Abstract
Background and Aims:
Whether gastric emptying tests predict longitudinal outcomes in patients with symptoms of gastroparesis is unclear. We aimed to determine whether baseline gastric emptying tests and gut motility parameters could impact longitudinal symptom(s) and quality of life (QOL) in a prospective, observational cohort study of patients with symptoms of gastroparesis.
Methods:
One hundred fifty patients with gastroparesis symptoms underwent simultaneous scintigraphy (GES) and wireless motility capsule (WMC) measurement of gastric emptying and other motility parameters. Patient Assessment of Upper Gastrointestinal Symptoms and Quality of Life were administered at baseline, and 3 and 6 months after testing. Multivariable generalized linear marginal models were fit to determine which baseline parameters predict longitudinal changes in symptoms and QOL.
Results:
Overall upper GI symptoms and QOL scores were moderate in severity at baseline and significantly improved over 6 months. Clinical variables, including female gender, harder stools by Bristol stool form score, and presence of functional dyspepsia (FD) by Rome III criteria, were predictive of more severe upper GI symptoms. Even after controlling for these clinical factors, delayed gastric emptying by GES or WMC was associated with worse symptom severity and QOL scores. Low gastric and elevated small bowel contractile parameters by WMC were also independently associated with more severe upper GI symptoms and worse QOL scores.
Conclusions:
Baseline features, including demographic and clinical variables, delayed gastric emptying and abnormal gastrointestinal contractility, were independent predictors of more severe longitudinal symptoms and worse quality of life outcomes. These factors may help to risk stratify patients and guide treatment decisions. ClinicalTrials.gov no: NCT02022826.
Keywords: Gastric emptying, gastrointestinal motility, longitudinal outcomes, scintigraphy, wireless motility capsule
INTRODUCTION:
Gastric motility studies are frequently performed to investigate symptoms suggestive of gastroparesis. However, previous studies reported poor correlation between rates of gastric emptying and symptoms.1,2 As such, current guidelines do not recommend routine motility studies for patients with chronic dyspeptic symptoms.1 However, prior studies were plagued by heterogeneity in the methodology in evaluating gastric emptying and reporting severity of upper GI symptoms.3 Indeed, a recent systematic review suggested that delayed gastric emptying, when measured correctly, is associated with increased symptom severity.4 Despite this, it is still unclear to what extent, and in which populations, gastric motility tests are helpful in predicting outcomes and/or guiding management.
Furthermore, while previous studies have significantly improved our knowledge of clinical and demographic features associated with outcomes in gastroparesis,5 these studies were performed predominantly in patients with known delayed gastric emptying. It is unclear if similar features are associated with longitudinal outcomes in patients with symptoms, but no prior diagnosis, of gastroparesis.
In a prospective, multicenter study of patients with suspected gastroparesis, we aimed to determine whether baseline factors, including demographic/clinical features, gastric emptying by gastric emptying scintigraphy (GES) and/or wireless motility capsule (WMC), as well as gastric and small bowel contractility by WMC were predictive of longitudinal changes in gastroparesis symptoms and quality of life.
METHODS:
Study Population:
We performed a prospective, observational cohort study of 167 adult subjects with ≥2 typical symptoms of gastroparesis (nausea/vomiting/retching, fullness/early satiety, bloating/abdominal distention, upper abdominal discomfort/pain) for ≥12 weeks. Subjects were recruited prospectively at 10 academic and community centers in the US from 2013 to 2016 (see Supplemental Methods).6 Subjects underwent gastric emptying testing at baseline followed by treatment recommendations as per the judgment of the treating physician.7 Subjects were seen in follow-up study visits at 3 and 6 months to determine changes in symptom scores and quality of life.
Assessment of Gastrointestinal Transit and Contractile Parameters:
All subjects underwent simultaneous GES and WMC testing at baseline (see Supplemental Methods).6 Patients were instructed to discontinue opioids, cannabinoids, prokinetics, or other medications that may influence gastrointestinal motility for at least 72h prior to gastric emptying testing. Delayed gastric emptying by GES was defined by >10% retention at 4 hours.8 Delayed gastric emptying time (GET) by WMC was defined as >5 hours for passage of the capsule into the duodenum.9 Generalized and global transit delays were defined as delays in at least two or all three gastrointestinal regions, respectively (stomach, small bowel, and/or colon).
Number of contractions (Ct) and motility index (MI) by WMC were quantified in the hour before and after GET to determine gastric and small bowel contractile parameters.6
Quantification of Outcomes:
We have reported portions of this study (ClinicalTrials.gov: NCT02022826), including validation of WMC.6,7 This is a subsequent analysis from the data of the original 150 patients previously included in a separate manuscript looking at the influence of motility test results on management decisions.7 This current manuscript aimed to evaluate the influence of baseline factors on longitudinal outcomes, which were a priori planned study endpoints (Supplemental Methods).
Primary outcomes:
The primary outcomes were changes in upper gastrointestinal symptom severity and quality of life scores. Upper gastrointestinal symptom severity was quantified by validated measures, including Patient Assessment of Upper Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM)10 and Gastroparesis Cardinal Symptom Index (GCSI),11 which range from 0 (no symptoms) to 5 (most severe). The GCSI is validated for use in gastroparesis but symptoms of gastroparesis are also found in large subsets of dyspeptic patients with normal gastric emptying.11 The Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life (PAGI-QOL), a validated questionnaire ranging from 0 (poor) to 5 (excellent), was utilized to quantify changes in quality of life.12 All surveys were administered at baseline and then repeated at 3 and 6 months.
Secondary Outcomes:
Secondary outcomes included changes in individual symptom scores by GCSI or PAGI-SYM subscales, including nausea/vomiting/retching, postprandial fullness/early satiety, bloating/abdominal distention, and upper abdominal pain.
