | 0.065 | (0.020) |
| Household income | 0.81 | 0.65 | 0.10 | — | 0.211 | (0.009) |
| Step 2: parent ACE score | 0.12 | 0.14 | 0.07 | 0.76 | 0.383 | 0.004 |
| Predicting parent PTSD symptoms | ||||||
| Unadjusted model | ||||||
| Step 1: parent ACE score | 1.04 | 0.37 | 0.20 | 7.79 | 0.006 | 0.040 |
| Adjusted model | — | — | — | 2.88 | 0.011 | 0.102 |
| Step 1: covariates | — | — | — | 3.29 | 0.008 | 0.097 |
| Site | 3.85 | 2.67 | 0.11 | — | 0.151 | (0.012) |
| Parent age | 0.27 | 0.18 | 0.12 | — | 0.137 | (0.013) |
| Parent gender | 1.72 | 3.49 | 0.04 | — | 0.623 | (0.001) |
| Parent race/ethnicity | 0.002 | 2.51 | <0.01 | — | 0.999 | (<0.001) |
| Household income | 6.72 | 1.91 | 0.27 | — | 0.001 | (0.073) |
| Step 2: parent ACE score | 0.37 | 0.40 | 0.08 | 0.86 | 0.357 | 0.005 |
n=192 for unadjusted models with parent depressive and anxiety symptoms, n=163 for adjusted models with parent depressive and anxiety symptoms; n=188 for unadjusted model with parent PTSD symptoms, n=159 for adjusted model with parent PTSD symptoms; Focus and reference groups of covariates are listed under “Data Preparation and Analysis.”
ACEs indicates adverse childhood experiences; b, unstandardized b-weight; sr2, squared semi-partial correlation; β, standardized beta-weight; PTSD, posttraumatic stress disorder.
The omnibus test for the adjusted model of parent ACEs predicting parent depressive symptoms was significant, with parent ACE score and covariates accounting for 22.6% of the total variance in parent depressive symptoms (R2=0.226). The addition of parent ACE score in step 2 significantly increased the predictive power of the model, and parent ACE score was a significant independent predictor of parent depressive symptoms while controlling for covariates (ΔR2=0.032, P=0.013). In addition, the covariates of site and household income significantly predicted parent depressive symptoms, accounting for a unique 9.4% and 7.4% of the variance, respectively. No other covariates were significant predictors of parent depressive symptoms.
The omnibus test for the adjusted model of parent ACEs predicting parent anxiety symptoms was significant, with parent ACE score and covariates accounting for 8.7% of the total variance in parent anxiety symptoms (R2=0.087). However, the addition of parent ACE score in step 2 did not significantly increase the predictive power of the model, and parent ACE score was not a significant independent predictor of parent anxiety symptoms while controlling for covariates (ΔR2=0.004, P=0.383). Instead, covariates accounted for the majority of the variance in the model (R2=0.082). In particular, the covariate of site significantly predicted parent anxiety symptoms, accounting for a unique 4.1% of the variance. No other covariates were significant predictors of parent anxiety symptoms.
The omnibus test for the adjusted model of parent ACEs predicting parent PTSD symptoms was significant, with parent ACE score and covariates accounting for 10.2% of the total variance in parent PTSD symptoms (R2=0.102). However, the addition of parent ACE score in step 2 did not significantly increase the predictive power of the model, and parent ACE score was not a significant independent predictor of parent PTSD symptoms while controlling for covariates (ΔR2=0.005, P=0.357). Instead, covariates accounted for the majority of the variance in the model (R2=0.097). In particular, household income significantly predicted parent PTSD symptoms, accounting for a unique 7.3% of the variance. No other covariates were significant predictors of parent PTSD symptoms.
Parent ACEs to Youth Pain
To examine associations between parent ACEs and youth pain at follow-up, linear regression analyses were conducted. Results of the unadjusted and adjusted analyses are reported in Table 6. In all models, parent ACE score was not a significant independent predictor of youth pain intensity or youth pain interference. The only covariates that were significantly associated with youth pain was data collection site and youth age, both of which significantly predicted youth pain interference. Specifically, youth from the IWK (vs. ACH) and older (vs. younger) youth reported significantly higher pain interference.
TABLE 6.
