Abstract
Background
Access to forms of dialysis, kidney transplantation (Tx) and comprehensive conservative management (CCM) for patients with end-stage kidney disease (ESKD) varies across European countries. Attitudes of nephrologists, information provision and decision-making may influence this access and nephrologists may experience several barriers when providing treatments for ESKD.
Methods
We surveyed European nephrologists and kidney transplant surgeons treating adults with ESKD about factors influencing modality choice. Descriptive statistics were used to compare the opinions of professionals from European countries with low–, middle– and high–gross domestic product purchasing power parity (GDP PPP).
Results
In total, 681 professionals from 33 European countries participated. Respondents from all GDP categories indicated that ∼10% of patients received no information before the start of renal replacement therapy (RRT) (P = 0.106). Early information provision and more involvement of patients in decision-making were more frequently reported in middle- and high-GDP countries (P < 0.05). Professionals’ attitudes towards several treatments became more positive with increasing GDP (P < 0.05). Uptake of in-centre haemodialysis was sufficient to 73% of respondents, but many wanted increased uptake of home dialysis, Tx and CCM. Respondents experienced different barriers according to availability of specific treatments in their centre. The occurrence of barriers (financial, staff shortage, lack of space/supplies and patient related) decreased with increasing GDP (P < 0.05).
Conclusions
Differences in factors influencing modality choice when providing RRT or CCM to adults with ESKD were found among low-, middle- and high-GDP countries in Europe. Therefore a unique pan-European policy to improve access to treatments may be inefficient. Different policies for clusters of countries could be more useful.
Keywords: chronic haemodialysis, chronic renal insufficiency, dialysis, kidney transplantation, peritoneal dialysis
Key Learning Points
What is already known about this subject?
Access to renal replacement therapy (RRT) modalities and comprehensive conservative management (CCM) for patients with end-stage kidney disease (ESKD) varies across European countries.
Limited access to the preferable treatment for ESKD may influence patient survival and quality of life and may lead to increased healthcare expenditures.
Most studies about factors influencing modality choice, such as those on nephrologists’ attitudes towards modalities, barriers experienced, information provision to patients and decision-making, have been conducted in high-income countries.
What this study adds?
We surveyed European nephrologists and kidney transplant surgeons and found differences in factors influencing modality choice among professionals from low–, middle– and high–gross domestic product countries.
Most but not all nephrologists had a positive attitude towards all modalities and wanted greater uptake of home dialysis, kidney transplantation and CCM.
European nephrologists experienced different barriers (patient related, lack of staff or supplies, financial) according to the availability of specific treatments in their centre.
What impact this may have on practice or policy?
A pan-European policy to improve access to treatments for patients with ESKD may be inefficient and different policies for clusters of countries could be more effective.
Support of all nephrologists to increase uptake of home dialysis, kidney transplantation or CCM cannot be assumed.
Education of both patients and healthcare professionals and policy measures may help to overcome several barriers for the provision of RRT and CCM.
INTRODUCTION
Kidney transplantation (Tx) offers superior quality of life and survival compared with dialysis for patients with ESKD [1, 2]. Unfortunately not all patients with ESKD who are suitable for Tx will receive a donor kidney. The large differences in Tx rates in Europe, varying from 2.6 per million population (pmp) in Ukraine to 103.2 pmp in Catalonia (Spain) [3], as well as data from the recently published Global Kidney Health Atlas (GKHA), showing limited access to Tx for patients in several European countries [4], suggest substantial room for improvement.
Several patients with ESKD are medically unsuitable to receive a kidney transplant and depend on a form of dialysis. Out-centre haemodialysis (OCHD), home haemodialysis (HHD) or peritoneal dialysis (PD) may be the preferred treatments over in-centre haemodialysis (ICHD) for these patients. Additionally, CCM may be an appropriate alternative, especially for elderly patients and those with severe comorbidities [5]. However, besides reduced access to Tx, patients in several European countries also have limited access to some forms of dialysis and CCM [3, 4].
The preferable treatment for patients with ESKD differs from individual to individual, but limited access to this preferable treatment may influence patient survival and quality of life. Moreover, it may lead to increased healthcare expenditures, as ICHD is usually more expensive than home dialysis or Tx [6–9].
To improve access to treatment modalities for patients with ESKD in Europe, information on factors influencing modality choice by nephrologists is needed. So far, most studies about these factors, such as those on barriers [10–13], nephrologists’ attitudes towards modalities [14], information provision [15] and decision-making have been conducted in high-income countries. European countries, however, show major economic differences and have different healthcare systems [4, 16]. This can affect the availability of treatments, which in turn may influence nephrologists’ views and experiences with barriers [17, 18].
The aim of this study was therefore to investigate information provision to patients, decision-making style and external pressure experienced, attitudes towards and satisfaction with uptake of different treatments and barriers as experienced by nephrologists and kidney transplant surgeons treating adult patients with ESKD in Europe. We compared the responses from professionals from countries with low–, middle– and high–gross domestic product purchasing power parity (GDP PPP).
MATERIALS AND METHODS
Development of the survey
We designed a survey in English using Limesurvey [19], based on results from a previously performed systematic review on barriers for nephrologists to provide RRT or CCM [20] and on input from nephrologists and a kidney patients’ advocate. Respondents received questions based on answers they provided to earlier questions in the survey. They were able to review and change their answers until submission of the survey. Six nephrologists from different countries tested the survey and we modified the survey based on their feedback. The nephrologist survey was conducted within the Effect of Differing Kidney Disease Treatment Modalities and Organ Donation and Transplantation Practices on Health Expenditure and Patient Outcomes (EDITH) project [21]. The complete survey and detailed methods using the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist [22] can be found in the Supplementary Methods.
Participants and data collection
The survey was promoted and distributed by national societies of nephrology, the European Renal Association—European Dialysis and Transplantation Association (ERA-EDTA) and the European Society of Organ Transplantation (ESOT). All European nephrologists and kidney transplant surgeons (including those in training) who treated adult patients with ESKD were eligible to participate. The EDITH nephrologist survey was publicly accessible from 14 March 2019 until 19 May 2019.
Ethical aspects
The Medical Ethics Review Committee of the Amsterdam University Medical Center, location Academic Medical Center in Amsterdam, The Netherlands, waived the need for ethical approval (W18_279#18.323). In addition, representatives or national societies were consulted about the need for additional ethical approval in their country. All individual respondents provided online written informed consent (see the Introduction section of the EDITH nephrologist survey, provided as Supplementary data, Item S1).
Data analysis
Results from participants from countries for which additional ethical approval was not needed or from countries where additional approval was received before the start of the survey were included in the final analysis. In addition, respondents needed to have completely answered all mandatory questions. Analyses were performed for all respondents together and according to country income. We categorized the European countries into three income groups by using tertiles based on GDP PPP (further indicated as GDP, Supplementary data, Table S1) 2016 data from the World Bank [23]. Note that we did not use the World Bank income classification itself [24] as this would result in three groups of unequal size (i.e. 2 countries with lower-middle income, 10 countries with upper-middle income and 28 countries with high income). Data of individual countries cannot be shared publicly due to the privacy of individuals that participated in the study. We used Fisher’s exact tests (with Monte Carlo simulation because of the size of the dataset) and Kruskal–Wallis tests to compare categorical and continuous outcomes between the GDP tertiles. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 26 (IBM, Armonk, NY, USA) [25].
