Abstract
Cells of the vascular wall are exquisitely sensitive to changes in their mechanical environment. In healthy vessels, mechanical forces regulate signaling and gene expression to direct the remodeling needed for the vessel wall to maintain optimal function. Major diseases of arteries involve maladaptive remodeling with compromise or loss of these homeostatic mechanisms. Whereas homeostasis invokes negative feedback loops at multiple scales to mediate mechanobiological stability, disease progression occurs via positive feedback that generates mechanobiological instabilities. In this review, we focus on the cell biology, wall mechanics, and regulatory pathways associated with arterial health and how changes in these processes lead to disease. We discuss how positive feedback loops arise via biomechanical and biochemical means. We conclude that inflammation plays a central role in overriding homeostatic pathways and suggest future directions for addressing therapeutic needs.
Keywords: mechanotransduction, feedback, fibrosis, hypertension, aneurysms, atherosclerosis
1. Introduction
The essential function of the vascular system, transport of oxygen and nutrients to and waste from the tissues, is aided by its remarkable ability to adapt to changing conditions. Examples abound. Sustained increases in cardiac output cause central arteries to enlarge, which reduces the resistance to flow and thus workload on the heart (1). Normal tissue growth or locally increased metabolic activity trigger angiogenesis, which increases local supplies of blood to match demand (2). In these and many similar examples, we recognize that transporting blood throughout the body is fundamentally a mechanical process. Consequently, to maintain the integrity of the vasculature itself and to enable appropriate adaptations, cells of the vascular wall sense and respond to diverse mechanical stimuli. Endothelial cells (ECs) of the intima appear to sense mainly wall shear stresses resulting from blood flow, while smooth muscle cells (SMCs) of the media and fibroblasts (FBs) of the adventitia sense mainly changes in intramural stress that result from local changes in blood pressure or axial loading. Mechanical stimuli thus guide vascular remodeling processes that mediate homeostasis.
Vessels vary in composition, structure, and function along the vascular tree, which determines both the forces that they experience and how they grow (change mass) and remodel (change structure). The largest arteries are highly elastic, which enables them to distend and then recoil to augment pulsatile blood flow while resisting the highest pressures within the vasculature. Medium-sized arteries are muscular, which enables them to control regional flow via vasoactive changes in caliber. The smallest arteries (arterioles), exhibit strong vasoactive potential and are under the control of both the endothelium and sympathetic nervous system; by constricting or dilating, they control blood flow to end-organs and tissues as well as set the mean arterial pressure experienced by proximal vessels. Capillaries, the smallest blood vessels, where gas and nutrient exchange occur, are thin-walled and minimally vasoactive. The venous system similarly consists of small (venules), medium-sized, and large veins, which return blood to the heart to circulate again. This review focuses primarily on large arteries, which are sites of vascular diseases such as aneurysms and atherosclerosis.
We first introduce basic concepts in arterial mechanics and mechanobiology and then briefly review components of the arterial tree – different cell types and extracellular matrix (ECM) as well as differences in structure and function by region – and how they form an integrated system. We then focus on local mechanobiological functions that are driven by feedback control mechanisms, particularly those contributing to homeostasis versus pathogenesis. Whereas homeostasis is driven by negative feedback, disease generally involves insidious positive feedback loops that drive aberrant remodeling (3). We conclude by discussing opportunities for understanding better the mechanobiology using multiscale approaches, both experimental and computational. We approach this problem by borrowing ideas of equilibrium and stability from mechanics as a conceptual framework for understanding both homeostasis and its failure in disease.
2. Basic arterial mechanics and mechanobiology
The large arteries form a curved, tapering, branching network (Figure 1). Blood flow within these central arteries is pulsatile due to the cardiac cycle, with pressure pulsations amplified along the human aorta due to its spatially varying geometry and properties. This pulsatility dampens in the muscular arteries and especially in the arterioles, resulting in steady flow within the capillaries and veins (4). Notwithstanding the importance of pulsatility, mean values of flow-induced wall shear stress τw and pressure-induced circumferential intramural stress σθ provide considerable insight. They are often written (5)
| (1,2) |
where Q is the flow, P the distending pressure, a the luminal radius, h the thickness of the wall, and μ the viscosity of the blood, often assumed Newtonian as appropriate at high shear rates in large vessels. Copious experimental findings have shown that the mean values of these two hemodynamically induced stresses tend to be maintained near normal (i.e., homeostatic) set-points in response to modest perturbations in blood flow and pressure (1; 6). In particular, if ε denotes a fold-change in mean flow and γ a fold-change in mean pressure, restoring mean wall shear stress and circumferential stress toward normal requires that (7)
| (3,4) |
where the subscript “o” denotes an original homeostatic value. Consequently, wall shear stress can be efficiently controlled via changes in luminal radius, including rapid vasoactive regulation or more permanent remodeling over longer periods. Intramural stress can similarly be controlled by changes in radius and wall thickness. A vessel is considered locally mechano-adapted if changes in luminal radius and wall thickness restore the mean values of these two stresses toward normal; the vessel is otherwise mechanically maladapted. Less is known about the control of mean axial wall stress, σz = f/πh(2a + h), where f is axial force, but associated changes in axial stretch tend to be amongst the fastest adaptations in many cases (8). Although these simple relations provide considerable insight, details of the nonlinear mechanics are often needed to understand general cases of homeostasis and pathogenesis. Further details on the mechanics of the vascular wall, both normal and diseased, can be found elsewhere (5; 9).
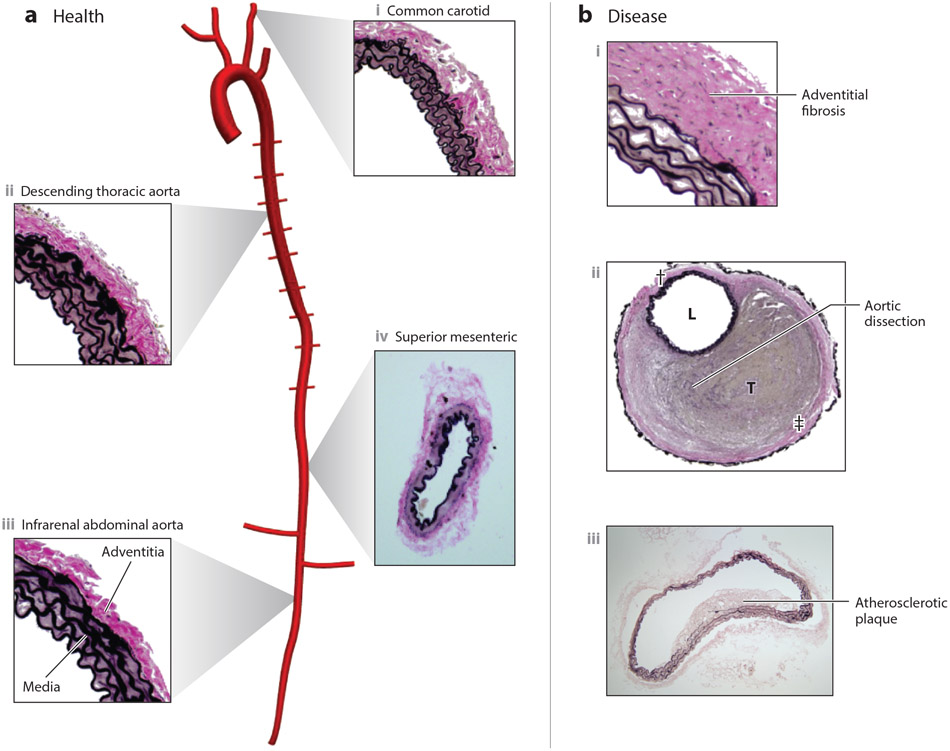
Figure 1.
(a) Overview of central arteries of the mouse vasculature reconstructed from a microCT image, showing the aorta, brachiocephalic, common carotid, subclavian, intercostal, and renal arteries, with representative histological images of portions of (i) a healthy common carotid artery, (ii) descending thoracic aorta, and (iii) infrarenal abdominal aorta (all elastic) as well as (iv) superior mesenteric artery (muscular). Note the nearly concentric elastic lamellar structures in the media of the elastic arteries (elastin shown black) in contrast to the smooth-muscle rich media of the muscular artery. Note, too, the abundant collagen (pink) in the adventitia of both types of arteries. Arterial microstructure arises during development and is maintained in health via a continual replacement of cells that die and matrix that degrades, accomplished via homeostatic processes driven by negative feedback. Resident macrophages can participate in the homeostatic removal of dead cells, debris, and damaged matrix. (b) In contrast, cross-sections are shown for three different pathologies that affect the large arteries: (i) hypertension induced adventitial fibrosis, (ii) contained rupture with a thrombus (T) filled false lumen, with L denoting the true lumen and daggers the adventitia, and (iii) an early atherosclerotic lesion in the intima of the abdominal aorta. Such maladaptive remodeling or catastrophic events are driven and/or preceded by compromised homeostasis, often via positive feedback.
3. Components of the Arterial Wall
3.1. Endothelial Cells (ECs).
ECs line the inner surface of all blood vessels and form a selective permeability barrier between the blood and tissues (10); they modulate and guide inflammation by controlling the transmigration of immune cells from the bloodstream to tissues (11); and they are the main sensors of flow-induced wall shear stress, which allows them to influence SMC contractility and thus luminal caliber. Many excellent reviews are available on EC mechanobiology and mechanisms of flow sensing and consequences thereof (12; 13).
EC responses to flow vary with magnitude and pattern as befits a physiological regulator (Table 1). Flows at magnitudes near the physiological target stabilize the vasculature, inducing EC alignment in the direction of flow and suppressing EC cycle progression as well as inflammatory and remodeling pathways (14-16). Modest changes in flow drive resultant adaptive remodeling (inward for lower flow, outward for higher flow) that restores shear stress to proper levels (Eqn. 3), which terminates the remodeling via homeostatic control. These flow-regulated remodeling processes often involve activation of inflammatory pathways such as NF-kB (Figure 2a), leading to the expression of leukocyte recruitment genes (17-19). These effects are consistent with remodeling processes that require the participation of inflammatory cells, often monocytes and macrophages (20; 21). Consistent with these notions, lower than normal shear stress typically leads to reduced production of the vasodilator nitric oxide (NO) and increased production of the vasoconstrictor endothelin-1 (ET-1) to induce vasoconstriction, whereas higher than normal shear stress leads to an up-regulation of NO and down-regulation of ET-1 to induce vasodilatation (22; 23). ECs also respond to cyclic circumferential wall strain, but the literature is sparse and the effects less dramatic than those for flow. Finally, flows in branching or highly curved regions of the arterial tree can result in regions of low and multidirectional flow, often simply called disturbed flow (24). Such flows induce mild but chronic activation of inflammatory pathways that may represent a state of futile flow-dependent remodeling (25). In the absence of other risk factors, this state appears neutrally stable but predisposed to pathological remodeling as discussed below.
