Abstract
Non-directed kidney donors can initiate living donor chains that end to patients on the waitlist. We compared 749 National Kidney Registry (NKR) waitlist chain end transplants to other transplants from the NKR and the Scientific Registry of Transplant Recipients between February 2008 and September 2020. Compared to other NKR recipients, chain end recipients were more often older (53 vs 52 years), black (32% vs. 15%), publicly insured (71% vs. 46%), and spent longer on dialysis (3.0 vs. 1.0 years). Similar differences were noted between chain end recipients and non-NKR living donor recipients. Black patients received chain end kidneys at a rate approaching that of deceased donor kidneys (32% vs. 34%). Chain end donors were older (52 vs 44 years) with slightly lower glomerular filtration rates (93 vs. 98 mL/min/1.73 m2) than other NKR donors. Chain end recipients had elevated risk of graft failure and mortality compared to control living donor recipients (both p<0.01), but lower graft failure (p=0.03) and mortality (p<0.001) compared to deceased donor recipients. Sharing non-directed donors among a multicenter network may improve the diversity of waitlist patients who benefit from living donation.
INTRODUCTION
Up to one third of patients in need of renal replacement therapy who have identified a willing living donor may be incompatible due to blood type or human leukocyte antigen (HLA) sensitization.1,2 Kidney paired donation (KPD) has evolved over recent years to provide a solution for these incompatible pairs, and is now responsible for 14.5% of living donor transplants in the United States.3 Non-directed donors have the potential to unlock multiple donor-recipient matches not previously possible due to traditional reciprocal KPD matching requirements, and thus initiate chains of paired exchanges.4,5 At eventual chain termination, the “extra” kidney is donated to a waitlist patient who otherwise does not have a willing living donor.
These beneficiaries of chain end kidneys, who would otherwise have continued to wait for a deceased donor offer, have not been well characterized. Allocation to individual patients on the waitlist is left to the discretion of the chain end transplant center. Thus, it is necessary to study center or KPD network behaviors to characterize waitlist chain end recipients. The National Kidney Registry (NKR) has facilitated over 4600 living donor transplants to date through 100 participating transplant centers, and is the largest KPD network in the world.6 Recent work has shown that NKR recipients are more often black, women, older, highly immunized, or have public insurance compared to other (non-NKR) living donor recipients.7,8 It is not clear what demographic similarities or differences exist among NKR waitlist chain ends and other recipients. In this study we aim to characterize these patients and identify whether current chain end allocation favors any demographic over another, and whether chain end candidates are being selected to create an unmonitored advantage compared to other waitlist candidates. We also aim to identify chain end donor qualities. Finally, we compare post-transplant outcomes of waitlist chain end recipients to other living as well as deceased donor kidney transplant recipients.
MATERIALS AND METHODS
The National Kidney Registry
This study used data from the National Kidney Registry, which is a nonprofit, 501(c) organization that facilitates kidney paired donations in the United States. The NKR network currently comprises 100 transplant centers and its data processing and policies have been previously described.8–10 Priority for allocation of chain end kidneys is given to member centers that have previously started chains and is described in the NKR Medical Board policies.6 The clinical and research activities of this study are consistent with the Declaration of Helsinki and Declaration of Istanbul. Using the NKR, we identified 4,174 cross-validated living donor kidney transplants facilitated by the NKR between February 2008 and September 2020.
National Registry Data Source
This study also used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in January 2021. The SRTR data system includes data on all donors, waitlist candidates, and transplant recipients in the US, submitted by members of the Organ Procurement and Transplantation Network (OPTN), and has been previously described.11 The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Using SRTR, we identified 209,668 kidney-only recipients who underwent kidney transplant between February 2008 and September 2020 (including the 4,174 National Kidney Registry transplants). There were 135,847 deceased donor and 73,821 living donor kidney transplants included in the study population. All recipients were followed for post-transplant outcomes through December 31, 2020 (minimum 3 months of complete follow-up).
Data Linkage
Data on kidney paired donation transplants facilitated by the National Kidney Registry were linked to the SRTR using unique, encrypted person-level identifiers; they were cross-validated using redundantly captured characteristics (transplant center, transplant date, donor blood type, donor sex, recipient blood type, and recipient sex). As a result of cross-validation, 4,174 of 4,238 (98%) living donor kidney transplants facilitated by the National Kidney Registry were included in the study population.
