Abstract
Background:
Chronic graft-versus-host disease (cGVHD), a potentially debilitating complication of hematopoietic cell transplantation, confers increased risk for mortality. While treatment decisions rely on an accurate assessment of disease activity/severity, validated methods of assessing cutaneous cGVHD activity/severity appear to be limited.
Objective:
We aimed to identify and evaluate current data on the assessment of disease activity/severity in cutaneous cGVHD
Study Design:
Using modified PRISMA methods, we performed critical literature review for relevant articles.
Results:
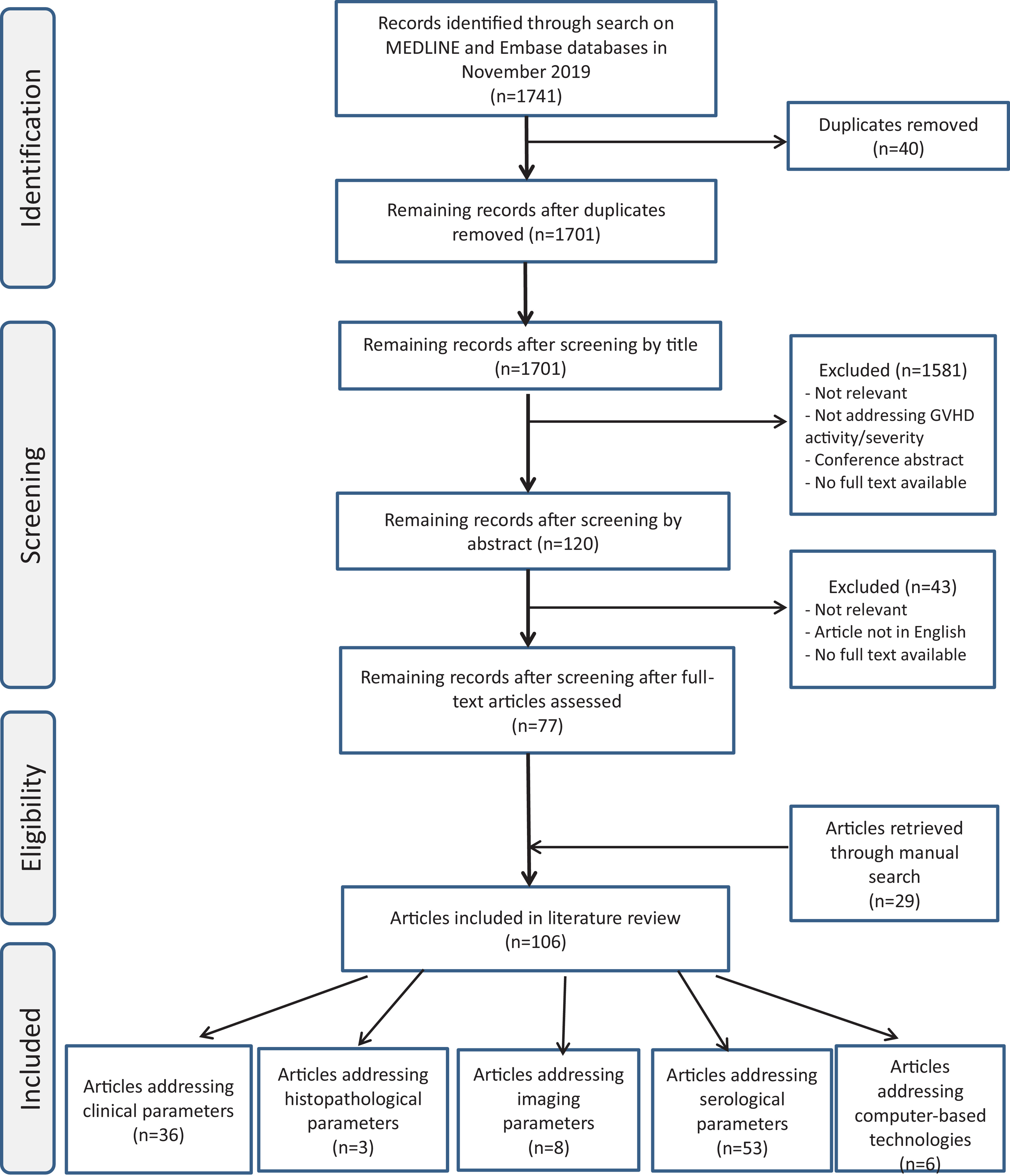
Literature search identified 1741 articles, of which 1701 were excluded as duplicates or failure to meet inclusion criteria. Of all included studies (n=106), 39 (37%) addressed clinical and/or histopathologic parameters, 53 (50%) serologic parameters, 8 (7.5%) imaging parameters and 6 (5.5%) computer-based technologies.
Conclusions:
The only formally validated metric is the NIH consensus scoring system. The currently validated measure of disease activity/severity assessment in cutaneous cGVHD is founded on clinical assessment alone. Lack of an objective marker for cGVHD necessitates further studies. The potential contributions of serologic, imaging and/or computer-based technology are warranted.
Keywords: Graft-versus-host disease, GVHD, sclerotic GVHD, monitoring, disease activity
Introduction
Graft-vs-host disease (GVHD), a common complication of allogeneic hematopoietic cell transplant (HCT), confers substantial risk for morbidity, mortality and diminished quality of life.1 Typically developing a few months after HCT, chronic GVHD (cGVHD) most frequently involves the skin and mucosal surfaces, although multiple organs may be affected, including the eyes, lungs, liver and GI tract.2,3 Cutaneous cGVHD can be classified clinically as sclerotic or non-sclerotic (Fig 1, 2).3 Although sclerotic cGVHD (ScGVHD) is not acutely life-threatening, widespread involvement may lead to considerable functional disability. In addition, skin sclerosis can be associated with ulceration,4 poor wound healing, and increased risk of infection.5 Scarring alopecia or nail dystrophy also can result.6
Figure 1. Dyspigmentation, brawny erythema, and sclerosis in a patient with sclerotic chronic graft-versus-host disease.
