Summary
COVID-19 vaccines are safe and highly effective, but some individuals experience unpleasant reactions to vaccination. As the majority of adults in the United States have received a COVID-19 vaccine this year, there is an unprecedented opportunity to study the genetics of reactions to vaccination via surveys of individuals who are already part of genetic research studies. Here, we have queried 17,440 participants in the Helix DNA Discovery Project and Healthy Nevada Project about their reactions to COVID-19 vaccination. Our genome-wide association study identifies an association between severe difficulties with daily routine after vaccination and HLA-A∗03:01. This association was statistically significant only for those who received the Pfizer-BioNTech vaccine (BNT162b2; n = 3,694; p = 4.70E−11; OR = 2.07 [95% CI 1.67–2.56]), and showed a smaller effect size in those who received the Moderna vaccine (mRNA-1273; n = 3,610; p = 0.005; OR = 1.32 [95% CI 1.09–1.59]). In Pfizer-BioNTech recipients, HLA-A∗03:01 was associated with a 2-fold increase in risk of self-reported severe difficulties with daily routine following vaccination. The effect was consistent across ages, sexes, and whether the person had previously had a COVID-19 infection. The reactions experienced by HLA-A∗03:01 carriers were driven by associations with chills, fever, fatigue, and generally feeling unwell.
Keywords: COVID, COVID-19, vaccination, HLA, SARS-CoV-2, HLA-A, HLA-A∗03:01, GWAS
Introduction
Less than 1 year after the first publication of a SARS-CoV-2 sequence, COVID-19 vaccines were developed, clinically tested, and authorized to be administered to the general population. Within months, hundreds of millions of adults worldwide were vaccinated, and rates of hospitalization among vaccinated individuals dropped precipitously; potential side effects were mild.1 During the clinical trials of mRNA vaccines (Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273), both local reactions such as pain at the injection site, and systemic symptoms such as fatigue, fever, chills, and myalgia, were observed in some participants.2,3 Both clinical trials showed that only a small fraction of these reactions could be categorized as severe, and most of the more severe reactions followed the second dose. These studies raise the question of which factors explain the interindividual variability of reactions following COVID-19 vaccination.
Younger age and a personal history of SARS-CoV-2 infection prior to vaccination are two factors leading to increased reactogenicity to the vaccine.4 However, these factors alone do not explain the large interindividual variability in the degree of severity of the reaction following COVID-19 vaccination. The aim of this study is therefore to identify factors associated with reactions following COVID-19 vaccination. Similarly to our hypothesis that genetic factors play a role in the severity of COVID-19 disease,5,6 we hypothesized that genetic factors help explain differences in reactions following COVID-19 vaccination. To test our hypothesis, we administered online surveys to 17,440 participants from the Helix DNA Discovery Project and the Healthy Nevada Project asking about their vaccination status and their reactions following the vaccine. These surveys included questions about four local and 20 systemic symptoms, as well as the overall severity of the reaction and its impact on daily routine in the days following vaccination. These participants were previously sequenced with the Helix Exome+ assay, which allowed us to perform rare and common variant genetic associations.7
Subjects and methods
Cohort and survey
We administered an online survey in June and September of 2021 and received responses from 8,125 Helix DNA Discovery Project participants and 9,315 Healthy Nevada Project (HNP) participants (Table 1).8,9 These are unselected Helix customers and patients in the Renown Health System who chose to consent to participate in research projects and respond to our survey. The survey takes approximately 15 min to complete and can be found in the supplemental information. The participants in this cohort are aged 18 to 89+ years, 65% are female, and 85% are of European genetic ancestry (Table 1). The Helix DNA Discovery Project study was reviewed and approved by the Western Institutional Review Board. The HNP study was reviewed and approved by the University of Nevada, Reno Institutional Review Board. The procedures followed were in accordance with ethical standards, and proper informed consent was obtained.
Table 1.
