Abstract
The emergence and rapid global spread of the new Delta and, more recently, Omicron variants of SARS-CoV-2 pose a daunting public health emergency. Being an RNA virus, the Covid-19 virus is continuing to mutate, resulting in the emergence of new variants with high transmissibility, such as the recently discovered Omicron variant. In this paper, we consider the conditions that may facilitate viral mutations and the emergence of variants with the ability to evade immunity. Here, we have discussed the importance of vaccination with the currently available vaccines. These vaccines are highly effective at preventing serious disease, hospitalization, and death from Covid-19. However, the antibody response induced by these vaccines is short-lasting and there are reports of breakthrough infections. A stable and persistent interaction between T follicular helper cells and germinal center B cells is needed for robust B cell memory response. We discussed the potential reasons behind the breakthrough infections and underscored the importance of developing better second-generation vaccines that may not necessitate frequent booster immunizations and are preventive in nature. This may involve the development of multivalent vaccines and creating vaccines against other viral proteins including conserved proteins. Vaccine hesitancy remains a notable hurdle for implementing vaccination. Furthermore, we recommend different approaches to increase vaccine acceptance, which is a critical translational component of a successful vaccine strategy. These perspectives on overcoming the pandemic's current challenges provide strategies to contain SARS-CoV-2 globally.
Keywords: RNA virus, Mutation, Variants, Breakthrough infections, Second-generation vaccines, Vaccine hesitancy, Transparency, Global vaccination
1. Introduction
About twenty-two months have passed since the first official infection was reported in Wuhan. Globally, SARS-CoV-2 infections continue to rage, though the levels are variable and erratic in different parts of the world.
The decline in infection rates following the introduction of vaccines in conjunction with efforts around masking and social distancing have raised hopes that the Coronavirus can be contained [1]. However, the continuous emergence of new variants of the virus [2,3], the short-lived nature of vaccine-induced immunity [4,5], sporadic reports of breakthrough infections [6,7], and sustained vaccine hesitancy [8,9] pose considerable challenges to our efforts in ending the current pandemic.
Furthermore, since the Coronavirus is an RNA virus, the virus will undergo mutation and the emergence of new variants will readily occur, as we have already seen. As the latest variant spreads, scientists continue to gather critical evidence-based data on Omicron induced pathogenesis and the mechanisms behind it. The rapidly spreading Omicron variant could dangerously stress healthcare systems, even if the risk of severe disease or death is relatively low for individuals; a small fraction of a very large number is still a large number, so the population-level threat is very real.
As demonstrated with the Delta and Omicron variants, new mutations can impact the transmissibility of the SARS-CoV-2 virus and the hosts’ immunity [10,11]. Having considered these important biological attributes in the host-virus relationship, this paper highlights what approaches should be undertaken in the present stage of the pandemic to stop the spread of the virus and to curtail the level of danger the virus may pose over time.
2. Mutations and transmission
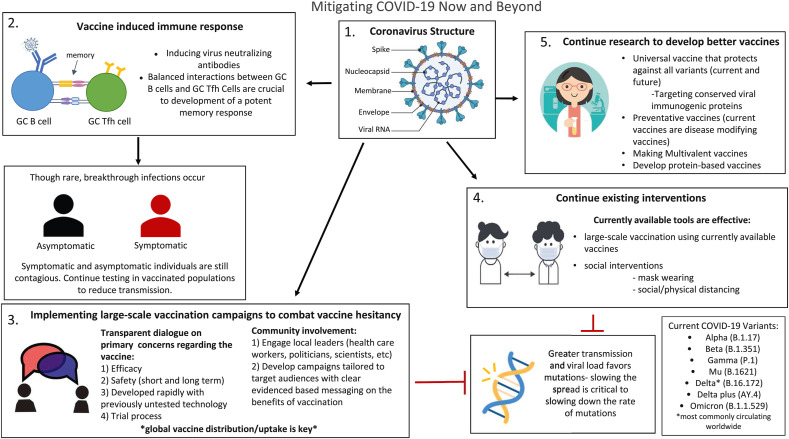
Novel sets of mutations result in the emergence of ‘variants of concern’; mutations that impact a virus' characteristics, including transmissibility and antigenicity, may come about in response to the changing immune profile of the human hosts. Many of the recent variants of SARS-CoV-2 are associated with increased transmission, which has raised questions about the nature and rates of mutations in viruses [10]. The less the virus spreads, the fewer opportunities it has to mutate [12,13]. SARS-CoV-2 could continue to evolve in ways that both accelerate viral transmission and reduce vaccine effectiveness. Additionally, infected people can transmit the virus even before the symptoms become apparent. Furthermore, many individuals who have been vaccinated but later become infected may not show symptoms and unknowingly can continue to transmit the virus to other people [14,15], a challenging situation to blunt transmission (Fig. 1 ). If we abandon strategies to reduce the spread and let the virus spread unmitigated then we will likely be in a pandemic situation for years to come.