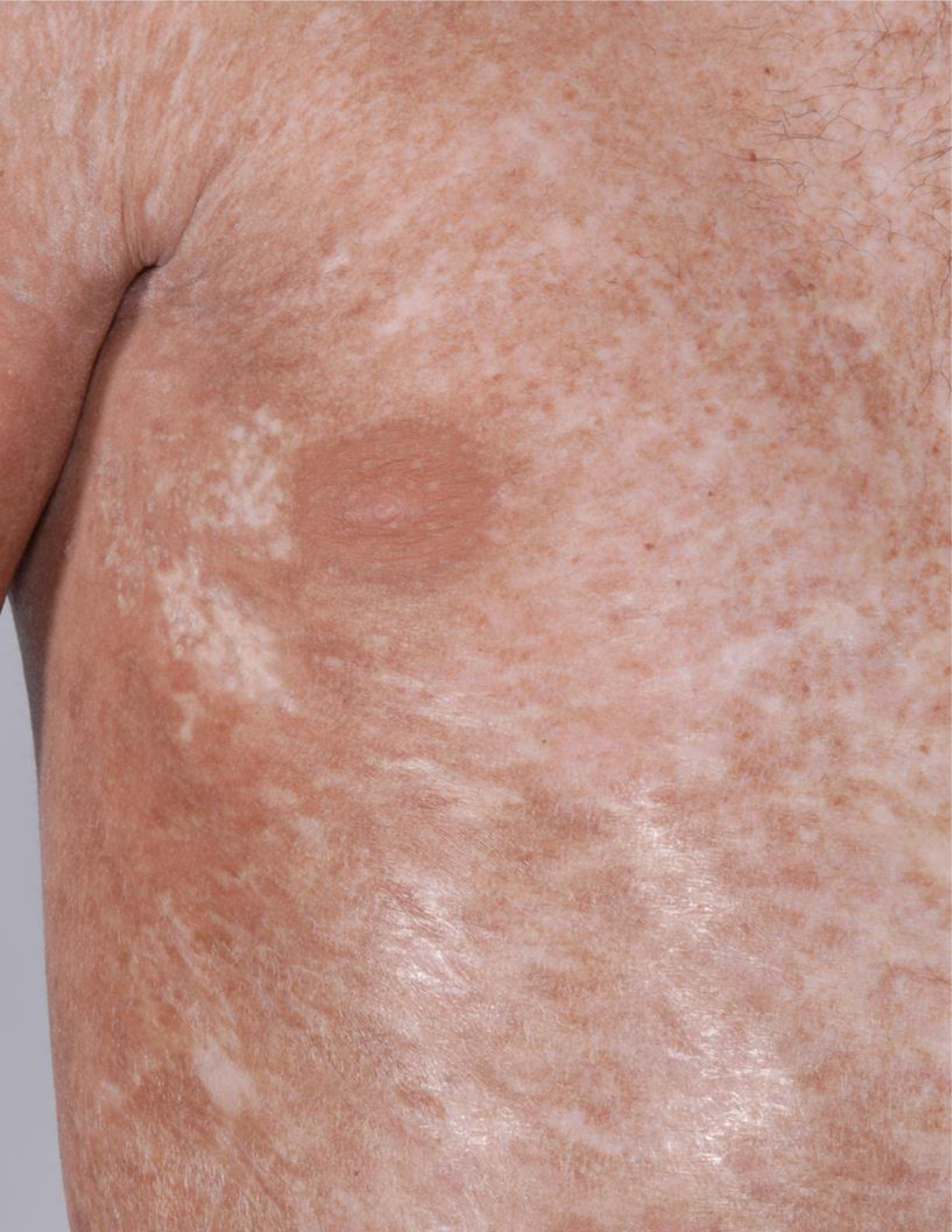
Figure 2. Induration and erythema of the arm in a fasciitis-like presentation of chronic graft-versus-host disease.
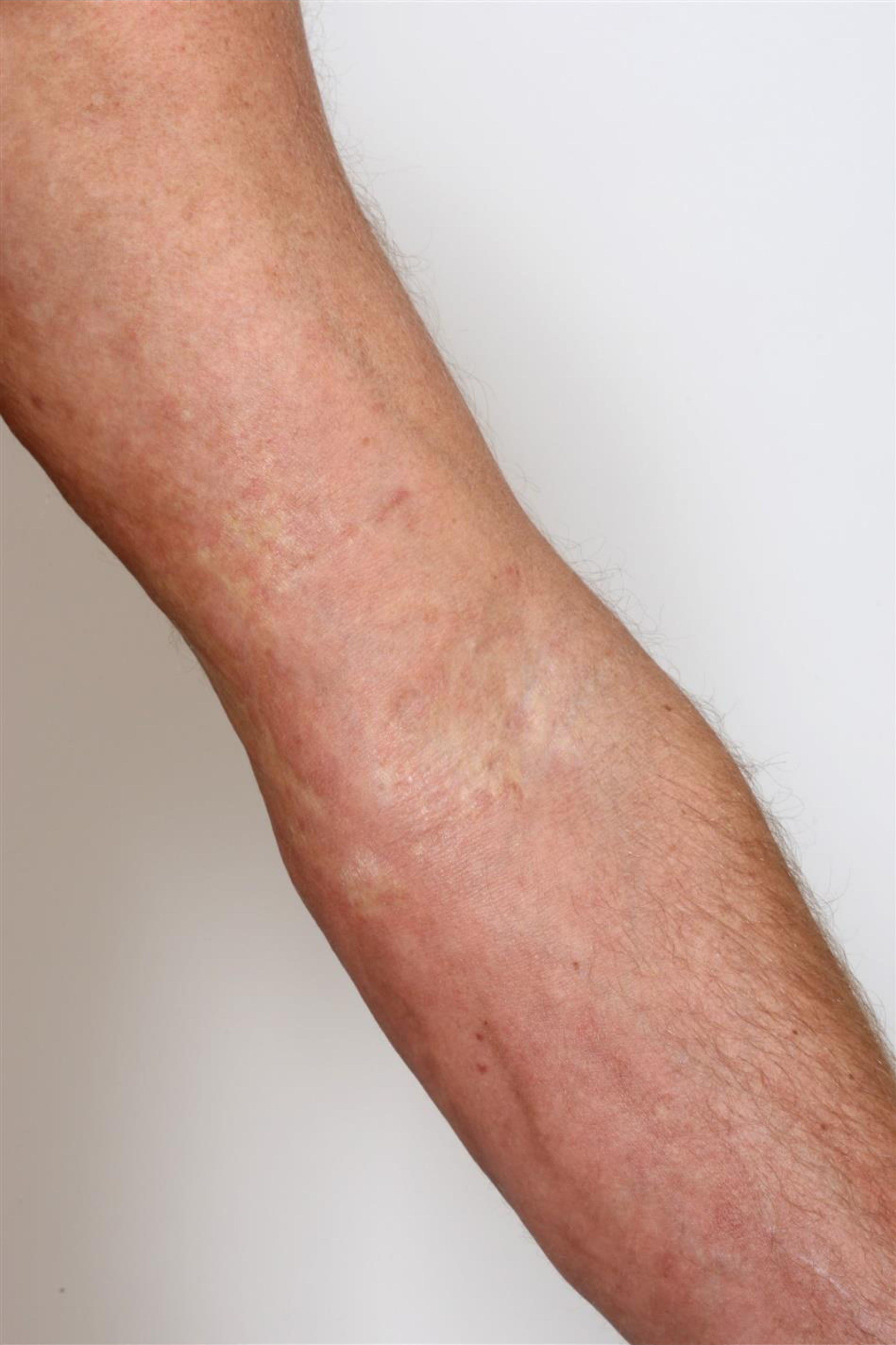
ScGVHD can be difficult to manage, not only due to lack of effective treatments along with their potential adverse effects and high costs, but also due to difficulty with assessing disease activity. Differentiation of active disease versus inactive sequelae can be challenging.7,8 Ideally, medication titration would be guided by reliable, reproducible measures of disease activity.
We aimed to critically evaluate the current literature to assess the utility of various methods, including clinical, histopathologic, serologic, imaging, and computer-based parameters, of accurately measuring disease activity and severity in cutaneous cGVHD.