Other Assessments:
Patients completed questionnaires at baseline to determine the presence of functional gastrointestinal disorders by Rome III criteria, including functional bowel disorders, functional dyspepsia (FD), and functional nausea/vomiting/belching disorders.13 Bristol Stool Form Scale (BSFS) questionnaires were completed at baseline and 3 months to measure changes in stool consistency.14
Statistical Analysis:
Subjects were included for analysis if they had gastric emptying data from GES or WMC available for review and completed surveys to quantify symptoms and QOL. Baseline characteristics were compared using t-tests for continuous data, one-way analysis of variance (ANOVA) and Tukey’s test for post-hoc analyses for continuous data from more than two groups, and Fisher’s exact test for categorical variables. All analyses were performed using R (v3.6.1). A two-tailed P-value < .05 was considered significant.
Several exploratory methods were employed to model gastric and small bowel contractility parameters by WMC, including as continuous variables; above/below 5th percentile value for healthy controls;15 above/below median values; and grouped into quartiles (<25th percentile values referred to as low Ct or MI, between 25th-75th percentile values referred to as normal Ct or MI, and >75th percentile values referred to as high Ct or MI) to explore the best model fit.
Unadjusted and adjusted linear regression models were fit to assess for multiple and possibly confounding variables that associate with outcomes. Generalized estimating equations (GEE) with an auto-regressive correlation structure were employed using the R package geepack (v1.2.1)16 to estimate population-averaged effects while controlling for correlated data from repeated measures in individual subjects and clustering effects by different centers. Covariates with a P-value <.10 by unadjusted analyses were included in multivariable models. Final multivariable models were selected by a backward stepwise regression method which resulted in the lowest quasi-likelihood under the independence model information criteria (QIC) goodness-of-fit statistic.17 Regression results were reported as coefficients with 95% confidence intervals (CIs) representing the mean change in symptom or QOL scores over a 6-month period. Sensitivity analyses were also performed to determine whether treatment effects and/or center-specific variability influenced results from the final multivariable model. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS:
Baseline Characteristics:
Of 167 subjects enrolled in the study (Supplemental Figure 1), 150 subjects had baseline GES or WMC results available and provided symptom and QOL scores (Table 1). One-hundred eighteen subjects (78.7%) were female while 107 (71.3%) were non-diabetic. One-hundred nine subjects (75.7%) met Rome III criteria for functional dyspepsia (FD). Thirty-six subjects (24.2%) had delayed gastric emptying by GES (>10% retention at 4h) and 53 (35.6%) had delayed gastric emptying time (GET > 5h) by WMC. The 25th, median, and 75th percentile values for gastric and small bowel Ct and MI are shown in Table 1.
Table 1.
Baseline Characteristics
| Suspected Gastroparesis N=150 subjects |
|
|---|---|
| Age, median (IQR) | 44.5 (22.8) |
| Female gender, n (%) | 118 (78.7) |
| BMI (kg/m2), median (IQR) | 27.2 (10.7) |
| Etiology, n (%) | |
| Infectious prodrome, n (%) | 17 (11.3) |
| Duration of symptoms (months), median (IQR) | 36 (53.5) |
| Marijuana use, n (%) | 11 (7.3) |
| Opioid use, n (%) | 18 (12.0) |
| Rome III criteria for FBD, n (%) | 117 (80.7) |
| Rome III criteria for FD, n (%) | 109 (75.7) |
| Rome III criteria for N/V/Belching Disorders, n (%) | 86 (59.7) |
| Baseline BSFS (range 1–7), median (IQR) | 4.0 (3) |
| Delayed GES, n (%) | |
| Severely delayed GES (> 35% retention at 4h), n (%) | 11 (7.4) |
| Delayed GET, n (%) | 53 (35.6) |
| Severely delayed GET (GET > 12h), n (%) | 21 (14.1) |
| Delayed SBTT, n (%) | 33 (22.6) |
| Delayed CTT, n (%) | 45 (30.8) |
| Generalized transit delay (transit delays in ≥2 regions), n (%) | 17 (11.6) |
| Global transit delay (transit delays in all 3 regions), n (%) | 8 (5.5) |
| Gastric Ct, median (IQR) | 48.0 (59.5) |
| Gastric MI, median (IQR) | 11.4 (2.5) |
| Small bowel Ct, median (IQR) | 111.0 (127.5) |
| Small bowel MI, median (IQR) | 12.6 (2.7) |
BMI, body mass index; BSFS, Bristol Stool Form Scale; Ct, contraction frequency; CTT, colonic transit time by wireless motility capsule; FBD, functional bowel disorder by Rome III criteria; FD, functional dyspepsia by Rome III criteria; GES, gastric emptying scintigraphy; GET, gastric emptying time by wireless motility capsule; MI, motility index; N/V/Belching, functional nausea/vomiting and/or belching disorder by Rome III criteria; SBTT, small bowel transit time by wireless motility capsule.
Longitudinal Changes in Upper Gastrointestinal Symptoms and Quality of Life:
In the entire cohort, overall GCSI scores were moderate in severity at baseline and decreased significantly over time (P<.0001 by ANOVA) (Supplemental Figure 2A). Patients showed moderate impairment in QOL at baseline but improved significantly over time (P=.0004 by ANOVA) (Supplemental Figure 2B). GCSI scores showed a strong negative correlation with PAGI-QOL scores (r=−0.75, P<.0001).
Variables Associated with Overall GCSI Scores:
Demographic Variables:
Female patients and presence of FD by Rome III criteria were associated with worse overall GCSI scores (Figure 1A–B). Other demographic variables including gastroparesis etiology, body mass index (BMI), marijuana or opioid use (Supplemental Figure 3A–D) were not associated with changes in overall GCSI scores.
Figure 1. Female gender, presence of functional dyspepsia, and delayed gastric emptying are associated with worse overall GCSI scores longitudinally.
