Abstract
Background
Fatigue is a common and disabling symptom in people with a primary brain tumour (PBT). The effectiveness of interventions for treating clinically significant levels of fatigue in this population is unclear.
Objectives
To assess the effectiveness and safety of pharmacological and non‐pharmacological interventions for adults with PBT and high levels of fatigue.
Search methods
In March 2016, we searched the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO and CINAHL and checked the reference lists of included studies. We also searched relevant conference proceedings, searched for ongoing trials via ClinicalTrials.gov and contacted major co‐operative groups with trials in this area.
Selection criteria
We included randomised controlled trials (RCTs) that investigated any pharmacological or non‐pharmacological intervention in adults with PBT and fatigue, where fatigue was the primary outcome measure. We restricted inclusion specifically to studies that enrolled only participants with clinically significant levels of fatigue.
Data collection and analysis
Three review authors (JD, SYK, DC) independently evaluated search results, extracted data from selected studies and carried out a bias risk assessment. We extracted data on fatigue, cognition, mood, quality of life and adverse events outcomes.
Main results
We identified nine studies. We excluded eight of these as they did not restrict participation to people with high fatigue. The single eligible trial investigated the use of modafinil compared to placebo. Although this study found a significant improvement over time in the primary outcome of fatigue, the improvement occurred after both modafinil and placebo with no significant difference in response between the two groups. The included trial did not reach its planned recruitment target and therefore may not, in practice, have been adequately powered to detect a difference. The trial was at a low risk of bias across most areas. There was an unclear risk of bias related to the use of mean imputation because the investigators did not analyse the impact of imputation on the results.
Authors' conclusions
There was insufficient evidence to draw reliable and generalisable conclusions regarding potential effectiveness or harm of any pharmacological or non‐pharmacological treatments for fatigue in people with PBT. More research is needed on how best to treat people with brain tumours with high fatigue.
Plain language summary
Interventions for the management of fatigue in adults with a primary brain tumour
Background
A primary brain tumour (PBT) is a cancer that began in the brain rather than spread from other parts of the body. Fatigue (tiredness) is common in people with a PBT. This may be due to the tumour, its treatment or to the use of other medicines such as antiepileptic drugs (which are used to treat epilepsy seizures). It may also occur with other symptoms such as sleep disturbance, thinking problems and emotional distress. Treatments to help manage fatigue may improve a person's quality of life, their ability to tolerate cancer treatment (which themselves are associated with fatigue), and their ability to carry out social and day‐to‐day activities.
Study characteristics
In March 2016, we searched five medical databases. We found one clinical trial that was eligible for inclusion; the trial investigated the medicine modafinil in 37 adults with PBT and high levels of fatigue. People in the study received six weeks of modafinil followed by a one‐week washout period and six further weeks of placebo, or vice versa. The washout period aims to allow time for any effects of the first treatment to wear off before the new one gets started.
Key findings
The one included trial found no evidence of a difference between modafinil and placebo in treating fatigue. It is possible that this could be due to the trial not reaching its planned number of participants. Several other studies investigated the management of fatigue too, but in these studies high fatigue was not essential for participation. We do not currently know whether any treatments are effective in the management of people with PBT and high fatigue.
Quality of the evidence
With only one included trial, the overall quality of evidence was low. More high‐quality studies are needed that enrol adults with BPT and high fatigue.
Summary of findings
Summary of findings for the main comparison. Modafinil compared with placebo for fatigue in people with a primary brain tumour.
| Modafinil compared with placebo for fatigue in people with a primary brain tumour | ||||||
|
Patient or population: people with a primary brain tumour Settings: hospital, outpatient Intervention: modafinil Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Modafinil | |||||
|
Fatigue ‐ concentration problems Sub‐scale from Checklist Individual Strength Scale from: 0 to 35 (follow‐up: 6 weeks) |
The mean concentration problem score ranged across control groups from 15.91 to 23.91 points | The mean concentration problem score in the intervention groups was 1.06 lower (‐3.18 to 1.06 lower) |
‐ | 37 (1) | ⊕⊕⊝⊝ low | Higher scores indicate a high level of concentration problems |
|
Fatigue ‐ reduced motivation Sub‐scale from Checklist Individual Strength Scale from: 0 to 28 (follow‐up: 6 weeks) |
The mean reduced motivation score ranged across control groups from 10.22 to 19.48 points | The mean reduced motivation score in the intervention groups was 0.48 lower (‐2.93 to 1.97 lower) |
‐ | 37 (1) | ⊕⊕⊝⊝ low | Higher scores indicate lower motivation |
|
Fatigue ‐ reduced activity Sub‐scale from Checklist Individual Strength Scale from: 0 to 21 (follow‐up: 6 weeks) |
The mean reduced activity score ranged across control groups from 3.84 to 21.34 points | The mean reduced activity score in the intervention groups was 1.01 lower (‐5.64 to 3.62 lower) |
‐ | 37 (1) | ⊕⊕⊝⊝ low | Higher scores indicate lower activity |
|
Fatigue ‐ fatigue severity Sub‐scale from Checklist Individual Strength Scale from: 0 to 56 |
The mean fatigue severity score ranged across control groups from 34.06 to 36.22 points | The mean fatigue severity score in the intervention groups was 0.22 lower (‐0.79 to 0.35 lower) |
‐ | 37 (1) | ⊕⊕⊝⊝ low | High scores indicate a high level of fatigue |
|
Adverse events (follow‐up: 6 weeks) |
Low‐risk population | RR 2.79, 95% CI 0.59 to 13.16 | 37 (1) | ⊕⊕⊝⊝ low | ‐ | |
| ‐ | 30 per 100 (1 to 180) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Fatigue is one of the most common symptoms experienced by people with cancer. The reported prevalence rates for cancer‐related fatigue in the clinical trial setting is in the range of 70% to 80% (Lawrence 2004; Lovely 1999). Cancer‐related fatigue is "a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer that is not proportional to recent activity and interferes with usual functioning" (NCCN 2014). Fatigue is also a common adverse effect of cancer treatment (Roscoe 2002), occurs across various cancer types (Stone 2000), and persists in disease‐free survivors (Servaes 2007). As the scientific knowledge about cancer‐related fatigue expands, there is increasing recognition on the importance of its effective management (Goedendorp 2009).
Prevalence of fatigue in primary brain tumours
Prevalence estimates suggest that as in other cancer populations, fatigue is an extremely common problem in people with a primary brain tumour (PBT). In one study, 96% of people with high‐grade glioma reported moderate or severe fatigue (Fox 2007). Studies enrolling mixed high‐grade and low‐grade tumour populations estimated that up to 42% of people with PBT had fatigue (Pelletier 2002). Fatigue remains troublesome throughout the course of survivorship, from the 12 months following PBT diagnosis (Molassiotis 2010), to more than eight years after diagnosis (Struik 2009). Fatigue in PBT has been studied both as a primary outcome (Armstrong 2010; Lovely 1999), and as a secondary outcome related to symptoms such as depression (Rooney 2011), poor quality of life (Kvale 2009), and sleep‐wake disturbances (Miaskowski 2011).
Associated clinical variables
Fatigue in the setting of PBT has multiple potential causes including primary treatments of the tumour, secondary symptomatic treatments, and the physical and emotional consequences of the diagnosis (Armstrong 2012). Up to 80% of people undertaking cranial radiotherapy report fatigue (Lovely 1999). Although it is rarely the only possible cause, radiotherapy in particular may exacerbate fatigue by endocrine (hormone) dysfunction when the irradiated area encroaches upon the hypothalamus or pituitary gland. The hypothalamic‐pituitary‐adrenal axis feedback system is responsible for controlling the secretion of many hormones that regulate many body processes, including sleep (Arlt 1997; Taphoorn 1995).
Fatigue is also a recognised adverse effect of many medications that may be taken by people with PBT, including chemotherapy, anticonvulsant drugs (Lu 2009; Maschio 2008; Struik 2009), and corticosteroids (Drappatz 2007; Hinds 2007). Fatigue is further associated with sleep disturbance, cognitive complaints, depression and anxiety (Armstrong 2010; Fox 2007; Pelletier 2002), and this cluster of symptoms may significantly influence people's quality of life (Fox 2007). Symptom clustering can make the presence of fatigue difficult to distinguish from other symptoms such as depression (Rooney 2011).
The relationship between histological tumour grade and fatigue remains unclear. Some authors find fatigue to be more common in high‐grade than in low‐grade tumours (Salo 2002), whereas other authors do not (Armstrong 2010; Pelletier 2002). Regardless, the wide range of possible causes suggest that fatigue is best investigated as a multifactorial symptom alongside these other associated issues (Armstrong 2010).
