Abstract
This study aims to evaluate the impact of French national lockdown of 55 days on brain metabolism of patients with neurological disorders. Whole‐brain voxel‐based PET analysis was used to correlate 18F‐FDG metabolism to the number of days after March 17, 2020 (in 95 patients; mean age: 54.3 years ± 15.7; 59 men), in comparison to the same period in 2019 before the SARS‐CoV‐2 outbreak (in 212 patients; mean age: 59.5 years ± 15.8; 114 men), and to the first 55 days of deconfinement (in 188 patients; mean age: 57.5 years ± 16.5; 93 men). Lockdown duration was negatively correlated to the metabolism of the sensory‐motor cortex with a prevailing effect on the left dominant pyramidal tract and on younger patients, also including the left amygdala, with only partial reversibility after 55 days of deconfinement. Weak overlap was found with the reported pattern of hypometabolism in long COVID (<9%). Restriction of physical activities, and possible related deconditioning, and social isolation may lead to functional disturbances of sensorimotor and emotional brain networks. Of note, this metabolic pattern seems distinct to those reported in long COVID. Further longitudinal studies with longer follow‐up are needed to evaluate clinical consequences and relationships on cognitive and mental health against functional deactivation hypothesis, and to extend these findings to healthy subjects in the context of lockdown.
Keywords: brain metabolism, COVID‐19, deconfinement, deconditioning, FDG‐PET, lockdown, long COVID, physical activities, pyramidal tract, SARS‐CoV‐2
Lockdown duration is negatively correlated to the metabolism of the sensory‐motor cortex with a prevailing effect on the left dominant pyramidal tract and on younger patients, also including the left amygdala, with only partial reversibility after 55 days of deconfinement. Weak overlap is found with the reported pattern of hypometabolism in long COVID (<9%). Restriction of physical activities, and possible related deconditioning, and social isolation may lead to functional disturbances of sensorimotor and emotional brain networks.
1. INTRODUCTION
More than 3 billion people worldwide were confined to their home to limit the spread of the COVID‐19 outbreak (Alleaume, Verger, Peretti‐Watel, & COCONEL Group, 2021). In France, a national lockdown was decreed between March 17 and May 11, 2020. During this 55‐day period, only people whose jobs were considered as essential could go to work, physical activity was restricted within 1 km of one's home, and all “nonelective” medical procedures were suspended (Gonzalez, Taïeb, Guedj, Le Coz, & Cammilleri, 2021).
While physical activity is supposed to promote “brain health” through plasticity and neuroprotection (Boecker & Drzezga, 2016), and that evidence is growing on the negative impact of lockdown for cognitive and mental functioning (Alleaume et al., 2021; Fiorenzato, Zabberoni, Costa, & Cona, 2021; Ganesan et al., 2021), we aim to evaluate the effect of lockdown duration on brain metabolism of patients with neurological disorders. Whole‐brain voxel‐based PET analysis was used to correlate 2‐18F‐fluoro‐2‐deoxy‐D‐glucose (18F‐FDG) metabolism to the number of days after March 17, 2020, in comparison to the same period in 2019 before the SARS‐CoV‐2 outbreak, and to the first 55 days of deconfinement, in patients clinically explored by PET in our department during these three periods.
2. MATERIALS AND METHODS
This research project retrospectively includes data acquired in clinical settings after information of patients and absence of opposition (MR‐004 French research type). APHM institutional board approved this study on March 11, 2021, with the following reference: PADS21‐84.
In the context of the national French lockdown of 55 days between March 17 and May 11, 2020, 95 adult patients (mean age: 54.3 years ± 15.7; 59 men) underwent 18F‐FDG brain PET in our nuclear medicine department for the following indications: cognitive/behavioral impairment (n = 49), glioma (n = 41), and focal epilepsy (n = 5), with 47 identified focal morphological lesions. Distribution of indications among time was similar between patients (p = .40, Jonckheere–Terpstra test). To compare data to the same period from the previous year, between March 17 and May 11, 2019, the same camera was used to perform 18F‐FDG brain PET on 212 adult patients (mean age: 59.5 years ± 15.8; 114 men) for cognitive/behavioral impairment (n = 145), glioma (n = 45), and focal epilepsy (n = 22), with 58 identified focal morphological lesions. During the first 55 days of deconfinement, the same camera was used to perform 18F‐FDG PET on 188 adult patients (mean age: 57.5 years ± 16.5; 93 men) for cognitive/behavioral impairment (n = 127), glioma (n = 42), and focal epilepsy (n = 19), with 47 identified morphological lesions. The characteristics of the three groups of patients are detailed in Table 1.
TABLE 1.