Methods
We used modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to perform a critical literature review of disease activity assessment in chronic GVHD affecting the skin. An electronic search of MEDLINE and Embase databases was performed in April 2021, using the following key words: “graft-versus-host disease”, “GVHD”, “stem cell transplant”. Each term was searched individually and in combination with terms including, “disease activity”, “disease severity”, and “monitoring”. In addition, relevant literature cited in identified articles was reviewed for possible inclusion. All article abstracts were reviewed manually by a dermatologist (HS) for relevance. Inclusion criteria included availability of full-text versions through our institution’s electronic library or interlibrary exchange and being written in English. Due to the nature of available literature, findings were reported descriptively. For the purposes of this study, we defined disease activity to mean evidence of ongoing inflammation and severity to indicate the intensity of disease activity.
Results
Literature search identified 1741 articles, of which 1701 were excluded as duplicates or failure to meet inclusion criteria. Of all included studies (n=106), 39 (37%) addressed clinical and/or histopathologic parameters, 53 (50%) serologic parameters, 8 (7.5%) imaging parameters and 6 (5.5%) computer-based technologies. Defining disease activity and severity in cutaneous cGVHD can be challenging. For instance, erythema is regarded as sign of active disease whereas pigmentary changes are not. Disease severity refers to intensity of disease activity and can be inferred from patient-reported outcomes like Lee symptom scale.9,10
Table 1 outlines previously reported methods for assessing disease activity and severity.
Table 1.
Possible serum biomarkers in chronic graft-versus-host disease.
| Parameter/Technique | Level of Evidence | Reference |
|---|---|---|
| Clinical parameters | ||
| 2014 NIH skin scoring system | Expert opinion | 8,9,11–16 |
| Itching as a part of patient-reported score | Prospective study (575 patients) | 12 |
| Lee cGVHD Symptom Scale | Prospective study (107 patients) | 12,17 |
| Vienna Skin Score | Prospective study (16 patients) | 18 |
| Skin erythema | Cross-sectional (193 patients) | 9 |
| Adnexal involvement | Retrospective, case-controlled study (36 patients) | 19,20 |
| original Rodnan total skin thickness score | Prospective study (147 scleroderma patients) | 21 |
| modified Rodnan total skin thickness score | Prospective study | 22 |
| Periorbital pigmentation | Case series (7 patients) | 23 |
| Grip strength | Prospective study (584 patients) | 24 |
| 2-minute walk test | Prospective study (584 patients) | 24 |
| Durometer | Prospective, case-controlled studies (13 patients) | 25–28 |
| Myoton device | Cross-sectional study (10 patients) | 28,29 |
| Histopathological parameters | ||
| Histolopathological grading | Retrospective study (120 skin biopsies) | 30 |
| Serological parameters | ||
| Eosinophilia | Retrospective studies (237 patients), (142 patients) | 31–33 |
| Autoantibodies | Retrospective studies (>200 patients) | 34 |
| Platelet-derived growth factor | Cross-sectional study (39 patients) | |
| CXCL9 | Prospective studies (17, 67, 211 patients) | 35–37 |
| CXCL10 | Prospective studies (170, 115 patients) | 38–40 |
| CXCL11 | Prospective study (49, 115, 211 patients) | 36,39,41 |
| CXCR3+CD4+ T cells | Prospective study (46 patients) | 42 |
| BAFF | Retrospective and prospective studies (104, 115 patients) | 39,43–47 |
| CD19+CD21low B Cells | Prospective study (70 patients) | 47–50 |
| ST2 | Prospective study (67 patients) | 37 |
| Elafin | Prospective study (22 patients) | 51 |
| Matrix metalloproteinase | Prospective study (67 patients) | 37 |
| Osteopontin | Prospective study (67 patients) | 37,52 |
| soluble CD163 | Prospective study (167 patients) | 53 |
| MICA | Prospective study (116 patients) | 54 |
| TNF-α | Prospective study (30 patients) | 55 |
| TGF-beta | Prospective study (66, 31 patients) | 56,57 |
| AIF-1 | Prospective study (31 patients) | 57 |
| n-10 | Prospective study (57 patients) | 58 |
| n-15 | Prospective study (153 patients) | 59 |
| n-17 | Retrospective study (51 patients) | 60,61 |
| n-6 | Retrospective study (51 patients) | 37,61,62 |
| IL1ra | Prospective study (98 patients) | 63,64 |
| Soluble IL-2R | Prospective study (27 patients) | 65 |
| Adiponectin | Prospective study (34 patients) | 66,67 |
| lactoperoxidase, lactotransferrin | Case series (10 patients) | 68 |
| Prolactin | Prospective study (236 patients) | 69 |
|
Branched-chain amino acids:
leucine, isoleucine and sulfur-containing metabolite (cystine) |
Prospective study (18 patients) | 70 |
| Imaging parameters | ||
| 20-MHz ultrasonography | Case series (5 patients) | 71 |
| Acoustic radiation force impulse (ARFI) and shear wave elasticity imaging (SWEI) | Case report (5 patients) | 72 |
| Magnetic resonance imaging (MRI) | Case series (16 patients) | 73 |
| MRI with PET | Case series (6 patients) | 74 |
| optical coherence tomography (OCTA) | Case series (1 patient) | 75, |
| Computer-based technologies | ||
| Assessing erythema in 3D photography | Case report (1 patient) | 76 |
| Crowdsourcing for assessing extent of skin involvement | Case report (1 patient) | 77 |
| Machine-learning for patient stratification, based on phenotype severity | Retrospective study (339 patients) | 78 |
| eGVHD App | Prospective study (78 physicians) | 79 |
Clinical Parameters
In 2005, the National Institutes of Health (NIH) Consensus Conference developed criteria and a practical worksheet to assist with the diagnosis and scoring of cGVHD of all major organ systems.80 After several years of implementation of original criteria, these guidelines were refined in 2014.8 Regarding documentation of cutaneous involvement, the scoring system evaluates extent of involvement by body surface area (BSA) and degree of sclerosis, with a mechanism to account for immobility or ulceration. Other skin features, including hyperpigmentation, hypopigmentation, poikiloderma, severe or generalized pruritus, hair involvement or nail involvement, are documented without BSA estimation.9 Assessment of joint and fascial involvement from ScGVHD was added, with photographic range-of-motion (ROM) included as an exploratory measure. While this scoring system represents a useful attempt to quantify disease severity in cutaneous cGVHD, the ability of the tool to distinguish between active, ongoing inflammation and inactive sclerosis has been challenged.81 For example, although erythema, a proxy for inflammation, is included in the BSA skin score calculation, it can be challenging to assess erythema in patients with poikiloderma due to admixed pigmentary changes. Even more challenging can be evaluation of dermal, subcutaneous or fascial disease only, when determination of activity relies on acute edema, loss of range of motion, involvement of new anatomic sites, and/or other organ involvement.
