Abstract
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by SARS-CoV-2 infection and is associated with both acute and chronic disorders affecting the nervous system. Acute neurological disorders affecting patients with COVID-19 range widely from anosmia, stroke, encephalopathy/encephalitis, and seizures to Guillain–Barré syndrome. Chronic neurological sequelae are less well defined although exercise intolerance, dysautonomia, pain, as well as neurocognitive and psychiatric dysfunctions are commonly reported. Molecular analyses of CSF and neuropathological studies highlight both vascular and immunologic perturbations. Low levels of viral RNA have been detected in the brains of few acutely ill individuals. Potential pathogenic mechanisms in the acute phase include coagulopathies with associated cerebral hypoxic-ischaemic injury, blood–brain barrier abnormalities with endotheliopathy and possibly viral neuroinvasion accompanied by neuro-immune responses. Established diagnostic tools are limited by a lack of clearly defined COVID-19 specific neurological syndromes. Future interventions will require delineation of specific neurological syndromes, diagnostic algorithm development and uncovering the underlying disease mechanisms that will guide effective therapies.
Keywords: SARS-CoV-2, COVID-19, nervous system, encephalopathy, stroke
Balcom et al. outline the neurological syndromes associated with COVID-19 in adults, including both acute and chronic disorders of the central and peripheral nervous systems. They describe the changes observed in CSF and brain tissues, and explore the underlying neuropathogenic mechanisms.
Introduction
Since its discovery in Wuhan, China in late 2019, coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 3 million deaths (https://covid19.who.int/) and placed unprecedented pressure on social, economic and health care systems worldwide. Many survivors of the acute infection experience persistent and incapacitating neurological symptoms, which can have socio-economic and personal consequences.1 It is thus imperative that there is a thorough understanding of the evolving clinical syndromes and the underlying pathophysiological mechanisms, enabling rational therapeutic interventions to be expeditiously deployed.
Retrospective cohort studies from around the world report neurological signs and symptoms such as headache, altered mental status, seizures and stroke, in over a third of patients during the acute phase of the illness,2-4 positioning SARS-CoV-2 as an emerging neuro-pathogen. A similar proportion of infected individuals develop a post-infectious viral syndrome with diverse neuropsychiatric manifestations. Viral infections cause neurological impairments through multiple mechanisms,5 including direct infection of neurons, glia or endothelial cells within the nervous system resulting in acute cell death, as observed in herpes simplex virus type-1 (HSV-1) encephalitis.6 Alternatively, viruses, e.g. the human immunodeficiency virus type-1 (HIV-1), can persist in cellular reservoirs within the central (CNS) and perhaps peripheral (PNS) nervous system resulting in chronic inflammation and insidious progressive neurological damage.7 Among non-neurotropic viruses such as influenza and other respiratory viruses, systemic infection is associated with inflammation, metabolic and hormonal derangements, with vascular injury resulting in neurological disease.8 The host immune responses triggered during or following viral infections can also result in autoimmune damage of neural tissues, as observed in the PNS [e.g. Guillain–Barré syndrome (GBS)] and in the CNS [e.g. acute disseminated encephalomyelitis (ADEM) or acute transverse myelitis (ATM)]. Each of these mechanisms are implicated in SARS-CoV-2 infection and are addressed next. Of note, a multisystem inflammatory syndrome in children (MIS-C) has been described in paediatric cohorts with COVID-19; several cohort studies of children infected with SARS-CoV-2 report neurological disorders resembling those observed in adults including headache, encephalopathy, demyelinating disorders and stroke.9–13 This review provides an update on neurological manifestations of COVID-19 that concentrates on adults while also examining contemporary evidence for the neuropathogenic mechanisms implicated in SARS-CoV-2 infection (Fig. 1) and their relationship to current and potential therapies (Table 1).
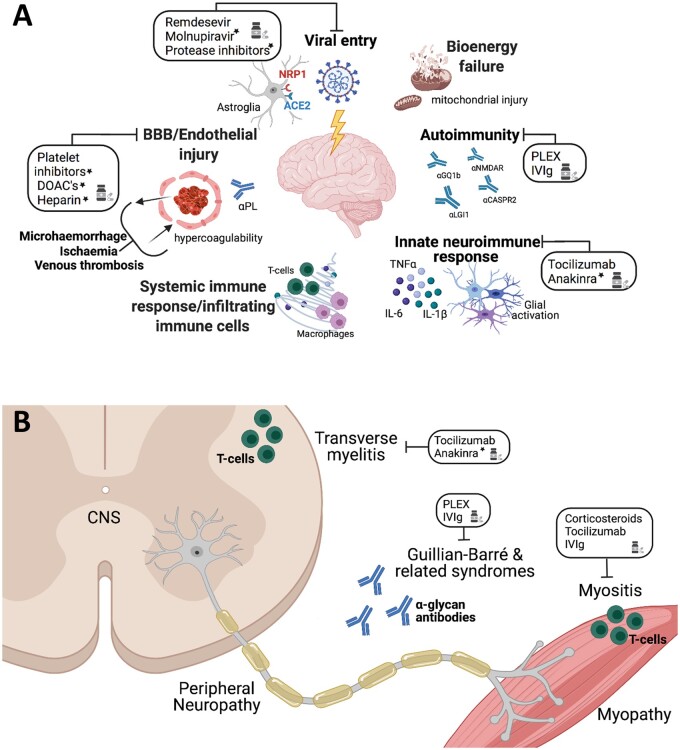
Figure 1.
Potential mechanisms of acute neurological disease in COVID-19. (A) Multiple pathogenic processes result in injury to the brain during COVID-19 including vascular abnormalities resulting in thromboembolism, microhaemorrhage and endotheliopathy with associated antiphospholipid antibodies (αPL) and disruption of the blood–brain barrier (BBB) leading to bioenergy failure, autoantibodies (e.g. αGQ1b, α-NMDA-R, α-CASPR2 and αLGI2) that target a range of neural antigens, and neuroinvasion with infection of neurons and astrocytes via ACE2 as well as associated systemic inflammation and innate neuroimmune responses (cytokine, chemokine, protease and reactive oxygen species production and release by microglia and astrocytes). Therapeutic interventions that have been reported or proposed are indicated with an asterisk. (B) In the PNS and spinal cord, GBS associated with anti-glycan antibodies (αGL), T-cell mediated transverse myelitis, as well as myositis have been reported in patients with COVID-19 that may be responsive to different therapies. PLEX = plasma exchange.
Table 1.
