Abstract
Over the past decade, Pfizer has focused efforts to improve its research and development (R&D) productivity. By the end of 2020, Pfizer had achieved an industry-leading clinical success rate of 21%, a tenfold increase from 2% in 2010 and well above the industry benchmark of ∼11%. The company had also maintained the quality of innovation, because 75% of its approvals between 2016 and 2020 had at least one expedited regulatory designation (e.g., Breakthrough Therapy). Pfizer’s Signs of Clinical Activity (SOCA) paradigm enabled better decision-making and, along with other drivers (biology and modality), contributed to this productivity improvement. These laid a strong foundation for the rapid and effective development of the Coronavirus 2019 (COVID-19) vaccine with BioNTech, as well as the antiviral candidate Paxlovid™, under the company’s ‘lightspeed’ paradigm.
Keywords: R&D, Productivity, Success rate, Pharmaceutical, Drug development, COVID-19, Vaccine
Introduction
The cost of failure represents a significant proportion of all R&D development costs (60%),1 making success rate a crucial driver of R&D productivity. Mid- and late-stage success rates are crucial for R&D productivity because of the high cost of studies, with average costs estimated at US$30 million, US$70 million, and US$310 million per study in Phase I, II, and III, respectively,1 as well as long development timelines in these Phases. Over the past decade, Pfizer has lagged peers on success rates and, hence, launched focused efforts to improve overall R&D success rates and productivity. We recently published early signs of a turnaround, with a step change in Phase II success rates.2 In that paper, we disclosed a 53% Phase II success rate (calculated at the time of submission with data through August 2020) over a 3-year rolling average at the end of 2020 compared with our historic low of 5% at the end of 2016 and a peer benchmark of ∼30%. Here, we demonstrate stronger and more durable signs of an R&D turnaround for Pfizer, with data on success rate improvements in each phase of clinical development over a longer duration of time. We also show that higher success rates were not achieved at the cost of scientific innovation.
We have previously shared our strategy2 to improve R&D productivity with an emphasis on three key aspects: (i) biology: a deeper understanding and emphasis on science: sharpening our focus on therapeutic areas in which we had deep expertise, strong scientific foundations, and capabilities has enabled us to achieve strong success rates and advance innovative programs; (ii) modalities: diversification to expand the druggable space: extending our breadth of modalities while maintaining our core capabilities in small molecules enabled Pfizer to expand the repertoire of potential drug targets that could be pursued in its core areas; and (iii) decision-making: enhanced objective metrics: greater adoption of enhanced, objective, and quantitative methods, including elevating the importance of key scientific quality metrics, such as 3 Pillars for Proof of Mechanism (POM) and Early Signal of Efficacy (ESOE),2 has enabled Pfizer to rapidly progress strong programs while stopping weaker programs earlier.
In our prior publication,2 we shared examples of how a strong foundation in biology and a diversified modality toolbox had significant impact on our recent productivity improvements. Here, we discuss in more detail how certain aspects of decision-making have improved Pfizer’s productivity, with relevant supporting examples.
Pfizer’s journey to improve R&D productivity
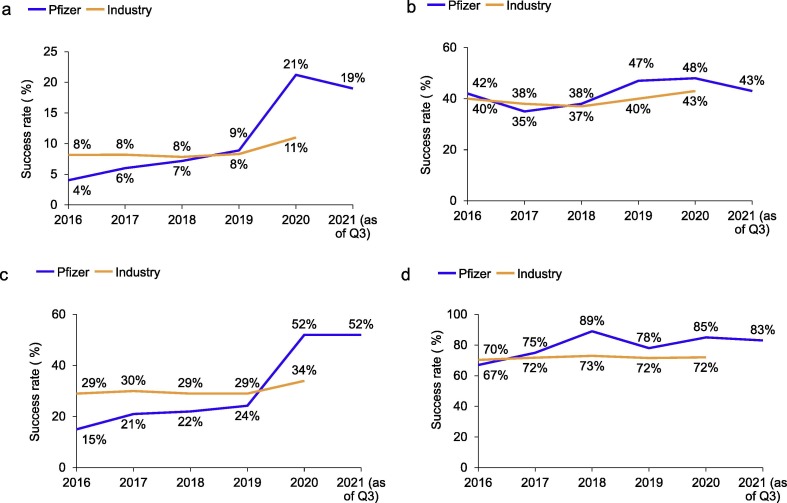
During the first half of the past decade, Pfizer’s success rates had consistently lagged industry benchmarks: in 2010, its end-to-end success rate was at 2%, less than half of the industry benchmark of 5% in the same year.3 By the end of 2020, Pfizer had achieved an end-to-end [First-in-Human (FIH) to regulatory approval] clinical success rate of 21%3 (Fig. 1 a), with the interim 2021 success rate showing a similar trend at 19%. Our interim 2021 Phase II success rate also remained steady at 52%. The end-to-end success rate represents a tenfold increase in Pfizer’s performance over a decade-long journey and positions Pfizer as the current success rate leader, with an industry high of 21% (peer average at 11%) as of year-end 2020.3 Over the past decade, from 2010 to 2020, the industry average has ranged from 5% to 11%.3
Figure 1.