Study and cohort information
| Sample size | 17,440 |
| Median age (range) | 58 (18–89+) |
| No. of females (%a) | 10,402 (64.9%) |
| Genetic ancestry, n (%a) | |
| African | 264 (1.6%) |
| East Asian | 301 (1.9%) |
| European | 13,643 (84.9%) |
| Hispanic | 1,391 (8.7%) |
| South Asian | 60 (0.4%) |
| Other/mixed ancestry | 403 (2.5%) |
| Number with COVID-19 vaccination (%) | |
| Pfizer-BioNTech | 8,041 (46.1%) |
| Moderna | 7,086 (40.6%) |
| J&J | 790 (4.5%) |
| Other/unsure | 227 (1.3%) |
| With positive COVID-19 test before vaccination | 1,280 (7.3%) |
| Number reporting vaccine reactions (%) | |
| 4: extreme difficulties/unable to perform daily routine | 1,029 (8.0%) |
| 3: severe difficulties with daily routine | 1,237 (9.6%) |
| 2: moderate difficulties with daily routine | 2,597 (20.2%) |
| 1: mild difficulties with daily routine | 3,502 (27.2%) |
| 0: no difficulties with daily routine | 4,500 (35.0%) |
The total here is adjusted to remove individuals who do not have their sex and ethnicity available: this demographic information was collected separately and was not yet available for some individuals for this round of analysis.
Respondents indicated whether they had been vaccinated and which vaccine type they had received. They rated the severity of their vaccine side effects as indicated in Table 1. They also answered questions about 24 specific side effects that can occur after COVID-19 vaccination (see supplemental survey). There were 3,323 individuals who took the survey in both June and September, updating their COVID-19 vaccination status and infection status. For these individuals, the highest severity score and all reactions were used as a phenotype, regardless of in which survey either were reported.
Genotyping
DNA samples were sequenced and analyzed at Helix using the Exome+ assay as previously described.10 Imputation of common variants was performed by pre-phasing samples and then imputing. Pre-phasing was performed using reference databases, which include the 1,000 Genomes Phase 3 data. This was followed by genotype imputation for all 1,000 Genomes Phase 3 sites with minor allele frequency (MAF) ≥ 1% that have genotype quality values less than 20. Imputation results were then filtered for quality (genotype probability ≥ 0.95) so that only high-precision imputed variant calls were reported.7 Genotype processing was performed in Hail 0.2.54-8526838bf99f.11
HLA types for A, B, C, DPB1, DQA1, DQB1, and DRB1 were imputed using HIBAG with the default recommendations.12 Individual genotypes were imputed using the model for the appropriate genetic ancestry for each individual.12 Probabilities higher than 0.5 were used as genotype calls.
Genetic analysis
We used regenie for the genetic analysis.13 In brief, this method builds a whole-genome regression model using common variants to account for the effects of relatedness and population stratification, and it accounts for situations where there is an extreme case-control imbalance, which can lead to test statistic inflation with other analysis methods. We used leave-one-out cross-validation, and to accommodate complete separation and case-control imbalances we used the approximate Firth p value when the logistic regression p value was less than 0.01. The covariates we included were age, sex, age∗sex, age∗age, sex∗age∗age, ten principal components (PCs), type of COVID-19 vaccine, whether the individual had COVID-19 prior to being vaccinated, and bioinformatics pipeline version. We only utilized phenotypes with at least 50 cases, with a minimum minor allele count cutoff of 5.
As previously described, a representative set of 184,445 coding and noncoding LD-pruned, high-quality common variants were identified for building PCs and the whole-genome regression model.10 Each genetic ancestry group was analyzed separately: African, East Asian, European, Hispanic, and South Asian.