The ability of the 2014 NIH scoring system to reliably identify patients with severe cGVHD was validated in subsequent studies. Specifically, Moon et al. demonstrated in a cohort study of 425 patients that the NIH scoring correctly identified high-risk patients (i.e. lower overall survival) using the method. Subsequently, the authors successfully validated the NIH global scoring system of cGVHD in severe disease.14
The Chronic GVHD Consortium evaluated patient-reported skin scores and outcomes, such as the 2005 NIH skin scoring scale, 2006 NIH skin response scale, Vienna Skin Scale, Johns Hopkins sclerosis and fasciitis scales, and the Lee Symptoms skin subscale. This large, multicenter study included a prospectively assembled cohort of 458 patients.15 Results demonstrated that an NIH skin score of 3 (most severe) correlated with increased risk for mortality and non-relapse mortality. Also, patients who experienced worsening of their NIH skin score had worse overall mortality than those with stable skin disease.15 In addition, another study compared NIH criteria and other tools to clinician- and patient-reported response measures in a cross-sectional prospective study of 193 patients with moderate-to-severe cGVHD. This study noted that the presence and extent of erythema was associated with active disease and poor survival. 81 The authors cautioned that the degree and extent of scored erythema can be affected by topical medications; hence, the effect of skin-directed treatment should be taken into account.81
Inter-rater agreement of the 2005 NIH scoring system was tested in several studies. Mitchell et al. evaluated 34 hemato-oncologists who received standardized training in skin scoring (2.5 hours) then assessed 25 cGVHD patients in an ambulatory clinic.16 Results were then compared with transplant experts’ ratings. Results showed a fair level of inter-rater agreement on skin manifestations, with regards to erythema and sclerosis. While this study demonstrated the feasibility of NIH for clinical application, it also required standardized training of raters. However, these findings were limited by lack of control group. 16 Another study involved assessment by 6 HCT transplant physicians and 4 dermatologists of 8 patients using NIH scoring system skin-specific variables, ROM severity grading, and a body site skin sclerosis grade (SSG); with a sclerosis score ranging from 0 to 3. Results were correlated with patient-reported severity scores and quality-of-life metrics.13 This study demonstrated reasonable inter-rater agreement for ROM, a finding attributed to the incorporation of a detailed visual aid with the 2014 NIH scoring sheet. Limitations of this study included a small number of patients, lack of information regarding the experience level of assessors, few details regarding the training guides provided to them, and lack of validation of the SSG grading used.
Other cGVHD scoring systems have been proposed previously. Specifically, Lee et al. developed a symptom scale of 30 items in a patient self-administered questionnaire to evaluate the effects of cGVHD on skin, vitality, pulmonary status, nutritional status, psychological functioning, ocular symptoms, and oral symptoms.17 It also predicted overall survival and non-relapse mortality.12 While shown to be easily understood and accepted by patients, the Lee cGVHD Symptom Scale emphasizes patient-reported symptoms rather than disease activity. The Vienna Skin Score was found to have reasonable reproducibility in a pilot validation study involving 16 patients examined by 4 physicians 3 separate times over 2 days.18 However, this scoring system also does not differentiate active disease from disease sequelae. In a large scale-study performed on 575 patients, Palmer et al demonstrated that itching could be a predictor of disease activity and treatment outcome. 12
A recent systematic review of patient-reported outcome measures (PROM) revealed that Human Activity Profile, Lee Symptom Scale and NIH Eleven Point Scale were the most reliable in cGVHD.82
The Rodnan total skin thickness score, which assesses skin thickness by palpation, is validated for use in systemic sclerosis and has since been modified for use in morphea.21,22 The utility of this scoring system in the assessment of ScGVHD is not well explored.
A clinical clue reported to correlate with increased severity of overall cGVHD is involvement of the hair follicle or nails,19 sites that are usually sequestered from the immune system and that, when affected, may indicate more aggressive skin disease.20 In an analysis of 7 patients with ScGVHD, periorbital pigmentation was observed as an initial manifestation in 3 cases, and so it has been proposed as a predictor for extensive ScGVHD; 23 however, larger validation studies are required.
Several attempts have been made to quantitatively measure the severity of skin and soft tissue sclerosis. Myotonometry is a non-invasive method to study soft tissue mechanical properties that works by delivering a brief mechanical impulse and measuring dampening of oscillatory tissue response. In a study of 14 cGVHD patients compared to 10 healthy controls, myotonometry proved to be effective in measuring skin stiffness, and thereby degree of sclerosis.29 A durometer is a non-invasive handheld device used to determine surface hardness by measuring the amount of force required to produce an indentation, to evaluate skin hardness.25–28 In a study of 7 cGVHD patients, both myotometry and durometry exhibited high inter-observer reproducibility.28 However, performance between the two modalities varied based on anatomic site of measurement.28 Larger studies involving serial measurements over time and correlation with other measures of clinical disease activity are required.
While dermoscopy, a rapid non-invasive tool, is extensively employed in the diagnosis of many dermatologic diseases, its utility in GVHD remains limited. One study correlated dermoscopic findings with histopathologic features in 15 patients with cGVHD. The most common findings in cGVHD were granularity, scaling, linear vessels, and white patchy areas. Granularity corresponds to melanophages whereas the white areas are related to increased dermal collagen.83 Further studies are needed to establish the dermoscopic features of GVHD along with the potential use for monitoring disease activity and severity.
Other reported disease activity assessment methods include hand grip strength (HGS) and 2-minute walk test (2MWT)11,24 pertain more to extracutaneous manifestations of cGVHD. The recent 2021 NIH consensus criteria emphasized on the patients’ role in monitoring and reporting their symptoms which would be helpful in determining the disease activity, severity as well as response to treatment.84 This can be facilitated using mobile application or online platforms.85
Histopathological parameters
Microscopic features of cGVHD can include pandermal sclerosis with periadnexal lymphoplasmacytic aggregates and vacuolar interface changes, considered helpful to support a clinical diagnosis of cGVHD.8 In a recent study, no correlation was observed between histopathological grading of GVHD and survival.30
Imaging parameters
Several studies involving the measurement of skin thickness radiographically in ScGVHD have been performed.71,72,74,86,87 One study found an inverse correlation between skin thickness, as quantified by two-dimensional 20-MHz B-mode B-scan ultrasonography, and clinical response to therapy in 5 patients with cGVHD.71 Another study using acoustic radiation force impulse (ARFI) and shear wave elasticity imaging (SWEI), a method allowing for simultaneous assessment of tissue thickness and stiffness, showed increased skin stiffness in sclerotic compared to unaffected skin in a patient with localized ScGVHD.72
In a study of 16 patients with cGVHD, magnetic resonance imaging (MRI) was employed to evaluate subcutaneous or fascial involvement.73 MRI was able to detect deeper and more extensive involvement than could be appreciated by physical examination alone. Another group of investigators combined MRI with fluorodeoxyglucose (FDG)-positron emission tomography (PET) to monitor disease activity in 6 patients with ScGVHD.74 The study found that MRI could detect pathological changes in the deep soft tissues, and the addition of contrast allowed estimation of the degree of inflammation. 74
Optical coherence tomography (OCTA), a non-invasive imaging modality using near-infrared light, has been explored in various dermatological diseases.88 It has been employed to visualize capillary-level vascular and structural features within skin in vivo, a function that has shown to correlate with vascular and structural changes in morphea75 and cGVHD.87 Recently, Chen et al. investigated the utility of OCTA in 7 patients with cGVHD, where they described hyperkeratosis, epidermal hyperplasia as well as reduced depth of light transmission in those patients. These findings correlated with the severity of cutaneous cGVHD when measured by the Vienna Skin Scale. The decrease in the depth of light transmission was attributed to inflammatory cellular infiltrate with more severe disease. Of note, these findings varied with body sites which may necessitate the need for body site-specific criteria when assessing patients with cGVHD. Authors also followed up one patient after receiving treatment and detected improvement by OCTA paralleling the clinical improvement. 89 However, larger scales studies are warranted to evaluate its utility in monitoring cGVHD.
