Significance
Megafauna strongly influence vegetation structure, and population declines can alter ecosystem functioning. Overhunting of grazing megafauna is argued to have driven the collapse of widespread, northern steppe-tundra and its replacement by woody vegetation at the end of the ice age. However, in Alaska and Yukon, mammoth and horse became extinct around the time that steppe-tundra was replaced by shrub tundra, leaving it unclear whether this vegetation change caused, or was caused by, reduced megafauna populations. Comparison of accurately dated pollen records with a radiocarbon-dated bone chronology shows that shrubs began expanding before grazer populations declined. This indicates that climate was the primary control of steppe-tundra persistence and that climate-driven vegetation change may pose threats to faunal diversity in the future.
Keywords: megafauna, eastern Beringia, keystone species, palaeoecology, steppe-tundra
Abstract
The collapse of the steppe-tundra biome (mammoth steppe) at the end of the Pleistocene is used as an important example of top-down ecosystem cascades, where human hunting of keystone species led to profound changes in vegetation across high latitudes in the Northern Hemisphere. Alternatively, it is argued that this biome transformation occurred through a bottom-up process, where climate-driven expansion of shrub tundra (Betula, Salix spp.) replaced the steppe-tundra vegetation that grazing megafauna taxa relied on. In eastern Beringia, these differing hypotheses remain largely untested, in part because the precise timing and spatial pattern of Late Pleistocene shrub expansion remains poorly resolved. This uncertainty is caused by chronological ambiguity in many lake sediment records, which typically rely on radiocarbon (14C) dates from bulk sediment or aquatic macrofossils—materials that are known to overestimate the age of sediment layers. Here, we reexamine Late Pleistocene pollen records for which 14C dating of terrestrial macrofossils is available and augment these data with 14C dates from arctic ground-squirrel middens and plant macrofossils. Comparing these paleovegetation data with a database of published 14C dates from megafauna remains, we find the postglacial expansion of shrub tundra preceded the regional extinctions of horse (Equus spp.) and mammoth (Mammuthus primigenius) and began during a period when the frequency of 14C dates indicates large grazers were abundant. These results are not consistent with a model of top-down ecosystem cascades and support the hypothesis that climate-driven habitat loss preceded and contributed to turnover in mammal communities.
In northern high latitudes, the widespread extinction of Quaternary megafauna (animals weighing >44 kg) and disappearance of the steppe-tundra biome they inhabited is used as an important example of top-down ecosystem cascades, where human hunting of keystone species led to profound changes in vegetation structure at the end of the Pleistocene (15 thousand years before 1950 [15 ka] to 11.7 ka) (1–5). This hypothesis, however, is not well tested, and it is unclear whether the relative timing of megafauna extinctions and vegetation change is consistent with a top-down model. Resolving this question is an important part of understanding how past ecosystems functioned and may help predict how modern high-latitude ecosystems will respond to climate-driven vegetation change, current declines in large mammal species, or their deliberate reintroduction.
In eastern Beringia (modern-day Alaska and the Yukon interior) (Fig. 1), Late Pleistocene megafauna extinctions broadly coincided with an expansion of shrub tundra vegetation including dwarf and tall-shrub species of birch (Betula nana and Betula glandulosa) and willow (Salix spp.) (6). Prior to these events, herds of grazing megafauna occupied a biome termed the mammoth steppe (7–9) or steppe-tundra (10, 11), which has no widespread modern analog. This novel, dry environment supported diverse plant communities, dominated by grasses, sedges, Artemisia spp., and a range of other forbs (8, 12–16). Sometime between 16 ka and 13 ka, woody shrub species began to expand across eastern Beringia, coupled with the development of peatlands and organic soil horizons (14, 17, 18). Lake sediment records show that the expansion of shrubs was rapid in many cases, and, although pollen influx data suggest herbaceous plant taxa continued to form an important part of the vegetation community, the abundance of Betula and Salix pollen (often >50% of the pollen sum) indicates that eastern Beringia became increasingly dominated by woody vegetation during this period (19–22).
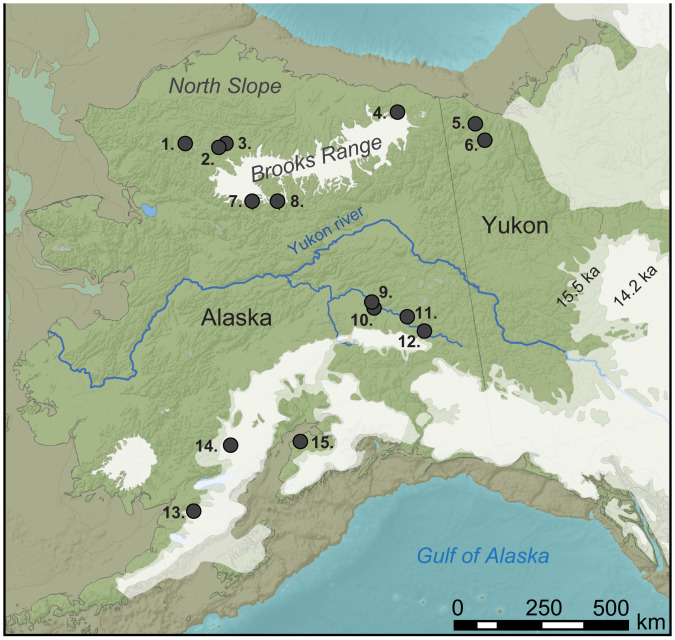
Fig. 1.
Eastern Beringia during the Late Pleistocene. Ice limits (14.2 and 15.5 ka) are redrawn from Dalton et al. (80). Lake sediment records reanalyzed in this study are numbered and include the following: 1, Burial Lake (81); 2, Tukuto Lake (82); 3, Lake of the Pleistocene (18); 4, Okpilak Lake (44); 5, Trout Lake (55); 6, Hanging Lake (54); 7, Ruppert Lake (83); 8, Xindi Lake (84); 9, Harding Lake (45); 10, Birch Lake (19); 11, Lost Lake (21); 12, Jan Lake (47); 13, Idavain Lake (20); 14, Beaver Lake (46); and 15, Discovery Pond (85).
