Abstract
The quantitative real-time polymerase chain reaction (qRT-PCR) is the most sensitive and commonly used technique for gene expression studies in biological systems. However, the reliability of qRT-PCR results depends on the selection of reference gene(s) for data normalization. Horse gram (Macrotyloma uniflorum) is an important legume crop on which several molecular studies have been reported. However, the stability of reference genes has not been evaluated. In the present study, nine candidate reference genes were identified from horse gram RNA-seq data and evaluated in two horse gram genotypes, HPK4 and HPKM317 under six abiotic stresses viz. cold, drought, salinity, heat, abscisic acid and methyl viologen-induced oxidative stress. The results were evaluated using geNorm, Bestkeeper, Normfinder and delta-delta Ct methods and comprehensive ranking was assigned using RefFinder and RankAggreg software. The overall result showed that TCTP was one of the most stable genes in all samples and in genotype HPK4, while in HPKM317 profilin was most stably expressed. However, PSMA5 was identified as least stable in all the experimental conditions. Expression of target genes dehydrin and early response to dehydration 6 under drought stress was also validated using TCTP and profilin for data normalization, either alone or in combination, which confirmed their suitability for qRT-PCR data normalization. Thus, TCTP and profilin genes may be used for qRT-PCR data normalization for molecular and genomic studies in horse gram.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01104-0.
Keywords: Bestkeeper, Delta-delta CT, geNorm, Housekeeping gene, Macrotyloma uniflorum, Normfinder, Rank aggregation, RefFinder
Introduction
In biological systems, gene expression analysis provides valuable insights into the complex mechanisms regulating growth and developmental and response to biotic and abiotic stresses. Quantitative real-time polymerase chain reaction (qRT-PCR) is one of the most powerful, quantitative methods for analyzing gene expression. It is often used to validate global gene expression data produced by RNA-seq experiments. Considering the sensitivity and specificity of the qRT-PCR technique, MIQE (Minimum information for Publication of Quantitative Real Time PCR Experiment) guidelines have been framed to ensure accuracy and reproducibility of the data (Udvardi et al. 2008; Bustin et al. 2009). According to these guidelines, reliable quantification of gene expression by qRT-PCR requires the standardization and fine tuning of many factors like amount and quality of initial RNA samples, reverse transcription efficiency, cDNA quantity and quality and transcriptional activity of the tissues or cells analyzed (Huggett et al. 2005; Nolan et al. 2006; Derveaux et al. 2010). One of the mandates to ensure accurate analysis is data normalization using suitable reference gene (s) with stable expression under given experimental conditions and tissue types (Brunner et al. 2004). In general, housekeeping genes (HKG) like 18S rRNA, actin, tubulin, EF1α, GAPDH (glyceraldehyde 3 phosphate dehydrogenase), polyubiquitin etc., are used as reference genes (Czechowski et al. 2005; Gutierrez et al. 2008). However, many reports have demonstrated that the commonly used HKGs are not always reliable for the normalization of qRT-PCR data, since their expression levels may vary in different cells, tissues, organs and other experimental conditions. Therefore, it is essential to identify the most stable reference gene(s) in each species, sample type and experimental condition. For example, in soybean, nearly eleven research groups have assessed expression stability of HKGs under different experimental conditions (Le et al. 2012; Ma et al. 2013; Li et al. 2012; Libault et al. 2008; Hu et al. 2009; Bansal et al. 2015; Gao et al. 2017; Nakayama et al. 2014; Kulcheski et al. 2010; Jian et al. 2008). Similarly, in pigeon pea, Sinha et al. (2015a, b) have identified UBC, GAPDH and IF4α as most stably expressed HKGs under heat, salt and drought stresses. Thus, it is always advisable to standardize the suitable reference gene for each experimental sample and condition before performing qRT-PCR based gene expression study.
Horse gram is an underutilized legume crop of tropics and subtropics, which is cultivated mainly for nutritious seeds, green leafy vegetables, sprouts, feed and fodder (Chahota et al. 2013). Horse gram produces seeds rich in vitamins and minerals and it can be cultivated in areas with very low rainfall (Chahota et al. 2013). Considering its medicinal properties and potential to thrive under dryland conditions, the US National Academy of Sciences has identified horse gram as a potential food crop for the future (National Research Council 1979). Despite being a valuable crop, the genomic information of horse gram is scarce. However, some studies have reported identification of horse gram genes involved in stress tolerance and their deployment using transgenic technology. For e.g., Drought-responsive genes were identified through transcriptome analysis of drought tolerant (M-249) and drought sensitive (M-191) genotypes (Bhardwaj et al. 2013a). The overexpression of horse gram NAC genes (Pandurangaiah et al. 2013, 2014) and HSP gene (Masand and Yadav 2016) imparted stress tolerance in plants and bacteria. Despite being an important legume crop, no study has been performed to identify the stably expressed reference gene(s), which may be used for qRT-PCR data normalization in horse gram.
Recently, RNA-seq data have been used for the selection and validation of stable reference genes in many plant species (Yim et al. 2015; Gong et al. 2016; Pombo et al. 2017; Zhou et al. 2017). An advantage of this method is that it allows the identification of a number of stably expressed genes across the samples, rather than only traditional HKGs. In the present study, genes having minimal expression variance across the RNA-seq data of horse gram (Bhardwaj et al. 2013a) were identified on the basis of coefficient of variance (CV). Nine potential reference genes viz., aquaporin (PIP 2), profilin, proteasome (PSMA5), 26S protease regulatory subunit (26S), translation initiation factor (IF5A4), RNA polymerase (RNAp), small nuclear ribo-nucleoprotein G (SnRNPG), ADP ribosylation factor (ADPRF2) and translationally controlled tumour protein (TCTP) were identified. Their expression stability was evaluated in two horse gram genotypes, HPK 4 and HPKM 317 under various abiotic stress conditions viz. salinity (NaCl, 200 mM), drought (by withholding irrigation), cold (4 °C), heat (42 °C), abscisic acid (ABA, 100 mM) and methyl viologen (MV, 5 mM), for different regimes like 1 h, 6 h and 24 h of treatment, except for drought stress which was imposed for 24 h and 48 h and heat stress where samples were collected after 1 h and 6 h of stress and 24 h of recovery. All the data were evaluated by statistical software viz., geNorm, NormFinder, BestKeeper and delta-delta Ct method. A comprehensive ranking was also performed using RefFinder and Rank Aggregation methods. Finally, the selected reference genes were validated through target gene expression analysis that was compared between most stable and unstable reference genes.
Materials and methods
Plant growth conditions and stress treatments
The seeds of horse gram genotypes HPK 4 and HPKM 317 were obtained from C.S.K. Himachal Pradesh Agricultural University, Palampur, India. The seeds were surface sterilized with 1% sodium hypochlorite (Sigma Aldrich) for 5 min., followed by thorough rinsing with sterile distilled water. The seeds were inoculated in earthen pots containing soil: sand mixture (3:1). The plants were grown for nearly 3 weeks after which they were subjected to various stress conditions as reported earlier (Sinha et al. 2019). Briefly, salinity (NaCl, 200 mM), drought (water withheld), cold (4 °C), heat (42 °C), abscisic acid (ABA, 100 mM) and methyl viologen (MV, 5 mM) were applied in independent pots in three biological replicates. For all the stresses, leaf samples were harvested after 1 h, 6 h and 24 h of treatment, except for drought stress, where the leaf samples were harvested after 24 h and 48 h after withholding water and for heat stress where leaf samples were harvested after 1 h and 6 h of stress and 24 h of recovery. Thus, in total thirty-six samples including all the stress treatments and control samples for both the genotypes were harvested. The samples were stored in liquid nitrogen till further processing.
