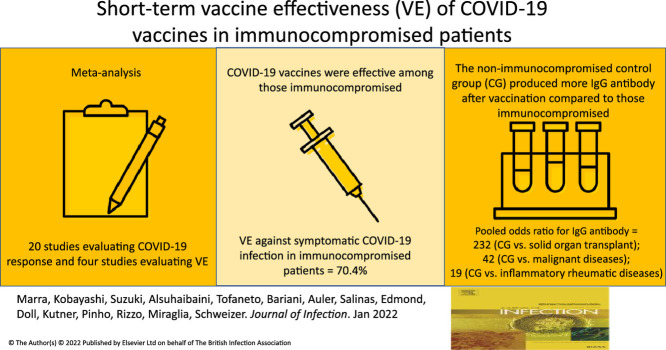
Abstract
Objectives
We aimed to assess the short-term effectiveness of COVID-19 vaccines among immunocompromised patients to prevent laboratory-confirmed symptomatic COVID-19 infection.
Methods
Systematic review and meta-analysis. We calculated the pooled diagnostic odds ratio [DOR] (95% CI) for COVID-19 infection between immunocompromised patients and healthy people or those with stable chronic medical conditions. VE was estimated as 100% x (1-DOR). We also investigated the rates of developing anti-SARS-CoV-2 spike protein IgG between the 2 groups.
Results
Twenty studies evaluating COVID-19 vaccine response, and four studies evaluating VE were included in the meta-analysis. The pooled DOR for symptomatic COVID-19 infection in immunocompromised patients was 0.296 (95% CI: 0.108–0.811) with an estimated VE of 70.4% (95% CI: 18.9%- 89.2%). When stratified by diagnosis, IgG antibody levels were much higher in the control group compared to immunocompromised patients with solid organ transplant (pOR 232.3; 95% Cl: 66.98–806.03), malignant diseases (pOR 42.0, 95% Cl: 11.68–151.03), and inflammatory rheumatic diseases (pOR 19.06; 95% Cl: 5.00–72.62).
Conclusions
We found COVID-19 mRNA vaccines were effective against symptomatic COVID-19 among the immunocompromised patients but had lower VE compared to the controls. Further research is needed to understand the discordance between antibody production and protection against symptomatic COVID-19 infection.
Keywords: COVID-19 vaccine, Effectiveness, Immunocompromised patients, Meta-analysis
Graphical abstract
Background
The first coronavirus disease 19 (COVID-19) vaccine was authorized by the U.S. Food and Drug Administration (FDA) on December 11, 2020 for prevention of severe illness or death. That mRNA vaccine demonstrated an efficacy of 95%1 and humoral and cellular responses were triggered within 1 week after the second dose.2 Subsequently, eight more vaccines have been authorized after phase III trials.3
Previous studies evaluated vaccine effectiveness (VE) among individuals who were healthy or had stable chronic medical conditions1. Since immunocompromised patients were excluded from trials conducted early in this pandemic, there is less data on immunocompromised patients compared with other patient populations. Due to growing concern over a poor response to vaccination among immunocompromised patients who are particularly at risk for severe disease, and some evidence for the benefit of booster doses4 the U.S. FDA gave emergency use authorization for an additional dose of COVID-19 vaccines for immunocompromised people on August 12, 2021.5
Recently, some studies provided real-world data on VE in people with immunocompromising conditions.6 , 7 Other studies evaluated the humoral immune response among these patients.8 Studies suggested that immunocompromised patients who received COVID-19 vaccines might not develop high neutralizing antibody titers or be as protected against severe COVID-19 outcomes as are immunocompetent patients.9 , 10 Vaccine responsiveness in patients who were receiving an immunosuppressor drug therapy exhibited impaired serological immune responses.9 , 11 Though there is growing evidence that VE and immune response among immunocompromised patients seem lower than in healthy people, limited data are available.4 , 6 , 8 Given higher complication and mortality rates from COVID-19,12 it is important to quantify vaccine effectiveness and assess whether this group is capable of producing neutralizing antibodies.
We aimed to review the literature on the impact of COVID-19 vaccination on neutralizing antibodies and the short-term effectiveness of COVID-19 vaccines among immunocompromised patients to prevent laboratory-confirmed symptomatic COVID-19 infection.
Methods
Systematic literature review and inclusion and exclusion criteria
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement13 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.14 This study was registered on Prospero (https://www.crd.york.ac.uk/PROSPERO/) on 6/17/2021 (registration number CRD42021261306). Institutional Review Board approval was not required. Immunocompromised patients were defined as those treated with immunosuppressive medication (e.g., corticosteroids, chemotherapy, or other immunosuppressive medications), solid organ transplant, hematopoietic stem cell transplant, HIV, thalassemia, or active cancer (current cancer, in treatment, or received diagnosis within last 12 months).15
Inclusion criteria for studies in this systematic literature review were as follows: original research manuscripts; published in peer-reviewed, scientific journals; involved vaccinated immunocompromised patients and vaccinated healthy control group or other vaccinated control group with similar clinical conditions; conducted in acute care settings or nursing homes that evaluated the effectiveness of COVID-19 vaccine in immunocompromised people after phase III COVID-19 vaccine clinical trials in immunocompetent participants; and observational study design. The literature search was limited to December 1, 2019 to August 10, 2021. Randomized clinical trials (phase III), commentaries, studies with overlapping patients, studies in pediatric populations, and studies from non-peer reviewed studies (e.g., MedRxiv) were excluded. Studies in which there was no comparison between vaccinated immunocompromised patients and vaccinated control groups, evaluating just one dose of COVID-19 vaccine, and those in which no VE data were published were also excluded.
Search strategy
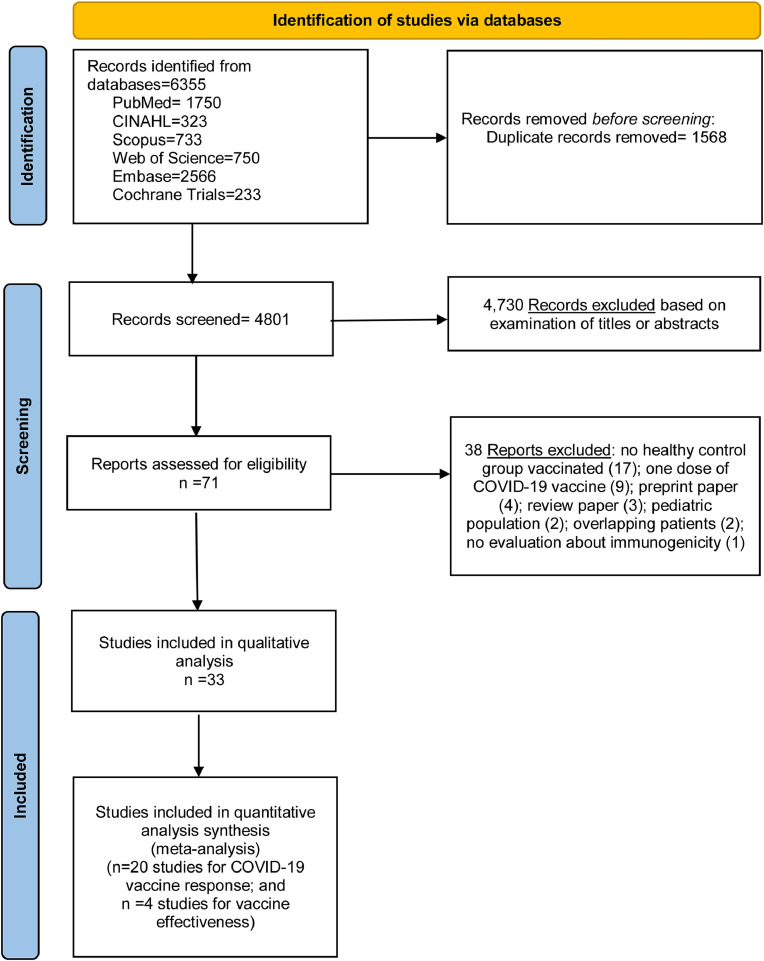
We performed literature searches in PubMed, Cumulative Index to Nursing and Allied Health (CINAHL), Embase (Elsevier Platform), Cochrane Central Register of Controlled Trials, Scopus, and Web of Science. The entire search strategy is described in Supplementary Appendix 1. We reviewed the reference lists of retrieved articles to identify studies that were not identified from the preliminary literature searches. After applying exclusion criteria, we reviewed 71 papers, 33 of which met the inclusion criteria and were included in the systematic literature review [Fig. 1 ].