RESULTS
General and professional characteristics
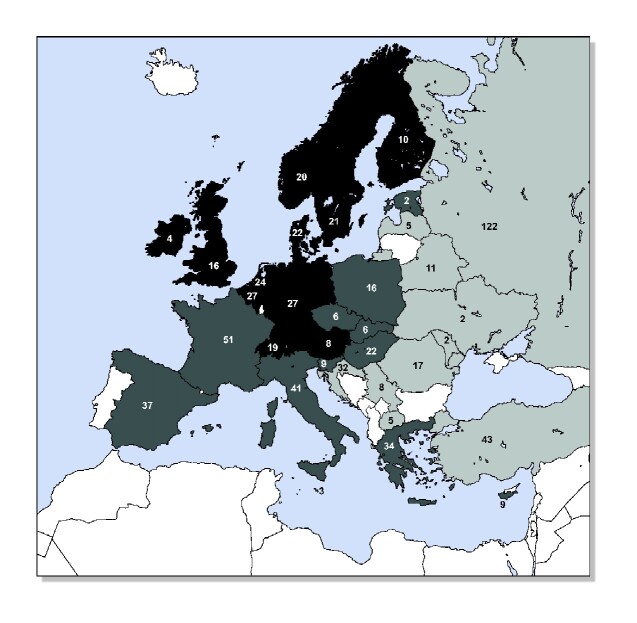
We included 681 respondents (54.9% male) from 33 countries in Europe (Figure 1 and Supplementary data, Figure S1). Of these, 31.0% were <40 years of age, 52.6% were between 41 and 60 years and 16.4% were ≥61 years. Most respondents were practicing nephrologists (86.5%). Of all respondents, 56.8% practiced in an academic centre, 91.9% practiced in an urban area and 78.2% practiced in a public centre (Table 1). ICHD was available in 95.4% of the centres while HHD was the least-available treatment (36.6%).
FIGURE 1.
Country of origin of respondents. Light grey countries (GDP <US$26 618): Belarus, Croatia, Latvia, Moldova, North Macedonia, Romania, Russia, Serbia, Turkey and Ukraine. Middle grey countries (GDP US$26 618–42 357): Cyprus, Czech Republic, Estonia, France, Greece, Hungary, Italy, Malta, Poland, Slovak Republic, Slovenia and Spain. Dark grey countries (GDP >US$42 357): Austria, Belgium, Denmark, Finland, Germany, Ireland, The Netherlands, Norway, Sweden, Switzerland and the UK. Blank countries: no ethical approval obtained or no answer; Albania, Bulgaria, Iceland, Kosovo*, Lithuania, Luxembourg, Montenegro, Portugal. No respondents: Bosnia and Herzegovina. N is the number of respondents per country. *This designation is without prejudice to positions on status and is in line with United Nations Security Council Resolution 1244/99 and the International Court of Justice opinion on the Kosovo declaration of independence.
Table 1.
General and professional characteristics of respondents
| Characteristics | All respondents (N = 681) | GDP lowest tertile * (n = 247) | GDP middle tertile * (n = 236) | GDP highest tertile * (n = 198) | P-value** |
|---|---|---|---|---|---|
| Sex (male), % | 54.9 | 48.2 | 57.6 | 60.1 | 0.024 |
| Age categories (years), % | <0.001 | ||||
| <40 | 31.0 | 41.3 | 29.2 | 20.2 | |
| 41–60 | 52.6 | 48.2 | 52.5 | 58.1 | |
| ≥61 | 16.4 | 10.5 | 18.2 | 21.7 | |
| Professional background, % | 0.078 | ||||
| Nephrologist | 86.5 | 86.6 | 89.4 | 82.8 | |
| Nephrologist in training | 4.4 | 2.4 | 3.4 | 8.1 | |
| Internal medicine specialist | 5.4 | 7.3 | 3.8 | 5.1 | |
| Kidney transplant surgeon | 3.7 | 3.6 | 3.4 | 4.0 | |
| Working in academic centre, % | 56.8 | 53.3 | 53.0 | 65.7 | 0.010 |
| Working in urban centre, % | 91.9 | 92.7 | 95.3 | 86.9 | 0.006 |
| Working in public centre, % | 78.2 | 71.3 | 76.5 | 88.8 | <0.001 |
| Centre size (number of patients on dialysis, with a functioning kidney transplant or on CCM), % | <0.001 | ||||
| <50 | 6.3 | 10.0 | 5.5 | 2.8 | |
| 50–100 | 18.3 | 26.5 | 17.4 | 9.4 | |
| 101–200 | 22.9 | 24.7 | 24.2 | 19.3 | |
| >200 | 52.5 | 38.8 | 53.0 | 68.5 | |
| Treatment available in centre, % | |||||
| ICHD | 95.4 | 92.3 | 95.3 | 99.5 | <0.001 |
| OCHD | 45.5 | 23.6 | 43.4 | 74.7 | <0.001 |
| HHD | 36.6 | 10.4 | 31.5 | 74.7 | <0.001 |
| PD | 79.6 | 62.8 | 83.1 | 96.0 | <0.001 |
| LTX | 53.8 | 32.0 | 57.6 | 75.8 | <0.001 |
| DTX | 56.7 | 36.5 | 61.4 | 75.8 | <0.001 |
| CCM | 75.6 | 61.3 | 77.1 | 91.4 | <0.001 |
For the GDP classification of individual countries, see Figure 1.
P-value calculated with Fisher’s exact test to compare GDP tertiles.
Respondents from low-GDP countries were more often women, were younger and were more often working in non-public centres (Table 1; P < 0.05). In centres in low-GDP countries, treatments other than ICHD were less often available compared with centres in middle- or high-GDP countries (P < 0.001).
Information provision to patients
In total, 72.1% of the respondents reported providing information on all available modalities, not only on those suitable for that specific patient (Table 2). Most respondents provided information about ICHD (97.4%), PD (86.9%), living kidney donor transplantation (LTx) and deceased kidney donor transplantation (DTx) (82.8% and 85.7%, respectively) but less often about CCM (65.5%), OCHD (47.3%) or HHD (40.4%). According to respondents, patients commonly received information from the nephrologist (98.3%), nurse (72.1%) and brochures or booklets (63.6%). Respondents estimated that 31.2% of the patients received information >1 year before the start of RRT and 10.3% of the patients received no information before the start of RRT.
Table 2.
