Abstract
State of the art on the valorisation of C1 carbon sources obtained either from natural or anthropogenic origins as a key challenge for the circular economy.
Introduction
Life is supported by a small number of elements of the periodic table, i.e. between 25 and 28 elements according to different criteria. While all of them are relevant, carbon is the key element that explains life in our planet, even though elemental carbon cannot be used directly as a carbon source by any organism. Carbon must be previously bound to other elements, forming small or very large molecules to become metabolizable by the living beings. In fact, life arose from the combination of small molecules such as hydrogen (H2), water (H2O), ammonia (NH3), hydrogen sulfide (H2S), carbon monoxide (CO), carbon dioxide (CO2), methane (CH4), formaldehyde (H2CO) or hydrocyanic acid (HCN), within others. Several of them contain a single carbon atom and form part of a group of molecules named as C1 compounds. Interestingly, all these prebiotic small molecules still remain in the biosphere, and some of them are quite abundant and useful to support life, remaining many micro‐ and macroorganisms able to use them as carbon and/or energy sources. The aim of this editorial is to analyse the present and future prospects and the valorization of C1 carbon sources obtained either from natural or anthropogenic origin through microbial biotechnology as a key challenge for the ‘Green Deal’ and the circular economy.
To focus this analysis, it is important to define the scope of C1 compounds. Although C1 compounds are usually defined as substances that contains a single carbon atom, some authors extend this scope to those compounds that contain carbon atoms without C‐C bonds. Among the first, we can consider CO, CO2, CH4, H2CO, methanol (CH3OH), formic acid (HCOOH), methylamine (CH3NH2), methanethiol (CH3SH), different halomethanes (e.g. CHCl3) and others. Among the latter, we can list, for instance, dimethyl or trimethyl amines, dimethylsufides, dimethylsulfoxide, dimethylsulfone, dimethylformamide and others. Special mention should be made of soluble or insoluble mineral carbonates that can be converted into CO2 under specific environmental conditions and then used by some organisms as a carbon source (Kral et al., 2014).
On the other hand, we should consider that not all C1 compounds are equally applicable as potential feedstock for industrial scale biomanufacturing mainly due to their limited availability. The most relevant C1 compounds for biotech purposes include CO2, CO, CH4, HCOOH and CH3OH. Significantly, CO2 and CH4 are greenhouse gases that are increasing their concentration in the atmosphere due to the large current anthropogenic activity, and consequently, developing biotechnology processes aimed at their sequestration and transformation is essential for planet survival. Additionally, other anthropogenic gases such as power plant flue gas, steel mill gas, anaerobic digestion‐derived biogas, synthesis gas (syngas) and others produced by gasification of organic waste are abundant, rich in C1 substrates (i.e. CO, CO2, CH4) and, therefore, useful to develop different bioprocesses. Finally, HCOOH or CH3OH derived from catalytic processing of CO2 or from other sources are liquid substances more amenable than C1 gases to transportation and more affordable for a microbial utilization, due to their higher water solubility.
Except CO2, the other relevant C1 compounds mentioned above can be used both as carbon and energy sources. Therefore, metabolizing CO2 requires an additional energy source that can be provided by light, H2, CO, electricity or some organic and inorganic compounds. Although some of these C1 compounds can be metabolized by plants and animals, this analysis will be focussed only in the biotech processes based on C1‐utilizing microbes including bacteria, fungi, microalgae and archaea. Native and synthetic C1 assimilation pathways have been used to validate the transformation of C1 compounds to biofuels, and biobased chemicals or even to food and feed (single cell protein) as industrially promising manufacturing procedures, but a deeper understanding of the governing mechanisms of C1 metabolic pathways is needed to develop most efficient C1‐based biotech processes (Jiang et al., 2021).
Finally, we have to consider that a circular bioeconomy based on C1 compounds has the potential to sustainably produce a large number of compounds, at the same time that can contribute to reduce accumulation of C1 greenhouse and waste gases responsible of climate change, such as CO2 and CH4. Moreover, technologies that facilitate treatments of all kind of organic waste by gasification followed by carbon capture and conversion of gases into useful products will help also to mitigate climate change by enabling a circular carbon economy (Fackler et al., 2021a; Wood et al., 2021). Figure 1 shows a scheme of the most relevant biomanufacturing processes that can be carried out using C1 compounds and that will be briefly reviewed hereinafter.
Fig. 1.
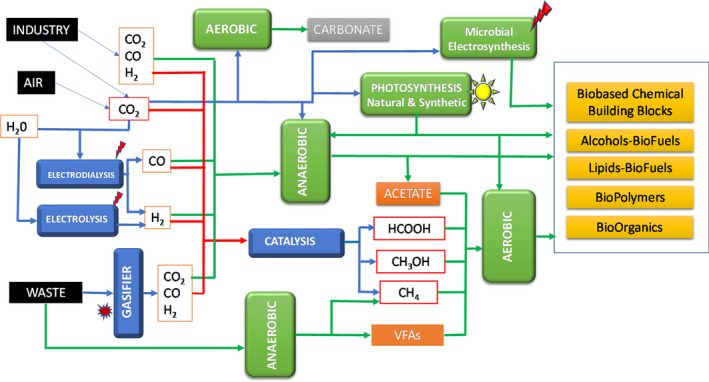
Main biomanufacturing processes that can be carried out using C1 compounds.
Fermentations of C1 gases
Syngas fermentation
We currently have at our disposal a collection of more than 100 isolated anaerobic bacteria named acetogens that synthesize acetyl‐CoA from CO or from CO2 plus H2 (Bengelsdorf et al., 2016; Takors et al., 2018; Müller, 2019; Jin et al., 2020; Katsyv and Müller, 2020; Lemaire et al., 2020; Bourgade et al., 2021). These organisms use CO and CO2 as substrates for the methyl or carbonyl branches of the Wood–Ljungdahl pathway that produce acetyl‐CoA as metabolic precursor. Acetogens can grow using syngas that is mainly composed of CO2, CO and H2, a mixture of CO2 and H2 or only CO. These microorganisms have been used to produce acetate, ethanol, 2,3‐butanediol or butyrate as the most relevant products although others such as acetone or butanol can also be produced from syngas by genetic modifications (Minton et al., 2016; Jin et al., 2020; Bourgade et al., 2021). In this sense, several industrially useful acetogenic bacteria have been already modified using synthetic biology tools, such as Clostridium ljungdahlii (Köpke et al., 2010; Molitor et al., 2016; Zhang et al., 2020a), Clostridium autoethanogenum (Liew et al., 2016; Fackler et al., 2021b), Acetobacterium woodii (Straub et al., 2014) or Moorella thermoacetica (Kita et al., 2013; Kato et al., 2021).
Acetogenic bacteria can be used not only to capture CO2 or CO produced as contaminants by anthropogenic activities, but also as a biological alternative to transform syngas into valuable products within the gasification stream of a chemical refinery. To reduce the volume of organic waste (or biomass) generated in cities or industries, it is possible to construct biorefineries that transform organic waste into syngas through different gasification processes (Chan et al., 2021; Fackler et al., 2021b). Up to now, syngas has been further transformed into different chemicals and fuels of industrial interest by using Fischer–Tropsch catalytic procedures. However, more recently, several research approaches from the academia and the industry (e.g. LanzaTech, IneosBio, Coskata) have demonstrated that syngas can be alternatively transformed into valuable products by using bacterial syngas fermentations (Molitor et al., 2017; Bengelsdorf et al., 2018; De Tissera et al., 2019). However, the efficiency of syngas fermentation is still low and needs to be improved to compete with chemical catalysis (Phillips et al., 2017; Sun et al., 2019; Geinitz et al., 2020).
CO metabolism
CO, one component of syngas, is a highly toxic compound for most living beings, but there are many microbes which can deal with its toxicity and use it as a carbon and energy source (Robb and Techtmann, 2018; Cordero et al., 2019; Duan et al., 2021). Curiously, CO occurs at relatively high concentration in Mars’ atmosphere, and it represents a focal point for astrobiological research (King, 2015). CO oxidation coupled to the generation of energy for growth is achieved by aerobic and anaerobic bacteria, and archaea, belonging to the physiological groups of aerobic carboxydotrophic, facultatively anaerobic phototrophic, and anaerobic acetogenic, methanogenic or sulfate‐reducing bacteria. However, not all microbes that metabolize CO are able to grow only using 100% CO. Within the aerobic CO‐oxidizing microorganisms, we can categorize two major groups, the carboxydotrophs and the carboxydovores (Cordero et al., 2019). While carboxydotrophs grow chemolithoautotrophically with CO as the sole energy and carbon source when present at elevated concentrations, carboxydovores represent a broader group of bacteria and archaea which oxidize CO at low concentrations, and in contrast to carboxydotrophs require organic carbon to grow. The possibility that CO can be used by some bacteria to convert it into a variety of chemicals and to generate bio‐H2 is also promoting a new field of research (Revelles et al., 2016, 2017; Robb and Techtmann, 2018; Rodríguez et al., 2021).
