Summary
Background
The dominant effect of age on COVID-19 mortality obscures the impact of other risk factors. Although the elderly is at a greater risk of severe disease and death due to COVID-19, the interaction of obesity and age was not carefully assessed. This analysis is especially critical for prioritizing groups to receive COVID-19 vaccination.
Methods
Starting with 1,120,767 unvaccinated individuals registered in a Brazilian surveillance system, we selected 313,898 hospitalized COVID-19 patients aged 20 to 89 who had a BMI ≥ 25 kg/m2 and cardiovascular diseases (CVD) or diabetes, as well as individuals with no risk factors associated with severe COVID-19. Patient data were stratified by age, obesity, BMI, and comorbidities, and subsequently, subjected to crude and adjusted odds ratio, hazard ratio, and Kaplan–Meier curves. Disease outcomes were invasive and non-invasive ventilatory support, intensive care unit (ICU) admission, and death.
Findings
Obesity alone is a risk factor for in-hospital mortality and is more significant than cardiovascular disease and diabetes. Furthermore, obesity, cardiovascular disease, and diabetes increase the risk of severity and death by COVID-19 more significantly in young adults than in the elderly. When categorizing patients by obesity classes, the severity of obesity was found to be associated with a higher risk of admission to the ICU and death from COVID-19 than the non-obese young adults or elderly population.
Interpretation
Our findings highlight the increased risk of severe COVID-19 on the Brazilian obese youth. As SARS-CoV-2 may become a recurrent seasonal infection, future vaccination campaigns against COVID-19 should prioritize obese young individuals.
Fundings
This work was supported by the Brazilian National Council for Scientific and Technological Development (grant number 313662/2017-7 and 307356/2017-5; the São Paulo Research Foundation (grant numbers 2018/14933-2); and CAPES.
Research in context.
Evidence before this study
Age and comorbidities such as diabetes, hypertension, and obesity significantly increase the risk of developing severe COVID-19. However, it remains unclear whether these comorbidities impact the youth and elderly differently in countries with high prevalence of obese young adults.
Added value of this study
We performed a comprehensive retrospective study on the Brazilian population to evaluate the contribution of obesity along with other comorbidities on the increased risk of severe COVID-19 in the elderly and the young. The values of our study are the following: (1) We used a large sample (N = 313,898) of hospitalized patients with laboratory-confirmed COVID-19; (2) We covered the entire pre-vaccination period in Brazil (February 16, 2020 to January 17, 2021); (3) We collected the information on comorbidities and BMI at the time of admission.
Implications of all the available evidence
Considering the potential seasonality of SARS-CoV-2 outbreaks, our results reveal a clear impact of obesity on the severity of COVID-19 in young adults. Therefore, the findings provide strong support for obese youth to be considered a high-priority group for receiving COVID-19 vaccines.
Alt-text: Unlabelled box
Introduction
Brazil is one of the countries with the highest number of COVID-19 cases, with 22,012,150 infections and 612,587 deaths until November 21, 2021.1 The lack of proportional mass vaccination among Brazilian states partially explains the high rate of transmission of the SARS-CoV-2 virus even 18 months after the first case was reported in the country.2
When vaccination against COVID-19 finally started in Brazil, vaccine availability was scarce, received in several batches and unevenly distributed between states and cities. Similarly to what was seen in other counties, the federal government needed to establish priority groups to receive the vaccines first. Age was defined as one of the significant risk factors for severe COVID-19 very early in the pandemic.3 Therefore, the elderly population was the first to receive vaccines against COVID-19. This measure was essential to reduce deaths in this age group.3 Associations between various cardiovascular risk factors and the risk of a poor outcome among those hospitalized with COVID-19 have also been described.4,5 In Brazil, vaccinating people with such comorbidities, even if they were young, was prioritized.6 Given that vaccines are only available to a small fraction of the population, it is unclear whether prioritizing young people with comorbidities is efficient.
Obesity was epidemic in Brazil before COVID-19. The prevalence of obesity among Brazilian adults aged 20 or older increased from 12.2 to 26.8% from 2003 to 2019.7Such high obesity rates in Brazil create a high health and economic burden. It has been projected that the prevalence cases of obesity-related diseases, such as stroke, hypertension, coronary heart disease, osteoarthritis and diabetes may double by 2050.8 We hypothesize that excess weight in young individuals may be associated with greater risk for severe COVID-19 when compared to young individuals with normal weight.