Total RNA extraction and first strand cDNA synthesis
The RNA extraction and first strand cDNA synthesis were performed as reported earlier (Sinha et al. 2019). Briefly, 100 mg of leaf samples were frozen in liquid nitrogen and RNA was extracted using IRIS solution (Ghawana et al. 2011). The RNA samples were quantified using Biospectrometer (Eppendorf) and the quality of RNA was assessed by formaldehyde gel electrophoresis (2.2 M, 1.5%). Prior to cDNA synthesis, 1 µg of RNA was treated with DNase I (Thermo Scientific) to remove genomic DNA contamination. First strand cDNA synthesis was performed using DNase treated RNA in 20 µl reaction volume using verso cDNA synthesis kit (Thermo Scientific). Each cDNA sample was further diluted ten times in autoclaved Type I milli Q water (Millipore).
Selection of potential reference genes and designing of primers
The drought-responsive transcriptome database of two horse gram genotypes with contrasting drought tolerance (Bhardwaj et al. 2013a) was used to identify candidate reference genes. From this database, we selected genes that exhibited minimal expression variance across the libraries representing a coefficient of variation (CV) lower than 5%. Initially, thirteen potential reference genes, namely 60S RPL (60 S ribosomal protein L), 14–3-3, epsilon 1, ubiquitin, PIP 2, profilin, PSMA5, 26S, IF5A4, RNAp, SnRNPG, ADPRF2 and TCTP were selected. The selected contig sequences were searched through BLASTn against viridiplantae and homologous sequences were aligned with CDS of matching sequences. The aligned contig sequences were then used for primer designing using Primer Express Software 3.0.1 (Applied Biosystems), using default parameter (except amplicon size modified to 80–120 bp). Out of the resulting primer pair, the one with the least penalty was selected. The primer specificity was screened by PCR amplification of target genes from horse gram cDNA.
qRT-PCR analysis and determination of PCR efficiencies
The real-time PCR analyses of the genes in given thirty-six samples were performed using StepOne Plus Real-Time PCR system (Applied Biosystems) with standard cycling conditions: initial denaturation—95 °C, 10 min; (denaturation—95 °C, 15 s, annealing and extension—60 °C, 60 s) * 40 cycles; followed by melt curve analysis (melting temperature from 60 to 95 oC) for determining specificity of amplification. Each reaction was performed in 10 µl reaction mixtures containing 250 ng of cDNA and DyNAmo Flash SYBR Green qPCR mix (Thermo Scientific), and 200 nM of each primer. The standard curve was prepared for each primer pair by qRT-PCR using serially diluted cDNA samples. The PCR efficiency was determined by the formula E = (10–1/slope − 1)* 100%. The Ct values of the genes were determined using three independent biological replicates with three technical replicates in each case.
Analysis of reference gene expression stability
The Ct values for all the genes under all experimental conditions were evaluated using statistical algorithms like delta-delta Ct method. It uses raw Ct values to evaluate stable expression of one gene with respect to other genes (Silver et al. 2006). It compares the relative expression (△Ct) of pair of genes within each sample (Silver et al. 2006; Schmitten et al. 2008) Delta Ct depends on CV value of independent genes, thus presenting a very preliminary data BestKeeper analysis determines the suitable reference gene according to the coefficient of correlation between candidate genes. It uses the raw Ct value to determine the coefficient of variation (CV) and standard deviation (SD) (CV ± SD). The gene with the lowest CV and SD values has highest expression stability. (Pfaffl et al. 2014; https://www.gene-quantification.de/bestkeeper.html) It determines the optimal reference genes by employing pairwise correlation analysis of all the pairs of tested genes geNorm analysis performs an average pairwise variation of each gene compared with other genes and determines gene expression stability (M) value. The recommended threshold level for M value is 1.5, genes with M value less than 1.5 are stably expressed and the gene with the lowest M value is considered as the most stably expressed (Vandesompele et al. 2002; https://genorm.cmgg.be/), it provides ideal reference genes with identical expression ratios irrespective of the experimental conditions. Normfinder transforms raw Ct values by 2−△Ct, where lowest Ct sample is used as calibrator (△Ct = each corresponding Ct value—minimum Ct value). It generates average expression stability value (S-value) and calculates the most reliable gene based on intra and intergroup variations of a sample set. The gene with the lowest S value is considered as the stable (Andersen et al. 2004; https://moma.dk/normfinder-software). Although, NormFinder is considered more robust than other software, it has limitations while working with small sample sizes. Thus, it is also liable that different software yields different results. The consensus ranking of the genes was performed using Reffinder integrates four commonly used analytical programs like geNorm, NormFinder, BestKeeper and delta Ct method, to generate comprehensive ranking of the stable expression of genes (Xie et al. 2012; http://leonxie.esy.es/RefFinder/). Another comprehensive approach used for ranking the genes is RankAggreg, which is based on R package for weighted rank aggregation (Pihur et al. 2009). It uses the data of four individual algorithms: delta Ct, BestKeeper, geNorm and NormFinder. On the basis of individual rank attribution, it performs a consensus ranking of the genes.
Validation of identified stable reference genes
The genes that were sorted by the above-mentioned statistical software as most stable and unstable genes were further validated using target gene expression analysis. In the present study △△Ct method was adopted to analyse the expression of known drought stress inducible genes, dehydrin and ERD6 (early response to dehydration 6) genes. These genes were identified from horse gram transcriptome database (Bhardwaj et al. 2013a) as mentioned in above sections and primers were designed using primer express software 3.0.1 (Applied Biosystems). These genes were induced in response to drought stress in plants (Brini et al. 2007; Kiyosue et al. 1998) and hence in the present study, these genes were analyzed and compared on drought stressed horse gram samples, only. The expression was compared using most stable genes either alone or in combination and least stable expressed genes. The differential gene expression of target genes in drought stress samples was compared to their control counterparts with respect to different reference genes using Relative Expression Software Tool (REST) (Pfaffl et al. 2004).
Results
Determination of primer specificity and PCR efficiency
The drought-responsive transcriptome database of two horse gram genotypes with contrasting drought tolerance (Bhardwaj et al. 2013a) was used to identify candidate reference genes. From this database, genes exhibiting minimal expression variance representing coefficient of variation (CV) lower than 5% across the libraries were selected. Surprisingly, the RNA-seq data had some common HKGs like actin, tubulin α, tubulin β, 60S ribosomal protein, 40S ribosomal protein, elongation factor, glyceraldehyde 3 phosphate dehydrogenase, but their CV values were very high and hence they were not selected. On contrary, thirteen genes selected on the basis of CV (less than 5%) were, 60S RPL (60 S ribosomal protein L), 14–3-3, epsilon 1, ubiquitin, aquaporin (PIP 2), profilin, proteasome (PSMA5), 26 S protease regulatory subunit (26S), translation initiation factor (IF5A4), RNA polymerase (RNAp), small nuclear ribo-nucleoprotein G (SnRNPG), ADP ribosylation factor (ADPRF2) and translationally control tumour protein (TCTP). As mentioned in the above section thirteen candidate reference genes exhibiting stable expression in RNA-seq data were selected for determining their stable expression in varied experimental conditions (Online Resource 1). The primer pairs were initially screened for specific amplifications by RT-PCR and followed by qRT-PCR melt curve analysis using horse gram cDNA. The appearance of a single band of expected amplicon size in gel electrophoresis and single major curve in melt curve analysis indicated specific amplification (Online Resource 2, 3). Out of thirteen selected genes, specific amplification was obtained for only nine genes, namely PIP 2, profilin, PSMA5, 26S, IF5A4, RNAp, SnRNPG, ADPRF2 and TCTP. Thus, these nine genes were used for further analysis in all the experimental conditions. The FPKM values, CV values, BLASTn homology results and standard curve analysis results of these nine genes are listed in Table 1. For all the nine genes, the PCR efficiencies and correlation coefficient (R2) ranged from 96.5 to 123% and 0.92 to 0.99, respectively which was within the acceptable range (80–120%) of MIQE guidelines (Bustin et al. 2009).
Table 1.