Statistical Analysis
Descriptive Statistics
All analyses were performed using Stata 16/MP for Linux (College Station, Texas). Descriptive statistics describe donor, recipient, and transplant characteristics among NKR chain end and control group transplants. In order to assess the post-transplant outcomes of death-censored graft failure and mortality, we plotted the inverse of the Kaplan-Meier survival curve (representing the cumulative incidence) and compared groups using the log-rank test. A two-sided α of 0.05 is typically used to indicate a statistically significant difference. For Kaplan-Meier plots, we compared National Kidney Registry chain end recipient outcomes to outcomes of (1) NKR non-chain end recipients, (2) control living donor kidney transplant recipients identified through SRTR, (3) control kidney paired donation recipients, and (4) deceased donor recipients. Control kidney paired donation recipients reported receiving a kidney through paired donation or a non-directed donor but were not linked to the National Kidney Registry. These recipients participated through either a local/region system or another multicenter network such as the Alliance for Paired Donation; however, we cannot systematically identify the paired donation system through the national registry.
Statistical Modeling
In order to produce unbiased estimates of the hazard ratio between chain ends recipients and control recipients, we used statistical models to control for potential confounders. In order to account for confounders, we used inverse probability of treatment weighting.12,13 The hazard ratio was estimated using Cox regression stratified by transplant center to account for center-level differences. The recipient model adjusted for recipient factors of female sex, black race, Hispanic ethnicity, age, BMI>30 kg/m2, diabetes, hypertension, history of transplant, college education, year of transplant, public insurance status, hepatitis C (HCV), preemptive transplant or time on dialysis, estimated glomerular filtration rate (eGFR), and type of induction. The recipient and donor model adjusted for the recipient factors above as well as the following donor factors: BMI>30 kg/m2, female sex, black race, Hispanic ethnicity, age, eGFR, and Living Kidney Donor Profile Index (LKDPI)/ Kidney Donor Profile Index (KDPI). A parsimonious model was developed using the F-test for goodness of fit. The parsimonious model adjusted for recipient sex, recipient black race, recipient age, recipient BMI>30 kg/m2, donor BMI>30 kg/m2, recipient diabetes, history of previous transplant, donor sex, donor Hispanic ethnicity, year of transplant, recipient public insurance status, recipient HCV, donor age, preemptive transplant, recipient eGFR, type of induction, number of HLA mismatches, and LKDPI/KDPI.
Handling of Missingness
There were low levels of missingness (<10%) among characteristics used in the statistical analyses. Characteristics with missingness included: BMI, college education, PRA at transplant, number of HLA mismatches, cold ischemia time, public insurance status, HCV, eGFR, LKDPI/KDPI, and type of induction. Using a missing-at-random assumption, missing values were imputed using multiple imputation by chained equations to avoid potential information bias.
RESULTS
Study Population Demographics
The demographics of 749 chain end transplant recipients identified during the study period are displayed in Table 1. These recipients were transplanted across a geographic diversity of centers throughout the United States (Figure 1). Compared to other NKR transplant recipients, NKR chain end recipients were more often black (31.6% vs 15.0%), older (median age 53.0 vs. 52.0), spent more time on dialysis (median 3.0 vs. 1.0 years), had public insurance (70.9% vs. 46.4%), or had co-morbidities such as diabetes (27.0% vs. 18.5%) and hypertension (21.5% vs. 14.5%). NKR chain end recipients were less often female (39.3% vs 47.2%), college educated (59.6% vs 68.6%), Hispanic (10.5% vs 11.4%), preemptive (16.0% vs. 28.1%) or repeat (11.3% vs. 23.7%) transplant recipients compared to other NKR transplant recipients. Only 6.4% of chain end recipients were highly sensitized with a panel reactive antibody (PRA) score >80% compared to 20.9% of other NKR recipients. Furthermore, chain end recipients experienced delayed graft function more commonly than other NKR recipients (7.6% vs. 4.2%). Comparisons between NKR chain end recipients and control living donor transplant recipients hold similar patterns to the comparisons with other NKR recipients.
Table 1.
Characteristics of National Kidney Registry Chain End and Control Transplants (Feb 2008-September 2020).
