Abstract
Aims
The aim of this article was to compare rates of all-cause death at 10 years following coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) in patients with or without diabetes.
Methods and results
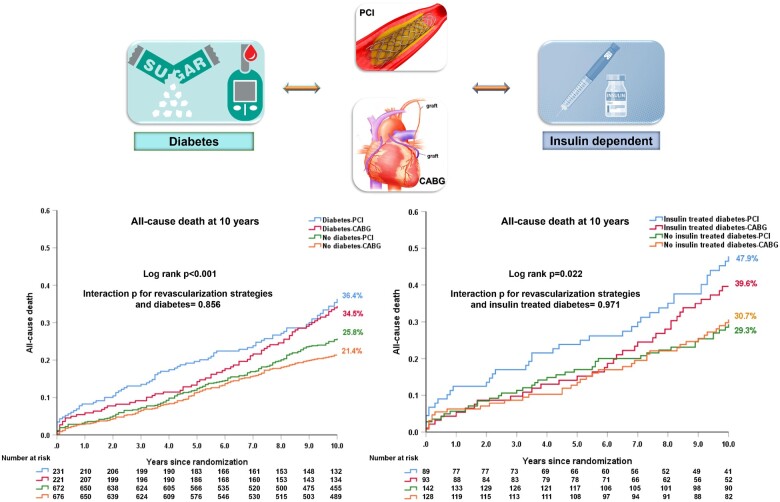
The SYNTAXES study evaluated up to 10-year survival of 1800 patients with three-vessel disease (3VD) and/or left main coronary artery disease (LMCAD) randomized to receive either PCI or CABG in the SYNTAX trial. Ten-year all-cause death according to diabetic status and revascularization strategy was examined. In diabetics (n = 452), the risk of mortality was numerically higher with PCI compared with CABG at 5 years [19.6% vs. 13.3%, hazard ratio (HR): 1.53, 95% confidence interval (CI): 0.96, 2.43, P = 0.075], with the opposite seen between 5 and 10 years (PCI vs. CABG: 20.8% vs. 24.4%, HR: 0.82, 95% CI: 0.52, 1.27, P = 0.366). Irrespective of diabetic status, there was no significant difference in all-cause death at 10 years between patients receiving PCI or CABG, the absolute treatment difference was 1.9% in diabetics (PCI vs. CABG: 36.4% vs. 34.5%, difference: 1.9%, 95% CI: −7.6%, 11.1%, P = 0.551). Among insulin-treated patients (n = 182), all-cause death at 10 years was numerically higher with PCI (47.9% vs. 39.6%, difference: 8.2%, 95% CI: −6.5%, 22.5%, P = 0.227).
Conclusions
The treatment effects of PCI vs. CABG on all-cause death at 10 years in patients with 3VD and/or LMCAD were similar irrespective of the presence of diabetes. There may, however, be a survival benefit with CABG in patients with insulin-treated diabetes. The association between revascularization strategy and very long-term ischaemic and safety outcomes for patients with diabetes needs further investigation in dedicated trials.
Trial registration
SYNTAX: ClinicalTrials.gov reference: NCT00114972 and SYNTAX Extended Survival: ClinicalTrials.gov reference: NCT03417050.
Keywords: All-cause death, Coronary artery bypass grafting, Diabetes, Percutaneous coronary intervention, SYNTAX
Graphical Abstract
See page 68 for the editorial comment for this article ‘Is there equivalence between PCI and CABG surgery in long-term survival of patients with diabetes? Importance of interpretation biases and biological plausibility’, by W.E. Boden, R. De Caterina, and D.P. Taggart, https://doi.org/10.1093/eurheartj/ehab445.
Introduction
Cardiovascular disease (CVD) is a major comorbidity and affects nearly a third of diabetics.1 Diabetes is associated with worse outcomes after coronary revascularization and has been identified as an independent predictor of adverse events in patients with CVD.2
The first randomized trial dedicated to diabetics (CARDia) demonstrated that percutaneous coronary intervention (PCI) had an increased rate of the composite primary endpoint of all-cause death, myocardial infarction (MI) and stroke at 12 months compared with coronary artery bypass grafting (CABG) surgery.3 Subsequently, the FREEDOM trial showed that CABG was superior to drug-eluting stents (DES) for the composite primary endpoint of death, stroke, and MI at 5 years.4 Similarly, the diabetes subgroup analysis of the SYNTAX study reported that PCI resulted in higher rates of 5-year MACCE (major adverse cardiovascular and cerebrovascular event: a composite endpoint of all-cause death, cerebrovascular accident, MI, or repeat revascularization), compared with CABG, which was driven by a higher rate of repeat revascularization.5 Moreover, a recent pooled analysis of individual patient data demonstrated that diabetes had a significant treatment interaction between PCI and CABG for 5-year all-cause mortality.6 Based on these findings in this specific subgroup, current guidelines recommend CABG as the preferred revascularization procedure in patients with diabetes, especially for those with multivessel coronary artery diseases (CAD).7 Most available studies have limited follow-up of only 5 years; however, the BARI trial, which reported outcomes at 10 years, demonstrated that CABG conferred a survival benefit over PCI with balloon angioplasty in patients with diabetes and multivessel disease [59%: two-vessel disease and 41%: three-vessel disease (3VD)].8 Whether this benefit remains in these patients when CABG is compared with PCI with DES remains to be established.
A suitable population to address this outstanding question comes from the SYNTAXES study, which established 10-year survival status in 94% of the 1800 patients with de novo 3VD and/or left main coronary artery disease (LMCAD) who were originally randomized to CABG or PCI in the SYNTAX trial.9 The aims of the present study were therefore (i) to evaluate the association between diabetes and all-cause death at 10 years; (ii) to examine the specific impact of diabetes with insulin dependence on all-cause death at 10 years; (iii) to investigate the 10-year treatment effect on survival of PCI vs. CABG, according to diabetes, in patients with complex CAD.
Methods
Study design
The design and the primary results of the SYNTAX study have been reported previously.10–12 Briefly, all-comer patients with de novo 3VD and/or LMCAD deemed to be eligible for both PCI and CABG were enrolled and randomized to either CABG (n = 897) or PCI (n = 903) with TAXUS DES (Boston Scientific, Marlborough, MA, USA). The SYNTAX trial completed patient follow-up at 5 years.12 The SYNTAXES study was an investigator-driven initiative that extended follow-up and aimed to evaluate vital status at up to 10 years.9 The German Heart Research Foundation (GHF, Frankfurt am Main, Germany) funded the extended follow-up, which was performed in accordance with local regulations of each participating centre and complied with the declaration of Helsinki.
Study endpoints
The present analysis is a pre-specified sub-study of the SYNTAXES study.9 The primary endpoint was all-cause death at 10 years. The secondary endpoint was all-cause death at maximum available follow-up. Vital status was confirmed by contact with medical care personnel or by electronic healthcare record review and national death registry. The aim of the study was to examine the impact of pharmacologically (non-insulin or insulin) treated diabetes (categorized at the time of randomization), on subsequent all-cause death at 10 years. The impact of all diabetes (pharmacological and diet-controlled) on all-cause death at 10 years was performed as a sensitivity analysis.
The following exploratory analyses were performed: elderly (>70 years old), anatomical SYNTAX score tertiles (≤22, 23–32, or ≥33), disease type (3VD or LMCAD), impact of haemoglobin A1c (HbA1c); C-reactive protein (<2, or ≥2), residual SYNTAX score (rSS = 0, >0–4, >4–8, and >8), type of revascularization (single or multiple arterial bypass graft) and optimal medical therapy. Finally, we applied SYNTAX score II 2020 to the diabetic and non-diabetic population.13
Statistical analysis
Continuous variables are shown as mean ± standard deviations and are compared using Student’s t-tests or Mann–Whitney U test. Categorical variables are reported as percentages and numbers and are compared using χ2 tests, or Fisher’s exact test when appropriate.
