Abstract
Introduction.
Esophageal adenocarcinoma (EAC) has increased in incidence in Western countries, and its poor prognosis necessitates the development of novel therapeutics. We previously reported the potential of conditionally replicative adenoviruses (CRAd) as a novel therapeutic treatment for this disease. To further augment the therapeutic effectiveness of our cyclooxygenase-2 (Cox2) controlled CRAd in EAC, we inserted an interferon alpha (IFN) transgene into the viral genome that is expressed upon viral replication. In this manuscript, we analyze the cytotoxic and oncolytic effects of an IFN-expressing oncolytic adenovirus in EAC and the role of the Cox2 promoter in providing for selective replication in human tissues.
Methods.
An infectivity-enhanced IFN-expressing CRAd (5/3 Cox2 CRAd ΔE3 ADP IFN) and other control viruses were first tested in vitro with cell lines. For the in vivo study, EAC xenografts in nude mice were treated with a single intratumoral dose of virus. An ex vivo analysis with live tissue slices was conducted using surgically resected EAC patient specimens.
Results.
Expression of IFN significantly enhanced the cytotoxic and oncolytic effect of a Cox2-promoter controlled CRAd. This virus showed significant tumor growth suppression in a xenograft model. Furthermore, in human EAC samples, the promoter-controlled virus demonstrated selective replication in cancerous tissues, leaving normal esophageal tissue unaffected.
Conclusion.
An IFN-expressing CRAd driven by the Cox2 promoter has strong oncolytic effects as well as cancer-specific replication. Our novel vector possesses critical characteristics that make it a potential candidate for clinical translation to treat EAC.
Esophageal cancer is the seventh most common malignancy in the world.1 It is an aggressive neoplasm with a 5-year overall survival rate of approximately 20%.2 For 2020, there are estimated to be approximately 18,440 new diagnoses of esophageal cancer in the USA and an estimated 16,170 deaths from this disease.3 In recent years, there has been a tremendous shift in the predominant histologic subtype (especially in Western countries) as evidenced by a sixfold increase in the incidence of esophageal adenocarcinoma (EAC) in the USA from 1975 to 2001,4 and EAC is now the most common histology, accounting for approximately 60% of new diagnoses in the USA.5 The etiology of this change in the predominant histologic subtype is likely multifactorial and related to gastroesophageal reflex disease, obesity, and infection with the bacteria Helicobacter pylori.6 A key characteristic that makes esophageal cancer so difficult to treat is the large percentage of patients who are found to have advanced disease at the time of initial presentation,1 in part because the esophagus does not have a serosal layer to serve as a barrier to other nearby structures within the abdomen.7 Only one-third of patients are candidates for surgical resection, and even for those that do undergo surgery, there is a high rate of local and distant recurrences.8 Despite advances in chemotherapy, surgical techniques, and perioperative care, there have only been modest improvements in overall survival in recent years.9 Furthermore, EAC are far less responsive to radiotherapy when compared to squamous cell carcinomas.10 Due to the limited effectiveness of currently available therapies, the development of novel treatment modalities is needed.
Interferon alpha (IFN), a type 1 interferon, is a well-studied cytokine with a multitude of effects including antitumor, antiangiogenic, and immunomodulatory properties.11 Additionally, it has been shown to sensitize cancer cells to chemotherapy and radiation.12,13 IFN has been used in the treatment of many solid tumors (including EAC) by systemic delivery;14–16 however, one of the main difficulties with its use is the associated dose-limiting systemic toxicities. In addition, it has a short half-life following administration that limits its overall bioavailability.17
A promising way to overcome the shortcomings of systemic IFN delivery is through local expression of the cytokine through the use of an oncolytic virus. These viruses have been emerging as novel anticancer therapeutics, spurred on by the Food and Drug Administration (FDA) approval of talimogene laherparepvec (T-VEC).18 Oncolytic viruses can be modified to allow for selective infection and replication within cancer cells. In addition, transgenes can be inserted into the viral genome, which are then expressed upon viral replication. Many groups, including our own, have incorporated IFN into the construct of an oncolytic adenovirus. We have shown that, by incorporating the human IFN transgene into the E3 region of the adenovirus genome, high concentrations of IFN can be achieved at the local tumor site.19–21
To enhance the safety and usability of oncolytic viruses in the clinic, they must be able to selectively replicate in cancer cells, as the natural tropism for wild-type adenovirus for the liver can result in hepatotoxicity.22 Cyclooxygenase 2 (Cox2) is known to be expressed in a variety of gastrointestinal adenocarcinomas, including EAC.23 Our group previously reported the design of a conditionally replicating adenovirus (CRAd) with the Cox2 promoter that selectively replicates within EAC cells, where the infectivity of this virus was further enhanced by incorporating a chimeric Ad5/Ad3 fiber24
In this study, we investigated the in vitro and in vivo effects of an IFN-expressing oncolytic virus that is driven by the Cox2 promoter in EAC cells. Furthermore, we analyzed the replication selectivity of this virus in fresh human tissues using a tissue slice culture system to further highlight its potential for clinical translation.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
Two human EAC cell lines (OE19 and OE33) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Herndon, VA). The OE19 and OE33 cell lines were obtained from the European Collection of Authenticated Cell Cultures (ECACC). All cell lines were cultured with DMEM supplemented with 10% (V/V) fetal bovine serum and 1% penicillin–streptomycin mixture (100 and 100 μg/mL, respectively). They were maintained as adherent monolayers at 37°C in a humidified incubator with 5% CO2.