Methods of measuring fatigue
Many tools have been developed to measure fatigue in people with cancer (Jean‐Pierre 2007); each instrument relies on subjective patient report. The Brief Fatigue Inventory (Mendoza 1999) has been used in several studies including brain tumour correlation studies (Kim 2012), and clinical trials (Gehring 2012). Other measurement tools validated for use in cancer include the Functional Assessment of Cancer Therapy ‐ Fatigue (Yellen 1997), the Cancer‐Related Fatigue Distress Scale (Holley 2000), the Fatigue Assessment Questionnaire (Glaus 1998), the Revised Piper Fatigue Scale (Piper 1998), and the Multidimensional Fatigue Symptom Inventory (Stein 2004). Several general and brain tumour‐specific quality of life measures also assess fatigue, such as the Functional Assessment of Cancer Therapy ‐ Brain (FACT‐Br) (Cella 1993), the MD Anderson Symptom Inventory Brain Tumour Module (MDASI‐BT) (Armstrong 2006), the European Organization of Research and Treatment Quality of Life Questionnaire‐C30 (EORTC QLQ‐C30) (Ringdal 1993), and the World Health Organization Quality of Life assessment (WHOQOL) (WHOQOL Group 1995). With many different tools available caution is needed when synthesising data in a systematic review or meta‐analysis.
Description of the intervention
In this review, we included pharmacological and non‐pharmacological interventions for fatigue in PBT. We defined pharmacological interventions as a drug, given by any route at any therapeutic dose, with the primary intention of treating fatigue. Such drugs could include psychostimulants and antidepressants. We defined non‐pharmacological interventions and general strategies as any psychological or behavioural treatment with the primary aim of improving fatigue in PBT. These interventions could, for example, include physical activity, cognitive or behavioural therapies and psychosocial interventions.
How the intervention might work
Studies have started to explore interventions aimed at improving and alleviating symptoms of fatigue (e.g. Cramp 2012; Goedendorp 2009; Minton 2013).
Pharmacological interventions
Pharmacological treatments might reduce fatigue by acting on critical neurotransmitter pathways. For example, the central nervous system stimulant, methylphenidate, could enhance neural signal processing by increasing concentrations of dopamine and noradrenaline (norepinephrine) (Volkow 2002). Similarly, the central nervous system stimulant, modafinil, may enhance the effect of dopamine, associated with wakefulness and motivation (Young 2010).
Non‐pharmacological interventions
Psychological interventions may improve fatigue by introducing and reinforcing adaptive coping strategies (Armstrong 2012). This approach can be effective through the use of cognitive therapy, which identifies negative or maladaptive thoughts/beliefs, challenges them and replaces them with more helpful and realistic alternatives (Beck 1979).
These strategies could be used alongside behavioural interventions such as exercise. Exercise may improve fatigue in people with PBT by increasing mental and physical stamina. A reduction in fatigue could be achieved through a balance between activity and rest (Winningham 1992). Excessive rest could promote muscle wasting and decreasing cardiorespiratory fitness, adding to the perception of fatigue (Dimeo 2001). By increasing functional capacity, exercise could reduce fatigue (NCCN 2014), while alleviating anxiety and improving mood (Dimeo 2001).
Why it is important to do this review
Fatigue is consistently the single most frequently reported symptom in studies of people with PBT. Therefore, there is a pressing need to search for trials in this area systematically to generate a high‐quality review of interventions for fatigue in people with PBT. With survival times for low‐grade PBT typically measured in years, and survival times for certain subgroups of people with high‐grade PBT gradually increasing, there is great potential benefit in establishing which interventions are effective for fatigue. Effective interventions could improve quality of life, yet the multifactorial nature of fatigue (potentially including neuroendocrine, neuroimmune and psychosocial causes) makes it a symptom that can be particularly difficult to treat (Bowe 2012).
A clear synthesis of the evidence for the effectiveness of managing fatigue in PBT is currently lacking. This review will answer a clinically useful research question: what are the effective interventions for managing fatigue in adults with a PBT?
Objectives
To assess the effectiveness and safety of pharmacological and non‐pharmacological interventions for adults with PBT and high levels of fatigue.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any intervention for the management of fatigue in adults with PBT, in which fatigue was (one of) the primary or secondary therapeutic outcome(s). Due to the prediction that there may currently be few RCTs that satisfy the inclusion criteria, we planned to include a narrative description of relevant excluded RCTs in the Excluded studies section. This was intended to provide valuable information about interventions that may warrant further investigation.
Types of participants
We included studies that evaluated the effect of interventions on adults (aged 18 years or older) with high self reported fatigue (defined by a pre‐established cut‐off using a questionnaire, validated measure, or presence/absence report), and with a histological diagnosis of PBT at any stage in their illness. Following discussion, we excluded studies that recruited non‐fatigued participants. We reasoned that the clinically relevant question of how to treat high fatigue required a strict focus on studies that enrolled people with high fatigue.
Types of interventions
Pharmacological interventions
For pharmacological interventions, we aimed to investigate the effectiveness of any drug, given by any route and at any therapeutic dose, with the intention of treating fatigue in PBT. For ethical reasons, RCTs of psychoactive drugs may not necessarily include a placebo arm. In order to increase the relevance of the review, we included studies without a placebo arm, provided that the study randomly allocated participants to a control group (e.g. treatment as usual, another active drug or allocation to a waiting list).
Non‐pharmacological interventions
For psychological interventions, we aimed to study any cognitive treatment given with the aim of improving fatigue in PBT. For behavioural interventions, we aimed to investigate the effectiveness of any behavioural or social treatment given for the improvement of fatigue in PBT; this may have included exercise and energy management techniques. We included RCTs in which the control group was allocated to treatment as usual or to a waiting list.
Types of outcome measures
Primary outcomes
Fatigue at study endpoint.
Due to potential differences in effectiveness endpoints between the different interventions, we aimed to analyse both short‐term and long‐term effects of these interventions, where the data were available.
High fatigue may be summarised categorically as 'present' or 'absent' (e.g. in response to a clinical interview), or else quantified ordinally on a rating scale assessing fatigue using cut‐offs defined by the measure used. Such rating scales can be specific to fatigue, or may assess fatigue as part of a wider symptom screen (e.g. as part of quality of life). We included studies in which fatigue was self reported using any validated method. Due to the subjective nature of fatigue, we did not include studies using clinician‐reported or relative‐ or carer‐reported measures, because these may not be a true reflection of the person's symptoms.
If fatigue was measured by a rating scale, we aimed to quantify its improvement with respect to the recommended scale threshold for 'caseness'. If possible, we also aimed to record the total number of people reaching 'non‐fatigued' status.
Secondary outcomes
General functioning, including quality of life measurements (e.g. Health Related Quality of Life questionnaire), and depression (e.g. Hospital Anxiety and Depression Scale) and cognitive outcomes (e.g. Addenbrooke's Cognitive Examination ‐ Revised) according to validated measures.
Overall survival (OS) and progression‐free survival (PFS).
Adverse events as described by Katz 2012. Adverse event occurrence: clinical adverse events; any serious adverse event as defined by any medical occurrence in any participant that resulted in a dose reduction or treatment discontinuation, which did not necessarily have causal relationship with the treatment. The International Conference on Harmonisation Guidelines defines serious adverse events as any event that may jeopardise the person or require an intervention to prevent it (ICH‐GCP 1997). This includes any important medical event that: was life‐threatening, led to death, resulted in significant or persistent disability or congenital anomaly/birth defect, or required inpatient hospitalisation or prolongation of existing hospitalisation, which may have jeopardised the person or required intervention to prevent it.
If possible and appropriate, we aimed to combine outcomes in a meta‐analysis. The secondary outcomes were not criteria for eligibility for this review, but were outcomes that we noted and reviewed.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2016), MEDLINE (1950 to March 2016), EMBASE (1980 to March 2016), PsycINFO (1974 to March 2016) and CINAHL (1982 to March 2016), (Appendix 1, Appendix 2; Appendix 3; Appendix 4). We did not apply language or date restrictions in any of the searches.
Searching other resources
Unpublished and grey literature
We searched online databases of registered clinical trials to identify ongoing trials. We also approached the major co‐operative trial groups active in this area.
Handsearching
We handsearched the reference lists of included studies and previous systematic reviews. We handsearched the latest journal and conference materials in 2014 and 2015 from the following sources:
Annual Meeting of the European Society of Medical Oncology (ESMO);
Annual meeting of the European Association of Neuro‐Oncology (EANO);
Annual meeting of the World Federation of Neuro‐Oncology (WFNO);
Annual Meeting of the American Society of Clinical Oncology (ASCO);
Annual Meeting of the Society for Neuro‐Oncology (SNO);
Annual Meeting of the Society for Behavioral Medicine (SBM);
Annual Meeting of the American Psychosocial Oncology Society (APOS);
Annual Meeting of the International Psycho‐Oncology Society (IPOS);
Annual Meeting of the Multinational Association of Supportive Care in Cancer (MASCC).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searches to the reference management database EndNote. We removed duplicates and three review authors (JD, SYK, DC) independently examined the remaining references. The review authors were not blinded to the authors or affiliations of the studies. We excluded those studies that clearly did not meet the inclusion criteria and we obtained copies of the full text of potentially relevant references. Three review authors (JD, SYK, DC) independently assessed the eligibility of retrieved papers. We resolved disagreements by discussion and documented the reasons for exclusion.