Success rates of first-in-human (FIH) studies to approval, Pfizer versus industry, 2016–Q3 2021. (a) Cumulative success rates of FIH (defined as Phase I) through Approval. Cumulative FIH to Approval success rate was calculated as the product of Phase I, Phase II, Phase III, and Approval success rates. For both Pfizer success rate and industry benchmarks, a 3-year rolling cohort was used for Phase I and 5-year rolling cohorts for subsequent phases (e.g., 5-year rolling cohort of 2019 represents the 2015–2019 5-year rolling average). The success rate of a specific phase was defined as the percentage of new molecular entities (NMEs) successfully transitioning from that phase to start of the next phase (or registration in the case of Phase III) divided by the total number of outcomes in that phase in any given year. Peer benchmarks were calculated using a similar methodology. The 2021-as-of-end-of-Q3 data point represents either the 3-year rolling average from October 1, 2018 through September 30, 2021 for Phase I outcomes, or the 5-year rolling average from October 1, 2016 through September 30, 2021 for outcomes of later phases. (b) Success rates of Phase I NMEs. The Phase I success rate was defined as transition from Phase I to Phase II and calculated using 3-year rolling averages. Sample sizes for Phase I were: N = 36 (2016), N = 40 (2017), N = 42 (2018), N = 36 (2019), N = 25 (2020), and N = 23 (2021 as of end of Q3). (c) Success rates of Phase II NMEs. The Phase II success rate was defined as transition from Phase II to Phase III and calculated using 5-year rolling averages. Sample sizes for Phase II were: N = 34 (2016), N = 34 (2017), N = 32 (2018), N = 33 (2019), N = 25 (2020), N = 25 (2021 as of end of Q3). (d) Cumulative success rates of Phase III and Approval NMEs. Cumulative success rate of Phase III and Approval NMEs was calculated as the product of Phase III and Approval success rates. The Phase III success rate was defined as the transition from Phase III to first regulatory submission in a major market. Approval success rate was defined as the transition from registration to Approval. All were calculated using 5-year rolling averages. Sample sizes for Phase III were: N = 9 (2016), N = 8 (2017), N = 9 (2018), N = 9 (2019), N = 13 (2020), N = 12 (2021 as of end of Q3); and for registration were: N = 7 (2016), N = 6 (2017), N = 9 (2018), N = 8 (2019), N = 8 (2020), N = 10 (2021 as of end of Q3). To declare success at each stage, the program needed to meet both technical and strategic thresholds. The latter was met, wherever feasible, when the overall efficacy/safety profile was superior to the most relevant standard of care (a higher bar than placebo). Specifically, for declaration of proof of concept (POC), which often coincides with the conclusion of Phase II studies, the program needed to meet criteria for starting Phase III development, including the potential of breakthrough value to patients, filling unmet needs, as well as assessment of commercial value, competitive landscape, resources required, and risks.
Pfizer’s high end-to-end clinical success rate was primarily driven by high late- and mid-stage successes.2 The late-stage success rate for Pfizer (85% as of year-end 2020) was moderately higher compared with 72% for the industry (Fig. 1d). By comparison, the Phase II success rate (52% for Pfizer as of year-end 2020) was nearly 50% higher than that of the industry at 34% and represented a more than threefold increase for Pfizer over the past 5 years (15% in 2016, based on a 5-year rolling average)3 (Fig. 1c). In 2015, we made the tough decision to not progress 13 Phase II new molecular entities (NMEs). Some of these programs were in areas that we were exiting for strategic reasons, whereas others were discontinued because of unfavorable Phase II readouts. The former allowed us to have a sharper focus on our current five therapeutic areas in which our success rates have been higher. Since then, we have seen steady improvement in our Phase II success rates, and with the 5-year rolling average ending in 2020, we observed a large numerical (∼37%) improvement, reflecting the progress we have made since 2016.
Additionally, Pfizer’s success rate in Phase I was comparable to that of the industry (48% for Pfizer as of year-end 2020 compared with 43% for the industry) (Fig. 1b). We have set an internal goal of ∼40% or greater for Phase I success: this enables us to pursue innovative first-in-class programs while maintaining peer-comparable industry-level success. As previously discussed,2 we have implemented the SOCA paradigm, which leverages POM and/or ESOE to enable early-stage decision making and attrition when it is more cost effective. From our experience since 2016, Phase I attrition was driven by a variety of factors, including technical drivers [efficacy, safety, and pharmacokinetic/pharmacodynamics (PK/PD)], strength of value proposition and disease area focus. For Pfizer’s 2016–2020 Phase I cohort, attrition was mainly driven by efficacy and significantly less so by safety (data not shown). During the same period, Pfizer’s preclinical success rates are comparable to industry benchmarks (data not shown).