Time-to-event Kaplan–Meier estimates with the log-rank test were used to compare PCI and CABG in patients with and without diabetes, and to compare diabetes vs. no diabetes in PCI and CABG groups. Hazard ratio (HR) with 95% confidence interval (CI) was assessed on the basis of the Cox proportional regression. The mean restricted life expectancy was estimated by the area under the survival curve between 0 and 10 years.8 The adjusted cubic spline was used to show the association between HbA1c and the risk of all-cause death at 10 years. Multivariable analyses were performed in the Cox proportional hazards regression model to evaluate whether pharmacologically treated or insulin-treated diabetes was an independent predictor of all-cause death at 10 years. The following covariates were included: age, gender, body mass index (BMI), current smoking, hypertension, peripheral vascular disease (PVD), chronic obstructive pulmonary disease, creatinine clearance (mL/min), left ventricular ejection fraction (as categorical: good ≥50%, moderate: 30–49%, and poor: <30%), anatomical SYNTAX score, prior MI, and stroke. All these variables were selected based on the previous knowledge of their association with clinical outcomes.14 All analyses were performed using SPSS Statistics, version 25 (IBM Corp., Armonk, 281 NY, USA), and R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). A P-value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Out of 1800 patients, 511 had diabetes, of which 59 were treated by diet alone, and of the remaining 452 treated pharmacologically, 182 were on insulin. The median maximum follow-up was 11.2 (interquartile range: 7.7, 12.1) years. Baseline characteristics according to diabetes are shown in Supplementary material online, Table S1. Compared with patients without diabetes, patients with diabetes were more frequently female, had more comorbidities (dyslipidaemia, PVD, previous stroke, carotid artery disease, and congestive heart failure), had a higher BMI, EuroSCORE, Parsonnet SCORE, more frequently had 3VD, with more lesions treated, whereas they were less likely to be current smokers. By randomization, baseline characteristics according to revascularization strategy were generally well balanced in diabetic and non-diabetic patients (Table 1).
Table 1.
Baseline characteristics according to diabetes and revascularization strategies
| No diabetes |
P-value | Diabetes |
P-value | |||
|---|---|---|---|---|---|---|
| PCI (n = 672) | CABG (n = 676) | PCI (n = 231) | CABG (n = 221) | |||
| Age (years) | 65.2 ± 9.9 | 64.7 ± 9.9 | 0.356 | 65.2 ± 9.1 | 65.6 ± 9.3 | 0.637 |
| Male sex | 78.1 (525/672) | 81.7 (552/676) | 0.106 | 71.4 (165/231) | 70.6 (156/221) | 0.844 |
| Body mass index (kg/m2) | 27.6 ± 4.5 | 27.4 ± 4.3 | 0.445 | 29.5 ± 5.4 | 29.4 ± 5.1 | 0.736 |
| Glycated haemoglobin (%) | 5.8 ± 0.5 | 5.8 ± 0.6 | 0.830 | 7.4 ± 1.3 | 7.3 ± 1.1 | 0.598 |
| Metabolic syndrome | 30.2 (203/672) | 28.8 (195/676) | 0.193 | 58.9 (136/231) | 55.2 (122/221) | 0.546 |
| Hypertension | 67.0 (450/672) | 63.6 (430/676) | 0.196 | 74.5 (172/231) | 65.2 (144/221) | 0.031 |
| Dyslipidaemia | 77.6 (520/670) | 75.9 (509/671) | 0.447 | 81.9 (185/226) | 81.2 (177/218) | 0.857 |
| Current smoker | 19.6 (132/672) | 23.8 (160/671) | 0.062 | 15.2 (35/231) | 16.4 (36/219) | 0.708 |
| Previous MI | 31.4 (209/665) | 34.9 (233/668) | 0.181 | 33.3 (76/228) | 30.6 (67/219) | 0.535 |
| Previous stroke | 3.4 (23/670) | 4.2 (28/671) | 0.479 | 5.2 (12/229) | 6.8 (15/219) | 0.474 |
| Previous TIA | 3.6 (24/671) | 5.1 (34/670) | 0.178 | 6.5 (15/230) | 5.0 (11/218) | 0.504 |
| Previous CAD | 7.7 (52/672) | 7.0 (47/676) | 0.580 | 9.1 (21/231) | 12.7 (28/221) | 0.221 |
| PVD | 6.8 (46/672) | 9.6 (65/676) | 0.064 | 15.6 (36/231) | 13.6 (30/221) | 0.545 |
| COPD | 7.0 (47/672) | 9.2 (62/676) | 0.143 | 10.4 (24/231) | 9.5 (21/221) | 0.753 |
| Impaired renal function | 18.6 (125/672) | 15.7 (106/676) | < 0.001 | 18.2 (42/231) | 19.5 (43/221) | 0.003 |
| Creatinine clearance (mL/min) | 86.4 ± 34.2 | 85.6 ± 28.2 | 0.648 | 87.5 ± 39.3 | 85.5 ± 33 | 0.586 |
| LVEF (%) | 59.6 ± 12.6 | 58.3 ± 13.2 | 0.163 | 57.5 ± 13.7 | 58.0 ± 13.1 | 0.736 |
| Congestive heart failure | 3.3 (22/669) | 4.2 (28/665) | 0.375 | 6.1 (14/229) | 8.8 (19/215) | 0.274 |
| Clinical presentation | 0.877 | 0.164 | ||||
| Silent ischemia | 14.7 (99/672) | 13.8 (93/676) | 12.1 (28/231) | 18.1 (40/221) | ||
| Stable angina | 57.3 (385/672) | 58.0 (392/676) | 55.8 (129/231) | 54.8 (121/221) | ||
| Unstable angina | 28.0 (188/672) | 28.3 (191/676) | 32.0 (74/231) | 27.1 (60/221) | ||
| EuroSCORE | 3.7 ± 2.6 | 3.7 ± 2.7 | 0.755 | 4.0 ± 2.7 | 4.0 ± 2.7 | 0.971 |
| Parsonnet score | 7.6 ± 6.9 | 7.4 ± 6.7 | 0.596 | 11.1 ± 6.5 | 11.5 ± 6.4 | 0.584 |
| Disease extent | 0.556 | 0.733 | ||||
| 3VD | 58.5 (393/672) | 60.1 (406/676) | 66.2 (153/231) | 64.7 (143/221) | ||
| LMCAD | 41.5 (279/672) | 39.9 (270/676) | 33.8 (78/231) | 35.3 (78/221) | ||
| Disease location | 0.658 | 0.883 | ||||
| LMCAD only | 5.1 (34/672) | 6.2 (42/675) | 3.5 (8/231) | 3.2 (7/221) | ||
| LMCAD +1VD | 8.0 (54/672) | 8.6 (58/675) | 5.6 (13/231) | 5.9 (13/221) | ||
| LMCAD +2VD | 12.8 (86/672) | 12.6 (85/675) | 11.3 (26/231) | 9.5 (21/221) | ||
| LMCAD +3VD | 15.6 (105/672) | 12.6 (85/675) | 13.4 (31/231) | 16.7 (37/221) | ||
| 2VD | 1.8 (12/672) | 1.8 (12/675) | 2.2 (5/231) | 3.2 (7/221) | ||
| 3VD | 56.7 (381/672) | 58.2 (393/675) | 64.1 (148/231) | 61.5 (136/221) | ||
| Anatomical SYNTAX score | 28.3 ± 11.5 | 28.9 ± 11.5 | 0.326 | 28.6 ± 11.5 | 29.5 ± 10.9 | 0.396 |
| No. of lesions | 4.3 ± 1.8 | 4.3 ± 1.8 | 0.720 | 4.5 ± 1.8 | 4.6 ± 1.7 | 0.492 |
| Any total occlusion | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.283 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.933 |
| Any bifurcation | 0.7 ± 0.5 | 0.7 ± 0.4 | 0.443 | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.566 |
| No. of stents | 4.6 ± 2.2 | — | 4.7 ± 2.3 | — | ||
| TSL per patient | 85.5 ± 47.5 | — | 89.0 ± 49.3 | — | ||
| Off—pump CABG | — | 14.2 (96/676) | — | 14.5 (32/221) | ||
| LIMA use | — | 83.0 (561/676) | — | 78.3 (173/221) | ||
| No. of total conduits | — | 2.8 ± 0.7 | — | 2.8 ± 0.7 | ||
| No. of arterial conduits | — | 1.4 ± 0.6 | — | 1.4 ± 0.7 | ||
| No. of venous conduits | — | 1.4 ± 0.9 | — | 1.4 ± 0.9 | ||
| Complete revascularization | 59.3 (395/666) | 64.0 (425/664) | 0.078 | 49.1 (113/230) | 60.7 (125/206) | 0.016 |
Metabolic syndrome defined as at least three of the following: (i) waist circumference >102 cm in males, >88 cm in females; (ii) triglycerides ≥150 mg/dL; (iii) high-density lipoprotein <40 mg/dL in males, <50 mg/dL in females; (iv) blood pressure ≥130/85 mmHg; and (v) fasting glucose ≥110 mg/dL. Impaired renal function defined as a calculated creatinine clearance <60 mL/min. Glycated haemoglobin was core laboratory reported.
