Abstract
Background
Place-based inequalities, such as exposure to violence and access to nutritious food and clean water, may contribute to human immunodeficiency virus (HIV)-associated cognitive impairment. In this study, we investigated neighborhood effects on cognition in children and adolescents with HIV in Lusaka, Zambia.
Methods
We conducted a prospective cohort study of 208 children with perinatally acquired HIV (ages 8–17) and 208 HIV-exposed uninfected controls. Participants underwent neuropsychological testing and interviews assessing socioeconomic status. Geographic regions with clusters of participants with HIV and cognitive impairment were identified using quantitative geographic information systems (QGIS) and SaTScan. Associations between location of residence and cognitive function were evaluated in bivariable and multivariable regression models. Mediation analysis was performed to assess direct and indirect effects of location of the residence on cognitive impairment.
Results
Residence in Chawama, one of the poorest neighborhoods in Lusaka, was significantly associated with cognitive impairment in participants with HIV (odds ratio 2.9; P = .005) and remained significant in a multivariable regression model controlling for potential confounders. Mediation analysis found that 46% of the cognitive effects of residence in Chawama were explained by higher rates of malnutrition, lower school attendance, and poorer self-reported health.
Conclusions
Place-based socioeconomic inequality contributes to cognitive impairment in Zambian children and adolescents with HIV. Neighborhood effects may be mediated by concentrated poverty, malnutrition, limited access to education and health care, and other yet unknown environmental factors that may be potentially modifiable.
Keywords: child health, global health, HIV, infectious diseases, Zambia
Neighborhood of residence is associated with cognitive impairment in children and adolescents with HIV in Lusaka Province, Zambia. Geospatial analysis is an important global health tool to understand how place-based socioeconomic determinants affect disease sequelae.
Worldwide more than 1.8 million children are infected with human immunodeficiency virus (HIV), and 20%–50% of these children are cognitively impaired [1, 2]. Cognitive impairment in children with HIV persists despite combined antiretroviral therapy (ART) [3, 4]. Childhood cognitive impairment likely impacts social participation, ART adherence, school performance, and future job participation, making early detection, intervention, and identification of prevention measures crucial [5, 6]. The plurality of childhood HIV infection and cognitive impairment occurs in sub-Saharan Africa [2], although it has been minimally studied in these regions.
Structural factors, such as socioeconomic status (SES), are strong predictors of cognitive outcomes in children with HIV [5, 7–10]. Geographical analysis of these structural factors may reveal place-based inequalities in the distribution of societal resources, exposure to violence, environmental risks, and access to nutritious food and clean water [10–12] which contribute to cognitive impairment—potentially modifiable risk factors. Although geographic information systems (GIS) and spatial analysis have been widely applied to HIV research in Africa [13–16], GIS has not been utilized in the study of HIV-associated cognitive impairment in children and adolescents in the African setting.
The HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) study seeks to understand the cognitive outcomes of children and adolescents with perinatally acquired HIV in Lusaka, Zambia. HIV is highly prevalent in Zambia with 12% of individuals aged 15–49 or approximately 960 000 people affected [17, 18], with an estimated 62 000 children currently living with HIV [17]. A previous HANDZ analysis found constituency clustering of neurocysticercosis cases with lack of access to clean water and modern toilet facilities [19]. The aim of this study was to identify place-based socioeconomic inequalities associated with cognitive impairment in Zambian children and adolescents with perinatally acquired HIV.
METHODS
Study Design, Setting, and Participants
HANDZ is a prospective cohort study that explores cognitive and psychiatric outcomes among children and adolescents living with HIV and HIV-exposed, uninfected (HEU) controls in Lusaka Province, Zambia [20]. HANDZ study is based in the Lusaka Province that contains the capital city and is 1 of the 10 Zambian provinces. The Lusaka Province contains 13 socioeconomically diverse constituencies, which are large neighborhoods with single-member representation in the National Assembly of Zambia [21]. Briefly, children and adolescents with perinatally acquired HIV (ages 8–17) were recruited from the Pediatric Center of Excellence (PCOE) in Lusaka, Zambia, a major outpatient pediatric HIV care referral center. Participants with HIV were included if treated with ART for longer than 1 year and excluded if they had a known history of CNS infection [20]. HEU controls were recruited from Lusaka neighborhoods by a community health worker using a stratified sampling method to ensure approximately equal age and sex distribution [20]. The HEU group provided a local normative sample for cognitive tests and served as a comparison group for rates of cognitive impairment.
Data Collection
Each participant completed a demographic questionnaire, standardized interviews, and comprehensive neuropsychological testing. Participants were seen at baseline and subsequently every 3 months, with a median of 2 years of follow-up completed at the time of this analysis.
Measurements
Comprehensive neuropsychological testing was performed using a combination of the National Institutes of Health Toolbox—Cognition Battery and standard pencil-and-paper neuropsychological tests on a quarterly and annual basis, respectively [20]. Cognitive impairment was defined using a Global Deficit Score (GDS) approach [20]. Domain-specific deficit scores were calculated based on standard deviations below the mean performance of the control population, then domain-specific deficit scores were averaged to create the GDS. By convention, cognitive impairment was defined as a GDS score of greater than or equal to 0.5 [22]. SES was measured using an adaptation of the UNICEF Multiple Indicator Cluster Survey (MICS4) [23]. Individual SES variables of prespecified importance (maternal education, electricity, access to running water, presence of a flush toilet, food security, income, and possession index) were combined to form an SES index (SESI) ranging from 0 to 12. Negative life events (eg, hospitalization, exposure to violence or abuse, and illness or death of a family member) were measured using an instrument designed for the HANDZ study, the Negative Life Event Questionnaire (NLEQ), and summed into a Negative Life Event Index [20]. The components of the SES Index and Negative Life Event Index are listed in Supplementary Table 1.
