Abstract
In recent years, cancer immunotherapy has been observed in numerous preclinical and clinical studies for showing benefits. However, due to the unpredictable outcomes and low response rates, novel targeting delivery approaches and modulators are needed for being effective to more broader patient populations and cancer types. Compared to synthetic biomaterials, extracellular vesicles (EVs) specifically open a new avenue for improving the efficacy of cancer immunotherapy by offering targeted and site-specific immunity modulation. In this review, the molecular understanding of EV cargos and surface receptors, which underpin cell targeting specificity and precisely modulating immunogenicity, are discussed. We review unique properties of EVs in terms of their surface markers, intravesicular contents, intrinsic immunity modulatory functions, and pharmacodynamic behavior in vivo with tumor tissue models, highlighting key indications of improved precision cancer immunotherapy. Novel molecular engineered strategies for reprogramming and directing cancer immunotherapeutics, and their unique challenges are also discussed to illuminate EV’s future potential as a cancer immunotherapeutic biomaterial.
Keywords: Extracellular Vesicles, Exosomes, Delivery Biomaterials, Cancer Immunotherapy
Graphical abstract
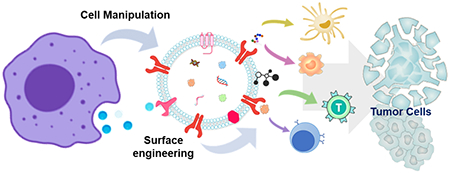
Cancer immunotherapy is the game changer in treating cancers. However, only a small population of patients could respond well, which highlights the urgent need of novel immunity modulators. Extracellular vesicles as the natural biomaterial open a new avenue for improving the efficacy of cancer immunotherapy by offering targeted and site-specific immunity modulation.
1. Introduction
Cancer immunotherapy, as a next-generation cancer treatment strategy, is currently attracting great attention[1]. Important breakthroughs within this field, including chimeric antigen receptor T-cell (CAR-T) therapy[2, 3], immune checkpoint blockade therapy [4–6] (e.g. anti-cytotoxic T lymphocyte antigen 4 (CLTA4), anti-programmed cell death 1 (PD-1), and anti-programmed cell death ligand 1 (PD-L1)), have further promoted massive efforts in exploring progressively more efficient immunotherapeutic strategies. However, the major challenge in the development of novel cancer immunotherapy still lies in the limited dosages, constrained by inherent life-threatening side-effects often due to uncontrolled immunity modulation. In particular, rapid elimination and degradation, non-specific distribution, and insufficient antigen uptake or presentation of immunotherapy agents during the delivery process are reported as the key hurdles in immunity activation[7]. Therefore, delivering immunotherapeutic agents to specific tissues or cells for precisely inducing anti-tumor immunity is of great importance in improving efficacious immunotherapy. Biomaterials, including nanoparticles, biodegradable implants, biomaterial scaffolds, and cell-derived extracellular vehicles (EVs), have been widely used for delivering a variety of bioactive cargos and immunotherapeutic agents[8–10]. Increasing interests are centering towards leveraging biomaterials as immunomodulatory agents. Different from synthetic biomaterials, EVs are natural nanovesicles secreted from a variety of cells, which are particularly attractive for dual acting as both delivery platforms and immunomodulatory agents.
EVs are heterogeneous groups of membrane-bound vesicles. These consist of exosomes, microvesicles (MVs), and apoptotic bodies, which all are secreted by most living cells[11]. Among those vesicles, endosome-originated exosomes (30-150nm in diameter) and cytoplasmic membrane-originated MVs (larger than 100nm) have been widely investigated. Due to substantial size overlap, assigning EVs to a specific biogenesis pathway remains extraordinarily challenging. Thus, in this paper, the generic term: EVs, will be used in compliance with the 2018 guidelines from the International Society of Extracellular Vesicles (“MISEV”)[12]. Depending on the cell of origin, EVs carry a characteristic composition of bioactive molecules, including proteins, lipids (e.g. cholesterols, ceramides), nucleic acids (e.g. DNAs and RNAs) and metabolites (e.g. amino acids and ATP). EVs can transfer these bioactive molecules from donor cells to recipient cells, acting as a novel mode of intercellular communication[13]. The bioactive molecules effectively alter the biological response and phenotypic features of the recipient cells, thereby playing an important role in numerous physiological and pathological processes, such as immunity regulation, signal transduction, and tumorigenesis[14]. However, successful EV-mediated intercellular communication requires delivery of the bioactive molecules and precise docking onto the target cells. The surface of EVs are enriched with numerous transmembrane proteins, such as tetraspanins, integrin, and intercellular adhesion molecule-1 (ICAM-1), which grant EVs the exceptional ability to target specific cells or tissues[13]. In addition to their intrinsic targeting abilities, EVs offer significant advantages over synthetic biomaterials, including excellent biocompatibility, prolonged circulation time, flexible drug loading abilities, and controllable biological properties, positioning EVs more advantageously for drug delivery purposes[15]. Thus, EVs demonstrate great potential as drug delivery systems to improve cancer immunotherapy, which has attracted a few review efforts to cover different challenging aspects, such as the tumor cell interaction with immune cell-derived EVs[16], therapeutic delivery[17], isolation and engineering of therapeutic EVs[18, 19], and fundamentals of tumor-derived EVs[20]. However, an in-depth understanding of EV intrinsic immunomodulatory function and their impact on using it as a delivery system has not been discussed, and our review intends to fill up this gap, focusing on the intrinsic role of EVs in immunomodulation, including the active and suppressive role within cancer immunity.
Moreover, EVs that are secreted by specific cells, including cancer cells, immune cells, and other non-immune host cells, may possess immunoregulatory potential while concurrently serving as a drug delivery system[21]. However, the immunoregulatory function of EVs derived from different cell origins are distinct, resulting in antigen presentations for either immune activation, suppression, or immune tolerance. For instance, EVs derived from dendritic cells (DCs) can stimulate the immune response by trafficking functional major histocompatibility complexes (MHC) and co-stimulatory molecules that activate antigen-specific T cell and B cell responses[22, 23]. DC-derived EVs (DEVs) pulsed with tumor peptides are capable of eradicating established murine tumors in a T cell-dependent manner[24]. Meanwhile, using tumor-derived EVs, as a source of neoantigens for internalization by DCs, could cross-prime CD8+ T cells, leading to the rejection of syngeneic and allogeneic murine tumors[25]. Thus, the EVs with immunoregulatory abilities can operate as potent therapeutics to develop cancer immunotherapy. Currently, several EV-based therapies have reached clinical trials, with three completed Phase I clinical trials and one completed Phase II clinical trial as summarized in Table 1.
Table 1.
The summary of clinical trials of EVs-based cancer therapies in recent five years.
| Disease | Phase/Number of Patients | EV Sources | Manipulation | Therapeutic Outcomes |
|---|---|---|---|---|
| Completed Trials | ||||
| Metastatic Melanoma[26] | Phase I/ n = 15 | imDCs, autologous | Pulsed with MAGE3 peptides | Safe, well tolerated; 2 stable disease, 1 minor response, 1 partial response, 1 mixed response |
| Colon cancer[27] | Phase I/ n = 40 | Ascites, autologous | Loaded with CEA± GM-CSF | Safe, well tolerated, 1 stable disease, 1 minor response |
| NSCLC[28] | Phase I/ n = 4 | imDC, autologous | Pulsed with MAGE-A3, -A4, -A10, -3DP04 peptides | Safe, well tolerated, 9 completed therapy, 2 with initial progression, 2 without progression for >12 months |
| NSCLC[29] [NCT01159288] |
Phase II/ n = 41 | mDCs, autologous | Pulsed with MAGE-A1, -A3, NYESO-1, MAGE-A3-DP04, EBV peptides | 22 completed therapy, 7 with stable disease (>4 months), primary endpoint (50%) not reached |
| Ongoing Trials | ||||
| Malignant ascites and pleural effusion [NCT02657460] |
Phase II/ n = 90 | Malignant pleural effusion | Loaded with methotrexate | Recruiting |
| Malignant ascites and pleural effusion [NCT01854866] |
Phase II/ n = 30 | Tumor derived | Loaded with chemotherapeutics | Unknown status |
| Malignant ascites [NCT03230708] |
Phase 1/2/ n = 18 | Erythrocytes, autologous | Loaded with methotrexate | Unknown status |
| Colon cancer | Phase I/ n = 35 | Plant derived | Loaded with Curcumin | Active, not recruiting |
| Metastasis pancreatic cancer [NCT0368631] | Phase I/ n = 28 | MSCs, allogeneic | KrasG12D siRNA (iExosomes) | Recruiting |
| Malignant pleural effusion | N/A/ n = 248 | Not reported | Loaded with methotrexate | Recruiting |
Notes: CEA: Carcinoembryonic antigen; NSCLC: Non-small cell lung cancer
Although great efforts have been devoted to understanding the intrinsic biological function of EVs, their potential to be leveraged or engineered for advanced cancer immunotherapy remains largely unexplored. In efforts to boost the efficacy of various immunotherapies, while circumventing immunoresistance and reducing off-target side-effects, rational engineering of EVs for advanced cancer immunotherapy is greatly required. In this review, we shed light on the application of EVs as drug delivery systems and immunoregulatory agents for targeting modulation of anti-tumor immunity. We begin with a description of the unique EV properties as a natural biomaterial, discussing resulting advantages over other synthetic biomaterials, such as liposomes and micelle nanoparticles. Secondly, we discuss the intrinsic immunoregulatory function of EVs regarding the immune system corresponding to the tumor microenvironment. We also discuss current strategies for circumventing and/or overcoming the immunosuppressive function, with a summary of published EV markers from the past 20 years, as well as their different sources in cells, for guiding proper selection of modulation targets. Lastly, we highlight several state-of-art applications of EVs in targeted cancer immunotherapy, including modifying and engineering EVs for significantly promoting delivery efficacy and immunostimulatory functions. The future potential and challenges are discussed with manufacturing and clinical translation practices.
2. EVs as an Advanced Delivery Biomaterial
Due to the intrinsic phospholipid bilayer, EVs are generally stable in maintaining their structural integrity for prolonged periods of time in vivo. Sokolova et al. [30] reported that EVs stored in phosphate-buffered saline at −20 °C were stable for longer times than EVs stored at 4 °C and 37 °C, indicating that storage temperature is a key factor in maintaining EV stability. Kalra et al. stored LIM1863 colon cancer cell derived-EVs in plasma at 37, 4, −20 and −80 °C. The lower storage temperatures ensured higher stability, with the EVs that were stored at −80 °C exhibiting the greatest stability[31] and retained the clinical usability after 5 months of storage[32]. In addition to EVs’ self-stability, EVs also can protect the endogenous or exogenous protein and RNA cargos from enzymatic degradation (e.g., protases and RNases), which leads to excellent stability of drugs during in vivo delivery. For instance, Curcumin self-assembling into the lipid layer of EVs via hydroscopic interactions, result in increased drug stability, solubility and bioavailability[33].
For intravenously administrated EV-based drug delivery systems, the delivery efficiency to the target cells is highly related to the in vivo circulation time of the system. Compared to synthetic nanoparticles, EVs with their intrinsic properties are more likely to possess longer circulation times without further optimization or modification. Synthetic nanoparticle delivery systems are limited to rapid clearance and non-specific biodistribution due to rapid opsonization-mediated recognition and phagocytosis by mononuclear phagocyte system (MPS)[34–36]. Several factors, including size, shape, surface charge, and surface property, are reported to play important roles in determining the opsonization, and subsequent MPS capture in these systems[37]. For instance, positively charged nanoparticles are more likely to absorb and aggregate with plasma proteins, contributing to their rapid MPS capture and limited circulation time[38]. In contrast, EVs essentially possess a negative surface charge due to their phospholipid bilayer, which is beneficial in reducing protein absorption, leading to prolonged circulation time[39]. Additionally, EV surface proteins could help evading MPS recognition and clearance[40–43]. An example is CD47, an integrin-associated transmembrane glycoprotein. CD47 plays a critical role in evading macrophage phagocytosis through the interaction of CD47’s extracellular domain with CD172a (also known as signal regulatory protein-α, SIRPα) on the macrophages, leading to the activation of the “don’t-eat-me” signal transduction pathway[44]. Moreover, isolated EVs can be functionalized with CD47 by specific conjugation strategies, resulting in an increased MPS-escaping ability[45].