Fig. 1.
Literature search for articles on COVID-19 vaccine effectiveness among immunocompromised patients.
Data abstraction and quality assessment
Titles and abstracts of all articles were screened to assess whether they met inclusion criteria. The reviewers (ARM, TK, HS, MAA, BMT, LMB, and MAA) abstracted data for each article. Reviewers resolved disagreements by consensus.
The reviewers abstracted data on study design, population and setting, study period (weeks or months), number of patients (immunocompromised vs. the control group), the total number of participants who produced neutralizing antibodies after one or two doses between immunocompromised vs. the control group, the mean or the median antibody levels after one or two doses among immunocompromised and the control groups, humoral and cellular immunity studies, and the immunosuppressive drugs used in each study. The FDA recommends defining the COVID-19 endpoint as virologically confirmed SARS-CoV-2 infection accompanied by symptoms.16 For that reason, we have defined the primary outcome as symptomatic COVID-19 infection.
Risk of bias was assessed using the Downs and Black scale.17 Reviewers followed all questions from this scale as written except for question #27 (a single item on the Power subscale, scored 0 to 5), which was changed to a yes or no. For the analysis, we classified the studies as good (19–23 of 28 possible points), or fair (14–18 points of 28 possible points) quality. Two authors performed component quality analysis independently, reviewed all inconsistent assessments, and resolved disagreements by consensus.18
Statistical analysis
To meta-analyze the extracted data, COVID-19 vaccine response was assessed using a random-effects model to estimate pooled odds ratios and 95% confidence intervals with weights as described by DerSimonian and Laird.19 We performed stratified analyses of the associations between anti-SARS-CoV-2 spike protein IgG production after two doses of COVID-19 vaccine between immunocompromised patients and the control group. We also performed stratified analyses among studies in patients with solid organ transplants, with malignancy or with inflammatory rheumatic diseases, respectively, in studies that evaluated neutralizing antibodies after COVID-19 vaccine, and in studies classified as good vs. fair per the Downs and Black score. In our stratified analyses we did not include studies that did not report the absolute number of patients that produced anti-SARS-CoV-2 spike protein IgG after the second vaccine dose. We did not include in our meta-analysis studies where only mean or the median antibody levels were reported. Heterogeneity between studies was evaluated with I2 estimation and the Cochran Q statistic test. We used the Cochrane Review Manager version 5.3.
We also calculated the pooled diagnostic odds ratio [DOR] (95% confidence interval) for symptomatic COVID-19 between vaccinated immunocompromised patients and vaccinated healthy controls or other vaccinated controls with similar clinical conditions. VE was estimated as 100% x (1-DOR). We performed statistical analysis using R version 4.1.0 with mada package version 0.5.4.20 Analogous to the meta-analysis of the odds ratio methods for the DOR, an estimator of random effects model following the approach of DerSimonian and Laird is provided by mada package.20 For the meta-analysis of estimates of COVID-19 VE, we used a bivariate random effects model, adopting a similar concept of calculating diagnostic accuracy, which enables simultaneous pooling of sensitivity and specificity with mixed-effect linear modeling while allowing for the trade-off between them.21 , 22 Heterogeneity between studies was also evaluated with I2 estimation and the Cochran Q statistic test. Publication bias was assessed using funnel plots.
Results
Characteristics of included studies
Thirty-three studies met the inclusion criteria23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 and were included in the final review (Fig. 1). All of these studies were non-randomized, of which, twenty-seven were prospective cohort studies,24 , 25 , 27 , 28 , 30, 31, 32, 33, 34, 35, 36, 37 , 39 , 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 and six were retrospective cohort studies.23 , 26 , 29 , 38 , 40 , 55 The majority of them (32 studies) evaluated the Pfizer/BioNTech mRNA COVID-19 vaccine.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 , 53, 54, 55 Six of these studies also analyzed the Moderna mRNA COVID-19 vaccine28 , 32 , 33 , 40 , 53 , 55 and another also analyzed the AstraZeneca COVID-19 vaccine42. Just one study evaluated the Coronavac COVID-19 vaccine.52 None of the studies evaluated the VE for the Johnson & Johnson/Janssen vaccine.
The majority of the studies included in our review were conducted in Israel (nine studies),23 , 27 , 29 , 31 , 34 , 37 , 38 , 47 , 48 following by the United States (six studies),33 , 35 , 40 , 53, 54, 55 Germany (five studies),32 , 41 , 49, 50, 51 France (four studies),25 , 26 , 30 , 43 Italy (two studies),39 , 45 the United Kingdom (two studies),42 , 46 and Czech Republic,36 Denmark,24 Lithuania,44 Spain,28 and Turkey52 with one study each. All studies were performed between December 2020 and May 202123, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55. Eleven studies evaluated solid organ transplant recipients26 , 30 , 34 , 36 , 41 , 45 , 47, 48, 49, 50, 51, being two studies of them evaluated hemodialysis patients.26 , 30 Eight studies evaluated patients with malignant diseases,25 , 33 , 37, 38, 39 , 42 , 44 , 46 six studies evaluated patients with inflammatory rheumatic diseases,24 , 27 , 31 , 32 , 35 , 52 two studies evaluated patients with inflammatory bowel diseases,40 , 55 two studies evaluated patients with chronic kidney failure on hemodialysis,28 , 43 one study evaluated patients with multiple sclerosis,23 and one study evaluated HIV patients.54 The definition of immunocompromised condition was not reported in two studies.29 , 53
Studies varied on their reporting of characteristics of the serological tests, including when they were performed, cutoff levels for antibody positivity, and the type of serological test analysis performed (Supplementary Appendix 2). Eight studies did not report the cut-off level for their specific assay.29 , 32 , 36 , 40 , 46 , 47 , 49 , 53 Three studies did not use serological tests to determine vaccine effectiveness.29 , 40 , 53 The cellular immunity investigation was performed in 10 studies with different approaches26 , 28 , 35 , 36 , 45 , 46 , 49, 50, 51 , 54 (Supplementary Appendix 2). Twenty-three studies did not report any cellular immune investigation.23, 24, 25 , 27 , 29, 30, 31, 32, 33, 34 , 37, 38, 39, 40, 41, 42, 43, 44 , 47 , 48 , 52 , 53 , 55
Four studies evaluated the variants of concerns (VOC) in some of patients’ samples.44 , 46 , 53 , 54 One study found that HIV patients and the healthy control group had similar levels of neutralizing antibodies to the vaccine strain spike protein and spike proteins from VOC including, alpha (B.1.1.7), beta (B.1.351), and gamma (P.1) strains.54 One study detected neutralization assays of VOC alpha lineage.46 Another one studied seven patients with hematological malignancies with breakthrough infection detecting mutations of the alpha (B.1.1.7 strain) variant44. Only one study performed genomic surveillance detecting the SARS-CoV-2 (alpha), beta, and gamma variants, where alpha variant was the most common lineage.53 The majority of the included studies did not perform genomic surveillance.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 , 45 , 47, 48, 49, 50, 51, 52 , 55
Regarding the quality assessment scores of the 33 included studies, more than half of the studies (22 studies) were considered good (19–23 of 28 possible points) per the Downs and Black quality tool.24 , 27, 28, 29 , 31, 32, 33, 34, 35, 36, 37, 38 , 40 , 41 , 43 , 44 , 46 , 48 , 51, 52, 53 , 55 Eleven studies were considered fair (14–18 points),23 , 25 , 26 , 30 , 39 , 42 , 45 , 47 , 49 , 50 , 54 and no study was considered poor quality (<14 points).