Geographic Analysis
Each participant’s location of residence was approximated using Google Maps and OpenStreetMap. Estimated latitude/longitude coordinates and shapefiles of the Lusaka constituencies were overlayed in maps generated by quantitative GIS (QGIS) software (version 3.2.0) [24]. The geospatial relationship of prespecified socioeconomic factors was visualized. Distance between PCOE and the participants’ residence was calculated using the HubDistance tool in QGIS. To ensure the confidentiality and privacy of participants in the HANDZ study, participant points were enlarged, and constructed maps were zoomed out to view the entire city of Lusaka without specific landmarks.
Statistical Methods
Geographic clustering analysis was performed with a spatial statistics software, SaTScan (version 9.6; https://www.satscan.org) using a Bernoulli model [25]. Maximum spatial cluster sizes were set at less than 50% of the population at risk within a circular window, default SaTScan parameters. Likelihood ratios were calculated for each cluster. Cluster analysis was not performed on the HEU sample, as these participants were recruited from specific constituencies, thus clustering detected in HEU participants could be an artifact of the location of recruitment.
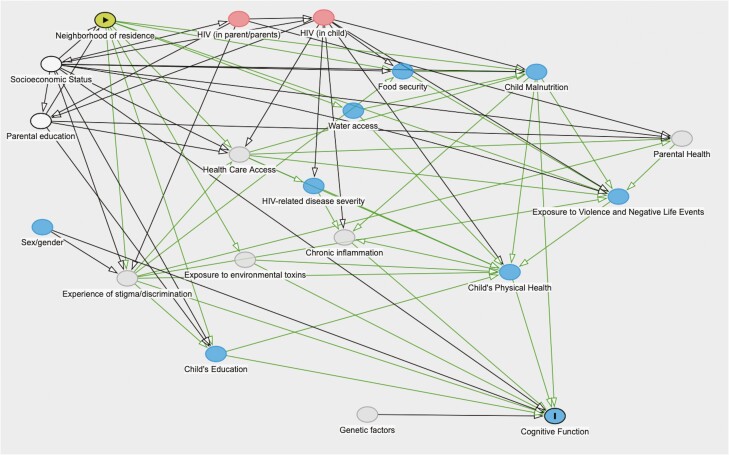
Additional statistical analyses were conducted using Stata 16.1 (College Station, TX). Chi-squared tests evaluated differences in dichotomous variables, t-test statistics for normally distributed continuous variables, and Kruskal-Wallis ranks for non-normally distributed continuous or ordinal variables. Constituencies identified as having clusters of participants with cognitive impairment using SaTScan with a significance of <=0.2 were evaluated with bivariable and multivariable logistic regression models. Two separate logistic regression models were fit; in the first, we adjusted for other SES variables and parental education in order to estimate the total causal effect of neighborhood of residence. In the second, we adjusted for confounding variables as well as all measured potential mediating variables in order to estimate the “direct” effect of neighborhood of residence. Using Dagitty (V. 3.0, http://www.dagitty.net), directed acyclic graphs (DAGs) were used to generate a causal model and select which variables to include in multivariable models (see Figure 1; Supplementary Table 2) [26, 27]. Mediation analysis using the “ldecomp” package in Stata was used to evaluate direct and indirect effects of neighborhood of residence on cognitive impairment [28]. Neighborhood of residence was used as the primary exposure variable, with each potential mediating variable chosen based on the DAG. P-values of <= .05 were considered significant in regression models.
Figure 1.
A directed acyclic graph (DAG) model of how socioeconomic status (SES) and neighborhood of residence influence cognitive impairment in Zambian children with HIV. This model implies that total effect of neighborhood of residence on cognition may be estimated by controlling for other SES variables and parental education. Testable implications of this model are that effects may be mediated through malnutrition, access to education, and exposure to violence and other negative life events.
Ethics Statement
This study was approved by the institutional review boards of the University of Zambia (reference #004-08-17), the University of Rochester (protocol #00068985), and the National Health Research Authority of Zambia. Verbal and written parental permission were obtained from the parents of all participants who participated. Verbal and written assent was obtained from all participants aged 12 years and older.
RESULTS
Demographic and Socioeconomic Trends
Demographic and socioeconomic indicators of HANDZ participants with HIV are described in Table 1. The median age of participants with HIV was 12, and there were roughly equal numbers of males and females. Participants with HIV were more likely to have cognitive impairment than HEU controls (34% vs 19%, P = .001). Statistically significant risk factors for cognitive impairment among participants with HIV in the bivariable analysis included self-reported poor health, low SESI, lack of access to indoor toilets, and running water. In addition, cognitively impaired participants with HIV were more likely to have growth stunting (52% vs 22%, P < .001) and to not attend school (14% vs 4%, P = .01). Although not statistically significant, among cognitively impaired participants with HIV, fewer had electricity in their home (74% vs 81%), and a greater number had a history of malnutrition (33% vs 29%) or severe malnutrition (26% vs 18%). Aerial distance to PCOE was not statistically different between cognitively impaired or unimpaired participants with HIV.