Methods
Study Design and Data Source
This study is a retrospective analysis of publicly available data extracted from the Influenza Epidemiological Surveillance Information System, SIVEP-Gripe, (Sistema de Informação Epidemiológica da Gripe). It is a nationwide surveillance system established by the Brazilian Ministry of Health to monitor cases of Severe Acute Respiratory Syndrome (SARS).9 The SIVEP-Gripe is the central repository for compulsory notifications regarding SARS by COVID-19 or other etiologies admitted in Brazil's public and private health systems. Furthermore, this databank includes socio-demographic information, self-reported symptoms, comorbidities, respiratory support use, date of hospital admission, date of entry to intensive care unit (ICU), clinical and laboratory exams, and in-hospital outcomes.
The criteria used by the Ministry of Health of Brazil to consider a case as SARS are dyspnea/respiratory discomfort or persistent pressure in the chest, O2 saturation being less than 95% under normal ventilation conditions, or blushing of the lips or face in patients with respiratory condition accompanied by at least two of the following symptoms: fever, chills, sore throat, headache, cough, runny nose, and olfactory or taste disturbances.9 Since we included only patients with COVID-19 as a defined cause of SARS in this study, throughout the text, we have used only the term COVID-19 to characterize our study population.
Study population and outcomes
From an initial sample of 1,120,767 patients registered in SIVEP-Gripe8 in the pre-vaccination period in Brazil, between February 16, 2020 and January 17, 2021, we analyzed data from 313,898 adult patients aged 20 to 89 years who were admitted to hospital due to SARS by COVID-19 and whose diagnosis was confirmed by polymerase chain reaction testing (RT-PCR) or antigenic tests. The study's inclusion and exclusion criteria are detailed in Supplementary Figure 1, which presents the profile of our study population. Inclusion criteria were patients without any comorbidity or patients with obesity or CVD, diabetes, or any combination of these risk factors (Supplementary Figure 1). The SIVEP-Gripe database considers the following conditions or comorbidities as a risk factor: cardiovascular disease (CVD), diabetes mellitus, overweight/obesity (body mass index [BMI] > 25 kg/m2), chronic respiratory disease (including asthma), hepatopathy, chronic kidney pathology, immune deficiency, genetic syndrome, renal pathology, and others (hematologic disease, neurology disorder, cancer, rheumatologic disease, and drug addiction). CVD also includes individuals with atherosclerotic diseases, as well as those that had stroke, myocardial infarction, arrhythmias, thrombosis, hypertension, congestive heart failure, and dyslipidemia. We reviewed these comorbidities to define our study group and divided the patients into two groups: 1) 164,119 patients without risk factors (BMI < 24.9 kg/m2 or without comorbidities) and 2) 149,779 patients with any composition of the following characteristics: overweight or obesity (BMI ≥ 25 kg/m2), Diabetes, CVD, or any combination of these risk factors. Any other comorbidity as well as pregnant or puerperal women were excluded (Supplementary Figure 1).
The SIVEP-Gripe database only provides BMI information for patients who have been classified as obese. Therefore, we classified obese patients as overweight (BMI 25 to 29.9 kg/m2), obesity class I (BMI 30 to 34.9 kg/m2), obesity class II (BMI 35 to 39.9 kg/m2), and obesity class III (BMI > 40 kg/m2). Patients who received non-invasive respiratory support or invasive respiratory support were compared with those who did not receive any respiratory support. The outcomes were analyzed, separately, as follows: ICU admission, non-invasive respiratory support, invasive respiratory support (intubation), and death due to COVID-19.
Statistical analysis
After defining the comorbidities, we evaluated the association between death rates, ICU admission, and non-invasive respiratory support to each age subgroup by calculating the crude odds ratio (OR) and the adjusted OR using multiple logistic regression. The factors adjusted in the analyses were race, education, region. The group of people without comorbidities was used as the reference control.