Proposed neuropathogenic mechanisms in SARS-CoV-2 infection
| Acute neurological syndromes | Proposed mechanisms | References | Proposed therapies |
|---|---|---|---|
| Anosmia/ageusia | Direct infection of olfactory bulb Inflammation of olfactory tract | Meinhardt et al.,14 Lu et al.15 | None |
| Stroke | Hypercoagulability/endothelial damage | Hernández-Fernández et al.,16 Goshua et al.,17 Yaghi et al.18 |
Prophylactic anticoagulation is currently under investigation; no clear guidelines to date Successful treatment with thrombolysis and mechanical thrombectomy reported |
| Encephalitis | Viral neuro-invasion | Nampoothiri et al.,19 Meinhardt et al.14 | Favourable responses to systemic corticosteroids, tocilizumab, and PLEX are observed in a subset of cases |
| Disrupted blood–brain barrier | Alexopoulos et al.20 | ||
| Autoimmunity | Cao et al.,21 Guilmot et al.,22 Pilotto et al.23,24 | ||
| Encephalopathy |
Metabolic dysfunction Hypoxia/ischaemia Cerebral microthrombi Cytokine storm (systemic) |
Bryce et al.,25 Antony and Haneef,26 Lee et al.,27 Lin et al.28 | Generally supportive, reported success with tocilizumab in case reports |
| Peripheral neuropathy | Critical illness neuropathy | Cabañes-Martínez et al.29 | Supportive |
| Molecular mimicry (GBS and variants) | Dalakas,30 Temme et al.31 | Standard therapy: IVIg, PLEX | |
| Myositis |
Bioenergetic dysfunction Immune-mediated myositis |
Beydon et al.,32 Dalakas,30 Zhang et al.33 | Favourable responses to steroids, IVIg and tocilizumab reported |
| Chronic neurological sequelae | |||
| Fatigue |
Chronic neuroinflammation Neuroendocrine dysfunction Persistent respiratory and cardiac damage |
Pandharipande et al.,34 Raman et al.,35 Mongioì et al.36 | None |
| Cognitive impairment |
Chronic neuroinflammation Frontoparietal hypometabolism |
Blazhenets et al.,37 Guedj et al.38 | None, demonstrated to improve over months |
| Depression/altered mood | Stress (isolation, post-traumatic stress) | Rogers et al.39 | No specific therapies proposed or tested for post-COVID-19 patients |
PLEX = plasma exchange.
Acute neurological syndromes
Anosmia and ageusia were among the first focal neurological symptoms described in COVID-19, and generated interest in SARS-CoV-2’s potential neurotropism.40 Anosmia was reported in 5–35% of hospitalized patients, and may be higher among non-hospitalized patients with COVID-19.41 In some cases, it is the sole reported symptom, or persists far beyond the acute respiratory symptoms, negatively influencing the quality of life of survivors. Infection of the nasal mucosa and sustentacular cells with dissemination throughout olfactory nerve projections is one proposed mechanism of neuropathogenesis in COVID-19.14 Viral RNA has also been found in the olfactory bulb at post-mortem of some patients, although its association with neurological injury is not established.14
Altered mental status is a commonly reported neurological finding associated with COVID-19 hospitalization. Abnormalities in EEG in COVID-19 related encephalopathy correlate with disease severity.26 Frontal slowing is the most common pattern observed and has been proposed as a biomarker for COVID-19 encephalopathy.26 Seizures are uncommon among COVID-19 patients; in a retrospective cohort of 1043 patients, 0.7% developed seizures in hospital and an even smaller proportion of those seizures occurred outside the context of pre-existing epilepsy42 although a recent retrospective study indicated that epileptiform abnormalities are frequently detected (48.7%) of hospitalized patients with COVID-19.28 Altered mental status, coma and seizures in COVID-19 are almost certainly multifactorial but can be stratified into metabolic/non-inflammatory versus inflammatory (e.g. encephalitis) categories.
Non-inflammatory encephalopathy
Many patients with COVID-19 and altered mental status (e.g. lethargy, confusion, coma) have clinical courses complicated by hypoxia, renal failure, electrolyte disturbances, sedating medications and underlying comorbidities. A third of critically ill patients with COVID-19 present with encephalopathy, often with frontal lobe-associated features,43,44 either at the onset of illness or during the course of hospitalization, and encephalopathy is associated with increased mortality and poor functional outcome.37,45,46 Although encephalopathy has been reported in COVID-19 patients at all ages, patients beyond the sixth decade of life and those with pre-existing neurologic conditions (stroke, dementia, Parkinson’s disease) are most affected, particularly in the context of severe respiratory illness.45,47 While encephalopathy in the aforementioned circumstances is probably multifactorial, reports of patients with encephalopathy in the absence of severe respiratory illness suggest other possible mechanisms including bioenergetic failure and vascular dysfunction in SARS-CoV-2 infection.48 The association between encephalopathy and morbidity exists independently of respiratory disease, similar to that observed in sepsis-associated encephalopathy.49 Encephalopathy in non-COVID-19 patients is attributed to mitochondrial dysfunction, excitotoxicity and macro- or micro-ischaemic injury.49 One recent post-mortem analysis in New York revealed cerebral microthrombi in a subset of 67 hospitalized, deceased COVID-19 patients.25 Small, subcortical ischaemic events may result in confusion and cognitive dysfunction.25 Patients can also manifest multifocal cerebral microhaemorrhages or vascular leakage (Fig. 2) due to compromise of the cerebral endothelial cells.50,51 Radiographic findings in COVID-associated encephalopathy include non-specific white matter hyperintensities, diffusion restriction, microhaemorrhage and leptomeningeal enhancement.50,52 In one study of intensive care unit (ICU) patients with COVID-19 and encephalopathy, bilateral frontotemporal hypoperfusion was evident in all patients who underwent perfusion imaging for altered mental status,53 although this finding was disputed and has not been replicated.54 Surprisingly, brain MRI was normal in up to 46% of patients with COVID-19 and associated encephalopathy.52 In another study, patients with COVID-19 and cognitive impairment showed decreased metabolism in the fronto-parietal regions on fluorodeoxyglucose (FDG)-PET scans.46 Indeed, correlations between neuropathology and brain MRI findings (including normal imaging) have yet to be established.
Figure 2.
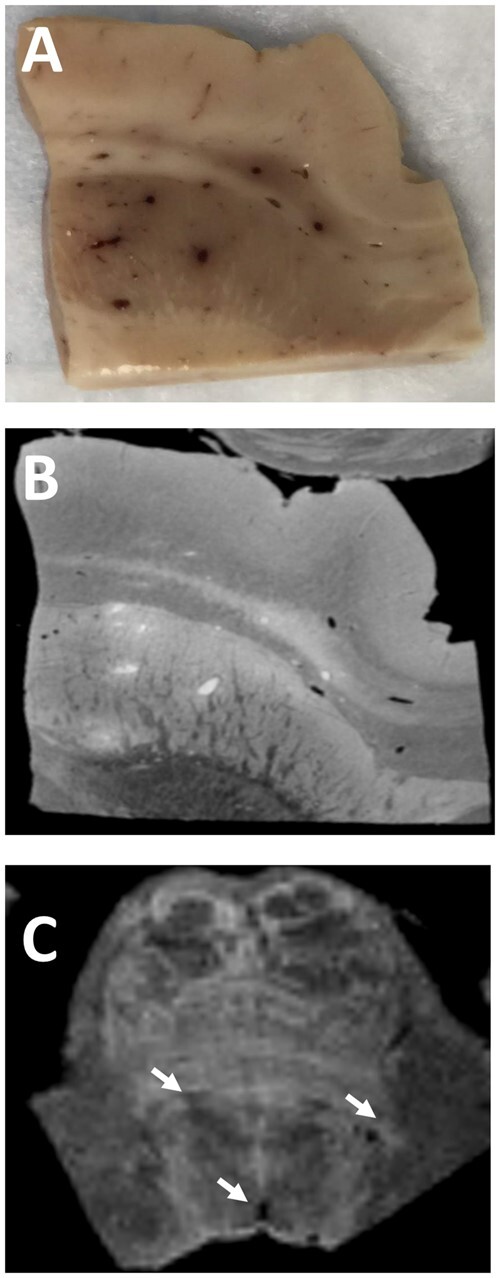
Microvascular diseases with COVID-19. (A) Multiple congested blood vessels and microhaemorrhages are observed in the basal ganglia at post-mortem. (B) MRI of the same block of tissue shows hyper and hypointense signals corresponding to the blood vessels in A. The hyperintense signals represent fibrin clots while the hypointense signals are microhaemorrhages. (C) MRI of the pons shows similar punctate hypointense signals (arrows).
