Abstract
We have amplified and sequenced the 5.8S and 28S ribosomal DNA genes and intergenic regions of Paracoccidioides brasiliensis, strain Pb01. Using primers specifically designed for both ribosomal DNA regions, we were able to discriminate between P. brasiliensis and other human pathogenic fungi by PCR. The use of this molecular marker could be important for paracoccidiodomycosis diagnosis and ecological and molecular epidemiological studies of P. brasiliensis in Latin America.
The thermal dimorphic fungus Paracoccidioides brasiliensis is the causal agent of paracoccidiodomycosis (PCM), a common human mycosis in Latin America. Epidemiological data show a broad geographic distribution in the Central and South America, from Mexico to Argentina, occurring mainly in Colombia, Venezuela, and Brazil. In areas where PCM is highly endemic, the disease incidence is estimated to be approximately 1 to 3 clinical cases per 100,000 inhabitants per year (15). The infection incidence shows high prevalence in the South-Central Brazil.
The defense mechanism against P. brasiliensis is the consequence of an efficient cellular immune response in the human host. PCM reactivation is related to immune deficiency, which occurs in AIDS patients (7, 11, 12), patients undergoing cancer treatment (10, 20), and transplant patients (21, 22). The AIDS infection has been disseminated in urban areas of South-Central Brazil, where P. brasiliensis has high infection prevalence (11).
The identification of P. brasiliensis is based on the morphological characteristics of fungus from lesions found on a patient. Depending on the histopathological pattern small forms of P. brasiliensis may be mistaken for other fungal infections (9). The serologic diagnosis has been extensively used, but some patients present low levels or an absence of detectable antibodies (3). The identification of a specific antigen for P. brasiliensis has been a main goal in South America (2, 13, 14). The antigenic serodiagnosis approach has been used, but the most important P. brasiliensis antigen, gp43, disappears from circulation during treatment (13). Antigenic diagnosis has also been performed using urine samples (16).
Molecular markers in the 28S ribosomal DNA region have been described for other pathogenic fungi (8, 18, 19). The primer specificity described for P. brasiliensis (5, 6) must yet be tested to evaluate the PCR diagnosis efficiency. The development of a specific molecular marker for P. brasiliensis PCR identification would be useful for diagnosis and therapeutic or epidemiological studies. In this paper we describe the cloning and sequencing of a 5.8S ribosomal DNA fragment and the molecular identification of P. brasiliensis by PCR.
A P. brasiliensis Pb01 isolate was used in this work (M.R.R. Silva Collection Instituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás, Goiânia, Brazil). Yeast cultures were grown at 36°C in semisolid Fava-Neto's medium (4) for 7 days under continuous subculturing. DNA was prepared as described by Borges et al. (1). Briefly, frozen cells were broken by mechanical maceration followed by the addition of Tris-spermidine buffer (40 mM Tris-HCl [pH 8.0], 4 mM spermidine, 10 mM EDTA, 0.1 M NaCl, 10 mM β-mercaptoethanol, and 0.1% sodium dodecyl sulfate). Two phenol extractions and one chloroform extraction were performed. The DNA was precipitated with 2.5 volumes of a solution containing ethanol and 0.3 M NaCl, centrifuged and resuspended in Tris-EDTA buffer (20 mM Tris-HCl [pH 8.0], 0.1 mM EDTA). The primers ITS1 (5′ - TCC GTA GGT GAA CCT GCG G - 3′; melting point [Tm] = 71.9°C) and ITS4 (5′ - TCC TCC GCT TAT TGA TAT GC - 3′; Tm = 66.2°C) were described by White et al. (23). The primers UNI-R (5′ - GGT CCG TGT TTC AAG ACG - 3′; Tm = 66.8°C) and UNI-F (5′-GCA TAT CAA TAA GCG GAG GAA AAG - 3′; Tm = 70.5°C) were described by Haynes et al. (8). The primers OL5 (5′ - TGT GAC GAA GCC CCA TAC G - 3′; Tm = 69.7°C) and OL3 (5′ - CTC AGC GGG CAC TT 3′ Tm = 59,6°C) were designed in this work. All primers were synthesized by DNA Agency and analyzed by the Oligo 4.0 program to verify the homodimer, secondary structure formation, and annealing temperature for each. PCR was performed with a 50-μl reaction mixture containing the following (per reaction): 25 ng of genomic DNA; 1× Taq buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.4], 1.5 mM MgCl2); a 0.25 mM (concentration of each deoxynucleoside triphosphate); a 2 μM concentration of each primer (ITS1, ITS4, OL5, UNI-R), except that OL3 was used at 3.5 μM; 2 U of Taq polymerase (Cenbiot-RS; Biotechnology Center, Rio Grande do Sul, Porto Alegre, Brazil). The reaction mixture was overlaid with 25 μl of mineral oil. Amplifications were performed in an MJ Research Mini-Cycler, and the PCR program was as follows: 95°C for 2 min and 35 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min 30 s followed by a 10-min final extension at 72°C. The PCRs using primers OL3 and UNI-R were performed under conditions the same as those described above, except that the annealing temperature was 57.5°C. Ten microliters of the mixture was analyzed by electrophoresis on 1.5% agarose gels and visualized by ethidium bromide (0.5 μg ml−1). DNA fragment cloning was carried out by using a PCR Superscript kit (Stratagene). Plasmid DNA was extracted as described by Sambrook et al. (17). The clones were analyzed by digestion with the restriction enzymes XhoI and NotI. DNA sequencing was performed on an automated DNA sequencer. Analysis of the similarity between DNA of P. brasiliensis and other fungi ribosomal DNA sequences from the GenBank database was performed by a Genetics Computer Group program. The sequences were from the following organisms (accession numbers in parentheses): Aspergillus fumigatus (m60301), Blastomyces dermatitidis (m55624), Candida albicans (x71088), Coccidioides immitis (u18360), Histoplasma capsulatum 1 (y13997), Histoplasma capsulatum 2 (y13400), and Saccharomyces cerevisiae (k01048).
