Abstract
Lifelong bilingualism is associated with delayed dementia onset, suggesting a protective effect on the brain. Here, we aim to study the effects of lifelong bilingualism as a dichotomous and continuous phenomenon, on brain metabolism and connectivity in individuals with Alzheimer's dementia. Ninety‐eight patients with Alzheimer's dementia (56 monolinguals; 42 bilinguals) from three centers entered the study. All underwent an [18F]‐fluorodeoxyglucose positron emission tomography (PET) imaging session. A language background questionnaire measured the level of language use for conversation and reading. Severity of brain hypometabolism and strength of connectivity of the major neurocognitive networks was compared across monolingual and bilingual individuals, and tested against the frequency of second language life‐long usage. Age, years of education, and MMSE score were included in all above mentioned analyses as nuisance covariates. Cerebral hypometabolism was more severe in bilingual compared to monolingual patients; severity of hypometabolism positively correlated with the degree of second language use. The metabolic connectivity analyses showed increased connectivity in the executive, language, and anterior default mode networks in bilingual compared to monolingual patients. The change in neuronal connectivity was stronger in subjects with higher second language use. All effects were most pronounced in the left cerebral hemisphere. The neuroprotective effects of lifelong bilingualism act both against neurodegenerative processes and through the modulation of brain networks connectivity. These findings highlight the relevance of lifelong bilingualism in brain reserve and compensation, supporting bilingual education and social interventions aimed at usage, and maintenance of two or more languages, including dialects, especially crucial in the elderly people.
Keywords: Alzheimer disease, default mode network, fluorodeoxyglucose F18, multilingualism, positron‐emission tomography
We investigated the neural mechanisms underlying the ability of bilingual senior individuals to better cope with pathology and symptoms of Alzheimer dementia, as compared to matched monolingual individuals with Alzheimer dementia. By using FDG‐PET to measure brain glucose metabolism and connectivity, we found evidence of neural reserve on brain hypometabolism and compensatory effects on brain metabolic connectivity, predominantly in the left hemisphere. In both cases, effects of lifelong bilingualism on the brain were linearly associated with the degree of second language use, that is, the higher the second language use, the higher the neural reserve/compensatory effect on brain metabolism and connectivity, respectively.
1. INTRODUCTION
Extended longevity for older age is associated with higher prevalence of cognitive decline and prospective dementing disorders, with the accompanying social and healthcare burden. The consequences of these projections suggest the need for maintaining brain health and cognitive efficiency across the lifespan, enabling older adults to function independently for longer.
Normal aging is characterized by neurophysiological and neuroanatomical changes of the brain (Ziegler, Dahnke, & Gaser, 2012). Considering the relative lack of success in developing effective pharmacological therapies to halt neurocognitive decline and prospective dementing illnesses (Massoud & Gauthier, 2010), a precise societal responsibility consists in identifying ecologically valid conditions and interventions aimed at promoting healthy aging through cognitive and neural reserve mechanisms. Activities such as physical exercise and cognitive training are the promising candidates to mitigate the effects of brain aging on daily functioning (Barulli & Stern, 2013; Cabeza et al., 2018), and lifestyle (e.g., volunteering, Mediterranean diet) and psychosocial factors (e.g., education, socioeconomic status, occupational attainment, and social engagement) are associated with a reduced or delayed expression of dementia symptoms and underlying brain pathology (Baumgart et al., 2015; Winblad et al., 2016). Crucially, recent observations have pointed that speaking two or more languages may foster healthy aging by promoting cognitive and neural reserve (Bialystok, 2021; Perani & Abutalebi, 2015), resulting in milder cognitive effects of stroke (Alladi et al., 2016), delayed‐onset dementia (Alladi et al., 2013; Bialystok, Craik, & Freedman, 2007), and protective role against the expression of other neurodegenerative disorders (Voits, Pliatsikas, Robson, & Rothman, 2020). One conjecture for this bilingual effect is that, bilingual individuals, compared to monolinguals, are in need to develop an increased ability to control languages, solving linguistic competition to keep the languages separated and avoid context‐oriented interference (Calabria, Costa, Green, & Abutalebi, 2018). This would enhance a set of cognitive functions and top‐down processes including selective attention and executive abilities, as it was shown for senior bilinguals compared to age‐matched monolinguals (Bialystok, Craik, & Luk, 2012; Valian, 2015). Of note, Valian argues that it is plausible to observe better performance in elderly bilinguals versus age‐matched monolinguals when compared to younger bilinguals versus younger monolinguals, as many behavioral studies report. Her line of argumentation is that younger subjects are so much exposed to many other cognitively challenging activities that any bilingual effect may be masked or canceled out. Once individuals age and retire, they are usually less exposed to multiple other activities and hence bilingualism may make a difference. (Valian, 2015). Language control emerges from the integration of distinct cortical and subcortical systems associated with domain‐general executive functions and, in bilinguals, because of the extended usage of these systems, the brain connections are more strongly developed (Abutalebi & Green, 2016). Structural neuroimaging studies comparing bilingual to monolingual seniors have shown a positive association between lifelong bilingual experience and gray and white matter plastic changes in areas traditionally related to language acquisition and processing as well as in the neural network for language control (Abutalebi et al., 2014; Abutalebi et al., 2015a; Coggins, Kennedy, & Armstrong, 2004; García‐Pentón, Pérez Fernández, Iturria‐Medina, Gillon‐Dowens, & Carreiras, 2014; Luk, Bialystok, Craik, & Grady, 2011; Olsen et al., 2015; Pliatsikas et al., 2020). As some of the regions and circuits of the language control network, such as the prefrontal cortices, are most vulnerable to aging, bilingual experience may protect the brain against atrophy and prospective age‐related disease (Bialystok et al., 2012; Hervais‐Adelman, Moser‐Mercer, & Golestani, 2011), either because sufficient neural substrate remains to support normal function or because compensatory strategies are employed to maintain and optimize performance (Perani & Abutalebi, 2015).
Consistent with these findings, bilinguals with neurodegenerative brain diseases, such as Alzheimer's dementia (AD) show a later onset of the symptoms and suffer to a lesser extent from associated cognitive deficits. For example, some studies have reported a delay of 4–5 years in the onset of AD symptoms in bilinguals as compared to age‐matched monolinguals (Alladi et al., 2013; Bialystok et al., 2007; Bialystok, Craik, Binns, Ossher, & Freedman, 2014; Chertkow et al., 2010; Craik & Bialystok, 2010; Woumans et al., 2015; see Anderson, Hawrylewicz, & Grundy, 2020 for a meta‐analysis). Additionally, using multiple languages from early life seems to protect against mild cognitive impairment, a sign of brain decline often observed before AD diagnosis (Perquin et al., 2013). A recent study (Smirnov et al., 2019) has also shown a significant correlation between the thickness of a core cognitive control region (i.e., anterior cingulate cortex) and naming performance in the nondominant language in bilingual AD patients, whereas no such correlation was found in healthy controls. More importantly, studies have shown that bilinguals with AD tend to exhibit more extended brain atrophy than monolinguals when cognitive performance is matched across the groups (Schweizer, Ware, Fischer, Craik, & Bialystok, 2012).
One of the most accurate tools to study the influence of cognitive reserve upon brain disorders is PET and especially fluorodeoxyglucose (FDG)‐PET. FDG‐PET has been successfully employed to study neurodegenerative diseases, and a growing body of research has provided convincing evidence for highly specific patterns of FDG‐PET hypometabolism in distinct dementia conditions, even before brain atrophy may become manifest (Perani, 2014; Perani et al., 2014).
