Abstract
Introduction
Immunotherapy efficacy data on appendiceal cancer (AC) from clinical trials does not exist, due to AC incidence of 0.97 per 100,000. The goal of this study was to preclinically explore the application of immunotherapy in treating AC in a personalized organoid model.
Methods
Patient tumor organoids (PTO) were fabricated using unsorted tumor cells with and without enrichment with patient matched immune components derived from peripheral blood leukocytes, spleen, or lymph nodes (iPTOs). Organoids were cultured for 7 days, followed by treatment with immunotherapy (Pembrolizumab, Ipilimumab, Nivolumab), and assessed for treatment efficacy.
Results
Between September 2019 and May 2021, 26 patients were enrolled in the study. Successful testing conducted in 19/26 (73.1%) patients, with 13/19 (68.4%) and 6/19 (31.6%) patients, having low grade (LGA) and high grade appendiceal (HGA) primaries, respectively. Immunotherapy response, with increased expression of Granzyme B and Caspase 3 and decreased expression of CK20 and ATP activity, was exhibited in 4/19 (21.1%) Pembrolizumab treated and 2/19 (10.5%) Nivolumab treated iPTOs. Post immunotherapy cellular viability, in responding HGA organoids to Pembrolizumab, decreased to less than 15% (p < 0.05). LGA iPTO treatment responses were observed in Pembrolizumab and Nivolumab, with an 8% - 47.4% (p < 0.05) viability compared to controls. Ipilimumab showed no efficacy in the examined cohort.
Conclusions
Immunotherapy shows measurable efficacy in AC organoids. Information derived from immunocompetent organoids may be applied in selecting patients for clinical trial enrollment in rare diseases where preclinical models of disease are lacking.
Keywords: organoids, appendiceal cancer, immunotherapy
Introduction
Appendiceal cancer (AC) is a rare tumor with an estimated incidence of 0.97 per 100,000 people in the United States1. Peritoneal dissemination in AC, increases treatment complexity and makes control of disease inherently more challenging. Surgical management of diffuse peritoneal involvement relies on cytoreductive surgery (CRS) with heated intraperitoneal chemotherapy (HIPEC)2.
The utility of systemic chemotherapy for low grade appendiceal neoplasms has been debated and there are few studies examining this outcome3. Patient-derived tumor organoids (PTOs) have been demonstrated as reliable ex-vivo models to study a variety of cancers such as appendiceal cancer, peritoneal mesothelioma, and colorectal cancer with peritoneal metastases4–7. Application of PTOs in metastatic colorectal and gastroesophageal cancer enrolled in phase I/II clinical trials, recapitulated patient response to chemotherapy with an 88% positive predictive value and 100% negative predictive value8.
Immunotherapy is a rapidly growing area of research in cancer drug development with promising results for a variety of cancers including colorectal, esophageal, and melanoma9–11. Since 2017, checkpoint inhibitors (CPIs), have gained widened FDA approval for treatment of solid tumors in patients with high tumor mutational burden (TMB-H) and patients whose tumors demonstrate mismatch repair deficiency (dMMR), or phenotypic evidence of microsatellite instability (MSI-H)12–14. Despite broader FDA approval, the rarity of appendiceal cancer makes accrual in immunotherapy clinical trials exceedingly difficult. Immunotherapy efficacy data in AC is currently limited to a single case report15. In addition, preclinical platforms such as patient-derived xenograft (PDX) models and cell lines, either do not exist for the majority of rare diseases or are associated with a timeframe of deployment that is not aligned with the clinical needs of the patient.
Herein, we utilized PTOs as a platform to study the efficacy of immunotherapy in appendiceal cancer organoids from patients presenting with peritoneal dissemination. We hypothesized, that AC PTOs can be reproducibly applied to generate preclinical immunotherapy efficacy data, with the potential to broaden drug indications, while defining a focused personalized approach in clinical trial design in an orphan primary.