RNA-seq details, genetic identity and qRT-PCR parameters of selected candidate reference genes and target genes
| Gene name | Abbreviation | FPKM value | CV (%) | E-value | Identity (%) | Amplicon size (bp) | Correlation coefficient (R2) | PCR efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| Proteasome | PSMA5 | 54.31 | 4.74 | 0 | 95.24 | 80 | 105.8 | 0.95 |
| RNA polymerase | RNAp | 49.16 | 3.51 | 0 | 94.55 | 80 | 120.6 | 0.97 |
| ADP ribosylation Factor 2 | ADPRF2 | 407.79 | 4.86 | 0 | 97.8 | 80 | 96.5 | 0.95 |
| Translational Initiation Factor 5A4 | IF5A4 | 418.10 | 3.29 | 0 | 95.24 | 80 | 102 | 0.95 |
| Profilin | – | 199.61 | 4.31 | 8e−167 | 93.43 | 80 | 115 | 0.92 |
| Small nuclear ribo-nucleoprotein G | SNRNPG | 165.36 | 5.56 | 1e−110 | 97.92 | 80 | 109.1 | 0.94 |
| 26 S protease regulatory subunit | 26 S | 127.06 | 3.71 | 0 | 94.22 | 80 | 123 | 0.95 |
| Aquaporin | PIP 2 | 902.86 | 4.88 | 0 | 95.05 | 80 | 122.4 | 0.98 |
| Translationally control tumour protein | TCTP | 164.26 | 5.14 | 0 | 94.8 | 80 | 111.1 | 0.99 |
Determination of expression stability of candidate genes
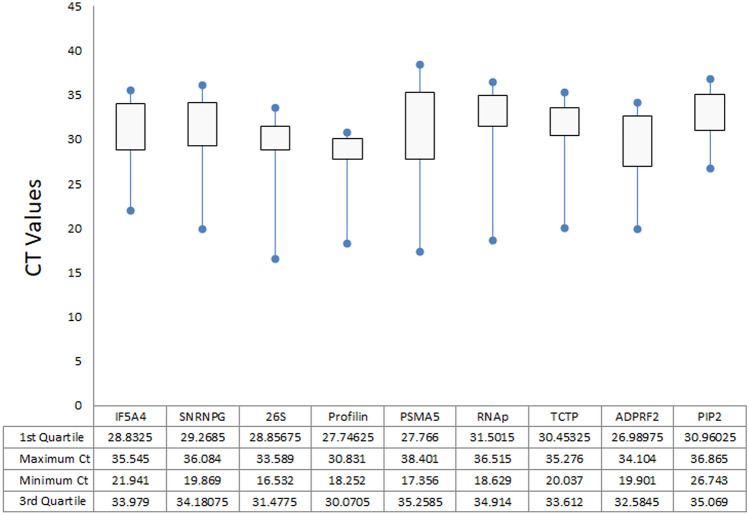
Ct values for all the nine candidate reference genes were obtained through qRT-PCR in all the thirty-six experimental conditions i.e., cold, salinity, heat, drought, ABA and MV treatments and both the genotypes HPK 4 and HPKM 317. The Ct values of the samples ranged widely between 16.5 ± 2.4 to 38.4 ± 3.2 and hence suggest broad variations in transcript abundance of these genes (Online Resource 4). A heat map showing mean Ct values of individual genes is also plotted (Online Resource 5). The threshold cycle values help in understanding the expression level of genes across all samples. The most highly expressed gene was profilin, with an average Ct value of 28.01 ± 3.21 and the least expressed was PIP 2, with Ct value of 32.81 ± 2.81. The other genes were moderately expressed with mean Ct value ranging from 29.2 ± 4.2 to 31.3 ± 5.5. Interquartile range was calculated across all the samples, to identify the Ct dispersal value of all the genes. The lowest Ct dispersal value (which is the difference between the third quartile and first quartile) was found to be for profilin (2.3) followed by 26S (2.6) and TCTP (3.1). The highest dispersal value was found to be for PSMA5 (7.5) followed by ADPRF2 (5.6) and IF5A4 (5.1; Fig. 1). Additionally, PIP 2 showed the least variation in transcript level with the lowest coefficient of variance (CV; 8.5%), followed by profilin (11.6%) and IF 5A4 (11.8%); thereby indicating a stable expression. In contrast, PSMA5 had highest CV value of 17.8%, followed by RNAp (15.7%), 26S and SnRNPG (14.6%). The CV values of other genes ranged between 12.4 and 12.9%. Dispersal range of Ct values and CV values, provide an indication of gene expression stability, however, to get a better insight and cross validation of the analysis, other statistical algorithm like delta Ct, geNorm, NormFinder, BestKeeper, and comprehensive algorithms like RefFinder and RankAggreg were also used; as discussed in following sections.
Fig. 1.
Ct value of each candidate reference genes in all samples. The box represents 25 and 75 percentile range. The line across the box indicates the median. Asterisk represents maximum and minimum value
Delta-delta Ct
For analyzing delta delta Ct value, the mean SD values of △Ct between one gene and other eight genes were calculated and the gene with lower mean SD value was considered as stably expressing. The mean SD values of all the samples, genotypes HPK 4 and HPKM 317 are shown in Table 2. As per the mean SD value, PSMA5 was identified as the most stable gene under all experimental conditions and genotypes HPK4 and HPKM 317, with mean SD values of 2.50, 2.54 and 2.45, respectively. This was followed by PIP 2 and Profilin in all the samples and HPK4, with mean SD values of 2.59 and 2.63 in all the samples and 2.62 and 2.76 in HPK 4, respectively. However, in HPKM 317 genes ranking, second and third ranking genes were profilin and PIP 2 with mean SD values 2.49 and 2.51, respectively. However, under all the three conditions, the least stable gene was TCTP with mean SD values of 2.89, 3.04 and 2.75, respectively.
Table 2.
Ranking and expression stability value (SD) of nine candidate reference genes in horse gram as calculated by delta-delta Ct
| Rank | All samples | HPK4 | HPKM 317 | |||
|---|---|---|---|---|---|---|
| Gene | Mean SD | Gene | Mean SD | Gene | Mean SD | |
| 1 | PSMA5 | 2.50 | PSMA5 | 2.54 | PSMA5 | 2.45 |
| 2 | PIP2 | 2.59 | PIP2 | 2.62 | Profilin | 2.49 |
| 3 | Profilin | 2.63 | Profilin | 2.76 | PIP2 | 2.51 |
| 4 | RNAp | 2.75 | RNAp | 2.87 | RNAp | 2.64 |
| 5 | 26S | 2.79 | ADPRF2 | 2.89 | 26S | 2.64 |
| 6 | ADPRF2 | 2.81 | 26S | 2.93 | SnRNPG | 2.66 |
| 7 | IF5A4 | 2.84 | IF5A4 | 2.98 | IF5A4 | 2.69 |
| 8 | SnRNPG | 2.85 | SnRNPG | 3.04 | ADPRF2 | 2.72 |
| 9 | TCTP | 2.89 | TCTP | 3.04 | TCTP | 2.75 |
BestKeeper analysis
In the present study, on the basis of the raw Ct value of the all the nine genes for all experimental conditions, CV and SD values were determined. The analysis was further performed in three batches: first all the samples were compared; followed by independent analysis for genotypes HPK4 and HPKM 317, respectively, so as to overcome any biasness owing to genotypic variation.
It was observed that the most stable reference gene for all the samples was PIP 2 with CV ± SD value of 6.98 ± 2.3, followed by profilin and TCTP with values 8.55 ± 2.4 and 9.31 ± 2.8, respectively. Whereas, the least stable gene was PSMA5 with CV ± SD value 14.8 ± 4.6. For HPK4, the most stable gene was again PIP2 with CV ± SD value 7.6 ± 2.5, followed by TCTP and profilin with values 8.9 ± 2.8 and 9.1 ± 2.5, respectively. For the genotype HPKM 317, the most stable gene was again PIP 2 with value 6.4 ± 2.1, followed by profilin and ADPRF2 with CV ± SD values 7.9 ± 2.2 and 8.1 ± 2.4, respectively. The least stable gene for both the genotypes was PSMA5 with values 15.8 ± 4.9 and 13.7 ± 4.3, for HPK4 and HPKM 317, respectively (Table 3).
Table 3.