Table 1.
Physiological levels of shear stress promote arterial stabilization, associated with low NF-kB and low expression of inflammatory mediators, high activation of Smad1/5 and Notch, moderate activation of eNOS and production of NO, and low cell proliferation. Flow-induced shear stress below physiological levels promotes inward remodeling in association with high activation and signaling by NF-kB, ET-1 and Smad2/3 as well as low eNOS/NO. Shear stress above physiological levels promotes outward remodeling in association with high NF-kB, higher eNOS/NO, cell proliferation and suppression of the stabilization pathways.
| Shear stress: | Reduced | Physiological | Elevated |
| Signals: | Smad2/3 NF-kB ET-1 | Smad1/5 eNOS/NO | NF-kB eNOS/NO |
| Outcome: | Inward remodeling | Homeostatic | Outward remodeling |
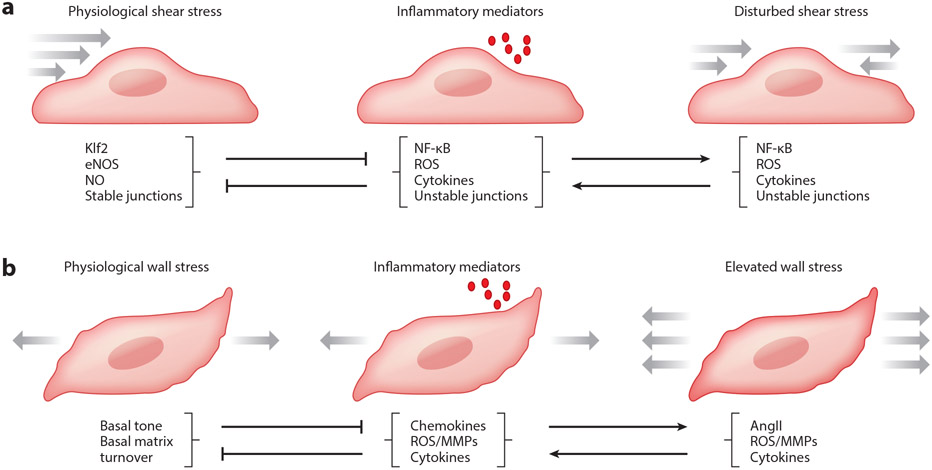
Figure 2.
(a) Schema of endothelial cells subjected to mechanical and/or inflammatory stimuli. Physiological levels of wall shear stress induced by blood flow activate multiple pathways that oppose inflammation and remodeling, most prominently Klf2, which mediates expression of anti-inflammatory, anti-thrombotic, and anti-oxidative genes. One important downstream gene is eNOS, which catalyzes the synthesis of NO, which promotes junctional stability, suppresses SMC contraction and proliferation, and suppresses activation of leukocytes and platelets. By contrast, disturbed flow or inflammatory mediators activate the transcription factor NF-kB and downstream genes, inducing reactive oxygen species (ROS) production and destabilizing junctions. NF-kB reduces Klf2 expression, and ROS react with NO to generate peroxynitrite. High levels of inflammatory mediators over long periods suppress expression of flow sensors such as PECAM and VE-cadherin, which inhibit flow responses. Disturbed flow also sensitizes ECs to inflammatory factors, amplifying these responses. (b) Schema of smooth muscle cells subjected to mechanical and/or inflammatory stimuli. Physiological levels of intramural stresses arising largely from blood pressure stimulate continual turnover of cells and matrix to maintain the structure and function of the arterial wall despite inevitable cell death and matrix degradation over long periods in maturity. Both elevated wall stress, as in hypertension, and inflammatory cell infiltration can disrupt normal processes and lead to maladaptive remodeling or diverse disease states (cf. Figure 1). The latter are driven via the increased production or presence of chemokines (such as MCP-1), cytokines (such as TGFβ, IL-6, IL-17a, and TNF-α), and matrix metalloproteinases (such as MMP-1, 2, 9, 12).
3.2. Smooth Muscle Cells (SMCs).
Excellent reviews of vascular SMCs and their roles in arterial development, aging, and disease can be found elsewhere (26-28). SMCs were long thought to exhibit either a mature/contractile phenotype that mediates vasoconstriction or an embryonic/synthetic where they proliferate, migrate, and elaborate ECM constituents of the developing arterial wall. More recent work, however, shows that SMCs adopt a wider range of phenotypes depending on artery type and disease. For SMCs in elastic arteries, contractile proteins appear to play a minor role in setting the caliber but instead enable the SMCs to mechanically probe the physical state of the ECM and to mediate matrix synthesis and assembly within the mechanically stressed artery wall. This “matrix phenotype” enables a primary function of an elastic artery: to store elastic energy during systole and to use this energy during diastole to recoil the vessel and augment antegrade and retrograde flow while maintaining appropriate stiffness and strength. In muscular arteries, SMCs appear to respond primarily to changes in endothelial-derived vasoactive molecules, including wall shear stress-regulated NO and ET-1, thus allowing appropriate vasoregulation of flow. Importantly, wall shear stress tends to be regulated best in muscular arteries, with actomyosin-induced contractility driving luminal caliber to control flow. In arterioles, SMCs respond strongly to changes in both sympathetic signaling and endothelial derived vasoactive molecules, thereby allowing central nervous system control of peripheral resistance and thus mean blood pressure, as required in orthostatic changes, control of body temperature, and fight-or-flight situations. Smooth muscle contractility in arterioles is also subject to a unique mechanosensitive (myogenic) response whereby the cells contract against increasing pressure-induced intramural stress. Hence, even the “contractile phenotype” exhibited by SMCs in muscular arteries and arterioles differs by degree.
Importantly, perturbation of hemodynamic load in healthy vessels leads to modulation of SMC phenotype to promote homeostasis; in contrast, such phenotypic changes are ill-controlled in disease and can lead to maladaptations (Figure 2b). In either case, SMCs are highly sensitive to changes in applied loads, which activate a wide range of cytoplasmic signals and gene expression events (29-31) to modulate SMC structure and phenotype. Integrin mediated adhesions appear to be major transducers in this setting though N-cadherin, which links SMCs to each other in the vessel wall, can also experience changes in mechanical loads and contribute to mechanically guided regulation (32). In general, cyclic strains (or stresses) at magnitudes that mimic physiological values (~5-10% for circumferential strains) promote physiologic SMC phenotypes. Conversely, higher than normal values of cyclic strain (or stress) typically activate inflammatory pathways (33; 34). Such responses presumably occur in adaptive remodeling but are central in pathological settings. Hypertension, for example, initially increases wall stress and strain, which results in thickening of the wall via SMC hypertrophy or hyperplasia and ECM deposition to restore the strain/stress toward normal values.
3.3. Fibroblasts (FBs).
Despite the paucity of information on adventitial FBs, there is nonetheless increasing recognition of the importance of these cells in arterial homeostasis and disease (35-37), and considerable information is available for FBs from other tissues (38; 39). Briefly, vascular FBs establish and maintain the adventitia, which consists primarily of fibrillar collagen I plus accessory proteins and proteoglycans, including decorin and biglycan. These proteoglycans are critical in collagen fibrillogenesis, and loss of biglycan has been shown to render large arteries susceptible to aneurysm and dissection (40; 41). Fibroblasts are highly sensitive to both stretch and ECM stiffness, with multiple changes in gene expression, including increased synthesis of fibrillar collagens and other matrix proteins (42-44). FBs from most tissue sources also integrate signals from mechanical loading and soluble factors such as transforming growth factor-beta (TGF-β), angiotensin II (AngII), and interleukin 17a to determine the rates of collagen synthesis, secretion, and turnover (45; 46).
3.4. Extracellular matrix (ECM).
Arterial ECM consists of myriad proteins, glycoproteins, and glycosaminoglycans that collectively endow the wall with site-specific functionality (47). Whereas all blood vessels have an intimal layer with ECs adhered to an underlying basement membrane consisting primarily of laminin and type IV collagen, the medial and adventitial layers differ with artery type. The medial layer of elastic arteries consists of alternating layers of elastic laminae that delimit monolayers of SMCs embedded within a matrix consisting largely of collagens (primarily types III and V) and glycosaminoglycans (primarily versican); the number of such elastic laminae decreases with luminal caliber and thus distance from the heart. The adventitial layer of elastic arteries consists primarily of undulated collagen I, with small amounts of admixed elastic fibers, proteogylcans, and, in thick vessels, a vasa vasorum (a network of small blood vessels that perfuse the tissue). In contrast, the medial layer of muscular arteries consists primarily of many layers of contractile SMCs embedded within sparse collagens and glycosaminoglycans and delimited by a prominent internal elastic layer and an external elastic layer (except in intracranial arteries). Again, the adventitial layer consists primarily of type I collagen. Thus “elastic” arteries consist of abundant elastic lamellae to store elastic energy. Interestingly, elastic fibers (consisting of ~90% elastin as a core and ~10% associated glycoproteins such as the fibrillins and fibulins) emerged evolutionarily with the appearance of a high pressure, pulsatile circulatory system (47). In contrast, “muscular” arteries contain abundant SMCs that contract and relax to regulate local blood flow. Elastic energy storage and smooth muscle contractility represent two primary arterial functions that dominate in different parts of the arterial tree.
