Abstract
Initial romantic attraction (IRA) refers to a series of positive reactions (such as feelings of exhilaration and compulsive thinking) toward desirable potential partners, usually at initial or early‐stage encounters when no close relationship has yet been established. After decades of effort, the evolutionary value and key characteristics of IRA are well understood. However, the brain mechanisms associated with IRA are unclear. To address this question, we simulated a mate selection platform similar to that of Tinder. When participants assessed their romantic interest in potential partners on the platform, their electroencephalogram (EEG) signals were recorded in real time. The behavioral data demonstrated that IRA to ideal potential partners mainly reflects the dimensions of arousal and domination. The main study finding was that processing of the individual preference faces that resulted in IRA was associated with a decrease in power in the alpha and lower beta bands over the posterior and anterior sensor clusters; this occurred between 870 and 2,000 ms post‐stimulus. Key findings regarding event‐related potentials (ERPs) sensitive to individual stimuli preferences were replicated. The results support the hypothesis that brain oscillations in the alpha and lower beta range may reflect modulation in cortical activity associated with individual mate preferences.
Keywords: event‐related desynchronization, event‐related potential, individual preference, mate selection, romantic attraction
Initial romantic attraction (IRA) refers to a series of positive reactions (such as feelings of exhilaration and compulsive thinking) toward desirable potential partners, usually at initial or early‐stage encounters when no close relationship has yet been established. After decades of effort, the evolutionary value and key characteristics of IRA are well understood. However, the brain mechanisms associated with IRA are unclear. The main study finding was that processing of the individual preference faces that resulted in IRA was associated with a decrease in power in the alpha and lower beta bands over the posterior and anterior sensor clusters; this occurred between 870 and 2,000 ms post‐stimulus.

1. INTRODUCTION
Attraction to a person during an initial encounter is generally the first step or the initial motivation to develop a romantic relationship (Gerlach & Reinhard, 2018; Olderbak, Malter, Wolf, Jones, & Figueredo, 2017). This attraction has inconsistently been referred to as romantic attraction (Asendorpf, Penke, & Back, 2011; Fisher, Aron, Mashek, Li, & Brown, 2002; Gerlach & Reinhard, 2018), initial attraction (Olderbak et al., 2017), initial romantic attraction (IRA; Finkel, Eastwick, & Matthews, 2007), romantic interest at zero acquaintance (Asendorpf et al., 2011), and love at first sight (Grant‐Jacob, 2016; Zsok, Haucke, De Wit, & Barelds, 2017). Here, the term IRA is used to describe this attraction. The IRA has a complex and multifaceted structure. It refers to a series of positive reactions toward ideal potential romantic partners, mainly including feelings of exhilaration, compulsive thinking, focused attention, and a craving for emotional union (Finkel et al., 2007; Fisher, 1998; Fisher et al., 2002; Fisher, Aron, & Brown, 2005; Gerlach & Reinhard, 2018). As one of the major emotion‐motivation systems that humans display during mating and reproduction, the evolutionary value of IRA facilitates mate choice and enables individuals to focus their mating energy on specific potential mates (Fisher, 1998; Fisher et al., 2002; Fisher et al., 2005; Gerlach & Reinhard, 2018).
The 1960s and 1970s witnessed the heyday of research on IRA. During this time, psychologists conducted a great deal of fruitful research and discovered a variety of factors (such as physical attractiveness [PA] and similarity) that predict the experience of romantic attraction (Asendorpf et al., 2011; Conroy‐Beam & Buss, 2016; Cooper, Dunne, Furey, & O'Doherty, 2012; Finkel et al., 2007; Olderbak et al., 2017; Zsok et al., 2017). Since the early 1980s, however, interest has shifted, and scholars have begun to direct their attention and resources to the study of ongoing romantic relationships. This has indirectly contributed to a prolonged downturn in academic interest in the study of IRA. Recently, however, this trend has started to reverse (Finkel et al., 2007). One of the important reasons for this shift is that, with the advent of the Internet era, an increasing number of young people tend to use efficient and convenient Internet tools (such as Tinder) to find desirable partners. While participating in online dating platforms, people often make subjective decisions about whether to further develop a potential relationship with a potential partner based only on first impressions (i.e., IRA). In this dating scenario, the importance of IRA in mating and reproduction is so important that it cannot be ignored. Tracking neural responses while the participant makes a preferential decision (i.e., mate choice) may, therefore, provide a unique window into the mental processes underlying subjective decision‐making. However, to our best knowledge, most research on IRA has been conducted at the behavioral level, and the brain activity and neural pathways associated with IRA are almost entirely unresearched.
The essence of IRA is a series of positive responses to ideal potential mates, including positive emotional responses (such as feelings of exhilaration and a craving for emotional union; Fisher, 1998; Fisher et al., 2002; Fisher et al., 2005; Gerlach & Reinhard, 2018). Previous studies have demonstrated that stimuli triggering IRA are emotionally salient and elicit a strong positive reaction based on individual mate preferences (Hajcak, MacNamara, & Olvet, 2010). Although there is a lack of research on the brain mechanisms mediating IRA, there is an abundance of research on other positive reactions elicited by individual preferences. For instance, a large number of ERP studies have reported that the processing of individual preference stimuli is strongly associated with N200 over the frontal‐central neural regions (occurring between 180 and 400 ms post‐stimulus) and an enhanced late positive potential (LPP) over the centroparietal regions (occurring approximately 400–1,000 ms post‐stimulus; Fu Guo, Wang, Liu, & Jin, 2016; Goto et al., 2019; Hu et al., 2020; Ma, Qian, Hu, & Wang, 2017). The N200 response is suggested to be associated with bottom‐up stimulus‐driven (automatic) attention capture (Fu Guo et al., 2016; Goto et al., 2017; Goto et al., 2019; Hu et al., 2020; Patel & Azzam, 2005), while LPP is believed to reflect top‐down motivation‐driven attention allocation (Fu Guo et al., 2016; Goto et al., 2017; Goto et al., 2019; Liu, Huang, McGinnis‐Deweese, Keil, & Ding, 2012). In contrast, changes in neural oscillations induced by processing individual preference stimuli are less well understood, and the results reported in the current literature are vague. For instance, among the few studies examining the processing of individual preference stimuli, some studies have reported that individual preference stimuli (such as favorite foods) can induce decreases in the power of the lower beta band (13–20 Hz; Al‐Ozzi, Botero‐Posada, Lopez Rios, & Hutchison, 2021; Tashiro et al., 2019), while other studies have reported that individual preference stimuli (i.e., preferred faces) cause a decrease in alpha power (but not beta power) through event‐related desynchronization (ERD; Kang, Kim, Cho, & Kim, 2015). Therefore, the currently ambiguous question regarding whether the processing of individual preference stimuli is related to alpha‐ERD or beta‐ERD responses needs to be further investigated.
