Abstract
In lowland rice ecosystems stagnant flooding or partial submergence has a significant negative impact on important yield attributing traits resulting in substantial grain yield reduction. Genetics of this stress is not yet studied intensively. Rashpanjor (IC 575321), a landrace from India, was identified and used as the tolerant donor for stagnant flooding and was crossed with high yielding variety Swarna to develop the RIL population for the present investigation. Yield and yield attributing traits of 180 F2:8 lines in rainfed non-stressed and stressed (stagnant flooding with 45 ± 5 cm standing water) conditions were recorded in the wet season of 2018 and stress susceptibility and tolerance indices of yield component traits were deduced. Homo-polymorphic high-quality SNPs between two parents derived from genotyping by sequencing were employed and 17 putative QTLs for plant height, shoot elongation, panicle number, grain weight, panicle length in control and stagnant flooding conditions were identified. Tolerance and susceptibility indexes for these traits were detected in chromosomes 1, 3, 4, 5, 6, 10, 11, and 12 with PVE ranging from 6.53 to 57.89%. Two major QTLs clusters were found for stress susceptibility index of grain and panicle weight on chromosome 1 and plant height in non-stress condition and stress tolerance index of elongation ability on chromosome 3. Putative functional genes present either in associated non-synonymous SNPs or inside the QTL regions were also predicted. Some of them were directly associated with ethylene biosynthesis and encoding auxin responsive factors for better adaptation under stagnant flooding and also coded for different transcription factors viz. NAC domain-binding protein, WRKY gene family, and MYB class known for ROS scavenging and production of metabolites to enhance tolerance to stagnant flooding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01107-x.
Keywords: Genetic mapping, Genome sequencing, Partial submergence, Single nucleotide polymorphism (SNP), Waterlogging tolerance
Introduction
In the era of global climate change, the increasing occurrence of adverse weather events is continuously challenging agricultural production worldwide. Excess rainfall and sudden downpour due to vagaries of nature led to frequent flood events and hydrological disasters globally. According to FAO reports (2017), annual flood events were just 30 per year in 1970s, which was doubled to more than 60 in 1980s and skyrocketed to about 180 in 2000s and currently at a peak of 246 after 2006. The low-lying ecology of southeast Asia is particularly vulnerable to such hydrological disasters accounting for more than 50 billion USD income loss globally (FAO reports 2017). Flooding stress is prevalent almost everywhere in the globe, but rice production suffers a heavy setback due to flooding in southeast Asia (Pathak et al. 2021). Germination and plant establishment is severely inhibited if flooding occurs after seeding (Vijayan et al. 2018). For the majority of rice cultivars, severe plant damage occurs under complete inundation of plant canopy beyond three days in case of complete submergence (Panda et al. 2006). In the case of partial submergence, water stagnation on the field with around 50 cm depth reduces yield due to reduction of panicle-bearing tillers, as well as increased sterility and degeneration of spikelets (Kuanar et al. 2017). If submergence due to flash flooding is taken into consideration along with water stagnation, around 50% i.e., 48.7 million hectares of land in the south and southeast Asian countries is always under threat of flooding due to complete/partial inundation or a combination of both. Out of these, about 9.8 million hectares i.e., 20% of the land belongs to the medium-depth category, where water stagnation varies between 25 and 50 cm depth continuously for a month duration or more (Singh et al. 2011; Sarkar 2016).
During flash flooding (generally shorter in duration), submergence tolerant rice genotypes restrict the internode elongation by adopting the quiescence mechanism to preserve the non-structural carbohydrate reserve, which facilitates post-submergence survival and regeneration (Fukao et al. 2019). But, the tolerance mechanism to prolonged stagnant flooding (partial submergence) is not so straightforward. Unlike complete submergence or anaerobic germination process, plant mortality is not the prime factor governing tolerance in this case (Kuanar et al. 2017). If at least 5% of the plant canopy remains above the water surface, the rice plant does not show any sign of mortality. Here, reduction in grain yield is far more important, which is found proportional to the depth of water stagnation (Sarkar 2016). Again, a water stagnation beyond 50 cm as seen in the growing environments of deep-water rice, shoot elongation is the most critical trait for survival and tolerance (Kende et al. 1998). But it is not obligatory for medium depth (30–50 cm) stagnant flooding tolerant rice plants (Sarkar 2016). Phenotype suitable for medium depth condition is necessarily different from the deep-water condition for achieving high yield. Unlike germination stage oxygen deficiency (anaerobic germination) or submergence tolerance, studies on stagnant flooding tolerance are scarce. Although, a few publications on the agro-physiological response towards stagnant flooding in rice are available (Kato et al. 2014; Kuanar et al. 2017), however, reports on QTL/genes associated with high yield under stagnant flooding conditions is rare, baring a report by Singh et al. (2017). Presently, the genetic basis of tolerance to stagnant flooding stress is still elusive. Therefore, in the present study, an attempt was made to identify QTL(s) associated with yield and yield parameters under medium-depth (45–50 cm) stagnant flooding conditions. Rashpanjor (IC 575321), a popular landrace from the coastal area with unique tolerance to stagnant flooding and salinity was crossed with popular susceptible cultivar, Swarna, and the RIL population was phenotyped for yield and yield attributing traits under both control and stagnant flooding conditions. Further, a highly efficient and cost-effective sequence-based genotyping approach called genotyping-by-sequencing (GBS) was used for simultaneous genome-wide SNP discovery and genotyping. The QTLs and associated SNPs for component traits related to stagnant flooding tolerance were detected and putative functional genes were delineated in those QTLs region.
Materials and method
Plant materials
Rashpanjor was identified as tolerant to stagnant flooding, salinity, and stagnant flooding with saline water (Pradhan et al. 2019). On the other hand, Swarna (MTU 7029) is high yielding popular variety in India but susceptible to all these stress conditions. We generated F1s from the cross between Swarna and Rashpanjor (IC-575321) and through the single seed descent (SSD) method developed a RIL (recombinant inbred line) population with 180 F2:8 lines.
Phenotyping for stagnant flooding tolerance
One hundred and eighty RILs developed from Swarna/Rashpanjor cross along with parents (Swarna and Rashpanjor) were planted in the field screening tanks (40 × 10 m each) without flooding stress and with optimum soil moisture conditions in the wet season of 2018. Stagnant flooding stress was imposed on one set of plants following standard procedures (Kuanar et al. 2017). The stress was started with 20 cm water depth after 45 days of sowing and gradually raised to 45–50 cm with a 5 cm/day rise. The stress was continued for around 60 days (since imposition) till 10–15 days before harvesting (Fig. S1). For each control and flooding stress condition two field tanks were used, which served as two independent replicates in our study. In each tank two 3 m line of each genotype was planted at 20 cm row to row and 15 cm plant to plant distance. Elongation ability (EL) was measured based on the difference of plant height before stress and at the time of harvesting. Plant height (cm) (PH), panicle number/plant (PN), grain weight/sq m (g) (GW), panicle weight (g) (PW), grain weight/panicle (g) (PGW), harvest index (HI) were measured from 10 randomly selected plants under stress (S) and non-stress (NS) condition.
The stress tolerance index (STI) and stress susceptibility index (SSI) were measured for all traits (Fernandez 1992).
Here Yp and Ys are yield (or yield traits) under non-stress (NS) and stress (S), respectively while is the mean yield (or yield traits) of the experiment under non-stress (NS) (Fischer and Maurer 1978)
(Ysi = yield or yield attributing traits under stress, Ypi = yield or yield attributing traits under non-stress, Ys and Yp are average yield/yield traits under stress and normal condition, respectively).
Genotyping through sequencing (GBS)
The genotyping by sequencing of the RIL population and their parents were outsourced (Nucleome Informatics Pvt. Ltd. Hyderabad). Briefly, plant DNA was extracted using the CTAB method from 5 plants of each RILs and parents (Murray and Thompson 1980). The Agarose gel electrophoresis approach was used for testing DNA integrity and purity along with NanoDrop 2000 spectrophotometric assessment. Qubit 3.0 Fluorometric quantification was used to measure the concentration of the genomic DNA of the samples. OD260/280 ratio between 1.8 and 2.0 and genomic DNA of 1.5 µg was used for generating the GBS libraries. The genomic DNA of each sample was digested respectively with MseI and HaeIII based on the in-silico evaluation results. After cluster preparation, high-throughput DNA sequencing was performed on the Illumina-HiSeq platform, Qubit®4.0 Fluorometer was firstly used to determine the concentration of the library. After dilution to 1 ng/µl, the Agilent®2100 Bioanalyzer was used to assess the insert size. And finally, the quantitative real-time PCR (qPCR) was performed to detect the effective concentration of each library. Libraries were considered to be passed if the size selected libraries had an effective concentration of more than 2 nM, and were passed for sequencing.
