Abstract
Background
Lymphomas are the third most common malignancy in childhood. Cure rates are high but have reached a plateau. Therefore new treatment modalities should be developed. Antibody therapy is a successful new treatment option in adult lymphoma. However, none of the therapeutic antibodies available for adults with cancer have been approved for treatment of paediatric lymphoma.
Objectives
To assess the efficacy of antibody therapy for childhood lymphoma in terms of survival, response and relapse rates, compared with therapy not including antibody treatment. To assess quality of life and the occurrence of adverse effects caused by antibody therapy treatment in children compared with therapy not including antibody treatment.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 10), MEDLINE in PubMed (from 1945 to October 2014), EMBASE in EMBASE.com (from 1980 to October 2014) and reference lists of relevant articles. Furthermore, we searched conference proceedings abstracts of SIOP, ASCO and ASH for studies from 2009 to 2013), and the World Health Organization (WHO) ICTRP portal and ClinicalTrials.gov for ongoing trials.
Selection criteria
Randomised controlled trials and controlled clinical trials comparing conventional therapy with antibody therapy in children with lymphoma.
Data collection and analysis
Two authors independently performed the study selection.
Main results
We found no studies meeting the inclusion criteria of the review.
Authors' conclusions
At this moment, it is not possible to draw evidence‐based conclusions regarding clinical practice. Phase I and II studies show a positive effect of using antibody therapy in childhood lymphoma. Further research is needed to evaluate and implement antibody therapy for paediatric lymphoma.
Keywords: Child; Humans; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Lymphoma; Lymphoma/drug therapy
Plain language summary
Antibody therapies for lymphoma in children
Review question
The objective of this review was to assess the efficacy of the treatment of lymphoma in children with antibody therapy in terms of survival, relapse rates and response to treatment, compared with therapy not including antibody treatment. Furthermore, it aimed to evaluate the effects of antibody therapy on quality of life and side effects.
Background
Lymphomas are the third most common cancer of childhood. They are cancer of the lymphatic system, which is part of the immune system and protects the body from infection. They often present as painless masses, accompanied by signs and symptoms resulting from local compression, as well as other signs and symptoms, such as fever and weight loss. Cure rates are high, exceeding 80%, but over the past years a plateau has been reached. Furthermore, cure rates for recurrent disease are dramatically lower. The long term effects of chemotherapy (chemicals used to treat cancer) are of great concern. Therefore, new treatments must be developed. Antibodies are produced by our bodies to help fight infection. Treatment with antibodies (antibody therapy) is a successful new treatment option in adults with lymphoma. However, none of the therapeutic antibodies available for adults with cancer have been approved for treatment of paediatric lymphomas. Monoclonal antibodies are proteins that recognise specific proteins on the surface of our body's cells. This binding could be used as a therapy for cancer. Binding of the antibody could result in direct cell death, or could mark the cells that need to be cleared by our body using the immune system.
Search date
8 October 2014.
Study characteristics
We included only studies comparing the use of antibody therapy to the standard care in identical groups of children.
Study funding sources
We included no studies in our analysis.
Key results
We found no studies. The authors analysed 27 publications investigating the safety and tolerability of two antibody therapies, rituximab and brentuximab vedotin, in children with various types of lymphoma. These trials indicated that antibody therapy is safe to use in children and is well tolerated. Furthermore, there seems to be a positive effect on survival rates. To further evaluate the effects randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) must be performed.
Use of statistics
We performed no analyses.
Quality of the evidence
We found no studies.
Background
Description of the condition
Lymphoma is the third most common malignancy of childhood. It often presents as painless mass, accompanied by signs and symptoms resulting from local compression, as well as systemic signs and symptoms, such as fever and weight loss (Young 2000). Lymphoma comprises non‐Hodgkin's lymphoma, Hodgkin's lymphoma and post‐transplantation lymphoproliferative disease. Approximately 7% of all childhood malignancies are accounted for by non‐Hodgkin's lymphoma (Gross 2007). Non‐Hodgkin's lymphoma is more common in children older than 10 years, with a peak incidence between 15 and 19 years of age (Bickert 2002).
In paediatric non‐Hodgkin's lymphoma, there are four major subtypes that account for about 90% of childhood non‐Hodgkin's lymphoma: Burkitt's lymphoma, diffuse large B‐cell lymphoma, precursor T‐ and B‐cell lymphoblastic lymphoma, and anaplastic large cell lymphoma (Gross 2007; Jaglowski 2009; Miles 2007).
Burkitt's lymphoma accounts for about 40% of non‐Hodgkin's lymphoma in children and is rare in adults (Gross 2007; Miles 2012). Current treatment with combined chemotherapy, including methotrexate, doxorubicin, cyclophosphamide, prednisolone and vincristine results in cure rates over 90% in limited disease (Miles 2007; Miles 2012), and five‐year event‐free survival of 85% to 90% in advanced‐stage disease (Miles 2012). Cure rates for endemic Burkitt's lymphoma in low‐income countries are usually significantly lower (less than 50%) due to limited resources (Hesseling 2013).
Diffuse large B‐cell lymphoma makes up for 10% of all childhood non‐Hodgkin's lymphoma (Gross 2007). Standard chemotherapy results in cure rates of over 90% in limited disease (Miles 2007).
T‐ and B‐cell lymphoblastic lymphoma accounts for approximately 30% of non‐Hodgkin's lymphoma in children and young adults (Cairo 2005; Gross 2007; Miles 2012). Lymphoblastic lymphoma originates from immature or precursor lymphoid cells, similar to acute lymphatic leukaemia. The similarity between lymphoblastic lymphoma and acute lymphatic leukaemia results in similar chemotherapy strategies. Current cure rates are 80% to 85% (Miles 2007).
Anaplastic large‐cell lymphoma is a T‐cell lymphoma and is responsible for 10% to 15% of paediatric non‐Hodgkin's lymphomas (Cairo 2005; Gross 2007). Initial response to the intensive and short chemotherapy regimens is over 80%; however, relapse is a significant clinical problem and occurs in 25% to 30% of the patients within two years (Brugieres 2009; Miles 2007).
Hodgkin's lymphoma accounts for 30% of the lymphomas diagnosed in children. Hodgkin's lymphoma is divided into classical Hodgkin's lymphoma and nodular lymphocyte‐predominant Hodgkin's lymphoma. Using current multi‐agent chemotherapy regimens, cure rates excess 90% for early‐stage and 80% for advanced‐stage disease (Bickert 2002; Daw 2011). In early‐stage disease, relapse rates are about 10%, in advanced stage disease, around 25% of the patients.
Post‐transplantation lymphoproliferative disease is one of the immunodeficiency‐associated lymphoproliferative disorders. It occurs after solid organ or haematopoietic stem cell transplantation and is associated with Epstein‐Barr virus infection and T‐lymphocyte depletion (Sandrini 2010). The first step in treatment is reduction of immune suppression. Anti‐CD20 antibodies (e.g. rituximab), chemotherapy and radiotherapy are other treatment options. There is no consensus treatment, however, treatment with rituximab plays a crucial role.
Description of the intervention
Cure rates for most paediatric lymphomas are high. This is the result of constant improvement of multi‐agent chemotherapy. However, a plateau has been reached. Modulation of conventional therapy is unlikely to significantly improve outcomes any further (Capitini 2010; Rossig 2011). In addition, long‐term adverse effects of current therapies are significant, including reduced fertility and development of secondary malignancies (Capitini 2010; Rossig 2011).
Furthermore, recurrent non‐Hodgkin's lymphoma and Hodgkin's lymphoma in children are still difficult to treat. In classical Hodgkin's lymphoma, in children with refractory disease, event‐free survival is (mean ± standard deviation) 35 ± 9% and overall survival is 67 ± 11%; in children with early relapse, event‐free survival is 76 ± 10% and overall survival is 48 ± 11%; and in children with late relapse, event‐free survival is 89 ± 7% and overall survival is 80 ± 10% (Gorde‐Grosjean 2012). For non‐Hodgkin's lymphoma, these numbers are lower, with an initial response of 42% and a five‐year survival rate of 23% (Bickert 2002).
Therefore, development of new better targeted therapeutic agents is needed to overcome long‐term toxicity and to improve cure rates further. Targeted therapy using monoclonal antibodies is one of these new, very promising approaches.
The most successful example of antibody‐based cancer immunotherapy is rituximab. Rituximab is a chimeric antibody directed against the CD20 antigen (Cioc 2008; van Meerten 2011). CD20 is mainly found on pre‐B and mature B‐lymphocytes (Castillo 2008), and is expressed in almost all cases of Burkitt's lymphoma and diffuse large B‐cell lymphoma (Miles 2007). Rituximab has been used since 1996 in adult follicular lymphoma. It improves the overall response rate and overall survival (Schulz 2007; van Meerten 2011), and is now the standard component of first‐line therapy in adults (van Meerten 2011). Since the success of rituximab, several other monoclonal antibodies against CD20 have been developed. Ofatumumab, veltuzumab, ocrelizumab and aftuzumab are examples of these new‐generation anti‐CD20 antibodies (van Meerten 2011).
How the intervention might work
Monoclonal antibodies are immunoglobulins that recognise specific antigens expressed on the surface of target cells (van Meerten 2011). Since the mid‐2000s, they have been successfully applied as cancer therapy in adults (Scott 2012; Weiner 2010). Anti‐tumour effects are due to four major mechanisms: first, complement‐dependent cytotoxicity resulting in membrane damage and osmotic lysis, second, activation of antibody‐dependent cellular cytotoxicity via NK‐cell‐mediated cytolysis (Grupp 2008), third, recognition of antibody‐coated cells by antigen‐presenting cells (e.g. macrophages and dendritic cells) inducing phagocytosis with consequent lysosomal degradation and antigen presentation (Janeway 2001), leading to, fourth, induction of adaptive anti‐cancer T‐cell immunity. Finally, direct anti‐tumour effects can occur by blocking growth factor receptors necessary for tumour cell survival (Grupp 2008). Conjugation of antibodies to cytotoxic drugs or radionuclides can further increase their efficacy (Grupp 2008; Weiner 2010).
Why it is important to do this review
In adults, monoclonal antibody cancer therapy has shown very promising results (Capitini 2010). The prototypic antibody, rituximab, received approval from the U.S. Food and Drug Administration (FDA) for treatment of relapsed or refractory low‐grade or follicular B‐non‐Hodgkin's lymphoma in adults in 1997. However, none of the therapeutic antibodies available for adults with cancer have been approved for treatment of paediatric cancers (MacDonald 2010; Meyer‐Wentrup 2013).
Many differences between adult and paediatric oncology need to be taken into account when a successful adult therapy is considered for children with cancer. First, lymphoma biology differs between adult and childhood lymphomas (Deffenbacher 2012; Meinhardt 2010; Murphy 1980; Rossig 2011). Second, the spectrum of childhood malignancies varies greatly from that in adults (Rossig 2011). Furthermore, cure rates are much higher in childhood than in adult cancer (Meinhardt 2010; Rossig 2011). Smaller numbers of children with cancer and superior therapy outcome make it more difficult to include children in phase II/III trials (Rossig 2011). Taken together, this makes extrapolating treatment results of antibody‐based lymphoma therapy in adults to children with lymphoma difficult. Therefore, this review aims to summarise and assess the available data on antibody‐based therapy of lymphoma in children.
Objectives
To assess the efficacy of antibody therapy for childhood lymphoma in terms of survival, response and relapse rates, compared with therapy not including antibody treatment. To assess quality of life and the occurrence of adverse effects caused by antibody therapy treatment in children compared with therapy not including antibody treatment.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and controlled clinical trials using antibody therapy in childhood lymphoma treatment.
Types of participants
Children under the age of 18 years at the time of diagnosis, with newly diagnosed, relapsed or refractory Hodgkin's lymphoma (classic Hodgkin's lymphoma, nodular lymphocyte predominant Hodgkin's lymphoma (also called nodular paragranuloma), or lymphoproliferative disease (Epstein‐Barr virus‐lymphoma and post‐transplantation lymphoproliferative disease) or non‐Hodgkin's lymphoma (Burkitt's lymphoma, T‐cell lymphoma (anaplastic large cell lymphoma), B‐cell lymphoma, diffuse large B‐cell lymphoma, follicular lymphoma, Burkitt's‐like lymphoma). We only included a study that also included non‐eligible participants if information for the subgroup of eligible participants was available.
Types of interventions
We wanted to compare the treatment results of antibody therapy with the results of standard therapy (in most cases chemotherapy). The adult experience has shown that antibodies are usually added to standard chemotherapy regimens instead of being given as a single agent. Therefore, our analysis also included studies in which standard chemotherapy was compared to standard chemotherapy plus antibody therapy.
We included the following antibody (related) therapies used to treat childhood lymphoma, regardless of dosage, intensity, frequency and duration:
rituximab;
brentuximab vedotin;
blinatumomab;
epratuzumab;
veltuzumab;
ofatumumab;
epratuzumab;
ibritumomab;
tositumomab;
alemtuzumab;
galiximab;
dacetuzumab;
apolizumab;
anti‐CD27;
mogamulizumab;
visilizumab;
otelixizumab;
muromonab‐CD3;
thymocyte antibody.
Types of outcome measures
Outcome measures were part of the study inclusion eligibility criteria, and thus we included studies that measured at least one of our outcomes of interest. We found no studies in which we needed to contact the authors that did not report relevant outcomes in order to ascertain if outcomes were not measured rather than not reported.
Primary outcomes
Remission (partial, complete), relapse rate, event‐free and overall survival, and adverse effects for each lymphoma type and treatment option separately.
Adverse effects of antibody therapy mentioned in the included studies, such as: immunosuppression (febrile neutropenia or use of antibiotics or infection), prolonged B‐cell depletion (longer than six months), progressive multi‐focal leukoencephalopathy, infusion reactions, skin changes, pulmonary toxicity, renal failure (due to acute cell lysis), hepatotoxicity, cardiac toxicity, seizures.
Defining remission and relapse is difficult in paediatric lymphoma. Study protocols for different lymphoma subtypes use different definitions. We intended to report the definitions for remission and relapse applied by the individual studies, when reported and interpret the treatment results in view of these definitions.
We intended to report all adverse effects, including those scored by using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) (NCI 2010), the World Health Organization (WHO) criteria for toxicity of treatment or any other scoring system.
Secondary outcomes
Quality of life.
Search methods for identification of studies
We did not impose language restrictions and we will update our literature searches every two years.
Electronic searches
We searched the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 10) (Appendix 1);
MEDLINE via PubMed (from 1945 to 8 October 2014) (Appendix 2); and
EMBASE via embase.com/ (from 1980 to 8 October 2014) (Appendix 3).