CO2 capture
Since CO2 cannot be used as the sole carbon and energy source, all organisms that capture CO2 require an additional source of energy (Claassens et al., 2016; Claassens, 2017; Hu et al., 2018; Kumar et al., 2018; Liang et al., 2020). In the case of acetogenic bacteria, CO2 is captured by reduction using the energy provided by CO or H2. Nevertheless, some acetogenic bacteria can also use electric energy from a cathode to fix CO2 by a process called microbial electrosynthesis (MES) (Dessì et al., 2021) (see below).
On the other hand, chemolithoautotrophic bacteria can use H2 or inorganic compounds as electron donors for energy requirement and growth using CO2 as a carbon source. One example of such bacteria is Cupriavidus necator that is able to grow and produce different industrial products using CO2 and H2 under aerobic conditions (Li et al., 2020; Nangle et al., 2020; Panich et al., 2021). Methanotrophs can also sequester CO2 and transform it into CH3OH using H2 or an organic compound as energy source. Therefore, methanotrophs are used as cell factories for the production of a wide range of high‐value products (Sahoo et al., 2021).
In addition to H2, there are other compounds used by microbes as electron donors (Gargaud, 2011). In this sense, the most common sulfur compounds utilized as electron donors by denitrifying bacteria (e.g. Thiobacillus, Thiomicrospira) are hydrogen sulfide (H2S), elemental sulfur (S0), sulfite (SO3 −2) and thiosulfate (S2O3 2−). The aerobic oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+) is an energy‐yielding reaction, used by some prokaryotes to conserve energy (e.g. Ferroglobus). The most common nitrogen compounds used as electron donors for energy conservation are NH3 (e.g. Nitrosomonas, Nitrosospira, Nitrosococcus and Nitrosolubus) and nitrite (NO2−) (e.g. Nitrobacter, Nitrospira and Nitrococcus). A special case of nitrogen‐oxidizing microorganisms corresponds to those capable of carrying out the anoxic oxidation of NH3, a process known as anamox. In this case, the electron acceptor is NO2−, and the product of the metabolic reaction in addition to proton motive force is the generation of N2. This metabolic reaction is carried out by a special type of microorganisms belonging to the Planctomycetes phylum of bacteria.
Phototrophic bacteria utilize light as energy source to capture CO2 (Choi et al., 2019; Naduthodi et al., 2021). It is well known that oxygenic phototrophic cyanobacteria as well as the eukaryotic microalgae, algae and plants use CO2 as a carbon source and many reviews have been devoted to show the utility of these bacteria for biotechnological purposes (Singh et al., 2018; Veaudor et al., 2020; Leong et al., 2021; Sarma et al., 2021). In addition, anoxygenic phototrophic bacteria can also use CO2 to grow and have been utilized for biotech purposes (George et al., 2020).
Geologic sequestration of CO2, i.e. carbon capture and storage (CCS), is one strategy to reduce the emission of greenhouse gases. Mineralization of CO2 into CaCO3 is possible if the equilibrium of the reaction of Ca2+ with CO3 2− is moved to the formation of CaCO3 under a saturation state. This is achieved in the presence of sufficient dissolved Ca2+ at alkaline pH and in the presence of a nucleation substrate. Microbes have been shown to enhance CaCO3 precipitation (microbiologically induced calcium carbonate precipitation, MICP) via cation adsorption to negatively charged functional groups on microbe surfaces and by metabolically driven changes in the solution chemistry, which increase mineral saturation and induce nucleation (Castro‐Alonso et al., 2019). In general, two metabolic pathways are involved in this biomineralization, i.e. the autotrophic and the heterotrophic pathways (Görgen et al., 2020). Autotrophic precipitation of carbonates includes oxygenic and anoxygenic photosynthesis, and non‐methylotrophic methanogenesis. In the heterotrophic pathway, two processes are reported involving sulfur and nitrogen cycles respectively. The microbial induced carbonate precipitation has been biotechnologically used for biocementation of materials (Reddy and Sumit, 2018). Moreover, MICP was investigated for crack repair and the surface treatment of various types of construction materials (Joshi et al., 2017; Lee and Park, 2018; Seifan and Berenjian, 2018). Fungi and bacteria can be used in these processes (Menon et al., 2019).
However, most interestingly, a number of researches are currently focussed on the creation of new synthetic organisms able to capture CO2 using HCOOH or light, opening new frontiers in this field (Woo, 2017; François et al., 2020; Liang et al., 2020; Satanowski and Bar‐Even, 2020; Satanowski et al., 2020). Rewiring Escherichia coli for CO2 fixation to convert it into sugar may enable diverse biotechnological applications (Antonovsky et al., 2017; Flamholz et al., 2020). An E. coli recombinant strain was created to use CO2 and HCOOH, and although it still required glucose to grow, authors anticipated that with some additional modifications, it could grow only using CO2 and HCOOH (Bang and Lee, 2018; Bang et al., 2021). A similar approach had been also carried out using a different metabolic strategy to capture CO2 in combination with a complex organic energy source (e.g. glycerol and xylose) (Antonovsky et al., 2016; Kerfeld, 2016). Interestingly, the hypothesis was demonstrated, and very recently, a new E. coli autotrophic recombinant was constructed by laboratory evolution able to capture CO2 using HCOOH as the only source of energy (Gleizer et al., 2019).
On the other hand, light‐driven CO2 sequestration has been achieved in E. coli by using self‐assembled cadmium sulfide nanoparticles (Hu et al., 2021). Biohybrids had been also investigated in other organisms (Nichols et al., 2015; Zhang and Tremblay, 2017; Guo et al., 2018; Ding et al., 2019; Dogutan and Nocera, 2019; Kumar et al., 2019; Sahoo et al., 2020) (see below).
Finally, as a proof of concept, a complex in vitro system with 17 enzymes was generated to sequester CO2 (Schwander et al., 2016). An example of how protein engineering and synthetic biology could assist in this mission is the new‐to‐nature glycolyl‐CoA carboxylase created by combining rational design, high‐throughput microfluidics and microplate screens that improved its catalytic efficiency by three orders of magnitude to match the properties of natural CO2‐fixing enzymes (Scheffen et al., 2021). Moreover, enzymes can also be used in combination with electrochemistry for CO2 capture (see below).
Methane fermentation
CH4 can be obtained from natural sources, such as wetlands or animal digestion, along with many anthropogenic activities such as the use of anaerobic digesters (methanogens and biogas) or by thermogenic processes. However, the largest reservoir of CH4 is under the seafloor in the form of CH4 clathrates. Natural gas is approximately 90% CH4. Therefore, it is normal to found many organisms capable of oxidizing CH4 in the biosphere that are known as methanotrophs utilizing CH4 as the source of carbon and energy. All aerobic methanotrophs oxidize CH4 to CO2 through a common enzymatic cascade. This oxidation process produces CH3OH, CH2O and HCOOH as reaction intermediates. Methanotrophs are therefore excellent candidates for CH4 sequestration (Sahoo et al., 2021). These capabilities enable them as cell factories for a wide range of high‐value products (Nguyen et al., 2021). In this sense, methanotrophs have been used to synthesize polyhydroxyalkanoates for plastic sector, single cell proteins for feeding animals and lipids for biofuel production (Wang et al., 2020).