The main aim of the present study was to determine the brain oscillatory responses associated with IRA. To investigate the brain activity associated with IRA, we simulated a Tinder‐like partner selection platform. Participants were asked to use the dating platform as if they were selecting desirable potential partners. Their brain electrical signals were recorded as they viewed photos of each potential partner and simultaneously assessed their IRA in that potential partner through self‐reported scale responses. Specifically, during the electroencephalogram (EEG) recording task, we asked heterosexual participants to rate photos of opposite‐sex potential partners on two dimensions: an IRA rating scale (based on the question “How much would you like to date this person?”) as well as a zero‐acquaintance rating scale (based on the question “Have you ever seen the person in the photo before?”). The IRA scale was used to assess IRA on the basis that (in humans) craving emotional union with a potential partner is one of the main characteristics of IRA (Fisher, 1998; Fisher et al., 2002). Therefore, if an individual makes a choice in the context of speed dating or online dating, including wanting to date a potential partner again, this choice can be taken as a sign of IRA (Cooper et al., 2012; Finkel et al., 2007; Gerlach & Reinhard, 2018). Following the EEG recording task, the participants were asked to perform a separate multi‐rating task using the same set of photos arranged in a random order. In each self‐paced trial, participants rated photos with respect to a series of characteristics, including arousal, dominance, valence, and PA. Among them, arousal dimension is used to characterize the intensity of emotional arousal, valence dimension is used to characterize the positive and negative emotional experience, and dominance dimension is used to characterize whether the individual is in the state of self‐control or controlled (Mehrabian, 1980, 1996).
Based on previous research (Cooper et al., 2012; Fisher, 1998; Gerlach & Reinhard, 2018; Fisher et al., 2002), we hypothesized that the IRA measure would be most associated with two measures of arousal and dominance. Furthermore, according to previous research (Schubring & Schupp, 2019, 2021), we predicted alpha and beta power decreases for individual preference faces resulting in IRA as compared to individual preference faces that not resulting in IRA during passive viewing conditions. Previous studies have found that individual preference stimuli are closely related to N200 and LPP; therefore, N200 and LPP were used as experimental manipulation checks in this study. In general, we aimed to replicate individual preference modulation of the N200 and LPP components and analyzed induced responses to reveal their representation in the time‐frequency domain.
2. MATERIALS AND METHODS
Both the auxiliary experiment and the main experiment were approved by the Ethical Review Committee of Southwest University, and all participants provided their written informed consent prior to study participation.
2.1. Auxiliary experiment
2.1.1. Participants
Sixty student volunteers with a mean age of 21.4 years (standard deviation [SD] = 2.6) were recruited on the campus of Southwest University. Participants received payment for participating in the study. All participants reported normal or corrected‐to‐normal visual acuity and had no history of psychiatric or neurological disorders, as confirmed by a screening interview.
2.1.2. Stimuli task and design
According to data reported in previous studies, the induction rate of romantic attraction is quite low (approximately a few percent on average; Zsok et al., 2017). To obtain relatively stable results, we needed to obtain a sufficient number of trials (approximately 30 per person) in which IRA was successfully induced. After conducting a rough estimation, we found that to achieve this goal, each participant would have to be exposed to stimuli thousands of times or more. Since this was clearly untenable, finding ways to increase the IRA induction rate was our first challenge. Numerous studies have reported that consensus judgments of the PA of a potential mate are good predictors of an individual's popularity with members of the opposite sex (i.e., the probability of being chosen by the opposite sex; Asendorpf et al., 2011; Gerlach & Reinhard, 2018; Olderbak et al., 2017), especially in the context of short‐term mate selection(Cooper et al., 2012; Zsok et al., 2017). In particular, Zsok et al. found that the IRA induction rate increased several times for each unit increase in the level of PA (Zsok et al., 2017). Thus, in the current study, we planned to increase the average evoked rate of IRA by increasing the proportion of stimuli with high consensus PA.
To increase the proportion of highly attractive stimuli, we first focused on downloading thousands of high‐resolution personal portrait photos from high‐definition copyright business photo gallery websites (such as VEER [https://www.veer.com/] and Hummingbird net [http://bbs.fengniao.com/]) and standardized these portraits (face and hair only; size, 839 × 1,080 pixels). Second, to minimize potential confounding, 1,600 photos were chosen from the normalized portrait photos, including 800 female portraits; these were generally similar in face orientation and expression as well as comparable in background complexity. Third, the level of PA of each face was assessed using a nine‐point Likert scale (ranging from “1—not at all” to “9—extremely”) in response to the question “How physically attractive is this person?” It is important to note that male participants rated only female faces, while female participants rated only male faces. Finally, we averaged the attractiveness ratings for each face across all opposite‐sex participants to obtain consensus facial attractiveness ratings.
2.2. Main experiment
2.2.1. Participants
One hundred and thirty single student volunteers, including 65 male volunteers, were recruited on the campus of Southwest University (mean age, 21.1 years [SD = 2.9]). No participants had a history of psychiatric or neurological disorders, as confirmed by a screening interview.
2.2.2. Stimuli task and design
In the natural environment, the proportion of individuals with high, average, and low attractiveness should conform to the normal distribution. However, in this study, we deliberately increased the proportion of highly attractive individuals to increase the mean induction rate of IRA. The ratio of high, average, and low PA stimuli was adjusted to 0.25:0.6:0.15. To achieve the goal of 30 romantic attraction reports per person, participants would have to be exposed to different stimuli 300 to 400 times. Ultimately, 360 photos were selected as the stimulus material for the main experiment for each gender from among 800 photos of women and 800 photos of men. The 360 photos of men were divided into three categories: high‐attractiveness stimuli (mean = 6.7, SD = 0.36), average‐attractiveness stimuli (mean = 5.1, SD = 0.29), and low‐attractiveness stimuli (mean = 3.6, SD = 0.33). The 360 photos of women were also divided into three categories: high‐attractiveness stimuli (mean = 6.9, SD = 0.33), average‐attractiveness stimuli (mean = 5.2, SD = 0.25), and low‐attractiveness stimuli (mean = 3.9, SD = 0.31). Regardless of gender, the proportions of high‐, average‐, and low‐attractiveness stimuli were approximately 0.25, 0.60, and 0.15, respectively.