During the same time period, mammal communities in eastern Beringia underwent some of the most profound changes to occur in the region since at least the end of the last interglacial (Marine Isotope Stage 5e), 115 ka. Of the 13 megafauna taxa present in eastern Beringia immediately prior to 15 ka, only seven survived in situ beyond the Pleistocene (steppe bison, Bison priscus; caribou, Rangifer tarandus; wapiti, Cervus canadensis; muskox, Ovibos moschatus; wolf, Canis lupus; grizzly bear, Ursus arctos; and sheep, Ovis). The remaining taxa (caballine/stout-legged horses, Equus; stilt-legged horses, Haringtonhippus; woolly mammoth, Mammuthus primigenius; saiga antelope, Saiga tatarica; lion, Panthera spelaea; and short-faced bear, Arctodus simus), along with smaller mammals such as the arctic ground squirrel (Urocitellus parryii), became regionally extinct throughout large areas between 15.0 ka and 11.7 ka, leaving behind a comparatively impoverished mammal community (6, 23). The arrival of moose (Alces alces), an obligate browser, in eastern Beringia shortly after 15 ka (24) marks the beginning of a shift from the grazer community of the steppe-tundra toward a community of mixed-feeding megafauna species better adapted to a shrub tundra environment.
The broad chronological overlap between the timing of shrub expansion and turnover in mammal populations has led numerous authors to hypothesize that habitat loss was a key driver of Late Pleistocene extinctions in eastern Beringia (8, 25–27). These authors argue that the Betula- and Salix-dominated shrub tundra was inhospitable to grazing megafauna because low-growing shrubs develop strong antiherbivory compounds, making them inedible or toxic to many mammals that lack a rumen to aid digestion (28). Other researchers have suggested that the decline in populations of grazing megafauna preceded shrub expansion, and that the spread of shrub tundra was caused by the resulting reduction in browsing pressure, vegetation trampling, and snow clearance (2, 29). These studies argue that grazers, and particularly megaherbivores (mammals of >1,000 kg), such as mammoth, acted as keystone species and were essential to the continuation of the steppe-tundra (1). In this case, human-caused “overkill” (30) or the compounded impacts of humans (e.g., burning, hunting, or simply their presence) in a dynamic ecosystem are advanced as the causes of megafauna extinctions. Finally, it is also possible that both of these processes reinforced one another, and the disappearance of the steppe-tundra was caused by both bottom-up and top-down pressures, or even that there was no causal relationship between the megafauna declines and shrub expansion. All of these hypotheses remain largely untested and continue to be controversial, in part because human arrival patterns, hunting preferences, and population size are largely unknown (31).
In eastern Beringia, it is difficult to distinguish between these alternative hypotheses because the regional timing and spatial pattern of Late Pleistocene shrub expansion is poorly resolved, despite more than 50 y of detailed paleoecological study (14, 32–35). This uncertainty is principally due to the difficulty in accurately dating lake sediments from high latitudes (36–38). Terrestrial plant macrofossil remains are often rare in these depositional environments, and many pioneering paleoenvironmental studies are founded on chronologies based on radiocarbon (14C) dates derived from bulk sediment or aquatic macrofossils. This is particularly common for lake records obtained before the routine availability of accelerator mass spectrometer radiocarbon (AMS 14C) dating, when larger samples were required. Radiocarbon dates from bulk sediment or aquatic macrofossils are often imprecise or contaminated by old carbon (SI Appendix, Text), and, as a result, chronologies developed in this way are unreliable.
To assess the chronology of shrub expansion and megafauna community turnover in eastern Beringia, we reanalyzed 15 lake sediment records for which AMS 14C dating of terrestrial macrofossils is available (SI Appendix, Figs. S1 and S6–S9). We developed Bayesian age–depth models for each study site, and compared the results with a new database of published 14C dates from plant macrofossils, megafauna remains, and arctic ground squirrel middens (Materials and Methods and SI Appendix, Text). In each pollen record, we define the beginning of shrub tundra expansion as the first sustained increase (replicated in three or more consecutive pollen samples) in Betula pollen above pre-15-ka background values, which are typically <5% of the pollen sum (SI Appendix, Fig. S2 and Text). In most cases, this expansion represents an increase to >20% of the pollen sum, and, where they are available, we use pollen influx data to support this timing (SI Appendix, Fig. S3). In some records, Salix pollen increases in abundance before Betula by as much as 1,200 y, and, in these cases, we consider the taxa separately (Fig. 2 and SI Appendix, Figs. S2 and S3). We define the timing of Salix expansion as the first sustained increase in Salix pollen above background values (see above). In most cases, this expansion represents an increase to >15% of the pollen sum. This approach is conservative. It provides minimum ages for the beginning of shrub expansion, as the true increase in shrub pollen above these thresholds is likely to lie between sampling points (i.e., would have an older assigned age). In records with high-resolution sampling [e.g., Birch Lake (19)], this difference is small; however, in most records, sampling resolution is ≤1 pollen spectrum every 10 cm, which may represent >500 y of sediment accumulation (SI Appendix, Table S1). With this approach, we aim to establish whether shrub expansion began prior to turnover in megafauna communities, as predicted by Guthrie (6, 8), or after populations of keystone species collapsed, as suggested by Zimov et al. (2, 29). As these hypotheses predict events in the opposite order, it allows us to assess whether the Late Pleistocene extinction of grazing megafauna species was a response to, or the cause of, steppe-tundra decline.
Fig. 2.