Fermentation of C1 liquids
Common challenges associated with C1 gas fermentation systems are gas‐to‐liquid mass transfer limitations and lower solubility of the gaseous substrates. This problem does not exist when using C1 liquids, such as CH3OH or HCOOH. Therefore, CH3OH is considered a promising C1 feedstock adding its great availability from different sources (Pirola et al., 2018; Simon Araya et al., 2020). However, CH3OH can inhibit the growth of microorganisms under aerobic conditions, mainly because of the high reactivity of its toxic downstream metabolite H2CO. Methylotrophs, including bacteria, such as Bacillus methanolicus, and yeasts, such as Pichia pastoris, can use CH3OH as a carbon and energy source. With some exceptions such as P. pastoris, the use of native methylotrophic microorganisms suffers from the drawbacks of poor genetic availability and low metabolic yield, and therefore, engineering non‐native methylotrophic microbes has been used to convert methanol into value‐added products (Zhang et al., 2019; Zhan et al., 2021). Different bioengineering efforts have shown that these recombinant organisms can be engineered to convert CH3OH into biofuels and other commodity chemicals (Bennett et al., 2018; Chistoserdova, 2018; Antoniewicz, 2019; Zhu et al., 2020). Engineering CH3OH metabolic pathways have been mainly carried out in E. coli, Saccharomyces cerevisiae and Corynebacterium glutamicum. However, to date, none of engineered strains can grow on CH3OH as the sole carbon source.
On the other hand, HCOOH can be efficiently produced via electrochemical or photochemical catalytic conversion of CO2, and it can be directly used as an organic carbon source by microorganisms (Yishai et al., 2016; Cotton et al., 2020). HCOOH has recently been suggested as an industrial feedstock, although bio‐production based on this carbon source is still not commercially mature (Satanowski and Bar‐Even, 2020). Consequently, the construction of efficient HCOOH‐assimilation pathways in microorganisms is essential for the utilization of cheap, renewable C1 compounds (Mao et al., 2020; Tuyishime and Sinumvayo, 2020; Bang et al., 2021). Natural microorganisms that possess HCOOH utilization pathways mainly use two strategies to grow on HCOOH as the sole carbon source. In the first one, HCOOH is completely oxidized to generate CO2 and reducing equivalents, being Calvin–Benson–Bassham (CBB) cycle an example of this type. In the second one, not all HCOOH is oxidized into CO2, while some is directly assimilated via the central metabolism. CBB cycle (reductive pentose‐phosphate cycle) discovered in C. necator is the only natural pathway for autotrophic growth on HCOOH, and thus, initial metabolic engineering of HCOOH utilization was mainly concentrated in this pathway. However, new synthetic alternative HCOOH utilization pathways have been recently investigated (Claassens et al., 2020; Mao et al., 2020). Currently, only some engineered strains of E. coli have been able to grow on HCOOH as the sole carbon source although the low cell density and specific growth rate need further improvement (Yishai et al., 2018; Bang et al., 2020; Kim et al., 2020).
Electrocatalysis
As remarked above, MES is emerging as a promising technology to improve the microbial utilization of C1 compounds (Chu et al., 2020; Dessì et al., 2021). The first proof‐of‐concept experiment of MES was conducted in 2010 showing that homoacetogens can produce extracellular acetate and 2‐oxobutyrate from CO2 with electrons delivered from a graphite electrode (Nevin et al., 2010). Since then, many hybrid electro‐biochemical systems have been developed (Li et al., 2012; Hwang et al., 2015; Bajracharya et al., 2017; Gimkiewicz et al., 2017; Jang et al., 2018; Le et al., 2018; Tashiro et al., 2018; Yuan et al., 2019; Hegner et al., 2020).
Nevertheless, electric energy can be also used to synthesize C1 by chemical catalysis and upgraded via microbial fermentation to produce biobased chemicals. In this sense, electrocatalysis represents an attractive strategy with a huge potential in the field of biomanufacturing. The high efficiencies and rates of electrochemical catalysis can be combined with the high selectivity and access to complex end products of microbial catalysis. The electrochemical CO2 reduction renders HCOOH or CO that, as stated above, can be used as carbon and energy sources from many microorganisms (Jin et al., 2021; Park et al., 2021). On the other hand, H2 can be generated by electrolysis of H2O and used as an energy source to grow. Moreover, the co‐electrolysis of CO2 and H2O can render at the same time CO and H2, this is, a syngas equivalent (Lu et al., 2020). Syngas can also be catalytically transformed into methanol suitable for methylotrophs.
Finally, enzyme based electro‐catalysed production of HCOOH from CO2 has received great attention (Srikanth et al., 2014, 2017; Zhang et al., 2016; Schlager et al., 2017; Jayathilake et al., 2019). Effective oxygen tolerant biocatalysts capable of utilizing electrons supplied from a cathode are being sought to render biocatalytic HCOOH production from CO2 feasible. Bioelectrochemical CO2 reduction with enzymes or whole‐cell biocatalysts is generally characterized by a high selectivity of products and a high energy efficiency with a small overpotential to drive the desired reaction.
Future prospects
The utilization of C1 raw materials is crucial for establishing a sustainable circular carbon economy. C1 compounds are envisioned as ideal resources for both the chemical industry and the biotechnological sector. Probably, the truly sustainable feedstock for a circular carbon economy is CO2 not only because its conversion to chemicals and fuels represents a sustainable solution for reducing greenhouse gas emissions, but also because it is abundant and can be obtained from different sources. Although direct CO2 capture from air will result in a net removal from the atmosphere, this process possesses technical and economic problems because it is highly dilute, only about 400 ppm, i.e. 100–300 times more dilute than in gas‐ and coal‐fired power plants. The estimated cost of capturing CO2 from air ranged from $300 to $1000 per ton. Thus, alternatively, the industrial production of chemicals from CO2 should consider the use of CO2 high‐volume waste as raw material (Bui et al., 2018).
Solar energy is envisioned as the most suitable renewable energy source to reduce CO2 and provide a sustainable system. Besides the firstly discovered Calvin–Benson–Bassham (CBB) cycle, other five natural CO2 fixation pathways have been described, i.e. the Wood‐Ljungdahl pathway, the reductive TCA cycle, the dicarboxylate/4‐hydroxybutyrate cycle, the 3‐hydroxypropionate bicycle and the 3‐hydroxypropionate/4‐hydroxybutyrate cycle. Refining the efficiencies of the native pathways as well as the design of synthetic pathways will provide new opportunities to improve the assimilation efficiencies of CO2. Developing new artificial autotrophic microorganisms, and especially phototrophic ones, for reinforcing carbon capture utilization (CCU) should be consider a key target in the next years. However, the use of artificial autotrophic cell factories still requires additional improvements in the CO2 fixation pathways, in order to solve compartmentalization, and to decide the best host as well as to reduce the cost of power supply. The increasing number of genomic and metagenomic sequences can help in this task, since it will allow finding by data mining better enzymes and pathways to improve the efficiency. In the same way, solar‐powered electrochemical reduction in CO2 and H2O to syngas, coupled to bacterial fermentation, can be also considered as an alternative to the sustainable production of useful chemicals (Haas et al., 2018).
Although it has been demonstrated that syngas, CO or CO2 can be directly transformed at industrial scale by acetogenic fermentation in useful alcohols (e.g. ethanol, butanediol), the main product generated by acetogenic bacteria is acetate. The potential of acetate to become a next‐generation platform substrate for its further fermentation into value‐added bioproducts has been underexplored so far (Kiefer et al., 2021; Kim et al., 2021).
Attractive platforms involving photomixotrophic metabolism in cyanobacteria can provide unparalleled improvements in yield for the conversion of CO2. Of particular interest is the ability to combine CO2 with other C1 compounds such as CH4 or chemically produced CH3OH and HCOOH (Kanno et al., 2017; Singh et al., 2018).
The creation of artificial bacterial consortia to improve the efficiency of C1 conversion into chemicals is a promising alternative (Hays et al., 2017). Strategies involving co‐cultivation of methanotrophic and oxygenic photosynthetic bacteria in biogas have been already explored (Van der Ha et al., 2012; Hill et al., 2017). An engineered Synechococcus elongatus able to convert CO2 into secreted sucrose can be used in co‐culture with other bacteria to generate biotechnological applications (Löwe et al., 2017; Weiss et al., 2017; Fedeson et al., 2020; Zhang et al., 2020b).
A proof‐of‐concept experiment conducted by Cheng et al. (2009) demonstrated that a biocathode enriched with the methanogenic archaea Methanobacterium palustre can store electricity in the form of CH4. In this CH4‐producing bioelectrochemical system (BES), CO2 and electrical energy are converted into CH4, using electrodes that supply either electrons or H2 to the archaea (Blasco‐Gómez et al., 2017). This technology is referred to as bioelectrochemical power‐to‐gas (BEP2G) and considered as a way of storing renewable surplus electricity (Geppert et al., 2016), i.e. CH4 generated with excess renewable power that cannot be fed into the electric grid can be directly stored in the existing gas infrastructures.