To calculate the survival curves, we considered the time (in days) between the final outcome and the onset of symptoms. Furthermore, we considered death as an event and hospital discharge as a non-event. Patients with missing data at any point were not considered in the survival analysis. To estimate the survival curves, we used the Kaplan–Meier method, and to compare the curves, we used the Gehan-Breslow-Wilcoxon test (non-parametric test analogous to the Log-Rank test) since the curves along time presented inversions and overlaps. Risk rates over time and Hazzard Ratio (HR) were carried over from the risk model proportionally to the COX, with adjustment for race, education level, and region.
The results were estimated using PRISMA (version 9.1.2) and SPSS (version 20), and the significance level was assumed to be 5%.
Ethical aspects
SIVEP-Gripe is a publicly available database which contains no personal information about patients. Therefore, the analysis was based on open data principles and did not require ethical approval in Brazil.10
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Results
The study population comprised of 313,898 Brazilian adults (20–89y) who were hospitalized with COVID-19 from February 16, 2020, to January 17, 2021. These dates cover the period between the first cases of COVID-19 in Brazil and the beginning of vaccination against the disease. Patients with COVID-19 were predominantly male (60%), white (40%), 60–89y (50%), had high school education level (12%), and were from the southeast region of Brazil (51%) (Table 1).
Table 1.
Sociodemographic characteristics of patients included in the study.
| Characteristics | All Patients | Deaths | P value* |
|---|---|---|---|
| N= 313,898 (column %) | N=94,775 (% of deceased patients in relation to all patients of the group) | ||
| Sex | 0.37993 | ||
| Male | 187,220 (60) | 56,638 (30) | |
| Female | 126,678 (40) | 38,137 (30) | |
| Age group | |||
| 20 to 39 y | 43,230 (14) | 3,926 (9) | < 0.00001 |
| 40 to 59 y | 114,737 (36) | 21,140 (18) | < 0.00001 |
| >= 60 y | 155,931 (50) | 69,709 (55) | reference |
| Self-reported race/ethnicity | |||
| White | 123,571 (39,7) | 33,919 (27) | < 0.00001 |
| Black/ Brown | 116,488 (37) | 40,715 (35) | reference |
| Asian | 3,396 (1) | 1,067 (32) | 0.00005 |
| Indigenous | 898 (0,3) | 350 (39) | 0.01189 |
| Misinformed | 69,545 (22) | 18,704 (27) | < 0.00001 |
| Education Level | |||
| Illiterate | 6,458 (2,5) | 3,444 (53) | < 0.00001 |
| Up to High-School | 48,765 (15,5) | 18,284 (38) | reference |
| High School | 38,069 (12) | 8,632 (23) | < 0.00001 |
| University or College | 19,678 (6) | 3,476 (18) | < 0.00001 |
| Missing information | 200,928 (64) | 60,939 (30) | |
| Brazilian Region | |||
| Southeast | 160,516 (51) | 44,831 (28) | reference |
| South | 45,190 (14,4) | 10,305 (23) | < 0.00001 |
| Northeast | 53,729 (17) | 21,935 (41) | < 0.00001 |
| North | 27,003 (8,6) | 10,731 (40) | < 0.00001 |
| Central-West | 27,460 (9) | 6,973 (25) | < 0.00001 |
Chi-square test.
We performed four separate analyses using as primary outcome: (i) ICU admission, (ii) use of non-invasive support, (iii) use of invasive ventilatory support, and (iv) death. We then compared the adjusted odds ratios for each primary outcome for patients identified as obese (N = 8,834), patients with CVD (N = 56,079), patients with diabetes (N = 24,535), patients with CVD + diabetes (N = 41,646), with obesity + CVD (N = 8,759), with obesity + diabetes (N = 2,178), and with obesity + CVD + diabetes (N = 7,748). The reference group comprised patients with no other risk factors (N = 164,119). Initially, we assessed the interplay between age and obesity using a multiple logistic regression model with BMI/age as concurrent variables. Considering age and BMI categories together in the model, we found that the interaction between them was often associated with all primary outcomes (Supplementary Table I). Likewise, when we considered BMI or age alone, the association with primary outcomes remains, thereby justifying the age stratification analyses (Supplementary Table I and II).