Methods
Tissue and whole blood specimens were obtained from 26 patients with appendiceal cancer with peritoneal dissemination who underwent CRS/HIPEC procedures between September 2019 and May 2021. Specimens were obtained in accordance to Wake Forest Baptist Medical Center guidelines and under an institution approved IRB protocol. Specimens were placed in Roswell Park Memorial Institute (RPMI) media and transferred to the Wake Forest Organoid Research Center (WFORCE) for processing within a 2 hour targeted framework from surgical resection.
Tumor Procurement and Processing
Once specimens were received in the laboratory, tumors were washed in phosphate-buffered saline with 100 U/mL penicillin-streptomycin, 5 μg/mL gentamicin, and 5 μg/mL amphotericin B for two 5-minute cycles. A portion of each specimen was saved for whole tissue histology. The remaining specimen portions were minced finely and placed in a 15 mL conical in a 3 mL solution of Dulbecco’s Modified Eagle’s Medium (DMEM) with 100,000 cytidine deaminase (CDA) units per mL collagenase HA (001–1050; VitaCyte, Indianapolis, IN), 22,000 narcissus pseudonarcissus agglutinin (NPA) units per mL protease (003–1000; VitaCyte, Indianapolis, IN), and 50 mM n-acetyl L-cysteine (A9165; Sigma-Aldrich, St. Louis, MO) per gram of tissue for up to 120 minutes under agitation at 37°C. Upon complete tissue dissolution, enzymatic digestion was terminated with 5 mL cold DMEM-10. The resultant tumor solution was filtered through a 100-micron pore-size vacuum filtration kit (SCNY00100; MilliporeSigma, Burlington, MA) and centrifuged to isolate a cell pellet. Supernatant was removed and the cell pellet resuspended with Red Blood Cell Lysis Buffer (Abcam, Cambridge, UK) according to company protocol. Lysis buffer was discarded, and the cell pellet resuspended and counted using a NucleoCounter NC-200 (Chemometec, Denmark). Whole blood was obtained from patients for processing and retrieval of immunocompetent cells using Ficoll-Paque PLUS and corresponding protocol (GE Healthcare, Chicago, US). Normal lymph nodes from two patients and normal spleen tissue from one patient were obtained for additional comparative analysis with blood-derived immunocompetent cells. Lymph nodes and spleen were processed similarly to whole tissue as described above.
Organoid Fabrication & Culture
The tumor cell pellet was resuspended with the thiol-modified hyaluronan/heparin (Heprasil®; Advanced Biomatrix, San Diego, CA) and methacrylated collagen (PhotoCol®; Advanced Biomatrix, San Diego, CA) solution in a 1:3 volume ratio at a cell density of 10 million cells per mL. Patient-derived tumor organoids (PTOs) were then created by seeding 5 μL of the hydrogel/cell mixture into individual wells of a 96-well non-tissue culture treated plate and then photocrosslinked by exposure to ultraviolet light (365 nm, 18W/cm2) from a BlueWave 75 V.2 UV spot lamp (Dymax Corp., Torrington, CT) for 2 seconds to form crosslinking via thiol-modified hyaluronan/heparin and methacrylated collagen. PTOs were cultured in 200 μL media containing DMEM-F12 with 5% FBS, 1% penicillin-streptomycin, 1% L-glutamine, 50 ng/mL EGF (PHG 0313; ThermoFisher Scientific), 10 μM Y-27632 (S1049) with media changes after 3–4 days.
Immune-enhanced PTOs (iPTOs) were created by combining the immunocompetent cells from each patient’s corresponding whole blood (blood iPTOs) and spleen or nodal lymph tissue (lymph iPTOs) in a ratio ranging from 1:1 to 1:10 according to cell yield with tumor cells and seeded onto plates as described above. The organoids in addition to tumor and CD8+ cells, contain CD4+ and APC cells, as well as stroma as described previously4,6,7. Organoids were cultured for 7 days prior to treatment.