Information provision, decision-making and external pressure
| All respondents (N = 681) | GDP lowest tertile * (n = 247) | GDP middle tertile * (n = 236) | GDP highest tertile * (n = 198) | P-value ** | |
|---|---|---|---|---|---|
| Information provision about all treatments available in the centre, % | 72.1 | 81.7 | 71.2 | 62.3 | <0.001 |
| Patients receive information about, % | |||||
| ICHD | 97.4 | 96.5 | 97.6 | 98.3 | 0.544 |
| OCHD | 47.3 | 23.3 | 48.6 | 73.3 | <0.001 |
| HHD | 40.4 | 14.4 | 37.5 | 73.9 | <0.001 |
| PD | 86.9 | 73.8 | 89.9 | 98.3 | <0.001 |
| LTX | 82.8 | 69.3 | 83.2 | 97.7 | <0.001 |
| DTX | 85.7 | 72.8 | 88.0 | 97.7 | <0.001 |
| CCM | 65.5 | 51.0 | 67.8 | 79.5 | <0.001 |
| Source of information, % | |||||
| Nephrologist | 98.3 | 99.5 | 97.6 | 97.7 | 0.249 |
| Kidney transplant surgeon | 19.0 | 19.3 | 16.4 | 21.6 | 0.422 |
| Other doctor (e.g. general practitioner, other medical specialist) | 19.7 | 29.7 | 17.4 | 10.8 | <0.001 |
| Nurse | 72.1 | 46.0 | 79.2 | 93.8 | <0.001 |
| Other kidney patients | 48.5 | 52.0 | 44.9 | 48.9 | 0.363 |
| Brochure/booklet | 63.6 | 56.4 | 53.1 | 84.1 | <0.001 |
| Website/internet | 45.1 | 49.0 | 36.7 | 50.6 | 0.009 |
| Timing of information provision, mean % per category | |||||
| More than 12 months before start of RRT | 31.2 | 23.1 | 34.8 | 37.0 | <0.001 |
| 4–12 months before start of RRT | 28.4 | 23.5 | 30.7 | 31.7 | <0.001 |
| 1–3 months before start of RRT | 17.7 | 21.2 | 15.9 | 15.3 | <0.001 |
| <1 month before start of RRT | 12.5 | 18.0 | 10.0 | 8.4 | <0.001 |
| No information before start of RRT | 10.3 | 14.2 | 8.6 | 7.6 | 0.106 |
| Style of modality decision-making, % | <0.001 | ||||
| Patient alone | 7.8 | 8.1 | 9.4 | 5.7 | |
| Patient with input from doctor | 32.5 | 25.8 | 40.9 | 30.5 | |
| Together | 48.5 | 47.0 | 42.4 | 57.5 | |
| Doctor with input from patient | 9.4 | 14.6 | 6.9 | 6.3 | |
| Doctor alone | 0.0 | 0.0 | 0.0 | 0.0 | |
| Decision left to doctor | 1.7 | 4.5 | 0.5 | 0.0 | |
| Experiencing external pressure, % | 36.9 | 40.7 | 34.5 | 35.5 | 0.435 |
| Source of pressure, % | |||||
| Family of the patient | 88.1 | 85.1 | 91.3 | 88.1 | 0.524 |
| Opinion of colleagues | 45.0 | 55.4 | 42.0 | 35.6 | 0.060 |
| Opinion of supervisor | 19.3 | 29.7 | 18.8 | 6.8 | 0.003 |
| Opinion of other medical specialists | 42.6 | 47.3 | 47.8 | 30.5 | 0.084 |
| Hospital management | 21.8 | 32.4 | 15.9 | 15.3 | 0.024 |
| Insurers | 10.9 | 16.2 | 8.7 | 6.8 | 0.178 |
For the GDP classification of individual countries, see Figure 1.
P-values calculated with Fisher’s exact test and Kruskal–Wallis test to compare GDP tertiles on categorical and continuous outcomes.
Respondents from low- and middle-GDP countries more often provided information only about modalities available in their centre than respondents from high-GDP countries (81.7%, 71.2% and 62.3%, respectively; P < 0.001; Table 2). In general, respondents from low-GDP countries less often provided information about OCHD, HHD, PD, LTx, DTx and CCM than respondents from middle- or high-GDP countries (P < 0.001). Next to nephrologists, nurses more often gave information in middle- and high-GDP countries, while doctors other than nephrologists more frequently gave information in low-GDP countries (P < 0.001). In low-GDP countries, patients tended to receive information closer to the start of RRT (P < 0.001), but the percentage of patients starting RRT without having received any information did not differ significantly between the GDP categories (low 14.2%, middle 8.6% and high 7.6%; P = 0.106).
Decision-making
In total, 48.5% of the respondents reported that the decision on modality choice was shared between doctor and patient, whereas 32.5% reported that patients decided with input from the doctor and 9.4% reported that the doctor decided with input from the patient. In addition, according to the respondents, 7.8% of patients made the decision alone and 1.7% left the decision to their doctor (Table 2). None of the respondents reported making decisions without influence from the patient. Respondents from low-GDP countries tended to report more key involvement of the doctor in decision-making (P < 0.001).
External pressure
In total, 36.9% of all respondents experienced external pressure when providing RRT or CCM (Table 2). The sources of that external pressure included the family of the patient (88.1%), colleagues (45.0%), other medical specialists (42.6%), hospital management (21.8%), supervisors (19.3%) and insurers (10.9%). The prevalence of external pressure was similar across the GDP categories (P = 0.435), but respondents from low-GDP countries experienced more pressure from supervisors and hospital management (P < 0.05).
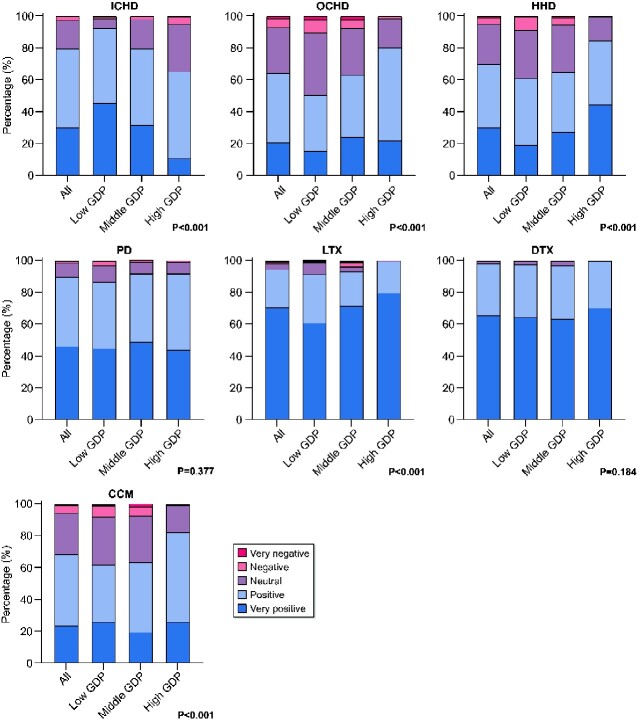
Attitudes towards different RRT modalities and CCM
On a 5-point scale, 97.8% of the respondents were positive or very positive about DTx and 94.3% about LTx (Figure 2). With respect to dialysis modalities, respondents were positive or very positive about PD (89.8%) followed by ICHD (79.6%), HHD (69.6%) and OCHD (64.1%). In total, 68.3% of the respondents were positive or very positive about CCM. Few respondents reported a negative or very negative attitude towards treatment modalities. Respondents from low-GDP countries tended to be more positive about ICHD and less positive about OCHD, HHD, LTx and CCM than those from middle- and high-GDP countries (P < 0.001). Attitudes towards PD and DTx did not differ between the GDP categories (P > 0.05) (Supplementary data, Table S2).