3VD, three-vessel disease; CABG, coronary artery bypass grafting; CAD, carotid artery disease; COPD, chronic obstructive pulmonary disease; LMCAD, left main coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; TIA, transient ischaemic attack; TSL, total stent length.
All-cause death according to diabetes
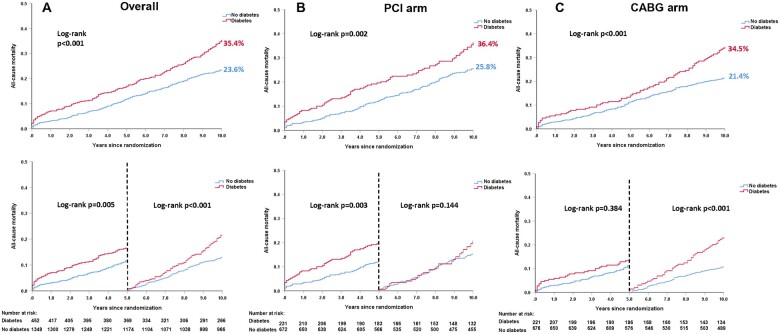
Overall compared with patients without diabetes, those with pharmacologically treated diabetes had a higher risk of all-cause death at 10 years (35.4% vs. 23.6%, adjusted HR: 1.58, 95% CI: 1.27, 1.95, P < 0.001, Figure 1A, Table 2). Similar results were observed for all-cause death at maximum follow-up (Table 2, Supplementary material online, Figure S1). Results were similar when including the 59 patients with diet-controlled diabetes (Supplementary material online, Figures S2 and S3).
Figure 1.
Kaplan–Meier curves for all-cause death at 10 years according to diabetes. (A) All-cause death at 10 years according to diabetes in the overall cohort. (B) All-cause death at 10 years according to diabetes in the percutaneous coronary intervention arm. (C) All-cause death at 10 years according to diabetes in the coronary artery bypass grafting arm. Event rates represent Kaplan–Meier estimates. Note: As Kaplan–Meier estimates, the rate is not the same as the ratio of the numerator and denominator.
Table 2.
Impact of diabetes on all-cause death according to treatment strategies
| Diabetes (n = 452) | No diabetes (n = 1348) | P -value | Unadjusted HR (95% CI) | Unadjusted P-value | Adjusted HR (95% CI) | Adjusted P-value | |
|---|---|---|---|---|---|---|---|
| 10 years | |||||||
| Overall | 35.4% (152) | 23.6% (308) | <0.001 | 1.61(1.32–1.95) | <0.001 | 1.58(1.27–1.95) | <0.001 |
| PCI | 36.4% (80) | 25.8% (168) | 0.002 | 1.53(1.17–2.00) | 0.002 | 1.54(1.15–2.06) | 0.003 |
| CABG | 34.5% (72) | 21.4% (140) | <0.001 | 1.70(1.28–2.26) | <0.001 | 1.65(1.19–2.28) | 0.003 |
| Maximum follow-up | |||||||
| Overall | 60.7% (187) | 35.4% (381) | <0.001 | 1.66(1.40–1.98) | <0.001 | 1.67(1.38–2.02) | <0.001 |
| PCI | 51.2% (94) | 37.5% (209) | 0.001 | 1.49(1.16–1.89) | 0.001 | 1.55(1.19–2.01) | 0.001 |
| CABG | 67.0% (93) | 32.2% (172) | <0.001 | 1.88(1.46–2.42) | <0.001 | 1.85(1.38–2.47) | <0.001 |
Percentage of deaths at a given time point, based on Kaplan–Meier estimates (number of deaths). The number of patients entered into the multivariable Cox model was 87.2% (1570/1800) patients in the overall population, 90.0% (813/903) patients in the PCI arm, and 84.4% (757/897) patients in the CABG arm.
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
After adjustment for baseline confounders, pharmacologically treated diabetes was an independent predictor of all-cause death at 10 years in the overall cohort (HR: 1.58, 95% CI: 1.27, 1.95, P < 0.001), the PCI arm (HR: 1.54, 95% CI: 1.15, 2.06, P = 0.003), and the CABG arm (HR: 1.65, 95% CI: 1.19, 2.28, P = 0.003, Supplementary material online, Table S2), with poorer outcomes in those receiving insulin (Supplementary material online, Table S3).
All-cause death according to diabetes and revascularization strategy
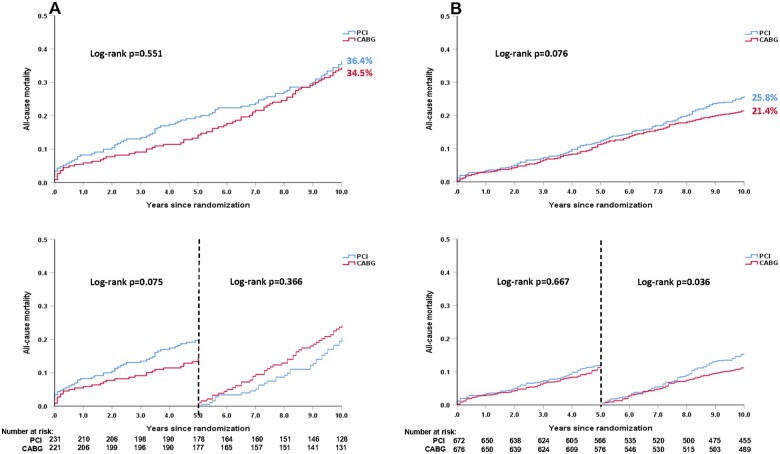
In non-diabetics, there was no significant absolute treatment difference in the risk of death at 10 years (PCI 25.8% vs. CABG 21.4%, difference: 4.4%, 95% CI: −0.2%, 9.0%, P = 0.076, Figure 2B, Supplementary material online, Table S4). The mean restricted life expectancy in non-diabetics was 8.89 and 8.74 years in patient receiving CABG and PCI, respectively (P = 0.076).
Figure 2.
Kaplan–Meier curves for all-cause death at 10 years according to treatment strategies in patients with (A) and without (B) diabetes. (A) All-cause death at 10 years according to treatment strategies in patients with diabetes. (B) All-cause death at 10 years according to treatment strategies in patients without diabetes. Event rates represent Kaplan–Meier estimates. Note: As Kaplan–Meier estimates, the rate is not the same as the ratio of the numerator and denominator.
In diabetics, all-cause death at 10 years occurred in 80 (36.4%) patients in the PCI arm and 72 (34.5%) patients in the CABG arm (difference: 1.9%, 95% CI: −7.6%, 11.1%, P = 0.551, Figure 2A, Table 3 and Supplementary material online, Table S4). Landmark analyses showed that the risk of mortality was numerically higher with PCI compared with CABG at 5 years (19.6% vs. 13.3%, HR: 1.53, 95% CI: 0.96, 2.43, P = 0.075), with the opposite seen between 5 and 10 years (PCI vs. CABG: 20.8% vs. 24.4%, HR: 0.82, 95% CI: 0.52, 1.27, P = 0.366, Figure 2A). The mean restricted life expectancy for diabetic patients treated with CABG and PCI was, respectively, 8.41 and 8.08 years (P = 0.551). The treatment effect of PCI vs. CABG on mortality at 10 years was not statistically different according to the presence of diabetes (P -interaction = 0.856, Table 3), with similar findings at maximum follow-up (Table 3 and Supplementary material online, Table S4 and Figure S4) and when including diet-controlled diabetics (Supplementary material online, Figures S5 and S6).
Table 3.
Treatment effect (percutaneous coronary intervention vs. coronary artery bypass grafting) on all-cause death in diabetic and non-diabetic patients
| PCI (n = 903) | CABG (n = 897) | Unadjusted HR (95% CI) | Unadjusted P-value | Adjusted HR (95% CI) | Adjusted P-value | P -interaction | |
|---|---|---|---|---|---|---|---|
| 10 years | |||||||
| Diabetes | 36.4% (80) | 34.5% (72) | 1.10 (0.80–1.52) | 0.551 | 1.15 (0.80–1.65) | 0.440 | 0.856 |
| No diabetes | 25.8% (168) | 21.4% (140) | 1.23 (0.98–1.53) | 0.076 | 1.30 (1.01–1.66) | 0.041 | |
| Maximum follow-up | |||||||
| Diabetes | 51.7% (94) | 67.3% (93) | 1.00 (0.75–1.33) | 0.991 | 1.06 (0.77–1.47) | 0.712 | 0.394 |
| No diabetes | 37.9% (209) | 33.3% (172) | 1.26 (1.03–1.55) | 0.024 | 1.34 (1.07–1.68) | 0.010 | |
Percentage of deaths at a given time point, based on Kaplan–Meier estimates (number of deaths). The number of patients entered into the multivariable Cox model was 87.3% (1177/1348) patients in non-diabetic group and 86.9% (393/452) patients in diabetic group, respectively. Test of interaction is on adjusted Cox proportional hazards model.