Results
A minority of individuals have severe difficulties with daily routine following COVID-19 vaccination
We received survey responses from 8,125 Helix participants and 9,315 HNP participants in June and September 2021. As a control, we first checked whether we observed the same trends as reported in the clinical trials of the Pfizer-BioNTech, Moderna, and J&J COVID-19 vaccines. The large majority of participants in these clinical trials did not have a personal history of SARS-CoV-2 infection. To assess the frequency of reactions in our cohort, we therefore restricted our initial analysis to participants who reported no previous infection by SARS-CoV-2. Our results were in agreement with what was previously reported.2,3,14 For the Pfizer-BioNTech vaccine, we observed that only 2.7% of individuals (152 of 5,590) had severe or extreme difficulties with their daily routine after the first dose, and 10.2% (569 of 5,583) had severe or extreme difficulties after the second dose (Figure S1). For the Moderna vaccine, we observed that 4.4% of individuals (243 of 5,575) had severe or extreme difficulties after the first dose, and this number grew to 19.5% (1,083 of 5,558) after the second dose (Figure S1). For the J&J vaccine, we observed that 17.7% (80 of 452) had severe difficulties after the single dose (Figure S1). Moreover, we observed a higher incidence of severe difficulties in respondents 18–55 years old compared with those older than 55 (Figure S1). These analyses supported the observation that there are large differences in reactions to COVID-19 vaccination between individuals and validated our self-reported survey as a reliable tool to investigate the genetic basis of these differences.
GWAS identifies HLA region associated with strong side effects after COVID-19 vaccination
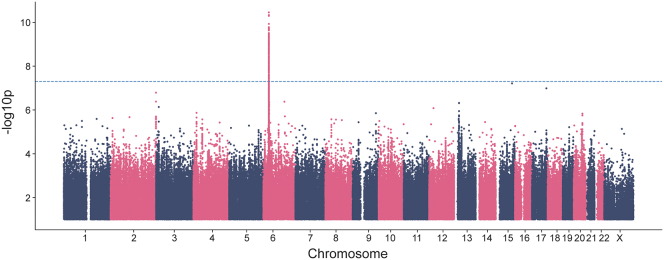
To test our hypothesis that genetic variation drives some of the differences seen in vaccine reaction, we performed a genome-wide association study (GWAS) using a phenotype of extreme or severe difficulties with daily routine (1,709 cases) compared with no or mild difficulties (6,203 controls). Individuals reporting moderate difficulties were excluded from the GWAS (Table 1). Our analysis of 12,602,603 SNPs identified 188 genome-wide significant variants on chromosome 6 (p < 5E−8; Figures 1 and S2). The lead SNP was rs144943243/chr6:29820015:AAAAT:A, p = 3.51E−11. This variant is in a region of AAAT repeats found upstream of HLA-G (MIM: 142871), with a MAF of 24% in the European genetic ancestry subset of our cohort.
Figure 1.
Manhattan plot for main phenotype of severe/extreme difficulties with daily routine against mild or no difficulties following any vaccination event with Pfizer-BioNTech, Moderna, J&J, or other COVID-19 vaccines. The lambda GC was 1.07 (Figure S2).
We next analyzed imputed HLA types against the phenotype to identify whether a specific HLA type was associated with the GWAS signal. We identified a significant association with HLA-A∗03:01 (p = 5.00E−11), which had a MAF of 15% in the European genetic ancestry subset of our cohort. The distribution of HLA-A∗03:01 across the globe is shown in Figure S3.15 A regression including both this HLA type and rs144943243 identified similar signals for each variant, with rs144943243 retaining a better p value in a joint analysis. The subsequent analyses assessing the impact of the genetic variant by phenotype led to similar results for rs144943243 and HLA-A∗03:01. In this paper, we have decided to show the results for HLA-A∗03:01, a common allele for the well-studied HLA-A gene (MIM: 142800).