Characteristics of the three groups of patients
| Lockdown | 2019 control period | Deconfinement | |
|---|---|---|---|
| Duration of inclusion in days | 55 | 55 | 55 |
| Total number of patients | 95 | 212 | 188 |
| Percentage of men | 62% | 54% | 49% |
| Mean age in years (SD) | 54.3 (15.7) | 59.5 (15.8) | 57.5 (16.5) |
| Percentage of patients with morphological lesion | 49% | 27% | 25% |
| Percentage of PET for cognitive/behavioral impairment | 52% | 68% | 68% |
| Percentage of PET for glioma | 43% | 21% | 22% |
| Percentage of PET for focal epilepsy | 5% | 10% | 10% |
Using SPM8 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, University College, London, UK), the whole‐brain voxel‐based correlation to the number of days after March 17 was first studied in interaction between the two groups of patients (year 2020 vs. 2019), after spatial normalization, smoothing (full width at half maximum of 8 mm), and global proportional scaling of 18FDG‐PET images. A quite similar SPM methodological approach was previously proposed to study the relationship between gray matter volume and the amount of time spent as a taxi driver in London using MRI (Maguire et al., 2000). The p‐voxel threshold was fixed to .001, and the correction for multiple comparisons was applied at the cluster level using the family‐wise error (FWE) method (p < .05, FWE‐corrected). Age, sex, and the presence of focal morphological lesion (yes/no) were added as covariables in the statistical model to control the possible effects. We secondarily tested the effect of the different variables included in the SPM model as well as the possible impact of distinct neurological disorders (cognitive/behavioral impairment, glioma, and focal epilepsy) using ANOVA (SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp and R Version 4.0.2). A complementary analysis was also performed by excluding patients with glioma and more largely patients with focal morphological lesion. Finally, the metabolic found cluster was extracted using MarsBaR (http://marsbar.sourceforge.net/) and compared between the three groups of patients (2019 control period, lockdown period, and deconfinement period) using ANOVA and post hoc tests, and correlations were checked by Pearson's test (SPSS).
3. RESULTS
In comparison to the 212 patients from the control period of 2019, the duration of the lockdown was negatively correlated to the metabolism of the left precentral gyrus in the 95 patients explored during the confinement (peak T score = 4.63; p cluster = .048 FWE‐corrected; k = 626; r = −.35 vs. r = .19 for 2019, both significant using Pearson's test; age, sex, and the presence of focal morphological lesions are considered as nuisance variables in this first SPM model; Figure 1a,b). Findings were strengthened after the exclusion of patients with glioma (r = −.44 vs. .22, both significant using Pearson's test, with 54 and 167 patients for the lockdown and 2019 control periods, respectively) as well after the exclusion of patients with brain focal morphological lesions (r = −.40 vs. .25, both significant using Pearson's test, with 48 and 154 patients for the lockdown and 2019 control periods, respectively).
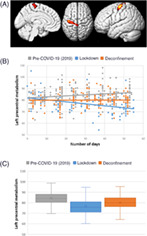
FIGURE 1.