Fig. 1.
Mitigating Covid-19 Now and Beyond. 1. Structure of Covid-19 coronavirus; 2. Vaccine induced immune response: inducing virus-neutralizing antibody response, which is short-lasting and efficient interactions between the germinal center (GC) B cells and GC T follicular helper (Tfh) cells are crucial to induce a potent memory response. Breakthrough infections may still occur in a few individuals after vaccination so testing should be continued to reduce transmission 3. Implementing large-scale vaccination campaigns to combat vaccine hesitancy: the necessity of transparent dialogue with the involvement of trusted community leaders conveying clear evidenced-based messages are critical strategies to combat vaccine hesitancy. Global vaccine coverage is important to contain the Covid pandemic 4. Continuing existing interventions: the currently available tools of vaccination, mask-wearing, and social distancing are proven to be effective and should be continued to reduce transmission and the rate of mutation 5. Continue research to develop better vaccines (second generation): new universal vaccines targeting conserved immunogenic or other viral proteins can protect against current and future variants, additionally, creating preventative, multivalent, or protein-based vaccines may confer better protection.
Viruses mutate all the time, so it is not surprising to see new versions emerge. The mutations - Y145H and A222V - have been found in various coronavirus lineages since the pandemic's beginning. An accumulation of mutations that significantly alter the properties of a virus lineage characterize the emergence of a new variant. As of December 2021, there are five variants of SARS-CoV-2 spreading among global populations: the Alpha Variant (formerly called the UK Variant and referred to as B.1.1.7), the Beta Variant (formerly called the South Africa Variant and referred to as B.1.351), the Gamma Variant (formerly called the Brazil Variant and referred to as P.1), the Delta Variant (formerly called the India Variant and referred to as B.1.617.2) and the Omicron Variant (formerly called the South Africa Variant and referred to as B.1.1.529). All are reported to have significantly higher transmission rates than earlier lineages (Fig. 1).
Reports demonstrate that the currently dominant Delta variant can replicate perhaps 1000 times as much as its predecessors and this particular variant has immune-evasive properties [16]. Research so far indicates that antibodies developed for the original strain (Wuhan strain) of the coronavirus may only be one half or a third as effective against the Delta variant [17]. Even more concerning, individuals infected by the Delta variant are shedding a lot more virus. When the viral dose on the receiving end is larger, this may impact the host's immunity against the virus [18]. Recent studies indicate that the current vaccines developed against the spike protein of the initial virus maintain a variable degree of efficacity against different variants [19]. Even for the Delta variant, it appears that the spike protein remains relatively stable although some minor modifications may occur. Yet another variant that some are calling “Delta Plus” (referred to as AY.4.2) has been found in the UK, which may spread more easily than regular Delta [2]. Although regular Delta still accounts for most Covid infections in the UK, cases of “Delta Plus” have been increasing. The latest official data suggest 6% of Covid cases are of this type, though there is no evidence yet that it causes worse illness. It is not yet known if current vaccines would be effective against the Delta Plus sub-variant.
In the last month, a new variant, named Omicron, which shows numerous mutations in the spike protein and is considered by the WHO as a “variant of concern”, was discovered in South Africa [20]. This variant has 32 mutations on the spike protein, which the virus uses as an entryway into our body's cells and is the target for currently used vaccines. The Omicron variant has 10 mutations in the receptor-binding domain (RBD) compared to just two for the Delta variant that swept the world. Furthermore, preliminary research from the University of Hong Kong suggests that the Omicron variant infects and replicates 70 times faster than its predecessors in the human bronchus, but the infection in the lung is significantly lower [21]. This data is currently under peer review for publication, but this data supports observations that this variant is more transmissible but causes less severe disease. The governments and media in several countries have already reported the spread of this variant in different parts of the world. Scientists are not yet aware whether current vaccines will be effective against this variant, nor do they know how dangerous it will turn out to be. We are expecting some clear answers in the coming weeks.
The impact of vaccination on the transmission of virus remains unclear [22]. Recent data showed that vaccinated people who became infected with the Delta variant can carry as much virus in their nose as unvaccinated people [23]. In contrast, vaccinated people might remain infectious for a shorter period when compared to unvaccinated individuals who are infected with the Delta variant. Delta viral loads were similar for both groups for the first week of infection but declined quickly after day 7 in vaccinated individuals [24].