The number of stimuli used in the main experiment was substantially reduced by the aforementioned strategy, but 360 was still a large number of stimuli for the participants to process. If participants were required to view 360 stimuli continuously over a short period, they were more likely to experience esthetic fatigue, which may have influenced the experimental effect. Therefore, to minimize the probability of or delay esthetic fatigue, the 360 photos of women (or men) were first evenly distributed into two sessions according to attractiveness level. Specifically, 180 photos were included in each session, and the proportions of high‐, average‐, and low‐attractiveness photos in each session were approximately 0.25, 0.6, and 0.15, respectively. Second, the participants waited for at least 24 hr between sessions 1 and 2. Third, the 180 photos in each session were evenly distributed into three runs according to attractiveness rating scores; that is, 60 photos were included in each session, and the proportions of high‐, average‐, and low‐attractiveness photos in each session were approximately 0.25, 0.6, and 0.15, respectively. The stimuli in each run were presented in random order and presented only once. Finally, a break was provided between every two runs, during which participants viewed serene landscapes while listening to soothing music over the course of 5–6 min. The experiment was conducted in a dark and quiet environment to allow the participants to focus on the stimuli.
For each session, participants first performed an EEG recording task (see the upper part of Figure 1a). The trial was structured as shown in the upper part of Figure 1b. First, a black fixation cross appeared in the center of a white computer screen for 1,000 ms, followed by a face appearing for 3,000 ms. The participants were then asked to rate their response to the question, “How much would you like to date this person?” using a four‐point rating scale (“not at all,” “a little,” “somewhat,” or “very much”). Then, they were asked, “Have you ever seen the person in the photo before?” (responses: “no,” “not sure,” or “yes”). Finally, there was a 2,000 ms blank screen.
FIGURE 1.
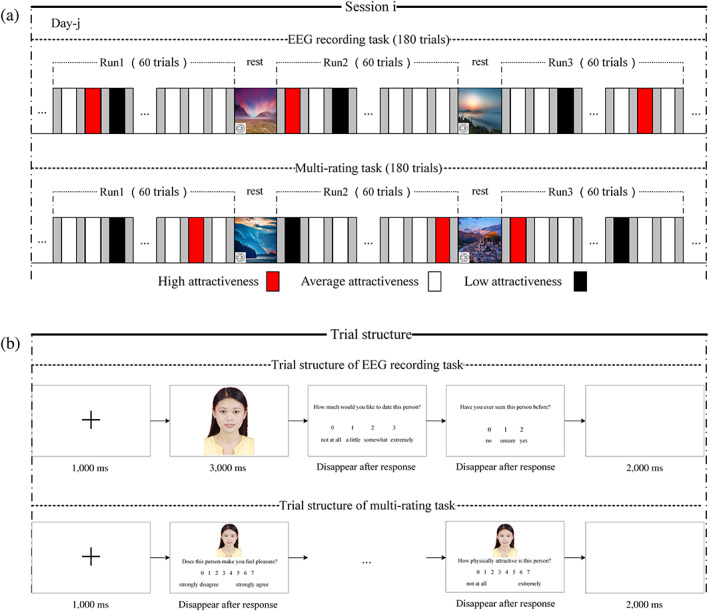
Experimental paradigm and trial structure. (a) Experimental paradigm. The upper part of the figure is the paradigm of the EEG recording task, and the lower part is the paradigm of the multi‐rating task. (b) Trial structure. The upper part of the figure is the trial structure of the EEG recording, and the lower part is the trial structure of the multi‐rating recording
Following the EEG recording task in each session (and after removing the EEG recording device), the participants performed a separate multi‐rating task (see the lower part of Figure 1a) using the same set of photos arranged in random order. In each self‐paced trial (Figure 1b), participants rated photos with respect to a series of characteristics‐based according to seven‐point scale, including four ratings of potential romantic desirability: “Does this person make you feel pleasure?” “Does this person make you feel exciting?” “Does this person make you feel controlled?” and “How physically attractive is this person?”
2.2.3. Data acquisition and analysis
EEG recording
The EEG analog signals were recorded using a 128‐channel BioSemi ActiveTwo system (BioSemi Inc., Heerlen, The Netherlands) with a 24‐bit analog‐to‐digital conversion. The 128 electrodes were equally spaced on an electrode cap and customized with an integrated primary amplifier. Data were filtered online with a 0.16–100 Hz band‐pass filter and sampled at 500 Hz.
Data processing
The EEG data were re‐referenced offline to average all channels after rejecting bad segments and interpolating bad traces, and the band‐pass was filtered between 0.1 and 40 Hz (12 dB/octave) and corrected for eye movements using the algorithm developed by Gratton, Coles, and Donchin (1983). An independent component analysis (ICA) procedure was used to correct the EEG deflections resulting from eye movements and blinks (Perry, Rubinsten, Peled, & Shamay‐Tsoory, 2013). The remaining artifact rejection criteria were minimum and maximum baseline‐to‐peak 75 to +75 mV with a maximum allowed voltage skip (gradient) of 50 mV (Langeslag, Jansma, Franken, & Van Strien, 2007). The data were split into epochs from 200 ms before the presentation of the stimulus to 2,000 ms after stimulus onset. EEG data analysis was conducted using the open‐source MATLAB signal processing toolbox FieldTrip and in‐house functions via MATLAB (MathWorks, Natick, MA; Oostenveld, Fries, Maris, & Schoffelen, 2011).
EEG epochs were categorized according to rating scores (ranging from “0—not at all” to “3—very much”) based on the question “How much would you like to date this person?” (Cooper et al., 2012; Zsok et al., 2017). Epochs with a rating score of 2 or 3 were assigned to the IRA engendered condition, and epochs with a rating score of 0 were assigned to the IRA un‐engendered condition. To minimize ambiguity, epochs with a rating score of 1 were excluded (mean = 19.78, SD = 3.12). Epochs corresponding to faces the participants had seen or were not sure they had seen were excluded (mean = 1.93, SD = 2.54). The mean number of accepted epochs per participant under the IRA engendered condition was 34.58 (SD = 3.38), and the mean number of accepted epochs per participant under the IRA un‐engendered condition was 303.71 (SD = 6.31). To solve the serious mismatch between the two conditions in terms of the number of trials, a number of accepted epochs under the IRA un‐engendered condition were randomly selected to allow the participants to focus on the stimuli to match the number of accepted epochs under the IRA engendered condition.
2.3. Behavioral and EEG analysis
2.3.1. Behavioral analysis
The behavioral data were analyzed using Statistical Package for the Social Sciences SPSS12 software (https://www.ibm.com/cn-zh/analytics/spss-statistics-software; SPSS, Inc., Chicago, IL). First, simple linear regression was used to model the relationship between IRA scores and consensus physical attraction scores. We calculated the mean elicitation rate of IRA among high‐, average‐, and low‐PA stimuli. Furthermore, simple regression analyses were implemented to examine the relationship between IRA scores and scores for four characteristics (arousal, valence, dominance, and PA). All variables for all models were Z‐scored over the entire group before being entered into the model.