Inflammatory encephalitis
A minority of patients with COVID-19 encompass established diagnostic criteria for infectious encephalitis.23 There are convincing reports of encephalitis-like presentations associated with elevated levels of soluble IL-6, IL-18, TNF-α, CXCL10 and markers of glial and astrocyte activation in CSF.20,24,55,56 Radiological findings associated with COVID-19 meningoencephalitis include mesial temporal lobe T2/FLAIR hyperintensities, varying from punctate to diffuse in subcortical white matter, the brainstem and claustrum, often accompanied by cerebral oedema.57–59 Case reports indicate that COVID-19 can present with an ADEM phenotype,52,60 including oculomotor dysfunction, seizures and coma. Others have reported cases of acute necrotizing haemorrhagic encephalopathy that present initially with symmetric lesions in the thalami and are thought to be cytokine mediated.61 Some patients may present with isolated pseudotumor cerebri/benign intracranial hypertension presumably from meningitis.62,63 Opsoclonus-myoclonus syndrome, which has been observed in association with infections such as Epstein–Barr virus (EBV), chikungunya and Mycoplasma pneumoniae, has also been reported in patients with COVID-19, including those with mild respiratory disease.64 Most patients with COVID-19 and opsoclonus-myoclonus syndrome had partial recovery at 4 weeks after treatments including pulse steroids, intravenous immunoglobulin (IVIg) and anti-epileptic medications.64–66
For presumed COVID-19 associated encephalitis, favourable therapeutic responses to corticosteroids and plasma exchange (PLEX) were observed in a subset of patients, although factors predicting a beneficial therapeutic response remain to be defined.21,67 Whether the neuroinflammation observed clinically, neuroradiologically and neuropathologically is due to direct viral invasion, para-infectious or autoimmune processes remains unknown. In most COVID-19 cases with encephalitis, SARS-CoV-2 RNA is not detectable in CSF via PCR with reverse transcription (RT-PCR), favouring an immune-mediated mechanism of disease.21,22,55,68
Cerebrovascular disease
Patients with COVID-19 have an increased rate of stroke compared to other disease cohorts, with higher NIHSS scores compared to non-COVID-19 associated stroke.16 Over half of strokes among patients with COVID-19 are cryptogenic, with a higher proportion of large vessel occlusions.18 Some series have reported higher than expected rates of posterior circulation strokes (35.3%).16,18 Hypercoagulability induced by systemic and focal inflammation has been implicated in COVID-19 associated strokes that include both arterial and venous thromboembolic events.69 Cerebral venous sinus thrombosis (CVST) among patients with COVID-19 can also occur with an abnormally activated prothromboplastin time (aPTT) and elevated D-dimer levels.70,71 COVID-19 associated CVST has an estimated in-hospital mortality of 40% in a cohort that included non-ventilated patients.70 In fact, CVST represents 4% of cerebrovascular complications in COVID-19 with an estimated frequency of 0.08% among hospitalized patients.70 In comparison, CVST accounts for 0.5–1% of all strokes among non-COVID-19 patients and occurs in ∼2–5 per million people each year (0.0002–0.0005%).72 Elevated D-dimer levels are more common among COVID-19 patients presenting with both ischaemic and haemorrhagic stroke and are associated with higher all-cause mortality.18 As there is an increased risk of thromboembolism during COVID-19, multiple studies have compared standard dose thromboprophylaxis to high and intermediate-dose prophylactic anticoagulation in hospitalized patients with COVID-19. The largest of these trials, INSPIRATION randomized clinical trial found no difference in all-cause mortality or venous thromboembolic events in patients treated with standard versus intermediate-dose thromboprophylaxis in critically ill patients with COVID-19,73 in contrast to earlier retrospective studies showing potential benefit in ICU patients.74 Interim unpublished results of the multiplatform merged randomized control trial (mpRCT) ATTACC/REMAP-CAP/ACTIV-4A found similar results in the critically ill cohort as well as a signal towards harm with therapeutic anticoagulation.75 Interestingly, this study found improved survival in moderately ill patients with COVID-19 treated with intermediate-dose anticoagulation, suggesting severity of disease may be important in determining appropriate thromboprophylaxis in hospitalized patients.76,77 Further studies are required to determine whether prophylactic anticoagulation specifically reduces the risk of stroke in COVID-19, particularly because of reports of haemorrhagic stroke in hospitalized patients while receiving therapeutic anticoagulation.78 Indeed, the risk of haemorrhagic stroke is higher than predicted among COVID-19 patients79 and is associated with elevated serum ferritin.16 Similarly, microhemorrhages50 and acute haemorrhagic necrotizing encephalitis have been reported in patients with COVID-19.61 Outcomes including risk of death and duration of hospitalization following intracerebral or subarachnoid haemorrhages are worse among patients with COVID-19.80
Recent reports of vaccine-induced thrombotic thrombocytopaenia (VITT) following administration of adenovirus vector-based COVID-19 vaccines have raised concern.81–83 As of 4 April 2021, there had been 169 cases of VITT-CVST reported to the European Medicines Agency out of 34 million doses administered of the ChAdOx1 nCoV-19 (AstraZeneca) vaccine, with an incidence of VITT estimated at 1 per 100 000 exposures.81 The reported rate of VITT-CVST after administration of 6.86 million doses of the Ad26.COV2.S adenoviral vector vaccine (Johnson & Johnson/Janssen) was 0.87 cases per million (0.000087%).84,85 Most cases occurred in females <50 years of age, and most patients displayed high levels of antibodies to platelet factor 4 (PF4)-polyanion complexes, prompting comparisons to heparin-induced thrombotic thrombocytopaenia.86 The precise pathophysiology of VITT is unknown to date; simultaneous thrombosis and thrombocytopaenia in VITT has only been reported for adenoviral vector vaccines although there have been five possible reports of CVST with normal platelet counts among 4 million doses of the Moderna mRNA vaccine.87 Among 54 million doses of the Pfizer-BioNTech mRNA vaccine, there have been 35 reports of CNS thrombosis without thrombocytopaenia (0.00006%).87 CSVT is a serious but rare condition associated with SARS-CoV-2 vaccination,88 but there remains a consensus among health authorities that the benefits of widespread vaccination outweigh the potential risks, particularly when one considers the rate of thrombosis in COVID-19 infection.
Acute PNS disorders
Autoimmune polyradiculoneuropathies such as GBS or Miller-Fisher syndrome have been reported in patients with SARS-CoV-2 infection, with and without respiratory symptoms.89 These disorders can be triggered by systemic infections and have been reported in patients with other coronavirus infections such as Middle East respiratory syndrome (MERS) and SARS-CoV-1.30 Most case reports of GBS in COVID-19 describe the common syndrome of ascending weakness, areflexia with supporting CSF and nerve conduction studies, and are of the acute inflammatory demyelinating polyneuropathy (AIDP) type. Disease onset is between 5 and 10 days after acute COVID-19 symptoms (including anosmia, respiratory and gastrointestinal symptoms), which in the ICU settings helps distinguish GBS from critical illness neuropathy that appears later in disease course.20,30,90 Patients with COVID-19-associated GBS respond to standard treatments (e.g. IVIg, PLEX) although how COVID-19 affects treatment responsiveness remains uncertain.30 Of note, a UK epidemiological cohort study showed rates of GBS have fallen during the current pandemic,91 probably resulting from increased public health efforts that have reduced transmission of more common infectious triggers.