Several early clinical trials have evaluated the effects of autologous DC-EVs for cancer immunotherapy and allogeneic mesenchymal stem cell derived EVs for regenerative and anti-inflammatory applications[46]. The majority of these trials reported only mild side effects, indicating that the administration of EVs from these specific cell sources are safe and well tolerated[47]. EVs are also secreted into circulation by all cell phenotypes within the body, leading to an extreme abundance in blood and plasma, with notable concentrations as high as 1010 EVs per mL. Amazingly, seriously adverse immune reactions following plasma and blood transfusions were infrequently reported, suggesting these allogeneic EVs are considerably safe[48]. These clinical trial efforts are establishing an excellent biocompatible delivery platform centered on using EVs
3. Intrinsic Immunological Role of EVs
The innate immune system and the adaptive immune system are critical mechanisms for regulating immune responses[49]. They control tumor initiation, progression, and metastasis. Although the intercellular communication between immune cells and tumor cells has been reported by engaging interleukins, chemokines, interferons, and other signaling molecules[50], accumulated studies suggest that EVs secreted from both immune cells and non-immune cells play distinct roles in regulating tumor immunity to form a cancer-immunity cycle (Figure 1), including antigen presentation, antigen transfer, innate and adaptive immune activation, immune suppression, and anti-inflammatory effects[51, 52]. Thus, rationally selecting EV populations with their intrinsic immunomodulatory function is crucial for delivering immunotherapeutic agents, which can further potentiate therapeutic efficacy.
Figure 1.
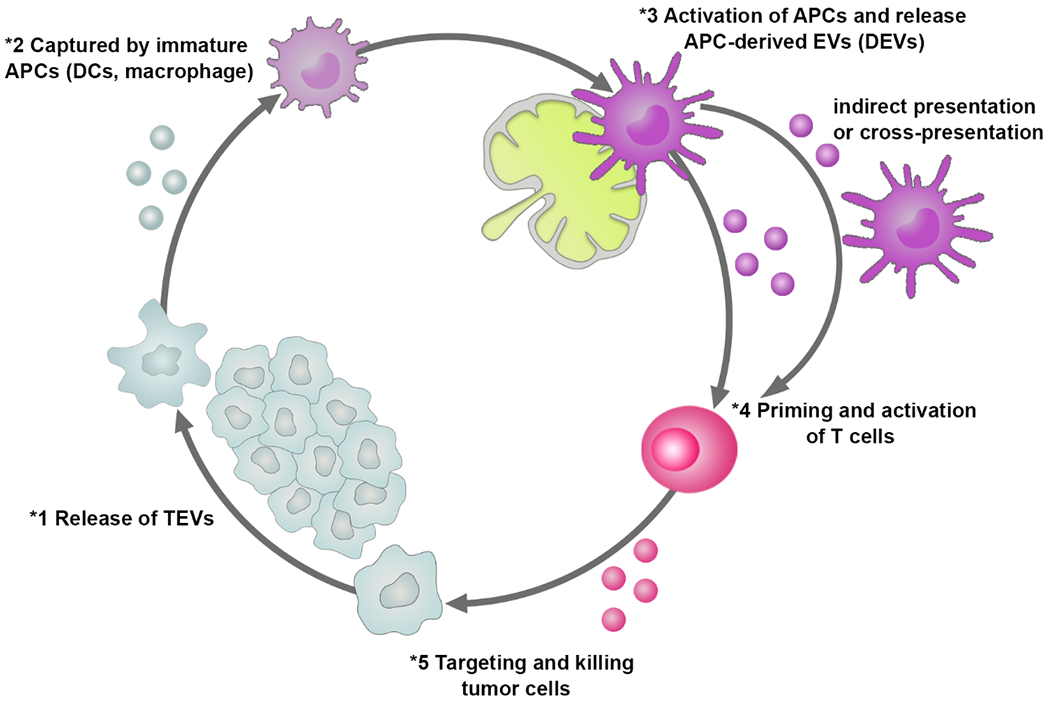
The role of EVs derived from either tumor cells or immune cells in regulating cancer-immunity cycle.
3.1. EVs as Mediators for Innate Immunity
Both tumor cell-derived EVs (TEVs) and immune cell-derived EVs play a positive role in regulating innate anti-tumor immunity. TEVs bearing heat shock protein 70 (HSP70) and other specific antigens can directly activate nature killer cells to promote the production of tumor necrosis factor-α (TNF-α) within macrophages[53, 54]. In addition, TEVs can promote the conversion of immune cells, such as DCs and macrophages, into pro-inflammatory cells at tumor-draining lymph nodes. This conversion leads to an increased production of pro-inflammatory cytokines (i.e. IL-6, IL-12, and interferon (IFN)-γ), while reducing anti-inflammatory cytokines (i.e., IL-10)[55].
Contrastively, EVs derived from natural killer (NK) cells, macrophages, and DCs have been described as pro-inflammatory mediators via paracrine messengers acting on the innate immune system[56]. DC-EVs are reported to express HLA-B-associated transcript 3 (BAT3), TNF superfamily members (TNF, TRAIL, Fas ligand (FasL)) as well as IL-15R, which can bind directly to corresponding surface receptors (NKG2D receptor) on NK cells[55, 57, 58]. The binding affinity of NK cells directly enhances their cytotoxic activity. Macrophage-derived EVs may play multiple roles in regulating innate immunity via either inducing macrophage differentiation or modulating the proliferation of myeloid cells[59]. Moreover, EVs released by pathogen infected macrophages (e.g., Toxoplasma or Mycobacterium tuberculosis-infected[60], lipopolysaccharide (LPS)-stimulated[61], or oxidized low-density lipoprotein (oxLDL)-stimulated[62]) were reported to induce maturation of DCs, also promoting pro-inflammatory cytokine secretion.
3.2. EVs as Mediators for Adaptive Immunity
To ensure an effectively adaptive anticancer immune response, a series of stepwise cellular events are initiated and expanded iteratively[63, 64] as shown in Figure 1. The cycle of tumor-immune cell interactions was generally considered to be manipulated by immune cells, including antigen presenting cells (APCs) and T cells against tumor cells. Recently accumulated evidence has indicated that immune cell derived EVs may play a similar role in procedurally maintaining the cancer-immunity cycle as their parent cells. TEVs containing various antigens from their parent tumor cells can be taken up by APCs, horizontally transferring to differently surrounding APCs and subsequently presenting to T cells, thereby activating anti-tumor immune responses[14, 20, 51, 65]. For example, Wolfers et al. first reported that TEVs could effectively cross-prime cancer-specific cytotoxic T lymphocyte (CTL) immunity on syngeneic and allogeneic established mouse tumors[25] during this antigen presentation process.
APC (e.g. DCs and macrophages)-derived EVs were found to express MHC-I, MHC-II, and T cell co-stimulatory molecules (e.g. CD80, CD86) on their surfaces for direct presentation to effector T cells (naïve CD4+ T cells or CD8+ T cells) , establishing immunity activation both in vitro and in vivo[66, 67]. It is worth mentioning: The efficiency of T cell activation by APC-derived EVs is highly relevant to the maturation status of APCs[68]. For instance, EVs secreted by mature DCs are more effective in inducing T cell activation in vitro and in vivo, than those secreted by immature DCs[69]. Immature DCs secreted EVs can only induce effector anti-tumor responses when co-injected with adjuvants or pre-loaded into recipient DCs. APC-derived EVs, unlike their parent cells, displayed a limited ability to directly induce T cell priming in vivo[23], partially due to an internalization by surrounding APCs through horizontally transferring MHC-antigen complexes. As a consequence, these antigen-bearing APCs can present to specific T cells, acting as an indirect or cross-presentation bearer[70–72].
In addition to APC-derived EVs, B cell-derived EVs are also involved in the presentation of antigenic activation of T cells. Raposo et al. reported that the Epstein-Barr virus-transformed B cells secreted EVs carrying antigen-MHC- II complexes, which could be presented to CD4+ T cells for immune-activation[73]. Subsequently, activated antigen-specific T cells will clone, proliferate, and migrate from lymph nodes into the circulatory system, thereby targeting tumors for specific killing. Alternatively, activated effector T cells may also secrete EVs carrying T cell receptors (TCR) to specifically recognize cognate tumor cells, or stimulating autologous resting T cells, further enhancing the immune response[74]. Valadi et al. reported that CD3+ T cell-derived EVs were involved in the stimulation and proliferation of resting CD3+ T cells, as well as CD8+ T cells, when combined with IL-2[75].
3.3. EVs-Mediated Immune Suppression and Evasion
Although TEVs can have indefinite immune-activating potential, conjunctively, they play a critical role in evading immune surveillance and suppressing anti-tumor immune responses: Resulting in pro-tumorigenesis, angiogenesis, and metastasis[76]. Specifically, TEVs carrying NKG2K ligands or transforming growth factor β1 (TGF-β1) on their surfaces can reduce NKG2D receptor expression on both NK cells and CD8+ T cells, leading to the neutralization of innate immune surveillance and adaptive immune response[77–79]. Other regulatory factors, such as FasL[80], TRAIL (TNF-related apoptosis-inducing ligand)[81, 82], and PD-L1[83–85], are also presented on the surface of TEVs and are involved in inhibiting T-cell proliferation, infiltration, and response by inducing the apoptosis of T cells or accelerating T cell exhaustion. Additionally, TEVs play an important role in promoting regulatory T (Treg) cell expansion by transferring TGF-β, a critical cytokine that mediates the suppression of CD8+ T cells and the proliferation of Foxp3+ Treg[86]. Moreover, tumor-derived EVs are shown to inhibit the differentiation of myeloid precursors into DCs, while also promoting the differentiation of myeloid-derived suppressor cells (MDSCs) through the interaction of HSP72 on EVs with TLR2 on MDSCs[87–89]. Similarly, TEVs enriched with miRNA, such as miR-21-3p, miR-125b-5p, and miR-181d-5p, potentially induce the polarization from anti-tumor M1-like tumor-associated macrophages (TAMs) to pro-tumor M2-like TAMs[90]. However, it should be noted that the role of TEVs in suppressing anti-tumor immunity is heterogeneous and highly dependent on cancer type, genomic characteristics, and apparent stage[30]. Apart from TEVs, EVs derived from other cells may also possess the potential to suppress the anti-tumor immune response. For instance, EVs derived from immature DCs have been reported to suppress peripheral immune responses in transplanted models and in mice with autoimmune diseases[91, 92]. EVs derived from T cells[93, 94], MDSCs[95, 96], and M2-like TAMs[97–99] were also shown notably functioning in suppressing the anti-tumor immune response. For example, EVs derived from CD4+ T cells inhibited the CD8+ CTL response and anti-tumor activity against OVA-expressing B16 melanoma[100]. Further, EVs derived from activated CD8+ T cell were reported to express FasL, which could promote the invasion of the murine melanoma cell line B16 and the Lewis lung cancer cell line via Fas signaling pathways[101]. In another study, Xie et al. reported that EVs derived from CD8+ T cells can be endocytosed by APCs through MHC-I/TCR interactions, inhibiting DCs mediated antigen-specific CD8+ CTL responses[102]. In summary, developing an EV-based delivery system or immunotherapeutic platform requires a deep understanding of EV intrinsic immunomodulatory effects for precise control of their in vivo behavior.