Serologic parameters
In 2014, the cGVHD Biomarker Working Group gave recommendations regarding identification and validation of potential biomarker candidates prior to qualification for clinical application.90 Several attempts have been made to identify clinically useful diagnostic and prognostic serologic biomarkers for cGVHD (Supplementary Table). However, none is specific to cutaneous cGVHD activity.
Evidence for the prognostic value of serum eosinophilia, a marker of Th2 immunity, in cGVHD has been mixed.31–33 C-reactive protein, a marker of overall inflammation, was found to be elevated in patients with active and severe cGVHD in a cohort of 189 patients.91 The relationship between platelet count and disease activity appears unclear. Specifically, complications and treatment-related mortality have been reported with thrombocytopenia92 while more severe forms of cGVHD were associated with thrombocytosis.91
Several studies have noted an association between certain chemokines, particularly CXCL10 and its biologic mediators, and the presence and severity of cGVHD.35,38,40,93 Given the role of B-cells in the development of cGVHD, researchers have investigated B-cell-activating factor (BAFF) as a biomarker for cGVHD.41,43,45,46 In a study of 104 patients, BAFF levels were higher in patients with active cGVHD. Another study of 46 patients showed reduction in serum BAFF levels after treatment with high-dose prednisone 43 and extracorporeal electrophoresis.45 In addition, BAFF levels seem to correlate directly with non-relapse mortality.94 Larger prospective validation studies of the most promising predictors of cGVHD disease activity are required.
The clinical relevance of circulating of autoantibodies, such as anti-nuclear antibodies, in patients with cGVHD has been investigated. A cohort study of 121 patients found that the presence of autoantibodies conferred more favorable survival outcomes.95 Though another study of 65 patients showed no correlation with cGVHD activity and outcomes.34 In addition, two larger studies, each involving over 200 patients, found no association with autoantibodies specific cGVHD disease manifestations.96 Among the most commonly studied autoantibodies were antinuclear antibody (ANA) and rheumatoid factor (RF).
Elafin, a serine protease inhibitor, secreted by keratinocytes. It has immunomodulatory and antiproliferative function favoring the development of a Th1response. While increased levels of epidermal elafin is associated with poor prognosis of lichenoid cGVHD, elafin was not detectable in sclerotic cGVHD.51 When measured on days 15 and 30 post-transplant, elafin was not associated with occurrence of cGVHD, non-relapse mortality, therapy-resistant GVHD, or overall survival. However, levels of elafin were elevated in patients with severe skin acute GVHD.97
Another potential biomarker for cGVHD is prolactin, a polypeptide hormone, not only secreted by pituitary gland but also by T lymphocytes with various immunoregulatory functions.98 Salas and colleagues studied prolactin in 255 patients with cGVHD and they found that patients with elevated prolactin levels were 6.4 times more likely to have active cGVHD compared to patients with normal levels. However, prolactin levels did not correlate with overall survival.69 Although these results support the use of prolactin as an indicator for activity in cGVHD, no solid association can be made owing to the retrospective design of this study. Larger prospective studies are warranted to prove the reliability of prolactin.
While the use of serologic biomarkers to assess disease activity would be attractive, there are several practical limitations, including: (a) clinical heterogeneity, (b) laboratory assay variation, (c) inadequate patient numbers included in studies, and (d) variable effect of immunosuppressive therapy or infection on biomarker levels.99
Clinical trials of preemptive interventions or novel drugs based on GVHD biomarkers are warranted.
Computer-based technologies
Computer assisted estimation of BSA involvement, a crucial component of the 2014 NIH skin score, has been recognized as a practice gap.8,81 Tkaczyk et al. investigated an objective means to assess the extent and severity of erythema in 3D photographs, a method that offered the potential to increase accuracy of BSA estimation over that from 2D photographs. After undergoing standardized training, 6 raters were asked to delineate areas of erythema in 3D photos of one patient. Annotated images were evaluated by software that could calculate BSA. While this method was shown to be efficient in tracking erythema, raters disagreed on extent and degree of erythema, likely due to the indistinct margins of erythema and/or differences in visual perception.76 Application on larger scale is required to fully understand the utility of this technology. The same group of investigators studied crowdsourcing, or employment of multiple non-expert individuals to complete independent tasks, to assess the extent of skin involvement by cGVHD.77 Crowdsourcing was shown to be an efficient method in delineating the extent of skin involvement in cGVHD patients. Machine-learning has also been used to risk-stratify patients with cGVHD based on phenotype.100. Based on the subcomponents of the NIH Consensus Criteria, disease manifestations were divided into 7 clusters based on every organ involvement. Then, computer analysis classified 339 patients into various clusters, which could then subsequently predict clinical outcomes. 100
Aiming to easily assess severity of cGVHD among health care providers, an online application has been used eGVHD App (www.uzleuven.be/egvhd). Using this low-cost, readily available tool encourages clinicians to systematically evaluate patients and score the disease severity.79,101,102 The recent 2021 NIH consensus criteria suggested the incorporation of such tools in the electronic health record for better disease assessment.84
Measures used in Clinical trials
The use of the 2005 and 2014 NIH consensus criteria proved to be useful for developing better structured clinical trials. they helped in approving a novel drug, ibrutinib by the FDA in 2017.103 When compared, the 2014 NIH consensus criteria were better than the 2005 criteria in classification of organ involvement in 284 patients with cGVHD.104 Therefore, recent clinical trials employed the 2014 NIH response criteria as primary end-point to measure overall survival. For example, these measures were used in trials for ibrutinib103 and pomalidomide for resistant cases of cGVHD.105 In addition to the most recent drug belumosudil (KD025) which showed substantial improvement in overall survival rate and quality-of-life with reduced corticosteroids doses and limited toxicity. 106 This highlights the necessity of having a valid tool to measure disease activity and severity as it helps in clinical trials and discovering novel therapies.
Conclusion
This critical literature analysis revealed several prior efforts to use clinical, histopathologic, imaging, serologic, or computer-based methods to assess disease activity and severity of cGVHD. However, the only well-validated method is the NIH clinical scoring system, which depends on clinical information only. While currently used by dermatologists in clinical practice, the 2014 NIH criteria are helpful for assessing skin disease yet the need for a more objective marker is warranted. As per the 2021 NIH recommendation for clinical trials, the use of optical coherence tomography and myoton device was highly supported. 84
Future studies may evaluate the additive value of incorporating other biomarkers, whether histopathologic, imaging, serologic, or computer-based, into clinical scoring systems to optimally assess disease activity and severity in cGVHD.
Supplementary Material
Figure 3. Flowchart indicating article inclusion/exclusion, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Highlights.