2.3.2. EEG analysis
Frequency analysis
EEG frequency analyses focused on induced brain activity. Therefore, in the first step, the average ERP of each condition was calculated and subtracted from the single trials examining this condition. In the second step, the spectral power of the underlying brain oscillations was calculated using time‐frequency representation (TFR) based on the wavelet transform. The TFR was obtained through a five‐cycle complex Morlet wavelet (Lindsen, Jones, Shimojo, & Bhattacharya, 2010). Sliding windows were advanced in 12 ms and 1 Hz increments to estimate changes in power over time and frequency in the alpha (8–13 Hz), lower beta (13–20 Hz), and higher beta (20–30 Hz) frequency ranges. For each of the standard frequency bands, we calculated the spectral power by averaging the constituent frequencies.
Statistics
A non‐parametric cluster‐based permutation test was used to determine the statistical significance of the main effects for each condition (Lindsen et al., 2010; Maris & Oostenveld, 2007). In short, this procedure clusters adjacent t values in the three‐dimensional space of time, frequency, and electrode into a single summed cluster test statistic. Clusters were formed based on at least two neighbors that reached a cluster‐forming threshold of p < .05. These clusters were tested against a Monte Carlo approximation of the test statistic, which was formed by randomly shuffling the data for 5,000 permutations and reporting the proportion of random shuffles that were larger than the observed cluster test statistic as a cluster p value. These steps were repeated for the cluster‐level statistics. Finally, a data‐driven unsupervised hierarchical clustering algorithm was used to cluster the topography of the t values at each major time point.
ERP analysis
ERPs were determined by averaging the two‐second segmented trials separately in each condition, referred to as a −100 to 0 ms prestimulus baseline. Afterward, the ERPs were subjected to the same cluster analyses as the frequency analyses, albeit with one fewer dimension (i.e., no frequency dimension).
3. RESULTS
3.1. Behavioral data
We first examined the extent to which the IRA measure was related to the consensus PA measure and calculated the average evoked rates of IRA for stimuli with different levels of consensus PA. The results showed that the IRA measure was moderately positively correlated with the consensus PA measure in a simple linear regression model (B = 0.32, p < .01); The results of the romantic attraction‐evoked rate calculation further confirmed this conclusion (i.e., the higher the potential partner's PA level, the more likely the participants were to be romantically interested in the potential partner). Specifically, the average induction rates of IRA for faces with low attractiveness, average attractiveness, and high attractiveness were 0.00, 0.06, and 0.25, respectively.
Next, we examined the extent to which the IRA was driven by subjective judgments of arousal, PA, dominance, and valence. We found that when arousal, valence, dominance, and PA measures were included separately in a simple linear regression model, IRA was statistically significantly related to all four measures (IRA‐arousal: B = 0.75, p < .01; IRA‐valence: B = 0.67, p < .01; IRA‐dominance: B = 0.73, p < .01; IRA‐PA: B = 0.71, p < .01). Because the four measures of arousal, valence, dominance, and PA were also highly correlated with each other (all R > 0.61, all p < .01), partial correlation models were used to examine the unique contribution of each measure. Partial correlations revealed that the measures of arousal and dominance ratings accounted for the majority of the variance underpinning IRA ratings (mean within‐participant IRA‐arousal partial correlation = .29, p < .001; mean within‐participant IRA‐dominance partial correlation = .23, p < .001; mean within‐participant IRA‐valence partial correlation = .21, p < .001; mean within‐participant IRA‐PA partial correlation = .14, p < .001).
3.2. TFR analysis
When TFRs for individual preference faces that resulted in IRA were contrasted with TFRs for faces that did not result in IRA, we found statistically significant differences in the alpha and low beta frequency bands (see Figures 2 and 3).
FIGURE 2.
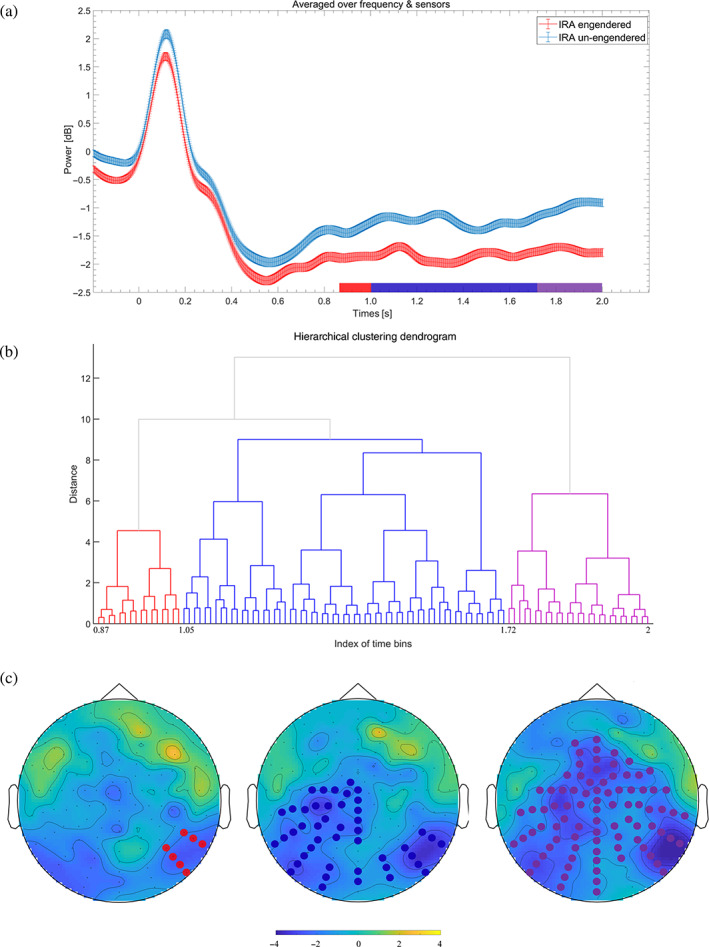
Illustration of alpha event‐related desynchronization (alpha‐ERD). Individual preference faces that resulted in IRA elicited a stronger alpha‐ERD than faces that did not result in IRA. (a) The time course of the alpha‐ERD cluster. Values were averaged over the respective sensors (see (c)). (b) A dendrogram depicting hierarchical clustering. Each node represents a topography of t values at a significant time point. (c) The topography of t values averaged across the significant time bins for each sub‐cluster (see (a,b))
FIGURE 3.