HLA-A∗03:01 association is specific to vaccine type
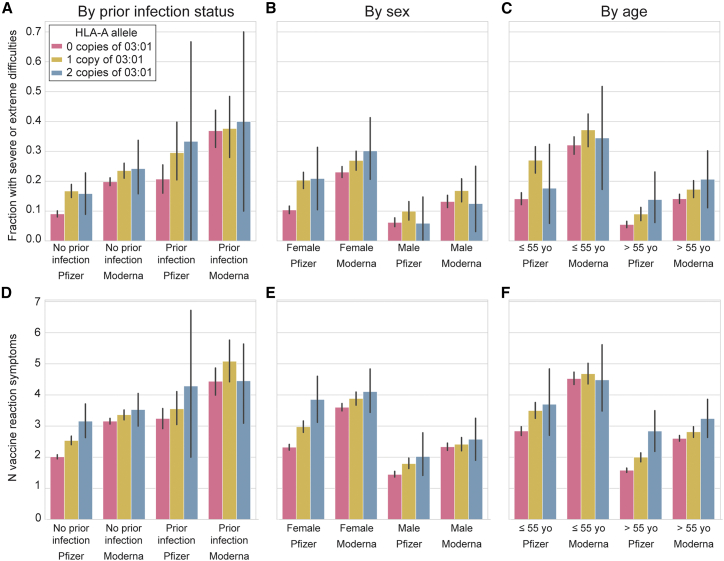
HLA-A∗03:01 had an odds ratio (OR) of 1.6 for individuals to experience severe/extreme difficulties with daily routine after vaccination as opposed to mild or no difficulties. We found that this effect was additive, with individuals with one copy of HLA-A∗03:01 having a phenotype intermediate to those with no or two copies (Figure 2). We found the effect of this variant to track similarly across age, sex, and whether the person had a history of SARS-CoV-2 infection prior to vaccination (Figures 2 and S4). However, our analyses beyond European (p = 5.00E−11, OR = 1.6) and Hispanic (p = 2.93E−04, OR = 2.7) genetic ancestries were underpowered to identify associations (Table S1). We also observed that most of the effect seemed to occur at the second dose for two-dose vaccines (Figure S5), and severe difficulties subsided within 2 days for 66% of those who experienced severe difficulties (Figure S6).
Figure 2.
Risk of vaccine side effects by HLA-A∗03:01 genotype and vaccine type
(A and D) Broken down by whether they had COVID-19 prior to vaccination.
(B and E) Broken down by sex (COVID-19 prior to vaccine excluded).
(C and F) By age (COVID-19 prior to vaccine excluded).
Top row (A–C) shows fraction with severe or extreme difficulties. Bottom row (D–F) shows number of vaccine reaction symptoms per person. Only European genetic ancestry with Pfizer-BioNTech or Moderna is shown. Error bars are 95% confidence intervals.
Importantly, we identified that the association signal came mostly from reactions to the Pfizer-BioNTech vaccine (HLA-A∗03:01 p = 4.70E−11, OR = 2.07 [95% confidence interval (CI) 1.67–2.56]; best Pfizer-BioNTech SNP chr6:29945053:T:C/rs2571381 p = 1.16E−12), with apparently much less impact on Moderna vaccine response (p = 0.005, OR = 1.32 [95% CI 1.09–1.59]) despite similar sample sizes for analysis (Pfizer 552 cases versus 3,142 controls; Moderna 1,018 cases versus 2,592 controls). Because the main phenotype of severe/extreme difficulties with daily routine after vaccination was more common in Moderna recipients than in Pfizer-BioNTech recipients, we also analyzed the reactions in Moderna recipients by restricting the cases to only those who reported extreme difficulties (n = 473) and the controls to those who reported no difficulties (n = 1,425). The CIs for the ORs from this analysis overlapped those from the analysis of Pfizer recipients, although the p value lacked statistical significance (p = 0.008, OR = 1.49 [95% CI 1.11–1.99]). The sample sizes for those who received J&J or other vaccinations were too low to clearly assess an association. Detailed counts of reaction severity split by genetic ancestry, HLA-A∗03:01 status, and vaccine type are reported in Table S1.