Cutaneous chronic graft-versus-host disease (cGVHD) can be difficult to manage, not only due to lack of effective treatments along with their potential adverse effects and high costs, but also due to difficulty with assessing disease activity.
There is unmet need for reliable and reproducible measures of disease activity/severity which will affect treatment plan.
This study found that the NIH consensus scoring system, based entirely on clinical data, is the only well-validated metric of disease activity/severity.
The potential value of imaging, serologic, and/or computer-based methods of disease activity/severity assessment requires further exploration.
Acknowledgments
Funding source: This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Dr. Tkaczyk was supported by a Career Development Award from the United States Department of Veterans Affairs Clinical Sciences R&D Service (IK2 CX001785).
Footnotes
Conflicts of interest: Authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inamoto Y, Storer BE, Petersdorf EW et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood 2013; 121: 5098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program 2008: 134–41. [DOI] [PubMed] [Google Scholar]
- 3.Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. Pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol 2012; 66: 515.e1–18; quiz 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jachiet M, de Masson A, Peffault de Latour R et al. Skin ulcers related to chronic graft-versus-host disease: clinical findings and associated morbidity. Br J Dermatol 2014; 171: 63–8. [DOI] [PubMed] [Google Scholar]
- 5.Martires KJ, Baird K, Steinberg SM et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood 2011; 118: 4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavand S, Lehman JS, Hashmi S et al. Cutaneous manifestations of graft-versus-host disease: role of the dermatologist. Int J Dermatol 2017; 56: 131–40. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers CJ, Burge S, Scarisbrick J et al. More than skin deep? Emerging therapies for chronic cutaneous GVHD. Bone Marrow Transplant 2013; 48: 323–37. [DOI] [PubMed] [Google Scholar]
- 8.Jagasia MH, Greinix HT, Arora M et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21: 389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Wolff D, Kitko C et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant 2015; 21: 984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood 2017; 129: 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavletic SZ, Martin P, Lee SJ et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response criteria working group report. Biology of Blood and Marrow Transplantation 2006; 12: 252–66. [DOI] [PubMed] [Google Scholar]
- 12.Palmer J, Chai X, Pidala J et al. Predictors of survival, nonrelapse mortality, and failure-free survival in patients treated for chronic graft-versus-host disease. Blood 2016; 127: 160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardones AR, Sullivan KM, Green C et al. Interrater Reliability of Clinical Grading Measures for Cutaneous Chronic Graft-vs-Host Disease. JAMA Dermatol 2019; 155: 833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JH, Sohn SK, Lambie A et al. Validation of National Institutes of Health Global Scoring System for Chronic Graft-Versus-Host Disease (GVHD) According to Overall and GVHD-Specific Survival. Biology of Blood and Marrow Transplantation 2014; 20: 556–63. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsohn DA, Kurland BF, Pidala J et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood 2012; 120: 2545–52; quiz 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell SA, Jacobsohn D, Thormann Powers KE et al. A multicenter pilot evaluation of the National Institutes of Health chronic graft-versus-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant 2011; 17: 1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkel EC, Mitchell SA, Lee SJ. Content Validity of the Lee Chronic Graft-versus-Host Disease Symptom Scale as Assessed by Cognitive Interviews. Biol Blood Marrow Transplant 2016; 22: 752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greinix HT, Pohlreich D, Maalouf J et al. A single-center pilot validation study of a new chronic GVHD skin scoring system. Biol Blood Marrow Transplant 2007; 13: 715–23. [DOI] [PubMed] [Google Scholar]
- 19.Huang JT, Duncan CN, Boyer D et al. Nail dystrophy, edema, and eosinophilia: harbingers of severe chronic GVHD of the skin in children. Bone Marrow Transplant 2014; 49: 1521–7. [DOI] [PubMed] [Google Scholar]
- 20.Naftulin JS, Penzi LR, Manatis-Lornell A et al. Longstanding alopecia and nail dystrophy are associated with more severe overall chronic graft-versus-host disease in adults. Bone Marrow Transplant 2019; 54: 469–72. [DOI] [PubMed] [Google Scholar]
- 21.Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum 1979; 22: 130–40. [DOI] [PubMed] [Google Scholar]
- 22.Khanna D, Furst DE, Clements PJ et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. Journal of scleroderma and related disorders 2017; 2: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chosidow O, Bagot M, Vernant JP et al. Sclerodermatous chronic graft-versus-host disease. Analysis of seven cases. J Am Acad Dermatol 1992; 26: 49–55. [DOI] [PubMed] [Google Scholar]
- 24.Pidala J, Chai X, Martin P et al. Hand grip strength and 2-minute walk test in chronic graft-versus-host disease assessment: analysis from the Chronic GVHD Consortium. Biol Blood Marrow Transplant 2013; 19: 967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falanga V, Bucalo B. Use of a durometer to assess skin hardness. Journal of the American Academy of Dermatology 1993; 29: 47–51. [DOI] [PubMed] [Google Scholar]
- 26.Seyger MMB, van den Hoogen FHJ, Boo Td et al. Reliability of two methods to assess morphea: Skin scoring and the use of a durometer. Journal of the American Academy of Dermatology 1997; 37: 793–6. [DOI] [PubMed] [Google Scholar]
- 27.Merkel PA, Silliman NP, Denton CP et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Care & Research 2008; 59: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Wang L, Vain A et al. Interobserver Reproducibility of the Myoton and Durometer Devices to Measure Skin Stiffness and Hardness in Chronic Cutaneous Graft-Versus-Host Disease Patients. Blood 2019; 134: 4515-. [Google Scholar]
- 29.Dellalana LE, Chen F, Vain A et al. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Skin Research and Technology 2019; 25: 289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogenes MCH, Te Boome LCJ, van der Valk DC et al. Clinical versus histological grading in the assessment of cutaneous graft versus host disease. Eur J Med Res 2019; 24: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortensen KB, Gerds TA, Bjerrum OW et al. The prevalence and prognostic value of concomitant eosinophilia in chronic graft-versus-host disease after allogeneic stem cell transplantation. Leuk Res 2014; 38: 334–9. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Popradi G, Xu W et al. Peripheral blood eosinophilia has a favorable prognostic impact on transplant outcomes after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 471–82. [DOI] [PubMed] [Google Scholar]
- 33.Huang JT, Lehmann L, Duncan C et al. Eosinophilia, edema, and nail dystrophy: Harbingers of severe chronic graft versus host disease of the skin in children. Biology of Blood and Marrow Transplantation 2014; 1): S171–S2. [DOI] [PubMed] [Google Scholar]