FIGURE 2.
Attitude towards different RRT modalities and CCM. For the GDP classification of individual countries, see Figure 1. P-values calculated with Fisher’s exact test to compare GDP tertiles.
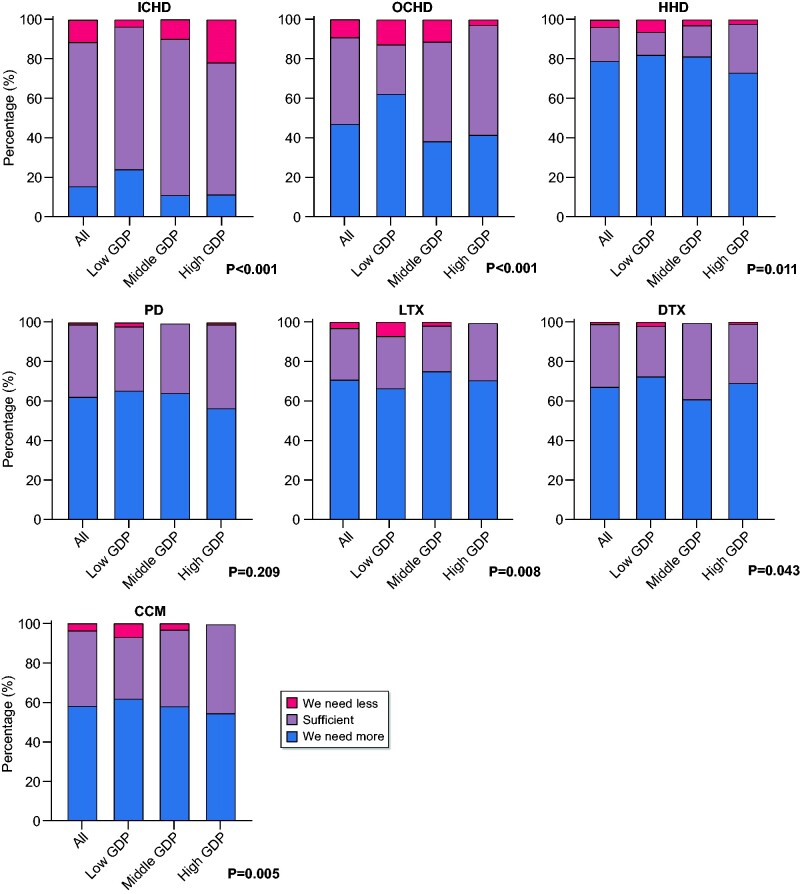
Uptake of different RRT modalities and CCM
A majority of respondents thought that the uptake of HHD (78.8%), LTx (70.7%), DTx (67.0%), PD (62.2%) and CCM (58.0%) should be increased. In total, 47.1% of the respondents wanted to increase the uptake of OCHD. Most respondents (72.9%) thought that the current uptake of ICHD was sufficient and >25% were satisfied with the current uptake of LTx and DTx (Figure 3 and Supplementary data, Table S3). Respondents from middle- and high-GDP countries were more likely to report sufficient uptake of OCHD, HHD and CCM. The proportion of respondents eager to increase the uptake of ICHD, OCHD, HHD and CCM decreased somewhat with increasing GDP.
FIGURE 3.
Uptake of different RRT modalities and CCM. For the GDP classification of individual countries, see Figure 1. P-values calculated with Fisher’s exact test to compare GDP tertiles.
Barriers if treatments are unavailable
Barriers for ICHD are not reported because only 22 respondents reported no ICHD in their centre. If HHD or PD were unavailable, the most reported barriers were lack of supportive staff (71.9% and 57.9%, respectively) and practical aspects (79.9% and 67.0%, respectively). If kidney transplantation was unavailable, lack of donors was the most reported barrier for both LTx (64.1%) and DTx (56.4%), whereas lack of supportive staff was the most reported barrier for CCM (59.2%). Knowledge or attitude of the nephrologist was the least reported barrier for HHD (27.7%), PD (21.3%), LTx (12.4%) and DTx (10.7%) (Figure 4 and Supplementary data, Table S4).
FIGURE 4.
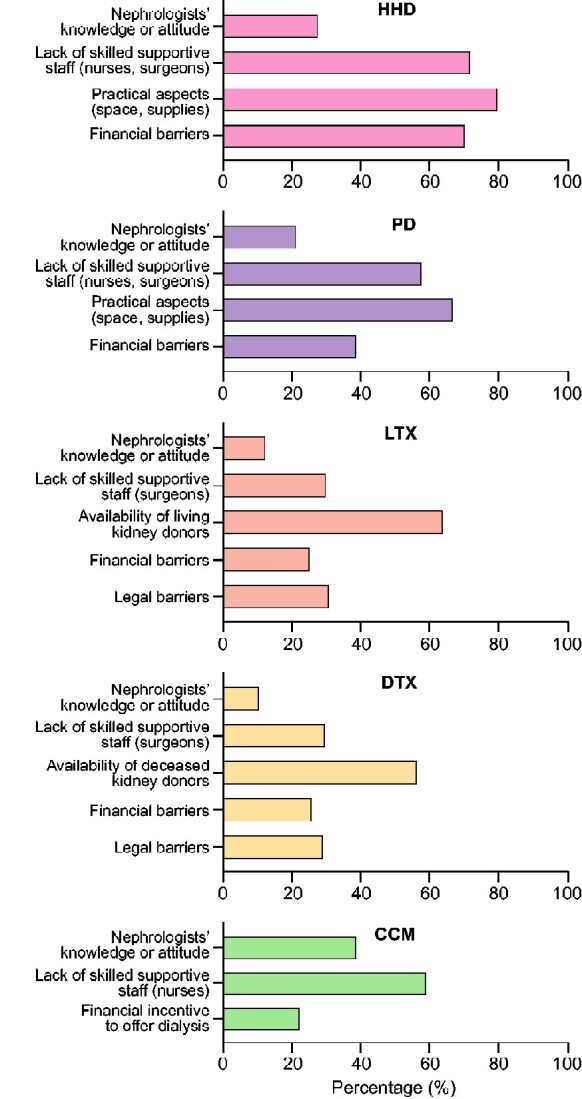
Barriers if treatments are unavailable—all respondents.
Respondents from low-GDP countries reported more financial barriers for HHD and both forms of Tx (P < 0.001). They also reported being more limited by the nephrologists’ knowledge or attitude about LTx (P < 0.05) and by legal barriers and a lack of donors for Tx (P < 0.01). In addition, they more often reported a financial incentive to offer dialysis instead of CCM (P < 0.01) (Supplementary data, Table S4).
