Abstract
Aims
Cardiovascular magnetic resonance (CMR) imaging is a key diagnostic tool for the evaluation of patients with suspected cardiac tumours. Patient management is guided by the CMR diagnosis, including no further testing if a mass is excluded or if only a pseudomass is found. However, there are no outcomes studies validating this approach.
Methods and results
In this multicentre study of patients undergoing clinical CMR for suspected cardiac tumour, CMR diagnoses were assigned as no mass, pseudomass, thrombus, benign tumour, or malignant tumour. A final diagnosis was determined after follow-up using all available data. The primary endpoint was all-cause mortality. Among 903 patients, the CMR diagnosis was no mass in 25%, pseudomass in 16%, thrombus in 16%, benign tumour in 17%, and malignant tumour in 23%. Over a median of 4.9 years, 376 patients died. Compared with the final diagnosis, the CMR diagnosis was accurate in 98.4% of patients. Patients with CMR diagnoses of pseudomass and benign tumour had similar mortality to those with no mass, whereas those with malignant tumour [hazard ratio (HR) 3.31 (2.40–4.57)] and thrombus [HR 1.46 (1.00–2.11)] had greater mortality. The CMR diagnosis provided incremental prognostic value over clinical factors including left ventricular ejection fraction, coronary artery disease, and history of extracardiac malignancy (P < 0.001).
Conclusion
In patients with suspected cardiac tumour, CMR has high diagnostic accuracy. Patients with CMR diagnoses of no mass, pseudomass, and benign tumour have similar long-term mortality. The CMR diagnosis is a powerful independent predictor of mortality incremental to clinical risk factors.
Keywords: Cardiac magnetic resonance, Cardiac masses, Cardiac tumours, Cardio-oncology, Diagnosis, Prognosis
Graphical Abstract
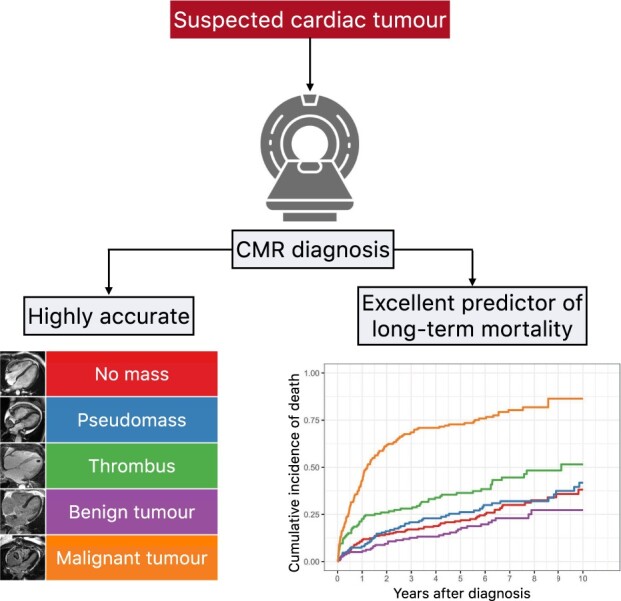
In patients with suspected cardiac tumour, CMR has high diagnostic accuracy and is an excellent independent predictor of long-term mortality.
See page 81 for the editorial comment for this article ‘When tissue and outcomes are the issue. Cardiac magnetic resonance for patients with suspected cardiac tumours’, by S. Giusca, S. Kelle, and G. Korosoglou, https://doi.org/10.1093/eurheartj/ehab625.
Introduction
Expert consensus documents recommend cardiovascular magnetic resonance (CMR) imaging as a key diagnostic tool in the evaluation of patients with suspected cardiac tumours.1–3 Patient management is often guided by the CMR diagnosis, including anticoagulation if a thrombus is found and no further testing if a mass is excluded or if only a pseudomass—a prominent normal structure or common variant—is detected. However, the American College of Cardiology Foundation Expert Consensus Document on CMR, while indicating that CMR is ‘appropriate’ for the evaluation of a suspected cardiac mass, recognizes that ‘no existing guidelines are established for the evaluation of a cardiac mass with CMR’.2 This statement likely reflects, in part, the dearth of evidence demonstrating the prognostic value of CMR for this purpose.
Investigations to date of the use of CMR in the evaluation of cardiac masses have several limitations. Nearly all are small single-centre studies that typically focus on imaging characteristics and the diagnostic accuracy of CMR for differentiating between various types of cardiac tumours.4–8 Often, studies only include patients with pathology-proven cardiac tumours,4 , 6 , 8–10 yet these represent only a small fraction of patients with suspected cardiac tumours who undergo CMR.
There is a paucity of studies of patients with suspected cardiac tumour that correlate the imaging diagnosis with outcomes. For example, although CMR is widely used to exclude a cardiac mass, there are no studies with systematic follow-up of patients in whom a cardiac mass has been excluded to determine their long-term outcomes, including whether a cardiac mass is subsequently diagnosed during follow-up. Hence, the aim of this multicentre study was to determine the prognostic value of the ‘real-world’ CMR diagnosis in patients clinically referred to CMR for suspected cardiac tumour.
Methods
Study design and cohort
Four geographically diverse medical centres in the USA participated in this observational study. The University of Minnesota Medical Center served as the data coordinating centre, using a cloud-based data aggregation system (CloudCMR software, Heart Imaging Technologies, Durham, NC, USA) as described previously.11 The cloud-based system contained clinical data, finalized CMR reports, and full Digital Imaging and Communications in Medicine image datasets (all de-identified) submitted by the participating centres. Institutional review board approval was obtained at each participating centre.
Between January 2003 and December 2014, consecutive patients 18 years of age or older referred to CMR for suspected cardiac tumour were enrolled. To avoid a preponderance of patients with cardiac thrombus in our study, patients with a clear, pre-CMR diagnosis of cardiac thrombus and with low diagnostic suspicion for a cardiac tumour were not enrolled. Patients were excluded if CMR examinations were incomplete (n = 6) or if they had cardiac implantable electronic devices (n = 5). A total of 935 patients formed the study cohort. No patients were excluded for poor image quality.