The transmission of SARS-CoV-2 is not yet seasonal. We have seen in several warmer countries, like India and Iran, that increased infection has not necessarily occurred during winter outbreaks. Yet, evidence suggests that dry winter air improves the stability and transmission of respiratory viruses, and respiratory-tract immune defenses might be impaired by inhaling dry air in the colder weather [25,26]. Moreover, people are more likely to stay indoors in the winter, where virus transmission through droplets is a bigger risk. These factors may make containment in the Northern Hemisphere this winter more difficult.
Many countries do not have the infrastructure and skilled personnel to perform systematic virus genome analysis and thereby could miss the detection of new variants of SARS-CoV-2. There may be many more variants circulating globally, which we may be unaware of, and which would impact the transmission rate in different parts of the world. It is important for primo or breakthrough infections that Covid detection tests should be done properly especially in the wake of the frequent emergence of new virus variants. The PCR test searches for traces of the virus's genetic material in samples. Usually, the PCR test targets a sequence of three parts on the spike protein of the virus to confirm the presence of an infection. In a recent observation, one of these PCR targets was increasingly coming back negative in samples from areas of England with rapidly rising case numbers, while the other two targets in the tests continued to work. It is unusual to see two of the test sequences working but a third not. When scientists investigated, they found that the virus in these samples had mutated – a deletion at the H69 and V70positions was determined to have caused the failed tests [27].
3. SARS-CoV-2 and host immunity
There is limited data on the timeline for SARS-CoV-2 immunity from natural infections and the timeline for the immunity induced by vaccines, especially in the face of emerging variants. Factors such as the history of exposure, hosts determinants, and viral dynamics, are likely to impact the timeline for immunity. Many factors could account for the persistence of immune protection despite declines in antibodies. Part of the story may have to do with memory B cells—immune cells that hang around, sometimes for decades, for the specific purpose of quickly restarting our antibody response when a familiar pathogen reappears. T cells, which also continue to circulate long after infection, also play a role by hunting for infected cells (Haque & Pant, in preparation). These and other systems come online quickly upon reinfection: essentially, a little protection may go a long way. As mentioned above, while vaccinated and unvaccinated people have similar viral loads at first, the amount of circulating virus declines much faster in those who have been immunized [24].
However, even when immune responses stay robust, viruses can mutate. Mutating viruses may express antigenic epitopes that will not be sufficiently recognized by antibodies and viruses may not bind to the antibodies generated in an individual with the same affinity. The variant may escape the host's immunity and can infect other individuals irrespective of their immunity to the initial virus type. However, a robust memory response with sufficient breadth of recognition may be able to react to the variant [28]. Such memory response will have both specificity and the capacity to adapt to the potential divergence of its targets.
T cells are as equally as important as B cells in immunity to SARS-CoV-2. Preliminary studies have recently documented T cells specific to SARS-CoV-2 in people with acute Covid-19 and in those recovering from infection [29,30]. Furthermore, T cells play an important role in maintaining efficient immunity to B cell-mediated humoral responses. It is important that the function of T follicular helper (Tfh) cells from the lymph nodes of SARS-CoV-2-infected individuals is not impaired. With the proper help of Tfh cells to germinal center (GC)-B cells, a stable and persistent B cell memory response is likely to develop [31]. In an infection primarily controlled by antibodies, protective immunity depends on the quantity and quality of available antibody, memory B cells, and memory Tfh cells. Understanding how GC-Tfh cell function is crucial and could provide critical information about the mechanisms leading to the development and maintenance of effective anti-SARS-CoV-2 antibodies (Fig. 1).
Preliminary data from the Imperial College of London demonstrates that the Omicron variant is largely able to evade immunity from past infections or vaccine doses [32]. In the absence of updated vaccines specifically targeting the mutant form of the virus, boosting could be effective in helping the immune system recognize the variant [33]. Recent observations describe the expansion of T cells after stimulation with spike peptides indicate that booster vaccination could increase the frequency of virus-specific T cells [34]. B and T cells are the components of the immune system expanded by booster immunizations and could increase the chances of immune cells seeing through the virus's disguise. In cases of uncertainty and the absence of redesigned vaccines, boosting is the quickest way to enhance protection though it may not contain immune escaping variants as efficiently as targeted vaccines.
The immune system has multiple specialized defenses that can be deployed strategically and dynamically against emerging variants (Haque & Pant, in preparation). The virus has some attributes, like immune evasion and changing its antigenic repertoire through mutation, but compared to the human immune system, these viral adaptations are likely to be much less efficient. Vaccines utilize the ability of the highly evolved human immune system to respond to and remember encounters with pathogen antigens. However, as long as there are people who are not vaccinated, the virus is circulating in any part of the world, and variants continue to emerge, the threat of resurgence and more waves of this pandemic remain.