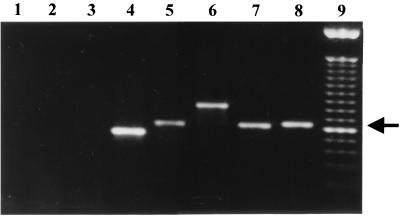
The primers ITS1 and ITS4, corresponding to intergenic sequences flanking the 5.8S ribosomal DNA, were tested with the P. brasiliensis genome and five other fungus genomes. Figure 1 shows the results of PCR with genomic DNA from A. fumigatus, C. albicans, C. immitis, S. cerevisiae, H. capsulatum, and P. brasiliensis amplified by primers ITS1 and ITS4. The 649-bp amplified fragment of P. brasiliensis is shown in Fig. 1, lane 8. As can be seen there was no amplification without P. brasiliensis DNA or with human DNA (Fig. 1, lanes 1 and 2, respectively). All tested fungus DNA reacted positively with primers ITS1 and ITS4, except the A. fumigatus DNA. The PCRs showed amplified fragments of different sizes (Fig. 1, lanes 4 to 8), which can be explained by the fact that the sizes of the intergenic regions may change from one organism to another. A. fumigatus DNA reaction showed no amplification in these conditions; however, we were able to amplify the ribosomal 5.8S region of this fungi when we used primer concentrations four times higher (data not shown). Also, the PCR reaction using DNA from 20 different P. brasiliensis isolates showed amplified fragments of sizes identical to those obtained with Pb01 (data not shown).
FIG. 1.
PCR using primers ITS1 and ITS4. Lanes: 1, primers alone; 2, human DNA; 3, A. fumigatus DNA; 4, C. albicans DNA; 5, C. immitis DNA; 6, S. cerevisiae DNA; 7, H. capsulatum DNA; 8, P. brasiliensis Pb01 DNA; 9, 100-bp molecular marker. The 649-bp P. brasiliensis fragment is indicated by an arrow.
Figure 2A shows the 649-bp sequenced region corresponding to the complete sequence of 5.8S ribosomal DNA plus partial sequences of 28S and 18S ribosomal DNA and intergenic sequences. A similarity analysis was performed comparing this sequence with other 5.8S ribosomal DNA sequences obtained from the GenBank database. The least-related region was chosen to design a specific reverse primer named OL5 (Fig. 2B).
FIG. 2.
(A) Complete sequence of 5.8S, including intergenic regions and partial sequences of 18S and 28S regions of P. brasiliensis; (B) Analysis of similarity to sequences from other fungi. Primers ITS1 and ITS4 are bolded and the P. brasiliensis-specific primer OL5 is underlined.
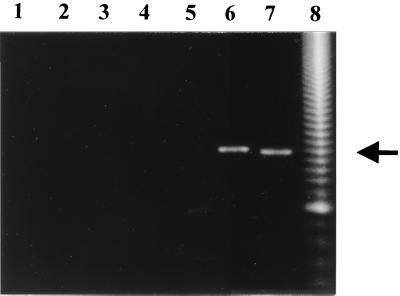
In order to verify whether OL5 was really a P. brasiliensis-specific primer, PCR analysis was performed. As shown in Fig. 3, there was no amplification when human DNA was used as the template (Fig. 3, lane 1). All the other fungus DNA reacted negatively with primers OL5 and ITS1, except that P. brasiliensis generated a 496-bp fragment (Fig. 3, lane 7) and H. capsulatum gave a cross-reaction-amplified fragment of approximately 500 bp (Fig. 3, lane 6). All reactions using DNA of different isolates of P. brasiliensis generated fragments of the same size (data not shown).