Oncolytic Adenoviruses
The IFN-expressing oncolytic adenoviruses (Fig. 1) were based on human adenovirus type 5 (Ad5) and contained the “ΔE3-ADP” structure as we described previously.19,20,22,25 Briefly, most of the adenovirus E3 genes were deleted with the exception of the adenovirus death protein (ADP), which is designed to facilitate augmented viral spread and oncolysis; furthermore, the human IFN-alpha gene was then placed in the deleted region, where its expression is controlled by the adenovirus major late promoter in a viral replication dependent manner.21,26,27
FIG. 1.

Schematic of the oncolytic adenovirus. The replication of virus was controlled by the Cox2 promoter which was inserted upstream of the adenovirus E1 region as a tissue-specific promoter. Also, an expression cassette containing the adenovirus death protein (ADP) and the interferon alpha (IFN) transgene was inserted into place within the E3 region. For one of the control viruses, the luciferase transgene was used in place of IFN. Finally, the virus has the 5/3 chimeric fiber to enhance infectivity in cancer cells
With the exception of the Ad5 wild-type virus, all other viruses involved in these studies included a 5/3-modified fiber.24,28 In the CRAd (5/3-Cox2-ΔE3-ADP-IFN), the adenovirus E1 region was placed under control of the Cox2 tumor-specific promoter.
The human IFN coding plasmid was kindly provided by Dr. Aoki (National Cancer Center Research Institute, Tokyo, Japan)29 As control viruses, the identical adenoviruses expressing the firefly luciferase (Luc) gene have been used.19,24,25
All viruses were propagated with the A549 cell line, purified by double cesium chloride density gradient ultra-centrifugation, and dialyzed against phosphate-buffered saline (PBS) with 10% glycerol.19,20 The adenoviruses were titrated by plaque forming assay, and the viral particle (vp) number was measured spectrophotometrically.19,20 Viral structure was confirmed by polymerase chain reaction for the presence of the Cox2 promoter, the fiber structure, and the absence of contamination with a mutant replication competent adenovirus as described previously.19,20,24,30
Qualitative Cytocidal Effect
Two human EAC cell lines (OE19 and OE33) were plated in 12-well plates, and viral infection was performed the next day using a titer of 10 vp/cell. Viruses included two CRAds (5/3 Cox2 ΔE3 ADP IFN and 5/3 Cox2 ΔE3 ADP Luc), a nonselective IFN-expressing control (5/3 ΔE3 ADP IFN), and a wild-type control (Ad5 Wt). At various time points following viral infection, the cells were fixed with 10% buffered formalin for 10 min, stained with 1% crystal violet in 70% ethanol for 20 min, washed three times with tap water, and allowed to air dry as described previously.31,32
Quantitative Analysis of Cell Viability
Two human EAC cell lines (OE19 and OE33) were cultured in 96-well plates (3000 cells/well), and subsequently infected with adenoviral viruses 24 h thereafter at 10 vp/cell. The number of surviving cells was measured by using a colorimetric method (Cell Titer Aqueous One Solution Cell Proliferation Assay: Promega; Madison, WI) according to the manufacturer’s instructions. Absorbance attributable to living cells was measured at a wavelength of 490 nm in a FLUOstar Omega spectrophotometer (BMG Labtech, Ortenberg, Germany) as described previously.31,32 The number of infected, living cells at each time point was normalized to the number of uninfected living cells.
In Vivo Tumor Analyses
OE19 EAC cells (1 × 106 cells in 100 μL PBS) were inoculated subcutaneously into the flanks of female athymic nude mice (Charles River Laboratories) to generate tumors. When the tumor nodules achieved a diameter of approximately 6–8 mm, virus was injected intratumorally at a dose of 1.0 × 1010 vp/tumor. The tumor diameter was measured twice per week with calipers. The tumor volume was calculated using the following formula: tumor volume = (width2 × length)/2. All procedures were carried out according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
In a separate experiment for hexon staining, nude mice with treated tumors (as above) were sacrificed 5 days after viral injection. Tumors were then harvested and fixed with buffered formaldehyde. The expression of adenoviral hexon protein in the tumor was analyzed with immunostaining as described previously.30,33,34 All slides were scanned at 10× magnification using the objective lens on a Nikon Eclipse TS100 microscope (Nikon, Meleville, NY). For adenoviral copy number analysis, 25 mg of each tumor was utilized for DNA extraction using the QIAmp DNA Mini Kit (Qiagen). Quantitative viral copy number was obtained by TaqMan RT-PCR with adenoviral E4 primers.24,30
Tissue Slicer Experiments
Deidentified remnant surgical specimens of biopsy-proven human EAC and nearby normal esophageal tissue were obtained within 1 h of resection. They were immediately placed in phosphate-buffered saline on ice and transported to the laboratory. Immediately thereafter, a 5-mm tissue core was prepared and placed into the Krumdieck tissue slicer.35 Multiple slices (200 μm in thickness) were created and placed into culture. DMEM/F12 1:1 medium supplemented with 20% fetal bovine serum, 1% penicillin–streptomycin, 1% amphotericin B, and dexamethasone was used for tissue slices. For this experiment, the tissues were derived from one patient and analyses performed in triplicate. Experiments were carried out using protocols approved by the Institutional Review Board and the Bionet Tissue Procurement Service at the University of Minnesota.
