Significance
This research provides a transformative hypothesis for the chemistry of the atmospheric cloud layers of Venus while reconciling decades-long atmosphere anomalies. Our model predicts that the clouds are not entirely made of sulfuric acid, but are partially composed of ammonium salt slurries, which may be the result of biological production of ammonia in cloud droplets. As a result, the clouds are no more acidic than some extreme terrestrial environments that harbor life. Life could be making its own environment on Venus. The model’s predictions for the abundance of gases in Venus’ atmosphere match observation better than any previous model, and are readily testable.
Keywords: Venus, clouds, atmospheric chemistry, astrobiology, habitability
Abstract
The atmosphere of Venus remains mysterious, with many outstanding chemical connundra. These include the unexpected presence of ∼10 ppm O2 in the cloud layers, an unknown composition of large particles in the lower cloud layers, and hard to explain measured vertical abundance profiles of SO2 and H2O. We propose a hypothesis for the chemistry in the clouds that largely addresses all of the above anomalies. We include ammonia (NH3), a key component that has been tentatively detected both by the Venera 8 and Pioneer Venus probes. NH3 dissolves in some of the sulfuric acid cloud droplets, effectively neutralizing the acid and trapping dissolved SO2 as ammonium sulfite salts. This trapping of SO2 in the clouds, together with the release of SO2 below the clouds as the droplets settle out to higher temperatures, explains the vertical SO2 abundance anomaly. A consequence of the presence of NH3 is that some Venus cloud droplets must be semisolid ammonium salt slurries, with a pH of ∼1, which matches Earth acidophile environments, rather than concentrated sulfuric acid. The source of NH3 is unknown but could involve biological production; if so, then the most energy-efficient NH3-producing reaction also creates O2, explaining the detection of O2 in the cloud layers. Our model therefore predicts that the clouds are more habitable than previously thought, and may be inhabited. Unlike prior atmospheric models, ours does not require forced chemical constraints to match the data. Our hypothesis, guided by existing observations, can be tested by new Venus in situ measurements.
Venus is often called Earth’s sister planet because of its similar mass and size to Earth. Yet, owing, in part, to the greenhouse effect from its massive CO2 atmosphere, Venus’s surface temperature is higher than 700 K—too hot for life of any kind. The Venusian surface is therefore a complete contrast to Earth’s temperate surface and rich surface biosphere. Nonetheless, scientists have been speculating on Venus as a habitable world for over half a century (1–7). Such speculations are based on the Earth-like temperature and pressure at the altitudes of 48 km to 60 km above the surface (8, 9).
Venus is perpetually shrouded in an ∼20-km-deep layer of clouds, including the temperate atmosphere layers at 48 km to 60 km. The prevailing consensus is that the clouds of Venus are made from droplets of concentrated sulfuric acid. This conclusion is inferred from the presence of small amounts of sulfuric acid vapor in the atmosphere (10, 11) and the refractive index of cloud droplets (12, 13). While the clouds are often described as “temperate” or “clement,” such a statement is misleading when it comes to habitability. If the cloud particles are actually made of concentrated sulfuric acid, then it is difficult to imagine how life chemically similar to life on Earth could survive (7, 14). Specifically, the aggressive chemical properties of sulfuric acid and the extremely low atmospheric water content (14, 15) are orders of magnitude more acidic and 50 to 100 times drier than any inhabited extreme environment on Earth.
Overview of Venusian Atmosphere Anomalies
Despite over 50 y of remote and local observation, Venus’s atmosphere has a number of lingering anomalies with either poor model fits or no explanations (16, 17).
One such long-standing mysterious feature of the atmosphere, which is not well explained by current atmospheric chemistry models, is the abundance profile of water vapor and SO2 in and above the cloud layers (17–19).
Observations show that H2O persists throughout the atmosphere, while the SO2 is observed in parts per million abundances below the clouds and parts per billion abundance above the clouds. Yet, expectations are very different. The primary source of SO2 and H2O in the atmosphere of Venus is volcanism. As the gases are released from volcanoes, they are uniformly mixed vertically throughout the atmosphere. At very high altitudes in the atmosphere, around 70 km, SO2 and H2O are efficiently destroyed by ultraviolet (UV) radiation. However, the observed SO2 and H2O abundance profiles deviate from the uniform distribution, notably, such that SO2 shows significant depletion in the cloud layers and H2O is present above the cloud layers.
Previous consensus models explained the SO2 profile by suggesting that SO2 is photochemically oxidized to SO3, which then reacts with water to form sulfuric acid in the clouds:
However, as there is 5× more SO2 than H2O, this chemistry should strip all the water out of the cloud layer, and additionally react with and prevent water from reaching and accumulating above the clouds as well, while only reducing SO2 by 20%, not the 99.9% observed (20). Previous models provide a numerical fix to match the observations, arbitrarily removing SO2 or artificially keeping the water abundance constant (21, 22).
Another mystery is the presence of O2 in the clouds (23, 24), as there is no known process for O2 formation in the cloud layers (discussed further below). Finally, the SO2, O2, and H2O anomalies, together with other trace atmospheric gas abundances, form a chemical disequilibrium in the clouds of Venus (25–27).
A more tentative but intriguing anomaly is that of the detection of NH3 in and below the cloud layers. NH3 was tentatively detected both by the Venera 8 chemical probe (28) and in reanalyzed Pioneer Venus (Pioneer 13) data (27). The reanalysis of Pioneer Venus data showed additional N species (NOx), suggesting further chemical disequilibrium in the cloud layers. The cloud particles themselves also contain many unknowns. The largest particles, predominant in the lower cloud decks [called Mode 3 particles (29)], may have a substantial solid component, implying that they cannot be exclusively made of liquid concentrated sulfuric acid (30).
Some additional anomalies that are not directly relevant to this work, such as the “unknown UV absorber” (31) and the possible presence of methane (32) or phosphine (33, 34), have all been suggested as signs of life in the clouds.
How the Rimmer et al. Model Resolves the SO2 and H2O Abundance Conundrum
Recently, Rimmer et al. (20) proposed a mechanism to explain the depletion of SO2 in the atmospheric cloud layers, as well as the vertical abundance profile of H2O in and above the clouds. If a base is present inside the cloud sulfuric acid droplets, SO2 will dissolve in the liquid droplets (by reaction with OH−) to form sulfite. The base (B), therefore traps the SO2 inside the cloud droplet as sulfite (HSO3−),
In summary, the equilibrium of the reaction
is pulled to the right of the above equation, and S(IV) species are trapped as sulfite salts through reaction with the base. Thus, SO2 is depleted in the cloud layer, compared to the model with no bases. Eventually, the cloud droplets rain down to lower atmosphere layers, and the salts dissociate due to higher temperatures, releasing SO2.
Water is consumed in the sulfite-forming reaction, but is recycled into the lower atmosphere on breakdown of the sulfites, which provides a mechanism to explain the water vapor abundance profile through the clouds. Some water is removed from the cloud layer, but, because it is replenished by recycling from below the clouds, the water removal is not absolute, and so some water remains at the cloud top and in the atmosphere above the clouds. Thus the Rimmer et al. (20) model predicts that both SO2 and H2O will be present above the clouds but at substantially lower abundance than they are below the clouds, in agreement with observation.
The formation of the sulfite salt within a droplet effectively neutralizes the acid in the droplet, with the very important outcome that some of the cloud droplets are much less acidic than previously thought, with a pH between −1 and 1 (20), instead of an acidity of approximately −11 (on the Hammett acidity scale). If correct, the revised pH range of some droplets has a significance for the habitability of the clouds of Venus that cannot be overstated. Such a pH range is habitable by terrestrial extremophiles (35), as compared to the acidity of concentrated sulfuric acid in which all terrestrial life, and most terrestrial biochemicals, would be destroyed (14).
We argue that the identity of any droplet-neutralizing base is unknown. Rimmer et al. (20) adopted NaOH as a model base for their calculations, but noted that iron oxides are a more physically realistic possibility. In principle, minerals that can absorb SO2 could be delivered to the clouds from Venusian volcanic eruption, from wind lofting of dust, or from meteoritic infall. However, it has not been demonstrated that such mechanisms could deliver the very high amount of ∼20 tonnes per second flux of mineral salts (specifically iron oxides) required (20).
We are motivated to extend ref. 20’s analysis with the hypothesis that the neutralizing base that is capturing SO2 is locally generated in the clouds. We postulate that NH3 is the neutralizing agent for the Venusian cloud droplets, trapping SO2 and thus explaining the drop in SO2 abundance across the clouds. We are additionally motivated by the tentative in situ observations of NH3 in the Venus cloud layers, from both Venera 8 chemical assay (28) and Pioneer Venus probe mass spectrometry (27). If present, NH3 observations cannot yet be readily explained through any known abiotic planetary processes (36). We therefore also explore the possibility that the NH3 is biologically produced.
Results
Ammonia as a Neutralizing Agent in the Venusian Cloud Droplets.