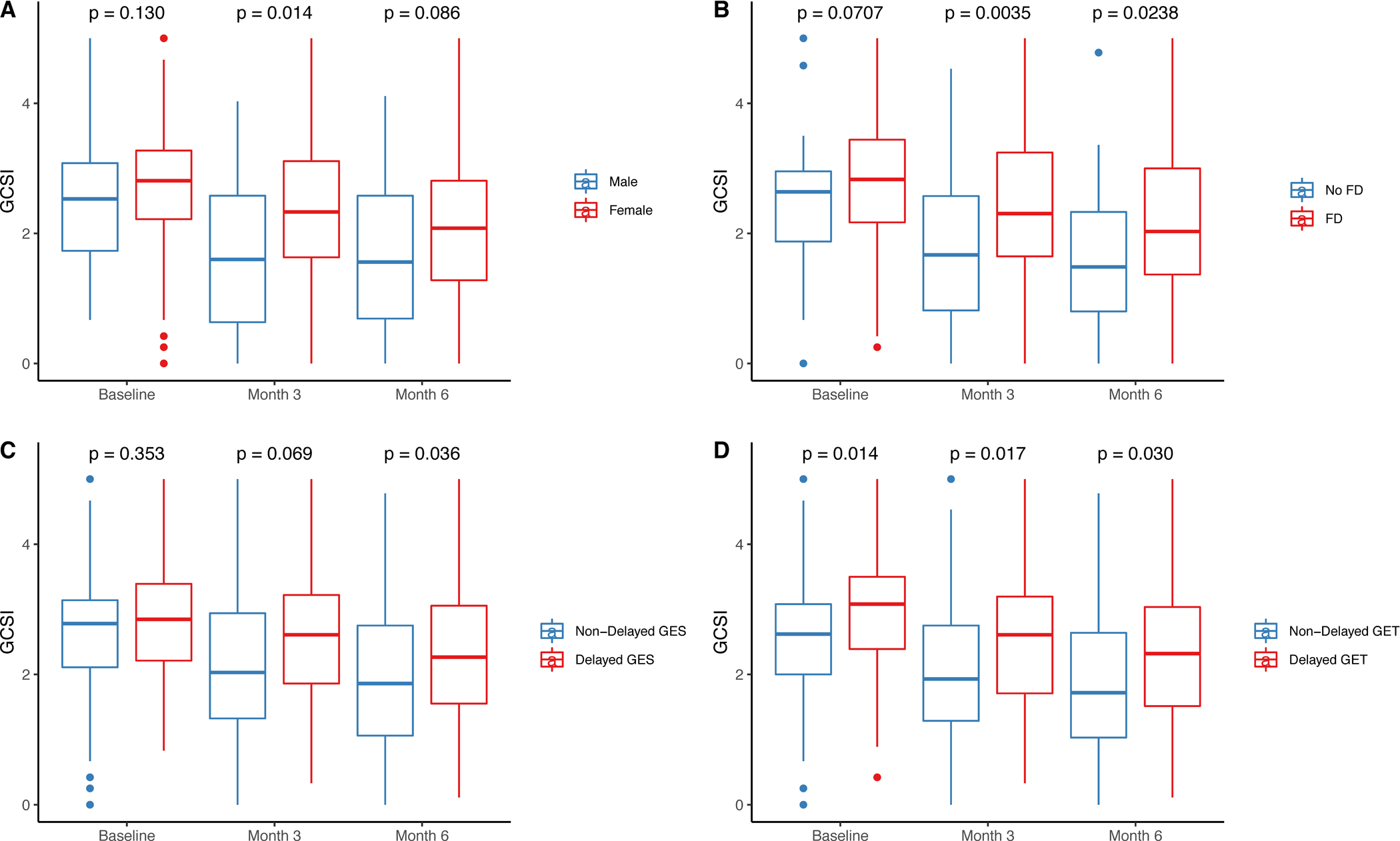
(A) Females (red) showed more severe GCSI scores at 3 months (P=.01) and 6 months (P=.09) compared with males (blue). (B) Functional dyspepsia (FD) by Rome III criteria (red) was associated higher GCSI scores at baseline (P=.07), 3 months (P=.004) and 6 months (P=.02) compared with non-FD patients (blue). (C) Patients with delayed gastric emptying by GES (red) have higher GCSI scores at 3 months (P=.07) and 6 months (P=.04) compared with non-delayed GES (blue). (D) Patients with delayed gastric emptying time (GET) by WMC (red) show higher GCSI scores at baseline (P=.01), 3 months (P=.02), and 6 months (P=.03) compared with non-delayed GET (blue).
Gastrointestinal Transit Measures:
Delayed gastric emptying by GES and delayed GET by WMC were associated with worse overall GCSI scores (Figure 1C–D). SBTT and CTT (Supplemental Figure 4A–B) were not associated with differences in upper gastrointestinal symptom severity. Global, but not generalized, transit delays showed differences in overall GCSI scores compared to delayed GET and/or no transit delays (Supplemental Figure 4C–D).
Gastrointestinal Contractile Parameters:
Patients with low gastric Ct or gastric MI (< 25th percentile) showed more severe overall GCSI scores compared to patients with normal gastric Ct or MI (between 25th and 75th percentile) (Supplemental Figure 5A–B).
Patients with small bowel Ct and MI above median values had worse overall GCSI scores compared to those with small bowel Ct or MI below median values (Supplemental Figure 5C–D).
Primary Outcomes:
Predictors of Longitudinal Changes for Overall GCSI Scores:
Unadjusted Estimates for Overall GCSI Scores:
Several candidate variables were identified by unadjusted GEE models for inclusion into the final multivariable model. Male gender (P<.05), increased BSFS (P=.02), and small bowel MI below median (P=.03) were associated with greater improvements in overall symptoms (Table 2, Supplemental Figure 6A). Presence of FD by Rome III criteria (P=.009), two transit measures (delayed gastric emptying by GES [P=.02] and WMC [P=.003]), and three contractile parameters (low gastric Ct [P=.01], low gastric MI [P=.04], and high small bowel MI [P=.03]) were associated with worse longitudinal GCSI scores.