Specific vaccine reaction phenotypes are associated with HLA-A∗03:01
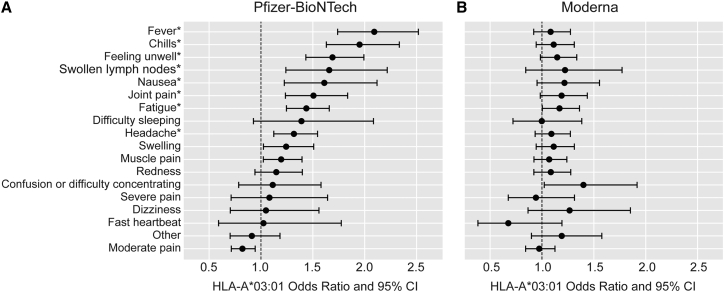
To understand the severity scores more deeply, we next analyzed the individual symptoms that participants reported as occurring after receiving the vaccine, split into Pfizer-BioNTech and Moderna subsets. We identified that in Pfizer-BioNTech recipients, HLA-A∗03:01 was most strongly associated with increased risk of fever (p = 1.76E−14), chills (p = 6.15E−13), feeling unwell (p = 5.73E−10), and fatigue (p = 5.60E−07) after receiving the vaccine (Figure 3). The ORs for these reactions ranged from 1.44 to 2.09 (95% CIs from 1.25 to 2.52), with 41% of those with one HLA-A∗03:01 copy who received a Pfizer vaccine having at least two of these four symptoms, 27% of those with two copies, and 19% of those with no copies. Associations with joint pain, headache, swollen lymph nodes, and nausea were less predictive but still statistically significant after correction for test multiple phenotypes (p < 0.001). In contrast, none of these symptoms were significantly associated with HLA-A∗03:01 in Moderna recipients, and the CIs did not overlap with those for Pfizer recipients for fever, chills, or feeling unwell (p values 0.04–0.34, ORs 1.08–1.22, 95% CIs from 0.84 to 1.77; Figure 3).
Figure 3.
Odds ratios and 95% confidence intervals (CI) for specific vaccine responses in an additive genetic analysis (regenie) of HLA-A∗03:01 in European ancestry individuals (n = 9,636)
(A) ∗p < 0.001 (signficant after correction for testing multiple phenotypes) in Pfizer-BioNTech recipients.
(B) No associations were significant in Moderna recipients.
Discussion
Here we identified an HLA type, HLA-A∗03:01, with a strong association with reactions to COVID-19 vaccines. We find that, all else being equal, individuals with this HLA type who received the Pfizer-BioNTech vaccine are approximately twice as likely to have severe or extreme difficulties with their daily routine following COVID-19 vaccination. Chills and fever were the two specific side effects that were most enriched in individuals carrying one or two copies of HLA-A∗03:01 compared with individuals with two other HLA-A alleles. This association was present in the Helix DNA Discovery cohort as well as the HNP cohort. We find this association to trend across age groups, sex, and whether the person had a personal history of COVID-19 prior to vaccination, all of which are known to be associated with severity of vaccine reaction. We find that the effect of this variant was much stronger for participants who had received the Pfizer-BioNTech vaccine than for those who had received the Moderna vaccine. Our sample size for J&J and other vaccines were too low for adequate analysis power. Importantly, 23andMe published on their blog (accessed on December 1, 2021) that HLA-A∗03:01 was also the strongest genetic association with COVID-19 vaccine reaction in their cohort with a p value of 2.0E−205. No additional details regarding the effect by vaccine type, dose, or symptoms were yet available.
HLA-A∗03:01 is the third most frequent HLA-A allele in the population.15 HLA-A∗03:01 was reported to be associated with hemochromatosis (MIM: 235200) in the UK Biobank cohort.21 However, this association could be due to the fact that HLA-A3/B7 is the ancestral haplotype from which the HFE (MIM: 613609) p.C282Y pathogenic variant originated.22 Neither HLA-A∗03:01 nor the other top hits from our GWAS, such as rs2571381, have been associated with COVID-19 severity or susceptibility to infection.5,16 This allele was also not reported to be associated with non-COVID vaccine reactions based on our literature search. However, HLA-A∗03:01 was reported to be associated with oxcarbazepine-induced maculopapular eruption in a small study in the Uighur Chinese population.17 The HLA-A∗03 allele was also reported to be associated with poor response to immune checkpoint blockade in cancer.18 The mechanism behind these associations remains unclear.