FDG‐PET offers the unique capability to measure resting‐state brain metabolism, which is a direct index of synaptic function and density (Attwell & Iadecola, 2002; Magistretti, Pellerin, Rothman, & Shulman, 1999). In individuals with AD and mild cognitive impairment, higher education and occupation (as proxies of cognitive reserve) correlate with more severe hypometabolism in temporo‐parietal areas and in the precuneus (Garibotto et al., 2008; Perneczky et al., 2006) and, in addition, with increased metabolism in the dorsolateral prefrontal cortex, suggesting a compensatory mechanism against AD‐related cerebral neurodegeneration (Morbelli et al., 2013). Unfortunately, despite its growing importance, it is surprising that only two FDG‐PET studies investigated the role of bilingualism as a proxy of cognitive reserve upon brain metabolism in individuals with AD. The study of Perani et al. (2017) revealed that cerebral hypometabolism was more severe for bilingual subjects, who, despite severe brain hypometabolism, were in average 4–5 years older than their monolingual peers and performed even better on standard neuropsychological testing, meaning that they had increased cognitive reserve and were able to compensate for the cerebral damage to a greater extent than monolinguals. Perani et al. (2017) also reported increased connectivity in the executive control and default mode networks (DFNs) in AD bilinguals compared to monolinguals. Another study utilizing FDG‐PET (Kowoll et al., 2016) held similar results: bilingual seniors diagnosed with MCI and probable AD showed significantly higher levels of glucose hypometabolism in frontotemporal and parietal regions and in the left cerebellum, compared to monolingual peers matched for age, gender, and disease severity.
In the present study, we modeled bilingualism with two complementary approaches, as either a dichotomous or a continuous phenomenon. We employed FDG‐PET to evaluate brain glucose metabolism and brain dysfunction in large series of bilingual and monolingual individuals with AD to investigate the neural effects of lifelong bilingualism. According to the brain reserve hypothesis, we expected more severe cerebral hypometabolism in the group of bilinguals with AD in comparison to the monolingual AD patients, at comparable levels of dementia severity. We also investigated metabolic connectivity (Yakushev, Drzezga & Habeck, 2017; Sala & Perani, 2019), addressing distinct neurocognitive networks and crucially the language and the executive networks, in bilingual as compared to monolingual AD subjects, and we tested whether the effect of bilingualism on either brain metabolism and network connectivity is dependent on the frequency of lifelong usage of the second language. We hypothesize that metabolic connectivity will be stronger in bilingual compared to monolingual individuals with AD due to neural reserve and neural compensation. Of note, the present study replicates the architecture of a previous one from our group (Perani et al., 2017), however, in an independent sample of bilingual individuals (and partially independent sample of monolingual individuals), recruited in a multicentric research project. In details, differently from the previous study, the present cohort of bilingual individuals spoke different languages (French/German/Italian/English, in the group from HUG Geneva, or German/Italian, in the group from Bozen Central Hospital). As such, results of the current study are expected to be more generalizable (increased external validity) compared to the previous one. In addition, we refined the methodological approach concerning metabolic connectivity analyses, by complementing cross‐group modeling (comparison based on visual assessment) with a continuous approach, similar to the well‐established psychophysiological modeling (fully quantitative assessment). As such, we expect metabolic connectivity results included in the current study to be very robust.
2. MATERIAL AND METHODS
2.1. Participants
Ninety‐eight individuals with AD (56 monolinguals and 42 bilinguals) were selected in three centers: the San Raffaele Hospital, Milan (n = 40; all monolinguals; 19 males and 21 females), the Bozen Central Hospital, South Tyrol (n = 17; all bilinguals; 2 males and 15 females), Italy, and the Geneva University Hospital, Geneva, Switzerland (n = 41; 16 monolinguals and 25 bilinguals; 16 males and 25 females). All individuals were diagnosed with probable AD according to the National Institute on Aging–Alzheimer's Association consensus criteria (McKhann GM, et al. 2011), confirmed at clinical follow‐up. All individuals underwent the Mini‐mental State Examination (MMSE) and a thorough assessment of cognitive functions, including memory, language, visuospatial, and executive functions. The clinical diagnosis was supported by the presence of the AD typical cerebral hypometabolism pattern, semi‐quantitatively assessed at the single‐subject level (Sala et al., 2020). For bilinguals, the choice of language used for cognitive assessment was made by the neurologists and neuropsychologists on the basis of language dominance and patients' compliance.
All bilingual individuals with AD permanently resided in Bozen, South Tyrol (n = 17, German/Italian bilingual speakers), a bilingual region in northern Italy, or in Geneva (n = 25, French/German/Italian/English bilingual speakers). A language background questionnaire was used to assess the age of acquisition for each language in the bilingual group and the level of language use for conversation and reading. Specifically, the following scale was used for conversation and reading levels: for all monolinguals, the index was set to 1 = never; for bilinguals, the index of second language (L2) use was set to either: 2 = less than every month; 3 = every month; 4 = every week; 5 = every day. Based on these data, an index of L2 use was computed in each patient, by multiplying the scores for conversation and reading of L2. Thus, the resulting variable ranges from 1 to 25.
As in the previous study, the cohort of monolingual AD patients was selected on the basis of a complete monolingual education and exposure and no speaking of dialects.
The cohort of multi/bilinguals included in this study is completely independent from the one used in a previous published study (Perani et al., 2017), while there is partial overlap with this previous study for what concerns the cohort of monolinguals.
Mean and standard deviations of demographic and cognitive features were calculated for monolingual and bilingual groups and reported in Table 1. We tested for differences in age, years of education, MMSE score, and disease duration between bilingual and monolingual patients, performing a two independent sample t tests. Differences in the distribution of gender between the two groups were tested with a chi‐square test.
TABLE 1.
Descriptive statistics
| N | Age | Education | Gender (F/M) | MMSE | Disease duration (months) | |
|---|---|---|---|---|---|---|
| Bilinguals | 42 | 72.1 ± 8.7 | 12.5 ± 4.5 | 28/14 | 20.5 ± 5.3 | 2.62 ± 2.44 |
| Monolinguals | 56 | 72.7 ± 6.2 | 10.8 ± 4.0 | 33/23 | 20.4 ± 5.0 | 2.31 ± 1.51 |
| p‐value | p = .71 | p = .06 | p = .43 | p = .93 | p = .44 |
Note: p‐values were derived from independent sample t‐tests or chi‐squared test.
Abbreviations: MMSE, Mini‐mental State Examination.
The study was approved by the San Raffaele Scientific Institute, and University of Geneva ethical committees. The study was performed in compliance with the Declaration of Helsinki.
2.2. FDG‐PET acquisition and pre‐processing
All individuals underwent a FDG‐PET imaging session using 3D PET/CT scans: multi‐ring GE Discovery STE in Milan, Philips Gemini Time‐of‐Flight in Bozen, and Siemens PET/CT in Geneva. All subjects were fasted for at least 6 hr and their measured blood glucose level before radiopharmaceutical injection of 18F‐FDG was <120 mg/dl. Scan static acquisition started 30–45 min after the injection and lasted for 15 min, in accordance with the EANM guidelines (Varrone et al., 2009). Image reconstruction was performed by means of an OSEM (ordered subset expectation maximization) algorithm. The co‐registered CT was used for attenuation correction of the signal and the dedicated software integrated in the PET scans was used for scatter correction.
2.3. FDG‐PET SPM analysis (comparisons of monolingual and bilingual individuals)
Each single‐subject FDG‐PET image was analyzed by means of an optimized single‐subject procedure (Della Rosa et al., 2014; Perani et al., 2014), allowing the comparison of each AD case to a large dataset of healthy controls. Briefly, FDG‐PET images were first spatially normalized to a dementia‐specific FDG‐PET template (Della Rosa et al., 2014) and smoothed by means of an isotropic 3D Gaussian Kernel (full‐width‐half‐maximum: 8 mm). Then, the warped and smoothed images entered a voxel‐level statistical comparison with a large dataset of neurologically intact controls selected from the Nuclear Medicine San Raffaele Hospital and the EADC (European Alzheimer's Disease Consortium, http://www.eadc.info/) databases. Age was entered as nuisance variable in this analysis in order to exclude its effect. The comparison generated single Statistical Parametric Mapping (SPM) t‐maps of regional hypometabolism with level of significance set at p < .05 family‐wise error (FWE) correction for multiple comparisons. Each individual SPM t‐map was evaluated by neuroimaging experts.