GBS data analysis
Reads are initially passed through a QC pipeline using Trimmomatic v0.36 using default parameters to remove low-quality sequences and to trim adapters (Bolger et al. 2014). Reads were then mapped to the reference genome using the parameters BWA v0.7.8-r455 “mem -t 4 -k 32 -M” and the alignments were reported in a BAM format using SAMTOOLS v0.1.19-44428cd (Li and Durbin 2009). An average mapping rate of 96% was observed amongst the samples. Metrics like enzyme cut ratios were based on the conservancy of restriction enzyme sites were observed to be around 94% and the enzyme digested ratio was around 97%. The ratios were calculated by taking the read count over the total clean reads. Depth profiling of the mapped reads was performed using “samtools depth” command. Subsequently, we called SNPs using SAMTOOLs v1.8 (Li et al. 2009) using the parameter ‘mpileup -m 2 -F 0.002 -d 1000’ and filtered for having an MQ > 20 and DP > 4. The filtered SNPs were finally annotated using Annovar release from 2013Aug23 at default parameters (Wang et al. 2010).
Linkage map and QTL detection
Linkage mapping was done using the ‘MAP’ option in QTL IciMappingV4 (http://www.isbreeding.net). For identification of the main effect of additive QTL for each trait, the inclusive composite interval mapping was done using ‘ICIM-ADD’ functions of the software (Meng et al. 2015). Permutation tests (1000 permutations, 95% confidence level, 1 cM interval) were performed for each trait to detect putative QTLs.
Prediction of probable functional genes inside QTLs
Associated genes for abiotic stress tolerance especially for stagnant flooding tolerance were downloaded along with their physical position from Rice Annotation Project Database (Sakai et al. 2013) and Oryzabase (Kurata and Yamazaki 2006). The genes located inside the QTL interval region or near to peak marker position were considered to be probable causative genes for increased stagnant flooding tolerance. Functions of the QTL-linked genes were further determined using Rice Genome Annotation Project Database (Kawahara et al. 2013) and Rice Annotation Project Database (Sakai et al. 2013). An in silico expression profile of functional genes located within the detected QTL regions was performed using the root, shoot, leaf, and panicle specific gene expression data generated for rice cv. Nipponbare which is available at the RiceXPro database (RXP_0012) (http://ricexpro.dna.affrc.go.jp/) (Kawahara et al. 2013).
Results
Phenotyping of RIL population
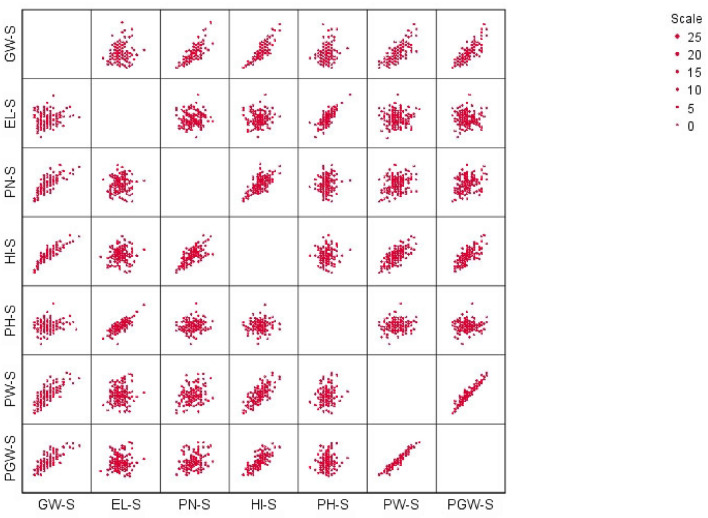
Analysis of variance depicted that the RIL population for all traits under normal and stagnant flooding conditions significantly (p < 0.05) differed. Mean GY, PN, HI were significantly reduced in stagnant flooding conditions with 47.21%, 34.51%, and 33.63%, respectively. On the other hand, significantly higher (25%) mean shoot elongation (EL) was observed under this stress condition (Supplementary Table S1). Under both stress (SF) and control (normal) conditions GW positively and significantly (P < 0.01) correlated with PN, PGW, PW, and HI. In both cases, EL showed a positive and significant correlation with PH. Only under SF, EL had a positive association with GW at a lower (p < 0.05) significant level (Table 1). Scatterplot (Fig. 1) graphically presented the linear or non-linear relationship among observed phenotypic traits under stagnant flooding conditions of 180 RILs and validated the correlation among them. Phenotyping of this population for each trait under stagnant flooding stress (S) and non-stress (NS) conditions revealed that most of the parameters except stress susceptibility index for elongation (SSI-EL) followed normal or near to normal distribution (Fig. 2) with near-unity skewness (Table 2). Hence, they were found suitable for QTL analysis.
Table 1.
Correlation coefficient matrix under normal (Control) and waterlogging (SF) condition of RIL population derived from Swarna × Rashpanjor cross
| Traits | Condition | PH | EL | PN | PW | HI | PGW | GW |
|---|---|---|---|---|---|---|---|---|
| PH | Control | 1.000 | ||||||
| SF | 1.000 | |||||||
| EL | Control | 0.801 | 1.000 | |||||
| SF | 0.783 | 1.000 | ||||||
| PN | Control | − 0.091 | − 0.132 | 1.000 | ||||
| SF | 0.096 | 0.075 | 1.000 | |||||
| PW | Control | 0.057 | 0.041 | 0.159 | 1.000 | |||
| SF | 0.151 | 0.091 | 0.289 | 1.000 | ||||
| HI | Control | − 0.100 | − 0.076 | 0.412 | 0.659 | 1.000 | ||
| SF | 0.058 | 0.085 | 0.691 | 0.725 | 1.000 | |||
| PGW | Control | 0.046 | 0.030 | 0.161 | 0.981 | 0.676 | 1.000 | |
| SF | 0.119 | 0.070 | 0.333 | 0.960 | 0.769 | 1.000 | ||
| GW | Control | − 0.073 | − 0.069 | 0.691 | 0.777 | 0.727 | 0.789 | 1.000 |
| SF | 0.159 | 0.123 | 0.772 | 0.767 | 0.882 | 0.812 | 1.000 |
Here PH plant height, EL elongation, PN: panicle number/plant, GW grain weight/sq m, PW panicle weight, PGW: grain weight/panicle, SF stagnant flooding stress
r value, p < 0.01: 0.209, p < 0.05: 0.159
Fig. 1.
Scatterplot based on different contributing traits under stagnant flooding condition
Fig. 2.
Distribution of mapping population from Swarna/Rashpanjor for different contributing traits under stagnant flooding condition
Table 2.