4. Vaccines and breakthrough infections
While the Covid-19 vaccines show variable efficacity against the various SARS-CoV-2 isolates, they remain our best option for protection. Of note, there is not a single vaccine in use currently that is 100% protective. We know from initial trials from both mRNA vaccines and the Johnson & Johnson adenovirus vaccine that the study environment was different because the patients were carefully selected. After the FDA gave its emergency use authorization (EUA), and it was given to the general population, we found that the real-world effectiveness of the vaccine is lower at around 90%. This indicates that there may be inherent aspects of the vaccine which react with the patients and their unique characteristics [35], such as comorbidities. Patients with immunosuppressed status may react in a different way and may not produce the same antibody response as other healthy individuals who got the vaccine.
Israel has one of the highest vaccination rates in the world, and yet, is experiencing another Covid surge [36]. One plausible answer is that as the pandemic's pace decelerated, Israel relaxed almost all social-distancing measures; even in a highly protected society, more contact almost always means more infections. However, another explanation has to do with how we analyze the numbers. Israel has an extremely high rate of vaccination: nearly eighty percent of the over-twelve population is immunized. If we take the underlying immunization rate into account, the vaccines appear to be nearly seventy percent effective at preventing hospitalization.
In some cases, after vaccination, the virus gains a foothold, multiplies, and challenges vaccine primed immune systems, inflicting “breakthrough” infections [6,37]. The development of breakthrough infections depends on the amount of virus a vaccinated person is exposed to, antibody levels, affinity, and avidity of antibodies to the circulating variant. The combination of these three factors affects the virus’ ability to establish disease in a vaccinated person [6,18]. Further studies are required to calculate the true risk of breakthrough infection; however, this is difficult to ascertain without knowing how many vaccinated people have actually been exposed to the virus. Indeed, more data is needed to determine what percentage of healthy individuals are contracting breakthrough infections and what underlying factors are involved.
The key question is whether the establishment of a virus in vaccinated individuals translates into a substantial weakening of immunity. A recent study done in Australia shows that a vaccinated person's antibody levels fall to around twenty percent of the typical post-infection level, however, protection against symptomatic infection drops to fifty percent. Protection against severe disease, however, doesn't fall to fifty percent until antibodies wane to just three percent of post-infection levels [38].
Most breakthrough infections appear to be mild because people who are vaccinated maintain some immunity and a majority of these breakthrough cases are going to be asymptomatic. According to the data, reported by CDC and other publications, it seems like around 25%–30% of the patients who have had breakthrough infections are completely asymptomatic [37,39]. Breakthrough infections could contribute to viral spread in a community because reinfected individuals (with or without symptoms) continue to harbor different levels of virus that are transmissible. It is just one more way for the virus to find and infect unvaccinated people (Fig. 1).
In rare cases, breakthrough infections may lead to persistent symptoms. Only a few studies have investigated how common or severe long Covid may be after breakthrough infections. The persistence of symptoms following breakthrough infections is likely to be rare because breakthrough infections are uncommon to begin with and typically shorter in duration. In one study in Israel, about seven of 36 people with breakthrough infections had persistent symptoms for more than six weeks [6]. And in a survey of Covid-19 survivors, 24 of 44 people with symptomatic breakthrough infection reported enduring problems [40].
Interestingly, breakthrough infections may offer an unexpected advantage. If individuals get through a breakthrough infection relatively unscathed, they are likely to develop more robust protection against variants. The breakthrough infection is likely to act as a booster shot, strengthening our immune system's ability to recognize and fight the virus.
It is important to note that studying all breakthrough infections presents serious data-collection challenges for both conceptual and practical reasons. The CDC relies on passive and voluntary reporting of infections, but many cases, especially those that are mild or asymptomatic, are never reported. To track every breakthrough infection, researchers can use cohort studies, which follow a defined group of people over time. This approach has an obvious advantage, in that we can test everyone—even those without symptoms. But it also has a critical limitation: one can never be quite certain how applicable the study's findings are to other people, in other settings, at other times.
Finally, the evolution of the disease and the immune response in these breakthrough infections may help in understanding the mechanisms of pathogenesis and the identification of protection correlates. It is noteworthy that the limited knowledge about which antibodies are protective, which immune responses are needed for protection, and how to enhance the right immune responses, particularly in the older and immunocompromised populations, are important considerations to avoid breakthrough infections.