One author (VMZ) designed the strategies with help of a librarian, and the same author ran the search.
Searching other resources
We screened references of the included studies and reviews. We searched conference proceedings abstracts of the International Society of Paediatric Oncology (SIOP), the American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH) for studies from 2009 to 2013. Furthermore, we searched the WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/) portal and ClinicalTrials.gov (clinicaltrials.gov/) for ongoing trials (8 October 2014). The search strategies are illustrated in Appendix 4 and Appendix 5. One author (VMZ) ran the searches of the ongoing trials databases.
Data collection and analysis
Selection of studies
After removing duplicates, two authors (VMZ, FMW) independently screened the titles and abstract against our inclusion and exclusion criteria. We resolved disagreements by discussion. Thereafter, the two authors (VMZ, FMW) retrieved the full‐text of each potentially relevant article for further assessment. Third party arbitration was not necessary.
We included a flow chart of the search and selection results in this review (Figure 1), and we clearly stated details of the reasons for exclusion of any study considered for the review in the Characteristics of excluded studies table.
1.
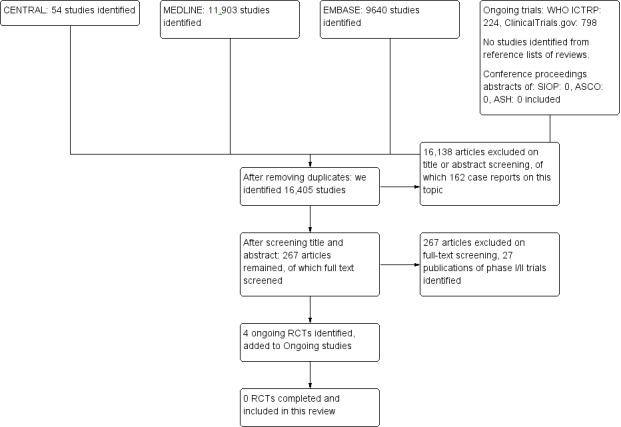
Flow diagram search.
Data extraction and management
Since we identified no eligible studies, data extraction by two independent authors (VMZ, FMW) using a specially developed data extraction sheet was not performed.
Assessment of risk of bias in included studies
If eligible studies had been identified, two authors (VMZ, FMW) would have independently assessed the risk of bias of each included study against the following key criteria.
Selection bias: random sequence generation, allocation concealment.
Performance bias: blinding of participants, blinding of personnel, other potential threats to validity for each outcome separately.
Detection bias: blinding of outcome assessors for each outcome separately, other potential threats to validity for each outcome separately.
Attrition bias: incomplete outcome data for each outcome separately.
Reporting bias: selective outcome reporting.
This is in accordance with methods recommended by Cochrane and the Cochrane Childhood Cancer Group (Higgins 2011; Kremer 2008). We would have used the following judgements: 'low risk of bias', 'high risk of bias' or 'unclear risk of bias' (either lack of information or uncertainty over the potential for bias). We would have presented the results in the 'Characteristics of included studies' table (Higgins 2011). If necessary, we would have consulted a third author (SCG) to resolve disagreements.
Regardless of the outcome of the assessment of risk of bias, we would have used all studies in the analyses. However, we would have taken into account the risk of bias in included studies when we interpreted the review's results.
However, since we identified no eligible studies, this was not applicable.
Measures of treatment effect
Treatment effect could not be measured because there were no eligible studies.
Dealing with missing data
It was not necessary to contact trial authors with regard to study selection, data extraction or risk of bias assessment.
Assessment of heterogeneity
We identified no eligible studies. As a result assessment of heterogeneity was not applicable.
Assessment of reporting biases
We identified no eligible studies. As a result assessment of reporting bias was not applicable.
Data synthesis
We identified no eligible studies. As a result, data analyses could not be performed.
Subgroup analysis and investigation of heterogeneity
We planned to analyse the following subgroups:
lymphoma type;
newly diagnosed versus relapsed/refractory lymphoma (to investigate if there was a difference in outcome when the antibody therapy was used as a first‐line treatment or if it was only appropriate in relapsed/refractory lymphoma);
dosage regimens (high versus low) and frequency of doses (this analysis aimed to identify if there was a difference in outcome when another treatment regimen was used);
therapy combinations (e.g. with radiotherapy versus without radiotherapy; with surgery versus without surgery; different types of chemotherapy to investigate if there was an optimal treatment to which antibody therapy could be added).
However, we identified no eligible studies. As a result, we did not perform subgroup analysis and investigation of heterogeneity.
Sensitivity analysis
We identified no eligible studies. As a result, we did not perform a sensitivity analysis.
Results
Description of studies
Results of the search
We conducted the electronic literature search on 8 October 2014.
We identified 16,405 references from the initial electronic literature search after removing duplicates (Figure 1). By screening title and abstract, we excluded 16,138 articles, most of these articles discussed other haematological conditions, such as leukaemia, and were therefore excluded from our systematic review. Other reasons to exclude the articles were: study concerning an adult population, ex vivo/laboratory study, animal study, review, diagnostic or prognostic study, other intervention or case reports.
We analysed the full text of the remaining 267 articles but none of these articles met our inclusion criteria. We excluded most studies for not being RCTs, other reasons were: a different disease, different intervention or an adult population, 27 excluded publications of 22 original studies were phase I/II trials addressing our research question.
We identified four ongoing clinical trials ; two trials research rituximab in non‐Hodgkin's lymphoma (NCT01516580; NCT01595048), and two trials studying brentuximab vedotin in anaplastic large cell lymphoma (NCT01979536) or non‐Hodgkin's lymphoma (NCT02166463). Results are expected in 2019 and 2020. See Characteristics of ongoing studies table.
We found no eligible studies by searching the conference proceedings of SIOP, ASCO and ASH, or by checking reference lists of relevant reviews.
Unfortunately, we found no eligible studies. However, four clinical trials are still ongoing of which the results are expected in a few years.
Excluded studies
We excluded 267 studies after full‐text assessment according to the eligibility criteria of this review, 27 of these studies are in the scope of this review but did not meet our eligibility criteria (see Characteristics of excluded studies table and Table 1; Table 2; Table 3; Table 4; Table 5).
1. Study characteristics phase I/II trials B‐NHL.
| Data extraction | Reference | |||||
| Cairo 2009 | Cairo 2010 | Goldman 2009a | Goldman 2009b | Griffin 2009 | ||
| Diagnosis | Mature B‐NHL, stage III/IV or BM+/CNS+ Unclear if newly diagnosed, relapsed or refractory disease |
Mature B‐NHL, stage III/IV or BM+/CNS+ Unclear if newly diagnosed, relapsed or refractory disease |
Mature B‐NHL, stage III/IV Unclear if newly diagnosed, relapsed or refractory disease |
Mature B‐NHL, BM+/CNS+ Unclear if newly diagnosed, relapsed or refractory disease |
Mature B‐NHL, relapsed/refractory | |
| Number of participants | 48 stage III/IV and 36 BM+/CNS+ | 48 stage III/IV and 42 BM+/CNS+ | 48 stage III/IV (7 in sub‐pilot, 41 in pilot) | 42 BM+/CNS+ | 6 DLBCL, 12 BL (2 not eligible) | |
| Age median (range) | (1‐23 years) | Not reported | 11 years (1‐23) | 9.5 years | (5‐20 years) | |
| Intervention | Rituximab (375 mg/m2), day ‐2 + day 0 COPADM2, day 1 + 2 CYM, or day 1 CYVE, Or day ‐2, day 0 COPADM1 |
Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYM1+2, or day 0 CYVE1+2 (sub‐pilot: day ‐2 + day 0 COPADM2, day 0 CYM1+2 or day 0 CYVE1+2) |
Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYM1+2 (sub‐pilot: day ‐2 + day 0 COPADM2, day 0 CYM1+2) |
Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYVE1+2 | Rituximab (375 mg/m2), day ‐2 + day 0 course 1+2, day 1 course 3 | |
| Chemotherapy | FAB/LMB 96 B4 or C1 | FAB/LMB 96 B4 or C1 | FAB/LMB 96 B4 | FAB/LMB 96 C1 | Ifosfamide 3000 mg/m2 days 3+4+5, etoposide 100 mg/m2 days 3+4+5, carboplatin 635 mg/m2 day 3. IT: MTX and cytarabine (dose age dependent) days 3+10+17 of courses 1+2 | |
| Outcome | Remission/ relapse |
Not reported | Not reported | Relapse: 4 of 48 | CR: 41 of 42 Progression: 1 of 42 |
CR: 7 of 20 PR: 5 of 20 SD: 2 of 20 PD: 6 of 20 |
| EFS | 2‐year EFS 93% (95% CI 86% to 100%) | Stage III/IV: 2‐year EFS 93% BM+/CNS+: 2‐year EFS 86% |
2‐year EFS 93% (95% CI 86% to 100%) | Not reported | Not reported | |
| OS | Not reported | Stage III/IV: 2‐year OS 96% BM+/CNS+: 2‐year OS 88% |
Not reported | Not reported | 2‐year OS 40% (responders: 2‐year OS 65%) | |
| Adverse effects | No toxicity related to rituximab | No SAE related to rituximab, 2 toxic deaths in BM+/CNS+ group | Toxicity: grade III/IV febrile neutropenia/ infections and grade III/IV mucositis | 2 toxic deaths, grade III/IV mucositis (lower than in FAB/LMB 96), grade III/IV neutropenia/infection (lower than in FAB/LMB 96) | Severe myelosuppression (reversible), infections, grade 2‐4 allergic reactions in 6 of 41 treatment courses | |
| Quality of life | Not reported | Not reported | Not reported | Not reported | Not reported | |
BL: Burkitt's lymphoma.
BM: bone marrow.
CI: confidence interval.
CNS: central nervous system.
CR: complete remission (defined as complete disappearance of all measurable or evaluable lesions, no blasts in the bone marrow or in the cerebrospinal fluid).
DLBCL: diffuse large B‐cell lymphoma.
EFS: event‐free survival (defined as minimum time to death from any cause, relapse, progressive disease or second malignancy measured from diagnosis).
FAB/LMB 96: treatment protocol for people with mature B‐cell lymphoma. Standard treatment was similar to that of group B in LMB89 (except the elimination of vincristine on day 6 in the second induction course of cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), methotrexate (COPADM) course). The pre‐phase COP consisted of low doses of cyclophosphamide, Oncovin (vincristine) and prednisone. People with at least a 20% response at day 7 received the first induction course, COPADM1 (cyclophosphamide 1.5 g/m2, Oncovin, prednisone, Adriamycin, high‐dose methotrexate (HDMTX 3 g/m2 in 3‐hour infusion with intrathecal MTX)). As soon as possible following recovery, participants received the second induction course COPADM2, in which the cyclophosphamide dose was doubled (3 g/m2 divided in 6 fractions administered every 12 hours). After 2 consolidation courses named CYM (cytarabine, HDMTX) treatment concluded with one maintenance course M1 (cyclophosphamide, Oncovin, prednisone, Adriamycin, HDMTX). Response to treatment was defined at 3 time points. The first evaluation was performed after COP at day 7. Participants with tumour reduction < 20% (poor responder to COP) were switched to the more intensive group C regimen including higher‐dose methotrexate (8 g/m2), high‐dose Ara‐C and VP‐16, and were not eligible for randomisation. The second evaluation was performed after the first COPADM course and participants were randomised if there was no disease progression. The third evaluation was performed after the first consolidation CYM course.
NHL: non‐Hodgkin's lymphoma.
OS: overall survival (defined as time to death from any cause, measured from the time of diagnosis).
PD: progressive disease (defined as any progression of > 25% in the product of the 2 largest diameters of any measurable lesion, appearance of new lesions, appearance or re‐appearance of lymphoma cells in bone marrow or cerebrospinal fluid).
PR: partial remission (defined as 20% to 99% reduction in the product of the 2 largest diameters of measurable lesions).
SAE: serious adverse effect (defined as an undesirable medical event that at any dose is fatal, life‐threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly, is medically significant, or a combination of these).
SD: stable disease (defined as persistence of tumour with tumour volume unchanged or with increase insufficient to classify as progression).
2. Study characteristics phase I/II trials B‐NHL, part 2.
| Data extraction | Reference | |||||
| Goldman 2011a | Goldman 2011b | Frazer 2012 | Goldman 2013 | Goldman 2014 | ||
| Diagnosis | Mature B‐NHL, stage III/IV or BM+/CNS+ Unclear if newly diagnosed, relapsed or refractory disease |
Mature B‐NHL, newly diagnosed, stage III/IV or BM+/CNS+ | B‐NHL: CNS + BL, newly diagnosed | Mature B‐NHL, newly diagnosed, stage III/IV | Mature B‐NHL: BM+/CNS + BL, newly diagnosed | |
| Number of participants | 41 stage III/IV and 40 BM+ /CNS+ | 41 stage III/IV and 37 BM+ /CNS+ | 15 | 7 sub‐pilot, 38 pilot (56% BL, 22% DLBCL, 9% MPBCL) | 4 sub‐pilot, 36 pilot | |
| Age median (range) | Not reported | Not reported | Not reported | 11 years (1‐23) | 11 years (3‐23) | |
| Intervention | Rituximab (375 mg/m2) 2 doses in induction cycles, 1 dose in consolidation cycles | Rituximab (375 mg/m2), 2 doses in induction cycles, 1 dose in consolidation cycles | Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYVE1+2 | Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYM1+2 (sub‐pilot: day ‐2 + day 0 COPADM2, day 0 CYM1+2) |
Rituximab (375 mg/m2), day ‐2 + day 0 COPADM1+2, day 0 CYVE1+2 (sub‐pilot: day ‐2 + day 0 COPADM2, day 0 CYVE1+2) |
|
| Chemotherapy | FAB/LMB 96 B4 or C1 | FAB/LMB 96 B4 or C1 | FAB/LMB 96 C1 | FAB/LMB 96 B4 | FAB/LMB 96 C1 | |
| Outcome | Remission/ relapse | stage III/IV: relapse: 1 in 41 BM+/CNS+: 2 in 40 DOD, and 2 toxic deaths |
Not reported | Remission: 14 in 15 (93%) | Response rate after induction: sub‐pilot 75% (95% CI 30% to 95%), pilot 89% (95% CI 73% to 95%) CR: 98% Relapse: 3 participants < 34 months |
Not reported |
| EFS | Stage III/IV: 3‐year EFS 93% (95% CI 79% to 98%) BM+/CNS+: 3‐year EFS 89% (95% CI 73% to 98%) |
Stage III/IV: 3‐year EFS 95% (95% CI 80% to 99%) BM+/CNS+: 3‐year EFS 89% (95% CI 73% to 98%) |
Not reported | 3‐year EFS all 45 participants 93% (95% CI 79% to 98%), pilot 95% (95% CI 80% to 99%) | 3‐year EFS all 40 participants 90% (95% CI 76% to 96%), 15 CNS+ participants 93% (95% CI 61% to 99%) | |
| OS | Not reported | Not reported | Not reported | 3‐year OS pilot 95% (95% CI 83% to 99%) | Not reported | |
| Adverse effects | Not reported | Not reported | None | Mucositis, infections, pain | Not reported | |
| Quality of life | Not reported | Not reported | Not reported | Not reported | Not reported | |
BL: Burkitt's lymphoma.