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Impact of insulin treatment on all-cause death
At 10 years, all-cause death occurred in 75 (43.6%) insulin-treated and 77 (30.0%) non-insulin-treated patients (adjusted HR: 1.59, 95% CI: 1.10%, 2.29%, P = 0.014, Supplementary material online, Table S5).
Patients receiving insulin had a non-significant numerically higher all-cause death at 10 years with PCI vs. CABG (47.9% vs. 39.6%, difference: 8.2%, 95% CI: −6.5%, 22.5%, P = 0.227, Table 4 and Supplementary material online, Table S4), with no significant heterogeneity of treatment effect (P -interaction = 0.971, Table 4). The mean restricted life expectancy in these patients was possibly longer with CABG than PCI; however, the differences were not statistically significant (8.24 vs. 7.55 years, P = 0.230) due to the limited sample size (n = 182) and restricted power.
Table 4.
Treatment effect (percutaneous coronary intervention vs. coronary artery bypass grafting) on all-cause death in insulin-treated and non-insulin agent-treated diabetic patients
| PCI (n = 231) | CABG (n = 221) | Unadjusted HR (95% CI) | Unadjusted P-value | Adjusted HR (95% CI) | Adjusted P-value | P -interaction | |
|---|---|---|---|---|---|---|---|
| 10 years | |||||||
| Insulin | 47.9% (40) | 39.6% (35) | 1.32 (0.84–2.08) | 0.227 | 1.40 (0.81–2.42) | 0.229 | 0.971 |
| Non-insulin agents | 29.3% (40) | 30.7% (37) | 0.98 (0.63–1.53) | 0.920 | 1.17 (0.70–1.95) | 0.547 | |
| Maximum follow-up | |||||||
| Insulin | 57.8% (44) | 71.9% (39) | 1.31 (0.85–2.01) | 0.224 | 1.27 (0.76–2.13) | 0.359 | 0.757 |
| Non-insulin agents | 48.7% (50) | 69.3% (54) | 0.83 (0.57–1.22) | 0.351 | 1.06 (0.68–1.65) | 0.798 | |
Percentage of deaths at a given time point, based on Kaplan–Meier estimates (number of deaths). The number of patients entered into the multivariable Cox model was 85.6% (231/270) patients in the non-insulin agent-treated diabetes group and 89.0% (162/182) patients in the insulin-treated diabetes group. Test of interaction is on adjusted Cox proportional hazards model.
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Impact of age
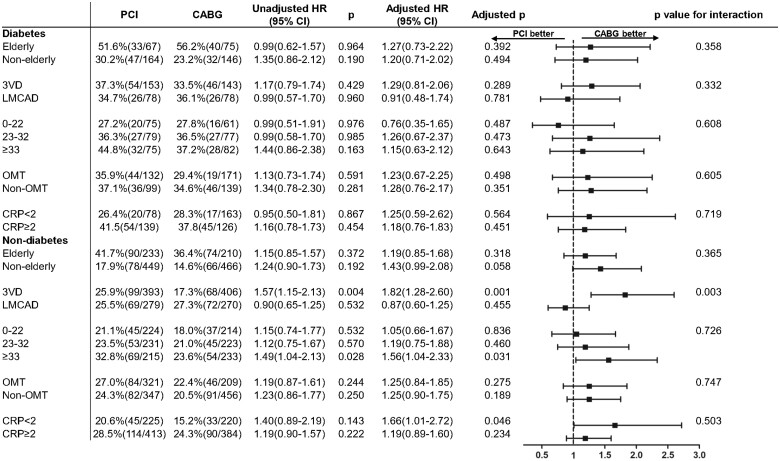
No significant interaction between revascularization mode and age on mortality at 10 years was observed amongst diabetics (P -interaction = 0.358) and non-diabetics (P -interaction = 0.365, Figure 3).
Figure 3.
All-cause death at 10 years in the percutaneous coronary intervention and coronary artery bypass grafting arms among diabetic or non-diabetic patients stratified by subgroups.
Impact of haemoglobin A1c on all-cause death
The spline curve in the overall population showed that an HbA1c of 6.0% had the lowest HR for all-cause death at 10 years, so this was used as the reference value (Supplementary material online, Figure S7). The adjusted cubic spline model showed a U-shaped relationship between HbA1c and all-cause death at 10 years in the overall population (Supplementary material online, Figure S7A) and the PCI arm (Supplementary material online, Figure S7B), whilst the relationship in the CABG arm was linear (Supplementary material online, Figure S7C).
Anatomical SYNTAX score subgroups
In diabetic patients, there were no significant differences in all-cause death at 10 years and at maximum follow-up between PCI and CABG groups in any anatomical SYNTAX score tertile (Supplementary material online, Figure S8). In non-diabetic patients with SYNTAX scores ≥33, all-cause death was significantly higher with PCI at 10 years (32.8% vs. 23.6%, adjusted HR: 1.56, 95% CI: 1.04, 2.33) and at maximum follow-up (38.1% vs. 29.0%, adjusted HR: 1.59, 95% CI: 1.09, 2.32, Supplementary material online, Figure S9).
Three-vessel disease and left main coronary artery disease subgroups
In diabetic patients with 3VD, PCI and CABG had comparable mortality at 10 years (37.3% vs. 33.5%, adjusted HR: 1.29, 95% CI: 0.81, 2.06, P = 0.289, Supplementary material online, Figure S10A and Table S6). Landmark analysis showed that mortality was significantly higher with PCI at 5 years (19.8% vs. 11.3%, adjusted HR: 2.27, 95% CI: 1.14, 4.52, P = 0.020), whereas it was numerically higher with CABG between 5 and 10 years (21.7% vs. 24.3%, adjusted HR: 0.70, 95% CI: 0.36, 1.37, P = 0.295). In non-diabetic patients with 3VD, the risk of mortality was significantly higher with PCI at 5 years (12.8% vs. 9.3%, HR: 2.04, 95% CI: 1.22, 3.41, P = 0.007) and 10 years (25.9% vs. 17.3%, adjusted HR: 1.82, 95% CI: 1.28, 2.60, P = 0.001, Supplementary material online, Table S7).
In patients with LMCAD, there was no significant difference in mortality at 10 years between PCI and CABG among patients with diabetes (34.7% vs. 36.1%, adjusted HR: 0.91, 95% CI: 0.48, 1.74, P = 0.781, Supplementary material online, Table S6, Figure S10B) or without (25.5% vs. 27.3%, adjusted HR: 0.87, 95% CI: 0.60, 1.25, P = 0.455, Supplementary material online, Table S7). Results at maximum follow-up are shown in Supplementary material online, Figure S11 and Tables S6 and S7. Ten-year mortality according to SYNTAX score tertiles and revascularization strategies in diabetic and non-diabetic patients with 3VD/LMCAD are shown in Supplementary material online, Figures S12 and S13.
Impact of residual SYNTAX score
The rSS was available in 890 (98.6%) patients in the PCI cohort and was significantly higher in diabetic compared with non-diabetic patients (5.92 ± 8.27 vs. 3.97 ± 6.22, P < 0.001). The percentages of diabetic patients in the sub-categories of rSS = 0, >0 to 4, >4 to 8, and >8 group were 20.5%, 26.6%, 26.3%, and 37.3%, respectively (P < 0.001). The risk of mortality at 10 years was significantly higher with an rSS > 8 compared with an rSS ≤ 8, for both diabetic (61.2% vs. 28.7%, P < 0.001) and non-diabetic patients (43.8% vs. 22.8%, P < 0.001, Supplementary material online, Figure S14).
Impact of type of revascularization (single or multiple arterial bypass grafts)
In patients with diabetes, there was no significant difference in all-cause death at 10 years between patients receiving a single (SAG) or multiple arterial bypass graft (MAG) or PCI (P = 0.432, Supplementary material online, Figure S15).
Among patients with diabetes, there were no significant treatment-by-subgroup interactions for C-reactive protein or optimal medical therapy for mortality at 10 years (Figure 3).