Results From Linear Regression Analyses of Parent ACEs Predicting Youth Pain at Follow-up
| Predictor Variables | b | SE−b | β | ΔF | P | ΔR2 (sr2) |
|---|---|---|---|---|---|---|
| Predicting youth pain intensity | ||||||
| Unadjusted model | ||||||
| Step 1: parent ACE score | −0.07 | 0.07 | −0.07 | 0.86 | 0.354 | 0.005 |
| Adjusted model | — | — | — | 0.66 | 0.728 | 0.040 |
| Step 1: covariates | — | — | — | 0.50 | 0.832 | 0.027 |
| Site | 0.14 | 0.59 | 0.02 | — | 0.806 | (0.001) |
| Parent age | −0.01 | 0.04 | −0.02 | — | 0.871 | (<0.001) |
| Parent gender | 0.22 | 0.72 | 0.03 | — | 0.757 | (0.001) |
| Parent race/ethnicity | 0.06 | 0.56 | 0.01 | — | 0.915 | (<0.001) |
| Household income | −0.25 | 0.40 | −0.06 | — | 0.536 | (0.003) |
| Youth age | 0.13 | 0.09 | 0.13 | — | 0.163 | (0.015) |
| Youth gender | 0.40 | 0.45 | 0.08 | — | 0.375 | (0.006) |
| Step 2: parent ACE score | −0.12 | 0.09 | −0.13 | 1.73 | 0.191 | 0.013 |
| Predicting youth pain interference | ||||||
| Unadjusted model | ||||||
| Step 1: parent ACE score | 0.38 | 0.33 | 0.09 | 1.35 | 0.248 | 0.009 |
| Adjusted model | — | — | — | 2.47 | 0.016 | 0.139 |
| Step 1: covariates | — | — | — | 2.81 | 0.010 | 0.138 |
| Site | 7.22 | 2.68 | 0.23 | — | 0.008 | (0.051) |
| Parent age | −0.13 | 0.18 | −0.07 | — | 0.447 | (0.004) |
| Parent gender | −2.97 | 3.35 | −0.08 | — | 0.377 | (0.006) |
| Parent race/ethnicity | 0.47 | 2.53 | 0.02 | — | 0.854 | (<0.001) |
| Household income | 1.86 | 1.85 | 0.09 | — | 0.319 | (0.007) |
| Youth age | 0.88 | 0.40 | 0.20 | — | 0.030 | (0.034) |
| Youth gender | 3.31 | 2.02 | 0.14 | — | 0.104 | (0.019) |
| Step 2: parent ACE score | 0.18 | 0.40 | 0.04 | 0.21 | 0.648 | 0.001 |
n=163 for unadjusted model with pain intensity, n=136 for adjusted model with pain intensity; n=158 for unadjusted model with pain interference, n=131 for adjusted model with pain interference; focus and reference groups of covariates are listed under “Data Preparation and Analysis.”
ACE indicates adverse childhood experiences; b, unstandardized b-weight; sr2, squared semi-partial correlation; β, standardized beta-weight.
Mediation Analyses
Mediation analyses examined the indirect effect of parent ACEs on youth interference at follow-up through parent depressive symptoms and parent PTSD symptoms at baseline. Results of the unadjusted and adjusted analyses are displayed in Table 7 and Figure 1. While controlling for covariates, the indirect effect of parent ACEs on youth pain interference was not statistically significant for either model (ie, through parent depressive symptoms or PTSD symptoms). All other paths were also not statistically significant when covariates were included in the model.
TABLE 7.
Results From Mediation Analyses of Parent ACEs Predicting Youth Pain Interference at Follow-up through Parent Depressive or PTSD Symptoms
| Path | b | 95% CI | SE−b | β | P |
|---|---|---|---|---|---|
| Parent depressive symptoms | |||||
| Unadjusted model | |||||
| Parent ACE score → Parent depressive symptoms (a) | 0.33 | 0.10, 0.57 | 0.12 | 0.22 | 0.005 |
| Parent depressive symptoms → Youth pain interference (b) | 0.52 | 0.08, 0.95 | 0.22 | 0.19 | 0.020 |
| Parent ACE score → Youth pain interference (c’) | 0.22 | −0.44, 0.87 | 0.33 | 0.05 | 0.516 |
| Parent ACE score → Parent depressive symptoms → Youth pain interference (ab) | 0.17 | 0.02, 0.43 | 0.11 | 0.04 | — |
| Adjusted model | |||||
| Parent ACE score → Parent depressive symptoms (a) | 0.23 | −0.03, 0.49 | 0.13 | 0.16 | 0.088 |
| Parent depressive symptoms → Youth pain interference (b) | 0.38 | −0.16, 0.91 | 0.27 | 0.13 | 0.164 |
| Parent ACE score → Youth pain interference (c') | 0.09 | −0.69, 0.88 | 0.40 | 0.02 | 0.816 |
| Parent ACE score → Parent depressive symptoms → Youth pain interference (ab) | 0.09 | −0.03, 0.29 | 0.08 | 0.02 | — |
| Parent PTSD symptoms | |||||
| Unadjusted model | |||||
| Parent ACE score → Parent PTSD symptoms (a) | 1.14 | 0.31, 1.98 | 0.42 | 0.21 | 0.008 |
| Parent PTSD symptoms → Youth pain interference (b) | 0.13 | 0.01, 0.25 | 0.06 | 0.17 | 0.034 |
| Parent ACE score → Youth pain interference (c') | 0.27 | −0.39, 0.93 | 0.33 | 0.07 | 0.419 |
| Parent ACE score → Parent PTSD symptoms → Youth pain interference (ab) | 0.15 | 0.01, 0.36 | 0.09 | 0.04 | — |
| Adjusted model | |||||
| Parent ACE score → Parent PTSD symptoms (a) | 0.27 | −0.70, 1.23 | 0.49 | 0.05 | 0.588 |
| Parent PTSD symptoms → Youth pain interference (b) | 0.10 | −0.05, 0.24 | 0.07 | 0.11 | 0.194 |
| Parent ACE score → Youth pain interference (c') | 0.16 | −0.63, 0.94 | 0.40 | 0.04 | 0.693 |
| Parent ACE score → Parent PTSD symptoms → Youth pain interference (ab) | 0.03 | −0.07, 0.17 | 0.06 | 0.01 | — |
n=159 for unadjusted model with parent depressive symptoms, n=132 for adjusted model with parent depressive symptoms; n=157 for unadjusted model with parent PTSD symptoms, n=130 for adjusted models with parent PTSD symptoms. Adjusted models include the following covariates: data collection site, parent age, parent gender, parent race/ethnicity, household income, youth age, and youth gender. The focus and reference groups of covariates are listed under “Data Preparation and Analysis.” 95% CI for indirect effects (path ab) are based on 5000 bootstrap samples.