EV-mediated immune suppression critically impedes cancer immunotherapy. Especially for TEVs, the robust strategies for overcoming or circumventing the potential immunosuppression might be beneficial for an elevated anti-tumor immunotherapy. It has been reported that blocking PD-L1 can reserve the immunosuppressive effect of PD-L1+ TEVs[83] [103]. However, whether the binding of anti-PD-L1 antibodies to PD-L1+ TEVs can affect the binding of antibodies to PD-L1 on the tumor cell surface remains unclear. Thus, more precise manipulation in reducing or removing the expression of PD-L1 on TEVs is highly desired. Poggio et al. demonstrated that deleting two important exosomal biogenesis genes (Rab27a and nSMNase2) can suppress exosomal PD-L1 expression, leading to a significant anti-tumor immune response and memory immunization [85]. Furthermore, Li et al. demonstrated that GW4869 and Nexinhib-20, two small molecular inhibitors towards Rab27a and nSMNase2, could also suppress exosomal PD-L1, leading to similar in vivo antitumor effects in MC38 models [104]. Such removal of PD-L1+ EVs could directly contribute to enhanced antitumor immunity. However, it should be noted that small molecule inhibitors might not guarantee the removal of PD-P1+ EVs in all cell lines. Due to robust adaptability, simplicity and efficiency, CRISPR-Cas9 technology has been of great interest for modulating gene-specific immunosuppression[105]. On the other hand, EVs derived from mature DCs have been shown to possess high potency towards priming CTL and inducing anti-tumor immunity, leading to the clinical utility of stimulated DC EVs in cancer immunotherapy [69]. For example, EVs derived from IFN-γ-matured DCs exhibited a stronger antitumor Th1 immune response than those derived from immature DCs, regardless of isolation methods and size[106]. Due to the antitumor function of M1-like TAMs, EVs derived from M1-like TAMs are also reported to potentiate antitumor immunity [107, 108]. Cheng et al. found that EVs derived from M1-like, not M2-like, enhanced activity of a lipid calcium phosphate nanoparticle-encapsulated Trp2 vaccine, resulting in a stronger antigen-specific cytotoxic T cell response [107].
4. EV Delivery for Tissue and Cell Targeting
EVs have intrinsic tissue and cell-targeting capabilities due to the expression of a broad spectrum of membrane proteins or molecules on their surfaces, such as intergrins, tetraspanins, lactadherin, and immune-related molecules[109]. It should be noted that the organotropic and tumor-targeting properties of certain EVs depend on their composition and origin[109, 110]. For instance, Hoshino et al. reported that EVs derived from different tumor cells exhibit different integrin expression profiles, which could mediate tumor metastasis to different organ sites. EVs expressing integrin α6β6 and integrin α6β1 can target lung-resident fibroblasts and epithelial cells. In contrast, EVs expressing integrin ανβ5 can specifically target liver-resident Kupffer cells[111]. Tetraspanins are a superfamily of 33 transmembrane proteins, which are also overexpressed on the surface of EVs for cellular targeting. Rana S et al. reported that tetraspanin8 interacts with integrin α4 to form span8-integrin α4 complexes, which could drive EVs trafficking towards CD54-expressing endothelial and pancreatic cells[112]. Studies also demonstrated that CD63-expressing EVs specifically bind to neuronal and glial cells, whereas CD63-negative EVs only reside in the dendrites of neurons[112, 113]. In addition, EVs derived from immune or tumor cells are also endowed with insidious capabilities for targeting immune cells: For example, DC EVs carry multiple proteins from the parent cells, including MHC-I, MHC-II, lactadherin, tetraspanins, integrins, ICAM-1, milk fat globule-epidermal growth factor 8 (MFG-E8), and costimulatory and adhesion molecules, such as CD80, CD88, CD83 and CD40[114]. DC EVs have been widely proven to be capable of targeting and presenting antigens to CTLs, leading to the activation of an immune response[72, 115, 116]. DC EVs were also found to transfer these immune-related molecules to cognate DCs for maturation[117]. Similarly, EVs derived from CTLs[75], NK T cells[118], or chimeric antigen receptor T (CAR-T) cells[119] exhibited tumor cell-targeting abilities for precise killing. We summarize the specific EV markers reported from the past 20 years for tissue and cell targeting in Figure 2, which illustrates the exponentially growing interests, particularly in tumor or blood associated EVs with more significant markers identified, such as CD63, TGF-β, and CD9. The more representative EV surface markers specific to disease type and tissue tropism are summarized in Table 2.
Figure 2.
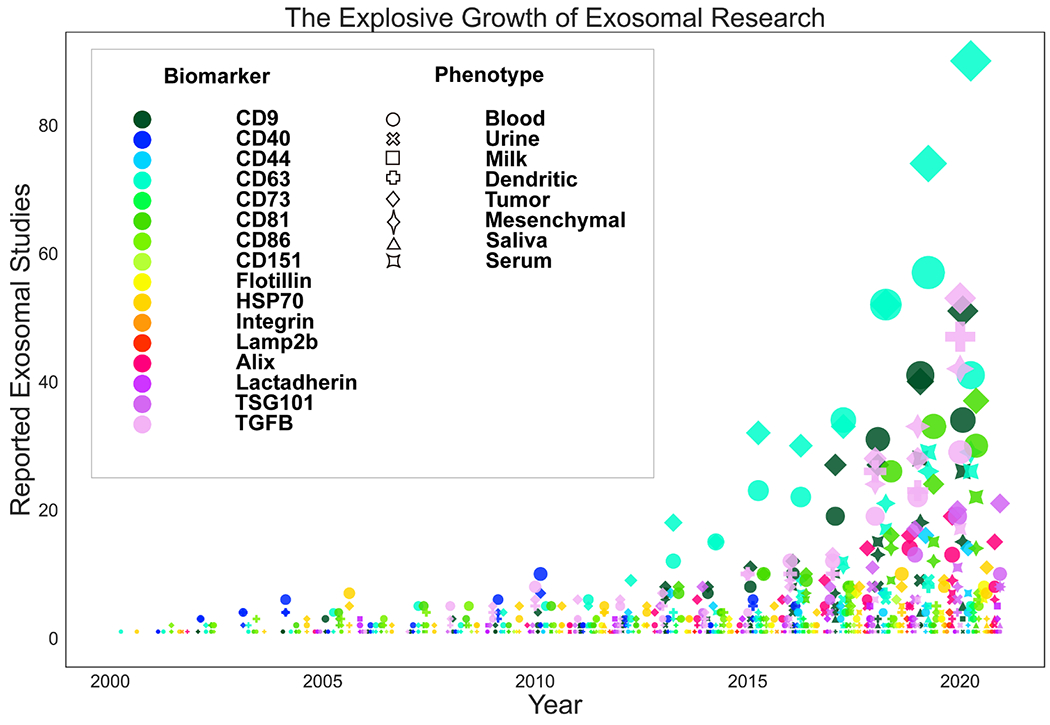
Summary of published EV markers in past 20 years from different sources in cell and tissue targeting.
Table 2.
Selected example list of reported EV surface markers corresponding to tissue tropism in different diseases.
| Disease | Cell/Tissuetropism | EV Surface Biomarkers |
|---|---|---|
| Melanoma | Heterogenous Tumor | FasL, Perforin, CD63, Alix[118] |
| DCs | A2/MART 1 Tetramer, CD11c, i-A, CD80, CD86, CD40 and H-2D, MA2.1, Integrin αvβ3/αvβ5, Lactadherin, OVA, LFA-1/CD54, CCR7, DEC205, TLR4, TLR9, MyD88, DC-Sign[120–122] | |
| T, B cell / Spleen | OVA/SIINFEKL, CD9, 54, 81, 80, 86[23] | |
| DCs / Spleen | Cyclophosphamide, MART1[123] | |
| NK Cells, DCs | MAGE 3 A1/B35, MAGE3.247-258DPO4[26] | |
| Liver, Spleen, Breast | Lamp-2b/αv specific iRGD peptide[124] | |
| Pancreatic Adenocarcinoma | BMC/Lung, Tissue, Kidney, Pancreas | Clusterin, GDF15, Neuropilin, Beta Actin, Thrombosposin CD9, CD151, CD49e, CD63, Tspan8 and B4, CD49f, CD621, CD104, CD54[112] |
| Neuroblastoma | Glial cells, Neuronal Soma | CTF, GAL4, CD63, Flot-1, APP[113] |
| Plasmacytoma | DCs | MFG-E8/Lacadherin, MAC-1a/b, CD9, MHC1/II, CD86, CD80, CD88, CD83, CD40, ICAM-1, Lamp2b, Syntenin, Gi2a, Alix, TPx,14-3-3,Galectin-3, Hsc73, Hsp84, RabGDI, Rap1B, Rab7, Annexins, I,II,IV,V,VII, Gag, EF1a, Actin, Tubulin, Cofillin, Profilin I, EIF-4a[114] |
| HIV-1 | DCs (CD11+) | HLA-DR1, CD1b, CXCR4- or CCR5- cells, CD9, CD63[115] |
| Unspecific | DCs | ICAM-1, LFA1, I-Ab/HY Peptide[117] |
|
Lymphatic Leukemia |
CD4+ and CD8+ w/ IL-2 | CD3, IL-2, CD9, CD63, CD81[75] |
| Brain, Kidney, Liver | Lamp2b, RVG, MSP, FLAG[125] | |
| Malignant B-cells, T-cells | EBV-gp350+, CD154, CD21, B1FNR1, CD154, HSP70, TSG101, CD63, GM1[126] | |
| Chronic Myeloid Leukemia | Liver, Spleen, Kidney, Tumor | IL-3/CD-123, ALIX, CD81, TSG101[127] |
| T-cell Lymphoma | B-cells | HLA, pMAGE-A33, Lactadherin[128] |
| Mouse Lymphoma | NK Cells, Liver, Lung, Spleen | α-Galactosyceramide, OVA, CD-1d[129] |
| T-cell hybridoma | Tumor | MFG-E8, OVA, Lactadherin, HSC70, TSG101, CD9[130] |
| Breast Cancer | Tumor Xenografts | Dicer/Actin, CD9, CD63, CD81, Flotillin-1, TSG101[49] |
| Lung, Colon, Tumor Xenografts | Oncolytic Adenovirus fused to CD40L, Paclitaxel TSG101, CD63, CD9[109] |
|
| Hepatocarcinoma | Liver, Spleen, Breast | Lamp-2b fused with an av integrin iRGD peptide[124] |
| DCs | Human HER2, nt 1-1953/nt 1-2025/AAV, Lactadherin[131] | |
| Unspecified | Unspecified | Lamp-2b fused with an αvβ integrin iRGD peptide[124] |
| Prostate Cancer | Tumor Tissue | Lactadherin[132] |
| Fibrosarcoma | Tumor | MFG-E8, OVA, Lactadherin, HSC70, TSG101, CD9[130] |
| Pancreatic Ductal Adenocarcinoma | Tumor, liver, skin, kidneys, lungs | Oxaliplatin and Galactin, Lactadherin, Annexin V, CD63, Alix, HSP70, CD81[133] |
| Acute Lung Inflammation / LPS Septic Shock | Liver, Lung, Kidney, Spleen, CD11b+Gr-1+ cells | TGS101, CD81[134] |
| Chronic Acute Inflammation | Spleen, Liver, DCs, Macrophages, Kupffer cells, | IL-4, FasL, CD11b, CD71, CD86, CD178[135] |
| Lung Cancer | NK cells | MAGE-A3 and MAGE-A4, MAGE-3DPO4, MHC1/2, CD1a-d, CD86, CD9, C37, CD53, CD81, CD82[28] |
Maximizing designed targeting efficiency and specificity strategies, EVs can be genetically engineered, post-isolation, to introduce certain tissue- or cell type-specific targeting ligands on their surface at the cellular level[136]. The EV producing cells can be transfected with expression plasmids that encode EV transmembrane proteins, fused with a target of interest (e.g. ligand/homing peptide/signaling peptide). The relevant technique was first reported by Alvarez-Erviti et al to transfect DCs, with the plasmid encoding a lysosome-associated membrane glycoprotein 2 (Lamp-2b) fused with rabies viral glycoprotein (RVG), a central nervous system-specific peptide that can specifically recognize acetylcholine receptors[125]. The external leaflet of the secreted EVs were enriched with Lamp-2b-RVG, these EVs were then loaded with therapeutic siRNA via electroporation after post-isolation. In vivo studies showed that the RVG-carried EVs could efficiently cross the blood-brain barrier (BBB), delivering to neurons and glia cells following intravenous injection, eventually resulting in significantly higher gene silencing than compared to non-targeted EVs [125]. The targeting peptides on EV surfaces, such as EBV glycoprotein 350 for targeting CD19+ B cells, has been widely studies since then[126], including the iRGD fused with Lamp-2b for targeting αν-integrins and neuropilins of tumors[124], and interleukin 3 (IL-3) fused with Lamp-2b for targeting IL3 receptors on chronic myeloid leukaemia cells[127]. In addition to Lamp-2b, other transmembrane proteins have been used, including tetraspanins (CD63, CD9, CD81), glycosyl-phosphatidyl-inositol (GPI), platelet-derived growth factor receptors (PDGFRs), and lactadhein C1C2 domain[136, 137]. Among these, lactadherin’s C1C2 domain, which can bind non-covalently to membrane phospholipids, has been widely used as a fusion partner[128]. However, researches were more interested in using lactadherin’s C1C2 domain to fuse with antigens or soluble proteins for targeting immune cells, such as T cells[130–132] and B cells[128].