Data extraction and management
Data extraction
For included trials, we extracted data as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Three review authors (JD, SYK, DC) independently extracted data onto a data extraction form specially designed for the review.
We extracted data on the following:
article details (author, year of publication, journal citation, country and language);
intervention (characteristics, e.g. drug name, dose and duration);
study design and methodology (including inclusion and exclusion criteria, assignment process and timing of measurements);
population demographics (e.g. age, gender and marital status) and total number involved;
details of participants' health status (including tumour pathology and treatment details).
dichotomous and continuous outcome measures (fatigue, cognitive functioning, quality of life, depression and adverse events);
risk of bias.
Where possible, we extracted all data relevant to an intention‐to‐treat analysis, in which participants are analysed in groups to which they are assigned.
Data management
We collated and entered data into Review Manager 5 (RevMan 2014).
Continuous data
For continuous outcomes (e.g. fatigue scales, cognitive tests and measures, depression measures, quality of life measures), we expressed the treatment effect as a mean difference (MD) with 95% confidence interval (CI). We extracted post‐intervention data to calculate the MD, the final value and standard deviation (SD) of the outcome of interest, and the number of participants assessed in each treatment arm at the end of follow‐up. If more than one trial was eligible, and trials measured outcomes on the same scale, we aimed to express treatment effect as an MD, with 95% CIs. If trials measured outcomes on different scales, we aimed to express treatment effect as standardised mean differences (SMDs) between treatment arms, with 95% CIs.
Dichotomous data
For dichotomous outcomes (e.g. high or low fatigue), we extracted the number of participants in each treatment arm who experienced the outcome of interest, at baseline and at study endpoint. We aimed to dichotomise fatigue using validated thresholds. We noted the time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). This included assessment of:
selection bias: random sequence generation and allocation concealment;
performance bias: blinding of participants and personnel (participants and treatment providers);
detection bias: blinding of outcome assessment;
performance bias: participants received similar care out with the intervention they received;
attrition bias: incomplete outcome data;
reporting bias: selective reporting of outcomes
other possible sources of bias.
See Appendix 5 for full description of each risk of bias area. Three review authors (JD, SYK, DC) applied the 'Risk of bias' tool independently and resolved differences by discussion. We summarised results in a 'Risk of bias' summary table. We aimed to interpret the results of any meta‐analyses in light of the findings with respect to risk of bias. We judged and reported all bias criteria in terms of 'low', 'high' or 'unclear' risk of bias. We classified criteria as having an 'unclear' risk of bias where insufficient information was provided, or when there was uncertainty over the potential for bias. We contacted authors to clarify uncertainties, if possible. It was noted that blinding may not have been possible for all treatment comparisons, particularly with respect to any non‐pharmacological interventions such as exercise.
Measures of treatment effect
For continuous data, we used MDs or SMDs as appropriate. For dichotomous data, we calculated the risk ratio (RR).
Dealing with missing data
We did not impute missing outcome data for the primary outcome. For the primary outcome, if data were missing, or only imputed data were reported, we contacted trial authors to request data on the outcomes among participants who were assessed.
We included details of missing data in the narrative summary and 'Risk of bias' table, and stated whether authors examined the extent to which the missing data could have altered the results of the review.
Assessment of heterogeneity
Assessment of heterogeneity was not possible because only one trial was eligible for inclusion in the review.
We planned to assess heterogeneity between studies by visual inspection of forest plots (including the presence of outliers and a poor overlap of CIs), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). We planned to investigate and report heterogeneity according to Higgins 2011.
Assessment of reporting biases
Three review authors (JD, SYK, DC) reviewed and recorded reporting biases.
We aimed to examine funnel plots to assess the potential for small‐study effects, such as publication bias, if the meta‐analysis included more than 10 trials.
Data synthesis
We planned to pool data for meta‐analysis using Review Manager 5 if studies were comparable with respect to participants, interventions and outcomes (RevMan 2014). We intended to combine studies at the level of the intervention itself (e.g. psychostimulant, cognitive behavioural therapy, exercise) rather than broad categories (e.g. pharmacological, psychological, behavioural). Had a meta‐analysis been possible, we planned to carry it out as follows.
We planned to pool the MDs between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale and at the same primary study endpoint, otherwise we planned to pool SMDs.
For dichotomous data, we intended to use risk ratios (RRs) and 95% CIs.
We intended to use random‐effects models with inverse variance weighting for all meta‐analyses, with 95% CIs (DerSimonian 1986).
For dichotomous data for adverse events, we planned to pool RRs.
We intended to note the time points at which outcomes were collected and reported.
However, data synthesis was not possible as only one trial was eligible for inclusion in the review.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses comparing changes in scale score studies using identical scales, where appropriate. We also intended to perform subgroup analyses according to World Health Organization (WHO) tumour grade (low grade/high grade) and interventions delivered only during treatment/only during follow‐up.
However, subgroup analysis and investigation of heterogeneity was not possible because only one trial was eligible for inclusion in the review.
Sensitivity analysis
We planned to involve all review authors in determining whether sensitivity analysis would be required, under the guidance of Higgins 2011.
We intended to consider the following factors as possible sources of heterogeneity across studies.
Differing study quality (high or low levels of risk of bias).
Different classes of drugs.
Dosage or scheduling differences.
We planned to identify additional possible types of sensitivity analyses during the conduct of the review.
However, sensitivity analysis was not carried out as only one trial was eligible for inclusion in the review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies tables.
Results of the search
Figure 1 shows details of the search.
1.
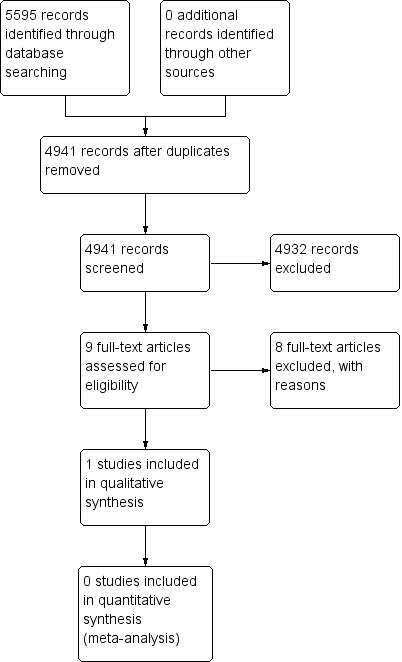
Study flow diagram.
We found 4941 citations when searching electronic databases and following de‐duplication of the results. The results were narrowed to nine articles upon screening of the titles and abstracts. One trial was eligible for inclusion in the review (Boele 2013). Eight studies did not meet our inclusion criteria for analysis due to the lack of high self reported fatigue as a necessary inclusion criterion (Butler 2007; Gehring 2009; Gehring 2012; Kaleita 2006; Lee 2014; Locke 2008; Shaw 2006; Shaw 2015). We identified no additional studies when searching conference proceedings and the reference list of the single included trial. We identified no additional studies when contacting experts in the field. There was one ongoing trial (Umphrey 2013).
Included studies
For detailed information see Characteristics of included studies table.
We found one eligible trial. Boele 2013 investigated the use of modafinil in treating fatigue in people with PBT compared with a placebo intervention.
Participant demographics
The study recruited 37 of the estimated 64 required participants from three neuro‐oncology centres in The Netherlands. Their mean age was 48.16 years (SD 12.02). Participants had a meningioma (32.4%), low‐grade glioma (37.8%) or high‐grade glioma (29.7%), with the majority having had surgery (94.6%), without further radiotherapy (56.8%) or chemotherapy (78.4%). There were more women (62.2%) than men (37.8%). All participants were required to have experienced high fatigue, determined using a cut‐off above 27 on the Checklist Individual Strength (CIS). The authors obtained ethical approval and registered the trial with a clinical trials database. All participants gave informed consent. The study recorded adverse events.
Intervention characteristics
Modafinil (2‐benzhydrylsulfinylethanamide) is a wakefulness‐promoting drug that targets fatigue, cognitive functioning and mood. The study used a dose escalation, washout and cross‐over method for both arms and participants received either modafinil then placebo or placebo then modafinil. It included a dose reduction and withdrawal technique if participants experienced adverse events.
Primary and secondary outcomes
The study assessed the primary outcome measure of fatigue using the CIS. It included secondary subjective measures of depression, health‐related quality of life and everyday cognitive functioning. Cognitive functioning was assessed using a neuropsychological test battery to assess verbal memory, working memory, attention, executive function and psychomotor speed. Assessments were carried out at baseline, six weeks and 12 weeks.