Further, a voxel‐wise two‐independent‐sample t‐test was applied to identify brain hypometabolism patterns overall in bilingual and monolingual groups as compared to healthy controls. The analysis was run separately for the two groups. The analysis was corrected for age, education, and MMSE scores. The p‐value was set at p < .05 with FWE correction for multiple comparisons with a minimum cluster extent (Ke) of 100 voxels.
Subsequently, a direct comparison of contrast images in monolingual and bilingual individuals was performed to identify between‐group differences using whole‐brain voxel‐wise two‐independent‐sample t‐tests. Age, years of education, and MMSE score were included as nuisance variables. The significance level was set at p < .001 at voxel level, and p < .05 at cluster level, uncorrected for multiple comparisons with minimal Ke was equal to 100 voxels.
2.4. Regression analyses (bilingualism as continuous phenomenon)
We applied whole‐brain voxel‐level multiple regressions to test the extent to which the index of L2 use predicted brain glucose metabolism in the entire patient group (N = 98). Nuisance covariates were included into the statistical model: age, education, and MMSE scores. The significance level was set at p < .001 at voxel level, and p < .05 at cluster level, uncorrected for multiple comparisons with Ke ≥100 voxels.
2.5. FDG‐PET metabolic connectivity analysis in monolingual and bilingual individuals
We further carried out a brain metabolic connectivity analysis with the specific aim to investigate resting‐state metabolic networks in both monolingual and bilingual groups. The core assumption of this analysis is that brain regions whose glucose metabolism is correlated at rest are functionally associated (Horwitz, Duara, & Rapoport, 1984). In this study, we applied seed‐based interregional correlation analysis using a voxel‐wise SPM procedure as described in Lee et al. (2008), to investigate differences in metabolic connectivity of resting state brain networks in AD bilinguals and monolinguals. Namely, we considered the bilateral executive control network, the language network and the anterior DMN (aDMN; Lee et al., 2008). We hypothesized a different involvement of these networks in bilinguals and monolinguals.
First, seed regions were defined from a functional atlas of resting state networks (as defined by Shirer, Ryali, Rykhlevskaia, Menon, and Greicius (2012) [findlab.stanford.edu/functional_ROIs.html]). Namely, for the executive control network, three seeds bilaterally were chosen: (a) the middle frontal and superior frontal gyri, (b) the inferior parietal lobules and angular gyrus, and (c) the caudate nucleus; for the language network : (a) the left angular gyrus, (b) the left middle temporal cortex, and (c) the left inferior frontal gyrus; for the aDMN: the anterior cingulate cortex (ACC) and medial frontal cortex (Shirer et al., 2012). Then, intensity normalization to the global mean was applied on the warped and smoothed FDG‐PET images, and mean FDG uptake was extracted from the seeds, separately for the monolingual and bilingual individuals. The extracted mean seed counts were set as variables of interest in a multiple regression model, testing the extent to which they predicted brain voxel‐wise metabolic activity in the two groups (p < .001 at voxel level, and p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100). Age, years of education, and MMSE score were included as nuisance variables.
2.6. Interaction between metabolic connectivity and index of L2 use (bilingualism as continuous phenomenon)
Across all individuals with AD, we implemented a voxel‐wise General Linear Model to each resting‐state network (executive control network, language network, aDMN), testing whether the interaction between mean seed uptakes (S) and index of use for L2 (L) predicted metabolism in each voxel (Y) of the corresponding large‐scale network (i), according to the following equation:
This model allows to test whether metabolic connectivity between the seed and each voxel in the rest of the brain is modulated by index of use for L2. Age, education, and MMSE scores were included as nuisance variables. We tested whether the interaction between mean seed FDG uptake (S) and index of use for L2 (L) presented a positive slope (β3 > 0), specifically measuring increased connectivity, thus testing for compensatory effects of index use of L2. Results were considered significant at p < .001 at voxel level, and p < .05 at cluster level (uncorrected for multiple comparisons; Ke ≥100 voxels).
Further, and in order to test the specificity of our findings, we computed this same model for a series of “control” networks, both in the sensory and motor domains (namely, the primary visual and sensorimotor networks) and in the cognitive domain (namely, the salience and posterior DMN), again based on the FINDlab's atlas.
All statistical analyses were performed using the MATLAB (http://it.mathworks.com/products/matlab/) (Mathworks Inc., Sherborn, MA) based software SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/SPM12/).
3. RESULTS
3.1. Descriptive statistics
Descriptive statistics of demographic variables (means and SDs) and their comparison are reported in Table 1. There were no significant differences for any demographic and clinical variable (p > .05).
3.2. FDG‐PET SPM group patterns and between group comparisons (monolingual and bilingual individuals)
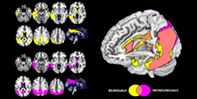
Analyses of brain hypometabolism revealed both similarities and differences between bilingual and monolingual groups with AD. Specifically, both groups showed extensive hypometabolism in temporo‐parietal associative cortices, the PCC (posterior cingulate cortex) and precuneus consistent with the typical pattern found in individuals with AD. Of note, bilinguals presented additional hypometabolism in the left inferior frontal gyrus, left superior middle and inferior temporal gyrus, insula and ACC (all results are reported at p < .05 FWE_corrected for multiple comparisons; Ke ≥100; Figure 1).
FIGURE 1.
Brain hypometabolism in monolingual (purple) and bilingual (yellow) patients with probable AD. Commonalities in brain hypometabolism of the two groups are represented in pink. All results are shown at p < .05 FWE and with Ke ≥100. Images are displayed in neurological convention (the left side of the brain at left in the figure), with results overlaid on a high‐resolution MRI standard template to illustrate commonalities and differences of cerebral hypometabolic patterns between the two groups
The direct comparison between groups confirmed the more severe left hemispheric hypometabolism in bilinguals encompassing the left superior temporal gyrus, insula, and Rolandic frontal operculum (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100). Monolinguals did not report more severe hypometabolism than bilinguals in any brain region (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100).
3.3. Regression: Index of L2 use (bilingualism as continuous phenomenon)
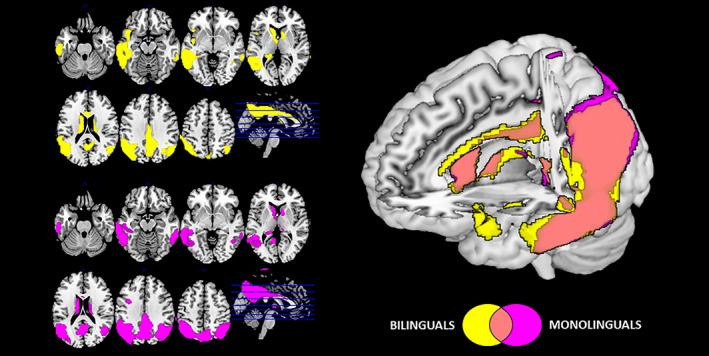
The index of L2 use significantly and negatively predicted brain metabolism in the left hemisphere, namely, in the inferior frontal gyrus, frontal and parietal operculum, insula, and supramarginal gyrus (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100—Figure 2). In other words, higher indexes of L2 use correlate with more pronounced patterns of hypometabolism in these regions.
FIGURE 2.