Statistical Methods
Differences in viral infectivity and replication, cell viability, and relative tumor volumes of in vivo treatment groups were analyzed using Student’s t-test. A logarithmic transformation was used for the in vivo tumor study. The results were considered statistically significant when a two-tailed p value was less than 0.05. Data are expressed as a mean ± standard deviation, unless otherwise noted.
RESULTS
In Vitro Cytocidal Effects of EAC-Targeted CRAds
To demonstrate the cytocidal effects of the viruses, qualitative analyses were performed with crystal violet staining at multiple time points following viral infection (Fig. 2). The staining was done at an early time point when 5/3 COX2 ΔE3 ADP IFN started to show oncolysis (day 4–5) as well as at a late time point when the Ad5 Wt (fiber unmodified) virus started to show oncolysis (day 7–8). The 5/3 Cox2 ΔE3 ADP IFN virus demonstrated a strong cytocidal effect and resulted in almost complete elimination of the cells at the later time point in all cell lines. The 5/3 Cox2 ΔE3 ADP Luc virus (identical virus without IFN) had a limited cytotoxic effect across all cell lines, even at the later time point. A comparison of the cell-killing effects of these two viruses demonstrated the profound cytotoxic effects of the IFN transgene. When compared with the Ad5 wild-type virus, the 5/3 Cox2 ΔE3 ADP IFN virus had a markedly improved cytotoxic effect. Compared with a nonselective IFN-expressing virus without a tissue-specific promoter (5/3 ΔE3 ADP IFN), the 5/3 Cox2 ΔE3 ADP IFN virus showed a slight delay in oncolysis but eventually displayed a strong cytotoxic effect in all cell lines that were tested.
FIG. 2.

Qualitative analysis of cytotoxicity (crystal violet assay). Four different viruses (5/3 ΔE3 ADP IFN, 5/3 Cox2 ΔE3 ADP IFN, 5/3 Cox2 ΔE3 ADP Luc, and Ad5 Wt) were analyzed to assess their cell killing effects in two EAC cell lines (OE19 and OE33). The plates were analyzed at two time points following viral infection at 10 viral particles (vp) per cell: the first time point was when the 5/3 Cox2 ΔE3 ADP IFN started to show oncolysis, and the second time point was when the Ad5 Wt virus started to show oncolysis. The 5/3 ΔE3 ADP IFN virus killed almost all of the cells at an early time point in all cell lines tested. The 5/3 Cox2 ΔE3 ADP IFN virus (identical aside from the addition of a tissue-specific promoter) had a slightly less potent cytocidal effect at earlier time point, but still killed the majority of cells by the later time point in all cell lines tested. Following infection with the 5/3 Cox2 ΔE3 ADP Luc virus, most cells still remained alive at the end of the experiment
