Abstract
Background and Objective:
Toenail metal concentrations can be used as an effective biomarker for exposure to environmental toxicants. Typically toenail clippings are measured ex vivo using inductively-coupled plasma mass spectrometry (ICP-MS). X-ray fluorescence (XRF) toenail metal measurements done on intact toenails in vivo could be used as an alternative to alleviate some of the disadvantages of ICP-MS. In this study, we assessed the ability of using XRF to measure toenail metal concentrations in real time without having to clip the toenails (i.e. in vivo) in two occupational settings for exposure assessment of manganese and mercury.
Materials and Methods:
The portable XRF method used a 3-minute in vivo measurement of toenails prior to clipping and was assessed against ICP-MS measurement of toenail clippings taken immediately after the XRF measurement and work history for a group of welders (n=16) assessed for manganese exposure and nail salon workers (n=10) assessed for mercury exposure.
Results and Conclusions:
We identified that in vivo XRF metal measurements were able to discern exposure to manganese in welders and mercury in nail salon workers. We identified significant positive correlations between ICP-MS of clippings and in vivo XRF measures of both toenail manganese (R= 0.59, p=0.02) and mercury (R= 0.74, p<0.001), as well as between in vivo XRF toenail manganese and work history among the welders (R=0.55, p=0.03). We identified in vivo XRF detection limits to be 0.5 μg/g for mercury and 2.6 μg/g for manganese. Further work should elucidate differences in timing of exposure using the in vivo XRF method over toenail clippings and modification of measurement time and x-ray setting to further decrease the detection limit. In vivo portable XRF measurements can be used to effectively measure toenail Mn and Hg in occupational participants in real time during study visits and at a fraction of the cost.
1. Introduction
Metal exposures have been consistently associated with increased risk for adverse health effects such as neurodegeneration, cardiovascular disease, motor dysfunction, and developmental disorders.[1–10] Exposure to metals is commonly measured using concentrations of those metals in blood.[11, 12] Metals have a relatively short biologic half-life of around 30 days in blood. Blood specimens are analyzed using an expensive procedure known as inductively coupled plasma mass spectrometry (ICP-MS).[13, 14] Manganese is an essential element, but can have neurotoxic effects at high levels of exposure typical of certain occupations like welders (as well as possible toxic effects at too low levels).[6, 12, 15] Hg, which is solely toxic in the body, having neurotoxic effects and demonstrated effects on the cardiovascular system.[8, 16, 17] That and the logistical requirements of drawing and storing blood specimens make blood metal concentrations a costly and time-consuming method of measuring metal exposures in large cohorts or for occupational health monitoring.
Metal concentrations in toenail clippings are an alternative biomarker of metal exposures,[17–20] and even can offer some advantages over blood metal measurements. Metal concentrations in toenail clipping samples reflect exposures in the time period about 6 months to 1.5 years prior to the clipping, meaning that toenail metals can serve as a slightly more cumulative marker of exposure than blood metals.[19, 21] The exposure window of Hg in toenails is less well-specified, but from the typical growth rate can be assumed to be approximately one year worth of exposure. The exposure window captured in toenail clippings is lagged somewhat because the growth rate of the toenails means that it takes time for the nail bed to grow deposited metals to the clipped portion of the nail. There are conveniences of using toenail clippings rather than blood samples, such as the ability to store them at room temperature and they take up little space. They are also easy to collect, and it is simpler to get many repeated samples on an individual thereby improving the estimation of more cumulative exposures. In addition, the collection can be done by anyone and can even be done by the participant and mailed into a central study collection site. However, if clippings need to be acquired at a specific time or study visit, subjects may not have enough toenail growth to provide adequate clipping sample total weight for a thorough analysis, which may lead to data loss if a sample is never collected or mismatch of timing of sample collection with other study data. This has the potential for causing reduced participation rates and increasing relative uncertainties of exposure assessment, and so compromising the exposure health relationship under study. In terms of the laboratory analysis, the typical approach to quantifying metals in toenails requires laboratory procedures similar to that of blood samples. However, newer exposure assessment measurement modalities present alternative strategies to address these disadvantages of using toenails as a biomarker in environmental studies.
X-ray fluorescence (XRF) technologies can provide an alternative to alleviate the problem of toenail sample size and costly analytical methods.[22–27] Portable XRF systems can be brought onto work sites, are less expensive for analysis than typical ICP-MS procedures, and measurements of a given participant can be completed in minutes. Importantly, the portable XRF raises the possibility of measuring the toenail in vivo without the need for clipping, which could greatly enhance data collection and reduce participant loss because of lack of sample for exposure assessment. Measuring toenails still on the toe affords benefits in measuring multiple time points of exposure in real time from a single study visit by measuring across the nail surface or measuring different toenails.
Prior studies have investigated the use of XRF for manganese, arsenic, and selenium in toenails, but these studies were restricted to toenail clippings and did not attempt in vivo measurements.[22–25] We previously used phantom toenails to calibrate the portable XRF for use with in vivo measurements of manganese (Mn) and mercury (Hg)[26], but this approach has not been tested on actual human in vivo toenails. The goal of this study was to evaluate and validate the use of portable XRF in vivo measurements (i.e. on live participants with nail on toe) in comparison with ICP-MS analysis of toenail clippings specifically for Mn and Hg. We set our investigation in data from a group of welders and nail salon workers. Welders typically have high manganese exposures and many studies have been shown to that effect in the past. Exposures of nail salon workers are relatively unknown, but a recent study found high Hg exposures, although these could result from cultural and dietary habits rather than being specifically occupational. We chose Mn and Hg as our previous work provided detailed analysis procedures for these two metals specifically.[26] We also investigated the temporality of in vivo XRF measured exposure by exploring the associations of XRF and ICP-MS measurements with work history. The temporality is likely to be affected by the amount and position of toenail measured using each method and the toenail growth associated with those areas (i.e. clipped toenail further from the nail bed reflects exposures longer in the past than an in vivo measurement taken closer to the nail bed), which highlights the potential for longer exposure window assessed via in vivo measurements of toenail with multiple measurements. Finally, we determine the relative utility of in vivo XRF for measurement of toenail metals in population studies of occupational and environmental health.
2. Methods
2.1. Study Population
The male welding participants (n=16) were recruited from a local Boilermaker union in Quincy, MA. The day-to-day occupational focus was welding for these participants. The female nail salon workers (n=10) were recruited from several nail salons around the Boston area. Approval was obtained from Harvard T.H. Chan School of Public Health (HSPH) IRB and the HSPH Radiation Safety Committee prior to the start of both studies. Signed consent forms were received from all participants. Participants filled out questionnaires to assess work history. Toenail metals were measured in vivo via portable XRF, and then toenail clippings were collected and measured for metal concentrations.
2.2. Portable XRF Measurements
The portable XRF used in this study was the Thermo Niton XL3t GOLDD+ (Thermo Fischer Billerica, MA), the same model as used in previous studies detailing calibration methods for Mn and Hg toenail measurements.[26] We used x-ray tube settings of 50 kV and 40 μA with a transmission-type silver anode and silver and iron filter. We calibrated the device using slab phantoms of epoxy resin doped with Mn and Hg, soft tissue phantoms made from Lucite, and bone phantoms made from plaster-of-Paris as done in our previous calibration study.[26, 28, 29]
For in vivo toenail metal measurements using the portable XRF, we cleaned the toenails before each measurement using alcohol swabs and any nail polish was removed prior to measurement. We placed the device in the center of the participants toenail and used the device’s camera to identify the end of the toenail. The measurement was made in the center near the edge of the toenail where it was clipped. The center of the big toenail was chosen as the easiest to measure with the most optimal geometry. The XRF requires a flat surface against the nail, which is much more difficult to do on the other nails or if not center on the nail itself. We estimated a maximum skin dose of about 28.7 mSv to a 1 cm2 area and maximum total body effective dose of 2.0 μSv for the 3-minute measurement, which is equivalent to about 1/50th the radiation dose of a typical chest x-ray.[30]
We used a spectral analysis to identify Mn and Hg counts as shown in our previous work.[26, 31–33] Briefly, traditional peak fitting using Matlab was used to distinguish the Mn and Hg counts from background. Surrounding background peaks for both Mn, which had a large iron peak influencing background, and Hg, which had a large tungsten peak influencing background, were fitted to disentangle the Mn and Hg net counts. The iron peak was from the coherent scattering of the filtration of the initial x-ray, and the tungsten peak was from the collimation and shielding used in the x-ray device.Net counts were related back to concentration in μg/g using our calibration lines from doped phantom measurements as shown in Figure 1 for Mn (R=0.99).