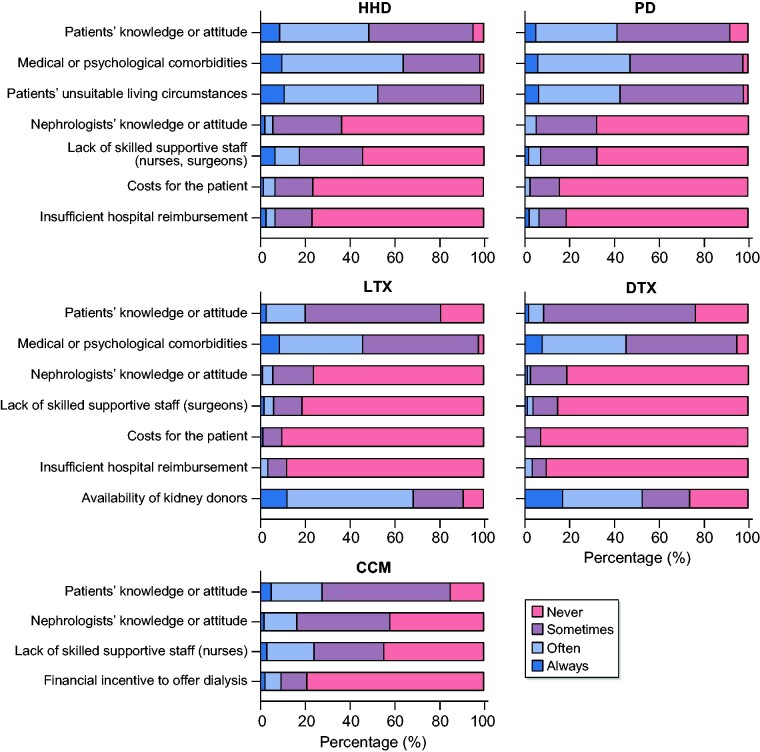
Barriers if treatments are available
When HHD or PD were available, the most frequently experienced barriers for our respondents were on the patient’s level (knowledge or attitude, medical or psychological comorbidities and unsuitable living circumstances) (Figure 5 and Supplementary data, Table S5). The most frequently experienced barrier to offering Tx was a lack of donors; also, the patients’ medical or psychological comorbidities and patients’ knowledge or attitude made it more difficult to offer Tx. Costs for patients or insufficient hospital reimbursement were the least frequently experienced barriers when HHD, PD or Tx were available. When offering CCM, the patients’ knowledge or attitude was the most frequently experienced barrier, followed by the nephrologists’ knowledge or attitude and lack of skilled staff, whereas a financial incentive to offer dialysis was least frequently reported.
FIGURE 5.
Barriers if treatments are available—all respondents.
Generally, barriers were more frequently experienced in low-GDP countries. However, medical or psychological comorbidities for HHD and PD were experienced with similar frequency in low-, middle- and high-GDP countries (P > 0.05).
DISCUSSION
To study factors influencing treatment modality choice for ESKD, we conducted a large survey among 681 nephrologists and kidney transplant surgeons from 33 European countries. We found significant differences in information provision to patients, style of decision-making, external pressure experienced, attitudes towards and satisfaction with uptake of treatments between European countries with low, middle and high GDP. In addition, we identified which barriers most frequently impede nephrologists when offering HHD, PD, LTx, DTx or CCM.
Information provision
Nephrologists from low-GDP countries less often provided information about treatments other than ICHD, which is in line with the limited availability of home dialysis and Tx in their countries [3]. Furthermore, in low-GDP countries, doctors other than nephrologists were more often involved in information provision, which may be due to a smaller workforce of nephrologists in low-GDP countries [26]. Consistent with previous studies, we found that in middle- and high-GDP countries, nurses were more frequently involved in information provision [27, 28]. According to experts [29, 30], information provision about RRT and CCM preferably starts 12 months before the estimated start of dialysis, but in our study this was reported by nephrologists for only 31% of their patients. Timely information provision, discussing various treatments and using various information sources increases patients’ satisfaction with information and may lead to a better-informed choice [31, 32]. This may also improve the uptake of OCHD, HHD, PD and pre-emptive Tx [28, 32, 33].
Decision-making
Usually our respondents made decisions about RRT or CCM together with their patients as suggested by expert groups and in guidelines [34, 35]. However, some experts suggest that nephrologists should encourage patients to choose OCHD, HHD or PD because the group of patients suitable for these treatments is larger than the current group receiving it [34, 35]. The style of decision-making should also be adjusted to patients’ preferences, as they may prefer their nephrologist to be informative, interpretative or rather paternalistic [36–38].
External pressure
When choosing a modality, nephrologists not only take into account the patient’s opinion, but often face the opinions of the patient’s family, other medical specialists, hospital management and insurers. The main sources of external pressure for our respondents were the family of the patient, colleagues and other medical specialists, but we did not investigate their influence on the choice of specific modalities. Other studies show that offering CCM is particularly associated with external pressure, caused by expectations of dialysis from other medical specialists and family [39–41]. Only a few studies have reported limited to moderate influence of other medical specialists on the choice of dialysis modality [13, 42]. Only 4% of our respondents in Europe experienced external pressure from insurers regarding modality choice. This seems a more frequent problem in the USA, as several studies from that country have reported insurance-related disparities in vascular access type and patients’ access to PD and pre-emptive Tx [43–45].
Attitudes towards and uptake of different RRT modalities and CCM
Many surveys have investigated the attitudes towards dialysis modalities, but we also studied the nephrologists’ attitudes towards Tx and CCM, which have rarely been studied in a quantitative manner [14, 40, 46]. Our findings provide an interesting perspective on the current uptake of these treatments. Despite nephrologists’ positive attitude and wish for a greater uptake of home dialysis in our study, only 13% of incident RRT patients and 5% of prevalent RRT patients in Europe are treated with PD, and the incidence and prevalence of HHD are even lower [3].
Moreover, in 2017, only 37% of European patients with ESKD were living with a functioning kidney transplant [3] and the availability of kidney Tx should be increased according to experts’ opinion and the European Commission [47–49]. Although the vast majority of our respondents wanted increased transplant activities, surprisingly >25% were satisfied with the current rate of kidney transplants, regardless of GDP category. Therefore one cannot assume that all nephrologists would encourage increased uptake of Tx and we suggest considering nephrologists’ opinions when creating a supportive culture for Tx.
Barriers
Respondents experienced different barriers to introduce or use certain treatment options in their centre. Consistent with other studies, financial barriers often limited the deployment of HHD and PD and also of LTx and DTx [50–52], in particular if the treatment was unavailable. Set-up costs for HHD and PD programmes (e.g. for training staff and purchasing supplies) can have a greater impact in countries where ICHD costs are low due to low staffing costs. Costs for patients and hospital reimbursement were seldom limiting the provision of available treatments, as insurance coverage and adequate reimbursement were usually present. However, reimbursement for ICHD may be so high that it serves as a financial disincentive for other treatments [39].
For available treatments, respondents were mostly limited by patient-related barriers. Patients’ lack of knowledge or negative attitudes was frequently experienced, in line with barriers found in high-income countries by the GKHA [17]. Patients’ comorbidity was also a major barrier, but apparently nephrologists do not always agree on absolute and relative contraindications [10, 11, 53]. Remarkably, in both lower- and higher-income countries, patients’ living circumstances were often considered as a barrier for home dialysis, in Europe as well as worldwide [54–56].