Ranking and expression stability value (CV ± SD) of nine candidate reference genes in horse gram as calculated by BestKeeper
| Rank | All samples | HPK4 | HPKM 317 | |||
|---|---|---|---|---|---|---|
| Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | |
| 1 | PIP2 | 6.98 ± 2.29 | PIP2 | 7.56 ± 2.49 | PIP2 | 6.41 ± 2.10 |
| 2 | Profilin | 8.55 ± 2.40 | TCTP | 8.92 ± 2.80 | Profilin | 7.89 ± 2.23 |
| 3 | TCTP | 9.31 ± 2.89 | Profilin | 9.11 ± 2.53 | ADPRF2 | 8.11 ± 2.49 |
| 4 | IF5A4 | 9.68 ± 3.04 | 26S | 9.77 ± 2.85 | IF5A4 | 8.56 ± 2.70 |
| 5 | 26S | 9.73 ± 2.84 | IF5A4 | 10.72 ± 3.36 | TCTP | 9.70 ± 2.99 |
| 6 | ADPRF2 | 10.56 ± 3.15 | SnRNPG | 10.95 ± 3.37 | 26S | 9.73 ± 2.85 |
| 7 | RNAp | 10.64 ± 3.40 | RNAp | 11.23 ± 3.55 | RNAp | 9.95 ± 3.22 |
| 8 | SnRNPG | 11.43 ± 3.53 | ADPRF2 | 12.59 ± 3.67 | SnRNPG | 11.83 ± 3.67 |
| 9 | PSMA5 | 14.79 ± 4.61 | PSMA5 | 15.85 ± 4.89 | PSMA5 | 13.75 ± 4.33 |
NormFinder analysis
In the present study the gene with lowest S value for all the samples was TCTP followed by profilin and IF5A4 with S values of 0.042, 0.043 and 0.067, respectively. For genotype HPK 4 also, the most stable gene was TCTP with S values 0.025, followed by SnRNPG and profilin with S values 0.029 and 0.043, respectively. For genotype HPKM 317, the gene profilin had lowest S value of 0.044, followed by TCTP and 26S, with S values 0.049 and 0.071, respectively. Among all the three experimental samples viz. all samples, HPK 4 and HPKM 317, the least stable gene was PSMA5 with a maximum S value of 0.170, 0.204 and 0.134, respectively (Table 4).
Table 4.
Ranking and expression stability value (S) of nine candidate reference genes in horse gram as calculated by NormFinder
| Rank | All samples | HPK4 | HPKM 317 | |||
|---|---|---|---|---|---|---|
| Gene | S value | Gene | S value | Gene | S value | |
| 1 | TCTP | 0.042 | TCTP | 0.025 | Profilin | 0.044 |
| 2 | Profilin | 0.043 | SnRNPG | 0.029 | TCTP | 0.049 |
| 3 | IF5A4 | 0.067 | Profilin | 0.043 | 26S | 0.071 |
| 4 | SnRNPG | 0.073 | IF5A4 | 0.064 | IF5A4 | 0.072 |
| 5 | 26S | 0.081 | 26S | 0.092 | ADPRF2 | 0.075 |
| 6 | ADPRF2 | 0.089 | ADPRF2 | 0.097 | RNAp | 0.080 |
| 7 | RNAp | 0.090 | RNAp | 0.101 | SnRNPG | 0.101 |
| 8 | PIP2 | 0.129 | PIP2 | 0.140 | PIP2 | 0.120 |
| 9 | PSMA5 | 0.170 | PSMA5 | 0.204 | PSMA5 | 0.134 |
geNorm analysis
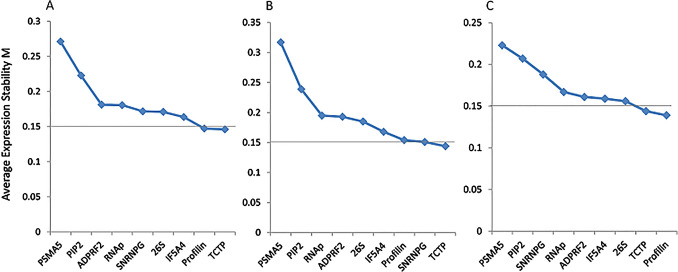
In the present analysis, all the genes were found to have M value lower than 1.5 (Fig. 2). The most stable gene for all samples was TCTP with M value 0.146 followed by profilin with M value 0.147. Likewise, for genotype HPK 4 also, the TCTP had lowest M value of 0.144, followed by SnRNPG, with M value of 0.151. However, for genotype HPKM 317, the most stable gene was profilin with lowest M value of 0.139, followed by TCTP, with M value of 0.144. Whereas, the least stable gene under all experimental conditions was PSMA5, with M value of 0.271, 0.317 and 0.223 for all samples, HPK 4 and HPKM 317, respectively (Table 5).
Fig. 2.
Gene expression stability values (M) and ranking of nine reference genes as assayed by geNorm in all sample types (A), HPK 4 genotypes (B) and HPKM 317 genotype (C). The least stable genes are on the left and the most stable genes on the right
Table 5.
Ranking and expression stability value (M) of nine candidate reference genes in horse gram as calculated by geNorm
| Rank | All samples | HPK4 | HPKM 317 | |||
|---|---|---|---|---|---|---|
| Gene | M value | Gene | M value | Gene | M value | |
| 1 | TCTP | 0.146 | TCTP | 0.144 | Profilin | 0.139 |
| 2 | Profilin | 0.147 | SnRNPG | 0.151 | TCTP | 0.144 |
| 3 | IF5A4 | 0.163 | Profilin | 0.154 | 26S | 0.156 |
| 4 | 26S | 0.171 | IF5A4 | 0.168 | IF5A4 | 0.159 |
| 5 | SnRNPG | 0.172 | 26S | 0.185 | ADPRF2 | 0.161 |
| 6 | RNAp | 0.181 | ADPRF2 | 0.193 | RNAp | 0.167 |
| 7 | ADPRF2 | 0.182 | RNAp | 0.195 | SnRNPG | 0.188 |
| 8 | PIP2 | 0.222 | PIP2 | 0.239 | PIP2 | 0.207 |
| 9 | PSMA5 | 0.271 | PSMA5 | 0.317 | PSMA5 | 0.223 |
The geNorm also determines an optimal number of reference gene(s) required for normalization among all or in an individual sample. It is done by pairwise variation (Vn/Vn+1) between sequential normalization factors (NFn and NFn+1). A pairwise cut off value below 0.15 indicates that an additional internal reference gene is not required for qRT-PCR data normalization. Our analysis showed that among all the three experimental conditions, using two genes is sufficient for qRT-PCR data normalization as V2/3 is < 0.15 and including third gene (V3/4), does not make any significant difference in the threshold values as shown in Fig. 3.
Fig. 3.
Pairwise variation (V) of the candidate gene as calculated by geNorm in all sample types, HPK 4 and HPKM 317 genotypes. Vn/Vn + 1 was used to ascertain the optimal number of reference genes
Comprehensive stability ranking
As the analyses mentioned above are based on different algorithms, thus they may identify different genes with the highest expression stability. For example, among all the experimental conditions, delta Ct analysis identified PSMA5as most stably expressed, followed by PIP 2 and profilin. BestKeeper identified PIP 2 as the most stable gene, whereas geNorm and NormFinder identified TCTP as the most stable gene. Similarly, while comparing genotypes individually; in HPK 4 the most stable gene was TCTP as per geNorm and NormFinder, whereas according to BestKeeper, the most stable gene was PIP 2. Likewise, in HPKM 317, the most stable gene according to geNorm and NormFinder was profilin, whereas, as per BestKeeper, the most stable gene was PIP 2. Thus, it becomes difficult to recognise the best reference gene that can be used for qRT-PCR data normalization. Therefore, comprehensive ranking is required to identify the most stable gene. In the present study, RefFinder analysis and RankAggreg protocol were used for comprehensive ranking.