5. Developing next-generation vaccines
Our greatest fear is that, in the face of variants or time, vaccine efficacy might decline. The anti-Covid vaccines that are currently in use weaken the link between cases and hospitalization/deaths [41]. These vaccines are particularly beneficial for vulnerable individuals who have comorbidities or have weakened immunity [42]. However, efforts should continue to develop the next generation of vaccines that are preventive in nature and can better confront emerging variants. The creation of vaccines that target the conserved nucleocapsid (N) protein of SARS-CoV-2 may be effective against all current and future variants. N proteins of many coronaviruses are expressed abundantly during infection and are highly immunogenic, stimulating both anti-viral antibody responses and T-cell activity [43]. Another possibility will be to develop a multivalent vaccine incorporating both new and old forms of the spike protein in a single jab (Fig. 1). The RBD protein could also be a target of interest although its immunogenicity is compromised by its small molecular size [44]. It is possible to overcome this limitation by increasing antigen size by fusing RBD with an Fc domain or by multimerization (RBD-dimer or RBD-trimer). The clinical trials of these redesigned vaccines would be challenging and depend on whether ‛correlates of protection’ are clearly defined. The testing of these vaccines will be slow as the first-generation vaccines are being deployed worldwide.
Most scientists agree that the vaccines are efficient but caution that if a new super variant comes along that is resistant to vaccines, vaccines will need to be redesigned quickly [35]. The Delta variant may be more infectious, but deaths to cases ratios are comparable to deaths caused by its predecessors. Thus, until the R0 number dips below 1, mask-wearing and social distancing need to stay in place.
5.1. Vaccine hesitancy interferes with vaccine success
One of the critical components for a successful vaccine is the translational implementation, which involves the effective distribution and acceptance of the vaccine by the population for whom the vaccine is made (Fig. 1). Vaccine non-accessibility, and hesitancy could compromise the global Covid-19 response. Vaccine misinformation on social media has greatly exacerbated hesitancy as it is affecting confidence in current vaccines on a previously unprecedented scale.
Depending on the country, there is a variable percentage of the population that has declined to be vaccinated, which is markedly higher than previous vaccines hesitancy rates against other infectious diseases [45]. In general, vaccine acceptance is higher in low-income countries than the high-income countries [45]. It is thought that the recent high rate of death in Russia is related to the rejection of vaccines by the population. Despite access being the main issue affecting global vaccine coverage, a considerable focus is currently on the challenges posed by the anti-vaccination movement.
Many metrics and indices measure vaccine acceptance and hesitancy globally [46,47]. In the case of Covid vaccines, we list six main reasons why people aren't getting vaccinated: they are concerned about the efficacity of the vaccine, the safety and/or side effects of the vaccine, they do not believe that they need it, they don't trust the vaccine since the vaccines were developed so quickly, they don't trust the pharmaceutical companies and the government, or they don't think Covid is a big threat. Public health policymakers and scientists have not been able to pointedly address these underlying concerns for vaccine hesitancy.
Different approaches are necessary to address these concerns. The vaccine hesitancy or vaccine intent is likely to change over time. Messaging and incentives must be tailored to address the varying concerns of different groups. A recent study in the USA highlights key differences in vaccine hesitancy by race and age subgroups. For example, younger black people were more hesitant than younger white people, while the reverse is true in older populations. Generally, Covid-19 vaccine hesitancy was higher among young (ages 18–24), non-Asian people, and less educated (≤high school diploma) adults with a history of a positive Covid-19 test [48]. Understanding the trends over the last several months is really important for projecting what may happen in the next couple of months. New strategies and incentives have to be defined accordingly.
The conflation of vaccine hesitant groups as anti-vax hinders the process of dialogue with them. As mentioned previously, vaccine hesitancy has been exacerbated by the proliferation of misinformation via social media and other outlets. Scientists and public health officials can get ahead of this by monitoring and analyzing misinformation that is circulating in their communities. By monitoring and evaluating these theories, public health messaging can combat misinformation and fill in knowledge gaps with accurate, clear, and easy-to-find information. In an earlier publication concerning the implementation of HPV vaccination programs in Sub-Saharan Africa, we discussed how a productive dialogue could be developed by involving the target community [49]. We propose that the Covid-19 mass vaccination programs could progress further when local hospital physicians, nurses, local politicians, newspapers and other media outlets, social workers, and other trusted local leaders participate in vaccine dialogue and implementation. They can make a positive and meaningful contribution to the efforts in the acceptance of the vaccination by providing evidence-based data and transparent information (Fig. 1). There is no denying that the current FDA-approved Covid-19 vaccines were made in a short period of time and used technology that had hardly been applied before so it is important to discuss the pros and cons of the technology used for developing each vaccine [35]. Total transparency regarding the trial process in different age groups is required and will help to mitigate confusion and mistrust. No human wants to die, and self-preservation is an instinctive reaction. We need to emphasize how the currently available vaccines could save lives, and to be explicit in the recognition that they are not 100% protective and that breakthrough infections and the rare chance of death may occur with this or any vaccine that is commonly administered. Scientists must acknowledge that the long-term side effects of vaccines remain unknown but also emphasize that no large-scale life-threatening severe effects have been noted since the beginning of mass vaccinations about twelve months ago. Such an approach may help in strengthening trust in these vaccines. People have understandably developed “pandemic fatigue” and want to return to normalcy. Officials and health workers must emphasize that the currently available vaccines will accelerate this process because these vaccines allow more people to become naturally infected without developing severe disease and avoiding hospitalization. Officials must make it abundantly clear that these vaccines are the only available tools to achieve these goals presently.