Outcomes measures – antibody response and vaccine effectiveness
Antibody response (anti-SARS-CoV-2 spike protein IgG)
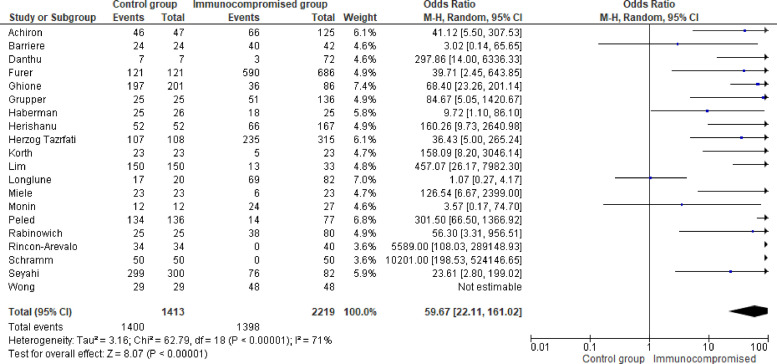
Among 33 studies identified for the systematic literature review, 30 studies evaluated the COVID-19 vaccine response with anti-SARS-CoV-2 spike protein IgG after the second dose.23, 24, 25, 26, 27, 28 , 30, 31, 32, 33, 34, 35 , 37, 38, 39 , 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 Of them, 10 studies reported only mean or median of anti-SARS-CoV-2 spike protein IgG, but they did not report positive rates24, 26, 27, 28, 32, 36, 39, 44, 50, 54. Twenty studies reported positive rates of anti-SARS-CoV-2 spike protein IgG with a total of 2219 immunocompromised patients, and were included in the meta-analysis.23 , 25 , 30 , 31 , 33, 34, 35 , 37 , 38 , 41, 42, 43 , 45, 46, 47, 48, 49 , 51 , 52 , 55 The positive rate ranged from 0% to 100%. Among 2219 immunocompromised patients, 63.0% developed anti-SARS-CoV-2 spike protein IgG compared to 99.1% (1400/1413) in the healthy control group. The control group had significantly higher odds of developing anti-SARS-CoV-2 spike protein IgG compared to immunocompromised patients (pooled odds ratio 58.18; 95% confidence interval [95% Cl]: 21.61–156.61) [Fig. 2 ]. After performing a stratified analysis, the pooled odds ratio for developing neutralizing antibodies among the control group was 181.9 (95% Cl: 22.76–1453.93) compared to immunocompromised patients. [Supplementary Appendix 3, Fig. 3]. With regards to the stratified analysis by immunocompromising conditions, the proportion of patients who developed anti-SARS-CoV-2 spike protein IgG changed to 25.2% in patients with solid organ transplant, 68.0% with malignancy, and 86% with inflammatory rheumatic diseases. The pooled odds ratio (pOR) for developing the anti-SARS-CoV-2 spike protein IgG production was higher in the control group compared to those immunocompromised patients with solid organ transplant at (pOR 232.3; 95% Cl: 66.98–806.03), those with malignant diseases (pOR 42.0; 95% Cl: 11.68–151.03), and to those with inflammatory rheumatic diseases (pOR19.06; 95% Cl: 5.00–72.62) [Table 2, Supplementary Appendix 3, Figs. 4–6].
Fig. 2.
Forest plot of COVID-19 vaccine response (anti-SARS-CoV-2 spike protein IgG) after two doses of COVID-19 vaccine [n = 20 studies] with control group and immunocompromised condition group. Odds ratios (OR) were determined with the Mantel-Haenszel random-effects method. Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel.
Table 2.
Subset analyses evaluating the association between COVID-19 vaccine response (anti-SARS-CoV-2 spike protein IgG) after two doses of COVID-19 vaccine with control group and immunocompromised condition (20 studies)*.
| Subset | Number of Studies Included | Pooled Odds Ratio, M-H, Random, comparing Control Group with Immunocompromised Group (95% CI) | I2 test for heterogeneity |
|---|---|---|---|
| Anti-SARS-CoV-2 Spike protein IgG | 20 | 58.18 (21.61, 156.61) | 71% |
| Neutralizing antibodies | 3 | 181.92 (22.76, 1453.93) | 55% |
| Anti-SARS-CoV-2 Spike protein IgG in control group compared with solid organ transplant patients | 8 | 232.35 (66.98, 806.03) | 35% |
| Anti-SARS-CoV-2 Spike protein IgG in control group compared with patients with malignant diseases | 6 | 42.00 (11.68, 151.03) | 47% |
| Anti-SARS-CoV-2 Spike protein IgG in control group compared with patients with inflammatory rheumatic diseases | 3 | 19.06 (5.00, 72.62) | 0% |
| Studies with 19–23 of 28 points (D&B, Good) | 13 | 36.35 (10.59, 124.77) | 73% |
| Studies with 14–18 of 28 points (D&B, Good) | 7 | 136.80 (31.97, 585.29) | 54% |
CI=Confidence Interval; D&B=Downs & Black score; M-H=Mantel-Haenszel; Random=Random-effects method.
*Reasons for not including the other 13 studies in the meta-analysis: there are no raw numbers to perform the COVID-19 vaccine response (produced anti-SARS-CoV-2 spike protein IgG) after two doses of COVID-19 vaccine for immunocompromised patients.
After stratifying by the risk of bias (Downs and Black score) there was still a difference in antibody response for the control group. The pooled odds ratio for developing anti-SARS-CoV-2 spike protein IgG among the control group was 36.5 (95% Cl: 10.59–124.77) compared to the immunocompromised group in studies with good Downs and Black score (19–23 of 28 points) and was 136.80 (95% Cl: 31.97–585.29) in studies with fair Downs and Black score (14–18 of 28 points) [Table 2, Supplementary Appendix 3, Fig. 7].
Vaccine effectiveness
Four studies with a total of 42,821 patients evaluated VE against symptomatic COVID-19 infection among immunocompromised patients vaccinated with 2 doses.29 , 36 , 40 , 53 VE ranged from 62.9% to 80.4% and all four studies were included for the meta-analysis. The pooled DOR for symptomatic COVID-19 infection was 0.296 (95% CI: 0.108–0.811) with an estimated VE of 70.4% (95% CI: 18.9%- 89.2%). The result of this meta-analysis was homogeneous for symptomatic COVID-19 (heterogeneity I2=0%) [Table 3].