Quality of success: Ensuring progression of innovative programs
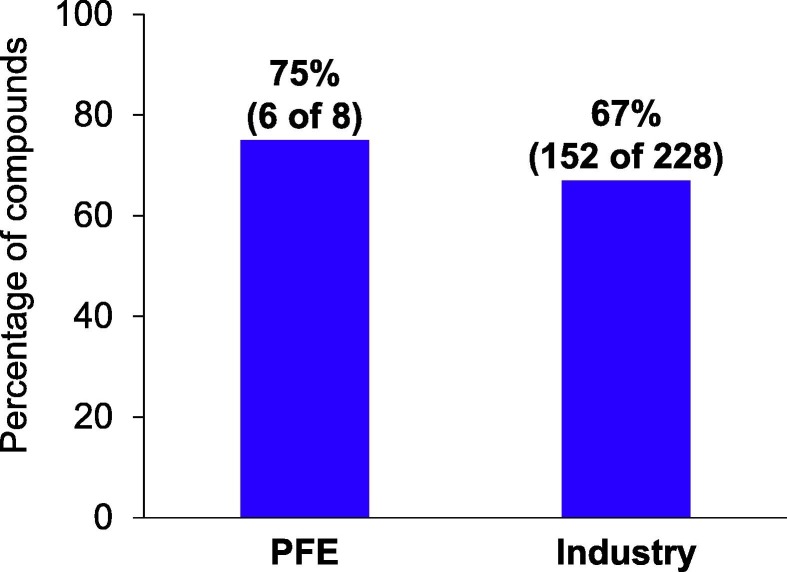
Quality of innovations is a key parameter that can be optimized to enhance R&D productivity in addition to success rates. As a surrogate of innovation, we assessed the proportion of Pfizer’s US Food and Drug Administration (FDA) Center of Drug Evaluation and Research (CDER) NME and novel Biologics License Application (BLA) approvals that received at least one regulatory expedited designation4 from the FDA as important therapeutic advances over existing treatment options (regulatory expedited designations include Breakthrough Therapy, Priority Review, Fast Track, and Accelerated Approval) over the past 5 years. Of Pfizer’s NME/novel BLA approvals from 2016 to 2020, 75% received at least one regulatory designation, compared with 67% for industry peers (Fig. 2 ). This suggests that higher success rates were achieved without sacrificing quality of innovation.
Figure 2.
Percentage of new molecular entities (NMEs) with at least one regulatory expedited designation, from 2016 to 2020. Regulatory designations include Breakthrough Therapy, Priority Review, Fast Track, and Accelerated Approval. This was calculated using a 5-year rolling average and excluded designations from the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) to be consistent. As a result, Comirnaty vaccine for COVID-19 was excluded from this analysis. Data from Pfizer internal tracking and FDA Center for Drug Evaluation and Research.
Attrition drivers in lifecycle management
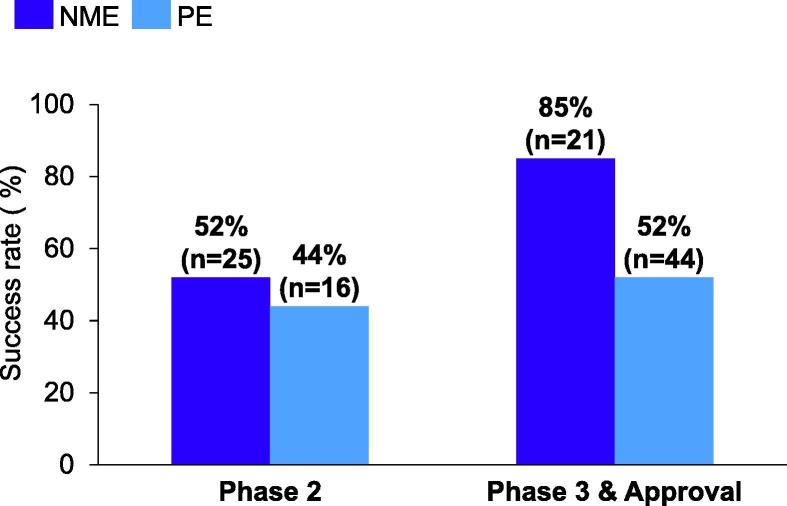
Success rates for NMEs are crucial metrics to gauge outcomes for innovative new molecules. However, success rates for product extensions (PEs) also impact R&D productivity because PE programs also require substantial time and investment to progress. PE programs represented over one-third of our total Phase II readouts and approximately two-thirds of Phase III/Approval readouts between 2016 and 2020 (Fig. 3 ). Pfizer’s PE success rates in each stage of development are comparable to those of NMEs, with the exception of Phase III. Here, the PE failure rate was higher in part because of failures of our antiprogrammed death-ligand 1 (PD-L1) Bavencio (avelumab, co-developed with Merck KGaA) programs in oncology. Although Bavencio succeeded in Phase III pivotal studies and registrations in four indications [accelerated and full approval in Merkel cell carcinoma; urothelial cancer, second line in 2017, first line in 2020; renal cell carcinoma, first line, in combination with the kinase inhibitor Inlyta (axitinib)], it failed in six Phase III studies encompassing four tumor types (gastric cancer, ovarian cancer, non-small cell lung cancer, and locally advanced squamous cell carcinoma of the head and neck) (Fig. 3). In this case, we were a late entrant into the PD-1/PD-L1 market and decided with our partner, Merck KGaA, to focus on less immunogenic tumor types. Although these indications presented the highest unmet needs, they also had the highest risks, given the less proven benefit of immunological therapies in these areas. Since then, we have been increasingly emphasizing the need to focus on therapies with novel targets or novel designs, as well as precision medicine opportunities wherever appropriate so we can provide the most benefits for patients in need.5
Figure 3.