Negative correlation between index of L2 use and FDG‐PET glucose metabolism across all patients (p < .001 uncorrected at voxel level, p < .05 at cluster level; Ke ≥100; yellow). Result at p < .01 uncorrected at voxel level are also shown (red). Images are displayed in neurological convention (the left side of the brain at left in the figure), with results overlaid on a high‐resolution MRI standard template
3.4. FDG‐PET metabolic connectivity (monolingual and bilingual individuals)
3.4.1. Executive control network
Using the bilateral parietal seeds (i.e., superior lobules and angular gyrus), we found significant metabolic connectivity between these parietal and contiguous regions, such as the cuneus, precuneus, and the PCC, in bilinguals only. Similarly, the bilateral middle and superior frontal gyri seeds showed significant metabolic connectivity with orbitofrontal regions, in bilinguals only. Further, metabolic connectivity of the frontal seeds resulted also enhanced in bilinguals with the left angular gyrus and the left inferior parietal lobe. Using the bilateral caudate nucleus as seed, a different network in bilinguals as compared with monolinguals emerged, encompassing the anterior, middle, and posterior cingulate cortex, and with additional connections with the dorsolateral prefrontal cortex, orbitofrontal cortex and right inferior frontal gyrus (pars orbitalis) in (p < .01 at voxel level, FDR‐corrected for multiple comparisons, p < .05 at cluster level; Ke ≥100—Figure 3).
FIGURE 3.
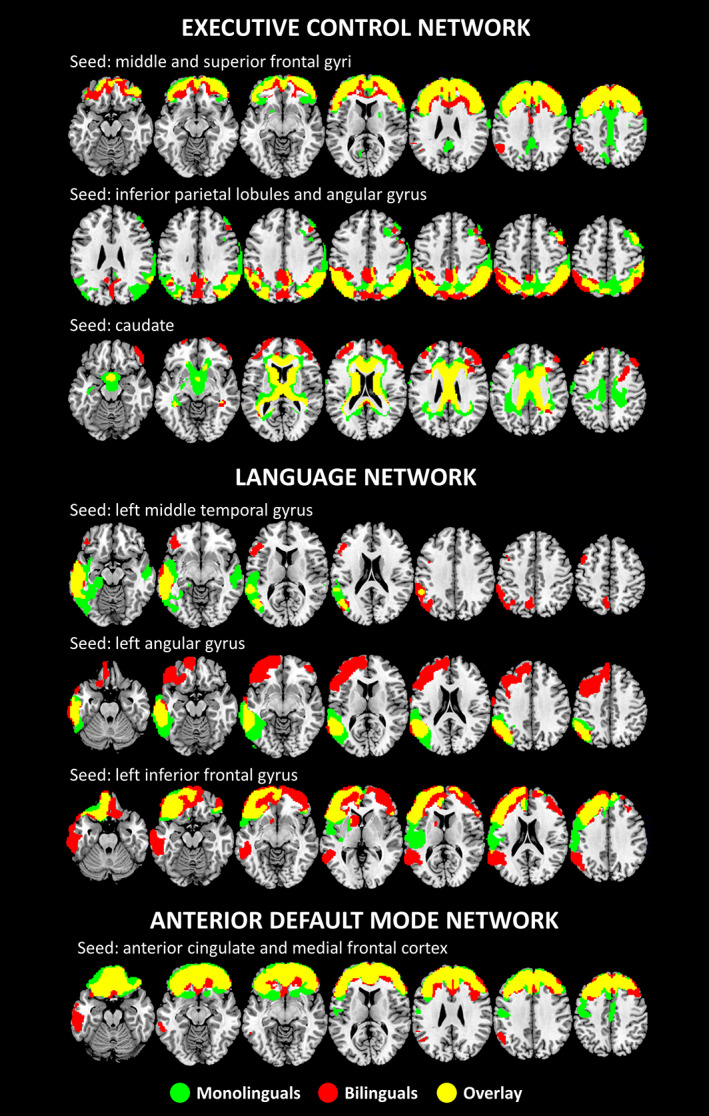
Results of the metabolic connectivity analysis in the executive control, the language and aDMNs (see text for details). All results are shown at p < .01 (FDR‐corrected for multiple comparisons) at voxel level, p < .05 at cluster level; Ke ≥100. Images are displayed in neurological convention (the left side of the brain is shown at left in the figure), with results overlaid on a high‐resolution MRI standard template
3.4.2. Language network
We found a more extended pattern of metabolic connectivity in bilinguals than monolinguals between the left angular gyrus (seed), the left dorsolateral prefrontal cortex, and the left orbitofrontal cortex. Similarly, when considering the left inferior frontal gyrus as seed, bilinguals showed significant metabolic connectivity between this region and the left angular gyrus, the left supramarginal gyrus, the left middle and superior temporal gyrus, the left inferior temporal gyrus, the left temporal pole, and the left caudate. The connectivity between the inferior frontal gyrus and the frontal lobe was enhanced as well, specifically in the dorsolateral prefrontal and the orbitofrontal cortex, bilaterally. Finally, the middle temporal gyrus, in bilinguals only, showed a strong connectivity with the left inferior frontal gyrus (pars triangularis, opercularis, and orbitalis), the left supramarginal gyrus, the left parietal lobe, and the left precuneus (p < .01 at voxel level, FDR‐corrected for multiple comparisons, p < .05 at cluster level; Ke ≥100—Figure 3).
3.4.3. Anterior default mode network
For the aDMN, significant metabolic connectivity was found between the frontal seed regions (i.e., ACC/medial frontal cortex) and dorsolateral prefrontal cortex in both monolingual and bilingual groups. In bilinguals, the seed regions were in addition significantly connected with the left inferior and middle temporal gyrus, the left temporal pole, the left supramarginal gyrus, and the left angular gyrus (p < .01 at voxel level, FDR‐corrected for multiple comparisons, p < .05 at cluster level, Ke ≥100—Figure 3).
3.5. Interactions between metabolic connectivity and index of L2 use (bilingualism as continuous phenomenon)
3.5.1. Executive control network
For the executive control network, the index of L2 use modulated connectivity between: (a) the frontal seed and the left inferior and middle temporal gyrus, and the left supramarginal gyrus; (b) the parietal seed with the left inferior temporal gyrus. The index of L2 use was not associated to the metabolic connectivity in the network with the caudate nuclei as seeds (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100—Figure 4).
FIGURE 4.