Data collection
The study used a cross‐over trial design, therefore they collected data for each participant on completion of the modafinil and placebo treatment schedules. Of 155 eligible participants, 39 participants met the inclusion criteria and agreed to participate. Two participants dropped out prior to randomisation leaving 37 participants in the trial, of whom 25 completed both treatment schedules and had all outcomes measured. Imputation was carried out for missing values for those who completed questionnaires and neuropsychological assessments.
Statistical analyses
The trial used a within‐participants design to determine differences between modafinil and placebo test scores. It used a Wilcoxon signed‐rank tests as no data were normally distributed. No corrections were carried out to account for multiple statistical testing.
Excluded studies
For detailed information see Characteristics of excluded studies table.
We found eight studies that included fatigue as a primary or secondary outcome measure, but we excluded them as high fatigue was not a necessary inclusion criterion for participation. Three studies investigated an intervention in people with brain tumours undergoing radiotherapy (Butler 2007; Lee 2014; Shaw 2015). Five studies evaluated an intervention in people with brain tumours not on active treatment (Gehring 2009; Gehring 2012; Kaleita 2006; Locke 2008; Shaw 2006).
Studies of people undergoing radiotherapy
Butler and co‐authors evaluated the use of d‐threo‐methylphenidate hydrochloride in a double‐blind randomised placebo‐controlled clinical trial in people with primary metastatic brain tumours receiving radiotherapy. Participation was not limited to people with fatigue. The study enrolled 68 participants. Using the Functional Assessment of Cancer Therapy ‐ Fatigue sub scale (FACT‐F), there were no differences between groups in measures of fatigue eight weeks after the completion of radiotherapy (P value = 0.64) (Butler 2007).
Lee and co‐authors presented an update of their randomised, placebo‐controlled pilot trial of armodafinil at the 2014 ASCO annual conference, which included 77 people undergoing radiotherapy. Participation was not limited to people with fatigue. This ongoing study included measures of fatigue, mood and quality of life. There were significant improvements in fatigue at 42 days using the Brief Fatigue Inventory (Wilcoxon P value = 0.008) (Lee 2014).
Shaw and co‐authors conducted a double‐blind placebo‐controlled study of armodafinil on fatigue in people undergoing cranial irradiation. Fatigue was not a necessary inclusion criterion. The study enrolled 54 participants, and measured fatigue and day‐time sleepiness. There were no significant differences in outcome measures between groups at the end of radiotherapy or at a four‐week follow‐up compared to baseline. However, in a post‐hoc analysis, there was an improvement in fatigue in participants with higher baseline fatigue, as measured by the FACIT‐F (Shaw 2015).
Studies of people not on active tumour treatment
Gehring and co‐authors investigated the use of a cognitive rehabilitation programme in people with glioma in a randomised wait‐list controlled trial. Participation was restricted to people with subjective and objective cognitive deficits, rather than fatigue. The study enrolled 140 adults, and measured cognition, fatigue, quality of life and community integration. Using the Multidimensional Fatigue Inventory, people in the intervention arm reported lower mental fatigue at six months (P value = 0.026), compared to baseline, but not activity (P value = 0.82) or motivation (P value = 0.063) (Gehring 2009).
Gehring and co‐authors enrolled 24 people with brain tumours in an open‐label randomised pilot trial comparing methylphenidate and modafinil. Participation was not limited to people with fatigue. The primary outcome measure was cognitive function. Other outcome measures included fatigue, sleep disturbance, mood and quality of life. In a post‐hoc analysis that combined the treatment groups, there was a beneficial effect on fatigue at four weeks (P value = 0.04) compared to baseline, as measured using the Brief Fatigue Inventory (Gehring 2012).
Kaleita and co‐authors conducted a double‐blind randomised dose‐controlled trial of modafinil on cognition, mood and fatigue in people with brain tumours. The study did not restrict participation to people with fatigue. There were 30 participants in the study. There were improvements in fatigue, using the Fatigue Severity Scale, at eight (P value = <0.0001) and 12 weeks (P value = 0.0003) after modafinil initiation compared to baseline (Kaleita 2006).
Locke and co‐authors reported the feasibility of a cognitive rehabilitation and problem‐solving programme in 19 people with PBT. Participation was not restricted to people with fatigue. The study included measures of fatigue, cognition, mood and quality of life. The study used the Brief Fatigue Inventory to assess fatigue. There were no statistical analyses, but most participants in both groups had only mild fatigue (Locke 2008).
Shaw and co‐authors evaluated 24 people with a brain tumour enrolled to a single‐arm open‐label study of donepezil. Participation was not limited to people with fatigue. The study recorded cognition, mood, fatigue and quality of life. There was an improvement in fatigue at 24 weeks using the Profile of Mood States Fatigue Subscale (P value = 0.03) (Shaw 2006).
Risk of bias in included studies
Three review authors (JD, SYK, DC) independently assessed the included trial using the Cochrane 'Risk of bias' tool (Higgins 2011). Where risk of bias was unclear, we contacted the author for clarification. Following discussion, we reached agreement on 'Risk of bias' scores. Figure 2 shows the risk of bias summary.
2.
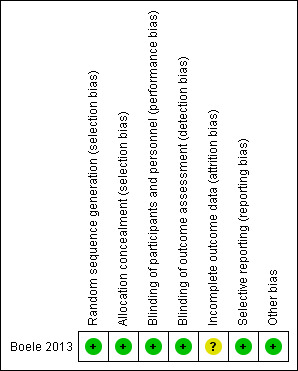
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The trial was at a low risk of bias, using a pharmacy randomisation system to allocate participants to each treatment arm. This was confirmed via correspondence to be through the use of a computer randomisation system.
Blinding
The trial was at a low risk of bias. Participants, treating physicians and researchers were blind to treatment allocation.
Incomplete outcome data
The trial was at an unclear risk of bias. The study reported that 12 participants dropped out: a similar number of participants dropped out between time‐point 1 and 2 (Modafinil arm; n = 4, placebo arm; n = 3); more participants dropped out of the placebo arm (n = 4) than the modafinil arm (n = 1) between time‐point 2 and 3. It was unclear why participants dropped out of each group, therefore we contacted the author for correspondence to request clarification. Five participants dropped out of the trial due to adverse events while receiving modafinil; three participants received modafinil first, two participants received modafinil second. Two participants dropped out of the trial due to adverse events while receiving placebo. The study used mean imputation where missing values were present in attempted questionnaires or neuropsychological assessments. They did not carry out an analysis to determine whether imputation or missing data could have altered the results of the study.
Selective reporting
The trial was at a low risk of bias; all outcomes appear to have been reported.
Other potential sources of bias
We did not identify any additional sources of bias.
Effects of interventions
See: Table 1
We found one eligible trial that investigated the use of modafinil in treating fatigue in people with PBT compared with a placebo intervention (Boele 2013).
Primary outcome
Fatigue at study endpoint
There was no significant difference in fatigue measures between modafinil and placebo score for concentration problems (MD ‐1.06, 95% CI ‐3.18 to 1.06), reduced motivation (MD ‐0.48, 95% CI ‐2.93 to 1.97), reduced activity (MD ‐1.01, 95% CI ‐5.64 to 3.62) or fatigue severity (MD ‐0.22, 95% CI ‐0.79 to 0.35) (Analysis 1.1).
1.1. Analysis.
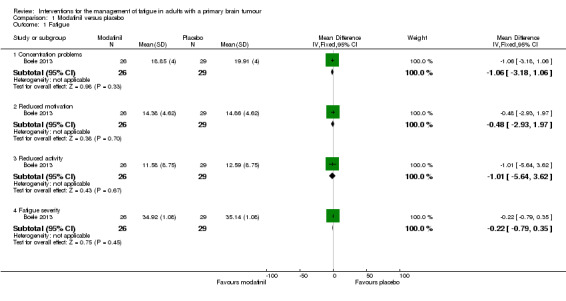
Comparison 1 Modafinil versus placebo, Outcome 1 Fatigue.
Secondary outcomes
Cognitive functioning
A significant difference was found between modafinil and placebo scores in attentional functioning (MD ‐0.03, 95% CI ‐0.05 to ‐0.01). There were no significant differences in objective cognitive functioning (verbal memory, MD 0.26, 95% CI ‐0.05 to 0.57; working memory, MD 0.07, 95% CI ‐0.04 to 0.18; information processing, MD 0.17, 95% CI ‐1.19 to 1.53; executive function, MD 0.14, 95% CI ‐9.33 to 9.61; psychomotor speed, MD 0.10, 95% CI ‐0.26 to 0.46) (Analysis 1.2), subjective cognitive functioning (MD 1.62, 95% CI ‐0.74 to 3.98) (Analysis 1.3), depression (MD 0.19, 95% CI ‐1.33 to 1.71) (Analysis 1.4) or quality of life (physical, MD 1.34, 95% CI ‐20.11 to 22.79; mental, MD ‐1.40, 95% CI ‐4.84 to 2.04) (Analysis 1.5).