FIG. 3.
PCR amplification using primers ITS1 and OL5. Lanes: 1, human DNA; 2, A. fumigatus DNA; 3, C. albicans DNA; 4, C. immitis DNA; 5, S. cerevisiae DNA; 6, H. capsulatum DNA; 7, P. brasiliensis Pb01 DNA; 8, 50 bp molecular marker. The P. brasiliensis 496-bp fragment is indicated by an arrow.
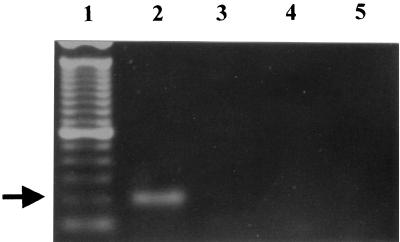
We also used fungal universal primers UNI-F and UNI-R, which amplify a region corresponding to the 28S ribosomal DNA. The PCR result showed an amplified fragment of 617 bp for P. brasiliensis (data not shown). This fragment was cloned, and the complete sequence was the same as that described by Sandhu et al. (GenBank accession number U81263) (19). We performed an analysis for similarity with other fungal sequences available from GenBank and designed a P. brasiliensis-specific primer in this region, designated OL3. PCR using OL3 and UNI-R generated a 203-bp fragment only when P. brasiliensis DNA was used as the template. These primers were able to discriminate between P. brasiliensis and H. capsulatum as can be seen in Fig. 4, lanes 2 and 3.
FIG. 4.
PCR discrimination between P. brasiliensis and H. capsulatum using primers OL3 and UNI-R. Lanes: 1, 100-bp molecular marker; 2, P. brasiliensis Pb01 DNA; 3, H. capsulatum DNA; 4, human DNA; 5, primers alone. The P. brasiliensis-specific 203-bp fragment is indicated by an arrow.
PCR has been effective for the detection of a great variety of microorganisms and it may be useful in PCM diagnosis. The analysis of these ribosomal fragments for similarity to six other sequences from human pathogenic fungi allowed us to design the molecular markers for PCR identification of P. brasiliensis, OL5 for the 5.8S ribosomal region and OL3 for the 28S ribosomal region. In this work, the results showed that the primers ITS1 and OL5 were able to identify P. brasiliensis, including different isolates. There was no PCR cross-reaction with four other pathogenic fungi or with human DNA, but there was a cross-reaction with H. capsulatum. To discriminate between P. brasiliensis and H. capsulatum we used the primers OL3 and UNI-R. Thus, we propose double PCR using primer pairs ITS1-OL5 and OL3-UNI-R for the specific identification of the pathogenic fungus P. brasiliensis. Although morphological and serological P. brasiliensis diagnosis has been performed, PCR would be an important tool to detect the fungus in patients with negative serologic reactions, where the antibody and/or antigen concentration are low, making it difficult to determine the best course of therapy for the patient. The primer pairs ITS1-OL5 and OL3-UNI-R could be used for PCM diagnosis and for molecular, epidemiological, and ecological P. brasiliensis studies, since PCR is a sensitive, specific, and rapid method.
Nucleotide sequence accession number.
The nucleotide sequence determined in this study has been submitted to GenBank under accession number AF092903.
Acknowledgments
This work was supported by grants from PADCT/CNPq, CNPq, FAP/DF, FUB and Fundação FUNAPE/UFG.
We thank George S. Deepe, Jr., for providing the DNA from H. capsulatum and C. immitis. We also thank Thaís Cristine de Araújo for providing the DNA from P. brasiliensis isolates.