ACE indicates adverse childhood experiences; b, unstandardized b-weight; β, standardized beta-weight; CI, confidence interval; PTSD, posttraumatic stress disorder.
FIGURE 1.
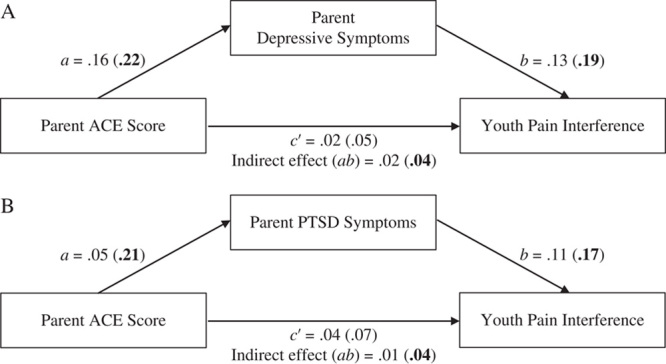
Mediation models for parent ACEs predicting youth pain interference through (A) parent depressive symptoms and (B) parent PTSD symptoms. Standardized coefficients for each path of the adjusted or unadjusted (in parentheses) models are presented. Bolded coefficients represent significant paths (P<0.05 or 95% CI not crossing 0). ACEs indicates adverse childhood experiences; PTSD, posttraumatic stress disorder.
DISCUSSION
Research has shown that parents’ physical and mental health is integrally related to the functioning of youth with chronic pain.1–5 This study extends the literature by examining the potential intergenerational cascade from parent ACEs to parent physical and mental health to child pain in a clinical sample of youth with chronic pain. As expected, parent ACEs significantly predicted parent chronic pain status and depressive symptoms. Contrary to our hypotheses, parent ACEs did not significantly predict parent anxiety or PTSD symptoms when key sociodemographic variables were controlled in the model. Parent ACEs were also not significantly related to youth pain intensity or pain interference, either directly or indirectly through parent health. These findings suggest an intergenerational cascade from parent ACEs to parent health to child pain was not present in the current sample.
Consistent with research showing that ACEs confer risk for chronic pain12,16–18,68 and depressive symptomatology,8,10,69 parent ACEs significantly predicted parent chronic pain status and parent depressive symptoms in the current study. However, parent ACEs accounted for only about 3% of the variance in both parent chronic pain status and parent depressive symptoms when sociodemographic characteristics were controlled, suggesting that ACEs are only one of many predictors of these health outcomes. Despite high rates of clinically elevated anxiety symptoms among parents in this study, parent ACEs were not significantly related to parent anxiety symptoms. These findings are consistent with previous studies that have found generally stronger associations between ACEs and depressive symptoms than ACEs and anxiety symptoms.9,10 Parent ACEs were also not significantly related to parent PTSD symptoms while controlling for sociodemographic variables. Only 5.2% of parents met the clinical cut-off for PTSD in the current study, which is less than a study from the United States that found 20% of parents of youth with chronic pain met the cut-off for PTSD.5 Thus, clinically elevated PTSD symptoms may not have been as prevalent in the current sample as in previous studies that have found associations between ACEs and PTSD symptoms.13,70–72 We also assessed current PTSD symptoms; given the time that had elapsed for many parents since their childhood, any PTSD symptoms associated with ACEs may have decreased. Indeed, only 6.8% of parents identified an ACE as the traumatic event that still bothers them. Similar to a previous study with a population-based sample,67 a stronger association may have been found between parent ACEs and lifetime PTSD.