1.2. Analysis.
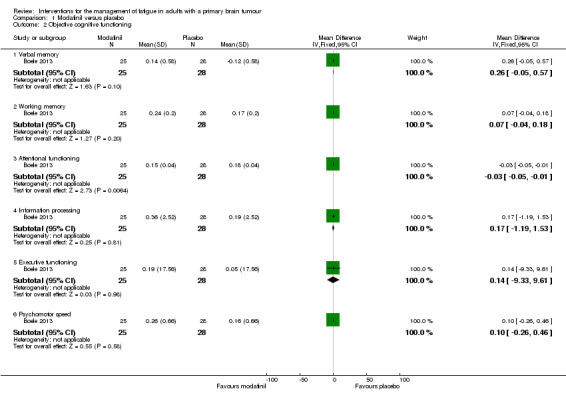
Comparison 1 Modafinil versus placebo, Outcome 2 Objective cognitive functioning.
1.3. Analysis.
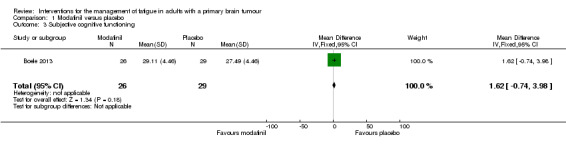
Comparison 1 Modafinil versus placebo, Outcome 3 Subjective cognitive functioning.
1.4. Analysis.
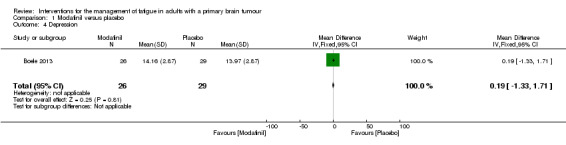
Comparison 1 Modafinil versus placebo, Outcome 4 Depression.
1.5. Analysis.
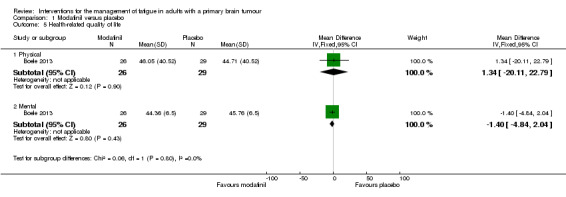
Comparison 1 Modafinil versus placebo, Outcome 5 Health‐related quality of life.
Adverse events
Reported adverse events included tingling sensations, depressive feelings or behaviours, nervousness, dizziness, vertigo, headaches, loss of appetite and seizures. Five participants dropped out of the trial due to adverse events while receiving modafinil; two participants dropped out of the trial due to adverse events while receiving placebo. There was no difference in adverse events reported between groups (RR 2.79, 95% CI 0.59 to 13.16) (Analysis 1.6).
1.6. Analysis.
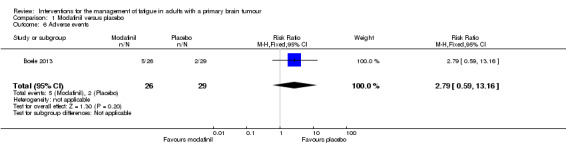
Comparison 1 Modafinil versus placebo, Outcome 6 Adverse events.
Discussion
The aim of this review was to determine the effectiveness of interventions to treat high fatigue in people with PBT. We included one randomised controlled cross‐over trial comparing the effect of modafinil to placebo (Boele 2013).
Summary of main results
Boele 2013 recruited 37 participants and used a cross‐over design to compare modafinil and placebo across three centres in The Netherlands. The washout period between treatments was one week. Since the half‐life of modafinil is 10 to 12 hours, the washout period was likely adequate. There was no significant difference in fatigue between modafinil and placebo groups. This finding was difficult to interpret because the trial failed to reach its recruitment target and may have lacked power to exclude a false‐negative result. There were improvements in fatigue severity and motivation in both modafinil and placebo conditions compared with baseline.
Overall completeness and applicability of evidence
We could only include one RCT examining the effectiveness of an intervention to treat fatigue in adults with PBT. The trial included people with glioma and meningioma tumours and, therefore, may be representative across these brain tumour types. All participants were fatigued at baseline and, therefore, results could potentially generalise to people with fatigue. However, since this trial had low accrual and high attrition, and restricted follow‐up to 12 weeks, overall the applicability of the evidence was limited.
We excluded eight studies that reported fatigue outcomes, but which enrolled a general population of people with brain tumours rather than restricting eligibility to people who were highly fatigued. We excluded these studies in order that our conclusions could be readily applied to a clinically relevant problem. However, we recognised that the excluded studies contained valuable data. More research is needed into whether the interventions investigated specifically benefit highly fatigued people with PBT, who are the ones most likely to require treatment in clinic.
Quality of the evidence
See Figure 2.
This review summarised the current evidence for the effect of pharmacological and non‐pharmacological interventions for the treatment of fatigue in adults with PBT. There was only one trial eligible for inclusion in the review. The included trial was at a low risk of bias across most areas, with an unclear risk of bias with respect to incomplete outcome data. Low accrual and high attrition limited the generalisability of this trial and taken together, the overall quality of evidence is currently low.
Potential biases in the review process
We searched five databases extensively, which included published studies and the most recent conference proceedings. We also searched the reference list of the included trial. Though we thoroughly handsearched the literature and searched online databases for unpublished and grey literature, and contacted known experts in the field to determine any further unpublished studies that may be eligible, we may have nevertheless failed to identify all eligible studies, specifically those that have not been published.
Agreements and disagreements with other studies or reviews
Authors of two narrative reviews on this topic also highlighted the lack of high‐quality evidence for treatment, noting favourable effectiveness of interventions in the general cancer population (Armstrong 2012; Schiff 2014).
We found one ongoing Phase III double‐blind placebo‐controlled RCT using armodafinil for the treatment of fatigue in people with high‐grade gliomas that aims to include only people with fatigue (Umphrey 2013). This study will hopefully offer more evidence for inclusion in a future update.
Authors' conclusions
Implications for practice.
At present, the effectiveness of any treatment for high fatigue in people with primary brain tumours is unclear. As detailed above only one trial met our pre‐defined inclusion criteria with 37 participants. This limits the conclusions that can be drawn. Other trials enrolling a general population of people with brain tumour suggest a potential benefit of certain treatments, but these data are difficult to generalise to clinical practice and require further study.
In the wider cancer field, one Cochrane review about drug therapy for the management of cancer‐related fatigue estimated the effect of several drugs including psychostimulants, hematopoietic growth factors, antidepressants and progestational steroids. Psychostimulants showed a small but significant improvement in fatigue over placebo (Z = 2.83; P value < 0.01). The conclusions were based on small samples (Minton 2010). The differences in the results from this review may be due to the inclusion of only people with PBT, that only one study met our pre‐defined inclusion criteria and that modafinil was not one of the drugs included in the cancer‐related review. The widely used National Comprehensive Cancer Network (NCCN) guidelines recommend identifying treatable contributory factors including sleep disturbance, anaemia, pain, emotional distress, nutritional deficiencies, poor functional status, medication and co‐morbidities (NCCN 2014). Given the relative lack of solid evidence, if a person with PBT and fatigue starts pharmacological treatment for fatigue, it may be advisable to use close follow‐up to help detect and manage adverse effects.
Implications for research.
Randomised controlled trials are necessary to address the benefits and risks of using pharmacological and non‐pharmacological interventions. These should be appropriately powered. Important research questions may include whether any intervention focusing on decreasing fatigue in people with primary brain tumours:
is effective in treating high fatigue;
has clinically significant effects on depression and cognition;
has clinically significant pharmacokinetic interactions with tumour‐related treatment (antiepileptic drugs, chemotherapy);
has a clinically significant effect on survival.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2016 | Amended | Author contact details updated |
Notes
None.
Acknowledgements
With thanks to the Cochrane Neuro‐Oncology Group for their contribution and continued support and feedback throughout the editorial process.