We propose NH3 as the only plausible neutralizing base that can be generated in situ in the clouds from gas-phase components (see SI Appendix, section 1 for further details on potential neutralizing agents in the cloud layers). The presence of NH3, as with any neutralizing base, leads to chemistry that results in the SO2 depletion in the clouds and the observed H2O abundance profile, and is consistent with a subset of Mode 3 particles being nonspherical (i.e., not liquid) and not composed of pure concentrated sulfuric acid. The presence of NH3 may also solve the otherwise unexplained presence of O2 in the clouds, especially if the source of NH3 is biological.
To support our hypothesis that NH3 could explain the presence of O2 within the clouds, we first explore the limited number of possible chemical reactions that could lead to the formation of NH3 in the Venusian atmosphere cloud layer conditions (Table 1).
Table 1.
Free energy per mole for NH3-generating reactions under Venus cloud conditions
| Reaction | Free energy of reaction (kJ/mol) | Free energy required per mole of surplus NH3 (kJ/mol) | Water consumed per surplus NH3 | |
| 1 | 4N2(aq) + 11H2O(l) → 2NH4+OH−(aq) + 3NH4+NO3−(aq) | 1,730 to 2,024 | 865 to 1,012 | 6.5 |
| 2 | N2(aq) + 8H2O(l) → 2NH4+OH−(aq) + 3H2O2(aq) | 1,203 to 1,471 | 602 to 736 | 4 |
| 3 | 2N2(aq) + 10H2O(l) → 4NH4+OH−(aq) + 3O2(aq) | 1,000 to 1,306 | 262 to 343 | 2.5 |
| 4 | 4N2(aq) + 17H2O(l) + 3HCl(aq) → 5NH4+OH−(aq) + 3NH4+ClO4−(aq) | 1,364 to 1,634 | 273 to 323 | 3.4 |
| 5 | N2(aq) + 6H2O(l) + 3SO2(aq) → (NH4+)2SO42-(aq) + 2H2SO4(aq) | 1,193 to 1,313 | N/A | N/A |
Free energies of NH3-producing reactions are calculated from refs. 83–85. Ranges are minimum to maximum over a range of pH = −3 to pH = +4 and temperature from 2 °C to 115 °C. Concentrations of SO2 and H2O are as described in ref. 34. O2 fractional abundance is assumed to be 10−6. Table columns are as follows. First column: reaction number. Second column: possible chemical reaction that produces NH3. Third column: free energy of reaction assuming that NH3 is accumulated to 2 molar concentration. For the fourth and fifth columns, values were calculated in terms of “surplus NH3,” which is the amount of NH3 synthesized as NH4OH. Fourth column: free energy per mole of “surplus NH3” produced. Fifth column: number of water molecules consumed per “surplus” NH3. Reaction 3 (bold type), which produces molecular oxygen as an oxidized byproduct, is the most efficient, in both its use of energy and its use to water. We note that reaction 4 could produce hypochlorite, chlorite, or chlorate as an oxidized product, but, as perchlorate is relatively stable and is the weakest oxidizing agent, we have shown this reaction for illustration only. Reaction 5 generates more acid than it consumes, and so cannot be a source of the base which neutralizes H2SO3. We also note that reaction 1 and reaction 4 (reactions making nitrate and perchlorate, respectively) clouds also alternatively explain the presence of O2. Nitrate and perchlorate would “rain out” and decompose to N2 and O2 or HCl, Cl2, and O2, respectively, below the clouds. In situ measurements of NOx and ClO4 abundance in the clouds could rule out these reactions as a potential source of indirect formation of O2.
The most abundant source of nitrogen atoms in the atmosphere of Venus is N2 gas, so, to make NH3, N2 must be reduced to NH3. The reduction of N2 to form NH3 requires a source of hydrogen atoms, and a source of electrons (reducing equivalents). Hydrogen atoms are rare in the atmosphere of Venus. The most abundant gas-phase source of hydrogen atoms in the atmosphere of Venus is H2O, followed by HCl. In order to generate reducing equivalents, some species must be oxidized. Species available to be oxidized include CO, OCS, SO2, N2, H2O, and HCl. Phosphorus, if present, will be overwhelmingly present as H3PO4 (34); neither H3PO4 or CO2 can be further oxidized.
The most energy- and water-efficient NH3-producing reaction (reaction 3 in Table 1) also produces molecular oxygen. We choose reaction 3, 2N2(aq) + 10H2O(l) → 4NH4+OH−(aq) + 3O2(aq), as the basis for our model for two reasons, Firstly, parsimony leads us to prefer a reaction that uses the smallest amount of rare materials (H2O and energy). Secondly, reaction 3 is the only NH3-forming reaction that directly produces O2 in the clouds (Table 1), whose detection is one of the anomalies we wish to explain (discussed below); the other reactions produce different oxidized species which would not be observed but which would also produce O2 on breakdown, and at the cost of greater energy consumption.
A key question is what NH3 production rate (by reaction 3) is needed for maintaining the low SO2 abundance, as compared to expected equilibrium values in the atmospheric cloud layers. We base the SO2 production rate on the rate at which SO2 would be replenished into the clouds by mixing from below, and hence the rate at which it must be removed from the clouds. The flux is ∼1011 tonnes per year NH3, which is on the order of photosynthetic production of O2 on Earth (see Materials and Methods). This flux is calculated assuming that NH3 is only produced to sequester SO2, and that only NH3 sequesters SO2. If other species contribute to removing SO2, whether hydroxide salts, iron oxides, or other species, the NH3 production will be accordingly lower. Any byproduct of SO2 sequestration must have a flux of ∼1011 tonnes per year at the bottom of the clouds, based on the SO2 depletion within the clouds. A flux of 1011 tonnes per year is consistent, to within an order of magnitude, with the mass loss at the bottom of the clouds from rainout of Mode 3 particles from our calculations (SI Appendix, section 2).
All of the NH3-producing reactions in the Venusian atmosphere conditions are highly endergonic (Table 1), and so must be coupled to an energy source if the reactions are to produce net, “surplus” NH3. There are several energy sources that could, in principle, drive the production of NH3. Lightning falls short by many orders of magnitude of the necessary rate of production of NH3 (SI Appendix, section 7.1 and Table S3), and is very unlikely to produce both NH3 and O2 simultaneously. Similarly, UV photochemistry is unlikely to produce NH3 in more than trace amounts (SI Appendix, section 7.2), although we note that the photochemistry of nitrogen species in concentrated sulfuric acid has not been explored. Volcanic sources of NH3 on Earth are closely associated with organic deposits, including coal, and also are quantitatively insufficient, even based on terrestrial rates of volcanic NH3 production, which are likely to be much higher than any plausible NH3 production on Venus (SI Appendix, section 7.3 and Fig. S4).
The ability to couple chemical energy to drive endergonic reactions is a universal characteristic of life, and, specifically, the use of energy to drive the reduction of N2 to NH3 in an oxidizing environment is widely found in terrestrial organisms (37, 38). We should therefore consider the possibility that living organisms in the clouds of Venus are making NH3. All of the NH3-producing reactions presented in Table 1 consume water, which is a rare resource in the clouds of Venus. The energy expended and water molecules consumed in the process of making NH3 must be balanced by an equally powerful benefit to the organism for this apparently wasteful chemistry. Neutralizing the acid to make the droplets habitable is a clear benefit.
We discuss the other, possibly insuperable barriers to the concept of life in the Venusian clouds below. Here we only note that the presence of life could explain the observed presence of NH3 and O2, and later show that it could explain the observed vertical abundances of H2O and SO2 within and above the atmospheric cloud layers, and the semisolid nature of Mode 3 particles. An additional consequence of the NH3 cloud droplet chemistry is that the pH of cloud particles with dissolved NH3 must have a pH between −1 and 1, as first shown by Rimmer et al. (20) for NaOH (Fig. 1).
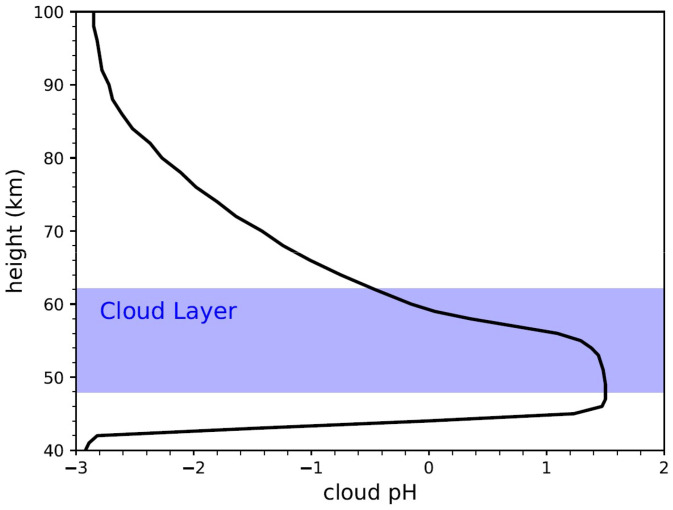
Fig. 1.