Apart from EVs intrinsic or engineered targeting traits, intravenously administrated EVs may also demonstrate passive targeting mechanisms due to their potential to cluster at tumor sites via the enhanced permeability and retention (EPR) effect. As a consequence, EV-based delivery systems may have the potential to deliver immunoregulatory agents to specific cells within a tumor site. It should be noted that the application of the EPR effect is highly dependent on both the pathological condition of the tumor, such as its neovasculature density, and the physicochemical properties of EVs, including size and shape[138]. The rapid development of tumors is often accompanied by the formation of neovasculatures, which are often immature and irregular, with large intercellular pores being potentially leaky between the endothelial cells (10-1000 nm)[139]. Therefore, near-spherical EVs with a size of 30-150 nm can extravasate from the intercellular pores and diffuse into the interstitial space of the tumor. Meanwhile, the absence of functional lymphatic vessels, in most tumors, contributes to EVs entrapment and retention, referred to as the EPR effect[140]. However, this mechanism has lower cell-targeting specificity and may cause non-specific distribution with unwanted off-target effects. Therapeutic potential when employing the EPR effect can be further improved through rational designs of EV-based delivery systems or combining the EPR effect with active-targeting functionality.
Lymph nodes (LNs) are a primary organ of the immune system, containing a large fraction of immune cells, such as T cells, B cells, and APCs[141]. LNs provide a specialized microenvironment to gather immunogenic information from peripheral tissues, regulating the adaptive immune response of the body[142]. Unsurprisingly, LNs are attractive therapeutic targets for the treatment of a variety of unmet clinical needs, including cancer. One of the most widely used immunotherapies based on LNs-targeting delivery is the conventional vaccine, which can be localized to LNs-resident APCs or lymphocytes after reaching the LNs. Conventional vaccines are usually administrated peripherally, in which free antigens and/or adjuvants are transported from interstitial spaces to downstream LNs via lymphatic vessels[143]. However, several challenges, including rapid degradation, limited circulation time, and the unique physiology of LNs may hinder high concentrations of therapeutics from reaching LNs. Encouragingly, EV-based vaccines are a promising alternative due to their improved delivery performance. Similar to the size-dependent EPR effect for tumor targeting, the delivery of EVs-based vaccines to LNs is also size-dependent. The LNs are more sensitive to the hydrodynamic size of EVs, owing to the unique structure of the lymphatics coupled with its size-restrictive reticular network. It has been reported that small molecules (< 20 kDa) are typically rapidly cleared into the blood following injection, whereas larger molecules (> 20 kDa) and particles (10-100 nm in diameter) drain into the lymphatics from interstitial spaces. Even larger particles, with diameters > 100 nm, are likely to become trapped in the interstitial matrix due to limited lymphatic access[144, 145]. Therefore, particles with diameters of 10-100 nm may ensure efficiently precise delivery to LNs. Considering that the intrinsic size range of EVs are 30-150 nm, which also overlaps with an ideal delivery size range, EV-based vaccines have the potential to be selectively delivered to the LNs in a size-dependent manner. Moreover, as aforementioned, the presence of surface molecules can further affect targeted delivery to cell subtypes within LNs, as well as enhance cellular uptake.
5. EV Delivery for Targeting Immunity Modulation
The immunoregulatory function of EVs derived from different cell origins are distinct, which could result in different in vivo behavior, promoting usage as therapeutic agents or delivery platforms. Further molecular engineering of those EVs could reprogram EV functions to develop novel and advanced cancer immunotherapy. The molecular engineering approaches, as well as the five major reprogramming pathways are summarized in Figure 3 and discussed respectively in the subsequent sub-headings.
Figure 3.
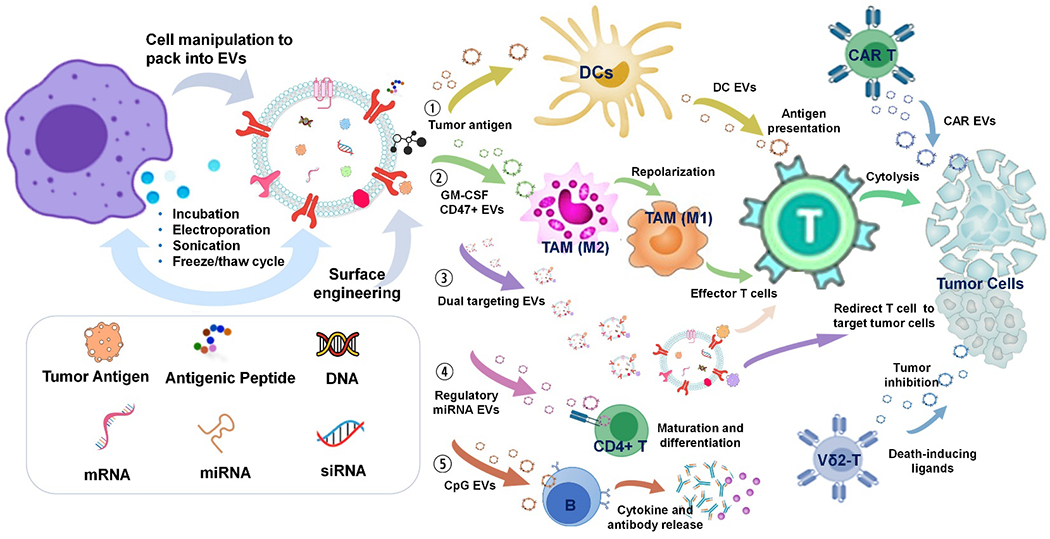
The schematic illustration of EVs for reprogramming tumor immunity. Number 1-5 indicate the five major EV reprograming pathways in regulating tumor immunity.
5.1. DC-derived EVs for immunomodulation
Cancer vaccines using tumor antigens[146], antigenic peptides[147], DNA[148], mRNA[149] or adjuvants[150] have been widely explored as promising options for cancer immunotherapy. However, their clinical outcomes vary, mainly because of limited immunoactivation and poor delivery efficacy[151–153]. Therefore, improving immunity potency and delivery efficacy for developing diverse vaccines is of great importance. Tailoring biomaterials (e.g. liposome, nanoparticle, scaffold) and autologous or allogeneic cells as vaccine delivery platforms has recently been proven to improve the therapeutic benefits[8, 154, 155]. Specifically, DC-based vaccines have emerged as the personalized cancer vaccine[156, 157]. Notably, Sipuleucel-T, an autologous DC therapy, was the first FDA-approved therapeutic cancer vaccine for prostate cancer in 2010[158]. However, the clinical implementation of Sipuleucel-T was hampered due to the manufacturing complexity associated many issues[159, 160]. EVs derived from APCs, especially DCs, have the potential as the therapeutic cell-free vaccines to surrogate their parent DCs, owing to the unique surface molecule composition, as illustrated in Figure 3 pathway 1. Pioneering work by Zitvogel et al. demonstrated that EVs derived from DCs, pulsed with antigen peptides, induced potent anti-tumor CD8+ T cell responses in murine mastocytoma P815 and mammary carcinoma TS/A tumor models, resulting in the regression of established tumors[24]. This study illustrates the potential of DC EVs as cell-free delivery platforms for vaccine development, while circumventing the bottleneck currently existing in DCs-based vaccine production. Additionally, preclinical studies also showed that peptides have a higher antitumor efficacy when carried by EVs[121, 123]. However, the antitumor efficiency of DC-EV-based vaccines are limited in some patients with cancers[26, 28, 29]. The heterogeneity of ex vivo expanded DC-EVs combined with the maturation status of parent DCs mainly account for the poor therapeutic benefit of DC-EV-based therapy: Long withstanding challenges include generating highly homogeneous DC-EVs, and obtaining mature parent DCs, of which are usually asynchronously incomplete in maturation.[161]. Furthermore, their small size relates to rapid dispersion caused by Brownian motion, consequently impeding their recognition and docking on T cell receptors. Indeed, enhanced T cell activation can be pursued when EVs are immobilized at high concentrations, such as with latex beads. Additionally, the expression of immune-related molecules, such as MHC-I, MHC-II, CD86, CD80, and cytokines carried by EVs, is much lower than parent cells, which may require a large number of peptide-MHC complexes per EV and/or high-level EVs secretion[14]. These requirements along with designated cargo packaging are still missing reliable supporting techniques.
5.2. Targeted Delivery of Nucleic acids for immunomodulation
EVs are also natural carriers of nucleic acids, including message RNAs (mRNA), miRNA, small interfering RNAs (siRNA)[162, 163] and DNA, which are used to relay intracellular signals. The nucleic acids, mostly RNAs carried by EVs, were reported as promising biomarkers significantly interplaying tumor-immunity microenvironment[164]. More importantly, not only are nucleic acids in TEVs found to manipulate the immune response, but those in EVs secreted from immune cells also counteractively mediate tumor responses[164, 165]. In order to modulate the delivery of therapeutic EVs for the purpose of immune regulation, EVs can be engineered with tumor targeting ligands via post-purification: Chemical modifications or cellular surface engineering methods[124, 166–168]. Surface modified EVs can be tailored for targeted delivery of synthetic drugs and exogenous nucleic acids, which significantly reduces the toxicity and avoids the potential degradation or binding to other proteins. Particularly, the therapeutic nucleic acids, such as mRNAs and miRNAs carried by EVs, can effectively mediate development of immune cells. For instance, miR-155 and miR-326 were demonstrated to regulate the differentiation and maturation of CD4+ T cells[169, 170], while miR-20, and miR-124 demonstrated anti-tumor T cell responses, while miR-19 showed NF-κB mediated inflammation[171–173]. Moreover, CpG oligonucleotides, which agonize TLR-9 receptors on B cells, can also be conjugated with EVs to boost anti-tumor immunity[174]. It was also found that streptavidin-lactadherin modified EVs, self-assembled with biotinylated CpG DNA, were internalized into TLR-9 presenting DC2.4 cells, which subsequently contributed to increasing cytokine release, initiating the immune response to adjuvants by dermal-resident APCs in vivo[175]. Therapeutic nucleic acids carried by EVs with surface tailored properties for targeted delivery have shown tremendous advantages in gene therapy[176, 177], which also will be an unmatched delivery system for advancing cancer immunotherapy. However, loading nucleic acids into EVs generally require different transfection strategies like electroporation, sonication, chemical transfection, which may potentially cause irreversible damage on EV membranes, and thereby influencing the recognition of EVs by recipient cells. Also, it remains unclear if enzymes carried by EVs can degrade nucleic acids. An unanswered question is if EVs can efficiently escape from lysosomes after cellular uptake through endocytosis or phagocytosis.
5.3. EV Targeting modulation of Tumor-Associated Macrophages
TAMs have been known in M2-like phenotype for promoting tumor growth by inducing immunosuppressive tumor microenvironments[178, 179]. In order to maximize the potential of macrophages for cancer immunotherapy, targeted modulation of TAMs by repolarizing pro-tumoral M2-like TAMs to M1-like TAMs is an emerging strategy, as shown in Figure 3 pathway 2. The EV surface CD47 interaction with SIRPα on the macrophage has been reported as preventing the capture and clearance from MPS[180]. Lv et al. proposed a hybrid nanoparticle, created by fusing CD47-expressing EVs with a thermosensitive liposome, for delivering chemotherapeutics and immunoregulatory agents to metastatic peritoneal cancer cells[181]. The CD47-expressed EVs produced from genetically engineered fibroblasts were capable of efficiently escaping from MPS capture after entering into systemic circulation. The granulocyte-macrophage colony-stimulating factor (GM-CSF) and docetaxel-loaded, genetically engineered EV-thermosensitive liposomes hybrid nanoparticles (G/D-gETL NPs) could preferentially accumulate in tumor sites, via the EPR effect, and release cargos under hypothemia conditions in hyperthermic intraperitoneal chemotherapy (HIPEC)[181]. As a consequence, the release of GM-CSF promoted the repolarization of M2-like TAMs to M1-like TAMs, leading to the improved presentation of antigens to effector T cells. Moreover, CD47 carried by gETL NPs interacted with SIRPα on macrophages, which further improves macrophage-mediated tumor cell phagocytosis. The targeted repolarization of M2-like TAMs into M1-like TAMs could restore the anti-tumor immunity, establishing an essential role in cancer immunotherapy. Although specific engineering ensures a CD47 outer coating, the biodistribution of this smart EVs-based system, after intravenous injection, was still mainly concentrated in liver. Moreover, the enhanced permeability and retention (EPR) effect-based passive targeting delivery of this EVs-based system to tumor site might not guarantee high TAM-targeting specificity when compared to ligand-mediated active targeting. It is highly expected that TAM targeting specificity could be further developed for a more robust and specific immunomodulation.