Myalgia and weakness occur in 30–50% of hospitalized patients with COVID-19,92,93 and are frequently reported by non-hospitalized patients.94 While myalgia is a common symptom during many viral illnesses, the mechanism by which SARS-CoV-2 infection causes debilitating muscle pain and weakness is unknown. Myositis and rhabdomyolysis as a complication of COVID-19 are well recognized with elevated serum creatine kinase (>10 000) levels as a common finding which correlates with mortality in hospitalized patients.92 There have also been multiple reports of muscle oedema demonstrated on MRI.32,33 While other viruses such as influenza are known to directly invade skeletal myocytes in vitro,95 to date there is no evidence for infection of skeletal myocytes with SARS-CoV-2.29,33 Myositis in COVID-19 could be triggered by host immune responses to the virus. Muscle biopsies from COVID-19 patients show perivascular inflammation33 including a case of type 1 interferonopathy associated myopathy in a young patient with SARS-CoV-2 infection.96 There are reports of COVID-19 patients with elevated creatine kinase levels and muscle weakness who respond to immunosuppression including high dose glucocorticoids33 as well as IVIg,30 prompting comparisons to immune-mediated myositis. There is also a recent report of a patient with proximal and bulbar weakness in COVID-19 with positive anti-SSA and SAE-1 antibodies who was successfully treated with the humanized monoclonal antibody against the IL-6 receptor, tocilizumab.33 While these neurological syndromes are observed in the acute setting, they have the capacity to exert long-term effects, as described next.
Patients who develop severe COVID-19 pneumonia often require prolonged ICU care. As expected, critical illness polyneuropathy (CIP)29 and myopathy (CIM)97 have been reported as complications of SARS-CoV-2 infection. While the pathophysiological mechanisms underlying CIM and CIP are unknown, both disorders are assumed to result from microcirculatory and metabolic changes brought on by severe physiological stress.98 Based on electrophysiological and pathological studies, there is no evidence that COVID-19 associated CIP/CIM has distinctive features, and treatment to date has been supportive.29 In fact, the lasting neurological consequences of prolonged hospitalization with or without intensive care and the associated interventions for patients with COVID-19 remain unclear.
Chronic neurological sequelae
The long-term neurological impact of COVID-19 is uncertain, but it is already apparent that a range of signs and symptoms emerge among patients hospitalized with COVID-19 while non-hospitalized patients also exhibit neurological disorders that arise after the acute COVID-19 illness phase (Fig. 3). The lingering or delayed neurological syndromes have been termed long COVID or post-acute sequelae of SARS-CoV-2 (PASC)99 and are composed of a wide range of symptoms and signs including neurocognitive symptoms with associated impaired performance on neuropsychological testing.100 Of note, neurocognitive and mood alterations among ICU survivors are well recognized phenomena, often attributed to sedating medications as well as systemic inflammation and neuronal injury.34 Notably, these ICU-related effects can confound the evaluation of chronic sequelae among survivors of severe acute COVID-19. A study evaluating patients with COVID-19 at 2–3 months post-hospitalization (approximately a third of patients required ICU) reported that those patients reported significantly higher rates of depressive symptoms and decreased quality of life compared to age- and comorbidity-matched controls.35 Moreover, abnormalities in visuospatial and executive function were detected among COVID-19 survivors compared to controls when assessed by the Montreal Cognitive Assessment tool (MoCA), recapitulating clinical experience of patients with post-COVID-19 who report apathy, short-term/working memory difficulties and ‘brain fog’ after SARS-CoV-2 infection.39,101 A recent study of patients post-COVID-19 without hospitalization reported ‘brain fog’, headache, anosmia, dysgeusia and myalgia as the predominant persisting symptoms.102 Over half of hospitalized COVID-19 patients report significant fatigue months after discharge, particularly among those who required admission to the ICU.35 Similarly, persistent psychological distress is reported by half of hospitalized patients with COVID-19-related ICU admission as well as those COVID-19 patients not requiring the ICU.103 A retrospective cohort analysis of over 200 000 patients in the UK found that 12.8% of patients with COVID-19 received a new neurological or psychiatric diagnosis in the 6 months after initial infection.104 In the same study, nearly half of ICU-COVID-19 survivors had a neurological or psychiatric illness at 6-month follow-up, of which half were new diagnoses. Of note, frontotemporal FDG hypometabolism reported for acute COVID-19, discussed previously, was also observed among COVID-19 patients with cognitive symptoms >3 weeks after initial illness, accompanied by brainstem and thalamus hypometabolism in ‘long COVID’ patients, compared to controls.38 A separate study of eight patients in the subacute and chronic stages of recovery from COVID-19 observed a similar pattern of bilateral frontoparietal hypometabolism, which resolved at the 6-month follow-up assessment and was accompanied by improved MoCA scores.37 FDG-PET imaging is a potentially useful research tool although it is not validated for diagnosis of COVID-19 related neurocognitive impairments, which require clinical evaluation. Future studies of cognitive impairment in COVID-19 survivors must take into account the fact that hospitalization for any infection is associated with an increased 10-year risk of dementia, particularly vascular dementia and Alzheimer’s disease.105
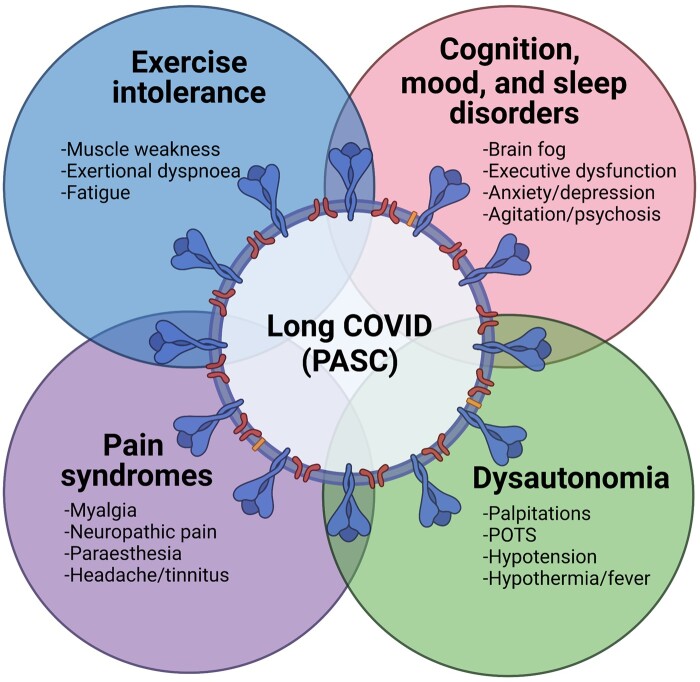
Figure 3.
Chronic neurological sequelae of COVID-19. Several long-term neurological syndromes result from SARS-CoV-2 among hospital- and community-treated patients, termed long COVID or post-acute sequelae of COVID-19 (PASC). These syndromes include neurocognitive, mood and sleep disorders, dysautonomia, diverse pain syndromes, as well as marked exercise intolerance and fatigue. These protracted syndromes remain to be fully defined in longitudinal cohort studies.
Patients with COVID-19 also develop autonomic instability that manifests as tachycardia, postural hypotension, hypertension, postural orthostatic tachycardia syndrome, low-grade fever with associated bowel, bladder or sexual dysfunctions.106,107 Cardiac MRI of COVID-19 survivors at 2–3 months after symptom onset showed evidence of fibrosis and inflammation, which was correlated with serum inflammatory markers (e.g. CRP, calcitonin),35 possibly accounting for the exercise intolerance reported by patients.