Mean, range, skewness, kurtosis and normality test (W-test) with p value of different parameters under stagnant flooding stress (S) and non-stress (NS) and stress susceptibility index (SSI) and stress tolerance index (STI) in 150 RILs derived from Swarna × Rashpanjor cross
| Trait name | Mean | Skewness | Kurtosis | Minimum | Maximum | W-test | P value |
|---|---|---|---|---|---|---|---|
| PH-NS | 154.266 cm | − 0.019 | 0.116 | 120.600 cm | 187.200 cm | 0.985 | 7.34E−01 |
| PH-S | 166.312 cm | 0.557 | 1.845 | 136.950 cm | 220.000 cm | 0.980 | 4.20E−01 |
| EL-NS | 46.287 cm | − 0.060 | 0.222 | 2.267 cm | 88.233 cm | 0.991 | 9.65E−01 |
| EL-S | 58.328 cm | 0.316 | 0.504 | 20.350 cm | 108.267 cm | 0.992 | 9.81E−01 |
| PN-NS | 172.714 | 0.403 | 0.709 | 80.000 | 300.000 | 0.974 | 1.28E−01 |
| PN-S | 114.294 | 0.080 | − 0.265 | 29.000 | 217.333 | 0.982 | 5.58E−01 |
| PW-NS | 1.924 g | 0.174 | − 0.013 | 0.707 g | 3.036 g | 0.975 | 1.66E−01 |
| PW-S | 1.640 g | 0.281 | 0.058 | 0.565 g | 2.946 g | 0.979 | 3.41E−01 |
| HI-NS | 30.834 | 0.245 | − 0.543 | 15.712 | 47.041 | 0.970 | 5.40E−02 |
| HI-S | 20.417 | 0.128 | 0.370 | 3.5629 | 41.313 | 0.982 | 5.31E−01 |
| PGW-NS | 1.611 g | 0.185 | − 0.129 | 0.500 g | 2.640 g | 0.976 | 1.87E−01 |
| PGW-S | 1.292 g | 0.404 | − 0.136 | 0.440 g | 2.450 g | 0.964 | 1.13E−02 |
| GW-NS | 281.453 g | 0.827 | 0.532 | 82.400 g | 652.333 g | 0.945 | 1.10E−05 |
| GW-S | 151.728 g | 1.045 | 1.821 | 13.180 g | 468.333 g | 0.938 | 4.77E−07 |
| SSI-PH | 1.030 | 0.589 | 3.839 | − 2.461 | 5.388 | 0.970 | 4.62E−02 |
| SSI-EL | 1.498 | 10.620 | 120.340 | − 1.664 | 53.919 | 0.266 | 0.00E+00 |
| SSI-PN | 0.999 | 0.327 | 0.244 | − 0.593 | 2.322 | 0.977 | 2.46E−01 |
| SSI-PW | 0.142 | − 0.406 | 2.464 | − 0.611 | 0.650 | 0.953 | 2.18E−04 |
| SSI-HI | 1.008 | 0.595 | 0.223 | 0.021 | 2.591 | 0.962 | 4.88E−03 |
| SSI-PGW | 0.987 | − 0.672 | 2.996 | − 3.390 | 3.784 | 0.949 | 4.14E−05 |
| SSI-GW | 1.002 | − 0.776 | 3.730 | − 1.201 | 1.989 | 0.964 | 9.53E−03 |
| STI-PH | 1.087 | 0.615 | 1.264 | 0.784 | 1.723 | 0.973 | 1.17E−01 |
| STI-EL | 1.328 | 0.838 | 0.907 | 0.039 | 3.740 | 0.953 | 2.47E−04 |
| STI-PN | 0.694 | 0.975 | 1.627 | 0.096 | 2.189 | 0.947 | 2.47E−05 |
| STI-PW | 0.885 | 0.794 | 0.342 | 0.151 | 2.080 | 0.937 | 2.98E−07 |
| STI-HI | 0.692 | 0.858 | 0.942 | 0.075 | 1.900 | 0.946 | 1.44E−05 |
| STI-PGW | 0.851 | 0.875 | 0.278 | 0.108 | 2.158 | 0.920 | 0.00E+00 |
| STI-GW | 0.606 | 1.706 | 3.196 | 0.018 | 2.864 | 0.831 | 0.00E+00 |
PH plant height, EL elongation, PN panicle number/plant, GW grain weight/sq m, PW panicle weight, PGW grain weight/panicle
GBS data analysis and linkage mapping
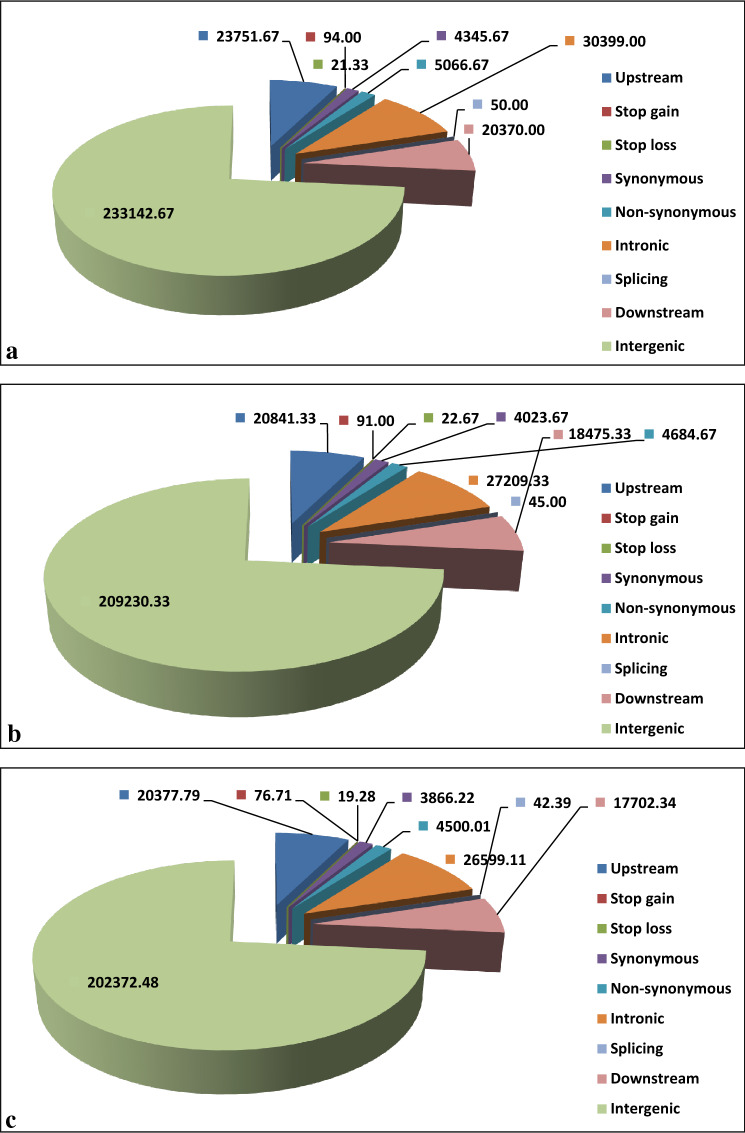
We generated high-quality GBS data through sequencing using Illumina-HiSeq platform, Qubit®4.0, and subsequent processing using different softwares. The total mapped read of all genotypes sequenced in the study was 8.2 × 108 bases. The average mapping rate was 96.7% in the RIL population with an average depth of 11X (Table 3). All different types of SNPs present in the exonic region such as synonymous, non-synonymous, stop loss, stop gain, and in the intronic, intergenic, upstream, and downstream regions were detected (Fig. 3). Average 2.8 million SNPs were found in parents and RILs. Around 10 lakhs of polymorphic SNPs were detected between parents which included both homo and hetero-polymorphic SNPs. But a majority of them were having more than an 80% gap. Only 500 homo-polymorphic SNPs were detected with less than 50% gap (Supplementary Table S2). These SNPs were filtered with less than 30% gap and X2 testing. Finally, 153 high-quality SNPs were employed for mapping. The map distance covered was 2134.9 cM which was distributed among 12 rice chromosomes.
Table 3.
Total read, unread, mapping rate in GBS of parents and RIL population
| Sample | Total reads | Mapped reads | Unread | read (%) | unread (%) | Tag number | Mapping rate (%) | Average depth (X) | Range of depth (X) |
|---|---|---|---|---|---|---|---|---|---|
| Swarna | 5,116,471 | 4,802,954 | 313,516.33 | 93.61 | 6.39 | 681,652.33 | 93.61 | 9.78 | 8.1–12.9 |
| Rashpanjor | 5,454,487 | 5,270,752 | 183,734.33 | 96.64 | 3.36 | 617,485.67 | 96.64 | 11.43 | 9.99–12.88 |
| RIL | 5,456,602 | 5,280,579 | 176,022.69 | 96.76 | 3.24 | 616,176.61 | 96.76 | 11.41 | 5.88–16.51 |
Fig. 3.
Type of SNPs and their average numbers found in a. Swarna, b. Rashpanjor and c. RIL population
Identification of QTLS
Out of a pool of high-quality SNP markers, 153 homo-polymorphic markers between Swarna and Rashpanjor, and their phenotyping data were employed in QTL analysis using ‘ICIM-ADD’ functions of IciMappingV4 (http://www.isbreeding.net) software (Meng et al. 2015). From this, 17 QTLs for traits viz. EL, PH, PN, GW, PW, and PGW were detected under stress and non-stress conditions which, are listed in Table 4, along with the STI and SSI of those traits. Two major additive QTLs were identified for EL (qEL-NS-1.1) and GW (qGW-NS-10.1) with 9.8% and 54.68% PVE in LOD scores of 2.72 and 2.52, respectively. Five additive putative QTLs were identified for plant height (PH), elongation (EL), panicle number/plant (PN), and grain weight/sq m (GW) in stress and non-stress condition with 6.5% PVE (qPN-NS-4.1) to 33.54% PVE (qPH-NS.3.1) in LOD score of 2.12 to 2.46. Five putative QTLs for stress susceptibility index (SSI) for plant height, panicle weight, grain weight/panicle, and grain weight/sq m were detected with 6.57% PVE (qSSI-PH-6.1) to 12.99% PVE (qSSI-GW-1.1). For stress tolerance index (STI) another five putative QTLs were found for elongation and grain weight with from 7.5% PVE (qSTI-EL-11.1) to 57% PVE (qSTI-GW-10.1). One pleiotropic QTL was identified on chromosome 1 between SNP 48 and SNP 49 for SSI-PW, SSI-PGW, and SSI-GW. Another pleiotropic QTL for PH and EL (qPH-NS-3.1, qEL-NS- 3.1, qSTI-EL-3.1) was detected on chromosome 3 (Fig. 4).
Table 4.