Pfizer’s new molecular entities (NMEs) versus product extensions (PE) success rates for Phase II, Phase III, and Approval from 2016 to 2020. Success rates are defined as in Fig. 1, for NME and PE cohorts separately. N is the total number of NME or PE programs for that stage within the time period analyzed. These were calculated using 5-year rolling averages. Data from Pfizer pipeline analysis.
Dissecting our successes and failures
Culture shift to objective decision-making
As discussed in our previous publication,2 we built the 3 Pillar paradigm based on our retrospective 2005–2009 analysis6 and stipulated that, for a development candidate to have the potential to elicit the desired pharmacological effect over the necessary period of time, three fundamental elements needed to be demonstrated: exposure at the site of action (Pillar 1); binding to the pharmacological target (Pillar 2); and expression of pharmacological activity from the site of action (Pillar 3). Our current SOCA framework is an evolution of 3 Pillars by using either POM and/or ESOE prospectively as stage gates for further clinical investments. Each early clinical development program is expected to have a SOCA strategy and prespecified target values reviewed and agreed upon ahead of initiating the relevant studies, according to the concepts of Model Informed drug development (MIDD).
A similar pillar concept has also been implemented as part of the AstraZeneca five ‘R’s framework7 (in which it is defined as ‘right tissue’ – drug exposure, pharmacological activity in the target organ, and appropriate understanding of PK/PD). The 5R framework also refers to promoting truth-seeking experiments applying and refining the principles of quantitative decision criteria advocated by Lalonde et al. 8 at Pfizer. The SOCA paradigm also has common traits with the Lilly Chorus model.9 The ‘quick-win, fast-fail model’ of Chorus is in fact meant to answer quickly and efficiently the crucial questions that can lead to a go/no-go decision or significantly increase the confidence in the investigational product (while minimizing investments in parallel). At Pfizer, we have taken the step to apply this paradigm systematically across the entire early drug development portfolio.
Case study: oral glucagon-like peptide-1 Danuglipron (PF-06882961) for type 2 diabetes mellitus and obesity: an example of acceleration based on ESOE data with precedented pharmacology and MIDD
Danuglipron is the first oral small-molecule glucagon-like peptide-1 (GLP-1) receptor agonist being developed for the treatment of type 2 diabetes mellitus (T2DM) and obesity. Multiple peptidic GLP-1R agonists are approved for T2DM,10 offering strong confidence in translation to clinical benefit. Before entering human studies, we agreed with the portfolio governance body to conduct the multiple dose study directly in patients with T2DM to gather dose responses for glucose lowering (considered both as one of 3 Pillars and an ESOE for SOCA declaration) and as go/no-go for further development. We started testing in patients based on a model-based meta-analysis of glycated hemoglobin (HbA1c) for antidiabetic agents that was used to set the target value for go/no-go against approved injectable GLP-1 agonists at the time. The selected target of 1% placebo-adjusted reduction in HbA1c was back translated using a MIDD approach11 from mean daily glucose (MDG) changes that could be monitored in a smaller and shorter dose escalation study. In fact, after establishing the safety, tolerability, and PK of danuglipron in healthy participants (NCT03309241), the multiple ascending dose (MAD) was conducted as a Phase Ib 28-day study in participants with T2DM on a background therapy of metformin (NCT03538743) and assessed MDG as the key PD measure to accelerate decision-making. The results of the study12 and the quantitative analyses were key drivers of the accelerated investments for both obesity and T2DM moving from multiple dose escalation to Phase Iib, thereby reducing the timelines by 12 months.