Obesity alone is undoubtedly an aggravating factor for COVID-19 severity when considering ICU admission, ventilatory support, and death (Figure 1 and Supplementary Tables III-V). Indeed, for all age intervals, patients who were only obese were at a higher risk for dying from COVID-19 than patients who had only CVD (P < 0.0001; Supplementary Table VI). Furthermore, for 20–39y and 40–59y age groups, the risks were equivalent to patients that had only diabetes (P > 0.05; Supplementary Table VI). The risk of mortality for obese patients was more prominent in younger patients: OR 3.70, CI 3.13–4.37 for 20–39 y; OR 2.41, CI 2.14–2.72 for 40–59 y and OR 1.41, CI 1.19–1.66) for ≥ 60 y. Obesity combined with diabetes or CVD in young patients leads to a 7.24 times (CI 5.14–10.18) higher risk of mortality from COVID-19 than in young patients without comorbidities (Figure 1). Obesity was found to be a risk factor using crude or adjusted odds ratios or hazard ratios (Supplementary Tables III-V).
Figure 1.
Isolated and associated obesity are outstanding risks factors for COVID-19 severity and mortality in young patients. Comparison of isolated obesity, CVD, and diabetes and the association of obesity with these two comorbidities with non-obese patients without comorbidities. Adjusted odds ratios for ICU admission, use of non-invasive and invasive ventilatory support, and deaths (Supplementary Tables III-V). Age 20–39y: Without comorbidity N = 33,994; Obesity N = 3,091; CVD N = 2,150; Obesity + CVD N = 1,008; Diabetes N = 1,646; Obesity + Diabetes N = 314; CVD + Diabetes N = 612; Obesity + CVD + Diabetes N = 415. Age 40–59y: Without comorbidity N = 64,952; Obesity N = 4,181; CVD = 15,870; Obesity + CVD N = 4,077; Diabetes N = 9,202; Obesity + Diabetes N = 1,017; CVD + Diabetes N = 9,370; Obesity + CVD + Diabetes N = 3,068. Age ≥ 60y: Without comorbidity N = 62,171; Obesity N = 1,614; CVD N = 37,396; Obesity + CVD N = 4,280; Diabetes N = 13,683; Obesity + Diabetes N = 842; CVD + Diabetes N = 30,784; Obesity + CVD + Diabetes N = 5,161.
A Kaplan-Meier survival analysis was performed to investigate the median survival time – the smallest time at which the survival probability drops to 50% or below. For hospitalized patients aged 20–39y it was estimated to be 180 days for patients without risk factors, 39 days for obesity, 49 days for CVD, 34 days for CVD + obesity, 55 days for diabetes, and 29 days for diabetes + obesity (Figure 2). These effects, although less prominent, are maintained for the population aged 40–59y: median survival time equal to 45 days for patients without risk factors; 36 days for obese; 40 days for CVD; 33 days for CVD + obese; 35 days for diabetes, and 32 days for diabetes + obesity patients. For the older population, the median survival time for all conditions was equivalent and in the range of 25–28 days (Supplementary Table VII).
Figure 2.
Kaplan–Meier survival curves of COVID-19 patients from symptoms onset till discharge or death. Adjusted hazard ratios for survival probability using Kaplan–Meier. Number at risk and censored patients at each timepoint are supplied (Supplementary Table VII). Overall survival of hospitalized patients without risk factors (black solid line); with obesity only (purple dashed line); with CVD only (red solid line); with CVD and Obesity (red dashed line); with Diabetes only (blue solid line); and with Diabetes and Obesity (blue dashed line). Comparisons were performed using the Gehan-Breslow-Wilcoxon test. The P-value were < 0.05 for all comparisons. Age 20–39y: Without comorbidity N = 33,994; Obesity N = 3,091; CVD N = 2,150; Obesity + CVD N = 1,008; Diabetes N = 1,646; Obesity + Diabetes N = 314; CVD + Diabetes N = 612; Obesity + CVD + Diabetes N = 415. Age 40–59y: Without comorbidity N = 64,952; Obesity N = 4,181; CVD = 15,870; Obesity + CVD N = 4,077; Diabetes N = 9,202; Obesity + Diabetes N = 1,017; CVD + Diabetes N = 9,370; Obesity + CVD + Diabetes N = 3,068. Age ≥ 60y: Without comorbidity N = 62,171; Obesity N = 1,614; CVD N = 37,396; Obesity + CVD N = 4,280; Diabetes N = 13,683; Obesity + Diabetes N = 842; CVD + Diabetes N = 30,784; Obesity + CVD + Diabetes N = 5,161.