One potential mechanism for the association we see is that COVID-19 vaccines activate CD8+ T cells more strongly in carriers of an HLA-A∗03:01 allele, leading to stronger symptoms especially after the second dose. This hypothesis could explain the difference of effect size we observed between the Pfizer-BioNTech and Moderna vaccines, because data from clinical trials suggest that the Pfizer-BioNTech vaccine elicits a stronger CD8+ T cell response than the Moderna vaccine.19,20 These two vaccines have identical spike protein sequences but may have different adjuvants. Another hypothesis to explain the difference between the two vaccines is that the higher dose of the Moderna vaccine leads to a stronger innate immune activation, which would be independent of the role of the HLA-A∗03:01 allele. This stronger innate immune activation may dilute the effect of HLA-A∗03:01 in recipients of the Moderna vaccine. Another possibility is simply that the higher prevalence of vaccine reactions in Moderna recipients makes the signal more difficult to identify in that subgroup (Figures 2, S1, and S5). Replication of the result in other cohorts, with results broken down by COVID-19 vaccine types, will be informative in hypothesizing further about potential mechanisms leading to these reactions.
One limitation of our study is that the HLA calls were imputed by HIBAG as opposed to being directly genotyped.12 These imputed values are affected by ancestry, linkage disequilibrium patterns, and SNP quality, and it is expected that a portion of the imputed HLA types are incorrect. For this reason, it is also not certain that HLA-A∗03:01 is the causal allele. Of note, the lowest p value we obtained for the main phenotype for Pfizer-BioNTech recipients was chr6:29945053:T:C/rs2571381 (p = 1.16E−12), and the lowest p value obtained for any phenotype was for fever in Pfizer-BioNTech recipients at chr6:29945765:T:G/rs2499 (p = 1.72E−16). Functional experiments looking at which epitopes from the spike protein sequence in the vaccines bind to and are presented by HLA-A∗03:01 may also help us to understand the mechanism and reveal which HLA-A allele or amino acid change is causal.23
To our knowledge, this study is one of few reports on genetic associations with reactogenicity after non-live-attenuated vaccines. A few studies have also investigated the genetics of response to vaccines by looking at the levels of antibodies after a certain time period in vaccine recipients, often identifying associations with HLA genes.24,25 Other studies have elucidated why some patients presented with a rare life-threatening disease following vaccinations with live-attenuated vaccines.26 For example, genetic defects in the interleukin-12-dependent interferon-γ pathway cause BCGitis after BCG vaccination.27 The difficulty of collecting appropriate phenotype information on the reaction or immune response to a vaccine with standard medical data or electronic health records could be one reason to explain the small number of these studies. This study highlights the importance of continuing to survey and engage participants who are enrolled in ongoing genetic research projects. Another example is a recent study based on self-reported symptoms of COVID-19, which showed that many COVID-19 symptoms such as fatigue or anosmia were heritable.28 The result of these genetic studies could be highly valuable in educating and preparing anxious vaccine recipients about what to expect. Additionally, results from GWAS of common variants, and even more so from rare variant studies, allow better understanding of our immune system in response to SARS-CoV-2 infection or in response to COVID-19 vaccination.5,29, 30, 31 These studies will act like a compass, showing the way toward personalized vaccines.
Lastly, it is important to emphasize that our study looked at self-reported severe difficulties with daily routine shortly after receiving the vaccine. These reactions were symptoms such as chills or fever, which cannot be compared with the severity of COVID-19 disease experienced by many individuals. COVID-19 vaccines have consistently been shown to be safe and very effective in preventing hospitalizations and life-threatening disease following SARS-CoV-2 infections.2,3,14,32
Data code and availability
The top 10,000 associations from the main analysis are available in Table S2. The HLA type association results are available in Table S3. The HNP data are available to qualified researchers upon reasonable request and with permission of the Institute for Health Innovation (IHI) and Helix. Researchers who would like to obtain the raw genotype data related to this study will be presented with a data user agreement which requires that no participants will be re-identified and no data will be shared between individuals or uploaded onto public domains. The IHI encourages and collaborates with scientific researchers on an individual basis. Examples of restrictions that will be considered in requests to data access include but are not limited to (1) whether the request comes from an academic institution in good standing and will collaborate with our team to protect the privacy of the participants and the security of the data requested, (2) type and amount of data requested, (3) feasibility of the research suggested, and (4) amount of resource allocation for the IHI and Renown Hospital required to support the collaboration. Any correspondence and data availability requests related to HNP should be addressed to J.J.G. (Joe.Grzymski@dri.edu) or Craig Kugler (Craig.Kugler@dri.edu).