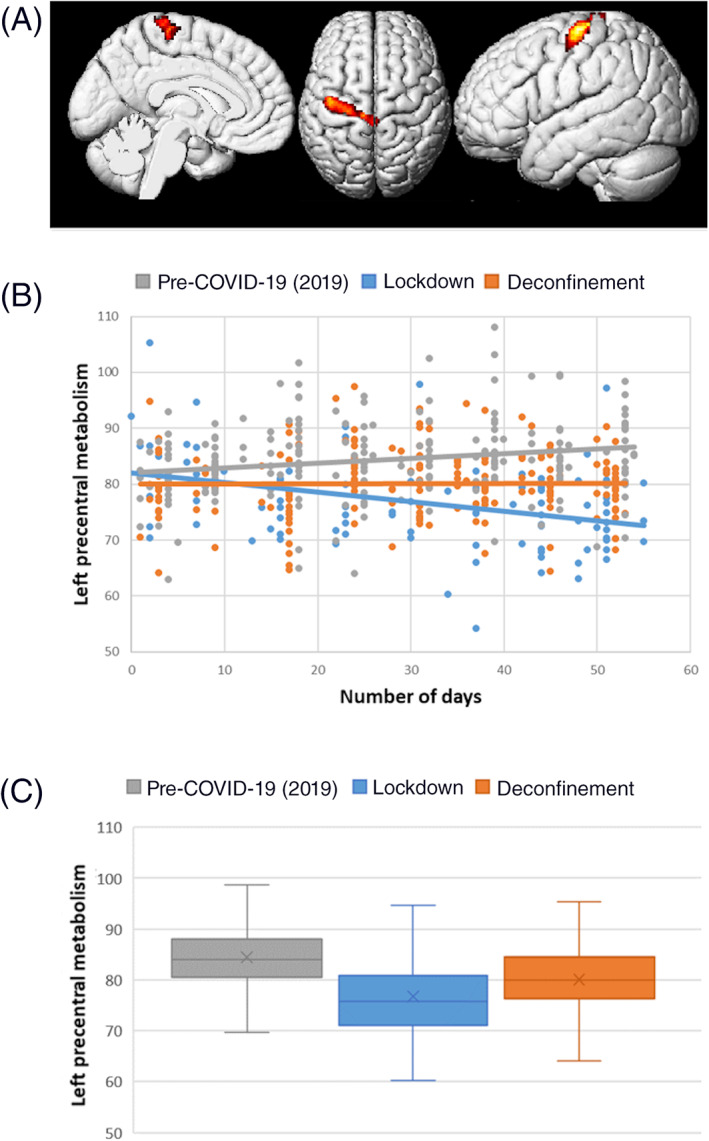
Correlation between brain metabolism and lockdown duration in comparison to the pre‐COVID‐19 period (independently of age, sex, and the presence of focal morphological lesions). The lockdown duration is negatively correlated to the metabolism of the left precentral gyrus in comparison to the pre‐COVID‐19 period. The brain localization of this cluster is presented on a 3D‐MRI view on panel (a) (p cluster = .048 FWE‐corrected). Distribution of brain metabolic values of this cluster is presented among the three groups of patients according to the number of days on panel (b), confirming the negative correlation during the lockdown (r = −.35, p < .001; Pearson's test). On panel (c), the mean metabolic value of the left primary cortex is significantly improved during the deconfinement period in comparison to the lockdown period (p < .001, ANOVA post hoc test) but still significantly reduced during the deconfinement period in comparison to the pre‐COVID‐19 period in 2019 (p < .001, ANOVA post hoc test)
A broader relationship was found with the group × age interaction (the two groups correspond to the lockdown and control periods). The lockdown duration was then negatively correlated to the metabolism of a large sensorimotor network prevailing in the dominant cerebrum in the 95 patients explored during the confinement, including the pre/postcentral cortex, thalamus, midbrain/pons, and contralateral cerebellum, and also negatively correlated to the metabolism of the left amygdala, again in comparison to the 212 patients from the control period of 2019, with major effect on younger patients (peak T score = 5.84; p cluster <.022 FWE‐corrected; k = 11,954; sex and presence of focal morphological lesions are considered as nuisance variables and the positive correlation with age as an explanatory variable in interaction with the two groups; Figure 2). No significant results were obtained with inverse contrasts or by limiting the multiple comparison of the analysis to the left language regions (BA22, BA44, and BA45) or olfactory regions (Guedj et al., 2021). No interaction was found with the other variables (sex; neurological disorder: cognitive/behavioral impairment, glioma, and focal epilepsy).
FIGURE 2.
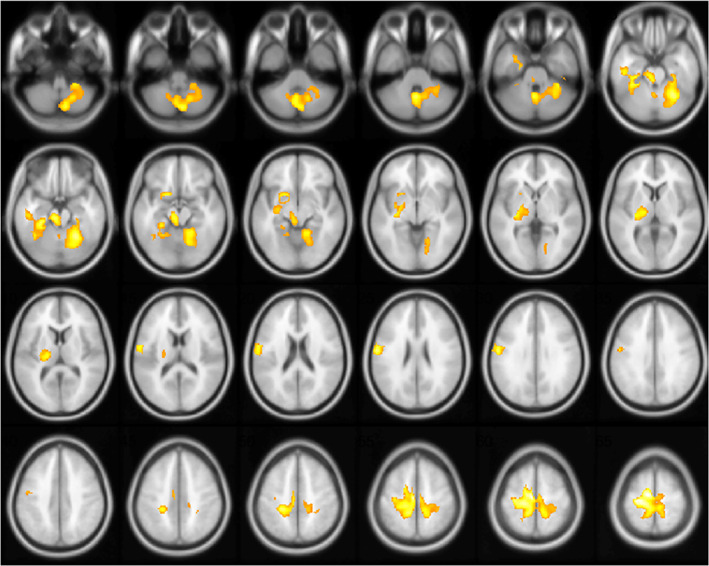
Correlation between brain metabolism and lockdown duration using group × age interaction (independently of sex and presence of focal morphological lesions). The positive correlation with age is included as an explanatory variable in interaction with the two groups corresponding to the lockdown and control periods. The lockdown duration is negatively correlated to the metabolism of a large sensorimotor network prevailing in the dominant cerebrum, including the pre/postcentral cortex, thalamus, midbrain/pons, and contralateral cerebellum as well as the left amygdala, with major effect on younger patients (p cluster <.022 FWE‐corrected; the left hemisphere is on the left side)
The metabolic cluster of the left primary motor cortex identified at the previous step was extracted, and no significant correlation was found with the number of days of deconfinement in patients explored after May 11, 2020 (r = .06, Pearson's test; Figure 1b).