| NKR Chain End | Other NKR | Living Donor | Non-NKR KPD | Deceased Donor | |
|---|---|---|---|---|---|
| N | 749 | 3425 | 69647 | 6034 | 135847 |
| Recipient Characteristics | |||||
| Female, % | 39.3 | 47.2 | 37.2 | 42.5 | 39.2 |
| Black, % | 31.6 | 15.0 | 12.7 | 13.3 | 33.6 |
| Hispanic, % | 10.5 | 11.4 | 15.2 | 14.5 | 17.9 |
| Median (IQR) Age, years | 53.0 (41.0-60.0) | 52.0 (40.0-61.0) | 50.0 (36.0-60.0) | 50.0 (39.0-60.0) | 55.0 (43.0-63.0) |
| Preemptive Transplant, % | 16.0 | 28.1 | 35.9 | 29.9 | 10.6 |
| Median (IQR) Years on Dialysis | 3.0 (1.1-4.8) | 1.0 (0.0-2.4) | 0.5 (0.0-1.6) | 0.9 (0.0-2.3) | 3.6 (1.5-5.9) |
| Median (IQR) BMI, kg/m2 | 26.5 (23.5-31.0) | 26.8 (23.3-30.9) | 27.2 (23.6-31.4) | 27.5 (23.8-31.7) | 28.0 (24.3-32.1) |
| College Educated, % | 59.6 | 68.6 | 61.5 | 64.7 | 48.1 |
| Public Insurance, % | 70.9 | 46.4 | 42.4 | 45.5 | 77.5 |
| Diabetes, % | 27.0 | 18.5 | 21 | 21.3 | 28.8 |
| Hypertension, % | 21.5 | 14.5 | 15.7 | 15.3 | 24.2 |
| Hepatitis C, % | 2.0 | 1.5 | 2.1 | 2.0 | 5.5 |
| Previous Transplant, % | 11.3 | 23.7 | 11.2 | 16.4 | 14.0 |
| PRA>80 at Transplant, % | 6.4 | 20.9 | 3.8 | 9.7 | 16.4 |
| Median (IQR) eGFR Pre-transplant, mL/min per 1.73 m2 | 7.4 (5.2-10.6) | 8.0 (5.6-11.7) | 8.9 (6.2-12.9) | 8.3 (5.7-12.0) | 6.7 (4.9-9.5) |
| Antibody Depleting Induction, % | 64.5 | 69.0 | 63.5 | 74.0 | 73.5 |
| Delayed Graft Function, % | 7.6 | 4.2 | 3.1 | 3.7 | 27.8 |
| Donor Characteristics | |||||
| Female, % | 64.8 | 63.3 | 62.7 | 63.9 | 39.1 |
| Black, % | 7.1 | 8.5 | 10.5 | 8.4 | 13.6 |
| Hispanic, % | 9.3 | 9.7 | 14.7 | 13.1 | 14.5 |
| Median (IQR) Age, years | 52.0 (41.0-58.0) | 44.0 (34.0-53.0) | 42.0 (33.0-52.0) | 44.0 (34.0-52.0) | 41.0 (29.0-52.0) |
| Median (IQR) BMI, kg/m2 | 26.1 (23.4-28.8) | 26.1 (23.3-29.0) | 26.7 (23.9-29.8) | 26.6 (23.8-29.7) | 27.2 (23.7-31.7) |
| Median (IQR) eGFR, mL/min per 1.73 m2 | 93.1 (81.7-103.0) | 97.6 (86.0-109.0) | 98.9 (85.9-110.8) | 97.1 (84.4-109.0) | 88.6 (58.9-112.6) |
| Median (IQR) LKDPI or KDPI, % | 19.8 (5.5-35.6) | 12.4 (−1.2-26.6) | 12.7 (−1.2-27.5) | 13.6 (0.2-28.0) | 46.8 (25.5-68.6) |
| Blood Type O, % | 2.3 | 46.7 | 64.9 | 55.2 | 47.4 |
| 1 Renal Vein, % | 88.5 | 88.2 | |||
| 2 Renal Veins, % | 5.1 | 3.7 | |||
| 3 Renal Veins, % | 0.4 | 0.2 | |||
| Missing Vein Information, % | 6 | 7.9 | |||
| 1 Renal Artery, % | 70.1 | 72.4 | |||
| 2 Renal Arteries, % | 21.2 | 18.6 | |||
| 3 Renal Arteries, % | 3.1 | 1.4 | |||
| Missing Artery Information, % | 6 | 7.9 | |||
| Transplant Characteristics | |||||
| Zero HLA mismatch, % | 0.4 | 0.9 | 6.9 | 0.7 | 6.7 |
| 1 HLA mismatch, % | 0.9 | 2.0 | 4.6 | 0.9 | 1.2 |
| 2 HLA mismatch, % | 4.7 | 6.4 | 14.9 | 4.9 | 4.7 |
| 3 HLA mismatch, % | 12.4 | 16.8 | 25.3 | 13.6 | 13.8 |
| 4 HLA mismatch, % | 26.3 | 26.2 | 16.4 | 26.8 | 27.2 |
| 5 HLA mismatch, % | 36.7 | 31.1 | 20.0 | 32.7 | 31.5 |
| 6 HLA mismatch, % | 17.2 | 14.7 | 11.0 | 18.5 | 14.8 |
| Median (IQR) cold ischemia time, hours | 6.4 (1.4-9.8) | 9.3 (6.3-12.5) | 1.0 (0.7-1.9) | 1.4 (0.9-3.1) | 16.5 (11.3-22.3) |
Figure 1. Distribution of NKR Chain End Recipient Centers.
