Abstract
West Nile virus was recovered from the brain of a red-tailed hawk that died in Westchester County, N.Y., in February 2000. Multiple foci of glial cells, lymphocytes, and a few pyknotic nuclei were observed in the brain. Three to 4 days after inoculation of Vero cells with brain homogenates, cytopathic changes were detected. The presence of West Nile virus antigen in fixed cells or cell lysates was revealed by fluorescent antibody testing or enzyme-linked immunosorbent assay, respectively. Furthermore, Reverse transcriptase-PCR with primers specific for the NS3 gene of West Nile virus resulted in an amplicon of the expected size (470 bp). Electron microscopy of thin sections of infected Vero cells revealed the presence of viral particles approximately 40 nm in diameter, within cytoplasmic vesicles. The demonstration of infection with the West Nile virus in the dead of the winter, long after mosquitoes ceased to be active, is significant in that it testifies to the survival of the virus in the region beyond mosquito season and suggests another route of transmission: in this case, prey to predator.
West Nile fever emerged in the northeastern United States last summer, and thousands of birds, 11 horses, and 7 humans died (1–3). During the peak of the outbreak we studied over 300 crows and other birds. West Nile virus (WNV) was isolated and positively identified from the brain of the first crow necropsied in Connecticut (1) and from many others thereafter. The virus was closely related to two Romanian (1) and one Israeli isolate (4). In Connecticut the virus was recovered from Culex pipiens and Aedes vexans mosquitoes (1). As winter set in, the number of avian cases decreased considerably. Nevertheless, we continued to accession and study birds that were found dead during the winter months.
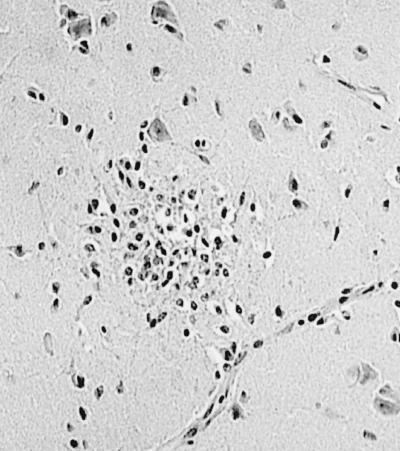
Among these birds was a red-tailed hawk that died on 6 February 2000 in Westchester County, N.Y. This bird was not emaciated and lacked the calvarial hemorrhage we had learned to associate with West Nile fever in crows. Brain and other tissues were frozen and stored for later virological examination or fixed in 10% neutral buffered formalin and routinely processed for histological examination. The brain contained numerous foci of encephalitis, characterized by aggregates of 10 to 20 cells, mostly glial cells and some lymphocytes; at the centers of these foci were 1 or 2 pyknotic nuclei, and faint vacuolar disruption of the neuropil was observed (Fig. 1).
FIG. 1.
Focus of glial cells, lymphocytes, and a few pyknotic nuclei in the cerebrum (histological examination). Magnification, ×350.
For virus isolation, Vero cells inoculated with brain homogenates were cultured in Dulbecco's minimal essential medium containing 10% fetal bovine serum and antibiotics at 37°C in a 5% CO2 atmosphere. After 3 days a visible diffuse cytopathic effect occurred. The cultures were harvested and centrifuged at 600 × g for 10 min. The cell pellets were lysed with phosphate-buffered saline (PBS), pH 7.2, containing 0.01% sodium dodecyl sulfate and 1% Triton X-100. Twofold dilutions of the lysates made in carbonate-bicarbonate buffer, pH 9.6, were incubated overnight at 4°C in 96-well enzyme-linked immunosorbent assay plates. The wells were coated with PBS containing 5% dry nonfat milk to block nonspecific binding. After incubation at room temperature with ascitic fluid containing antibodies specific for WNV (American Type Culture Collection, Rockville, Md.) and washes with PBS containing 0.05% Tween 20, an anti-mouse horseradish peroxidase conjugate was applied for 1 h, and the plates were then washed again. 2,2′-Azinobis(3-ethylbenzthiazolinesulfonic acid (ABTS) microwell peroxidase substrate (Kirkegard & Perry Laboratories, Gaithersburg, Md.) was added, and the plates were read in a microplate reader. Specific and dose-response reactions were detected in infected cell wells. Those treated with the control ascitic fluid and uninfected Vero cell controls were negative.