The main objective of the so call third‐generation‐(3G)‐biorefineries is to use cell factories to convert renewable energies and CO2 into chemicals, searching for routes for biomanufacturing chemicals in a carbon‐neutral manner. However, there are still many trends and key challenges for future advancement to make them competitive with the petroleum industry (Liu et al., 2020). Within this challenge, the design of efficient CO2 reduction systems by mimicking the mechanism of natural photosynthesis using semiconducting nanomaterials interfaced with electroactive bacteria in a photosynthetic microbial electrosynthesis system opens a revolutionary alternative (Xu et al., 2020; Gupta et al., 2021).
Finally, although the opportunities offered by the bioeconomy linked to the use of C1 compounds are wide and very promising, today there are still few companies that have started or are exploring the implementation of these biotechnological processes to industrial scale (Teixeira et al., 2018). Table 1 tries to summarize some examples of the main industrial approaches without pretending to be exhaustive. While CH3OH was explored years ago at industrial scale to produce single cell protein by bacteria or yeasts, the C1 liquids, neither CH3OH nor HCOOH are currently being used at industrial scale as feedstock to produce materials of commercial interest through fermentation. Interestingly, Feedstocks United (Netherlands) has developed a new technology that uses trioxane derived by chemical synthesis from C1 compounds as feedstock for microbial fermentation, exemplifying that there are still other options to be explored in the field of C1 biotechnology. All this leads to the conclusion that we are facing a large scenario of opportunities and strategies for biotechnological companies to face the challenge posed by the Green Deal in the coming years.
Table 1.
List of companies that use C1 compounds as raw materials for microbial fermentation. Some industrial alliances are shown in parentheses.
| C1 compound | Company | Final product |
|---|---|---|
| Syngas | LanzaTech (Basf, Global Bioenergies, Evonik, ArcelorMittal, Aemetis, IndianOil, Swayana) | Ethanol, butanediol, chemicals |
| Syngas | Ineos Bio (New Planet Bioenergy) | Ethanol |
| Syngas | Coskata (Synata Bio) | Ethanol |
| CH4 | Newlight Technologies | Polyhydroxyalkanoates |
| CH4 | Mango Materials | Polyhydroxyalkanoates |
| CH4 | Calysta (BP, Cargill, NouriTech) | Protein, chemicals |
| CH4 | Unibio | Protein |
| CH4 | Industrial Microbes | Methanol |
| CH4 | MBP Titan (formerly Intrexon) | Protein, chemicals |
| CH4 | NatureWorks (Calysta) | Lactic acid |
| CO2 | Deep Branch | Protein |
| CO2 | Solar Foods | Protein |
| CO2 | Air Protein | Protein |
| CO2 | Novo Nutrients | Protein |
| CO2 | Kiverdi | Protein |
| CO2 | White Dog Labs | Protein |
| CO2 | OPX Biotechnologies (Cargill) | Biofuels |
| CO2 | Trelys | Amino acids |
| CO2 | BioMason (Novo Holdings) | Biocementation |
| CO2 | BioCement Technologies Inc. | Biocementation |
| CO2 | Basilisk | Self‐healing concrete |
| HCOOH‐electro CO2 | Ginkgo Bioworks | Biofuels |
Conflict of interest
None declared.
Microbial Biotechnology (2021) 15(1), 228–239
Funding information
Comunidad de Madrid (Grant / Award Number: 'S2018/BAA‐4532'). H2020 Environment, (Grant / Award Number: 'H2020‐LC‐GD‐2020‐3. Ref. 101037031') Ministerio de Ciencia e Innovación, (Grant / Award Number: 'RTI2018‐095584‐B‐C41‐42‐43‐44'). H2020 Energy, (Grant / Award Number: 'H2020‐EU.3.2.4.2. Ref. 101000790','H2020‐LC‐SC3‐2019‐NZE‐RES‐CC. Ref. 884208').
References
- Antoniewicz, M.R. (2019) Synthetic methylotrophy: strategies to assimilate methanol for growth and chemicals production. Curr Opin Biotechnol 59: 165–174. 10.1016/j.copbio.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Antonovsky, N. , Gleizer, S. , Noor, E. , Zohar, Y. , Herz, E. , Barenholz, U. , et al. (2016) Sugar synthesis from CO2 in Escherichia coli . Cell 166: 115–125. 10.1016/j.cell.2016.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonovsky, N. , Gleizer, S. , and Milo, R. (2017) Engineering carbon fixation in E. coli: from heterologous RuBisCO expression to the Calvin‐Benson‐Bassham cycle. Curr Opin Biotechnol 47: 83–91. 10.1016/j.copbio.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Bajracharya, S. , Vanbroekhoven, K. , Buisman, C.J.N. , Strik, D.P.B.T.B. , and Pant, D. (2017) Bioelectrochemical conversion of CO2 to chem icals: CO2 as a next generation feedstock for electricity‐driven bioproduction in batch and continuous modes. Faraday Discuss 202: 433–449. 10.1039/c7fd00050b. [DOI] [PubMed] [Google Scholar]
- Bang, J. , Ahn, J.H. , Lee, J.A. , Hwang, C.H. , Kim, G.B. , Lee, J. , and Lee, S.Y. (2021) Synthetic formatotrophs for one‐carbon biorefinery. Adv Sci (Weinh) 8: 2100199. 10.1002/advs.202100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang, J. , Hwang, C.H. , Ahn, J.H. , Lee, J.A. , and Lee, S.Y. (2020) Escherichia coli is engineered to grow on CO2 and formic acid. Nat Microbiol 5: 1459–1463. 10.1038/s41564-020-00793-9. [DOI] [PubMed] [Google Scholar]
- Bang, J. , and Lee, S.Y. (2018) Assimilation of formic acid and CO2 by engineered Escherichia coli equipped with reconstructed one‐carbon assimilation pathways. Proc Natl Acad Sci USA 115: E9271–E9279. 10.1073/pnas.1810386115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengelsdorf, F.R. , Beck, M.H. , Erz, C. , Hoffmeister, S. , Karl, M.M. , Riegler, P. , et al. (2018) Bacterial anaerobic synthesis gas (Syngas) and CO2+H2 fermentation. Adv Appl Microbiol 103: 143–221. 10.1016/bs.aambs.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Bengelsdorf, F.R. , Poehlein, A. , Linder, S. , Erz, C. , Hummel, T. , Hoffmeister, S. , et al. (2016) Industrial acetogenic biocatalysts: A comparative metabolic and genomic analysis. Front Microbiol 7: 1036. 10.3389/fmicb.2016.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, R.K. , Steinberg, L.M. , Chen, W. , and Papoutsakis, E.T. (2018) Engineering the bioconversion of methane and methanol to fuels and chemicals in native and synthetic methylotrophs. Curr Opin Biotechnol 50: 81–93. 10.1016/j.copbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Blasco‐Gómez, R. , Batlle‐Vilanova, P. , Villano, M. , Balaguer, M.D. , Colprim, J. , and Puig, S. (2017) On the edge of research and technological application: A critical review of electromethanogenesis. Int J Mol Sci 18: 874. 10.3390/ijms18040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgade, B. , Minton, N.P. , and Islam, M.A. (2021) Genetic and metabolic engineering challenges of C1‐gas fermenting acetogenic chassis organisms. FEMS Microbiol Rev 45: fuab008. 10.1093/femsre/fuab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui, M. , Adjiman, C.S. , Bardow, A. , Anthony, E.J. , Boston, A. , Brown, S. , et al. (2018) Carbon capture and storage (CCS): the way forward. Energy Environ Sci 11: 1062. 10.1039/c7ee02342a. [DOI] [Google Scholar]
- Castro‐Alonso, M.J. , Montañez‐Hernandez, L.E. , Sanchez‐Muñoz, M.A. , Macias Franco, M.R. , Narayanasamy, R. , and Balagurusamy, N. (2019) Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and molecular concepts. Front Mat 6: 126. 10.3389/fmats.2019.00126. [DOI] [Google Scholar]
- Chan, Y.H. , Syed Abdul Rahman, S.N.F. , Lahuri, H.M. , and Khalid, A. (2021) Recent progress on CO‐rich syngas production via CO2 gasification of various wastes: a critical review on efficiency, challenges and outlook. Environ Pollut 278: 116843. 10.1016/j.envpol.2021.116843. [DOI] [PubMed] [Google Scholar]