The ECM also serves instructional roles. Binding of cells to ECM proteins through integrins regulates a vast array of intracellular signals and functions (48). Different matrix proteins such as fibronectin (which preferentially binds α5β1) and collagen (which preferentially binds α2β1 or α1β1) can drive phenotypic changes, with fibronectin generally promoting a pro-inflammatory phenotype and intact collagen an anti-inflammatory phenotype (49). The ECM also serves as a rich repository for growth factors, cytokines, and proteases, which enables diverse regulatory roles (50). Importantly, cytokines such as TGFβ and proteases such as the matrix metalloproteinases (MMPs) are secreted in matrix-bound latent forms. Activation of latent TGFβ bound within the matrix can be controlled by both matrix composition and matrix mechanics, with the former enabling cells to actively pull on the latency complex to liberate the active TGFβ dimer and the latter allowing a similar mechanical opening of the latency complex (51). Matrix degradation products can also drive phenotypic changes, often stimulating an inflammatory / degradative cell phenotype (28; 52; 53). Degradation of ECM by specific proteinases (e.g., MMP-1 for collagen, MMP-2,9 for denatured collagen, and MMP-12 for elastin) can expose cryptic sites that bind distinct receptors (54; 55) or can liberate fragments having cryptic sites that can then bind cell receptors and modulate cell phenotypes (56; 57). Degradation of collagens and other ECM proteins can influence the function of ECs, for example, regulating angiogenesis. Clearly, active cell mechanics, passive matrix mechanics, and biochemical mechanisms all contribute to ECM regulation of biological processes.
4. Mechanical Homeostasis
The concept of homeostasis was introduced by the physiologist Walter Cannon in the 1920s as an extension of the notion of a stable internal environment (mileu interieur) introduced by Claude Bernard in the 1870s. Homeostasis is the process by which cells, tissues, and organs maintain key variables within defined ranges (alternatively, near target values or set-points). Classical examples include maintenance of core body temperature or pH in interstitial fluids. Homeostasis exists at all scales in the vasculature, from sub-cellular to cellular to tissue levels as reflected by regulated values of stress at both focal adhesions and the level of the entire wall (58). The aforementioned responses of healthy arteries to sustained modest changes in blood flow and pressure, reflected by Eqns. 3 and 4, are manifestations of tissue-level mechanical homeostasis.
Mechanical homeostasis requires an input (mechanical stimulus) and an output (biological response) that is ultimately resolved via negative feedback by a system that consists of a collection of functional cells and ECM (Figure 3a). For arteries, mechanical inputs include the hemodynamic and axial loads that act on the vessel. Combining standard equations of vascular mechanics with knowledge of the geometry and material properties allows calculation of the mechanical state in terms of continuum metrics such as stress, strain, stiffness, or energy. Although the mechanics dictates the values of these mechanical quantities, comparisons to set-points requires that the cells sense these values, which may or may not reflect the actual state. That is, whereas the actual mechanical state alone dictates the outcome in mechanics, as, for example, rupture of the arterial wall when stress exceeds strength, in mechanobiology it is not the mechanical state but rather what the cell perceives that state to be that determines outcome based on comparisons to the desired set-points. Hence, “states,” “sensors,” and “set-points” are equally important in determining possible deviations from homeostatic targets that dictate subsequent cellular responses. Such responses reflect the underlying cellular phenotype, which often changes, and typically lead to reorganization or turnover of the ECM. Designating the effector cells and ECM herein as the “system,” it can be modeled at various levels, from gene regulatory networks to cell signaling to phenomenological models of tissue growth and remodeling. Multi-scale models that capture both mechanisms and manifestations especially useful.
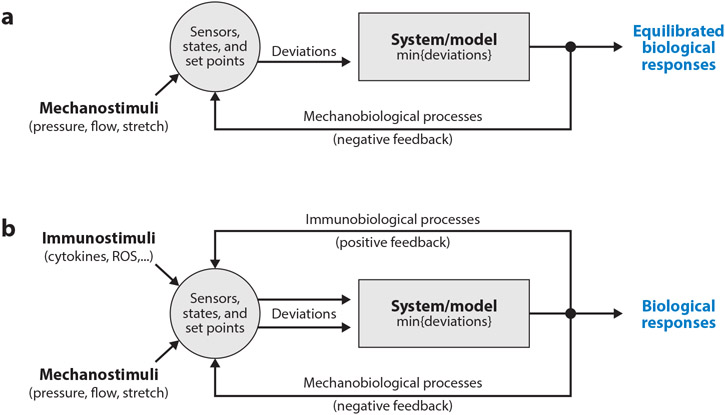
Figure 3.
(a) Schema of key components of mechanical homeostasis in arteries. Mechanical stimuli (pressure, flow, and axial stretch) combine with local geometry and properties to define the mechanical state, which can be calculated using equations of continuum biomechanics. Cells must sense this state and compare perceived values against homeostatic targets, or set-points. Any deviation in mechanical state from the set-point is then used by the effector cells to modify their phenotype and/or the extracellular matrix (collectively the system to be modeled). Various models, both phenomenological and mechanistic, single-scale and multi-scale, can be used to determine the associated biological response, which can affect the geometry and material properties and hence the mechanical state. Continued negative feedback can maintain or restore the homeostatic state. (b) Although (para-) inflammation can contribute to the negative feedback that is dominated in maturity by the mechanobiology, chronic inflammation often drives a positive feedback that exacerbates the perturbation and drives disease progression. Various cell types can contribute to this disease-promoting inflammation, including monocytes/macrophages and T-cells though also ECs, SMCs, or FBs that undergo a phenotypic modulation towards an inflammatory (or degradative) phenotype.
As noted above, sustained increases or decreases in blood pressure tend to drive thickening or thinning of the arterial wall to actively return intramural stress toward a target value (7; 59; 60). Yet, such mechano-adaptations exhibit considerable regional differences that illustrate differences in mechanism and disease susceptibility. Elastic arteries tend to increase in caliber due, in part, to their elastic response to the increased pressure in the absence of a strongly opposing vasoactive response, which reflects their SMC matrix phenotype that primarily supports mechano-sensing and mechano-regulation of ECM. Muscular arteries tend to maintain their caliber due to their SMC contractile phenotype. Arterioles, in contrast, tend to decrease in caliber in hypertension (61; 62) due to an exuberant (myogenic) vasoconstriction in the absence of a strong elastic response, reflecting their specialized SMC contractile phenotype. This distinctive myogenic constriction to an increased pressure is thought to protect against end-organ microcirculatory damage by reducing the ability of the pulse pressure wave to propagate into the organ.
A central principle is that long (usually days to weeks (1), though in some cases detectable after only 4 hours (63)) periods of vasoconstriction or vasodilatation enable remodeling to alter the baseline vessel geometry and properties. This process is often referred to as entrenchment. Remodeling of both cytoskeleton and ECM contribute to this process, the latter often via crosslinking through tissue transglutaminase during the vasoactive period. Similar entrenchment has been observed ex vivo in tissue equivalents, with associated changes in cross-linked ECM generating what is sometimes referred to as a residual matrix tension (64; 65) during the cells attempt to promote a tensional homeostasis (65; 66). Further support for the coupled roles of vascular tone and matrix turnover in remodeling derives from studies in mice wherein deletion of eNOS, an enzyme catalyzing the production of the vasodilator NO, blocks long-term flow-dependent vessel remodeling (67).
Although the mechanics and physiological principles behind mechano-regulation of wall thickness are relatively well understood, how constituent cells actually sense intramural stress is emphatically not. It is notable that the mean wall shear stress in the human aorta is on the order of 1.5 Pa whereas the mean circumferential intramural stress is on the order of 150 kPa, 5 orders of magnitude higher. Intramural stresses on the order of 150 kPa can be generated by fully contractile SMCs within muscular arteries and perhaps arterioles, but probably not by SMCs involved in sensing and regulating the matrix. It thus appears that SMCs in large arteries are stress shielded, with the ECM bearing most of the load. It has long been thought that elastic fibers bear most of the intramural stress at normal blood pressures while collagen fibers bear more at higher pressures (68). This distribution is thought to maximize energy storage at physiological loads, consistent with a recent bi-layered stress analysis (69). Only in cases of acute supra-physiologic increases in blood pressure, as in weight lifting or fight-or-flight responses, does the collagen of the adventitia likely engage and carry the higher loads so as to protect the more vulnerable underlying SMCs and elastic fibers. Dramatic sustained elevation in blood pressure can engage the adventitia, however, resulting in an increased deposition of collagen, sometimes fibrotic. Hence, it seems that ECs, SMCs, and FBs have different homeostatic set-points, which can vary with region, and that these different values enable the different cell types to work together to promote a robust, integrated vessel and vascular network. As noted above, it is not understood exactly how the intramural cells sense their local mechanical environment. It may be that the intramural cells – SMCs of the media and FBs of the adventitia – sense mechanical loading through their own cyclic deformations due to intramural strain. Alternatively, they may sense forces indirectly via integrins and changes in the mean or cyclic stress within the elastic and collagen fibers, which necessarily balance much of the applied force.
5. An Integrated Framework with Negative Feedback
Whereas mechanical homeostasis in healthy arteries is characterized by negative feedback that restores the preferred state following a perturbation, risk factors such as hypertension and vascular aging, and major diseases of arteries, including aneurysms and atherosclerosis, often associate with either compromised homeostasis or positive feedback loops that promote pathological processes (70). A constrained mixture model for soft tissue growth and remodeling (G&R) reveals three key classes of constitutive relations that are needed to model mechanical homeostasis and its biomechanical consequences in the vasculature (71): the rates at which new structural constituents are produced and incorporated within an extant cell or tissue (denoted mα > 0, where α = 1,2, …, N denotes N individual families of structurally significant constituents), the rates at which these structural constituents are removed (denoted rα > 0), often via cell death or matrix degradation, and the typically nonlinear and anisotropic mechanical properties of each constituent (often captured by a stored energy function Wα > 0). Contained within the stored energy function are additional modulators of homeostasis, including the effective pre-stretch (or pre-stress) at which new structural constituents are incorporated within extant matrix and their orientations and cross-link density. If one uses a heredity integral approach rather than a rate-based approach (72), one can alternatively prescribe a survival function qα rather than a removal function. In this case, the three requisite constitutive functions are mα(τ), qα(s,τ), Wα(τ), where qα(s,τ) ∈[0,1] accounts for the finite half-life of each constituent that is produced at G&R time τ ∈ [0,s] and survives to current time s. The normal half-life of vascular collagen is on the order of 70 days, though it can be as low as 7 days in hypertension (73). Remarkably, the normal half-life of vascular elastin is on the order of 50 years (47), though loss of elastic fiber integrity is accelerated in diseases such as aneurysms and atherosclerosis. Genetic diseases that affect elastic fibers, including Marfan syndrome resulting from mutations to the FBN1 gene, also reduce the half-life of elastin. These very different constituent- and state-specific half-lives suggest the utility of mixture models. Reorganization of the cytoskeleton or ECM as part of remodeling in the absence of mass turnover can also be captured via such functions since remodeled constituents are effectively newly produced from ones removed.