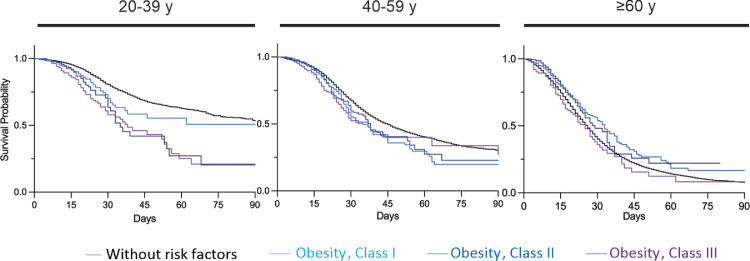
We next stratified patients with BMI information available (N = 3,772) as overweight (N = 178 cases), class I obesity (N = 1,738), class II obesity (N = 984), and class III obesity (N = 872) (Figure 3). The grade of obesity did not affect the severity and deaths of elderly patients (class I obesity compared with class II or class III, P >0.05). However, in the youngest population, the grade of obesity was significantly associated with increased mortality (class I obesity compared with class III, P < 0.0001; class II compared with class III, P = 0.008; Figure 3, Supplementary Tables VIII-X). The median survival time for patients aged 20–39y was estimated to be 180 days for patients without risk factors, 63 days for class I obese, 35 days for class II obese, and 38 days for class III obese patients (Supplementary Table XI). For the intervals of 40–59y and ≥ 60y, there was no correlation of severity with obesity (Figure 4 and Supplementary Table XI).
Figure 3.
COVID 19 severity and mortality increases with the increase of BMI in young patients. Comparisons of obese patients (Class I, BMI 30–34.9 kg/m2, Class II (BMI 35–39.9 kg/m2; and Class III (BMI ≥ 40 kg/m2) with non-obese patients without comorbidities. Adjusted odds ratios for ICU admission, use of non-invasive and invasive ventilatory support, and deaths (Supplementary Tables VIII-X). Age 20–39y: Obese Class I N = 588; Obese Class II N = 391; Obese Class III N = 362. Age 40–59y: Obese Class I N = 861; Obese Class II N = 459; Obese Class III N = 394. Age ≥ 60y: Obese Class I N = 289; Obese Class II N = 134; Obese Class III N = 116.
Figure 4.
Kaplan–Meier survival curves of patients with different BMI classes from symptoms onset till discharge or death. Adjusted hazard ratios for survival probability using the Kaplan–Meier method. Number at risk and censored patients at each timepoint are supplied (Supplementary Tables XI). Overall survival of hospitalized patients without risk factors (black solid line); Obesity Class I (light blue); Obesity Class II (blue); Obesity Class III (purple). Comparisons were performed using the Gehan-Breslow-Wilcoxon test. The P-value were <0.05 for all comparisons. Age 20–39y: Obese Class I N = 588; Obese Class II N = 391; Obese Class III N = 362. Age 40–59y: Obese Class I N = 861; Obese Class II N = 459; Obese Class III N = 394. Age ≥ 60y: Obese Class I N = 289; Obese Class II N = 134; Obese Class III N = 116.
Discussion
In this study, we demonstrated that obesity alone, or when associated with CVD and diabetes, considerably increases the risk for COVID-19 severity in young adults. Obesity was found to be an independent risk factor for in-hospital mortality and more critical than CVD and diabetes. However, it is important to emphasize that our findings cannot be extrapolated to those with COVID-19 who remained in the ambulatory and did not require hospitalization. In Brazil, the triad obesity, CVD, and diabetes represent a major public health issue.11 CVD has been the leading cause of hospitalization and mortality since the 1960s, and the increasing prevalence of obesity and diabetes mellitus is also a cause for concern.11 In addition, even prior to the COVID-19 pandemic, studies have shown that both all-cause mortality and cause-specific mortality were higher in younger individuals with excess weight than in older individuals with similar weight.12,13
BMI is commonly used to measure obesity grades in clinical settings and research studies. For our study, this information was only available for 3,772 of a total of 8,834 obese patients (obesity alone). When obese patients were stratified by BMI and compared to non-obese individuals, a strong association between obesity grades and ICU admission and deaths from COVID-19 in young patients was evident. Such association was found to be weaker in 40–59y patients, and it disappeared in patients ≥ 60y. Unfortunately, we did not find BMI values < 18 kg/m2 and we found very few BMI values 25–29.9 kg/m2. This prevented us from assessing the severity of COVID-19 in underweight and overweight patients.