Predicted pH profile of cloud particles. The blue shaded region shows the altitude where clouds are present, from 48 km to 62 km. Note that the plot extends above and below the cloud tops because there are plausibly cloud particle populations that extend down to the altitude where sulfuric acid is sublimated, and up into the mesosphere where sulfuric acid aerosol evaporation may explain the anomalous SO2 inversion at 80 km to 100 km. Our model provides no constraints on the composition of the mesospheric particles, which may well be composed of pure sulfuric acid.
The Flux of NH3 Is within the Plausible Biomass Production.
The flux of NH3 needed to achieve the neutralization effect is not prohibitive for a realistic biomass within the cloud droplets. We calculate the biomass required by this model as follows. The production of 1011 tonnes per year is equivalent to 3·109 g NH3 per second. Several species of cyanobacteria fix nitrogen at an average rate of ∼4·10−7 g per g wet weight biomass per second (39–41). If life is present in the clouds of Venus, it will not be terrestrial life; however, if we take these terrestrial organisms as precedent, 1011 tonnes per year would be produced by ∼8·1015 g wet weight of organism. While this mass might appear significantly high, it is ∼1/2,000 (0.05%) the biomass of the Earth (42). This mass translates to ∼1.5% of the mass of cloud particles in the lower 5 km of the cloud deck (25).
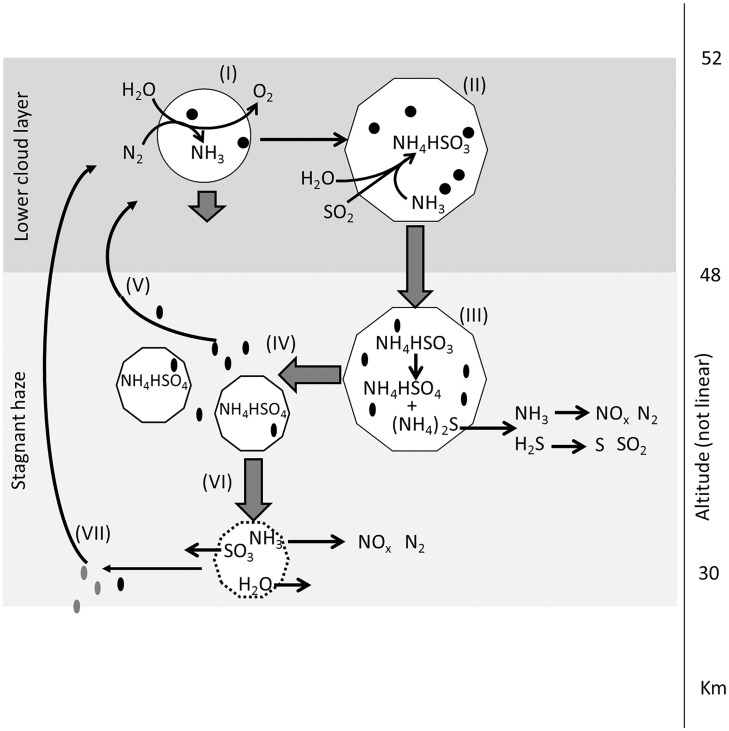
Our model for the production of NH3 by life is summarized in Fig. 2.
Fig. 2.
Ammonia cycle in the atmosphere of Venus. See SI Appendix, section 10 for details. I: NH3 is produced locally in the clouds from atmospheric N2 and H2O (Table 1) by metabolically active microorganisms (black dots) inhabiting cloud droplets (white circle). II: The production of NH3 in the droplet raises the droplet pH to −1 to 1 (from −11 on the Hammett acidity scale) by trapping the SO2 and H2O in the droplet as ammonium hydrogen sulfite (NH4HSO3). The production of sulfite salts in the droplet leads to the formation of a large, semisolid (and hence nonspherical) Mode 3 particle (white decagon). III: The Mode 3 particle settles out of the clouds where ammonium sulfite disproportionates to ammonium sulfate and ammonium sulfide; the latter decomposes to H2S and NH3, which, in turn, undergo photochemical reactions to a variety of products. IV: Disproportionation and gas release break up the Mode 3 particles into smaller haze particles and microorganism spores (black ovals), some of which return to the cloud layer (V). VI: The ammonium sulfate particles fall farther below the cloud decks, where ammonium sulfate decomposes to SO3, NH3, and H2O. VII: Spores released at this stage may be unviable (gray ovals), but any surviving could also be eventually transported back to the clouds.
Toward a Resolution of Venus Atmospheric Anomalies.
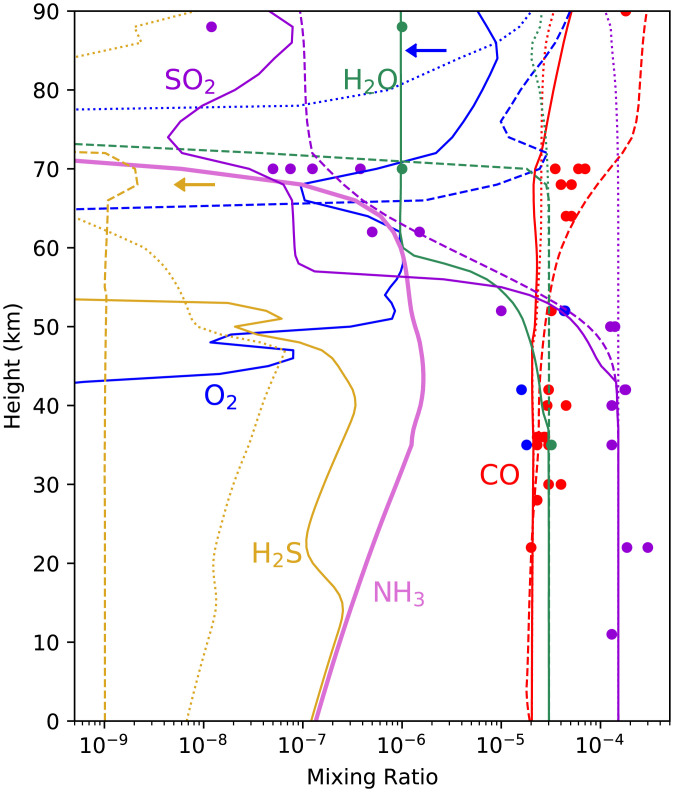
The incorporation of NH3 in our photochemistry model of the Venusian atmosphere produces profiles of atmospheric gases that match the observed abundances of some atmospheric gases better than existing models of Venus’s atmosphere. Although NH3 is an input to our model, no existing Venus photochemical models include NH3 (e.g., refs. 22 and 43). In Figs. 3 and 4, we show a summary of the output of the modeling with NH3 included, compared to the same model run without NH3 and O2 input, the latter as reported in ref. 20. The atmospheric photochemistry of the clouds was modeled as described in refs. 20 and 34, and is summarized in Materials and Methods. Specifically, our model better explains, compared to previous models, 1) the observed disequilibria in the clouds of Venus; 2) the measured, but subsequently ignored, abundances of O2 in the clouds; 3) the abundance profile of water vapor; 4) the tentative detections of NH3 by Venera 8 and Pioneer Venus probes; and 5) the abundance profile of SO2 through the cloud layers. To demonstrate how well our model with NH3 fits the measured data, we show three model results in Figs. 3 and 4: one model with NH3, one model without NH3 but with an unphysical arbitrary depletion rate of SO2 (a fix common among other models in order to fit the data), and one model without NH3 and without any artificial chemical constraints.
Fig. 3.
Venus atmosphere abundance profiles of key molecular species. The x axis is the gas fraction by volume, called the mixing ratio. The y axis is altitude above the surface in kilometers. The lines are gas mixing ratios from our models: with NH3 chemistry (solid lines), without NH3 chemistry (dotted lines; model in ref. 20), and without NH3 but with an arbitrary removal rate for SO2 in the cloud layers tuned to fit the data (dashed lines; model in refs. 20 and 34). The colored circles show a representative subset of collated remote and in situ data (error bars not shown) from refs. 20 (their table 4) and 33 (their supplementary table S3). Key is that the baseline model predicts no NH3 or H2S above the 1-ppb level. Models with NH3 chemistry have very different H2O, SO2, O2, and H2S values at some altitudes than models without NH3 chemistry, and improve the match to observational data. The main takeaway is that the model without NH3 and without the SO2 arbitrary removal rate (dotted line) fits the cloud layer data very poorly, whereas the model with NH3 (with no arbitrary constraints; solid line) fits the data much better. The boundary conditions for surface abundance in the photochemical model are listed in SI Appendix, Table S6.