In this study, socioeconomic disparities mattered. Specifically, annual household income was a significant predictor of parent chronic pain status, parent depressive symptoms, and parent PTSD symptoms. This finding may reflect the impact these health conditions can have on the ability to work full-time. However, research has also shown that socioeconomic disparities increase risk for these health conditions.73–78 Importantly, ACEs are also related to adult socioeconomic status.79,80 Although annual household income was not a significant direct predictor of youth pain in the current study, socioeconomic status may play an important role in the cascade from parent ACEs to parent health to child chronic pain.81,82 For example, socioeconomic status may moderate an association between parent ACEs and child chronic pain, with parent ACEs more strongly predicting child outcomes in families of lower (vs. higher) socioeconomic status. To better elucidate their role, future research should incorporate indicators of socioeconomic status into models of intergenerational transmission of risk as explanatory (vs. control) variables.
Contrary to hypotheses, parent ACEs did not directly predict youth pain intensity or pain interference. Moreover, in mediation models, parent ACEs were not indirectly related to youth pain interference through parent depressive or PTSD symptoms. These findings suggest that parent ACEs are not directly or indirectly related to the severity of chronic pain and impairment in youth when key sociodemographic factors are considered. Instead, parent ACEs may be more related to the development of child chronic pain, possibly through parent chronic pain status or depressive symptoms. Research has shown that children whose parents have chronic pain are at risk for developing their own chronic pain.83 In the current study, almost one-third of parents with chronic pain reported that their pain began before the child was born. Thus, at least for some families, parent chronic pain may have contributed to the onset of the child’s chronic pain, likely through biological (eg, epigenetics, alterations in prenatal HPA axis functioning) and psychosocial (eg, parent modeling of pain behaviors) mechanisms.84 A recent study with a sample of mothers with chronic pain and their preadolescent children also demonstrated that parent ACEs were indirectly related to child depressive symptoms through maternal depressive symptoms, and suggested that parent ACEs may confer risk for adolescent-onset chronic pain, through elevated depressive symptoms in the parent and child.20 In this way, parent chronic pain status and parent depressive symptoms may play a mediating role in the relation between parent ACEs and the development of child chronic pain; however, prospective research is needed to test this hypothesis.
This study also investigated the simple mediating roles of 4 parent risk factors on youth pain. However, the indirect effect of parent ACEs on child chronic pain may involve other factors. First, parent ACEs may impact the broader functioning of youth with chronic pain, such as their mental health or quality of life. In fact, previous research with community-based samples and high-risk populations (eg, low-income women, mothers with chronic pain) has shown that parent ACEs are significantly related to children’s internalizing and externalizing mental health symptoms, with parent mental health symptoms acting as a mediator.19,20,24,26,85 Studies have also found that parent health indirectly relates to the functioning of youth with chronic pain through various cognitive and behavioural factors (eg, parent catastrophizing about child pain, parent protective responses to child pain).2,53 ACEs may impact these factors, either directly or indirectly through parent experiences with their own health.2,15,53,86 Lastly, individuals exposed to adversities in childhood report significantly more stressful or adverse events as adults,33,87,88 and exposure to adverse events in adulthood has been shown to mediate the association between ACEs and adult physical health.33 Thus, parent lifetime exposure to adverse events may contribute to child chronic pain, possibly by increasing parent risk for poor health or child exposure to stressful life events. Further research examining more comprehensive models is needed to better understand the potential pathways from parent ACEs to the development and maintenance of child chronic pain and impairment.
Importantly, the absence of a relationship between parent ACEs and youth pain may also reflect resiliency in these families. Research has shown that protective factors, such as safe and supportive relationships, can buffer the negative impact of ACEs on health outcomes in parents and their children.36,89 Future research is needed to examine the potential moderating role of protective factors in the intergenerational transmission of risk from parent ACEs to child chronic pain. This includes factors at various levels of the child’s ecology, including community-level factors (eg, social support), parent factors (eg, positive parent-child relationship), and child factors (eg, adaptive coping skills).90,91
Although further investigation in this emerging area is needed, research to date suggests that ACEs are common among parents of youth with chronic30 but may not contribute to the pain experience of youth with chronic pain. These findings have important clinical implications for health care providers that work with these families. Specifically, providers should be aware that many parents and children37,92 have trauma histories and thus should consider adopting trauma-informed approaches in their practice to avoid retraumatizing their patients.93,94 However, a direct assessment of parent ACEs does not appear to be indicated in the treatment of youth with chronic pain. Instead, it may unnecessarily distress or retraumatize parents.