The spectrum of symptoms described in long COVID has prompted comparisons with myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). Indeed, the overlap in symptoms between post-acute COVID-19 syndromes and ME/CFS is remarkable for the shared symptomatology including fatigue, autonomic instability, post-exertional myalgia or weakness as well as neurocognitive impairments.36,102,108 Nonetheless, other viral illnesses (e.g. Dengue, West Nile disease, mononucleosis) are also associated with substantial disabilities that resemble the previous symptom complex. The precise diagnosis and management of neurological symptoms in long COVID is an emerging area of study, which is in evolution as more studies become available. Important caveats in considering persistent or delayed neurological disorders related to COVID-19 include the contribution of comorbid illnesses and their associated therapies to neurological disease as well as the potential for uncovering previously unrecognized illnesses.109
Laboratory analyses of nervous system tissues and fluids
Analyses of CSF from patients with COVID-19 vary widely depending on the associated neurological disorder although pleocytosis, especially lymphocytic, and elevated protein110 are common findings, particularly among patients with other features of encephalitis. The IgG index is increased in many patients with COVID-19 together with the presence of antiviral and antiviral receptor (e.g. ACE2) antibodies, indicative of intrathecal synthesis.20,111 In contrast, viral RNA is infrequently detected in CSF using standard RT-PCR protocols,110,112 although the timing of the CSF collection in relation onset is often not reported. Host innate immune responses were also apparent in CSF from patients with COVID-19 based on reports of neopterin and β2-microglobulin detection in CSF.113 Similarly, several chemokines and cytokines in CSF have shown to be associated with COVID-19-related neurological disease (e.g. encephalitis) including IL-8, TNF-α, IL-6 as well as neural cell type-specific markers (e.g. GFAP, neurofilament and tau).24 However, a specific diagnostic profile in CSF for COVID-19 associated neurological disease awaits definition. Antibodies associated with autoimmune encephalitis have been reported concurrently with SARS-CoV-2 infection, including anti-GD1b, -NMDA-R22,114,115 and -CASPR2.22 While these reports are intriguing, a direct link between SARS-CoV-2 infection and the development of these autoantibodies has not been established. Interestingly, there are emerging reports of non-neurological autoimmune disorders including psoriatic arthritis,116 rheumatoid arthritis117 and immune thrombocytopenic purpura118 developing after COVID-19.119 Possible explanations for this phenomenon include transient immunosuppression during acute viral illness, including suppression of regulatory T and B cells resulting in impaired self-tolerance, as has been suggested in other viral infections.120 In susceptible individuals, the process of immune reconstitution following COVID-19 may ‘unmask’ autoimmune conditions, including multiple sclerosis and neuromyelitis optica spectrum disorders.121,122 In contrast, other groups have proposed that T-cell exhaustion might contribute to autoimmune neuropathogenesis in COVID-19.123
As with CSF studies, autopsy-based neuropathological findings are diverse. Several variables need to be considered in interpreting the neuropathological findings including the presence and severity of prior or concurrent comorbidities, duration in ICU and ventilator support, concomitant therapies and the circumstances of death. Moreover, for many neuropathological reports of COVID-19, a corresponding clinical phenotype was not observed or reported. Nevertheless, reports range from the findings of absent neuropathology124 to hypoxic/ischaemia changes, acute infarction and haemorrhagic lesions with endotheliitis.51 ADEM- and ATM-like findings have been observed in select cases.60,125 Post-mortem studies of patients with ADEM-associated COVID-19 report periventricular inflammation, characterized by foamy macrophages and axonal injury.60,126 Conversely, other neuropathological studies have identified lymphocyte-predominant inflammation in the meninges, brainstem and perivascular spaces27 with significant neuronal and axonal loss.127 Meningoencephalitis, haemorrhagic posterior reversible encephalopathy syndrome, as well as diffuse leukoencephalopathy and microhaemorrhages have also been reported.51,128,129 While a number of post-mortem studies indicate there is a paucity of immune cell infiltration within the neuroaxis,47,51,130 recent studies have found marked microglial activation and CD8+ T cells in the brainstem and cerebellum.68,131 In fact, one study reported pan-encephalitis in a cohort of patients with severe pulmonary-associated COVID-19.127 Microscopy in larger studies (n = 43) have described diverse findings including astrogliosis with activated microglia and infiltrating T cells in brain parenchyma, together with ischaemic lesions in a subset of patients.68,132,133 In one post-mortem study using imaging mass cytometry, distinct neuropathological features within the brainstem and olfactory bulb of COVID-19 patients were identified, including microglial nodules, CD8+ T-cell infiltration, and increased ACE2 expression in blood vessels.131 These findings were not as pronounced in control patients who had been on ECMO but did not have COVID-19. Nevertheless, some authors have commented that collectively the neuropathological findings, especially microglia activation in COVID-19 resemble that observed in patients with hypoxia and sepsis.132,134
Mechanisms of neurological disease
Multiple putative mechanisms of disease have been proposed for COVID-19 induced nervous system disorders135 including coagulopathies as well as virus-associated host responses. Indeed, it is probable that specific pathogenic processes underlie the individual neurological presentations associated with COVID-19 in both the CNS (Fig. 1A) and the PNS (Fig. 1B). We review the different proposed mechanisms next.
Cerebrovascular disease/bioenergy failure
Microvascular injury characterized by thinning of the basal lamina of endothelial cells, fibrinogen leakage and microhaemorrhages has been described in the brainstem and olfactory bulb of deceased COVID-19 patients corresponding to visible MRI changes.27 These observations are also complemented by other neuroimaging studies in which cerebral infarction was the most common finding on conventional brain MRI.50 Most post-mortem analyses have shown signs of thrombotic microangiopathy and endothelial injury with minimal evidence of prototypic vasculitis.16 This pattern is suggestive of endotheliitis. Although there have been several case reports of CNS vasculitis associated with COVID-19, none have confirmed the diagnosis histologically.136 A cohort of patients with stroke and COVID-19 in Wuhan, China, showed elevated serum levels of IL-6,137 IL-8 and TNF-α, a finding that has been replicated in several subsequent studies.18 Both IL-8 and TNF-α promote the release of von Willebrand factor, a marker of endothelial damage that is elevated in both ICU and non-ICU patients with COVID-19,17 while IL-6 inhibits cleavage of von Willebrand factor leading to accumulation of multimers that promote platelet aggregation.16 These changes are bolstered by findings of damaged cerebral blood vessels or endotheliitis that was associated with extravasation of fibrinogen.27 These mechanisms of disease are highly plausible because of the frequency of coagulation-related events during COVID-19. Indeed, neuroimaging studies point to abnormal energy metabolism, shown by reduced FDG detection in frontal lobes of patients with acute COVID-19.138
Viral neuroinvasion
SARS-CoV-2 infects respiratory cells via engagement of the angiotensin-converting enzyme 2 (ACE2) receptor,15,139,140 with a higher binding affinity than other coronaviruses such as SARS-CoV-1. The ACE2 receptor is present on type II alveolar and respiratory epithelial cells, cardiomyocytes, neurons141 and astrocytes.140,142 This receptor is also present in pericytes and smooth muscle cells of cerebral blood vessels and is expressed in the thalamus, cerebellum and brainstem nuclei of humans.143–145 After binding to ACE2, cleavage of the spike (S) protein of SARS-CoV-2 by transmembrane serine protease 2 (TMPRSS2) facilitates cell entry.146 Alternative docking receptors including neuropilin-1 (NRP1)147 and basigin (BSG)/CD147148 are found at higher levels in the CNS. Similarly, alternative proteases including furin and cathepsin might permit viral entry in cells with low levels of TMPRSS2 expression (e.g. brain).149