A fundamental relation from mass balance for a constrained mixture defines changes in wall composition via evolving constituent-specific apparent mass densities (71), namely:
| (5) |
where total mass density ρ(s) = ∑ρα(s). Note that Qα(s) = qα(s,0), with Qα(0) = 1. As a simple illustrative example, consider the following functional forms for rates of production and subsequent survival,
| (6,7) |
where is an original homeostatic rate of production (mass per volume per time) and is an original homeostatic rate of removal (units of inverse time, in this first-order type kinetic decay), both constant in health in maturity and defined individually for each structurally significant constituent; it can be shown that to ensure balanced turnover within homeostatic states. Importantly, the basal rates can be modulated by deviations in the mechanical state from its homeostatic target, here illustrated for deviations Δ in a scalar measure of intramural stress σ, (e.g., first invariant or magnitude) from its set-point σo, with Kα > 0 constituent-specific gain-type parameters. For example, noting advantages of normalizing these deviations to focus on non-dimensional values, let Δσ = ((1 – δ)σ – σo)/σo, where the parameter δ ∈ [0,1[ accounts for the ability of the effector cell to sense the mechanical state: δ = 0 if the cell can sense the actual stress σ and δ = 1 if the cell can no longer sense the stress. Intermediate values of δ capture degrees to which mechano-sensing is compromised. In summary, in the context of Figure 3a, the state is determined by solving linear momentum balance to find the stress σ, the sensor incorporates the factor δ, and the set-point is given by σo.
In this formulation, tissue maintenance is captured by balanced rates of production and removal in a normal, unchanging mechanical state (i.e., Δσ = 0). Eqns. 6-7 show further that when the stress σ deviates from its homeostatic value (i.e., Δσ ≠ 0), the rates of production and removal change in an attempt to hasten restoration of the stress back toward the homeostatic target (i.e., within a particular tolerance), with the effective rates returning towards original values once the stress relaxes to its homeostatic value. Hence, this system demonstrates negative feedback regulation; in the context of Figure 3a, Eqns. 6-7 are part of the model that defines the responsive system. The necessity of negative feedback in mechanical homeostasis in the vasculature was recognized early on by Taber (74) and has been enforced in all such models since. Taber and others used a finite kinematic growth model rather than the constrained mixture model noted above, which means that growth laws were prescribed in terms of deformations, not mass turnover (75), yet enforcement of negative feedback remains a common feature. Moreover, different metrics (stress, strain, stiffness, energy, and so forth) can be used to drive the feedback loops, noting again that the precise “set-point” variables remain unknown in many cases, though mean intramural stress is a convenient metric since it only requires knowledge of the current geometry and applied loads (Eqn. 1), not past configurations as needed to compute strain or strain energy. Wall shear stress also appears to be a particularly good candidate in endothelial mechanobiology (15), noting that these values can be computed easily from hemodynamic simulations whereas it is difficult to compute micro-scale endothelial deformations, stiffness, or energy storage.
When the illustrative constitutive relation above for mass production is augmented with a similar negative feedback term for flow-induced wall shear stress, as, for example,
| (8) |
the associated computational models recover ideal mechano-adaptations as defined by Equations 3-4 (71) as well as salient features of many disease conditions. Here, are gain-type parameters that modulate effects of deviations in wall shear stress from homeostatic values, where and the index o again denotes the original homeostatic target or set-point. The negative sign associated with this shear term accounts for the observation that NO (which is upregulated in ECs in increased flows, when Δτw > 0) decreases matrix production by SMCs and FBs whereas ET-1 (which is upregulated in ECs in decreased flows, when Δτw < 0) increases matrix production by SMCs and FBs (76; 77). Relative magnitudes of the stress differences (Δσ and Δτw) and associated gains ( and ) dictate the differential effects of altered intramural and wall shear stress, both regionally and in different conditions.
6. Positive Feedback Loops in Vascular Disease
6.1. Local-Global Effects.
Central artery stiffening, a key risk factor for many cardiovascular diseases (78), introduces positive feedback via effects on global hemodynamics, specifically pulse wave velocity (PWV). Whereas decreased arteriolar radii increase peripheral resistance and thereby increase mean arterial pressure, including in hypertension, increases in the structural stiffness of the central (elastic) arteries increases central pulse pressures and the propagation of the pulse wave (via the PWV) into the microcirculation of end organs (62). Albeit limited theoretically (79), the following relation provides considerable intuition:
| (9) |
where E is a linearized material stiffness of the wall, ρf the mass density of the fluid (blood), and a and h are again luminal radius and wall thickness. This Moens-Korteweg equation reveals that it is mainly the structural stiffness of the wall, Eh, and luminal radius a that govern the speed at which the pressure pulse propagates within central arteries. Recall from above that if blood pressure increases in a central artery by γ-fold without a change in blood flow (ε = 1), then restoration of the local mean intramural stress requires a γ-fold increase in wall thickness without a change in luminal radius. By Moens-Korteweg, however, this will also increase PWV unless material stiffness decreases via reverse wall remodeling, which either tends not to occur or to be insufficient. Without such remodeling or a compensatory change in luminal radius (which would alter local shear stress regulation if ε = 1), this local mechano-adaptation will increase PWV, which in turn will cause the reflected pressure wave to return to the central vasculature earlier in the cardiac cycle, thus augmenting the central pulse pressure (4). That is, an insidious positive feedback loop can arise wherein local adaptations via mechanical homeostasis to increased pressure cause global changes in hemodynamics that increase the pressure that drives the local attempt of mechano-adaptation (80). In addition, an increase in proximal blood pressures can elicit baroreceptor resetting (81), effectively reducing baroreceptor sensitivity. A structurally stiffer wall will also distend less with each cardiac cycle, thus reducing a possible cyclic strain mechano-stimulus, which can affect SMC phenotype and possibly even EC phenotype. Endothelial dysfunction, that is, reduced availability of the vasodilator and inflammation inhibitor NO, is common in large arteries in hypertension and aging (82-84). Hence, locally adaptive thickening of the arterial wall in hypertension can have negative consequences that shift the control toward positive feedback and loss of mechanical homeostasis.
6.2. Inflammation.
It is increasingly realized that inflammation plays key roles in vascular conditions and diseases (85-87), including aging, abdominal aortic aneurysms, and atherosclerosis (Figure 3b). For example, very low wall shear stress can upregulate leukocyte adhesion molecule expression by ECs and promote local inflammation (88; 89). The recruited inflammatory cells release chemokines and cytokines that contribute to inflammatory activation of ECs and SMCs and further recruitment of inflammatory cells, often leading to a positive feedback loop and a pathologic neointima (Figure 1b), as in atherosclerosis (85). Activated inflammatory cells can similarly populate the adventitia, sometimes leading to adventitial fibrosis (Figure 1b) and thus overall structural stiffening (90; 91), as seen in aortic aging and hypertension (45; 92). The media tends to be immuno-protected (93) until the internal and/or external elastic laminae are compromised, at which time the media can become inflamed as in some aneurysms, particularly of the abdominal aorta (94). Although resident macrophages participate, many inflammatory cells, including neutrophils, monocytes, and T-cells, reach target arteries by transport via the lumen or the vasa vasorum and similarly via the lymphatic system, including that within perivascular fat (95; 96).
Inflammation plays key roles in diverse remodeling processes, not just in infections and tissue injury (97; 98). Cells of the monocyte/macrophage lineage in particular are essential for many aspects of arterial remodeling, including homeostatic removal of dead cells and cellular debris or partially degraded and damaged ECM (21; 22). The term para-inflammation has been used to delineate the homeostatic function of inflammation (98). Yet, because the immune system developed to fight infections, it often takes priority over other biological processes whose contribution to survival is less acute. Inflammation can thus dramatically modify other biological processes. In particular, inflammatory signals, typically chemokines and cytokines but also proteases, can significantly alter the gain and rate parameters and set-points (cf. Eqn. 8) inherent to homeostatic processes, including mechanical (98). Altered gain and rate parameters can accelerate responses to stimuli, thus facilitating the needed response; altered homeostatic targets can similarly promote an opportune response, as in adaptations (99). The term adaptive homeostasis has been used to describe beneficial changes that facilitate such adaptations (100).
The close relationship between vascular function and immunity is often critical in other ways. Tissue inflammation leads to the production of cytokines that act on the endothelium to recruit leukocytes to fight infection or repair damaged tissue (101; 102). The endothelium is thus an essential participant in immune function by directing leukocyte traffic. Inflammation also acts on the endothelium to increase permeability to plasma proteins such as complement and antibodies that participate in immunity (10; 11). Many inflammatory signals affect vascular permeability. Inflammatory signals also affect NO production and/or availability to control vessel tone and local blood flow (103). One pernicious mechanism involves reactive oxygen species (ROS) generated during inflammation that combine with NO to produce peroxynitrite; this process thus replaces a vasodilator and inflammatory suppressor with a vasoconstrictor and inflammatory activator (104). Inflammation thus modulates vascular function in ways that override normal homeostatic functions.
The proposal that homeostatic systems with greater potential for adaptability can also have greater disease susceptibility (98) underscores another potential for inflammation in vascular disease progression. Changes in set-point that are induced by inflammation may lead, for example, to a vicious positive feedback cycle that promotes chronic inflammation (Figure 3b). Indeed, the seemingly irreversible effects of hypertension on central artery structure and properties may result, in part, from such a process. Recent studies using a mouse model of induced hypertension suggest that changes in thoracic aorta structure, properties, and stresses reverse slightly but largely persist after the hypertensive stimulus (exogenous AngII) is removed, apparently due to a persistent low-level inflammation (91; 105). Note, therefore, that the renin-angiotensin system is a central node in blood pressure control. Chronic infusion of AngII is, accordingly, a common method of inducing hypertension in rodents. This peptide is a potent vasoconstrictor, first thought primarily to increase total peripheral resistance via arteriolar constriction. Yet, exogenous AngII has myriad effects on the vasculature, including stimulation of a pro-inflammatory state in large arteries (106-108). For example, exogenous angiotensin promotes FB-macrophage interactions that involve the pro-inflammatory cytokine IL-6, which then promotes adventitial fibrosis (109). In the case of the illustrative constitutive relations noted above for the production and removal of constituents α (equations 6-7), inflammation can be captured by adding another stimulus to the mass production term, namely
| (10) |
mainly in the adventitia where inflammatory cells infiltrate, but also in the media. Here, Δρφ ∈ [0,1] is a normalized measure of inflammatory cell burden, typically CD4+ T-cells in the adventitia of angiotensin models of hypertension, but CD68+ macrophages and other cell types as well. If the inflammation resolves, Δρφ → 0, the stimulus reverts back to mechanobiological alone; if inflammation persists (Δρφ > 0), the rate of production is permanently altered even if mechanical stresses return to normal.