Data available in the cloud-based system were supplemented by additional data collection at each participating site. This included a comprehensive medical history at the time of the CMR study and data collected during follow-up including details of clinical management (e.g. anticoagulation therapy, radiation therapy, chemotherapy, surgery) and pathology results from either biopsy or surgical specimens. In patients found to have either no mass or a pseudomass by CMR, follow-up clinical data were reviewed (including follow-up imaging studies if performed) to determine if patients were subsequently diagnosed with a cardiac mass. Patients were also followed for the primary endpoint of all-cause mortality. The cloud-based system included mortality data, which was automatically determined every 3 months by comparing patient identifiers (only available locally) to the US Social Security Death Index. Where available, the Social Security Death Index mortality data were supplemented with information from other locally available data sources such as the hospital electronic medical record and the state’s vital records department. Data collection ended in December 2018.
Cardiovascular magnetic resonance protocol
Patients were scanned on 1.5 or 3 T scanners (Siemens, Malvern, PA, USA) with phased array coil systems. Sites used the same standardized protocol as described by the Society for Cardiovascular Magnetic Resonance (SCMR).12–14 The SCMR protocol comprises (i) steady-state free precession (SSFP) cine imaging, (ii) bright-blood (SSFP) and dark-blood (HASTE) single-shot morphological imaging, (iii) T1- and T2-weighted fast spin-echo imaging with and without fat saturation, (iv) first-pass perfusion imaging during the administration of gadolinium contrast, and (v) two sets of late gadolinium enhancement (LGE) imaging: one with inversion time set to null normal myocardium and the other with inversion time set longer (long-TI LGE) to null thrombus (∼500–550 ms at 1.5 T, 850–900 ms at 3 T).13–17 Also, as described by the SCMR protocol,13 , 14 optional sequences such as post-contrast cine imaging (often helpful for small mobile masses) and serial LGE imaging (to distinguish hypoperfused tumour necrotic core from thrombus) were performed as needed. Specific details of the CMR pulse sequences are provided in Supplementary material online.
Cardiovascular magnetic resonance diagnosis and final diagnosis
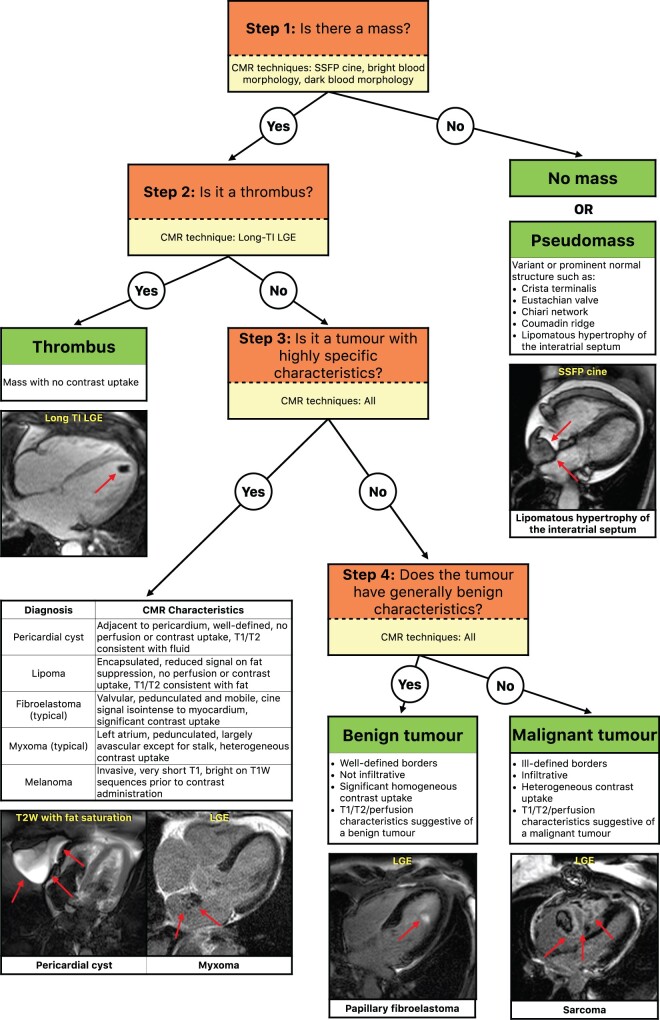
Experienced CMR physicians (all with SCMR-Level III training) at each participating centre performed clinical interpretations within 1 day following CMR. An algorithm of the approach to interpretation used by the physicians is provided in Figure 1. In brief, the interpretation was based on conventional imaging features described in the literature;9 , 18 , 19 however, a stepwise approach was emphasized with certain diagnoses more dependent on specific CMR techniques than others. For example, differentiating thrombus from tumour (e.g. Figure 1 ‘Step 2’) was based on long-TI LGE images.
Figure 1.
Stepwise algorithm used for cardiovascular magnetic resonance interpretation of patients with suspected cardiac tumour. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; SSFP, steady-state free precession.
Exclusion of a cardiac mass (‘no mass’) required the absence of a mass or any structure that could have been mistaken for a mass on prior imaging. A ‘pseudomass’ was diagnosed when physicians noted a prominent normal structure (Eustachian valve, crista terminalis, etc.), a variant of a normal structure (lipomatous hypertrophy of the interatrial septum, epicardial fat, etc.), or a non-mass pathology (hiatal hernia) that could have been mistaken for a cardiac mass on prior imaging. A thrombus was diagnosed based on its location within the cardiac cavity, and tissue characteristics consistent with a homogeneous, avascular mass. This determination was made on post-contrast LGE images obtained with a long inversion time showing a homogeneously dark mass with no contrast uptake. A tumour was diagnosed and determined to be benign or malignant, based on general features such as location (intracavitary, intramural, or epicardial), morphology (rounded, irregular), border definition at the interface with the normal myocardium (well-defined, infiltrating), perfusion characteristics (hypoperfused, hypervascular), and late contrast uptake (homogeneous, heterogeneous), as well as sequence-specific characteristics, suggesting that the mass was composed of fat (fat suppression sequences) or cystic fluid (T1 and/or T2 weighting consistent with fluid).