6. Conclusion
We often fail to consider that the Covid-19 still is a pandemic and billions of people around the world have yet to receive a single dose of vaccine. Only 8.1% of people in low-income countries have received at least one dose whereas almost 60–80% of people are fully vaccinated in developed countries [50]. Wealthy nations secured the “lion's share” of vaccines before they had even been proven to work, and many African countries have been left behind with only 6.5% of the population being fully vaccinated. These numbers underline the disparity in vaccine equity between wealthy and low-income countries. Equitable vaccine implementation is in everyone's interest and could avoid further impacts to the global economy and the myriad humanitarian crises that have been propagated and/or exacerbated by this pandemic.
If the virus is not contained in other countries, the risk of the emergence of a more serious variant threatens places with high and low vaccination rates alike. It should be noted that the Delta variant had emerged in India at a time when a large section of that population was not vaccinated. The only way to end this pandemic is to get enough people vaccinated so we can reduce the speed of new variants emerging and spreading.
In the face of skepticism about vaccination, a clear and transparent response from the scientific community about the existing knowledge gaps and strategies is required. Access to vaccines is still one of the greatest obstacles; improving infrastructure, continuing education, and enhancing community engagement will be essential for global vaccine distribution and uptake. Improving delivery platforms that eliminate the need for a cold chain could contribute to the wider deployment of vaccines.
There are many possible outcomes. At one extreme is the most optimistic scenario, in which new-generation Covid-19 vaccines are effective against all SARS-CoV-2 variants (including those that may yet emerge) and viral control is pursued effectively in every country in a coordinated effort to achieve global control.
Author contributions
A.H. conceived the concept and design of the paper. A.B.P. participated in the revision process and contributed to the preparation of the Figure.
Declaration of competing interest
The authors have no competing interests to declare. All authors concur with the submission of the manuscript and none of the materials included in this manuscript have been published or are under consideration for publication elsewhere. The authors received no specific funding for this work.
References
- 1.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan S.R., Spratt A.N., Cohen A.R., Naqvi S.H., Chand H.S., Quinn T.P., Lorson C.L., Byrareddy S.N., Singh K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021;124:102715. doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell F., Archer B., Laurenson-Schafer H., Jinnai Y., Konings F., Batra N., Pavlin B., Vandemaele K., Van Kerkhove M.D., Jombart T., Morgan O., le Polain de Waroux O. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 5.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., Valluri S.R., Pan K., Angulo F.J., Jodar L., McLaughlin J.M. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I., Zavitan M., Zuckerman N., Bar-Chaim A., Kreiss Y., Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.S. Gazit, R. Shlezinger, G. Perez, R. Lotan, A. Peretz, A. Ben-Tov, D. Cohen, K. Muhsen, G. Chodick, T. Patalon, Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections, (n.d.). 10.1101/2021.08.24.21262415. [DOI] [PMC free article] [PubMed]
- 8.Machingaidze S., Wiysonge C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021;27:1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 9.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. COVID-19 Genomics UK (COG-UK) Consortium, SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauring A.S., Malani P.N. JAMA; 2021. Variants of SARS-CoV-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., Pohlmann A., King J., Steiner S., Kelly J.N., Portmann J., Halwe N.J., Ulrich L., Trüeb B.S., Fan X., Hoffmann B., Wang L., Thomann L., Lin X., Stalder H., Pozzi B., de Brot S., Jiang N., Cui D., Hossain J., Wilson M.M., Keller M.W., Stark T.J., Barnes J.R., Dijkman R., Jores J., Benarafa C., Wentworth D.E., Thiel V., Beer M. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- 13.Flower T.G., Buffalo C.Z., Hooy R.M., Allaire M., Ren X., Hurley J.H. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2021785118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shastri J., Parikh S., Aggarwal V., Agrawal S., Chatterjee N., Shah R., Devi P., Mehta P., Pandey R. Severe SARS-CoV-2 breakthrough reinfection with Delta variant after recovery from breakthrough infection by Alpha variant in a fully vaccinated health worker. Front. Med. 2021;8 doi: 10.3389/fmed.2021.737007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2021. C. for Disease Control, Prevention, Others, Interim Public Health Recommendations for Fully Vaccinated People.https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/grc-747228 [Google Scholar]