The review was funded in part by a grant from the European Association of Neuro‐Oncology.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
1 exp Fatigue/ 2 Lethargy/ 3 Asthenia/ 4 (fatigue* or lassitude or weary or weariness or tired or exhausted or exhaustion or lethargy or asthenia or ((lack* or loss*) adj5 energy)).mp. 5 1 or 2 or 3 or 4 6 exp Brain Neoplasms/ 7 exp Glioma/ 8 Meningioma/ 9 (brain adj5 (tumor* or tumour* or carcinoma* or malignan* or neoplas* or cancer*)).mp. 10 (glioma* or astrocytoma* or oligodendroglioma* or ependymoma* or medulloblastoma* or meningioma*).mp. 11 6 or 7 or 8 or 9 or 10 12 5 and 11
Key:
mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier
Appendix 2. EMBASE search strategy
1 exp fatigue/ 2 lethargy/ 3 asthenia/ 4 (fatigue* or lassitude or weary or weariness or tired or exhausted or exhaustion or lethargy or asthenia or ((lack* or loss*) adj5 energy)).mp. 5 1 or 2 or 3 or 4 6 exp brain tumor/ 7 exp glioma/ 8 exp meningioma/ 9 (brain adj5 (tumor* or tumour* or carcinoma* or malignan* or neoplas* or cancer*)).mp. 10 (glioma* or astrocytoma* or oligodendroglioma* or ependymoma* or medulloblastoma* or meningioma*).mp. 11 6 or 7 or 8 or 9 or 10 12 5 and 11
Key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 3. PsycINFO search strategy
1 fatigue/ 2 exp asthenia/ 3 (fatigue* or lassitude or weary or weariness or tired or exhausted or exhaustion or lethargy or asthenia or ((lack* or loss*) adj5 energy)).mp. 4 1 or 2 or 3 5 brain neoplasms/ 6 glioma/ 7 (glioma* or astrocytoma* or oligodendroglioma* or ependymoma* or medulloblastoma* or meningioma*).mp. 8 5 or 6 or 7 9 4 and 8
Key:
mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor: [Fatigue] explode all trees #2 MeSH descriptor: [Lethargy] explode all trees #3 MeSH descriptor: [Asthenia] explode all trees #4 (fatigue* or lassitude or weary or weariness or tired or exhausted or exhaustion or lethargy or asthenia or ((lack* or loss*) near/5 energy)) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Brain Neoplasms] explode all trees #7 MeSH descriptor: [Glioma] explode all trees ˜#8 MeSH descriptor: [Meningioma] this term only #9 (brain near/5 (tumor* or tumour* or carcinoma* or malignan* or neoplas* or cancer*)) #10 (glioma* or astrocytoma* or oligodendroglioma* or ependymoma* or medulloblastoma* or meningioma*) #11 #6 or #7 or #8 or #9 or #10 #12 #5 and #11
Appendix 5. 'Risk of bias' assessment tool
Random sequence generation
Low risk of bias, e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers.
High risk of bias, e.g. participants assigned to treatments on basis of date of birth, clinic identity number or surname, or no attempt to randomise participants.
Unclear risk of bias, e.g. not reported, information not available.
Allocation concealment
Low risk of bias, e.g. where the allocation sequence could not be foretold.
High risk of bias, e.g. allocation sequence could be foretold by patients, investigators or treatment providers.
Unclear risk of bias, e.g. not reported.
Blinding of participants and personnel
Assessment of blinding was restricted to pharmacological interventions, since it would not be possible to blind participants and treatment providers to the non‐pharmacological interventions.
Low risk of bias, if participants and personnel were adequately blinded.
High risk of bias, if participants were not blinded to the intervention that the participant received.
Unclear risk of bias, if this was not reported or unclear.
Blinding of outcomes assessors
Low risk of bias, if outcome assessors were adequately blinded.
High risk of bias, if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias, if this was not reported or unclear.
Performance bias
Low risk of bias, e.g. both groups were followed on similar schedules of neurological examinations and brain imaging.
High risk of bias, e.g. each group was followed according to different schedules.
Unclear risk of bias, e.g. not reported or incomplete reporting of outcome data.
We recorded the proportion of participants whose outcomes were not reported at the end of the study.
Incomplete outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome according to the following.
Low risk of bias, if less than 20% of participants were lost to follow‐up, and reasons for loss to follow‐up were similar in both treatment arms.
High risk of bias, if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms.
Unclear risk of bias, if loss to follow‐up was not reported.
Selective reporting of outcomes
Low risk of bias, e.g. review reported all outcomes specified in the protocol.
High risk of bias, e.g. it was suspected that outcomes were selectively reported.
Unclear risk of bias, e.g. it was unclear whether outcomes were selectively reported.
Other bias
Low risk of bias, if we did not suspect any other source of bias and the trial appeared to be methodologically sound.
High risk of bias, if we suspected that the trial was prone to an additional bias.
Unclear risk of bias, if we were uncertain whether an additional bias may have been present.
Data and analyses
Comparison 1. Modafinil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatigue | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Concentration problems | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐3.18, 1.06] |
| 1.2 Reduced motivation | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐2.93, 1.97] |
| 1.3 Reduced activity | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐5.64, 3.62] |
| 1.4 Fatigue severity | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.79, 0.35] |
| 2 Objective cognitive functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Verbal memory | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.05, 0.57] |
| 2.2 Working memory | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.04, 0.18] |
| 2.3 Attentional functioning | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 2.4 Information processing | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐1.19, 1.53] |
| 2.5 Executive functioning | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐9.33, 9.61] |
| 2.6 Psychomotor speed | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.26, 0.46] |
| 3 Subjective cognitive functioning | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 1.62 [‐0.74, 3.98] |
| 4 Depression | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐1.33, 1.71] |
| 5 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Physical | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 1.34 [‐20.11, 22.79] |
| 5.2 Mental | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.84, 2.04] |
| 6 Adverse events | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.59, 13.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boele 2013.
| Methods | Double‐blind randomised placebo‐controlled trial, parallel arm | |
| Participants |
Inclusion criteria: aged ≥ 18 years; diagnosed with a histologically confirmed glioma or meningioma; no signs of tumour recurrence in the last 6 months; fatigue self reported > 27 on the Checklist Individual Strength Exclusion criteria: history of psychiatric disease or symptoms; expected adverse interactions between prescribed medications and modafinil; unable to communicate in Dutch Number randomised: modafinil: 20; placebo: 17 Follow‐up: 12 weeks Setting: 3 centres in The Netherlands |
|
| Interventions | 2 treatment arms of 6 weeks Arm 1 treatment schedule: Week 1: oral modafinil 200 mg per day taken in divided does (100 mg upon waking, 100 mg at lunch) Week 2‐6: oral modafinil 400 mg per day taken in divided doses (200 mg upon waking, 200 mg at lunch) Week 7: washout period Week 8‐12: matched placebo Arm 2 treatment schedule: Week 1‐6: matched placebo Week 7: washout period Week 8: oral modafinil 200 mg per day taken in divided does (100 mg upon waking, 100 mg at lunch) Week 9‐12: oral modafinil 400 mg per day taken in divided doses (200 mg upon waking, 200 mg at lunch) |
|
| Outcomes | Fatigue (Checklist Individual Strength) Depression (Center for Epidemiologic Studies Depression Scale) Health‐related quality of life (Medical Outcomes Study Short‐Form Health Survey) Subjective cognitive functioning (Medical Outcomes Study subjective cognitive functioning scale) Objective cognitive functioning (Rey Auditory Verbal Learning Test, Memory Comparison Test, Stroop Colour Word Test, Letter Digit Substitution Test, Concept Shifting Test, Categorical Word Fluency Test, Concept Shifting Test) |
|
| Notes | Mean imputation used where missing values were present in questionnaires or neuropsychological assessments No corrections for multiple statistical testing carried out |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A pharmacy randomization system was used to assign participants". Confirmed via correspondence |
| Allocation concealment (selection bias) | Low risk | "A pharmacy randomization system was used to assign participants". Confirmed via correspondence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, treating physicians, and researchers were blind to treatment allocation". Confirmed via correspondence |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, treating physicians, and researchers were blind to treatment allocation". Confirmed via correspondence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how much imputation may have affected the result as sensitivity analysis was not carried out to determine whether missing data altered the results of the review Similar number of participants dropped out between time points 1 and 2 (modafinil arm; n = 4, placebo arm; n = 3), more participants dropped out of placebo arm (n = 4) than modafinil arm (n = 1) between time points 2 and 3. Mean imputation was used where missing values were present in questionnaires or neuropsychological assessments. Details of adverse events per group confirmed through correspondence with the lead author. Five participants dropped out of the trial due to adverse events while receiving modafinil. Details per participant were:
Two participants dropped out of the trial due to adverse events while receiving placebo. Details per participant were:
|
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Butler 2007 | Fatigue was not a necessary inclusion criterion |
| Gehring 2009 | Fatigue was not a necessary inclusion criterion |
| Gehring 2012 | Fatigue was not a necessary inclusion criterion |
| Kaleita 2006 | Fatigue was not a necessary inclusion criterion |
| Lee 2014 | Fatigue was not a necessary inclusion criterion |
| Locke 2008 | Fatigue was not a necessary inclusion criterion |
| Shaw 2006 | Fatigue was not a necessary inclusion criterion. No control group |
| Shaw 2015 | Fatigue was not a necessary inclusion criterion |
Characteristics of ongoing studies [ordered by study ID]
Umphrey 2013.