Table 1.
Demographics and Socioeconomic Indicators of Participants With HIV: Impaired vs Unimpaired
| Variable | Impaired (n = 70) | Unimpaired (n = 136) | P-Value |
|---|---|---|---|
| Mean age (+/− SD) | 11.9 +/− 2.2 | 12.4 +/− 2.4 | .140* |
| Female (f/m) | 51% (36/34) | 41% (56/80) | .161** |
| Nadir CD4+ count, n = 188 | 547 | 598 | .313* |
| Mean viral load, mean copies per mL+/− SD, n = 180 | 6896 +/− 18439 | 2124 +/− 10 053 | .026* |
| History of WHO Stage 4, n = 194 | 66% (37/29) | 41% (53/75) | .052** |
| % ART non-adherent in last year % (yes/no), n = 201 | 3% (2/67) | 8% (11/121) | .226** |
| History of malaria, % (yes/no) | 60% (42/28) | 64% (87/49) | .649** |
| History of TB, % (yes/no) | 39% (27/43) | 31% (42/94) | .279** |
| No. of known hospitalizations, mean +/− SD | 1.4 +/− 1.2 | 1.7 +/− 1.9 | .192* |
| Stunted % (yes/no), n = 204 | 52% (36/33) | 22% (30/105) | <.001** |
| Aerial distance to PCOE (in km) n = 201 mean +/− SD | 9.0 +/− 7.4 | 8.5 +/− 7.3 | .667* |
| Median Socioeconomic Status Index, n = 191 | 5 | 6 | <.001*** |
| Low Socioeconomic Status Index (Socioeconomic Status Index <= 2), n = 191 | 20% (13/51) | 9% (12/115) | .036** |
| Self-reported poor health (yes/no), n = 204 | 17% (12/57) | 7% (9/126) | .027** |
| Running water (yes/no) | 33% (23/47) | 54% (74/62) | .003** |
| Flush toilet (yes/no) | 24% (17/53) | 46% (62/74) | .003** |
| Electricity (with/without) | 74% (52/18) | 81% (110/26) | .274** |
| Malnutrition (yes/no) | 33% (23/47) | 29% (40/96) | .611** |
| Severe malnutrition (yes/no) | 26% (18/52) | 18% (25/111) | .220** |
| Mean negative life events, n = 199 | 1.76 | 1.52 | .647*** |
| Negative life events >=4 (yes/no) n = 199 | 15% (10/57) | 9% (12/120) | .237** |
| Not in school | 14% (10/60) | 4% (6/130) | .012** |
Abbreviations: WHO, World Health Organization; ART, antiretroviral therapy; TB, tuberculosis; PCOE, Pediatric Center of Excellence, Lusaka, Zambia.
*Two-sample t-test.
**Fisher’s exact.
***Kruskal-Wallis.
Socioeconomic differences between constituencies are summarized for participants with HIV in Table 2. Noted constituencies with low median SESI were Chawama and Kabwata. In Chawama, Katuba, and Lusaka Central, more than half of participants with HIV were GDS impaired. Additionally, constituency size and census population data are also shown [29, 30]. In Chawama, Kanyama, Katuba, and Munali, more than 28% of participants with HIV reported severe malnutrition.
Table 2.
Demographic, SES, and HIV Characteristics by Lusaka Constituency for HANDZ Participants With HIV
| Chawama | Chilanga | Chongwe | Kabwata | Kanyama | Katuba | Lusaka Central | Mandevu | Matero | Munali | |
|---|---|---|---|---|---|---|---|---|---|---|
| n, observations | 37 | 20 | 6 | 27 | 40 | 3 | 7 | 32 | 13 | 19 |
| Mean age +/− SD | 10.7 +/− 2.6 | 11.1 +/− 2.4 | 10.4 +/− 1.8 | 11.3 +/− 2.5 | 12.2 +/− 2.3 | 11.3 +/− 2.3 | 12 +/− 2 | 12.6 +/− 2.1 | 11.9 +/− 2.3 | 11.9 +/− 2.2 |
| Median SES Index | 5 | 3.5 | 8 | 8 | 6 | 5 | 7 | 6 | 8 | 7 |
| Running water %, yes/no | 22%, 8/28 | 40%, 8/12 | 80%, 4/1 | 26%, 20/7 | 35%, 14/26 | 33%, 1/2 | 86%, 6/1 | 44%, 14/18 | 85%, 11/2 | 58%, 11/8 |