An indirect fluorescent antibody test was done on infected Vero cells (grown in chamber slides) which had been fixed 3 days postinoculation with 2% paraformaldehyde containing 0.1% Triton X-100 in PBS. The fixed cells were treated with PBS containing 2% bovine serum albumin to block nonspecific binding, ascitic fluid specific for WNV was applied as primary antibody for 1 h at room temperature, the cells were washed, and anti-mouse immunoglobulin G fluorescein isothiocyanate conjugate was applied for 1 h. After another washing, the slides were mounted and examined. Positive fluorescence was observed in infected cells treated with anti-WNV antibodies but not in those treated with control ascitic fluid or in uninfected cells. Reverse transcriptase-PCR analysis of infected Vero cell culture fluids using a primer set for the NS3 gene (3) revealed a band of the expected size (470 bp). Together these results provided compelling evidence that the virus isolated from the red-tailed hawk was indeed WNV.
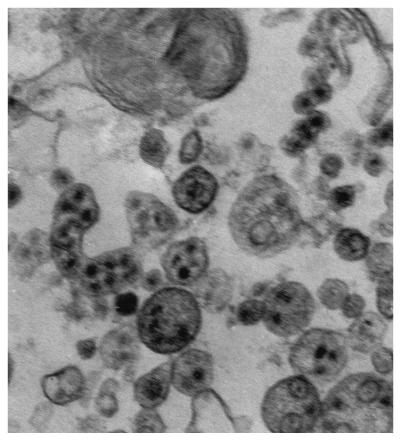
A second isolation of virus from brain tissue, followed by ELISA, conducted at the Connecticut Agricultural Experiment Station, confirmed these findings. Additionally, for electron microscopy, infected Vero cell cultures were fixed for 2 h at 4°C in 4% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). The cells were washed in buffer, removed with a scraper, postfixed in 1% osmium tetroxide for 1 h, and centrifuged. The cell pellet was dehydrated in ethyl alcohol, cleared in propylene oxide, and embedded in Embed-812. Thin sections were cut on a Reichert Ultracut ultramicrotome, mounted on copper grids, stained with uranyl acetate and lead citrate, and examined using a Zeiss EM 10 microscope. Virions approximately 40 nm in size and morphologically consistent with WNV were demonstrated within cytoplasmic vesicles (Fig. 2).
FIG. 2.
Hawk isolate of WNV within membrane-bound vesicles and free in the cytoplasm of Vero cells. The virions are 40 to 45 nm in diameter, spherical, and enveloped (image obtained by electron micrography). Magnification, ×64,000.
There are several potential explanations for our findings. These include the following: (i) that WNV was transmitted by an infected arthropod vector during midwinter, (ii) that the hawk acquired the virus from a vector earlier and the virus remained latent until it caused the death of this animal, or (iii) that the hawk acquired the infection by killing and eating an infected reservoir host. Given the acute course of West Nile fever, the acute nature of the brain lesions, and the time of the year when the hawk died, we favor the last explanation. Oral infection with WNV has previously been reported in mice: adult females that ate infected sucklings died, as did others following oral inoculation with WNV suspensions (5). Our observations and hypothesis about transmission in the hawk invite experimental testing and raise important questions about the nature and distribution of reservoir hosts.
Acknowledgments
We thank Joseph French and Robert Leavitt for bringing the bird to our attention and Giovanni Rompato, Kimberly Holmes, Bonnie Hamid, and Jodie Correia for excellent technical support.
REFERENCES
- 1.Anderson J F, Andreadis T G, Vossbrinck C R, Tirrel S, Wakem E M, French R A, Garmendia A E, Van Kruiningen H J. Isolation of West Nile virus from mosquitoes, crows and a Cooper's hawk in Connecticut. Science. 1999;286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 2.Asnis D, Conetta R, Waldman G, Texeira A, McNamara T, et al. Outbreak of West Nile-like viral encephalitis New York, 1999. Morbid Mortal Weekly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- 3.Briese T, Jia X-Y, Huang C, Grady L I, Lipkin W I. Identification of a Kunjin/West Nile like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- 4.Lancioti R S, Roehrig J T, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe K E, Crabtree M B, Scherret J H, Hall R A, MacKenzie J S, Cropp C B, Panigraphy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage H M, Stone W, McNamara T, Gubler D J. Origin of West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 5.Oderola H A, Oduye O O. West Nile virus infection of adult mice by oral route. Arch Virol. 1977;54:251–253. doi: 10.1007/BF01314791. [DOI] [PubMed] [Google Scholar]