RefFinder analysis
For all the samples, the most stable gene was found to be profilin, however it was ranked second by geNorm, NormFinder and BestKeeper (Table 6). This variation in ranking is expected as it uses raw Ct value without considering the PCR efficiency of genes. Moreover, it is performed by calculating the geometric mean of each candidate gene, lower geomean indicates stable expression. For both the genotype HPK 4 and HPKM 317, TCTP was identified as the most stable gene, similar to geNorm and NormFinder, but in contrast to BestKeeper that identified PIP 2 as the most stable gene. However, for all the samples, and individual genotypes HPK 4 and HPKM 317, the least stable gene was PSMA5 as identified by other software also.
Table 6.
Ranking order of the genes as determined by RefFinder analysis
| Ranking order (Better-Good-Average) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| All samples | |||||||||
| Delta Ct | Profilin | TCTP | 26S | IF5A 4 | SnRNPG | ADPRF 2 | RNAp | PIP2 | PSMA5 |
| BestKeeper | PIP 2 | Profilin | 26S | TCTP | IF5A 4 | ADPRF 2 | RNAp | SnRNPG | PSMA5 |
| Normfinder | Profilin | TCTP | 26S | IF5A 4 | SnRNPG | ADPRF 2 | RNAp | PIP2 | PSMA5 |
| geNorm | Profilin/TCTP | 26S | IF5A 4 | RNAp | SnRNPG | ADPRF2 | PIP2 | PSMA5 | |
| Comprehensive ranking | Profilin | TCTP | 26S | IF5A 4 | PIP 2 | SnRNPG | ADPRF2 | RNAp | PSMA5 |
| Geomean values | 1.19 | 2.00 | 3.00 | 4.23 | 4.76 | 5.89 | 6.24 | 6.44 | 9.00 |
| HPK 4 genotype | |||||||||
| Delta Ct | TCTP | SnRNPG | Profilin | IF5A4 | 26 S | ADPRF 2 | RNAp | PIP 2 | PSMA5 |
| BestKeeper | PIP2 | Profilin | TCTP | 26S | IF5A4 | SnRNPG | RNAp | ADPRF2 | PSMA5 |
| Normfinder | TCTP | SnRNPG | Profilin | IF5A4 | 26S | ADPRF 2 | RNAp | PIP 2 | PSMA5 |
| geNorm | Profilin/TCTP | SnRNPG | IF5A4 | 26 S | RNAp | ADPRF 2 | PIP 2 | PSMA5 | |
| Comprehensive ranking | TCTP | Profilin | SnRNPG | IF5A4 | 26 S | PIP2 | ADPRF 2 | RNAp | PSMA5 |
| Geomean values | 1.32 | 2.06 | 2.91 | 4.23 | 4.73 | 4.76 | 6.70 | 6.74 | 9.00 |
| HPKM 317 genotype | |||||||||
| Delta Ct | Profilin | TCTP | 26 S | ADPRF 2 | IF5A4 | RNAp | SnRNPG | PIP 2 | PSMA5 |
| BestKeeper | PIP 2 | Profilin | ADPRF 2 | IF5A4 | 26 S | TCTP | RNAp | SnRNP G | PSMA5 |
| Normfinder | Profilin | TCTP | 26 S | ADPRF 2 | IF5A4 | RNAp | SnRNP G | PIP 2 | PSMA5 |
| geNorm | Profilin/ADPRF 2 | IF5A4 | 26 S | TCTP | RNAp | SnRNPG | PIP 2 | PSMA5 | |
| Comprehensive ranking | Profilin | ADPRF 2 | TCTP | 26 S | IF5A4 | PIP 2 | RNAp | SnRNPG | PSMA5 |
| Geomean values | 1.19 | 2.63 | 3.31 | 3.66 | 4.16 | 4.76 | 6.24 | 7.24 | 9.00 |
RankAggreg protocol
For the present data, BruteForce method was employed and on the basis of spearman correlation coefficient, it assigned appropriate weight to an individual gene and calculated geometric mean of the weight, thus providing an overall comprehensive ranking. For all the experimental conditions, the most stably expressing gene was PIP2 followed by profilin and TCTP, for genotype HPK 4 the most stable gene was again PIP 2, followed by TCTP and profilin. For HPKM 317, the most stable gene was also PIP 2 followed by profilin and ADPRF 2. However, the least stable gene was PSMA5 for all the three experimental conditions (Table 7). The optimal stability ranking list for each experimental condition was created as shown in Fig. 4.
Table 7.
Ranking order of the genes as determined by Rank Aggreg using Brute Force algorithm and Spearman distance
| Optimal ranking | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 | Rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| All samples | TCTP | Profilin | IF5A4 | SnRNPG | 26 S | ADPRF 2 | RNAp | PIP 2 | PSMA5 |
| HPK 4 | TCTP | SnRNPG | Profilin | IF5A4 | 26 S | ADPRF2 | RNAp | PIP 2 | PSMA5 |
| HPKM 317 | Profilin | TCTP | 26S | IF5A4 | ADPRF 2 | RNAp | SnRNP G | PIP 2 | PSMA5 |
Fig. 4.
Rank aggregation of genes lists using the Monte Carlo algorithm. The rank aggregation result is shown in a plot in which genes are ordered based on their ranking position according to each stability measurement (Gray line). The mean ranking position of each gene is shown in black, and the model computed by the Monte Carlo algorithm in red line
Reference gene validation
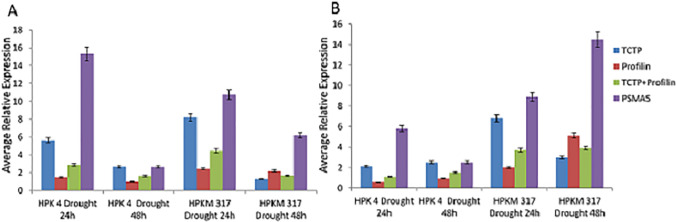
The results obtained from all the statistical software showed that the most stable gene was TCTP and Profilin under various experimental conditions, whereas proteasome (PSMA5) was the least stable under all the experimental conditions. The observation was further validated through qRT-PCR analysis. Therefore, expression analysis of two drought-responsive genes, dehydrin and ERD 6 was performed by △△Ct in drought stressed samples. The data normalization was performed using two stable reference genes viz. TCTP and profilin either individually or in combination (TCTP + profilin) and one least stable gene PSMA5 (Fig. 5). It was observed that in the case of dehydrin, the average expression under drought (24 h) for genotype HPK4 was nearly 5.6, 1.5 and 2.8-folds, when data was normalized with TCTP, profilin and TCTP + profilin, respectively. However, using PSMA5 the data varied to as high as 15.3. Similarly, under drought (48 h), the average expression ranged from 2.7, 0.9 and 1.6, using TCTP, profilin and TCTP + profilin, respectively. Whereas, the relative fold change value was 2.6 using PSMA5 as the reference gene. Similar observations were made for genotype HPKM 317, wherein case of drought (24 h), average fold change values were 8.2, 2.4 and 4.5, when data was normalized with TCTP, profilin and TCTP + profilin, respectively; whereas, the value was as high as 10.7 when the data was normalized with PSMA5. Under drought (48 h), dehydrin expression in HPKM 317 values were 1.2, 2.1 and 1.6 using TCTP, profilin and TCTP + profilin, respectively; whereas, the average expression was 6.1 with reference gene PSMA5. Hence, it was observed that the expression values varied vividly using PSMA5 and were not in accordance with the data observed with reference to stably expressed reference genes.
Fig. 5.
Validation of reference genes under drought stress conditions. Relative expression of dehydrin (A) and ERD 6 (B) genes as determined by △△Ct method and data normalization was done using stable candidate reference genes: TCTP, Profilin, TCTP + Profilin and least stable candidate reference genes: PSMA5. Error bars represent standard error
Likewise, the average relative expression of ERD 6 under drought (24 h) in genotype HPK4 with reference to TCTP, profilin and TCTP + profilin was 2.1, 0.56 and 1.1, respectively (Fig. 5B); Whereas, with reference to PSMA5, the expression value was as high as 5.6. Similarly, under drought (48 h), the values with reference to TCTP, profilin and TCTP + profilin were 2.5, 0.9 and 1.9, respectively, whereas with reference to PSMA5, the value was 2.5. Likewise, for genotype HPKM 317, the values were 6.8, 2.1 and 3.7 with reference to TCTP, profilin and TCTP + profilin, respectively under drought (24 h). Whereas, with reference to PSMA5, the value was as high as 8.9. In case of drought (48 h), with reference to TCTP, profilin and TCTP + profilin the values were 2.9, 5.1 and 3.9, respectively, whereas with reference to PSMA5, the value was as high as 14.5.