Case study: tumor necrosis factor α-like ligand 1A (PF-06480605) for inflammatory bowel disease: example of increased confidence in translation for a new mechanism of action
Tumor necrosis factor α-like ligand 1A (TL1A) is a potential therapeutic target for inflammatory bowel disease (IBD) and PF-06480605 is a first-in-class monoclonal antibody targeting TL1A. The single ascending dose (SAD)/MAD study in healthy participants showed that both single and multiple doses of PF-06480605 were safe, well tolerated, and demonstrated dose-dependent target engagement [as measured by increases in total soluble TL1A (sTL1A)].13 The study also established the potential impact on PK and PD at low doses from the development of antidrug antibodies and/or neutralizing antibodies.
To prove its potential in IBD, PF-06480605 was then evaluated in an ESOE study (NCT02840721) for SOCA declaration in patients with moderate to severe ulcerative colitis (UC) at one high dose selected based on sTL1A PK/PD modeling to provide >95% target coverage. Given the novel mechanism, severity of the disease, challenge in recruiting patients because of other trials, low preference for placebo-control and shorter treatment duration (week 14), the challenge was to design a trial that was fast to execute with optimal patient benefit to validate the mechanism or derisk large investments early. Hence, the study used a Simon’s two-stage design14 (typically used in oncology) to allow flexibility to stop early for futility if the effect was inferior to that of its competitors. Objective endpoints (endoscopic improvement and remission) were chosen to support a priori for go/no-go decision-making at the interim (stage one) as well as final analysis, with meta-analysis used to set target values. Instead of including a placebo control arm, we leveraged quantitative understanding of placebo and drug effect from internal IBD data matched with propensity score. Given that there was no placebo, it helped the team to recruit patients faster compared with a placebo-controlled trial, yet allowed us to make informed decisions to transition swiftly to the next stage of development.
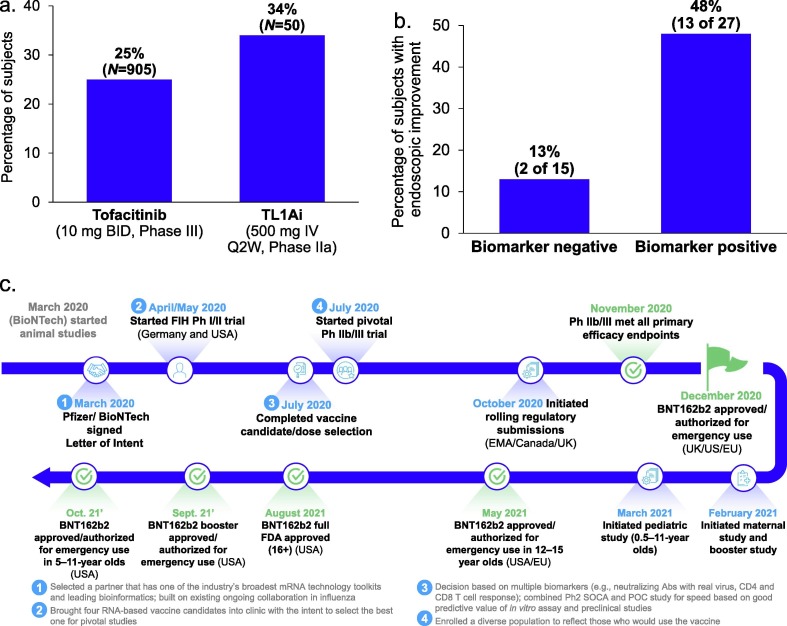
PF-06480605 has since passed stage one and, based on the final analysis (Fig. 4 a), demonstrated significant efficacy in participants with moderate to severe UC.15 Fig. 4a shows the estimate of the endoscopic improvements at week 14 for a single-arm Phase IIa study. To understand the effect in a similar population, historical Phase III tofacitinib data were used. Propensity score matching of multiple baseline characteristics created a tofacitinib data set used to interpolate the week 14 effect based on week 8 induction data and month 2 and month 12 maintenance study data in the matched population. This comparison showed that 34% of patients in the TL1A study experienced endoscopic improvements compared with 25% in the tofacitinib comparison study, representing a relative increase of 36%. Additionally, TL1A might be able to differentiate on safety from tofacitinib based on available early data (data not shown). This is another example in which we were able to make informed decisions using a combined quantitative PK/PD/biomarker modeling approach along with a nimble study design (without a placebo arm in a new mechanism). This also helped us to swiftly move into the next stage of development (estimated time saving of 9 months) with high expected probability of success with sufficient derisking.
Figure 4.
Title. (a) Final analysis of endoscopic improvement at Week 14 between a single-arm Phase IIa study of tumor necrosis factor α-like ligand 1A (TL1A) and a propensity score matched population from tofacitinib Phase III studies. Graph is for illustrative purposes rather than a head-to-head study, no direct comparisons can be made, propensity score weighted analysis. Endoscopic improvement = Mayo Score < 1. Data on file, interpolated endoscopic improvement at week 14, data longitudinally modeled from week 8 induction data to week 14 based on month 2 and month 12 extension data. (b) Analysis of a potential biomarker-driven patient selection strategy to improve efficacy based on a Phase IIa study of TL1A. Biomarkers were undisclosed. (c) Timeline of our ‘lightspeed’ journey to achieve the first approval of coronavirus 2019 (COVID-19) vaccine.