Putative QTLs for different grain yield and yield attributing traits under waterlogging stress (S) and non-stress (NS) condition and stress susceptibility index (SSI) and stress tolerance index (STI) for waterlogging stress identified through SNP genotyping of RIL population derived from Swarna × Rashpanjor cross
| Trait | Condition/index | Ch. number | Position (cM) | QTL name | Left Marker | Right Marker | LOD | PVE (%) | Additive | Nearest SNP | Position (Mb) | Gene/non-coding region of nearest SNP | Function of gene |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | NS | 3 | 74 | qPH-NS.3.1 | SNP130 | SNP112 | 2.45 | 33.5353 | − 7.2845 | SNP112 | 3.775291 | Os03g0170900 | Sucrose transport protein SUT1 |
| S | 5 | 98 | qPH-S.5.1 | SNP190 | SNP196 | 2.24 | 6.8337 | 4.9925 | SNP190 | 1.859866 | Os05g0132300 | Hypothetical protein | |
| SSI | 6 | 0 | qSSI-PH-6.1 | SNP232 | SNP215 | 2.23 | 6.5761 | − 0.2453 | SNP232 | 18.9041 | Intergenic region | ||
| EL | NS | 1 | 97 | qEL-NS-1.1 | SNP23 | SNP11 | 2.72 | 9.8351 | 4.5552 | SNP23 | 24.82973 | Os01g0592900 | Regulator of telomere elongation helicase 1 homolog |
| NS | 3 | 65 | qEL-NS-3.1 | SNP130 | SNP112 | 2.44 | 22.9294 | − 6.8637 | SNP130 | 29.62379 | Os03g0713400 | NADH dehydrogenase | |
| S | 11 | 212 | qEL-S-11.1 | SNP432 | SNP450 | 2.2916 | 9.537 | − 4.6522 | SNP450 | 25.76171 | Os11g0615200 | SSXT family protein | |
| STI | 1 | 153 | qSTI-EL-1.1 | SNP47 | SNP48 | 2.2929 | 8.57 | − 0.217 | SNP47 | 42.18993 | Os01g0923600 | Ethylene-induced calmodulin-binding protein 4-like | |
| STI | 3 | 78 | qSTI-EL-3.1 | SNP130 | SNP112 | 2.3647 | 23.3951 | − 0.318 | SNP112 | 3.775291 | Os03g0170900 | Sucrose transport protein SUT1 | |
| STI | 11 | 213 | qSTI-EL-11.1 | SNP432 | SNP450 | 2.2771 | 7.9747 | − 0.2057 | SNP450 | 25.76171 | Os11g0615200 | SSXT family protein | |
| STI | 12 | 107 | qSTI-EL-12.1 | SNP488 | SNP498 | 2.0515 | 15.4926 | − 0.2605 | SNP498 | 20.3287 | Intergenic region | ||
| PN | NS | 4 | 141 | qPN-NS-4.1 | SNP185 | SNP186 | 2.12 | 6.5317 | 10.3181 | SNP186 | 33.80545 | Os04g0655000 | Curculin like (Mannose –binding) lectin domain containing protein |
| SSI | 12 | 169 | qSSI-PN-12.1 | SNP496 | SNP501 | 2.1711 | 6.8919 | 0.1661 | SNP501 | 26.37656 | Os12g0616400 | Similar to Isoform 2 of Transcription factor PCF8 | |
| GW | NS | 10 | 214 | qGW-NS-10.1 | SNP389 | SNP407 | 2.5197 | 54.6806 | 87.2539 | SNP389 | 9.647341 | Intergenic region | |
| SSI | 1 | 198 | qSSI-GW-1.1 | SNP48 | SNP49 | 2.2332 | 12.9923 | − 0.1581 | SNP49 | 42.89045 | Os01g0936100 | Transmembrane receptor protein serine/threonine kinase activity | |
| STI | 10 | 209 | qSTI-GW-10.1 | SNP389 | SNP407 | 2.1459 | 57.8947 | 0.4844 | SNP389 | 9.647341 | Intergenic region | ||
| PW | SSI | 1 | 206 | qSSI-PW-1.1 | SNP48 | SNP49 | 2.104 | 6.5974 | − 0.049 | SNP49 | 42.89045 | Os01g0936100 | Transmembrane receptor protein serine/threonine kinase activity |
| PGW | SSI | 1 | 206 | qSSI-PGW-1.1 | SNP48 | SNP49 | 2.1161 | 6.634 | − 0.2747 | SNP49 | 42.89045 | Os01g0936100 | Transmembrane receptor protein serine/threonine kinase activity |
Here, PH plant height, EL elongation, PN panicle number/plant, GW grain weight/sq m, PW panicle weight, PGW grain weight/panicle, S stress, NS non-stress, SSI stress susceptibility index, STI stress tolerant index
Fig. 4.
Linkage map showing 17 QTLs using SNP based genotyping and agro-morphology based phenotyping of RIL population derived from Swarna × Rashpanjor cross
QTLs governing various phenotypic traits under stress and non-stress conditions
QTLs for PH
Two QTLs were detected which were responsible for plant height, qPH-NS.3.1 and qPH-S.5.1 for non-stress and stress conditions, respectively with 33.6% and 6% PVE, respectively. The nearest SNP (SNP 112) of qPH-NS.3.1 was located in the gene, Os03g0170900 controlling Sucrose transport protein SUT1. Another QTL for stress susceptible index for plant height (qSSI-PH-6.1) was found with 6% PVE and associated SNP was located in intergenic region.
QTLs for elongation ability
Two QTLs, qEL-NS-1.1, qEL-NS-3.1 were detected for shoot elongation with PVE of 9.84% and 22.93%, respectively in non-stress conditions. The nearest SNPs, SNP 23 and SNP130 located in genes, Os01g0592900 and Os03g0713400, respectively which coded for regulating the telomere elongation helicase 1 homolog and NADH dehydrogenase, respectively. Two co-localized QTLs for shoot elongation under stagnant flooding, qEL-S-11.1 and qSTI-EL-11.1 had the nearest marker (SNP 450) located on gene Os11g0615200 yielded SSXT family protein. Another two QTLs, qSTI-EL-1.1 and qSTI-EL-3.1 had nearest markers, SNP47 and SNP 112, explaining 8.57% and 23.4% PVE. SNP 47 was located in the gene, Os01g0923600 encoding ethylene-induced calmodulin-binding protein. The qSTI-EL-12.1 was located in chromosome 12 and explained 15.49% PVE. Whereas, qSTI-EL-3.1 was co-localized with qPH-NS.3.1 and the nearest marker SNP 112 was located in Os03g0170900 controlling Sucrose transport protein SUT1.
QTLs for panicle number
One QTL (QPN-NS-4.1) was identified in chromosome 4 for the trait panicle and two flanking markers SNP185 and SNP186 were present at the border of this region. The nearest marker SNP 186 is located in Os04g0655000 gene encoding curculin like (Mannose-binding) lectin domain-containing protein. In chromosome 12, a QTL, qSSI-PN-12.1 was identified with nearest marker SNP501 located in Os12g0616400 gene encoding Isoform 2 of Transcription factor PCF8.
QTLs for grain weight
The qGW-NS-10.1 and qSTI-GW-10.1 were co-localized with 54 and 57% PVE and the nearest marker SNP 389 was located in an intergenic region. A pleiotropic QTL was detected for qSSI-PW-1.1, qSSI-PGW-1.1, qSSI-GW-1.1, and nearest marker SNP 49 was located in Os01g0936100 encoding transmembrane receptor protein serine/threonine kinase activity.
Bioinformatic analysis for locating putative functional genes inside the detected QTL region and in-silico expression analysis
Inside the nine identified QTLs in the present study, we found several functional genes related to abiotic stress tolerance especially for stagnant flooding tolerance including phytohormone biosynthesis, carbohydrate metabolism, and nutrient uptake pathway genes along with some key transcription factors known to play a crucial role in abiotic stress tolerance (Table 5). Among these, inside QTL qPH-NS.3.1, qSTI-EL-3.1 we found three important genes viz. LOC_Os03g06520.1, LOC_Os03g06654.1, and LOC_Os03g08500.1 which encodes putative sulfate transporter 3.1, disulfide oxidoreductase/ monooxygenase/ oxidoreductase and ethylene-responsive element-binding protein 2, respectively. Similarly, inside qPH-S.5.1QTL region we got another two important putative genes LOC_Os05g04170.1 and LOC_Os05g04510.1 encoding ACS-like protein and S-adenosyl methionine synthetase 1, respectively, which play a key role in ethylene biosynthesis in plants. Besides, we also found a putative gene LOC_Os03g52460.1 inside the QTL qEL-NS-3.1 which encodes the large subunit of the enzyme glucose-1-phosphate adenylyltransferase required for adenylation of a glucose molecule to ADP-Glucose, an important precursor for starch biosynthesis in plants. Interestingly, we identified two auxin response factor family proteins encoding putative genes LOC_Os04g56850.2 and LOC_Os12g41390.1 inside the QTL qPN-NS-4.1 and qSSI-PN-12.1, respectively which might play a crucial role in constitutive aerenchyma formation as preadapted stagnant flooding tolerance trait in rice. Apart from this, we also identified different transcription factors (TFs) like NAC-domain containing TFs, WRKY family proteins, MYB family TFs, and one universal stress protein family gene, which are known to play a crucial role under different kinds of abiotic stresses in rice and other plants.