To assess the accuracy of the CMR diagnosis, all participating centres were instructed to determine a ‘final diagnosis’ for each patient using all available clinical data during the follow-up period, including follow-up imaging studies, pathology data, clinical course, and outcome. In patients with CMR diagnoses of no mass or pseudomass, follow-up included determination of whether a thrombus or tumour was later diagnosed. Similarly, follow-up in cases of thrombus included determination of response to anticoagulation, such as resolution of the mass. When available, the pathology diagnosis of the cardiac mass was assumed as the reference standard.
Data coordinating centre and core lab interpretations
The data coordinating centre verified the completeness of data submitted by the participating sites. An investigator at the centre reviewed clinical reports on the CloudCMR system and assigned each study patient to one of the five categories based on the clinical CMR interpretation: (i) no mass, (ii) pseudomass, (iii) thrombus, (iv) benign tumour, and (v) malignant tumour. Diagnoses that did not fit into any of the first five categories were categorized as ‘Other’. The assignment was done blinded to all clinical data after the CMR, including mass-directed clinical management and outcome. In patients interpreted to have pseudomass or benign tumour, the specific type was noted. In patients diagnosed to have thrombus, the location was noted. Specific types of malignant tumours were not categorized since CMR features of different malignant tumours overlap significantly, and the clinical history of an extracardiac malignancy often guides the interpretation of the specific type of malignant cardiac tumour. In instances where thrombus was noted covering or overlying a tumour, the CMR diagnosis was tumour. Tumours with variable aggressiveness (thymoma, carcinoid, etc.) were classified as malignant based on the implication for patient management.
To test the ‘stand-alone’ value of CMR interpretations independent of all associated clinical information, centralized core lab interpretations were made in a random subgroup of 200 study patients. Two expert investigators at the data coordinating centre reviewed CMR images on the CloudCMR system blinded to the clinical report and all other clinical data, and by consensus assigned patients to one of the categories. Cases of no mass and pseudomass were categorized together as ‘no mass-or-pseudomass’ because pre-CMR clinical information may increase the likelihood of a CMR diagnosis of pseudomass. For instance, the knowledge that CMR was requested for a tumour suspected along the posterior wall of the right atrium increases the likelihood that a physician would interpret a prominent crista terminalis as pseudomass, whereas a physician blinded to that information is more likely to interpret the CMR as showing no mass.
Statistical analyses
Continuous, normally distributed data were expressed as mean ± standard deviation, and between-group comparisons were made using two-sample t-tests. The median and the interquartile range were used to summarize non-normally distributed, continuous data, and between-group comparisons were made using Wilcoxon tests. Comparisons of discrete variables between groups were made using χ 2 test or Fisher’s exact test. Kaplan–Meier survival analyses and Cox proportional hazards regression analyses were used to evaluate the relationships between CMR diagnostic categories and death. Pairwise unadjusted comparisons between CMR diagnostic categories were made using the log-rank test with P-value adjustment for multiple testing using the Benjamini and Hochberg method.20 Covariates for inclusion in the multivariable Cox regression models were selected a priori and included age, sex, hypertension, hyperlipidaemia, diabetes, smoking, coronary artery disease (CAD), left ventricular ejection fraction (LVEF) from CMR, and history of extracardiac malignancy. The assumption of proportional hazards was assessed by plotting the scaled Schoenfeld residuals for each independent variable against time; these correlations were found to be non-significant for all variables included in the multivariable models. All tests were two-tailed. A P-value of <0.05 was used to denote statistical significance. Analyses were performed using R, version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study cohort consisted of 935 consecutive adult patients from four centres (Duke University Medical Center, n = 384; Houston Methodist Hospital, n = 266; University of Minnesota Medical Center, n = 171; and Virginia Commonwealth University Medical Center, n = 114). Thirty-two (3%) patients had a CMR diagnosis of ‘Other’ (Supplementary material online, Table S1). Among these, 14 had valve-associated vegetations, but otherwise, the possible diagnoses were heterogeneous, and these 32 patients were excluded from further analyses.
Baseline patient characteristics
Clinical and imaging characteristics at the time of CMR of the remaining 903 patients are shown in Table 1. The median age was 60 years, and 46% of patients were male. Cardiac risk factors were prevalent: 59% had hypertension, 40% had a history of smoking, and 27% had CAD. Nearly one-third of patients had a diagnosis of extracardiac malignancy (32%). Among imaging studies preceding the CMR, echocardiography was the most common (78%), followed by computed tomography (25%).
Table 1.