5.4. EV Dual-Targeting modulation of T Cells and Tumor Cells
Surface engineering EVs to simultaneously express ligands for targeting both T cells and tumor cells can effectively elicit cytotoxicity of T cells towards specific tumor cells. Cheng et al. developed synthetic multivalent antibodies (svFc) tailored EVs by engineering HEK293 cells to secret EVs carrying svFc specific to T-cell CD3 and epidermal growth factor receptor (EGFR) from tumor cells[167], which can bridge T cellular anti-tumor responses against the EGFR-expressing breast cancer cells. Similarly, engineered EVs carrying svFc specific to CD3 and human epidermal growth factor receptor 2 (HER2) exhibited excellent anti-tumor immunity against HER2+ breast cancer cells in vitro and in vivo[182]. The effectiveness of dual-targeting engineered EVs open up novel possibilities for designing EVs to re-direct T cell cytotoxicity towards specific tumor cells, which could improve targeted cancer immunotherapy and reduce off-target effects, as illustrated in Figure 3 pathway 3. However, the in vivo efficiency of these EVs with dual-targeting functionality to bridge together T cells and tumor cells needs further investigation. Also, if the effector T cells were dysfunctional or apoptotic, their enrichment around tumor cells may impede the function of other activated effector T cells like immune recognition and subsequent response. Therefore, a more specific engineering strategy ensuring targeting activated effector T cells is highly recommended. Furthermore, to realize clinical applications, in-depth characterization of exosomal composition may be required for developing therapeutic EVs with improved efficacy and reduction in side effects.
5.5. Engineering T-cell Derived EVs for Targeted Immunomodulation
The most common strategies for engineering EVs with intravesicular cargos are exogenous and endogenous loading[18, 183]. Endogenous loading functions through genetical modification of their parental cells for specific cargo packaging into the EV secretary pathway. Reports showing exogenous loading was mostly performed by co-incubating cargos with isolated EVs under chemical transfection, electroporation, sonication, or freeze/thaw cycles[15, 163]. Genetically engineered T cells expressing a chimeric antigen receptor (CAR) or T-cell receptor (TCR) are rapidly emerging as promising treatment options for a broad range of cancers, especially CD19+ B cell malignancies[184–187]. However, the associated life-threatening toxicity is significant, such as high fever, hypertension, hypoxia, and/or multiorgan toxicity, and CAR-T-related encephalopathy syndrome (CRES)[188]. Thus, CAR-T or TCR-T cells derived EVs are catching great attentions as alternatives to their parent cells. Lentiviral vectors can be used to transfect primary human T cells with Cetuximab scFv (termed CAR-T-CTX) or Trastuzumab svFv (CAR-T-TTZ), with results demonstrating that CAR-T CTX or CAR-T TTZ could efficiently lyse EGFR+ cancer cells or HER2+ cancer cells[189]. By isolating EVs from these engineered T cells using ultracentrifugation, it was found that EVs carry parent CARs on their surface, mostly in the form of exosomes. Similar to CAR-T cells, these CAR-EXO-CTX or CAR-EXO-TTZ notably expressed perforin or granzyme B, which is crucial for cytolytic activity[189]. The CAR-EXO-CTX or CAR-EXO-TTZ also demonstrated strong cytotoxic effects on EGFR-expressing cells or HER2-expressing cells respectively. Most importantly, the CAR EVs specifically targeted EGFR+ or HER2+ tumor cells after intravenous injection, inhibiting tumor growth in a dose-dependent manner[189]. Given that tumor cells may inactivate CAR-T cells via a variety of immunosuppressive signaling pathways, such as PD-L1/PD-1, an in vivo study was conducting, by adding recombinant PD-L1 to CAR exosomes which showed no significant influence on cytolytic activity, indicating the rare expression of PD-1 on these CAR exosomes. There was very limited CRS side effect observed. EVs derived from genetically engineered T cells abstract functionality from their parent cells, cytotoxically affecting cancer cells, and are less vulnerable to suppression.
γδ-T cells are innate-like T cells with lytic activities that are not restricted by MHCs[190]. The Vδ1 type T cells are mainly located in mucosal and epithelial tissues, while Vδ2-T cells exist in the peripheral blood and lymphoid organs[191]. As reported, either the adoptive transfer of ex vivo phosphoantigens-expanded Vδ2-T cells or the direct administration of phosphoantigens to active Vδ2-T cells in vivo, could efficiently control Epstein-Barr virus (EBV)-induced B cell lymphoproliferative disorder (EBV-LPD)[192]. It has been hypothesized that Vδ2-T cell-derived EVs may possess the potential to inhibit tumor proliferation. Wang et al. reported that EVs derived from phosphoantigen-expanded Vδ2-T cells carry death-inducing ligands (FasL and TRAIL), an activating receptor for nature killer cells (NKG2D), and other immune-related molecules (e.g. MHC class I and II, CD80 and CD86)[193]. In vivo studies demonstrated that, after intraperitoneally injection into mice for 24 h, Vδ2-T derived exosomes targeted EBV-transformed B lymphoblastoid cell lines (EBV-LCL) with much higher levels of accumulation than control exosomes. More importantly, the anti-tumor effect of Vδ2-T-Exos using an EBV-induced B cell lymphoma model was also shown as significantly reducing the incidence of tumors. Interestingly, it observed that antitumor effect of Vδ2-T-Exos was mediated by activating FasL and TRAIL signal pathways, as well as by inducing EBV antigen-specific CD4+ and CD8+ T cell expansion, indicating EVs derived from activated γδ-T cells hold great promise in modulating antitumor immune responses. Compared with cell-based therapy, cell-free EVs-based therapy has several advantages in clinical applications. CAR-EVs may have a lower risk of toxicities compared to parent CAR-T cells, such as cytokine release syndrome (CRS), which is characterized by high fever hypotension, hypoxia and/or multiorgan toxicity[194, 195]. Another advantage, EVs-based immunotherapy may be more resistive to the immunosuppressive tumor microenvironment[196]. The manufacturing process of these cell-free vesicles is also safer than that of living CAR-T cells. However, it is still difficult to compare the killing efficiency between CAR-T cells and CAR-EVs, because they possess different natural mechanisms even though they have a similar function. Different from CAR-T cell-based treatment, the exact number of CAR-EVs normalized in equivalent protein content is controversial based on current methods[197]. Moreover, the efficiency of Vδ2-T-EVs was only evaluated in the context of EBV-associated cancers; whether Vδ2-T-EVs would be effective in other cancers remains to be determined. These aforementioned engineering strategies are tailorable and applicable for investigation of a variety of ligand-receptor pathways in immunomodulation, further unlocking the effective targeting ability of T cell derived EVs.
6. Challenge and Prospective
EVs are emerging as promising drug delivery biomaterials, involving extensive applications ranging from gene therapy, regenerative medicine, to immunotherapy and cancer vaccines. By harnessing the power of EVs, scientists have made tremendous strides in modulating the immune response for improving therapeutic outcomes. However, the majority of recent research investigations on EVs are from either animal models or 2D planar cell cultures. Given that 3D culture systems are recognized as more advanced in physiologically relevance than 2D culture systems, a growing body of studies are highlighting the distinct profiles of EVs secreted from 3D culture systems which are more accurately reflect the in vivo situation[198]. Increased secretion of EVs, upregulation of microRNAs, and downregulation of proteins was observed in 3D spheroid models composed of human gastric cancer cell lines (MKN45 and MKN74) in comparison to 2D conditions[199]. These results reveal the effect of cellular architecture on the release and content of EVs[200, 201]. Similarly, Thippabhotla et al. also demonstrated that EV miRNAs derived from 3D cervical cell culture displayed a 96% similarity to in vivo circulating EVs derived from cervical cancer patient plasma, while only a 80% similarity was observed in 2D culture[202]. Beyond just content and concentration, Kim et al. also conducted a comparative study on EVs-mediating immunomodulatory functions using 2D- and 3D-cultured mesenchymal stem/stromal cell-derived extracellular vesicles (MSC-EVs)[203]. In vitro analysis identified that EVs, derived from 3D cultured MSCs, performed better on restraining the secretion of pro-inflammatory cytokines (IFN-g and IL-6) while stimulating the production of immunoregulatory cytokines (TGF-b1 and IL-10)[204]. Taken together, EV production is highly sensitive to their secretion-by-cell-culture system and apparent conditions implemented. EV sources also could introduce significant variables. Therefore, such variables pose substantial challenges in EV manufacturing and purification.
Currently, there is no standardized isolation and purification methods for preparing homogeneous EVs yet. Although ultracentrifugation, polymer precipitation, or column filtration isolation methods can provide high recovery yield of EV, such yields are irrelevant to homogenous or highly specific EV molecular components. Highly specific isolation methods are much more advanced in terms of isolation precision to EV subtypes and specificity to pathogenesis-relevant markers, such as filtration combined with SEC[205], immunoaffinity capture-based techniques[206], and microfluidics-based isolation techniques[207–209]. The ideal isolation technique is desired with homogenous molecular cargos, reproducible, high-yielding and throughput, and scape up capability. Thus, careful considerations should be taken while choosing an isolation method with the cell sources and the nature of molecular cargos in mind.
Due to the constrains from the above EV isolation methods, the clinical translation of EV-based drug delivery and therapies is hampered consequently. Several attempts have been made to develop production and purification using GMP standard protocols, as well as characterization procedures of GMP-grade EVs[32, 210–212], which requires sterilized manufacturing process in producing EVs with sufficient and consistent therapeutic payloads. The batch-to-batch variation is the focal point, requiring rigorous validation. Currently, amounts of efforts are contributed to a more ideal strategy meeting all the criteria for large-scale GMP-grade EVs manufacturing in pursuit of good scalability, reproducibility, safety, potency, and purity of EVs for precision cancer immunotherapy. As shown in Table 2, including three completed Phase I clinical trials and one completed Phase II clinical trial, there is no approved EV-based therapy yet for clinical use, possibly due to the nature of heterogeneity within the production of EVs and intra-exosomal compositions. Particularly, in light of COVID-19 pandemic needs, two clinical trials were registered on March 2020 for investigating mesenchymal stem cell secreted EVs as therapy for reducing pulmonary inflammation and their safety (ClinicalTrials.gov Identifier: NCT04276987 and NCT04313647). Despite this significant progression, timely large-scale manufacturing in meeting emerging clinical needs is still challenging, mainly due to the complication on isolation and purification of heterogeneous EVs from a tremendous upscale, attempting to consistently output a production of population-based dosages. Therefore, to further translate EVs to the bedside applications, more multidisciplinary technologies and collaborations are highly expected in the near future.
Acknowledgements
This project is supported by NIH NIGMS MIRA award 1R35GM133794 and USDA-NIFA 2017-67021-26600 to Dr. Mei He
Biographies
Author Biographies
Dr. Shaobo Ruan obtained his Ph.D. from the Sichuan University, China in 2018. Since then, he joined Dr. He research group at the University of Florida school of pharmacy in USA. His research interests include nanoparticles and EVs, drug delivery and therapeutic development focusing on cancer immunotherapy.