- 34.Hao B, Gao S, Sang YW et al. Potential value of autoantibodies as biomarkers of chronic graft-versus-host disease after allogeneic stem cell transplantation. J Zhejiang Univ Sci B 2019; 20: 849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitko CL, Levine JE, Storer BE et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood 2014; 123: 786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu Zaid M, Wu J, Wu C et al. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood 2017; 129: 162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Storer BE, Kushekhar K et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. J Clin Oncol 2016; 34: 2583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kariminia A, Holtan SG, Ivison S et al. Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood 2016; 127: 3082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed SS, Wang XN, Norden J et al. Identification and validation of biomarkers associated with acute and chronic graft versus host disease. Bone Marrow Transplant 2015; 50: 1563–71. [DOI] [PubMed] [Google Scholar]
- 40.Paczesny S, Abu Zaid M. CXCL10: most consistent cGVHD biomarker? Blood 2016; 127: 2950–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohmann EM, Fehn U, Holler B et al. Altered immune reconstitution of B and T cells precedes the onset of clinical symptoms of chronic graft-versus-host disease and is influenced by the type of onset. Ann Hematol 2017; 96: 299–310. [DOI] [PubMed] [Google Scholar]
- 42.Croudace JE, Inman CF, Abbotts BE et al. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood 2012; 120: 4246–55. [DOI] [PubMed] [Google Scholar]
- 43.Sarantopoulos S, Stevenson KE, Kim HT et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clinical cancer research : an official journal of the American Association for Cancer Research 2007; 13: 6107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakim FT, Rehman N, Dickinson J et al. Elevated BAFF Is Correlated with Inflammatory Processes in Chronic Graft Versus Host Disease and Supports Increases in Transitional B Cells. Blood 2008; 112: 465-. [Google Scholar]
- 45.Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood 2011; 118: 6446–9. [DOI] [PubMed] [Google Scholar]
- 46.Whittle R, Denney H, Alfred A et al. Circulating B-cell activating factor level predicts likelihood of chronic GvHD flare and probability of successful steroid taper during extracorporeal photopheresis therapy. Bone Marrow Transplant 2012; 1): S63. [Google Scholar]
- 47.Li X, Gao Q, Feng Y et al. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br J Haematol 2019; 184: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greinix HT, Kuzmina Z, Weigl R et al. CD19+CD21low B Cells and CD4+CD45RA+CD31+ T Cells Correlate with First Diagnosis of Chronic Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation 2015; 21: 250–8. [DOI] [PubMed] [Google Scholar]
- 49.Greinix HT, Pohlreich D, Kouba M et al. Elevated numbers of immature/transitional CD21-B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant 2008; 14: 208–19. [DOI] [PubMed] [Google Scholar]
- 50.Lawitschka A, Gueclue ED, Januszko A et al. National Institutes of Health–Defined Chronic Graft-vs.-Host Disease in Pediatric Hematopoietic Stem Cell Transplantation Patients Correlates With Parameters of Long-Term Immune Reconstitution. Front 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruggen MC, Petzelbauer P, Greinix H et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. J Invest Dermatol 2015; 135: 999–1006. [DOI] [PubMed] [Google Scholar]
- 52.Zhao F, Zhang Y, Wang H et al. Blockade of osteopontin reduces alloreactive CD8+ T cell-mediated graft-versus-host disease. Blood 2011; 117: 1723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inamoto Y, Martin PJ, Paczesny S et al. Association of Plasma CD163 Concentration with De Novo-Onset Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant 2017; 23: 1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boukouaci W, Busson M, Peffault de Latour R et al. MICA-129 genotype, soluble MICA, and anti-MICA antibodies as biomarkers of chronic graft-versus-host disease. Blood 2009; 114: 5216–24. [DOI] [PubMed] [Google Scholar]
- 55.Skert C, Damiani D, Michelutti A et al. Kinetics of Th1/Th2 cytokines and lymphocyte subsets to predict chronic GVHD after allo-SCT: results of a prospective study. Bone Marrow Transplant 2009; 44: 729–37. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Zhai Z, Xu X et al. Decrease of CD4(+)CD25(+) regulatory T cells and TGF-beta at early immune reconstitution is associated to the onset and severity of graft-versus-host disease following allogeneic haematogenesis stem cell transplantation. Leuk Res 2010; 34: 1158–68. [DOI] [PubMed] [Google Scholar]
- 57.Wu JM, Thoburn CJ, Wisell J et al. CD20, AIF-1, and TGF-B in graft-versus-host disease: A study of mRNA expression in histologically matched skin biopsies. Mod Pathol 2010; 23: 720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirayama M, Azuma E, Nakagawa-Nakazawa A et al. Interleukin-10 spot-forming cells as a novel biomarker of chronic graft-versus-host disease. Haematologica 2013; 98: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pratt LM, Liu Y, Ugarte-Torres A et al. IL15 levels on day 7 after hematopoietic cell transplantation predict chronic GVHD. Bone Marrow Transplant 2013; 48: 722–8. [DOI] [PubMed] [Google Scholar]
- 60.Klimczak A, Suchnicki K, Sedzimirska M et al. Diverse Activity of IL-17+ Cells in Chronic Skin and Mucosa Graft-Versus-Host Disease. Arch Immunol Ther Exp (Warsz) 2019; 67: 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dander E, Balduzzi A, Zappa G et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation 2009; 88: 1261–72. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson AN, Chang K, Kuns RD et al. IL-6 dysregulation originates in dendritic cells and initiates graft-versus-host disease via classical signaling. Blood 2019;. [DOI] [PubMed] [Google Scholar]
- 63.Pidala J, Sigdel TK, Wang A et al. A combined biomarker and clinical panel for chronic graft versus host disease diagnosis. The journal of pathology. Clinical research 2017; 3: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cullup H, Dickinson AM, Cavet J et al. Polymorphisms of interleukin-1α constitute independent risk factors for chronic graft-versus-host disease after allogeneic bone marrow transplantation. British Journal of Haematology 2003; 122: 778–87. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi S, Imamura M, Hashino S et al. Clinical relevance of serum soluble interleukin-2 receptor levels in acute and chronic graft-versus-host disease. Leuk Lymphoma 1997; 28: 159–69. [DOI] [PubMed] [Google Scholar]
- 66.Nakasone H, Terasako-Saito K, Yamazaki R et al. Impact of high-/middle-molecular-weight adiponectin on the synthesis and regulation of extracellular matrix in dermal fibroblasts. Exp Hematol 2014; 42: 261–73. [DOI] [PubMed] [Google Scholar]
- 67.Nakasone H, Binh PN, Yamazaki R et al. Association between serum high-molecular-weight adiponectin level and the severity of chronic graft-versus-host disease in allogeneic stem cell transplantation recipients. Blood 2011; 117: 3469–72. [DOI] [PubMed] [Google Scholar]
- 68.Bassim CW, Ambatipudi KS, Mays JW et al. Quantitative salivary proteomic differences in oral chronic graft-versus-host disease. J Clin Immunol 2012; 32: 1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salas MQ, Ezzat S, Lam W et al. Prolactin, a potential biomarker for chronic GVHD activity. Eur J Haematol 2021; 106: 158–64. [DOI] [PubMed] [Google Scholar]