| Severe malnutrition %, yes/no | 35%, 13/24 | 15%, 3/17 | 0%, 0/6 | 4%, 1/26 | 28%, 11/29 | 33%, 1/2 | 14%, 1/6 | 19%, 6/26 | 8%, 1/12 | 32%, 6/13 |
| Mean negative life events | 2.2 | 1.4 | 2.3 | 1.6 | 1.6 | 2.3 | 2.1 | 1.1 | 1 | 1.2 |
| Not in school %, yes/no | 20%, 6/24 | 13%, 2/13 | 0%, 0/5 | 5%, 1/19 | 12%, 3/23 | 0%, 0/2 | 0%, 0/5 | 5%, 1/20 | 0%, 0/10 | 0%, 0/16 |
| History of WHO Stage 4 %, yes/no | 53%, 19/17 | 39%, 7/11 | 17%, 1/5 | 54%, 14/12 | 51%, 20/19 | 100%, 1/0 | 29%, 2/5 | 45%, 14/17 | 31%, 4/9 | 47%, 9/10 |
| Stunted %, yes/no | 35%, 12/22 | 30%, 6/14 | 0%, 0/6 | 33%, 9/18 | 33%, 13/27 | 25%, 1/3 | 0%, 0/4 | 10/22 | 42%, 5/7 | 37%, 7/12 |
| Distance to PCOE, kma | 4.8 | 22.5 | 22.5 | 4.1 | 7.8 | 16.2 | 5.8 | 7.6 | 7.9 | 6.4 |
| GDS impaired %, yes/no | 55.9%, 19/15 | 47.4%, 9/10 | 16.7%, 1/5 | 22.2%, 6/21 | 37.5%, 15/25 | 66.7%, 2/1 | 57.1%, 4/3 | 25%, 8/24 | 15.4%, 2/11 | 21.1%, 4/15 |
| Area (square km)a | 55 | 1336 | 2537 | 46 | 98 | 1722 | 110 | 54 | 40 | 52 |
| Total population, 2000 Census [26] | 139 998 | 56 673 | 100 281 | 89 556 | 170 803 | 56 628 | 99 431 | 219 285 | 189 480 | 176 150 |
| Total population, 2010 Census [27] | 187 565 | 107 051 | 192 303 | 174 338 | 364 655 | 79 306 | 117 097 | 358 788 | 282 734 | 261 975 |
| Calculated population density 2000, people/km2 | 2545 | 42 | 40 | 1947 | 1743 | 33 | 904 | 4061 | 4737 | 3388 |
| Calculated population density 2010, people/km2 | 3410 | 80 | 76 | 3790 | 3721 | 46 | 1065 | 6644 | 7068 | 5038 |
Abbreviations: HANDZ, HIV-Associated Neurocognitive Disorders in Zambia; WHO, World Health Organization; GDS, global deficit scale; PCOE, Pediatric Center of Excellence, Lusaka, Zambia; SES, socioeconomic status. QGIS, quantitative geographic information systems.
aComputed in QGIS.
Geographic Analysis
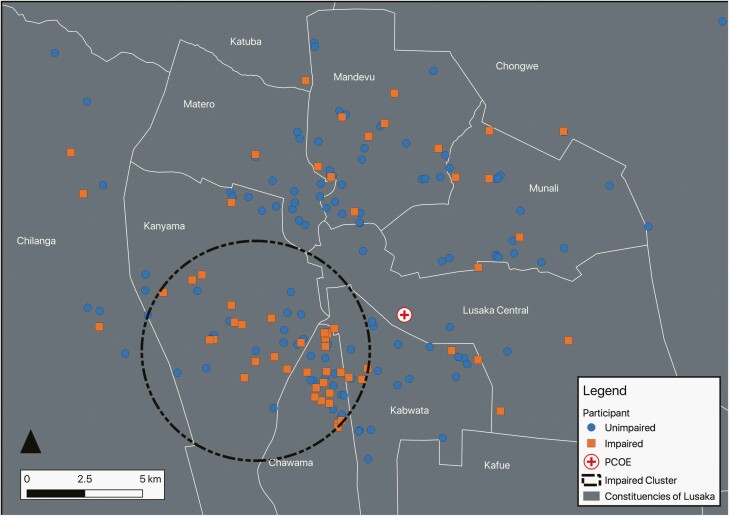
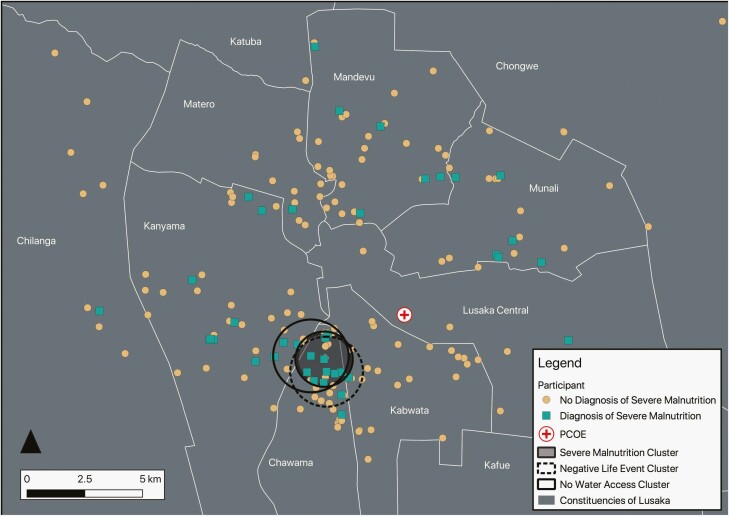
Given that several constituencies had low numbers of participants with HIV (see Table 2), we performed a geographic clustering analysis independent of administrative boundaries to identify clusters of cognitive impairment and socioeconomic disparity. Clustering analysis demonstrated a grouping of cognitively impaired participants with HIV in regions that overlap with the Chawama and Kanyama constituencies (see Figure 2). The prevalence of cognitive impairment among participants with HIV in this 76 km2 area was 52.2%, compared with 34% over the entire geographic area (observed/expected ratio of 1.50; relative risk 2.03; P = .128). Similar geographic clustering was observed with the socioeconomic variables: lack of access to running water, greater than or equal to 4 negative life events, severe malnutrition, and low school attendance (see Figure 3; Supplementary Figures 1 and 2). These clusters qualitatively overlapped with the Chawama constituency. There was no geographically significant increased prevalence of malaria or tuberculosis infection history (not shown). Tabulated results of the clustering analyses are displayed in Supplementary Table 3.