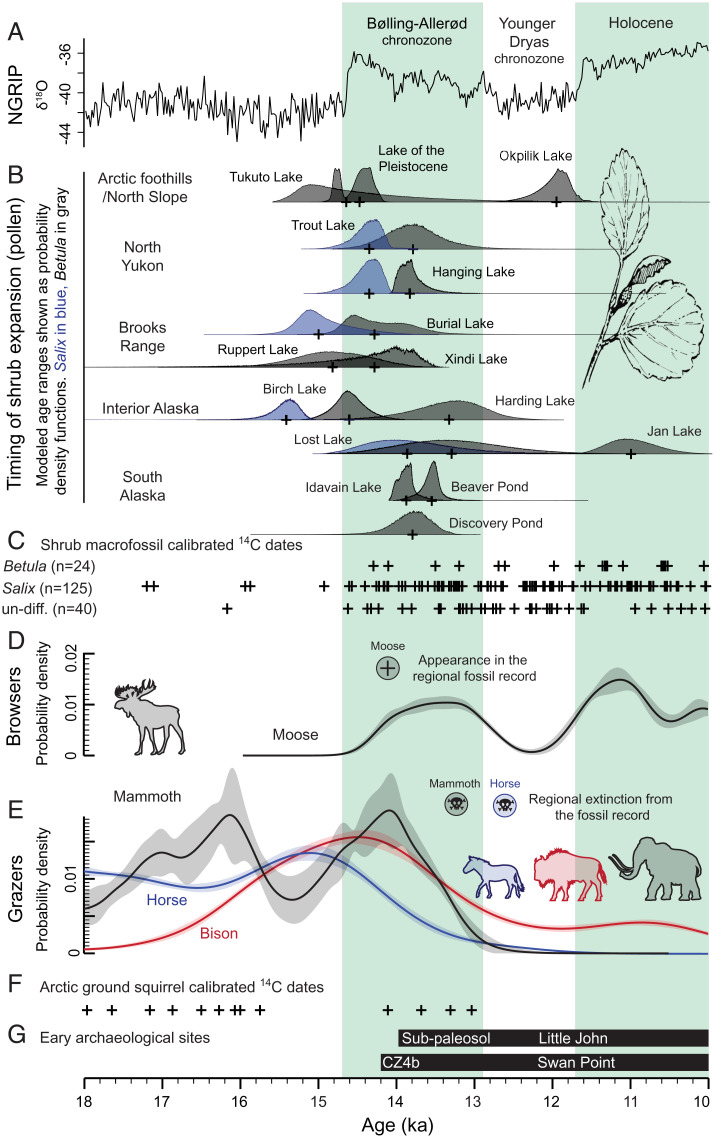
(A) North Greenland ice core project (NGRIP) δ18O record (86). (B) Modeled, calibrated age ranges (shown as probability density functions) for the beginning of Salix (shown in blue when clearly defined) and Betula (shown in gray) expansion from lake sediment records reanalyzed in this study. The medians of calibrated modeled dates are indicated by black crosses. (C) The median of calibrated 14C age ranges from shrub macrofossils in eastern Beringia. (D) Kernel Density Estimation modeled distributions (mean and 1σ uncertainty) for calibrated 14C dates from moose in eastern Beringia (sum probability distributions shown in SI Appendix, Fig. S3). (E) Kernel Density Estimation modeled distributions (mean and 1σ uncertainty) for calibrated 14C dates from horse, bison, and mammoth in eastern Beringia (sum probability distributions shown in SI Appendix, Fig. S3). (F) The median of calibrated 14C age ranges from arctic ground squirrel middens in eastern Beringia. (G) Periods of human occupation at archaeological sites in the Tanana River Valley, Alaska (70).
Study Area—Eastern Beringia
During the Late Pleistocene, eastern Beringia remained largely unglaciated (Fig. 1) (39); however, it was biologically isolated from the rest of North America by the coalesced Laurentide and Cordilleran ice sheets until 13.5 ka to 13.2 ka (40). Lowered eustatic sea levels during this cold stage exposed the shallow continental shelf between northwest North America and northeast Asia, forming the Bering Land Bridge. Due to the expanded landmass and the orographic effects of the cordillera (Alaska Range), eastern Beringia was hypercontinental and arid during Marine Isotope Stage 2 (29 ka to 14 ka), as evidenced by widespread eolian deposits (41) and the scarcity of lake sediment records extending beyond 15 ka (8, 32). The expanded land mass also meant that eastern Beringia was biologically connected with Asia—a connection that served as the intercontinental land bridge allowing old-world terrestrial mammals, including humans, to colonize North America (8, 34). Megafauna that exploited this migration route during the Late Pleistocene include bison, which reached North America during Marine Isotope Stage 6 (195 ka to 135 ka) (42), and moose, which arrived from Asia shortly after 15 ka (24).
Results and Discussion
The Chronology of Shrub Expansion in Eastern Beringia.
Several lines of evidence indicate that, in some areas of eastern Beringia, Salix spp. expanded and were widespread on the landscape before Betula spp. In six of the reanalyzed pollen records, Salix is abundant from the lowermost sample (SI Appendix, Fig. S2). In a further five records, Salix pollen clearly increases prior to Betula by as much as 1,200 y (Fig. 2). In two other records, Salix appears to increase ahead of Betula, but the detail of these early rises becomes obscured by large increases in Betula abundance (SI Appendix, Fig. S2). Salix spp. produce substantially less pollen than Betula, yet the Late Pleistocene macrofossil record is dominated by Salix remains, which occur prior to the earliest 14C-dated Betula macrofossils (Fig. 2).* It is therefore likely that Salix formed a greater component of the shrub tundra vegetation than is evident from palynology. Salix spp. can tolerate cooler and drier conditions than Betula (43) and may have responded to postglacial warming before other shrub species. This paleoecological evidence for early expansion of Salix suggests that the Late Pleistocene vegetation transition took place in several waves, each of which would have impacted megafauna differently.
The expansion of Betula, as defined by pollen values, began prior to, or soon after, 14 ka in 10 of the 15 reanalyzed AMS 14C–dated lake sediment records (Fig. 2 and SI Appendix, Fig. S2). The remaining five pollen records, which place the onset of Betula expansion after this date, lie at high elevation where shrubs are likely to have expanded later (e.g., Okpilak Lake; 720 m) (44), have limited dating control through this period (e.g., Lost Lake) (21), or are affected by lower sampling resolution (e.g., Harding Lake and Beaver Lake) (45, 46). This infrequent sampling results in younger age estimates, as the true Betula increase lies between sampling points. An exception is Jan Lake (47) where Betula pollen does not become abundant until ca. 11 ka. While this site meets our dating criteria, it has atypical lithostratigraphy and biostratigraphy, as well as substantial age reversals in the lowermost meter of sediment; therefore, it may provide a less reliable record than the other pollen records in our database. The ca. 14-ka timing of shrub expansion in eastern Beringia is consistent with the earliest 14C-dated remains of moose (an obligate browser, and indicator of an abundance of tall shrubs on the landscape) from interior Alaska and the North Slope (6, 23, 24), and an abrupt increase in the frequency of 14C dates from shrub macrofossils (Fig. 2). These multiproxy paleoecological data place the regional onset of shrub expansion during the first half of the Bølling–Allerød Interstadial (14.6 ka to 12.9 ka) (hereafter referred to as the Bølling–Allerød chronozone).