Table 3.
Meta-analyses evaluating the COVID-19 Vaccine Effectiveness among immunocompromised patients (4 studies*).
| Studies Included (n) | Immunocompromised patients (n) | Pooled Diagnostic Odds Ratio [DOR] (95% CI) | I2 test for heterogeneity | Vaccine Effectiveness* (95%CI) | |
|---|---|---|---|---|---|
| All studies evaluating vaccinated immunocompromised patients (two doses) and symptomatic COVID-19 | 4 | 42,821 | 0.296 (0.108, 0.811) | 0% | 70.4% (18.9%, 89.2%) |
CI=Confidence Interval.
*Vaccine Effectiveness was estimated as 100% x (1-DOR).
[Chodick 2021; Havlin 2021; Khan 2021; Tenforde 2021*]
*We have opted to include in our meta-analysis Tenforde 2021 CID [50] because in Tenforde 2021 MMWR study [67] there are no raw numbers to perform the vaccine effectiveness for immunocompromised patients.
Publication bias
We assessed publication bias by creating funnel plots of studies evaluating COVID-19 vaccine response with anti-SARS-CoV-2 spike protein IgG (Supplementary Appendix 3, Fig. 8). Aside from studies with extreme ORs (<1 or >10), studies were reasonably balanced around the pooled OR, and studies with null results were included. Thus, there was little evidence of publication bias.
Discussion
Based on studies evaluating short-term VE between December 2020 and May 2021, this systematic literature review and meta-analysis showed that COVID-19 vaccines (primarily the mRNA COVID-19 vaccines) decrease symptomatic COVID-19 infection with a VE of 70.4% in immunocompromised patients. This number was lower compared to VE in the general population reported in the randomized trials1 , 56 in a noncontrolled setting,57 and also in a recent meta-analysis among healthcare workers (HCWs).58 We also found that a wide range of anti-SARS-CoV-2 spike protein IgG development has been reported after two doses of COVID-19 vaccines among those immunocompromised and the rate of response was significantly lower compared to the control group in these studies.
There is no test to quantify the level of immunosuppression in an immunocompromised patient. However in our meta-analysis we were able to identify that immunocompromised patients with a variety of underlying conditions, produced lower levels of anti-SARS-CoV-2 spike protein IgG after two doses of COVID-19 vaccine in comparison to a non-immunocompromised control group.23 , 25 , 30 , 31 , 33, 34, 35 , 37 , 38 , 41, 42, 43 , 45, 46, 47, 48, 49 , 51 , 52 , 55 The pooled OR for developing the antibody is significantly higher among people in the healthy or stable condition group (i.e., control group) compared to those with solid organ transplant (pOR=232.3), malignant diseases (pOR=42.0), and inflammatory rheumatic diseases (pOR=19.1). This might represent the severity of the immunosuppression for each different diagnosis category.
Immunocompromised patients have a higher incidence of persistent SARS-CoV-2 infection, possibly representing an important reservoir for the emergence of novel viral variants.59 , 60 SARS-CoV-2 has been recovered in viral culture from immunocompromised patients several months after their primary infection61 , 62 signifying that certain individuals may be able to transmit the virus beyond the period of their acute illness.62 Real-world observational studies demonstrated that vaccination of the most vulnerable immunosuppressed population is not fully protective and therefore suggests the need for a third COVID-19 vaccine in immunocompromised patients as well as other protective measures (facial masks and social distancing) until more data on short- and long-term vaccine effectiveness is obtained.57 , 58 Prior studies demonstrated chronic kidney disease patients undergoing hemodialysis have more IgG antibody levels after receiving COVID-19 vaccines than kidney transplant recipients.26 , 30 Also, a recent European cohort study of patients with hemato-oncological diseases and a control group of HCWs suggested that patients with cancer developed lower antibody, and those receiving chemotherapy and B cell-targeting agents showed a particularly impaired serological response.9 This could suggest that the immunosuppressant therapy may be a critical factor implicated in this lack of humoral response.
For the humoral response, the most utilized and reported method was IgG antibody titers. These could be total antibody levels or levels against specific structural proteins, such as spike (S) or membrane proteins of SARS-CoV-2. The antibody response can be reported as positive or negative based on the manufacturer's criteria, actual titers, or relative titers as ratio to an internal control.63 Measurement of neutralizing capability against live viruses or pseudo-viruses is more reflective of the robustness of humoral response because it directly measures the capability to suppress viral growth.64 However, the U.S. FDA does not recommend antibody testing for SARS-CoV-2 to determine immunity or protection from COVID-19, especially among those who are vaccinated.65 In fact, our study showed extremely variable level of antibody response ranging from 0 to 100% among immunocompromised patients, yet the VE was moderately high at 70%. Further research is needed to understand the discordance between antibody production and protection against symptomatic COVID-19 infection.
Our study had several limitations. First, we only included observational studies for the meta-analysis, which are subject to multiple biases.66 However, this is the most common type of study in the infection prevention literature.66 Second, since we estimated the VE based on only short-term durations, we could not evaluate the long term VE or need for a third vaccine dose. One recent study published after our systematic search ended evaluated the long term VE among those immunocompromised and reported the effectiveness of mRNA vaccination against COVID-19 hospitalization was lower (77%) among immunocompromised individuals than among immunocompetent individuals (90%) over nine months.10 There is a need for longer-term observational studies to assess sustained immune response and VE. Third, each study adopted different serological tests to quantify antibody response to SARS-CoV-2 after COVID-19 vaccine among immunocompromised patients.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 Fourth, we do not have any data to evaluate how good enough were cellular immunity to prevent severe disease or mortality among immunocompromised populations. This could represent that many of the studies reviewed were challenging due to lack of information regarding the intensity of immunosuppression or capture of the incidence of COVID-19 outside the hospital. Fifth, our systematic review has not included studies that detected the delta variant, which contributed to the majority of recent breakthrough infections around the world.67 , 68 We need more studies on the SARS-CoV-2 variants of concerns (VOC) that have multiple spike protein mutations and appear to be more infectious or cause more disease than other circulating SARS-CoV-2 variants.69 One recent study performed genomic surveillance detecting the new SARS-CoV-2 delta variant, alpha variant, and other variants.70 It was not included in our systematic review because there is an overlapping of patients in this study with another study,53 and we were unable to extract data for the meta-analysis to calculate VE for symptomatic COVID-19 infection. Sixth, different definitions were used in different studies for immunocompromising conditions. There may also be diagnostic overlap since immunocompromised patients can have multiple comorbidities. Finally, the results of our meta-analysis should be interpreted with caution, particularly since only four studies were included to calculate the COVID-19 VE among immunocompromised patients. Additionally, there was considerable heterogeneity in the identified studies, and there was not enough data to run additional stratified analysis for asymptomatic COVID-19, or COVID-19 breakthrough infections.
We found that the COVID-19 mRNA vaccines were moderately effective against symptomatic COVID-19 among immunocompromised patients. More studies are needed to evaluate VE of other COVID-19 vaccines, COVID-19 breakthrough post-vaccination, VE against new variants, and to better understand the clinical significance of anti-SARS-CoV-2 spike protein IgG antibody levels in immunosuppressed populations (Table 1 ).
Table 1.