Figure 1.
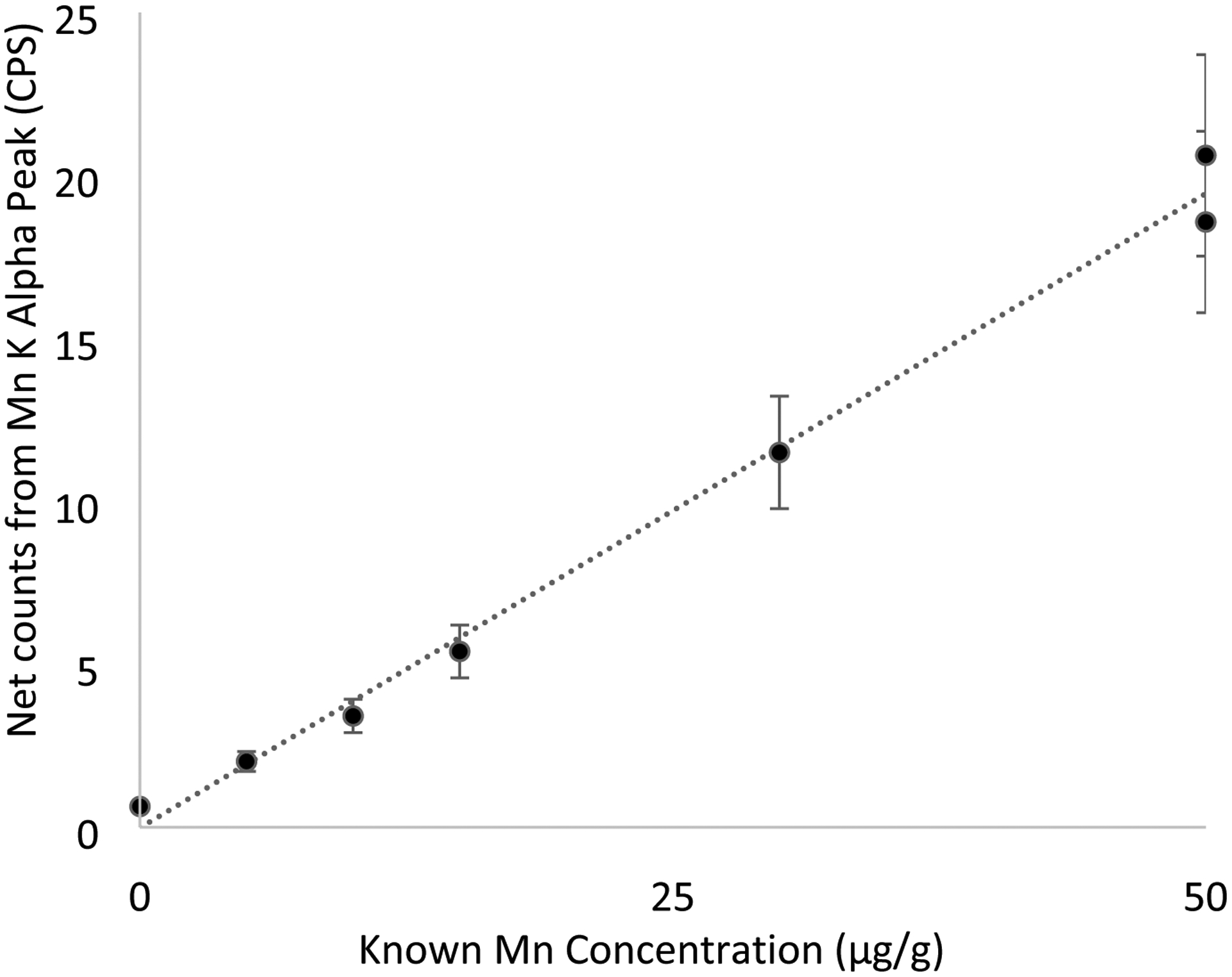
Calibration line for Mn relating phantom measurements in counts per second to total concentration.
2.3. ICP-MS Toenail Metal Measurements
We asked participants to not paint or trim their toenails for a few days before the study visit. We (or the participant) cut the nails into a paper envelope using cleaned stainless-steel toenail clippers. The clippings were collected from all ten toes after the XRF measurement on the big toe. Ten toes were used as this would better encapsulate a larger window of exposure time, as each toenail has a different length and corresponding time window of exposure reflected. Previous studies that use toenails as an exposure biomarker typically used clippings from all ten toes.
The toenail clipping samples were prepared for analysis as described in our previous works.[34, 35] Nail salon workers and welders were analyzed by different labs using the same procedures. Briefly, the samples were cleaned using a 1% triton solution of deionized (DI) water and were sonicated for 30-minutes and vortexed for 2–3 seconds before the solution was removed. Then, a new 1% triton and DI water solution was added, and samples were again sonicated and vortexed before the solution was removed. The clippings were then rinsed with DI water and vortexed three times. Acetone was added to the clippings and samples were again sonicated and vortexed before the acetone was removed. Finally, samples were rinsed three times with DI water, vortexed once more, and either freeze-dried overnight or dried in a hood over 48 days.
The clippings were digested in 3 mL of trace metal-grade nitric acid and 1 mL of hydrogen peroxide at room temperature for 48 hours. Then the samples were diluted with 6 mL of DI water prior to analysis. ICP-MS analysis was done using an internal standard of 45Sc, 89Y, and 159Tb. For further quality control and assessment, we used ERM-DB001, a certified standard for human hair, varying concentrations of a continuous calibration standard, digestion duplicates, and NIST1643f standard for trace elements in water. Our calibration standards had recovery on average of 90%+ for all metals, and the NIST1643f standard had an average recovery for all metals of 90%+.
2.4. Work History
Work history was established in the welders (n=16) via a questionnaire. Participants were asked how many hours they worked in the past week, month, and year. Participants were also asked the estimated percentage of time they spent wearing respirators during their work, which would heavily influence overall exposure. Finally, welders were asked total years working on the job.
For nail salon workers, we determined via questionnaire the total hours worked per week, years worked as a nail technician, personal protective equipment use (gloves and respirator), and work habits (washing hands and etc.) that would influence occupational exposure.
2.5. Statistical Analysis
Pearson correlations were computed in R to determine relationships between exposure assessment methods, work history, and exposure. Distributions were found to be normal, and Spearman correlations gave similar results. We also used linear regressions as a further check when both variables were normally distributed and to obtain beta values when appropriate for reporting. Uncertainty in portable XRF measurements was derived from a combination of background and net counts as output from the fitting in Matlab for each metal measured. The uncertainty identified from the spectral fitting is reflective of two standard deviations or approximately the 95% confidence interval in the in vivo XRF toenail metal measurements. Negative measurements are possible with XRF and should be included as negatives in the analyses to prevent bias from normalizing uncertainty estimates. In epidemiological health studies, it has been shown that excluding those values below a limit of detection can reduce efficiency and result in biased effect estimates for relations between the exposure and health outcomes.[36–38] Because Mn is primarily an occupational exposure for welders, we focused only on the welders for our Mn analyses. Nail salon workers had a higher exposure to Hg, although likely not occupational, so we restricted to nail salon workers in the Hg analysis. Attempted Mn measurements in nail salon workers and Hg measurements in welders were below the detection limit of the device for Mn and Hg respectively, leading to more noise in the relationships identified.