- Cheng, S. , Xing, D. , Call, D.F. , and Logan, B.E. (2009) Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol 43: 3953–3958. 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- Chistoserdova, L. (2018) Applications of methylotrophs: can single carbon be harnessed for biotechnology? Curr Opin Biotechnol 50: 189–194. 10.1016/j.copbio.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Choi, Y.Y. , Patel, A.K. , Hong, M.E. , Chang, W.S. , and Sim, S.J. (2019) Microalgae bioenergy with carbon capture and storage (BECCS): an emerging sustainable bioprocess for reduced CO2 emission and biofuel production. Biores Technol Rep 7: 1001270. 10.1016/j.biteb.2019.100270. [DOI] [Google Scholar]
- Chu, N. , Liang, Q. , Jiang, Y. , and Zeng, R.J. (2020) Microbial electrochemical platform for the production of renewable fuels and chemicals. Biosens Bioelectron 150: 111922. 10.1016/j.bios.2019.111922. [DOI] [PubMed] [Google Scholar]
- Claassens, N.J. (2017) A warm welcome for alternative CO2 fixation pathways in microbial biotechnology. Microb Biotechnol 10: 31–34. 10.1111/1751-7915.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassens, N.J. , Bordanaba‐Florit, G. , Cotton, C.A.R. , De Maria, A. , Finger‐Bou, M. , Friedeheim, L. , et al. (2020) Replacing the Calvin cycle with the reductive glycine pathway in Cupriavidus necator . Metab Eng 62: 30–41. 10.1016/j.ymben.2020.08.004. [DOI] [PubMed] [Google Scholar]
- Claassens, N.J. , Sousa, D.Z. , Dos Santos, V.A. , de Vos, W.M. , and van der Oost, J. (2016) Harnessing the power of microbial autotrophy. Nat Rev Microbiol 14: 692–706. 10.1038/nrmicro.2016.130. [DOI] [PubMed] [Google Scholar]
- Cordero, P.R.F. , Bayly, K. , Man Leung, P. , Huang, C. , Islam, Z.F. , Schittenhelm, R.B. , et al. (2019) Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J 13: 2868–2881. 10.1038/s41396-019-0479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton, C.A. , Claassens, N.J. , Benito‐Vaquerizo, S. , and Bar‐Even, A. (2020) Renewable methanol and formate as microbial feedstocks. Curr Opin Biotechnol 62: 168–180. 10.1016/j.copbio.2019.10.002. [DOI] [PubMed] [Google Scholar]
- De Tissera, S. , Köpke, M. , Simpson, S.D. , Humphreys, C. , Minton, N.P. , and Dürre, P. (2019) Syngas biorefinery and syngas utilization. Adv Biochem Eng Biotechnol 166: 247–280. 10.1007/10_2017_5. [DOI] [PubMed] [Google Scholar]
- Dessì, P. , Rovira‐Alsina, L. , Sánchez, C. , Dinesh, G.K. , Tong, W. , Chatterjee, P. , et al. (2021) Microbial electrosynthesis: Towards sustainable biorefineries for production of green chemicals from CO2 emissions. Biotechnol Adv 46: 107675. 10.1016/j.biotechadv.2020.107675. [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Bertram, J.R. , Eckert, C. , Bommareddy, R.R. , Patel, R. , Conradie, A. , et al. (2019) Nanorg microbial factories: Light‐driven renewable biochemical synthesis using quantum dot‐bacteria nanobiohybrids. J Am Chem Soc 141: 10272–10282. 10.1021/jacs.9b02549. [DOI] [PubMed] [Google Scholar]
- Dogutan, D.K. , and Nocera, D.G. (2019) Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis. Acc Chem Res 52: 3143–3148. 10.1021/acs.accounts.9b00380. [DOI] [PubMed] [Google Scholar]
- Duan, H. , He, P. , Shao, L. , and Lü, F. (2021) Functional genome‐centric view of the CO‐driven anaerobic microbiome. ISME J 15: 2906–2919. 10.1038/s41396-021-00983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler, N. , Heffernan, J. , Juminaga, A. , Doser, D. , Nagaraju, S. , González‐García, R.A. , et al. (2021) Transcriptional control of Clostridium autoethanogenum using CRISPRi. Synth Biol (Oxf) 6: ysab008. 10.1093/synbio/ysab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler, N. , Heijstra, B.D. , Rasor, B.J. , Brown, H. , Martin, J. , Ni, Z. , et al. (2021) Stepping on the gas to a circular economy: accelerating development of carbon‐negative chemical production from gas fermentation. Annu Rev Chem Biomol Eng 12: 439–470. 10.1146/annurev-chembioeng-120120-021122. [DOI] [PubMed] [Google Scholar]
- Fedeson, D.T. , Saake, P. , Calero, P. , Nikel, P.I. , and Ducat, D.C. (2020) Biotransformation of 2,4‐dinitrotoluene in a phototrophic co‐culture of engineered Synechococcus elongatus and Pseudomonas putida . Microb Biotechnol 13: 997–1011. 10.1111/1751-7915.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamholz, A.I. , Dugan, E. , Blikstad, C. , Gleizer, S. , Ben‐Nissan, R. , Amram, S. , et al. (2020) Functional reconstitution of a bacterial CO2 concentrating mechanism in Escherichia coli . eLife 9: e59882. 10.7554/eLife.59882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François, J.M. , Lachaux, C. , and Morin, N. (2020) Synthetic biology applied to carbon conservative and carbon dioxide recycling pathways. Front Bioeng Biotechnol 7: 446. 10.3389/fbioe.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaud, M. (ed) (2011) Encyclopedia of Astrobiology. Berlin, Heidelberg, Germany: Springer‐Verlag. [Google Scholar]
- Geinitz, B. , Hüser, A. , Mann, M. , and Büchs, J. (2020) Gas fermentation expands the scope of a process network for material conversion. Chem Ing Tech 92: 1665–1679. 10.1002/cite.202000086. [DOI] [Google Scholar]
- George, D.M. , Vincent, A.S. , and Mackey, H.R. (2020) An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable resource recovery. Biotechnol Rep (Amst) 28: e00563. 10.1016/j.btre.2020.e00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert, F. , Liu, D. , van Eerten‐Jansen, M. , Weidner, E. , Buisman, C. , and Ter Heijne, A. (2016) Bioelectrochemical power‐to‐gas: state of the art and future perspectives. Trends Biotechnol 34: 879–894. 10.1016/j.tibtech.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Gimkiewicz, C. , Hegner, R. , Gutensohn, M.F. , Koch, C. , and Harnisch, F. (2017) Study of electrochemical reduction of CO2 for future use in secondary microbial electrochemical technologies. Chemsuschem 10: 958–967. 10.1002/cssc.201601675. [DOI] [PubMed] [Google Scholar]
- Gleizer, S. , Ben‐Nissan, R. , Bar‐On, Y.M. , Antonovsky, N. , Noor, E. , Zohar, Y. , et al. (2019) Conversion of Escherichia coli to generate all biomass carbon from CO2 . Cell 179: 1255–1263.e12. 10.1016/j.cell.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görgen, S. , Benzerar, K. , Skouri‐Panet, F. , Gugger, M. , Chauvat, F. , and Cassier‐Chauvat, C. (2020) The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discover Mat 1: 2. 10.1007/s43939-020-00001-9. [DOI] [Google Scholar]
- Guo, J. , Suástegui, M. , Sakimoto, K.K. , Moody, V.M. , Xiao, G. , Nocera, D.G. , and Joshi, N.S. (2018) Light‐driven fine chemical production in yeast biohybrids. Science 362: 813–816. 10.1126/science.aat9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, P. , Noori, M.T. , Núñez, A.E. , and Verma, N. (2021) An insight into the bioelectrochemical photoreduction of CO2 to value‐added chemicals. iScience 24: 102294. 10.1016/j.isci.2021.102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ha, D. , Nachtergaele, L. , Kerckhof, F.‐M. , Rameiyanti, D. , Bossier, P. , Verstraete, W. , and Boon, N. (2012) Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ Sci Technol 46: 13425–13431. 10.1021/es303929s. [DOI] [PubMed] [Google Scholar]
- Haas, T. , Krause, R. , Weber, R. , Demler, M. , and Schmid, G. (2018) Technical photosynthesis involving CO2 electrolysis and fermentation. Nat Catalysis 1: 32–39. 10.1038/s41929-017-0005-1. [DOI] [Google Scholar]