We find no evidence for a spatial pattern of expansion in either Betula or Salix, suggesting that woody taxa expanded from glacial refugia where local conditions provided adequate snow cover for winter insulation and summer moisture (14, 35). However, the lack of a spatial pattern may be an artifact of limited site availability—particularly in eastern Yukon (Fig. 1). The available evidence implies a gradual fragmentation of steppe-tundra habitat across eastern Beringia as isolated shrub communities expanded and coalesced. A coexistence of steppe-tundra with expanding shrub tundra is supported by the youngest arctic ground squirrel nests, which are indicators of steppe-tundra vegetation and deep active layers (13, 15). These date to almost 1,000 y after the beginning of postglacial shrub expansion (Fig. 2) and demonstrate that steppe-tundra persisted in favorable areas (e.g., well-drained soils) for some time after the postglacial warming began.
Evidence for Bottom-Up, Climate-Driven Controls of Steppe-Tundra Persistence.
The expansion of shrub tundra began during a period when the frequency of 14C-dated remains from grazers is high, and >700 y before mammoth (ca. 750 y) and horse (ca. 1,350 y) disappear from the fossil record (Fig. 2 and SI Appendix, Fig. S4). This demonstrates that a reduction of grazing megafauna populations was not a prerequisite for postglacial shrub expansion in eastern Beringia. Instead, the timing of shrub expansion corresponds with both Arctic-wide and regional warming and/or wetting trends that occurred during the Bølling–Allerød chronozone. These findings indicate that the role of keystone herbivores was secondary to the role of climate in preserving the steppe-tundra ecosystem and are inconsistent with the hypothesis that a decline in ecosystem-maintaining herbivore populations preceded the expansion of shrub tundra and drove ecosystem cascades (2, 29).
While our results do not support a top-down model of ecosystem cascades at the end of the Pleistocene, there is ample evidence for bottom-up controls on megafauna population dynamics that support the hypothesis that the demise of the steppe-tundra ecosystem was a climate driven-process:
-
1)
The pattern of megafauna community turnover suggests Late Pleistocene mammals were responding to vegetation change, not driving it. Populations of preferential grazers (horse and mammoth) decline and disappear from the fossil record independent of more mixed feeders, several of which occupy Alaska and the Yukon today (wapiti, musk ox, caribou, and reintroduced bison). For example, the number of 14C dates from horse species declines early in the Bølling–Allerød chronozone (Fig. 2), and shortening metacarpal lengths (48) suggest that these taxa underwent a reduction in body size during the same period. Modern horses are mostly obligate grazers, and, although woody plant macrofossils have been recovered from the tooth pits of fossil Alaska horses, the diet of these animals is suggested to have been heavily reliant on the graminoid and forb vegetation of the steppe-tundra (8). Conversely, the number of 14C dates from steppe bison remains high until ca. 13.6 ka, and the species persisted into the Holocene along with other mixed feeders (Fig. 2 and SI Appendix, Fig. S2). Evidence from tooth-wear analysis and observations of extant wood bison (Bison bison athabascae) suggest that the steppe bison had a broader herbivorous diet that included seasonal browsing—allowing the species to better survive the expansion of woody shrub taxa (49, 50).
-
2)
The expansion of Salix shrubs coincides with increases in the frequency of 14C-dated remains from steppe bison and wapiti (Fig. 2 and SI Appendix, Fig. S4). Instead of suppressing shrub expansion, as predicted in a top-down model, these taxa appear to have benefited from this vegetation change and may have browsed on nutrient-rich, spring willow leaves (6).
-
3)
Prior to the Pleistocene–Holocene transition, steppe-tundra persisted in eastern Beringia throughout periods when grazing megafauna populations fluctuated (26). For example, evidence from molecular sequences and the fossil record suggest that the number of steppe bison declined during the Last Glacial Maximum (22 ka to 12 ka) (6, 23, 51), and this taxon is rare in our database prior to ca. 15.8 ka (Fig. 2 and SI Appendix, Fig. S4).
-
4)
In other areas of Beringia, vegetation does not appear to have been affected by the presence or absence of megafauna. For example, pollen from Lake El’gygytgyn, northeast Siberia, demonstrates that shrub tundra expanded during previous interglacial periods when megafauna were unaffected by human hunting pressure (52).
Although our results do not support top-down cascades as the initial trigger for shrub expansion, it is possible that the expansion of shrub tundra caused a positive feedback in which an increase in unpalatable, woody shrubs fragmented suitable habitat for grazing megafauna, reducing populations and thus the effects of trampling and snow clearance, promoting further shrub expansion.
If the loss of keystone species was not the primary driver of steppe-tundra biome collapse, then why did it disappear? The magnitude and duration of Late Pleistocene climate change is likely to have driven the expansion of shrub tundra and demise of the steppe-tundra (53). Evidence from chironomid assemblages (54–56), isotopic bone measurements (δ15N) (57), paleolake levels (58, 59), and the onset of aquatic sedimentation in many basins that were dry prior to 15 ka (34) demonstrate that eastern Beringia became both warmer and wetter 16,000 y to 13,000 y ago. These changes are likely to be linked with rising sea levels and a northward shift in storm tracks that increased the frequency and intensity of maritime air masses penetrating the region (11, 60). The result was a weakening of continental aridity and less frequent eolian disturbances (57), which had previously raised summer soil temperatures and maintained nutrient levels in the steppe-tundra ecosystem (8, 25). Guthrie (6) and Mann et al. (26) argued that increased warmth and moisture would allow for an initial expansion of megafauna populations as vegetation became more productive. This trend would have been reversed as woody vegetation began to outcompete graminoid communities and paludification took hold across the landscape (“death by peat” hypothesis) (26). The patterns in the frequency of 14C-dated remains from grazers, and particularly mammoth and bison, appear to support this hypothesis; however, the increase in the number of 14C dates is subtle, and we are hesitant to overinterpret our results, because of potential for bias or gaps in the fossil record.
The Role of Keystone Herbivores in Maintaining the Steppe-Tundra Biome.
There is clear evidence that the extirpation and/or reintroduction of megaherbivores can exert strong, top-down influences on ecosystems (5, 51, 61–63), yet these effects appear to have been insufficient to suppress the expansion of shrub tundra during the Late Pleistocene. Therefore, it is pertinent to ask what role megaherbivores played in maintaining the steppe-tundra, and why the presence of these taxa did not prevent shrub expansion.