Acknowledgments
Funding was provided to Desert Research Institute (DRI) by the Nevada Governor's Office of Economic Development. Funding was provided to the Renown Institute for Health Innovation by Renown Health and the Renown Health Foundation. We acknowledge the entire Helix Bioinformatics team for their contributions to the production exome sequencing pipeline. We thank C. Clinton, K.T. Farley, and E. Levin for their contribution running the Helix DNA Discovery Project and the work with the IRB. We thank all of the genomic representatives of the HNP. We thank Renown Health and DRI marketing for helping to launch the HNP. We thank J. Fellay for comments on the manuscript.
Declaration of interests
A.B., K.M.S.B., S.W., M.I., W.L., N.L.W., and E.T.C. are employees of Helix.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2021.100084.
Web resources
regenie: https://rgcgithub.github.io/regenie/.
HIBAG: https://bioconductor.org/packages/release/bioc/html/HIBAG.html.
OMIM: https://omim.org/.
23andMe: https://blog.23andme.com/23andme-research/reaction-to-covid-vaccine/
Supplemental information
References
- 1.Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova J.-L., Su H.C., COVID Human Genetic Effort A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181:1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helix (2019)Helix’s Exome+ Performance White Paper.
- 8.Lu J., Bowes J. Introducing the Helix DNA Discovery Project. 2018. https://blog.helix.com/helix-dna-discovery-project/
- 9.Grzymski J.J., Elhanan G., Smith E., Rowan C., Slotnick N., Dabe S., Schlauch K., Read R., Metcalf W.J., Lipp B., et al. Population health genetic screening for tier 1 inherited diseases in Northern Nevada: 90% of at-risk carriers are missed. BioRxiv. 2019 doi: 10.1101/650549. [DOI] [Google Scholar]
- 10.Cirulli E.T., White S., Read R.W., Elhanan G., Metcalf W.J., Tanudjaja F., Fath D.M., Sandoval E., Isaksson M., Schlauch K.A., et al. Genome-wide rare variant analysis for thousands of phenotypes in over 70,000 exomes from two cohorts. Nat. Commun. 2020;11:542. doi: 10.1038/s41467-020-14288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hail Team Hail 0.2 54-8526838bf99f (Github) https://github.com/hail-is/hail
- 12.Zheng X., Shen J., Cox C., Wakefield J.C., Ehm M.G., Nelson M.R., Weir B.S. HIBAG-HLA genotype imputation with attribute bagging. Pharmacogenomics J. 2014;14:192–200. doi: 10.1038/tpj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbatchou J., Barnard L., Backman J., Marcketta A., Kosmicki J.A., Ziyatdinov A., Benner C., O’Dushlaine C., Barber M., Boutkov B., et al. Computationally efficient whole genome regression for quantitative and binary traits. Nat. Genet. 2020;53:1097–1103. doi: 10.1038/s41588-021-00870-7. [DOI] [PubMed] [Google Scholar]
- 14.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Galarza F.F., McCabe A., Santos E.J.M.D., Jones J., Takeshita L., Ortega-Rivera N.D., Cid-Pavon G.M.D., Ramsbottom K., Ghattaoraya G., Alfirevic A., et al. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020;48:D783–D788. doi: 10.1093/nar/gkz1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kousathanas A., Pairo-Castineira E., Rawlik K., Stuckey A., Odhams C.A., Walker S., Russell C.D., Malinauskas T., Millar J., Elliott K.S., et al. Whole genome sequencing identifies multiple loci for critical illness caused by COVID-19. medRxiv. 2021 doi: 10.1101/2021.09.02.21262965. [DOI] [Google Scholar]