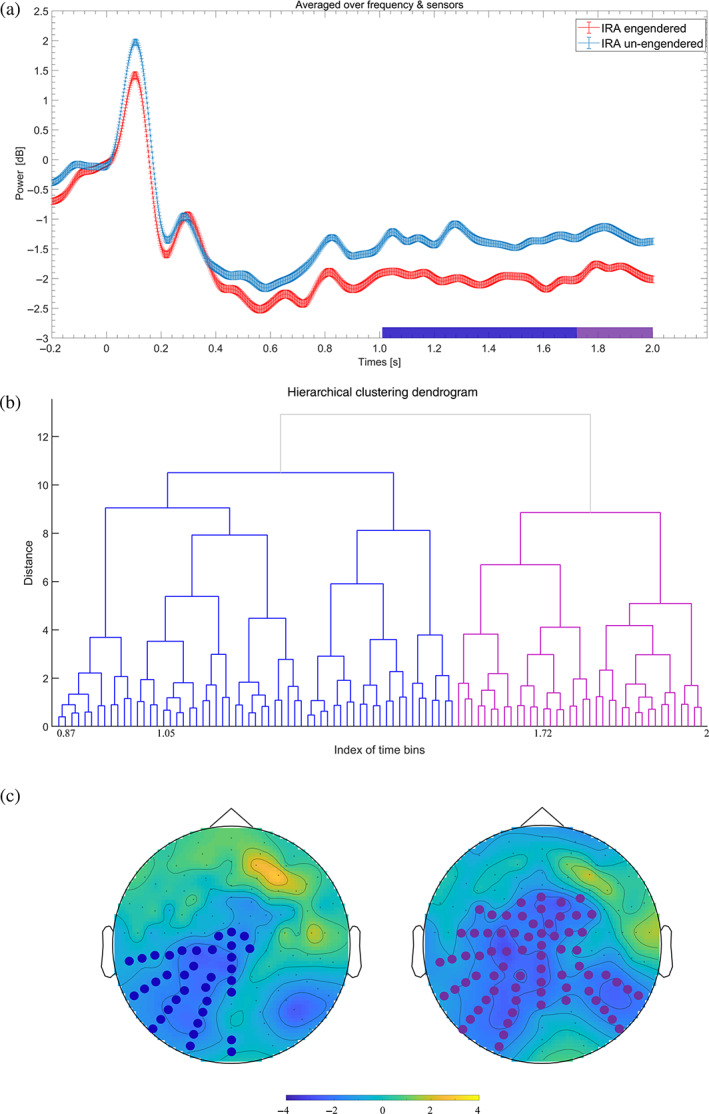
Illustration of lower beta event‐related desynchronization (beta‐ERD). Individual preference faces that resulted in IRA elicited a stronger lower beta‐ERD than faces that did not result in IRA. (a) The time course of the lower beta‐ERD cluster. Values were averaged over the respective sensors (see (c)). (b) A dendrogram depicting hierarchical clustering. Each node represents a topography of t values at a significant time bin. (c) The topography of t values averaged across the significant time bins for each sub‐cluster (see (a,b))
As shown in Figure 2a, the effect of alpha‐ERD (p < .001) reached statistical significance within 0.87–2 s. Furthermore, based on hierarchical clustering and scrutiny of the present distributions, the topography of the t values over all statistically significant time bins was grouped into three clusters, as shown in Figures 2b,c: the first sub‐cluster effect (between 0.87 and 1 s) appeared over the right temporoparietal junction area, the second sub‐cluster effect (between 1 and 1.72 s) was found over the extended parietooccipital and left central sensor regions, and the third sub‐cluster effect (between 1.72 and 2 s) appeared over the extended posterior, central, and frontal regions.
As shown in Figure 3a, the effect of beta‐ERD (p < .05) reached statistical significance within 1.02–2 s. Based on hierarchical clustering and scrutiny of the present distributions, the topography of the t values over all significant time bins were grouped into two clusters, as shown in Figures 3b,c. The first sub‐cluster effect (between 1.02 and 1.71 s) appeared over the left parietooccipital regions. The second sub‐cluster effect (between 1.71 and 2 s) was found over the extended posterior, central, and frontal regions.
3.3. ERP analysis
As shown in Figures 4, statistically significant main effects for each condition were observed in the two clusters. In the first cluster (p < .001), individual preference faces that resulted in IRA, as compared to faces that did not result in IRA, elicited a more positive N200 response over frontal‐central sensor sites between 186 and 350 ms. In the second cluster (p < .001), individual preference faces that resulted in IRA, as compared to faces that did not result in IRA, elicited enhanced LPP over frontal, central, and parietal sensor sites between 368 and 2,000 ms.
FIGURE 4.
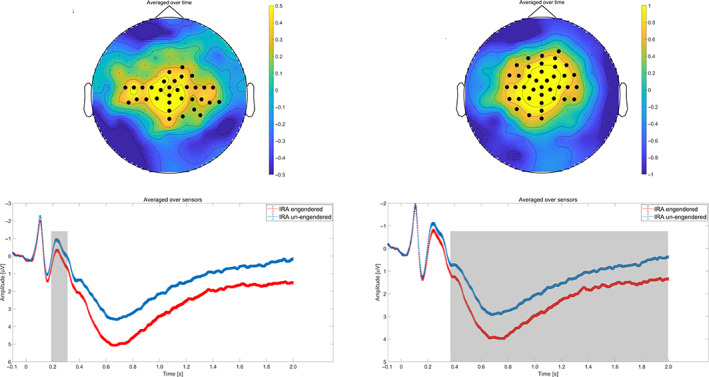
Illustration of condition modulation for the ERP response. (a) Illustration of condition modulation for N200. The topography of t values (upper left). The time course of the N200 (bottom left). Gray shaded areas represent significant time interval. (b) Illustration of condition modulation for late positive potential (LPP). The topography of t values (upper right). The time course of the LPP (bottom right). Gray shaded areas represent the significant time intervals
4. DISCUSSION
IRA refers to a series of positive reactions toward ideal potential romantic partners (Fisher et al., 2005; Fisher, 1998; Gerlach & Reinhard, 2018; Fisher et al., 2002). IRA evolved to facilitate mate choice, thus enabling individuals to focus their mating energy on specific potential mates. Although the evolutionary value and key characteristics of IRA are well understood, little is known about electrical brain responses during the formation of IRA. The primary goal of the present study was to identify brain oscillations associated with IRA. Our main finding is that the processing of faces that resulted in IRA based on individual mate preference was associated with a decrease in power in the alpha and lower beta bands. This effect was strongest between approximately 0.87–2 s post‐stimulus and could be observed over the lateral occipital complex and vertex regions. These findings suggest that the generation of IRA is associated with power decreases in the alpha and lower beta bands, which are considered to reflect cortical activation (Liu et al., 2012; Tashiro et al., 2019).
In view of previous contradictory findings regarding alpha and lower beta power modulations associated with individual preference stimuli processing, several ERP components were incorporated in the present study to ensure the accurate interpretation of findings (Al‐Ozzi et al., 2021; Kang et al., 2015; Tashiro et al., 2019). Previous findings have demonstrated that the N200 and LPP response components are consistently and robustly modulated by individual preference stimuli (Fu Guo et al., 2016; Goto et al., 2017; Goto et al., 2019; Ma et al., 2017). Replicating N200 and LPP modulations to individual preference faces that resulted in IRA with predicted polarity, topography, and latency served as a within‐study check that the experimental manipulation was successful. Specifically, individual preference faces that resulted in IRA elicited a more positive N200 response over the frontal‐central regions and a larger LPP component over the centroparietal and frontal regions as compared to faces that did not result in IRA. In general, we replicated established findings related to individual preference stimulus processing; consistent with the previous literature supports the significance of our primary findings related to brain oscillations induced by individual preference faces resulting in IRA.