- Hays, S.G. , Yan, L.L.W. , Silver, P.A. , and Ducat, D.C. (2017) Synthetic photosynthetic consortia define interactions leading to robustness and photoproduction. J Biol Eng 11: 4. 10.1186/s13036-017-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegner, R. , Neubert, K. , Kroner, C. , Holtmann, D. , and Harnisch, F. (2020) Coupled electrochemical and microbial catalysis for the production of polymer bricks. Chemsuschem 13: 5295–5300. 10.1002/cssc.202001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E.A. , Chrisler, W.B. , Beliaev, A.S. , and Bernstein, H.C. (2017) A flexible microbial co‐culture platform for simultaneous utilization of methane and carbon dioxide from gas feedstocks. Bioresour Technol 228: 250–256. 10.1016/j.biortech.2016.12.111. [DOI] [PubMed] [Google Scholar]
- Hu, G. , Li, Y. , Ye, C. , Liu, L. , and Chen, X. (2018) Engineering microorganisms for enhanced CO2 sequestration. Trends Biotechnol 37: 532–547. 10.1016/j.tibtech.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Hu, G. , Li, Z. , Ma, D. , Ye, C. , Zhang, L. , Gao, C. , et al. (2021) Light‐driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals. Nat Catal 4: 395–406. 10.1038/s41929-021-00606-0. [DOI] [Google Scholar]
- Hwang, H. , Yeon, Y.J. , Lee, S. , Choe, H. , Jang, M.G. , Cho, D.H. , et al. (2015) Electro‐biocatalytic production of formate from carbon dioxide using an oxygen‐stable whole cell biocatalyst. Bioresour Technol 185: 35–39. 10.1016/j.biortech.2015.02.086. [DOI] [PubMed] [Google Scholar]
- Jang, J. , Jeon, B.W. , and Kim, Y.H. (2018) Bioelectrochemical conversion of CO2 to value added product formate using engineered Methylobacterium extorquens . Sci Rep 8: 7211. 10.1038/s41598-018-23924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayathilake, B.S. , Bhattacharya, S. , Vaidehi, N. , and Narayanan, S.R. (2019) Efficient and selective electrochemically driven enzyme‐catalyzed reduction of carbon dioxide to formate using formate dehydrogenase and an artificial cofactor. Acc Chem Res 52: 676–685. 10.1021/acs.accounts.8b00551. [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Hernández Villamor, D. , Peng, H. , Chen, J. , Liu, L. , Haritos, V. , and Ledesma‐Amaro, R. (2021) Metabolic engineering strategies to enable microbial utilization of C1 feedstocks. Nat Chem Biol 17: 845–855. 10.1038/s41589-021-00836-0. [DOI] [PubMed] [Google Scholar]
- Jin, S. , Bae, J. , Song, Y. , Pearcy, N. , Shin, J. , Kang, S. , et al. (2020) Synthetic biology on Acetogenic bacteria for highly efficient conversion of C1 gases to biochemicals. Int J Mol Sci 21: 7639. 10.3390/ijms21207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S. , Hao, Z. , Zhang, K. , Yan, Z. , and Chen, J. (2021) Advances and challenges for the electrochemical reduction of CO2 to CO: From fundamentals to industrialization. Angew Chem Int Ed 60: 2–24. 10.1002/anie.202101818. [DOI] [PubMed] [Google Scholar]
- Joshi, S. , Goyal, S. , Mukherjee, A. , and Reddy, M.S. (2017) Microbial healing of cracks in concrete: a review. J Ind Microbiol Biotechnol 44: 1511–1525. 10.1007/s10295-017-1978-0. [DOI] [PubMed] [Google Scholar]
- Kanno, M. , Carroll, A.L. , and Atsumi, S. (2017) Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat Commun 8: 14724. 10.1038/ncomms14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, J. , Takemura, K. , Kato, S. , Fujii, T. , Wada, K. , Iwasaki, Y. , et al. (2021) Metabolic engineering of Moorella thermoacetica for thermophilic bioconversion of gaseous substrates to a volatile chemical. AMB Express 11: 59. 10.1186/s13568-021-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyv, A. , and Müller, V. (2020) Overcoming energetic barriers in acetogenic C1 conversion. Front Bioeng Biotechnol 8: 621166. 10.3389/fbioe.2020.621166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfeld, C.A. (2016) Rewiring Escherichia coli for carbon‐dioxide fixation. Nat Biotechnol 34: 1035–1036. 10.1038/nbt.3693. [DOI] [PubMed] [Google Scholar]
- Kiefer, D. , Merkel, M. , Lilge, L. , Henkel, M. , and Hausmann, R. (2021) From acetate to bio‐based products: Underexploited potential for industrial biotechnology. Trends Biotechnol 39: 397–411. 10.1016/j.tibtech.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Lindner, S.N. , Aslan, S. , Yishai, O. , Wenk, S. , Schann, K. , and Bar‐Even, A. (2020) Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat Chem Biol 16: 538–545. 10.1038/s41589-020-0473-5. [DOI] [PubMed] [Google Scholar]
- Kim, Y. , Lama, S. , Agrawal, D. , Kumar, V. , and Park, S. (2021) Acetate as a potential feedstock for the production of value‐added chemicals: Metabolism and applications. Biotechnol Adv 49: 107736. 10.1016/j.biotechadv.2021.107736. [DOI] [PubMed] [Google Scholar]
- King, G.M. (2015) Carbon monoxide as a metabolic energy source for extremely halophilic microbes: implications for microbial activity in Mars regolith. Proc Natl Acad Sci USA 112: 4465–4470. 10.1073/pnas.1424989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita, A. , Iwasaki, Y. , Sakai, S. , Okuto, S. , Takaoka, K. , Suzuki, T. , et al. (2013) Development of genetic transformation and heterologous expression system in carboxydotrophic thermophilic acetogen Moorella thermoacetica . J Biosci Bioeng 115: 347–352. 10.1016/j.jbiosc.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Kopke, M. , Held, C. , Hujer, S. , Liesegang, H. , Wiezer, A. , Wollherr, A. , et al. (2010) Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc Natl Acad Sci USA 107: 13087–13092. 10.1073/pnas.1004716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral, T.A. , Birch, W. , Lavender, L.E. , and Virden, B.T. (2014) Potential use of highly insoluble carbonates as carbon sources by methanogens in the subsurface of Mars. Planet Space Sci 101: 181–185. 10.1016/j.pss.2014.07.008. [DOI] [Google Scholar]
- Kumar, M. , Sahoo, P.C. , Srikanth, S. , Bagai, R. , Puri, S.K. , and Ramakumar, S.S.V. (2019) Photosensitization of electro‐active microbes for solar assisted carbon dioxide transformation. Bioresour Technol 272: 300–307. 10.1016/j.biortech.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Kumar, M. , Sundaram, S. , Gnansounou, E. , Larroche, C. , and Thakur, I.S. (2018) Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour Technol 247: 1059–1068. 10.1016/j.biortech.2017.09.050. [DOI] [PubMed] [Google Scholar]
- Le, Q.A.T. , Kim, H.G. , and Kim, Y.H. (2018) Electrochemical synthesis of formic acid from CO2 catalyzed by Shewanella oneidensis MR‐1 whole‐cell biocatalyst. Enzyme Microb Technol 116: 1–5. 10.1016/j.enzmictec.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Lee, Y.S. , and Park, W. (2018) Current challenges and future directions for bacterial self‐healing concrete. Appl Microbiol Biotechnol 102: 3059–3070. 10.1007/s00253-018-8830-y. [DOI] [PubMed] [Google Scholar]
- Lemaire, O.N. , Jespersen, M. , and Wagner, T. (2020) CO2‐fixation strategies in energy extremophiles: what can we learn from acetogens? Front Microbiol 11: 486. 10.3389/fmicb.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, Y.K. , Chew, K.W. , Chen, W.H. , Chang, J.S. , and Show, P.L. (2021) Reuniting the biogeochemistry of algae for a low‐carbon circular bioeconomy. Trends Plant Sci 26: 729–740. 10.1016/j.tplants.2020.12.010. [DOI] [PubMed] [Google Scholar]