Table 2.
Unadjusted and Adjusted Estimates for Predicting Longitudinal Changes in Overall GCSI Scores
| Unadjusted Estimates | Adjusted Estimates | |||
|---|---|---|---|---|
| Mean Difference in Total GCSI over 6-Months (95% CI) | P-value | Mean Difference in Total GCSI over 6-Months (95% CI) | P-value | |
| Variables Associated with Improvement in GCSI Scores Over 6-Months | ||||
| Male gender | −0.41 (−0.81, −0.01) | <.05 | −0.45 (−0.85, −0.06) | .03 |
| Small bowel MI below median value (< 12.7) | −0.33 (−0.62, −0.04) | .03 | −0.59 (−0.88, −0.30) | .0001 |
| BSFS (per every 1-unit increase) | −0.08 (−0.14, −0.01) | .02 | −0.08 (−0.14, −0.01) | .02 |
| Variables Associated with Worsening of GCSI Scores Over 6-Months | ||||
| Delayed GES | 0.39 (0.06, 0.73) | .02 | ||
| Delayed GET | 0.44 (0.15, 0.73) | .003 | 0.45 (0.15, 0.75) | .004 |
| Meets Rome III criteria for FD | 0.49 (0.12, 0.85) | .009 | 0.40 (0.07, 0.72) | .02 |
| Gastric Ct below 25th percentile (< 27) (compared to Ct between 25th and 75th percentile) | 0.43 (0.11, 0.76) | .01 | 0.55 (0.22, 0.88) | .001 |
| Gastric MI below 25th percentile (< 10.1) (compared to MI between 25th and 75th percentile) | 0.37 (0.02, 0.72) | .04 | ||
| Small bowel MI above 75th percentile (> 13.8) (compared to MI between 25th and 75th percentile) | 0.41 (0.05, 0.78) | .03 | ||
BSFS, Bristol stool form scale; Ct, Contraction frequency; FD, functional dyspepsia; GCSI, gastroparesis cardinal symptom index; GES, gastric emptying scintigraphy; GET, gastric emptying time by wireless motility capsule; MI, motility index.
Final Multivariable Model for Overall GCSI Scores:
A final multivariable model was comprised of three variables associated with greater improvement in overall GCSI scores, including male gender [P=.03], small bowel MI below median value [P=.0001], and increased BSFS [P=.02] (Table 2, Supplemental Figure 6B, Figure 2A). Additionally, three variables were associated with worse GCSI scores longitudinally, including delayed GET [P=.004], presence of FD [P=.02], and low gastric Ct [P=.001].
Figure 2. Predictors of overall upper GI symptom severity and quality of life scores by final multivariable models.
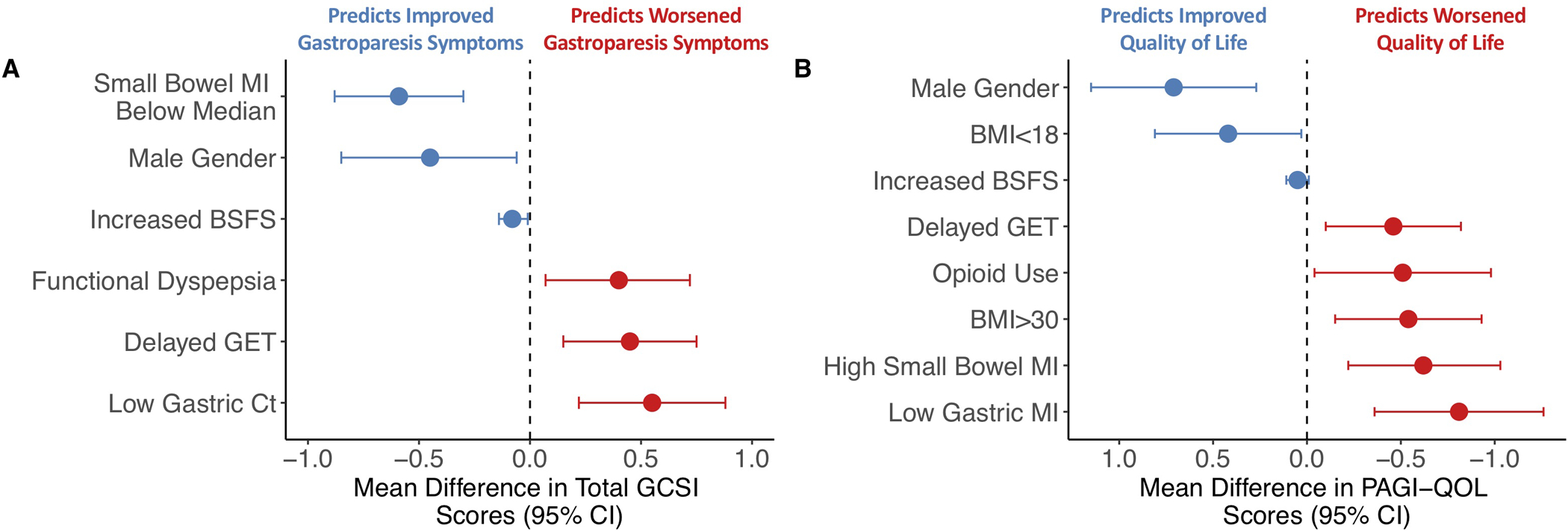
(A) Estimated mean change in overall GCSI scores with 95% confidence intervals (CI) based on final multivariable model. Negative values indicate factors predicting improvement (blue) while positive values indicate worsening (red) of overall GCSI scores. (B) Estimated mean differences (circles) in quality of life with accompanying 95% CIs based on final multivariable model. Positive values indicate factors predicting improvement (blue) while negative values indicate worsening (red) of PAGI-QOL scores.