Table 5.
Putative functional genes inside QTLs region
| Sl no | QTL name | Position (MB) | Gene inside and adjacent to QTL region | Gene function |
|---|---|---|---|---|
| 1 | qPH-NS.3.1, qSTI-EL-3.1 | 3.775291 | LOC_Os03g06520.1 | Sulfate transporter 3.1, putative, expressed |
| LOC_Os03g06654.1 | Disulfide oxidoreductase/ monooxygenase/ oxidoreductase, putative, expressed | |||
| LOC_Os03g08500.1 | Ethylene-responsive element binding protein 2, putative, expressed | |||
| 2 | qPH-S.5.1 | 1.859866 | LOC_Os05g04170.1 | ACS-like protein, putative, expressed |
| LOC_Os05g04510.1 | S-adenosylmethionine synthetase 1, putative, expressed | |||
| LOC_Os05g04820.1 | MYB2, putative, expressed | |||
| 3 | qEL-NS-1.1 | 24.82973 | LOC_Os01g43650 | OsWRKY11—Superfamily of rice TFs having WRKY and zinc finger domains, expressed |
| 4 | qEL-NS-3.1 | 29.62379 | LOC_Os03g51110.1 | MYB52, putative, expressed |
| LOC_Os03g52460.1 | Glucose-1-phosphate adenylyltransferase large subunit 3, chloroplast precursor, putative, expressed | |||
| LOC_Os03g52860.1 | lipoxygenase 2, putative, expressed | |||
| LOC_Os03g53050.1 | WRKY transcription factor 21, putative, expressed | |||
| LOC_Os03g55164.1 | OsWRKY4—Superfamily of rice TFs having WRKY and zinc finger domains, expressed | |||
| 5 | qPN-NS-4.1 | 33.80545 | LOC_Os04g55560.1 | AP2 domain containing protein, expressed |
| LOC_Os04g55800.1 | Sulfate transporter 3.3, putative, expressed | |||
| LOC_Os04g49110.1 | chitin-inducible gibberellin-responsive protein 2, putative, expressed | |||
| LOC_Os04g56850.2 | Auxin response factor family protein, expressed | |||
| LOC_Os04g57340.1 | Ethylene-responsive transcription factor 3, putative, expressed | |||
| 6 | qSSI-PN-12.1 | 26.37656 | LOC_Os12g16790.1 | Calmodulin, putative, expressed |
| LOC_Os12g41390.1 | OsSAUR57—Auxin-responsive SAUR gene family member, expressed | |||
| LOC_Os12g41680.1 | NAC domain-containing protein 21/22, putative, expressed | |||
| LOC_Os12g42210.1 | Glutathione-regulated potassium-efflux system protein kefB, putative, expressed | |||
| 7 | qSSI-PW-1.1, qSSI-PGW-1.1, qSSI-GW-1.1 | 42.89045 | LOC_Os01g71990.1 | Pyrroline-5-carboxylate reductase, putative, expressed |
| LOC_Os01g72690.1 | NAD kinase 1, putative, expressed | |||
| 8 | qSTI-EL-1.1 | 42.18993 | LOC_Os01g71990.1 | Pyrroline-5-carboxylate reductase, putative, expressed |
| 9 | qSTI-EL-12.1 | 20.3287 | LOC_Os12g31710.1 | Universal stress protein family protein, expressed |
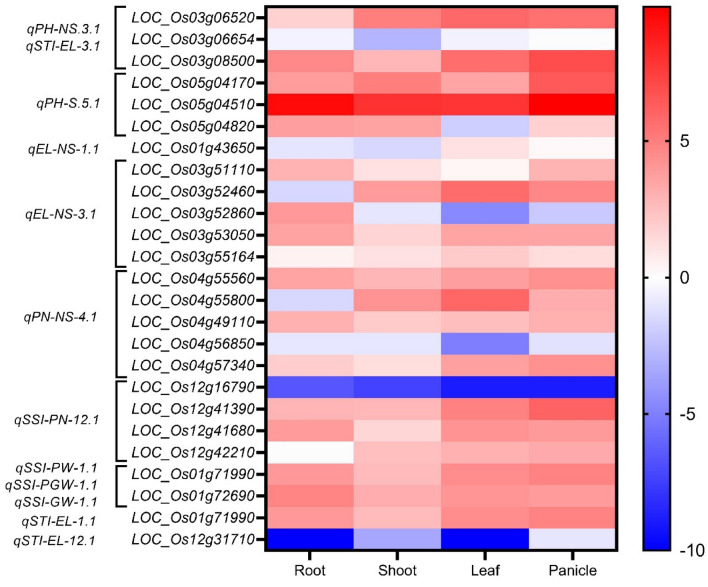
Using the RiceXPro database (RXP_0012) an expression heat map was generated to compare the gene expression profile of the 24 probable functional genes located inside 12 QTLs in leaf, shoot, root, and panicle-specific tissues at 14–126 days after transplanting (DAT) and in seed (embryonic and endosperm) -specific tissues at 14 days after flowering (DAF), respectively. The heat map (Fig. 5) showed very high up-regulation of LOC_Os05g04510 and very high down-regulation of Os12g16790 in all tissues. Most of the selected genes were up-regulated in all tissues. LOC_Os03g08500.1 encoding ethylene-responsive element-binding protein 2 was upregulated in the leaf and panicle, while LOC_Os12g41390.1 encoding Auxin-responsive SAUR gene family was upregulated in all tissues.
Fig. 5.
The Heat map depicting expression profiles of selected 23 putative functional genes in root, leaf, shoot, and panicle-specific tissues. (Note: The X-axis represents the source of sample used to generate the expression data while the Y-axis represents hierarchical clustering pattern)
Discussion
Despite the semi-aquatic nature of rice plants, excess water stress at different growth stages was found to be detrimental for crop growth and productivity (Sarkar et al. 2019). Genotypes tolerant to complete submergence stress, in general, are not tolerant to partial submergence or stagnant flooding (Singh et al. 2017). Kuanar et al. (2019) reported that submergence tolerant genotypes such as Swarna-Sub1 and Savitri-Sub1 showed high degrees of susceptibility under medium-depth stagnant flooding as compared to their parent materials Swarna and Savitri. Marker aided backcross breeding (MAB) improved the breeding efficiency in developing stress-tolerant rice varieties (Singh et al. 2017). A few numbers of submergence tolerant (Sub1) varieties were developed using this approach (Kuanar et al. 2019). On the other hand, varieties adapted in deep-water conditions have a fast internode elongation trait. The discovery of the SNORKEL genes, SK1 and SK2 for rapid elongation could support the development of varieties for deep-water flooding conditions (Hattori et al. 2009). Stagnant flooding (SF) with 25–50 cm of water depth often found to be stagnated in the field for several weeks to a few months in lowland ecologies (Singh et al. 2011). Varieties with facultative elongation ability are required in this ecology, hence deep-water rice do not perform well in this ecology mainly due to the problem of lodging and consequent reductions in yield and grain quality (Kato et al. 2014; Vergara et al. 2014). Rice genotypes tolerant to flooding stress at the germination stage can withstand the stress through overexpression of amylolytic and fermentative enzymes, greater ethylene production, and increased coleoptile growth (Vijayan et al. 2018). Whereas rice genotypes capable to tolerate complete submergence restrict ethylene production vis-a-vis gibberellins biosynthesis, and limit the use of non-structural carbohydrates through suppressing amylolytic enzyme activity (Chakraborty et al. 2021a). Therefore, QTLs/genes for stagnant flooding tolerance could be different from that of submergence or deep-water stresses.
Stagnant flooding or waterlogging tolerance is also a complex trait, controlled by many genes including some with small effects (Zhou 2010). Except for one study, no report on QTL analysis of this trait was found in rice (Singh et al. 2017). But many QTLs for waterlogging tolerance in barley have been reported (Zhang et al. 2016). Different traits were used in different studies, such as leaf scoring system, aerenchyma formation, and other agronomic traits (Zhang et al. 2016). However, the lack of suitable donors and accurate phenotyping techniques remained the main challenge for improving stagnant flooding tolerance in crop plants. In the present investigation, we developed a RIL population using a landrace Rashpanjor, collected from the coastal region of Odisha, India, as the tolerant donor. Higher aerenchyma formation, moderate elongation, and low yield reduction of this genotype under stagnant flooding conditions were observed (Chakraborty et al. 2021b). The population for most of the observed traits was found normally distributed which signified its suitability for mapping (Chattopadhyay et al. 2020). GW positively and significantly (P < 0.01) correlated with PN, PGW, PW, HI, and EL under stagnant flooding conditions (Table 1) which asserted with the previous study done by Kuanar et al (2017). Singh et al (2017) also observed a similar kind of observation that PN, HI, and EL significantly influenced grain yield in rice under stagnant flooding.