| Trial name or title | Armodafinil in Reducing Cancer‐Related Fatigue in Patients with High Grade Glioma |
| Methods | Phase III double‐blind placebo‐controlled randomised controlled trial. Participants were randomly assigned to receive one of two doses of armodafinil or placebo for 8 weeks |
| Participants |
Inclusion criteria: aged ≥ 18 years; glioblastoma multiforme, anaplastic astrocytoma, gliosarcoma or anaplastic oligodendroglioma; clinically stable (stable/improved Karnofsky Performance Status compared to the prior month); completed radiotherapy > 21 days and ≤ 24 months prior to enrolment; ≥ 6 score on the worst fatigue question of the Brief Fatigue Inventory; previous surgery (gross total or sub‐total resection) or biopsy; negative serum pregnancy test done ≤ 7 days prior to registration; ability to complete questionnaire(s) by themselves or with assistance, Eastern Cooperative Oncology Group Performance Status 0, 1, 2 or 3; provide informed written consent; willing to return to enrolling institution for follow‐up (during the active monitoring phase of the study); stable dose of corticosteroid ≤ 28 days prior to registration Exclusion criteria: history of hypersensitivity to other psychostimulants; history of steroid psychosis; history of/currently taking medications for attention deficit hyperactivity disorder, severe anxiety disorder, schizophrenia or substance abuse by patient record or self report, or both; currently taking medications to treat fatigue including psychostimulants, antidepressants, acupuncture (antidepressants used to treat items other than fatigue (such as hot flushes or depression) were allowed if the person had been on a stable dose for ≥ 30 days and planned to continue for the duration of the trial); anticipating surgery; laboratory evidence of hypothyroidism with an elevated thyroid‐stimulating hormone concentration in the blood > 5.0 mlU/L; profound anaemia (haemoglobin < 10 g/dL) ≤ 28 days prior to registration; clinical depression per physician discretion; active/history of Tourette's syndrome or tic disorder, glaucoma, intractable epilepsy or uncontrolled seizure disorder; history of myocardial infarction, unstable angina, left ventricular hypertrophy or mitral valve prolapse syndrome; use of strong or moderate inhibitors of cytochrome P450 3A4 ≤ 7 days prior to registration; use of medications or substances that are inducers of cytochrome P450 3A4 ≤ 7 days prior to registration Follow‐up: 8 weeks Setting: 92 centres in the USA |
| Interventions |
Arm 1: armodafinil 150 mg Arm 2: armodafinil 250 mg Arm 3: matched placebo |
| Outcomes | Participant‐reported fatigue (Brief Fatigue Inventory) Patient‐Reported Outcomes Measurement Information System Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events Cognitive functioning (Symbol Digit Modalities Test, Controlled Oral Word Association, Trail Making Test, Functional Assessment of Cancer Therapy ‐ Cognitive Function) Quality of life (linear analogue self assessment) |
| Starting date | 2013 |
| Contact information |
Study Chair: Alyx Umphrey Mayo Clinic Rochester Minnesota MN 55905 USA +1 507 538 7623 |
| Notes | ClinicalTrials.gov Identifier: NCT01781468 Current status: recruiting participants as of November 2015 |
IU: international units.
Differences between protocol and review
For clarification, we changed the following sentence "We include details of missing data in the narrative summary and 'Risk of bias' table, alongside an assessment of the extent to which the missing data could have altered the results of the review" in the protocol, to "We included details of missing data in the narrative summary and 'Risk of bias' table, and stated whether authors examined the extent to which the missing data could have altered the results of the review" in the full review. This was to clarify that we had not planned to carry out any formal analyses but to assess the extent to which the missing data could have altered the results by reviewing any assessments carried out by the included studies.
Contributions of authors
| Contribution | Author |
| Draft the review | All authors |
| Develop and run the search strategy | JD, SYK, and Cochrane staff |
| Obtain copies of trials | JD, SYK, DC |
| Select which trials to include (3 people) | JD, SYK, DC, with advice from all authors as needed |
| Extract data from trials (3 people) | JD, SYK, DC |
| Enter data into Revview Manager 5 | JD, SYK, DC |
| Carry out the analysis | JD, SYK, DC |
| Interpret the analysis | All authors |
| Draft the final review | All authors |
| Update the review | All authors |
Sources of support
Internal sources
No sources of support supplied
External sources
The review was funded in part by a grant from the European Association of Neuro‐Oncology, Italy.
Declarations of interest
Julia Day ‐ none known. Shlomit Yust‐Katz ‐ none known. David Cachia ‐ none known. Jeffrey Wefel ‐ none known. Lior H Katz ‐ none known. Ivo Tremont ‐ none known. Terri Armstrong ‐ none known. Helen Bulbeck ‐ none known. Alasdair G Rooney ‐ none known.
Edited (no change to conclusions)
References
References to studies included in this review
Boele 2013 {published data only}
- Boele FW, Douw L, Groot M, Thuijl HF, Cleijne W, Heimans JJ, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro‐Oncology 2013;15(10):1420‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Butler 2007 {published data only}
- Butler JM, Case LM, Atkins J, Frizzell B, Sanders G, Griffin P, et al. A phase III, double‐blind, placebo‐controlled prospective randomized clinical trial of d‐threo‐methylphenidate HCl in brain tumor patients receiving radiation therapy. International Journal of Radiation Oncology *Biology *Physics 2007;69(5):1496‐501. [DOI] [PubMed] [Google Scholar]
Gehring 2009 {published and unpublished data}
- Gehring K, Sitskoorn MM, Gundy CM, Sikkes SAM, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. Journal of Clinical Oncology 2009;27(22):3712‐22. [DOI] [PubMed] [Google Scholar]
Gehring 2012 {published data only}
- Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, et al. A randomised trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. Journal of Neuro‐Oncology 2012;107(1):165‐74. [DOI: 10.1007/s11060-011-0723-1] [DOI] [PubMed] [Google Scholar]
Kaleita 2006 {published data only}
- Kaleita TA, Wellisch DK, Graham CA, Steh B, Nghiemphu P, Ford JM, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. Journal of Clinical Oncology 2006;24(18S):1503. [Google Scholar]
Lee 2014 {published and unpublished data}
- Lee E, Muzikansky A, Kesari S, Wong E, Fadul C, Reardon DA, et al. A randomized, placebo‐controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro‐Oncology 2014;16(Suppl 5):v206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Locke 2008 {published data only}
- Locke DEC, Cerhan JH, Wu W, Malec JF, Clark MM, Rummans TA, et al. Cognitive rehabilitation and problem‐solving to improve quality of life of patients with primary brain tumors: a pilot study. Journal of Supportive Oncology 2008;6(8):383‐91. [PubMed] [Google Scholar]
Shaw 2006 {published data only}
- Shaw EG, Rosdhal R, D'Agostino RB, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology 2006;24(9):1415‐20. [DOI] [PubMed] [Google Scholar]
Shaw 2015 {published and unpublished data}
- Page BR, Shaw EG, Lu L, Bryant D, Grisell D, Lesser GJ, et al. Phase II double‐blind placebo‐controlled randomized study of armodafinil for brain radiation‐induced fatigue. Neuro‐Oncology 2015;0:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Umphrey 2013 {published data only}
- Armodafinil in Reducing Cancer‐Related Fatigue in Patients with High Grade Glioma. Ongoing study 2013.