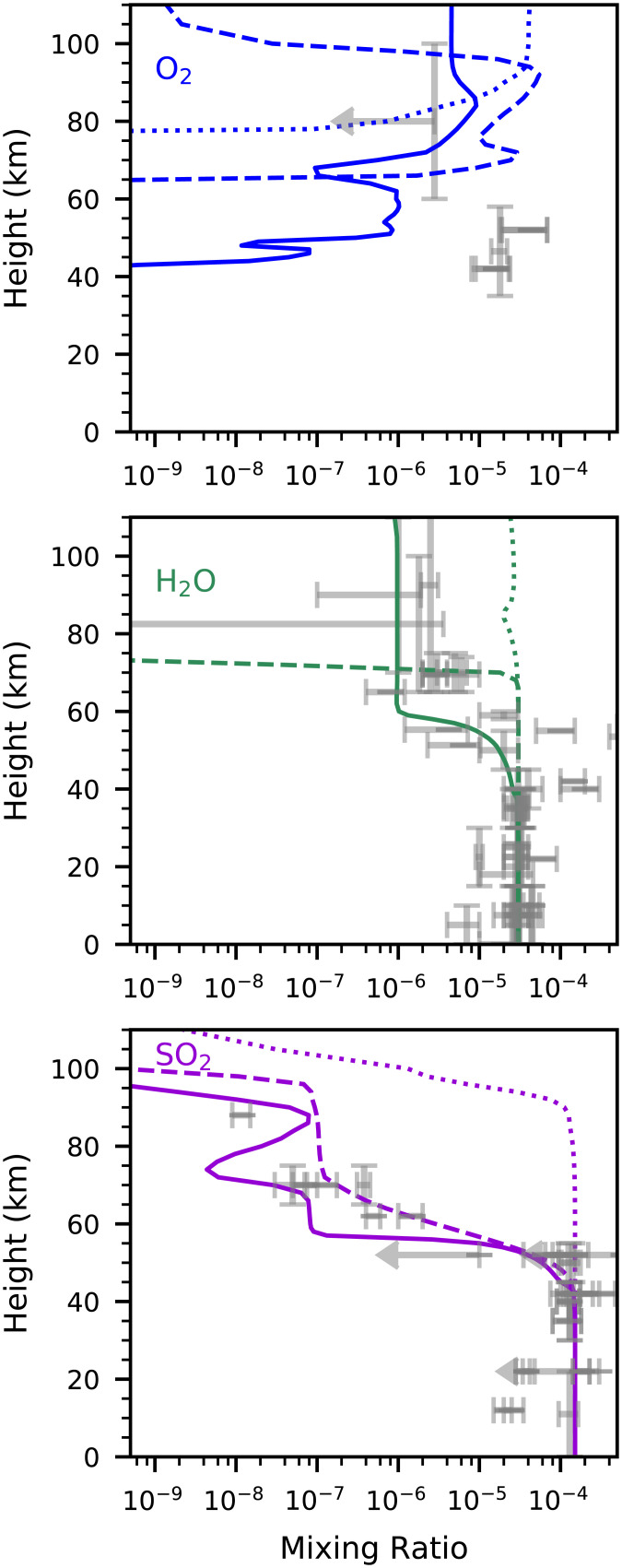
Fig. 4.
Venus atmosphere abundance profiles of three molecular species. The x axis is the gas fraction by volume, called the mixing ratio. The y axis is altitude above the surface in kilometers. The lines are gas mixing ratios from our models: with NH3 chemistry (solid lines), without NH3 chemistry (dotted lines; model in ref. 32), and without NH3 but with an arbitrary removal rate for SO2 in the cloud layers tuned to fit the data (dashed lines; model in refs. 20 and 34). Gray points with error bars are data from observations tabulated in ref. 20. (Top) O2. Our model with NH3 chemistry improves upon both the long-standing problem of presence and overabundance of O2 in the upper atmosphere and the presence of O2 in the cloud layers. (Middle) H2O. Our model with NH3 chemistry supports the presence of water vapor above the cloud layer (>80 km). (Bottom) SO2. Our models with NH3 chemistry (solid line) and without NH3 chemistry but with arbitrary constraints on SO2 (dashed line) both provide a fit to observed values throughout the atmosphere except for the top (>85 km). Key is that the model without NH3 and without the SO2 arbitrary removal rate (dotted line) fits the cloud layer data very poorly, whereas the model with NH3 (with no arbitrary constraints; solid line) fits the data much better.
We now turn to each relevant atmosphere anomaly, first reviewing the data, and then how the presence of NH3 helps resolve the anomaly.
O2 in the clouds is a natural outcome of NH3 production.
Our model provides an explanation for the presence of O2 in the Venus cloud layers. O2 has been measured via in situ measurements (44, 45). The Pioneer Venus gas chromatography (GC) reported 43.6 ppm molecular oxygen (O2) in the clouds at 51.6 km, 16 ppm below the clouds at 41.7 km, and no detection of oxygen at 21.6 km (23). The Venera 14 GC detected 18 ppm O2 average between 35 and 58 km (24). [The Large Neutral Mass Spectrometer (LNMS) on Pioneer Venus showed a signal of 32 amu, but this was attributed to O2 ions formed from reaction of CO2 in the mass spectrometer (46), and therefore was considered unreliable. However, we emphasize that this uncertainty about the source of O2 is specific to mass spectrometry (47).] We also note that several ground-based observations attempted to provide upper limits for the abundance of O2 above the clouds (48, 49). The spectroscopic searches for O2 have been subjected to varying interpretations (16, 17) and are claimed to be difficult to reconcile with the in-cloud O2 abundance detected by both Pioneer Venus and Venera probes, because one expects to observe a gradient of O2 from above to below the clouds. Such discrepancies can only ultimately be resolved by new in situ measurements of O2 in the clouds of Venus.
In the past, the validity of O2 has been challenged based on thermodynamics. Initial studies of the atmosphere of Venus in the 1970s and 1980s assumed the atmosphere was at thermodynamic equilibrium. One author discounted O2 as follows (44): “We therefore conclude, that either we have to accept a strong disequilibrium state among CO, SO2, O2 and H2O in the lower atmosphere of Venus, or discard at least one of the measurements in order to save the assumption of thermodynamic equilibrium. The latter course is our preferred one.” Some subsequent studies followed this argument (17, 23, 36), although not all (50), and the author himself modified his opinion in a subsequent paper (45). By now, it has been accepted for over two decades that the atmosphere of Venus is not at thermodynamic equilibrium (25, 26, 51, 52), although Venus’s atmosphere is not as far from disequilibrium as Earth’s atmosphere is (51, 52). Recently, the reanalysis of the Pioneer Venus data showed the atmosphere was farther from equilibrium than previously thought, due the presence of a range of reduced gases (27). Still, the cause of the Venus atmosphere thermodynamic disequilibrium is one of the unsolved problems in Venus science (17).
If the chemistry of NH3 production is the source of O2, then our model predicts on order 1 ppm O2 in the cloud level between about 50 and 60 km; 1 ppm is 20-fold lower than the measured values (23, 24). However, the value of 1 ppm at lower altitudes is far greater (15 orders of magnitude) than predicted by our and other photochemistry models that exclude NH3. While there are no known nonbiological processes that could produce O2 locally in the clouds of Venus, we note, for future work, that other biological processes such as oxygenic photosynthesis could also be contributing to the overall O2 budget in the clouds.
It has been suggested that O2 could also be produced by lightning, which is consistent with O2’s presence in and below but not above the clouds (53). Lightning and coronal discharge can produce O2 in a CO2 + N2 atmosphere (54). A thermodynamic-based calculation suggests that the amount of O2 possibly produced by lightning is four to five orders of magnitude too low to explain the observations (SI Appendix, section 8.1 and Table S4). However, the efficiency of the production of O2 by lightning could be tested experimentally on Earth. It is possible that all the O2 detections summarized above were made as spacecraft fell through high-intensity storm regions (55), but it seems an unlikely coincidence for two or three separate probes to experience storms. In addition, any NH3 present in the clouds would be destroyed by the lightning, and only trace amounts would reform (SI Appendix, section 7.1). The thermal decomposition of H2SO4 to O2 and SO2 has been suggested as an industrial process (56), but it is unlikely under Venus conditions (SI Appendix, section 8.2 and Fig. S5).
At altitudes above the cloud level (∼62 km), no O2 has been detected, strongly suggesting a fractional abundance of less than 10−7 (48). Yet, all existing photochemical models predict significant molecular oxygen above the clouds (e.g., ref. 22) due to the instability of CO2 to photolysis. CO2 is dissociated into CO and O, which cannot rapidly recombine because the recombination reaction is spin forbidden. Some alternative pathway, involving, for example, OH chemistry, sulfur chemistry, or chlorine chemistry, is required to restore CO2 (see ref. 18), but none of these pathways are sufficient to draw above-cloud O2 below 1 ppm (22). This mismatch between the extremely low observed O2 levels above the clouds and the higher predicted levels is a well-known conundrum of Venus’s cloud layer chemistry. Our model provides a partial solution by predicting a reduced O2 level above the clouds compared to the same model without NH3 (Figs. 3 and 4).
Model output H2O and SO2 abundance profiles are consistent with observations.
Our photochemistry model with NH3 production is, together with the model it is based on (20), consistent with the observed H2O and SO2 abundance profiles in and above the clouds.