In the present study, we observed a mean reduction of 33% in PN. Under stagnant flooding panicle number decreased due to the suppression of tiller growth or mortality of tiller either due to low underwater penetration of light or lack of sufficient oxygen supply (Kato et al. 2014; Bhaduri et al. 2020). Our investigation also agreed with previous researchers that tiller number per square meter, panicle number per square meter, panicle weight, single panicle weight, and harvest index were greatly affected by stagnant flooding (Voesenek and Bailey-Serres 2015). Singh et al (2017) reported a 52.1% decrease in the mean grain yield (GY) in a mapping population for stagnant flooding tolerance. We also found a reduction of 46% in mean yield which was in congruence with the observation made by Kato et al. (2014) where yield under stress was reduced by 47% across genotypes. For the identification of stable QTLs, many types of molecular markers have been used. In rice large set of SSR and SNP markers were used for the detection of QTLs for abiotic stress tolerance (Chattopadhyay et al. 2020). A more recently developed and still evolving technique is GBS first introduced by Elshire et al. (2011). This technique uses a methylation-sensitive restriction enzyme for DNA fragmentation to generate reduced representation libraries of the genome followed by highly multiplexed sequencing of mapping populations. GBS has been employed in detecting QTLs in rice for abiotic stress tolerance (Yadav et al. 2019). For the present investigation employing the GBS approach, we identified a large set of SNPs (Fig. 3). But most of them could not be used in mapping due to gap and heterozygosity in parents and RIL population which is a limitation also observed in some of the previous studies (Yadav et al. 2019).
In this present study finally, around 153 SNPs located in the genic and non-genic regions were taken for construction of the linkage map. Seventeen putative QTLs were detected for PH, EL, PN, GW, PGW, and PW under stress and non-stress conditions (Table 4). Except for PH, all these traits were significantly correlated with GY under stagnant flooding conditions. QTLs were distributed in chromosomes 1, 3, 4, 5, 6, 10, 11, and 12 (Fig. 4). Among 7 QTLs for shoot elongation, one (qSTI-EL-12.1) was located on chromosome 12, and eventually, genes for elongation ability (Snorkel 1 and 2) under deep water conditions were also found at a similar position. The rest of them were located on different chromosomes. Two of them were found pleiotropic with a QTL for PH on chromosome 3. Other QTL clusters were detected on chromosome 11 for EL, chromosome 1 for GW, PG, and PGW, and chromosome 10 for also GW. In an earlier study, consistent QTL for grain yield was detected over the environments on chromosome 1 of rice (Lei et al. 2018). Only one report of QTLs for stagnant flooding tolerance was available where 38 and 46 QTLs were identified related to days to flowering, grain yield, harvest index, flag leaf length, leaf sheath length, shoot elongation rate, plant height, panicle length, and survival rate under stress and control conditions, respectively. Clusters of QTLs were detected especially on chromosomes 3 and 5 (Singh et al. 2017). We have also found one QTL cluster on chromosome 3 with qPH-NS.3.1, qEL-NS-3.1, and qSTI-EL-3.1. The nearest SNPs, SNP 112 and SNP 130 were located in genes encoding Sucrose transport protein SUT1 and NADH dehydrogenase. As the name suggests sucrose transport protein SUT1 takes part in carbohydrate transport. The sucrose/H+ symporter takes part in partitioning carbohydrates from source to sink in temporary storage organs such as stems, roots during vegetative stage while at ripening stage to seeds (Griffiths et al. 2016). NADH dehydrogenase takes part in two important biological processes viz. electron transport chain in generating ATP and production of oxidized NAD and H+ from reduced NADH. The end products are used in different metabolic activities in plant development and mitigating different environmental stresses (Sweetman et al. 2019). Cumulative action of these two genes probably helps better partitioning of sucrose in rice under stagnant flooding conditions.
Another QTL cluster on chromosome 1 with qSSI-PW-1.1, qSSI-PGW-1.1, qSSI-GW-1.1 had the nearest marker, SNP 49 located on gene Os01g0936100 encoding for transmembrane receptor protein serine/threonine kinase activity. Receptor-like kinases (RLK) appear to play as a central processing unit by accepting signal either internally or externally and based on the reception of the signal an output signal is derived which control the overall plant growth and defense system. Overall gene expression and consequently plant adaptation to a specific environment are supposed to be controlled by RLK (Afzal et al. 2008). A reference database search based on the nine identified QTL regions revealed the existence of many important functional genes which might had a direct role in excess water stress tolerance in rice as suggested in many previous reports. The identified genes (Table 5) in these QTL regions, showed that five genes (LOC_Os03g08500.1, LOC_Os05g04170.1, LOC_Os05g04510.1, LOC_Os04g55560.1, and LOC_Os04g57340.1) are directly associated with ethylene biosynthesis, reiterating the essential role of ethylene in waterlogging tolerance in rice (Sasidharan and Voesenek 2015). Among these five genes, two encoding ACS-like proteins (LOC_Os05g04170.1) and S-adenosyl methionine synthetase 1 (LOC_Os05g04510.1) are directly involved in ethylene biosynthesis, whereas the other three encodes either ethylene-responsive element-binding protein or ethylene response factor (ERF) viz. AP2 class of proteins. ACC oxidase and ACC synthase mediated ethylene biosynthesis is a key inducible response in hypoxic roots and water submerged plant parts (Drew et al. 2000). This inducible ethylene production and its subsequent accumulation in underwater plant tissue initiate the lysigenous inducible aerenchyma formation via a coordinated process of programmed cell death in rice (Steffens et al. 2011), wheat (Yamauchi et al. 2014), and maize (Gunawardena et al. 2001). However, the role of ethylene in constitutive aerenchyma formation as a pre-adaptive hypoxia tolerant trait was also established by Yukiyoshi and Karahara (2014) in rice roots and Chakraborty et al. (2021b) in rice stem and leaf sheaths. Besides the crucial role of aerenchyma formation, other ERFs such as APETALA2/Ethylene-Responsive Factor (AP2/ERF) superfamily of transcription factors (TFs) is known to regulate diverse stress responses from tillering and panicle branching in rice (Qi et al. 2011) to triggering SNORKEL1 (OsSK1) and SNORKEL2 (OsSK2) gene expression resulting in waterlogging-induced internode elongation in rice (Hattori et al. 2009).
Besides ethylene, auxin is another phytohormone that play a crucial role in stress-induced response in rice and other plants under stagnant flooding conditions (Yamauchi et al. 2019; Zhang et al. 2021). Recently, Yamauchi et al. (2019) reported that Auxin/indole-3-acetic acid protein (AUX/IAA; IAA) and auxin response factor (ARF)-mediated signaling are essential for lateral root formation and development of constitutive aerenchyma in rice. Both these traits are hugely important for pre-adaptive response and ensuring hypoxia preparedness in rice. Interestingly, we found two genes LOC_Os04g56850.2 (located inside QTL qPN-NS-4.1) and LOC_Os12g41390.1 (located inside the QTL qSSI-PN-12.1) encoding auxin response factor family protein and OsSAUR57—Auxin-responsive SAUR gene family member, respectively, which may make the waterlogging tolerant plants preadapted to flooding stress even before the onset of the stress. Yield sustenance is the most important agronomic factor for waterlogging tolerance in rice (Kuanar et al. 2017). Efficient carbohydrate metabolism and starch biosynthesis under waterlogging stress are thus very crucial tolerance responses for plants (Lothier et al. 2020). We observed the presence of a functional gene of the large subunit of glucose-1-phosphate adenylyltransferase which is involved in biosynthesis of ADP-Glucose, an important metabolite for starch biosynthesis in plants (Cui et al. 2020). Apart from this, found few genes of sulfur transport/metabolism in plants that might play a key role in sulfur uptake and metabolism (Gallardo et al. 2014) and hypoxia-induced sulpho-lipid biosynthesis in plants (Klecker et al. 2014). Besides, sulfur also plays a vital role in maintaining chloroplast structural and functional integrity (i.e., Photosystem II). Gene inside QTL region which are involved in sulfur transport, improve pigment level and the formation of chloroplast protein which are involved in regulation of redox and photosynthetic electron transport is an adaptive response in rice tolerant to stagnant flooding (Panda et al. 2019).