Hence, the average expression values of drought responsive genes dehydrin and ERD6, were quite comparable when calculated with reference to stably expressing genes TCTP and profilin or TCTP and profilin together (TCTP + profilin). However, the expression values obtained with respect to PSMA5, a gene with least stable expression, varied enormously and randomly, emphasizing the importance of selecting stably expressed reference gene(s).
Discussion
With the advent of next generation sequencing technologies, the RNA-seq approach has emerged as a powerful tool to perform global gene expression profiling not only in model organisms but also in organisms for which no genomic information is available. To validate the results obtained by RNA-seq experiments, qRT-PCR analysis of genes of interest is routinely performed. Moreover, qRT-PCR approach is also routinely used for functional genomics studies. RNA-seq studies also provide an opportunity to scan a wide range of candidate reference genes along with novel reference genes, which are stably expressed at the whole transcriptomic level (Marcolino-Gomes et al. 2015; Huang et al. 2019; Liang et al. 2020). Before such reports, house-keeping genes (HKGs) were often used as reference genes for qRT-PCR based gene expression studies (Liang et al. 2018; Ciesielska and Staczek 2018). However, it is evident that the expression of HKGs may vary considerably among biological samples. Thus, the selection of stably expressed reference gene(s) for each biological experiment is crucial for obtaining reliable results. In the present study, candidate reference genes were selected from horse gram transcriptome data by Bhardwaj et al. (2013a) on the basis of CV values. Additionally, gene expression abundance was also considered while selecting, such that genes with low expressional levels may be avoided. In summary, the final list of genes selected included traditional HKG viz. PSMA5, RNA polymerase (RNAp), ADP ribosylation factor (ADPRF2), and translation initiation factor (IF5A4) along with some novel genes viz. profilin, small nuclear ribo-nucleoprotein G (SnRNPG), 26 S protease regulatory subunit (26S), aquaporin (PIP 2), and translationally control tumour protein (TCTP). It is also worthwhile to mention here that the mean FPKM value of these selected genes was from 49.15 to 902.86, which was in accordance with Zhao et al. (2017) that suggested FPKM value of 50–1000, as the cut-off threshold for reference gene selection from RNA-seq data.
In the present study, the majority of the selected candidate contigs showed homology to plant genes, related to basic biological processes, cellular components, or molecular functions. The traditional HKGs, shortlisted in the present study includes, proteasome (PSMA5) subunit α type 5 (PSMA5) belonging to the PSMA5 family proteins and is commonly used as HKG in animal and human models (Szabo et al. 2004). Another gene is eukaryotic translational initiation factor (IF5A-4), which is involved in the formation of ribosome pre-initiation complex during translation. Various isoforms of IF are widely accepted reference genes and are regularly used for qRT-PCR data normalization (Wang and Lu 2016; Chao et al. 2016). Similarly, ADP ribosylation factor 2 is the member of ARF family of GTP-binding proteins of Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, with six highly conserved members in mammalian cells. These genes have also been screened previously as reference genes (Wang et al. 2016; Huang et al. 2019). Likewise, DNA dependent RNA polymerase II, IV and V subunits are involved in transcription, however, RNA pol IV and V have been reported to perform RNA dependent DNA methylation (Haag and Pikaard 2011). RNA polymerase II has been screened as one of the stably expressed reference genes in apple (Kumar and Singh 2015).
Another set of genes include novel candidate reference genes, like Profilin, which is a low molecular weight, ubiquitously present, actin monomer binding protein and organises actin polymerisation (Ramchandran et al. 2001; Sun et al. 2013). It has been screened earlier also as a potent reference gene in rice (Wang et al. 2016). Similarly, small nuclear ribonuclear protein G (SnRNPG) is the protein-RNA complex composed of specific snRNP-associated proteins along with small nuclear RNAs (snRNAs). The SnRNPGs are mainly involved in spliceosome activity (Ohtani 2018). There is no previous report using SnRNPG as a reference gene. Another gene 26S protease regulatory subunit is the proteolytic component of the ubiquitin (Ub)-dependent proteolytic system (UPS). It degrades the functional proteins that have been covalently linked to polyUb chain, therefore negatively controlling the protein abundance, particularly signalling and metabolic pathway proteins (as reviewed by Kurepa et al. 2009). Although, many works have screened ubiquitin conjugating enzyme complexes (UBC) for stable expression, however till date no report could be found for 26 S proteasomal complex as the reference gene. Likewise, aquaporins (PIP 2) are transmembrane proteins, which form channels in intracellular and plasma membranes to facilitate the movement of water and other solutes. Many studies demonstrating the role of aquaporins in maintaining water homeostasis in plants under environmental stress conditions like drought, salinity, low temperatures have been reported (reviewed by Kapilan et al. 2018). It may emphasise the constitutive expression of aquaporin genes, during environmental stress, so as to maintain the water balance, without affecting plant metabolic activities. Another novel gene identified was translationally control tumour protein (TCTP) which is known to be highly conserved among many eukaryotes and involved in a variety of cellular activities like microtubule stabilisation, calcium binding activities and apoptosis. However, no further reports regarding the use of TCTP as a reference gene could be found. These genes may express stably under stress conditions, to help plants combat the stress situations.
It is also worth mentioning that some of the novel candidate reference genes analyzed in the present study have been reported to exhibit variable expression under environmental stress conditions in poplar (Zhao et al. 2017). However, we analyzed their expression stability considering the fact that these genes have been selected from RNA-seq data of horse gram on the basis of consistent Log2FC values and minimal expression variance (CV < 5%) under drought stress.
Stability index
As can be seen in the present data the combined analysis using geNorm, NormFinder and RankAggreg suggested that the most stable gene for all samples and genotype HPK4 is TCTP, however, in genotype HPKM 317 the most stable gene was profilin. However, BestKeeper identified PIP 2 as the most stable gene for all the samples as well as for both the genotypes. Although, the PSMA5 gene was identified as the least stable for all the samples, ranking last in the entire analysis. However, on contrary, delta Ct identified PSMA5 as having minimum CV value (most stable), thereby emphasising the use of robust statistical algorithms for calculating stable expressing reference genes and not merely the use of CV value parameters.
Another important analysis of the study was to compare genotypic variation between gene expression and it also showed remarkable observations. For both the genotypes, geNorm and NormFinder gave consistently similar results. In case of HPK4, the most stable gene identified by geNorm, NormFinder and RankAggreg was TCTP followed by SnRNPG and profilin, whereas the least stable gene was PSMA5. For the genotype HPKM 317 also geNorm, NormFinder and Rank Aggreg data were similar, the most stable gene was profilin followed by TCTP and 26S and the least stable gene was again PSMA5. The data also reveals the difference in stable gene expression between two genotypes, which may be attributed to the contrasting physiological responses of the genotypes, where HPK4 is a drought tolerant genotypes (Bhardwaj et al., 2013b), while, HPKM 317 is a drought susceptible genotype. Similar, observations have also been reported in the previous studies (Tong et al. 2009; Mafra et al. 2012; Sinha et al. 2019). According to these stability analyses, many of the novel candidate genes, TCTP, profilin and SnRNPG exhibited indeed greater expression stability than traditional HKGs: IF5A4, PSMA5, RNAp and ADPRF2. Such results have also been obtained previously (Tang et al. 2019; Marcolino-Gomes et al. 2015). The results are also consistent with Ct values and range of interquartile. The genes with too high (PSMA5) or too low (PIP 2) expression abundance as well as wide interquartile range (ADPRF 2) are not suitable to be used as reference genes as can be corroborated with previous results (Liang et al. 2020).