Zachary Greenberg is a Ph.D. student in the University of Florida School of Pharmacy. He obtained his master’s degree in 2020 from the University of Florida with major in Material Science and Engineering, and bachelor’s degree in 2017 from the University of Florida with major in Chemistry. His research interests include the targeted molecular engineering of extracellular vesicle.

Dr. Xiaoshu Pan obtained her Ph.D. from the University of Florida with major in Biochemistry in 2020. Since then, she joined Dr. He research group at the University of Florida school of pharmacy for developing gene therapy and exosome delivery.

Dr. Pei Zhuang obtained her Ph.D. from the Nanyang Technological University, Singapore in 2019, with the major in Mechanical Engineering. Since then, she joined Dr. He research group at the University of Florida school of pharmacy for developing 3D tissue model for exosome delivery.

Nina Erwin is a Ph.D. student in the University of Florida School of Pharmacy. She obtained her bachelor’s degree in 2021 from the University of Florida with major in Material Science and Engineering. Her research interests include the targeted drug delivery in developing cancer immunotherapy.

Dr. Mei He is an assistant professor from the Department of Pharmaceutics, College of Pharmacy at the University of Florida. She obtained her PhD degree from the University of Alberta and postdoc training from the University of California, Berkeley. Dr. He is the Editorial Board member of Pharmaceutics and The American Association of Pharmaceutical Scientists (AAPS) Scientific Programming Committee Track Leader, as well as the Advisory Board Member of journal Lab on Chip (LOC). She Received NIH Maximizing Investigator’s Research Award for Early Stage Investigators in 2019. She also received the LOC Emerging Investigator Award in 2019 and LOC Outstanding Reviewer in 2018 and 2020 by the Royal Society of Chemistry. Dr. He’s innovation leads to more than a dozen of patents and translated for commercialization through her startup company Clara Biotech. Dr. He’s research interests include exosome-based precision therapeutics and delivery strategy in precision cancer medicine.

Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Reference
- [1].Mellman I, Coukos G, Dranoff G, Cancer immunotherapy comes of age, Nature 480(7378) (2011) 480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mullard A, FDA approves fourth CAR-T cell therapy, Nat Rev Drug Discov 20(3) (2021) 166. [DOI] [PubMed] [Google Scholar]
- [3].Newick K, O’Brien S, Moon E, Albelda SM, CAR T Cell Therapy for Solid Tumors, Annu Rev Med 68 (2017) 139–152. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Q, Chen Y, Bai X, Liang T, Immune Checkpoint Blockade Therapy for Hepatocellular Carcinoma: Clinical Challenges and Considerations, Front Oncol 10 (2020) 590058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang N, Wei L, Ye M, Kang C, You H, Treatment Progress of Immune Checkpoint Blockade Therapy for Glioblastoma, Front Immunol 11 (2020) 592612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wieder T, Eigentler T, Brenner E, Rocken M, Immune checkpoint blockade therapy, J Allergy Clin Immunol 142(5) (2018) 1403–1414. [DOI] [PubMed] [Google Scholar]
- [7].Arcangeli S, Mestermann K, Weber J, Bonini C, Casucci M, Hudecek M, Overcoming key challenges in cancer immunotherapy with engineered T cells, Curr Opin Oncol 32(5) (2020) 398–407. [DOI] [PubMed] [Google Scholar]
- [8].Zhang R, Billingsley MM, Mitchell MJ, Biomaterials for vaccine-based cancer immunotherapy, J Control Release 292 (2018) 256–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gong N, Sheppard NC, Billingsley MM, June CH, Mitchell MJ, Nanomaterials for T-cell cancer immunotherapy, Nat Nanotechnol 16(1) (2021) 25–36. [DOI] [PubMed] [Google Scholar]
- [10].Riley RS, June CH, Langer R, Mitchell MJ, Delivery technologies for cancer immunotherapy, Nat Rev Drug Discov 18(3) (2019) 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kalluri R, LeBleu VS, The biology, function, and biomedical applications of exosomes, Science 367(6478) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Witwer KW, Soekmadji C, Hill AF, Wauben MH, Buzas EI, Di Vizio D, Falcon-Perez JM, Gardiner C, Hochberg F, Kurochkin IV, Lotvall J, Mathivanan S, Nieuwland R, Sahoo S, Tahara H, Torrecilhas AC, Weaver AM, Yin H, Zheng L, Gho YS, Quesenberry P, Thery C, Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility, J Extracell Vesicles 6(1) (2017) 1396823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mathieu M, Martin-Jaular L, Lavieu G, Thery C, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication, Nat Cell Biol 21(1) (2019) 9–17. [DOI] [PubMed] [Google Scholar]
- [14].Robbins PD, Morelli AE, Regulation of immune responses by extracellular vesicles, Nat Rev Immunol 14(3) (2014) 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhu Q, Heon M, Zhao Z, He M, Microfluidic engineering of exosomes: editing cellular messages for precision therapeutics, Lab Chip 18(12) (2018) 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yong T, Li X, Wei Z, Gan L, Yang X, Extracellular vesicles-based drug delivery systems for cancer immunotherapy, J Control Release 328 (2020) 562–574. [DOI] [PubMed] [Google Scholar]
- [17].Nazimek K, Bryniarski K, Perspectives in Manipulating EVs for Therapeutic Applications: Focus on Cancer Treatment, Int J Mol Sci 21(13) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO, El Andaloussi S, Advances in therapeutic applications of extracellular vesicles, Sci Transl Med 11(492) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang L, Liu J, Engineered drug-loaded cells and cell derivatives as a delivery platform for cancer immunotherapy, Biomater Sci 9(4) (2021) 1104–1116. [DOI] [PubMed] [Google Scholar]
- [20].Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L, Zhou F, Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy, Adv Sci (Weinh) 6(24) (2019) 1901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Witwer KW, Wolfram J, Extracellular vesicles versus synthetic nanoparticles for drug delivery, Nature Reviews Materials 6(2) (2021) 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Admyre C, Johansson SM, Paulie S, Gabrielsson S, Direct exosome stimulation of peripheral human T cells detected by ELISPOT, Eur J Immunol 36(7) (2006) 1772–81. [DOI] [PubMed] [Google Scholar]
- [23].Naslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S, Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity, J Immunol 190(6) (2013) 2712–9. [DOI] [PubMed] [Google Scholar]
- [24].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S, Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat Med 4(5) (1998) 594–600. [DOI] [PubMed] [Google Scholar]
- [25].Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L, Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming, Nat Med 7(3) (2001) 297–303. [DOI] [PubMed] [Google Scholar]
- [26].Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L, Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial, J Transl Med 3(1) (2005) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G, Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer, Mol Ther 16(4) (2008) 782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK, A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, J Transl Med 3(1) (2005) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N, Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology 5(4) (2016) e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nam GH, Choi Y, Kim GB, Kim S, Kim SA, Kim IS, Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy, Adv Mater 32(51) (2020) e2002440. [DOI] [PubMed] [Google Scholar]
- [31].Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S, Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma, Proteomics 13(22) (2013) 3354–64. [DOI] [PubMed] [Google Scholar]
- [32].Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R, Generation and testing of clinical-grade exosomes for pancreatic cancer, JCI Insight 3(8) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG, A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes, Mol Ther 18(9) (2010) 1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Owens DE 3rd, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int J Pharm 307(1) (2006) 93–102. [DOI] [PubMed] [Google Scholar]
- [35].Hoshyar N, Gray S, Han H, Bao G, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, Nanomedicine 11(6) (2016) 673–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Simpson CA, Salleng KJ, Cliffel DE, Feldheim DL, In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles, Nanomedicine 9(2) (2013) 257–63. [DOI] [PubMed] [Google Scholar]
- [37].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat Biotechnol 33(9) (2015) 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS, The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles, Biomaterials 32(13) (2011) 3435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Midekessa G, Godakumara K, Ord J, Viil J, Lattekivi F, Dissanayake K, Kopanchuk S, Rinken A, Andronowska A, Bhattacharjee S, Rinken T, Fazeli A, Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability, ACS Omega 5(27) (2020) 16701–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kibria G, Ramos EK, Lee KE, Bedoyan S, Huang S, Samaeekia R, Athman JJ, Harding CV, Lotvall J, Harris L, Thompson CL, Liu H, A rapid, automated surface protein profiling of single circulating exosomes in human blood, Sci Rep 6 (2016) 36502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW, Proteomic analysis of microvesicles derived from human mesenchymal stem cells, J Proteome Res 11(2) (2012) 839–49. [DOI] [PubMed] [Google Scholar]
- [42].Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A, High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources, J Extracell Vesicles 5 (2016) 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].de Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, Vader P, Schiffelers RM, Drug Delivery with Extracellular Vesicles: From Imagination to Innovation, Acc Chem Res 52(7) (2019) 1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP, Role of CD47 as a marker of self on red blood cells, Science 288(5473) (2000) 2051–4. [DOI] [PubMed] [Google Scholar]
- [45].Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, Zhang Y, Liu Y, Li J, Pu K, Xie HY, Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy, Angew Chem Int Ed Engl 59(5) (2020) 2018–2022. [DOI] [PubMed] [Google Scholar]
- [46].Marar C, Starich B, Wirtz D, Extracellular vesicles in immunomodulation and tumor progression, Nat Immunol (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P, Extracellular vesicles as drug delivery systems: Why and how?, Adv Drug Deliv Rev 159 (2020) 332–343. [DOI] [PubMed] [Google Scholar]
- [48].Witwer KW, Wolfram J, Extracellular vesicles versus synthetic nanoparticles for drug delivery, Nat. Rev. Mater. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sirisinha S, Evolutionary insights into the origin of innate and adaptive immune systems: different shades of grey, Asian Pac J Allergy Immunol 32(1) (2014) 3–15. [PubMed] [Google Scholar]
- [50].Ferrari D, McNamee EN, Idzko M, Gambari R, Eltzschig HK, Purinergic Signaling During Immune Cell Trafficking, Trends Immunol 37(6) (2016) 399–411. [DOI] [PubMed] [Google Scholar]
- [51].Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W, Exosomes and their roles in immune regulation and cancer, Semin Cell Dev Biol 40 (2015) 72–81. [DOI] [PubMed] [Google Scholar]
- [52].Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY, Biological roles and potential applications of immune cell-derived extracellular vesicles, J Extracell Vesicles 6(1) (2017) 1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G, Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells, Cancer Res 65(12) (2005) 5238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A, Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages, J Immunol 180(6) (2008) 4299–307. [DOI] [PubMed] [Google Scholar]
- [55].Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N, Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha, PLoS One 4(3) (2009) e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O, Biological properties of extracellular vesicles and their physiological functions, J Extracell Vesicles 4 (2015) 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E, Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function, PLoS One 3(10) (2008) e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL, Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands, Oncoimmunology 1(7) (2012) 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB, Macrophage microvesicles induce macrophage differentiation and miR-223 transfer, Blood 121(6) (2013) 984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS, Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo, Blood 110(9) (2007) 3234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Lopez E. Aradillas, Alexander GM, Sacan A, Fortina P, Ajit SK, Functional significance of macrophage-derived exosomes in inflammation and pain, Pain 155(8) (2014) 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Truman JP, Al Gadban MM, Smith KJ, Jenkins RW, Mayroo N, Virella G, Lopes-Virella MF, Bielawska A, Hannun YA, Hammad SM, Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release, Immunology 136(1) (2012) 30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity 39(1) (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [64].Chen DS, Mellman I, Elements of cancer immunity and the cancer-immune set point, Nature 541(7637) (2017) 321–330. [DOI] [PubMed] [Google Scholar]
- [65].Whiteside TL, Stimulatory role of exosomes in the context of therapeutic anti-cancer vaccines, Biotarget 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M, MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells, Immunol Lett 89(2-3) (2003) 125–31. [DOI] [PubMed] [Google Scholar]