- 16.Riemersma K.K., Grogan B.E., Kita-Yarbro A., Halfmann P.J., Segaloff H.E., Kocharian A., Florek K.R., Westergaard R., Bateman A., Jeppson G.E. Others, Shedding of infectious SARS-CoV-2 despite vaccination. medRxiv. 2021 doi: 10.1371/journal.ppat.1010876. https://www.medrxiv.org/content/10.1101/2021.07.31.21261387v4.full?s=03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore J.P., Offit P.A. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 18.Kawasuji H., Takegoshi Y., Kaneda M., Ueno A., Miyajima Y., Kawago K., Fukui Y., Yoshida Y., Kimura M., Yamada H., Sakamaki I., Tani H., Morinaga Y., Yamamoto Y. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K., Koirala A., Nicolopoulos K., Chiu C., Wood N., Britton P.N. Vaccines for COVID-19: where do we stand in 2021? Paediatr. Respir. Rev. 2021;39:22–31. doi: 10.1016/j.prrv.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Update on Omicron. https://www.who.int/news/item/28-11-2021-update-on-omicron (n.d.)
- 21.HKUMed finds Omicron SARS-CoV-2 can infect faster and better than Delta in human bronchus but with less severe infection in lung. https://www.med.hku.hk/en/news/press/20211215-omicron-sars-cov-2-infection (n.d.)
- 22.Subbaraman N. How do vaccinated people spread Delta? What the science says. Nature. 2021;596:327–328. doi: 10.1038/d41586-021-02187-1. [DOI] [PubMed] [Google Scholar]
- 23.Christensen P.A., Olsen R.J., Long S.W., Subedi S., Davis J.J., Hodjat P., Walley D.R., Kinskey J.C., Ojeda Saavedra M., Pruitt L., Reppond K., Shyer M.N., Cambric J., Gadd R., Thakur R.M., Batajoo A., Mangham R., Pena S., Trinh T., Yerramilli P., Nguyen M., Olson R., Snehal R., Gollihar J., Musser J.M. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2021 doi: 10.1016/j.ajpath.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chia P.Y., Xiang Ong S.W., Chiew C.J., Ang L.W., Chavatte J.-M., Mak T.-M., Cui L., Kalimuddin S., Chia W.N., Tan C.W., Ann Chai L.Y., Tan S.Y., Zheng S., Pin Lin R.T., Wang L., Leo Y.-S., Lee V.J., Lye D.C., Young B.E. bioRxiv; 2021. Virological and Serological Kinetics of SARS-CoV-2 Delta Variant Vaccine-Breakthrough Infections: a Multi-Center Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mecenas P., da Rosa Moreira Bastos R.T., Vallinoto A.C.R., Normando D. Effects of temperature and humidity on the spread of COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan S., Jiang S.-C., Li Z.-L. Do humidity and temperature impact the spread of the novel coronavirus? Front. Public Health. 2020;8:240. doi: 10.3389/fpubh.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bal A., Destras G., Gaymard A., Marlet J., Stefic K., Regue H., Semanas Q., d'Aubarde C., Billaud G., Laurent F., Gonzales C., Valette M., Bouscambert M., Gaudy-graffin C., Lina B., Morfin F., Josset L. bioRxiv; 2020. Two-step Strategy for the Identification of SARS-CoV-2 Variant of Concern 202012/01 and Other Variants with Spike Deletion H69-V70, France, August to December 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quast I., Tarlinton D. B cell memory: understanding COVID-19. Immunity. 2021;54:205–210. doi: 10.1016/j.immuni.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., Wullimann D.J., Kammann T., Emgård J., Parrot T., Folkesson E., Karolinska COVID-19 Study Group. Rooyackers O., Eriksson L.I., Henter J.-I., Sönnerborg A., Allander T., Albert J., Nielsen M., Klingström J., Gredmark-Russ S., Björkström N.K., Sandberg J.K., Price D.A., Ljunggren H.-G., Aleman S., Buggert M. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson N., Ghani A., Cori A., Hogan A., Hinsley W. Others, report 49: growth, population distribution and immune escape of Omicron in England. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-49-Omicron (n.d.)
- 33.Moderna announces preliminary booster data and updates strategy to address Omicron variant. https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx (n.d.)