Sensitivity Analysis to Determine Influence of Therapies and/or Center-Specific Variability on Final Model for Overall GCSI scores:
No significant changes were observed in the final model after adjusting for use of different therapies, including prokinetic agents, neuromodulators, anti-emetics, laxatives, and dietary therapies, as well as center-specific variability (Supplemental Table 1).
Predictors of Longitudinal Changes in QOL:
Unadjusted Estimates for PAGI-QOL Scores:
Candidate predictors associated with greater QOL improvements, included male gender [P=.09] and increased BSFS [P=.08] by unadjusted GEE models (Table 3, Supplemental Figure 6A). Four clinical variables (obese BMI [P=.06], opioid use [P=.06], and presence of FD [P=.003] and N/V/belching disorders [P<.10] by Rome III criteria), two transit measures (delayed gastric emptying by GES [P=.01] and WMC [P=.09]), and three contractile variables (low gastric Ct [P=.01] or MI [P=.01], and high small bowel MI [P=.08]) were associated with worse longitudinal QOL scores.
Table 3.
Unadjusted and Adjusted Estimates for Predicting Longitudinal Changes in Quality of Life Scores
| Unadjusted Estimates | Adjusted Estimates | |||
|---|---|---|---|---|
| Mean Difference in PAGI-QOL over 6-Months (95% CI) | P-value | Mean Difference in PAGI-QOL over 6-Months (95% CI) | P-value | |
| Variables Associated with Improvement in Quality of Life Scores Over Time | ||||
| Male gender | 0.43 (−0.07, 0.92) | .09 | 0.71 (0.27, 1.15) | .002 |
| BSFS (per every 1-unit increase) | 0.06 (−0.007, 0.12) | .08 | 0.05 (−0.01, 0.11) | <.10 |
| BMI < 18 (compared to 18 ≤ BMI ≤ 30) | 0.42 (0.03, 0.81) | .03 | ||
| Variables Associated with Worsening of Quality of Life Scores Over Time | ||||
| Opioid use | −0.47 (−1.00, 0.05) | .06 | −0.51 (−0.98, −0.04) | .03 |
| BMI > 30 (compared to 18 ≤ BMI ≤ 30) | −0.38 (−0.78, 0.02) | .06 | −0.54 (−0.93, −0.15) | .007 |
| Meets Rome III criteria for FD | −0.63 (−1.04, −0.22) | .003 | ||
| Meets Rome III criteria for N/V/belching disorders | −0.32 (−0.69, 0.06) | <.10 | ||
| Delayed GES | −0.53 (−0.94, −0.12) | .01 | ||
| Delayed GET | −0.31 (−0.66, 0.05) | .09 | −0.46 (−0.82, −0.10) | .01 |
| Gastric Ct below 25th percentile (< 27) (compared to Ct between 25th and 75th percentile) | −0.54 (−0.95, −0.13) | .01 | ||
| Gastric MI below 25th percentile (< 10.1) (compared to MI between 25th and 75th percentile) | −0.54 (−0.97, −0.12) | .01 | −0.81 (−1.26, −0.36) | .0004 |
| Small bowel MI above 75th percentile (> 13.8) (compared to MI between 25th and 75th percentile) | −0.39 (−0.82, 0.05) | .08 | −0.62 (−1.03, −0.22) | .003 |
BMI, body mass index (kg/m2); BSFS, Bristol stool form scale; Ct, contraction frequency; FD, functional dyspepsia by Rome III criteria; GES, gastric emptying scintigraphy; GET, gastric emptying time measured by wireless motility capsule; MI, motility index; PAGI-QOL, patient assessment of upper gastrointestinal disorders-quality of life.
Final Multivariable Model for PAGI-QOL Scores:
A final multivariable model identified male gender (P=.002), increased BSFS (P<.10), and underweight BMI (P=.03) were associated with greater QOL improvements (Table 3, Supplemental Figure 6B, Figure 2B). Opioid use (P=.03), obese BMI (P=.007), delayed gastric emptying by WMC (P=.01), and two contractility parameters (low gastric MI [P=.0004], high small bowel MI [P=.003]) were associated with worse longitudinal QOL scores.
Secondary Outcomes:
Predictors of Longitudinal Changes in Individual Symptoms:
Candidate predictors based on unadjusted GEE models for individual GCSI subscores are presented in Supplemental Tables 2–5 and Supplemental Figure 6A.
GCSI-Nausea/Vomiting Subscale:
A final multivariable model revealed greater improvements in nausea/vomiting subscores with high gastric MI (P=.02) while marijuana use (P=.006), presence of FD (P=.03), delayed WMC GET (P=.006), and high small bowel MI (P=.02) were associated with worse nausea/vomiting scores (Supplemental Table 2, Supplemental Figure 6B, Figure 3A).
Figure 3. Predictors of individual gastroparesis symptom scores by final multivariable models.