SYNTAX score II 2020 for predicting death at 10 years in patients with and without diabetes
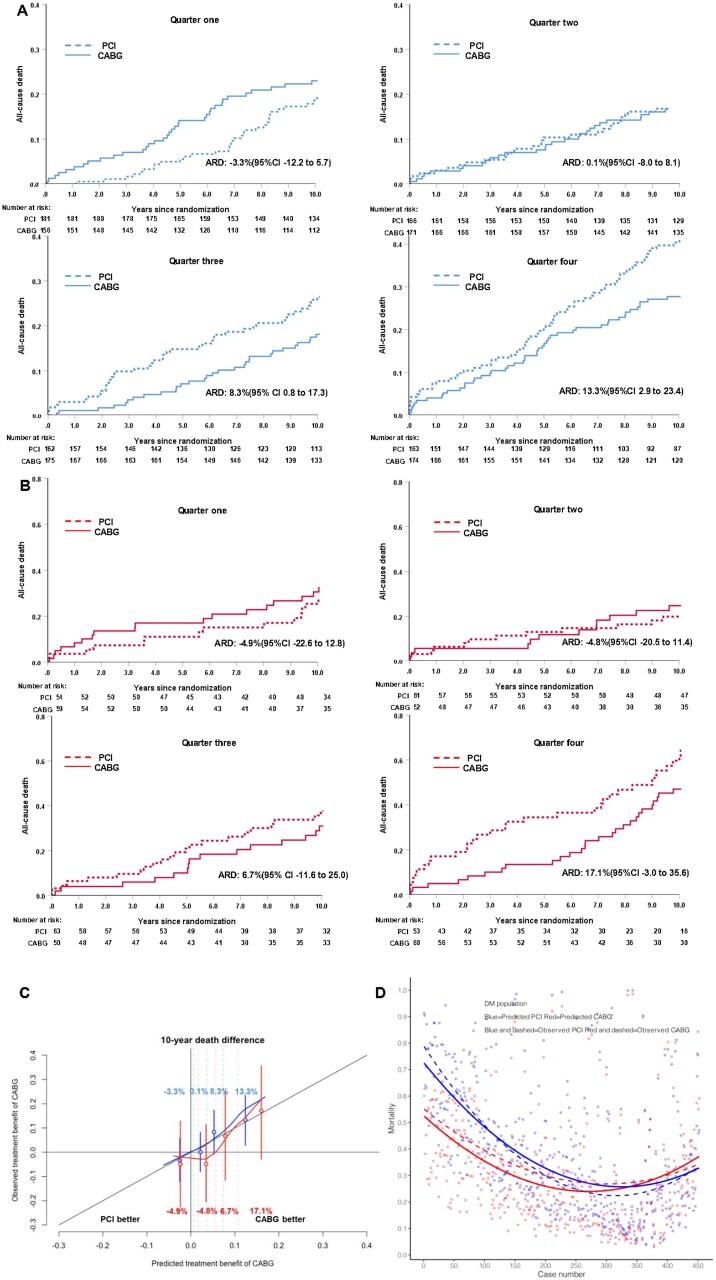
The ability of the SYNTAX score II 2020 to predict rates of all-cause death at 10 years after PCI or CABG was equally valuable in diabetic and non-diabetic patients (Supplementary material online, Figure S16). Figure 4C shows that for both diabetics (red curves) and non-diabetics (blue curves), the absolute risk difference in mortality (treatment benefit of CABG over PCI) curves was not only well-calibrated, but also largely overlapped in the same range of predicted and observed mortality. Additionally, Figure 4A and B displays the absolute risk differences in mortality for each quarter of the diabetic and non-diabetic population together with their respective Kaplan–Meier curves.
Figure 4.
Kaplan–Meier plots showing the observed vs. predicted treatment benefit of coronary artery bypass grafting over percutaneous coronary intervention according to the SYNTAX score II 2020 in predicted benefit quarters in non-diabetic population (A) and diabetic population (B), and calibration plot (C) showing the observed vs. predicted treatment benefit (absolute difference in mortality between coronary artery bypass grafting and percutaneous coronary intervention) in patients with diabetes (red line) and without diabetes (blue line). The percentages in red or blue figuring in the illustration are the absolute risk differences between coronary artery bypass grafting and percutaneous coronary intervention in each quarter for the diabetic and non-diabetic population. Vertical dashed lines represent quartiles, and the solid red or blue lines represent the mean value and 95% CI of the observed absolute risk differences between coronary artery bypass grafting and percutaneous coronary intervention in each quartile. (D) The individual difference between the predicted mortality (solid lines) by SYNTAX Score II 2020 after either percutaneous coronary intervention or coronary artery bypass grafting as well as the individual observed mortality (dashed lines) in diabetic patients. Blue solid line represents the predicted mortality after percutaneous coronary intervention; Red solid line represents the predicted mortality after coronary artery bypass grafting; Blue dashed line represents the observed mortality after percutaneous coronary intervention; Red dashed line represents the observed mortality after coronary artery bypass grafting.
Figure 4D shows ranked individual differences (n = 452) in predicted mortalities for diabetic patients undergoing either PCI (blue solid line) or CABG (red solid line). Actually, 338 patients have higher predicted mortality after PCI than after CABG, then in the ranking order a crossover point in predicted mortalities (equipoise) is reached: beyond that point, the predicted mortality following PCI of the remaining patients (n = 114) becomes lower than the predicted mortality after CABG.
The dashed line in Figure 4D depicts in a spline regression (LOESS), the observed mortality either after PCI or CABG. Notably, the dashed lines depicting the observed mortalities following either PCI or CABG crossover around the 200th ranked patient suggesting an equipoised vital prognosis after either PCI or CABG for that specific patient. The remaining 252 patients had higher observed mortality after surgery compared with PCI. In contrast to the neutral ‘average treatment effect’ observed in diabetics at 10 years with either CABG or PCI, the SYNTAX score II 2020 clearly identifies individuals who derive a treatment survival benefit from either CABG or PCI.
Discussion
The present study was a pre-specified subgroup analysis of the SYNTAXES study, in which we assessed all-cause death at 10 years after PCI with first-generation DES vs. CABG as a function of pharmacologically treated diabetes, with or without insulin (Graphical abstract). The main findings are:
The treatment effects of PCI versus CABG on all-cause death at 10 years in 3VD/LMCAD patients with pharmacologically treated diabetes and insulin-treated diabetes.
The treatment effects of PCI vs. CABG on all-cause death at 10 years were similar irrespective of the presence of diabetes. In this limited sample size with restricted power, insulin-treated patients undergoing PCI had a numerically higher mortality compared with those undergoing CABG.
Compared with non-diabetics, pharmacologically treated diabetics had a higher risk of all-cause death at 10 years after PCI or CABG, with poorer outcomes amongst insulin vs. non-insulin-treated patients. After adjustment for baseline confounders, pharmacologically treated and insulin-treated diabetes were both independent predictors of 10-year mortality.
There was a U-shaped relationship between HbA1c and all-cause death at 10 years in the overall population and the PCI arm, whereas a linear relationship was observed in the CABG arm.
Amongst diabetics, there were no significant differences in all-cause death at 10 years between PCI and CABG in any anatomical SYNTAX score tertile.
In patients with diabetes and 3VD, the overall risk of mortality at 10 years was comparable between PCI and CABG; however, it was numerically higher with PCI in the highest (≥33) SYNTAX score tertile.
The SYNTAX score II 2020 further endorses our general contention that—as an ‘average treatment effect’—differences in all-cause death between CABG and PCI in diabetic patients at 10 years are minor, whereas individualized predicted and observed mortality clearly identify individuals who benefit either from CABG or PCI.
Previous studies have been unable to conclusively establish whether PCI or CABG offers the best long-term survival for patients with diabetes and multivessel CAD. The BARI trial was the first to report a significant 10-year survival benefit with CABG over PCI with balloon angioplasty amongst 353 diabetic patients with multivessel CAD, however, the benefit diminished somewhat overextended follow-up.8 In the FREEDOM study, the benefit with CABG over PCI for all-cause death at 5 years was only marginally significant (P = 0.049),4 however, considering that the trial was not powered for all-cause death, this result could be considered hypothesis-generating.15 In the FREEDOM Follow-On study,16 which extended follow-up in 943 of the original 1900 patients cohort, the estimated rate of mortality at 8 years was 23.7% and 18.7% in the PCI-DES and CABG group, respectively (unadjusted HR: 1.32, 95% CI: 0.97, 1.78, P = 0.076), with the HR remaining unchanged after adjustment.