This study had limitations that should be noted. First, concerns have been raised about using self-report measures to retrospectively assess ACEs (eg, impact of recall biases95). Specifically, it has been suggested that the current mental or physical health status of the respondent could influence his or her responses on the ACE measure and a review of the relevant literature found that adults tend to underestimate their experiences of childhood adversity.96 Second, the time period between baseline and follow-up in the current study was relatively brief (ie, 3 mo) and may not have been able to capture longitudinal associations between parent health and child chronic pain. Third, our sample was limited to youth with clinically-significant chronic pain; thus, results may not generalize to youth who are not receiving tertiary treatment for chronic pain either because their pain is less severe and does not require tertiary treatment or because they face barriers in accessing tertiary treatment. The current sample was also predominately White, female (with the majority of parents being mothers), and of higher socioeconomic status. Future research that examines the role of parent ACEs in pediatric chronic pain in a more diverse sample is needed to explicate the relevance of the current findings to a broader population.
This was the first study to examine the relation between parent ACEs and child chronic pain in a clinical sample of youth with chronic pain. Our findings revealed that parent ACEs significantly predicted parent chronic pain status and parent depressive symptoms, but not parent anxiety symptoms, parent PTSD symptoms, or youth pain, when key sociodemographic factors were controlled. Contrary to our hypothesis, these results suggest that an intergenerational cascade from parent ACEs to parent health to child chronic pain was not present in the current sample. Further research is needed to more comprehensively examine the role of parent ACEs in the development and maintenance of child chronic pain. Specifically, research that identifies neurobiological and psychosocial mechanisms mediating a potential association is needed. Since ACEs are not deterministic of poor outcomes, with protective factors moderating the intergenerational impact of ACEs,36,90,97,98 research that identifies both risk and protective factors will be crucial for designing interventions that halt the continuation of poor health outcomes across generations.
ACKNOWLEDGMENTS
The authors thank the parents and youth who participated in this study.
Footnotes
This research was supported by research funding awarded to M.N. from the Vi Riddell Pediatric Pain Initiative, (Calgary, AB) Alberta Children’s Hospital Foundation and Alberta Children’s Hospital Research Institute, (Calgary, AB) and the Canadian Institutes of Health Research Strategy for Patient-Oriented Research “Chronic Pain Network (Hamilton, ON).” J.K.B. is supported by graduate studentship awards from Alberta Innovates and the Canadian Institutes of Health Research. S.M. is supported by the Canada Research Chairs program. K.O.Y. is supported by the Ronald and Irene Ward Chair in Pediatric Brain Injury from the Alberta Children’s Hospital Foundation. A.L.S. is supported by T32 GM 108554 from the National Institutes of Health. The authors declare no conflict of interest.
Contributor Information
Jaimie K. Beveridge, Email: jaimie.beveridge@ucalgary.ca.
Keith O. Yeates, Email: kyeates@ucalgary.ca.
Sheri Madigan, Email: sheri.madigan@ucalgary.ca.
Amanda L. Stone, Email: amanda.l.stone@vumc.org.
Anna C. Wilson, Email: longann@ohsu.edu.
Janice E. Sumpton, Email: janice.sumpton@gmail.com.
Sabrina Salberg, Email: sabrina.salberg@monash.edu.
Richelle Mychasiuk, Email: richelle.mychasiuk@monash.edu.
Melanie Noel, Email: melanie.noel@ucalgary.ca.
REFERENCES
- 1.Beveridge JK, Neville A, Wilson AC, et al. Intergenerational examination of pain and posttraumatic stress disorder symptoms among youth with chronic pain and their parents. Pain Reports. 2018;3:e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone AL, Bruehl S, Smith CA, et al. Social learning pathways in the relation between parental chronic pain and daily pain severity and functional impairment in adolescents with functional abdominal pain. Pain. 2018;159:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law EF, Blume H, Palermo TM. Longitudinal impact of parent factors in adolescents with migraine and tension-type headache. Headache. 2020;60:1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccleston C, Crombez G, Scotford A, et al. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–229. [DOI] [PubMed] [Google Scholar]