The present study had limitations. First, our descriptive analysis did not try to established a causal relationship between the comorbidities and COVID-19 severity or death. By stratifying individuals by age and comorbidities, we aimed to assess if the association between comorbidities and COVID-19 severity or death was different in young and elderly patients. Second, the classification of individuals as being obese was not done in a uniform and standardized manner nationwide. In general, patients were classified as obese in four situations: (1) patients self-reported that they were obese; (2) the healthcare employee assumed whether the patient was obese based on visual inspection; (3) the healthcare employee took the weight and height of the patient, calculated the BMI but did not report the BMI value; and (4) the healthcare employee took the weight and height of the patient, calculated and reported the BMI value. Moreover, as expected for any large, nationwide, retrospective database, some variables (including obesity) may be underestimated. To further address this limitation, we performed two independent and complementary analyses: one utilizing all patients classified as obese, regardless if the BMI value was reported, and one utilizing only those with BMI values.
Although the association between obesity and COVID-19 severity has been evaluated in other high-quality studies,4,14, 15, 16 our retrospective study covers the entire country of Brazil during the pre-vaccination period. By using a government database created exclusively for monitoring SARS, we leveraged the following: (1) a large sample size of hospitalized patients with laboratory-confirmed COVID-19; (2) information on comorbidities and BMI collected at the time of hospital admission, therefore simultaneously with the laboratory diagnosis of COVID-19; (3) the specific contribution of obesity, CVD, and diabetes to the risk of severe COVID-19, (4) stratification by BMI and age, and (5) analysis of ICU admission, ventilatory support, and death.
The specific contribution of increased morbidity and mortality from COVID-19 in young obese people may offer clues to formulate mechanisms determining the severity of the disease. Factors linked to obesity, such as overexpression of angiotensin-converting enzyme 2 (ACE2), inflammation, and cytokine storm have been considered the mechanism behind the increased morbidity and mortality.17, 18, 19 However, these do not help explain the higher susceptibility of young obese patients. Curiously, in an old experimental study, it was revealed that obesity in young animals leads to overexpression of ACE2.20 Future integrated analysis of biochemical and metabolic differences along life-related to adipose tissue accumulation could be a helpful strategy to define new mechanisms for COVID-19 severity.
A significant challenge for middle-income countries with considerable territorial extension, such as Brazil, is implementing a vaccination program with sufficient coverage and speed to mitigate the effects of the COVID-19 pandemic. Studies have shown that prioritizing young adults minimizes transmissibility, while prioritizing older adults reduces mortality.21 Vaccination in Brazil started on January 17, 2020, prioritizing the elderly and expanding to younger people with comorbidities in May 2021. To date (December 1, 2021), over 120 million Brazilians have already been fully vaccinated, and, in some States, immunization of adolescents 12 years or older has started.2 Consequently, a reduction in COVID-19 hospitalizations and deaths can only be seen in Brazilian older adults that have been administered the full vaccination regimen.22 When the authors wrote this paper, more than 22 million Brazilians had been infected with SARS-CoV-2, and almost 613,000 had died. Our data provides crucial epidemiological information about the impact of COVID-19 on the obese young that must be considered in future vaccination coverage strategies, especially considering the possible seasonality of SARS- CoV-2.
Contributors
MD, AC, HIN and SS performed the analyses. AC and HIN coordinated the study and wrote the manuscript with inputs from all of the co-authors.
Declaration of Interests
The authors declare no conflict of interest.