- [67].Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y, Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL, J Immunol 179(8) (2007) 5024–32. [DOI] [PubMed] [Google Scholar]
- [68].Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N, Dendritic cell-derived exosomes for cancer immunotherapy: what’s next?, Cancer Res 70(4) (2010) 1281–5. [DOI] [PubMed] [Google Scholar]
- [69].Segura E, Amigorena S, Thery C, Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses, Blood Cells Mol Dis 35(2) (2005) 89–93. [DOI] [PubMed] [Google Scholar]
- [70].Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S, Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes, Nat Immunol 3(12) (2002) 1156–62. [DOI] [PubMed] [Google Scholar]
- [71].Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE, Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition, J Immunol 180(5) (2008) 3081–90. [DOI] [PubMed] [Google Scholar]
- [72].Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L, Dendritic cell-derived exosomes for cancer therapy, J Clin Invest 126(4) (2016) 1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, B lymphocytes secrete antigen-presenting vesicles, J Exp Med 183(3) (1996) 1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schorey JS, Cheng Y, Singh PP, Smith VL, Exosomes and other extracellular vesicles in host-pathogen interactions, EMBO Rep 16(1) (2015) 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wahlgren J, Tde L. Karlson, Glader P, Telemo E, Valadi H, Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling, PLoS One 7(11) (2012) e49723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rajagopal C, Harikumar KB, The Origin and Functions of Exosomes in Cancer, Front Oncol 8 (2018) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT, Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes, Cancer Res 70(2) (2010) 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, Wikstrom P, Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion, PLoS One 9(9) (2014) e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mincheva-Nilsson L, Baranov V, Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance, Semin Cancer Biol 28 (2014) 24–30. [DOI] [PubMed] [Google Scholar]
- [80].Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S, Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles, J Exp Med 195(10) (2002) 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, Burdek M, Botti L, Arioli I, Cova A, Mauri G, Vergani E, Bianchi B, Della Mina P, Cantone L, Bollati V, Zaffaroni N, Gianni AM, Colombo MP, Huber V, TNF-Related Apoptosis-Inducing Ligand (TRAIL)-Armed Exosomes Deliver Proapoptotic Signals to Tumor Site, Clin Cancer Res 22(14) (2016) 3499–512. [DOI] [PubMed] [Google Scholar]
- [82].Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L, Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape, Gastroenterology 128(7) (2005) 1796–804. [DOI] [PubMed] [Google Scholar]
- [83].Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W, Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response, Nature 560(7718) (2018) 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, Pan J, Qi C, Tumor exosomes block dendritic cells maturation to decrease the T cell immune response, Immunol Lett 199 (2018) 36–43. [DOI] [PubMed] [Google Scholar]
- [85].Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R, Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory, Cell 177(2) (2019) 414–427 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL, Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes, J Immunol 183(6) (2009) 3720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z, Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes, J Extracell Vesicles 6(1) (2017) 1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG, Tumor exosomes inhibit differentiation of bone marrow dendritic cells, J Immunol 178(11) (2007) 6867–75. [DOI] [PubMed] [Google Scholar]
- [89].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F, Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells, J Clin Invest 120(2) (2010) 457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X, Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype, Cancer Lett 435 (2018) 80–91. [DOI] [PubMed] [Google Scholar]
- [91].Yang X, Meng S, Jiang H, Zhu C, Wu W, Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats, J Surg Res 171(2) (2011) 826–32. [DOI] [PubMed] [Google Scholar]
- [92].Li X, Li JJ, Yang JY, Wang DS, Zhao W, Song WJ, Li WM, Wang JF, Han W, Zhang ZC, Yu Y, Cao DY, Dou KF, Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model, PLoS One 7(8) (2012) e44045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xie Y, Zhang X, Zhao T, Li W, Xiang J, Natural CD8(+)25(+) regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma, Biochem Biophys Res Commun 438(1) (2013) 152–5. [DOI] [PubMed] [Google Scholar]
- [94].Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, Lombardi G, CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function, Eur J Immunol 43(9) (2013) 2430–40. [DOI] [PubMed] [Google Scholar]
- [95].Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, Xu H, Wang S, Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9, Adv Sci (Weinh) 6(18) (2019) 1901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rashid MH, Borin TF, Ara R, Piranlioglu R, Achyut BR, Korkaya H, Liu Y, Arbab AS, Critical immunosuppressive effect of MDSCderived exosomes in the tumor microenvironment, Oncol Rep 45(3) (2021) 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y, Wang X, Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer, Cancer Immunol Res 6(12) (2018) 1578–1592. [DOI] [PubMed] [Google Scholar]
- [98].Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G, M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer, Cancer Res 79(1) (2019) 146–158. [DOI] [PubMed] [Google Scholar]
- [99].Yang F, Wang T, Du P, Fan H, Dong X, Guo H, M2 bone marrow-derived macrophage-derived exosomes shuffle microRNA-21 to accelerate immune escape of glioma by modulating PEG3, Cancer Cell Int 20 (2020) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhang H, Xie Y, Li W, Chibbar R, Xiong S, Xiang J, CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity, Cell Mol Immunol 8(1) (2011) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J, Activated T cell exosomes promote tumor invasion via Fas signaling pathway, J Immunol 188(12) (2012) 5954–61. [DOI] [PubMed] [Google Scholar]
- [102].Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar R, Qureshi M, Xiang J, Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity, J Immunol 185(9) (2010) 5268–78. [DOI] [PubMed] [Google Scholar]
- [103].Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C, Rooj AK, Krasemann S, Carter BS, Chen CC, Steed T, Treiber J, Rodig S, Yang K, Nakano I, Lee H, Weissleder R, Breakefield XO, Godlewski J, Westphal M, Lamszus K, Freeman GJ, Bronisz A, Lawler SE, Chiocca EA, Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles, Sci Adv 4(3) (2018) eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li XQ, Liu JT, Fan LL, Liu Y, Cheng L, Wang F, Yu HQ, Gao J, Wei W, Wang H, Sun GP, Exosomes derived from gefitinib-treated EGFR-mutant lung cancer cells alter cisplatin sensitivity via up-regulating autophagy, Oncotarget 7(17) (2016) 24585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Doudna JA, Charpentier E, Genome editing. The new frontier of genome engineering with CRISPR-Cas9, Science 346(6213) (2014) 1258096. [DOI] [PubMed] [Google Scholar]
- [106].Tkach M, Kowal J, Zucchetti AE, Enserink L, Jouve M, Lankar D, Saitakis M, Martin-Jaular L, Thery C, Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes, EMBO J 36(20) (2017) 3012–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cheng L, Wang Y, Huang L, Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node, Mol Ther 25(7) (2017) 1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Li Z, Suo B, Long G, Gao Y, Song J, Zhang M, Feng B, Shang C, Wang D, Exosomal miRNA-16-5p Derived From M1 Macrophages Enhances T Cell-Dependent Immune Response by Regulating PD-L1 in Gastric Cancer, Front Cell Dev Biol 8 (2020) 572689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Garofalo M, Villa A, Rizzi N, Kuryk L, Rinner B, Cerullo V, Yliperttula M, Mazzaferro V, Ciana P, Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice, J Control Release 294 (2019) 165–175. [DOI] [PubMed] [Google Scholar]
- [110].Yang Y, Hong Y, Cho E, Kim GB, Kim IS, Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery, J Extracell Vesicles 7(1) (2018) 1440131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature 527(7578) (2015) 329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rana S, Yue S, Stadel D, Zoller M, Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection, Int J Biochem Cell Biol 44(9) (2012) 1574–84. [DOI] [PubMed] [Google Scholar]
- [113].Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, Chatellard C, Sadoul R, Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons, Cell Mol Life Sci 75(4) (2018) 757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S, Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles, J Immunol 166(12) (2001) 7309–18. [DOI] [PubMed] [Google Scholar]
- [115].Wiley RD, Gummuluru S, Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection, Proc Natl Acad Sci U S A 103(3) (2006) 738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE, Donor dendritic cell-derived exosomes promote allograft-targeting immune response, J Clin Invest 126(8) (2016) 2805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Segura E, Guerin C, Hogg N, Amigorena S, Thery C, CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo, J Immunol 179(3) (2007) 1489–96. [DOI] [PubMed] [Google Scholar]
- [118].Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC, Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma, Theranostics 7(10) (2017) 2732–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tang XJ, Sun XY, Huang KM, Zhang L, Yang ZS, Zou DD, Wang B, Warnock GL, Dai LJ, Luo J, Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy, Oncotarget 6(42) (2015) 44179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L, Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells, J Immunol 172(4) (2004) 2126–36. [DOI] [PubMed] [Google Scholar]
- [121].Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L, Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection, J Immunol 172(4) (2004) 2137–46. [DOI] [PubMed] [Google Scholar]
- [122].Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J, Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity, Immunology 120(1) (2007) 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jeze G, Lemonnier F, Zitvogel L, Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines, J Immunol 176(5) (2006) 2722–9. [DOI] [PubMed] [Google Scholar]
- [124].Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G, A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials 35(7) (2014) 2383–90. [DOI] [PubMed] [Google Scholar]
- [125].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nat Biotechnol 29(4) (2011) 341–5. [DOI] [PubMed] [Google Scholar]
- [126].Ruiss R, Jochum S, Mocikat R, Hammerschmidt W, Zeidler R, EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells--a new option for the treatment of B-CLL, PLoS One 6(10) (2011) e25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P, Cirrincione G, Giavaresi G, Monteleone F, Fontana S, De Leo G, Alessandro R, Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth, Theranostics 7(5) (2017) 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, Khine S, Le Pecq JB, Exosome Display technology: applications to the development of new diagnostics and therapeutics, Blood Cells Mol Dis 35(2) (2005) 158–68. [DOI] [PubMed] [Google Scholar]
- [129].Wagner AK, Gehrmann U, Hiltbrunner S, Carannante V, Luu TT, Naslund TI, Brauner H, Kadri N, Karre K, Gabrielsson S, Soluble and Exosome-Bound alpha-Galactosylceramide Mediate Preferential Proliferation of Educated NK Cells with Increased Anti-Tumor Capacity, Cancers (Basel) 13(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C, Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses, Cancer Res 68(4) (2008) 1228–35. [DOI] [PubMed] [Google Scholar]
- [131].Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY, Osada T, Hobeika A, Delcayre A, Le Pecq JB, Morse MA, Clay TM, Lyerly HK, Increasing vaccine potency through exosome antigen targeting, Vaccine 29(50) (2011) 9361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A, Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy, Cancer Res 71(15) (2011) 5235–44. [DOI] [PubMed] [Google Scholar]
- [133].Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, Chu Y, Sun T, Jiang C, Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment, Biomaterials 268 (2021) 120546. [DOI] [PubMed] [Google Scholar]
- [134].Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, Miller G, Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance, Nat Med 23(5) (2017) 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD, Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4, J Immunol 179(4) (2007) 2242–9. [DOI] [PubMed] [Google Scholar]
- [136].Antimisiaris SG, Mourtas S, Marazioti A, Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery, Pharmaceutics 10(4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Mentkowski KI, Snitzer JD, Rusnak S, Lang JK, Therapeutic Potential of Engineered Extracellular Vesicles, AAPS J 20(3) (2018) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC, Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology, Cancer Res 73(8) (2013) 2412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Danhier F, To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?, J Control Release 244(Pt A) (2016) 108–121. [DOI] [PubMed] [Google Scholar]
- [140].Fang J, Nakamura H, Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Adv Drug Deliv Rev 63(3) (2011) 136–51. [DOI] [PubMed] [Google Scholar]