An additional innovation being pursued in the development of PF-06480605 is the identification of a potential biomarker (undisclosed), motivated by its relevance to TL1A based on literature and external databases. This potential biomarker achieved nominal significance by identifying potential responders in the Phase IIa UC trial. Of all trial subjects, 64% were biomarker positive, 48% of whom experienced endoscopic improvements, versus only 13% in the biomarker negative group (Fig. 4b). Although the validity of this biomarker is yet to be seen in the upcoming Phase IIb trial, this encouraging analysis represents step 1 in our 3-step precision medicine strategy (i.e., ‘candidate biomarker identification’ at SOCA). We are currently seeking ‘validation’ at POC as step 2 in our strategy and, if validated, will plan to seek ‘confirmation’ at Pivotal trials as step 3.
Case study: anti-myostatin antibody domagrozumab (PF-06252616) for Duchenne muscular dystrophy: a failure when the SOCA model was not used
Domagrozumab is a humanized anti-myostatin antibody that was in development for Duchene muscular dystrophy (DMD), a rare, severe muscular dystrophy with high unmet medical need. Myostatin is a protein controlling excessive muscle growth and, hence inhibition of myostatin was hypothesized to exert anabolic effects to preserve or reduce the rate of muscle function decline in DMD. This example illustrates how progressing the molecule in absence of a clear POM or SOCA led to failure in the proof-of-concept (POC) trial. The FIH of domagrozumab was a Phase I single and multiple dose-escalating study in healthy participants to evaluate its safety, tolerability, PK, and PD (NCT01616277). Prospective application of modeling approaches enabled us to bridge domagrozumab PK and target coverage (measured as total myostatin concentration) from adult healthy participants to pediatric patients with DMD,16 showing that the mechanism would have been fully tested at safe and tolerable doses. We evaluated evidence of an anabolic effect as POM using the percentage change from baseline in lean body mass (LBM) measured by dual-energy X-ray absorptiometry (DEXA) and a Bayesian decision rule with a target value greater than 1.5% and at least a 70% probability that the posterior mean difference from placebo was greater than 0. However, the study did not demonstrate robust downstream pharmacology. A hint of pharmacological activity was observed, but not to the level prespecified for success. To not lose momentum among mounting competitive pressure, we decided to initiate a Phase II POC trial (NCT02310763) to investigate the safety and efficacy of domagrozumab in boys with DMD. Results from this POC trial demonstrated no significant efficacy of the treatment groups over the placebo groups. The development of domagrozumab was terminated after a longer and more expensive trial, the objective of which would have been achieved with a shorter and faster ESOE study.
Case study: Pfizer-BioNTech COMIRNATY vaccine for COVID-19: R&D turnaround at Pfizer laid a strong foundation for lightspeed progression with several learnings to be applied to future programs
The R&D turnaround at Pfizer, especially our efforts to enable objective and nimble decision-making, coupled with capability investments that enable innovation, speed, and success, laid a strong foundation for the ‘lightspeed’ progression of our COVID-19 vaccine. Pfizer’s vaccine portfolio has historically had a strong end-to-end development success rate, which is among industry leaders: 56% for Pfizer as of year-end 2020, calculated based on similar methodology as in Fig. 1a, versus the industry benchmark of 33–39% for vaccines17., 18. (sample sizes for Pfizer: Phase I, 7; Phase II, 5; Phase III/Approval, 3). Over the years, we have made several investments in technological infrastructure (e.g., cutting-edge clinical, serological, and diagnostic facilities) that allows us to generate clinical data quickly, as well as system upgrades, which enable us to capture, analyze, and report clinical safety data in real time.