Interestingly, previously both nephrologists and nurses have reported a lack of knowledge about home dialysis, which in turn may influence the education and training of patients [57–59]. Therefore it is suggested to provide education about home dialysis to all nephrology professionals, including those mainly working with ICHD patients, as they may have many opportunities to discuss home dialysis with their patients [57, 60]. More education could also help to dispel misperceptions and create a supportive culture for home dialysis among all staff [61, 62].
Although the actual size of the donor shortage has rarely been studied, a lack of donors was the most frequently experienced barrier in our survey to offering kidney Tx. Barriers on various levels cause a lack of donors. Patients with ESKD are reluctant to ask potential living donors due to multiple influencing factors such as fear for the donor’s health [47, 63]. Healthcare professionals, among others, may have difficulties discussing organ donation with families or may miss potential donors [64, 65]. Furthermore, legislation (e.g. with respect to the consent system, donation after circulatory death or unrelated living donors), lack of coordination, poor infrastructure and limited awareness of organ donation and transplantation among the general public are associated with a shortage of both living and deceased kidney donors [47, 50, 66].
Strengths and limitations
We conducted a large European survey among 681 nephrologists and kidney transplant surgeons from both Western and Eastern European countries. We studied a range of factors influencing modality choice and are among the first to study differences in these factors between European countries with low, middle and high GDP.
Our results should be interpreted with some caution. First, our results may suffer from selection bias, as nephrologists who were not members of a society of nephrology or who had limited access to the internet may have been less likely to participate. To maximize the group of potential respondents and respect the anonymity of respondents in the context of the General Data Protection Regulation, potential respondents were not directly contacted by the research team. Therefore we do not know who our respondents are. Moreover, the characteristics of the non-respondents are unknown. As a result, we were unable to determine whether the sample is representative. In addition, nephrologists interested in the topic may have been more likely to respond to our survey. Comparison of our sample with the Kidney Health for Life survey [67] suggests underrepresentation of nephrologists working in the private sector. Although several nephrologists assessed and improved the comprehensibility of our survey prior to sending it out, language or cultural barriers may have caused a different understanding of questions and responses. Previous studies suggest that perceptions of professionals may be more positive than patient experiences [personal communication, R. Vanholder, 2017 European Kidney Health Alliance (EKHA) Questionnaire on patient choice]. Due to our relatively limited number of responses per country, we were unable to compare individual countries. Furthermore, the availability of treatments on the centre level may not reflect the availability in a country and good cooperation between centres with different expertise may reduce experienced barriers. Finally, clustering of countries using GDP PPP is a commonly used method to compare countries, but within these clusters there may exist important variations. For example, our low-GDP tertile includes both Ukraine, having the lowest European kidney transplant rate (3 pmp), and Croatia, having one of the highest kidney transplant rates (51 pmp). However, this variation also provides opportunities, as countries with comparable financial conditions but different uptake of treatments could learn from each other and exchange best practices.
CONCLUSION
Our survey among European nephrologists and kidney transplant surgeons showed that many factors influencing treatment modality choice for adults with ESKD differed among low-, middle- and high-GDP countries. Information provision and decision-making could be optimized in certain countries, bearing in mind that patients in different countries might have different needs and wishes. Limited availability of OCHD, HHD, PD and Tx may hamper optimal information provision and decision-making, creating a vicious circle: due to limited availability of treatment modalities, nephrologists may not discuss treatment options with patients, which may lead to the perception that patients are not interested, which in turn keeps availability low.
Nephrologists and kidney transplant surgeons usually have a positive attitude towards and want greater uptake of most treatments that are currently less accessible for patients. Nevertheless, we cannot presume that all nephrologists support this view, as for example >25% of our respondents were satisfied with the uptake of Tx, although there is room for improvement in almost all European countries. Our respondents were notably limited by healthcare system–related barriers (space and supplies, financial, legal), particularly if a treatment was unavailable in their centre or country. Patient-related barriers (knowledge, housing, comorbidity) were most frequently experienced when a treatment was already available. Healthcare system-related barriers and patient-related barriers as well as nephrologist-related barriers (knowledge, attitude) could be targeted by policy measures and proper education.
The results of this survey suggest that factors influencing modality choice, including barriers, for providing RRT and CCM to patients with ESKD, differ across GDP tertiles. Therefore a single European policy may not be effective. Besides variations in GDP, European countries show variations in other characteristics (e.g. healthcare organization and legislation) that may influence uptake of RRT and CCM as well. Therefore we suggest that measures to improve access to treatment modalities for patients with ESKD should be tailored to clusters of countries with similar characteristics so countries can learn from each other and exchange best practices.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all the nephrologists and kidney transplant surgeons who filled out the EDITH nephrologist Survey. In addition, they would like to thank all colleagues who pre-tested the survey, provided advice about the ethical approval in their country or helped to distribute the survey in their country or personal network. Among others, we are grateful for support from Austria (R. Kramar, R. Oberbauer), Belarus (K. Komissarov), Belgium (F. Collart, J. De Meester), Croatia (I. Bubic, M. Bušić, M. Dragović and S. Živčić Ćosić), Cyprus (K. Ioannou), Czech Republic (I. Rychlik and V. Tesař), Denmark (J. Heaf and S. Schwartz Sørensen), Estonia (M. Rosenberg-Ots), Finland (P. Finne and V. Rauta), Germany (M. Lingemann and C. Wanner), Greece (T. Apostolou, E. Dounousi and G. Moustakas), Hungary (O. Deme, S. Mihaly and G. Reúsz), Ireland (W. Plant), Italy (G. Brunori, C. Carella, P. di Ciaccio and M. Postorino), Latvia (H. Cernevskis and A. Petersons), Malta (J. Buttigieg), Moldova (A. Tanase), The Netherlands (F. van Ittersum and S. Logtenberg), North Macedonia (G. Spasovski and O. Stojceva-Taneva), Norway (A. Åsberg, M. Dahl Solbu and A. Varberg Reisæter), Poland (S. Dudzicz and M. Nowicki), Romania (L. Gârneaţă and L. Tuta), Russia (A. Andrusev and H. Zakharova), Serbia (R. Naumovic), Slovakia (V. Spustova), Slovenia (J. Buturovic Ponikvar and D. Kovac), Spain (C. Alberich, J. Comas, M. Ferrer Alamar, B. Mahillo and M. del Pino y Pino), Sweden (M. Evans), Switzerland (P. Ambühl and U. Huynh-Do), Turkey (M. Arici and N. Seyahi), Ukraine (M. Kolesnyk) and the UK (S. Fraser and G. Lipkin) and from the following organisations: EKITA (I. Bellini and R. Langer), ERA-EDTA (F. Trebelli), EuroPD (S. Davies) and Eurotransplant (P. Branger, M. van Meel and U. Samuel).
FUNDING
R.W.d.J., K.J.J. and V.S.S. report grants from the European Union (PP-01-2016) and from the ERA-EDTA during the conduct of the study. This work was presented as an oral presentation at the 56th ERA-EDTA Congress (Budapest, 13–16 June 2019). The content of this article represents the views of the authors only and is their sole responsibility; it cannot be considered to reflect the views of the European Commission or any other body of the European Union. The European Commission does not accept any responsibility for use that may be made of the information it contains.