Patient characteristics—overall and by cardiovascular magnetic resonance interpretation
| CMR diagnosis |
P-value | ||||||
|---|---|---|---|---|---|---|---|
| All patients (n = 903) | No cardiac mass (n = 236) | Pseudomass (n = 149) | Thrombus (n = 146) | Benign tumour (n = 159) | Malignant tumour (n = 213) | ||
| Age (years), median (IQR) | 60 (47–69) | 57 (45–68) | 67 (57–73) | 55 (43–65) | 63 (51–72) | 58 (46–66) | <0.001 |
| Male sex, n (%) | 412 (45.6) | 119 (50.4) | 41 (27.5) | 87 (59.6) | 51 (32.1) | 114 (53.5) | <0.001 |
| CAD risk factors | |||||||
| Hypertension, n (%) | 535 (59.2) | 141 (59.7) | 101 (67.8) | 94 (64.4) | 102 (64.2) | 97 (45.5) | <0.001 |
| Hyperlipidaemia, n (%) | 405 (44.9) | 112 (47.5) | 69 (46.3) | 89 (61.0) | 72 (45.3) | 63 (29.6) | <0.001 |
| Diabetes mellitus, n (%) | 181 (20.0) | 49 (20.8) | 35 (23.5) | 40 (27.4) | 33 (20.8) | 24 (11.3) | 0.003 |
| Smoking, n (%) | 359 (39.8) | 82 (34.7) | 73 (49.0) | 69 (47.3) | 54 (34.0) | 81 (38.0) | 0.008 |
| Known CAD, n (%) | 239 (26.5) | 54 (22.9) | 34 (22.8) | 71 (48.6) | 40 (25.2) | 40 (18.8) | <0.001 |
| Known extracardiac malignancy, n (%) | 286 (31.7) | 47 (19.9) | 27 (18.1) | 32 (21.9) | 23 (14.5) | 157 (73.7) | <0.001 |
| CMR LVEF, median (IQR) | 60 (55–65) | 60 (55–65) | 61 (55–65) | 45 (25–60) | 65 (60–68) | 60 (55–65) | <0.001 |
| Symptoms and signs, n (%) | |||||||
| Dyspnoea | 307 (34.0) | 60 (25.4) | 44 (29.5) | 60 (41.1) | 37 (23.3) | 106 (49.8) | <0.001 |
| Chest pain | 156 (17.3) | 29 (12.3) | 30 (20.1) | 24 (16.4) | 30 (18.9) | 43 (20.2) | 0.16 |
| Palpitations | 30 (3.3) | 11 (4.7) | 4 (2.7) | 3 (2.1) | 4 (2.5) | 8 (3.8) | 0.68 |
| Oedema | 51 (5.6) | 12 (5.1) | 8 (5.4) | 19 (13.0) | 5 (3.1) | 7 (3.3) | 0.001 |
| Arrhythmia | 72 (8.0) | 21 (8.9) | 17 (11.4) | 14 (9.6) | 10 (6.3) | 10 (4.7) | 0.14 |
| Pre-syncope | 19 (2.1) | 5 (2.1) | 0 (0.0) | 6 (4.1) | 3 (1.9) | 5 (2.3) | 0.14 |
| Syncope | 36 (4.0) | 17 (7.2) | 3 (2.0) | 6 (4.1) | 6 (3.8) | 4 (1.9) | 0.045 |
| Thromboembolism | 84 (9.3) | 33 (14.0) | 9 (6.0) | 19 (13.0) | 19 (11.9) | 4 (1.9) | <0.001 |
| Other symptoms | 250 (27.7) | 51 (21.6) | 34 (22.8) | 26 (17.8) | 36 (22.6) | 103 (48.4) | <0.001 |
| None | 207 (22.9) | 54 (22.9) | 40 (26.8) | 39 (26.7) | 42 (26.4) | 32 (15.0) | 0.025 |
| Imaging preceding CMR, n (%) | |||||||
| Echocardiography | 706 (78.2) | 206 (87.3) | 136 (91.3) | 119 (81.5) | 123 (77.4) | 122 (57.3) | <0.001 |
| Computed tomography | 222 (24.6) | 22 (9.3) | 13 (8.7) | 14 (9.6) | 46 (28.9) | 127 (59.6) | <0.001 |
| Plain radiography | 21 (2.3) | 4 (1.7) | 1 (0.7) | 0 (0.0) | 4 (2.5) | 12 (5.6) | 0.003 |
| Coronary angiography | 35 (3.9) | 12 (5.1) | 2 (1.3) | 14 (9.6) | 3 (1.9) | 4 (1.9) | <0.001 |
| Positron emission tomography | 36 (4.0) | 10 (4.2) | 0 (0.0) | 1 (0.7) | 1 (0.6) | 24 (11.3) | <0.001 |
| None | 60 (6.6) | 12 (5.1) | 7 (4.7) | 22 (15.1) | 6 (3.8) | 13 (6.1) | <0.001 |
CAD, coronary artery disease; CMR, cardiovascular magnetic resonance; IQR, interquartile range; LVEF, left ventricular ejection fraction. P-values in bold are <0.05.
The CMR diagnosis was no mass in 236 (25%), pseudomass in 149 (16%), thrombus in 146 (16%), benign tumour in 159 (17%), and malignant tumour in 213 (23%). Patients with pseudomass were like those with no mass in nearly all characteristics except sex; patients in the pseudomass group were disproportionately female (72%), whereas those with no mass were evenly distributed by sex (50% women). The most common types of pseudomass were lipomatous hypertrophy of the interatrial septum, prominent epicardial fat, prominent Eustachian valve, prominent crista terminalis, and hiatal hernia, accounting for 66% of all cases (Supplementary material online, Table S2).
Patients with cardiac thrombus formed the youngest group, with the lowest proportion of women, the lowest mean LVEF, and the highest prevalence of CAD. Thrombus was most often located in the left ventricle (50%) and right atrium (34%) (Supplementary material online, Table S3). Patients with right atrial thrombus were more likely to have a diagnosis of extracardiac malignancy than those with left ventricular thrombus (41% vs. 10%; P < 0.001). Approximately 20% of patients in each of the diagnostic groups of no mass, pseudomass, and thrombus had extracardiac malignancy; yet despite this high prevalence of extracardiac malignancy, a cardiac tumour was excluded by CMR in these diagnostic groups.
Patients with a CMR diagnosis of benign tumour were disproportionately female (68%) whereas those with malignant tumour were evenly distributed by sex (46% women). The most common types of benign tumour were myxomas (42%), papillary fibroelastomas (23%), and pericardial cysts (22%) (Supplementary material online, Table S4). The benign (cardiac) tumour group had the lowest rate of extracardiac malignancy (15%). While patients with a CMR diagnosis of malignant (cardiac) tumour had the highest prevalence of extracardiac malignancy, notably, 26% of this cohort had no known extracardiac malignancy at the time of CMR.