3. Results
3.1. Participant characteristics
Welder’s age and work history data are summarized in Table 1. Eleven (73%) of welders reported wearing respirators on the job. Many of the participants were new to welding, as shown by the median job years of 3. Nail salon workers were identified as 75% Asian, for whom cultural fish consumption habits may lead to the higher Hg exposures observed, as occupational history data did not correlate well with exposure[39]. The nail salon worker exposures are discussed at length in Ceballos et. Al. 2021.[39]
Table 1.
Age, job years, and work hours for welders in our study (n=16).
| Median | 25th Percentile | 75th Percentile | |
|---|---|---|---|
| Welders n=16 | |||
| Age | 34 | 27 | 44 |
| Total Years on the Job | 3 | 2 | 9 |
| Estimated Number of Job Hours in the Past Month | 18.0 | 8.8 | 42.5 |
| Estimated Number of Job Hours in the Past Year | 250 | 92.5 | 500 |
| Nail Salon Workers n=10 | |||
| Age | 37 | 26 | 44 |
| Total Years on the Job | 8 | 2.4 | 16 |
| Estimated Number of Job Hours per Week | 40 | 30 | 49 |
3.2. In vivo Toenail Spectra
Figure 2 and 3 below show the spectra with background fitted gaussians for tungsten and iron and corresponding Hg and Mn peaks respectively. This demonstrates the feasibility of the in vivo peak fitting functionality.
Figure 2.
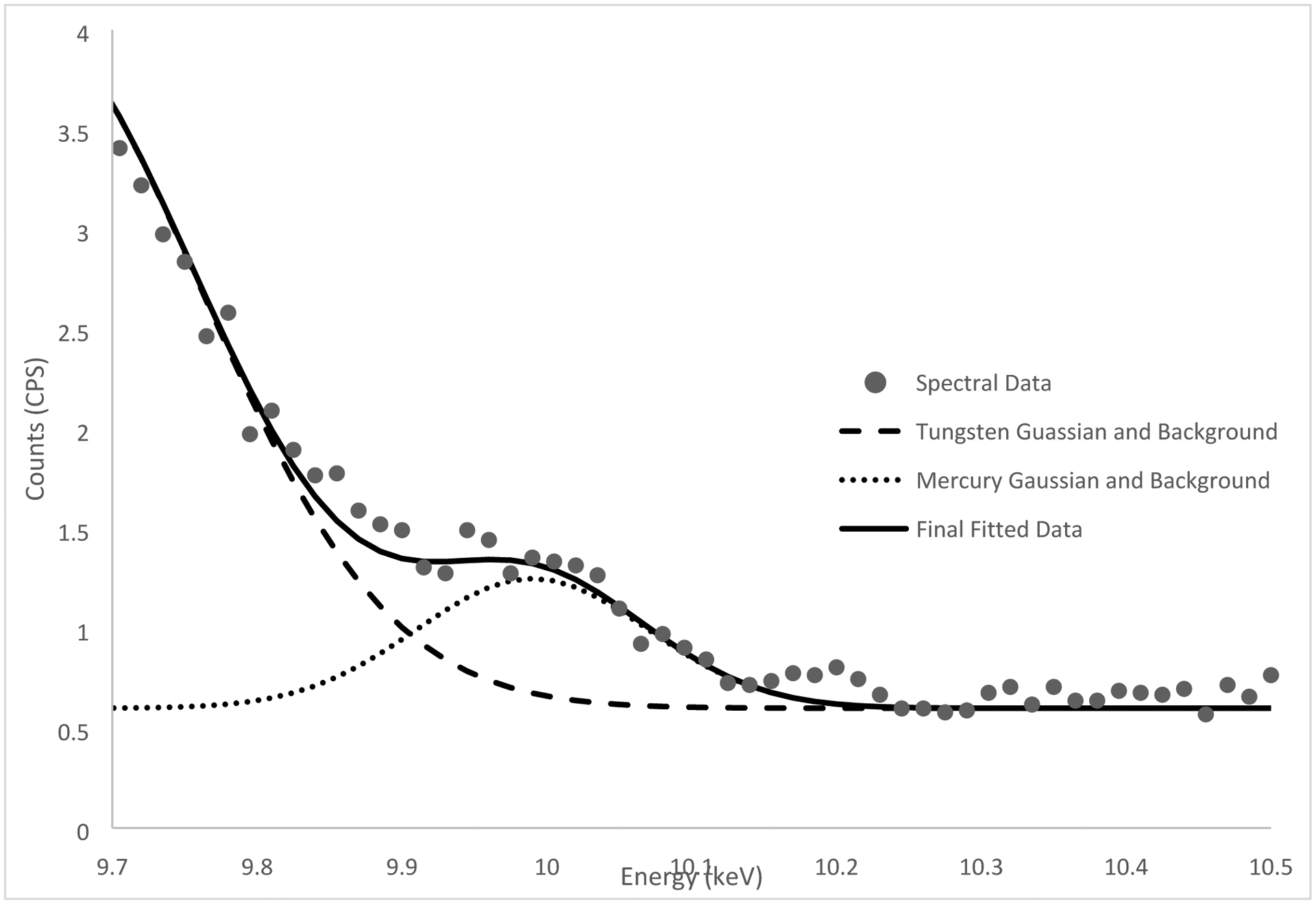
Mercury peak fitting (9.99 keV mercury alpha peak) of an in vivo measurement for a nail salon worker.
Figure 3.
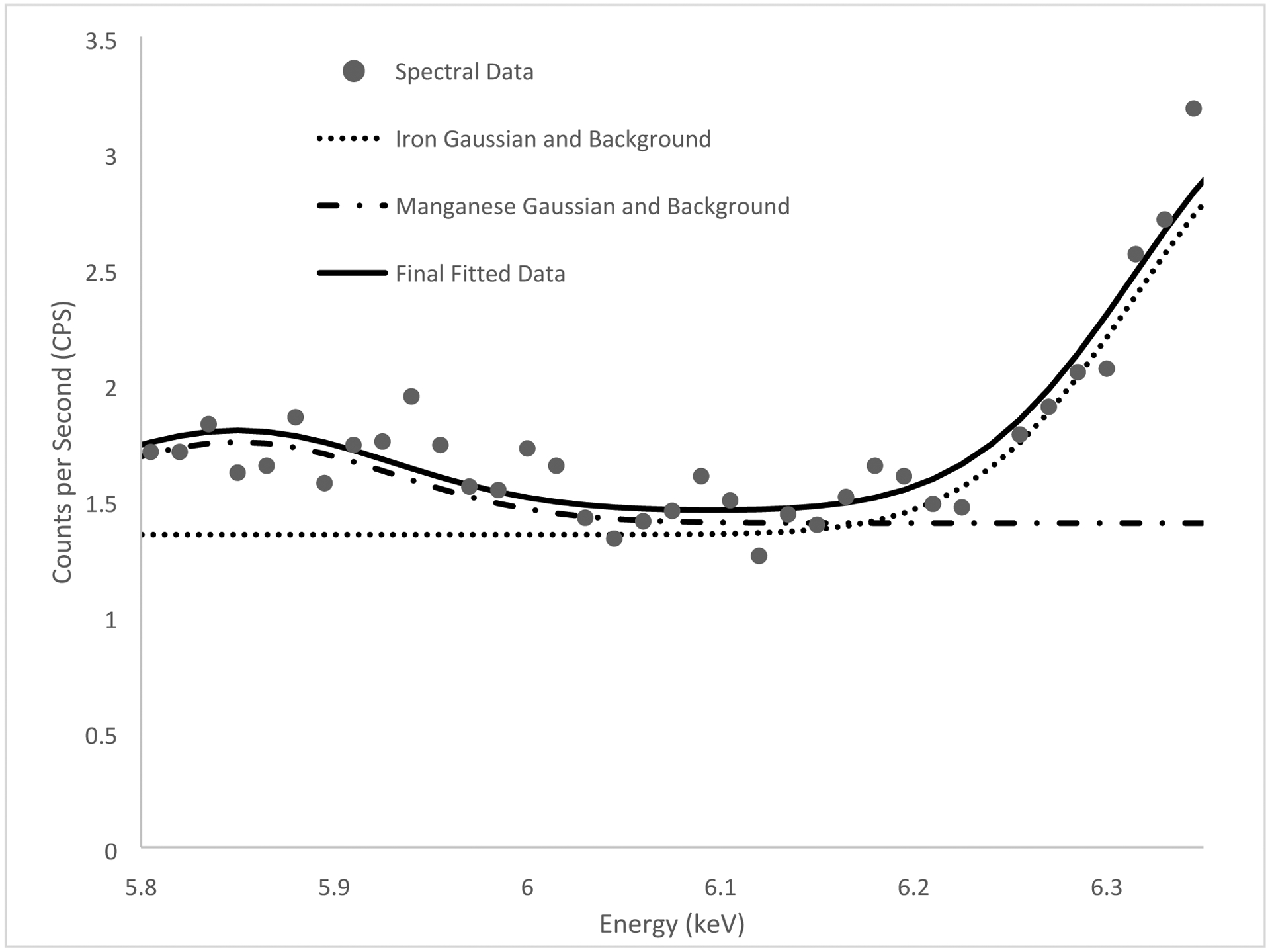
Manganese peak fitting (5.90 keV manganese alpha peak) of an in vivo measurement for a welder.