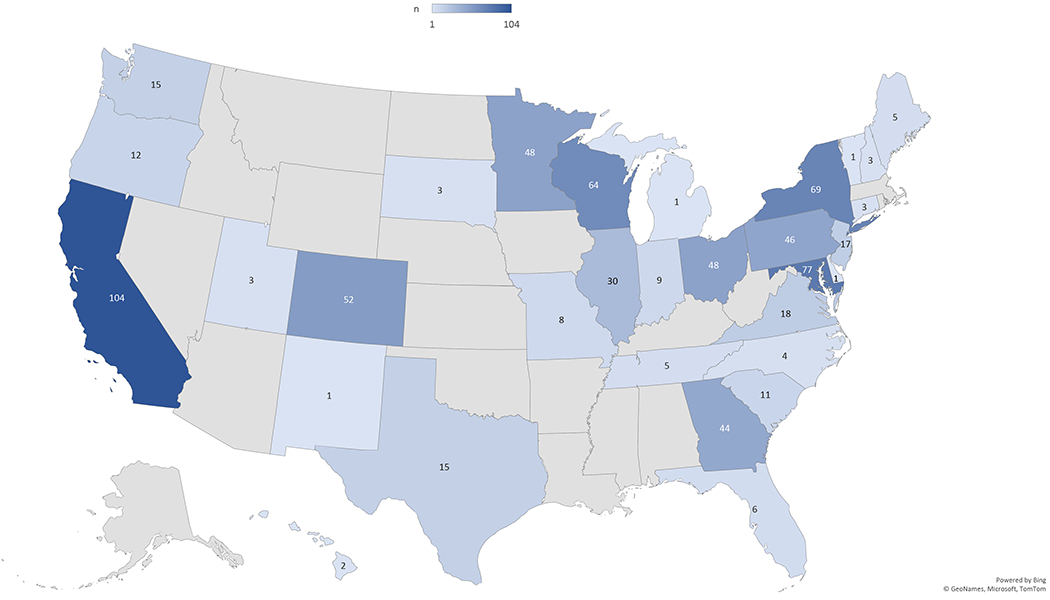
A United States map demonstrates the geographic distribution of NKR participating centers that have performed chain end transplants to waitlist patients. The number of chain end transplants per state is shown.
Compared to control deceased donor transplant recipients, NKR chain end recipients were younger (median age 53.0 vs. 55.0 years), spent less time on dialysis (3.0 vs 3.6 years), were more often preemptive transplant recipients (16.0% vs. 10.6%), and more had some college education (59.6% vs. 48.1%). NKR chain end recipients were less often black (31.6% vs. 33.6%), Hispanic (10.5% vs. 17.9%), had public insurance (70.9% vs. 77.5%), or were highly sensitized (PRA>80 6.4% vs. 16.4%). Unsurprisingly, deceased donor recipients experienced delayed graft function more commonly (27.8% vs 7.6%) and were more often a zero HLA mismatch to their donors (6.7% vs 0.4%) compared to chain end living donor recipients.
NKR chain end donors and other NKR donors were similar across many characteristics (Table 1). NKR chain end donors were more often older (median age 52.0 vs. 44.0), had lower pre-transplant estimated glomerular filtration rates (eGFR, median 93.1 vs. 97.6), had higher Living Kidney Donor Profile Index (LKDPI)14 scores (19.8% vs. 12.4%), and were less commonly blood type O (2.3% vs 46.7%) compared to other NKR donors. NKR chain end donor kidneys had multiple veins (5.5% vs. 3.9%) and arteries (24.3% vs. 20.0%) more often than other NKR kidneys. Comparisons between NKR chain end donors and control living donors hold similar patterns to the comparisons with other NKR donors. Compared to control deceased donors, NKR chain end donors were more often female (64.8% vs. 39.1%), older (median age 52.0 vs. 41.0), and had higher pre-transplant eGFR (median 93.1 vs 88.6). NKR chain end donors were less often black (7.1% vs. 13.6%), Hispanic (9.3% vs. 14.5%), or blood type O (2.3% vs. 47.4%).
Because blood type O living donors have the potential to facilitate additional transplants through propagating a chain rather than terminating to the waitlist, special focus was given to the characteristics of the 17 blood type O chain end donors to determine whether there were any obvious concerns regarding donor quality or anatomy that lead to chain termination. Type O chain end donors were less often female (35.3% vs 65.4%, p=0.01), but no other significant differences were noted in race, age, BMI, eGFR, LKDPI, or renal vascular anatomy (Table 2).
Table 2.
Characteristics of National Kidney Registry Chain End Blood Type O and Non-O Donors (February 2008-September 2020).