The primary finding of the present study is that the processing of faces that resulted in IRA is associated with a decrease in alpha and lower beta power over lateral occipital complex and vertex areas. Moreover, self‐reported data indicate that initial romantic interest in desirable potential partners is mainly driven by arousal and dominance rather than by valence and PA. This finding is consistent with the previously published viewpoint that, in humans, IRA is often characterized by feeling excited and compulsive thinking about desirable potential mates (Fisher et al., 2005; Fisher, 1998; Gerlach & Reinhard, 2018; Fisher et al., 2002). Similarly, previous findings reporting that power in the alpha and beta bands is inversely correlated with increasing stimulus arousal (De Cesarei & Codispoti, 2011; Schubring & Schupp, 2019, 2021). Thus, alpha‐ and lower beta‐ERD with respect to desirable potential partners are presumed to reflect the significance of preferred stimuli (Schubring & Schupp, 2019, 2021). Furthermore, research in the cognitive domain revealed that brain regions that are activated during a cognitive task exhibit alpha‐ and lower beta‐ERD, whereas alpha and lower beta synchronization occurs over brain regions associated with task‐irrelevant and potentially interfering processes. In particular, recent studies in the field of emotion have revealed that the desynchronization of alpha and low beta frequency bands may reflect cortical activation related to emotional stimulus significance (Schubring & Schupp, 2019, 2021). Accordingly, the lateral occipital complex and the vertex regions may play an important role in the processing of faces that resulted in IRA.
The lateral occipital complex, which mainly includes the fusiform face area (FFA), the lateral occipital cortex (LOC), and medially adjacent regions, plays an important role in human object recognition (Grill‐Spector, Kourtzi, & Kanwisher, 2001). Furthermore, this region may also participate in the processing of visual features before object recognition, such as the understanding of aesthetic features of objects, thus affecting the aesthetic judgment (Chatterjee, Thomas, Smith, & Aguirre, 2009; Grill‐Spector et al., 2001). For instance, the activities in the region have been found to be related not only to the aesthetic judgment of abstract images (Chatterjee et al., 2009; Jacobsen, Schubotz, Hofel, & Cramon, 2006), but also to the formation of painting preferences (Chatterjee et al., 2009; Oshin Vartanian, 2004). In particular, Chatterjee et al. found that attractive faces automatically activate this region, and the authors hypothesized that this region may act as a neural trigger for the universal effect of attractiveness in social interactions (Chatterjee et al., 2009). Thus, the decrease in alpha and lower beta power over the lateral occipital complex may reflect cortical activation associated with the processing of facial aesthetic characteristics and the formation of partner preferences.
It is well established that the vertex regions, which mainly includes the sensorimotor cortex, the supplementary motor cortex, and posterior parietal cortex, play an important role in motor control (Cohen & Andersen, 2002; Ferenc Matyas et al., 2010; Neuper, Wörtz, & Pfurtscheller, 2006; Rektor, Sochůrková, & Bočková, 2006; Whitlock, 2017). Furthermore, preparation, execution, and also imagination of movement produce alpha‐ and beta‐ERD over the sensorimotor region (Ferenc Matyas et al., 2010; Neuper et al., 2006; Rektor et al., 2006; Tashiro et al., 2019), the supplementary motor area, and posterior parietal cortex (Rektor et al., 2006; Szurhaj et al., 2003). Especially, many studies have demonstrated that IRA refers to a series of positive reactions toward ideal potential mates, including the desire and behavior to approach the other (Gerlach & Reinhard, 2018). Therefore, these convergent evidences suggest that alpha‐ and lower beta‐ERD over the vertex region may reflect secondary cortical activation related to motivational significance of preferred stimuli.
On the one hand, the present findings are consistent with this inference regarding the direction of power modulation; specifically, individual preference faces that resulted in IRA led to consistent reductions in alpha and lower beta power compared to faces that did not result in IRA. However, on the other hand, there was variability of effects across alpha and lower beta frequency categories regarding topography and timing points. Specifically, effect latency differed for alpha and lower beta frequency bands, appearing earlier in time for the alpha frequency band. In addition, although the topographic effect of both the alpha and lower beta bands included the extended posterior and anterior regions, the effect between 0.87 and 1 s appeared over the right temporoparietal junction sensor sites for the alpha frequency band only. Assuming that reduced alpha‐ and lower beta‐band oscillations reflects increased cortical excitability (Foxe & Snyder, 2011; Klimesch, 2012; Schubring & Schupp, 2019, 2021), one may speculate that the brain oscillation activities of the two frequency bands may play different roles in the processing of stimuli that resulted in IRA (Klostermann et al., 2007; Tashiro et al., 2019). Thus, future research needs to study the topography and latency of alpha/beta power decreases as a function of different types of preferred stimuli and combine EEG source analysis and functional neuroimaging to explore the generator of alpha/beta desynchronization induced by preferred stimuli.
In the present study, the picture contents were faces of the opposite sex that differed in the level of consensus PA. These differences are reflected in the self‐report data, which revealed a profound difference in the induction rate of IRA according to PA. Similar to previous findings, the induction rate of romantic attraction increased exponentially with an increase in attraction level (Zsok et al., 2017). Our results are consistent with previous findings demonstrating that consensus PA is indeed a significant predictor of romantic attraction (Asendorpf et al., 2011; Conroy‐Beam & Buss, 2016; Cooper et al., 2012; Finkel et al., 2007; Olderbak et al., 2017).
Let us offer a few critical remarks. First, as an exploratory study, this study used portrait photos rather than real people as stimuli to induce IRA based on feasibility considerations. The advantage of this approach is that by increasing the amount of stimulus, it can effectively solve the problem of insufficient trials in which IRA was successfully induced due to the low average induction rate. However, IRA in real life usually occurs in the environment that allows some meaningful interaction, but the types of stimuli used in this study did not allow participants to interact effectively with potential partners in the photos. This is a problem that needs to be paid attention to and solved in the follow‐up research. Second, while facial attractiveness has been proved to be a significant predictor of IRA (Asendorpf et al., 2011; Gerlach & Reinhard, 2018; Luo & Zhang, 2009; Olderbak et al., 2017), however, other studies have suggested that other factors, such as voice (Asendorpf et al., 2011; Feinberg, 2008), can also serve as cues for romantic attraction. Therefore, subsequent studies can also elicit and investigate the brain activity associated with IRA from other dimensions.