3.3. XRF Toenail Mn and Hg Analysis Summary
Table 2 summarizes the in vivo XRF toenail metal measurements and ICP-MS toenail clipping metal measurements from the welders and nail salon workers.
Table 2.
Toenail Mn and Hg measurement distribution in the welders (Mn measurements) and nail salon workers (Hg measurements) in our study.
| N | Group | Average | Standard Deviation | ||
|---|---|---|---|---|---|
| Mn | |||||
| ICP-MS (μg/g) | 16 | Welders | 1.1 | 1.00 | |
| XRF (μg/g) | 16 | Welders | 1.8 | 7.0 | |
| XRF Uncertainty (μg/g) | 16 | Welders | 2.7 | 2.8 | |
| Hg | |||||
| ICP-MS (μg/g) | 10 | Nail Salon | 0.92 | 0.4 | |
| XRF(μg/g) | 10 | Nail Salon | 1.7 | 0.25 | |
| XRF Uncertainty (μg/g) | 10 | Nail Salon | 0.44 | 0.39 |
3.4. Welders Toenail Mn XRF and ICP-MS Comparisons
Figure 4 shows the significant positive correlation between Mn measurements using in vivo portable XRF and ICP-MS (R = 0.58, Beta (95% CI) = 3.97 (2.52, 5.43) P=0.02).
Figure 4.
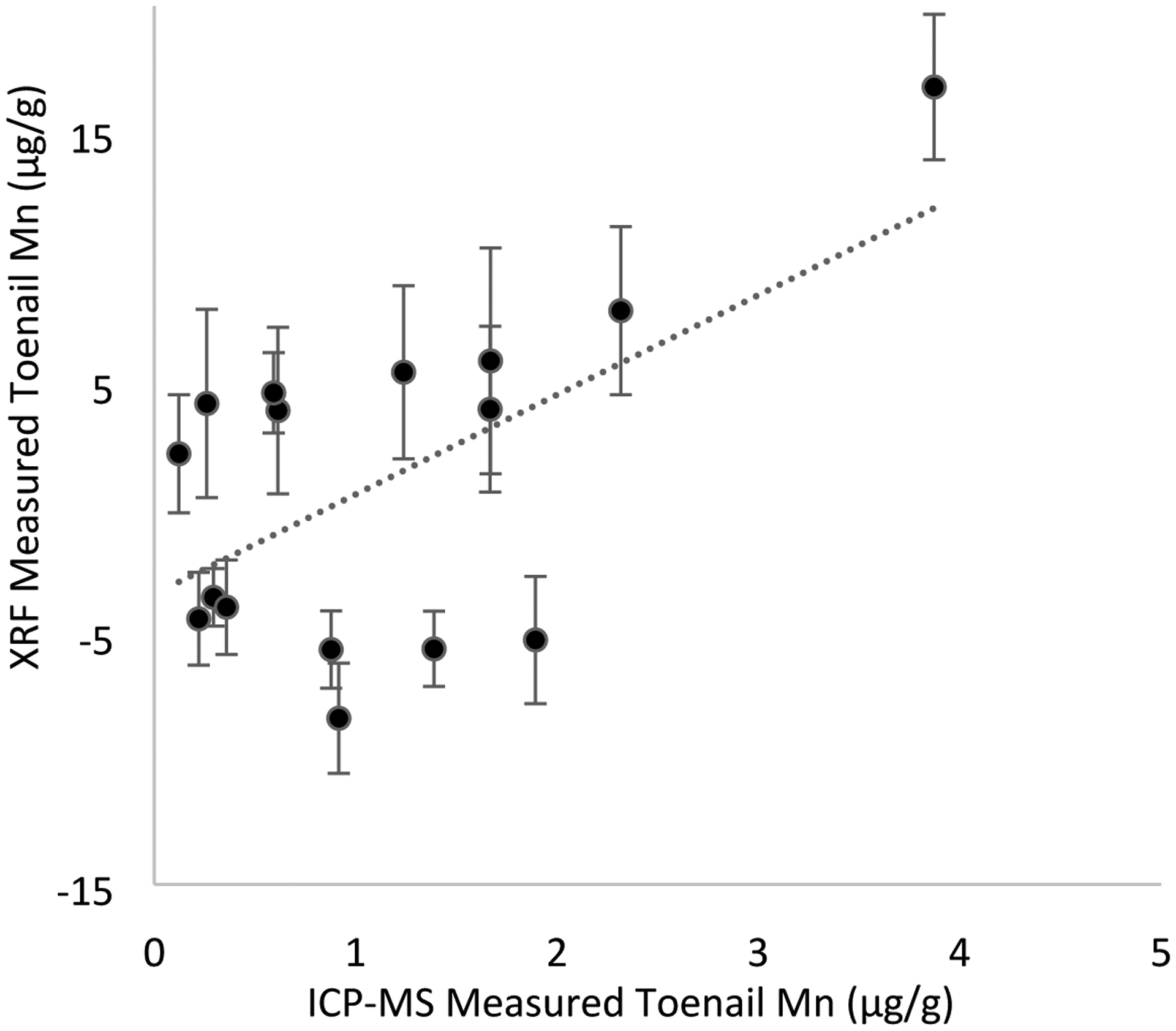
Significant positive relationship (R = 0.58, Beta (95% CI) = 3.97 (2.52, 5.43) P=0.02) between Mn measured via in vivo portable XRF (error bars represent two standard deviation uncertainty for each measurement) and ICP-MS on toenail clippings in welders (n=16). Regression results were from crude analyses.
3.5. Toenail Mn and Hg Relationships with Work History and Age
Figures 5 and 6 show the relationships between XRF measured Mn and ICP-MS measured Mn and welding work over the past month collected via self-report. For toenail Mn measured by XRF related to total hours worked over the past month we saw a significant relationship shown in Figure 5 (R = 0.55, Beta (95% CI) = 0.11 (0.07, 0.15), P=0.03). For toenail Mn measured by ICP-MS related to total hours worked over the past month we saw a significant relationship shown in Figure 6 (R = 0.75, Beta (95% CI) = 0.02 (0.015, 0.025), P=0.001). Mn measured via XRF or ICP-MS was not significantly associated with age of participants, respirator use, or total work hours estimated over the year.