We recently found that description of extreme adventitial fibrosis in an angiotensin-infusion mouse model (91) required the gain parameter for mass production to become a function of inflammation and similarly for the rate of mass removal parameter ; indeed, the intramural stress set-point also changed , with sub-script h denoting a new homeostatic state. Each of these changes are consistent with inflammation overriding a homeostatic process (98). Importantly, none of these model changes were needed to capture the observed mechano-adaptation of the minimally inflamed infrarenal abdominal aorta. Why such dramatic regional differences – fibrosis in the thoracic aorta and adaptation in the infrarenal aorta – arise in the same animal model is not clear, but regional variations in angiotensin II receptors (greater in the distal than the proximal aorta; (110)), adrenoceptors (111), and SMC phenotype may play roles. Other differences, including local hemodynamics (112) and perivascular tissue, particularly fat, could also contribute.
Not surprisingly, inflammation is also involved in the in vivo progression of tissue engineered vascular grafts that are implanted as biodegradable polymeric constructs (113). The presence of the polymer elicits a strong inflammatory response, which drives the early neotissue formation as the polymer degrades. Thereafter, neotissue formation is presumed to be driven largely by the mechanobiology, which enables good computational model descriptions and predictions of the geometry, composition, and properties of the evolving neovessel (114). There is a need, however, to determine if long-term persistent inflammation could also play a role, particularly given that the neotissue is not equivalent to normal vasculature (115). In multiple systems, ECM arising during injury responses or pathological situations contains altered proteins or other factors (perhaps antigens given roles of T-cells in adventitial fibrosis; (45)) that stimulate fibrosis. That is, inflammatory cells can stimulate the production of aberrant ECM by otherwise normal synthetic cells such as FBs, while aberrant ECM can also stimulate normal cells to produce additional aberrant ECM in the absence of inflammatory cells (116), thus establishing another type of positive feedback loop. Conversely FBs from fibrotic tissue can be returned to a non-fibrotic phenotype by exposing them to normal matrix (117). There is clearly a pressing need to better understand how pathologic changes in ECM promote positive or negative feedback.
6.3. Cellular phenotypic transitions
Different cell types or states show substantial differences in mechanosensing parameters and outputs. ECs align perpendicular to the direction of stretch, muscle cell types align parallel (118). Similar variability is observed in stiffness sensing. In general, less contractile cell types such as immune cells have a lower stiffness threshold, spreading and organizing their cytoskeleton on compliant substrates whereas more contractile cells remain round until stiffness reaches higher levels (119). Cell type also determines the signaling and gene expression pathways that depend on stiffness (120). In the case of flow-induced shear stress, ECs of different types (vascular vs. lymphatic) have different set-points (15), which likely extends to different subtypes of vessels (arterial, venous, capillary) or from different tissues. Vascular vs. lymphatic ECs exhibit drastically different responses to oscillatory flow, which is pro-inflammatory in arterial blood ECs but triggers valve morphogenesis in lymphatic ECs (121).
There is now strong evidence that both ECs and SMCs in diseased arteries undergo phenotypic transitions. Immunohistochemistry, fate mapping, and bulk RNAseq show that ECs in diseased vessels undergo graded transitions to mesenchymal phenotypes (Endo-MT; (122)). This process involves downregulation of EC-specific receptors such as PECAM-1, VE-cadherin, and VEGFR2 that are involved in shear stress mechanotransduction, and, thus, could inhibit or alter homeostasis. More recent single cell RNA sequencing of plaque ECs revealed clusters of cells with inflammatory or mesenchymal gene expression signatures and decreased expression of EC-specific genes involved in flow-sensing (123). One cell cluster exhibited a progenitor signature as well, suggestive of cell fate transitions. EC deletion of TGFβ receptors reduced the prevalence of these populations, supporting a role for Endo-MT. This type of altered EC phenotype is also likely a component of normal morphogenesis and remodeling. For example, sprouting of angiogenic ECs to form new blood vessels involves limited and transient Endo-MT (122). In pathological settings, however, these transitions are never resolved and can progress to more extreme phenotypes.
SMCs in diseased arteries have long been known to transition from a “contractile” phenotype with high expression of smooth muscle myosin, actin, and other lineage-specific cytoskeletal proteins to a “synthetic” phenotype characterized by increased proliferation, motility, and ECM production (53). Subsequent studies expanded this repertoire, demonstrating that a fraction of neointimal SMCs developed into macrophage-like cells with reduced expression of SMC-specific genes and upregulation of a subset of macrophage markers (124). Single cell RNAseq analyses in both human and mouse arteries revealed SMC-derived progenitor clusters that likely give rise to multiple fates, including chondrogenic and osteogenic phenotypes (125). In all cases, SMC-specific genes were downregulated. These events are again predicted to reflect or result in altered or defective mechanosensing.
6.4. Aneurysms and Atherosclerosis.
Aneurysms are focal dilatations of the arterial wall. Aneurysms in all locations exhibit fragmented or degraded elastic fibers, smooth muscle cell apoptosis, and fibrosis with concomitant stiffening; they also share aging and hypertension as major risk factors (126; 127). Abdominal aortic aneurysms (AAA) correlate with atherosclerosis and have a strong inflammatory component (128); they also frequently develop an intraluminal thrombus that exacerbates the inflammation, thus creating a positive feedback loop whereby increased dilatation can drive increased thrombosis. Disturbed flows and altered EC mechanobiology can contribute to the early inflammatory phenotype via the recruitment of leukocytes with subsequent changes in SMC phenotype. In intracranial aneurysms, regions of low flow correlate with regions of maximal enlargement (129). Aneurysm enlargement increases the pro-inflammatory reverse and transverse components of wall shear stress, accelerating disease. In contrast, thoracic aortic aneurysms (TAA) are more strongly linked with genetic mutations than inflammation, with mutations in genes for cytoskeletal (smooth muscle α-actin and smooth muscle myosin heavy chain) or ECM (fibrillin-1, a glycoprotein that associates with elastin to form elastic fibers as well as connecting SMCs to the elastic fibers) proteins. These causative mutations suggest that TAAs may arise, in part, from dysfunctional mechano-sensing or mechano-regulation of ECM by the SMCs (130; 131); referring to the equation Δσ = ((1 – δ)σ – σo)/σo, the parameter δ < 1 can model reduced mechano-sensing. In particular, if a cell perceives the stress to be lower than homeostatic, it may promote proteolytic degradation in an attempt to raise the (perceived) stress toward its normal value, thus increasing the vulnerability of the wall to dissection or rupture. Progressive fibrosis of the vessel may protect against rupture but also interfere with mechano-sensing, thus contributing to further pathological remodeling. Both cases, therefore, involve positive feedback mechanisms. The SMC phenotypic transitions seen in aneurysms, including reduced expression of SMC-specific cytoskeletal genes (132; 133), also likely interfere with sensing of mechanical forces and thus homeostasis.
Atherosclerosis is an inflammatory/metabolic disease of muscular and elastic arteries (134; 135), with intimal lesions characterized by accumulations of lipids, necrotic debris, calcification, and remodeled matrix. Disturbed flow at bends and branches within the arterial tree is a local risk factor (136; 137) due to activation of EC inflammatory pathways (10; 101). Leukocyte recruitment, increased junctional permeability that facilitates entry of lipoproteins into the vessel wall and oxidative stress establish positive feedback loops that increase inflammatory status and promote disease progression (Figure 2a). Encroachment of lesions into the lumen exacerbates abnormal flows, with high shear at the region of luminal narrowing and very low/disturbed shear on the downstream side. Luminal narrowing is, however, a relatively late event in plaque progression. Plaques at early stages trigger outward remodeling to preserve lumen diameter, so-called Glagov compensatory enlargement, driven in part by a stress shielding of SMCs that leads to a local medial atrophy together with conventional physiological remodeling in which high wall shear stress increases lumen diameter. Yet, at later stages, Glagov enlargement fails and the lumen continues to narrow. It has been speculated that the failure of outward remodeling may be due in part to stiffening of the adventitia or resistance due to the surrounding tissue, but the existence of aneurysms would seem to argue against this view. An alternative model is based on the Endo-MT driven decrease in VE-cadherin and PECAM in the ECs of atherosclerotic plaques. Loss of these receptors that mediate flow sensing (138) would seem to be a likely explanation for the compromised flow-dependent outward remodeling that, in the face of lesion growth, contributes to vessel narrowing.
7. From Mechanics to Modeling the Mechanobiology
7.1. Vascular Mechanics.
As noted above, quantifying the mechanical state of arteries is essential for understanding both health and disease. Advances in vascular mechanics over the past few decades (5; 9) have underscored the concepts of mechanical equilibrium and stability. For example, analysis of the nonlinear biomechanics of a saccular aneurysm in the brain (139) reveals that these lesions do not exhibit a limit point instability upon quasi-static inflation (i.e., the quasi-equilibrated states are mechanically stable) and moreover that they do not exhibit resonant instabilities during pulsatile loading (i.e., they are dynamically stable). Eliminating the possibility of unstable mechanical behaviors focuses attention on more relevant aspects of lesion enlargement, including matrix turnover that drives the enlargement of an aneurysm through a sequence of mechanically equilibrated states, primarily via collagen degradation and deposition. We recently suggested the applicability of these concepts to the mechanobiologically controlled dynamic enlargement of aneurysms (140) and to vascular mechanobiology in general (72; 141).