The greatest variance with our results, which showed no significant difference between PCI and CABG at 10 years amongst diabetics with multivessel CAD, comes from a recent propensity score matching analysis by Tam et al.,17 which reported significantly higher mortality with PCI compared with CABG at 8 years. These conflicting results may be explained by the differences between the trials designs. First, the study cohorts were different as Tam et al included patients with two-vessel disease or 3VD, whereas our study only included patients with 3VD and/or LMCAD. Secondly, they included patients with acute coronary syndrome (ACS), while only patients with stable CAD and unstable angina were included in SYNTAXES. Moreover, 22.9% of their patients received bare-metal stents. Finally, the proportion of incomplete revascularization in the PCI arm was higher in their study, which may partly contribute to the higher incidence of all-cause death in their PCI arm.18–20 In fact, after the exclusion of patients with ACS and those treated with bare-metal stents, Kaplan–Meier curves between PCI and CABG appear to converge, especially after 8 years, suggesting a diminishing treatment difference between PCI and CABG with very long-term follow-up.
At the time of the 5 years report of the SYNTAX study,12 investigators, surgeons, and interventionists were intuitively convinced that the diverging Kaplan–Meier curves for mortality would keep diverging; however, it now appears that our intuitive assumption was somewhat naïve and partially incorrect. Our landmark analysis showed that after 5 years the Kaplan–Meier survival curve was worse after CABG than PCI, although the differences were not statistically significant (Figure 2A). These results suggest that a temporal change in the survival benefit of CABG over PCI in diabetic patients. Notably, within the confines of our limited sample size that may not have adequate power, further research in adequately powered long-term studies are required.
In insulin-treated diabetics, PCI resulted in a numerically higher non-significant mortality at 10 years compared with CABG; however, no significant interaction was established within the limitations of our sample size. Similar results in all-cause death were reported in the insulin-treated diabetic subgroup from the FREEDOM study (PCI vs. CABG: 19% vs. 14.1%, HR: 1.19, 95% CI: 0.76, 1.85, P -interaction = 0.64),21 indicating that even their sample size of 1850 was too small to detect a differential treatment survival benefit with CABG over PCI between diabetics treated with or without insulin. Patients with insulin-treated diabetes appear to have a longer life expectancy following CABG compared with PCI, although the differences were not statistically significant (8.24 vs. 7.55 years, P = 0.230) due to limited sample size (n = 182) and restricted power. This reaffirms the need for large sample size investigations in this subset of patients.
In the BARI trial, 10-year survival among diabetic patients was higher following CABG with arterial grafting, compared with CABG using only vein grafts and PCI.8 However, in our study, no significant difference in all-cause death at 10 years was observed between patients with diabetes receiving PCI or either single or multiple arterial grafts (Supplementary material online, Figure S15). These results support our main findings that the survival benefit of CABG over PCI subsides over time (10 years); nevertheless, the convergence of the three survival curves (PCI, CABG with SAG or MAG) is striking. It is also remarkable that the survival curves in the non-diabetic cohort kept diverging over time, at least between patients with MAG and those either treated with PCI, or a combination of SAG and venous grafts. As a matter of fact, this observation is more worrisome than the one made in diabetic patients.
It could be argued that the convergence of Kaplan–Meier curves at 10 years is due to ageing; however, no significant interaction on mortality at 10 years was seen between revascularization mode and age (>70 or ≤70 years old) in patients with and without diabetes. In the entire population of SYNTAXES, a similar lack of significant interaction for age was also reported. Hence the convergence of the two Kaplan–Meier curves at 10 years cannot be solely explained by ageing. This observation could also be attributed to the late attrition of bypass grafts around the 7th year of follow-up affecting both SAG and MAG patients (Supplementary material online, Figure S15); however, only the attrition of venous grafts has been widely documented in the literature.22
The relationship between HbA1c and mortality following PCI23 , 24 or CABG25 , 26 is controversial. Currently, several guidelines recommend the assessment of HbA1c to help achieve better clinical outcomes; however, the threshold for implementing more stringent glycaemic control varies substantially.27 In the present analysis, a U-shaped relationship between HbA1c and mortality at 10 years was observed with PCI, whereas the relationship was linear with CABG. These results suggest that a threshold HbAc1 exists beyond which it should be prognostically unacceptable to treat patients with PCI. Unfortunately, our limited sample size did not permit any strong statistical inference or formal recommendations. High-quality randomized large-scale trials are needed to further investigate this important issue.
Prior studies established that CABG was preferred for those with intermediate or high SYNTAX scores.5 , 7 , 28 Notably, an observation from the FREEDOM trial was that when CABG is compared with PCI, diabetic status was more determinant of outcomes and vital prognosis, than the extent and complexity of CAD.29 Our results suggest that the anatomical SYNTAX score is not a determinant factor of fatal prognosis in diabetic patients in the SYNTAX study; however, following multivariable adjustment, it was associated with an increased risk of death in patients who received PCI, but not CABG (Supplementary material online, Table S2).
The anatomical SYNTAX score as well as the type of CAD (3VD or LMCAD) are ‘modifiers’, as labelled by epidemiologists, that have a profound interaction with other clinical characteristics and comorbidities, and deserve to be computed and incorporated into the calculation of the SYNTAX score II 2020.13 Therefore, if the anatomical SYNTAX score was not integrated into the SYNTAX score II 2020, and just interpreted in isolation, it would have no prognostic value for the Heart Team when deciding the optimal revascularization strategy of diabetic patients with complex CAD. In patients with diabetes and 3VD, the current guidelines recommend that PCI may be considered in patients with a SYNTAX score ≤22 (recommendation IIb for PCI), however, it is not recommended in patients with a SYNTAX score >22 (recommendation III).30
Our landmark analysis showed that in diabetic patients with 3VD, PCI compared with CABG had higher all-cause death at 5 years, with a reverse risk seen between 5 and 10 years. These 5-year results were in line with previous studies6; however, the survival benefit from CABG was seen to diminish between 5 and 10 years. In patients with diabetes and 3VD, PCI appeared to have a non-significant higher risk of all-cause death at 10 years compared with CABG. Consistent with a prior meta-analysis reporting survival up to 5 years,6 no significant between-group (PCI vs. CABG) difference in mortality at 5 years was observed in diabetics with LMCAD, with this absence of difference in vital outcome maintained up to 10 years and beyond (Supplementary material online, Figures S10B and S11B). Notably, with the limited sample size, these subgroup analyses may reduce the power of the analysis and increase the risk of Type 1 and 2 errors.
In non-diabetic patients with 3VD, we observed a higher risk of mortality with PCI over CABG at 5 years, which is inconsistent with the aforementioned meta-analysis.6 This finding could be due to a play of chance related to the smaller sample size than the pooled patient-level analysis. It could also be due to disparity in follow-up durations, which were a median of 3.8 years in the meta-analysis and 11.2 years in SYNTAXES. Moreover, in the meta-analysis, only 60% of patients had 3VD, unlike the current subgroup analysis where all patients had 3VD. Furthermore, the higher mortality at 10 years with PCI in non-diabetics with 3VD was mainly driven by patients with a SYNTAX score ≥33 (Supplementary material online, Figure S13A).
An rSS > 8 has been associated with increased short- and mid-term adverse events, including all-cause death.31 , 32 Recently, an observational study found that diabetes and an rSS > 8 contribute independently to late outcomes in STEMI patients with a follow-up of 3.6 years.33 In our analysis, compared with patients with rSS ≤ 8, patients with rSS > 8 had a significantly higher all-cause death at 10 years both in diabetic and non-diabetic patients. These results were in line with the 5-year results in the SYNTAX trial.32 Residual SYNTAX score is a post-procedural parameter, and therefore it is difficult to determine the specific risk and outcome a priori; however, if it is unlikely that complete or nearly complete revascularization (rSS ≤ 8) can be achieved, then CABG should be considered.
To further endorse our contention that treatment differences in vital prognosis between diabetic and non-diabetic patients at 10 years are not major, we applied the SYNTAX score II 2020 to the diabetic and non-diabetic population. We found that the ability of the SYNTAX score II 2020 to predict rates of all-cause death at 10 years following PCI or CABG was equally valuable in diabetic and non-diabetic patients. This is not surprising since the score is derived from the outcomes of SYNTAXES, but its applicability and accuracy in diabetics, as well as in non-diabetics, is a form of internal validation. Therefore, the SYNTAX score II 2020 has the capability to support revascularization decision-making in diabetic and non-diabetic patients with 3VD and/or LMCAD. However, we have to acknowledge that due to the limited sample size, the confidence intervals of the absolute risk difference for each quarter in the diabetic population are wide (Figure 4).