Clinical follow-up and final diagnoses
Patients were followed for a median of 4.9 years (interquartile range 1.6–7.3 years) with a total of 4285 patient-years of follow-up. Pathology of the cardiac mass was available in 226 patients, representing 47% of those with a CMR diagnosis of benign tumour and 60% of those with a CMR diagnosis of malignant tumour. The final diagnosis using all available clinical data during the follow-up period was no mass in 235, pseudomass in 149, thrombus in 145, benign tumour in 164, and malignant tumour in 210. The most common malignant tumours were sarcoma, lymphoma, and melanoma (Supplementary material online, Table S5).
The CMR diagnosis was accurate in 98.4% (889/903) of patients compared against the final diagnosis. The 14 patients with discordant CMR and final diagnoses are listed in Table 2. The lone patient with an incorrect CMR diagnosis of no mass had a small mobile mass with chaotic motion attached to the mitral valve chordal apparatus on echocardiography. The CMR report noted that although the small mass was not visualized, this was likely because of motion averaging. The patient subsequently had surgery that revealed a left ventricular papillary fibroelastoma. The CMR diagnosis of pseudomass was concordant with the final diagnosis in all cases. All four patients with an incorrect CMR diagnosis of thrombus had a final diagnosis of benign tumour, three of whom were myxomas. Of the five patients with an incorrect CMR diagnosis of benign tumour, two were believed to have myxoma but were found to have thrombus on the final diagnosis; two others had a final diagnosis of malignant tumour; however, the specific diagnoses of carcinoid tumour and teratoma have variable malignant potential. All four patients with an incorrect CMR diagnosis of malignant tumour had a final diagnosis of benign tumour, two of whom were haemangiomas that were hypervascular on perfusion imaging. Among 518 patients with a final diagnosis of thrombus or tumour, the long-TI sequence (Figure 1, ‘Step 2’) was accurate in 98.7% (512/519) in distinguishing thrombus from tumour. Among 374 patients with a final diagnosis of tumour, CMR was accurate in 98.4% (368/374) in distinguishing benign from malignant (Figure 1, ‘Steps 3 and 4’).
Table 2.
Patients with discordant cardiovascular magnetic resonance diagnosis and final diagnosis
| Patient number | CMR diagnosis | Final diagnosis |
|---|---|---|
| 1 | No mass | Benign tumour—papillary fibroelastoma (LV) |
| 2 | Thrombus (RA) | Benign tumour—calcified amorphous tumour |
| 3 | Thrombus (RA) | Benign tumour—myxoma |
| 4 | Thrombus (LA) | Benign tumour—myxoma |
| 5 | Thrombus (LA) | Benign tumour—myxoma |
| 6 | Benign tumour—myxoma (RA) | Thrombus |
| 7 | Benign tumour—myxoma (RA) | Thrombus |
| 8 | Benign tumour—encapsulated, non-specific (RA) | Thrombus (organized) |
| 9 | Benign tumour—unspecified benign cystic tumour | Malignant tumour—carcinoid tumour |
| 10 | Benign tumour—complex pericardial cyst | Malignant tumour—teratoma |
| 11 | Malignant tumour | Benign tumour—myxoma |
| 12 | Malignant tumour | Benign tumour—fibroma with hyalinization |
| 13 | Malignant tumour | Benign tumour—haemangioma |
| 14 | Malignant tumour | Benign tumour—haemangioma |
CMR, cardiovascular magnetic resonance; LA, Left Atrium; LV, Left Ventricle; RA, Right Atrium.
Mass-directed clinical management following CMR is detailed in Supplementary material online, Table S6. Eleven patients with a CMR diagnosis of pseudomass underwent surgical biopsy, which confirmed the CMR diagnosis. Most patients with a CMR diagnosis of thrombus received anticoagulation therapy (94%). Only patients with a CMR diagnosis of malignant tumour received chemotherapy and radiation therapy, but 12% had no cardiac tumour-specific treatment because of advanced disease.
In the subgroup of 200 patients with CMR interpretations that were performed blinded to all clinical information, there was high concordance between the interpretations with no clinical information and both the clinical CMR diagnosis and the final diagnosis (95% and 94% concordance, respectively; Supplementary material online, Table S7).
Long-term prognostic value and the incremental value of cardiovascular magnetic resonance
During follow-up, 376 patients died. Kaplan–Meier analysis (Figure 2) shows that patients with CMR diagnoses of pseudomass and benign tumour had similar long-term mortality to those with no mass, whereas those with thrombus and malignant tumour had greater mortality. The estimated cumulative 5-year mortality rates were 22% for no mass, 26% for pseudomass, 17% for benign tumour, 36% for thrombus, and 73% for malignant tumour.
Figure 2.
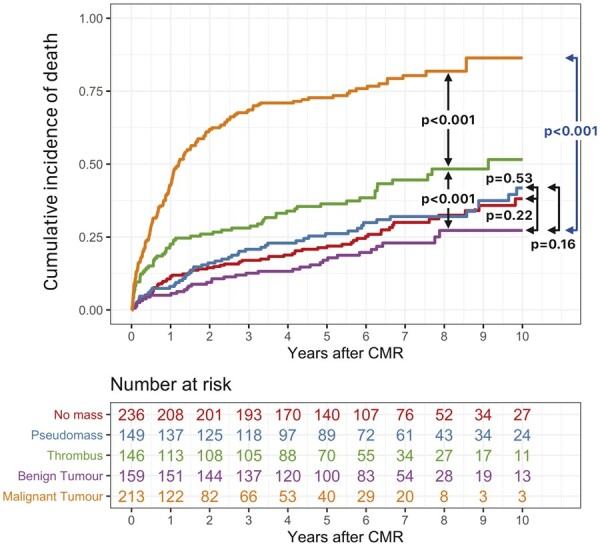
Kaplan–Meier curves for death during follow-up stratified by cardiovascular magnetic resonance diagnoses. CMR, cardiovascular magnetic resonance.
On Cox proportional hazards regression analysis, age [hazard ratio (HR) 1.09 (95% confidence interval 1.04–1.13) per 5-year increase], smoking [HR 1.37 (1.11–1.69)], CMR LVEF [HR 1.05 (1.01–1.10) per 5% decrease], extracardiac malignancy [HR 2.32 (1.81–2.97)], CMR diagnosis of thrombus [HR 1.46 (1.00–2.11) relative to CMR diagnosis of no mass], and CMR diagnosis of malignant tumour [HR 3.31 (2.40–4.57) relative to CMR diagnosis of no mass] were independently associated with mortality (Table 3).