We also observed the presence of two genes for pyrroline-5-carboxylate reductase in our identified QTLs (qSSI-PW-1.1 and qSTI-EL-1.1), which catalyzes a key step for proline biosynthesis in plants (Sekhar et al. 2007). Inside QTL cluster with qSSI-PW-1.1, qSSI-PGW-1.1, qSSI-GW-1.1, gene LOC_Os01g71990.1 encoded NAD kinase 1. Accumulation of proline is directly associated with the activity of NAD kinase (Ruiz et al. 2002). Although, there was no direct evidence reported for proline in waterlogging tolerance in rice, but its prominent role as reactive oxygen species (ROS) scavenger is well known under different environmental stresses in plants (Hayat et al. 2012). Functional genes for different transcription factors (TFs) viz. NAC domain-binding protein, WRKY gene family, and MYB class of TFs were found in the nine QTLs identified in the present study. Among these NAC-domain TFs are known to exhibit low-oxygen induced upregulation for the survival of Arabidopsis under hypoxic conditions (Christianson et al. 2009). qSSI-PN-12.1 had nearest marker SNP 501 located on the gene coding for a protein similar to Isoform 2 of Transcription factor PCF8 which was reported to be involved in plant growth and the regulation of secondary shoot formation (Kosugi and Ohashi 2002). In rice, NAC transcription factors are reported to positively regulate plant growth under drought and oxidative stress (Yuan et al. 2019). Similarly, the beneficial effect of different members of the WRKY gene family under excess water stress was also documented for Arabidopsis, sunflower, and other crops (Hsu et al. 2013; Raineri et al. 2015; Han et al. 2019). In rice, an R2-R3 type MYB transcription factor (OsMYB1) is reported to be associated with waterlogging tolerance (Deeba et al. 2017) and ROS scavenging (Yokotani et al. 2013).
To sum it up, we observed the presence of five genes (LOC_Os03g08500.1, LOC_Os05g04170.1, LOC_Os05g04510.1, LOC_Os04g55560.1, and LOC_Os04g57340.1) which are directly associated with ethylene biosynthesis. Among these, two encoding ACS-like proteins (LOC_Os05g04170.1) and S-adenosylmethionine synthetase 1 (LOC_Os05g04510.1) are directly involved in ethylene biosynthesis, whereas the other three encodes either ethylene-responsive element-binding protein or ethylene response factor (ERF) viz. AP2 class of proteins. Besides ethylene, we found two genes LOC_Os04g56850.2 (located inside QTL qPN-NS-4.1) and LOC_Os12g41390.1 (located inside the QTL qSSI-PN-12.1) encoding auxin response factor family protein and OsSAUR57—Auxin-responsive SAUR gene family member, respectively, which are related to constitutive aerenchyma formation and may preadapt rice plants for stagnant flooding tolerance. We found the presence of a functional gene of the large subunit of glucose-1-phosphate adenylyltransferase which involves in the biosynthesis of ADP-Glucose and a few genes of sulfur transport/metabolism in plants. We also observed the presence of two genes of pyrroline-5-carboxylate reductase in our identified QTLs (qSSI-PW-1.1 and qSTI-EL-1.1), which catalyzes a key step for proline biosynthesis in plants. Functional genes for different transcription factors (TFs) viz. NAC domain-binding protein, WRKY gene family, and MYB class of TFs were also found in the nine QTLs identified in the present study.
Conclusion
Stagnant flooding or partial submergence for a prolonged period at late vegetative and reproductive phases in rice causes substantial yield loss in lowland ecology. Like complete submergence where intensive research followed by identification and utilization of SUB1 QTL resulted in submergence tolerant rice, genetical and molecular dissection of stagnant flooding was very rare. We utilized a local landrace Rashpanjor in genetic mapping, which shows ~ 20% yield reduction under stagnant flooding stress as against 73% reduction in high yielding popular variety Swarna. From this 17 QTLs were identified for stagnant flooding tolerance. Among those two clusters of QTLs on chromosomes 3 and 1 for shoot elongation and grain yield, respectively, were unique. In the present study, we identified some of the associated SNPs with QTLs located on the functional genes encoding sucrose transport protein SUT1, ethylene-induced calmodulin-binding protein, NADH dehydrogenase, etc. which were reported to be directly or indirectly responsible for inducing tolerance to stagnant flooding. In addition, some probable functional genes either on or inside the QTLs region were found to be directly associated with ethylene biosynthesis, encoding small auxin response factor family protein stated for their role in stagnant flooding tolerance. Different transcription factors (TFs) viz. NAC domain-binding protein, WRKY gene family, and MYB class of TFs with their known function for abiotic stress tolerance were postulated in the present study. Further, these findings will guide in multiple donor-based detection and validation of robust QTLs and associated linked molecular markers for improvement of rice for stagnant flooding tolerance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank ICAR-National Rice Research Institute, Cuttack, Odisha, India and National Innovations on Climate Resilient Agriculture (NICRA, EAP-245) of Indian Council of Agricultural Research, New Delhi; for providing the necessary facilities and funding to carry out the research.
Author contributions
KC and RKS conceived the study and designed the experiment; PS, KoC and RKS did the phenotyping, KC did analysis of genotyping data, QTL identification and mapping, KoC and KC did reference genome search and identification of functional genes. KC, KoC and RKS drafted the manuscript. All the authors read and approved the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Krishnendu Chattopadhyay, Email: krishnenducrri@gmail.com.
Koushik Chakraborty, Email: koushikiari@gmail.com.
Prabhudatta Samal, Email: samalprabhudutt@gmail.com.
Ramani Kumar Sarkar, Email: rksarkarcrri@gmail.com.
References
- Afzal AJ, Wood AJ, Lightfoot DA. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol Plant-Microbe Inter. 2008;21:507–517. doi: 10.1094/MPMI-21-5-0507. [DOI] [PubMed] [Google Scholar]
- Bhaduri D, Chakraborty K, Nayak AK, Shahid M, Tripathi R, Behera R, Singh S, Srivastava AK. Alteration in plant spacing improves submergence tolerance in Sub1 and non-Sub1 rice (cv. IR64) by better light interception and effective carbohydrate. Funct Plant Biol. 2020;47:891–903. doi: 10.1071/FP19364. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Marc L, Bjoern U. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Guru A, Jena P, Ray S, Guhey A, Chattopadhyay K, Sarkar RK. Rice with SUB1 QTL possesses greater initial leaf gas film thickness leading to delayed perception of submergence stress. Ann Bot. 2021;127:251–265. doi: 10.1093/aob/mcaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Ray S, Vijayan J, Molla KA, Nagar R, Jena P, Mondal S, Panda BB, Shaw BP, Swain P, Chattopadhyay K, Sarkar RK. Preformed aerenchyma determines the differential tolerance response under partial submergence imposed by fresh and saline water flooding in rice. Physiol Plant. 2021;173(4):1597–1615. doi: 10.1111/ppl.13536. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K, Mohanty SK, Vijayan J, Marndi BC, Molla KA, Chakraborty K, Ray S, Sarkar RK. Genetic dissection of component traits for salinity tolerance at reproductive stage in rice. Plant Mol Biol Rep. 2020;7:1–7. [Google Scholar]
- Christianson JA, Wilson IW, Llewellyn DJ, Dennis ES. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 2009;149(4):1724–1738. doi: 10.1104/pp.108.131912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Li Y, Cui C, Huo Y, Lu G, Yang H. Proteomic and metabolic profile analysis of low-temperature storage responses in Ipomoea batata Lam. tuberous roots. BMC Plant Biol. 2020;20(1):1–5. doi: 10.1186/s12870-020-02642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeba F, Sultana T, Javaid B, Mahmood T, Naqvi SM. Molecular characterization of a MYB protein from Oryza sativa for its role in abiotic stress tolerance. Braz Arch Biol Technol. 2017;60:e17160352. [Google Scholar]
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5(3):123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6:e19379. doi: 10.1371/journal.pone.0019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2017). http://www.fao.org/3/i7876e/i7876e.pdf. Accessed 5 June 2021
- Fernandez GCJ (1992) Effective selection criteria for assessing plant stress tolerance. In: Proceedings of the international symposium on adaptation of vegetables and other food crops in temperature and water stress, 13–16 Aug 1992, Shanhua, Taiwan, pp 257–270
- Fischer R, Maurer R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust J Agric Res. 1978;29:897–912. [Google Scholar]
- Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM. Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front Plant Sci. 2019;10:340. doi: 10.3389/fpls.2019.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Courty PE, Le Signor C, Wipf D, Vernoud V. Sulfate transporters in the plant’s response to drought and salinity: regulation and possible functions. Front Plant Sci. 2014;5:580. doi: 10.3389/fpls.2014.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CA, Paul MJ, Foyer CH. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim Biophys Acta. 2016;1857:1715–1725. doi: 10.1016/j.bbabio.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena AH, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ 24(12): 1369–75.