For the quantitative MTS assay, the 5/3 Cox2 ΔE3 ADP IFN and 5/3 ΔE3 ADP IFN viruses resulted in the death of over 75% of the cells in both EAC cell lines at day 10 (Fig. 3). As these two viruses are identical with the exception of the tissue-specific promoter, the Cox2 promoter did not negatively impact the cytotoxic potential of the 5/3 Cox2 ΔE3 ADP IFN virus to any large degree. Additionally, the 5/3 Cox2 ΔE3 ADP Luc virus did not demonstrate much cell-killing effect, and approximately 70% of the cells were still alive at the second time point.
FIG. 3.
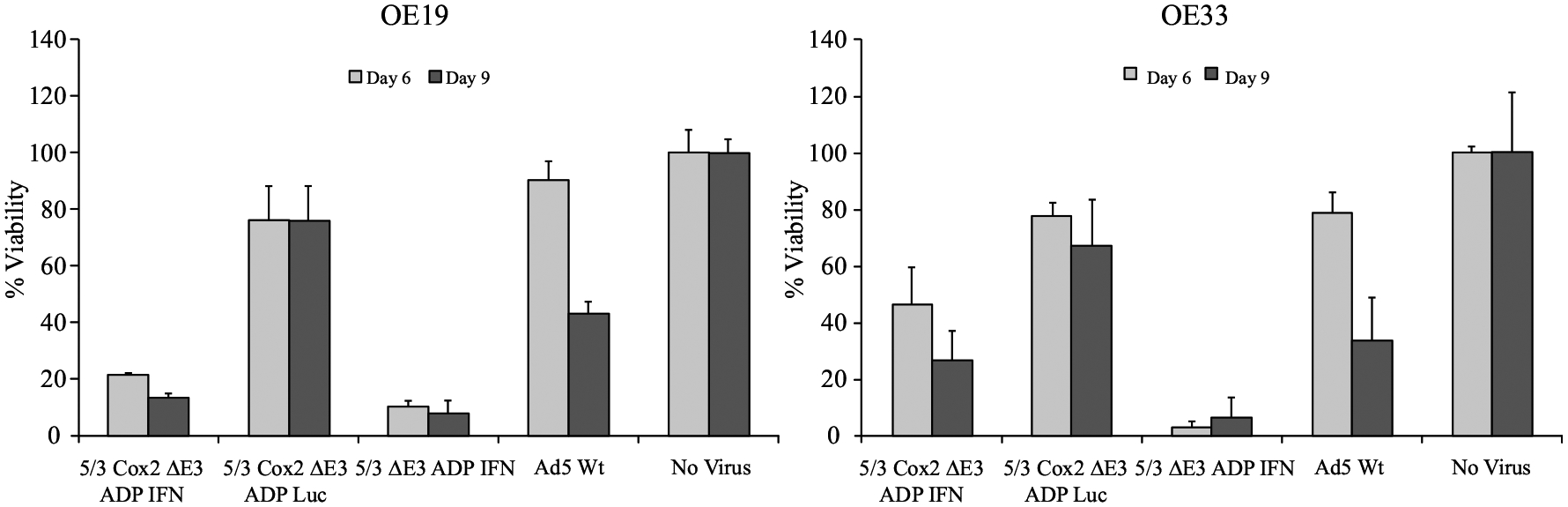
Quantitative analysis of cytotoxicity (MTS assay). The oncolytic effect of a nonselective IFN-expressing virus (5/3 ΔE3 ADP IFN), a selective IFN-expressing virus (5/3 Cox2 ADP IFN), a selective luciferase-expressing virus (5/3 Cox2 ΔE3 ADP Luc), and a wild-type adenovirus (Ad5 Wt) were compared in two different EAC cell lines (OE19 and OE33) with a quantitative viability assay. Data shown as percentage of living cells (with the living noninfected cells set to 100%). The 5/3 ΔE3 ADP IFN virus (nonselective) resulted in near-complete cell death at early time points. The 5/3 Cox2 ΔE3 ADP IFN virus still had a strong cytocidal effect by the later time point in the experiment. The luciferase-expressing virus did not have a strong cell-killing effect
These data indicate that addition of IFN greatly augmented cytotoxicity. Furthermore, the Cox2 promoter-controlled, IFN-expressing virus maintained a strong cytocidal effect.
Therapeutic Effect of Intratumoral Virus Injection and Viral Replication In Vivo
The in vivo antitumor effects of the 5/3 Cox2 ΔE3 ADP IFN virus were assessed with a nude mouse model using subcutaneous xenografts established with the OE 19 EAC cell line (Fig. 4). Both treatment groups (Ad5 Wt and 5/3 Cox2 ΔE3 ADP IFN, 1.0 × 1010 viral particles via intratumoral delivery) demonstrated tumor growth suppression when compared with the phosphate-buffered saline (PBS)-injected control group by day 6 after injection. At day 16, the 5/3 Cox2 ΔE3 ADP IFN group had significantly less tumor volume than the saline-injected control group (p < 0.05). More importantly, the 5/3 Cox2 ΔE3 ADP IFN was outperforming the wild-type virus and that difference was even more pronounced by the end of the experiment (p < 0.05 at day 22).
FIG. 4.
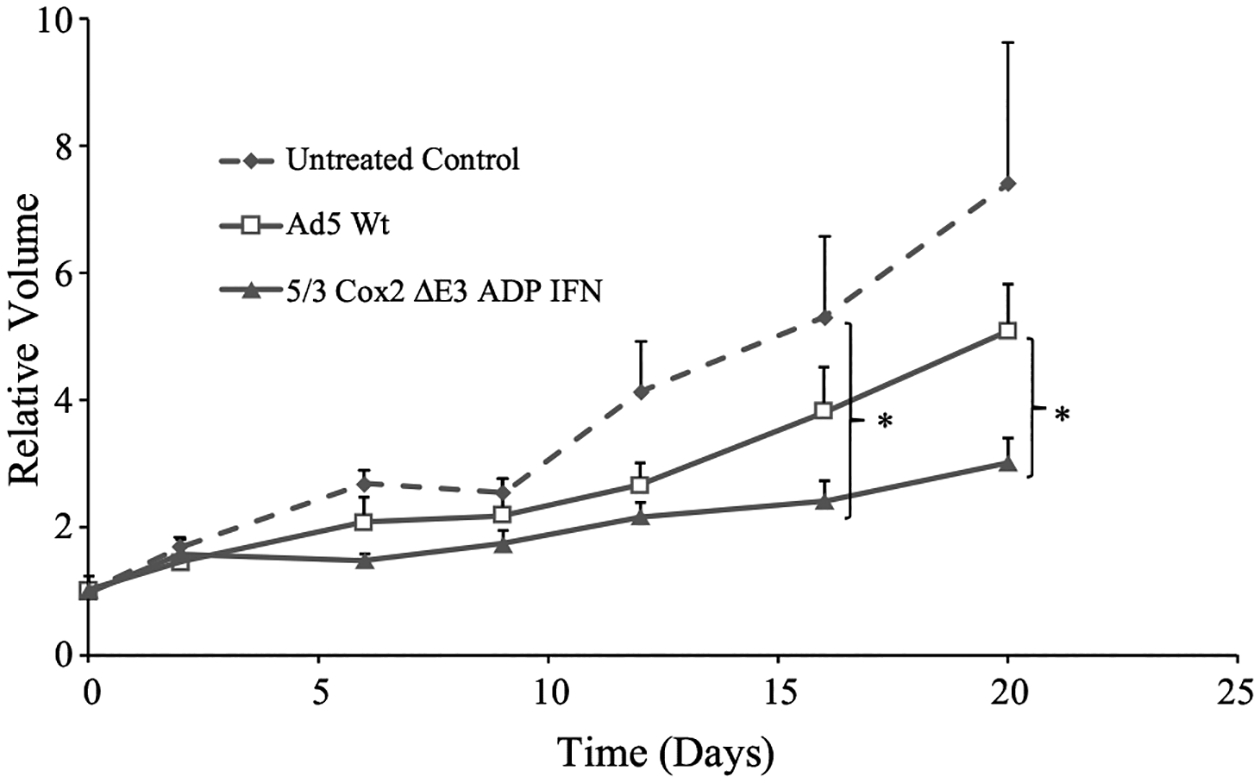
Oncolytic effect of IFN-expressing oncolytic adenovirus in EAC xenograft model. Subcutaneous xenografts of OE19 in nude mice were injected with one dose of adenovirus intratumorally (1 × 1010 vp/tumor). Tumor size is shown as volume relative to day 0 (viral treatment). The selective, interferon-expressing virus (5/3 Cox2 ΔE3 ADP IFN) (eight tumors) demonstrated significant tumor growth suppression when compared with the wild-type adenovirus (eight tumors) and the PBS control (six tumors) (p < 0.05). Student’s t test was used to compared volumes between treatment groups, and the error bars represent standard deviation