BM: bone marrow.
CI: confidence interval.
CNS: central nervous system.
CR: complete remission (defined as complete disappearance of all measurable or evaluable lesions, no blasts in the bone marrow or in the cerebrospinal fluid).
DLBCL: diffuse large B‐cell lymphoma.
DOD: died of disease.
EFS: event‐free survival (defined as minimum time to death from any cause, relapse, progressive disease or second malignancy measured from diagnosis).
FAB/LMB 96: treatment protocol for people with mature B‐cell lymphoma. Standard treatment was similar to that of group B in LMB89 (except the elimination of vincristine on day 6 in the second induction course of cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), methotrexate (COPADM) course). The pre‐phase COP consisted of low doses of cyclophosphamide, Oncovin (vincristine) and prednisone. Participants with at least a 20% response at day 7 received the first induction course, COPADM1 (cyclophosphamide 1.5 g/m2, Oncovin, prednisone, Adriamycin, high‐dose methotrexate (HDMTX 3 g/m2 in 3‐hour infusion with intrathecal MTX)). As soon as possible following recovery, participants received the second induction course COPADM2, in which the cyclophosphamide dose was doubled (3 g/m2 divided in 6 fractions administered every 12 hours). After 2 consolidation courses named CYM (cytarabine, HDMTX) treatment concluded with 1 maintenance course M1 (cyclophosphamide, Oncovin, prednisone, Adriamycin, HDMTX). Response to treatment was defined at 3 time points. The first evaluation was performed after COP at day 7. Participants with tumour reduction < 20% (poor responder to COP) were switched to the more intensive group C regimen including higher‐dose methotrexate (8 g/m2), high‐dose Ara‐C and VP‐16, and were not eligible for randomisation. The second evaluation was performed after the first COPADM course and participants were randomised if there was no disease progression. The third evaluation was performed after the first consolidation CYM course. High‐risk participants with bone marrow, CNS, or both disease received 2 identical consolidation courses, CYVE 1 + 2 (continuous infusion of high‐dose cytarabine and etoposide) after COP and COPADM1 and 2.
NHL: non‐Hodgkin's lymphoma.
OS: overall survival (defined as time to death from any cause, measured from the time of diagnosis).
PD: progressive disease (defined as any progression of > 25% in the product of the 2 largest diameters of any measurable lesion, appearance of new lesions, appearance or re‐appearance of lymphoma cells in bone marrow or cerebrospinal fluid).
PR: partial remission (defined as 20% to 99% reduction in the product of the 2 largest diameters of measurable lesions).
SAE: serious adverse effect (defined as an undesirable medical event that at any dose is fatal, life‐threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly, is medically significant, or a combination of these).
SD: stable disease (defined as persistence of tumour with tumour volume unchanged or with increase insufficient to classify as progression).
3. Study characteristics phase I/II trials B‐NHL, part 3.
| Data extraction | Reference | |||||
| Bilić 2009/Bilić 2010 | Fedorova 2009 | Samochatova 2009 | Meinhardt 2009/Reiter 2009/Meinhardt 2010 | Samochatova 2014 | ||
| Diagnosis | B‐NHL, newly diagnosed | B‐BHL: PMBLCL or B‐ALL (stage III/IV), newly diagnosed | Mature B‐NHL/B‐ALL, newly diagnosed | Mature B‐NHL, newly diagnosed | Mature B‐cell lymphoma, newly diagnosed | |
| Number of participants | 6 (BL, DLBCL, 1 ALL) | 28 | 61 | 136, 49 drop‐outs | 83 | |
| Age median (range) | (4‐16 years) | 14.3 years | 8.7 years | 10.4 years (1.5‐17.5) | 8.84 years (2.8‐16.9) | |
| Intervention | Rituximab (375 mg/m2), 5 days before every chemotherapy course | Rituximab (375 mg/m2), day 0 of courses 1‐4 of Russian protocol | Rituximab (375 mg/m2), day ‐1 of courses 1‐4 of chemotherapy | Rituximab (375 mg/m2), day ‐4 prior to chemotherapy | Rituximab (375 mg/m2), day ‐1 of courses 1‐4 of chemotherapy | |
| Chemotherapy | NHL‐BFM95 | Russian protocol | NHL‐BFM90, reduced MTX | B‐NHL‐BFM04 | Cytoreductive phase, followed by 6 short chemotherapy cycles, MTX dose reduction in first 2 cycles | |
| Outcome | Remission/relapse | CR 100% Relapse: 1 after 2 months (DOD) |
Remission: 25 participants (89%) Relapse: 3 participants (11%) |
CR 88% | Response rate: 41.4% (95% CI 31% to 52%) | Not reported |
| EFS | Not reported | 22 months EFS 79% (FFS 92%) (7 lymphoma participants: 86%) |
29 months EFS 90% (± 4%) (RFS 98% ± 2%) | Not reported | 84% ± 6% | |
| OS | Not reported | Not reported | 29 months OS 90% (± 4%) | Not reported | 82% ± 8% | |
| Adverse effects | Prolonged B‐cell depletion, mucositis, infections, severe BM aplasia, 1 participant intestinal peristaltic loss, 1 participant increased ureum and creatinine | Moderate infusion reactions, hypogammaglobulinaemia, late neutropenia, 3 toxic deaths | Not reported | Fatigue 13%, anaphylaxis 6%, infection 3%, S‐GOT/S‐GPT 10%, acute tumour lysis 7% | Headache, nausea, fever, urticarial rash, 1 x hypotensive, 5 x broncho‐obstruction | |
| Quality of life | Not reported | Not reported | Not reported | Not reported | Not reported | |
BL: Burkitt's lymphoma,
BM: bone marrow.
B‐NHL‐BFM04: prospective, stratified, non‐randomised multicentre observational trial based on NHL‐BFM90 and NHL‐BFM95. Depending on stage of disease and initial lactate dehydrogenase, participants received 2 to 7 courses of 5 days' duration. Courses were based on dexamethasone, methotrexate, ifosfamide, cyclophosfamide, cytarabine, etoposide, doxorubicine, vincristine and intrathecal therapy. The criteria for stratification, combination and number of therapy courses were unchanged to the previous trial NHL‐BFM95.
CNS: central nervous system.
CR: complete remission (defined as complete disappearance of all measurable or evaluable lesions, no blasts in the bone marrow or in the cerebrospinal fluid).
DLBCL: diffuse large B‐cell lymphoma.
EFS: event‐free survival (defined as minimum time to death from any cause, relapse, progressive disease or second malignancy measured from diagnosis).
NHL: non‐Hodgkin's lymphoma.
NHL‐BFM90 and NHL‐BFM95:2 treatment protocols for NHLs. 3 different subgroups were distinguished and stratified into 3 major therapy groups: 1. lymphoblastic lymphoma (LBL) (precursor T‐cell and B‐cell), 2. mature B‐cell neoplasms (Burkitt's lymphoma, B‐acute leukaemia (B‐AL), DLBCL and primary mediastinal large B‐cell lymphoma (PMLBL)) and 3. anaplastic large cell lymphoma (ALCL). For each of the 3 therapy groups, the back‐bone of the treatment strategy was almost similar throughout the 2 NHL‐BFM studies. Therapy for LBL was based on stage of disease and followed an acute lymphoblastic leukaemia (ALL)‐type regimen. Treatment consisted of a 9‐week induction protocol (prednisone, vincristine, daunorubicin, asparaginase, cyclophosphamide, cytarabine, 6‐mercaptopurine and intrathecal therapy), an 8‐week consolidation therapy (high‐dose methotrexate), a delayed 7‐week re‐intensification protocol (only for people with advanced‐stage disease including dexamethasone, vincristine, doxorubicin, asparaginase, cyclophosphamide, cytarabine, 6‐thioguanine and intrathecal therapy) and maintenance therapy up to a total treatment duration of 24 months. People with B‐cell NHL/B‐AL and ALCL received a pulse‐like therapy with 5‐day chemotherapy courses including dexamethasone, methotrexate, cytarabine, cyclophosphamide, ifosfamide, doxorubicin, etoposide, vincristine, vindesine and intrathecal therapy. The number of courses was mainly based on disease stage and tumour mass.
OS: overall survival (defined as time to death from any cause, measured from the time of diagnosis).
PD: progressive disease (defined as any progression of > 25% in the product of the 2 largest diameters of any measurable lesion, appearance of new lesions, appearance or re‐appearance of lymphoma cells in bone marrow or cerebrospinal fluid).
PR: partial remission (defined as 20% to 99% reduction in the product of the 2 largest diameters of measurable lesions).
SAE: serious adverse effect (defined as an undesirable medical event that at any dose is fatal, life‐threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly, is medically significant, or a combination of these).
SD: stable disease (defined as persistence of tumour with tumour volume unchanged or with increase insufficient to classify as progression).
S‐GOT: serum glutamic oxaloacetic transaminase.
S‐GPT: serum glutamic‐pyruvic transaminase.
4. Study characteristics phase I/II trials post‐transplant lymphoproliferative disease.
| Data extraction | Reference | ||||
| Gross 2009a | Gallego 2010 | Gross 2009b/Gross 2012 | Maecker‐Kolhoff 2011/Maecker‐Kolhoff 2012 | ||
| Diagnosis | Refractory PTLD | Newly diagnosed PTLD | Newly diagnosed PTLD | PTLD Unclear if newly diagnosed, relapsed or refractory disease |
|
| Number of participants | 54 | 6 | 55 | 44 | |
| Age median (range) | 1.8 years (0.1‐4.1) | 9 years (2.5‐17) | 9.7 years (0.8‐19.4) | Not reported | |
| Intervention | Rituximab 375 mg/m2 weekly, 6 cycles | Rituximab 375 mg/m2 weekly | Rituximab 375 mg/m2, days 1, 8 and 15 of each cycle, for total of 6 times | Weekly rituximab, > 25% reduction: 3 additional infusions | |
| Chemotherapy | Cyclophosphamide 600 mg/m2 IV x 1 day, prednisone 1 mg/kg IV x 5 days every 3 weeks for 6 cycles | LMB89 (compared to 8 participants with only LMB89) |
6 cycles every 3 weeks. Cyclophosphamide 600 mg/m2 day 1, prednisone 1 mg/kg twice a day or methylprednisolone 0.8 mg/kg twice a day on days 1‐5 | All others: mCOMP (15 participants) | |
| Outcome | Remission/relapse | CR 74% Overall response 87% |
Not reported | CR 69% (95% CI 56% to 81%) | CR 8/10 with rituximab, 13/15 with mCOMP |
| EFS | 2‐year EFS 77% (95% CI 64% to 90%) | 3 participants LMB 89 6 participants rituximab |
EFS 71% (95% CI 57% to 82%) | 2‐year EFS: 68% | |
| OS | 1‐year OS 83% (95% CI 71% to 95%) 2‐year OS 80% (95% CI 67% to 93%) |
6 participants LMB89 6 participants rituximab |
10 participants died of infection 7 participants died of disease |
2 year OS: 84% | |
| Adverse effects | Not reported | Not reported | SVT, neutropenia, fever | Transient hypogammaglobulinaemia | |
| Quality of Life | Not reported | Not reported | Not reported | Not reported | |
BL: Burkitt's lymphoma.
BM: bone marrow.
CI: confidence interval.
CNS: central nervous system.
CR: complete remission (defined as complete disappearance of all measurable or evaluable lesions, no blasts in the bone marrow or in the cerebrospinal fluid).
DLBCL: diffuse large B‐cell lymphoma.
EFS: event‐free survival (defined as minimum time to death from any cause, relapse, progressive disease or second malignancy measured from diagnosis).
IV: intravenous.
LMB89: treatment protocol for B‐cell (Burkitt's and large B‐cell) lymphoma with multi‐agent chemotherapy adapted to the tumour burden (stage, resection status, percentage of blasts in bone marrow and central nervous system (CNS) involvement) and early response to chemotherapy. Participants were classified into 3 risk groups. Group A (resected stage I and abdominal stage II) received 2 courses of vincristine, cyclophosphamide, doxorubicin and prednisone. Group B (participants not eligible for groups A or C) received 5 courses of chemotherapy with, in addition, high‐dose methotrexate, 3 g/m2 over 3 hours, cytarabine and intrathecal (IT) methotrexate. Group C (participants with CNS involvement) received 8 courses with, in addition, high‐dose methotrexate, 8 g/m2, high‐dose cytarabine, etoposide and triple IT chemotherapy.
mCOMP: moderate chemotherapeutic regimen to treat PTLD. Treatment consists of day 1 vincristine, prednisone and cyclophosphamide, and day 15 methotrexate; and is repeated every 4 weeks for 6 cycles.
NHL: non‐Hodgkin's lymphoma.
OS: overall survival (defined as time to death from any cause, measured from the time of diagnosis).
PD: progressive disease (defined as any progression of more than 25% in the product of the 2 largest diameters of any measurable lesion, appearance of new lesions, or appearance or re‐appearance of lymphoma cells in bone marrow or cerebrospinal fluid).
PR: partial remission (defined as 20% to 99% reduction in the product of the 2 largest diameters of measurable lesions).
PTLD: post‐transplant lymphoproliferative disease.
SAE: serious adverse effect (defined as an undesirable medical event that at any dose is fatal, life‐threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly, is medically significant, or a combination of these).
SD: stable disease (defined as persistence of tumour with tumour volume unchanged or with increase insufficient to classify as progression).
SVT: supraventricular tachycardia.