- Han C, Li J, Ma Y, Guo J, Guo X, Xu J. PlWRKY70: a Paeonia lactiflora transcription factor that sensitively responds to low-temperature, salt and waterlogging stresses. Can J Plant Sci. 2019;100:146–155. [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments. Plant Sign Behav. 2012;7:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Chou MY, Chou SJ, Li YR, Peng HP, Shiha MC. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell. 2013;25:2699–2713. doi: 10.1105/tpc.113.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Collard BC, Septiningsih EM, Ismail AM. Physiological analyses of traits associated with tolerance of long-term partial submergence in rice. AoB Plants. 2014;6:plu058. doi: 10.1093/aobpla/plu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, van der Knaap E, Cho HT. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecker M, Gasch P, Peisker H, Dörmann P, Schlicke H, Grimm B, Mustroph A. A shoot-specific hypoxic response of Arabidopsis sheds light on the role of the phosphate-responsive transcription factor phosphate starvation response1. Plant Physiol. 2014;165:774–790. doi: 10.1104/pp.114.237990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J. 2002;30:337–348. doi: 10.1046/j.1365-313x.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- Kuanar SR, Ray A, Sethi SK, Chattopadhyay K, Sarkar RK. Physiological basis of stagnant flooding tolerance in rice. Rice Sci. 2017;24:73–84. [Google Scholar]
- Kuanar SR, Molla KA, Chattopadhyay K, Sarkar RK, Mohapatra PK. Introgression of Sub1(SUB1) QTL in mega rice cultivars increases ethylene production to the detriment of grain-filling under stagnant flooding. Sci Rep. 2019;9:18567. doi: 10.1038/s41598-019-54908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata N, Yamazaki Y. Oryzabase. An integrated biological and genome information database for rice. Plant Physiol. 2006;140:12–17. doi: 10.1104/pp.105.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zheng HL, Wang JG, et al. Genetic dissection of rice (Oryza sativa L.) tiller, plant height, and grain yield based on QTL mapping and meta-analysis. Euphytica. 2018;214:109. [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothier J, Diab H, Cukier C, Limami AM, Tcherkez G. Metabolic responses to waterlogging differ between roots and shoots and reflect phloem transport alteration in Medicago truncatula. Plants. 2020;9:1373. doi: 10.3390/plants9101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Li H, Zhang L, et al. QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015;3:269–283. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D, Rao DN, Sharma SG, Strasser RJ, Sarkar RK. Submergence effects on rice genotypes during seedling stage: probing of submergence driven changes of photosystem 2 by chlorophyll a fluorescence induction O–J–I–P transients. Photosynthetica. 2006;44:69–75. [Google Scholar]
- Panda D, Ray A, Sarkar RK. Yield and photochemical activity of selected rice cultivars from Eastern India under medium depth stagnant flooding. Photosynthetica. 2019;57:1084–1093. [Google Scholar]
- Pathak H, Kumar M, Molla KA, Chakraborty K. Abiotic stresses in rice production: impacts and management. Oryza. 2021;58(4):103–125. [Google Scholar]
- Pradhan B, Chakraborty K, Prusty N, et al. Distinction and characterization of rice genotypes tolerant to combined stresses of salinity and partial submergence, proved by a high-resolution chlorophyll fluorescence imaging system. Funct Plant Biol. 2019;46:248–261. doi: 10.1071/FP18157. [DOI] [PubMed] [Google Scholar]
- Qi W, Sun F, Wang Q, Chen M, Huang Y, Feng YQ, Luo X, Yang J. Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol. 2011;157(1):216–228. doi: 10.1104/pp.111.179945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri J, Ribichich KF, Chan RL. The sunflower transcription factor HaWRKY76 confers drought and flood tolerance to Arabidopsis thaliana plants without yield penalty. Plant Cell Rep. 2015;34:2065–2080. doi: 10.1007/s00299-015-1852-3. [DOI] [PubMed] [Google Scholar]
- Ruiz JM, Sanchez E, Garcia PC, Lopez-Lefebre LR, Rivero RM, Romero L. Proline metabolism and NAD kinase activity in green bean plants subjected to cold-shock. Phytochemistry. 2002;59:473–478. doi: 10.1016/s0031-9422(01)00481-2. [DOI] [PubMed] [Google Scholar]
- Sakai H, Lee SS, Tanaka T, et al. Rice annotation project database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol. 2013;54:e6. doi: 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar RK, Chakraborty K, Chattopadhyay K, Ray S, Panda D, Ismail AM. Responses of rice to individual and combined stresses of flooding and salinity. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas J, editors. Advances in rice research for abiotic stress tolerance. Sawston: Woodhead Publishing; 2019. pp. 281–297. [Google Scholar]
- Sarkar RK (2016) Stagnant flooding tolerance in rice: endeavours and achievement. NRRI Research Bulletin No. 11, ICAR-National Rice Research Institute, Cuttack
- Sasidharan R, Voesenek LA. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015;169:3–12. doi: 10.1104/pp.15.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar PN, Amrutha RN, Sangam S, Verma DP, Kishor PK. Biochemical characterization, homology modeling and docking studies of ornithine δ-aminotransferase—an important enzyme in proline biosynthesis of plants. J Mol Graph Model. 2007;26(4):709–719. doi: 10.1016/j.jmgm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Singh S, Mackill DJ, Ismail AM. Tolerance of longer-term partial stagnant flooding is independent of the SUB1 locus in rice. Field Crops Res. 2011;121:311–323. [Google Scholar]
- Singh A, Carandang J, Gonzaga ZJC, Collard BC, Ismail AM, Septiningsih EM. Identification of QTLs for yield and agronomic traits in rice under stagnant flooding conditions. Rice. 2017;10:15. doi: 10.1186/s12284-017-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Geske T, Sauter M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011;190:369–378. doi: 10.1111/j.1469-8137.2010.03496.x. [DOI] [PubMed] [Google Scholar]
- Sweetman C, Waterman CD, Rainbird BM, Smith PMC, Jenkins CD, Day DA, Soole KL. AtNDB2 is the main external NADH Dehydrogenase in mitochondria and is important for tolerance to environmental stress. Plant Physiol. 2019;181:774–788. doi: 10.1104/pp.19.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara GV, Nugraha Y, Esguerra MQ, Mackill DJ, Ismail AM. Variation in tolerance of rice to long-term stagnant flooding that submerges most of the shoot will aid in breeding tolerant cultivars. AoB Plants. 2014;6:plu055. doi: 10.1093/aobpla/plu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan J, Senapati S, Ray S, Chakraborty K, Molla KA, Basak N, Pradhan B, Yeasmin L, Chattopadhyay K, Sarkar RK. Transcriptomic and physiological studies identify cues for germination stage oxygen deficiency tolerance in rice. Environ Exp Bot. 2018;147:234–248. [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol. 2015;206:57–73. doi: 10.1111/nph.13209. [DOI] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164–e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Sandhu N, Singh VK, Catolos M, Kumar A. Genotyping-by-sequencing based QTL mapping for rice grain yield under reproductive stage drought stress tolerance. Sci Rep. 2019;9:14326. doi: 10.1038/s41598-019-50880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot. 2014;65:261–273. doi: 10.1093/jxb/ert371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Tanaka A, Inahashi H, Nishizawa NK, Tsutsumi N, Inukai Y, Nakazono M. Fine control of aerenchyma and lateral root development through AUX/IAA-and ARF-dependent auxin signaling. Proc Natl Acad Sci. 2019;116:20770–20775. doi: 10.1073/pnas.1907181116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N, Ichikawa T, Kondou Y, Iwabuchi M, Matsui M, Hirochika H, Oda K. Role of the rice transcription factor JAmyb in abiotic stress response. J Plant Res. 2013;126:131–139. doi: 10.1007/s10265-012-0501-y. [DOI] [PubMed] [Google Scholar]
- Yuan X, Wang H, Cai J, Bi Y, Li D, Song F. Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol. 2019;19:1–9. doi: 10.1186/s12870-019-1883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukiyoshi K, Karahara I. Role of ethylene signalling in the formation of constitutive aerenchyma in primary roots of rice. AoB Plants. 2014;1:6. doi: 10.1093/aobpla/plu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou G, Shabala S, Koutoulis A, Shabala L, Johnson P, Li C, Zhou M. Identifcation of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor Appl Genet. 2016;129:1167–1177. doi: 10.1007/s00122-016-2693-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yu Z, Yao X, Chen J, Chen X, Zhou H, Lou Y, Ming F, Jin Y. Genome-wide identification and characterization of small auxin-up RNA (SAUR) gene family in plants: evolution and expression profiles during normal growth and stress response. BMC Plant Biol. 2021;21:1–4. doi: 10.1186/s12870-020-02781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S, editors. Waterlogging signaling and tolerance in plants. Berlin: Springer; 2010. pp. 267–285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.