Consider next another example of the importance of understanding mechanics in mechanobiology. Arteries are residually stressed; that is, there exists a self-equilibrating state of stress in the absence of external loading, which can be revealed by introducing a radial cut into an excised cylindrical segment. This residual stress field includes compressive circumferential and axial stresses in the inner portion of the wall and complementary tensile circumferential and axial stresses in the outer wall. Although these residual stresses are low (on the order of 3 kPa), they have a tremendous effect on the calculated transmural distribution of intramural stress under in vivo (axially stretched and pressurized) conditions, where biaxial stresses are on the order of 150 kPa or more (5). Indeed, when accounting separately for the two predominant structural layers of an elastic artery – the media and adventitia – a nonlinear analysis of intramural stress reveals two key findings (69). First, that the transmural distribution of stress is nearly homogenous separately in the media and adventitia and, second, that these stresses are naturally higher in the media than in the adventitia in an elastic artery under physiological loads. In other words, nonlinear analyses that account for the presence of residual stress reveal what appears to be separate homeostatic target values of stress in the media (sensed by smooth muscle cells) and adventitia (sensed by fibroblasts), with these stresses tending to be uniform within each layer rather than a decreasing monotonic function of radial location as expected in an inflated cylindrical tube. In hindsight, the existence of higher stresses in the media of an elastic artery should have been expected for it promotes its fundamental function, to store elastic energy during systole, since it is primarily the abundant elastic fibers of the media that store the energy. Importantly, however, this bilayered analysis of the wall also suggests that large acute increases in blood pressure result in a greater increase in intramural stress in the adventitia than in the media, thus allowing the stiffer and stronger collagen of the adventitia to engage and stress-shield the more vulnerable SMCs and elastic fibers of the media, again revealing an important structure-function relationship of these vessels. In summary, the multi-layered structure reflects the complex functionality of an elastic artery, to store and use elastic energy while withstanding the highest pressures in the vasculature. Toward this end, it is also important to recognize the different homeostatic target stresses for the different cell types (in the mouse aorta): ~6.5 Pa wall shear stress for endothelial cells, ~300 kPa circumferential stress for smooth muscle cells, and ~100 kPa circumferential stress for fibroblasts. The axial stress experienced by the smooth muscle cells and fibroblasts appear to be closer in magnitude, ~200 kPa, thus reminding us that the intramural cells are subject to biaxial stresses.
7.2. Modeling the mechanobiology.
There are advantages to couch the concept of mechanical homeostasis in terms of continuum mechanics and dynamical systems wherein one can exploit a large body of mathematical tools. Simply put, mechanical equilibrium requires that the sum of all forces is zero, typically resulting in a body that is static; mechanical stability implies that the body will return to this, or at least a nearby, equilibrium configuration following a mechanical perturbation. By analogy, one can postulate that mechanobiological equilibrium requires the sum of mass production and mass removal is zero (mα – rα = 0). Mechanobiological stability further requires that the system is stable against perturbations (72; 142). The simplest visual illustration of stable, neutrally stable, and unstable equilibria is, of course, that of a rigid sphere placed within a valley, on a flat plane, or on a hill (Figure 4). In each case the equilibrium state is the same (i.e., the reaction force is equal and opposite the weight of the sphere), yet the mechanical situations are very different – the sphere in the valley will return to its original equilibrium position when perturbed (asymptotically stable), the sphere on the flat plane will move to a new nearby equilibrium position (neutrally stable), and the sphere on the hill will move to another state far from the original one (unstable). Asymptotically stable responses can represent homeostatic responses; neutrally stable responses adaptive homeostasis; unstable responses cases of disease progression by positive feedback. Because of the need to evaluate responses to small perturbations, asymptotic expansions or the theory of small deformations superimposed on large are both appropriate frameworks for study. Such analyses have promise to describe and predict diverse vascular responses, including those that are homeostatic, adaptive, or maladaptive (Figure 4).
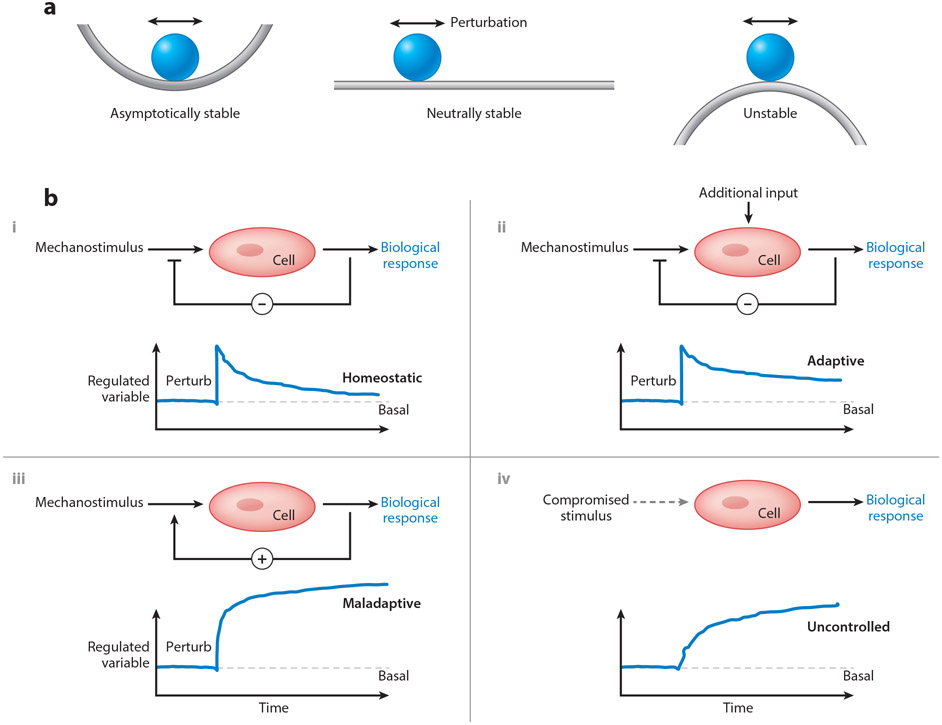
Figure 4.
(a) Conceptual representation of three common types of (in)stabilities: asymptotically stable, neutrally stable, and unstable. Imagine, respectively, that a perturbation in loading would cause the rigid sphere to move, returning to its original location on the left, finding a new nearby location in the middle, and falling far from its original position on the right. Note that neutral stability allows adaptivity in biology, but also increased vulnerability to instabilities that manifest as diseases. (b) Possible control systems relevant to arterial health and disease: three show responses to perturbations (vertical change in the regulated variable, such as stress or material stiffness) and one (lower right) in the context of compromised mechano-sensing. In particular, negative feedback (−) is restorative, as in cases of acute or modest sustained changes in hemodynamics (upper left), but can be over-ridden by additional stimuli, such as inflammation (upper right). In contrast, positive feed-back (+), as in adventitial fibrosis (cf. Figure 1), is typically maladaptive, moving the controlled variable from its basal value (horizontal dashed line). Finally, systems that rely on appropriate input can become maladaptive when the input signal is compromised or lost, as in dysfunctional mechanotransduction. Overall, negative feedback promotes asymptotic mechanobiological stability, negative feedback with additional non-mechanical stimuli represents neutral stability, and cases of positive feedback or compromised mechano-input represent instabilities.
Comparison of two recent constrained mixture-based G&R approaches for assessing mechanobiological stability reveals that the types of (in)stabilities that are admitted depends on the representation of the mechanobiological system. First, if one defines a system in terms of rate equations for position (mechanical) and mass production (mechanobiological), then the system admits either a neutral stability or an instability (141). Based on this approach, it was suggested that progressive abdominal aortic aneurysmal enlargement may represent a mechanobiological instability despite remaining mechanically stable. That this simulation suggested neutral stability implied, in turn, that such an aneurysmal enlargement could represent an attempted adaptation to an initiating insult, that is, a new stable mechanobiological state. Multiple factors were found that could stabilize AAA enlargement, including increased rates of collagen production, which was predicted computationally (143) and confirmed experimentally using an antago-miR29b treatment (142). The low rate of rupture of intracranial saccular aneurysms, at less than 0.1% per year (126), is also consistent with the concept of aneurysmal stability or adaptivity. Yet, second hits, such as intraluminal thrombosis in AAAs or dissections in TAAs, would compromise any attempted adaptation. Although adaptive homeostasis (97; 100) can be highly beneficial biologically, it can also expose a vulnerability to maladaptations characteristic of disease, which could explain continued enlargement of certain aneurysms until rupture.
Second, if one choses rate equations based on mass density and biaxial stresses for an elastic artery subjected to altered loading, asymptotic stability is possible in addition to neutral stability and instability (72). In particular, such a numerical artery can respond to a transient perturbation and return to its original state or it can respond to a sustained perturbation via geometric and compositional adaptations that restore the stresses towards normal. Many different parameters affect the stability, including the intrinsic material stiffness as well as magnitudes of the gain parameters that control ECM production and the rate parameters that control its degradation. Similar findings were reported for a stability analysis based on rates equations for total potential energy (144), which again suggested that increased rates of production, decreased rates of degradation or increased material stiffness can stabilize responses. Similar mechanobiological stability analyses can also be examined using the theory of kinematic growth (145).
8. Future Perspectives
Hypertension and vascular aging are key risk factors for diverse cardiovascular, renovascular, and neurovascular diseases. Atherosclerosis and aneurysms are but two examples of chronic diseases that involve pathological remodeling of the vessel wall, often over decades. As we have seen, these diseases arise through complex regulatory loops in which homeostatic control is corrupted or overcome by the appearance of positive feedback mechanisms. While physiological regulation is dominated by negative feedback that promotes stability in the face of perturbations, positive feedback often results in instabilities that promote continual pathological remodeling, that is, disease progression.
Past and many current efforts in vascular disease research have focused largely on inhibiting obvious steps in the disease etiology. For example, hypertension is widely treated using diuretics to reduce blood volume or inhibitors of the renin-angiotensin pathway or voltage-gated calcium channels to limit vasoconstriction. Statins, to inhibit cholesterol synthesis, are by far the most widely used medical treatment for atherosclerosis. Importantly, the clinical success of these inhibitors now appears to depend heavily on unintended consequences. Twenty years after their clinical introduction, statins were found to induce expression of the anti-inflammatory transcription factor Klf2, a major contributor to drug efficacy (146; 147). Similarly, inhibitors of the angiotensin pathway have anti-inflammatory benefits that substantially diminish disease progression independent of direct effects on SMC relaxation and pressure reduction (148). These fortuitous successes were accompanied, however, by a vastly larger number of drugs that failed in preclinical studies or clinical trials, many due to unanticipated harmful effects (149; 150). These results point toward the importance of achieving a more complete, systems-oriented understanding of the relevant homeostatic and disease processes and associated consequences of different classes of drugs.