- [141].Chandrasekaran S, King MR, Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy, Int J Mol Sci 15(11) (2014) 20209–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sainte-Marie G, The lymph node revisited: development, morphology, functioning, and role in triggering primary immune responses, Anat Rec (Hoboken) 293(2) (2010) 320–37. [DOI] [PubMed] [Google Scholar]
- [143].Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L, The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node, Immunity 22(1) (2005) 19–29. [DOI] [PubMed] [Google Scholar]
- [144].Schudel A, Francis DM, Thomas SN, Material design for lymph node drug delivery, Nat Rev Mater 4(6) (2019) 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Porter CJH, Trevaskis NL, Targeting immune cells within lymph nodes, Nat Nanotechnol 15(6) (2020) 423–425. [DOI] [PubMed] [Google Scholar]
- [146].Chiang CL, Coukos G, Kandalaft LE, Whole Tumor Antigen Vaccines: Where Are We?, Vaccines (Basel) 3(2) (2015) 344–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wei X, Chen F, Xin K, Wang Q, Yu L, Liu B, Liu Q, Cancer-Testis Antigen Peptide Vaccine for Cancer Immunotherapy: Progress and Prospects, Transl Oncol 12(5) (2019) 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lopes A, Vandermeulen G, Preat V, Cancer DNA vaccines: current preclinical and clinical developments and future perspectives, J Exp Clin Cancer Res 38(1) (2019) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].McNamara MA, Nair SK, Holl EK, RNA-Based Vaccines in Cancer Immunotherapy, J Immunol Res 2015 (2015) 794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Dubensky TW Jr., Reed SG, Adjuvants for cancer vaccines, Semin Immunol 22(3) (2010) 155–61. [DOI] [PubMed] [Google Scholar]
- [151].Goldman B, DeFrancesco L, The cancer vaccine roller coaster, Nat Biotechnol 27(2) (2009) 129–39. [DOI] [PubMed] [Google Scholar]
- [152].Tran T, Blanc C, Granier C, Saldmann A, Tanchot C, Tartour E, Therapeutic cancer vaccine: building the future from lessons of the past, Semin Immunopathol 41(1) (2019) 69–85. [DOI] [PubMed] [Google Scholar]
- [153].Bachmann MF, Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns, Nat Rev Immunol 10(11) (2010) 787–96. [DOI] [PubMed] [Google Scholar]
- [154].Liu Z, Ju XJ, Wang W, Xie R, Jiang L, Chen Q, Zhang YQ, Wu JF, Chu LY, Stimuli-Responsive Capsule Membranes for Controlled Release in Pharmaceutical Applications, Curr Pharm Des 23(2) (2017) 295–301. [DOI] [PubMed] [Google Scholar]
- [155].Strioga MM, Felzmann T, Powell DJ Jr., Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J, Schijns VE, Therapeutic dendritic cell-based cancer vaccines: the state of the art, Crit Rev Immunol 33(6) (2013) 489–547. [DOI] [PubMed] [Google Scholar]
- [156].Gilboa E, DC-based cancer vaccines J Clin Invest 117(5) (2007) 1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sahin U, Tureci O, Personalized vaccines for cancer immunotherapy, Science 359(6382) (2018) 1355–1360. [DOI] [PubMed] [Google Scholar]
- [158].Handy CE, Antonarakis ES, Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions, Future Oncol 14(10) (2018) 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, I.S. Investigators, Sipuleucel-T immunotherapy for castration-resistant prostate cancer, N Engl J Med 363(5) (2010) 411–22. [DOI] [PubMed] [Google Scholar]
- [160].Graff JN, Chamberlain ED, Sipuleucel-T in the treatment of prostate cancer: an evidence-based review of its place in therapy, Core Evid 10 (2015) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chow KV, Sutherland RM, Zhan Y, Lew AM, Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells, Immunol Cell Biol 95(3) (2017) 244–251. [DOI] [PubMed] [Google Scholar]
- [162].Jiang L, Vader P, Schiffelers RM, Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy, Gene Ther 24(3) (2017) 157–166. [DOI] [PubMed] [Google Scholar]
- [163].Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J, Extracellular vesicle-based drug delivery systems for cancer treatment, Theranostics 9(26) (2019) 8001–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].LeBleu VS, Kalluri R, Exosomes as a Multicomponent Biomarker Platform in Cancer, Trends Cancer 6(9) (2020) 767–774. [DOI] [PubMed] [Google Scholar]
- [165].Wan Z, Dong Y, Wei M, Gao X, Yang G, Zhang J, Liu L, Exosomes in Tumor Immunotherapy: Mediator, Drug Carrier, and Prognostic Biomarker, Adv Biosyst 4(11) (2020) e2000061. [DOI] [PubMed] [Google Scholar]
- [166].Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L, Tan W, Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy, Anal Chem 91(3) (2019) 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y, Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity, J Am Chem Soc 140(48) (2018) 16413–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].de Jong OG, Murphy DE, Mager I, Willms E, Garcia-Guerra A, Gitz-Francois JJ, Lefferts J, Gupta D, Steenbeek SC, van Rheenen J, El Andaloussi S, Schiffelers RM, Wood MJA, Vader P, A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA, Nat Commun 11(1) (2020) 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A, Requirement of bic/microRNA-155 for normal immune function, Science 316(5824) (2007) 608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G, MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis, Nat Immunol 10(12) (2009) 1252–9. [DOI] [PubMed] [Google Scholar]
- [171].Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF, Williams BR, A miR-19 regulon that controls NF-kappaB signaling, Nucleic Acids Res 40(16) (2012) 8048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wei J, Wang F, Kong LY, Xu S, Doucette T, Ferguson SD, Yang Y, McEnery K, Jethwa K, Gjyshi O, Qiao W, Levine NB, Lang FF, Rao G, Fuller GN, Calin GA, Heimberger AB, miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma, Cancer Res 73(13) (2013) 3913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Dews M, Fox JL, Hultine S, Sundaram P, Wang W, Liu YY, Furth E, Enders GH, El-Deiry W, Schelter JM, Cleary MA, Thomas-Tikhonenko A, The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors, Cancer Res 70(20) (2010) 8233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Xu Z, Chokkalingam N, Tello-Ruiz E, Wise MC, Bah MA, Walker S, Tursi NJ, Fisher PD, Schultheis K, Broderick KE, Humeau L, Kulp DW, Weiner DB, A DNA-Launched Nanoparticle Vaccine Elicits CD8(+) T-cell Immunity to Promote In Vivo Tumor Control, Cancer Immunol Res 8(11) (2020) 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Matsumoto A, Takahashi Y, Ariizumi R, Nishikawa M, Takakura Y, Development of DNA-anchored assembly of small extracellular vesicle for efficient antigen delivery to antigen presenting cells, Biomaterials 225 (2019) 119518. [DOI] [PubMed] [Google Scholar]
- [176].Zhuang J, Tan J, Wu C, Zhang J, Liu T, Fan C, Li J, Zhang Y, Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy, Nucleic Acids Res 48(16) (2020) 8870–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Xue VW, Wong SCC, Song G, Cho WCS, Promising RNA-based cancer gene therapy using extracellular vesicles for drug delivery, Expert Opin Biol Ther 20(7) (2020) 767–777. [DOI] [PubMed] [Google Scholar]
- [178].Ruffell B, Coussens LM, Macrophages and therapeutic resistance in cancer, Cancer Cell 27(4) (2015) 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P, Tumour-associated macrophages as treatment targets in oncology, Nat Rev Clin Oncol 14(7) (2017) 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD, CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells, Matrix Biol 37 (2014) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J, Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer, Adv Sci (Weinh) 7(18) (2020) 2000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, Epstein AL, Lenz HJ, Zhang Y, Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy, Mol Ther 28(2) (2020) 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].S ELA, Mager I, Breakefield XO, Wood MJ, Extracellular vesicles: biology and emerging therapeutic opportunities, Nat Rev Drug Discov 12(5) (2013) 347–57. [DOI] [PubMed] [Google Scholar]
- [184].Rosenberg SA, Restifo NP, Adoptive cell transfer as personalized immunotherapy for human cancer, Science 348(6230) (2015) 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Fesnak AD, June CH, Levine BL, Engineered T cells: the promise and challenges of cancer immunotherapy, Nat Rev Cancer 16(9) (2016) 566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC, CAR T cell immunotherapy for human cancer, Science 359(6382) (2018) 1361–1365. [DOI] [PubMed] [Google Scholar]
- [187].Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Pender BS, Hawkins RM, Vakil A, Steinmetz RN, Riddell SR, Maloney DG, Turtle CJ, High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy, Blood 134(7) (2019) 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ, Chimeric antigen receptor T-cell therapy - assessment and management of toxicities, Nat Rev Clin Oncol 15(1) (2018) 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, Ding M, Pan M, Ye X, Yang Y, Hu S, CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity, Nat Commun 10(1) (2019) 4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Silva-Santos B, Mensurado S, Coffelt SB, gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer, Nat Rev Cancer 19(7) (2019) 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Adams EJ, Gu S, Luoma AM, Human gamma delta T cells: Evolution and ligand recognition, Cell Immunol 296(1) (2015) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, Hu H, Lam KT, Chan GC, Yang Y, Chen H, Tsao GS, Bonneville M, Lau YL, Tu W, Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease, Cancer Cell 26(4) (2014) 565–76. [DOI] [PubMed] [Google Scholar]
- [193].Wang X, Xiang Z, Liu Y, Huang C, Pei Y, Wang X, Zhi H, Wong WH, Wei H, Ng IO, Lee PP, Chan GC, Lau YL, Tu W, Exosomes derived from Vdelta2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity, Sci Transl Med 12(563) (2020). [DOI] [PubMed] [Google Scholar]
- [194].Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL, Current concepts in the diagnosis and management of cytokine release syndrome, Blood 124(2) (2014) 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Brudno JN, Kochenderfer JN, Toxicities of chimeric antigen receptor T cells: recognition and management, Blood 127(26) (2016) 3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Syn NL, Wang L, Chow EK, Lim CT, Goh BC, Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges, Trends Biotechnol 35(7) (2017) 665–676. [DOI] [PubMed] [Google Scholar]
- [197].Sverdlov ED, Amedeo Avogadro’s cry: what is 1 microg of exosomes?, Bioessays 34(10) (2012) 873–5. [DOI] [PubMed] [Google Scholar]
- [198].Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D, Thomsen AR, Walter N, Khanduri R, Sanchez JC, Keller A, Oliveira C, Nazarenko I, 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles, Adv Sci (Weinh) 6(4) (2019) 1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Abdollahi S, Extracellular vesicles from organoids and 3D culture systems, Biotechnol Bioeng 118(3) (2021) 1029–1049. [DOI] [PubMed] [Google Scholar]
- [200].Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D, Thomsen AR, Walter N, Khanduri R, Sanchez J-C, Keller A, Oliveira C, Nazarenko I, 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles, Advanced Science 6(4) (2019) 1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Gao W, Liang T, He R, Ren J, Yao H, Wang K, Zhu L, Xu Y, Exosomes from 3D culture of marrow stem cells enhances endothelial cell proliferation, migration, and angiogenesis via activation of the HMGB1/AKT pathway, Stem Cell Res 50 (2020) 102122. [DOI] [PubMed] [Google Scholar]
- [202].Thippabhotla S, Zhong C, He M, 3D cell culture stimulates the secretion of in vivo like extracellular vesicles, Scientific Reports 9(1) (2019) 13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Kim H, Lee MJ, Bae E-H, Ryu JS, Kaur G, Kim HJ, Kim JY, Barreda H, Jung SY, Choi JM, Shigemoto-Kuroda T, Oh JY, Lee RH, Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation, Molecular Therapy 28(7) (2020) 1628–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, Kim JY, Barreda H, Jung SY, Choi JM, Shigemoto-Kuroda T, Oh JY, Lee RH, Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation, Mol Ther 28(7) (2020) 1628–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, von Strandmann E. Pogge, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J Extracell Vesicles 7(1) (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].He S.T. Nan, Zhong Cuncong, Greenberg Zachary, Xu Liang, Pessetto Ziyan, Godwin Andrew K., Zeng Yong, He Mei, Nano Pom-poms Prepared Highly Specific Extracellular Vesicles Expand the Detectable Cancer Biomarkers, BioRxiv (2021). [Google Scholar]
- [207].He M, Crow J, Roth M, Zeng Y, Godwin AK, Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology, Lab Chip 14(19) (2014) 3773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ, Isolation of exosomes from whole blood by integrating acoustics and microfluidics, Proc Natl Acad Sci U S A 114(40) (2017) 10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Liga A, Vliegenthart AD, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M, Exosome isolation: a microfluidic road-map, Lab Chip 15(11) (2015) 2388–94. [DOI] [PubMed] [Google Scholar]
- [210].Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB, Production and characterization of clinical grade exosomes derived from dendritic cells, J Immunol Methods 270(2) (2002) 211–26. [DOI] [PubMed] [Google Scholar]
- [211].Pachler K, Lener T, Streif D, Dunai ZA, Desgeorges A, Feichtner M, Oller M, Schallmoser K, Rohde E, Gimona M, A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles, Cytotherapy 19(4) (2017) 458–472. [DOI] [PubMed] [Google Scholar]
- [212].Andriolo G, Provasi E, Lo Cicero V, Brambilla A, Soncin S, Torre T, Milano G, Biemmi V, Vassalli G, Turchetto L, Barile L, Radrizzani M, Exosomes From Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method, Front Physiol 9 (2018) 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]