5. Study characteristics phase I/II trials, other.
| Data extraction | Reference | |||
| Cooney‐Qualter 2007 | Fanale 2011 | Locatelli 2013 | ||
| Diagnosis | Relapsed or refractory CD20+ NHL | CD 30+ haematological malignancies Unclear if newly diagnosed, relapsed or refractory disease |
Relapsed or refractory Hodgkin's lymphoma | |
| Number of participants | 5 | 9 | 14 | |
| Age median (range) | 5‐18 years | (12‐17 years) | 15 years (8‐18) | |
| Intervention | Rituximab 250 mg/m2 day 0 and 7, indium‐111 ibritumomab‐tiuxetan 5 mCi day 0, 90Y‐IT day 7 (0.4‐0.1 mCi/kg) | Brentuximab vedotin every 3 out of 4 weeks. 1 participant: 0.8 mg/kg, 1 participant 1.2 mg/kg. Or every 3 weeks, 1 participant: 1.2 or 1.8 mg/kg (6 participants) |
Brentuximab vedotin 1.8 mg/kg every 3 weeks for up to 16 cycles, until progression or unacceptable toxicity | |
| Chemotherapy | Cytarabine CNS prophylaxis, 3 x every 3 weeks, starting on day 0 | Not reported | None | |
| Outcome | Remission/relapse | Stabilisation on day 35 in 3 participants | CR 6 out of 9 (6‐12+ months, 1 participant relapse), stable disease 3 participants | CR 21% (95% CI 5% to 51%) PR 43% (95% CI 18% to 71%) ORR 64% (95% CI 35% to 87%) |
| EFS | Not reported | Not reported | Not reported | |
| OS | Not reported | Not reported | Not reported | |
| Adverse effects | No dose‐limiting toxicity | 3 participants: TAEs ≥ grade 3 (hyperaesthesia, leucocytopenia, neutropenia) | 12 of 16 (75%) participants had ≥ 1 AE, and 7 (44%) had grade ≥ 3 AEs. 4 SAEs in 3 participants were considered related to brentuximab vedotin: grade 3 hepatotoxicity and grade 3 febrile neutropenia (1 participant); grade 3 anaphylaxis (1 participant) and grade 3 pneumonia (1 participant) | |
| Quality of Life | Not reported | Not reported | Not reported | |
AE: adverse effect.
ALCL: anaplastic large cell lymphoma.
BM: bone marrow.
CI: confidence interval.
CNS: central nervous system.
CR: complete remission (defined as complete disappearance of all measurable or evaluable lesions, no blasts in the bone marrow or in the cerebrospinal fluid).
DLBCL: diffuse large B‐cell lymphoma.
EFS: event‐free survival (defined as minimum time to death from any cause, relapse, progressive disease or second malignancy measured from diagnosis).
NHL: non‐Hodgkin's lymphoma.
ORR: overall response rate (defined as sum of participants in complete and partial remission).
OS: overall survival (defined as time to death from any cause, measured from the time of diagnosis).
PD: progressive disease (defined as any progression of > 25% in the product of the 2 largest diameters of any measurable lesion, appearance of new lesions, or appearance or re‐appearance of lymphoma cells in bone marrow or cerebrospinal fluid).
PR: partial remission (defined as 20% to 99% reduction in the product of the 2 largest diameters of measurable lesions).
SAE: serious adverse effect (defined as an undesirable medical event that at any dose is fatal, life‐threatening, requires or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly, is medically significant, or a combination of these).
SD: stable disease (defined as persistence of tumour with tumour volume unchanged or with increase insufficient to classify as progression).
TAE: treatment‐associated adverse event (defined as any new undesirable medical occurrence or worsening of a pre‐existing medical condition in a person administered an investigational drug, whether or not a causal relationship with the treatment is suspected).
Risk of bias in included studies
We found no eligible studies.
Effects of interventions
We found no eligible studies.
Discussion
Summary of main results
This systematic review evaluated the current state of evidence on treatment of childhood lymphoma with antibody therapy. An RCT is the best study design to evaluate this research question. Our search did not identify any eligible studies evaluating antibody therapy in childhood lymphoma.
Overall completeness and applicability of evidence
At this stage no RCTs and controlled clinical trials on the effect of antibody therapy on childhood lymphoma are available. The literature that we came across while writing this systematic review focused mainly on children with post‐transplant lymphoproliferative disease or diffuse large B‐cell lymphoma treated with rituximab. There were no other lymphoma types and disease stages in children in the literature. Phase I/II trials of antibody therapy of childhood lymphoma showed a positive trend, although there is a risk of bias in all of these trials due to their study design. Many of these studies included participants not eligible for this review. No strong conclusions can be made based on these studies. Four randomised trials are currently being conducted, with results expected in 2019 and 2020.
In current practice, antibody therapy in children is only used in end‐stage disease or in children with very poor prognoses as a salvage therapy.
Quality of the evidence
The current literature shows no evidence on which a conclusion can be based regarding the objectives of this review. Since we found no trials eligible for this systematic review, we performed no quality analyses.
Potential biases in the review process
We performed an electronic literature search after which articles were screened by two authors independently. We attempted to ensure that we did not overlook any relevant articles by searching for studies using all synonyms and no filter for RCTs.
Nevertheless, there always remains a slight possibility that studies have been missed.
Authors' conclusions
Implications for practice.
Since no randomised controlled trials (RCTs) or controlled clinical trials (CCTs) evaluating the effect of antibody therapy in childhood lymphoma are available, we could make no definitive conclusions about their effects on anti‐tumour efficacy, adverse effects and quality of life. Based on the currently available evidence, we are unable to give recommendations for clinical practice.
Implications for research.
Antibody therapy has proven effective in adult lymphoma treatment. Phase I/II trials of antibody therapy of childhood lymphoma showed a positive trend. Further research is needed to assess the effects of antibody therapy in children and in childhood lymphoma. RCTs comparing current treatment regimens with additional antibody therapy in different types of childhood lymphoma, newly diagnosed as well as refractory disease, with adequate statistical power should be performed. Depending on which lymphoma is investigated, different types of antibody therapy should be evaluated and compared to conventional therapy. Remission (partial, complete), relapse rate, event‐free survival and overall survival should be reported, so that solid conclusions of efficacy of antibody treatment of childhood lymphoma can be drawn.
In addition, adverse effects for each lymphoma type and treatment option should be closely monitored and included in study publications.
Acknowledgements
Dr A Borker and an undisclosed person kindly agreed to peer review our manuscript. The editorial base of the Cochrane Childhood Cancer Group is funded by Stichting Kinderen Kankervrij (KiKa).
Appendices
Appendix 1. Search strategy for CENTRAL (via the "Advanced search" page)
1. For children we used: (child:ti,ab OR children:ti,ab OR childhood:ti,ab OR pediatric:ti,ab OR pediatrics:ti,ab OR paediatric:ti,ab OR paediatrics:ti,ab OR newborn:ti,ab OR new‐born:ti,ab OR neonate:ti,ab OR neonates:ti,ab OR infant:ti,ab OR baby:ti,ab OR toddler:ti,ab OR toddlers:ti,ab OR youngster:ti,ab OR adolescence:ti,ab OR adolescent:ti,ab OR teenage:ti,ab OR teenager:ti,ab OR puberty:ti,ab OR Schoolchild:ti,ab OR "School child":ti,ab OR Boy:ti,ab OR Boys:ti,ab OR Boyhood:ti,ab OR girl:ti,ab OR girls:ti,ab OR girlhood:ti,ab OR youth:ti,ab OR youths:ti,ab OR teen:ti,ab OR teens:ti,ab OR preschool:ti,ab OR "preschool child":ti,ab OR suckling:ti,ab OR sucklings:ti,ab OR juvenile:ti,ab OR juveniles:ti,ab)
2. For lymphoma we used: Lymphoma:ti,ab OR Lymphomas:ti,ab OR Hodgkin:ti,ab OR Hodgkin's:ti,ab OR Hodgkins:ti,ab OR Non‐hodgkin:ti,ab OR Non‐hodgkin's:ti,ab OR Non‐hodgkins:ti,ab OR "Non Hodgkin":ti,ab OR "Non hodgkin's":ti,ab OR "Non hodgkins":ti,ab OR Nonhodgkin:ti,ab OR Nonhodgkin's:ti,ab OR Nonhodgkins:ti,ab OR HD:ti,ab OR HL:ti,ab OR NHL:ti,ab OR NHD:ti,ab OR Burkitt:ti,ab OR Burkitt's:ti,ab OR Burkitts:ti,ab OR "Malignant Lymphogranuloma":ti,ab OR "Malignant Lymphogranulomas":ti,ab OR "Malignant lymphogranulomatosis":ti,ab OR "Lymphogranuloma maligne":ti,ab OR "Lymphogranuloma malignum":ti,ab OR Lymphogranulomatosis:ti,ab OR "Malignant Granuloma":ti,ab OR "Malignant Granulomas":ti,ab OR "Malignant granulomatosis":ti,ab OR "Reed hodgkin disease":ti,ab OR "Reed sternberg disease":ti,ab OR "Classic HL":ti,ab OR "Classical HL":ti,ab OR "Classic HD":ti,ab OR "Classical HD":ti,ab OR "NLP HD":ti,ab OR "NLPHD":ti,ab OR "NLP HL":ti,ab OR NLPHL:ti,ab OR "LP HL":ti,ab OR "LPHL":ti,ab OR "Nodular paragranuloma":ti,ab OR "Nodular paragranulomas":ti,ab OR BL:ti,ab OR BLL:ti,ab OR ALCL:ti,ab OR DLBCL:ti,ab OR "Giant follicular lymphosarcoma":ti,ab OR "Giant follicular lymphosarcomas":ti,ab OR "Giant follicle lymphosarcoma":ti,ab OR "Giant follicle lymphosarcomas":ti,ab OR "Giant follicular blastoma":ti,ab OR "Giant follicular blastomas":ti,ab OR "Giant follicle blastoma":ti,ab OR "Giant follicle blastomas":ti,ab OR "Giant follicular lymphoblastoma":ti,ab OR "Giant follicular lymphoblastomas":ti,ab OR "Giant follicle lymphoblastoma":ti,ab OR "Giant follicle lymphoblastomas":ti,ab OR "Brill‐Symmers Disease":ti,ab OR "Brill Symmers Disease":ti,ab OR "Lymphoproliferative disease":ti,ab OR "Lymphoproliferative disorder":ti,ab OR "Lymphoproliferative disorders":ti,ab OR "Lymphoproliferative syndrome":ti,ab OR "Lymphoproliferative syndromes":ti,ab OR Lymphoreticulosis:ti,ab OR "Immunoproliferative disease":ti,ab OR "Immune proliferative disease":ti,ab OR "Immunoproliferative disorder":ti,ab OR "Immunoproliferative disorders":ti,ab OR "Immune proliferative disorder":ti,ab OR "Immune proliferative disorders":ti,ab OR "Lymphomatoid Granulomatosis":ti,ab OR "Post‐transplant lymphoproliferative disease":ti,ab OR "Posttransplant lymphoproliferative disease":ti,ab OR "Post‐transplant lymphoproliferative disorder":ti,ab OR "Post‐transplant lymphoproliferative disorders":ti,ab OR "Posttransplant lymphoproliferative disorder":ti,ab OR "Posttransplant lymphoproliferative disorders":ti,ab OR PTLD:ti,ab OR (("Lymph node":ti,ab OR "Lymph nodes":ti,ab OR Lymphocytic:ti,ab OR Lymphoid:ti,ab) AND (tumor:ti,ab OR tumors:ti,ab OR tumour:ti,ab OR tumours:ti,ab OR malignancy:ti,ab OR malignancies:ti,ab OR Malignant:ti,ab OR Neoplasm:ti,ab OR Neoplasms:ti,ab OR leukemia:ti,ab OR leukemias:ti,ab))