A long-term goal for investigators of vascular biomechanics, cell signaling, immunobiology, matrix biology, and mechanobiology should be integrative quantitative models that are sufficiently rich to identify therapeutic targets for blocking disease processes without substantially impeding homeostatic processes. Progress over the past 20 years leads us to believe that reasonably detailed and accurate mechanobiological models are within reach. It may be optimistic, but it seems that a cell biological model that includes regulation of the principal signaling and gene expression pathways in ECs, SMCs, FBs, and immune cells that govern homeostasis is achievable. Considerable advances have been realized in allied fields, including cardiac mechanics and mechanobiology wherein logic-based cell signaling models have been developed that describe myriad empirical findings and thus promise to provide predictive capability (151). Such models, when extended across multiple interacting cell types having diverse phenotypes, should enable much better prediction of remodeling under conditions of interest as well as expected outcomes for potential therapeutic intervention.
Nonetheless, one further step is needed to solve the bigger problem – to synthesize knowledge across scales within a single modeling framework that includes gene regulation, cell signaling, and cell and tissue-level mechanical consequences. This may require integrating different modeling approaches (e.g., cell signaling, agent-based, differential equation-based) so that combined effects of forces on cell signaling and the effects of cells on force generation and matrix synthesis and assembly can be assessed. While their precise nature remains to be determined, a comprehensive approach of this general type is the basic requirement for understanding the multiscale physical and regulatory interactions that govern vascular homeostasis, adaptation, and disease. The benefit to both basic science and clinical research would be considerable.
Acknowledgments
This work was supported, in part, by grants from the US NIH (R01 HL105297, U01 HL146723, and P01 HL134605).
References
- 1.Dajnowiec D, Langille BL. 2007. Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodelling. Clin Sci (Lond) 113:15–23 [DOI] [PubMed] [Google Scholar]
- 2.Bikfalvi A 2006. Angiogenesis: health and disease. Ann Oncol 17 Suppl 10:x65–70 [DOI] [PubMed] [Google Scholar]
- 3.Humphrey JD, Dufresne ER, Schwartz MA. 2014. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15:802–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safar ME. 2010. Arterial aging--hemodynamic changes and therapeutic options. Nat Rev Cardiol 7:442–9 [DOI] [PubMed] [Google Scholar]
- 5.Humphrey JD. 2002. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer-Verlag; New York. XVI, 758 pp. [Google Scholar]
- 6.Davies PF. 2009. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6:16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey JD. 2008. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey JD, Eberth JF, Dye WW, Gleason RL. 2009. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzapfel GA, Ogden RW. 2010. Modelling the layer-specific three-dimensional residual stresses in arteries, with an application to the human aorta. J R Soc Interface 7:787–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claesson-Welsh L 2015. Vascular permeability--the essentials. Ups J Med Sci 120:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wettschureck N, Strilic B, Offermanns S. 2019. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol Rev 99:1467–525 [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Li YS, Chien S. 2014. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34:2191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. 2017. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol Med 23:850–68 [DOI] [PubMed] [Google Scholar]
- 14.Tsao PS, Buitrago R, Chan JR, Cooke JP. 1996. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation 94:1682–9 [DOI] [PubMed] [Google Scholar]
- 15.Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, et al. 2015. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. Elife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, et al. 2017. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 8:2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan S, Mohan N, Sprague EA. 1997. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol 273:C572–8 [DOI] [PubMed] [Google Scholar]
- 18.Mohan S, Mohan N, Valente AJ, Sprague EA. 1999. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am J Physiol 276:C1100–7 [DOI] [PubMed] [Google Scholar]
- 19.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, et al. 2000. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch 436:257–70 [DOI] [PubMed] [Google Scholar]
- 20.Yuan S, Yurdagul A Jr., Peretik JM, Alfaidi M, Al Yafeai Z, et al. 2018. Cystathionine gamma-Lyase Modulates Flow-Dependent Vascular Remodeling. Arterioscler Thromb Vasc Biol 38:2126–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaper W 2009. Collateral circulation: past and present. Basic Res Cardiol 104:5–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek A, Izumo S. 1992. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol 263:C389–96 [DOI] [PubMed] [Google Scholar]
- 23.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, et al. 1995. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 269:C1371–8 [DOI] [PubMed] [Google Scholar]
- 24.Chiu JJ, Wang DL, Chien S, Skalak R, Usami S. 1998. Effects of disturbed flow on endothelial cells. J Biomech Eng 120:2–8 [DOI] [PubMed] [Google Scholar]
- 25.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. 2016. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126:821–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens GK, Kumar MS, Wamhoff BR. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801 [DOI] [PubMed] [Google Scholar]
- 27.Haga JH, Li YS, Chien S. 2007. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40:947–60 [DOI] [PubMed] [Google Scholar]
- 28.Lacolley P, Regnault V, Segers P, Laurent S. 2017. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol Rev 97:1555–617 [DOI] [PubMed] [Google Scholar]
- 29.Leung DY, Glagov S, Mathews MB. 1976. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191:475–7 [DOI] [PubMed] [Google Scholar]
- 30.Wilson ESK, Ives HE. 1995. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. Journal of Clinical Investigation 96:2364–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldschmidt ME, McLeod KJ, Taylor WR. 2001. Integrin-mediated mechanotransduction in vascular smooth muscle cells: frequency and force response characteristics. Circ Res 88:674–80 [DOI] [PubMed] [Google Scholar]
- 32.Leckband DE, de Rooij J. 2014. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol 30:291–315 [DOI] [PubMed] [Google Scholar]
- 33.Cao W, Zhang D, Li Q, Liu Y, Jing S, et al. 2017. Biomechanical Stretch Induces Inflammation, Proliferation, and Migration by Activating NFAT5 in Arterial Smooth Muscle Cells. Inflammation 40:2129–36 [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Cao W, Cui J, Yu Y, Zhao Y, et al. 2018. Arterial Wall Stress Induces Phenotypic Switching of Arterial Smooth Muscle Cells in Vascular Remodeling by Activating the YAP/TAZ Signaling Pathway. Cell Physiol Biochem 51:842–53 [DOI] [PubMed] [Google Scholar]
- 35.Strauss BH, Rabinovitch M. 2000. Adventitial fibroblasts: defining a role in vessel wall remodeling. Am J Respir Cell Mol Biol 22:1–3 [DOI] [PubMed] [Google Scholar]
- 36.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, et al. 2001. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 89:1111–21 [DOI] [PubMed] [Google Scholar]
- 37.Gingras M, Farand P, Safar ME, Plante GE. 2009. Adventitia: the vital wall of conduit arteries. J Am Soc Hypertens 3:166–83 [DOI] [PubMed] [Google Scholar]
- 38.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–63 [DOI] [PubMed] [Google Scholar]
- 39.Hinz B 2010. The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–55 [DOI] [PubMed] [Google Scholar]
- 40.Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, et al. 2007. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 115:2731–8 [DOI] [PubMed] [Google Scholar]
- 41.Meester JA, Vandeweyer G, Pintelon I, Lammens M, Van Hoorick L, et al. 2017. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med 19:386–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiquet M, Gelman L, Lutz R, Maier S. 2009. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 1793:911–20 [DOI] [PubMed] [Google Scholar]
- 43.Janmey PA, Wells RG, Assoian RK, McCulloch CA. 2013. From tissue mechanics to transcription factors. Differentiation 86:112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herum KM, Lunde IG, McCulloch AD, Christensen G. 2017. The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart. J Clin Med 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Jing SRT, Kirabo Annet, Trott Daniel W, Saleh Mohamed A, Xiao Liang, Madhur Meena S, Chen Wei, Harrison David G. 2014. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circulation Research 114:616–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saini K, Cho S, Dooling LJ, Discher DE. 2020. Tension in fibrils suppresses their enzymatic degradation - A molecular mechanism for 'use it or lose it'. Matrix Biol 85-86:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagenseil JE, Mecham RP. 2009. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89:957–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphries JD, Chastney MR, Askari JA, Humphries MJ. 2019. Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol 56:14–21 [DOI] [PubMed] [Google Scholar]
- 49.Finney AC, Stokes KY, Pattillo CB, Orr AW. 2017. Integrin signaling in atherosclerosis. Cell Mol Life Sci 74:2263–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz GS, Wysocki A. 2009. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17:153–62 [DOI] [PubMed] [Google Scholar]
- 51.Hinz B 2015. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol 47:54–65 [DOI] [PubMed] [Google Scholar]
- 52.Tremble P, Damsky CH, Werb Z. 1995. Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol 129:1707–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez D, Owens GK. 2012. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95:156–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis GE. 1992. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun 182:1025–31 [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, et al. 2001. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol 154:1069–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells JM, Gaggar A, Blalock JE. 2015. MMP generated matrikines. Matrix Biol 44-46:122–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortega N, Werb Z. 2002. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci 115:4201–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphrey JD. 2008. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50:53–78 [DOI] [PubMed] [Google Scholar]
- 59.Matsumoto T, Hayashi K. 1994. Mechanical and dimensional adaptation of rat aorta to hypertension. J Biomech Eng 116:278–83 [DOI] [PubMed] [Google Scholar]
- 60.Wolinsky H, Glagov S. 1967. A lamellar unit of aortic medial structure and function in mammals. Circ Res 20:99–111 [DOI] [PubMed] [Google Scholar]
- 61.Intengan HD, Schiffrin EL. 2001. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38:581–7 [DOI] [PubMed] [Google Scholar]
- 62.Laurent S, Boutouyrie P. 2015. The structural factor of hypertension: large and small artery alterations. Circ Res 116:1007–21 [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Lemus LA, Hill MA, Meininger GA. 2009. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24:45–57 [DOI] [PubMed] [Google Scholar]
- 64.Marenzana M, Wilson-Jones N, Mudera V, Brown RA. 2006. The origins and regulation of tissue tension: identification of collagen tension-fixation process in vitro. Exp Cell Res 312:423–33 [DOI] [PubMed] [Google Scholar]
- 65.Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. 1998. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol 175:323–32 [DOI] [PubMed] [Google Scholar]
- 66.Mizutani T, Haga H, Kawabata K. 2004. Cellular stiffness response to external deformation: tensional homeostasis in a single fibroblast. Cell Motil Cytoskeleton 59:242–8 [DOI] [PubMed] [Google Scholar]
- 67.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. 1998. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101:731–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burton AC. 1954. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev 34:619–42 [DOI] [PubMed] [Google Scholar]