REFERENCES
- 1.Borges M I, Azevedo M O, Bonatelli R, Jr, Felipe M S S, Astolfi-Filho S. A practical method for the preparation of total DNA from filamentous fungi. Fungal Genet Newsl. 1990;37:10. [Google Scholar]
- 2.Camargo Z P, Unterkir Cher C, Travassos L R. Identification of antigenic polypeptides of P. brasiliensis by immunoblotting. J Med Vet Mycol. 1989;27:407–412. [PubMed] [Google Scholar]
- 3.Del-Negro G M B, Garcia N M, Rodrigues E G, Cano M I N, Aguiar M S M W, Lirio V S, Lacaz C S. The sensitivity, specificity and efficiency values of some serological tests used in the diagnosis of paracoccidiodomycosis. Rev Inst Med Trop Sao Paulo. 1991;33:277–280. doi: 10.1590/s0036-46651991000400006. [DOI] [PubMed] [Google Scholar]
- 4.Fava-Neto C. Contribuição ao estudo imunológico da blastomicose de Lutz. Rev Inst Adolfo Lutz. 1961;21:99. [Google Scholar]
- 5.Goldani L, Sugar A M. Short report: use of the polymerase chain reaction to detect Paracoccidioides brasiliensis in murine Paracoccidioidomycoses. Am J Trop Med Hyg. 1998;58:152–153. doi: 10.4269/ajtmh.1998.58.152. [DOI] [PubMed] [Google Scholar]
- 6.Goldani L, Maia A L, Sugar A M. Cloning and nucleotide sequence of a specific DNA fragment from Paracoccidioides brasiliensis. J Clin Microbiol. 1995;33:1652–1654. doi: 10.1128/jcm.33.6.1652-1654.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldani L Z, Martinez R, Landell G A M, Machado A A, Coutinho V. Paracoccidioidomycoses in a patient with acquired immunodeficiency syndrome. Mycopathologia. 1989;84:151–152. doi: 10.1007/BF00444027. [DOI] [PubMed] [Google Scholar]
- 8.Haynes K A, Westerneng T J, Fell J W, Moens W. Rapid detection and identification of pathogenic fungi by polymerase chain reaction amplification of large subunit ribosomal DNA. J Med Vet Mycol. 1995;33:319–325. doi: 10.1080/02681219580000641. [DOI] [PubMed] [Google Scholar]
- 9.Lacaz C S. Mycological diagnosis. In: Franco M, Lacaz C S, Restrepo-Moreno A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 339–344. [Google Scholar]
- 10.Leão R C, Mendes E. Paracoccidioidomycoses, neoplasia and associated infections. Allergol Immunopathol. 1980;8:185–188. [PubMed] [Google Scholar]
- 11.Marques S A, Conterno L O, Sgarbi L P, Villagra A M P C, Sabongi V P G, Bagatini E, Gonçalves V L C. Paracoccidiodomycosis associated with acquired immunodeficiency syndrome. Report of seven cases. Rev Inst Med Trop Sao Paulo. 1995;37:261–265. doi: 10.1590/s0036-46651995000300014. [DOI] [PubMed] [Google Scholar]
- 12.Marques S A, Shikanai-Yasuda M A. Paracoccidiodomycosis associated with immunosuppression, AIDS and cancer. In: Franco M, Lacaz C S, Restrepo-Moreno A, Del-Negro G, editors. Paracoccidiodomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 393–405. [Google Scholar]
- 13.Mendes-Gianini M J S, Bueno J P, Shikanai-Yasuda M A, Ferreira A W, Masuda A. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J Clin Microbiol. 1989;27:2842–2845. doi: 10.1128/jcm.27.12.2842-2845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puccia R, Schenkman S, Gorin P A J, Travassos L R. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun. 1986;53:199–206. doi: 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restrepo M. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. J Med Vet Mycol. 1985;23:323–334. [PubMed] [Google Scholar]
- 16.Salina M A, Shikanai-Yasuda M A, Mendes R P, Barraviera B, Giannini M J S M. Detection of circulating Paracoccidioides brasiliensis antigen in urine of paracoccidiodomycosis patients before and during treatment. J Clin Microbiol. 1998;36:1723–1728. doi: 10.1128/jcm.36.6.1723-1728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Sandhu G S, Kline B C, Stochman L, Roberts G. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhu G S, Aleff R A, Kline B C, Silva Lacaz C. Molecular detection and identification of Paracoccidioides brasiliensis. J Clin Microbiol. 1997;35:1894–1896. doi: 10.1128/jcm.35.7.1894-1896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severo L C, Londero A T, Geyer G R, Porto N S. Acute pulmonar paracoccidioidomycosis in an immunosuppressed patient. Mycopathologia. 1979;68:171–174. doi: 10.1007/BF00578525. [DOI] [PubMed] [Google Scholar]
- 21.Shikanai-Yasuda M A, Duarte M I S, Nunes D F, Lacaz C S, Sabagga E, Abdala E, Duarte A J S, Lopes M H. Paracoccidioidomycosis in a renal transplant recipient. J Med Vet Mycol. 1995;33:411–414. doi: 10.1080/02681219580000781. [DOI] [PubMed] [Google Scholar]
- 22.Sugar A M, Restrepo A, Stevens D A. Paracoccidioidomycosis in the immunosupressed host: report of a case and review of literature. Am Rev Respir Dis. 1984;129:340–342. [PubMed] [Google Scholar]
- 23.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, et al., editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. pp. 315–322. [Google Scholar]