Several anatomic routes of neuroinvasion by SARS-CoV-2 have been proposed. The integrity of the blood–brain barrier is compromised in multiple conditions associated with mortality in COVID-19, including hypertension, diabetes, smoking and stroke.150 Areas of increased vascular permeability or lack of blood–brain barrier, such as the pituitary and median eminence of the hypothalamus are also rich in ACE2, NRP1 and TMPRSS2, thus representing possible portals of entry into the CNS.19 SARS-CoV-2 infects nasal epithelium and perhaps olfactory bulb cells, presenting another entry portal to the CNS, as suggested for other coronaviruses.151,152 A recent post-mortem analysis of humans with COVID-19 detected SARS-CoV-2 by RT-PCR in neuroepithelium, the olfactory bulb, trigeminal ganglion and brainstem, albeit at low levels.14 Interestingly, olfactory nerves terminate in the frontal cortex as well as the hypothalamus and amygdala, structures that are implicated clinically, radiographically and electrographically in the neurological sequelae of COVID-19.19 The importance of the choroid plexus in the development of COVID-19 associated neurological disease in conjunction with neuroinflammation has been highlighted recently in a large study predicated on RNA deep sequencing of brain-derived single cell nuclei transcriptomes.153 The lack of evidence for productive infection of trafficking immune cells by SARS-CoV-2 to date makes a Trojan horse mechanism of neuroinvasion less likely. Nonetheless, viral proteins and RNA have been detected in CD68+ macrophages isolated from bronchoalveolar lavage of COVID-19 patients.154 SARS-CoV-2 RNA levels in brain tissue detected by RT-PCR are low and seemingly independent of the presence or absence of apparent neurological dysfunction and histopathological alterations.14,132 Immunodetection of SARS-Cov-2 viral antigens in neurons from autopsied patients with COVID-19 underscores the potential for direct viral invasion as an important disease determinant.155
Remdesivir, a nucleoside analogue that inhibits RNA-dependent replication of SARS-CoV-2, is the only direct antiviral agent approved for COVID-19 treatment despite preliminary results showing no impact on mortality or progression to mechanical ventilation.156 Molnupiravir is orally available nucleoside analogue that induces coronavirus lethal mutagenesis and is in phase 2 and 3 trials for treatment of COVID-19.157 A recent randomized control trial of the TMPRSS2 inhibitor, camostat mesylate, in hospitalized patients with COVID-19 did not have any impact on recovery, progression to ICU or mortality.158
Host neuroimmune responses
Post-infectious neuro-inflammation triggered by expression of viral antigens into the CNS is another proposed mechanism of encephalitis in COVID-19. While human data supporting this hypothesis are limited, a recently published study using a murine model showed a subunit of the SARS-CoV-2 spike protein (S1) crosses the BBB via absorptive transcytosis when administered intravenously and intra-nasally.159 Indeed, neuropathological studies demonstrate glia activation and occasional leucocyte infiltrates in patients with COVID-19 although the associated molecular pathways (e.g. cytokine, protease, or free radical release) induced are unclear. CSF studies suggest activation of innate immune responses with elevated levels of β2-microglobulin and neopterin and the presence of dedifferentiated monocytes.113,123 This is associated with increased levels of neurofilament suggesting neuronal injury.113 Autoimmune mechanisms including both antibody- as well as cell-mediated immune injury of neural tissue are also plausible, given the recognition of autoimmune processes in the systemic COVID-19 pathogenesis. The injury and loss of endothelial cells in arterioles, venules and capillaries represents another neuropathogenic avenue via disruption on the blood–brain barrier and through endothelia production of immune molecules160 in the lung, kidney and heart of patients with COVID-19. These latter events can be initiated by systemic immune activation as well as a coagulation diathesis. An important qualification to these mechanisms is that concurrent clinical events including systemic hypoxia-ischaemia might affect immune processes within the nervous system. Among patients with COVID-19 associated cerebrovascular disease, autoimmune processes have been directly implicated. For example, the contribution of antiphospholipid antibodies to ischaemic stroke in patients with COVID-19 is controversial. Zhang et al.161 described three COVID-19 patients with coagulopathy and multi-territory infarcts and anticardiolipin and anti-β2 microglobulin antibodies. Subsequent studies have reported lupus anticoagulant positivity in more than half of COVID-19 patients.162 Most case reports of antiphospholipid antibodies in COVID-19 do not include repeat assays 12 weeks apart, which is required for the diagnosis of antiphospholipid antibody syndrome. Transient elevation of lupus anticoagulant during systemic inflammation is common, and several infections are associated with false positive antiphospholipid assays, including HIV, hepatitis C virus and syphilis, making current reports of antiphospholipid antibodies in COVID-19 difficult to interpret.163
Similarly, autoimmunity is also incriminated in COVID-19 associated GBS; anti-ganglioside antibodies implicated in autoimmune polyradiculoneuropathies such as anti-Gq1b, -GM1164 and -GD1b antibodies have been reported in patients with COVID-19 presenting with cranial neuropathies, weakness, areflexia and sensory ataxia.22 Anti-ganglioside antibodies are most strongly associated with more aggressive axonal motor neuropathies and poorer functional outcomes compared to AIDP.165 The rare presence of these antibodies raises concern about potential molecular mimicry mediated by SARS-CoV-2 that could trigger autoimmune responses with important implications for vaccine safety. The spike (S) protein of SARS-CoV-2 is highly glycosylated; thus, the development of anti-glycan antibodies may be essential for an effective host immune response in COVID-19. In a microarray study of 800 human carbohydrate antigens, levels of anti-glycolipid antibodies associated with GBS, including GM1a, GD1a and GD1b significantly higher in COVID-19 patients compared to healthy controls. In this latter study, there was no direct correlation with antibody titre and clinical features of GBS. Anti-glycan antibodies are also observed in other viral and bacterial infections (HIV, EBV, Neisseria meningitidis31,165) as well as autoimmune diseases such as Crohn’s disease,166 and thus may merely be a marker of systemic inflammation. Of relevance, there were no reported cases of GBS in the three major COVID-19 vaccine trials.167–169
While randomized control trials demonstrate dexamethasone and tocilizumab improve respiratory outcomes in hospitalized patients, their effects on neurological disease in COVID-19 is presently supported only by case reports.67,170–172 A subset of COVID-19 associated encephalopathies are responsive to steroids and IVIg, and there is a single report of a young patient with encephalitis and SARS-CoV-2 (based on CSF lymphocytosis and T2/FLAIR hyperintensities on MRI), which resolved after treatment with IVIg and tocilizumab.173 In most cases with a positive response to immunosuppressive or modulatory therapy, SARS-CoV-2 was not detected in CSF, further supporting a para-infectious/immune-mediated basis for disease.
Future perspectives
Given the mounting impact of SARS-CoV-2 infection globally together with the increasing recognition of associated neurological disorders, it is imperative to define the types of COVID-19 related neurological syndrome, including those caused directly by viral infection versus those arising from systemic illness, the impact of different viral variants on neurological disease, as well as identifying informative diagnostic tools and effective therapies. GWAS studies have identified susceptibility genes for severe respiratory illness with COVID.174,175 Similar studies to identify host factors associated with neurological complications would also be useful. The long-term neurological sequelae of COVID-19 remain unclear and await delineation in longitudinal studies. The neurodevelopmental impacts of COVID-19 are also unknown in utero as well as in infants or adolescents; this issue could have substantial lasting effects that require further investigation. Finally, a more comprehensive understanding of the pathogenic mechanisms underpinning the neurological syndromes associated with COVID-19 will advance therapeutic options for affected patients.