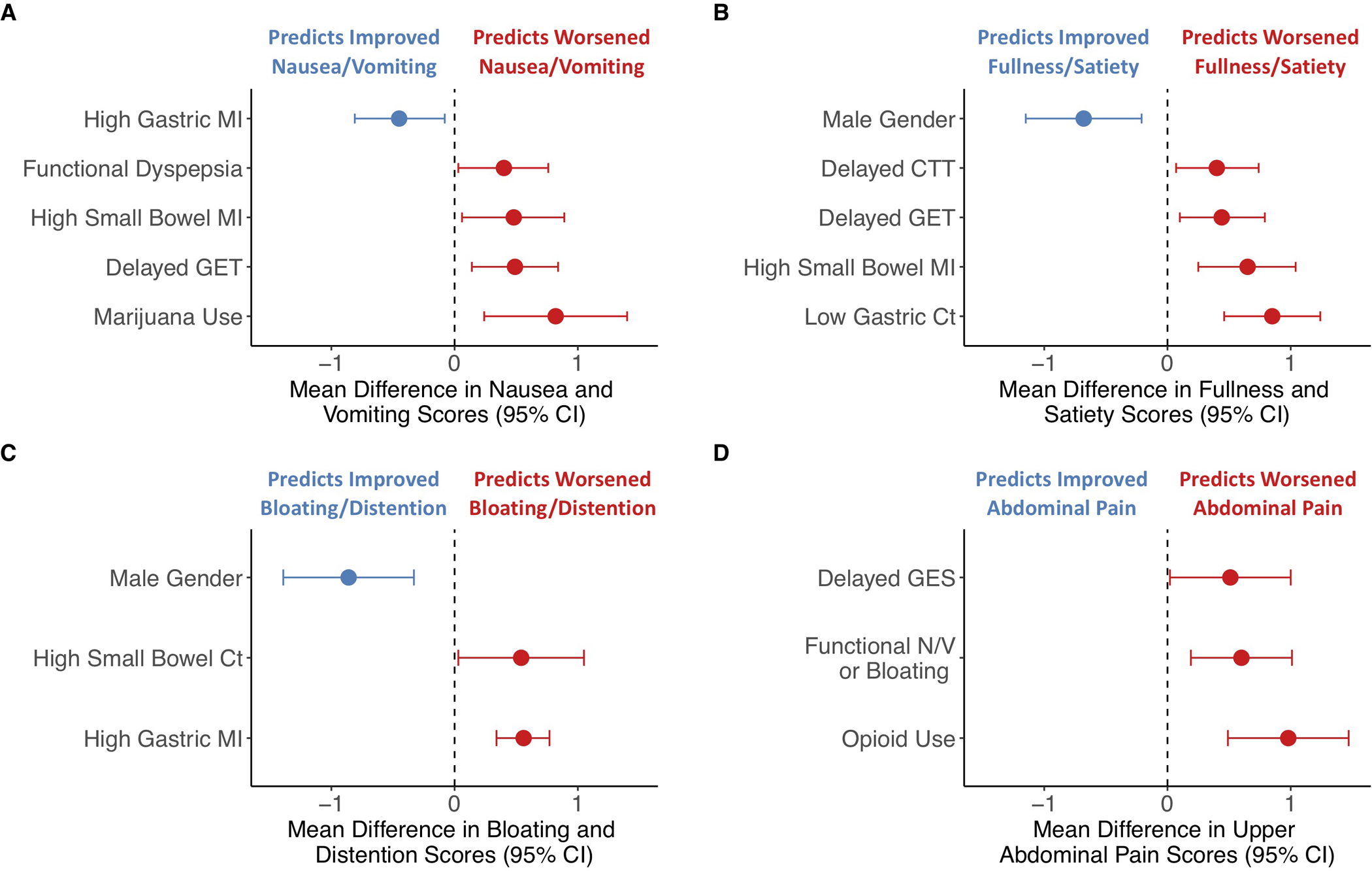
Estimated mean change in individual symptom severity scores with accompanying 95% CIs based on final multivariable models are shown including (A) nausea/vomiting; (B) fullness/satiety; (C) bloating/distention; and (D) upper abdominal pain. Negative values indicate factors predicting improvement (blue) while positive values indicate worsening (red) of individual gastroparesis symptoms.
GCSI-Fullness/Early Satiety Subscale:
A final multivariable model showed greater improvements in fullness/early satiety symptoms with male gender [P=.005] while worse fullness/early satiety subscores were observed with two transit measures (delayed WMC GET [P=.01] and CTT [P=.02]) and two contractility parameters (low gastric Ct [P<.0001], high small bowel MI [P=.001]) (Supplemental Table 3, Supplemental Figure 6B, Figure 3B).
GCSI-Bloating/Distention Subscale:
A final multivariable model including male gender [P=.002] was associated with greater improvement in bloating/distention subscores while increased gastric MI (P<.0001) and high small bowel Ct (P=.04) were associated with more severe bloating/distention (Supplemental Table 4, Supplemental Figure 6B, Figure 3C).
PAGI-SYM Upper Abdominal Pain Subscale:
A final multivariable model identified opioid use (P<.0001), presence of nausea/vomiting/belching disorders (P=.004) and delayed gastric emptying by GES (P=.04) were associated with more severe upper abdominal pain (Supplemental Table 5, Supplemental Figure 6B, Figure 3D).
DISCUSSION:
In this prospective investigation, we determined that baseline factors were associated with longitudinal symptom and QOL outcomes. Specifically, we found clinical variables, including gender, body mass, opioid/marijuana use, stool form, and functional dyspepsia status, were associated with longitudinal changes in symptom and QOL scores. However, even after controlling for these clinical variables, we found that delayed gastric emptying as well as abnormal gastrointestinal contractile parameters were independently predictive of worse upper gastrointestinal symptoms and QOL.
Because of the significant overlap in clinical presentations between gastroparesis and FD,1 the utility of performing motility testing has not been clearly defined. Similar to previous studies,1,2 we found that symptoms of gastroparesis do not accurately discriminate between normal and delayed gastric emptying as only ~36% of patients in our cohort had delayed gastric emptying while 76% of patients met criteria for FD. A prior study in FD demonstrated that delayed gastric emptying was independently associated with symptoms of postprandial fullness only but was not associated with quality of life measures.18 However, this study utilized a non-standardized definition for delayed gastric emptying (>6.3% scintigraphic retention) which may be overly sensitive. Furthermore, the study design was cross-sectional and could not determine whether delayed gastric emptying may predict longitudinal outcomes. In contrast, our analyses showed that patients satisfying Rome III criteria for FD have worse upper GI symptoms and QOL scores. However, even after adjusting for presence of FD, we found that delayed gastric emptying was associated with worse longitudinal outcomes. This suggests that gut motility testing provides clinically useful information independent from symptom-based criteria.