- 5.Noel M, Wilson AC, Lewandowski Holley A, et al. Post-traumatic stress disorder symptoms in youth with versus without chronic pain. Pain. 2016;157:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law EF, Fisher E, Howard WJ, et al. Longitudinal change in parent and child functioning after internet-delivered cognitive-behavioral therapy for chronic pain. Pain. 2017;158:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poppert Cordts KM, Stone AL, Beveridge JK, et al. The (parental) whole is greater than the sum of its parts: a multifactorial model of parent factors in pediatric chronic pain. J Pain. 2019;20:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 9.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Heal. 2017;2:e356–e366. [DOI] [PubMed] [Google Scholar]
- 11.Petruccelli K, Davis J, Berman T. Adverse childhood experiences and associated health outcomes: a systematic review and meta-analysis. Child Abus Negl. 2019;97:104127. [DOI] [PubMed] [Google Scholar]
- 12.Anda RF, Tietjen G, Schulman E, et al. Adverse childhood experiences and frequent headaches in adults. Headache. 2010;50:1473–1481. [DOI] [PubMed] [Google Scholar]
- 13.Atzl VM, Narayan AJ, Rivera LM, et al. Adverse childhood experiences and prenatal mental health: type of ACEs and age of maltreatment onset. J Fam Psychol. 2019;33:304–314. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br J Psychiatry. 2010;197:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neville A, Soltani S, Pavlova M, et al. Unravelling the relationship between parent and child PTSD and pediatric chronic pain: the mediating role of pain catastrophizing. J Pain. 2018;19:196–206. [DOI] [PubMed] [Google Scholar]
- 16.Olivieri P, Solitar B, Dubois M. Childhood risk factors for developing fibromyalgia. Open Access Rheumatol Res Rev. 2012;4:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Videlock EJ, Shih W, et al. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;28:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott KM, Von Korff M, Angermeyer MC, et al. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry. 2011;68:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke JE, Racine N, Plamondon A, et al. Maternal adverse childhood experiences, attachment style, and mental health: pathways of transmission to child behavior problems. Child Abus Negl. 2019;93:27–37. [DOI] [PubMed] [Google Scholar]
- 20.Dennis CH, Clohessy DS, Stone AL, et al. Adverse childhood experiences in mothers with chronic pain and intergenerational impact on children. J Pain. 2019;20:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lê-Scherban F, Wang X, Boyle-Steed KH, et al. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. 2018;141:e20174274. [DOI] [PubMed] [Google Scholar]
- 22.Madigan S, Wade M, Plamondon A, et al. Maternal adverse childhood experience and infant health: biomedical and psychosocial risks as intermediary mechanisms. J Pediatr. 2017;187:282–289. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell CG, Valentino K. Intergenerational effects of childhood trauma: evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreat. 2016;21:317–326. [DOI] [PubMed] [Google Scholar]
- 24.Plant DT, Pawlby S, Pariante CM, et al. When one childhood meets another—maternal childhood trauma and offspring child psychopathology: a systematic review. Clin Child Psychol Psychiatry. 2018;23:483–500. [DOI] [PubMed] [Google Scholar]
- 25.Racine N, Plamondon A, Madigan S, et al. Maternal adverse childhood experiences and infant development. Pediatrics. 2018;141:e20172495. [DOI] [PubMed] [Google Scholar]
- 26.Schickedanz A, Halfon N, Sastry N, et al. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics. 2018;142:e20180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Patel F, Rose-Jacobs R, et al. Mothers’ adverse childhood experiences and their young children’s development. Am J Prev Med. 2017;53:882–891. [DOI] [PubMed] [Google Scholar]
- 28.Thomas-Argyriou JC, Letourneau N, Dewey D, et al. The APrON Study Team. The role of HPA-axis function during pregnancy in the intergenerational transmission of maternal adverse childhood experiences to child behavior problems. Dev Psychopathol. 2021;33:284–300. [DOI] [PubMed] [Google Scholar]
- 29.Treat AE, Sheffield-Morris A, Williamson AC, et al. Adverse childhood experiences and young children’s social and emotional development: the role of maternal depression, self-efficacy, and social support. Early Child Dev Care. 2020;190:2422–2436. [Google Scholar]
- 30.Beveridge JK, Dobson KS, Madigan S, et al. Adverse childhood experiences in parents of youth with chronic pain: prevalence and comparison with a community-based sample. Pain Reports. 2020;5:e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhushan D, Kotz K, McCall J, et al. Roadmap for resilience: The California Surgeon General’s report on adverse childhood experiences, toxic stress, and health. Office of the California Surgeon General. 2020. Available at: https://osg.ca.gov/sg-report/.