Late Pleistocene megafauna may have exerted top-down influences through trampling and nutrient loading (via excretion); however, their dietary preferences meant that shrub vegetation was unlikely to be strongly affected by browsing pressure. Guthrie (8) notes that mammoth, horse species and steppe bison were all predominantly grazers, whose diets would have “favored shrub vegetation and woodlands, not the expansion of grasslands,” by consuming the grasses and forbs in competition with shrubs. Tooth-wear patterns (49), isotopic analyses (64), and plant macrofossil remains retrieved from tooth pits, dung samples, and frozen mummies (8, 65) all support this inferred dietary preference. In studies focused on extant grazing communities most similar to the megafauna of the steppe-tundra, large mammals have been shown to promote forb biodiversity through the selective grazing of grasses (61, 66). If this relationship was replicated in the Late Pleistocene ecosystem, then large grazers may well have had a keystone role promoting herbaceous diversity within the vegetation mosaic, yet have impacted shrub vegetation to a lesser degree than their closest extant relatives.
If mammoth, steppe bison, and horse species did exert strong top-down influences, population density is left as the remaining explanation as to why the presence of megaherbivores was insufficient to prevent climate-driven biome collapse. Quantitative estimations of Late Pleistocene megafauna populations in eastern Beringia are uncertain and range between 590 and 8,800 kg/km2 (23, 67–69). Although higher than the modern megaherbivore biomass in the region (e.g., North Slope, Alaska: 264 kg/km2) (23), these estimates are significantly lower than the megafauna biomass of the sub-Saharan African plains (4,270 kg/km2 to 23,500 kg/km2) (68), or in enclosure experiments, where the effects of ecosystem-maintaining herbivores are clearly observed (5, 62). Therefore, the weak top-down control in eastern Beringia may have been because of insufficient population densities. In contrast, Zimov et al. (29) report considerably higher population estimates from Duvanny Yar, Siberia, underlining that more precise biomass estimates are needed to robustly test this hypothesis.
Implications for Late Pleistocene Extinctions in Eastern Beringia.
The timing of shrub expansion has a bearing on Late Pleistocene extinctions in eastern Beringia, which have been attributed to both human hunting and climate change (2, 6, 29, 53). Our results do not refute overkill hypotheses; however, the expansion of shrub tundra ca. 14 ka suggests that regional extinctions of mammoth and horse took place during a period of climate-driven vegetation change, to which these taxa were poorly adapted. It is hard to imagine that these vegetation changes would not have stressed grazing megafauna populations, although some plasticity in the diet of horses has been inferred from δ15N values in 14C-dated bones (23). The first appearance of archaeological evidence in the Tanana Valley, Alaska, closely precedes declines in the abundance of 14C dates from both mammoth and bison (Fig. 2), and it is possible that humans had a compounding influence on population decline. However, archaeological evidence demonstrates that humans and grazing megafauna coexisted in eastern Beringia for at least 700 y (70), and possibly for several millennia (71, 72). Further, while grazers declined as shrubs expanded, browsers increased in abundance with seemingly little influence by human hunting, despite the presence of their remains in archaeological sites (70) (Fig. 2 and SI Appendix, Fig. S4). Therefore, any human influence on megafauna populations was likely gradual and taxa specific.
Implications for Present-Day Arctic “Greening.”
Our findings suggest that top-down controls were insufficient to prevent Late Pleistocene shrub expansion when megafauna biomass was likely to be higher than today (23). This finding indicates that extant mammals will have a limited effect on the current, climate-driven “greening” of the Arctic associated with shrub expansion (73), and that the reintroduction of megafauna, at densities similar to those that occupied eastern Beringia during the Late Pleistocene (assuming it were possible) would not restore the steppe-tundra biome, or prevent widespread permafrost degradation (74). Replicated pollen records show that Late Pleistocene shrub expansion occurred in several waves as, first, Salix and, then, Betula responded to rising temperature and/or precipitation (Fig. 2 and SI Appendix, Fig. S2). This pattern suggests shrub taxa that are currently dominant or expanding at high latitudes may be succeeded by more thermophilous species if the climate continues to warm. Finally, the close link between shrub expansion and loss of mammal biodiversity at the end of the Pleistocene indicates that current Arctic greening will have strong impacts on faunal community structures as ranges expand or contract (75).
Conclusions
The reanalysis of robustly dated pollen records and exclusion of datasets that present chronologies based on ambiguous bulk sediment or aquatic 14C dates shows that shrub tundra began to replace steppe-tundra in eastern Beringia around 14 ka—consistent with both the plant macrofossil record and the first appearance of moose. The fossil record suggests that keystone megaherbivore species (steppe bison and mammoth) were abundant at this time, and therefore the expansion of woody shrub vegetation was not driven by megafauna population decline and top-down ecological cascades but by a rapid, sustained shift to warmer and wetter conditions. In many areas, Salix spp. expanded and were widespread on the landscape before Betula, which is likely to represent the greater tolerance of some Salix species to low temperatures and aridity. These findings suggest that extant mammals will have a limited effect on current shrub expansion in the Arctic and that the reintroduction of megafauna, at densities similar to those which occupied eastern Beringia during the Late Pleistocene, will not restore the steppe-tundra biome.
Materials and Methods
This section provides an overview of the methods used in this study. Full details can be found in SI Appendix, Text.
Age–Depth Modeling.
We used the Bayesian statistical program OxCal version 4.4 (76) to establish age–depth models for Late Pleistocene pollen records where AMS 14C dating is available (SI Appendix, Fig. S1). This involved developing a P_Sequence depositional model for each record using the IntCal20 Northern Hemisphere calibration curve (77) and a variable K parameter (increments per unit length). Boundaries were placed at sharp transitions in the sediment stratigraphy where changes in accumulation rate are likely to have occurred. A general outlier model was applied with a 5% prior probability of any individual radiocarbon date being a statistical outlier (78). Where the agreement index for any individual 14C date fell below 60% (typically caused by an age reversal), we considered this date for manual rejection (78). Well-dated tephra isochrons (e.g., Aniakchak CFE II) were included in the age–depth models where the tephra deposit was robustly identified by the major–minor element composition of the volcanic glass. A description of each age–depth model is reported in SI Appendix, Text.
Kernel Density Estimations.
Temporal patterns in the frequency of 14C dates from megafauna remains were investigated using Kernel Density Estimations (KDE_Model) in OxCal version 4.4 (79). This method averages Markov Chain Monte Carlo simulations from the distribution of the underlying, calibrated 14C probability density functions. The approach smooths noise associated with peaks in sum probability and allows trends in the dataset to be more clearly identified. In order to test the impacts of calibration, which will cause 14C dates to correspond with near-identical calendar ages at steep parts of the curve and to become widely spaced, or even reversed, along plateaus, we analyzed a synthetic database of evenly spaced 14C dates (every 100 y) using the same methods (SI Appendix, Fig. S5).