Our findings offer additional insights into the association between delayed gastric emptying and longitudinal outcomes in patients with symptoms of gastroparesis. First, delayed gastric emptying by either WMC or GES showed similar effects by unadjusted analyses. Although they are not measuring identical physiologies of gastric emptying,19 delayed gastric emptying measured by either modality may have similar negative effects on longitudinal outcomes. Secondly, delayed gastric emptying by WMC was a more important predictor for changes in upper gastrointestinal symptom severity and QOL by multivariable models. We speculate this is related to the 10% additional diagnostic yield of WMC compared with GES previously reported by our group.6 Thirdly, the overall impact of gastric emptying delays on upper gastrointestinal symptom severity and QOL scores was relatively modest, which suggests that other variables, e.g. gastric accommodation or visceral hypersensitivity, are also important considerations in patients with symptoms of gastroparesis.20 However, a recent trial of the prokinetic medication prucalopride elicited similar improvements in symptoms,21 which suggest that our results are accurately estimating the negative impact of delayed gastric emptying on upper gastrointestinal symptoms.
Our results show important differences from those reported by the National Institutes of Health (NIH) Gastroparesis Consortium, which found moderate-severely delayed gastric emptying (>20% scintigraphic retention at 4h) was predictive of improved symptoms while no association was observed between gastric emptying and QOL scores.5 These discordant results likely relate to cohort differences between studies. Because the Consortium population included few patients with normal gastric emptying, it was not possible to define the impact of gastric emptying on long-term outcomes.22 Furthermore, the Consortium cohort likely represented a severe phenotype of gastroparesis as 15% of diabetic patients required gastric stimulator surgery, 10% of all patients required parenteral nutrition and 40% used chronic opioids. In contrast, only 12% of patients in our cohort reported opioid use, while the majority had normal gastric emptying with moderate baseline symptoms, and were less refractory on follow-up, which is more generalizable to patients seen in typical clinical practice.
Delayed gastric emptying was also associated with worse nausea/vomiting and fullness/early satiety. In contrast, bloating/distention did not associate with gastric emptying while abdominal pain was associated with delayed GES only. Presence of bloating23 and moderate-severe abdominal pain5 have been associated with worse gastroparesis outcomes. These findings suggest that delayed gastric emptying contributes more to symptoms of nausea/vomiting and fullness/early satiety compared to other cardinal symptoms of gastroparesis.
In contrast to prior studies which have not clearly identified the utility of measuring gastrointestinal contractility,24,25 our results show reduced gastric and elevated small bowel contraction parameters were associated with worse overall GCSI scores and QOL outcomes. Prior reports using antroduodenal manometry have similarly reported antral hypomotility and increased small intestinal resistance related to intestinal dysmotility in gastroparesis.26 Our findings suggest that abnormal upper gut contractility measures can predict differential outcomes in patients with symptoms of gastroparesis, separate from transit values. Furthermore, negative test results may be helpful in excluding dysmotility and may prompt investigation of alternative etiologies.
We found delayed CTT was associated with worsening fullness/satiety scores while increasing stool liquidity by the BSFS was associated with greater improvements in overall GCSI and QOL scores. This suggests gastroparesis symptoms are impacted by lower GI tract function and indicate a potential treatment target. However, as we utilized surveys specifically validated for upper GI disorders, the accuracy of our results estimating the effects of lower gut dysmotility is unclear.
Similar to previous reports,5 male gender was associated with greater improvements in overall GCSI and QOL scores. We further showed associations between cannabis use and worsening of nausea/vomiting as well as opioid use and worse pain and QOL outcomes, which have also been reported.27,28 However, we cannot determine causality with these findings as cannabinoid and/or opioid use may have been markers of more severe phenotypes for nausea/vomiting and abdominal pain, respectively. Furthermore, inferences should be taken with caution given the small number of subjects who reported cannabinoid use.
Our study had several strengths, including its large size, multicenter structure with a mix of academic and community centers, inclusion of previously uninvestigated patients with gastroparesis symptoms, use of validated transit methods, and collection of longitudinal data using standardized GI symptom and GI-specific QOL instruments. However, there were limitations. First, we did not examine the impact of treatment choices in these analyses. Given the uncertainty regarding the utility of motility testing, we felt it was necessary to first establish a causal relationship between transit/contractile parameters and longitudinal outcomes before we could analyze the impact of treatment effects. Although treatment effects may have influenced our results, we performed a sensitivity analysis, which showed similar results even after controlling for use of different therapies. Additionally, we were already seeing divergence in symptom scores at baseline prior to initiation of therapies in those with vs. without delayed gastric emptying by WMC. These observations suggest that we are accurately estimating the effects of baseline variables on longitudinal outcomes, independent from treatment effects. Secondly, while there were likely center-specific differences in clinical management, our results did not change after adjusting for center-specific variability, which suggests that our results are robust to clustering effects by centers. Thirdly, we did not characterize subtypes of FD, including epigastric pain and postprandial distress syndrome, in our cohort. Fourthly, we cannot determine the exact location of the capsule during the 1h period before/after GET, which may have influenced our contractility results. Fifthly, we could not assess whether there were differences in outcomes in severely vs. mildly delayed gastric emptying due to small sample sizes. Finally, other factors impacting clinical outcomes, including abnormalities in gastric accommodation, visceral hypersensitivity, and psychosocial contributors, were not examined in our study.5
In conclusion, our study showed that clinical factors, including female gender and functional dyspepsia, were associated with worse outcomes in patients with symptoms of gastroparesis. However, even after controlling for these clinical factors, delayed gastric emptying and abnormal upper gut motility were independently associated with worse longitudinal outcomes. Although not recommended in current guidelines,1 our data suggest that gastric motility tests may help to risk stratify and provide prognostic information. Future analyses will determine whether motility test results may predict response to different therapeutic options in specific patient subsets.