- 70.Alborghetti MR, Correa MEP, Whangbo J et al. Clinical Metabolomics Identifies Blood Serum Branched Chain Amino Acids as Potential Predictive Biomarkers for Chronic Graft vs. Host Disease. Frontiers in oncology 2019; 9: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottlober P, Leiter U, Friedrich W et al. Chronic cutaneous sclerodermoid graft-versus-host disease: evaluation by 20-MHz sonography. J Eur Acad Dermatol Venereol 2003; 17: 402–7. [DOI] [PubMed] [Google Scholar]
- 72.Lee SY, Cardones AR, Doherty J et al. Preliminary Results on the Feasibility of Using ARFI/SWEI to Assess Cutaneous Sclerotic Diseases. Ultrasound in medicine & biology 2015; 41: 2806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horger M, Boss A, Bethge W et al. MR findings in patients with disabling musculocutaneous chronic graft-versus-host disease. Skeletal Radiol 2008; 37: 885–94. [DOI] [PubMed] [Google Scholar]
- 74.Sauter AW, Schmidt H, Mantlik F et al. Imaging findings and therapy response monitoring in chronic sclerodermatous graft-versus-host disease: preliminary data of a simultaneous PET/MRI approach. Clin Nucl Med 2013; 38: e309–17. [DOI] [PubMed] [Google Scholar]
- 75.Su P, Cao T, Tang MBY et al. In Vivo High-Definition Optical Coherence Tomography: A Bedside Diagnostic Aid for Morphea. JAMA Dermatology 2015; 151: 234–5. [DOI] [PubMed] [Google Scholar]
- 76.Tkaczyk ER, Chen F, Wang J et al. Overcoming human disagreement assessing erythematous lesion severity on 3D photos of chronic graft-versus-host disease. Bone Marrow Transplant 2018; 53: 1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tkaczyk ER, Coco JR, Wang J et al. Crowdsourcing to delineate skin affected by chronic graft-vs-host disease. Skin Res Technol 2019; 25: 572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gandelman JS, Zic J, Dewan AK et al. The Anatomic Distribution of Skin Involvement in Patients with Incident Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant 2019; 25: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schoemans HM, Goris K, Van Durm R et al. The eGVHD App has the potential to improve the accuracy of graft-versus-host disease assessment: a multicenter randomized controlled trial. Haematologica 2018; 103: 1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filipovich AH, Weisdorf D, Pavletic S et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biology of Blood and Marrow Transplantation 2005; 11: 945–56. [DOI] [PubMed] [Google Scholar]
- 81.Curtis LM, Grkovic L, Mitchell SA et al. NIH response criteria measures are associated with important parameters of disease severity in patients with chronic GVHD. Bone Marrow Transplant 2014; 49: 1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kilgour JM, Wali G, Gibbons E et al. Systematic Review of Patient-Reported Outcome Measures in Graft-versus-Host Disease. Biol Blood Marrow Transplant 2020; 26: e113–e27. [DOI] [PubMed] [Google Scholar]
- 83.Kaminska-Winciorek G, Zalaudek I, Mendrek W et al. Dermoscopy of Cutaneous Graft-Versus-Host-Disease in Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Dermatol Ther (Heidelb) 2020; 10: 1043–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitko CL, Pidala J, Schoemans HM et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant Cell Ther 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Racioppi A, Dalton T, Ramalingam S et al. Assessing the Feasibility of a Novel mHealth App in Hematopoietic Stem Cell Transplant Patients. Transplant Cell Ther 2021; 27: 181.e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dumford K, Anderson JC. CT and MRI findings in sclerodermatous chronic graft vs. host disease. Clin Imaging 2001; 25: 138–40. [DOI] [PubMed] [Google Scholar]
- 87.Deegan AJ, Talebi-Liasi F, Song S et al. Optical coherence tomography angiography of normal skin and inflammatory dermatologic conditions. Lasers Surg Med 2018; 50: 183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt 2013; 18: 061224. [DOI] [PubMed] [Google Scholar]
- 89.Chen GL, Jeon M, Ross M et al. Optical Coherence Tomography for Quantifying Human Cutaneous Chronic Graft-versus-Host Disease. Transplant Cell Ther 2021; 27: 271.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paczesny S, Hakim FT, Pidala J et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant 2015; 21: 780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grkovic L, Baird K, Steinberg SM et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia 2012; 26: 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pulanic D, Lozier JN, Pavletic SZ. Thrombocytopenia and hemostatic disorders in chronic graft versus host disease. Bone Marrow Transplant 2009; 44: 393–403. [DOI] [PubMed] [Google Scholar]
- 93.Luft T, Schmidt K, Kellner KH et al. ATG and statins reduce incidence of severe chronic GVHD by distinct mechanisms involving CXCL9 and kynurenine catabolism. Blood 2015; 126 (23): 856. [Google Scholar]
- 94.Saliba RM, Sarantopoulos S, Kitko CL et al. B-cell activating factor (BAFF) plasma level at the time of chronic GvHD diagnosis is a potential predictor of non-relapse mortality. Bone Marrow Transplant 2017; 52: 1010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon JH, Lee SJ, Kim JG et al. Clinical significance of autoantibody expression in allogeneic stem-cell recipients. Transplantation 2009; 88: 242–50. [DOI] [PubMed] [Google Scholar]
- 96.Martires KJ, Baird K, Steinberg SM et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood 2011; 118: 4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solán L, Carbonell D, Muñiz P et al. Elafin as a Predictive Biomarker of Acute Skin Graft-Versus-Host Disease After Haploidentical Stem Cell Transplantation Using Post-Transplant High-Dose Cyclophosphamide. Front Immunol 2021; 12: 516078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marano RJ, Ben-Jonathan N. Minireview: Extrapituitary prolactin: an update on the distribution, regulation, and functions. Mol Endocrinol 2014; 28: 622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolff D, Greinix H, Lee SJ et al. Biomarkers in chronic graft-versus-host disease: quo vadis? Bone Marrow Transplant 2018; 53: 832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gandelman JS, Byrne MT, Mistry AM et al. Machine learning reveals chronic graft-versus-host disease phenotypes and stratifies survival after stem cell transplant for hematologic malignancies. Haematologica 2019; 104: 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schoemans HM, Goris K, Van Durm R et al. Accuracy and usability of the eGVHD app in assessing the severity of graft-versus-host disease at the 2017 EBMT annual congress. Bone Marrow Transplant 2018; 53: 490–4. [DOI] [PubMed] [Google Scholar]
- 102.Schoemans H, Goris K, Durm RV et al. Development, preliminary usability and accuracy testing of the EBMT ‘eGVHD App’ to support GvHD assessment according to NIH criteria-a proof of concept. Bone Marrow Transplant 2016; 51: 1062–5. [DOI] [PubMed] [Google Scholar]
- 103.Miklos D, Cutler CS, Arora M et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017; 130: 2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerep AZ, Broome J, Pirsl F et al. A large cohort comparison of the new 2014 national institutes of health chronic graft-versus-host disease staging criteria with the 2005 version in severely affected patients. Biology of Blood and Marrow Transplantation 2018; 24 (3 Supplement 1): S254. [Google Scholar]
- 105.Curtis LM, Ostojic A, Venzon DJ et al. A randomized phase 2 trial of pomalidomide in subjects failing prior therapy for chronic graft-versus-host disease. Blood 2021; 137: 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jagasia M, Lazaryan A, Bachier CR et al. ROCK2 Inhibition With Belumosudil (KD025) for the Treatment of Chronic Graft-Versus-Host Disease. J Clin Oncol; 0: JCO.20.02754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