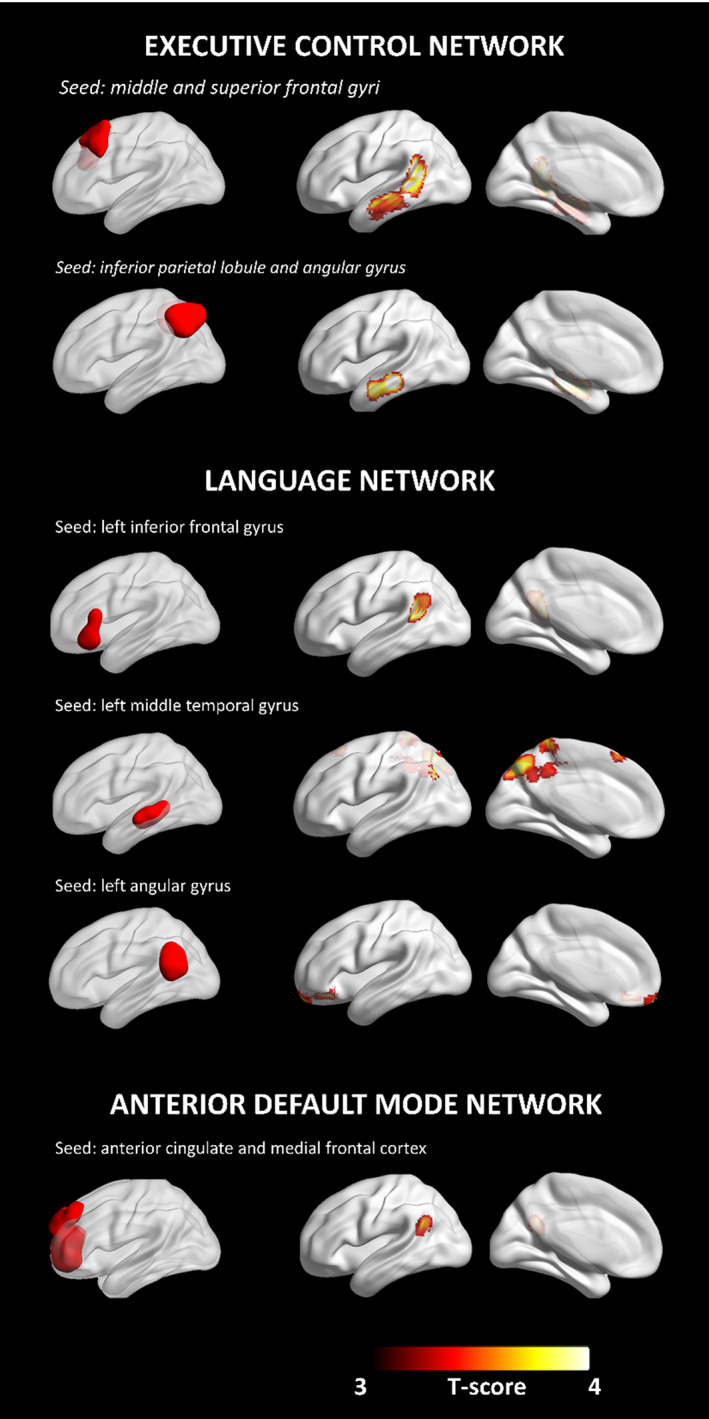
Modulation of metabolic connectivity by L2 use. Networks' seeds are shown on the left. Clusters whose connectivity with the network's seed is modulated by the index of L2 use are shown on the right. All results are shown at p < .001 at voxel level, p < .05 at cluster level (uncorrected for multiple comparisons; Ke ≥100). Images are displayed in neurological convention (the left side of the brain is shown at left in the figure), with results overlaid on a high‐resolution MRI standard template. BrainNet Viewer was used for rendering (Xia, Wang, & He, 2013)
3.5.2. Language network
For the Language Network, the index of L2 use modulated brain connectivity between the angular gyrus as seed and the left orbitofrontal cortex, the left inferior and middle frontal gyri. Considering the middle temporal gyrus as seed, the index of L2 use modulated metabolic connectivity between this region and multiple brain areas, namely the precuneus bilaterally, left cuneus, left middle and superior occipital gyri, right supplementary motor area, and paracentral lobule. Finally, the index of L2 use modulated metabolic connectivity between the left inferior frontal gyrus and the left superior and middle temporal gyri, and the angular gyrus (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100—Figure 4).
3.5.3. Anterior default mode network
For the aDMN, the index of L2 use modulated metabolic connectivity between the seeds (ACC/medial frontal cortex) and the left superior temporal and angular gyri (p < .001 at voxel level, p < .05 at cluster level, uncorrected for multiple comparisons; Ke ≥100—Figure 4).
Of note, we found no significant results at p < .001 voxel‐level, p < .05 cluster level, uncorrected for multiple comparisons, Ke = 100 voxels, in any of the other tested resting‐state networks (the primary visual, the sensorimotor, the salience and posterior DMNs).
4. DISCUSSION
In the present study, we investigated the neural mechanisms underlying the ability of bilingual senior citizens to better cope with pathology and symptoms of AD dementia, as compared to matched monolingual individuals with AD. To do so, we used different strategies, assessing the effects of lifelong bilingualism both as a dichotomous and continuous phenomenon, on brain metabolism and connectivity in individuals with AD. By using FDG‐PET to measure brain glucose metabolism, we showed that cerebral hypometabolism, of note in the left hemisphere, was significantly more severe and more extended in bilingual than monolingual individuals. Since our bilingual and monolingual individuals with AD show comparable levels of cognitive impairment, this result suggests a relevant cognitive reserve effect induced by speaking multiple languages upon the brain in bilinguals. Furthermore, we report increased connectivity in specific brain neurocognitive networks in bilingual as compared to monolingual individuals, with a predominant effect again in the left hemisphere. This suggests that the putative protective effect of bilingualism in AD is achieved through the modulation of spared and dysfunctional brain networks. Notably, we show that both the effect on brain hypometabolism (brain reserve) and brain metabolic connectivity (compensatory effect and neural reserve) are linearly associated with the degree of second language use, that is, the higher the second language use, the more severe the hypometabolism and the stronger the increase in metabolic connectivity. Altogether, our results can be understood in the context of two different neural mechanisms, that is, neural reserve and compensation, that have been called upon to explain how bilingualism protects the brain in aging and dementia (Perani & Abutalebi, 2015). These details will be discussed in the following sections.
Overall, as to our first finding, we report for both groups of individuals the typical pattern of brain hypometabolism associated to AD, that is, extensive hypometabolism in lateral parieto‐temporal regions, in the PCC and in the precuneus (Sala et al., 2020). Interestingly, despite reporting comparable scores on cognitive testing (Table 1) and being matched for the major demographic variables, bilinguals showed significantly more severe and extended hypometabolism over the left hemisphere in the superior temporal gyrus, insula, and Rolandic frontal operculum (Figure 1). Further, severity of hypometabolism linearly correlated with our index of L2 use, where more severe hypometabolism was observed in the individuals that most frequently used the second language (Figure 2). We interpret these results as suggesting a relevant brain reserve and neural compensation effects induced by speaking multiple languages upon the brain. We suggest a mechanistic explanation of such effect in AD, so that the lifelong usage of the second language, requiring “overuse” of specific brain functions and of the associated neurocognitive networks (Abutalebi & Green, 2016) induces plastic changes in the brain, and an increase of functional connectivity (cf. Kim et al., 2019). Lifelong bilingualism has also been reported associated with preservation of white matter (Abutalebi et al., 2014; Abutalebi et al., 2015a; Abutalebi et al., 2015b; Luk et al., 2011; Olsen et al., 2015) and gray matter density (Abutalebi et al., 2014; Abutalebi, Canini, et al., 2015; Bialystok, Abutalebi, Bak, Burke, & Kroll, 2016; Coggins et al., 2004; García‐Pentón et al., 2014; Li, Legault, & Litcofsky, 2014; Luk et al., 2011; Olsen et al., 2015; Pliatsikas et al., 2020). Altogether, these changes contribute to the build‐up of a brain reserve, conferring a higher resistance against the effects on aging and AD pathology on the brain, and of a cognitive reserve so that bilingual individuals are able to retain a similar degree of residual cognitive function, compared to monolingual individuals, in spite of presenting more severe and extended brain hypometabolism. Our results are also in line with the only two previous FDG‐PET studies, where similar differences in the severity of hypometabolism were observed in independent cohorts of bilingual individuals with AD (Kowoll et al., 2016; Perani et al., 2017).