| Type O Chain End | Non-Type O Chain End | p-value | |
|---|---|---|---|
| N | 17 | 732 | |
| Female, % | 35.3 | 65.4 | 0.01 |
| Black, % | 5.9 | 7.1 | 0.8 |
| Hispanic, % | 11.8 | 9.3 | 0.7 |
| Median (IQR) Age, years | 52.0 (39.0-56.0) | 52.0 (41.0-58.0) | 0.6 |
| Median (IQR) BMI | 27.0 (24.4-29.5) | 26.0 (23.4-28.7) | 0.3 |
| Median (IQR) eGFR, mL/min | 91.7 (82.7-111.0) | 93.1 (81.7-103.0) | 0.6 |
| Median (IQR) LKDPI, % | 14.6 (−2.2-29.4) | 19.9 (5.5-35.9) | 0.3 |
| 1 Renal Vein, % | 94.1 | 88.4 | 0.5 |
| 2 Renal Veins, % | 0.0 | 5.2 | 0.8 |
| 3 Renal Veins, % | 0.0 | 0.4 | 0.4 |
| Missing Renal Vein Information, % | 5.9 | 6.0 | >0.9 |
| 1 Renal Artery, % | 82.4 | 69.8 | 0.3 |
| 2 Renal Arteries, % | 5.9 | 21.6 | 0.1 |
| 3 Renal Arteries, % | 5.9 | 3.0 | 0.5 |
| Missing Renal Artery Information, % | 5.9 | 6.0 | >0.9 |
Post-Transplant Outcomes
We compared post-transplant outcomes for NKR chain ends to control living and deceased donor transplant recipients. The median (interquartile range) follow-up for death-censored graft failure and mortality was 3.0 (2.0-5.2) years for NKR chain end recipients, 5.6 (2.8-8.8) years for control living donor recipients, and 4.1 (1.8-7.2) years for control deceased donor recipients.
In unadjusted Kaplan-Meier analyses NKR chain end recipients had an elevated risk of death-censored graft failure and mortality compared to non-chain end NKR recipients (graft failure log rank p<0.01, mortality log rank p<0.01), control living donor kidney transplant recipients (graft failure log rank p<0.01), mortality log rank p<0.01), and non-NKR kidney paired donation recipients (graft failure log rank p=0.04, mortality log rank =0.02). Compared with control deceased donor recipients, NKR chain end recipients had lower death-censored graft failure (log rank p0.03) and mortality (log rank p<0.001) (Figures 2 and 3).
Figure 2. Death-censored graft failure cumulative incidence of National Kidney Registry chain end recipients.
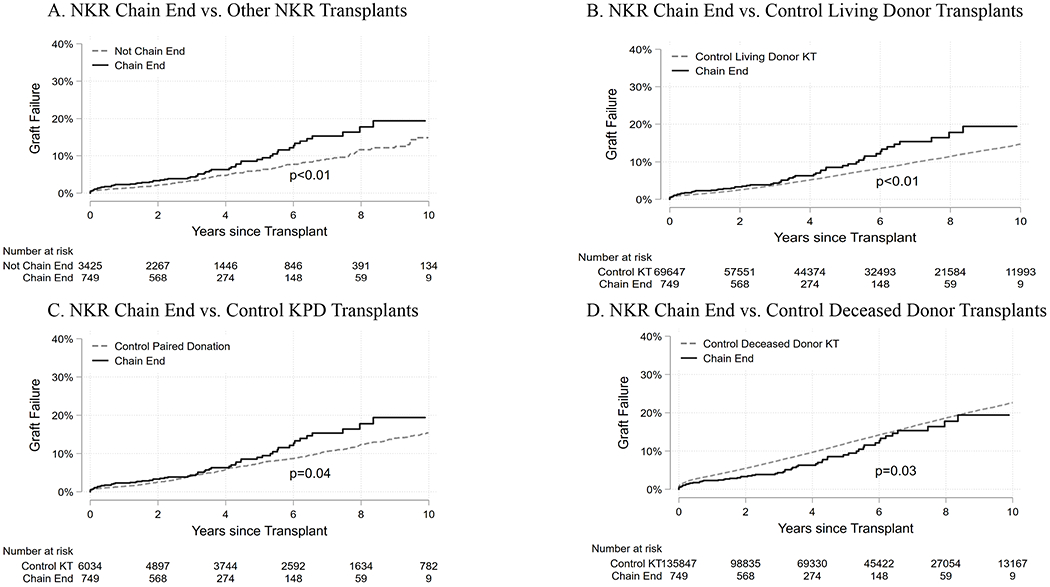
(A) Cumulative death-censored graft failure comparing NKR chain end recipients (solid line) to NKR non-chain end recipients (dashed line) during the study period. (B) Cumulative death-censored graft failure comparing NKR chain end recipients (solid line) to control living donor recipients (dashed line) identified through the SRTR during the study period. (C) Cumulative death-censored graft failure comparing NKR chain end recipients (solid line) to control kidney paired donation (KPD) recipients (dashed line) during the study period. (D) Cumulative death-censored graft failure comparing NKR chain end recipients (solid line) to control deceased donor transplant recipients identified through the SRTR (dashed line) during the study period.
Figure 3. Mortality cumulative incidence of National Kidney Registry chain end recipients.