Although there is equipoise in mortality between diabetics in SYNTAXES treated with PCI and CABG, and theoretically they may seem equally eligible to receive either treatment, the present analysis reflects an ‘average treatment effect’ based on a singled out comorbidity, namely diabetes. Nowadays, precision medicine tries to individualize the prognosis of patients taking into account multiple co-variables.34 In the SYNTAX score II 2020,13 diabetes is included as one of the prognostic indexes predicting the risk of all-cause death at 10 years that is also affected by so-called effect-modifiers (e.g. anatomical SYNTAX score and type of disease: 3VD or LMCAD). The endpoint in SYNTAXES was all-cause death only, and in the absence of data collection of MACCE in the last 5 years of follow-up, caution must be exerted about a simplistic interpretation on the equipoise of mortality. Although all-cause death may for the trialist be the ultimate unbiased comparative assessment between two revascularization approaches,35 from the patient’s viewpoint MACE and quality-adjusted life years (QUALY) are also very relevant outcomes.36
Limitations
Although the diabetes subgroup was pre-specified and randomization was stratified by the presence of diabetes,11 the present analyses did not have adequate statistical power and subgroup analyses may increase the risk of Type 1 and 2 errors. There was no formal correction for multiple testing for subgroup analyses of the trial, taking into account the post hoc nature of the analysis.37 The sophistication and number of the analysis may lead to the likelihood of spurious findings, and all reported findings should be considered strictly as exploratory and hypothesis-generating. To improve statistical efficiency/power, we performed a multivariable analysis in the present study; however, the inability to include all relevant confounders may cause bias that cannot be adjusted. Additionally, the SYNTAX trial enrolled patients with de novo 3VD and/or LMCAD, and our findings should not be extrapolated to general CAD patients or in patients with previous revascularization. The endpoint in the SYNTAXES study was all-cause death only, detailed causes for death were not collected. In the elderly patients, the long-term mortality likely includes a sizeable number of non-cardiac death. The therapies received in the last 5 years of follow-up such as revascularization procedures and pharmacological agents, as well as changes in diabetic status were not collected. However, all-cause death has been considered as the most robust and unbiased index for clinical assessment and is less likely to be affected by ascertainment bias.38 In the SYNTAXES study, only one measurement of HbA1c was available at enrolment, which cannot accurately reflect prior control of diabetes. Nevertheless, it is remarkable that this single measurement still has a long-term prognostic value. In future studies with larger sample sizes, multiple measurements of HbA1c should be recommended.
Since loss to follow-up may have potentially impacted on estimated treatment effects, it should be acknowledged that vital status was missing in 6% of patients. However, the drop-out rate was comparable between PCI and CABG. Notably, a previous systematic review that included trials published in five top general medical journals found that the median loss to follow-up was also 6% in 191 trials.39 Another limitation is that the diagnostic criteria of diabetes in the SYNTAX trial (2005–07) did not include HbA1c, which was only adopted by ADA in 2010.40 Finally, in the SYNTAX study, patients received PCI with first-generation DES, which are no longer commercially available, hence, our results are only partially applicable to contemporary new-generation DES. Obviously, patients did not benefit from new-generation anti-diabetic drugs such as GLP-1 receptor agonists and SGLT2 inhibitors or inhibitors of PCSK 9 which have all been shown to lower the risks of cardiovascular mortality.41–44 Although the very long-term data from SYNTAXES are important, we have to emphasize that they are not fully applicable to today’s patients, and the Task Force drawing future Guidelines should be warned to avoid strict recommendations with legal implications based only on ‘old’ data, because they are the only data available. Further investigations in dedicated large-scale trials in patients with diabetes on contemporary pharmacologic therapeutic regimens are warranted. However, it is unavoidable that the findings from long-term follow-up data are based on outdated technology, while the evidence for contemporary technology can be derived from studies with only short-term follow-up.
Conclusions
Diabetes was associated with an increased risk of all-cause death at 10 years in patients with 3VD and/or LMCAD who underwent either PCI or CABG. In diabetic patients with complex CAD, CABG did not lower the risk of all-cause death at 10 years compared with PCI, although diabetic patients on insulin may derive a survival benefit from CABG. The SYNTAX score II 2020 may identify diabetic patients who will benefit from either CABG or PCI.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The SYNTAX Extended Survival study was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). The SYNTAX trial was funded during 0–5 year follow-up by Boston Scientific Corporation (Marlborough, MA, USA). Both sponsors had no role in the study design, data collection, data analyses, and interpretation of the study data nor were involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom. This work, R.W., and C.G. are supported by Science Foundation Research Professorship Award (15/RP/2765).
Conflict of interest: F.B. reports speaker’s fees from Abiomed, Abbott, and Medtronic. S.J.H. reports to work as a full-time employee of Medtronic outside the scope of this work. S.J.’s institution has received research grants from Boston Sc, Abbot, Biotronik, Medtronic, Astra Zeneca, Bayer, Jansen, The MedCo, and has received lecture fees from Biotronik and Astra Zeneca. P.K. reports to work as an employee of Medtronic, outside the submitted work. P.W.S. reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. R.-J.v.G. reports grants and personal fees from Boston Scientific, Abbott Vascular, Astra Zeneca, and Amgen and grants from InfraRedx, outside the submitted work. All other authors have no disclosures.
Data availability
Data will be made available upon request in adherence with transparency conventions in medical research and through reasonable requests to the corresponding author.
Supplementary Material
Contributor Information
Rutao Wang, Department of Cardiology, Xijing Hospital, Changle West Road 127, Xi’an 710032, China; Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Radboud University Medical Center, Geert Grooteplein Zuid 8, 6525 GA Nijmegen, The Netherlands.
Patrick W Serruys, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Imperial College London, Exhibition Rd, London SW7 2BX, UK.
Chao Gao, Department of Cardiology, Xijing Hospital, Changle West Road 127, Xi’an 710032, China; Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Radboud University Medical Center, Geert Grooteplein Zuid 8, 6525 GA Nijmegen, The Netherlands.
Hironori Hara, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Kuniaki Takahashi, Department of Cardiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Masafumi Ono, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Hideyuki Kawashima, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland; Department of Cardiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Neil O’leary, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland.
David R Holmes, Department of Cardiology, Mayo ClinicSchool of Medicine, 200 First St. SW Rochester, MN 55905, USA.
Adam Witkowski, Department of Interventional Cardiology and Angiology, National Institute of Cardiology, ul. Alpejska 42, 04-628 Warsaw, Poland.
Nick Curzen, Cardiology Department, University Hospital Southampton, Coxford Rd, Southampton SO16 5YA, UK.
Francesco Burzotta, Institute of Cardiology, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy.
Stefan James, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjolds vag 14B SE-752 37, Uppsala, Sweden.
Robert-Jan van Geuns, Department of Cardiology, Radboud University Medical Center, Geert Grooteplein Zuid 8, 6525 GA Nijmegen, The Netherlands.
Arie Pieter Kappetein, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Dr Molewaterplein 40, 3015 GE Rotterdam, The Netherlands.
Marie-angele Morel, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland.
Stuart J Head, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Dr Molewaterplein 40, 3015 GE Rotterdam, The Netherlands.
Daniel J F M Thuijs, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Dr Molewaterplein 40, 3015 GE Rotterdam, The Netherlands.
Piroze M Davierwala, Department of Cardiac Surgery, Heart Centre Leipzig, Strumpelstrasse 39, Leipzig 4289, Germany.
Timothy O’Brien, Regenerative Medicine Institute, CURAM, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland.
Valentin Fuster, Division of Cardiology, Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicina at Mount Sinai School, 1 Gustave L. Levy Place, 10029-5674 New York, NY, USA.
Scot Garg, Department of Cardiology, East Lancashire Hospitals NHS Trust, Haslingden Rd, Blackburn BB2 3HH, Lancashire, UK.
Yoshinobu Onuma, Department of Cardiology, National University of Ireland, Galway (NUIG), University Road, Galway H91 TK33, Ireland.