More globally, the mean metabolic value of the left primary motor cortex was significantly improved during the deconfinement period in comparison to the lockdown period (80.3 ± 6.9 vs. 76.7 ± 8.3; p < .001, ANOVA post hoc test) but still significantly reduced during the deconfinement period in comparison to the pre‐COVID‐19 period in 2019 (80.3 ± 6.9 vs. 84.4 ± 7.2; p < .001, ANOVA post hoc test; Figure 1c).
4. DISCUSSION
This study shows the negative impact of the lockdown on the brain metabolism of the sensory‐motor cortex with a prevailing effect on the left dominant pyramidal tract and on younger patients, also including the left amygdala, with only partial reversibility after 55 days of deconfinement. Physicians in nuclear medicine should be aware of such possible functional effects on interpretation of metabolic brain PET imaging. Of note, this metabolic pattern seems distinct to those reported in long COVID (Guedj et al., 2021), mostly involving right limbic areas, and especially the olfactory regions with severe hypometabolisms (peak T score = 9.80 for long COVID vs. 4.63 and 5.84 for the two SPM analyses of the present study on lockdown). A direct comparison between SPM masks of metabolic changes here reported for lockdown and previously for long COVID shows no common voxel for the relationship with the lockdown duration, and 647 common voxels for the group × age interaction (corresponding to 8.5% of abnormalities reported in long COVID, and 5.4% of those found in the present study for this interaction). These common voxels correspond to part of the abnormalities reported in the brainstem and the cerebellum.
In line with the restriction of daily activities and social isolation, previous studies confirmed negative impact of lockdown on cognitive and mental health, with a prevailing effect in subjects under 45 years (Fiorenzato et al., 2021). The precentral metabolic cluster, negatively correlated to the lockdown duration in our study, precisely corresponds to the motor skill region of the lower and proximal upper limbs on Penfield's homunculus (Penfield & Boldrey, 1937). We interpret this finding as a consequence of the reduction in walking perimeter and physical activities over this period, and possibly the deconditioning. Using movement data of mobile phone users, Schlosser et al. interestingly confirmed that lockdown lead to pejorative mobility changes with a more local, clustered network and a moderation of the “small‐world” effect (Schlosser et al., 2020). On the other hand, while sedentary behavior is associated with elevated risks for developing dementia and that regular exercise training slows down age‐related brain atrophy (see Boecker & Drzezga, 2016, for review), few studies have so far investigated the metabolic PET impact of physical activities, with nevertheless preliminary evidence of increased metabolism in healthy subjects (Tashiro et al., 2001), in individuals at risk of Alzheimer's disease (AD; Dougherty et al., 2017), and in patients with mild AD (Castellano et al., 2017), especially within motor networks. Physical activities are also known to induce acute and sustained psychophysiological effects, including mood changes, stress reduction, and anxiolysis (Boecker & Drzezga, 2016). Moreover, the positive correlation found with the left precentral metabolism in the control period may suggest a seasonal effect with more physical activities with the more favorable spring weather.
Despite the large number of patients and the addition of two control groups, this study is limited by its retrospective design, especially the absence of clinical evaluation of daily activities and of cognitive/mental health, and the absence of longitudinal follow‐up. In this line, the reversible effect suggested during the deconfinement period is obtained in an independent group on a cross‐sectional analysis and not properly on a longitudinal follow‐up of individuals. The absence of screening of SARS‐CoV‐2 status among subjects can also be viewed as a limitation to better clarify the link with COVID‐19 and especially with long COVID. RT‐PCR was in fact mostly performed in most severe patients during the first wave of the outbreak in France, and consequently, patients were not systematically tested before their PET exam (http://www.senat.fr/compte-rendu-commissions/20201207/ce_covid.html#toc2). This limitation is relative, given the incidence of the disease during this period (much less than 1%). On the other hand, it is interesting to notice that the metabolic pattern here reported seems distinct from those of long COVID (Guedj et al., 2021). Finally, the suspension of “nonelective” medical procedures during the lockdown may constitute a bias of selection in a between‐group analysis; this risk is nevertheless limited on the correlation with the lockdown duration, especially because a similar distribution of indications was found among time between patients during the confinement.