- 69.Bellini C, Ferruzzi J, Roccabianca S, Di Martino ES, Humphrey JD. 2014. A microstructurally motivated model of arterial wall mechanics with mechanobiological implications. Ann Biomed Eng 42:488–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz MA, Vestweber D, Simons M. 2018. A unifying concept in vascular health and disease. Science 360:270–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valentin A, Humphrey JD. 2009. Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodelling. Philos Trans A Math Phys Eng Sci 367:3585–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Latorre M, Humphrey JD. 2019. Mechanobiological Stability of Biological Soft Tissues. J Mech Phys Solids 125:298–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nissen R, Cardinale GJ, Udenfriend S. 1978. Increased turnover of arterial collagen in hypertensive rats. Proc Natl Acad Sci U S A 75:451–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taber LA. 1998. A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng 120:348–54 [DOI] [PubMed] [Google Scholar]
- 75.Ambrosi D, Ben Amar M, Cyron CJ, DeSimone A, Goriely A, et al. 2019. Growth and remodelling of living tissues: perspectives, challenges and opportunities. J R Soc Interface 16:20190233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myers PR, Tanner MA. 1998. Vascular endothelial cell regulation of extracellular matrix collagen: role of nitric oxide. Arterioscler Thromb Vasc Biol 18:717–22 [DOI] [PubMed] [Google Scholar]
- 77.Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, et al. 2007. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol 26:625–32 [DOI] [PubMed] [Google Scholar]
- 78.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, et al. 2006. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–605 [DOI] [PubMed] [Google Scholar]
- 79.Demiray H 1992. Wave propagation through a viscous fluid contained in a prestressed thin elastic tube. International Journal of Engineering Science 30:1607–20 [Google Scholar]
- 80.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. 2016. Central Artery Stiffness in Hypertension and Aging: A Problem With Cause and Consequence. Circ Res 118:379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dampney RAL. 2017. Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: Description and Conceptual Model of Central Mechanisms. Front Neurosci 11:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai H, Harrison DG. 2000. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–4 [DOI] [PubMed] [Google Scholar]
- 83.Davignon J, Ganz P. 2004. Role of endothelial dysfunction in atherosclerosis. Circulation 109:III27–32 [DOI] [PubMed] [Google Scholar]
- 84.Endemann DH, Schiffrin EL. 2004. Endothelial dysfunction. J Am Soc Nephrol 15:1983–92 [DOI] [PubMed] [Google Scholar]
- 85.Virdis A, Schiffrin EL. 2003. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens 12:181–7 [DOI] [PubMed] [Google Scholar]
- 86.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. 2006. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med 259:351–63 [DOI] [PubMed] [Google Scholar]
- 87.Sprague AH, Khalil RA. 2009. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78:539–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szmitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S. 2003. Biomarkers of vascular disease linking inflammation to endothelial activation: Part II. Circulation 108:2041–8 [DOI] [PubMed] [Google Scholar]
- 89.Csiszar A, Wang M, Lakatta EG, Ungvari Z. 2008. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol (1985) 105:1333–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. 2016. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can J Cardiol 32:659–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Latorre M BM, Humphrey JD. 2019. Computational modeling suggests biomechanical mechanisms of maladaptative aortic remodeling in hypertension. Int J Engr Sci (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, et al. 2007. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50:219–27 [DOI] [PubMed] [Google Scholar]
- 93.Tellides G, Pober JS. 2015. Inflammatory and immune responses in the arterial media. Circ Res 116:312–22 [DOI] [PubMed] [Google Scholar]
- 94.Choke E, Cockerill G, Wilson WR, Sayed S, Dawson J, et al. 2005. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 30:227–44 [DOI] [PubMed] [Google Scholar]
- 95.Szasz T, Bomfim GF, Webb RC. 2013. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9:105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee HY, Despres JP, Koh KK. 2013. Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis 230:177–84 [DOI] [PubMed] [Google Scholar]
- 97.Medzhitov R 2008. Origin and physiological roles of inflammation. Nature 454:428–35 [DOI] [PubMed] [Google Scholar]
- 98.Kotas ME, Medzhitov R. 2015. Homeostasis, inflammation, and disease susceptibility. Cell 160:816–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mrosovsky N 1990. The physiology of change. Oxford University Press [Google Scholar]
- 100.Davies KJ. 2016. Adaptive homeostasis. Mol Aspects Med 49:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ley K 1996. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res 32:733–42 [PubMed] [Google Scholar]
- 102.Kreuger J, Phillipson M. 2016. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov 15:125–42 [DOI] [PubMed] [Google Scholar]
- 103.Chen JY, Ye ZX, Wang XF, Chang J, Yang MW, et al. 2018. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother 97:423–8 [DOI] [PubMed] [Google Scholar]
- 104.Pacher P, Beckman JS, Liaudet L. 2007. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. 2017. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. 1996. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98:2572–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Touyz RM. 2005. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens 14:125–31 [DOI] [PubMed] [Google Scholar]
- 108.Lacolley P, Safar ME, Regnault V, Frohlich ED. 2009. Angiotensin II, mechanotransduction, and pulsatile arterial hemodynamics in hypertension. Am J Physiol Heart Circ Physiol 297:H1567–75 [DOI] [PubMed] [Google Scholar]
- 109.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, et al. 2011. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J Vasc Res 48:261–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poduri A, Owens AP 3rd, Howatt DA, Moorleghen JJ, Balakrishnan A, et al. 2012. Regional variation in aortic AT1b receptor mRNA abundance is associated with contractility but unrelated to atherosclerosis and aortic aneurysms. PLoS One 7:e48462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallardo-Ortiz IA, Rodriguez-Hernandez SN, Lopez-Guerrero JJ, Del Valle-Mondragon L, Lopez-Sanchez P, et al. 2015. Role of alpha1D -adrenoceptors in vascular wall hypertrophy during angiotensin II-induced hypertension. Auton Autacoid Pharmacol 35:17–31 [DOI] [PubMed] [Google Scholar]
- 112.Trachet B, Renard M, De Santis G, Staelens S, De Backer J, et al. 2011. An integrated framework to quantitatively link mouse-specific hemodynamics to aneurysm formation in angiotensin II-infused ApoE −/− mice. Ann Biomed Eng 39:2430–44 [DOI] [PubMed] [Google Scholar]
- 113.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, et al. 2011. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25:4253–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khosravi R, Miller KS, Best CA, Shih YC, Lee YU, et al. 2015. Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng Part A 21:1529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelleher CM, McLean SE, Mecham RP. 2004. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62:153–88 [DOI] [PubMed] [Google Scholar]
- 116.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, et al. 2014. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124:1622–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marinkovic A, Liu F, Tschumperlin DJ. 2013. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 48:422–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoffman BD, Grashoff C, Schwartz MA. 2011. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475:316–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janmey PA, Fletcher DA, Reinhart-King CA. 2020. Stiffness Sensing by Cells. Physiol Rev 100:695–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georges PC, Janmey PA. 2005. Cell type-specific response to growth on soft materials. J Appl Physiol (1985) 98:1547–53 [DOI] [PubMed] [Google Scholar]
- 121.Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, et al. 2015. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125:3861–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Souilhol C, Harmsen MC, Evans PC, Krenning G. 2018. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res 114:565–77 [DOI] [PubMed] [Google Scholar]
- 123.Chen PY, Qin L, Li G, Wang Z, Dahlman JE, et al. 2019. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nat Metab 1:912–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, et al. 2015. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alencar GF, Owsiany KM, K S, Sukhavasi K, Mocci G, et al. 2020. The Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Humphrey JD, Taylor CA. 2008. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng 10:221–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Humphrey JD, Holzapfel GA. 2012. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 45:805–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakalihasan N, Limet R, Defawe OD. 2005. Abdominal aortic aneurysm. Lancet 365:1577–89 [DOI] [PubMed] [Google Scholar]
- 129.Boussel L, Rayz V, McCulloch C, Martin A, Acevedo-Bolton G, et al. 2008. Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39:2997–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. 2015. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res 116:1448–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, et al. 2017. Altered Smooth Muscle Cell Force Generation as a Driver of Thoracic Aortic Aneurysms and Dissections. Arterioscler Thromb Vasc Biol 37:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen PY, Qin L, Li G, Malagon-Lopez J, Wang Z, et al. 2020. Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell 26:542–57 e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li G, Wang M, Caulk AW, Cilfone NA, Gujja S, et al. 2020. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J Clin Invest 130:1233–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Libby P 2002. Inflammation in atherosclerosis. Nature 420:868–74 [DOI] [PubMed] [Google Scholar]
- 135.Hahn C, Schwartz MA. 2009. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ku DN, Giddens DP, Zarins CK, Glagov S. 1985. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5:293–302 [DOI] [PubMed] [Google Scholar]
- 137.Chiu JJ, Chien S. 2011. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, et al. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437:426–31 [DOI] [PubMed] [Google Scholar]
- 139.David G, Humphrey JD. 2003. Further evidence for the dynamic stability of intracranial saccular aneurysms. J Biomech 36:1143–50 [DOI] [PubMed] [Google Scholar]
- 140.Cyron CJ, Wilson JS, Humphrey JD. 2014. Mechanobiological stability: a new paradigm to understand the enlargement of aneurysms? J R Soc Interface 11:20140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cyron CJ, Humphrey JD. 2014. Vascular homeostasis and the concept of mechanobiological stability. Int J Eng Sci 85:203–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, et al. 2012. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 122:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilson JS, Baek S, Humphrey JD. 2013. Parametric study of effects of collagen turnover on the natural history of abdominal aortic aneurysms. Proc Math Phys Eng Sci 469:20120556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Satha G, Lindstrom SB, Klarbring A. 2014. A goal function approach to remodeling of arteries uncovers mechanisms for growth instability. Biomech Model Mechanobiol 13:1243–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Erlich A, Moulton DE, Goriely A. 2019. Are Homeostatic States Stable? Dynamical Stability in Morphoelasticity. Bull Math Biol 81:3219–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, et al. 2010. Statin-induced Kruppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J Clin Invest 120:1961–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, et al. 2005. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112:720–6 [DOI] [PubMed] [Google Scholar]
- 148.Brasier AR, Recinos A 3rd, Eledrisi MS. 2002. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22:1257–66 [DOI] [PubMed] [Google Scholar]
- 149.Wong CH, Siah KW, Lo AW. 2019. Estimation of clinical trial success rates and related parameters. Biostatistics 20:273–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takebe T, Imai R, Ono S. 2018. The Current Status of Drug Discovery and Development as Originated in United States Academia: The Influence of Industrial and Academic Collaboration on Drug Discovery and Development. Clin Transl Sci 11:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD, Omens JH. 2019. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol 16:361–78 [DOI] [PMC free article] [PubMed] [Google Scholar]