Summary of characteristics of studies included in the systematic literature review.
| First author, year, location | COVID-19 vaccine | Study design/D&B score (max score 28) | Study duration [dates] | Number of patients [immunocompromised vs. healthy controls] | Total or% participants with neutralizing antibodies [immunocompromised vs. healthy controls] |
Mean (SD) or median [IQR] antibody titers [immunocompromised vs. healthy control] |
Immunocompromised patients: Symptomatic COVID-19 (N) /No symptomatic COVID-19 (N) [vaccinated vs. unvaccinated] |
Immunosuppressive therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| After 1st dose | After 2nd dose | After 1st dose | After 2nd dose | After 1st dose | After 2nd dose | ||||||
| Achiron, 2021, Israel | Pfizer/ BioNTech |
Retrospective cohort 18 |
NR | 93 on treatment for MS Vs. 32 with untreated MS Vs. 47 healthy controls |
NR | 34 (32.3%) treatment for MS Vs. 32 (100%) with untreated MS Vs. 46 (97.9%) healthy controls |
NR | 7 (6.5–8.1) on cladribine, 0.27 (0.12–0.45) on Fingolimod and 0.29 (0.006–0.89) on Ocrelizum Vs. 8.1 (7.5–8.4) for untreated MS Vs. 7.4 (6.4–8.1) for healthy controls |
NR | NR | Claribine, Fingolimod, Ocrelizumab |
| Ammitzbøll, 2021, Aarhus, Denmark | Pfizer/ BioNTech |
Prospective cohort 21 |
4 months [Dec, 2020 Apr, 2021] | 61 patients with SLE Vs. 73 patients with RA |
NR | SLE patents: 89% (54/61) Vs. RA patients: 67% (49/73) |
NR | NR | NR | NR | Prednisone, Hydroxychloroquine, MMF, Azathioprine Rituximab |
| Barriere 2021, Nice, France |
Pfizer/ BioNTech |
Prospective cohort 18 |
2 months [Jan 18, 2021 - Mar 15, 2021] | 122 immunocompromised patients (solid tumors) Vs. 29 healthy controls |
58/122 Vs. 13/13 |
40/42 Vs. 24/24 |
0.52 UI/mL Vs. 21.6 UI/mL |
245.2 UI/mL Vs 2517 UI/mL |
NR | NR | NR |
| Bertrand, 2021, France | Pfizer/ BioNTech |
Retrospective cohort 17 |
2 months [Jan 2021 – Mar 2021] | 45 kidney transplant recipients Vs. 10 hemodialysis patients |
NR | NR | 311 AU/mL for kidney transplant recipients Vs. 178.9 AU/mL for HD patients |
671 AU/mL (IQR: 172–1523) for kidney transplant recipients Vs. 1052 AU/mL (IQR: 515–2689) for HD patients |
NR | NR | Tacrolimus, Belatacept |
| Braun-Moscovici, 2021, Israel | Pfizer/ BioNTech |
Prospective Cohort 22 |
NR | NR | NR | 227 (86%) immunocompromised [rheumatic inflammatory disease] who took the vaccine Vs. 24 (92.3%) - COVID19 recovered immunocompromised |
NR | 6764.27 AU/mL (9291.61) immunocompromised who took the vaccine Vs. 2044.8 AU/mL (4944.8) - COVID19 recovered immunocompromised |
NR | NR | conventional DMARDs, colchicine, nintedanib, biological/targeted DMARDs, and corticosteroids |
| Broseta, 2021, Spain | Pfizer/ BioNTech and Moderna |
Prospective cohort 21 |
11 weeks [Feb 3, 2021 – Apr 2021] |
10 HD patients on immunosuppression Vs. 165 HD patients without immunosuppression |
NR | 6 (60%) for patients on immunosuppression Vs. 161 (97.6%) for patients without immunosuppression |
NR | NR | NR | NR | Tacrolimus, eclizumab |
| Chodick,2021, Israel | Pfizer/ BioNTech |
Retrospective Cohort 21 |
2 months [Dec 19,2020 – Feb 20, 2021] |
25,459 immunocompromised patients with 2 doses Vs. 27,822 immunocompromised patients with 1 dose |
NR | NR | NR | NR | NR | 56/25,403 [vaccinated] Vs. 79/27,743 [unvaccinated] |
NR |
| Danthu, 2021, France | Pfizer/ BioNTech |
Prospective cohort 17 |
14 weeks [vaccine time: Feb 2, 2021 – Mar, 15, 2021; and 58 days of follow-up] |
74 kidney transplant patients Vs. 78 patients on HD Vs. 7 healthy controls |
NR | 3 (4.1%) for patients after kidney transplant, 59 (85.5%) for patients on HD, and 7 (100%) for healthy control | NR | 6.6 AU/mL (2.1–19) for patients on HD, 1082 AU/mL (735–1662) for healthy control. Titer not shown for transplant patients (only 3 were positive) | NR | NR | Antimetabolite, Steroid |
| Furer, 2021, Tel Aviv, Israel |
Pfizer/ BioNTech |
Prospective cohort 22 |
3 months [Dec 2020 – Mar 2021] | 686 patients with AIIRD Vs. 121 healthy controls |
NR | Immunocompromised: 86% (590/686) Vs. Healthy control: 100% (121/121) |
NR | Immunocompromised: S1/S2 IgG: 132.9 (+/- 91.7) BAU/mL Vs. Healthy controls S1/S2 IgG: 218.6 (+/- 82.06) BAU/mL |
NR | NR | Anti-CD20, Methotrexate, MMF, Interleukin 6 inhibitor, Janus kinase inhibitor, Glucocorticoids, Abatacept |
| Geisen, 2021, Kiel,,Germany. |
Pfizer/ BioNTech and Moderna |
Prospective cohort 19 |
6 weeks [NR] | 26 immunocompromised (patients with chronic inflammatory conditions, and immunosuppressive therapy) Vs. 42 healthy controls |
NR | Immunocompromised: 26(100% ) Vs. Healthy controls 42 (100%) |
NR | Immunocompromised: Anti-SARS-COV-2 IgG: 2053 BAU/mL (+- 1218); Neutralizing antibodies: 87.42% (+−17.94); IgA: 24.52 U/mL (+- 30.48) Vs. Healthy controls: Anti-SARS-COV-2 IgG: 2685 BAU/mL (+−1102);Neutralizing antibodies: 96.04% (+−1551); IgA: 47.65 U/mL (+−45.12) |
NR | NR | Biological DMARD; Conventional DMARD; Steroids (prednisolone) |
| Ghione, 2021, Buffalo, NY, United States |
Pfizer/ BioNTech and Moderna |
Prospective cohort 20 |
2 months [NR] |
65 lymphoma patients with treatment Vs. 21 Lymphoma patients without treatment Vs. 194 healthy controls |
NR | 36/86 Vs. 197/201 |
NR | 0.13 in patients with recent treatment 20.7 in patients with prior treatment |
NR | NR | Anti-CD20, Bruton tyrosine kinase, inhibitors based therapied, chimeric antigen receptor (CAR) T cell therapy |
| Grupper, 2021, Israel | Pfizer/ BioNTech |
Prospective cohort 23 |
NR | 136 kidney transplant patients Vs. 25 healthy controls |
NR | NR | NR | 5.9 (3.8–4.2) for kidney transplants Vs. 189 (141.1–248) for healthy controls |
NR | NR | Methylprednisolone + Basiliximab for induction; Calcineurin inhibitors + Mycophenolate mofetil + Prednisone for maintenance |
| Haberman, 2021, United States | Pfizer/ BioNTech |
Prospective cohort 19 |
4 months [Dec 23, 2020 – Mar 31, 2021] |
101 with immunemediated inflammatory diseases Vs. 26 healthy controls |