Figure 2.
Geographic clustering of cognitively impaired Zambian participants with HIV. This figure shows a 4.92-km-radius cluster of cognitively impaired participants with HIV. The prevalence of cognitive impairment among participants with HIV in this region was 52.2%, compared with the 34% of participants who were cognitively impaired over the entire geographic area. The cluster had an observed/expected ratio of 1.50, a log-likelihood ratio of 6.85, and a P-value of .128. Created on December 31, 2020—EPSG:20934 Arc 1950/UTM Zone 34S—Stanford Earthworks, Google Maps, OpenStreetMap, Central Statistical Office of Zambia. The color version of this figure is available in the online edition.
Figure 3.
Clusters of severe malnutrition, negative life events, and lack of water access among participants with HIV. This figure shows several sociodemographic indicators clusters in the constituency of Chawama. The 1.14-km-radius severe malnutrition cluster had a case rate of 62.5%, observed/expected ratio of 2.98, a log-likelihood ratio of 7.18, and a P-value of .087. The 1.57-km-radius negative life events cluster had a case rate of 46.2%, observed/expected ratio of 4.17, a log-likelihood ratio of 13.03, and a P-value of < .001. There were 2 clusters that showed no running water access. Cluster A was a 1.63-km-radius cluster that had a case rate of 10.7%, observed/expected ratio of 0.22, a log-likelihood ratio of 10.4, and a P-value of .003. Cluster B was a 1.24-km-radius cluster that had a case rate of 5%, observed/expected ratio of 0.1, a log-likelihood ratio of 10.1, and a P-value of .004. Created by A. B. on December 31, 2020, EPSG:20934 Arc 1950/UTM Zone 34S—Stanford Earthworks, Google Maps, OpenStreetMap, Central Statistical Office of Zambia. The color version of this figure is available in the online edition.
Regression Model and Mediation Analysis Results
Given that the cluster of cognitively impaired participants with HIV predominantly overlapped Chawama and Kanyama, these 2 constituencies were further examined in bivariable and multivariable regression models. Bivariable analysis revealed that residence in Chawama was significantly associated with cognitive impairment in participants with HIV (odds ratio [OR] 2.9; 95% confidence interval [CI], 1.4-6.2; P = .005). In participants with HIV, residence in Chawama remained significantly associated with cognitive impairment in a multivariable regression model controlling for SES and parental education (adjusted OR 3.2; 95% CI, 1.3-8.1; P = .01); and in a second model controlling for age, sex, SES, negative life events, malnutrition, growth stunting, and self-reported poor health (adjusted OR 2.7; 95% CI, 1.1-6.6; P = .04). Residence in Chawama was associated with having a CD4+ nadir of <=200 (OR 3.9; 95% CI, 1.3–11.0; P = .01) but was not associated with other HIV-specific disease measures such as WHO stage, mean viral load, or non-adherence with ART in the last year. Mediation analysis suggested that the effect of residence in Chawama was partially mediated through malnutrition, poor health, and lack of school attendance, but that these effects accounted for only 46% of the total effect. Residence in Kanyama was not significantly associated with cognitive impairment among participants with HIV (OR 1.2; 95% CI, 0.6–2.6; P = .6).
Discussion
In this prospective cohort study, we investigated the effects of place-based inequality on cognition in children and adolescents with HIV in Lusaka, Zambia. We identified a geographic region, centered on the Lusaka constituency Chawama, where socioeconomic disparities including the lack of running water, higher exposure to negative life events, severe malnutrition, and lower school attendance overlapped with regions of participants with HIV who were cognitively impaired. Residence in Chawama was significantly associated with cognitive impairment in participants with HIV. Notably, when we controlled for Chawama-specific socioeconomic disparity, Chawama residency remained a significant contributor to cognitive impairment suggesting other, unrecorded environmental drivers. Mediation analysis revealed that about half of the residency effect of Chawama might be explained by higher rates of malnutrition, decreased school attendance, and self-reported poorer health of participants with HIV. Our data also suggest that the geographic drivers of cognitive impairment in Chawama may be specific to HIV infection, given that cognitively impaired HEU controls were not clustered in the Chawama constituency.