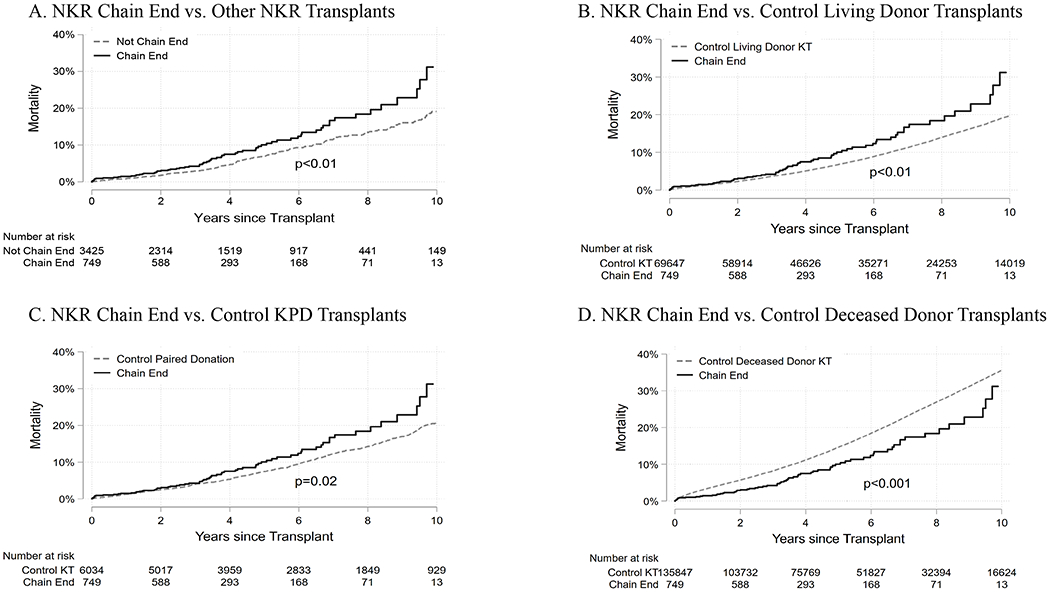
(A) Cumulative mortality comparing NKR chain end recipients (solid line) to NKR non-chain end recipients (dashed line) during the study period. (B) Cumulative mortality comparing NKR chain end recipients (solid line) to control living donor recipients (dashed line) identified through the SRTR during the study period. (C) Cumulative mortality comparing NKR chain end recipients (solid line) to control kidney paired donation (KPD) recipients (dashed line) during the study period. (D) Cumulative mortality comparing NKR chain end recipients (solid line) to control deceased donor transplant recipients identified through the SRTR (dashed line) during the study period.
In adjusted models, we also observed an increased risk of graft failure and mortality in chain end recipients compared to control living donor recipients (Table 3). The increased risk of mortality comparing chain ends recipients to control living donor recipients was attenuated when adjusted for donor and transplant factors. Compared to deceased donor transplant recipients, we observed no statistical difference in graft failure and a lower risk of mortality among chain end recipients. These models held similar outcomes when time on dialysis was substituted as a variable in place of pre-emptive transplant status (Supplemental Table).
Table 3.
Graft Failure and Mortality Outcomes Comparing NKR Chain End Recipients to non-NKR Living Donor and Deceased Donor Transplant Recipients.
| Chain End vs. Living Donor HR (95% CI) |
p-value | Chain End vs. Deceased Donor HR (95% CI) |
p-value | |
|---|---|---|---|---|
| Death-Censored Graft Failure | ||||
| Recipient Adjusted1 | 1.80 (1.32, 2.46) | <0.001 | 0.93 (0.69, 1.26) | 0.6 |
| Recipient + Donor Adjusted2 | 2.27 (1.37, 3.77) | <0.01 | 1.17 (0.73, 1.87) | 0.5 |
| Full Parsimonious Adjusted3 | 2.31 (1.38, 3.85) | <0.01 | 1.19 (0.74, 1.90) | 0.5 |
| Mortality | ||||
| Recipient Adjusted | 1.48 (1.12, 1.97) | <0.01 | 0.65 (0.49, 0.86) | <0.01 |
| Recipient + Donor Adjusted | 1.38 (0.92, 2.09) | 0.1 | 0.61 (0.43, 0.87) | <0.01 |
| Full Parsimonious Adjusted | 1.39 (0.91, 2.13) | 0.1 | 0.62 (0.43, 0.90) | 0.01 |
Adjusted for recipient factors: female sex, African American race, Hispanic ethnicity, age, BMI>30 kg/m2, diabetes, hypertension, history of transplant, college education, year of transplant, public insurance status, HCV, preemptive transplant, eGFR, and type of induction.
Adjusted for recipient factors above and donor factors: BMI>30 kg/m2, female sex, African-American race, Hispanic ethnicity, age, eGFR, and LKDPI/KDPI.
Adjusted for recipient sex, recipient African-American race, recipient age, recipient BMI>30 kg/m2, donor BMI>30 kg/m2, recipient diabetes, history of previous transplant, donor sex, donor Hispanic ethnicity, year of transplant, recipient public insurance status, recipient HCV, donor age, preemptive transplant, recipient eGFR, type of induction, number of HLA mismatches, and LKDPI/KDPI.