Data sharing
The processed database is freely available at Mendeley Data repository: “Discacciati_et_al_Database_COVID19_ Obesity_Brazil_pre_vaccination_period”, Mendeley Data, V1, doi: 10.17632/r3rtvz7zcj.1.
Fundings
This work was supported by the Brazilian National Council for Scientific and Technological Development (grant number 313662/2017-7 and 307356/2017-5; the São Paulo Research Foundation (grant numbers 2018/14933-2); and CAPES.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100167.
Contributor Information
Ana Campa, Email: anacampa@usp.br.
Helder I Nakaya, Email: helder.nakaya@einstein.br.
Appendix. Supplementary materials
References
- 1.Brasil MdS. Painel de casos de doença pelo coronavírus 2019 (COVID-19); 2021. Accessed on August 5, 2021. https://covid.saude.gov.br/.
- 2.Brasil MdS. COVID-19 vacinação. Doses aplicadas. Accessed on August 5, 2021. https://qsprod.saude.gov.br/extensions/DEMAS_C19Vacina/DEMAS_C19Vacina.html; 2021.
- 3.Victora PC, Castro PMC, Gurzenda S, Medeiros AC, França GVA, Barros PAJD. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: Analyses of routinely-collected data on vaccine coverage and mortality. EClinicalMedicine. [DOI] [PMC free article] [PubMed]
- 4.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr: Clin Res Rev. 2020;14(5):1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasil MdS. NOTA TÉCNICA N° 467/2021-CGPNI/DEIDT/SVS/MS. In: Transmissíveis DdIeD, editor. https://saude.rs.gov.br/upload/arquivos/202104/27181903-nota-tecnica-467-2021-cgpni-deidt-svs-ms.pdf; 2021.
- 7.Instituto Brasileiro de Geografia e Estatística . IBGE; Rio de Janeiro: 2020. Coordenação de Trabalho e Rendimento. Pesquisa nacional de saúde: 2019: atenção primária à saúde e informações antropométricas: Brasil /IBGE. [Google Scholar]
- 8.Rtveladze K, Marsh T, Webber L, et al. Health and economic burden of obesity in Brazil. PLoS One. 2013;8(7):e68785. doi: 10.1371/journal.pone.0068785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil MdS. SRAG 2020-severe acute respiratory syndrome database—including data from COVID-19. Accessed on February 08, 2021. In: DATASUS, editor. https://opendatasus.saude.gov.br/dataset/bd-srag-2020; 2021.
- 10.Brasil MdS. Conselho Nacional de Saúde (CNS). Resolução N° 510/216. https://bvsms.saude.gov.br/bvs/saudelegis/cns/2016/res0510_07_04_2016.html; 2021.
- 11.Ribeiro AL, Duncan BB, Brant LC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular Health in Brazil: Trends and Perspectives. Circulation. 2016;133(4):422–433. doi: 10.1161/CIRCULATIONAHA.114.008727. [DOI] [PubMed] [Google Scholar]
- 12.Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaskaran K, dos-Santos-Silva I, DA Leon, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. The Lancet Diabetes & Endocrinology. 2018;6(12):944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamer M, Kivimaki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M, Piernas C, Astbury NM, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vera-Zertuche JM, Mancilla-Galindo J, Tlalpa-Prisco M, et al. Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: a national observational study. Epidemiol Infect. 2021;149:e109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhaes KG. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front Endocrinol (Lausanne) 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Rourke RW, Lumeng CN. Pathways to Severe COVID-19 for People with Obesity. Obesity (Silver Spring) 2021;29(4):645–653. doi: 10.1002/oby.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos ESJC, Vasconcelos AP, Noma IHY, et al. Gene signatures of autopsy lungs from obese patients with COVID-19. Clin Nutr ESPEN. 2021;44:475–478. doi: 10.1016/j.clnesp.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Sandmann FG, Barnard RC, et al. Optimising health and economic impacts of COVID-19 vaccine prioritisation strategies in the WHO European Region. medRxiv. 2021 doi: 10.1016/j.lanepe.2021.100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in the elderly population during a Gamma variant-associated epidemic of COVID-19 in Brazil: A test-negative case-control study. medRxiv. 2021 doi: 10.1136/bmj.n2015. 2021.05.19.21257472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.