Neighborhood SES is foundational for childhood cognitive, social, and cultural development [11]. Social-interactive mechanisms including increased social disorder, less social cohesion, lack of safety, and structure of the family environment likely mediate this effect [11, 31–36]. Recent studies of Alzheimer’s disease and adult-onset mild cognitive impairment have highlighted the importance of environmental influences on cognition including the size and density of buildings, access to community centers and green spaces, proximity to hospitals, and the level of pollution [37, 38]. In individuals with HIV, social comorbidities such as poverty, lack of access to education, and exposure to trauma likely contribute to cognitive impairment [39, 40]. Additionally, chronic exposure to low-resource environments and trauma may diminish neurocognitive reserves worsening HIV-associated cognitive impairment [41]. Our results generally aligned with these previous studies with several indicators of low SES associated with cognitive impairment in our cohort as a whole (see Table 1), examples including lack of school attendance and lack of access to running water. An exception is the number of negative life events, a summary statistic of exposure to trauma and violence in our cohort, which was not associated with cognitive impairment in bivariable analysis (see Table 1). Additionally, despite high numbers of negative life events geographically clustering in Chawama (Figure 3), this variable was not a significant contributor in the mediation analysis. In a previous analysis of the HANDZ cohort by Molinaro et al [42], we know that negative life events are associated with depression, but in this study, the direct effect of negative life events on cognition was small and not statistically significant.
Chawama is a densely populated urban community that has experienced significant population growth in the past 2 decades (see Table 2) [29, 30, 43]. Chawama originated as a squatter settlement in the 1950s and lacks planned essential service infrastructure, including central water supply, sanitation service, and waste management; many residents have inadequate household income to finance these services independently [44]. Participants with HIV experiencing severe malnutrition and lack of access to running water clustered in Chawama (see Figure 3), data that were similar to a previous report from our group which identified low rates of flush toilets and running water overlapping with cases of neurocysticercosis in a similar region [19]. We hypothesize that the poor sanitary conditions of Chawama could contribute to the development of malnutrition by increasing exposure to infectious diseases of the gastrointestinal tract, a potentially potent contributor given the immunocompromised population. Other infectious disease vectors, including Ascaris lumbricoides, Giardia duodenalis, Schistosoma haematobium, and Vibrio cholerae, have also been reported in Chawama and could be contributing to malnutrition as well [44–46]. Although nutritional and vitamin deficiencies are common comorbidities in HIV-associated cognitive impairment, they have not been causally implicated [47]. Further research of constituency-based disease vector incidence and sanitary conditions is required to explore this hypothesis further.
Environmental pollutants may also play a role in the development of HIV-associated cognitive impairment. Dichlorodiphenyltrichloroethane (DDT), an organic pesticide and known neurotoxin, was applied in Chawama at least 3 times from 2002 to 2012 as part of a pilot study for malaria control [48], corresponding approximately with the perinatal period of the children and adolescents participating in our study. In 2012, significant levels of DDT and its metabolites were found in water and soil samples from Chawama, at much higher concentrations than maximum levels recommended by the World Health Organization. DDT and DDT metabolite exposure has been associated with altered cognitive development in children [49–51], as well as in the development of Alzheimer’s disease [52]. Soil pollutants like DDT could be particularly relevant given that geophagy is a known phenomenon in children and pregnant women in Lusaka [46, 53]. Additionally, the Chawama constituency includes industrially zoned areas including factories and cement production quarries which could generate other environmental pollutants [54]. More research into the relationship between HIV-associated cognitive impairment and environmental toxins is necessary.
Strengths of the study include its relatively large sample size and structured acquisition of comprehensive neuropsychological, socioeconomic, and clinicodemographic data of a rarely studied population.
This study also has several limitations. First, household geographic location was manually estimated using OpenStreetMap and Google maps, and although most parts of Lusaka are completely mapped, some areas may have been incompletely or inaccurately annotated. To reduce inaccurate participant mapping, any participant address location that could not be verified by HANDZ staff familiar with Lusaka was excluded. It is also possible that participants could have frequently moved throughout their childhood, confounding our ability to define longitudinal place-based risk; data on the frequency of moving between households were not collected. Second, given that the HANDZ study was not initially designed to look at constituency-based metrics, we did not have sufficient sampling of all constituencies to do comprehensive comparative analyses. Third, the SatScan analysis was limited in that only circular clusters could be detected, and its ability to detect small clusters may be limited [55]. Additionally, simply because we noted geographic clustering in the SatScan analysis does not imply that a biological connection between cases exists. Fourth, several potential key mediators were not measured as part of this study, including measures of healthcare access, stigma/discrimination, toxic exposures, and chronic inflammation. More comprehensive mediation analysis using structural equation models will be utilized in future publications. Fifth, it is possible that participants with HIV receiving their medical care at PCOE in Lusaka are of higher acuity or higher income than the surrounding parts of their neighborhoods given that PCOE is one of the only tertiary care referral centers for pediatric HIV in Lusaka. We believe the latter issue is less likely given that study participants were provided travel stipends to reach their appointments. In addition, within the confines of this study, we were unable to assess the effect of disease control because among the HANDZ participant population, HIV is generally very well controlled. Finally, although we hypothesize that our methods would be similarly useful in identifying high-risk neighborhoods in other locations, it is possible that Lusaka-specific contributions to HIV-associated cognitive impairment would not be applicable in other locations.