- 32.Deighton S, Neville A, Pusch D, et al. Biomarkers of adverse childhood experiences: a scoping review. Psychiatry Res. 2018;269:719–732. [DOI] [PubMed] [Google Scholar]
- 33.Min MO, Minnes S, Kim H, et al. Pathways linking childhood maltreatment and adult physical health. Child Abus Negl. 2013;37:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provençal N, Binder EB. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol. 2015;268:10–20. [DOI] [PubMed] [Google Scholar]
- 35.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232. [DOI] [PubMed] [Google Scholar]
- 36.Thomas JC, Letourneau N, Campbell TS, et al. Social buffering of the maternal and infant HPA axes: mediation and moderation in the intergenerational transmission of adverse childhood experiences. Dev Psychopathol. 2018;30:921–939. [DOI] [PubMed] [Google Scholar]
- 37.Nelson S, Beveridge JK, Mychasiuk R, et al. Adverse Childhood Experiences (ACEs) and internalizing mental health, pain, and quality of life in youth with chronic pain: a longitudinal examination. J Pain. 2021;22:1210–1220. [DOI] [PubMed] [Google Scholar]
- 38.Neville A, Griep Y, Palermo TM, et al. A “dyadic dance”: pain catastrophizing moderates the daily relationships between parent mood and protective responses and child chronic pain. Pain. 2020;161:1072–1082. [DOI] [PubMed] [Google Scholar]
- 39.Neville A, Jordan A, Beveridge JK, et al. Diagnostic uncertainty in youth with chronic pain and their parents. J Pain. 2019;20:1080–1090. [DOI] [PubMed] [Google Scholar]
- 40.Neville A, Jordan A, Pincus T, et al. Diagnostic uncertainty in pediatric chronic pain: nature, prevalence, and consequences. Pain Reports. 2020;5:e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neville A, Kopala-Sibley DC, Soltani S, et al. A longitudinal examination of the interpersonal fear avoidance model of pain: the role of intolerance of uncertainty. Pain. 2021;162:152–160. [DOI] [PubMed] [Google Scholar]
- 42.Pavlova M, Kopala-Sibley DC, Nania C, et al. Sleep disturbance underlies the co-occurrence of trauma and pediatric chronic pain: a longitudinal examination. Pain. 2020;161:821–830. [DOI] [PubMed] [Google Scholar]
- 43.Soltani S, van Ryckeghem DML, Vervoort T, et al. Attentional biases in pediatric chronic pain: an eye-tracking study assessing the nature of the bias and its relation to attentional control. Pain. 2020;161:2263–2273. [DOI] [PubMed] [Google Scholar]
- 44.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translation informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27:169–190. [DOI] [PubMed] [Google Scholar]
- 47.Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics (CT) Scales. J Marriage Fam. 1979;41:75–88. [Google Scholar]
- 48.Wyatt GE. The sexual abuse of Afro-American and White-American women in childhood. Child Abus Negl. 1985;9:507–519. [DOI] [PubMed] [Google Scholar]
- 49.Dube SR, Williamson DF, Thompson T, et al. Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abus Negl. 2004;28:729–737. [DOI] [PubMed] [Google Scholar]
- 50.Ford DC, Merrick MT, Parks SE, et al. Examination of the factorial structure of adverse childhood experiences and recommendations for three subscale scores. Psychol Violence. 2014;4:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frampton NMA, Poole JC, Dobson KS, et al. The effects of adult depression on the recollection of adverse childhood experiences. Child Abus Negl. 2018;86:45–54. [DOI] [PubMed] [Google Scholar]
- 52.Mersky JP, Janczewski CE, Topitzes J. Rethinking the measurement of adversity: moving toward second-generation research on adverse childhood experiences. Child Maltreat. 2017;22:58–68. [DOI] [PubMed] [Google Scholar]
- 53.Birnie KA, Heathcote LC, Bhandari RP, et al. Parent physical and mental health contributions to interpersonal fear avoidance processes in pediatric chronic pain. Pain. 2020;161:1202–1211. [DOI] [PubMed] [Google Scholar]
- 54.Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 56.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 57.Weathers FW, Litz BT, Keane TM, et al. The PTSD Checklist for DSM-5 with Life Events Checklist for DSM-5 and Criterion A [Measurement instrument]. 2013. Available at: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp.
- 58.National Center for PTSD. PTSD checklist for DSM-5 (PCL-5). Available at: https://www.ptsd.va.gov/professional/assessment/documents/PCL-5_LEC_criterionA.pdf.
- 59.Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489–498. [DOI] [PubMed] [Google Scholar]
- 60.Palermo TM, Witherspoon D, Valenzuela D, et al. Development and validation of the child activity limitations interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109:461–470. [DOI] [PubMed] [Google Scholar]
- 61.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. [DOI] [PubMed] [Google Scholar]
- 62.Kashikar-Zuck S, Carle A, Barnett K, et al. Longitudinal evaluation of Patient Reported Outcomes Measurement Information Systems (PROMIS) measures in pediatric chronic pain. Pain. 2016;157:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 64.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach, 2nd ed. New York, NY: Guilford Publications; 2017. [Google Scholar]
- 65.Valeri L, VanderWeele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobson K, Pusch D, Allan L, et al. The long shadow of adverse childhood events (ACEs): 2. Physical health outcomes in an adult community sample. Am J Prev Med Public Heal. 2020;6:39–49. [Google Scholar]
- 67.Sachs-Ericsson NJ, Sheffler JL, Stanley IH, et al. When emotional pain becomes physical: adverse childhood experiences, pain, and the role of mood and anxiety disorders. J Clin Psychol. 2017;73:1403–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. [DOI] [PubMed] [Google Scholar]