Drug Screens
Organoids were subsequently treated after 7 days of culture with 100 nM of Pembrolizumab (A2002, Selleckchem, Houston, TX), Ipilimumab (A2001, Selleckchem, Houston, TX) or Nivolumab (A2005, Selleckchem, Houston, TX). This drug concentration corresponds to dosing recommendations for a 70 kg patient. Media was aspirated from the wells and drug solutions mixed in culture media were added to each well individually. Organoids remained in drug-containing media solution for 3 days prior to endpoint viability assessment.
Organoid Viability Assessment
After 3 days of incubation in drug-containing media, organoids were assessed with LIVE/DEAD staining and CellTiter-Glo® 3D viability assays. LIVE/DEAD staining (L3224; Invitrogen, Carlsbad, CA) was performed according to the manufacture’s protocol and incubated at 37°C for 2 hours prior to imaging. Fluorescent imaging was performed on whole organoids using a Leica TCS LSI macro confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL). Images from red and green channels were overlaid and stacked in maximum projection.
Quantitative viability was assessed utilizing CellTiter-Glo® 3D Cell Viability Assay (G968B; Promega, Madison, WI). Half the media (100 μL) was removed from individual wells and 100 μL of ATP assay was added to each well, incubated at room temperature on a shaker for 30 minutes. Well contents were transferred to a Costar White Polystyrene 96 well Assay Plate (3912; Corning, NY) and analyzed with a Veritas Microplate Luminometer (Turner BioSystems, Sunnyvale, CA).
Organoid Tissue Characterization
Organoids were fixed for histology on days 1 and 10 of culture in 4% paraformaldehyde for 4 hours. Organoids were processed, paraffin embedded, and sectioned at 5-μm intervals for staining. Organoid sections were stained on glass microscope slides with hematoxylin and eosin (H&E).
Additional staining was performed with immunohistochemistry (IHC) to characterize programmed death ligand-1 (PD-L1), cluster of differentiation 8 (CD-8), cytokeratin 20 (CK-20), and granzyme B biomarker expression. Unstained slides underwent antigen retrieval in a pH 6 citrate buffer solution prior to blocking with Dako Protein Block for 30 minutes. Fluorescent IHC was performed by applying primary antibodies PD-L1 (ab205921, abcam, rabbit), CD-8 (ab4055, abcam, rabbit), CK-20 (MA5–13263, Invitrogen, mouse), and granzyme B (ab4059, abcam, rabbit), and cleaved caspase 3 (9661S, Cell Signaling Technologies, rabbit) to slides in ratios of 1:500, 1:200, 1:200, 1:100, 1:400 in Dako Antibody Diluent, respectively. After incubation for 1 hour, appropriate species reactive secondary Alexa Fluor 488 or Alexa Fluor 594 antibodies (Biotium, Fremont, CA) were applied to samples for 1 hour at a 1:1000 dilution. Sections were then incubated with DAPI for 5 minutes prior to finalization with coverslipping. An Olympus BX-63 upright fluorescent microscope (Olympus, Tokyo, Japan) was used to image the sections.
Cell Membrane Tracking
For Patient 21, prior to organoid encapsulation, unsorted tumor cells and immune cells were tagged using DIO (tumor cells) and DII (immune cells) fluorescent dyes (ThermoFisher) according to company protocol. Organoids were cultured, treated and imaged as described above. Fluorescent images were analyzed using ImageJ Fiji analysis software using red and green pixel analysis. The percentage ratio of total green to red pixel counts were obtained and tumor cell images were converted from green to yellow.