NR | NR | NR | 46,90125-694,528 units for Metothrexate group. 113,60825-737,310 units for the imunossupressed without metothrexate and 104,354 units (141–601,185) for healthy controls | NR | NR | disease-modifying antirheumatic drugs (TNF inhibitor) with or without Methotrexate |
| Havlin, 2021, Prague Czech Republic | Pfizer/ BioNTech |
Prospective cohort 19 |
NR | 48 LTR patients Vs. 33 LTR patients post-COVID-19 infection Vs. 10 healthy controls |
NR | LTR patients: 0% (0/48) Vs. LTR patients post-COVID-19: 85% (28/33) Vs. Healthy control: 100% (10/10) |
NR | NR | NR | 3/43 [vaccinated] Vs. 1/1 [unvaccinated] |
NR |
| Herishanu 2021 Tel-Aviv, Israel |
Pfizer/ BioNTech |
Prospective cohort 22 |
3 months [Dec 2020 – Feb 2021] |
167 immunocompromised (chronic lymphocytic leukemia) Vs. 52 healthy controls |
NR | Immunocompromised:39.5% (66/167) patients with CLL Vs. Healthy controls: 52 (100%) |
NR | Immunocompromised: Median: 0.824 U/ml (IQR: 0.4–167.3) Vs. Healthy controls: Median: 1084 U/mL (IQR: 128.9–1879) |
NR | NR | Anti-CD20 (rituximab or obinutuzumab), Venetoclax |
| Herzog Tzarfati, 2021, Israel | Pfizer/ BioNTech |
Retrospective cohort 21 |
NR | 315 immunocompromised (hematologic CA) Vs. 108 healthy controls |
NR | 74.6% for hematologic CA Vs. CIT (29%); single agent anti CD20 Ab (0%); BCL2i (25%); BTKi (40%), JAK2i (42%) Vs. 99.1% for controls |
85 (10.7–172) AU/mL - median | 157 (130–221) AU/mL - median | NR | NR | Chemotherapy, chemo-immunotherapy (CIT), single agent anti CD20, proteasome inhibitors, IMIDs, BCR-ABL TKI, BCL2 inhibitors, JAK2 inhibitors, BTK inhibitors |
| Iacono, 2021, Italy | Pfizer/ BioNTech |
Prospective Cohort 16 |
NR |
36 hematologic CA Vs. 72 healthy controls |
NR | NR |
NR |
2369.1 (0–3276.3) AU/mL in the immunosuppressed Vs. 8737.49 (398.9–967,280) AU/mL for healthy controls |
NR | NR | Rituximab, Paclitaxel + Transtuzumab, Corticosteroids |
| Khan, 2021, VA across the United States |
Pfizer/ BioNTech and Moderna |
Retrospective cohort 21 |
4 months [Dec 18, 2020 – April 20, 2021] |
7376 immunocomprimised patients [IBD] with vaccines Vs. 7211 immunocomprmised patients without vaccine |
NR | NR | NR | NR | NR | 14/7098 [vaccinated] Vs. 197/14,500 [unvaccinated] |
Mesalamine Thiopurine Anti TNF Vedolizumab Ustekinumab Tofacitinib Methotrexate steroid |
| Korth 2021 Kronach Germany |
Pfizer/ BioNTech |
Prospective cohort 19 |
2 months [Jan 2021 – Feb 2021] |
23 Immunocompromised: (KTR) Vs. 23 healthy controls |
NR | Immunocompromised: 22% (5/23) Vs. Healthy controls: 100% (23/23) |
NR | Immunocompromised: IgG: 50.9 (+/- 138.7) AU/mL Vs. Healthy controls IgG: 727.7 (+/- 151.3) AU/mL |
NR | NR | Tacrolimus, MMF, corticosteroids |
| Lim, 2021, UK | Pfizer/ BioNTech; AstraZeneca |
Prospective Cohort 18 |
NR | 119 immunocompromised patients: 44% (52/119) with lymphoma on treatment Vs. 150 healthy controls |
Immuno compromised: 9/31 (28%) Vs. healthy control: - |
Immuno compromised: 13/33 (39%) Vs. not on treatment: Hodgkin lymphoma (100%); aggressive B-cell non-Hodgkin lymphoma (81%); indolent B-cell non-Hodgkin lymphoma (89%) |
Immuno compromised: Vs. healthy control: Pfizer/ BioNTech: 172 BAU/mL (95% CI 109–272) AstraZeneca: 67 BAU/mL40-111 |
Immunocompromised: on treatment: 2.5 BAU/mL (95% CI 1.1–5.8) Vs. not on treatment: 141.8 BAU/mL (75.6–266.0); Hodgkin lymphoma: 652.2 BAU/mL (95% CI 604.7–703.4); aggressive B-cell non-Hodgkin lymphoma: 244.6 BAU/mL (31.12–1923); Vs. healthy control: Pfizer/ BioNTech: 2339 BAU/mL1,923-2,844 AstraZeneca: 199 BAU/mL (140–282) |
NR | NR | Anti-lymphoma therapy |
| Longlune, 2021, France | Pfizer/ BioNTech |
Prospective cohort 21 |
NR | 20 HD patients on immunosuppression, Vs. 92 HD patients without immunosuppression |
5 (4.5%) in total | 10 (58.8%) for patients on immunosuppression Vs. 76 (95.0%) for patients without immunosuppression |
NR | NR | NR | NR | mTOR inhibitor, Mycophenolic acid, steroid |
| Maneikis, 2021, Lithuania | Pfizer/ BioNTech |
Prospective cohort 21 |
3.5 months [Jan 8, 2021 – April 21, 2021] |
857 patients with hematologic CA Vs. 68 healthy controls |
NR | NR | NR | 6961 AU/mL (IQR: 1292–20,672) for hematologic CA Vs. 21,395 AU/mL (IQR: 14,831–33,553) for healthy controls |
NR | NR | Anti-CD20, Tyrosine kinase inhibitors, Nivolumab, Ruxolitinib, Venetoclax, Aregrelide or interferon |
| Miele, 2021, Palermo, Italy | Pfizer/ BioNTech |
Prospective cohort 18 |
4 months [Dec 2020 – Mar 2021] |
16 SOT patients Vs. 23 healthy controls |
NR | Immunocompromised:37% (6/16) Vs. Healthy control: 100% (23/23) |
NR | Mean: 87.32 AU/mL for SOT patients Vs. Mean: 233 AU/mL for healthy controls |
NR | NR | Tacrolimus, Everolimus, MMF, Corticosteroids |
| Monin 2021 London, UK |
Pfizer/ BioNTech |
Prospective cohort 21 |
2 months [Dec 8, 2020 – Feb 18, 2021] |
151 immunocompromised (solid and hematologic CA) Vs. 54 healthy controls |
Solid CA: Week 3: 21; 28%26-51; Week 5: 10; 30%17-47 Vs. Hematologic CA: Week 3: 8; 18%10-32 Week 5: 4; 11%4-25 Vs. Healthy controls: Week 3: 32; 94% (81–98); Week 5:18; 86%65-95 |
Solid CA: 18; 95% (75–99) Vs. Hematological CA:; 60%23-88 Vs. Healthy controls: 12; 100% (76–100) |
NR | NR | NR | NR | NR |
| Peled, 2021, Israel | Pfizer/ BioNTech |
Prospective cohort 16 |
NR | 77 patients after heart transplant Vs. 136 healthy controls |
NR | 8 (10.4%) for heart transplant patients Vs. 127 (93.4%) for healthy controls |
NR | NR | NR | NR | Calcineurin inhibitor, prednisone |
| Rabinowich, 2021, Tel Aviv-Yafo, Israel |
Pfizer/ BioNTech |
Prospective cohort 20 |
6 weeks [Dec 2020 – Jan 2021] |
80 in immunocompromised patients (liver transplant recipients) Vs. 25 healthy controls |
NR | (47.5%) 38/80 in immunocompromised patients Vs. (100%) 25/25 in healthy controls |
NR | Immunocompromised: 95.4 AU/ml (+- 92.4) Vs. 200.5 AU/ml (+−65.1) in healthy controls |
NR | NR | Prednisone, Tacrolimus/cyclosporin, Everlolimus, Azathioprine, MMF |
| Rincon-Arevalo, 2021, Berlin, Germany | Pfizer/ BioNTech |
Prospective cohort 18 |
7 weeks [NR] |
44 on dialysis Vs. 40 in kidney transplant patients Vs. 34 healthy controls |
NR | 30/44 in dialysis group Vs. 0/10 in transplant group |