References
- 1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011;32:2748–2757. [DOI] [PubMed] [Google Scholar]
- 3. Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010;55:432–440. [DOI] [PubMed] [Google Scholar]
- 4. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 5. Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ; on behalf of the SYNTAX Investigators. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013;43:1006–1013. [DOI] [PubMed] [Google Scholar]
- 6. Head SJ, Milojevic M, Daemen J, Ahn J-M, Boersma E, Christiansen EH, Domanski MJ, Farkouh ME, Flather M, Fuster V, Hlatky MA, Holm NR, Hueb WA, Kamalesh M, Kim Y-H, Mäkikallio T, Mohr FW, Papageorgiou G, Park S-J, Rodriguez AE, Sabik JF, Stables RH, Stone GW, Serruys PW, Kappetein AP. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939–948. [DOI] [PubMed] [Google Scholar]
- 7. Windecker S, Neumann FJ, Juni P, Sousa-Uva M, Falk V. Considerations for the choice between coronary artery bypass grafting and percutaneous coronary intervention as revascularization strategies in major categories of patients with stable multivessel coronary artery disease: an accompanying article of the task force of the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:204–212. [DOI] [PubMed] [Google Scholar]
- 8.BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007;49:1600–1606. [DOI] [PubMed] [Google Scholar]
- 9. Thuijs DJFM, Kappetein AP, Serruys PW, Mohr F-W, Morice M-C, Mack MJ, Holmes DR, Curzen N, Davierwala P, Noack T, Milojevic M, Dawkins KD, da Costa BR, Jüni P, Head SJ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–1334. [DOI] [PubMed] [Google Scholar]
- 10. Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR Jr, Mack MJ, van den Brand M, Morel MA, van Es GA, Kleijne J, Koglin J, Russell ME. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J 2006;151:1194–1204. [DOI] [PubMed] [Google Scholar]
- 11. Serruys PW, Morice M-C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 12. Mohr FW, Morice M-C, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR, Morel M-A, Dyck NV, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi K, Serruys PW, Fuster V, Farkouh ME, Spertus JA, Cohen DJ, Park S-J, Park D-W, Ahn J-M, Kappetein AP, Head SJ, Thuijs DJFM, Onuma Y, Kent DM, Steyerberg EW, van Klaveren D. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 2020;396:1399–1412. [DOI] [PubMed] [Google Scholar]
- 14. Pocock SJ, McMurray JJV, Collier TJ. Statistical controversies in reporting of clinical trials: art 2 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol 2015;66:2648–2662. [DOI] [PubMed] [Google Scholar]
- 15. Koskinas KC, Windecker S. Revascularization in complex multivessel coronary artery disease after FREEDOM. Is there an indication for PCI and drug-eluting stents? Herz 2016;41:224–232. [DOI] [PubMed] [Google Scholar]
- 16. Farkouh ME, Domanski M, Dangas GD, Godoy LC, Mack MJ, Siami FS, Hamza TH, Shah B, Stefanini GG, Sidhu MS, Tanguay JF, Ramanathan K, Sharma SK, French J, Hueb W, Cohen DJ, Fuster V; FREEDOM Follow-On Study Investigators. Long-term survival following multivessel revascularization in patients with diabetes: the FREEDOM follow-on study. J Am Coll Cardiol 2019;73:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tam DY, Dharma C, Rocha R, Farkouh ME, Abdel-Qadir H, Sun LY, Wijeysundera HC, Austin PC, Udell JA, Gaudino M, Fremes SE, Lee DS. Long-term survival after surgical or percutaneous revascularization in patients with diabetes and multivessel coronary disease. J Am Coll Cardiol 2020;76:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourassa MY, Holubkov R, Sopko G, Detre KM. Long-term outcome of patients with incomplete vs complete revascularization after multivessel PTCA. A report from the NHLBI PTCA Registry. Eur Heart J 1998;19:103–111. [DOI] [PubMed] [Google Scholar]
- 19. Hannan EL, Racz M, Holmes DR, King SB 3rd, Walford G, Ambrose JA, Sharma S, Katz S, Clark LT, Jones RH. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation 2006;113:2406–2412. [DOI] [PubMed] [Google Scholar]
- 20. Takahashi K, Serruys PW, Gao C, Ono M, Wang R, Thuijs D, Mack MJ, Curzen N, Mohr FW, Davierwala P, Milojevic M, Wykrzykowska JJ, de Winter RJ, Sharif F, Onuma Y, Head SJ, Kappetein AP, Morice MC, Holmes DR Jr. Ten-year all-cause death according to completeness of revascularization in patients with three-vessel disease or left main coronary artery disease: insights from the SYNTAX extended survival study. Circulation 2021;doi:10.1161/CIRCULATIONAHA.120.046289. [DOI] [PubMed] [Google Scholar]
- 21. Dangas GD, Farkouh ME, Sleeper LA, Yang M, Schoos MM, Macaya C, Abizaid A, Buller CE, Devlin G, Rodriguez AE, Lansky AJ, Siami FS, Domanski M, Fuster V; FREEDOM Investigators. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol 2014;64:1189–1197. [DOI] [PubMed] [Google Scholar]
- 22. Benedetto U, Raja SG, Albanese A, Amrani M, Biondi-Zoccai G, Frati G. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trialsdagger. Eur J Cardiothorac Surg 2015;47:59–65; discussion 65. [DOI] [PubMed] [Google Scholar]
- 23. Sharma PK, Agarwal S, Ellis SG, Goel SS, Cho L, Tuzcu EM, Lincoff AM, Kapadia SR. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2014;7:503–509. [DOI] [PubMed] [Google Scholar]
- 24. Zheng J, Cheng J, Zhang Q, Qi C, Wang T, Xiao X. Association between glycosylated hemoglobin level and cardiovascular outcomes in diabetic patients after percutaneous coronary intervention. Medicine (Baltimore) 2016;95:e3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tennyson C, Lee R, Attia R. Is there a role for HbA1c in predicting mortality and morbidity outcomes after coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg 2013;17:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Luo X, Jin X, Lv M, Li X, Dou J, Zeng J, An P, Chen Y, Chen K, Mu Y. Effects of preoperative HbA1c levels on the postoperative outcomes of coronary artery disease surgical treatment in patients with diabetes mellitus and nondiabetic patients: a systematic review and meta-analysis. J Diabetes Res 2020;2020:3547491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018;168:569–576. [DOI] [PubMed] [Google Scholar]
- 28. Hakeem A, Garg N, Bhatti S, Rajpurohit N, Ahmed Z, Uretsky BF. Effectiveness of percutaneous coronary intervention with drug-eluting stents compared with bypass surgery in diabetics with multivessel coronary disease: comprehensive systematic review and meta-analysis of randomized clinical data. J Am Heart Assoc 2013;2:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Esper RB, Farkouh ME, Ribeiro EE, Hueb W, Domanski M, Hamza TH, Siami FS, Godoy LC, Mathew V, French J, Fuster V. SYNTAX score in patients with diabetes undergoing coronary revascularization in the FREEDOM trial. J Am Coll Cardiol 2018;72:2826–2837. [DOI] [PubMed] [Google Scholar]
- 30. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 31. Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu K, Parise H, Mehran R, Serruys PW, Stone GW. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol 2012;59:2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, Holmes DR, Mack M, Morice MC, Stahle E, Colombo A, de Vries T, Morel MA, Dawkins KD, Kappetein AP, Mohr FW. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation 2013;128:141–151. [DOI] [PubMed] [Google Scholar]
- 33. Burgess SN, Juergens CP, Nguyen T, Leung M, Robledo KP, Thomas L, Mussap C, Lo STH, French JK. Diabetes and incomplete revascularisation in ST elevation myocardial infarction. Heart Lung Circ 2021;30:471–480. [DOI] [PubMed] [Google Scholar]
- 34. Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 2018;363:k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaudino M, Hameed I, Farkouh ME, Rahouma M, Naik A, Robinson NB, Ruan Y, Demetres M, Biondi-Zoccai G, Angiolillo DJ, Bagiella E, Charlson ME, Benedetto U, Ruel M, Taggart DP, Girardi LN, Bhatt DL, Fremes SE. Overall and cause-specific mortality in randomized clinical trials comparing percutaneous interventions with coronary bypass surgery: a meta-analysis. JAMA Intern Med. 2020;180:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stolker JM, Spertus JA, Cohen DJ, Jones PG, Jain KK, Bamberger E, Lonergan BB, Chan PS. Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation 2014;130:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, Devereaux PJ, Thabane L. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol 2017;46:746–755. [DOI] [PubMed] [Google Scholar]
- 38. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618–620. [DOI] [PubMed] [Google Scholar]
- 39. Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, Mulla S, Lamontagne F, Bassler D, Vera C, Alshurafa M, Katsios CM, Zhou Q, Cukierman-Yaffe T, Gangji A, Mills EJ, Walter SD, Cook DJ, Schunemann HJ, Altman DG, Guyatt GH. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ 2012;344:e2809. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62—S6 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 43. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 44. Handelsman Y, Lepor NE. PCSK9 inhibitors in lipid management of patients with diabetes mellitus and high cardiovascular risk: a review. J Am Heart Assoc 2018;7:e008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request in adherence with transparency conventions in medical research and through reasonable requests to the corresponding author.