Literature search strategy and selection criteria
Studies were selected from the peer-reviewed literature using NCBI and Google Scholar. We searched the databases using the following keywords: central and peripheral nervous systems, COVID-19, SARS-CoV-2, coronavirus, stroke, encephalopathy, neurocognitive impairment, hypercoagulability, encephalitis, neurologic infection, seizure and neuroinflammation. We also reviewed bibliographies of relevant articles. Non-peer-reviewed studies and single case reports were not included as references unless they were highly informative.
Acknowledgements
The authors thank Brittney Hlavay for creation of figures, Nathalie Arbour for helpful discussions, Rebecca Folkerth, New York University for providing the post-mortem tissue images and Govind Nair for providing the MR images of the brain.
Funding
No specific funding was received towards this work.
Competing interests
The authors report no competing interests.
Glossary
- COVID-19
coronavirus disease 2019
- CVST
cerebral venous sinus thrombosis
- ICU
intensive care unit
- IVIg
intravenous immunoglobulin
References
- 1. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. [DOI] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varatharaj A, Thomas N, Ellul MA, et al. ; CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Pol AN. Viral infection leading to brain dysfunction: More prevalent than appreciated? Neuron. 2009;64(1):17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: Pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balcom EF, Roda WC, Cohen EA, Li MY, Power C. HIV-1 persistence in the central nervous system: Viral and host determinants during antiretroviral therapy. Curr Opin Virol. 2019;38:54–62. [DOI] [PubMed] [Google Scholar]
- 8. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feldstein LR, Rose EB, Horwitz SM, et al. ; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin JE, Asfour A, Sewell TB, et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743:135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2020;24(2):168–175. [DOI] [PubMed] [Google Scholar]
- 15. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nampoothiri S, Sauve F, Ternier G, et al. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. bioRxiv. [Preprint] doi:10.1101/2020.06.08.139329 [Google Scholar]
- 20. Alexopoulos H, Magira E, Bitzogli K, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao A, Rohaut B, Le Guennec L, et al. ; CoCo-Neurosciences study group. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. 2020;143(12):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2020;268(3):751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pilotto A, Masciocchi S, Volonghi I, et al. ; SARS-CoV-2 related encephalopaties (ENCOVID) Study Group. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: The ENCOVID multicenter study. J Infect Dis. 2021;223(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pilotto A, Masciocchi S, Volonghi I, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: Evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021;73(9):e3019–e3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: Targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. [Preprint] doi:10.1101/2020.05.18.20099960 [Google Scholar]
- 26. Antony AR, Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2020;384(5):481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin L, Al-Faraj A, Ayub N, et al. Electroencephalographic abnormalities are common in COVID-19 and are associated with outcomes. Ann Neurol. 2021;89(5):872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol. 2020;131(12):2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Temme JS, Butler DL, Gildersleeve JC. Anti-glycan antibodies: Roles in human disease. Biochem J. 2021;478(8):1485–1509. [DOI] [PubMed] [Google Scholar]
- 32. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. Published online 23 April 2020. https://doi.org/10.1136/annrheumdis-2020-217573 [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62(3):E57–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mongioì LM, Barbagallo F, Condorelli RA, et al. Possible long-term endocrine-metabolic complications in COVID-19: Lesson from the SARS model. Endocrine. 2020;68(3):467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blazhenets G, Schroeter N, Bormann T, et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021;62(7):910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guedj E, Campion JY, Dudouet P, et al. F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pouga L. Encephalitic syndrome and anosmia in COVID-19: Do these clinical presentations really reflect SARS-CoV-2 neurotropism? A theory based on the review of 25 COVID-19 cases. J Med Virol. 2020;93(1):550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020;10(7):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anand P, Al-Faraj A, Sader E, et al. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilepsy Behav. 2020;112:107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toniolo S, Di Lorenzo F, Scarioni M, Frederiksen KS, Nobili F. Is the frontal lobe the primary target of SARS-CoV-2? J Alzheimers Dis. 2021;81(1):75–81. [DOI] [PubMed] [Google Scholar]
- 44. Pensato U, Muccioli L, Pasini E, et al. Encephalopathy in COVID-19 presenting with acute aphasia mimicking stroke. Front Neurol. 2020;11:587226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144(4):1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of COVID-19. N Engl J Med. 2020;383(10):989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heming N, Mazeraud A, Verdonk F, Bozza FA, Chrétien F, Sharshar T. Neuroanatomy of sepsis-associated encephalopathy. Crit Care. 2017;21(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gulko E, Oleksk ML, Gomes W, et al. MRI brain findings in 126 patients with COVID-19: Initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020;41(12):2199–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kantonen J, Mahzabin S, Mäyränpää MI, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30(6):1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology. 2020;95(13):e1868–e1882. [DOI] [PubMed] [Google Scholar]
- 53. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Helms J, Kremer S, Meziani F. More on neurologic features in severe SARS-CoV-2 infection. Reply. N Engl J Med. 2020;382(26):e110. [DOI] [PubMed] [Google Scholar]
- 55. Bodro M, Compta Y, Llansó L, et al. ; ‘Hospital Clínic Infecto-COVID-19’ and ‘Hospital Clínic Neuro-COVID-19’ groups. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Espíndola OM, Gomes YCP, Brandão CO, et al. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann Neurol. 2021;89(5):1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zuhorn F, Omaimen H, Ruprecht B, et al. Parainfectious encephalitis in COVID-19: ‘The Claustrum Sign’. J Neurol. 2020;268(6):2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Casez O, Willaume G, Grand S, et al. SARS-CoV-2 related encephalitis: MRI pattern of the olfactory tract involvement. Neurology. 2020;96(4):e645–e646. [DOI] [PubMed] [Google Scholar]
- 60. Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020;296(2):E119–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Silva MTT, Lima MA, Torezani G, et al. Isolated intracranial hypertension associated with COVID-19. Cephalalgia. 2020;40(13):1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verkuil LD, Liu GT, Brahma VL, Avery RA. Pseudotumor cerebri syndrome associated with MIS-C: A case report. Lancet. 2020;396(10250):532. [DOI] [PubMed] [Google Scholar]
- 64. Emamikhah M, Babadi M, Mehrabani M, et al. Opsoclonus-myoclonus syndrome, a post-infectious neurologic complication of COVID-19: Case series and review of literature. J Neurovirol. 2021;27(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Werner J, Reichen I, Huber M, Abela IA, Weller M, Jelcic I. Subacute cerebellar ataxia following respiratory symptoms of COVID-19: A case report. BMC Infect Dis. 2021;21(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following Coronavirus Disease 2019 (COVID-19). Mov Disord Clin Pract. 2020;7(8):974–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cani I, Barone V, D'Angelo R, et al. Frontal encephalopathy related to hyperinflammation in COVID-19. J Neurol. 2021;268(1):16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020;19(11):919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baldini T, Asioli GM, Romoli M, et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koralnik IJ, Tyler KL. COVID-19: A global threat to the nervous system. Ann Neurol. 2020;88(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: A retrospective population-based study. Stroke. 2016;47(9):2180–2182. [DOI] [PubMed] [Google Scholar]
- 73. Mazloomzadeh S, Khaleghparast S, Ghadrdoost B, et al. ; INSPIRATION Investigators. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tacquard C, Mansour A, Godon A, et al. ; French Working Group on Perioperative Hemostasis. Impact of high dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021;159(6):2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ATTACC, ACTIV-4a & REMAP-CAP. Multiplatform RCT: Results of Interim Analysis. 2021. Accessed 19 June 2021. https://nhlbi-connects.org/documents/mpRCT%20Interim%20Presentation.pdf