Definition of Treatment Response
Immunotherapy efficacy in organoids is currently undefined. Herein, we developed a conservative approach for considering an organoid to be responsive to immunotherapy, consisting of three distinct criteria that simultaneously must be met by iPTOs: 1) demonstrate a statistically significant reduction in cell viability when compared to iPTO untreated (control) organoids (Ex: iPTO control vs iPTO treated), and 2) demonstrate a statistically significant reduction in cell viability when comparing treated organoids from immune enhanced to the non-immune enhanced counter conditions (Ex: Pembrolizumab treated iPTO vs Pembrolizumab treated PTO), and 3) exhibit a post immunotherapy ATP viability < 50%.
The lower threshold of immunotherapy efficacy in organoids is unknown. Herein we arbitrarily selected 50% killing of the tumor as the lowest threshold suggestive of immunotherapy response. This number can be increased or decreased based on the desired tumor response in need to be studied or the kinetics and the tumor biology of every individual patient, demonstrating the plasticity of the platform.
Statistical Analysis
All data is expressed as mean ± standard deviation for each experimental group. Each treatment and condition combination consisted of 3 or more organoids for analysis. ATP assay values of treated organoids were standardized to condition-matched (iPTO or PTO) controls prior to statistical analysis. Upon review of the CellTiter-Glo® results, outlier ATP replicate values were removed by a committee of researchers to improve the rigor of the final analysis. Two sample t-tests were also used to assess whether cell viability values were different between immune enhanced and non-immune enhanced counter conditions. We intentionally chose a rigorous threshold for determining whether a particular organoid showed a response to immunotherapy. This threshold requires all three treatment response conditions (identified in the previous section) be met in order to consider an organoid as being a treatment response. This approach was used to reduce the probability of a type 1 error occurring. Specifically, the chance that the two t-tests described above would both be significant at p < 0.05 (rather than because both indicated evidence of a treatment response) would be 0.25%. Further, if the probability that post immunotherapy ATP viability is less than 50% were a random event (i.e., 50% chance that it would occur by chance) then the combined probability that all three events would occur simultaneously by chance would be 0.125% or 12.5 in 10,000. Drug screen studies were determined to be successful for a patient if untreated control PTOs demonstrated adequate viability at day 10 of culture, which coincided with termination of drug screens, and each condition had a counter control condition with adequate viability. Adequate viability is described as blank value of less than 1% of control condition. Statistical analysis was performed with GraphPad Prism (GraphPad Software Inc., USA) and a p value of < 0.05 was used as the threshold for statistical significance. Reported p values indicate significance to parts 1 and 2 of the treatment response definitions outlined above.
Results
Patient Characteristics
A total of 26 patients with appendiceal neoplasms were enrolled in the study, including 16/26 (61.5%) low grade and 10/26 (38.5%) high-grade appendiceal primaries (Table 1). Thirteen patients (50%) had prior systemic chemotherapy, while eight patients (30.8%) had no prior surgical or medical treatment. Genetic analysis was reported for nine patients with FoundationOne® sequencing panels16 and for one patient with STRATA. Both sequencing panels report PD-L1 expression by the Tumor Proportion Score (TPS) (Table 1). Similarly, microsatellite instability and mismatch repair testing were clinically performed on three patients, who were determined to be MMR-proficient. Patient demographic information, including race and ethnicity, are not reported to minimize the potential for patient identification given the rarity of this disease.
Table 1 –
Patient cohort demographics including sequencing analysis and prior surgical or medical treatments. All treatments listed occurred prior to index CRS/ HIPEC. Successful patient-derived tumor organoid fabrication (PTO) as defined by viable controls at 10 days of culture. Tumor mutations identified utilizing FoundationOne® CDx testing.