DISCUSSION
In this study of the largest kidney paired donation clearinghouse, we described the characteristics and outcomes of kidney transplant recipients who were awaiting a deceased donor transplant but were offered a living donor chain end kidney. These chain end recipients on average spent longer time on dialysis and more often had systemic conditions such as diabetes and hypertension compared to other living donor recipients. Furthermore, chain end recipients were more likely to be publicly insured and less likely to have a college level education, both of which are socioeconomic factors that are linked to disparities in transplant outcomes.15,16 Chain end donors were older and had slightly lower eGFRs (reflecting higher average LKDPI) compared to other living donors. Chain end donors appeared to have complex vascular anatomy more often than other NKR donors, the absolute difference was small. It is not clear whether the differences between chain end and other living donors result in clinically relevant impacts on donor organ quality. Nevertheless, the findings in this study suggest that chain end recipients experience an increased risk of graft failure and mortality (although this is attenuated in adjusted models) compared to other living donor recipients. Recipients of chain end donor kidneys had improved outcomes compared to other waitlist patients who received a deceased donor transplant.
While overall the characteristics of chain end recipients are much more similar to deceased donor recipients compared to other living donor recipients, the chain end recipient population does not exactly mirror the deceased donor recipient population. We hypothesize that this is partly explained by the fact that only a subset of transplant centers in the United States participate in the NKR and therefore the entire waitlist population is not represented. However, we also acknowledge that a subtle bias may exist towards selecting waitlist patients who exhibit predictors for improved transplant outcomes (e.g. patients with fewer co-morbidities). If such a bias does exist, it may be partly influenced by regulatory quality and outcome requirements to which individual transplant programs must adhere.
It is well established that living donor kidneys outperform deceased donor kidneys with lower rates of delayed graft function and acute rejection, and longer graft survival.17 It remains a concern in transplantation that racial minorities are much less likely to receive living donor kidneys compared to white patients with end stage renal disease.18,19 Furthermore, racial minorities have been substantially underrepresented even as recipients of non-directed living donor kidneys. From 1998 to 2008, before kidney paired donation programs were widely adopted, black patients benefited from only 19.5% of living non-directed kidney donations in the United States despite comprising 33% of the national waitlist, whereas white patients received 64.7% of living non-directed donor kidneys while representing only 42.3% of the waitlist.20 This disparity persisted from 2008-2015 when only 15% of non-directed living donor recipients were black.21 However, over this period the recipients may have represented a different patient population as paired donation and domino chains became more common.
Recent studies have shown that black patients receive living donor kidneys at higher rates through the NKR compared to other living donor recipients.7,8 Importantly and partially accounting for these rates, this present study demonstrates that black patients represent 32% of NKR chain end living donor kidney recipients chosen by participating centers, which approaches the national proportion of black waitlist patients. A feature of NKR is that it is comprised of many centers across the country representing urban, rural, suburban, academic, and community transplant centers. This allows for increased heterogeneity in the living donor pool as it is not limited to the demographics of one center’s referral base. Given that NKR encourages centers to share non-directed donors by allocating these programs with chain ends, improvement in the diversity of chain end recipients may continue to be seen with increased input of non-directed donors from regionally diverse centers. In other words, “laundering” through a multicenter exchange has potential to improve the equitable distribution of the valuable resource of non-directed donors. An increased participation in multicenter paired donation may push chain end recipient characteristics more closely towards the characteristics of the national waitlist.
Only seventeen (2.3%) chain end donors in this study were blood type O, again raising a concern that O waitlist patients may be disadvantaged in paired exchange programs.22 However, it has been shown that in transplant chains, more O recipients were transplanted than there were O non-directed donors who initiated chains.23 The ability to transplant hard to match patients through paired exchange removes them from competition for limited deceased donor organs on the waitlist. On the other hand, one could argue that ending a chain with a blood type O donor results in fewer overall transplants, as these donors could be used to propagate a chain rather than terminate to the waitlist. Based on internal chart review of a sample of these chain end O donors, several donated to the waitlist due to time constraints (i.e. the donor wanted to recover from surgery before a planned trip or family event). However, it is worth considering whether future paired donation policy should be directed towards maximizing the utilization of O donors.