Stability validation using target genes
To validate the results, the expression of two genes, dehydrins and ERD6 was analyzed in drought conditions using the most stable and least stable genes as reference genes. Both dehydrin and ERD6 are known to play important role in drought tolerance in plants (Brini et al. 2007; Kiyosue et al. 1998). The average relative expression of these genes obtained through △△Ct experiments was normalized with two most stable genes TCTP and Profilin, individually as well as in combination (TCTP + profilin), along with the least stable gene proteasome (PSMA5). It was observed that when the data were normalized using stably expressing genes as a reference, the expression values of the drought responsive genes during drought 24 h and 48 h in genotypes HPK4 and HPKM 317 were comparable and significant as elaborated in the results section. However, when the expression of these genes was normalized using the least stable gene proteasome (PSMA5), none of the values could be corroborated or correlated. Moreover, the data were also not in accordance with other reference genes, marking the irrelevant use of unstable genes for data normalization (Ma et al., 2016; Liang et al., 2020) and emphasizing the fact that the use of unstable genes for data normalization may generate very absurd results, thereby misinterpreting the results.
Conclusion
The present study intends to determine the stably expressing reference genes in horse gram. However, due to limited genomic information, the candidate genes were selected from RNA-seq data. It was observed that not only the traditional housekeeping genes rather some novel genes were also analyzed as stably expressing based on CV values of RNA-seq data. Expression stability of nine candidate genes was evaluated under drought, salinity, cold, heat, ABA and MV stress conditions. The Ct values were analyzed through statistical algorithms geNorm, NormFinder, BestKeeper and delta-delta Ct. The consensus results through RefFinder and RankAggreg revealed some novel reference genes like TCTP, profilin and SnRNPG as most stable genes and PSMA5 as least stable gene under experimental conditions. However, slight variation between genotypes was observed. The genes were further validated using target drought responsive genes: dehydrin and ERD6, which revealed similar average expression during different stress regimes when normalized with stably expressing genes: TCTP, profilin and TCTP + profilin whereas with reference to unstable gene PSMA5, the expression values varied, insignificantly and abruptly. The analysis thus establishes the relevance of using stable expressing genes for data normalization, The study provides significant insights towards the relevance of reference gene analysis in otherwise unexplored horse gram genomic studies, which is the first report in this species. The work may be further explored using more genotypes and varied environmental conditions, as well as miRNAs may also be included so as to get a broader aspect regarding reference genes in horse gram. However, similar works on more genotypes of horse gram under varied biotic stress conditions and miRNAs would definitely strengthen this insight.
Supplementary information
Acknowledgements
AKS acknowledges funding support by Indian Council of Agricultural Research, New Delhi in the form of projects IXX12585 and IXX12644. RS and PP acknowledges DBT-BioCARe project no. BT/PR30922/BIC/101/1184/2018 funded by DBT, India for financial support. MB acknowledges University Grant Commission (UGC), New Delhi for JRF. AR acknowledges Senior Research Associateship under scientists' pool scheme by Council of Scientific and Industrial Research (CSIR), India.
Declarations
Conflict of interest
Authors declare that they have No Conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Bansal R, Mittapelly P, Cassone BJ, Mamidala P, Redinbaugh MG, et al. Recommended reference genes for quantitative PCR analysis in soybean have variable stabilities during diverse biotic stresses. PLoS ONE. 2015;10:e0134890. doi: 10.1371/journal.pone.0134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj J, Chauhan R, Swarnkar MK, Chahota RK, Singh AK, Shankar R, Yadav SK. Comprehensive transcriptomic study on horse gram (Macrotyloma uniflorum): de novo assembly, functional characterization and comparative analysis in relation to drought stress. BMC Genomics. 2013;14:647. doi: 10.1186/1471-2164-14-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj J, Mahajan M, Yadav SK. Comparative analysis of DNA methylation polymorphism in drought sensitive (HPKC2) and tolerant (HPK4) genotypes of horse Gram (Macrotyloma uniflorum) Biochem Genet. 2013;51:493–502. doi: 10.1007/s10528-013-9580-2. [DOI] [PubMed] [Google Scholar]
- Brini F, Hanin M, Lumbreras V, Irar S, Pagès M, Masmoudi K. Functional characterisation of DHN-5, a dehydrin showing a differential phosphorylation pattern in two Tunisian durum wheat (Triticum durum Desf.) varieties with marked differences in salt and drought tolerance. Plant Sci. 2007;172:20–28. [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum Information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chahota RK, Sharma TR, Sharma SK, Kumar N, Rana JC. Horse gram. In: Singh M, Upadhyay HD, Bisht IS, editors. Genetic and genomic resources of grain legume improvement. Elsevier Inc; 2013. pp. 293–305. [Google Scholar]
- Chao J, Yang S, Chen Y, Tian WM. Evaluation of reference genes for quantitative real-time PCR analysis of the gene expression in laticifers on the basis of latex flow in rubber tree (Hevea brasiliensis Muell. Arg.) Front Plant Sci. 2016;7:1149. doi: 10.3389/fpls.2016.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A, Stączek P. Selection and validation of reference genes for qRT-PCR analysis of gene expression in Microsporum canis growing under different adhesion-inducing conditions. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-19680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derveaux S, Vandesompele J, Hellemans J. How to do successful gene expression analysis using real-time PCR. Methods. 2010;50:227–230. doi: 10.1016/j.ymeth.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gao M, Liu Y, Ma X, Shuai Q, Gai J, Li Y. Evaluation of reference genes for normalization of gene expression using quantitative RT-PCR under aluminum, cadmium, and heat stresses in soybean. PLoS ONE. 2017;12:e0168965. doi: 10.1371/journal.pone.0168965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghawana S, Paul A, Kumar H, Kumar A, Singh H, Bhardwaj PK, et al. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Res Notes. 2011;4:85–89. doi: 10.1186/1756-0500-4-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Yang Y, Chen Y, Shi J, Song Y, Zhang H, et al. LbCML38 and LbRH52, two reference genes derived from RNA-Seq data suitable for assessing gene expression in Lycium barbarum L. Sci Rep. 2016;6:37031. doi: 10.1038/srep37031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Pelloux J, Bellini C, Wuytswinkel OV. Towards a systematic validation of references in real time RT-PCR. Plant Cell. 2008;20:1734–1735. doi: 10.1105/tpc.108.059774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag JR, Pikaard CS. Multi-subunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nat Rev Mol Cell Biol. 2011;12:483–492. doi: 10.1038/nrm3152. [DOI] [PubMed] [Google Scholar]
- Hu R, Fan C, Li H, Zhang Q, Fu YF. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol. 2009;10:93. doi: 10.1186/1471-2199-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li S, Zhan A. Genome-wide identification and evaluation of new reference genes for gene expression analysis under temperature and salinity stresses in Cionasa vignyi. Front Genet. 2019;10:71. doi: 10.3389/fgene.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Jian B, Liu B, Bi Y, Hou W, Wu C, Han T. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Bio. 2008;9:59. doi: 10.1186/1471-2199-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapilan R, Vaziri M, Zwiazek JJ. Regulation of aquaporins in plants under stress. Biol Res. 2018;51:4. doi: 10.1186/s40659-018-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T, Abe H, Yamaguchi-Shinozaki K, Shinozaki K. ERD6, a cDNA clone for an early dehydration-induced gene of Arabidopsis, encodes a putative sugar transporter. Biochim Biophys Acta. 1998;1370:187–191. doi: 10.1016/s0005-2736(98)00007-8. [DOI] [PubMed] [Google Scholar]