Figure 5.
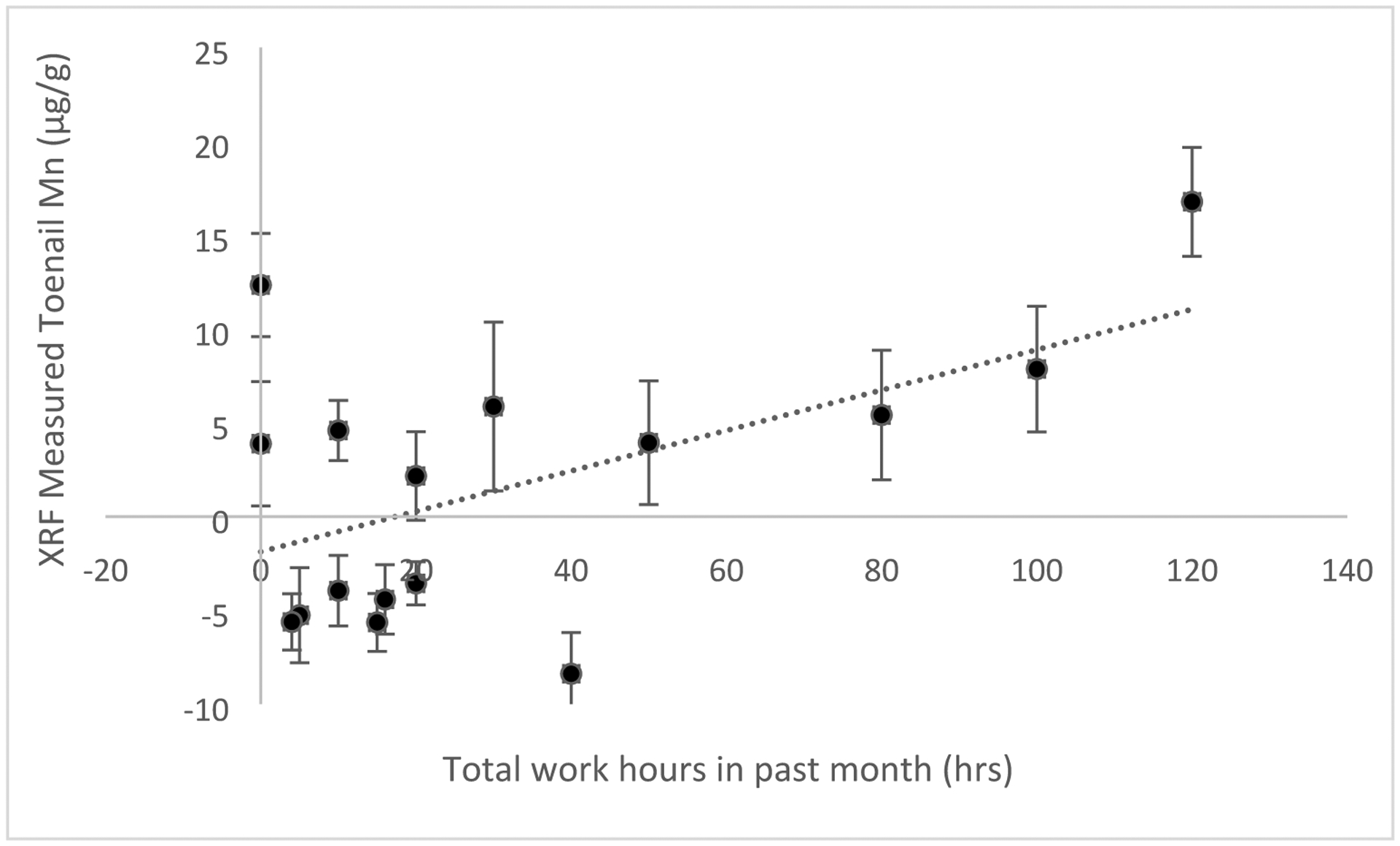
Toenail Mn measured by XRF in vivo (error bars reflect two standard deviation uncertainty for each measurement) related to total hours worked in welders over the month prior to measurement (R = 0.55, Beta (95% CI) = 0.11 (0.07, 0.15), P=0.03, n=16). Regression results were from crude analyses.
Figure 6.
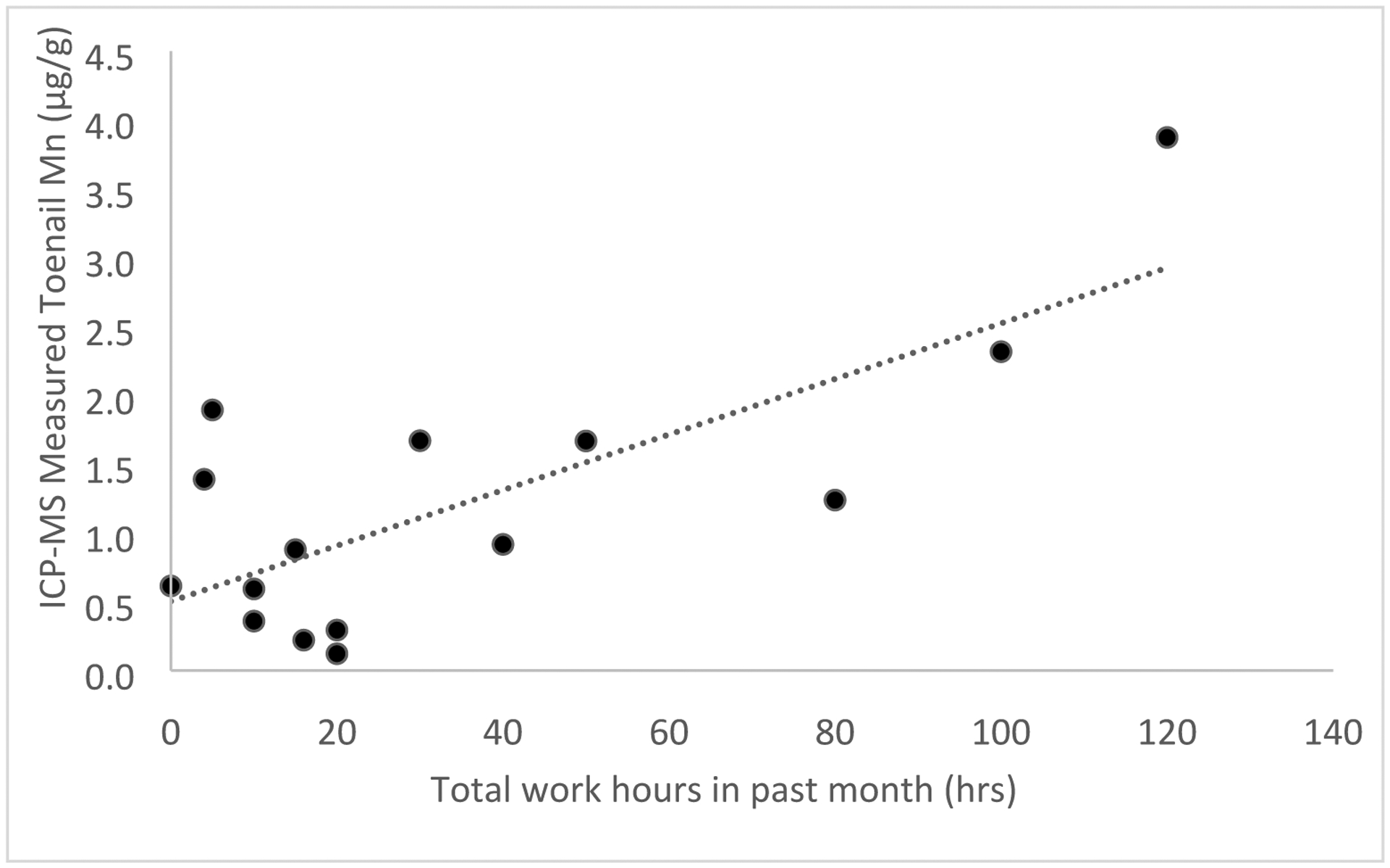
Relationship between toenail Mn measured by ICP-MS and total hours worked over the month prior to measurement in welders (R = 0.75, Beta (95% CI) = 0.02 (0.015, 0.025), P=0.001, n=16). Regression results were from crude analyses.