In a separate experiment with the same conditions, tumors were harvested from mice 5 days after virus injection and subsequently prepared for adenoviral hexon staining and adenoviral E4 copy number analysis (Fig.5a, b). As expected, there was no evidence of viral replication in the saline control group. The Ad5 Wt virus and the 5/3 Cox2 ΔE3 ADP IFN virus had comparable degrees of viral replication as demonstrated through similar hexon staining on the FITC and merge slides (Fig.5a) and nonsignificant differences in viral replication in the quantitative E4 copy number analysis (Fig.5b).
FIG. 5.
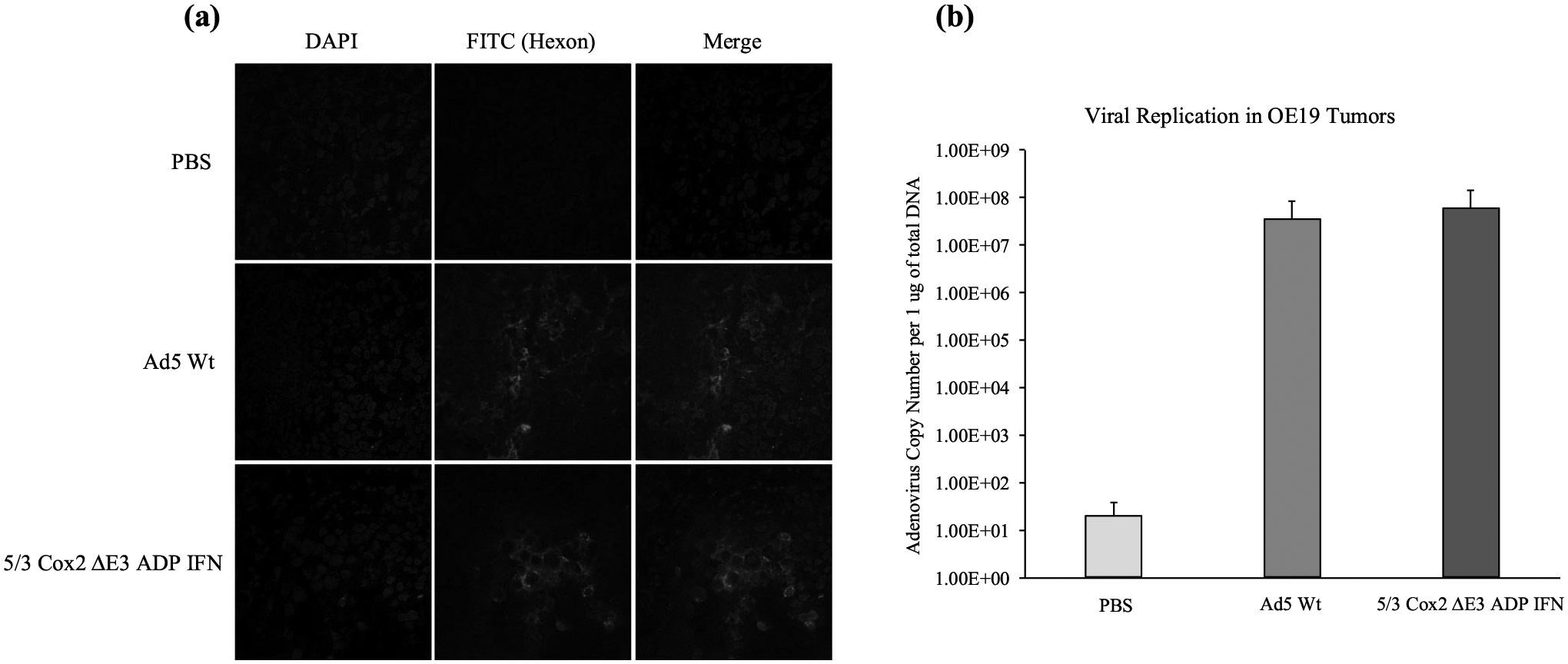
Viral protein (adenoviral hexon) expression and viral copy number analysis, a At day 5 following viral injection, expression of adenovirus hexon was assessed by immunostaining with FITC-labeled anti-hexon polyclonal antibody (counterstained with DAPI). Staining and sections were performed in at least two independent experiments. Green: adenovirus hexon protein; blue: nucleus (original magnification; ×60). b Adenoviral DNA copy number analysis performed by RT-PCR. There was no significant difference in viral replication observed between the Ad5 Wt vector and the Cox2 promoter-controlled virus. Error bars represent standard deviation
Ex Vivo Tissue Slice Experiment
Samples of biopsy-proven EAC (and nearby normal esophageal tissue) were prepared and placed into culture. Two viruses were used for this experiment, only differing in the presence or absence of the Cox 2 promoter (5/3 Cox2 ΔE3 ADP Luc, 5/3 ADP Luc). Forty-eight hours following viral infection, a luciferase assay was performed to analyze the degree of viral replication (Fig. 6). Here, the luciferase transgene in the adenovirus E3 region is expressed when virus is replicating and serves as a quantitative marker of the degree of adenovirus replication. For the EAC sample, there was a comparable degree of viral replication between both viruses as expected. In the normal (noncancerous) esophageal tissue sample, there was a statistically significant difference in the degree of viral replication (p < 0.05). Here, there was almost no replication of the 5/3 Cox2 ΔE3 ADP Luc virus and it was approximately 100-fold less than that of the 5/3 ADP Luc virus. This finding suggests that, in human tissue samples, the Cox2 promoter element adds a significant degree of selectivity to viral replication.