The database of 14C-dated bone samples includes material collected opportunistically over the course of a century from exposures and river bluffs, with little stratigraphic context. The variety and number of different collection sites suggests that high densities of 14C-dated bones indicate that mammal taxa were abundant on the landscape. The temporal distribution of these 14C dates may not be completely random, however, as bones are typically buried in floodplain deposits during periods of aggradation, which is a climate-controlled geomorphic process. It is also important to consider that the absence of evidence may not necessarily be robust evidence of absence. As a result, we are hesitant to infer subtle changes in population size from this dataset prior to the final decline in bone abundance, which is replicated in different depositional environments across eastern Beringia.
Supplementary Material
Acknowledgments
We are grateful to Nancy Bigelow, Yue Wang, Joshua Kurek, Darrell Kaufman, Scott Anderson, Petra Boltshauser-Kaltenrieder, Willy Tinner, César Morales, and Jesse Vermaire, who provided access to palaeoecological datasets from study sites in eastern Beringia. The project was supported by a Natural Sciences and Engineering Research Council Canada grant as part of the Future ArcTic Ecosystems (FATE) project. Ben Gaglioti was supported by NSF grant OPP-1850578. The comments from two anonymous reviewers helped to improve the clarity and focus of this manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107977118/-/DCSupplemental.
*M. E. Edwards et al., INQUA 2019 Meeting, October 25–31, 2019, Dublin, Ireland.
Data Availability
All study data are included in the article and/or supporting information. Previously published data were used for this work. (The supporting information for this manuscript includes a database of published radiocarbon dates. The original studies for these dates are cited, and there are no copyright issues.)
References
- 1.Owen-Smith N., Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology 13, 351–362 (1987). [Google Scholar]
- 2.Zimov S. A., et al. , Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am. Nat. 146, 765–794 (1995). [Google Scholar]
- 3.Johnson C. N., Ecological consequences of Late Quaternary extinctions of megafauna. Proc. Biol. Sci. 276, 2509–2519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi Y., et al. , Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker E. S., et al. , Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. U.S.A. 113, 847–855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guthrie R. D., New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Guthrie R. D., Paleoecology of the large-mammal community in interior Alaska during the late Pleistocene. Am. Midl. Nat. 79, 346–363 (1968). [Google Scholar]
- 8.Guthrie R. D., Origin and causes of the mammoth steppe: A story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia. Quat. Sci. Rev. 20, 549–574 (2001). [Google Scholar]
- 9.Williams J. W., Jackson S. T., Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (2007). [Google Scholar]
- 10.Schweger C. E., “Late Pleistocene vegetation of eastern Beringia: Pollen analysis of dated alluvium” in Paleoecology of Beringia, Hopkins D. M., et al., Eds. (Academic, New York, NY, 1982), pp. 95–112. [Google Scholar]
- 11.Anderson P. M., Late Quaternary vegetational change in the Kotzebue Sound area, northwestern Alaska. Quat. Res. 24, 307–321 (1985). [Google Scholar]
- 12.Goetcheus V. G., Birks H. H., Full-glacial upland tundra vegetation preserved under tephra in the Beringia National Park, Seward Peninsula, Alaska. Quat. Sci. Rev. 20, 135–147 (2001). [Google Scholar]
- 13.Zazula G. D., Froese D. G., Elias S. A., Kuzmina S., Mathewes R. W., Arctic ground squirrels of the mammoth steppe: Paleoecology of Late Pleistocene middens (∼24,000–29,450 14C yr BP), Yukon Territory, Canada. Quat. Sci. Rev. 26, 979–1003 (2007). [Google Scholar]
- 14.Anderson P. M., Edwards M. E., Brubaker L. B., Results and paleoclimate implications of 35 years of paleoecological research in Alaska. Developments in Quaternary Sciences 1, 427–440 (2004). [Google Scholar]
- 15.Blinnikov M. S., Gaglioti B. V., Walker D. A., Wooller M. J., Zazula G. D., Pleistocene graminoid-dominated ecosystems in the Arctic. Quat. Sci. Rev. 30, 2906–2929 (2011). [Google Scholar]
- 16.Willerslev E., et al. , Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506, 47–51 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Jones M. C., Yu Z., Rapid deglacial and early Holocene expansion of peatlands in Alaska. Proc. Natl. Acad. Sci. U.S.A. 107, 7347–7352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann D. H., Peteet D. M., Reanier R. E., Kunz M. L., Responses of an arctic landscape to late glacial and early Holocene climatic changes: The importance of moisture. Quat. Sci. Rev. 21, 997–1021 (2002). [Google Scholar]
- 19.Bigelow N. H., “Late Quaternary vegetation and lake level changes in central Alaska,” PhD thesis, University of Alaska Fairbanks, Fairbanks, AK (1997). [Google Scholar]
- 20.Brubaker L. B., Anderson P. M., Hu F. S., Vegetation ecotone dynamics in southwest Alaska during the late Quaternary. Quat. Sci. Rev. 20, 175–188 (2001). [Google Scholar]
- 21.Tinner W., et al. , Postglacial vegetational and fire history: Pollen, plant macrofossil and charcoal records from two Alaskan lakes. Veg. Hist. Archaeobot. 15, 279–293 (2006). [Google Scholar]
- 22.Edwards M. E., Brubaker L. B., Lozhkin A. V., Anderson P. M., Structurally novel biomes: A response to past warming in Beringia. Ecology 86, 1696–1703 (2005). [Google Scholar]
- 23.Mann D. H., Groves P., Kunz M. L., Reanier R. E., Gaglioti B. V., Ice-age megafauna in Arctic Alaska: Extinction, invasion, survival. Quat. Sci. Rev. 70, 91–108 (2013). [Google Scholar]
- 24.Guthrie R. D., New dates on Alaskan Quaternary moose, Cervalces-Alces—Archaeological, evolutionary, and ecological implications. Curr. Res. Pleistocene 7, 111–112 (1990). [Google Scholar]