- Li, H. , Opgenorth, P.H. , Wernick, D.G. , Rogers, S. , Wu, T.Y. , Higashide, W. , et al. (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335: 1596. 10.1126/science.1217643. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Xin, X. , Xiong, B. , Zhao, D. , Zhang, X. , and Bi, C. (2020) Engineering the Calvin‐Benson‐Bassham cycle and hydrogen utilization pathway of Ralstonia eutropha for improved autotrophic growth and polyhydroxybutyrate production. Microb Cell Fact 19: 228. 10.1186/s12934-020-01494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, B. , Zhao, Y. , and Yang, J. (2020) Recent advances in developing artificial autotrophic microorganism for reinforcing CO2 fixation. Front Microbiol 11: 592631. 10.3389/fmicb.2020.592631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, F. , Henstra, A.M. , Winzer, K. , Köpke, M. , Simpson, S.D. , and Minton, N.P. (2016) Insights into CO2 fixation pathway of Clostridium autoethanogenum by targeted mutagenesis. MBio 7: e00427‐16. 10.1128/mBio.00427-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Wang, K. , Chen, Y. , Tan, T. , and Nielsen, J. (2020) Third‐generation biorefineries as the means to produce fuels and chemicals from CO2 . Nat Catal 3: 274–288. 10.1038/s41929-019-0421-5. [DOI] [Google Scholar]
- Löwe, H. , Hobmeier, K. , Moos, M. , Kremling, A. , and Pflüger‐Grau, K. (2017) Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB . Biotechnol Biofuels 10: 190. 10.1186/s13068-017-0875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Shi, Y. , Meng, N. , Lu, S. , Yu, Y. , and Zhang, B. (2020) Electrosynthesis of syngas via the co‐reduction of CO2 and H2O. Cell Reports Physical Sci 1: 100237. 10.1016/j.xcrp.2020.100237. [DOI] [Google Scholar]
- Mao, W. , Yuan, Q. , Qi, H. , Wang, Z. , Ma, H. , and Chen, T. (2020) Recent progress in metabolic engineering of microbial formate assimilation. Appl Microbiol Biotechnol 104: 6905–6917. 10.1007/s00253-020-10725-6. [DOI] [PubMed] [Google Scholar]
- Menon, R.R. , Luo, J. , Chen, X. , Zhou, H. , Liu, Z. , Zhou, G. , et al. (2019) Screening of fungi for potential application of self‐healing concrete. Sci Rep 9: 2075. 10.1038/s41598-019-39156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton, N.P. , Ehsaan, M. , Humphreys, C.M. , Little, G.T. , Baker, J. , Henstra, A.M. , et al. (2016) A roadmap for gene system development in Clostridium . Anaerobe 41: 104–112. 10.1016/j.anaerobe.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor, B. , Kirchner, K. , Henrich, A.W. , Schmitz, S. , and Rosenbaum, M.A. (2016) Expanding the molecular toolkit for the homoacetogen Clostridium ljungdahlii . Sci Rep 6: 31518. 10.1038/srep31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor, B. , Marcellin, E. , and Angenent, L.T. (2017) Overcoming the energetic limitations of syngas fermentation. Curr Opin Chem Biol 41: 84–92. 10.1016/j.cbpa.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Müller, V. (2019) New horizons in acetogenic conversion of one‐carbon substrates and biological hydrogen storage. Trends Biotechnol 37: 1344–1354. 10.1016/j.tibtech.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Naduthodi, M.I.S. , Claassens, N.J. , D'Adamo, S. , van der Oost, J. , and Barbosa, M.J. (2021) Synthetic biology approaches to enhance microalgal productivity. Trends Biotechnol 39: 1019–1036. [DOI] [PubMed] [Google Scholar]
- Nangle, S.N. , Ziesack, M. , Buckley, S. , Trivedi, D. , Loh, D.M. , Nocera, D.G. , and Silver, P.A. (2020) Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator . Metab Eng 62: 207–220. 10.1016/j.ymben.2020.09.002. [DOI] [PubMed] [Google Scholar]
- Nevin, K.P. , Woodard, T.L. , Franks, A.E. , Summers, Z.M. , and Lovley, D.R. (2010) Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. MBio 1: e00103‐10. 10.1128/mBio.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, D.T.N. , Lee, O.K. , Nguyen, T.T. , and Lee, E.Y. (2021) Type II methanotrophs: a promising microbial cell‐factory platform for bioconversion of methane to chemicals. Biotechnol Adv 47: 107700. 10.1016/j.biotechadv.2021.107700. [DOI] [PubMed] [Google Scholar]
- Nichols, E.M. , Gallagher, J.J. , Liu, C. , Su, Y. , Resasco, J. , Yu, Y.I. , et al. (2015) Hybrid bioinorganic approach to solar‐to‐chemical conversion. Proc Natl Acad Sci USA 112: 11461–11466. 10.1073/pnas.1508075112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panich, J. , Fong, B. , and Singer, S.W. (2021) Metabolic engineering of Cupriavidus necator H16 for sustainable biofuels from CO2 . Trends Biotechnol 39: 412–424. 10.1016/j.tibtech.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Park, S. , Wijaya, D.T. , Na, J. , and Lee, C.W. (2021) Towards the large‐scale electrochemical reduction of carbon dioxide. Catalysts 11: 253. 10.3390/catal11020253. [DOI] [Google Scholar]
- Phillips, J.R. , Huhnke, R.L. , and Atiyeh, K.H. (2017) Syngas fermentation: a microbial conversion process of gaseous substrates to various products. Fermentation 3: 28. 10.3390/fermentation3020028. [DOI] [Google Scholar]
- Pirola, C. , Bozzano, G. , and Manenti, F. (2018) Chapter 3 ‐ Fossil or renewable sources for methanol production? In Methanol. Basile, A. , and Dalena, F. (eds). Elsevier, pp. 53–93. ISBN 9780444639035. 10.1016/B978-0-444-63903-5.00003-0. [DOI] [Google Scholar]
- Reddy, M.S. , and Sumit, J. (2018) Carbon dioxide sequestration on biocement‐based composites. In Woodhead Publishing Series in Civil and Structural Engineering. Pacheco‐Torgal, F. , Shi, C. , and Sanchez, A. P. (eds). Patiala, India: Thapar Institute of Engineering & Technology, pp. 225–243. 10.1016/B978-0-08-102444-7.00010-1. [DOI] [Google Scholar]
- Revelles, O. , Beneroso, D. , Menéndez, J.A. , Arenillas, A. , García, J.L. , and Prieto, M.A. (2017) Syngas obtained by microwave pyrolysis of household wastes as feedstock for polyhydroxyalkanoate production in Rhodospirillum rubrum . Microb Biotechnol 10: 1412–1417. 10.1111/1751-7915.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelles, O. , Tarazona, N. , García, J.L. , and Prieto, M.A. (2016) Carbon roadmap from syngas to polyhydroxyalkanoates in Rhodospirillum rubrum . Environ Microbiol 18: 708–720. [DOI] [PubMed] [Google Scholar]
- Robb, F.T. , and Techtmann, S.M. (2018) Life on the fringe: microbial adaptation to growth on carbon monoxide. F1000Res 7: 1981. 10.12688/f1000research.16059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Hernández‐Herreros, N. , García, J.L. , and Prieto, M.A. (2021) Enhancement of biohydrogen production rate in Rhodospirillum rubrum by a dynamic CO‐feeding strategy using dark fermentation. Biotechnol Biofuels 14: 168. 10.1186/s13068-021-02017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo, K.K. , Goswami, G. , and Das, D. (2021) Biotransformation of methane and carbon dioxide into high‐value products by methanotrophs: current state of art and future prospects. Front Microbiol 12: 636486. 10.3389/fmicb.2021.636486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo, P.C. , Pant, D. , Kumar, M. , Puri, S.K. , and Ramakumar, S.S.V. (2020) Material‐microbe interfaces for solar‐driven CO2 bioelectrosynthesis. Trends Biotechnol 38: 1245–1261. 10.1016/j.tibtech.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Sarma, S. , Sharma, S. , Rudakiya, D. , Upadhyay, J. , Rathod, V. , Patel, A. , and Narra, M. (2021) Valorization of microalgae biomass into bioproducts promoting circular bioeconomy: a holistic approach of bioremediation and biorefinery. 3 Biotech 11: 378. 10.1007/s13205-021-02911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satanowski, A. , and Bar‐Even, A. (2020) A one‐carbon path for fixing CO2: C1 compounds, produced by chemical catalysis and upgraded via microbial fermentation, could become key intermediates in the valorization of CO2 into commodity chemicals. EMBO Rep 21: e50273. 10.15252/embr.202050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satanowski, A. , Dronsella, B. , Noor, E. , Vögeli, B. , He, H. , Wichmann, P. , et al. (2020) Awakening a latent carbon fixation cycle in Escherichia coli . Nat Commun 11: 5812. 10.1038/s41467-020-19564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffen, M. , Marchal, D.G. , Beneyton, T. , Schuller, S.K. , Klose, M. , Diehl, C. , et al. (2021) A new‐to‐nature carboxylation module to improve natural and synthetic CO2 fixation. Nat Catalysis 4: 105–115. 10.1038/s41929-020-00557-y. [DOI] [Google Scholar]