SO2 and H2O have been observed on many occasions by remote campaigns, orbiters, and in situ probes (reviewed in refs. 20 and 26). For example, the Visible and Infrared Thermal Imaging Spectrometer instrument on board Venus Express observed a mean abundance of H2O and SO2, below the clouds at 30 km to 40 km, to be ∼30 ppm and ∼150 ppm, respectively (57). The observed abundances of H2O and SO2 just below the clouds are consistent between remote, orbiter, and in situ observations (20). Recall, that the 5× excess SO2 over H2O should strip all the water out of the cloud layer, and hence remove all water above the clouds as well, a solution that is not consistent with observations. The Rimmer et al. (20) model uses cloud chemistry (NH3 or mineral bases) to strip the SO2 in the clouds. As a result, water remains in the clouds and above the clouds, which agrees with the remote, orbiter, and in situ observations of a few parts per million of H2O above the cloud layers (reviewed in ref. 20).
Within the Venus cloud layers, there is substantial difference among measurements of water abundance in the clouds as summarized by ref. 20, which may represent varied local conditions.
In models previous to the one described here, water vapor is removed at the cloud tops by reaction with SO3 to form sulfuric acid, which then condenses out to form the cloud droplets. Since there is more below-cloud SO2 than H2O, all the H2O above the clouds is removed, some models even showing complete depletion of H2O (33). Yet, this depleted H2O above the clouds does not match observations which show plenty of water vapor above the clouds (Figs. 3 and 4). Models previous to ours solve this problem with physically unrealistic numerical fixes, either including excess H2O below the clouds (21) or fixing the H2O abundance to observed values such that any reactions involving H2O do not consume any H2O (22). Most models avoid the water vapor abundance problem altogether by restricting the calculations only to a section of the atmosphere, above the clouds or below the clouds.
Critically important is that our model without NH3 (33) must, similarly to other models, impose nonphysical constraints on SO2 chemistry in order to make the SO2 gas abundance profile fit observations, specifically by adding an arbitrary removal rate for SO2 in the clouds tuned to fit the data.
We emphasize that our model that includes NH3 or another base (20) is the only model known that avoids artificial fixes of SO2 and H2O. To further emphasize this point, Figs. 3 and 4 include our very poorly fitting model gas abundance profiles without NH3 and without the artificial SO2 removal rate.
Below the clouds, our photochemical model with NH3 predicts the same H2O abundance as models without NH3, including previous models (e.g., ref. 43).
NH3 in the clouds and below the cloud layers is consistent with tentative observations.
NH3 is a necessary input for our photochemistry model; indeed, the input of NH3 is the core assumption of our hypothesis. We therefore discuss the tentative observations of NH3 on Venus.
The Venera 8 descent probe reported the presence of NH3 in the lower atmosphere of Venus. The estimated amounts from the signal are large and varied from 0.01 to 0.1%. (For further discussion on the validity of the Venera 8 NH3 detection, see SI Appendix, section 6.) A recent reassessment of the Pioneer Venus LNMS has also provided suggestive evidence for the presence of NH3 and its oxidation products in gas phase in the cloud decks of Venus (27).
The Venera 8 observations were largely discounted at the time because NH3 is not likely to be present if Venus’s atmosphere is in thermodynamic equilibrium (36). At least one author supported the plausibility of the presence of NH3 in the cloud layers: Florensky et al. (50), in the late 1970s, argued that the upper parts of the Venus troposphere do not necessarily have to be in chemical equilibrium and could contain a number of minor chemical species, including NH3 (45).
An additional argument against the plausibility of NH3 is that an atmosphere containing sulfuric acid droplets cannot contain a significant amount of a free base; all of the base, in this case NH3, would be sequestered in the droplets as ammonium ions. However, if the clouds have a pH of >0 and contain significant ammonium salts, then partial pressures of >1 ppm of free ammonia gas are expected over those droplets in the lower clouds (Materials and Methods and SI Appendix, section 5).
Our model provides a mechanism for the release of NH3 below the clouds. As the droplets gravitationally settle out of the atmosphere to higher temperatures, the droplet evaporates, and NH3 is released through the thermal decomposition of ammonium sulfate and ammonium sulfite. NH3 is subsequently oxidized to NOx and N2 (Fig. 2). We note that a NOx signal has been identified in the Pioneer Venus LNMS reanalyzed data (27).
Mode 3 cloud particles.
Measurements by the Pioneer Venus and Venera Probes indicate that the Mode 3 particles might not be spherical, and that their composition differs from pure concentrated sulfuric acid. (See SI Appendix, section 3 for a brief discussion of the observational support for nonspherical particles.)
If NH3 is the main neutralizing agent of the sulfuric acid cloud droplets, then the Mode 3 cloud particles in the lower clouds must be supersaturated in ammonium salts, with a small liquid phase, and therefore are not liquid droplets of concentrated sulfuric acid. Thus, the mechanism proposed here predicts that the Mode 3 particles in the lower cloud are solid or semisolid, and hence likely to be nonspherical.
Specifically, the Mode 3 (largest) cloud particles in the lower cloud must be 9.3 molar to 18.1 molar in ammonium salts in order to provide sufficient downward transport of SO2 to produce the observed drop in SO2 concentration across the clouds (Materials and Methods and SI Appendix, section 5). Such concentrations are not implausible if the Mode 3 particles in the cloud are actually a semisolid slurry of ammonium salts and sulfuric acid.
We note that presence of NH3 creating nonspherical Mode 3 particles is consistent with the Mode 1 and/or Mode 2 particles being of quite different composition than the Mode 3 particles. If NH3 production were the result of biological activity, then life could be confined to the larger Mode 3 particles, which have more volume. If NH3 was produced by a nonbiological process, then it would be expected to apply to particles of all sizes, and not discriminate in favor of Mode 3 particles. However, the data on particle size and shape is consistent with Mode 1 and 2 particles being spherical (29).
Our model also explains the presence of the so-called stagnant haze layer below the cloud decks (30 km to 47 km altitude) (9). If the large Mode 3 particles are made of mostly solid ammonium sulfite and ammonium sulfate, then evaporation of any residual H2SO4 at the cloud base leaves dry solid particles. The subsequent thermal disproportionation of the remaining salts generates gas that shatters the particles at the cloud base (∼100 °C at ∼47 km), and the fragmented particles form the haze. The haze that settles down and is not mixed back up into the clouds decomposes at ∼200 °C at the bottom of the stagnant haze layer at ∼30 km (Fig. 2). The layered structure and the altitudes of the boundaries between the layers is therefore a natural consequence of the ammonia-based cloud chemistry. See also ref. 7 for a discussion of the composition of the haze layer.
H2S below the clouds.
We also note that our model predicts the presence of H2S below the clouds (Figs. 2 and 3). The presence of H2S is consistent with the tentative detection of H2S below the clouds by the Venera 14 GC (24), which is the only in situ measured abundance value for H2S. If NH3 is present in the Venus atmosphere, H2S is a result of disproportionation of NH4HSO3 that yields NH3, H2S, and H2O to the atmosphere below the clouds, and hence is a unique output of our model. H2S was also tentatively identified in the recent reanalysis of the Pioneer Venus LNMS data (27). H2S, however, is a known volcanic gas on Earth so it is likely produced by volcanoes on Venus as well.
Discussion
Our model provides a view of the habitability of Venusian clouds. Concentrated sulfuric acid would make the Venusian cloud environment both chemically aggressive and extremely dry (7, 14). Our model removes the issue of extreme acidity for a subset of cloud particles from consideration.
Our model implies that the Mode 3 cloud particles cannot be all composed of concentrated H2SO4. Instead, there has to be a population of cloud particles that are less acidic and have a higher pH (between −1 and 1) than concentrated sulfuric acid. Specifically, our model predicts that the Mode 3 cloud particles are semisolid ammonium sulfites and sulfates (Fig. 2) with a pH as high as one (Fig. 1). We emphasize that not all droplets need to contain semisolid ammonium sulfite and (if the NH3 is made by life) ammonia-producing microorganisms.
Relevant to the Mode 3 cloud particles is a recent, independent finding that the Mode 3 cloud particle composition is not primarily sulfuric acid, but instead is consistent with some particles being ammonium hydrogen sulfate (NH4HSO4), as also predicted in our analysis. Mogul et al. (58) base this finding on a reanalysis of the Pioneer Venus legacy data on the refractive index of the Venusian cloud droplets, independent of atmospheric chemistry. Also, independently from our work presented here, Mogul et al. (58) have described the potential for phototropic synthesis of NH3 to neutralize sulfuric acid cloud droplets, leading to the Mode 3 particle possibly containing NH4HSO4.
A pH of zero to one is within the range of environments known from Earth to be habitable and, in fact, to be inhabited. Life can grow in acid (pH = 0) aqueous environments (35), and microbial growth in solutions as acidic as a pH = −0.5 has been claimed (59). Furthermore, most of the Mode 3 particles have been detected at altitudes in the temperature range (60 °C to 80 °C), a range that overlaps with environments known to harbor thermophilic acidophiles on Earth (with life that can grow in temperatures up to 100 °C; e.g., refs. 60–63).