We did not find any significant relationships between work history, age, work behaviors, or personal protective equipment use and toenail Hg measured via ICP-MS or XRF.
3.6. Toenail Hg XRF and ICP-MS Comparison
Figure 7 shows the significant positive correlation between Hg measurements using in vivo portable XRF and ICP-MS (R = 0.74, Beta (95% CI) = 0.84 (0.64, 1.25), P=0.01).
Figure 7.
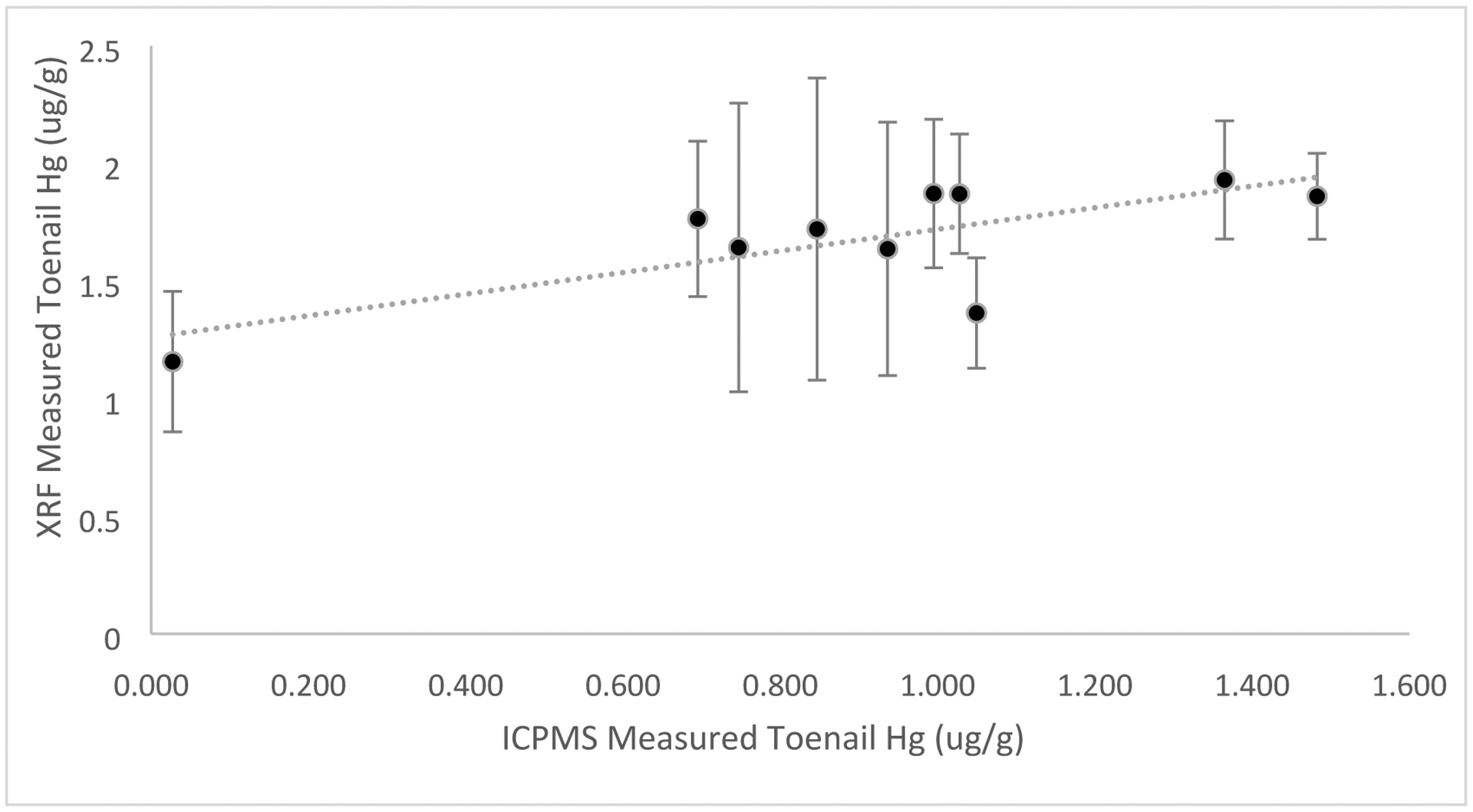
Correlation between Hg measured via in vivo portable XRF and ICP-MS on toenail clippings for nail salon workers (R = 0.74, Beta (95% CI) = 0.84 (0.64, 1.25), P=0.01, n=10). Regression results were from crude analyses.
4. Discussion
The significant correlations between ICPMS toenail clippings, work history, and XRF in vivo toenail measurements of Mn and Hg demonstrate the suitability of XRF for exposure assessment. The in vivo measurement reflected a different time period of exposure assessment from clippings, and potentially measure a shorter exposure time window than is typical with standard ten-toe clipping measurements via ICP-MS. The in vivo XRF measurements would be most suitable in studies with potential for high exposures, especially occupational exposures, but with increased measurement time would be suitable for evaluation of general population.
The correlation found between XRF and ICP-MS measured Mn and Hg exposure indicated a reasonable agreement between the two methods. In the welders, we were able to strengthen this association by relating the work history data to both ICP-MS and XRF Mn as well. The toenail Mn found in our study population would reflect a range typical of occupationally exposed individuals in comparison to other studies in the Boston area focusing on environmental exposures.[17] This correlation is perturbed by the increased uncertainty in the toenail metals measured via XRF. We expect the two measurements to be slightly different since they are measuring different parts of the toenail and measuring metals at different sections of the toenail would reflect different time periods of metal exposure. It is unclear as to how measuring slightly different portions of the toenail may affect the exposure time window reflected by an XRF measurement versus a typical toenail clipping assessment, which was shown previously to be about 6 months to 1.5 years in clippings for some metals.[19, 21] Another aspect potentially affected by a change in the toenail area assessed for metals would be the lag between exposure and measurement via XRF. This was previously found to be about 6 months based on work history data and estimated from known toenail growth, but could be slightly less with an XRF measurement that would potentially reflect exposure with less lag period.[19] This is a result of the in vivo XRF measurement averaging over an area closer to the root of the nail bed, which would reflect more recent exposures than the typical clipped portion of the nail alone.
Neither XRF nor ICPMS Mn measurements in the welders correlated well with estimated hours worked over the past year, which, for both, is not what was observed in previous studies.[19] This null finding may simply be due to the inaccurate recall of the hours worked over the year. It is unlikely but possible there is still contamination present on these nails. The cleaning procedure is extensive and has been used similarly for samples for years. Furthermore, there was a correlation with hours over the past month, suggestion contamination was not a significant issue in this regard. Nonetheless, further studies with extended follow-up to determine the precise timing of exposure in populations evaluated using in vivo XRF would be valuable.