- 25.Guthrie R. D., Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe (University of Chicago Press, 1990). [Google Scholar]
- 26.Mann D. H., et al. , Life and extinction of megafauna in the ice-age Arctic. Proc. Natl. Acad. Sci. U.S.A. 112, 14301–14306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conroy K. J., et al. , Tracking late-Quaternary extinctions in interior Alaska using megaherbivore bone remains and dung fungal spores. Quat. Res. 97, 99–110 (2020). [Google Scholar]
- 28.Guthrie R. D., “Mosaics, allelochemics, and nutrients: An ecological theory of late Pleistocene megafaunal extinctions” in Quaternary Extinctions: A Prehistoric Revolution, Martin P. S., Klein R. G., Eds. (University of Arizona Press, 1984), pp. 258–259. [Google Scholar]
- 29.Zimov S. A., Zimov N. S., Tikhonov A. N., Chapin III F. S., Mammoth steppe: A high-productivity phenomenon. Quat. Sci. Rev. 57, 26–45 (2012). [Google Scholar]
- 30.Martin P. S., “Prehistoric overkill: The global model” in Quaternary Extinctions: A Prehistoric Revolution, Martin P. S., Klein R. G., Eds. (University of Arizona Press, 1984), pp. 354–403. [Google Scholar]
- 31.Meltzer D. J., Overkill, glacial history, and the extinction of North America’s Ice Age megafauna. Proc. Natl. Acad. Sci. U.S.A. 117, 28555–28563 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heusser C. J., “A Pleistocene phytogeographical sketch of the Pacific Northwest and Alaska” in The Quaternary of the United States, Wright H. E., Frey D. G., Eds. (Princeton University Press, Princeton, NJ, 1965), pp. 469–483. [Google Scholar]
- 33.Ager T. A., “Late Quaternary environmental history of the Tanana valley, Alaska,” PhD thesis, The Ohio State University, Columbus, OH: (1975). [Google Scholar]
- 34.Hopkins D. M., “Aspects of the paleogeography of Beringia during the late Pleistocene” in Paleoecology of Beringia, Hopkins D. M., Matthews J. V., Schweger C. E., Young S. B., Eds. (Academic, 1982), pp. 3–28. [Google Scholar]
- 35.Brubaker L. B., Anderson P. M., Edwards M. E., Lozhkin A. V., Beringia as a glacial refugium for boreal trees and shrubs: New perspectives from mapped pollen data. J. Biogeogr. 32, 833–848 (2005). [Google Scholar]
- 36.Abbott M. B., Stafford T. W., Radiocarbon geochemistry of modern and ancient Arctic lake systems, Baffin Island, Canada. Quat. Res. 45, 300–311 (1996). [Google Scholar]
- 37.Oswald W. W., et al. , Effects of sample mass and macrofossil type on radiocarbon dating of arctic and boreal lake sediments. Holocene 15, 758–767 (2005). [Google Scholar]
- 38.Gaglioti B. V., et al. , Radiocarbon age‐offsets in an arctic lake reveal the long‐term response of permafrost carbon to climate change. J. Geophys. Res. Biogeosci. 119, 1630–1651 (2014). [Google Scholar]
- 39.Kaufman D. S., Young N. E., Briner J. P., Manley W. F., “Alaska palaeo-glacier atlas (version 2)” in Quaternary Glaciations–Extent and Chronology: A Closer Look, Ehlers J., Gibbard P. L., Hughes P. D., Eds. (Developments in Quaternary Sciences, Elsevier, 2011), vol. 15, pp. 427–445. [Google Scholar]
- 40.Heintzman P. D., et al. , Bison phylogeography constrains dispersal and viability of the Ice Free Corridor in western Canada. Proc. Natl. Acad. Sci. U.S.A. 113, 8057–8063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lea P. D., Waythomas C. F., Late Pleistocene eolian sand sheets in Alaska. Quat. Res. 34, 269–281 (1990). [Google Scholar]
- 42.Froese D., et al. , Fossil and genomic evidence constrains the timing of bison arrival in North America. Proc. Natl. Acad. Sci. U.S.A. 114, 3457–3462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigelow N. H., et al. , Climate change and Arctic ecosystems: 1. Vegetation changes north of 55° N between the last glacial maximum, mid‐Holocene, and present. J. Geophys. Res. D Atmospheres 108, 8170 (2003). [Google Scholar]
- 44.Finkenbinder M. S., Abbott M. B., Finney B. P., Stoner J. S., Dorfman J. M., A multi-proxy reconstruction of environmental change spanning the last 37,000 years from Burial Lake, Arctic Alaska. Quat. Sci. Rev. 126, 227–241 (2015). [Google Scholar]
- 45.Kaltenrieder P., Tinner W., Lee B., Hu F. S., A 16,000‐year record of vegetational change in south‐western Alaska as inferred from plant macrofossils and pollen. J. Quaternary Sci. 26, 276–285 (2011). [Google Scholar]
- 46.Oswald W. W., Gavin D. G., Anderson P. M., Brubaker L. B., Hu F. S., A 14,500-year record of landscape change from Okpilak Lake, northeastern Brooks Range, northern Alaska. J. Paleolimnol. 48, 101–113 (2012). [Google Scholar]
- 47.Carlson L. J., Finney B. P., A 13,000-year history of vegetation and environmental change at Jan Lake, east-central Alaska. Holocene 14, 818–827 (2004). [Google Scholar]
- 48.Guthrie R. D., Rapid body size decline in Alaskan Pleistocene horses before extinction. Nature 426, 169–171 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Rivals F., Solounias N., Mihlbachler M. C., Evidence for geographic variation in the diets of late Pleistocene and early Holocene Bison in North America, and differences from the diets of recent Bison. Quat. Res. 68, 338–346 (2007). [Google Scholar]
- 50.Kowalczyk R., et al. , Influence of management practices on large herbivore diet—Case of European bison in Białowieża Primeval Forest (Poland). For. Ecol. Manage. 261, 821–828 (2011). [Google Scholar]
- 51.Drummond A. J., Rambaut A., Shapiro B., Pybus O. G., Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 22, 1185–1192 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Lozhkin A. V., Anderson P. M., Vegetation responses to interglacial warming in the Arctic: Examples from Lake El’gygytgyn, Far East Russian Arctic. Clim. Past 9, 1211–1219 (2013). [Google Scholar]
- 53.Mann D. H., Groves P., Gaglioti B. V., Shapiro B. A., Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: The Plaids and Stripes Hypothesis. Biol. Rev. Camb. Philos. Soc. 94, 328–352 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurek J., Cwynar L. C., Vermaire J. C., A late Quaternary paleotemperature record from Hanging Lake, northern Yukon Territory, eastern Beringia. Quat. Res. 72, 246–257 (2009). [Google Scholar]