| Patient | Tumor Histologic Type & Grade | Tumor Mutation(s) | Prior Treatment(s) | Successful PTO Fabrication | Immune Component | Treatment Effect | Patient Response To Immunotherapy | Correlation? |
|---|---|---|---|---|---|---|---|---|
| 1 | High grade mucinous adenocarcinoma with signet ring cell features (HGA) | RAS | FOLFOX, FOLFIRI | No | PBMC | NR | N/A | |
| 2 | High grade mucinous adenocarcinoma with signet ring cell features (HGA) | KRAS, PD-L1 ≥ 1% | Xeloda | Yes | PBMC | NR | NR (Pembrolizumab) | Yes |
| 3 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | Nivolumab | N/A | |
| 4 | High grade adenocarcinoma, non-mucinous (HGA) | N/A | FOLFOX | Yes | PBMC, LN^ | Pembrolizumab* | N/A | |
| 5 | High grade adenocarcinoma, non-mucinous (HGA) | PD-L1 ≥ 1% | FOLFOX | No | PBMC | NR | N/A | |
| 6 | High grade mucinous adenocarcinoma with signet ring cell features (HGA) | Negative | Ileocecectomy, FOLFOX, CRS/HIPEC (MMC/Doxorubicin) | Yes | PBMC | Pembrolizumab | N/A | |
| 7 | Low grade mucinous adenocarcinoma (LGA) | PD-L1 ≥ 1% | FOLFOX, CRS/HIPEC, Camptosar/Avastin | Yes | PBMC | NR | N/A | |
| 8 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | NR | N/A | |
| 9 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | NR | N/A | |
| 10 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | FOLFOX | Yes | PBMC | NR | N/A | |
| 11 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | CRS/HIPEC (MMC) | Yes | PBMC | NR | N/A | |
| 12 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | Pembrolizumab | N/A | |
| 13 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | CRS/HIPEC (MMC) | Yes | PBMC | NR | N/A | |
| 14 | Low grade appendiceal mucinous neoplasm (LAMN) | Negative | CRS, Carboplatin/Paclitaxel | No | PBMC | NR | N/A | |
| 15 | High grade mucinous adenocarcinoma (HGA) | MMR proficient | CRS/HIPEC x2 (MMC & Oxaliplatin) | Yes | PBMC | NR | N/A | |
| 16 | High grade mucinous adenocarcinoma (HGA) | MMR proficient, PD-L1 ≥ 1% | CRS/HIPEC (MMC), FOLFOXIRI, CRS | No | PBMC | NR | N/A | |
| 17 | Low grade appendiceal mucinous neoplasm (LAMN) | KRAS, NRAS, TMB ≥ 10 Muts/Mb | Right hemicolectomy, FOLFOX, FOLFIRI | Yes | PBMC | NR | Receiving Pembrolizumab (NR) | Yes |
| 18 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | Diagnostic laparoscopy | Yes | Spleen | Pembrolizumab | N/A | |
| 19 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | CRS, CRS/HIPEC (MMC), FOLFOX | No | PBMC | NR | N/A | |
| 20 | High grade mucinous adenocarcinoma (HGA) | N/A | FOLFOX | No | PBMC | NR | N/A | |
| 21 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC, LN^ | NR | N/A | |
| 22 | Low grade mucinous adenocarcinoma (LGA) | N/A | CRS, CRS/HIPEC (MMC) | No | PBMC | NR | N/A | |
| 23 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | NR | N/A | |
| 24 | Low grade appendiceal mucinous neoplasm (LAMN) | N/A | N/A | Yes | PBMC | NR | N/A | |
| 25 | High grade mucinous adenocarcinoma with signet ring cell features (HGA) | KRAS | Appendectomy, FOLFOX | Yes | PBMC | Nivolumab | N/A | |
| 26 | High grade mucinous adenocarcinoma with signet ring cell features (HGA) | Negative | Small bowel resection, FOLFOX | Yes | PMBC | NR | N/A |
RAS – Rat sarcoma oncogene; KRAS – Kirsten rat sarcoma oncogene; NRAS – neuroblastoma rat sarcoma oncogene; PD-L1 – Programmed death ligand 1; MMR – mismatch repair proteins; TMB – tumor mutational burden; CRS – cytoreductive surgery; HIPEC – heated intraperitoneal chemotherapy; FOLFOX – folinic acid, fluorouracil, oxaliplatin; FOLFIRI – folinic acid, fluorouracil, irinotecan; MMC – mitomycin C; FOLFOXIRI – folinic acid fluorouracil, oxaliplatin, irinotecan. PBMC – Peripheral blood mononuclear cells; LN – lymph node.