AUTHORS’ CONTRIBUTIONS
R.W.d.J., K.J.J. and V.S.S. were responsible for the research idea, study design and statistical analysis. R.W.d.J., K.J.J., C.C., Z.A.M. and V.S.S. were responsible for the data acquisition. R.W.d.J., K.J.J., R.C.V., C.C., M.M., A.R., Z.A.M. and V.S.S. were responsible for the data analysis/interpretation. K.J.J., Z.A.M. and V.S.S. were responsible for the supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
R.W.d.J., K.J.J. and V.S.S. report grants from the European Union (PP-01-2016) and from the ERA-EDTA during the conduct of the study. A.R., C.C., M.M., R.C.V. and Z.A.M. have nothing to disclose.
Contributor Information
Rianne W de Jong, ERA-EDTA Registry, Department of Medical Informatics, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Kitty J Jager, ERA-EDTA Registry, Department of Medical Informatics, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Raymond C Vanholder, Nephrology Section, Department of Internal Medicine and Pediatrics, Ghent University Hospital, Ghent, Belgium; European Kidney Health Alliance (EKHA), Brussels, Belgium.
Cécile Couchoud, REIN Registry, Agence de la Biomédecine, Saint-Denis La Plaine, France.
Mark Murphy, The Irish Kidney Association CLG, Dublin, Ireland.
Axel Rahmel, Deutsche Stiftung Organtransplantation, Frankfurt am Main, Germany.
Ziad A Massy, INSERM U1018, Équipe 5, Centre de Recherche en Epidémiologie et Santé des Populations (CESP), Université Paris Saclay et Université Versailles Saint Quentin en Yvelines (UVSQ), Villejuif, France; Service de Néphrologie et Dialyse, Assistance Publique—Hopitaux de Paris (APHP), Hôpital Universitaire Ambroise Paré, Boulogne-Billancourt, France.
Vianda S Stel, ERA-EDTA Registry, Department of Medical Informatics, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
REFERENCES
- 1. Wolfe RA, Ashby VB, Milford EL et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 2. Cameron JI, Whiteside C, Katz J et al. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000; 35: 629–637 [DOI] [PubMed] [Google Scholar]
- 3. ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2017. Amsterdam, The Netherlands: Amsterdam UMC, location AMC, Department of Medical Informatics, 2019. [Google Scholar]
- 4. Bello AK, Levin A, Lunney M et al. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Global Burden of End-Stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care Across World Countries and Regions. Brussels: International Society of Nephrology, 2019 [Google Scholar]
- 5. Verberne WR, Geers AB, Jellema WT et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol 2016; 11: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarl J, Desatnik P, Peetz Hansson U et al. Do kidney transplantations save money? A study using a before–after design and multiple register-based data from Sweden. Clin Kidney J 2018; 11: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krahn MD, Bremner KE, de Oliveira C et al. Home dialysis is associated with lower costs and better survival than other modalities: a population-based study in Ontario. Perit Dial Int 2019; 39: 553–561 [DOI] [PubMed] [Google Scholar]
- 8. Mohnen SM, van Oosten MJM, Los J et al. Healthcare costs of patients on different renal replacement modalities—analysis of Dutch health insurance claims data. PLoS One 2019; 14: e0220800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Tol A, Lameire N, Morton RL et al. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol 2019; 14: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung B, Blake PG, Mehta RL et al. Attitudes of Canadian nephrologists toward dialysis modality selection. Perit Dial Int 1999; 19: 263–268 [PubMed] [Google Scholar]
- 11. Mendelssohn DC, Mullaney SR, Jung B et al. What do American nephrologists think about dialysis modality selection? Am J Kidney Dis 2001; 37: 22–29 [DOI] [PubMed] [Google Scholar]
- 12. Bouvier N, Durand PY, Testa A et al. Regional discrepancies in peritoneal dialysis utilization in France: the role of the nephrologist’s opinion about peritoneal dialysis. Nephrol Dial Transplant 2008; 24: 1293–1297 [DOI] [PubMed] [Google Scholar]
- 13. Desmet JM, Fernandes V, Des Grottes JM et al. Perceptive barriers to peritoneal dialysis implementation: an opinion poll among the French-speaking Belgian nephrologists. Clin Kidney J 2013; 6: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fluck RJ, Fouque D, Lockridge RS Jr.. Nephrologists’ perspectives on dialysis treatment: results of an international survey. BMC Nephrol 2014; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendelssohn DC, Toffelmire EB, Levin A. Attitudes of Canadian nephrologists toward multidisciplinary team-based CKD clinic care. Am J Kidney Dis 2006; 47: 277–284 [DOI] [PubMed] [Google Scholar]
- 16. Spasovski G, Rroji M, Vazelov E et al. Nephrology in the Eastern and Central European region: challenges and opportunities. Kidney Int 2019; 96: 287–290 [DOI] [PubMed] [Google Scholar]
- 17. van de Luijtgaarden MWM, Jager KJ, Stel VS et al. Global differences in dialysis modality mix: the role of patient characteristics, macroeconomics and renal service indicators. Nephrol Dial Transplant 2013; 28: 1264–1275 [DOI] [PubMed] [Google Scholar]
- 18. Lunney M, Alrukhaimi M, Ashuntantang GE et al. Guidelines, policies, and barriers to kidney care: findings from a global survey. Kidney Int Suppl 2018; 8: 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Limesurvey. LimeSurvey: An Open Source Survey Tool. Oxford University Press; http://www.limesurvey.org (20 August 2019, date last accessed) [Google Scholar]
- 20. de Jong RW, Stel VS, Heaf JG et al. Non-medical barriers reported by nephrologists when providing renal replacement therapy or comprehensive conservative management to end-stage kidney disease patients: a systematic review. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfz271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jager KJ, Stel VS, Branger P et al. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant 2018; 33: 560–562 [DOI] [PubMed] [Google Scholar]
- 22. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004; 6: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Bank. Indicators. https://data.worldbank.org/indicator (20 August 2019, date last accessed)
- 24.World Bank. World Bank Country and Lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (25 August 2020, date last accessed)
- 25. IBM. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM, ; 2017. [Google Scholar]
- 26. Osman MA, Alrukhaimi M, Ashuntantang GE et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl 2018; 8: 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prieto-Velasco M, Isnard Bagnis C, Dean J et al. Predialysis education in practice: a questionnaire survey of centres with established programmes. BMC Res Notes 2014; 7: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morton RL, Howard K, Webster AC et al. Patient INformation about Options for Treatment (PINOT): a prospective national study of information given to incident CKD stage 5 patients. Nephrol Dial Transplant 2011; 26: 1266–1274 [DOI] [PubMed] [Google Scholar]
- 29. Isnard Bagnis C, Crepaldi C, Dean J et al. Quality standards for predialysis education: results from a consensus conference. Nephrol Dial Transplant 2015; 30: 1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saggi SJ, Allon M, Bernardini J et al. Considerations in the optimal preparation of patients for dialysis. Nat Rev Nephrol 2012; 8: 381–389 [DOI] [PubMed] [Google Scholar]