FIG. 6.
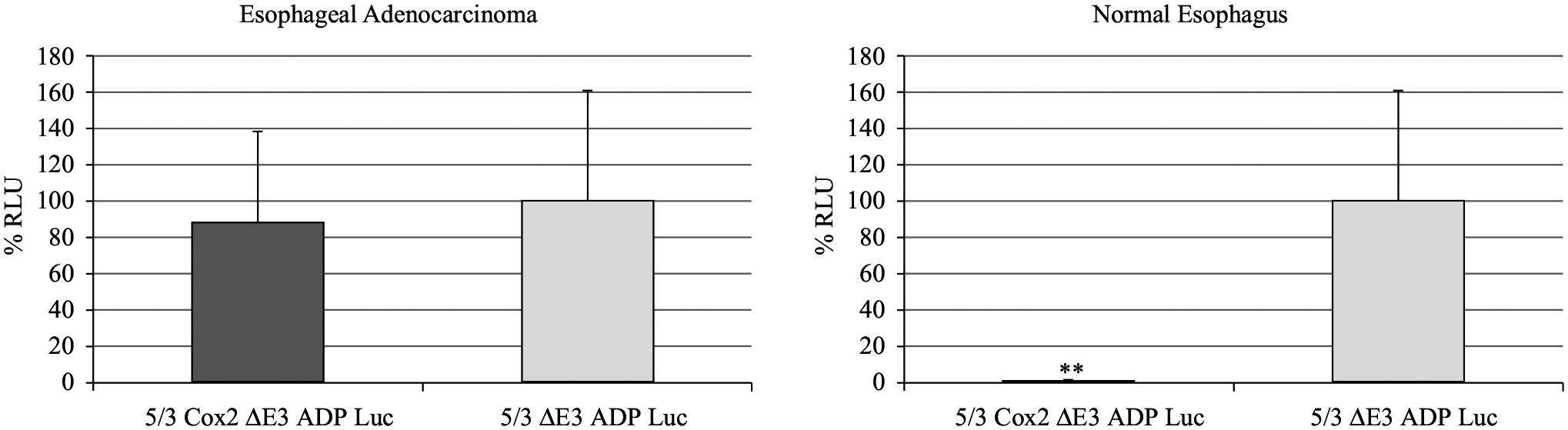
Replication selectivity in ex vivo experiments with tissue slices. Live samples of human EAC and nearby normal esophagus were obtained following surgical resection. They were subsequently sliced to thickness of 200 μm using the Krumdieck tissue slicer. Viral infection was performed with luciferase-expressing viruses (with and without Cox2 promoter) at strength of 100 vp/cell. After 48 h of incubation, luminescence was measured and the results expressed as percentage of relative light units (RLU) with respect to the 5/3 ΔE3 ADP Luc virus. Importantly, there was minimal replication with the promoter-controlled virus in the normal tissue, and this was significantly different when compared with the virus lacking promoter control (**p < 0.05)
DISCUSSION
Esophageal adenocarcinoma remains a difficult-to-treat solid tumor of the gastrointestinal tract despite surgical and chemotherapeutic advances in recent years.1,9 To that end, there is a great need for novel therapies that can serve as adjuncts to these standard approaches to treatment. The field of oncolytic virotherapy continues to grow and expand with an eye towards maximizing cytocidal and oncolytic potential, while minimizing the effects on surrounding normal cells.36 In these studies, we utilized an infectivity-enhanced adenovirus expressing IFN that was under the control of a Cox2 tissue-specific promoter to analyze both its cell-killing potential as well as its selectivity.
The coxsackie and adenovirus receptor is the primary receptor for adenovirus serotype 5, but it is poorly expressed on EAC cells.37,38 We previously demonstrated how the chimeric Ad5/Ad3 fiber augments adenovirus infectivity when compared with the Ad5 wild-type fiber and an RGD (arginine–glycine–aspartate) fiber in EAC cells,24 which is why the chimeric Ad5/3 fiber modification was chosen for these studies.