- Kulcheski FR, Marcelino-Guimaraes FC, Nepomuceno AL, Abdelnoor RV, Margis R. The use of microRNAs as reference genes for quantitative polymerase chain reaction in soybean. Anal Biochem. 2010;406:185–192. doi: 10.1016/j.ab.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Kumar G, Singh AK. Reference Gene validation for qRT-PCR based gene expression studies in different developmental stages and under biotic stress in apple. Sci Hortic. 2015;197:597–606. [Google Scholar]
- Kurepa J, Wang S, Li Y, Smalle J. Proteasome regulation, plant growth and stress tolerance. Plant Signal Behav. 2009;4:924–927. doi: 10.4161/psb.4.10.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Aldrich DL, Valliyodan B, Watanabe Y, Van HC, Nishiyama R, et al. Evaluation of candidate reference genes for normalization of quantitative RT-PCR in soybean tissues under various abiotic stress conditions. PLoS ONE. 2012;7:e46487. doi: 10.1371/journal.pone.0046487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fan CM, Zhang XM, Fu YF. Validation of reference genes for real-time quantitative PCR normalization in soybean developmental and germinating seeds. Plant Cell Rep. 2012;31:1789–1798. doi: 10.1007/s00299-012-1282-4. [DOI] [PubMed] [Google Scholar]
- Liang W, Zou X, Carballar-Lejarazú R, Wu L, Sun W, Yuan X, Wu X, et al. Selection and evaluation of reference genes for qRT-PCR analysis in Euscaphis konishii Hayata based on transcriptome data. Plant Methods. 2018;14:1–9. doi: 10.1186/s13007-018-0311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, He Z, Yu H, Wang E, Zhang X, Zhang B, et al. Selection and validation of reference genes for gene expression studies in Codonopsis pilosula based on transcriptome sequence data. Sci Rep. 2020;10:1362. doi: 10.1038/s41598-020-58328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Thibivilliers S, Bilgin DD, Radwan O, Benitez M, et al. Identification of four soybean reference genes for gene expression normalization. Plant Genome. 2008;1:44–54. [Google Scholar]
- Ma S, Niu H, Liu C, Zhang J, Hou C, Wang D. Expression stabilities of candidate reference genes for RT-qPCR under different stress conditions in soybean. PLoS ONE. 2013;8:e75271. doi: 10.1371/journal.pone.0075271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Xu S, Zhao Y, Xia B, Wang R. Selection and validation of appropriate reference genes for quantitative real-time PCR analysis of gene expression in Lycoris aurea. Front Plant Sci. 2016;7:536. doi: 10.3389/fpls.2016.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra V, Kubo KS, Alves-Ferreira M, Ribeiro-Alves M, Stuart RM, Boava LP, et al. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE. 2012;7:e31263. doi: 10.1371/journal.pone.0031263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolino-Gomes J, Rodrigues FA, Fuganti-Pagliarini R, Nakayama TJ, Ribeiro Reis R, Bouças Farias JR, et al. Transcriptome-wide identification of reference genes for expression analysis of soybean responses to drought stress along the day. PLoS ONE. 2015;10:e0139051. doi: 10.1371/journal.pone.0139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand S, Yadav SK. Overexpression of MuHSP70 gene from Macrotyloma uniflorum confers multiple abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol Biol Rep. 2016;43:53–64. doi: 10.1007/s11033-015-3938-y. [DOI] [PubMed] [Google Scholar]
- Nakayama TJ, Rodrigues FA, Neumaier N, Marcelino-Guimaraes FC, Farias JR, de Oliveira MC, et al. Reference genes for quantitative real-time polymerase chain reaction studies in soybean plants under hypoxic conditions. Genet Mol Res. 2014;13:860–871. doi: 10.4238/2014.February.13.4. [DOI] [PubMed] [Google Scholar]
- National Research Council . Tropical legumes: resources for the future. Washington, DC: The National Academies Press; 1979. [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Ohtani M. Plant snRNP Biogenesis: A perspective from the nucleolus and cajal bodies. Front Plant Sci. 2018;8:2184. doi: 10.3389/fpls.2017.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandurangaiah M, Reddy KE, Rao GL, Sivakumar M, Sudhakarbabu O, Nareshkumar A, et al. Cloning and expression analysis of MuNAC4 transcription factor protein from horsegram (Macrotyloma uniflorum (Lam.) Verdc.) conferred salt stress tolerance in Escherichia coli. Acta Physiol Plant. 2013;35:139–146. [Google Scholar]
- Pandurangaiah M, Rao GL, Sudhakarbabu O, Nareshkumar A, Kiranmai K, Lokesh U, et al. Overexpression of horsegram (Macrotyloma uniflorum Lam. Verdc.) NAC transcriptional factor (MuNAC4) in groundnut confers enhanced drought tolerance. Mol Biotechnol. 2014;56:758–769. doi: 10.1007/s12033-014-9754-0. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper- excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Pihur V, Datta S, Datta S. RankAggreg, an R package for weighted rank aggregation. BMC Bioinform. 2009;10:62. doi: 10.1186/1471-2105-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo M, Zheng Y, Fei Z, Martin GB, Rosli HB. Use of RNA-seq data to identify and validate RT-qPCR reference genes for studying the tomato-Pseudomonas pathosystem. Sci Rep. 2017;7:44905. doi: 10.1038/srep44905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Christensen H, Ishimaru Y, Dong CH, Chao-Ming W, Cleary AL, et al. Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis. Plant Physiol. 2001;124:1637–1647. doi: 10.1104/pp.124.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative Ct method. Nat Protoc. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Singh VK, Suryanarayana V, Krishnamurthy L, Saxena RK, Varshney RK. Evaluation and validation of housekeeping genes as reference for gene expression studies in pigeon pea (Cajanus cajan) under drought stress conditions. PLoS ONE. 2015;10:e0122847. doi: 10.1371/journal.pone.0122847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Saxena RK, Singh VK, Krishnamurthy L, Varshney RK. Selection and validation of housekeeping genes as reference for gene expression studies in pigeon pea (Cajanus cajan) under heat and salt stress conditions. Front Plant Sci. 2015;6:1071. doi: 10.3389/fpls.2015.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Sharma TR, Singh AK. Validation of reference genes for qRT-PCR data normalisation in lentil (Lens culinaris) under leaf developmental stages and abiotic stresses. Physiol Mol Biol Plants. 2019;25:123–134. doi: 10.1007/s12298-018-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Li S, Ren H. Profilin as a regulator of the membrane-actin cytoskeleton interface in plant cells. Front Plant Sci. 2013;4:512. doi: 10.3389/fpls.2013.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Perou CM, Karaca M, Perreard L, Quackenbush JF, Bernard PS, et al. Statistical modelling for selecting housekeeper genes. Genome Biol. 2004;5:R59. doi: 10.1186/gb-2004-5-8-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Chu L, Shu W, He X, Wang L, Lu M, et al. Selection and validation of reference genes for quantitative expression analysis of miRNAs and mRNAs in Poplar. Plant Methods. 2019;15:35. doi: 10.1186/s13007-019-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Gao Z, Wang F, Zhou J, Zhang Z. Selection of reliable reference genes for gene expression studies in peach using realtime PCR. BMC Mol Biol. 2009;10:71. doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van-Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lu S. Validation of suitable reference genes for quantitative gene expression analysis in Panax ginseng. Front Plant Sci. 2016;6:1259. doi: 10.3389/fpls.2015.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Yang J, Hu K, An B, Deng X, et al. Reliable selection and holistic stability evaluation of reference genes for rice under 22 different experimental conditions. Appl Biochem Biotechnol. 2016;179:753–775. doi: 10.1007/s12010-016-2029-4. [DOI] [PubMed] [Google Scholar]
- Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
- Yim AK-Y, Wong JW-H, Ku Y-S, Qin H, Chan T-F, Lam H-M. Using RNA-Seq data to evaluate reference genes suitable for gene expression studies in soybean. PLoS ONE. 2015;10:e0136343. doi: 10.1371/journal.pone.0136343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yang F, Feng J, Wang Y, Lachenbruch B, Wang J, et al. Genome-wide constitutively expressed gene analysis and new reference gene selection based on transcriptome data: a case study from poplar/canker disease interaction. Front Plant Sci. 2017;8:1876. doi: 10.3389/fpls.2017.01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Cong P, Tian Y, Zhu Y. Using RNA-seq data to select reference genes for normalizing gene expression in apple roots. PLoS ONE. 2017;12:e0185288. doi: 10.1371/journal.pone.0185288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.