5. SUMMARY
Although the evolutionary value and key characteristics of IRA appear to be clear and consistent, the brain responses associated with IRA remain largely unknown and unstudied. To address this issue, the present study evaluated induced frequency modulations according to individual preference faces that resulted in IRA during an online dating simulation. We identified four EEG components associated with subjective decision‐making regarding mate preference. Two brain oscillation effects (i.e., desynchronization effects in the alpha‐and lower beta‐frequency bands) were interpreted as reflecting cortical activation associated with the formation of a subjective mate preference. The replication of established ERP effects with respect to individual preference, specifically a more positive frontal‐central N200 and an enhanced LPP, likely reflect automatic attention capture and active attention allocation, respectively. Overall, these findings suggest that brain oscillations in the alpha‐ and lower beta‐band may reflect enhanced cortical activation associated with individual mate preferences.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Guangjie Yuan and Guangyuan Liu conceived the study. Guangjie Yuan designed and programmed the tasks. Guangjie Yuan contributed to data collection. Guangjie Yuan analyzed the composite behavioral data and EEG data. Guangjie Yuan interpreted the results. Guangjie Yuan wrote the article. Guangyuan Liu provided revisions to the article. All authors approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank all the participants and researchers involved in the experiment. This work was supported in part by the National Natural Science Foundation of China (No. 61472330 and No. 61872301).
Yuan, G. , & Liu, G. (2022). Mate preference and brain oscillations: Initial romantic attraction is associated with decreases in alpha‐ and lower beta‐band power. Human Brain Mapping, 43(2), 721–732. 10.1002/hbm.25681
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 61472330, 61872301
DATA AVAILABILITY STATEMENT
The data and code that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Al‐Ozzi, T. M. , Botero‐Posada, L. F. , Lopez Rios, A. L. , & Hutchison, W. D. (2021). Single unit and beta oscillatory activities in subthalamic nucleus are modulated during visual choice preference. The European Journal of Neuroscience, 53(7), 2220–2233. 10.1111/ejn.14750 [DOI] [PubMed] [Google Scholar]
- Asendorpf, J. B. , Penke, L. , & Back, M. D. (2011). From dating to mating and relating: Predictors of initial and long‐term outcomes of speed‐dating in a community sample. European Journal of Personality, 25(1), 16–30. 10.1002/per.768 [DOI] [Google Scholar]
- Chatterjee, A. , Thomas, A. , Smith, S. E. , & Aguirre, G. K. (2009). The neural response to facial attractiveness. Neuropsychology, 23(2), 135–143. 10.1037/a0014430 [DOI] [PubMed] [Google Scholar]
- Cohen, Y. E. , & Andersen, R. A. (2002). A common reference frame for movement plans in the posterior parietal cortex. Nature Reviews. Neuroscience, 3(7), 553–562. 10.1038/nrn873 [DOI] [PubMed] [Google Scholar]
- Conroy‐Beam, D. , & Buss, D. M. (2016). Do mate preferences influence actual mating decisions? Evidence from computer simulations and three studies of mated couples. Journal of Personality and Social Psychology, 111(1), 53–66. 10.1037/pspi0000054 [DOI] [PubMed] [Google Scholar]
- Cooper, J. C. , Dunne, S. , Furey, T. , & O'Doherty, J. P. (2012). Dorsomedial prefrontal cortex mediates rapid evaluations predicting the outcome of romantic interactions. The Journal of Neuroscience, 32(45), 15647–15656. 10.1523/JNEUROSCI.2558-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesarei, A. , & Codispoti, M. (2011). Affective modulation of the LPP and alpha‐ERD during picture viewing. Psychophysiology, 48(10), 1397–1404. 10.1111/j.1469-8986.2011.01204.x [DOI] [PubMed] [Google Scholar]
- Feinberg, D. R. (2008). Are human faces and voices ornaments signaling common underlying cues to mate value? Evolutionary Anthropology: Issues, News, and Reviews, 17(2), 112–118. 10.1002/evan.20166 [DOI] [Google Scholar]
- Ferenc Matyas, V. S. , Marbach, F. , Wacongne, C. , Barsy, B. , Mateo, C. , Aronoff, R. , & Petersen1., C. C. H. (2010). Motor control by sensory cortex. Science, 330(6008), 1240–1243. 10.1126/science.1195797 [DOI] [PubMed] [Google Scholar]
- Finkel, E. J. , Eastwick, P. W. , & Matthews, J. (2007). Speed‐dating as an invaluable tool for studying romantic attraction: A methodological primer. Personal Relationships, 14(1), 149–166. 10.1111/j.1475-6811.2006.00146.x [DOI] [Google Scholar]
- Fisher, H. , Aron, A. , & Brown, L. L. (2005). Romantic love: an fMRI study of a neural mechanism for mate choice. The Journal of Comparative Neurology, 493(1), 58–62. 10.1002/cne.20772 [DOI] [PubMed] [Google Scholar]
- Fisher, H. , Aron, A. , Mashek, D. J. , Li, H. , & Brown, L. (2002). Defining the Brain Systems of Lust, Romantic Attraction, and Attachment. Archives of Sexual Behavior, 31, 413–419. 10.1023/a:1019888024255 [DOI] [PubMed] [Google Scholar]
- Fisher, H. E. (1998). Lust, attraction, and attachment in mammalian reproduction. Human Nature, 9, 23–52. 10.1007/s12110-998-1010-5 [DOI] [PubMed] [Google Scholar]
- Foxe, J. J. , & Snyder, A. C. (2011). The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2, 154. 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Guo, Y. D. , Wang, T. , Liu, W. , & Jin, H. (2016). Applying event related potentials to evaluate user preferences toward smartphone form design. International Journal of Industrial Ergonomics, 54, 57–64. 10.1016/j.ergon.2016.04.006 [DOI] [Google Scholar]
- Gerlach, T. M. , & Reinhard, S. K. (2018). Personality and romantic attraction. In Encyclopedia of personality and individual differences (pp. 1–6). Cham, Switzerland: Springer. 10.1007/978-3-319-28099-8_717-2 [DOI] [Google Scholar]
- Goto, N. , Lim, X. L. , Shee, D. , Hatano, A. , Khong, K. W. , Buratto, L. G. , … Schaefer, A. (2019). Can brain waves really tell if a product will be purchased? Inferring consumer preferences from single‐item brain potentials. Frontiers in Integrative Neuroscience, 13, 19. 10.3389/fnint.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, N. , Mushtaq, F. , Shee, D. , Lim, X. L. , Mortazavi, M. , Watabe, M. , & Schaefer, A. (2017). Neural signals of selective attention are modulated by subjective preferences and buying decisions in a virtual shopping task. Biological Psychology, 128, 11–20. 10.1016/j.biopsycho.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Grant‐Jacob, J. A. (2016). Love at first sight. Frontiers in Psychology, 7, 1113. 10.3389/fpsyg.2016.01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton, G. , Coles, M. G. H. , & Donchin, E. (1983). A new method for off‐line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Grill‐Spector, K. , Kourtzi, Z. , & Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10–11), 1409–1422. 10.1016/S0042-6989(01)00073-6 [DOI] [PubMed] [Google Scholar]