On March 11, 2020, the WHO officially declared COVID-19 a global pandemic. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was an unknown virus and there were profound uncertainties over how to best prevent infection and the COVID-19 disease caused by it. Pfizer, along with its partner BioNTech, made the bold decision to select an mRNA approach to develop a vaccine against COVID-19. Although no vaccine or therapy had previously been licensed using an mRNA platform, a decade of preclinical and early clinical experience had generated a rich data set that demonstrated its ability to induce strong B cell, T cell, and innate immune responses. A second strong driver for the mRNA platform was its propensity for a very fast development timeline, which is crucial in any pandemic. On March 17, 2020, Pfizer and BioNTech signed a letter of intent to partner on the development of an mRNA vaccine against COVID-19. In just 221 days from the start of FIH studies in the USA, the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) achieved emergency use authorization from FDA, the first vaccine candidate to accomplish this (Fig. 4c). This is in comparison to the industry median of 9 years and 4 months for other vaccines.19
Several scientific and operational drivers enabled this ‘lightspeed’ timeline. Scientifically, we conducted preclinical experiments in parallel with clinical development to build confidence in approach. This enabled the faster timeline and rapid decision-making in selecting the best vaccine candidate for Phase III development. Multiple candidates were tested in Phase I/II POC studies, including BNT162b1, which encodes a secreted trimerized SARS-CoV-2 spike protein receptor-binding domain, and BNT162b2, which encodes a membrane-anchored SARS-CoV-2 full-length spike protein, stabilized in the prefusion conformation. Based on prespecified selection criteria, including breadth of immune response, good tolerability profile, as well as ease to manufacture, BNT162b2 was selected for the larger Phase II/III studies. Operationally, several factors enabled the rapid advancement of the vaccine. First, we selected an external partner with technical experience in the mRNA platform and a similar culture to Pfizer, which has a strong focus on science. This was the foundation for the fast and unbureaucratic decision-making. Second, we operated internally at Pfizer with a ‘one team mindset’, with strong and decisive leadership empowering colleagues to work through organizational silos. We developed an innovative fit-for-purpose governance process in which all key decision-makers met frequently to speed up the development process. Third, as another key to generate speed, we used a parallel as opposed to sequential R&D process and made large at-risk investments in R&D and manufacturing at a time when scientific data were still limited. Lastly, we funded an end-to-end budget for the program without resource constraints. All these operational drivers allowed the approval of a first-in-class vaccine for COVID-19 with a strong profile: 95% efficacy in preventing COVID-19 disease in individuals 16 years and older. We subsequently expanded the authorization to younger ages as well as boosting in certain populations to provide durable protection. As of November 2021, we have shipped 2 billion doses globally and rolled out the vaccine in 152 countries and territories.
The ‘lightspeed’ paradigm enabled the organization to leverage the foundation already in place and continue to push what could be further improved. Specifically, the ‘lightspeed’ designation enabled effective and nimble decision-making with a prioritized and streamlined governance process. In addition, this program was fully funded with an end-to-end budget from the inception and large-scale investments were made early during development to allow R&D activities to be pursued in parallel that would otherwise have required stage-gating. Although this paradigm might not be suitable for the entire portfolio, Pfizer has adopted certain key ‘lightspeed’ tenets for select programs in which further acceleration could provide significant benefits to patients more quickly. Another program that has benefited under this paradigm is our COVID-19 oral antiviral treatment candidate, Paxlovid, which has been shown to have significantly reduced hospitalization and death, based on an interim analysis of the Phase II/III Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness. We started this program in March 2020, shortly after the WHO declaration of the pandemic. In a year, by March 2021, we were ready to start the FIH study of the compound, compared with the typical duration of 4–5 years from early discovery to the start of the FIH study. Eight months later, we were able to demonstrate the clinical benefit of this compound and have since filed for Emergency Use Authorization in the USA.
Concluding remarks
The cost of failure remains a significant challenge for the biopharma industry. A decade ago, Pfizer began a journey to improve its R&D productivity. As discussed in our recent publication2, Pfizer placed emphasis on improving success rates in Phase II, in which we had the largest gap versus peers. By the end of 2020, Pfizer demonstrated a durable performance on Phase II success rates and achieved success rates in other stages of clinical development at levels moderately higher than its peers. This has translated into an industry-leading end-to-end clinical success rate of 21% (Fig. 1). Importantly, Pfizer has achieved a step-change in success rates while maintaining a high standard of innovation (Fig. 2), as indexed by approvals receiving at least one regulatory designation. One of the key drivers of our improved productivity has been a paradigm that enables early decision-making: being decisive to weed out failures early and taking calculated risks to accelerate ‘winners’. In parallel, we have also pursued several initiatives to harness the power of digital/artificial intelligence (AI). For example, we initiated an immuno-AI collaboration with CytoReason Ltd. to improve target and biomarker discovery in immunology and oncology. Based on public human ‘omics combined with our high-value clinical ‘omics data from clinical trials, the collaboration delivered insights into several projects (e.g., selecting potential indications for CD47 × PD-L1 bispecific antibody program in oncology), increased confidence in rationale for a CCR6 program for immunology, as well as routinely supporting target assessment of new discovery entries. Although these efforts are still in their early stages, we have viewed digital/AI as a driver to further improve our productivity. Pfizer’s R&D turnaround laid a strong foundation for the ’lightspeed’ progression of the Pfizer-BioNTech COVID-19 vaccine, with learnings from this paradigm being applied to other key programs in the portfolio (e.g., Paxlovid). We believe that many of the lessons shared here could be beneficial to other biopharmaceutical R&D organizations.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors are employed by Pfizer Inc and may hold Pfizer shares. M.D. is a member of the Board of Directors of Agilent Technologies and of the Vimian Group.