Supplementary Material
WHAT YOU NEED TO KNOW:
Background:
The utility of gastric motility testing in patients with symptoms of gastroparesis is unclear due to uncertain correlation with symptoms. As such, clinical guidelines currently do not recommend gastric motility testing for the majority of patients with chronic dyspeptic symptoms.
Findings:
Clinical factors, such as female gender, functional dyspepsia, and harder stools, were associated with worse longitudinal outcomes. After adjusting for these clinical factors, delayed gastric emptying as well as reduced gastric and elevated small bowel contractility were independently predictive of worse symptom scores and quality of life outcomes.
Implications for Patient Care:
Gastric motility testing may help to risk stratify and provide prognostic information to inform management in patients with symptoms of gastroparesis.
Acknowledgements:
The authors would like to acknowledge Dr. Richard Krause, MD, for his assistance with recruiting subjects for this study.
Support:
The parent study supporting the data and analyses included in this article was funded by Covidien. Covidien did not participate in study design, data interpretation, writing support, or other preparation assistance for this article. Grant support was also provided by the NIH grants KL2TR002241 (to AL) and AI124255 (to KR).
Disclosures:
AL, HP, RM, LN, JW, MS, BM, SR, BK, and WH received funding from Covidien to conduct this study. BK is a consultant for Gelesis, Ironwood Pharmaceuticals, Takeda, Alpha Waserman, Neurogastrx, Arena Pharmaceuticals, and CinRx Pharma and received research support from Vanda, Genzyme, and Covidien. The other authors have no financial, professional, or personal disclosures to report.
Abbreviations:
- BMI
body mass index
- BSFS
Bristol stool form scale
- Ct
contraction frequency
- CTT
colon transit time
- FD
functional dyspepsia
- GCSI
gastroparesis cardinal symptom index
- GEE
generalized estimating equation
- GES
gastric emptying scintigraphy
- GET
gastric emptying time
- MI
motility index
- PAGI-QOL
patient assessment of upper gastrointestinal disorders-quality of life
- PAGI-SYM
patient assessment of upper gastrointestinal disorders-symptom severity index
- SBTT
small bowel transit time
- WMC
wireless motility capsule.
REFERENCES:
- 1.Moayyedi PM, Lacy BE, Andrews CN, et al. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol 2017;112:988. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Yates K, Hasler WL, et al. Clinical Features of Idiopathic Gastroparesis Vary With Sex, Body Mass, Symptom Onset, Delay in Gastric Emptying, and Gastroparesis Severity. Gastroenterology 2011;140:101–115. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol 2013;108:1382–1391. [DOI] [PubMed] [Google Scholar]
- 4.Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2019;68:804–813. [DOI] [PubMed] [Google Scholar]
- 5.Pasricha PJ, Yates KP, Nguyen L, et al. Outcomes and Factors Associated With Reduced Symptoms in Patients With Gastroparesis. Gastroenterology 2015;149:1762–1774. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AA, Rao S, Nguyen LA, et al. Validation of Diagnostic and Performance Characteristics of the Wireless Motility Capsule in Patients With Suspected Gastroparesis. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasler WL, Rao SSC, McCallum RW, et al. Influence of Gastric Emptying and Gut Transit Testing on Clinical Management Decisions in Suspected Gastroparesis. Clin Transl Gastroenterol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456–1462. [DOI] [PubMed] [Google Scholar]
- 9.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008;27:186–196. [DOI] [PubMed] [Google Scholar]
- 10.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2004;13:1737–1749. [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): Development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res 2004;13:833–844. [DOI] [PubMed] [Google Scholar]
- 12.de la Loge C, Trudeau E, Marquis P, et al. Responsiveness and interpretation of a quality of life questionnaire specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol 2004;2:778–786. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2006;130:1377–1390. [DOI] [PubMed] [Google Scholar]
- 14.Heaton KW, Radvan J, Cripps H, et al. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 1992;33:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloetzer L, Chey WD, McCallum RW, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil 2010;22:527–533, e117. [DOI] [PubMed] [Google Scholar]
- 16.Højsgaard S, Halekoh U, Yan J. The R Package geepack for Generalized Estimating Equations. J Stat Softw 2005;15:1–11. [Google Scholar]
- 17.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57:120–125. [DOI] [PubMed] [Google Scholar]
- 18.Talley NJ, Locke GR, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut 2006;55:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008;20:311–319. [DOI] [PubMed] [Google Scholar]
- 20.Pasricha PJ, Camilleri M, Hasler WL, et al. White Paper AGA: Gastroparesis: Clinical and Regulatory Insights for Clinical Trials. Clin Gastroenterol Hepatol 2017;15:1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone F, Van den Houte K, Clevers E, et al. Prucalopride in Gastroparesis: A Randomized Placebo-Controlled Crossover Study. Am J Gastroenterol 2019;114:1265. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Wald A. Gastroparesis: Time for a Reappraisal? Gastroenterology 2015;149:1666–1669. [DOI] [PubMed] [Google Scholar]
- 23.Anaparthy R, Pehlivanov N, Grady J, et al. Gastroparesis and gastroparesis-like syndrome: response to therapy and its predictors. Dig Dis Sci 2009;54:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soffer E, Thongsawat S. Clinical value of duodenojejunal manometry. Its usefulness in diagnosis and management of patients with gastrointestinal symptoms. Dig Dis Sci 1996;41:859–863. [DOI] [PubMed] [Google Scholar]
- 25.Hasler WL, May KP, Wilson LA, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil 2018;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology 1986;91:94–99. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan T, Levinthal DJ, Li BUK, et al. Role of chronic cannabis use: Cyclic vomiting syndrome vs cannabinoid hyperemesis syndrome. Neurogastroenterol Motil 2019;31:e13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jehangir A, Parkman HP. Chronic opioids in gastroparesis: Relationship with gastrointestinal symptoms, healthcare utilization and employment. World J Gastroenterol 2017;23:7310–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