Remarkably, examples of life on Earth secreting NH3 to neutralize a droplet-sized acidic environment exist. Pathogens such as Mycobacterium tuberculosis and Candida albicans can neutralize the interior of phagosomes (acid-containing vesicles inside cells used for digestion of captured organic material) by secreting ammonia, thus evading destruction (64–66). Some plant pathogens also secrete ammonia to neutralize local pH in their target plant cells (67). By contrast, pond-dwelling acidophilic microorganisms adapt to low pH in other ways, because it is implausible for them to neutralize an entire river or pond.
Challenges to life in the Venus atmosphere remain. The extreme aridity of the Venus cloud environment has been well known for decades (e.g., ref. 68), having been often described (e.g., refs. 7, 14, and 34), and most recently reviewed in ref. 69, and remains a significant challenge to life as we know it. Our model predicts a water vapor abundance mixing ratio of 10−5 in the lower clouds, that is, a relative humidity of 0.02% (depending on temperature). This is ∼50-fold lower than the lowest water activity known to support life on Earth. (We note that terrestrial life can survive extremely hot and dry environments as spores or other inactive forms, as summarized in the legend to Fig. 2 and SI Appendix, section 10, but these are not actively growing, and to survive an ecosystem requires at least some cells or organisms to be actively growing.) The range of in-cloud water vapor abundance mixing ratios reported in the literature is very large (5 ppm to 0.2%), as summarized by ref. 20, which may represent the presence of more clement local conditions. All global models may therefore represent an average of extremely arid “desert” regions and much more humid “habitable” regions.
The extreme aridity is a reflection of the very low number density of hydrogen atoms in the Venusian atmosphere. The scarcity of H atoms argues against the presence of life. Terrestrial biochemicals are typically ∼50% hydrogen by atom number (as illustrated by the database of natural products compiled by ref. 70; SI Appendix, section 9 and Table S5). However, much of the water in a bacterial cell is derived from reactions of the metabolites within the cell (71–73). For example, under active growth of Escherichia coli, up to 70% of the intracellular water is generated during metabolism and not transported across the membrane from the outside environment (71). If there is life on Venus, it is therefore likely to have substantially different biochemistry from Earth’s, and, if it is based on water as a solvent, it is likely to have very different strategies for water accumulation and retention to combat extreme aridity of the clouds. We note, however, that the lack of hydrogen is not just a challenge for the habitability of Venus’s clouds but also a challenge for making detectable amounts of any hydrogen-saturated gas-phase species, such as NH3, by any mechanism, abiotic or biological.
We note that additional challenges such as nutrient scarcity or high energy requirements are comparatively less limiting than aridity; for an in-depth discussion of the challenges to life in the Venusian clouds, see ref. 7.
An origin for life on Venus is an open question. If life exists in the Venus clouds, it may have originated on the Venus surface and migrated into the clouds. One model of Venus’s evolution to its modern state suggests that Venus had clement surface conditions after formation, only to have entered the current greenhouse runaway after up to 3.5 billion years (74, 75). This model is dependent on a range of specific conditions but, if correct, suggests that Venus in the past had similar conditions to those under which life originated on Earth. If life emerged on the surface, terrestrial precedent suggests that some organisms would adapt to living some of the time in the clouds (reviewed in ref. 7). The microbial acid-neutralizing strategy provides a facile evolutionary path to Venusian cloud life. As the Venus surface became increasingly hot and uninhabitable, cloud dwelling would become a permanent lifestyle.
As the atmospheric chemistry changed to high acidity, the cloud-dwelling organisms would adapt by neutralizing their droplet habitats. A plausible evolutionary path is therefore suggested by the unique role of a droplet environment in the acid-neutralizing strategy, and the proposed history of Venus. We note, however, that, if life is the source of NH3 on Venus, it very likely does not resemble the elemental ratios of life on Earth and likely has a different biochemistry than life on our planet, specifically adapted to the unique challenges of the Venusian cloud environment.
The Venus low D/H ratio (76) and the possible existence of felsic rocks which form in the presence of water (77–80) imply the presence of past Venus oceans, yet the debate on whether or not Venus ever had oceans continues. Recently, Turbet et al. (81) demonstrated, with a three-dimensional (3D) global climate model, that Venus may have been too hot early on for water oceans to form. Their climate model shows that the steam atmosphere of early Venus never condensed on the planet’s surface to form liquid water oceans. Instead, according to the model, water vapor condensed on the nightside of the planet to form clouds that warmed the surface by absorbing and reemitting the planet’s outgoing infrared radiation (81). However, Turbet et al. (81) do state that a comprehensive sensitivity study is needed to quantitatively confirm their result, as cloud and atmospheric circulation feedbacks can vary nonlinearly and nonmonotonically with rotation period. The newly selected VERITAS and EnVision missions, as well as DAVINCI’s instruments, should solidify or rule out the possibility of the past water-rich era of Venus, by a combination of D/H measurements and multispectral imaging of the tesserae regions for mineral compositions.
Summary and Critical Future Measurements
Our hypothesis of locally produced NH3 in the Venus clouds explains a number of anomalies in the atmosphere and clouds of Venus. Our photochemical model of the consequences of NH3 production explains the SO2 depletion in the clouds and vertical abundance profile of H2O, building on the work of ref. 20, explains the presence of O2 in the clouds, supports the in situ detection of H2S below the clouds, and explains the nonspherical nature of Mode 3 particles. While the presence of other mineral bases could contribute, none of them can explain the parts per million levels of O2 in the clouds or the tentative presence of NH3. No definitive source for NH3 has been identified; in chemical terms, biological production is the most plausible, but the concept of life in the clouds of Venus remains controversial. Many of the in situ observations should be repeated for confirmation, and more model work is needed to fully resolve the vertical abundance profiles of relevant gases.
We must be careful not to fall for a conjunction fallacy. While life may explain the combined anomalies with some external assumptions, there may yet be a chemical explanation for each individual anomaly.
An in situ Venus probe can support or refute our proposed view of Venus as an inhabited planet with the following measurements.
Gases.
-
•
Establish the existence of NH3 and O2 in the cloud layers.
-
•
Measure the amounts of NOx to establish which NH3-destruction pathway dominates.
-
•
Determine the specific altitude-dependent abundance profiles of H2O, SO2, and H2S, ideally with day and night measurements to inform chemistry sources and sinks.
Cloud Particles.
-
•
Confirm the nonspherical, semisolid nature of Mode 3 cloud particles and identify them as ammonia salts.
-
•
Measure the pH of cloud particles, especially Mode 3 cloud particles
-
•
Detect organic molecules in cloud particles; if found exclusively in the larger particles, this would be an indicator of life.
Search for Life.
-
•
Analyze a large number of individual cloud particles, especially Mode 3, for morphological and chemical signs of life.
In the meantime, a public release of original data from the Russian Venera and Vega missions could enable further support or refutation of current models and predictions, and would provide needed context for future mission results.
We have presented an initial analysis of several sources for the NH3 on Venus. We have argued that biological production may be a potential source of both NH3 and O2 that we have identified that meets the quantitative requirements for NH3 production. Although the biomass required to make NH3 and O2 at the required rate is not unrealistic, at 0.05% of the total biomass on Earth and ∼1.5% of the total Venusian cloud mass, life in the clouds of Venus has been considered implausible because of very high acidity, very low water activity, and scarcity of hydrogen atoms. By predicting a Mode 3 particle pH of −1 to 1 due to neutralizing NH3, our work implies both that Venus clouds are more habitable than previously thought and, by the requirement of locally produced NH3, that clouds may be inhabited. We hope our work will encourage further studies into habitability and astrobiological potential of Venusian clouds.
Materials and Methods
Photochemical Model.
The details of the model are provided in SI Appendix, section 4. In summary, we employ a 1D Lagrangian photochemistry/diffusion code that follows a single parcel as it moves from the bottom to the top of the atmosphere. The temperature, pressure, and actinic UV flux are prescribed at each altitude in the atmosphere (20).
Calculation of Flux of Ammonia.
We calculate the flux of ammonia necessary to maintain the observed gradient of SO2 through the clouds following the method of Rimmer et al. (20). The goal is to explain the removal of nearly all of 3.5·1015 cm−3 of SO2 (1.5·10−4 bar at 300-K level of the atmosphere) that should be present from upward mixing from volcanic sources and recycled SO2. The time taken for SO2 to mix through the region 45 km to 65 km is calculated using Lee et al.’s (82) equation 7,
In other words, SO2 will be replenished in the atmospheric cloud layers in 8.25 Earth years, and this is the timescale that the presence of NH3 needs to remove SO2. The atmospheric scale height H0 ≈ 6.5 × 105 cm is the average scale height in the atmospheric cloud layers, = 2 × 106 cm is the distance between 45 and 65 km, and Kzz = 104 cm2⋅s−1 is the eddy diffusion coefficient throughout the atmospheric cloud layers. The flux (per square centimeter per second) of SO2 into the clouds is therefore given by
Recall that there is a one-to-one molar ratio for NH3 to remove SO2. Given the mass of NH3, is the flux rate above is equivalent of 1.1 × 1011 tonnes per year of NH3.