Table 3.
Cox multivariable analyses and the incremental value of cardiovascular magnetic resonance diagnosis for the prediction of all-cause death
| Covariates | All patients (n = 903) |
Patients without extracardiac malignancy (n = 617) |
Patients with extracardiac malignancy (n = 286) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (per 5-year increase) | 1.09 (1.04–1.13) | <0.001 | 1.15 (1.08–1.22) | <0.001 | 1.04 (0.98–1.10) | 0.20 |
| Male sex | 1.10 (0.88–1.37) | 0.40 | 1.13 (0.81–1.59) | 0.47 | 1.15 (0.85–1.55) | 0.37 |
| Hypertension | 1.02 (0.80–1.30) | 0.87 | 0.91 (0.63–1.32) | 0.63 | 1.09 (0.78–1.52) | 0.61 |
| Hyperlipidaemia | 0.80 (0.63–1.02) | 0.07 | 0.68 (0.48–0.95) | 0.026 | 0.95 (0.68–1.34) | 0.78 |
| Diabetes | 1.22 (0.94–1.59) | 0.13 | 1.31 (0.92–1.86) | 0.13 | 0.96 (0.63–1.45) | 0.84 |
| Smoking | 1.37 (1.11–1.69) | 0.004 | 1.50 (1.09–2.06) | 0.013 | 1.39 (1.03–1.87) | 0.029 |
| Coronary artery disease | 1.17 (0.91–1.51) | 0.22 | 1.21 (0.83–1.76) | 0.32 | 1.15 (0.80–1.65) | 0.46 |
| CMR LVEF (per 5% decrease) | 1.05 (1.01–1.10) | 0.019 | 1.09 (1.03–1.15) | 0.005 | 1.02 (0.95–1.09) | 0.58 |
| Extracardiac malignancy | 2.32 (1.81–2.97) | <0.001 | — | — | — | — |
| CMR diagnosis—no mass | Reference | NA | Reference | NA | Reference | NA |
| CMR diagnosis—pseudomass | 1.03 (0.70–1.51) | 0.90 | 1.02 (0.63–1.65) | 0.93 | 0.98 (0.51–1.91) | 0.96 |
| CMR diagnosis—thrombus | 1.46 (1.00–2.11) | 0.048 | 1.45 (0.90–2.35) | 0.13 | 1.18 (0.64–2.18) | 0.59 |
| CMR diagnosis—benign tumour | 0.77 (0.50–1.17) | 0.22 | 0.79 (0.47–1.31) | 0.36 | 0.81 (0.38–1.76) | 0.60 |
| CMR diagnosis—malignant tumour | 3.31 (2.40–4.57) | <0.001 | 5.40 (3.38–8.63) | <0.001 | 2.28 (1.45–3.57) | <0.001 |
| Incremental value testing | ||||||
| χ 2 | P-value | χ 2 | P-value | χ 2 | P-value | |
| Clinical model | 215.8 | <0.001 | 49.6 | <0.001 | 18.8 | 0.02 |
| Clinical model with CMR diagnosis | 299.8 | <0.001 | 106.9 | <0.001 | 45.2 | <0.001 |
| Likelihood ratio test comparing models with and without CMR diagnosis | <0.001 | <0.001 | <0.001 | |||
CI, confidence interval; CMR, cardiovascular magnetic resonance; HR, hazard ratio; LVEF, left ventricular ejection fraction; NA, Not applicable. P-values in bold are <0.05.
Patients with extracardiac malignancy compared to those without had different clinical variables associated with mortality. Only smoking predicted mortality in patients with extracardiac malignancy, whereas age, hyperlipidaemia, smoking, and CMR LVEF predicted mortality in patients without extracardiac malignancy. The CMR diagnosis of malignant tumour was predictive of mortality in both groups. The addition of the CMR diagnosis to a clinical model lacking the CMR diagnosis significantly increased the χ 2 statistic from 215.8 to 299.8 (P < 0.001) demonstrating incremental value. The CMR diagnosis had incremental value also in both patients with and without extracardiac malignancy.
Discussion
Cardiovascular magnetic resonance is one of the most used techniques for the assessment of patients with suspected cardiac tumours. While contemporary CMR can provide impressive anatomic, functional, and tissue characterization, there remains a large evidence gap between the demonstration of the feasibility of imaging and the demonstration of clinical benefit to patients. With concerns about the cost and the availability of CMR, outcomes data are essential to establish the value of CMR guidance for decision-making in these patients and to inform policymakers, clinicians, and patients.
The present study is the largest imaging study to date for the diagnosis of cardiac tumour and confirms the high accuracy of CMR previously reported in smaller cohorts in whom cardiac tumours were known to be present.5–8 More importantly, we found that CMR also has high accuracy in excluding a cardiac tumour. The significance of this finding is demonstrated by the observation that nearly half the CMRs (385/903) were requested to evaluate a suspected tumour in patients later found to have either no mass or pseudomass. However, proving that a tumour is truly absent, or is being mimicked by a pseudomass is inherently problematic, in part because such patients will not usually undergo biopsy or have pathology confirmation. In the absence of tissue confirmation, proving that CMR can be relied upon to definitively exclude a cardiac tumour required the multiyear follow-up performed in the present study. Our mean follow-up of nearly 5 years seems sufficient for any significant cardiac tumour to become apparent. Only a single small benign tumour (papillary fibroelastoma) not seen by CMR was later found during the 4285 patient-years of follow-up. These data validate for the first time the clinical practice of using CMR to exclude a cardiac tumour.