Additional references
Arlt 1997
- Arlt W, Hove U, Müller B, Reincke M, Berwiler U, Schwab F, et al. Frequency and frequently overlooked: treatment‐induced endocrine dysfunction in adult long‐term survivors or primary brain tumours. Neurology 1997;49(2):498‐506. [PUBMED: 9270585] [DOI] [PubMed] [Google Scholar]
Armstrong 2006
- Armstrong TS, Mendoza T, Gring I, Coco C, Cohen MZ, Eriksen L, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumour Module (MDASI‐BT). Journal of Neuro‐Oncology 2006;80(1):27‐35. [PUBMED: 16598415] [DOI] [PubMed] [Google Scholar]
Armstrong 2010
- Armstrong TS, Cron SG, Boloanos EV, Gilber MR, Kang DH. Risk factors for fatigue severity in primary brain tumour patients. Cancer 2010;116(11):2707‐15. [DOI: 10.1002/cncr.25018] [DOI] [PubMed] [Google Scholar]
Armstrong 2012
- Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro‐Oncology 2012;14(Suppl 4):iv65‐iv72. [DOI: 10.1093/neuonc/nos210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guildford Press, 1979. [Google Scholar]
Bowe 2012
- Bowe JE. Fatigue, brain, behavior, and immunity: summary of the 2012 Named Series on fatigue. Behavior and Immunity 2012;26(8):1220‐3. [10.1016/j.bbi.2012.08.009] [DOI] [PubMed] [Google Scholar]
Cella 1993
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570‐9. [PUBMED: 8445433 ] [DOI] [PubMed] [Google Scholar]
Cramp 2012
- Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323(7305):157‐62. [DOI: 10.1136/bmj.323.7305.157] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dimeo 2001
- Dimeo FC. Effects of exercise on cancer‐related fatigue. Cancer 2001;92(6 Suppl):1689‐93. [DOI] [PubMed] [Google Scholar]
Drappatz 2007
- Drappatz J, Schiff D, Kesari S, Norden AD, Wen P. Medical management of brain tumour patients. Radiotherapy Oncology 2007;100(1):131‐6. [DOI: 10.1016/j.ncl.2007.07.015] [DOI] [PubMed] [Google Scholar]
Fox 2007
- Fox SW, Lyon D, Ferace E. Symptom clusters in patients with high‐grade glioma. Journal of Nursing Scholarship 2007;39(1):61‐7. [PUBMED: 17393967] [DOI] [PubMed] [Google Scholar]
Glaus 1998
- Glaus A. Fatigue in patients with cancer. Analysis and assessment. Recent Results Cancer Research 1998;I44(I‐XI):1‐172. [PUBMED: 9551500] [PubMed] [Google Scholar]
Goedendorp 2009
- Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006953.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinds 2007
- Hinds PS, Hockenberry MJ, Gattuso JS, Srivastava DK, Tong X, Jones H, et al. Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer 2007;110(10):2321‐30. [PUBMED: 17926333] [DOI] [PubMed] [Google Scholar]
Holley 2000
- Holley SK. Evaluating patient distress from cancer‐related fatigue: an instrument development study. Oncology Nursing Forum 2000;27(9):1425‐31. [PUBMED: 11058974] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. CFR & ICH Guidelines 1997;1(1):19063‐2043. [Google Scholar]
Jean‐Pierre 2007
- Jean‐Pierre P, Figueroa‐Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer‐related fatigue: Implications for clinical diagnosis and treatment. The Oncologist 2007;12(Suppl 1):11‐21. [PUBMED: 17573452] [DOI] [PubMed] [Google Scholar]
Katz 2012
- Katz LH, Goldvaser H, Gafter‐Gvili A, Tur‐Kaspa R. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow‐responder adult patients. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD008516.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012
- Kim BR, Chun MH, Han EY, Kim D‐K. Fatigue assessment and rehabilitation outcomes in patients with brain tumours. Supportive Care in Cancer 2012;20(4):805‐12. [DOI: 10.1007/s00520-011-1153-5] [DOI] [PubMed] [Google Scholar]
Kvale 2009
- Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high‐grade brain tumour patients. Supportive Care in Cancer 2009;17(7):793‐9. [DOI: 10.1007/s00520-008-0551-9] [DOI] [PubMed] [Google Scholar]
Lawrence 2004
- Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute. Monographs 2004;32:40‐50. [DOI] [PubMed] [Google Scholar]
Lovely 1999
- Lovely MP, Miaskowski C, Dodd M. Relationship between fatigue and quality of life in patients with glioblastoma multiformae. Oncology Nursing Forum 1999;26(5):921‐5. [PUBMED: 10382191] [PubMed] [Google Scholar]
Lu 2009
- Lu Y, Yu W, Wang X. Efficacy of topiramate in adult patients with symptomatic epilepsy: an open–label, long‐term, retrospective observation. CNS Drugs 2009;23(4):351‐9. [PUBMED: 19374462] [DOI] [PubMed] [Google Scholar]
Maschio 2008
- Maschio M, Dinapoli L, Zarabla A, Pompili A, Carapella C, Pace A, et al. Outcome and tolerability of topiramate in brain tumour associated epilepsy. Journal of Neuro‐Oncology 2008;86(1):61‐70. [PUBMED: 17598071 ] [DOI] [PubMed] [Google Scholar]
Mendoza 1999
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85(5):1186‐96. [PUBMED: 10091805 ] [DOI] [PubMed] [Google Scholar]
Miaskowski 2011
- Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, et al. Sleep‐wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nursing 2011;34(4):255‐68. [DOI: 10.1097/NCC.0b013e3181f65d9b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minton 2010
- Minton O, Richardson A, Sharpe M, Stone P. Drug therapy for the management of cancer‐related fatigue. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD006704.pub2] [DOI] [PubMed] [Google Scholar]
Minton 2013
- Minton O, Berger A, Barsevick A, Cramp F, Goedendorp M, Mitchell SA, et al. Cancer‐related fatigue and its impact on functioning. Cancer 2013;119(S11):2124‐30. [DOI] [PubMed] [Google Scholar]
Molassiotis 2010
- Molassiotis A, Wilson B, Brunton L, Chaudhary H, Gattamaneni R, McBain C. Symptom experience in patients with primary brain tumours: a longitudinal exploratory study. European Journal of Oncology Nursing 2010;14(5):410‐6. [DOI: 10.1016/j.ejon.2010.03.001] [DOI] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network (NCCN). Cancer‐related fatigue. NCCN Clinical Practice Guidelines in Oncology 2014; Vol. Version 1.2014.
Pelletier 2002
- Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumour patients: the relative contribution of depression, fatigue, emotional distress and existential issues. Journal of Neuro‐Oncology 2002;57(1):41‐9. [PUBMED: 12125966 ] [DOI] [PubMed] [Google Scholar]
Piper 1998
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Sale: psychometric evaluation in women with breast cancer. Oncology Nursing Forum 1998;25(4):677‐87. [PUBMED: 9599351 ] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringdal 1993
- Ringdal GI, Ringdal K. Testing the EORTC Quality of Life Questionnaire on cancer patients with heterogeneous diagnoses. Quality of Life Research 1993;2(2):129‐40. [DOI] [PubMed] [Google Scholar]
Rooney 2011
- Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. Journal of the National Cancer Institute 2011;103(1):61‐76. [DOI: 10.1093/jnci/djq458] [DOI] [PubMed] [Google Scholar]
Roscoe 2002
- Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer 2002;10(4):329‐36. [PUBMED: 12029433] [DOI] [PubMed] [Google Scholar]
Salo 2002
- Salo J, Neimela A, Joukamaa M, Koivukangas J. Effect of brain tumour laterality on patients' perceived quality of life. Journal of Neurology Neurosurgery and Psychiatry 2002;72(3):373‐7. [PUBMED: 11861699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiff 2014
- Schiff D, Lee EQ, Nayak L, Norden AD, Reardon D, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro‐oncology 2014;0:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Servaes 2007
- Servaes P, Gielissen M, Verhagen S, Bleijenberg G. The course of severe fatigue in disease‐free breast cancer patients: a longitudinal study. Psycho‐oncology 2007;16(9):787‐95. [PUBMED: 17086555] [DOI] [PubMed] [Google Scholar]
Stein 2004
- Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the Multidimensional Fatigue Symptoms Inventory ‐ Short Form. Journal of Pain and Symptom Management 2004;27(1):14‐23. [DOI: 10.1016/j.jpainsymman.2003.06.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2000
- Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer‐related fatigue: inevitable, unimportant and untreatable? Results of a multi‐centre patient survey. Cancer Fatigue Forum. Annals of Oncology 2000;11(8):971‐5. [PUBMED: 11038033] [DOI] [PubMed] [Google Scholar]
Struik 2009
- Struik K, Klein M, Heimans JJ, Gielissen MF, Bleijenberg G, Taphoorn MJ, et al. Fatigue in low‐grade glioma. Journal of Neuro‐Oncology 2009;92(1):73‐8. [DOI: 10.1007/s11060-008-9738-7] [DOI] [PubMed] [Google Scholar]
Taphoorn 1995
- Taphoorn MJB, Heimans JJ, Veen EA, Karim ABMF. Endocrine functions in long‐term survivors of low‐grade supratentorial glioma treated with radiation therapy. Journal of Neuro‐Oncology 1995;25(2):97‐102. [PUBMED: 8543975] [DOI] [PubMed] [Google Scholar]
Volkow 2002
- Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. Journal of Attention Disorders 2002;6(Suppl 1):S31‐43. [PUBMED: 12685517] [DOI] [PubMed] [Google Scholar]
WHOQOL Group 1995
- The WHOQOL Group. The World Health Organization Quality of Life Assessment (WHOQOL): Position Paper from the World Health Organization. Social Science and Medicine 1995;41(10):1403‐9. [PUBMED: 8560308] [DOI] [PubMed] [Google Scholar]
Winningham 1992
- Winningham ML. How exercise mitigates fatigue: implications for people receiving cancer therapy. In: Carroll‐Johnson RM editor(s). The Biotherapy of Cancer V. Pittsburgh: Oncology Nursing Press, 1992:16‐21. [Google Scholar]
Yellen 1997
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management 1997;13(2):63‐74. [PUBMED: 9095563] [DOI] [PubMed] [Google Scholar]
Young 2010
- Young JW, Greyer MA. Action of modafinil‐increased motivation via the dopamine transporter inhibition and D1 receptors?. Biological Psychiatry 2010;67(8):784‐7. [DOI: 10.1016/j.biopsych.2009.12.015] [DOI] [PMC free article] [PubMed] [Google Scholar]