This interpretation is also supported by our second finding, that is, that bilingual individuals present increased metabolic connectivity specifically in neurocognitive networks (Figure 3) that sub‐serve timely coordination between language systems, so as to speak each language without interference from the other (Abutalebi & Green, 2016; Green & Abutalebi, 2013). We specifically observed increased metabolic connectivity in cortical areas belonging to the language, executive and aDMNs, with an increase in connectivity proportional to the frequency of second language use (Figure 4). In details: as for the Language Network, we found linearly increased connectivity between left inferior frontal gyrus and angular gyrus, and between left middle temporal gyrus, precuneus, and supplementary motor area. As for the executive control networks, we found increased connectivity between fronto‐parietal regions and inferior/middle temporal gyri and supramarginal gyrus, bilaterally. As for the aDMN, we found increased connectivity between ACC and left superior temporal and angular gyri. The temporal brain regions listed above are known to be involved in language processing, and in particular in semantic processing (Gleichgerrcht, Fridriksson, & Bonilha, 2015). The fronto‐parietal brain regions listed above have notably been previously described as part of the “bilingual” executive control network (Abutalebi & Green, 2016), representing the neural correlate of cognitive processes, such as selection of target response, inhibition of words from the nontarget language, speech monitoring (Abutalebi & Green, 2007; Costa, Miozzo, & Caramazza, 1999; Kroll, Bobb, & Wodniecka, 2006), and language switching (Green & Abutalebi, 2013), that are crucial to the bilingual experience (Abutalebi & Green, 2016). Accordingly, previous studies in healthy and cognitively intact bilingual individuals, have shown a greater intrinsic functional connectivity in executive, default mode (Berken, Chai, Chen, Gracco, & Klein, 2016; Grady, Luk, Craik, & Bialystok, 2015), and language networks (de Frutos‐Lucas et al., 2019), as well as higher axonal density in white matter tracts, connecting anterior and posterior components in the executive control network (Pliatsikas, DeLuca, Moschopoulou, & Saddy, 2017). Here, we extend these findings, showing that the enhancement of brain functional connectivity that is observed in healthy bilingual individuals in strategic neurocognitive networks, is still preserved in individuals with AD dementia. Our findings are also in line with those of a previous study of ours (Perani et al., 2017), in which we observed a more extensive pattern of metabolic connectivity in language, executive, and DMNs, again in bilingual individuals with AD. Here, we consistently replicate these findings in an independent cohort of bilinguals, and further expand them by proving that the degree of enhancement of metabolic connectivity is dependent on the frequency of L2 use (Figure 4). Altogether, these findings suggest that the bilingualism induces its neuroprotective effect in a dose‐dependent manner, with second language use progressively strengthening functional connections between fronto‐parieto‐temporal regions involved in language processing (Gleichgerrcht et al., 2015), executive control, and switching (Cole et al., 2013; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013).
We note that, together with bilingualism, other factors can contribute to brain plasticity in aging and AD, including education level (Garibotto et al., 2008; Malpetti et al., 2017), occupation (Garibotto et al., 2008; Malpetti et al., 2017), physical exercise (Ahlskog, Geda, Graff‐Radford, & Petersen, 2011), obesity (Malpetti et al., 2018) and others (Stern et al., 2018). Effects of age (Kakimoto et al., 2016) upon brain metabolism have also been reported. The control over confounding variables is hence a crucial step to identify the specific effect of bilingualism as cognitive reserve proxy over AD‐related brain hypometabolism, independently of other relevant factors (Bak, 2016). Many studies, for examples, reported more severe hypometabolism in AD in subject with higher education, who are able to compensate longer for brain neurodegeneration compared to subjects with lower education (Garibotto et al., 2008; Stern, 2012). To this regard, it must be noted that our cohorts of AD monolingual and bilingual individuals were matched for years of education, and also did not differ in terms of severity of cognitive impairment or demographic variables. Note that we still included years of education, severity of cognitive impairment and age as nuisance covariates in all the analysis, so as to be sure that the reported effects on brain metabolism and connectivity are indeed specifically due to bilingualism, and not to other proxies of cognitive reserve. Altogether, our findings suggest that the effect of speaking different languages in providing a protection against AD pathology goes well beyond those of age or education.
Finally, we note that bilingualism contributes to mechanisms of neural reserve and compensation in a graded fashion and not in accordance to an all‐or‐nothing principle. This is consistent with the view that bilingualism does not represent a dichotomous phenomenon (Grundy, 2020; Kroll & Bialystok, 2013), but can be better understood as a continuous entity. The presence of a proportionally increasing effect of bilingualism is of utmost importance for practical applications. As the beneficial effect of bilingualism in contrasting the consequences of AD is dependent on the degree of second language use, social programs and interventions should be aimed at preserving a high level of second or multiple language use in the elderlies, even after retirement, which usually coincides with a drop in second language use in favor of the native language (Abutalebi et al., 2014).
This study has some limitations. While the cohort of bilingual individuals included in this study was completely independent from the one published in a previous study (Perani et al., 2017), there was a partial overlap between the current and the previous study for what concerns the sample of monolinguals. We opted for inclusion of some of the previous subjects (monolingual groups only) to avoid increasing chances of false positive/false negative findings due to reliance on small samples of individuals, favoring robustness of the results over independence of the cohorts.
In conclusion, our study provides further support to the concept that bilingualism acts as a powerful proxy of neural and cognitive reserve, enhancing language control circuits in the brain, and conferring a protection against AD neurodegeneration, even in advanced disease stages. We conclude that, as prevention of dementia represents a top priority in the aging population, health policies aimed at fostering bilingual or multilingual education should be strongly promoted.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
ETHICS APPROVAL AND PATIENT CONSENT STATEMENT
Informed consent was obtained from each subject or relatives; the protocols conformed to the Ethical standards of the declaration of Helsinki for protection of human subjects and were approved by the San Raffaele Scientific Institute, University of Geneva and Azienda Sanitaria dell'Alto Adige Bolzano Ethical Committees.
Sala, A. , Malpetti, M. , Farsad, M. , Lubian, F. , Magnani, G. , Frasca Polara, G. , Epiney, J.‐B. , Abutalebi, J. , Assal, F. , Garibotto, V. , & Perani, D. (2022). Lifelong bilingualism and mechanisms of neuroprotection in Alzheimer dementia. Human Brain Mapping, 43(2), 581–592. 10.1002/hbm.25605
Funding information Italian Ministry of Health, Grant/Award Number: Ricerca Finalizzata Progetto Reti Nazionale AD NET‐2011‐02346784; Swiss National Science Foundation, Grant/Award Numbers: 320030_169876, 320030_185028; Velux foundation, Grant/Award Number: 1123
DATA AVAILABILITY STATEMENT
Data availability statement: Data used in preparation of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abutalebi, J. , Canini, M. , Della Rosa, P. A. , Green, D. W. , & Weekes, B. S. (2015). The neuroprotective effects of bilingualism upon the inferior parietal lobule: A Structural Neuroimaging Study in Aging Chinese Bilinguals. Journal of Neurolinguistics, 33, 3–13. [Google Scholar]