- 76. Kollias A, Kyriakoulis KG, Syrigos NK, Stergiou GS. Anticoagulation therapy in COVID-19: Is there a dose-dependent benefit? Thromb Res. 2021;199:19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moll M, Connors JM. When to use anticoagulation in COVID-19. Thromb Res. 2021;204:136–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dogra S, Jain R, Cao M, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mishra S, Choueka M, Wang Q, et al. Intracranial hemorrhage in COVID-19 patients. J Stroke Cerebrovasc Dis. 2021;30(4):105603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ravindra VM, Grandhi R, Delic A, et al. Impact of COVID-19 on the hospitalization, treatment, and outcomes of intracerebral and subarachnoid hemorrhage in the United States. PLoS One. 2021;16(4):e0248728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397(10285):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination - response from the manufacturer. N Engl J Med. 2021;384(20):1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Torjesen I. Covid-19: Risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ. 2021;373:n1005. [DOI] [PubMed] [Google Scholar]
- 89. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2020;268(7):2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2020;144(2):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Desdouits M, Munier S, Prevost MC, et al. Productive infection of human skeletal muscle cells by pandemic and seasonal influenza A(H1N1) viruses. PLoS One. 2013;8(11):e79628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Manzano GS, Woods JK, Amato AA. COVID-19-associated myopathy caused by Type I interferonopathy. N Engl J Med. 2020;383(24):2389–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bagnato S, Boccagni C, Marino G, Prestandrea C, D'Agostino T, Rubino F. Critical illness myopathy after COVID-19. Int J Infect Dis. 2020;99:276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhou C, Wu L, Ni F, Ji W, Wu J, Zhang H. Critical illness polyneuropathy and myopathy: A systematic review. Neural Regen Res. 2014;9(1):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. Apr 2021;27(4):601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Woo MS, Malsy J, Pöttgen J, et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2(2):fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nath A. Long-Haul COVID. Neurology. 2020;95(13):559–560. [DOI] [PubMed] [Google Scholar]
- 102. Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 ‘long haulers’. Ann Clin Transl Neurol. 2021;8(5):1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. [DOI] [PubMed] [Google Scholar]
- 104. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sipilä PN, Heikkilä N, Lindbohm JV, et al. Hospital-treated infectious diseases and the risk of dementia: A large, multicohort, observational study with a replication cohort. Lancet Infect Dis. 2021;21(11):1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 dysautonomia. Front Neurol. 2021;12:624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin Auton Res. 2021;31(3):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lo YL, Leong HN, Hsu LY, et al. Autonomic dysfunction in recovered severe acute respiratory syndrome patients. Can J Neurol Sci. 2005;32(2):264. [PubMed] [Google Scholar]
- 109. Brundin P, Nath A, Beckham JD. Is COVID-19 a perfect storm for Parkinson's disease? Trends Neurosci. 2020;43(12):931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lersy F, Benotmane I, Helms J, et al. Cerebrospinal fluid features in COVID-19 patients with neurologic manifestations: Correlation with brain MRI findings in 58 patients. J Infect Dis. 2020;223(4):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Achar A, Ghosh C. COVID-19-associated neurological disorders: The potential route of CNS invasion and blood-brain relevance. Cells. 2020;9(11):2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Destras G, Bal A, Escuret V, et al. ; COVID-Diagnosis HCL Study Group. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1(4):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Edén A, Kanberg N, Gostner J, et al. CSF biomarkers in patients with COVID-19 and neurologic symptoms: A case series. Neurology. 2021;96(2):e294–e300. [DOI] [PubMed] [Google Scholar]
- 114. Álvarez Bravo G, Ramió I Torrentà L. Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurologia. 2020;35(9):699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Monti G, Giovannini G, Marudi A, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Novelli L, Motta F, Ceribelli A, et al. A case of psoriatic arthritis triggered by SARS-CoV-2 infection. Rheumatology (Oxford). 2021;60(1):e21–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Perrot L, Hemon M, Busnel JM, et al. First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021;3(1):e6–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with COVID-19. N Engl J Med. 2020;382(18):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ehrenfeld M, Tincani A, Andreoli L, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). 2020;162(7):1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Heming M, Li X, Räuber S, et al. Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(1):164–175.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schaller T, Hirschbühl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Roman GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM): Clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol. 2021;12:653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, et al. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol. 2020;267(11):3157–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Franceschi AM, Ahmed O, Giliberto L, Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(7):1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jensen MP, Le Quesne J, Officer-Jones L, et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2020;47(1):17–25. [DOI] [PubMed] [Google Scholar]
- 131. Schwabenland M, Salié H, Tanevski J, et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54(7):1594–1610.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144(9):2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140(4):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat Rev Neurol. 2021;17(2):65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hanafi R, Roger PA, Perin B, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41(8):1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Qin C, Zhou L, Hu Z, et al. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke. 2020;51(7):2219–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Delorme C, Paccoud O, Kas A, et al. ; CoCo-Neurosciences study group and COVID SMIT PSL study group. COVID-19-related encephalopathy: A case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. 2020;27(12):2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. [DOI] [PubMed] [Google Scholar]
- 140. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu J, Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol Neurobiol. Published online 4 July 2020. doi: 10.1007/s10571-020-00915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen R, Wang K, Yu J, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brains. Front Neurol. 2021;11:573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Allen AM, Chai SY, Clevers J, McKinley MJ, Paxinos G, Mendelsohn FA. Localization and characterization of angiotensin II receptor binding and angiotensin converting enzyme in the human medulla oblongata. J Comp Neurol. 1988;269(2):249–264. [DOI] [PubMed] [Google Scholar]
- 144. Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26(2):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Allen AM, O’Callaghan EL, Mendelsohn FAO, Chai SY. Neuronal angiotensin. Encyclopedia Neurosci. 2009;697–702. [Google Scholar]
- 146. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang K, Chen W, Zhang Z, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pan H, Peto R, Henao-Restrepo AM, et al. ; WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gordon CJ, Tchesnokov EP, Schinazi RF, Gotte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297(1):100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gunst JD, Staerke NB, Pahus MH, et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with COVID-19-a double-blind randomized controlled trial. EClinicalMedicine. 2021;35:100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rhea EM, Logsdon AF, Hansen KM, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2020;24(3):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhang Y, Cao W, Jiang W, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med. 2020;383(3):288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cervera R, Asherson RA. Antiphospholipid syndrome associated with infections: Clinical and microbiological characteristics. Immunobiology. 2005;210(10):735–741. [DOI] [PubMed] [Google Scholar]
- 164. Dufour C, Co TK, Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre Syndrome - A case report, systemic review and implication for vaccine development. Brain Behav Immun Health. 2021;12:100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cutillo G, Saariaho AH, Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol. 2020;17(4):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kaul A, Hutfless S, Liu L, Bayless TM, Marohn MR, Li X. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: A systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(10):1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Muccioli L, Pensato U, Cani I, et al. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. 2020;349:577400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. RECOVERY Collaborative Group, Horby P, Lim WS. et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Freire-Álvarez E, Guillén L, Lambert K, et al. COVID-19-associated encephalitis successfully treated with combination therapy. Clin Infect Pract. 2020;7:100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pairo-Castineira E, Clohisey S, Klaric L, et al. Gen-COVID Investigators. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591(7848):92–98. [DOI] [PubMed] [Google Scholar]