NR | NR | NR | NR | MMF, Steroid, Calcineurin inhibitor |
| Sattler, 2021, Germany |
Pfizer/ BioNTech |
Prospective Cohort 17 |
NR | 39 immunocompromised (KTR) Vs. 26 patients with kidney failure on HD Vs. 39 healthy controls |
NR | Immunocompromised:IgG: 1 (2.6%); IgA: 4 (10.26%); Neutralizing antibodies: 0 Vs. Patients with kidney failure on hemodialysis: IgG: 22 (84.62%); IgA: 22 (84.62%); Neutralizing antibodies: 20 (76.92%) Vs. Healthy patients: IgG: 39 (100%); IgA: 38 (97.44%); Neutralizing antibodies: 39 (100%) |
NR | NR | NR | NR | Cyclosporin, Tacrolimus MMF, corticosteroids |
| Schramm, 2021, Germany | Pfizer/ BioNTech |
Prospective Cohort 19 |
NR | 50 cardiothoracic transplant recipients Vs. 50 healthy controls |
NR | NR | 96% below the cut off in the immunosuppressed group Vs. Abbott: 8241-149; Roche: 3312-75; Euroimmun: 62 27-100 in healthy controls |
90% below the cut off in the immunosuppressed group Vs. Abbott 1417 (732-2,589); Roche: >250; Euroimmun: >100 in healthy controls |
NR | NR | Calcineurin inhibitor, MMF |
| Seyahi, 2021, Instabul, Turkey |
Coronavac | Prospective cohort 22 |
15 weeks [Jan 14, 2021 – May 2, 2021] | Elderly immunocompromised [inflammatory rheumatic disease]: 22 elderly healthy control: 47 Vs. immunocompromised [inflammatory rheumatic disease]: hospital workers: 82 Vs. healthy controls (health care workers): 300 |
NR | 1 (14.3%) for RTX Vs. 22 (88%) for non-RTX biological agents Vs. 25 (92.6%) for conventional DMARDs- Vs. 16 (100%) for colchicine Vs. 29 (100%) for no treatment |
NR | NR | NR | NR | RTX, non-RTX biological agents-based regimen, conventional DMARDs-based regimen, colchicine |
| Tenforde,2021, United States |
Pfizer/ BioNTech and Moderna |
Prospective cohort 19 |
4 months [Mar 11, 2021 – May 5, 2021] |
254 in immunocompimised patients Vs. 958 healthy controls |
NR | NR | NR | NR | NR | 20/59 [vaccinated] Vs. 62/60 [unvaccinated] |
NR but definition for immunocompromised active solid organ CA (treated or newly diagnosed in the past 6 months), active hematologic CA (e.g., leukemia, lymphoma, myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, SOT, immunosuppressive medication, SLE, RA, psoriasis, scleroderma, or IBD |
| Woldemeskel, 2021, United States | Pfizer/ BioNTech |
Prospective cohort 14 |
NR | 12 patients with HIV Vs. 17 healthy controls |
NR | NR | NR | median 8.84 for HIV patients Vs. median 9.49 for healthy controls |
NR | NR | Antiretroviral therapy* |
| Wong, 2021, NY, United States | Pfizer/ BioNTech and Moderna |
Retrospective cohort 22 |
2 months [Dec 14, 2020 –Feb 12, 2021] | 48 patients with IBD receiving biologic therapies Vs. 14 HCWs and 29 healthy volunteers |
NR | Immunocompromised:26 IBD patients (100%) who received the 2nd vaccine dose had positive anti-RBD tests Vs. 100% in healthy controls |
NR | NR | NR | NR | TNF antagonist monotherapy, vedolizumab monotherapy, ustekinumab |
AIIRD=Autoimmune inflammatory rheumatic diseases; Anti-RBD=Anti receptor-binding domain; AU=Arbitrary units; BAU=Binding antibody units; BCL2 inhibitors= B-cell lymphoma 2; BCR-ABL TKI; BTK inhibitors= Bruton tyrosine kinase; CA=cancer; 95% CI=95% Confidence Interval; DMARDs=Disease modifying anti-rheumatic drugs; HD=Hemodialysis; IBD=Inflammatory Bowel Disease; IMIDs= Immune modulatory drugs; IQR=Interquartile range; JAK2= Janus kinase 2; KTR= Kidney transplant recipient; LTR=Lung transplant recipient; MDS=Myelodysplastic syndrome; MMF=Mycophenolate mofetil; MS=Multiple Sclerosis; mTOR= mammalian target of rapamycin; N=number reported; NR=Not reported; RA=Rheumatoid Arthritis; RTX=Rituximab; S=Spike; SCT=Stem Cell Transplantation; SD=Standard Deviation; SLE=Systemic Lupus Erythematosus; SOT=Solid organ transplant; TKI= Tyrosine kinase inhibitor; TNF=tumor necrosis factor; UI= Units; VE=Vaccine Effectiveness.
*All HIV patients were on antiretroviral therapy and had a median CD4+ T cell count on 913 cells/uL (range of 649 to 1678 cells/uL).
Funding
This study was not funded.
Conflict of Interest
All authors report no conflict of interest relevant to this article.
Acknowledgements
We thank Jennifer Deberg, MLS, from the Hardin Library for the Health Sciences, University of Iowa Libraries, for assistance with the search methods.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.12.035.
Appendix. Supplementary materials
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals [updated August 12, 2021; cited 2021 Aug 29]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
- 6.Boyarsky B.J., Ou M.T., Greenberg R.S., Teles A.T., Werbel W.A., Avery R.K., et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56–ee7. doi: 10.1097/TP.0000000000003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman J.D., Robinson P.C., Uldrick T.S., Ljungman P. COVID-19 in immunocompromised populations: implications for prognosis and repurposing of immunotherapies. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mair M.J., Berger J.M., Berghoff A.S., Starzer A.M., Ortmayr G., Puhr H.C., et al. Humoral immune response in hematooncological patients and health care workers who received SARS-CoV-2 vaccinations. JAMA Oncol. 2021:1–8. doi: 10.1001/jamaoncol.2021.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., et al. Effectiveness of 2-Dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - Nine states, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijenthira A., Gong I., Betschel S.D., Cheung M., Hicks L.K. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv. 2021;5(12):2624–2643. doi: 10.1182/bloodadvances.2021004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340–350. doi: 10.1093/cid/ciaa863. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Pilishvili T., Fleming-Dutra K.E., Farrar J.L., Gierke R., Mohr N.M., Talan D.A., et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel - 33 U.S. Sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, U.S. Food and drug administration, center for biologics evaluation and research. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. 2021.