In vitro studies demonstrated the profound effect of IFN expression on the cytocidal effect of the virus (Figs. 2,3). By comparing the 5/3 Cox2 ΔE3 ADP IFN and 5/3 Cox2 ΔE3 ADP Luc viruses (identical viruses with the exception of the transgene that is expressed), we clearly demonstrated this augmented cell killing with interferon. Furthermore, in the in vivo studies, the IFN-expressing virus demonstrated statistically significant tumor growth suppression compared with the saline control and the wild-type adenovirus (Fig. 4). Due to technical challenges with establishing orthotopic xenograft EAC tumors in mice and injecting therapeutics into these tumors, we chose to employ the subcutaneous tumor model as it is currently the most dependable model to represent EAC.
Cox2 is highly expressed on EAC cells,23 and our group previously reported the utility of using this promoter in the construct of adenoviruses directed towards esophageal cancer cells. Placing a promoter control element is critically important to increase tissue-specific replication as adenovirus with wild-type E1 regions have a natural tropism for the liver and have the potential to result in hepatoxicity.19 However, this is the first set of experiments in which the selectivity afforded by the Cox2 promoter has been combined with the oncolytic potential of IFN expression in an oncolytic adenovirus directed towards EAC.
The evaluation of oncolytic adenoviruses with human cancer xenografts has limitations in mice as murine cells are not permissive to human adenovirus replication.39,40 Additionally, Syrian golden hamsters are immunocompetent and semipermissive to adenovirus replication, but there is currently no syngeneic EAC model available.41 Therefore, we set out to demonstrate the selective replication resulting from a Cox2-controlled adenovirus in a scenario that would closely resemble a clinical situation. To do this, we employed a tissue-slice culture system using fresh specimens obtained from our university hospital operating room. The specimens were then infected with one of two viruses that only differed in the presence or absence of the Cox2 promoter (Fig. 6). As expected, both viruses replicated in the EAC samples. On the other hand, in the samples of normal esophageal tissue, there was a statistically significant decrease in the replication of the Cox2-controlled adenovirus when compared with the nonselective virus. In fact, there was only minimal replication (measured in RLU) with the Cox2-driven virus in these samples. Considering that syngeneic mouse models are not well suited to study oncolytic adenoviruses due to a lack of human adenovirus replication in mice and because the establishment of orthotopic models is extremely difficult for esophageal cancer due to anatomical features of this disease process, this ex vivo model has unique advantages for functionality analyses of oncolytic adenoviruses to fill the gaps of existing experimental systems.39,40,42
Adenoviral late genes, which include most of the viral structural proteins (e.g., hexon), are controlled by adenoviral major late promoter and are only expressed when the adenovirus is actively replicating. Therefore, adenoviral hexon staining was used to demonstrate active viral replication (Fig. 5). When compared with the Ad5 wild-type virus, there was comparable signal to the promoter-controlled IFN expressing virus. This signifies that the degree of viral replication is negligibly affected by the addition of the tissue-specific promoter into the adenoviral genome. Our group previously showed that, in EAC cells, adenoviruses with wild-type E1 promoters have a stronger cytocidal effect when compared with those with the Cox2 promoter inserted into the E1 region of the genome.24 Therefore, despite the similar early replication demonstrated by the hexon staining, the ADP overexpression and IFN cytokine more than compensate for any potential decrease in oncolytic effect caused by insertion of the Cox2 promoter.26,27,31 When this high degree of replication is combined with the selectivity demonstrated in the abovementioned experiment, there is great potential for a novel therapy that will have a strong cancer-killing effect through IFN expression while minimizing damage to surrounding normal tissues via a Cox2 promoter control mechanism (Fig. 6).
CONCLUSIONS
Our data demonstrate how an oncolytic adenovirus equipped with a Cox2 tissue-specific promoter can demonstrate selective replication in EAC in an ex vivo analysis using patient-derived tissues. Furthermore, infectivity enhancements with a Ad5/3 chimeric fiber and incorporation of the IFN transgene into the virus genome augment the cancer killing ability of the adenovirus. The data presented in this manuscript serve as a proof of concept that an IFN-expressing oncolytic adenovirus can be effective against EAC. While additional preclinical testing will be needed to guide clinical translation, we are encouraged by the therapeutic potential of this virus in the treatment of patients with EAC. Future studies will be geared towards performing preclinical toxicology studies in addition to testing this virus in combination with chemotherapy and radiation to further demonstrate its potential clinical benefits. A long-term goal is to develop an immunocompetent, replication-permissive animal model to analyze our IFN-expressing adenovirus in EAC.
ACKNOWLEDGMENT
The authors thank Dr. Kazunori Aoki (National Cancer Center Research Institute of Japan) for allowing us to use the human IFN-alpha encoding plasmid.
FUNDING
The project is partly supported by NIH/NCI R01CA228760-01A1 (MY/JD) and a University of Minnesota-VFW Surgical Oncology Research Award (C.J.L.).
Footnotes
DISCLOSURE None.