3. For antibody therapy we used: antibody:ti,ab OR antibodies:ti,ab OR mAb:ti,ab OR Immunotherapy:ti,ab OR "Immune therapy":ti,ab OR "Immunoglobulin therapy":ti,ab OR "Immunological therapy":ti,ab OR "Immunological treatment":ti,ab OR "Cancer immunotherapy":ti,ab OR "Tumor immunotherapy":ti,ab OR Immunoglobulin:ti,ab OR Immunoglobulins:ti,ab OR Rituximab:ti,ab OR "Anti‐CD20":ti,ab OR Mabthera:ti,ab OR Rituxan:ti,ab OR reditux:ti,ab OR rituxin:ti,ab OR "IDEC‐C2B8":ti,ab OR "Brentuximab vedotin":ti,ab OR Adcetris:ti,ab OR "anti‐CD30":ti,ab OR "cAC10‐vcMMAE":ti,ab OR "SGN‐35":ti,ab OR "SGN35":ti,ab OR "SGN 35":ti,ab OR "Anti‐CD19":ti,ab OR "MDX 1342":ti,ab OR "MDX‐1342":ti,ab OR "Anti‐MDX 1342":ti,ab OR "Anti‐MDX1342":ti,ab OR "Anti‐MDX‐1342":ti,ab OR blinatumomab:ti,ab OR "medi 538":ti,ab OR medi538:ti,ab OR "mt 103":ti,ab OR mt103:ti,ab OR epratuzumab:ti,ab OR "anti‐CD22":ti,ab OR "hLL2 agent":ti,ab OR LymphoCide:ti,ab OR "immu 1903":ti,ab OR immu1903:ti,ab OR "immu‐1903":ti,ab OR veltuzumab:ti,ab OR "anti‐CD20 IgG":ti,ab OR "ha 20":ti,ab OR ha20:ti,ab OR "ha‐20":ti,ab OR "immu 106":ti,ab OR immu106:ti,ab OR "immu‐106":ti,ab OR Ofatumumab:ti,ab OR Arzerra:ti,ab OR "HuMax‐CD20":ti,ab OR "HuMax CD20":ti,ab OR HuMaxCD20:ti,ab OR "humac CD20":ti,ab OR "gsk 1841157":ti,ab OR gsk1841157:ti,ab OR epratuzumab:ti,ab OR "epratuzumab y 90":ti,ab OR "epratuzumab yttrium y 90":ti,ab OR "epratuzumab tetraxetan yttrium y 90":ti,ab OR "epratuzumab Iodine‐131":ti,ab OR "90Y‐labeled ibritumomab tiuxetan":ti,ab OR "Ibritumomab tiuxetan":ti,ab OR Ibritumomab:ti,ab OR "yttrium‐90‐ibritumomab tiuxetan":ti,ab OR Zevalin:ti,ab OR "in‐111 zevalin":ti,ab OR "y‐90 zevalin":ti,ab OR zevaline:ti,ab OR "idec 129":ti,ab OR "idec y2b8":ti,ab OR idec129:ti,ab OR Tositumomab:ti,ab OR "Tositumomab I 131":ti,ab OR "tositumomab iodine‐131":ti,ab OR "iodine‐131 tositumomab":ti,ab OR "iodine‐131‐tositumomab":ti,ab OR "131I‐labeled tositumomab":ti,ab OR "Tositumomab‐I131":ti,ab OR Bexxar:ti,ab OR "bexxar dosimetric":ti,ab OR "bexxar i 131 dosimetric":ti,ab OR "131I anti‐B1":ti,ab OR Alemtuzumab:ti,ab OR Campath:ti,ab OR MabCampath:ti,ab OR "Campath‐1H":ti,ab OR Lemtrada:ti,ab OR "Campath 1G":ti,ab OR "Campath‐1G":ti,ab OR "Campath‐1‐G":ti,ab OR Campath1G:ti,ab OR "Campath 1M":ti,ab OR "Campath‐1M":ti,ab OR "Campath‐1‐M":ti,ab OR Campath1M:ti,ab OR Campath1H:ti,ab OR "Campath 1H":ti,ab OR "Campath‐1H":ti,ab OR "Campath‐1‐H":ti,ab OR "Campath 1":ti,ab OR "Anti‐CD52":ti,ab OR "ldp 103":ti,ab OR ldp103:ti,ab OR galiximab:ti,ab OR "Anti‐CD80":ti,ab OR "anti‐B7‐1 mAb":ti,ab OR "P‐16C10":ti,ab OR "IDEC‐114":ti,ab OR "idec 114":ti,ab OR idec114:ti,ab OR dacetuzumab:ti,ab OR "anti‐CD40":ti,ab OR "SGN 40":ti,ab OR "SGN40 cpd":ti,ab OR "SGN‐40":ti,ab OR SGN40:ti,ab OR "hu S2C6":ti,ab OR Apolizumab:ti,ab OR "Hu1D 10":ti,ab OR Hu1D10:ti,ab OR Remitogen:ti,ab OR "anti‐CD27 ":ti,ab OR "anti‐CCR4":ti,ab OR mogamulizumab:ti,ab OR "AMG 761":ti,ab OR AMG761:ti,ab OR "AMG‐761":ti,ab OR "KW 0761":ti,ab OR KW0761:ti,ab OR "KW‐0761":ti,ab OR "km 8761":ti,ab OR km8761:ti,ab OR "anti‐CD3":ti,ab OR "OKT‐3":ti,ab OR OKT3:ti,ab OR "OKT 3":ti,ab OR visilizumab:ti,ab OR "hu m 291":ti,ab OR "hu m291":ti,ab OR "hum 291":ti,ab OR hum291:ti,ab OR nuvion:ti,ab OR "SMART anti‐CD3":ti,ab OR Nuvion:ti,ab OR Otelixizumab:ti,ab OR "gsk 2136525":ti,ab OR "gsk2136525 ":ti,ab OR "trx 4":ti,ab OR trx4:ti,ab OR "Muromonab‐CD3":ti,ab OR "muromonab‐cd3":ti,ab OR orthoclone:ti,ab OR "anti‐thymocyte immunoglobulin":ti,ab OR "antithymocyte immunoglobulin":ti,ab OR "antithymocytic immunoglobulin":ti,ab OR "thymocyte antiserum":ti,ab OR "thymocyte isoantiserum":ti,ab OR "thymocyte serum":ti,ab OR "thymocyte isoantibody":ti,ab OR "anti thymocyte antiserum":ti,ab OR "antithymocyte antiserum":ti,ab OR "anti thymocyte serum ":ti,ab OR "antithymocyte serum":ti,ab OR "antithymic serum":ti,ab OR "antithymocytic serum":ti,ab OR "antithymus serum":ti,ab OR "anti thymocyte globulin":ti,ab OR "antithymocyte globulin":ti,ab OR "anti thymocytic globulin":ti,ab OR "antithymocytic globulin":ti,ab OR atg:ti,ab OR atgam:ti,ab OR thymoglobulin:ti,ab OR thymoglobuline:ti,ab OR "thymus antiserum":ti,ab
Final search 1 AND 2 AND 3
ti,ab = title, abstract
Appendix 2. Search strategy for PubMed
1. For children we used: (child[MeSH] OR paediatrics[MeSH] OR infant[MeSH] OR adolescent[MeSH] OR "preschool child"[MeSH] OR child[tiab] OR children[tiab] OR childhood[tiab] OR pediatric[tiab] OR pediatrics[tiab] OR paediatric[tiab] OR paediatrics[tiab] OR newborn[tiab] OR new‐born[tiab] OR neonate[tiab] OR neonates[tiab] OR infant[tiab] OR baby[tiab] OR toddler[tiab] OR toddlers[tiab] OR youngster[tiab] OR adolescence[tiab] OR adolescent[tiab] OR teenage[tiab] OR teenager[tiab] OR puberty[tiab] OR Schoolchild[tiab] OR "School child"[tiab] OR Boy[tiab] OR Boys[tiab] OR Boyhood[tiab] OR girl[tiab] OR girls[tiab] OR girlhood[tiab] OR youth[tiab] OR youths[tiab] OR teen[tiab] OR teens[tiab] OR preschool[tiab] OR "preschool child"[tiab] OR suckling[tiab] OR sucklings[tiab] OR juvenile[tiab] OR juveniles[tiab])
2. For lymphoma we used: Lymphoma[MeSH] OR "Lymphoproliferative disorders"[MeSH] OR Lymphoma[tiab] OR Lymphomas[tiab] OR Hodgkin[tiab] OR Hodgkin's[tiab] OR Hodgkins[tiab] OR Non‐hodgkin[tiab] OR Non‐hodgkin's[tiab] OR Non‐hodgkins[tiab] OR "Non Hodgkin"[tiab] OR "Non hodgkin's"[tiab] OR "Non hodgkins"[tiab] OR Nonhodgkin[tiab] OR Nonhodgkin's[tiab] OR Nonhodgkins[tiab] OR HD[tiab] OR HL[tiab] OR NHL[tiab] OR NHD[tiab] OR Burkitt[tiab] OR Burkitt's[tiab] OR Burkitts[tiab] OR "Malignant Lymphogranuloma"[tiab] OR "Malignant Lymphogranulomas"[tiab] OR "Malignant lymphogranulomatosis"[tiab] OR "Lymphogranuloma maligne"[tiab] OR "Lymphogranuloma malignum"[tiab] OR Lymphogranulomatosis[tiab] OR "Malignant Granuloma"[tiab] OR "Malignant Granulomas"[tiab] OR "Malignant granulomatosis"[tiab] OR "Reed hodgkin disease"[tiab] OR "Reed sternberg disease"[tiab] OR "Classic HL"[tiab] OR "Classical HL"[tiab] OR "Classic HD"[tiab] OR "Classical HD"[tiab] OR "NLP HD"[tiab] OR "NLPHD"[tiab] OR "NLP HL"[tiab] OR NLPHL[tiab] OR "LP HL"[tiab] OR "LPHL"[tiab] OR "Nodular paragranuloma"[tiab] OR "Nodular paragranulomas"[tiab] OR BL[tiab] OR BLL[tiab] OR ALCL[tiab] OR DLBCL[tiab] OR "Giant follicular lymphosarcoma"[tiab] OR "Giant follicular lymphosarcomas"[tiab] OR "Giant follicle lymphosarcoma"[tiab] OR "Giant follicle lymphosarcomas"[tiab] OR "Giant follicular blastoma"[tiab] OR "Giant follicular blastomas"[tiab] OR "Giant follicle blastoma"[tiab] OR "Giant follicle blastomas"[tiab] OR "Giant follicular lymphoblastoma"[tiab] OR "Giant follicular lymphoblastomas"[tiab] OR "Giant follicle lymphoblastoma"[tiab] OR "Giant follicle lymphoblastomas"[tiab] OR "Brill‐Symmers Disease"[tiab] OR "Brill Symmers Disease"[tiab] OR "Lymphoproliferative disease"[tiab] OR "Lymphoproliferative disorder"[tiab] OR "Lymphoproliferative disorders"[tiab] OR "Lymphoproliferative syndrome"[tiab] OR "Lymphoproliferative syndromes"[tiab] OR Lymphoreticulosis[tiab] OR "Immunoproliferative disease"[tiab] OR "Immune proliferative disease"[tiab] OR "Immunoproliferative disorder"[tiab] OR "Immunoproliferative disorders"[tiab] OR "Immune proliferative disorder"[tiab] OR "Immune proliferative disorders"[tiab] OR "Lymphomatoid Granulomatosis"[tiab] OR "Post‐transplant lymphoproliferative disease"[tiab] OR "Posttransplant lymphoproliferative disease"[tiab] OR "Post‐transplant lymphoproliferative disorder"[tiab] OR "Post‐transplant lymphoproliferative disorders"[tiab] OR "Posttransplant lymphoproliferative disorder"[tiab] OR "Posttransplant lymphoproliferative disorders"[tiab] OR PTLD[tiab] OR (("Lymph node"[tiab] OR "Lymph nodes"[tiab] OR Lymphocytic[tiab] OR Lymphoid[tiab]) AND (tumor[tiab] OR tumors[tiab] OR tumour[tiab] OR tumours[tiab] OR malignancy[tiab] OR malignancies[tiab] OR Malignant[tiab] OR Neoplasm[tiab] OR Neoplasms[tiab] OR leukemia[tiab] OR leukemias[tiab]))
3. For antibody therapy we used: Antibodies[MeSH] OR Immunotherapy[MeSH] OR "I‐131 anti‐B1 antibody"[MeSH] OR Rituximab[MeSH] OR "cAC10‐vcMMAE"[MeSH] OR "MDX‐1342" [MeSH] OR epratuzumab[MeSH] OR veltuzumab[MeSH] OR epratuzumab[MeSH] OR Ofatumumab[MeSH] OR Ibritumomab tiuxetan[MeSH] OR Alemtuzumab[MeSH] OR galiximab[MeSH] OR dacetuzumab[MeSH] OR Apolizumab[MeSH] OR mogamulizumab[MeSH] OR visilizumab[MeSH] OR Otelixizumab[MeSH] OR antibody[tiab] OR antibodies[tiab] OR mAb[tiab] OR Immunotherapy[tiab] OR "Immune therapy"[tiab] OR "Immunoglobulin therapy"[tiab] OR "Immunological therapy"[tiab] OR "Immunological treatment"[tiab] OR "Cancer immunotherapy"[tiab] OR "Tumor immunotherapy"[tiab] OR Immunoglobulin[tiab] OR Immunoglobulins[tiab] OR Rituximab[tiab] OR "Anti‐CD20"[tiab] OR Mabthera[tiab] OR Rituxan[tiab] OR reditux[tiab] OR rituxin[tiab] OR "IDEC‐C2B8"[tiab] OR "Brentuximab vedotin"[tiab] OR Adcetris[tiab] OR "anti‐CD30"[tiab] OR "cAC10‐vcMMAE"[tiab] OR "SGN‐35"[tiab] OR "SGN35"[tiab] OR "SGN 35"[tiab] OR "Anti‐CD19"[tiab] OR "MDX 1342"[tiab] OR "MDX‐1342"[tiab] OR "Anti‐MDX 1342"[tiab] OR "Anti‐MDX1342"[tiab] OR "Anti‐MDX‐1342"[tiab] OR blinatumomab[tiab] OR "medi 538"[tiab] OR medi538[tiab] OR "mt 103"[tiab] OR mt103[tiab] OR epratuzumab[tiab] OR "anti‐CD22"[tiab] OR "hLL2 agent"[tiab] OR LymphoCide[tiab] OR "immu 1903"[tiab] OR immu1903[tiab] OR "immu‐1903"[tiab] OR veltuzumab[tiab] OR "anti‐CD20 IgG"[tiab] OR "ha 20"[tiab] OR ha20[tiab] OR "ha‐20"[tiab] OR "immu 106"[tiab] OR immu106[tiab] OR "immu‐106"[tiab] OR Ofatumumab[tiab] OR Arzerra[tiab] OR "HuMax‐CD20"[tiab] OR "HuMax CD20"[tiab] OR HuMaxCD20 [tiab] OR "humac CD20"[tiab] OR "gsk 1841157"[tiab] OR gsk1841157[tiab] OR epratuzumab[tiab] OR "epratuzumab y 90"[tiab] OR "epratuzumab yttrium y 90"[tiab] OR "epratuzumab tetraxetan yttrium y 90"[tiab] OR "epratuzumab Iodine‐131"[tiab] OR "90Y‐labeled ibritumomab tiuxetan"[tiab] OR "Ibritumomab tiuxetan"[tiab] OR Ibritumomab[tiab] OR "yttrium‐90‐ibritumomab tiuxetan"[tiab] OR Zevalin[tiab] OR "in‐111 zevalin"[tiab] OR "y‐90 zevalin"[tiab] OR zevaline[tiab] OR "idec 129"[tiab] OR "idec y2b8"[tiab] OR idec129[tiab] OR Tositumomab[tiab] OR "Tositumomab I 131"[tiab] OR "tositumomab iodine‐131"[tiab] OR "iodine‐131 tositumomab"[tiab] OR "iodine‐131‐tositumomab"[tiab] OR "131I‐labeled tositumomab"[tiab] OR "Tositumomab‐I131"[tiab] OR Bexxar[tiab] OR "bexxar dosimetric"[tiab] OR "bexxar i 131 dosimetric"[tiab] OR "131I anti‐B1"[tiab] OR Alemtuzumab[tiab] OR Campath[tiab] OR MabCampath[tiab] OR "Campath‐1H"[tiab] OR Lemtrada[tiab] OR "Campath 1G"[tiab] OR "Campath‐1G"[tiab] OR "Campath‐1‐G"[tiab] OR Campath1G[tiab] OR "Campath 1M"[tiab] OR "Campath‐1M"[tiab] OR "Campath‐1‐M"[tiab] OR Campath1M[tiab] OR Campath1H[tiab] OR "Campath 1H"[tiab] OR "Campath‐1H"[tiab] OR "Campath‐1‐H"[tiab] OR "Campath 1"[tiab] OR "Anti‐CD52"[tiab] OR "ldp 103"[tiab] OR ldp103[tiab] OR galiximab[tiab] OR "Anti‐CD80"[tiab] OR "anti‐B7‐1 mAb"[tiab] OR "P‐16C10"[tiab] OR "IDEC‐114"[tiab] OR "idec 114"[tiab] OR idec114[tiab] OR dacetuzumab[tiab] OR "anti‐CD40"[tiab] OR "SGN 40"[tiab] OR "SGN40 cpd"[tiab] OR "SGN‐40"[tiab] OR SGN40[tiab] OR "hu S2C6"[tiab] OR Apolizumab[tiab] OR "Hu1D 10"[tiab] OR Hu1D10[tiab] OR Remitogen[tiab] OR "anti‐CD27 "[tiab] OR "anti‐CCR4"[tiab] OR mogamulizumab[tiab] OR "AMG 761"[tiab] OR AMG761[tiab] OR "AMG‐761"[tiab] OR "KW 0761"[tiab] OR KW0761[tiab] OR "KW‐0761"[tiab] OR "km 8761"[tiab] OR km8761[tiab] OR "anti‐CD3"[tiab] OR "OKT‐3"[tiab] OR OKT3[tiab] OR "OKT 3"[tiab] OR visilizumab[tiab] OR "hu m 291"[tiab] OR "hu m291"[tiab] OR "hum 291"[tiab] OR hum291[tiab] OR nuvion[tiab] OR "SMART anti‐CD3"[tiab] OR Nuvion[tiab] OR Otelixizumab[tiab] OR "gsk 2136525"[tiab] OR "gsk2136525 "[tiab] OR "trx 4"[tiab] OR trx4[tiab] OR "Muromonab‐CD3"[tiab] OR "muromonab‐cd3"[tiab] OR orthoclone[tiab] OR "anti‐thymocyte immunoglobulin"[tiab] OR "antithymocyte immunoglobulin"[tiab] OR "antithymocytic immunoglobulin"[tiab] OR "thymocyte antiserum"[tiab] OR "thymocyte isoantiserum"[tiab] OR "thymocyte serum"[tiab] OR "thymocyte isoantibody"[tiab] OR "anti thymocyte antiserum"[tiab] OR "antithymocyte antiserum"[tiab] OR "anti thymocyte serum "[tiab] OR "antithymocyte serum"[tiab] OR "antithymic serum"[tiab] OR "antithymocytic serum"[tiab] OR "antithymus serum"[tiab] OR "anti thymocyte globulin"[tiab] OR "antithymocyte globulin"[tiab] OR "anti thymocytic globulin"[tiab] OR "antithymocytic globulin"[tiab] OR atg[tiab] OR atgam[tiab] OR thymoglobulin[tiab] OR thymoglobuline[tiab] OR "thymus antiserum"[tiab]