Toenail Hg measured via ICP-MS on clipped nails or XRF in vivo, did not show any associations with work history or other measures of behaviors at work. However, we did show a correlation between in vivo XRF toenail Hg and toenail clipping Hg levels, which indicates the method was able to discriminate exposures well. Nail salon workers have a wide variety of potential exposures to metals or other toxicants, which will vary based on many different factors in their work environment.[40] A study of nail salon workers and nail polish impurities found a broad array of metals with potential exposure but did not specifically point to one definitive exposure that may impact workers health in this regard.[39, 41] Other than metals, nail salon workers have a variety of exposures to other potentially harmful chemicals such as plasticizers and phthalates.[39, 41–43] Welders have a straightforward Mn exposure relationship with work history, as anytime they are working there is the potential for Mn exposure.[19] Other exposures experienced by both groups would not interfere with the measurements from XRF as they are specific to the elements indicated. A potential limitation may also be residual confounding or recall bias in the work history relationships for either nail salon workers or welders, which could be influencing the results identified here. All but one (n=10) of the nail salon workers had toenail Hg levels above the detection limit of the XRF in vivo measurement, which indicates the XRF’s ability in exposure assessment in similar populations in the future. There was a substantial y-intercept, which would indicate some potential issues during the calibration procedures. This would mean there was an underestimating of the zero point, which could have been influenced by other metals present during in vivo measurements, but not accounted for in our calibration. It would be best to modify the calibration approach or quantify this difference to make the quantifiable results more comparable between ICP-MS and XRF in the future.
The main limitation of XRF use for in vivo measurements is the increased uncertainty relative to ICP-MS measurements. Of note, this study used 3-minute measurements, while a recent radiation dosimetry study indicated that much longer measurement times should be feasible, which would reduce the detection limits (and associated uncertainty in the measurement) by the square root of the increase in measurement time.[30] For example, if we did 6-minute measurements we would reduce our detection limits in this study for Mn from 2.6 μg/g to 1.8 μg/g while doubling our radiation dose. Our study on radiation dose indicated that even with a 10-minute measurement we would be more than 100 times lower than the ICRP limits for deterministic effects in skin and it would still represent a negligibly small effective dose with total effective dose less than that received in a typical day of natural background radiation. However, any of these limits would be substantially lower with toenail, given the fact that with a 1mm nail, almost 90% of the initial beam would be absorbed in the nail itself. Nail being dead tissue would not experience any measurable impact from the radiation dose. This decreased detection limit could open the possibility of studies in exposures typical of the general public. In addition, if populations were being assessed primarily for one metal, e.g. Mn or Hg alone, the device settings can be optimized to further reduce the detection limit.[26] The main limitation of portable XRF measurements is the detection limit (0.5 μg/g for Hg and 2.6 μg/g for Mn as derived from our in vivo measurements) for simultaneous measurement of metals, but optimization can be done to limit these issues in certain populations.
The detection limit combined with the range of samples present in the study presented an additional limitation. In the welders, if we removed the highest measurement the correlation between ICP-MS in clippings and XRF in vivo was reduced by half. Alternatively, if we removed the lowest measurement the correlation improved by 30%. The Mn detection seems to be more limiting particularly because of the characteristic x-ray proximity to tungsten characteristic x-rays. Tungsten is used as a component in the XRF itself, and thus presents a difficulty in fitting for this particular XRF, which has been discussed in previous studies.[26] This is why the calibration for Mn presented some issues with many results varying dramatically and including almost a third of the samples being negative. Again, the correlations with known work exposure history help to resolve some of these problems comparing two technically different biomarkers with clippings and in vivo XRF. In the nail salon workers, the range of samples is the main limitation. When the lowest sample is removed the correlation between clippings and in vivo reduced by a factor of two, but still remained significant. Again, we want to highlight the fact that the two measurement approaches, ICP-MS on clippings and XRF in vivo, are only expected to give a perfect correlation if the exposure remained consistent. This may not be a good assumption for welders, given the work history findings, but for nail salon workers with diet as a main predictor may be a slightly better assumption, as shown in other works evaluating toenail biomarkers over time.[19] However, we could not evaluate dietary factors in this study because they were not collected. This is further illustrated in our regression slopes. For Hg, the slope includes 1 in the 95% CI from our findings, but Mn does not. Thus, Hg seemed to provide a consistent biomarker in clippings and in vivo, whereas Mn had more discrepancies due to workplace exposures.
This work builds on many previous studies that have evaluated the feasibility of XRF for measurement of metals in toenail clippings or in vivo, but is the first study to show the distinct relationships between metals assessed in toenail clippings measured via ICP-MS and in vivo XRF on toenails.[22–27] It is clear that the XRF can be used effectively to measure Hg and Mn and likely other metals in toenails in vivo with some differences based on the background surrounding those peak areas and how easily they can be disentangled and calibrated, as evidenced by Figures 2 and 3. Previous studies focused on using the XRF for measurements of nail clippings primarily or in-lab phantoms for calibration of the device. This study opens the possibility of future analyses of metal exposures in vivo using XRF but will depend heavily on the population measured and the metals of interest to optimize results and detection limits of the device accordingly.
5. Conclusions
In vivo portable XRF measurements can be used to effectively measure toenail Mn and Hg in human participants in real time during study visits and at a fraction of the cost of typical chemical analyses. Toenail metals assessed via in vivo portable XRF reflect a different time period of exposure than is typical of metals assessed via toenail clipping measurements alone. A longer measurement time with the XRF will produce better results in the future and allow for potential assessment of typical general public environmental exposures using the in vivo technique.
Acknowledgments
The research described in this paper was supported by NIH/NIEHS 1R21 ES024700, NIEHS ES-000002, NIOSH 1K01 OH011648, NIOSH T42OH008416, NIH/NIEHS 2R25ES023635-04, Harvard Catalyst NIH UL1TR001102, NIEHS P42 ES007373, NCI Cancer Center Support Grant 5P30CA023108-37, Harvard Hoffman Program on Chemicals and Health, and Harvard JPB Environmental Health Fellowship. The authors would also like to thank the many different research assistants and research coordinators who helped with IRB, radiation safety approvals, and site visits.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References.