Calculation of Concentration of NH3 in Particles.
The concentration of salts in the cloud droplets can be estimated from the concentration necessary to provide the flux of NH3 as calculated above. The necessary flux of NH3 is dependent on the size of the particles, and hence the particles’ rate of fall. For a given particle size, we can calculate the rate of fall, and hence the volume of cloud material removed per second, and, from this, the concentration of salts in that volume needed to provide the flux calculated above. See SI Appendix, section 2 for the details on the calculation of the concentration of ammonium salts in the lower cloud particles.
Calculation of Concentration of Gaseous NH3 over Droplets.
The concentration of gaseous NH3 over an acid droplet containing dissolved NH4+ was calculated as follows. The fraction of total N species that is present as NH3 and as NH4+ can be calculated from the acid dissociation constant (pKa) of NH3 as the pH. The concentration of NH3 over solution can be calculated from the solution concentration and Henry’s constant (SI Appendix, section 5 and Fig. S3). Both pKa and Henry’s constant are dependent on temperature.
Supplementary Material
Acknowledgments
P.B.R. thanks the Simons Foundation for funding (Simons Collaboration on the Origins of Life [SCOL] Award 599634). S.S. thanks the Change Happens Foundation and Breakthrough Initiatives for partial funding of this work. We are grateful to Vladimir Krasnopolsky for useful discussions on the presence of O2 in the atmosphere of Venus.
Footnotes
Reviewers: D.G., Planetary Science Institute; and T.P., Institute of Astrophysics, Pontificia Universidad Catolica.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110889118/-/DCSupplemental.
Data Availability
Previously published data were used for this work (20). All other study data are included in the article and/or SI Appendix.
Change History
January 21, 2022: The byline has been updated.
References
- 1.Morowitz H., Sagan C., Life in the clouds of Venus? Nature 215, 1259–1260 (1967). [Google Scholar]
- 2.Cockell C. S., Life on Venus. Planet. Space Sci. 47, 1487–1501 (1999). [Google Scholar]
- 3.Schulze-Makuch D., Irwin L. N., Reassessing the possibility of life on Venus: Proposal for an astrobiology mission. Astrobiology 2, 197–202 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Schulze-Makuch D., Grinspoon D. H., Abbas O., Irwin L. N., Bullock M. A., A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 4, 11–18 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Limaye S. S., et al. , Venus' spectral signatures and the potential for life in the clouds. Astrobiology 18 (2018), 1181–1198. [DOI] [PMC free article] [PubMed]
- 6.Schulze-Makuch D., The case (or not) for life in the Venusian clouds. Life (Basel) 11, 255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seager S., et al. , The Venusian lower atmosphere haze as a depot for desiccated microbial life: A proposed life cycle for persistence of the Venusian aerial biosphere. Astrobiology 21, 1206–1223 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Krasnopolsky V. A., Photochemistry of the Atmospheres of Mars and Venus (Springer Science & Business Media, 2013). [Google Scholar]
- 9.Titov D. V., Ignatiev N. I., McGouldrick K., Wilquet V., Wilson C. F., Clouds and hazes of Venus. Space Sci. Rev. 214, 1–61 (2018). [Google Scholar]
- 10.Jenkins J. M., Steffes P. G., Hinson D. P., Twicken J. D., Tyler G. L., Radio occultation studies of the Venus atmosphere with the Magellan Spacecraft. 2. Results from the October 1991 experiments. Icarus 110, 79–94 (1994). [Google Scholar]
- 11.Imamura T., et al. , Initial performance of the radio occultation experiment in the Venus orbiter mission Akatsuki Akatsuki. Earth Planets Space 69, 137 (2017). [Google Scholar]
- 12.Young A. T., Are the clouds of Venus sulfuric acid? Icarus 18, 564–582 (1973). [Google Scholar]
- 13.Barstow J. K., et al. , Models of the global cloud structure on Venus derived from Venus Express observations. Icarus 217, 542–560 (2012). [Google Scholar]
- 14.Bains W., Petkowski J. J., Zhan Z., Seager S., Evaluating alternatives to water as solvents for life: The example of sulfuric acid. Life (Basel) 11, 400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izenberg N. R., et al. , The Venus life equation. Astrobiology 21, 1305–1315 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Mills F. P., Allen M., A review of selected issues concerning the chemistry in Venus’ middle atmosphere. Planet. Space Sci. 55, 1729–1740 (2007). [Google Scholar]
- 17.Krasnopolsky V. A., Chemical composition of Venus atmosphere and clouds: Some unsolved problems. Planet. Space Sci. 54, 1352–1359 (2006). [Google Scholar]
- 18.Yung Y. L., Demore W. B., Photochemistry of the stratosphere of Venus: Implications for atmospheric evolution. Icarus 51, 199–247 (1982). [Google Scholar]
- 19.Parkinson C. D., et al. , Distribution of sulphuric acid aerosols in the clouds and upper haze of Venus using Venus Express VAST and VeRa temperature profiles. Planet. Space Sci. 113–114, 205–218 (2015). [Google Scholar]
- 20.Rimmer P. B., et al. , Hydroxide salts in the clouds of Venus: Their effect on the sulfur cycle and cloud droplet pH. Planet. Sci. J. 2, 133 (2021). [Google Scholar]
- 21.Winick J. R., Stewart A. I. F., Photochemistry of SO2 in Venus’ upper cloud layers. J. Geophys. Res. Space Phys. 85, 7849–7860 (1980). [Google Scholar]
- 22.Bierson C. J., Zhang X., Chemical cycling in the Venusian atmosphere: A full photochemical model from the surface to 110 km. J. Geophys. Res. Planets 125, e2019JE006 (2020). [Google Scholar]
- 23.Oyama V. I., et al. , Pioneer Venus gas chromatography of the lower atmosphere of Venus. J. Geophys. Res. Space Phys. 85, 7891–7902 (1980). [Google Scholar]
- 24.Mukhin L. M., et al. , VENERA-13 and VENERA-14 gas chromatography analysis of the Venus atmosphere composition. Sov. Astron. Lett. 8, 216–218 (1982). [Google Scholar]
- 25.Esposito L. W., Bertaux J. L., Krasnopolsky V. A., Moroz V. I., Zasova L. V., Chemistry of lower atmosphere and clouds. Venus II, 415–458 (1997). [Google Scholar]
- 26.Johnson N. M., de Oliveira M. R. R., Venus atmospheric composition in situ data: A compilation. Earth Space Sci. 6, 1299–1318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogul R., Limaye S. S., Way M. J., Cordova J. A., Venus’ mass spectra show signs of disequilibria in the middle clouds. Geophys. Res. Lett. 48, e2020GL091327 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surkov Y. A., Andrejchikov B. M., Kalinkina O. M., On the content of ammonia in the Venus atmosphere based on data obtained from Venera 8 automatic station. Akademia Nauk SSSR Doklady 213, 296–298 (1973),. [Google Scholar]
- 29.Knollenberg R. G., Hunten D. M., The microphysics of the clouds of Venus: Results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. Space Phys. 85, 8039–8058 (1980). [Google Scholar]
- 30.Knollenberg R. G., Clouds and hazes. Nature 296, 18 (1982). [Google Scholar]
- 31.Limaye S. S., et al. , Venus’ spectral signatures and the potential for life in the clouds. Astrobiology 18, 1181–1198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donahue T. M., Hodges R. R. Jr., Venus methane and water. Geophys. Res. Lett. 20, 591–594 (1993). [Google Scholar]
- 33.Greaves J. S., et al. , Phosphine gas in the cloud decks of Venus. Nat. Astron. 5, 655–664 (2021). [Google Scholar]
- 34.Bains W., et al. , Phosphine on Venus cannot be explained by conventional processes. Astrobiology 21, 1277–1304 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Mirete S., Morgante V., González-Pastor J. E., “Acidophiles: Diversity and mechanisms of adaptation to acidic environments” in Adaption of Microbial Life to Environmental Extremes, Stan-Lotter H., Fendrihan F., Eds. (Springer, 2017), pp. 227–251. [Google Scholar]
- 36.Goettel K. A., Lewis J. S., Ammonia in the atmosphere of Venus. J. Atmos. Sci. 31, 828–830 (1974). [Google Scholar]
- 37.Reed S. C., Cleveland C. C., Townsend A. R., Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 42, 489–512 (2011). [Google Scholar]
- 38.Burris R. H., Roberts G. P., Biological nitrogen fixation. Annu. Rev. Nutr. 13, 317–335 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Sprőber P., Shafik H. M., Présing M., Kovács A. W., Herodek S., Nitrogen uptake and fixation in the cyanobacterium Cylindrospermopsis raciborskii under different nitrogen conditions. Hydrobiologia 506, 169–174 (2003). [Google Scholar]
- 40.Phlips E. J., Zeman C., Hansen P., Growth, photosynthesis, nitrogen fixation and carbohydrate production by a unicellular cyanobacterium, Synechococcus sp.(Cyanophyta). J. Appl. Phycol. 1, 137–145 (1989). [Google Scholar]
- 41.Vyas D., Kumar H. D., Nitrogen fixation and hydrogen uptake in four cyanobacteria. Int. J. Hydrogen Energy 20, 163–168 (1995). [Google Scholar]
- 42.Bar-On Y. M., Phillips R., Milo R., The biomass distribution on Earth. Proc. Natl. Acad. Sci. U.S.A. 115, 6506–6511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasnopolsky V. A., Chemical kinetic model for the lower atmosphere of Venus. Icarus 191, 25–37 (2007). [Google Scholar]
- 44.Von Zahn U., Kumar S., Niemann H., Prinn R., “Composition of the Venus atmosphere” in Venus, Hunte D. M., Colin L., Donahue T. M., Moroz V. M., Eds. (University of Arizona Press, 1983), pp. 299–430. [Google Scholar]
- 45.Von Zahn U., Moroz V. I., Composition of the Venus atmosphere below 100 km altitude. Adv. Space Res. 5, 173–195 (1985). [Google Scholar]
- 46.Hoffman J. H., Hodges R. R., Donahue T. M., McElroy M. B., Composition of the Venus lower atmosphere from the Pioneer Venus mass spectrometer. J. Geophys. Res. Space Phys. 85, 7882–7890 (1980). [Google Scholar]
- 47.Newton A. S., Some observations on the mass spectrum of CO2. J. Chem. Phys. 20, 1330–1331 (1952). [Google Scholar]
- 48.Trauger J. T., Lunine J. I., Spectroscopy of molecular oxygen in the atmospheres of Venus and Mars. Icarus 55, 272–281 (1983). [Google Scholar]
- 49.Mills F. P., A spectroscopic search for molecular oxygen in the Venus middle atmosphere. J. Geophys. Res. Planets 104, 30757–30763 (1999). [Google Scholar]
- 50.Florensky C. P., Volkov V. P., Nikolaeva O. V., A geochemical model of the Venus troposphere. Icarus 33, 537–553 (1978). [Google Scholar]
- 51.Catling D. C., Bergsman D., “Using atmospheric composition as a metric for detecting life on habitable planets“ in AGU Fall Meeting Abstracts (American Geophysical Union, 2009), B11E-06.