- Hajcak, G. , MacNamara, A. , & Olvet, D. M. (2010). Event‐related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hu, F. , Wu, Q. , Li, Y. , Xu, W. , Zhao, L. , & Sun, Q. (2020). Love at first glance but not after deep consideration: The impact of sexually appealing advertising on product preferences. Frontiers in Neuroscience, 14, 465. 10.3389/fnins.2020.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, T. , Schubotz, R. I. , Hofel, L. , & Cramon, D. Y. (2006). Brain correlates of aesthetic judgment of beauty. NeuroImage, 29(1), 276–285. 10.1016/j.neuroimage.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Kang, J. H. , Kim, S. J. , Cho, Y. S. , & Kim, S. P. (2015). Modulation of alpha oscillations in the human EEG with facial preference. PLoS One, 10(9), e0138153. 10.1371/journal.pone.0138153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch, W. (2012). Alpha‐band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann, F. , Nikulin, V. V. , Kuhn, A. A. , Marzinzik, F. , Wahl, M. , Pogosyan, A. , … Curio, G. (2007). Task‐related differential dynamics of EEG alpha‐ and beta‐band synchronization in cortico‐basal motor structures. The European Journal of Neuroscience, 25(5), 1604–1615. 10.1111/j.1460-9568.2007.05417.x [DOI] [PubMed] [Google Scholar]
- Langeslag, S. J. , Jansma, B. M. , Franken, I. H. , & Van Strien, J. W. (2007). Event‐related potential responses to love‐related facial stimuli. Biological Psychology, 76(1–2), 109–115. 10.1016/j.biopsycho.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Lindsen, J. P. , Jones, R. , Shimojo, S. , & Bhattacharya, J. (2010). Neural components underlying subjective preferential decision making. NeuroImage, 50(4), 1626–1632. 10.1016/j.neuroimage.2010.01.079 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Huang, H. , McGinnis‐Deweese, M. , Keil, A. , & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. 10.1523/jneurosci.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, S. , & Zhang, G. (2009). What leads to romantic attraction: Similarity, reciprocity, security, or beauty? Evidence from a speed‐dating study. Journal of Personality, 77(4), 933–964. 10.1111/j.1467-6494.2009.00570.x [DOI] [PubMed] [Google Scholar]
- Ma, Q. , Qian, D. , Hu, L. , & Wang, L. (2017). Hello handsome! Male's facial attractiveness gives rise to female's fairness bias in Ultimatum Game scenarios‐An ERP study. PLoS One, 12(7), e0180459. 10.1371/journal.pone.0180459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Mehrabian, A. (1980). Basic dimensions for a general psychological theory: Implications for personality, social, environmental, and developmental studies. Washington, D.C.: American Psychological Association. https://www.webofscience.com/wos/alldb/full‐record/WOS:A1982NN87800023 [Google Scholar]
- Mehrabian, A. (1996). Pleasure‐arousal‐dominance: A general framework for describing and measuring individual differences in Temperament. Current Psychology, 14(4), 261–292. 10.1007/BF02686918 [DOI] [Google Scholar]
- Neuper, C. , Wörtz, M. , & Pfurtscheller, G. (2006). ERD/ERS patterns reflecting sensorimotor activation and deactivation. In Event‐related dynamics of brain oscillations (Vol. 159, pp. 211–222). Cambridge, MA: Academic Press. [DOI] [PubMed] [Google Scholar]
- Olderbak, S. G. , Malter, F. , Wolf, P. S. A. , Jones, D. N. , & Figueredo, A. J. (2017). Predicting romantic interest at zero acquaintance: Evidence of sex differences in trait perception but not in predictors of interest. European Journal of Personality, 31(1), 42–62. 10.1002/per.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshin Vartanian, V. G. (2004). Neuroanatomical correlates of aesthetic preference of paintings. Neuroreport, 15(5), 893–897. 10.1097/00001756-200404090-00032 [DOI] [PubMed] [Google Scholar]
- Patel, S. H. , & Azzam, P. N. (2005). Characterization of N200 and P300: Selected studies of the event‐related potential. International Journal of Medical Sciences, 2(4), 147–154. 10.7150/ijms.2.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, A. , Rubinsten, O. , Peled, L. , & Shamay‐Tsoory, S. G. (2013). Don't stand so close to me: A behavioral and ERP study of preferred interpersonal distance. NeuroImage, 83, 761–769. 10.1016/j.neuroimage.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Rektor, I. , Sochůrková, D. , & Bočková, M. (2006). Intracerebral ERD/ERS in voluntary movement and in cognitive visuomotor task. In Event‐related dynamics of brain oscillations (pp. 311–330). Cambridge, MA: Academic Press. [DOI] [PubMed] [Google Scholar]
- Schubring, D. , & Schupp, H. T. (2019). Affective picture processing: Alpha‐ and lower beta‐band desynchronization reflects emotional arousal. Psychophysiology, 56(8), e13386. 10.1111/psyp.13386 [DOI] [PubMed] [Google Scholar]
- Schubring, D. , & Schupp, H. T. (2021). Emotion and brain oscillations: High arousal is associated with decreases in alpha‐ and lower beta‐band power. Cerebral Cortex, 31(3), 1597–1608. 10.1093/cercor/bhaa312 [DOI] [PubMed] [Google Scholar]
- Szurhaj, W. , Derambure, P. , Labyt, E. , Cassim, F. , Bourriez, J.‐L. , Isnard, J. , … Mauguière, F. (2003). Basic mechanisms of central rhythms reactivity to preparation and execution of a voluntary movement: A stereoelectroencephalographic study. Clinical Neurophysiology, 114(1), 107–119. 10.1016/S1388-2457(02)00333-4 [DOI] [PubMed] [Google Scholar]
- Tashiro, N. , Sugata, H. , Ikeda, T. , Matsushita, K. , Hara, M. , Kawakami, K. , … Fujiki, M. (2019). Effect of individual food preferences on oscillatory brain activity. Brain and Behavior: A Cognitive Neuroscience Perspective, 9(5), e01262. 10.1002/brb3.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, J. R. (2017). Posterior parietal cortex. Current Biology, 27(14), R691–R695. 10.1016/j.cub.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Zsok, F. , Haucke, M. , De Wit, C. Y. , & Barelds, D. P. H. (2017). What kind of love is love at first sight? An empirical investigation. Personal Relationships, 24(4), 869–885. 10.1111/pere.12218 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and code that support the findings of this study are available from the corresponding author upon reasonable request.