Immune-enhanced PTOs (iPTOs) generated separately for PBMC and LN.
Pembrolizumab effective under both PBMC and LN immune component conditions. N/A – information not available. NR – no treatment response. We refer to LGA and LAMN collectively as low grade appendiceal primaries (LGA).
Organoid characteristics and biofabrication timeline
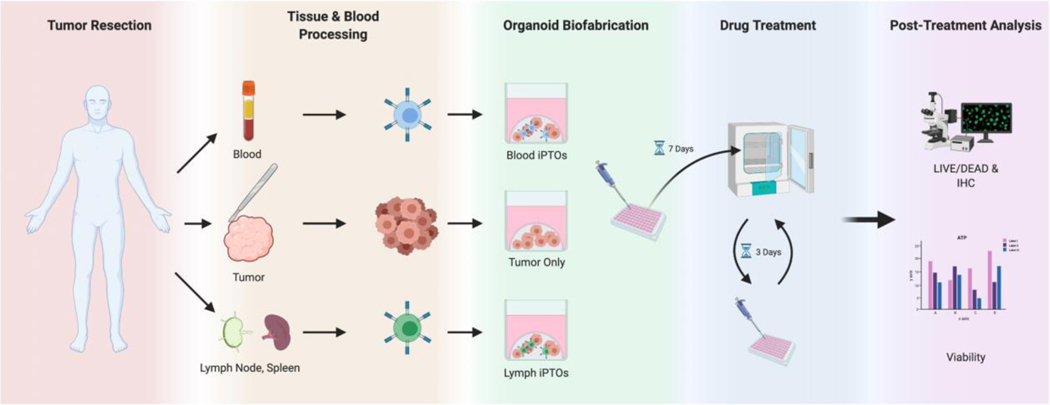
All specimens underwent initiation of an hour-long enzymatic digestion with collagenase, n-acetylcysteine, and protease within 2.5 hours from tissue procurement (Fig. 1). Cell counts were completed within an additional hour post digestion followed by stabilization of tumor cells in supportive ECM. The above sequence resulted in fabrication of immune system enhanced organoids with a time frame of less than 5 hours post specimen resection. iPTOs were enhanced with WBCs only in 23/26 (88.5%) cases, while normal spleen tissue only was used in 1/26 (3.8%) patients. Two patients (7.7%) had iPTOs made with both WBC and lymph nodes as separate comparative groups. PTOs without immune components were generated for comparative controls for all 26 tumor specimens.
Fig 1. Organoid Workflow Diagram.
Incorporating patient matched immune system into tumor organoids, followed by immunotherapy testing, and analysis. PBMC, lymph nodes or spleen, provided immune system elements for PTO enhancement(iPTOs). Blood iPTOs were enhanced with PBMCs. Lymph iPTOs were enhanced with lymph nodes or spleen tissue. PTOs were biofabricated utilizing a collagen-based ECM. After 7 days of incubation, PTOs and iPTOs were treated with Pembrolizumab, Ipilimumab, and Nivolumab containing media for 3 days, prior to analysis of immunotherapy efficacy. (Created with Biorender - biorender.com).