Final search 1 AND 2 AND 3
tiab = title, abstract
Appendix 3. Search strategy for EMBASE (EMBASE.com)
1. For children we used: child/exp OR pediatrics/exp OR infant/exp OR adolescent/exp OR 'preschool child'/exp OR Adolescence/exp OR Toddler/exp OR Newborn/exp OR Childhood/exp OR juvenile/exp OR girl/exp OR Boy/exp OR School child/exp OR child:ab,ti OR children:ab,ti OR childhood:ab,ti OR pediatric:ab,ti OR pediatrics:ab,ti OR paediatric:ab,ti OR paediatrics:ab,ti OR newborn:ab,ti OR new‐born:ab,ti OR neonate:ab,ti OR neonates:ab,ti OR infant:ab,ti OR baby:ab,ti OR toddler:ab,ti OR toddlers:ab,ti OR youngster:ab,ti OR adolescence:ab,ti OR adolescent:ab,ti OR teenage:ab,ti OR teenager:ab,ti OR puberty:ab,ti OR Schoolchild:ab,ti OR 'School child':ab,ti OR Boy:ab,ti OR Boys:ab,ti OR Boyhood:ab,ti OR girl:ab,ti OR girls:ab,ti OR girlhood:ab,ti OR youth:ab,ti OR youths:ab,ti OR teen:ab,ti OR teens:ab,ti OR preschool:ab,ti OR 'preschool child':ab,ti OR suckling:ab,ti OR sucklings:ab,ti OR juvenile:ab,ti OR juveniles:ab,ti
2 For lymphoma we used: Lymphoma/exp OR 'Lymphoproliferative disease'/exp OR Lymphoma:ab,ti OR Lymphomas:ab,ti OR Hodgkin:ab,ti OR 'Hodgkin/s':ab,ti OR Hodgkins:ab,ti OR Non‐hodgkin:ab,ti OR 'Non‐hodgkin/s':ab,ti OR Non‐hodgkins:ab,ti OR 'Non hodgkin':ab,ti OR 'Non hodgkin/s':ab,ti OR 'Non hodgkins':ab,ti OR Nonhodgkin:ab,ti OR 'Nonhodgkin/s':ab,ti OR Nonhodgkins:ab,ti OR HD:ab,ti OR HL:ab,ti OR NHL:ab,ti OR NHD:ab,ti OR Burkitt:ab,ti OR 'Burkitt/s':ab,ti OR Burkitts:ab,ti OR 'Malignant Lymphogranuloma':ab,ti OR 'Malignant Lymphogranulomas':ab,ti OR 'Malignant lymphogranulomatosis':ab,ti OR 'Lymphogranuloma maligne':ab,ti OR 'Lymphogranuloma malignum':ab,ti OR Lymphogranulomatosis:ab,ti OR 'Malignant Granuloma':ab,ti OR 'Malignant Granulomas':ab,ti OR 'Malignant granulomatosis':ab,ti OR 'Reed hodgkin disease':ab,ti OR 'Reed sternberg disease':ab,ti OR 'Classic HL':ab,ti OR 'Classical HL':ab,ti OR 'Classic HD':ab,ti OR 'Classical HD':ab,ti OR 'NLP HD':ab,ti OR 'NLPHD':ab,ti OR 'NLP HL':ab,ti OR NLPHL:ab,ti OR 'LP HL':ab,ti OR 'LPHL':ab,ti OR 'Nodular paragranuloma':ab,ti OR 'Nodular paragranulomas':ab,ti OR BL:ab,ti OR BLL:ab,ti OR ALCL:ab,ti OR DLBCL:ab,ti OR 'Giant follicular lymphosarcoma':ab,ti OR 'Giant follicular lymphosarcomas':ab,ti OR 'Giant follicle lymphosarcoma':ab,ti OR 'Giant follicle lymphosarcomas':ab,ti OR 'Giant follicular blastoma':ab,ti OR 'Giant follicular blastomas':ab,ti OR 'Giant follicle blastoma':ab,ti OR 'Giant follicle blastomas':ab,ti OR 'Giant follicular lymphoblastoma':ab,ti OR 'Giant follicular lymphoblastomas':ab,ti OR 'Giant follicle lymphoblastoma':ab,ti OR 'Giant follicle lymphoblastomas':ab,ti OR 'Brill‐Symmers Disease':ab,ti OR 'Brill Symmers Disease':ab,ti OR 'Lymphoproliferative disease':ab,ti OR 'Lymphoproliferative disorder':ab,ti OR 'Lymphoproliferative disorders':ab,ti OR 'Lymphoproliferative syndrome':ab,ti OR 'Lymphoproliferative syndromes':ab,ti OR Lymphoreticulosis:ab,ti OR 'Immunoproliferative disease':ab,ti OR 'Immune proliferative disease':ab,ti OR 'Immunoproliferative disorder':ab,ti OR 'Immunoproliferative disorders':ab,ti OR 'Immune proliferative disorder':ab,ti OR 'Immune proliferative disorders':ab,ti OR 'Lymphomatoid Granulomatosis':ab,ti OR 'Post‐transplant lymphoproliferative disease':ab,ti OR 'Posttransplant lymphoproliferative disease':ab,ti OR 'Post‐transplant lymphoproliferative disorder':ab,ti OR 'Post‐transplant lymphoproliferative disorders':ab,ti OR 'Posttransplant lymphoproliferative disorder':ab,ti OR 'Posttransplant lymphoproliferative disorders':ab,ti OR PTLD:ab,ti OR (('Lymph node':ab,ti OR 'Lymph nodes':ab,ti OR Lymphocytic:ab,ti OR Lymphoid:ab,ti) AND (tumor:ab,ti OR tumors:ab,ti OR tumour:ab,ti OR tumours:ab,ti OR malignancy:ab,ti OR malignancies:ab,ti OR Malignant:ab,ti OR Neoplasm:ab,ti OR Neoplasms:ab,ti OR leukemia:ab,ti OR leukemias:ab,ti))
3. For antibody therapy we used: Antibody/exp OR Immunotherapy/exp OR antibody:ab,ti OR antibodies:ab,ti OR mAb:ab,ti OR Immunotherapy:ab,ti OR 'Immune therapy':ab,ti OR 'Immunoglobulin therapy':ab,ti OR 'Immunological therapy':ab,ti OR 'Immunological treatment':ab,ti OR 'Cancer immunotherapy':ab,ti OR 'Tumor immunotherapy':ab,ti OR Immunoglobulin:ab,ti OR Immunoglobulins:ab,ti OR Rituximab:ab,ti OR 'Anti‐CD20':ab,ti OR Mabthera:ab,ti OR Rituxan:ab,ti OR reditux:ab,ti OR rituxin:ab,ti OR 'IDEC‐C2B8':ab,ti OR 'Brentuximab vedotin':ab,ti OR Adcetris:ab,ti OR 'anti‐CD30':ab,ti OR 'cAC10‐vcMMAE':ab,ti OR 'SGN‐35':ab,ti OR 'SGN35':ab,ti OR 'SGN 35':ab,ti OR 'Anti‐CD19':ab,ti OR 'MDX 1342':ab,ti OR 'MDX‐1342':ab,ti OR 'Anti‐MDX 1342':ab,ti OR 'Anti‐MDX1342':ab,ti OR 'Anti‐MDX‐1342':ab,ti OR blinatumomab:ab,ti OR 'medi 538':ab,ti OR medi538:ab,ti OR 'mt 103':ab,ti OR mt103:ab,ti OR epratuzumab:ab,ti OR 'anti‐CD22':ab,ti OR 'hLL2 agent':ab,ti OR LymphoCide:ab,ti OR 'immu 1903':ab,ti OR immu1903:ab,ti OR 'immu‐1903':ab,ti OR veltuzumab:ab,ti OR 'anti‐CD20 IgG':ab,ti OR 'ha 20':ab,ti OR ha20:ab,ti OR 'ha‐20':ab,ti OR 'immu 106':ab,ti OR immu106:ab,ti OR 'immu‐106':ab,ti OR Ofatumumab:ab,ti OR Arzerra:ab,ti OR 'HuMax‐CD20':ab,ti OR 'HuMax CD20':ab,ti OR HuMaxCD20:ab,ti OR 'humac CD20':ab,ti OR 'gsk 1841157':ab,ti OR gsk1841157:ab,ti OR epratuzumab:ab,ti OR 'epratuzumab y 90':ab,ti OR 'epratuzumab yttrium y 90':ab,ti OR 'epratuzumab tetraxetan yttrium y 90':ab,ti OR 'epratuzumab Iodine‐131':ab,ti OR '90Y‐labeled ibritumomab tiuxetan':ab,ti OR 'Ibritumomab tiuxetan':ab,ti OR Ibritumomab:ab,ti OR 'yttrium‐90‐ibritumomab tiuxetan':ab,ti OR Zevalin:ab,ti OR 'in‐111 zevalin':ab,ti OR 'y‐90 zevalin':ab,ti OR zevaline:ab,ti OR 'idec 129':ab,ti OR 'idec y2b8':ab,ti OR idec129:ab,ti OR Tositumomab:ab,ti OR 'Tositumomab I 131':ab,ti OR 'tositumomab iodine‐131':ab,ti OR 'iodine‐131 tositumomab':ab,ti OR 'iodine‐131‐tositumomab':ab,ti OR '131I‐labeled tositumomab':ab,ti OR 'Tositumomab‐I131':ab,ti OR Bexxar:ab,ti OR 'bexxar dosimetric':ab,ti OR 'bexxar i 131 dosimetric':ab,ti OR '131I anti‐B1':ab,ti OR Alemtuzumab:ab,ti OR Campath:ab,ti OR MabCampath:ab,ti OR 'Campath‐1H':ab,ti OR Lemtrada:ab,ti OR 'Campath 1G':ab,ti OR 'Campath‐1G':ab,ti OR 'Campath‐1‐G':ab,ti OR Campath1G:ab,ti OR 'Campath 1M':ab,ti OR 'Campath‐1M':ab,ti OR 'Campath‐1‐M':ab,ti OR Campath1M:ab,ti OR Campath1H:ab,ti OR 'Campath 1H':ab,ti OR 'Campath‐1H':ab,ti OR 'Campath‐1‐H':ab,ti OR 'Campath 1':ab,ti OR 'Anti‐CD52':ab,ti OR 'ldp 103':ab,ti OR ldp103:ab,ti OR galiximab:ab,ti OR 'Anti‐CD80':ab,ti OR 'anti‐B7‐1 mAb':ab,ti OR 'P‐16C10':ab,ti OR 'IDEC‐114':ab,ti OR 'idec 114':ab,ti OR idec114:ab,ti OR dacetuzumab:ab,ti OR 'anti‐CD40':ab,ti OR 'SGN 40':ab,ti OR 'SGN40 cpd':ab,ti OR 'SGN‐40':ab,ti OR SGN40:ab,ti OR 'hu S2C6':ab,ti OR Apolizumab:ab,ti OR 'Hu1D 10':ab,ti OR Hu1D10:ab,ti OR Remitogen:ab,ti OR 'anti‐CD27 ':ab,ti OR 'anti‐CCR4':ab,ti OR mogamulizumab:ab,ti OR 'AMG 761':ab,ti OR AMG761:ab,ti OR 'AMG‐761':ab,ti OR 'KW 0761':ab,ti OR KW0761:ab,ti OR 'KW‐0761':ab,ti OR 'km 8761':ab,ti OR km8761:ab,ti OR 'anti‐CD3':ab,ti OR 'OKT‐3':ab,ti OR OKT3:ab,ti OR 'OKT 3':ab,ti OR visilizumab:ab,ti OR 'hu m 291':ab,ti OR 'hu m291':ab,ti OR 'hum 291':ab,ti OR hum291:ab,ti OR nuvion:ab,ti OR 'SMART anti‐CD3':ab,ti OR Nuvion:ab,ti OR Otelixizumab:ab,ti OR 'gsk 2136525':ab,ti OR 'gsk2136525 ':ab,ti OR 'trx 4':ab,ti OR trx4:ab,ti OR 'Muromonab‐CD3':ab,ti OR 'muromonab‐cd3':ab,ti OR orthoclone:ab,ti OR 'anti‐thymocyte immunoglobulin':ab,ti OR 'antithymocyte immunoglobulin':ab,ti OR 'antithymocytic immunoglobulin':ab,ti OR 'thymocyte antiserum':ab,ti OR 'thymocyte isoantiserum':ab,ti OR 'thymocyte serum':ab,ti OR 'thymocyte isoantibody':ab,ti OR 'anti thymocyte antiserum':ab,ti OR 'antithymocyte antiserum':ab,ti OR 'anti thymocyte serum ':ab,ti OR 'antithymocyte serum':ab,ti OR 'antithymic serum':ab,ti OR 'antithymocytic serum':ab,ti OR 'antithymus serum':ab,ti OR 'anti thymocyte globulin':ab,ti OR 'antithymocyte globulin':ab,ti OR 'anti thymocytic globulin':ab,ti OR 'antithymocytic globulin':ab,ti OR atg:ab,ti OR atgam:ab,ti OR thymoglobulin:ab,ti OR thymoglobuline:ab,ti OR 'thymus antiserum':ab,ti
Final search 1 AND 2 AND 3
ab,ti = abstract, title ; / = Emtree term
Appendix 4. Search strategy for the WHO ICTRP search portal (via the "Advanced search" page)
The following strategy is used to search WHO ICTRP:
In Condition: (lymphoma)
In Intervention: antibody search up to 250 characters:
1. antibody OR antibodies OR mAb OR immunotherapy OR immune therapy OR immunological therapy OR immunological treatment OR immunoglobulin OR immunoglobulins OR rituximab OR anti‐CD20 OR mabthera OR rituxan OR reditux OR rituxin OR IDEC‐C2B8
2. brentuximab vedotin OR adcetris OR anti‐CD30 OR cAC10‐vcMMAE OR SGN 35 OR Anti‐CD19 OR MDX 1342 OR blinatumomab OR medi 538 OR mt 103 OR epratuzumab OR anti‐CD22 OR hLL2 agent OR lymphoCide OR immu 1903 OR veltuzumab OR anti‐CD20 IgG OR ha 20
3. immu 106 OR ofatumumab OR Arzerra OR HuMax CD20 OR humac CD20 OR gsk 1841157 OR epratuzumab OR ibritumomab OR zevalin OR zevaline OR idec 129 OR idec y2b8 OR tositumomab OR bexxar OR bexxar i 131 dosimetric OR 131I anti‐B1 OR alemtuzumab OR campath
4. MabCampath OR Lemtrada OR Anti‐CD52 OR ldp 103 OR galiximab OR Anti‐CD80 OR anti‐B7‐1 mAb OR P‐16C10 OR IDEC‐114 OR idec 114 OR dacetuzumab OR anti‐CD40 OR SGN 40 OR hu S2C6 OR apolizumab OR Hu1D 10 OR remitogen OR anti‐CD27 OR anti‐CCR4
5. mogamulizumab OR AMG 761 OR KW 0761 OR km 8761 OR anti‐CD3 OR OKT 3 OR visilizumab OR hu m 291 OR hum 291 OR nuvion OR SMART anti‐CD3 OR otelixizumab OR gsk 2136525 OR trx 4 OR muromonab‐CD3 OR orthoclone OR antithymocyte immunoglobulin
6. antithymocytic immunoglobulin OR thymocyte antiserum OR thymocyte isoantibody OR antithymocyte antiserum OR antithymocyte serum OR antithymic serum OR antithymocytic serum
7. antithymus serum OR antithymocyte globulin OR antithymocytic globulin OR atg OR atgam OR thymoglobulin OR thymoglobuline OR thymus antiserum
Tick off: Search for Clinical trials in Children.