Conclusions
GIS is an important global health tool to understand how place-based socioeconomic determinants affect disease sequelae. In this study, we demonstrated that neighborhood of residence is associated with cognitive impairment in children and adolescents with HIV in Lusaka Province, Zambia. This association appeared to be mediated by malnutrition, poor health, and educational factors, though it is likely that other unmeasured factors also contributed. These risk factors are important from a public health perspective, since they are potentially modifiable. Future interventional studies to improve cognitive outcomes in people living with HIV might target recruitment in neighborhoods at greatest risk for high rates of impairment.
Supplementary Material
Notes
Financial support . This work was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS117310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the URMC Office of Medical Education International Research Grant and grants from the University of Rochester Center for AIDS Research (CFAR), an NIH-funded program (grant number P30 AI 045008), the University of Rochester School of Medicine, and the McGowan Foundation.
Potential conflicts of interest. There are no relevant conflicts of interest to disclose for any authors related to this work.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. Global Factsheet. Accessed March 24 2020. https://aidsinfo.unaids.org.
- 2. Banks LM, Zuurmond M, Ferrand R, Kuper H. The relationship between HIV and prevalence of disabilities in sub-Saharan Africa: systematic review (FA). Trop Med Int Health 2015; 20:411–29. [DOI] [PubMed] [Google Scholar]
- 3. Boivin MJ, Barlow-Mosha L, Chernoff MC, et al. ; IMPAACT P1104s Study Team. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS 2018; 32:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nozyce ML, Lee SS, Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics 2006; 117:763–70. [DOI] [PubMed] [Google Scholar]
- 5. Cohen S, Ter Stege JA, Geurtsen GJ, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis 2015; 60:1111–9. [DOI] [PubMed] [Google Scholar]
- 6. Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnoses on long-term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. AIDS 2009; 23:1859–65. [DOI] [PubMed] [Google Scholar]
- 7. Mwanza-Kabaghe S, Mubanga E, Matafwali B, et al. Zambian preschools: a boost for early literacy. Engl Linguist Res 2015; 4:1–10. [Google Scholar]
- 8. Kandawasvika G, Gumbo F, Kuona P. HIV exposed uninfected children at school age: developing country context. Int J Virol AIDS 2016; 3:024. [Google Scholar]
- 9. Kabuba N, Menon JA, Franklin DR, et al. Effect of age and level of education on neurocognitive impairment in HIV positive Zambian adults. Neuropsychology 2018; 32:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bangirana P, John CC, Idro R, et al. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One 2009; 4:e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minh A, Muhajarine N, Janus M, et al. A review of neighborhood effects and early child development: how, where, and for whom, do neighborhoods matter? Health Place 2017; 46:155–74. [DOI] [PubMed] [Google Scholar]
- 12. Dean O, Buda A, Adams HR, et al. Brain magnetic resonance imaging findings associated with cognitive impairment in children and adolescents with human immunodeficiency virus in Zambia. Pediatr Neurol 2020; 102:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyda DC, Holzman SB, Berman A, et al. Geographic information systems, spatial analysis, and HIV in Africa: a scoping review. PLoS One 2019; 14:e0216388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chimoyi LA, Musenge E. Spatial analysis of factors associated with HIV infection among young people in Uganda, 2011. BMC Public Health 2014; 14:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kandala NB, Brodish P, Buckner B, et al. Millennium development goal 6 and HIV infection in Zambia: what can we learn from successive household surveys? AIDS 2011; 25:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandala NB, Campbell EK, Rakgoasi SD, et al. The geography of HIV/AIDS prevalence rates in Botswana. HIV AIDS (Auckl) 2012; 4:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UNAIDS. Zambia Data Factsheet. Accessed March 24 2020.https://aidsinfo.unaids.org.
- 18. Ministry of Health Zambia. Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: Final Report. Lusaka: Ministry of Health; 2019. [Google Scholar]
- 19. Buda A, Dean O, Adams HR, et al. Neurocysticercosis among Zambian children and adolescents with human immunodeficiency virus: a geographic information systems approach. Pediatr Neurol 2020; 102:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams HR, Mwanza-Kabaghe S, Mbewe EG, et al. The HIV-associated neurocognitive disorders in Zambia (HANDZ) study: protocol of a research program in pediatric HIV in sub-Saharan Africa. J HIV/AIDS Infect Dis 2019; 5:1–18. [Google Scholar]
- 21. National Assembly of Zambia. List of Constituencies by Province. Accessed March 24 2021. http://www.parliament.gov.zm/members/constituencies
- 22. Carey CL, Woods SP, Gonzalez R, et al. ; HNRC Group . Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26:307–19. [DOI] [PubMed] [Google Scholar]
- 23. United Nations Children’s Fund (UNICEF). Multiple Indicator Cluster Surveys (MICS). http://mics.unicef.org/. Accessed 1 March 2021.
- 24. QGIS Development Team. QGIS Geographic Information System. 3.2.0 ed: Open Source Geospatial Foundation Project. http://qgis.osgeo.org. Accessed 1 October 2020.
- 25. Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med 1995; 14:799–810. [DOI] [PubMed] [Google Scholar]
- 26. Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2016; 45:1887–94. [DOI] [PubMed] [Google Scholar]
- 27. Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011; 22:745. [DOI] [PubMed] [Google Scholar]
- 28. Buis ML. Direct and indirect effects in a logit model. Stata J 2010; 10:11–29. [PMC free article] [PubMed] [Google Scholar]
- 29. Central Statistical Office. 2000 Census of Population and Housing—National Analytical Report. 2003. Zambia Statistics Agency - Lusaka, Zambia. https://www.zamstats.gov.zm/index.php/publications. Accessed 1 February 2021.