In patients in whom a mass was present, CMR offers a unique ability to differentiate between various diagnostic possibilities, particularly thrombus vs. tumour. One important aspect of our study was the systematic investigation of the long-TI sequence (Figure 1, ‘Step 2’), a technique originally validated by Weinsaft et al.,15 and recommended in the standardized SCMR protocols to distinguish between thrombus and tumour,13 , 14 although there are limited data supporting this recommendation. Thrombus, owing to its avascular nature, is low in signal intensity on long-TI images and is readily differentiated from a neoplasm that has a vascular supply. In the present study, we found that the long-TI sequence was highly accurate (98.7%; 512/519) in distinguishing thrombus from tumour. The few inaccuracies were almost entirely in cases of myxomas mistaken for thrombi, or vice versa, because of the overlap in rare cases of imaging findings common to both. Specifically, the avascular appearance of thrombus may be mimicked by a rare gelatinous myxoma with no discernible vascularized stalk.21 Given its ease of use, it should be noted that long-TI imaging can be implemented on any CMR-capable scanner, without requiring additional software or hardware.
Cardiovascular magnetic resonance also correctly distinguished benign from malignant tumours, owing to its excellent soft tissue contrast and high spatial resolution. Notably, this distinction was highly accurate (98.4%; 368/374), even though many patients had a history of extracardiac malignancy, which would seemingly increase the odds of a cardiac mass being malignant. Almost 20% of patients in each of the CMR diagnostic groups of no mass, pseudomass, and thrombus had a history of extracardiac malignancy but were nonetheless correctly diagnosed. These data show that many patients with known malignancy are referred for CMR to evaluate a finding suggestive of cardiac involvement and that CMR can reliably exclude such involvement.
Outcomes data from our study demonstrate that patients with CMR diagnoses of no mass, pseudomass, and benign tumour have a similar prognosis. Although there are no prior studies with long-term follow-up of patients deemed to have either no mass or pseudomass, the 5-year mortality in our patients with benign tumours was 17%, like previous reports.22 Patients with a CMR diagnosis of malignant tumour had a significantly worse prognosis. Five-year mortality rates of 83–89% have been reported in cancer registry studies of primary malignant cardiac tumours.23 , 24 Our lower 5-year mortality rate of 73% likely reflects improvements in survival rates over time, as has been noted in these registries.24 Patients with a CMR diagnosis of thrombus had an outcome intermediate between those with benign tumour and those with malignant tumour. The worse prognosis relative to those with benign tumour likely reflects the finding that these patients frequently had coexisting CAD and decreased LVEF.16
Little data exist on independent predictors of mortality in patients with suspected cardiac tumours, beyond the tumour type itself. We found that age, smoking, CMR LVEF, and extracardiac malignancy were independently associated with long-term mortality in the study cohort. Whereas age, hyperlipidaemia, smoking, and CMR LVEF predicted mortality in the subgroup of patients without extracardiac malignancy—as might be expected since these are known risk factors for early mortality in many general populations—only smoking was associated with long-term mortality in the subgroup with extracardiac malignancy. The latter was likely secondary to a variety of factors, including early mortality in those with extracardiac malignancy, the virulence of smoking-related malignant tumours, a higher rate of tumour treatment failure in smokers, an increased risk of second primary cancers known to be caused by smoking,25 and cardiovascular disease. Importantly, the addition of the CMR diagnosis to a clinical model without the CMR diagnosis demonstrated incremental prognostic value for the whole cohort, and in both sub-cohorts of patients with and without extracardiac malignancy.
Limitations
We included patients referred for a clinical CMR and did not perform a head-to-head comparison of echocardiography vs. CMR in all comers with suspected cardiac tumour. Therefore, we do not have data on patients who may have had a definitive diagnosis on echocardiography and did not require a CMR. Nonetheless, our study describes a real-life cohort that exists in clinical practice, that is patients with a suspected cardiac tumour where echocardiography and/or other imaging results are equivocal or incomplete, necessitating a CMR. We also do not have data regarding primary malignant vs. metastatic tumours. Our primary endpoint was all-cause mortality, and we did not account for the cause of death. Thus, not all deaths were necessarily related to cardiac tumours. However, all-cause mortality is an important and appropriate study endpoint because it is objective, clinically relevant, and unbiased, which is often not the case for cause-specific mortality.26 While T1 and T2 mapping techniques likely provide additional tissue characterization of cardiac masses, the techniques were not clinically available for most of the study period. It is also important to note that T1 and T2 mapping are unlikely to add significantly to the key pathways by which CMR already provides value, namely the determination of whether a mass is present or absent and the delineation between thrombus and tumour (‘Steps 1 and 2’ in the Stepwise Algorithm). Moreover, CMR as performed in our study appears effective in distinguishing between benign and malignant tumours. This is highlighted by the high accuracy of CMR despite the lack of T1 and T2 mapping in our protocol, where T1 and T2 mapping may help us in identifying specific subtypes of benign or malignant tumours since some tumours such as melanoma often have unique T1 or T2 characteristics. This needs to be investigated in future studies.
Conclusions
In patients with suspected cardiac tumour, CMR has high diagnostic accuracy. The CMR diagnosis is a powerful predictor of mortality and is incremental to common clinical risk factors (Graphical Abstract). Our findings provide the first large-scale validation of a CMR-based approach to evaluate patients with suspected cardiac tumours.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Institutes of Health (K23HL132011 to C.S. and R01HL64726 to R.J.K.).
Conflict of interest: R.J.K. is a Board Member at Heart IT, LLC. All other authors declare no conflict of interest.
Data availability
The data underlying this article cannot be shared publicly due to privacy reasons. The de-identified study dataset will be made available on reasonable request to the corresponding author, subject to institutional and ethical committee approvals.
Supplementary Material
Contributor Information
Chetan Shenoy, University of Minnesota Medical Center, Cardiovascular Division, Department of Medicine, 420 Delaware St MMC 508, Minneapolis, MN, USA.
John D Grizzard, Virginia Commonwealth University Medical Center, 1250 E. Marshall Street, Richmond, VA, USA.
Dipan J Shah, Houston Methodist Hospital, 6550 Fannin St Suite 1901, Houston, TX, USA.