- Schlager, S. , Dibenedetto, A. , Aresta, M. , Apaydin, D.H. , Dumitru, L.M. , Neugebauer, H. , and Sariciftci, N.S. (2017) Biocatalytic and bioelectrocatalytic approaches for the reduction of carbon dioxide using enzymes. Energy Technol 5: 1–11. 10.1002/ente.201600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander, T. , Schada von Borzyskowski, L. , Burgener, S. , Cortina, N.S. , and Erb, T.J. (2016) A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354: 900–904. 10.1126/science.aah5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifan, M. , and Berenjian, A. (2018) Application of microbially induced calcium carbonate precipitation in designing bio self‐healing concrete. World J Microbiol Biotechnol 34: 168. 10.1007/s11274-018-2552-2. [DOI] [PubMed] [Google Scholar]
- Simon Araya, S. , Liso, V. , Cui, X. , Li, N.A. , Zhu, J. , Sahlin, S.L. , et al. (2020) A review of the methanol economy: the fuel cell route. Energies 13: 596. 10.3390/en13030596. [DOI] [Google Scholar]
- Singh, A.K. , Kishore, G.M. , and Pakrasi, H.B. (2018) Emerging platforms for co‐utilization of one‐carbon substrates by photosynthetic organisms. Curr Opin Biotechnol 53: 201–208. 10.1016/j.copbio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Srikanth, S. , Alvarez‐Gallego, Y. , Vanbroekhoven, K. , and Pant, D. (2017) Enzymatic electrosynthesis of formic acid through carbon dioxide reduction in a bioelectrochemical system: effect of immobilization and carbonic anhydrase addition. ChemPhysChem 18: 3174–3181. 10.1002/cphc.201700017. [DOI] [PubMed] [Google Scholar]
- Srikanth, S. , Maesen, M. , Dominguez‐Benetton, X. , Vanbroekhoven, K. , and Pant, D. (2014) Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour Technol 165: 350–354. 10.1016/j.biortech.2014.01.129. [DOI] [PubMed] [Google Scholar]
- Straub, M. , Demler, M. , Weuster‐Botz, D. , and Dürre, P. (2014) Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii . J Biotechnol 178: 67–72. 10.1016/j.jbiotec.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Sun, X. , Atiyeh, K.H. , Huhnke, R. , and Tunner, R.S. (2019) Syngas fermentation process development for production of biofuels and chemicals: a review. Biores Technol Rep 7: 100279. 10.1016/j.biteb.2019.100279. [DOI] [Google Scholar]
- Takors, R. , Kopf, M. , Mampel, J. , Bluemke, W. , Blombach, B. , et al. (2018) Using gas mixtures of CO, CO2 and H2 as microbial substrates: the do's and don'ts of successful technology transfer from laboratory to production scale. Microb Biotechnol 11: 606–625. 10.1111/1751-7915.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro, Y. , Hirano, S. , Matson, M.M. , Atsumi, S. , and Kondo, A. (2018) Electrical‐biological hybrid system for CO2 reduction. Metab Eng 47: 211–218. 10.1016/j.ymben.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Teixeira, L.V. , Moutinho, L.F. , and Romão‐Dumaresq, A.S. (2018) Gas fermentation of C1 feedstocks: commercialization status and future prospects. Biofuels Bioprod Bioref 12: 1013–1117. 10.1002/bbb.1912. [DOI] [Google Scholar]
- Tuyishime, P. , and Sinumvayo, J.P. (2020) Novel outlook in engineering synthetic methylotrophs and formatotrophs: a course for advancing C1‐based chemicals production. World J Microbiol Biotechnol 36: 118. 10.1007/s11274-020-02899-y. [DOI] [PubMed] [Google Scholar]
- Veaudor, T. , Blanc‐Garin, V. , Chenebault, C. , Diaz‐Santos, E. , Sassi, J.F. , Cassier‐Chauvat, C. , et al. (2020) Recent advances in the photoautotrophic metabolism of Cyanobacteria: biotechnological implications. Life 10: 71. 10.3390/life10050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Anb, Z. , and Wang, Z.W. (2020) Bioconversion of methane to chemicals and fuels by methane‐oxidizing bacteria. Adv Bioenergy 5: 169–247. 10.1016/bs.aibe.2020.04.005. [DOI] [Google Scholar]
- Weiss, T.L. , Young, E.J. , and Ducat, D.C. (2017) A synthetic, light‐driven consortium of cyanobacteria and heterotrophic bacteria enables stable polyhydroxybutyrate production. Metab Eng 44: 236–245. 10.1016/j.ymben.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Woo, H.M. (2017) Solar‐to‐chemical and solar‐to‐fuel production from CO2 by metabolically engineered microorganisms. Curr Opin Biotechnol 45: 1–7. 10.1016/j.copbio.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Wood, J.C. , Grové, J. , Marcellin, E. , Heffernan, J.K. , Hu, S. , Yuan, Z. , and Virdis, B. (2021) Strategies to improve viability of a circular carbon bioeconomy‐A techno‐economic review of microbial electrosynthesis and gas fermentation. Water Res 201: 117306. 10.1016/j.watres.2021.117306. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Xiu, Y. , Liu, F. , Liang, Y. , and Wang, S. (2020) Research progress in conversion of CO2 to valuable fuels. Molecules 25: 3653. 10.3390/molecules25163653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yishai, O. , Bouzon, M. , Döring, V. , and Bar‐Even, A. (2018) In vivo assimilation of one‐carbon via a synthetic reductive glycine pathway in Escherichia coli . ACS Synth Biol 7: 2023–2028. 10.1021/acssynbio.8b00131. [DOI] [PubMed] [Google Scholar]
- Yishai, O. , Lindner, S.N. , Gonzalez de la Cruz, J. , Tenenboim, H. , and Bar‐Even, A. (2016) The formate bio‐economy. Curr Opin Chem Biol 35: 1–9. 10.1016/j.cbpa.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Yuan, M. , Kummer, M.J. , and Minteer, S.D. (2019) Strategies for bioelectrochemical CO2 reduction. Chemistry 25: 14258–14266. 10.1002/chem.201902880. [DOI] [PubMed] [Google Scholar]
- Zhan, C. , Li, X. , Yang, Y. , Nielsen, J. , Bai, Z. , and Chen, Y. (2021) Strategies and challenges with the microbial conversion of methanol to high‐value chemicals. Biotechnol Bioeng 118: 3655–3668. 10.1002/bit.27862. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Chen, L. , Diao, J. , Song, X. , Shi, M. , and Zhang, W. (2020) Construction and analysis of an artificial consortium based on the fast‐growing cyanobacterium Synechococcus elongatus UTEX 2973 to produce the platform chemical 3‐hydroxypropionic acid from CO2 . Biotechnol Biofuels 13: 82. 10.1186/s13068-020-01720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Liu, J. , Ong, J. , and Li, S.F. (2016) Specific and sustainable bioelectro‐reduction of carbon dioxide to formate on a novel enzymatic cathode. Chemosphere 162: 228–234. 10.1016/j.chemosphere.2016.07.102. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhao, R. , Jia, D. , Jiang, W. , and Gu, Y. (2020) Engineering Clostridium ljungdahlii as the gas‐fermenting cell factory for the production of biofuels and biochemicals. Curr Opin Chem Biol 59: 54–61. 10.1016/j.cbpa.2020.04.010. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Yuan, X.‐J. , Zhang, C. , Zhu, L.‐P. , Mo, X.‐H. , Chen, W.‐J. , and Yang, S. (2019) Bioconversion of methanol into value‐added chemicals in native and synthetic methylotrophs. Curr Issues Mol Biol 33: 225–236. 10.21775/cimb.033.225. [DOI] [PubMed] [Google Scholar]
- Zhang, T. , and Tremblay, P.L. (2017) Hybrid photosynthesis‐powering biocatalysts with solar energy captured by inorganic devices. Biotechnol Biofuels 10: 249. 10.1186/s13068-017-0943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, T. , Zhao, T. , Bankefa, O.E. , and Li, Y. (2020) Engineering unnatural methylotrophic cell factories for methanol‐based biomanufacturing: Challenges and opportunities. Biotechnol Adv 39: 107467. 10.1016/j.biotechadv.2019.107467. [DOI] [PubMed] [Google Scholar]