- 30. Central Statistical Office. 2010 Census of Population and Housing—National Analytical Report. 2012. Zambia Statistics Agency - Lusaka, Zambia. https://www.zamstats.gov.zm/index.php/publications. Accessed 1 February 2021.
- 31. Singh GK, Ghandour RM. Impact of neighborhood social conditions and household socioeconomic status on behavioral problems among US children. Matern Child Health J 2012; 16:S158–69. [DOI] [PubMed] [Google Scholar]
- 32. O′Campo P, Caughy MO, Nettles SM. Partner abuse or violence, parenting and neighborhood influences on children’s behavioral problems. Soc Sci Med 2010; 70:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dupere V, Leventhal T, Crosnoe R, Dion E. Understanding the positive role of neighborhood socioeconomic advantage in achievement: the contribution of the home, child care, and school environments. Dev Psychol 2010; 46:1227–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flouri E, Tzavidis N, Kallis C. Area and family effects on the psychopathology of the Millennium Cohort Study children and their older siblings. J Child Psychol Psychiatry 2010; 51:152–61. [DOI] [PubMed] [Google Scholar]
- 35. Heberle AE, Thomas YM, Wagmiller RL, et al. The impact of neighborhood, family, and individual risk factors on toddlers’ disruptive behavior. Child Dev 2014; 85:2046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jeon L, Buettner CK, Hur E. Family and neighborhood disadvantage, home environment, and children’s school readiness. J Fam Psychol 2014; 28:718–27. [DOI] [PubMed] [Google Scholar]
- 37. Besser L, Galvin JE, Rodriguez D, et al. Associations between neighborhood built environment and cognition vary by apolipoprotein E genotype: multi-ethnic study of atherosclerosis. Health Place 2019; 60:102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerin E. Building the evidence for an ecological model of cognitive health. Health Place 2019; 60:102206. [DOI] [PubMed] [Google Scholar]
- 39. Tedaldi EM, Minniti NL, Fischer T. HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int 2015; 2015:641913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leserman J, Whetten K, Lowe K, et al. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med 2005; 67:500–7. [DOI] [PubMed] [Google Scholar]
- 41. Vance DE, Fazeli PL, Grant JS, et al. The role of neuroplasticity and cognitive reserve in aging with HIV: recommendations for cognitive protection and rehabilitation. J Neurosci Nurs 2013; 45:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Molinaro M, Adams HR, Mwanza-Kabaghe S, et al. Evaluating the relationship between depression and cognitive function among children and adolescents with HIV in Zambia. AIDS Behav 2021;1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shifa M. Profiling Multidimensional Poverty and Inequality in Kenya and Zambia at Sub-National Levels.SALDRU Working Papers. Southern Africa Labour and Development Research Unit, University of Cape Town; 2017:209.
- 44. Ndhlema D. An Environmental Profile of an Urban Squatter Settlement, Chawama Compound in Lusaka [BSc Dissertation]. Lusaka, Zambia: University of Zambia; 2000. [Google Scholar]
- 45. Tembo SJ, Mutengo MM, Sitali L, et al. Prevalence and genotypic characterization of Giardia duodenalis isolates from asymptomatic school-going children in Lusaka, Zambia. Food Waterborne Parasitol 2020; 19:e00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nchito M, Geissler PW, Mubila L, et al. The effect of iron and multi-micronutrient supplementation on Ascaris lumbricoides reinfection among Zambian schoolchildren. Trans R Soc Trop Med Hyg 2009; 103:229–36. [DOI] [PubMed] [Google Scholar]
- 47. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sipilanyambe Munyinda N, Michelo C, Sichilongo K. Linking environmental exposure with public health: dichlorodiphenyltrichloroethane extracted from soils and water of recently exposed communities of selected locations in Zambia. J Environ Public Health 2015; 2015:564189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaspar FW, Harley KG, Kogut K, et al. Prenatal DDT and DDE exposure and child IQ in the CHAMACOS cohort. Environ Int 2015; 85:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribas-Fitó N, Torrent M, Carrizo D, et al. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. Am J Epidemiol 2006; 164:955–62. [DOI] [PubMed] [Google Scholar]
- 51. Eskenazi B, Marks AR, Bradman A, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics 2006; 118:233–41. [DOI] [PubMed] [Google Scholar]
- 52. Yan D, Zhang Y, Liu L, Yan H. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep 2016; 6:32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shinondo C, Mwikuma G. Geophagy as a risk factor for helminth infections in pregnant women in Lusaka, Zambia. Med J Zambia 2008; 35:48–52. [Google Scholar]
- 54. Lusaka City Council and Environmental Council of Zambia. Lusaka City State of Environment Outlook Report. Lusaka, Zambia: UN Environment Program Document Repository; 2008. [Google Scholar]
- 55. Mathes RW, Lall R, Levin-Rector A, et al. Evaluating and implementing temporal, spatial, and spatio-temporal methods for outbreak detection in a local syndromic surveillance system. PLoS One 2017; 12:e0184419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.