Mahwash Kassi, Houston Methodist Hospital, 6550 Fannin St Suite 1901, Houston, TX, USA.
Michael J Reardon, Houston Methodist Hospital, 6550 Fannin St Suite 1901, Houston, TX, USA.
Marianna Zagurovskaya, Virginia Commonwealth University Medical Center, 1250 E. Marshall Street, Richmond, VA, USA.
Han W Kim, Duke University Medical Center, Duke Medical Pavilion, 10 Medicine Circle, Rm IE-58 Durham, NC 27710, USA.
Michele A Parker, Duke University Medical Center, Duke Medical Pavilion, 10 Medicine Circle, Rm IE-58 Durham, NC 27710, USA.
Raymond J Kim, Duke University Medical Center, Duke Medical Pavilion, 10 Medicine Circle, Rm IE-58 Durham, NC 27710, USA.
References
- 1. Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR; American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006;48:1475–1497. [DOI] [PubMed] [Google Scholar]
- 2. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK; American College of Cardiology Foundation Task Force on Expert Consensus Documents. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on expert consensus documents. Circulation 2010;121:2462–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leiner T, Bogaert J, Friedrich MG, Mohiaddin R, Muthurangu V, Myerson S, Powell AJ, Raman SV, Pennell DJ. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2020;22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colin GC, Symons R, Dymarkowski S, Gerber B, Bogaert J. Value of CMR to differentiate cardiac angiosarcoma from cardiac lymphoma. JACC Cardiovasc Imaging 2015;8:744–746. [DOI] [PubMed] [Google Scholar]
- 5. Pazos-Lopez P, Pozo E, Siqueira ME, Garcia-Lunar I, Cham M, Jacobi A, Macaluso F, Fuster V, Narula J, Sanz J. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging 2014;7:896–905. [DOI] [PubMed] [Google Scholar]
- 6. Patel R, Lim RP, Saric M, Nayar A, Babb J, Ettel M, Axel L, Srichai MB. Diagnostic performance of cardiac magnetic resonance imaging and echocardiography in evaluation of cardiac and paracardiac masses. Am J Cardiol 2016;117:135–140. [DOI] [PubMed] [Google Scholar]
- 7. Giusca S, Mereles D, Ochs A, Buss S, Andre F, Seitz S, Riffel J, Fortner P, Andrulis M, Schonland S, Katus HA, Korosoglou G. Incremental value of cardiac magnetic resonance for the evaluation of cardiac tumors in adults: experience of a high volume tertiary cardiology centre. Int J Cardiovasc Imaging 2017;33:879–888. [DOI] [PubMed] [Google Scholar]
- 8. Kassi M, Polsani V, Schutt RC, Wong S, Nabi F, Reardon MJ, Shah DJ. Differentiating benign from malignant cardiac tumors with cardiac magnetic resonance imaging. J Thorac Cardiovasc Surg 2019;157:1912–1922.e2. [DOI] [PubMed] [Google Scholar]
- 9. Beroukhim RS, Prakash A, Buechel ER, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R, Misra N, Moniotte S, Parks WJ, Powell AJ, Soriano BD, Srichai MB, Yoo SJ, Zhou J, Geva T. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol 2011;58:1044–1054. [DOI] [PubMed] [Google Scholar]
- 10. Mousavi N, Cheezum MK, Aghayev A, Padera R, Vita T, Steigner M, Hulten E, Bittencourt MS, Dorbala S, Di Carli MF, Kwong RY, Dunne R, Blankstein R. Assessment of cardiac masses by cardiac magnetic resonance imaging: histological correlation and clinical outcomes. J Am Heart Assoc 2019;8:e007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heitner JF, Kim RJ, Kim HW, Klem I, Shah DJ, Debs D, Farzaneh-Far A, Polsani V, Kim J, Weinsaft J, Shenoy C, Hughes A, Cargile P, Ho J, Bonow RO, Jenista E, Parker M, Judd RM. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48000 patient-years of follow-up. JAMA Cardiol 2019;4:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, Society for Cardiovascular Magnetic Resonance: board Of Trustees Task Force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols: 2013 update. J Cardiovasc Magn Reson 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008;52:148–157. [DOI] [PubMed] [Google Scholar]
- 16. Velangi PS, Choo C, Chen KA, Kazmirczak F, Nijjar PS, Farzaneh-Far A, Okasha O, Akcakaya M, Weinsaft JW, Shenoy C. Long-term embolic outcomes after detection of left ventricular thrombus by late gadolinium enhancement cardiovascular magnetic resonance imaging: a matched cohort study. Circ Cardiovasc Imaging 2019;12:e009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooks M, Okasha O, Velangi PS, Nijjar PS, Farzaneh-Far A, Shenoy C. Left ventricular thrombus on cardiovascular magnetic resonance imaging in non-ischaemic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2020; doi:10.1093/ehjci/jeaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grizzard JD, Ang GB. Magnetic resonance imaging of pericardial disease and cardiac masses. Cardiol Clin 2007;25:111–140, vi. [DOI] [PubMed] [Google Scholar]
- 19. Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology 2013;268:26–43. [DOI] [PubMed] [Google Scholar]
- 20. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 21. Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610–1617. [DOI] [PubMed] [Google Scholar]
- 22. Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 2008;118:S7–15. [DOI] [PubMed] [Google Scholar]
- 23. Sultan I, Bianco V, Habertheuer A, Kilic A, Gleason TG, Aranda-Michel E, Harinstein ME, Martinez-Meehan D, Arnaoutakis G, Okusanya O. Long-term outcomes of primary cardiac malignancies: multi-institutional results from the national cancer database. J Am Coll Cardiol 2020;75:2338–2347. [DOI] [PubMed] [Google Scholar]
- 24. Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors: a 40-year analysis of >500 patients. Circulation 2015;132:2395–2402. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 26. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy reasons. The de-identified study dataset will be made available on reasonable request to the corresponding author, subject to institutional and ethical committee approvals.