5. CONCLUSION
Restriction of physical activities, and possible related deconditioning, and social isolation may lead to functional disturbances of sensorimotor and emotional brain networks in patients with neurological disorders. The positive correlation found during the deconfinement period suggests reversibility, at least partially after 55 days. Of note, this metabolic pattern seems distinct to those reported in long COVID. Further longitudinal studies with longer follow‐up are needed to evaluate clinical consequences and relationships on cognitive and mental health against functional deactivation hypothesis and to extend these findings to healthy subjects in the context of lockdown.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Guedj, E. , Campion, J.‐Y. , Horowitz, T. , Barthelemy, F. , Cammilleri, S. , & Ceccaldi, M. (2022). The impact of COVID‐19 lockdown on brain metabolism. Human Brain Mapping, 43(2), 593–597. 10.1002/hbm.25673
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author on reasonable request.
REFERENCES
- Alleaume, C. , Verger, P. , Peretti‐Watel, P. , & COCONEL Group . (2021). Psychological support in general population during the COVID‐19 lockdown in France: Needs and access. PLoS One, 16(5), e0251707. 10.1371/journal.pone.0251707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker, H. , & Drzezga, A. (2016). A perspective on the future role of brain pet imaging in exercise science. NeuroImage, 131, 73–80. 10.1016/j.neuroimage.2015.10.021 [DOI] [PubMed] [Google Scholar]
- Castellano, C. A. , Paquet, N. , Dionne, I. J. , Imbeault, H. , Langlois, F. , Croteau, E. , … Cunnane, S. C. (2017). A 3‐month aerobic training program improves brain energy metabolism in mild Alzheimer's disease: Preliminary results from a neuroimaging study. Journal of Alzheimer's Disease, 56(4), 1459–1468. 10.3233/JAD-161163 [DOI] [PubMed] [Google Scholar]
- Dougherty, R. J. , Schultz, S. A. , Kirby, T. K. , Boots, E. A. , Oh, J. M. , Edwards, D. , … Okonkwo, O. C. (2017). Moderate physical activity is associated with cerebral glucose metabolism in adults at risk for Alzheimer's disease. Journal of Alzheimer's Disease, 58(4), 1089–1097. 10.3233/JAD-161067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenzato, E. , Zabberoni, S. , Costa, A. , & Cona, G. (2021). Cognitive and mental health changes and their vulnerability factors related to COVID‐19 lockdown in Italy. PLoS One, 16(1), e0246204. 10.1371/journal.pone.0246204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, B. , Al‐Jumaily, A. , Fong, K. N. K. , Prasad, P. , Meena, S. K. , & Tong, R. K. (2021). Impact of coronavirus disease 2019 (COVID‐19) outbreak quarantine, isolation, and lockdown policies on mental health and suicide. Frontiers in Psychiatry, 12, 565190. 10.3389/fpsyt.2021.565190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, S. , Taïeb, D. , Guedj, E. , Le Coz, P. , & Cammilleri, S. (2021). To what extent has the reorganization of nuclear medicine activities during the COVID‐19 pandemic fulfilled medical ethics? European Journal of Nuclear Medicine and Molecular Imaging, 48(1), 3–5. 10.1007/s00259-020-05008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj, E. , Campion, J. Y. , Dudouet, P. , Kaphan, E. , Bregeon, F. , Tissot‐Dupont, H. , … Eldin, C. (2021). F‐FDG brain PET hypometabolism in patients with long COVID. European Journal of Nuclear Medicine and Molecular Imaging, 48(9), 2823–2833. 10.1007/s00259-021-05215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A. , Gadian, D. G. , Johnsrude, I. S. , Good, C. D. , Ashburner, J. , Frackowiak, R. S. , & Frith, C. D. (2000). Navigation‐related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 4398–4403. 10.1073/pnas.070039597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield, W. , & Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60(4), 389–443. [Google Scholar]
- Schlosser, F. , Maier, B. F. , Jack, O. , Hinrichs, D. , Zachariae, A. , & Brockmann, D. (2020). COVID‐19 lockdown induces disease‐mitigating structural changes in mobility networks. Proceedings of the National Academy of Sciences of the United States of America, 117(52), 32883–32890. 10.1073/pnas.2012326117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro, M. , Itoh, M. , Fujimoto, T. , Fujiwara, T. , Ota, H. , Kubota, K. , … Sasaki, H. (2001). 18F‐FDG PET mapping of regional brain activity in runners. The Journal of Sports Medicine and Physical Fitness, 41(1), 11–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author on reasonable request.