Appendix 5. Search strategy for Clinicaltrial.gov (via the "Advanced search" page)
In Condition: (lymphoma)
In Intervention: antibody search up to 250 characters:
1. antibody OR antibodies OR mAb OR immunotherapy OR immune therapy OR immunological therapy OR immunological treatment OR immunoglobulin OR immunoglobulins OR rituximab OR anti‐CD20 OR mabthera OR rituxan OR reditux OR rituxin OR IDEC‐C2B8
2. brentuximab vedotin OR adcetris OR anti‐CD30 OR cAC10‐vcMMAE OR SGN 35 OR anti‐CD19 OR MDX 1342 OR blinatumomab OR medi 538 OR mt 103 OR epratuzumab OR anti‐CD22 OR hLL2 agent OR LymphoCide OR immu 1903 OR veltuzumab
3. anti‐CD20 IgG OR ha 20 OR immu 106 OR ofatumumab OR arzerra OR HuMax CD20 OR humac CD20 OR gsk 1841157 OR epratuzumab OR ibritumomab OR zevalin OR zevaline OR idec 129 OR idec y2b8 OR tositumomab OR bexxar OR bexxar i 131 dosimetric
4. 131I anti‐B1 OR alemtuzumab OR campath OR MabCampath OR lemtrada OR anti‐CD52 OR ldp 103 OR galiximab OR anti‐CD80 OR anti‐B7‐1 mAb OR P‐16C10 OR IDEC‐114 OR idec 114 OR dacetuzumab OR anti‐CD40 OR SGN 40 OR hu S2C6
5. apolizumab OR Hu1D 10 OR remitogen OR anti‐CD27 OR anti‐CCR4 OR mogamulizumab OR AMG 761 OR KW 0761 OR km 8761 OR anti‐CD3 OR OKT 3 OR visilizumab OR hu m 291 OR hum 291 OR nuvion OR SMART anti‐CD3 OR otelixizumab
6. gsk 2136525 OR trx 4 OR muromonab‐CD3 OR orthoclone OR antithymocyte immunoglobulin OR antithymocytic immunoglobulin OR thymocyte antiserum OR thymocyte isoantibody OR antithymocyte antiserum OR antithymocyte serum
7. antithymic serum OR antithymocytic serum OR antithymus serum OR antithymocyte globulin OR antithymocytic globulin OR atg OR atgam OR thymoglobulin OR thymoglobuline OR thymus antiserum
Tick off with Additional Criteria, Age Group: Child (birth‐17)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bilić 2009 | Not an RCT or CCT |
| Bilić 2010 | Not an RCT or CCT |
| Cairo 2009 | Not an RCT or CCT |
| Cairo 2010 | Not an RCT or CCT |
| Cooney‐Qualter 2007 | Not an RCT or CCT |
| Fanale 2011 | Not an RCT or CCT |
| Fedorova 2009 | Not an RCT or CCT |
| Frazer 2012 | Not an RCT or CCT |
| Gallego 2010 | Not an RCT or CCT |
| Goldman 2009a | Not an RCT or CCT |
| Goldman 2009b | Not an RCT or CCT |
| Goldman 2011a | Not an RCT or CCT |
| Goldman 2011b | Not an RCT or CCT |
| Goldman 2013 | Not an RCT or CCT |
| Goldman 2014 | Not an RCT or CCT |
| Griffin 2009 | Not an RCT or CCT |
| Gross 2009a | Not an RCT or CCT |
| Gross 2009b | Not an RCT or CCT |
| Gross 2012 | Not an RCT or CCT |
| Locatelli 2013 | Not an RCT or CCT |
| Maecker‐Kolhoff 2011 | Not an RCT or CCT |
| Maecker‐Kolhoff 2012 | Not an RCT or CCT |
| Meinhardt 2009 | Not an RCT or CCT |
| Meinhardt 2010 | Not an RCT or CCT |
| Reiter 2009 | Not an RCT or CCT |
| Samochatova 2009 | Not an RCT or CCT |
| Samochatova 2014 | Not an RCT or CCT |
CCT: controlled clinical trial; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01516580.
| Trial name or title | Intergroup Randomized Trial for Children or Adolescents With B‐Cell Non Hodgkin Lymphoma or B‐Acute Leukemia: Rituximab Evaluation in High Risk Patients |
| Methods | Randomised |
| Participants | Children or adolescents with B‐cell non‐Hodgkin's lymphoma or mature B‐cell leukaemia Burkitt's‐type |
| Interventions | Intervention 1: vincristine, cyclophosphamide, methotrexate, doxorubicin, cytarabine, ara‐C Intervention 2: rituximab, vincristine, cyclophosphamide, methotrexate, doxorubicin, cytarabine, ara‐C |
| Outcomes | Event‐free survival; survival; acute toxicity; long‐term toxicity |
| Starting date | December 2011 |
| Contact information | Catherine Patte, MD; telephone +33 1 42 11 41 76; catherine.patte@igr.fr Thomas Gross, MD; telephone 614 722 3552; thomas.gross@nationwidechildrens.org |
| Notes |
NCT01595048.
| Trial name or title | Combination Chemotherapy With or Without Rituximab in Treating Younger Patients With Stage III‐IV Non‐Hodgkin Lymphoma or B‐Cell Acute Leukemia |
| Methods | Randomised |
| Participants | Childhood B acute lymphoblastic leukaemia; childhood Burkitt's leukaemia; childhood diffuse large cell lymphoma; mediastinal (thymic) large B‐cell lymphoma; stage III childhood large cell lymphoma; stage IV childhood large cell lymphoma |
| Interventions | Rituximab; prednisone; etoposide; doxorubicin hydrochloride; cytarabine; vincristine sulphate; cyclophosphamide; methotrexate; methylprednisolone; leucovorin calcium; therapeutic hydrocortisone |
| Outcomes | Event‐free survival; survival; complete remission rate compared between arms by logistic regression; acute‐term and long‐term toxicity as assessed by the National Cancer Institute Common Terminology Criteria version 4.0 |
| Starting date | June 2012 |
| Contact information | Thomas Gross, MD; 614 722 3552; thomas.gross@nationwidechildrens.org |
| Notes |
NCT01979536.
| Trial name or title | Brentuximab Vedotin or Crizotinib and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II‐IV Anaplastic Large Cell Lymphoma |
| Methods | Randomised |
| Participants | Stage II childhood anaplastic large cell lymphoma; stage III childhood anaplastic large cell lymphoma; stage IV childhood anaplastic large cell lymphoma |
| Interventions | Brentuximab vedotin; crizotinib; dexamethasone; ifosfamide; methotrexate; cytarabine; etoposide; cyclophosphamide; doxorubicin hydrochloride |
| Outcomes | Occurrence of grade 3+ non‐haematological adverse events, using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0; event‐free survival |
| Starting date | November 2013 |
| Contact information | Eric Lowe; Children's Oncology Group |
| Notes |
NCT02166463.
| Trial name or title | Brentuximab Vedotin and Combination Chemotherapy in Treating Younger Patients With Newly Diagnosed Hodgkin Lymphoma |
| Methods | Randomised |
| Participants | Stage II childhood Hodgkin's lymphoma; stage III childhood Hodgkin's lymphoma; stage IV childhood Hodgkin's lymphoma |
| Interventions | Doxorubicin hydrochloride; bleomycin sulphate; vincristine sulphate; etoposide; prednisone; cyclophosphamide; brentuximab vedotin |
| Outcomes | Event‐free survival, where events include disease progression or relapse, second malignancy or death; proportion of participants experiencing grade 3+ peripheral neuropathy assessed by modified Balis scale |
| Starting date | March 2015 |
| Contact information | Sharon Castellino; Children's Oncology Group |
| Notes |
Differences between protocol and review
We did not contact experts in the field to inquire about randomised controlled trial (RCTs) or controlled clinical trials that we may have missed with our search as we had proposed in the study protocol. Experts in the field authored/co‐authored the publications on recent phase I/II studies that we included in the 'Additional tables' section of this review. In addition, they conducted the ongoing studies that we included in the 'Characteristics of ongoing studies' table. We are convinced that these expert authors would have mentioned any RCTs answering our research question in their articles, if they had knowledge of them. Therefore, we decided not to contact them in person, in addition to reporting the results of their studies and mentioning their ongoing trials.
Contributions of authors
VM de Zwart: designed and co‐ordinated the review, collected data, designed the search strategies, undertook the searches, screened the search results, organised retrieval of papers, screened retrieved papers against eligibility criteria, managed and collected data for the review, entered data into Review Manager 5, analysed and interpreted data (case studies and phase I/II trials), provided a methodological perspective and wrote the protocol and review.
SC Gouw: provided a methodological and clinical perspective, and provided general advice on the review.
FAG Meyer‐Wentrup: conceived the review, collected data, screened the search results, organised retrieval of papers, screened retrieved papers against eligibility criteria, provided a methodological and clinical perspective, and provided general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
KWF, Netherlands.
Friederike Meyer‐Wentrup was a KWF‐research fellow. Her salary and research expenses were paid for by the KWF.
Declarations of interest
The authors declare that there are no conflicts of interest.