- 31. Mehrotra R, Marsh D, Vonesh E et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68: 378–390 [DOI] [PubMed] [Google Scholar]
- 32. Friberg IO, Martensson L, Haraldsson B et al. Patients’ perceptions and factors affecting dialysis modality decisions. Perit Dial Int 2018; 38: 334–342 [DOI] [PubMed] [Google Scholar]
- 33. Manns BJ, Taub K, Vanderstraeten C et al. The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005; 68: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 34. Chan CT, Blankestijn PJ, Dember LM et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 96: 37–47 [DOI] [PubMed] [Google Scholar]
- 35. Warwick G, Mooney A, Russon L et al. Planning, Initiating and Withdrawal of Renal Replacement Therapy. Bristol, UK: The Renal Association, 2014 [Google Scholar]
- 36. Ladin K, Pandya R, Perrone RD et al. Characterizing approaches to dialysis decision making with older adults: a qualitative study of nephrologists. Clin J Am Soc Nephrol 2018; 13: 1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orsino A, Cameron JI, Seidl M et al. Medical decision-making and information needs in end-stage renal disease patients. Gen Hosp Psychiatry 2003; 25: 324–331 [DOI] [PubMed] [Google Scholar]
- 38. Emanuel EJ, Emanuel LL. Four models of the physician–patient relationship. JAMA 1992; 267: 2221–2226 [PubMed] [Google Scholar]
- 39. Grubbs V, Tuot DS, Powe NR et al. System-level barriers and facilitators for foregoing or withdrawing dialysis: a qualitative study of nephrologists in the United States and England. Am J Kidney Dis 2017; 70: 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ladin K, Pandya R, Kannam A et al. Discussing conservative management with older patients with CKD: an interview study of nephrologists. Am J Kidney Dis 2018; 71: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong SPY, Boyapati S, Engelberg RA et al. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis 2020; 75: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thamer M, Hwang W, Fink NE et al. US nephrologists’ recommendation of dialysis modality: results of a national survey. Am J Kidney Dis 2000; 36: 1155–1165 [DOI] [PubMed] [Google Scholar]
- 43. Lin E, Mell MW, Winkelmayer WC et al. Health insurance in the first 3 months of hemodialysis and early vascular access. Clin J Am Soc Nephrol 2018; 13: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perez JJ, Zhao B, Qureshi S et al. Health insurance and the use of peritoneal dialysis in the United States. Am J Kidney Dis 2018; 71: 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keith D, Ashby VB, Port FK et al. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol 2008; 3: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ludlow MJ, George CR, Hawley CM et al. How Australian nephrologists view home dialysis: results of a national survey. Nephrology 2011; 16: 446–452 [DOI] [PubMed] [Google Scholar]
- 47. Vanholder R, Stel VS, Jager KJ et al. How to increase kidney transplant activity throughout Europe—an advocacy review by the European Kidney Health Alliance. Nephrol Dial Transplant 2019; 34: 1254–1261 [DOI] [PubMed] [Google Scholar]
- 48.European Commission. Action plan on Organ Donation and Transplantation (2009–2015): Strengthened Cooperation between Member States, 2008. https://ec.europa.eu/health/ph_threats/human_substance/oc_organs/docs/organs_action_en.pdf (29 January 2020, date last accessed)
- 49.Joint Statement of the Thematic Network on Improving Organ Donation and Transplantation in the EU 2019. http://ekha.eu/wp-content/uploads/FINAL_14.01.2020_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (29 January 2020, datelast accessed)
- 50. Gan Kim Soon P, Lim SK, Rampal S et al. A qualitative examination of barriers and solutions to renal transplantation in Malaysia: key-informants’ perspective. PLoS One 2019; 14: e0220411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morita PP, Huynh K, Zakir A et al. Supporting the establishment of new home dialysis programs through the explore home dialysis program. Kidney Int Rep 2019; 4: 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jayanti A, Morris J, Stenvinkel P et al. Home hemodialysis: beliefs, attitudes, and practice patterns. Hemodial Int 2014; 18: 767–776 [DOI] [PubMed] [Google Scholar]
- 53. Jassal SV, Krishna G, Mallick NP et al. Attitudes of British Isles nephrologists towards dialysis modality selection: a questionnaire study. Nephrol Dial Transplant 2002; 17: 474–477 [DOI] [PubMed] [Google Scholar]
- 54. Tong A, Palmer S, Manns B et al. Clinician beliefs and attitudes about home haemodialysis: qualitative interview study. Nephrol Dial Transplant 2012; 27: ii281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Osterlund K, Mendelssohn D, Clase C et al. Identification of facilitators and barriers to home dialysis selection by Canadian adults with ESRD. Semin Dial 2014; 27: 160–172 [DOI] [PubMed] [Google Scholar]
- 56. Paudel K, Fan S, Sharma S et al. How to overcome barriers and start up new peritoneal dialysis programs—experience from Nepal. Nephrol Dial Transplant 2016; 31: i503–i503 [Google Scholar]
- 57. Firanek CA, Garza S, Gellens ME et al. Contrasting perceptions of home dialysis therapies among in-center and home dialysis staff. Nephrol Nurs J 2016; 43: 195–205 [PubMed] [Google Scholar]
- 58. Merighi JR, Schatell DR, Bragg-Gresham JL et al. Insights into nephrologist training, clinical practice, and dialysis choice. Hemodial Int 2012; 16: 242–251 [DOI] [PubMed] [Google Scholar]
- 59. Beaton TJ, Krishnasamy R, Toussaint ND et al. Nephrology training in Australia and New Zealand: a survey of outcomes and adequacy. Nephrology 2017; 22: 35–42 [DOI] [PubMed] [Google Scholar]
- 60. Chan CT, Wallace E, Golper TA et al. Exploring barriers and potential solutions in home dialysis: an NKF-KDOQI conference outcomes report. Am J Kidney Dis 2019; 73: 363–371 [DOI] [PubMed] [Google Scholar]
- 61. Metzger S. Home dialysis modalities: educational barriers to utilization. Nephrol Nurs J 2016; 43: 251–254 [PubMed] [Google Scholar]
- 62. Combes G, Sein K, Allen K. How does pre-dialysis education need to change? Findings from a qualitative study with staff and patients. BMC Nephrol 2017; 18: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Calestani M, Tonkin-Crine S, Pruthi R et al. Patient attitudes towards kidney transplant listing: qualitative findings from the ATTOM study. Nephrol Dial Transplant 2014; 29: 2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kosieradzki M, Jakubowska-Winecka A, Feliksiak M et al. Attitude of healthcare professionals: a major limiting factor in organ donation from brain-dead donors. J Transplant 2014; 2014: 296912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Witjes M, Kotsopoulos A, Herold IHF et al. The influence of end-of-life care on organ donor potential. Am J Transplant 2017; 17: 1922–1927 [DOI] [PubMed] [Google Scholar]
- 66. del Mar Lomero M, Johnson R, Coll E et al. Donation after circulatory death: an updated description of the European landscape. Transplantation 2018; 102: S386 [Google Scholar]
- 67.Kidney Health for Life. Chronic Kidney Disease Multinational Inventory. https://www.theisn.org/images/Initiatives/KH4L_-_CKD_Multinational_Inventory.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.