- 17.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alderson PGS HJ, editors. Assessment of study quality. 2004.
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Doebler P.. Meta-analysis of diagnostic accuracy with mada. 2017.
- 21.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Goto M., Ohl M.E., Schweizer M.L., Perencevich E.N. Accuracy of administrative code data for the surveillance of healthcare-associated infections: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(5):688–696. doi: 10.1093/cid/cit737. an official publication of the Infectious Diseases Society of America. [DOI] [PubMed] [Google Scholar]
- 23.Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammitzbøll C., Bartels L.E., Bøgh Andersen J., Risbøl Vils S., Elbaek Mistegård C., Dahl Johannsen A., et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021;3(9):622–628. doi: 10.1002/acr2.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. official journal of the European Society for Medical Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertrand D., Hamzaoui M., Lemée V., Lamulle J., Hanoy M., Laurent C., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun-Moscovici Y., Kaplan M., Braun M., Markovits D., Giryes S., Toledano K., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 28.Broseta J.J., Rodríguez-Espinosa D., Rodríguez N., Mosquera M.D.M., Marcos M., Egri N., et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571–581. doi: 10.1053/j.ajkd.2021.06.002. the official journal of the National Kidney Foundation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chodick G., Tene L., Rotem R.S., Patalon T., Gazit S., Ben-Tov A., et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danthu C., Hantz S., Dahlem A., Duval M., Ba B., Guibbert M., et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153–2158. doi: 10.1681/ASN.2021040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 32.Geisen U.M., Berner D.K., Tran F., Sümbül M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghione P., Gu J.J., Attwood K., Torka P., Goel S., Sundaram S., et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell directed therapies. Blood. 2021;138(9):811–814. doi: 10.1182/blood.2021012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben-Yehoyada M., Shashar M., et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberman R.H., Herati R., Simon D., Samanovic M., Blank R.B., Tuen M., et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10):1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havlin J., Svorcova M., Dvorackova E., Lastovicka J., Lischke R., Kalina T., et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40(8):754–758. doi: 10.1016/j.healun.2021.05.004. the official publication of the International Society for Heart Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herzog Tzarfati K., Gutwein O., Apel A., Rahimi-Levene N., Sadovnik M., Harel L., et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iacono D., Cerbone L., Palombi L., Cavalieri E., Sperduti I., Cocchiara R.A., et al. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021 doi: 10.1016/j.jgo.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan N., Mahmud N. Effectiveness of SARS-CoV-2 Vaccination in a Veterans Affairs Cohort of Patients With Inflammatory Bowel Disease With Diverse Exposure to Immunosuppressive Medications. Gastroenterology. 2021;161(3):827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korth J., Jahn M., Dorsch O., Anastasiou O.E., Sorge-Hädicke B., Eisenberger U., et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13(5) doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim S.H., Campbell N., Johnson M., Joseph-Pietras D., Collins G.P., O'Callaghan A., et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542–e5e4. doi: 10.1016/S2352-3026(21)00199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longlune N., Nogier M.B., Miedougé M., Gabilan C., Cartou C., Seigneuric B., et al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2021. [DOI] [PMC free article] [PubMed]
- 44.Maneikis K., Šablauskas K., Ringelevičiūtė U., Vaitekėnaitė V., Čekauskienė R., Kryžauskaitė L., et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–ee92. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miele M., Busà R., Russelli G., Sorrentino M.C., Di Bella M., Timoneri F., et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21(8):2919–2921. doi: 10.1111/ajt.16702. official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peled Y., Ram E., Lavee J., Sternik L., Segev A., Wieder-Finesod A., et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–762. doi: 10.1016/j.healun.2021.04.003. the official publication of the International Society for Heart Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rincon-Arevalo H., Choi M., Stefanski A.L., Halleck F., Weber U., Szelinski F., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60) doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 50.Sattler A., Schrezenmeier E., Weber U.A., Potekhin A., Bachmann F., Straub-Hohenbleicher H., et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14) doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schramm R., Costard-Jäckle A., Rivinius R., Fischer B., Müller B., Boeken U., et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021;110(8):1142–1149. doi: 10.1007/s00392-021-01880-5. official journal of the German Cardiac Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seyahi E., Bakhdiyarli G., Oztas M., Kuskucu M.A., Tok Y., Sut N., et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41(8):1429–1440. doi: 10.1007/s00296-021-04910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenforde M.W., Patel M.M., Ginde A.A., Douin D.J., Talbot H.K., Casey J.D., et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis. 2021 an official publication of the Infectious Diseases Society of America. [Google Scholar]
- 54.Woldemeskel B.A., Karaba A.H., Garliss C.C., Beck E.J., Wang K.H., Laeyendecker O., et al. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab648. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong S.Y., Dixon R., Martinez Pazos V., Gnjatic S., Colombel J.F., Cadwell K. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161(2):715–718. doi: 10.1053/j.gastro.2021.04.025. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marra A.R., Kobayashi T., Suzuki H., Alsuhaibani M., Tofaneto B.M., Bariani L.M., et al. The short-term effectiveness of coronavirus disease 2019 (COVID-19) vaccines among healthcare workers: A systematic literature review and meta-analysis. Antimicrobial Stewardship & Healthcare Epidemiology. 2021;1(1):E33. doi: 10.1017/ash.2021.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Truong T.T., Ryutov A., Pandey U., Yee R., Goldberg L., Bhojwani D., et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baang J.H., Smith C., Mirabelli C., Valesano A.L., Manthei D.M., Bachman M.A., et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis. 2021;223(1):23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case Study: prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183(7):1901–1912. doi: 10.1016/j.cell.2020.10.049. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aydillo T., Gonzalez-Reiche A.S., Aslam S., van de Guchte A., Khan Z., Obla A., et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020;383(26):2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D., Li J. Immunologic testing for SARS-CoV-2 Infection from the Antigen Perspective. J Clin Microbiol. 2021;59(5) doi: 10.1128/JCM.02160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luchsinger L.L., Ransegnola B.P., Jin D.K., Muecksch F., Weisblum Y., Bao W., et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J Clin Microbiol. 2020;58(12) doi: 10.1128/JCM.02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination: FDA safety communication.
- 66.Harris A.D., Lautenbach E., Perencevich E. A systematic review of quasi-experimental study designs in the fields of infection control and antibiotic resistance. Clin Infect Dis. 2005;41(1):77–82. doi: 10.1086/430713. an official publication of the Infectious Diseases Society of America. [DOI] [PubMed] [Google Scholar]
- 67.Del Rio C., Malani P.N., Omer S.B. Confronting the delta variant of SARS-CoV-2, Summer 2021. JAMA. 2021;326(11):1001–1002. doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 68.Lustig Y., Zuckerman N., Nemet I., Atari N., Kliker L., Regev-Yochay G., et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 2021;26(26) doi: 10.2807/1560-7917.ES.2021.26.26.2100557. bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. Clinical research ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tenforde M.W., Self W.H., Naioti E.A., Ginde A.A., Douin D.J., Olson S.M., et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.