New
References
References to studies excluded from this review
Bilić 2009 {published data only}
- Bilić E, Femeniç R, Rajiç L, Konja J, Simat M. Treatment of pediatric B‐NHL with protocol NHL‐BFM‐95 and rituximab: a single centre experience. Haematologica Meeting Reports 2009;3(5):31. [Google Scholar]
Bilić 2010 {published data only}
- Bilić E, Femenić R, Konja J, Simat M, Dubravcić K, Batinić D, et al. CD20 positive childhood B‐non Hodgkin lymphoma (B‐NHL): morphology, immunophenotype and a novel treatment approach: a single center experience. Collegium Antropologicum 2010;34(1):171‐5. [PubMed] [Google Scholar]
Cairo 2009 {published data only}
- Cairo MS, Lynch J, Harrison L, Perkins S, Sanger W, Gross TG, et al. Results of the addition of rasburicase and rituximab to the FAB/LMB96 chemotherapy backbone in children and adolescents with mature B‐NHL: a Children's Oncology Group report. Pediatric Blood and Cancer 2009;53(5):858. [Google Scholar]
Cairo 2010 {published data only}
- Cairo MS, Lynch J, Harrison L, Perkins S, Shiramizu B, Gross TG, et al. Safety, kinetics, and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B‐NHL: a Children's Oncology Group report. Journal of Clinical Oncology 2010;28(15s):Abstr 9536. [Google Scholar]
Cooney‐Qualter 2007 {published data only}
- Cooney‐Qualter E, Krailo M, Angiolillo A, Fawwaz RA, Wiseman G, Harrison L, et al. A phase I study of 90Yttrium‐Ibritumomab‐Tiuxetan in children and adolescents with relapsed/refractory CD20‐positive non‐Hodgkin's lymphoma: a Children's Oncology Group study. Clinical Cancer Research 2007;13(18 (Pt 2)):5652‐60s. [DOI] [PubMed] [Google Scholar]
Fanale 2011 {published data only}
- Fanale M, Franklin A, Radhakrishnan R, Termuhlen A, Gopal AK, Shustov A, et al. Complete remissions observed in a subset of pediatric patients with CD30‐expressing malignant lymphomas treated in clinical studies of brentuximab vedotin (SGN‐35). European Journal of Cancer 2011;47:S640. [Google Scholar]
Fedorova 2009 {published data only}
- Fedorova A, Belevtsev M, Vashkevich E, Savva N, Aleinikova O. Rituximab in the first line therapy of advanced B‐mature lymphoma and leukemia in children and adolescents: preliminary results. Haematologica Meeting Reports 2009;3(5):28‐9. [Google Scholar]
Frazer 2012 {published data only}
- Frazer JK, Goldman S, Smith L, Harrison L, Perkins SL, Cairo MS. Efficacy of rituximab plus FAB group C chemotherapy without CNS radiation in CNS‐positive pediatric Burkitt lymphoma/leukemia: a report from the Children's Oncology Group. Journal of Clinical Oncology 2012;30(15):9501. [Google Scholar]
Gallego 2010 {published data only}
- Gallego S, Llort A, Gros L, Sanchez de Toledo J Jr, Bueno J, Moreno A, et al. Post‐transplant lymphoproliferative disorders in children: the role of chemotherapy in the era of rituximab. Pediatric Transplantation 2010;14(1):61‐6. [DOI] [PubMed] [Google Scholar]
Goldman 2009a {published data only}
- Goldman S, Lynch J, Harrison L, VandeVen C, Gross T, Shiramizu B, et al. Rituximab combined with FAB group B4 therapy in children and adolescents with stage III/IV mature B‐NHL: a Children's Oncology Group report. Haematologica Meeting Reports 2009;3(15):83‐4. [Google Scholar]
Goldman 2009b {published data only}
- Goldman SC, Lynch J, Harrison L, Gross TG, Shiramizu B, Sanger W, et al. Preliminary results of the addition of rasburicase to the reduction cycle and rituximab to the induction and consolidation cycles of FAB group C chemotherapy in children and adolescents with advanced stage (bone marrow (plus or minus) CNS) mature B‐cell non‐Hodgkin lymphoma (B‐NHL): a Children's Oncology Group report. Blood 2009;114(22):Abstract 104. [Google Scholar]
Goldman 2011a {published data only}
- Goldman S, Smith L, Perkins SL, Shiramizu B, Gross T, Sanger W, et al. Outcome and pharmacokinetic (PK) analysis of adding rituximab to fab chemotherapy in children and adolescents with advanced mature B‐NHL/ leukemia: a Children's Oncology Group report. Pediatric Blood and Cancer 2011;57(5):737‐8. [Google Scholar]
Goldman 2011b {published data only}
- Goldman S, Galardy PJ, Smith L, Perkins SL, Shiramizu B, Gross T, et al. The efficacy of rasburicase and rituximab combined with FAB chemotherapy in children and adolescents with newly diagnosed stage III/IV, BM+ and CNS+ mature B‐NHL: a Children's Oncology Group report. Blood 2011;118(21):Abstract 2702. [Google Scholar]
Goldman 2013 {published data only}
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B‐cell non‐Hodgkin lymphoma: a Children's Oncology Group report. Leukemia 2013;27(5):1174‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 2014 {published data only}
- Goldman S, Smith L, Galardy P, Perkins S, Frazer JK, Sanger W, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow‐positive Burkitt lymphoma/leukaemia: a Children's Oncology Group report. British Journal of Haematology 2014;167(3):394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2009 {published data only}
- Griffin TC, Weitzman S, Weinstein H, Chang M, Cairo MS, Hutchison R, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B‐cell (CD20+) non‐Hodgkin lymphoma and mature B‐cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatric Blood and Cancer 2009;52(2):177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 2009a {published data only}
- Gross TG, Orjuela M, Hayashi R, Park JR, Perkins SL, Cairo MS, et al. Pediatric, refractory post‐transplant lymphoproliferative disease (PTLD) following solid organ transplantation (SOT): a Children's Oncology Group phase II study. Haematologica Meeting Reports 2009;3(5):74. [Google Scholar]
Gross 2009b {published data only}
- Gross TG, Orjuela MA, Perkins SL, Park JR, Lynch CJ, Cairo MS, et al. Preliminary results of low‐dose chemotherapy for pediatric patients with EBV(+), CD20(+) post‐transplant lymphoproliferative disease (PTLD) following solid organ transplantation (SOT): a Children's Oncology Group phase II study. Pediatric Transplantation 2009;13(1):109. [Google Scholar]
Gross 2012 {published data only}
- Gross TG, Orjuela MA, Perkins SL, Park JR, Lynch JC, Cairo MS, et al. Low dose chemotherapy and rituximab for PTLD: a Children's Oncology Group report. American Journal of Transplantation 2012;12(11):3069‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Locatelli 2013 {published data only}
- Locatelli F. Phase 1/2 study of brentuximab vedotin in pediatric patients with relapsed or refractory (R/R) Hodgkin lymphoma (HL) or systemic anaplastic large‐cell lymphoma (SALCL): preliminary phase 2 data for brentuximab vedotin 1.8 mg/kg in the HL study arm. Blood 2013;122(21):Abstract 4378. [Google Scholar]
Maecker‐Kolhoff 2011 {published data only}
- Maecker‐Kolhoff B, Meissner B, Zimmerman M, Kebelmann‐Betzing C, Henze G, Klein C. Post‐transplant lymphoproliferative disorders in pediatric solid organ transplant recipients ‐ interim analysis of trial Ped‐PTLD‐Pilot‐2005. Monatsschrift fur Kinderheilkunde 2011;159(4):399. [Google Scholar]
Maecker‐Kolhoff 2012 {published data only}
- Maecker‐Kolhoff B, Meissner B, Zimmerman M, Kebelmann‐Betzing C, Henze G, Klein C. Post‐transplant lymphoproliferative disorders in pediatric solid organ transplant recipients ‐ interim analysis of trial Ped‐PTLD‐Pilot‐2005. Transplantation 2012;94:25. [Google Scholar]
Meinhardt 2009 {published data only}
- Meinhardt A, Burkhardt B, Zimmerman M, Borkhardt A, Kontny U, Klingebiel T, et al. Results of a phase II window study on rituximab in newly diagnosed pediatric mature b‐cell non‐Hodgkin lymphoma (B‐NHL)/Burkitt's leukemia (B‐ALL). Haematologica Meeting Reports 2009;4(5):21. [Google Scholar]
Meinhardt 2010 {published data only}
- Meinhardt A, Burkhardt B, Zimmerman M, Borkhardt A, Kontny U, Klingebiel T, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B‐cell non‐Hodgkin's lymphoma and Burkitt leukemia. Journal of Clinical Oncology 2010;28(19):3115‐21. [DOI] [PubMed] [Google Scholar]
Reiter 2009 {published data only}
- Reiter A, Meinhardt A, Burkhardt B, Zimmerman M, Borkhardt A, Kontny U, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B‐cell non‐Hodgkin lymphoma (NHL). Journal of Clinical Oncology 2009;27(15):10000. [DOI] [PubMed] [Google Scholar]
Samochatova 2009 {published data only}
- Samochatova EV, Shelikhova LN, Myakova NV, Litvinov D, Belogurova MB, Gechina L, et al. Treatment of pediatric advance staged mature B‐NHL/B‐ALL with intensive chemotherapy+rituximab: results of 4 years multicenter study (04.2004‐05.2008). Haematologica Meeting Reports 2009;3(5):83. [Google Scholar]
Samochatova 2014 {published data only}
- Samochatova EV, Maschan AA, Shelikhova LN, Myakova NV, Belogurova MB, Khlebnikova OP, et al. Therapy of advanced‐stage mature B‐cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B‐NHL 2004M protocol): the results of a multicenter study. Journal of Pediatric Hematology/Oncology 2014;36(5):395‐401. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01516580 {published data only}
- NCT01516580. Intergroup randomized trial for children or adolescents with B‐cell non Hodgkin lymphoma or B‐acute leukemia: rituximab evaluation in high risk patients. clinicaltrials.gov/ct2/show/NCT01516580 (accessed 18 December 2015). [NCT01516580]
NCT01595048 {published data only}
- NCT01595048. Combination chemotherapy with or without rituximab in treating younger patients with stage III‐IV non‐Hodgkin lymphoma or B‐cell acute leukemia. clinicaltrials.gov/ct2/show/NCT01595048 (accessed 18 December 2015). [NCT01595048]
NCT01979536 {published data only}
- NCT01979536. Brentuximab vedotin or crizotinib and combination chemotherapy in treating patients with newly diagnosed stage II‐IV anaplastic large cell lymphoma. clinicaltrials.gov/ct2/show/NCT01979536 (accessed 18 December 2015). [NCT01979536]
NCT02166463 {published data only}
- NCT02166463. Brentuximab vedotin and combination chemotherapy in treating younger patients with newly diagnosed Hodgkin lymphoma. clinicaltrials.gov/ct2/show/NCT02166463 (accessed 18 December 2015). [NCT02166463]
Additional references
Bickert 2002
- Bickert BM. Treatment of common childhood malignancies. Journal of Pharmacy Practice 2002;15(1):42‐51. [Google Scholar]
Brugieres 2009
- Brugieres L. Anaplastic large cell lymphoma (ALCL): current management. Haematologica Meeting Reports 2009;3(5):40‐1. [Google Scholar]
Cairo 2005
- Cairo MS, Raetz E, Lim MS, Davenport V, Perkins SL. Childhood and adolescent non‐Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatric Blood and Cancer 2005;45(6):753‐69. [DOI] [PubMed] [Google Scholar]
Capitini 2010
- Capitini CM, MacKall CL, Wayne AS. Immune‐based therapeutics for pediatric cancer. Expert Opinion on Biological Therapy 2010;10(2):163‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo 2008
- Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Experimental Hematology 2008;36(7):755‐68. [DOI] [PubMed] [Google Scholar]
Cioc 2008
- Cioc AM, Vanderwerf SM, Peterson BA, Robu VG, Forster CL, Pambuccian SE. Rituximab‐induced changes in hematolymphoid tissues found at autopsy. American Journal of Clinical Pathology 2008;130(4):604‐12. [DOI] [PubMed] [Google Scholar]
Daw 2011
- Daw S, Wynn R, Wallace H. Management of relapsed and refractory classical Hodgkin lymphoma in children and adolescents. British Journal of Haematology 2011;152(3):249‐60. [DOI] [PubMed] [Google Scholar]
Deffenbacher 2012
- Deffenbacher KE, Iqbal J, Sanger W, Shen Y, Lachel C, Liu Z, et al. Molecular distinctions between pediatric and adult mature B‐cell non‐Hodgkin lymphomas identified through genomic profiling. Blood 2012;119(16):3757‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorde‐Grosjean 2012
- Gorde‐Grosjean S, Oberlin O, Leblanc T, Pacquement H, Donadieu J, Lambilliotte A, et al. Outcome of children and adolescents with recurrent/refractory classical Hodgkin lymphoma : a study from the Societe Francaise de Lutte contre le Cancer des Enfants et des Adolescents (SFCE). British Journal of Haematology 2012;158(5):649‐56. [DOI] [PubMed] [Google Scholar]
Gross 2007
- Gross TG, Termuhlen AM. Pediatric non‐Hodgkin's lymphoma. Current Oncology Reports 2007;9(6):459‐65. [DOI] [PubMed] [Google Scholar]
Grupp 2008
- Grupp SA, Verneris M, Sondel PM, Cooper LJN. Immunotherapy for pediatric cancer. Biology of Blood and Marrow Transplantation 2008;14(1):33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesseling 2013
- Hesseling P, Israels T, Harif M, Chantada G, Molyneux E. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource limited setting. Pediatric Blood and Cancer 2013;60(3):357‐62. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jaglowski 2009
- Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Seminars in Oncology 2009;36(5):381‐418. [DOI] [PubMed] [Google Scholar]
Janeway 2001
- Janeway CA, Travers P, Walport M, Shlomchick MJ. Immunobiology. 5th Edition. New York: Garland Science, 2001. [Google Scholar]
Kremer 2008
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2008, Issue 5. Art. No.: CHILDCA.
MacDonald 2010
- MacDonald T. Pediatric cancer: a comprehensive review. Part II: chemotherapy, monoclonal antibodies and tyrosine kinase inhibitors. Canadian Pharmacists Journal 2010;143(5):240‐7. [Google Scholar]
Meyer‐Wentrup 2013
- Meyer‐Wentrup F, Zwart V, Bierings M. Antibody therapy of pediatric B‐cell lymphoma. Frontiers in Oncology 2013;3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miles 2007
- Miles RR, Cairo MS, Satwani P, Zwick DL, Lones MA, Sposto R, et al. Immunophenotypic identification of possible therapeutic targets in paediatric non‐Hodgkin lymphomas: a Children's Oncology Group report. British Journal of Haematology 2007;138(4):506‐12. [DOI] [PubMed] [Google Scholar]
Miles 2012
- Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. British Journal of Haematology 2012;156(6):730‐43. [DOI] [PubMed] [Google Scholar]
Murphy 1980
- Murphy SB. Classification, staging and end results of treatment of childhood non‐Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Seminars in Oncology 1980;7(3):332‐9. [PubMed] [Google Scholar]
NCI 2010
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2010. Available from evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_8.5x11.pdf.
Rossig 2011
- Rossig C, Juergens H, Berdel WE. New targets and targeted drugs for the treatment of cancer: an outlook to pediatric oncology. Pediatric Hematology and Oncology 2011;28(7):539‐55. [DOI] [PubMed] [Google Scholar]
Sandrini 2010
- Sandrini S, Valerio F, Insalaco M. Kidney transplantation and lymphomas. Giornale Italiano di Nefrologia 2010;27 Suppl 50:S46‐50. [PubMed] [Google Scholar]
Schulz 2007
- Schulz H, Bohlius J, Skoetz N, Trelle S, Kober T, Reiser M, et al. Chemotherapy plus rituximab versus chemotherapy alone for B‐cell non‐Hodgkin's lymphoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003805.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2012
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature Reviews. Cancer 2012;12(4):278‐87. [DOI] [PubMed] [Google Scholar]
van Meerten 2011
- Meerten T, Hagenbeek A. Novel antibodies against follicular non‐Hodgkin's lymphoma. Best Practice & Research Clinical Haematology 2011;24(2):231‐56. [DOI] [PubMed] [Google Scholar]
Weiner 2010
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology 2010;10(5):317‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2000
- Young G, Toretsky JA, Campbell AB, Eskenazi AE. Recognition of common childhood malignancies. American Family Physician 2000;61(7):2144‐54. [PubMed] [Google Scholar]
References to other published versions of this review
de Zwart 2014
- Zwart V, Gouw SC, Meyer‐Wentrup FAG. Antibody therapies for lymphoma in children. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011181] [DOI] [PMC free article] [PubMed] [Google Scholar]