- 69.Poole JC, Dobson KS, Pusch D. Childhood adversity and adult depression: the protective role of psychological resilience. Child Abuse Negl. 2017;64:89–100. [DOI] [PubMed] [Google Scholar]
- 70.Leardmann CA, Smith B, Ryan MA. Do adverse childhood experiences increase the risk of postdeployment posttraumatic stress disorder in US Marines? BMC Public Health. 2010;10:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schalinski I, Teicher MH, Nischk D, et al. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu NS, Schairer LC, Dellor E, et al. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict Behav. 2010;35:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonathan C, Hearn L, Williams ACdeC. Socioeconomic status and the course and consequences of chronic pain. Pain Manag. 2013;3:159–162. [DOI] [PubMed] [Google Scholar]
- 74.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for postraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. [DOI] [PubMed] [Google Scholar]
- 75.DiGrande L, Perrin MA, Thorpe LE, et al. Posttraumatic stress symptoms, PTSD, and risk factors among lower Manhattan residents 2-3 years after the September 11, 2001 terrorist attacks. J Trauma Stress. 2008;21:264–273. [DOI] [PubMed] [Google Scholar]
- 76.Lorant V, Deliège D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157:98–112. [DOI] [PubMed] [Google Scholar]
- 77.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: findings from the National Health and Nutrition Examination Survey. Pain Med. 2014;15:1508–1521. [DOI] [PubMed] [Google Scholar]
- 79.Metzler M, Merrick MT, Klevens J, et al. Adverse childhood experiences and life opportunities: shifting the narrative. Child Youth Serv Rev. 2017;72:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schurer S, Trajkovski K, Hariharan T. Understanding the mechanisms through which adverse childhood experiences affect lifetime economic outcomes. Labour Econ. 2019;61:101743. [Google Scholar]
- 81.Braveman P, Barclay C. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;124:S163–S175. [DOI] [PubMed] [Google Scholar]
- 82.Font SA, Maguire-Jack K. Pathways from childhood abuse and other adversities to adult health risks: the role of adult socioeconomic conditions. Child Abus Negl. 2016;51:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higgins KS, Birnie KA, Chambers CT, et al. Offspring of parents with chronic pain: a systematic review and meta-analysis of pain, health, psychological, and family outcomes. Pain. 2015;156:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stone AL, Wilson AC. Transmission of risk from parents with chronic pain to offspring: an integrative conceptual model. Pain. 2016;157:2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haynes E, Crouch E, Probst J, et al. Exploring the association between a parent’s exposure to Adverse Childhood Experiences (ACEs) and outcomes of depression and anxiety among their children. Child Youth Serv Rev. 2020;113:105013. [Google Scholar]
- 86.Sieberg CB, Williams S, Simons LE. Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain? J Pediatr Psychol. 2011;36:1043–1051. [DOI] [PubMed] [Google Scholar]
- 87.Lampe A, Doering S, Rumpold G, et al. Chronic pain syndromes and their relation to childhood abuse and stressful life events. J Psychosom Res. 2003;54:361–367. [DOI] [PubMed] [Google Scholar]
- 88.Ports KA, Ford DC, Merrick MT. Adverse childhood experiences and sexual victimization in adulthood. Child Abuse Negl. 2016;51:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bethell C, Jones J, Gombojav N, et al. Positive childhood experiences and adult mental and relational health in a statewide sample: associations across adverse childhood experiences levels. JAMA Pediatr. 2019;173:e193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benzies K, Mychasiuk R. Fostering family resiliency: a review of the key protective factors. Child Fam Soc Work. 2009;14:103–114. [Google Scholar]
- 91.Sege RD, Harper Browne C. Responding to ACEs With HOPE: Health Outcomes From Positive Experiences. Acad Pediatr. 2017;17:S79–S85. [DOI] [PubMed] [Google Scholar]
- 92.Nelson S, Simons LE, Logan D. The incidence of adverse childhood experiences (ACEs) and their association with pain-related and psychosocial impairment in youth with chronic pain. Clin J Pain. 2018;34:402–408. [DOI] [PubMed] [Google Scholar]
- 93.Racine N, Killam T, Madigan S. Beyond the Adverse Childhood Experiences Questionnaire: trauma-informed care as a universal precaution. JAMA Pediatr. 2020;174:5–6. [DOI] [PubMed] [Google Scholar]
- 94.Substance Abuse and Mental Health Services Administration. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. 2014. Available at: https://ncsacw.samhsa.gov/userfiles/files/SAMHSA_Trauma.pdf.
- 95.Widom CS, Raphael KG, DuMont KA. The case for prospective longitudinal studies in child maltreatment research: commentary on Dube, Williamson, Thompson, Felitti, and Anda (2004). Child Abus Negl. 2004;28:715–722. [DOI] [PubMed] [Google Scholar]
- 96.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry Allied Discip. 2004;45:260–273. [DOI] [PubMed] [Google Scholar]
- 97.Schofield TJ, Lee RD, Merrick MT. Safe, stable, nurturing relationships as a moderator of intergenerational continuity of child maltreatment: a meta-analysis. J Adolesc Heal. 2013;53:S32–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Serbin LA, Karp J. The intergenerational transfer of psychosocial risk: mediators of vulnerability and resilience. Annu Rev Psychol. 2004;55:333–363. [DOI] [PubMed] [Google Scholar]