- 55.Irvine F., Cwynar L. C., Vermaire J. C., Rees A. B., Midge-inferred temperature reconstructions and vegetation change over the last ∼15,000 years from Trout Lake, northern Yukon Territory, eastern Beringia. J. Paleolimnol. 48, 133–146 (2012). [Google Scholar]
- 56.Rabanus-Wallace M. T., et al. , Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions. Nat. Ecol. Evol. 1, 125 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Gaglioti B. V., Aeolian stratigraphy describes ice-age paleoenvironments in unglaciated Arctic Alaska. Quat. Sci. Rev. 182, 175–190 (2018). [Google Scholar]
- 58.Abbott M. B., Finney B. P., Edwards M. E., Kelts K. R., Lake-level reconstruction and paleohydrology of Birch Lake, central Alaska, based on seismic reflection profiles and core transects. Quat. Res. 53, 154–166 (2000). [Google Scholar]
- 59.Finkenbinder M. S., et al. , A 31,000 year record of paleoenvironmental and lake-level change from Harding Lake, Alaska, USA. Quat. Sci. Rev. 87, 98–113 (2014). [Google Scholar]
- 60.Bartlein P. J., Anderson P. M., Edwards M. E., McDowell P. F., A framework for interpreting paleoclimatic variations in eastern Beringia. Quat. Int. 10, 73–83 (1991). [Google Scholar]
- 61.McNaughton S. K., Grazing lawns: Animals in herds, plant form, and coevolution. Am. Nat. 124, 863–886 (1984). [Google Scholar]
- 62.Asner G. P., Vaughn N., Smit I. P., Levick S., Ecosystem‐scale effects of megafauna in African savannas. Ecography 39, 240–252 (2016). [Google Scholar]
- 63.Olofsson J., et al. , Herbivores inhibit climate‐driven shrub expansion on the tundra. Glob. Change Biol. 15, 2681–2693 (2009). [Google Scholar]
- 64.Schwartz-Narbonne R., Longstaffe F. J., Metcalfe J. Z., Zazula G., Solving the woolly mammoth conundrum: Amino acid 15N-enrichment suggests a distinct forage or habitat. Sci. Rep. 5, 9791 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Geel B., et al. , Mycological evidence of coprophagy from the feces of an Alaskan Late Glacial mammoth. Quat. Sci. Rev. 30, 2289–2303 (2011). [Google Scholar]
- 66.Knapp A. K., et al. , The keystone role of bison in North American tallgrass prairie: Bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience 49, 39–50 (1999). [Google Scholar]
- 67.Bliss L. C., Richards J. H., “Present-day vegetation and ecosystems as a predictive tool for the arctic-steppe mammoth biome” in Paleoecology of Beringia, Hopkins D. M., Matthews J. V., Schweger C. E., Young S. B., Eds. (Academic, 1982), pp. 241–257. [Google Scholar]
- 68.Redmann R. E., “Production and diversity in contemporary grasslands” in Paleoecology of Beringia, Hopkins D. M., Matthews J. V., Schweger C. E., Young S. B., Eds. (Academic, 1982), pp. 223–239. [Google Scholar]
- 69.Matheus P. E., “Locomotor adaptations and ecomorphology of short-faced bears (Arctodus simus) in eastern Beringia” (Occasional Papers in Earth Science 7, Government of the Yukon, 2003). [Google Scholar]
- 70.Potter B. A., et al. , Early colonization of Beringia and Northern North America: Chronology, routes, and adaptive strategies. Quat. Int. 444, 36–55 (2017). [Google Scholar]
- 71.Haile J., et al. , Ancient DNA reveals late survival of mammoth and horse in interior Alaska. Proc. Natl. Acad. Sci. U.S.A. 106, 22352–22357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourgeon L., Burke A., Higham T., Earliest human presence in North America dated to the last glacial maximum: New radiocarbon dates from Bluefish Caves, Canada. PloS One 12, e0169486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sturm M., Racine C., Tape K., Climate change. Increasing shrub abundance in the Arctic. Nature 411, 546–547 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Zimov S. A., Essays on science and society. Pleistocene Park: Return of the mammoth’s ecosystem. Science 308, 796–798 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Zhou J., et al. , Enhanced shrub growth in the Arctic increases habitat connectivity for browsing herbivores. Glob. Change Biol. 26, 3809–3820 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Bronk Ramsey C., Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009). [Google Scholar]
- 77.Reimer P. J., et al. , The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020). [Google Scholar]
- 78.Bronk Ramsey C., Dealing with outliers and offsets in radiocarbon dating. Radiocarbon 51, 1023–1045 (2009). [Google Scholar]
- 79.Bronk Ramsey C., Methods for summarizing radiocarbon datasets. Radiocarbon 59, 1809–1833 (2017). [Google Scholar]
- 80.Dalton A. S., et al. , An updated radiocarbon-based ice margin chronology for the last deglaciation of the North American Ice Sheet Complex. Quat. Sci. Rev. 234, 106223 (2020). [Google Scholar]
- 81.Abbott M. B., Edwards M. E., Finney B. P., A 40,000-yr record of environmental change from Burial Lake in Northwest Alaska. Quat. Res. 74, 156–165 (2010). [Google Scholar]
- 82.Oswald W. W., Brubaker L. B., Anderson P. M., Late Quaternary vegetational history of the Howard Pass area, northwestern Alaska. Can. J. Bot. 77, 570–581 (1999). [Google Scholar]
- 83.McGowan S., et al. , Vegetation transitions drive the autotrophy–heterotrophy balance in Arctic lakes. Limnol. Oceanogr. Lett. 3, 246–255 (2018). [Google Scholar]
- 84.Higuera P. E., et al. , Frequent fires in ancient shrub tundra: Implications of paleorecords for arctic environmental change. PLoS One 3, e0001744 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaufman D. S., Anderson R. S., Hu F. S., Berg E., Werner A., Evidence for a variable and wet Younger Dryas in southern Alaska. Quat. Sci. Rev. 29, 1445–1452 (2010). [Google Scholar]
- 86.Johnsen J., et al. , Oxygen isotope and palaeotemperature records from six Greenland ice‐core stations: Camp Century, Dye‐3, GRIP, GISP2, Renland and NorthGRIP. J. Quaternary Sci. 16, 299–307 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. Previously published data were used for this work. (The supporting information for this manuscript includes a database of published radiocarbon dates. The original studies for these dates are cited, and there are no copyright issues.)