Acknowledgments
The authors sincerely thank Jasmine Williams-Dautovich, Rebecca Hamm, Jean Lee, Michael S. Vincent, Morris Birnbaum, and Michael Binks for their contributions to the manuscript.
References
- 1.Ringel M.S., Scannell J.W., Baedeker M., Schulze U. Breaking Eroom’s Law. Nature Rev Drug Discov. 2020;19:833–834. doi: 10.1038/d41573-020-00059-3. [DOI] [PubMed] [Google Scholar]
- 2.Wu S.S., Fernando K., Allerton C., Jansen K.U., Vincent M.S., Dolsten M. Reviving an R&D pipeline: a step change in the Phase II success rate. Drug Discov Today. 2020;26:308–314. doi: 10.1016/j.drudis.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Pharmaceutical Benchmarking Forum. KMR Group. https://kmrgroup.com/forums/ [accessed December 13, 2021].
- 4.Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. FDA. www.fda.gov/media/86377/download [accessed December 13, 2021].
- 5.Prigodich A.E., Wang S., Verhoest P., Warne N., Allerton C., Burkhardt J., et al. Innovation in breakthrough drugs and vaccines: development risk, patient impact, and value. Drug Discov Today. 2021;26:2232–2237. doi: 10.1016/j.drudis.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Morgan P., Van Der Graaf P.H., Arrowsmith J., Feltner D.E., Drummond K.S., Wegner S.D., et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Cook D., Brown D., Alexander R., March R., Morgan P., Satterthwaite, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nature Rev Drug Discov. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 8.Lalonde R.L., Kowalski K.G., Hutmacher M.M., Ewy W., Nichols D.J., Milligan P.A., et al. Model-based drug development. Clin Pharmacol Ther. 2007;82:21–32. doi: 10.1038/sj.clpt.6100235. [DOI] [PubMed] [Google Scholar]
- 9.Owens P.K., Raddad E., Miller J.W., Stille J.R., Olovich K.G., Smith N.V., et al. A decade of innovation in pharmaceutical R&D: the Chorus model. Nature Rev Drug Discov. 2015;14:17–28. doi: 10.1038/nrd4497. [DOI] [PubMed] [Google Scholar]
- 10.Aroda V.R. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20:22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 11.Kjellsson M.C., Cosson V.F., Mazer N.A., Frey N., Karlsson M.O. A model-based approach to predict longitudinal HbA1c, using early phase glucose data from type 2 diabetes mellitus patients after anti-diabetic treatment. J Clin Pharmacol. 2013;53:589–600. doi: 10.1002/jcph.86. [DOI] [PubMed] [Google Scholar]
- 12.Saxena A, Gorman D, Chidsey K, Buckeridge C, Kim AM, Bergman A. 353-OR: oral small molecule GLP-1R agonist PF-06882961 robustly reduces plasma glucose and body weight after 28 days in adults with T2DM. Diabetes. Published online June 2020. https://dx.doi.org/10.2337/db20-353-OR.
- 13.Banfield C., Rudin D., Bhattacharya I., Goteti K., Li G., Hassan-Zahraee M., et al. First-in-human, randomized dose-escalation study of the safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of PF-06480605 in healthy subjects. Br J Clin Pharmacol. 2020;86:812–824. doi: 10.1111/bcp.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 15.Danese S., Klopocka M., Scherl E.J., Romatowski J.A., Allegretti J.R., Peeva E., et al. 1027 Safety, tolerability, and efficacy of anti-tl1a antibody PF-06480605 in treatment of ulcerative colitis: the open-label, multicenter, phase 2a Tuscany study. Gastroenterology. 2020;158:S108–S110. [Google Scholar]
- 16.Bhattacharya I., Manukyan Z., Chan P., Heatherington A., Harnisch L. Application of quantitative pharmacology approaches in bridging pharmacokinetics and pharmacodynamics of domagrozumab from adult healthy subjects to pediatric patients with Duchenne muscular disease. J Clin Pharmacol. 2018;58:314–326. doi: 10.1002/jcph.1015. [DOI] [PubMed] [Google Scholar]
- 17.DiMasi J.A., Florez M.I., Stergiopoulos S., Peña Y., Smith Z., Wilkinson M., Getz K.A. Development times and approval success rates for drugs to treat infectious diseases. Clin Pharm Ther. 2019;107:324–332. doi: 10.1002/cpt.1627. [DOI] [PubMed] [Google Scholar]
- 18.Lo AW, Siah KW, Wong CH. Estimating Probabilities of Success of Vaccine and Other Anti-Infective Therapeutic Development Programs. Harvard Data Sci Rev. Published online May 14, 2021. http:/dx./doi.org/10.1162/99608f92.e0c150e8.
- 19.Global Trends in R&D. IQVIA Institute for Human Data Science. www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d. [accessed December 13, 2021].