- 17.Zhao T., Wang T.-T., Jia L., Wang F., Bahatibieke M., Liu W.-L., Ji Y., Sun L., Sun Y., Li H.-J., et al. The association between HLA-A∗03:01 and HLA-B∗07:02 alleles and oxcarbazepine-induced maculopapular eruption in the Uighur Chinese population. Seizure. 2020;81:43–46. doi: 10.1016/j.seizure.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Naranbhai V., Viard M., Dean M., Groha S., Braun D.A., Labaki C., Shukla S.A., Yuki Y., Shah P., Chin K., et al. HLA-A∗03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. Lancet Oncol. 2021;23:172–184. doi: 10.1016/S1470-2045(21)00582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes G., Tanigawa Y., DeBoever C., Lavertu A., Olivieri J.E., Aguirre M., Rivas M.A. Global biobank engine: enabling genotype-phenotype browsing for biobank summary statistics. Bioinformatics. 2019;35:2495–2497. doi: 10.1093/bioinformatics/bty999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacho A., Mancebo E., del Rey M.J., Castro M.J., Oliver D., García-Berciano M., González L., Morales P. HLA haplotypes associated with hemochromatosis mutations in the Spanish population. BMC Med. Genet. 2004;5:25. doi: 10.1186/1471-2350-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grifoni A., Sidney J., Vita R., Peters B., Crotty S., Weiskopf D., Sette A. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe. 2021;29:1076–1092. doi: 10.1016/j.chom.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung S., Roh E.Y., Park B., Lee Y., Shin S., Yoon J.H., Song E.Y. GWAS identifying HLA-DPB1 gene variants associated with responsiveness to hepatitis B virus vaccination in Koreans: independent association of HLA-DPB1∗04:02 possessing rs1042169 G - rs9277355 C - rs9277356 A. J. Viral Hepat. 2019;26:1318–1329. doi: 10.1111/jvh.13168. [DOI] [PubMed] [Google Scholar]
- 25.Png E., Thalamuthu A., Ong R.T.H., Snippe H., Boland G.J., Seielstad M. A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum. Mol. Genet. 2011;20:3893–3898. doi: 10.1093/hmg/ddr302. [DOI] [PubMed] [Google Scholar]
- 26.Pöyhönen L., Bustamante J., Casanova J.-L., Jouanguy E., Zhang Q. Life-threatening infections due to live-attenuated vaccines: early manifestations of inborn errors of immunity. J. Clin. Immunol. 2019;39:376–390. doi: 10.1007/s10875-019-00642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenauer-Kaligis E.G.R., de Boer T., Verreck F.A.W., van Voorden S., Hoeve M.A., van de Vosse E., Ersoy F., Tezcan I., van Dissel J.T., Sanal O., et al. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor β1 deficient patients and evidence for the existence of partial IL-12 receptor β1 deficiency. Eur. J. Immunol. 2003;33:59–69. doi: 10.1002/immu.200390008. [DOI] [PubMed] [Google Scholar]
- 28.Williams F.M.K., Freidin M.B., Mangino M., Couvreur S., Visconti A., Bowyer R.C.E., Le Roy C.I., Falchi M., Mompeó O., Sudre C., et al. Self-reported symptoms of COVID-19, including symptoms most predictive of SARS-CoV-2 infection, are heritable. Twin Res. Hum. Genet. 2020;23:316–321. doi: 10.1017/thg.2020.85. [DOI] [PubMed] [Google Scholar]
- 29.Colona V.L., Biancolella M., Novelli A., Novelli G. Will GWAS eventually allow the identification of genomic biomarkers for COVID-19 severity and mortality? J. Clin. Invest. 2021;131:e155011. doi: 10.1172/JCI155011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6:eabl4348. doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S.-Y., COVID Human Genetic Effort. Cobat A., Notarangelo L.D., Su H.C., Abel L., Casanova J.-L. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Med (N Y) 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.