REFERENCES
- 1.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. April 2 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2): 142–6. [DOI] [PubMed] [Google Scholar]
- 5.Then EO, Lopez M, Saleem S, et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020;11(2):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63(4):232–48. [DOI] [PubMed] [Google Scholar]
- 7.Quint LE, Bogot NR. Staging esophageal cancer. Cancer Imaging. 2008;8 Spec No A:S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulligan CR Jr. Multidisciplinary management of esophageal cancer. Surg Oncol Clin N Am. 2013;22(2):217–46. [DOI] [PubMed] [Google Scholar]
- 9.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11): 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. [DOI] [PubMed] [Google Scholar]
- 11.Decatris M, Santhanam S, O’Byrne K. Potential of interferon-alpha in solid tumours: part 1. BioDrugs. 2002;16(4):261–81. [DOI] [PubMed] [Google Scholar]
- 12.Holsti LR Mattson K, Niiranen A, et al. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1987;13(8):1161–6. [DOI] [PubMed] [Google Scholar]
- 13.Wadler S, Wersto R, Weinberg V, Thompson D, Schwartz EL. Interaction of fluorouracil and interferon in human colon cancer cell lines: cytotoxic and cytokinetic effects. Cancer Res. 1990;50(18):5735–9. [PubMed] [Google Scholar]
- 14.Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89(6–7):884–93. [DOI] [PubMed] [Google Scholar]
- 15.Kelsen D, Lovett D, Wong J, et al. Interferon alfa-2a and fluorouracil in the treatment of patients with advanced esophageal cancer. J Clin Oncol. 1992;10(2):269–74. [DOI] [PubMed] [Google Scholar]
- 16.Ilson DH, Sirott M, Saltz L, et al. A phase II trial of interferon alpha-2A, 5-fluorouracil, and cisplatin in patients with advanced esophageal carcinoma. Cancer. 1995;75(9):2197–202. [DOI] [PubMed] [Google Scholar]
- 17.Koeller JM. Biologic response modifiers: the interferon alfa experience. Am J Hosp Pharm. 1989;46(11 Suppl 2):S11–15. [PubMed] [Google Scholar]
- 18.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong L, Arrington A, Han J, et al. Generation of a novel, cyclooxygenase-2-targeted, interferon-expressing, conditionally replicative adenovirus for pancreatic cancer therapy. Am J Surg. 2012;204(5):741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong L, Davydova J, Brown E, Han J, Yamamoto M, Vickers SM. Delivery of interferon alpha using a novel Cox2-controlled adenovirus for pancreatic cancer therapy. Surgery. 2012;152(1): 114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salzwedel AO, Han J, LaRocca CJ, Shanley R, Yamamoto M, Davydova J. Combination of interferon-expressing oncolytic adenovirus with chemotherapy and radiation is highly synergistic in hamster model of pancreatic cancer. Oncotarget. 2018;9(26): 18041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davydova J, Yamamoto M. Oncolytic adenoviruses: design, generation, and experimental procedures. Curr Protoc Hum Genet. July 2013;Chapter 12:Unit 12 14. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59(1):198–204. [PubMed] [Google Scholar]
- 24.Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V, Yamamoto M. Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004;64(12):4319–27. [DOI] [PubMed] [Google Scholar]
- 25.Davydova J, Gavrikova T, Brown EJ, et al. In vivo bioimaging tracks conditionally replicative adenoviral replication and provides an early indication of viral antitumor efficacy. Cancer Sci. 2010;101(2):474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3–11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305(2):378–87. [DOI] [PubMed] [Google Scholar]
- 27.Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74(13):6147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70(10):6839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara H, Kobayashi A, Yoshida K, et al. Local interferon-alpha gene therapy elicits systemic immunity in a syngeneic pancreatic cancer model in hamster. Cancer Sci. 2007;98(3):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto M, Davydova J, Wang M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125(4): 1203–18. [DOI] [PubMed] [Google Scholar]
- 31.LaRocca CJ, Han J, Gavrikova T, et al. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157(5):888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaRocca CJ, Han J, Salzwedel AO, et al. Oncolytic adenoviruses targeted to Human Papilloma Virus-positive head and neck squamous cell carcinomas. Oral Oncol. 2016;56:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato-Dahlman M, Miura Y, Huang JL, et al. CD133-targeted oncolytic adenovirus demonstrates anti-tumor effect in colorectal cancer. Oncotarget. 2017;8(44):76044–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura Y, Yamasaki S, Davydova J, et al. Infectivity-selective oncolytic adenovirus developed by high-throughput screening of adenovirus-formatted library. Mol Ther. 2013;21(1):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby TO, Rivera A, Rein D, et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10(24):8697–703. [DOI] [PubMed] [Google Scholar]
- 36.LaRocca CJ, Warner SG. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin Transl Med. 2018;7(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders M, Rosch T, Kuster K, et al. Expression and function of the coxsackie and adenovirus receptor in Barrett’s esophagus and associated neoplasia. Cancer Gene Therapy. 2009;16(6):508–15. [DOI] [PubMed] [Google Scholar]
- 38.Heideman DA, Snijders PJ, Craanen ME, et al. Selective gene delivery toward gastric and esophageal adenocarcinoma cells via EpCAM-targeted adenoviral vectors. Cancer Gene Therapy. 2001;8(5):342–51. [DOI] [PubMed] [Google Scholar]
- 39.Ginsberg HS, Moldawer LL, Sehgal PB, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88(5):1651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan SJ, Gordon FC, Gregory DW, et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40(1):45–61. [DOI] [PubMed] [Google Scholar]
- 41.Wold WS, Toth K. Chapter three-Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res. 2012;115:69–92. [DOI] [PubMed] [Google Scholar]
- 42.Lee NP, Chan CM, Tung LN, Wang HK, Law S. Tumor xenograft animal models for esophageal squamous cell carcinoma. J Biomed Sci. 2018;25(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]