- 52.Krissansen-Totton J., Bergsman D. S., Catling D. C., On detecting biospheres from chemical thermodynamic disequilibrium in planetary atmospheres. Astrobiology 16, 39–67 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Chameides W. L., Walker J. C. G., Nagy A. F., Possible chemical impact of planetary lightning in the atmospheres of Venus and Mars. Nature 280, 820–822 (1979). [Google Scholar]
- 54.Nna Mvondo D., Navarro-González R., McKay C. P., Coll P., Raulin F., Production of nitrogen oxides by lightning and coronae discharges in simulated early Earth, Venus and Mars environments. Adv. Space Res. 27, 217–223 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Delitsky M. L., Baines K. H., Storms on Venus: Lightning-induced chemistry and predicted products. Planet. Space Sci. 113, 184–192 (2015). [Google Scholar]
- 56.General Atomics, “Decomposition of sulfuric acid using solar thermal energy” (Rep. GA-A-17573, US Department of Energy, 1985).
- 57.Marcq E., et al. , A latitudinal survey of CO, OCS, H2O, and SO2 in the lower atmosphere of Venus: Spectroscopic studies using VIRTIS‐H. J. Geophys. Res. Planets 113, E00B07 (2008). [Google Scholar]
- 58.Mogul R., Limaye S. S., Lee Y. J., Pasillas M., Potential for phototrophy in Venus’ clouds. Astrobiology 21, 1237–1249 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Schleper C., Puhler G., Klenk H.-P., Zillig W., Picrophilus oshimae and Picrophilus torridus fam. nov., gen. nov., sp. nov., two species of hyperacidophilic, thermophilic, heterotrophic, aerobic archaea. Int. J. Syst. Evol. Microbiol. 46, 814–816 (1996). [Google Scholar]
- 60.Carrizo D., Sánchez-García L., Rodriguez N., Gómez F., Lipid biomarker and carbon stable isotope survey on the Dallol hydrothermal system in Ethiopia. Astrobiology 19, 1474–1489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rojas-Gätjens D., et al. , Temperature and elemental sulfur shape microbial communities in two extremely acidic aquatic volcanic environments. Extremophiles 25, 85–99 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Belilla J., et al. , Hyperdiverse archaea near life limits at the polyextreme geothermal Dallol area. Nat. Ecol. Evol. 3, 1552–1561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gómez F., et al. , Ultra-small microorganisms in the polyextreme conditions of the Dallol volcano, Northern Afar, Ethiopia. Sci. Rep. 9, 7907 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song H., et al. , Expression of the ompATb operon accelerates ammonia secretion and adaptation of Mycobacterium tuberculosis to acidic environments. Mol. Microbiol. 80, 900–918 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gouzy A., et al. , Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog. 10, e1003928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vylkova S., Lorenz M. C., Phagosomal neutralization by the fungal pathogen Candida albicans induces macrophage pyroptosis. Infect. Immun. 85, e00832-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alkan N., Fluhr R., Sherman A., Prusky D., Role of ammonia secretion and pH modulation on pathogenicity of Colletotrichum coccodes on tomato fruit. Mol. Plant Microbe Interact. 21, 1058–1066 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Donahue T. M., Hodges R. R. Jr., Past and present water budget of Venus. J. Geophys. Res. Planets 97, 6083–6091 (1992). [Google Scholar]
- 69.Hallsworth J. E., et al. , Water activity in Venus’s uninhabitable clouds and other planetary atmospheres. Nat. Astron. 5, 665–675 (2021). [Google Scholar]
- 70.Petkowski J. J., Bains W., Seager S., An apparent binary choice in biochemistry: Mutual reactivity implies life chooses thiols or nitrogen-sulfur bonds, but not both. Astrobiology 19, 579–613 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Kreuzer-Martin H. W., Ehleringer J. R., Hegg E. L., Oxygen isotopes indicate most intracellular water in log-phase Escherichia coli is derived from metabolism. Proc. Natl. Acad. Sci. U.S.A. 102, 17337–17341 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreuzer-Martin H. W., Lott M. J., Ehleringer J. R., Hegg E. L., Metabolic processes account for the majority of the intracellular water in log-phase Escherichia coli cells as revealed by hydrogen isotopes. Biochemistry 45, 13622–13630 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Li H., et al. , Probing the metabolic water contribution to intracellular water using oxygen isotope ratios of PO4. Proc. Natl. Acad. Sci. U.S.A. 113, 5862–5867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Way M. J., et al. , Was Venus the first habitable world of our solar system? Geophys. Res. Lett. 43, 8376–8383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Way M. J., Del Genio A. D., Venusian habitable climate scenarios: Modeling Venus through time and applications to slowly rotating Venus‐like exoplanets. J. Geophys. Res. Planets 125, e2019JE006276 (2020). [Google Scholar]
- 76.Donahue T. M., Hoffman J. H., Hodges R. R., Watson A. J., Venus was wet: A measurement of the ratio of deuterium to hydrogen. Science 216, 630–633 (1982). [DOI] [PubMed] [Google Scholar]
- 77.Mueller N., et al. , Venus surface thermal emission at 1 μm in VIRTIS imaging observations: Evidence for variation of crust and mantle differentiation conditions. J. Geophys. Res. Planets 113, E00B17 (2008). [Google Scholar]
- 78.Basilevsky A. T., et al. , Geologic interpretation of the near-infrared images of the surface taken by the Venus Monitoring Camera, Venus Express. Icarus 217, 434–450 (2012). [Google Scholar]
- 79.Gilmore M. S., Glaze L. S., “The oldest rocks on Venus: The importance of tessera terrain for Venus exploration“ in AGU Fall Meeting Abstracts (American Geophysical Union, 2013), P34A-01.
- 80.Hashimoto G. L., et al. , Felsic highland crust on Venus suggested by Galileo near‐infrared mapping spectrometer data. J. Geophys. Res. Planets 113, E00B24 (2008). [Google Scholar]
- 81.Turbet M., et al. , Day-night cloud asymmetry prevents early oceans on Venus but not on Earth. Nature 598, 276–280 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Lee G., Helling C., Dobbs-Dixon I., Juncher D., Modelling the local and global cloud formation on HD 189733b. Astron. Astrophys. 580, A12 (2015). [Google Scholar]
- 83.Amend J. P., Shock E. L., Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and bacteria. FEMS Microbiol. Rev. 25, 175–243 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Gilliland A. A., Johnson W. H., Heats of formation of lithium perchlorate, ammonium perchlorate, and sodium perchlorate. J. Res. Natl. Bur. Stand., A Phys. Chem. 65A, 67–70 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer R., Köhler J., Homburg A., Explosives (John Wiley, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Previously published data were used for this work (20). All other study data are included in the article and/or SI Appendix.