- Abutalebi, J. , Canini, M. , Della Rosa, P. A. , Sheung, L. P. , Green, D. W. , & Weekes, B. S. (2014). Bilingualism protects anterior temporal lobe integrity in aging. Neurobiology of Aging, 35, 2126–2133. [DOI] [PubMed] [Google Scholar]
- Abutalebi, J. , & Green, D. (2007). Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics, 20, 242–275. [Google Scholar]
- Abutalebi, J. , & Green, D. W. (2016). Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Bilingualism, 19, 689–698. [Google Scholar]
- Abutalebi, J. , Guidi, L. , Borsa, V. , Canini, M. , Della Rosa, P. A. , Parris, B. A. , & Weekes, B. S. (2015). Bilingualism provides a neural reserve for aging populations. Neuropsychologia, 69, 201–210. [DOI] [PubMed] [Google Scholar]
- Ahlskog, J. E. , Geda, Y. E. , Graff‐Radford, N. R. , & Petersen, R. C. (2011). Physical exercise as a preventive or disease‐modifying treatment of dementia and brain aging. In Mayo Clinic proceedings (Vol. 86, pp. 876–884). Rochester, MN: Mayo Clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alladi, S. , Bak, T. H. , Duggirala, V. , Surampudi, B. , Shailaja, M. , Shukla, A. K. , … Kaul, S. (2013). Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology, 81, 1938–1944. [DOI] [PubMed] [Google Scholar]
- Alladi, S. , Bak, T. H. , Mekala, S. , Rajan, A. , Chaudhuri, J. R. , Mioshi, E. , … Kaul, S. (2016). Impact of bilingualism on cognitive outcome after stroke. Stroke, 47, 258–261. [DOI] [PubMed] [Google Scholar]
- Anderson, J. A. E. , Hawrylewicz, K. , & Grundy, J. G. (2020). Does bilingualism protect against dementia? A meta‐analysis. Psychonomic Bulletin & Review, 27, 952–965. [DOI] [PubMed] [Google Scholar]
- Attwell, D. , & Iadecola, C. (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25, 621–625. [DOI] [PubMed] [Google Scholar]
- Bak, T. H. (2016). Cooking pasta in La Paz: Bilingualism, bias and the replication crisis. Linguistic Approaches to Bilingualism, 6, 699–717. [Google Scholar]
- Barulli, D. , & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17, 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, M. , Snyder, H. M. , Carrillo, M. C. , Fazio, S. , Kim, H. , & Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population‐based perspective. Alzheimer's & Dementia, 11, 718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Berken, J. A. , Chai, X. , Chen, J.‐K. , Gracco, V. L. , & Klein, D. (2016). Effects of early and late bilingualism on resting‐state functional connectivity. The Journal of Neuroscience, 36, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok, E. (2021). Bilingualism: Pathway to cognitive reserve. Trends in Cognitive Sciences, 25, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok, E. , Abutalebi, J. , Bak, T. H. , Burke, D. M. , & Kroll, J. F. (2016). Aging in two languages: Implications for public health. Ageing Research Reviews, 27, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok, E. , Craik, F. I. M. , Binns, M. A. , Ossher, L. , & Freedman, M. (2014). Effects of bilingualism on the age of onset and progression of MCI and AD: Evidence from executive function tests. Neuropsychology, 28, 290–304. [DOI] [PubMed] [Google Scholar]
- Bialystok, E. , Craik, F. I. M. , & Freedman, M. (2007). Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia, 45, 459–464. [DOI] [PubMed] [Google Scholar]
- Bialystok, E. , Craik, F. I. M. , & Luk, G. (2012). Bilingualism: Consequences for mind and brain. Trends in Cognitive Sciences, 16, 240–250. 10.1016/j.tics.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. , Albert, M. , Belleville, S. , Craik, F. I. M. , Duarte, A. , Grady, C. L. , … Rajah, M. N. (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews. Neuroscience, 19, 701–710. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria, M. , Costa, A. , Green, D. W. , & Abutalebi, J. (2018). Neural basis of bilingual language control. Annals of the New York Academy of Sciences, 1426, 221–235. [DOI] [PubMed] [Google Scholar]
- Chertkow, H. , Whitehead, V. , Phillips, N. , Wolfson, C. , Atherton, J. , & Bergman, H. (2010). Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: Evidence from a bilingual community. Alzheimer Disease and Associated Disorders, 24, 118–125. [DOI] [PubMed] [Google Scholar]
- Coggins, P. E. , Kennedy, T. J. , & Armstrong, T. A. (2004). Bilingual corpus callosum variability. Brain and Language, 89, 69–75. [DOI] [PubMed] [Google Scholar]
- Cole, M. W. , Reynolds, J. R. , Power, J. D. , Repovs, G. , Anticevic, A. , & Braver, T. S. (2013). Multi‐task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, A. , Miozzo, M. , & Caramazza, A. (1999). Lexical selection in bilinguals: Do words in the bilingual's two lexicons compete for selection? Journal of Memory and Language, 41, 365–397. [Google Scholar]
- Craik, F. I. M. , & Bialystok, E. (2010). Bilingualism and aging: Costs and benefits. In L. Bäckman & L. Nyberg (Eds.), Memory, aging and the brain: A Festschrift in honour of Lars‐Göran Nilsson (pp. 115–131). London, England: Psychology Press. [Google Scholar]
- de Frutos‐Lucas, J. , López‐Sanz, D. , Cuesta, P. , Bruña, R. , de la Fuente, S. , Serrano, N. , … Marcos, A. (2019). Enhancement of posterior brain functional networks in bilingual older adults. Bilingualism: Language and Cognition, 23, 387–400. [Google Scholar]
- Della Rosa, P. A. , Cerami, C. , Gallivanone, F. , Prestia, A. , Caroli, A. , Castiglioni, I. , … Perani, D. (2014). A standardized [(18)F]‐FDG‐PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics, 12, 575–593. [DOI] [PubMed] [Google Scholar]
- García‐Pentón, L. , Pérez Fernández, A. , Iturria‐Medina, Y. , Gillon‐Dowens, M. , & Carreiras, M. (2014). Anatomical connectivity changes in the bilingual brain. NeuroImage, 84, 495–504. [DOI] [PubMed] [Google Scholar]
- Garibotto, V. , Borroni, B. , Kalbe, E. , Herholz, K. , Salmon, E. , Holtoff, V. , … Perani, D. (2008). Education and occupation as proxies for reserve in aMCI converters and AD: FDG‐PET evidence. Neurology, 71, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht, E. , Fridriksson, J. , & Bonilha, L. (2015). Neuroanatomical foundations of naming impairments across different neurologic conditions. Neurology, 85, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady, C. L. , Luk, G. , Craik, F. I. M. , & Bialystok, E. (2015). Brain network activity in monolingual and bilingual older adults. Neuropsychologia, 66, 170–181. 10.1016/j.neuropsychologia.2014.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. W. , & Abutalebi, J. (2013). Language control in bilinguals: The adaptive control hypothesis. Journal of Cognitive Psychology (Hove, England), 25, 515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy, J. G. (2020). The effects of bilingualism on executive functions: An updated quantitative analysis. Journal of Cultural Cognitive Science, 4, 177–199. [Google Scholar]
- Hervais‐Adelman, A. G. , Moser‐Mercer, B. , & Golestani, N. (2011). Executive control of language in the bilingual brain: Integrating the evidence from neuroimaging to neuropsychology. Frontiers in Psychology, 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, B. , Duara, R. , & Rapoport, S. I. (1984). Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. Journal of Cerebral Blood Flow and Metabolism, 4, 484–499. [DOI] [PubMed] [Google Scholar]
- Kakimoto, A. , Ito, S. , Okada, H. , Nishizawa, S. , Minoshima, S. , & Ouchi, Y. (2016). Age‐related sex‐specific changes in brain metabolism and morphology. Journal of Nuclear Medicine, 57, 221–225. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Jeon, S. G. , Nam, Y. , Soo, K. H. , Yoo, D. H. , & Moon, M. (2019). Bilingualism for dementia: Neurological mechanisms associated with functional and structural changes in the brain. Frontiers in Neuroscience, 13, 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowoll, M. E. , Degen, C. , Gorenc, L. , Küntzelmann, A. , Fellhauer, I. , Giesel, F. , … Schröder, J. (2016). Bilingualism as a contributor to cognitive reserve? Evidence from cerebral glucose metabolism in mild cognitive impairment and Alzheimer's disease. Frontiers in Psychiatry, 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, J. F. , & Bialystok, E. (2013). Understanding the consequences of bilingualism for language processing and cognition. Journal of Cognitive Psychology, 25, 497–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, J. F. , Bobb, S. C. , & Wodniecka, Z. (2006). Language selectivity is the exception, not the rule: Arguments against a fixed locus of language selection in bilingual speech. Bilingualism, 9, 119–135. [Google Scholar]