- [1].Shih RA, Hu H, Weisskopf MG, and Schwartz BS, “Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead,” Environ Health Perspect, vol. 115, pp. 483–92, Mar 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grashow R, Spiro A, Taylor KM, Newton K, Shrairman R, Landau A, et al. , “Cumulative lead exposure in community-dwelling adults and fine motor function: comparing standard and novel tasks in the VA normative aging study,” Neurotoxicology, vol. 35, pp. 154–61, Mar 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weisskopf MG, Sparrow D, Hu H, and Power MC, “Biased Exposure-Health Effect Estimates from Selection in Cohort Studies: Are Environmental Studies at Particular Risk?,” Environ Health Perspect, vol. 123, pp. 1113–22, Nov 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ji JS, Power MC, Sparrow D, Spiro A 3rd, Hu H, Louis ED, et al. , “Lead exposure and tremor among older men: the VA normative aging study,” Environ Health Perspect, vol. 123, pp. 445–50, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. , “Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility,” Environ Int, vol. 108, pp. 299–308, Nov 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bowler RM, Beseler CL, Gocheva VV, Colledge M, Kornblith ES, Julian JR, et al. , “Environmental exposure to manganese in air: Associations with tremor and motor function,” Sci Total Environ, vol. 541, pp. 646–654, Jan 15 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sureda A, Bibiloni MDM, Julibert A, Aparicio-Ugarriza R, Palacios-Le Ble G, Pons A, et al. , “Trace element contents in toenails are related to regular physical activity in older adults,” PLoS One, vol. 12, p. e0185318, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He K, Xun P, Liu K, Morris S, Reis J, and Guallar E, “Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study,” Diabetes Care, vol. 36, pp. 1584–9, Jun 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weisskopf MG and Weuve J, “Aging and Vulnerability to Environmental Chemicals: Age-related Disorders and Their Origins in Enviromental Exposures,” ed: The Royal Society of Chemistry, 2013. [Google Scholar]
- [10].Weisskopf MG, Weuve J, Nie H, Saint-Hilaire MH, Sudarsky L, Simon DK, et al. , “Association of cumulative lead exposure with Parkinson’s disease,” Environ Health Perspect, vol. 118, pp. 1609–13, Nov 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barbosa F Jr., Tanus-Santos JE, Gerlach RF, and Parsons PJ, “A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs,” Environ Health Perspect, vol. 113, pp. 1669–74, Dec 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J, et al. , “Biomonitoring of several toxic metal(loid)s in different biological matrices from environmentally and occupationally exposed populations from Panasqueira mine area, Portugal,” Environ Geochem Health, vol. 36, pp. 255–69, Apr 2014. [DOI] [PubMed] [Google Scholar]
- [13].Rabinowitz MB, “Toxicokinetics of bone lead,” Environ Health Perspect, vol. 91, p. 4, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Specht AJ, Lin Y, Weisskopf M, Yan C, Hu H, Xu J, et al. , “XRF-measured bone lead (Pb) as a biomarker for Pb exposure and toxicity among children diagnosed with Pb poisoning,” Biomarkers, vol. 21, pp. 347–52, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ellingsen DG, Chashchin M, Bast-Pettersen R, Zibarev E, Thomassen Y, and Chashchin V, “A follow-up study of neurobehavioral functions in welders exposed to manganese,” Neurotoxicology, vol. 47, pp. 8–16, Mar 2015. [DOI] [PubMed] [Google Scholar]
- [16].Hong SB, Im MH, Kim JW, Park EJ, Shin MS, Kim BN, et al. , “Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age children,” Environ Health Perspect, vol. 123, pp. 271–6, Mar 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. , “Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study,” Environ Health Perspect, vol. 120, pp. 98–104, Jan 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, et al. , “Toenail, blood, and urine as biomarkers of manganese exposure,” J Occup Environ Med, vol. 53, pp. 506–10, May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, and Cavallari JM, “Toenail metal concentration as a biomarker of occupational welding fume exposure,” J Occup Environ Hyg, vol. 11, pp. 397–405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johnson N, Shelton BJ, Hopenhayn C, Tucker TT, Unrine JM, Huang B, et al. , “Concentrations of arsenic, chromium, and nickel in toenail samples from Appalachian Kentucky residents,” J Environ Pathol Toxicol Oncol, vol. 30, pp. 213–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu AC, Allen JG, Coull B, Amarasiriwardena C, Sparrow D, Vokonas P, et al. , “Correlation over time of toenail metals among participants in the VA normative aging study from 1992 to 2014,” J Expo Sci Environ Epidemiol, Nov 27 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fleming DE, Nader MN, Foran KA, Groskopf C, Reno MC, Ware CS, et al. , “Assessing arsenic and selenium in a single nail clipping using portable X-ray fluorescence,” Appl Radiat Isot, vol. 120, pp. 1–6, Feb 2017. [DOI] [PubMed] [Google Scholar]
- [23].Fleming DE and Ware CS, “Portable x-ray fluorescence for the analysis of chromium in nail and nail clippings,” Appl Radiat Isot, vol. 121, pp. 91–95, Mar 2017. [DOI] [PubMed] [Google Scholar]
- [24].Fleming DEB, Gherase MR, and Anthonisen M, “Calibrations for measurement of manganese and zinc in nail clippings using portable XRF,” X-Ray Spectrometry, vol. 42, pp. 299–302, 2013. [Google Scholar]
- [25].Gherase MR and Fleming DE, “A calibration method for proposed XRF measurements of arsenic and selenium in nail clippings,” Phys Med Biol, vol. 56, pp. N215–25, Oct 21 2011. [DOI] [PubMed] [Google Scholar]
- [26].Zhang X, Specht AJ, Weisskopf MG, Weuve J, and Nie LH, “Quantification of manganese and mercury in toenail in vivo using portable X-ray fluorescence (XRF),” Biomarkers, vol. 23(2):154–160, pp. 1–7, Oct 10 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McIver DJ, VanLeeuwen JA, Knafla AL, Campbell JA, Alexander KM, Gherase MR, et al. , “Evaluation of a novel portable x-ray fluorescence screening tool for detection of arsenic exposure,” Physiol Meas, vol. 36, pp. 2443–59, Dec 2015. [DOI] [PubMed] [Google Scholar]
- [28].“<Nagata 2005. Cadmium and sex hormones.pdf>.”
- [29].Roy CW, Gherase MR, and Fleming DE, “Simultaneous assessment of arsenic and selenium in human nail phantoms using a portable x-ray tube and a detector,” Phys Med Biol, vol. 55, pp. N151–9, Mar 21 2010. [DOI] [PubMed] [Google Scholar]
- [30].Specht AJ, Zhang X, Goodman BD, Maher E, Weisskopf MG, and Nie LH, “A Dosimetry Study of Portable X-ray Fluorescence in Vivo Metal Measurements,” Health Phys, vol. 116, pp. 590–598, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dickerson AS, Rotem RS, Christian MA, Nguyen VT, and Specht AJ, “Potential Sex Differences Relative to Autism Spectrum Disorder and Metals,” Curr Environ Health Rep, vol. 4, pp. 405–414, Dec 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Specht AJ, Mostafaei F, Lin Y, Xu J, and Nie LH, “Measurements of Strontium Levels in Human Bone In Vivo Using Portable X-ray Fluorescence (XRF),” Appl Spectrosc, vol. 71, pp. 1962–1968, Aug 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Specht AJ, Parish CN, Wallens EK, Watson RT, Nie LH, and Weisskopf MG, “Feasibility of a portable X-ray fluorescence device for bone lead measurements of condor bones,” Sci Total Environ, vol. 615, pp. 398–403, Feb 15 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Specht AJ, Dickerson AS, Kponee-Shovein KZ, Nkpaa KW, and Weisskopf MG, “Toenail Metal Exposures in Fishermen from Bodo City, Nigeria,” Bull Environ Contam Toxicol, Nov 14 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Specht AJ, Kponee K, Nkpaa KW, Balcom PH, Weuve J, Nie LH, et al. , “Validation of x-ray fluorescence measurements of metals in toenail clippings against inductively coupled plasma mass spectrometry in a Nigerian population(),” Physiol Meas, vol. 39, p. 085007, Aug 31 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, and Hu H, “K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data,” Phys Med Biol, vol. 40, pp. 1475–85., 1995. [DOI] [PubMed] [Google Scholar]
- [37].Whitcomb BW and Schisterman EF, “Assays with lower detection limits: implications for epidemiological investigations,” Paediatr Perinat Epidemiol, vol. 22, pp. 597–602, Nov 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Specht AJ, Dickerson AS, and Weisskopf MG, “Comparison of bone lead measured via portable x-ray fluorescence across and within bones,” Environ Res, vol. 172, pp. 273–278, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ceballos DM, Young AS, Allen JG, Specht AJ, Nguyen VT, Craig JA, et al. , “Exposures in nail salons to trace elements in nail polish from impurities or pigment ingredients - A pilot study,” Int J Hyg Environ Health, vol. 232, p. 113687, Mar 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Borowska S and Brzoska MM, “Metals in cosmetics: implications for human health,” J Appl Toxicol, vol. 35, pp. 551–72, Jun 2015. [DOI] [PubMed] [Google Scholar]
- [41].Young AS, Allen JG, Kim UJ, Seller S, Webster TF, Kannan K, et al. , “Phthalate and Organophosphate Plasticizers in Nail Polish: Evaluation of Labels and Ingredients,” Environ Sci Technol, vol. 52, pp. 12841–12850, Nov 6 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ceballos DM, Craig J, Fu X, Jia C, Chambers D, Chu MT, et al. , “Biological and environmental exposure monitoring of volatile organic compounds among nail technicians in the Greater Boston area,” Indoor Air, vol. 29, pp. 539–550, Jul 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Craig JA, Ceballos DM, Fruh V, Petropoulos ZE, Allen JG, Calafat AM, et al. , “Exposure of Nail Salon Workers to Phthalates, Di(2-ethylhexyl) Terephthalate, and Organophosphate Esters: A Pilot Study,” Environ Sci Technol, vol. 53, pp. 14630–14637, Dec 17 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