- 34.Woldemeskel B.A., Garliss C.C., Blankson J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Invest. 2021;131 doi: 10.1172/JCI149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haque A., Pant A.B. vol. 8. Vaccines; Basel: 2020. (Efforts at COVID-19 Vaccine Development: Challenges and Successes). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. bioRxiv; 2021. Waning Immunity of the BNT162b2 Vaccine: A Nationwide Study from Israel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC . 2021. COVID-19 Vaccine Effectiveness.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Feffectiveness.html [Google Scholar]
- 38.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. medRxiv; 2021. What Level of Neutralising Antibody Protects from COVID-19?https://www.medrxiv.org/content/10.1101/2021.03.09.21252641v1.abstract [Google Scholar]
- 39.Kaiser Family Foundation . 2021. COVID-19 Vaccine Breakthrough Cases: Data from the States.https://www.kff.org/policy-watch/covid-19-vaccine-breakthrough-cases-data-from-the-states/ [Google Scholar]
- 40.Massey D., Berrent D., Krumholz H. bioRxiv; 2021. Breakthrough Symptomatic COVID-19 Infections Leading to Long Covid: Report from Long Covid Facebook Group Poll. [DOI] [Google Scholar]
- 41.Hippisley-Cox J., Coupland C.A., Mehta N., Keogh R.H., Diaz-Ordaz K., Khunti K., Lyons R.A., Kee F., Sheikh A., Rahman S., Valabhji J., Harrison E.M., Sellen P., Haq N., Semple M.G., Johnson P.W.M., Hayward A., Nguyen-Van-Tam J.S. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. doi: 10.1136/bmj.n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munro C. Covid-19: 40% of patients with weakened immune system mount lower response to vaccines. BMJ. 2021;374:n2098. doi: 10.1136/bmj.n2098. [DOI] [PubMed] [Google Scholar]
- 43.Dutta Noton K., Mazumdar Kaushiki, Gordy James T., Dutch Rebecca Ellis, The Nucleocapsid Protein of SARS–CoV-2: a Target for Vaccine Development, J. Virol. 94 (n.d.) e00647–20. 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed]
- 44.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solís Arce J.S., Warren S.S., Meriggi N.F., Scacco A., McMurry N., Voors M., Syunyaev G., Malik A.A., Aboutajdine S., Adeojo O., Anigo D., Armand A., Asad S., Atyera M., Augsburg B., Awasthi M., Ayesiga G.E., Bancalari A., Björkman Nyqvist M., Borisova E., Bosancianu C.M., Cabra García M.R., Cheema A., Collins E., Cuccaro F., Farooqi A.Z., Fatima T., Fracchia M., Galindo Soria M.L., Guariso A., Hasanain A., Jaramillo S., Kallon S., Kamwesigye A., Kharel A., Kreps S., Levine M., Littman R., Malik M., Manirabaruta G., Mfura J.L.H., Momoh F., Mucauque A., Mussa I., Nsabimana J.A., Obara I., Otálora M.J., Ouédraogo B.W., Pare T.B., Platas M.R., Polanco L., Qureshi J.A., Raheem M., Ramakrishna V., Rendrá I., Shah T., Shaked S.E., Shapiro J.N., Svensson J., Tariq A., Tchibozo A.M., Tiwana H.A., Trivedi B., Vernot C., Vicente P.C., Weissinger L.B., Zafar B., Zhang B., Karlan D., Callen M., Teachout M., Humphreys M., Mobarak A.M., Omer S.B. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021;27:1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.C. Betsch, P. Schmid, D.K. Heinemeier, L. Korn, C. Holtmann, R. Böhm, Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination, (n.d.). 10.31234/osf.io/ytb7w. [DOI] [PMC free article] [PubMed]
- 47.Gilkey M.B., Magnus B.E., Reiter P.L., McRee A.-L., Dempsey A.F., Brewer N.T. The Vaccination Confidence Scale: a brief measure of parents' vaccination beliefs. Vaccine. 2014;32:6259–6265. doi: 10.1016/j.vaccine.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Momplaisir F.M., Kuter B.J., Ghadimi F., Browne S., Nkwihoreze H., Feemster K.A., Frank I., Faig W., Shen A.K., Offit P.A., Green-McKenzie J. Racial/ethnic differences in COVID-19 vaccine hesitancy among health care workers in 2 large academic hospitals. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haque A., Kouriba B., ’diaye Aïssatou N., Pant A. Eliminating cervical cancer in Mali and Senegal, two sub-saharan countries: insights and optimizing solutions. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritchie H. Our World in Data; 2020, March 5. Coronavirus (COVID-19) Vaccinations - Statistics and Research.https://ourworldindata.org/covid-vaccinations [Google Scholar]