This study is limited by the lack of specificity regarding transplant center-specific chain end policies as well as center level of participation in donor sharing to the larger NKR paired donation pool. In accordance with OPTN policy, participating NKR centers have autonomy in the allocation of chain ends to their waitlist, and individual center allocation policies could result in chain end demographic variation. This study is also limited by the availability of data in the national registry related to the process of paired donation. Although it is possible to identify a portion of the paired donation transplants, we are not able to directly compare NKR transplants to transplants of specific paired donation networks. While a majority of chain end recipients in the United States are likely transplanted by the NKR, other systems or local chains may have different inferences from their practice. Furthermore, we acknowledge that outcomes may be slightly worse for chain end recipients compared to other living donor recipients. While several recipient factors are likely responsible, this could also in part be due to donor factors such as age. This raises concern as to whether waitlist patients would have improved graft survival and mortality by receiving a kidney immediately from a non-directed donor rather than at the end of a chain. Unfortunately we lack outcome data of waitlist patients who received a kidney straight from non-directed donors to make this comparison.
These limitations notwithstanding, chain end recipients had better outcomes compared to their peers on the waitlist who received deceased donor transplants. Inputting non-directed donors into a large paired donation system benefits not only patients with willing but incompatible donors, but it could also have the potential to diversify the opportunity for waitlist patients to benefit from non-directed donation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH’s National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID) grant numbers: T32HL007055 (Thomas) and K24AI144954 (Segev). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. MR is employed by the National Kidney Registry as Director of Research and Technology. MC, SMF, and JLV serve as unpaid members of the Medical Board for the National Kidney Registry with MC as Surgical Director. DS reports that Johns Hopkins University receives institutional support from the National Kidney Registry to provide analytical support for general research activities. The other authors of this manuscript have no conflicts of interest to disclose.
ABBREVIATIONS
- BMI
Body Mass Index
- CI
Confidence Interval
- eGFR
estimated Glomerular Filtration Rate
- HCV
hepatitis C
- HLA
Human Leukocyte Antigen
- HR
Hazard Ratio
- IQR
Interquartile Range
- KDPI
Kidney Donor Profile Index
- LKDPI
Living Kidney Donor Profile Index
- NKR
National Kidney Registry
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel Reactive Antibodies
- SRTR
Scientific Registry of Transplant Recipients
- US
United States
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA 2005;293:1883–90. [DOI] [PubMed] [Google Scholar]
- 2.Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis 2011;57:144–51. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am J Transplant 2020;20 Suppl s1:20–130. [DOI] [PubMed] [Google Scholar]
- 4.Melcher ML, Leeser DB, Gritsch HA, et al. Chain transplantation: initial experience of a large multicenter program. Am J Transplant 2012;12:2429–36. [DOI] [PubMed] [Google Scholar]
- 5.Gentry SE, Montgomery RA, Swihart BJ, Segev DL. The roles of dominos and nonsimultaneous chains in kidney paired donation. Am J Transplant 2009;9:1330–6. [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Registry. 2020. (Accessed August 4, 2020, at https://www.kidneyregistry.org/index.php.)
- 7.Flechner SM, Thomas AG, Ronin M, et al. The first 9 years of kidney paired donation through the National Kidney Registry: Characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant 2018;18:2730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeser DB, Thomas AG, Shaffer AA, et al. Patient and Kidney Allograft Survival with National Kidney Paired Donation. Clin J Am Soc Nephrol 2020;15:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holscher CM, Jackson K, Thomas AG, et al. Temporal changes in the composition of a large multicenter kidney exchange clearinghouse: Do the hard-to-match accumulate? Am J Transplant 2018;18:2791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbesey J, Thomas AG, Ronin M, et al. Early graft losses in paired kidney exchange: Experience from 10 years of the National Kidney Registry. Am J Transplant 2020;20:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand CM, Bowring MG, Thomas AG, et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med 2018;168:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massie AB, Leanza J, Fahmy LM, et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant 2016;16:2077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, et al. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol 2006;1:313–22. [DOI] [PubMed] [Google Scholar]
- 16.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol 2010;5:2276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cecka M Clinical outcome of renal transplantation. Factors influencing patient and graft survival. Surg Clin North Am 1998;78:133–48. [DOI] [PubMed] [Google Scholar]
- 18.Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS. Disparities in the utilization of live donor renal transplantation. Am J Transplant 2009;9:1124–33. [DOI] [PubMed] [Google Scholar]
- 19.Purnell TS, Luo X, Cooper LA, et al. Association of Race and Ethnicity With Live Donor Kidney Transplantation in the United States From 1995 to 2014. JAMA 2018;319:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segev DL, Montgomery RA. Regional and racial disparities in the use of live non-directed kidney donors. Am J Transplant 2008;8:1051–5. [DOI] [PubMed] [Google Scholar]
- 21.Kumar K, Holscher CM, Luo X, et al. Persistent regional and racial disparities in nondirected living kidney donation. Clin Transplant 2017;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodle ES, Daller JA, Aeder M, et al. Ethical considerations for participation of nondirected living donors in kidney exchange programs. Am J Transplant 2010;10:1460–7. [DOI] [PubMed] [Google Scholar]
- 23.Melcher ML, Veale JL, Javaid B, et al. Kidney transplant chains amplify benefit of nondirected donors. JAMA Surg 2013;148:165–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