- Lee, D. S. , Kang, H. , Kim, H. , Park, H. , Oh, J. S. , Lee, J. S. , & Lee, M. C. (2008). Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. European Journal of Nuclear Medicine and Molecular Imaging, 35, 1681–1691. [DOI] [PubMed] [Google Scholar]
- Li, P. , Legault, J. , & Litcofsky, K. A. (2014). Neuroplasticity as a function of second language learning: Anatomical changes in the human brain. Cortex, 58, 301–324. [DOI] [PubMed] [Google Scholar]
- Luk, G. , Bialystok, E. , Craik, F. I. M. , & Grady, C. L. (2011). Lifelong bilingualism maintains white matter integrity in older adults. The Journal of Neuroscience, 31, 16808–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr, Kawas, C. H., … Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti, P. J. , Pellerin, L. , Rothman, D. L. , & Shulman, R. G. (1999). Energy on demand. Science (80‐ ), 283, 496–497. [DOI] [PubMed] [Google Scholar]
- Malpetti, M. , Ballarini, T. , Presotto, L. , Garibotto, V. , Tettamanti, M. , & Perani, D. (2017). Gender differences in healthy aging and Alzheimer's dementia: A 18F‐FDG‐PET study of brain and cognitive reserve. Human Brain Mapping, 38, 4212–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpetti, M. , Sala, A. , Vanoli, E. G. , Gianolli, L. , Luzi, L. , & Perani, D. (2018). Unfavourable gender effect of high body mass index on brain metabolism and connectivity. Scientific Reports, 8, 12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud, F. , & Gauthier, S. (2010). Update on the pharmacological treatment of Alzheimers disease. Current Neuropharmacology, 8, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbelli, S. , Perneczky, R. , Drzezga, A. , Frisoni, G. B. , Caroli, A. , van Berckel, B. N. M. , … Nobili, F. (2013). Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: A European Alzheimer disease consortium project. Journal of Nuclear Medicine, 54, 894–902. [DOI] [PubMed] [Google Scholar]
- Olsen, R. K. , Pangelinan, M. M. , Bogulski, C. , Chakravarty, M. M. , Luk, G. , Grady, C. L. , & Bialystok, E. (2015). The effect of lifelong bilingualism on regional grey and white matter volume. Brain Research, 1612, 128–139. [DOI] [PubMed] [Google Scholar]
- Perani, D. (2014). FDG‐PET and amyloid‐PET imaging: The diverging paths. Current Opinion in Neurology, 27, 405–413. [DOI] [PubMed] [Google Scholar]
- Perani, D. , & Abutalebi, J. (2015). Bilingualism, dementia, cognitive and neural reserve. Current Opinion in Neurology, 28, 618–625. [DOI] [PubMed] [Google Scholar]
- Perani, D. , Della Rosa, P. A. , Cerami, C. , Gallivanone, F. , Fallanca, F. , Vanoli, E. G. , … Gianolli, L. (2014). Validation of an optimized SPM procedure for FDG‐PET in dementia diagnosis in a clinical setting. NeuroImage: Clinical, 6, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani, D. , Farsad, M. , Ballarini, T. , Lubian, F. , Malpetti, M. , Fracchetti, A. , … Abutalebi, J. (2017). The impact of bilingualism on brain reserve and metabolic connectivity in Alzheimer's dementia. Proceedings of the National Academy of Sciences, 114, 1690–1695. 10.1073/pnas.1610909114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky, R. , Drzezga, A. , Diehl‐Schmid, J. , Schmid, G. , Wohlschläger, A. , Kars, S. , … Kurz, A. (2006). Schooling mediates brain reserve in Alzheimer's disease: Findings of fluoro‐deoxy‐glucose‐positron emission tomography. Journal of Neurology, Neurosurgery, and Psychiatry, 77, 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perquin, M. , Vaillant, M. , Schuller, A. M. , Pastore, J. , Dartigues, J. F. , Lair, M. L. , & Diederich, N. (2013). Lifelong exposure to multilingualism: New evidence to support cognitive reserve hypothesis. PLoS One, 8, e62030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas, C. , DeLuca, V. , Moschopoulou, E. , & Saddy, J. D. (2017). Immersive bilingualism reshapes the core of the brain. Brain Structure & Function, 222, 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliatsikas, C. , Meteyard, L. , Veríssimo, J. , DeLuca, V. , Shattuck, K. , & Ullman, M. T. (2020). The effect of bilingualism on brain development from early childhood to young adulthood. Brain Structure & Function, 225, 2131–2152. 10.1007/s00429-020-02115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala, A. , Caprioglio, C. , Santangelo, R. , Vanoli, G. E. , Iannaccone, S. , Magnani, G. , & Perani, D. (2020). Brain metabolic signatures across the Alzheimer's disease spectrum. European Journal of Nuclear Medicine and Molecular Imaging, 47, 256–269. [DOI] [PubMed] [Google Scholar]
- Sala, A., & Perani, D. (2019). Brain molecular connectivity in neurodegenerative diseases: Recent advances and new perspectives using positron emission tomography. Frontiers in Neuroscience, 13, 617. 10.3389/fnins.2019.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, T. A. , Ware, J. , Fischer, C. E. , Craik, F. I. M. , & Bialystok, E. (2012). Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer's disease. Cortex, 48, 991–996. [DOI] [PubMed] [Google Scholar]
- Shirer, W. R. , Ryali, S. , Rykhlevskaia, E. , Menon, V. , & Greicius, M. D. (2012). Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cerebral Cortex, 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov, D. S. , Stasenko, A. , Salmon, D. P. , Galasko, D. , Brewer, J. B. , & Gollan, T. H. (2019). Distinct structural correlates of the dominant and nondominant languages in bilinguals with Alzheimer's disease (AD). Neuropsychologia, 132, 107131. 10.1016/j.neuropsychologia.2019.107131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. , Sepulcre, J. , Turner, G. R. , Stevens, W. D. , & Schacter, D. L. (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience, 25, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology, 11, 1006–1012. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. , Arenaza‐Urquijo, E. M. , Bartrés‐Faz, D. , Belleville, S. , Cantilon, M. , Chetelat, G. , … Vuoksimaa, E. (2018). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's & Dementia, 16, 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valian, V. (2015). Bilingualism and cognition: A focus on mechanisms. Bilingualism, 18, 47–50. [Google Scholar]
- Varrone, A. , Asenbaum, S. , Vander Borght, T. , Booij, J. , Nobili, F. , Nagren, K. , … Van Laere, K. (2009). EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. European Journal of Nuclear Medicine and Molecular Imaging, 36, 2103–2110. [DOI] [PubMed] [Google Scholar]
- Voits, T. , Pliatsikas, C. , Robson, H. , & Rothman, J. (2020). Beyond Alzheimer's disease: Can bilingualism be a more generalized protective factor in neurodegeneration? Neuropsychologia, 147, 107593. [DOI] [PubMed] [Google Scholar]
- Winblad, B. , Amouyel, P. , Andrieu, S. , Ballard, C. , Brayne, C. , Brodaty, H. , … Zetterberg, H. (2016). Defeating Alzheimer's disease and other dementias: A priority for European science and society. Lancet Neurology, 15, 455–532. [DOI] [PubMed] [Google Scholar]
- Woumans, E. , Santens, P. , Sieben, A. , Versijpt, J. , Stevens, M. , & Duyck, W. (2015). Bilingualism delays clinical manifestation of Alzheimer's disease. Bilingualism: Language and Cognition, 18, 568–574. [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushev, I., Drzezga, A., & Habeck, C. (2017). Metabolic connectivity: methods and applications. Current Opinion in Neurology, 30(6), 677–685. [DOI] [PubMed] [Google Scholar]
- Ziegler, G. , Dahnke, R. , & Gaser, C. (2012). Models of the aging brain structure and individual decline. Frontiers in Neuroinformatics, 6, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability statement: Data used in preparation of this study are available from the corresponding author upon reasonable request.