Organoid Characterization
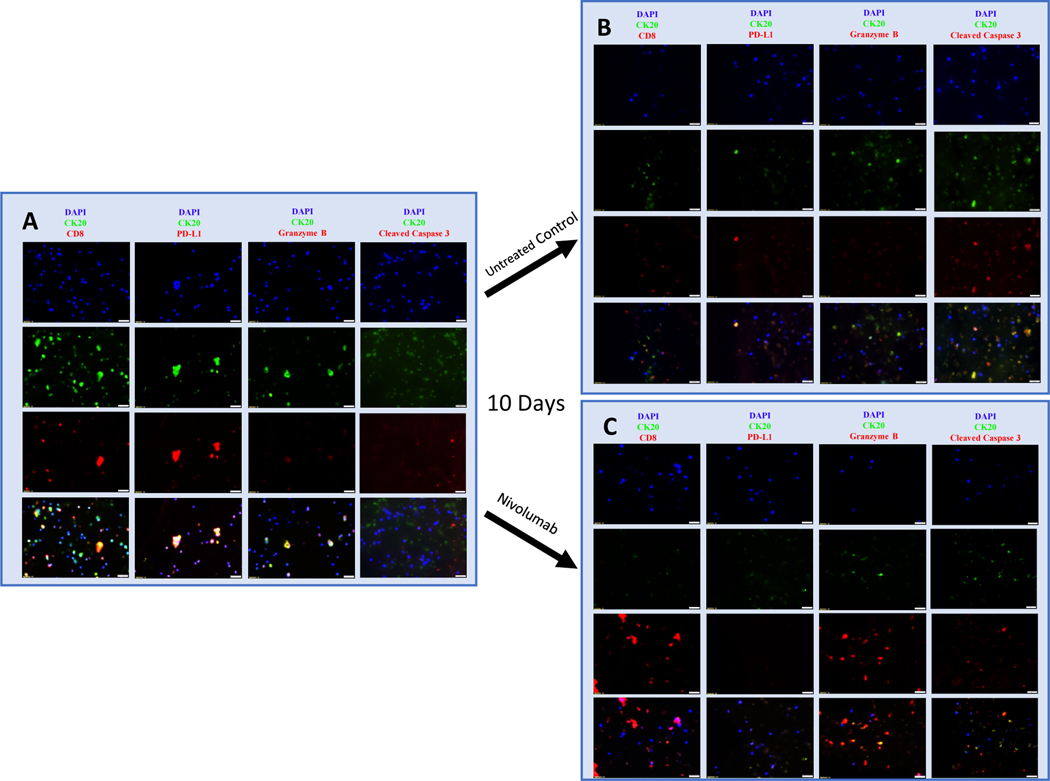
Fluorescent IHC was performed to characterize tumor cell and leukocyte interactions. Abundance of CK20 positive epithelial cells and CD8 positive T-cells were observed on day 1, indicating viable appendiceal tumor cells and immune cells co-cultured within iPTOs (Fig. 2A). The expression of PD-L1 was confirmed on CK20 positive appendiceal tumor cells along with no discernable granzyme B staining, indicating lack of CD8 T-cell mediated cytotoxic activity in the absence of CPIs (Fig 2A & B). At day 10, iPTOs responding to immunotherapy demonstrated increased granzyme B expression (red) and cleaved caspase 3 (red) with corresponding decrease in CK20 appendiceal tumor cells (green) (Fig. 2C & D).
Fig 2. Fluorescent Immunohistochemistry demonstrating CPI induced activation of CD8 positive T-Cells resulting in expression of Granzyme B, cleaved caspase 3 and CD8 T-cell mediated cytotoxicity against a low grade appendiceal primary (LAMN, Patient 3).
PBMC iPTOs at Day 1 (Panel A), untreated day 10 (Panel B), and Nivolumab treated day 10 (Panel C). The top row in each panel represents DAPI (blue), second row CK20 (green), and third row CD8/PD-L1/granzyme B/cleaved caspase 3 (red) staining. The fourth row in each panel represents the combined images. Images at 40x magnification. Scale bar represents 20 μm.
A: Day 1, untreated PBMC iPTOs demonstrate co-culture of CK20 positive appendiceal tumor cells (green) with CD8 positive T-cells (red), with no appreciable granzyme B or cleaved caspase 3 expression (red).
B: Day 10, untreated PBMC iPTOs demonstrate viable CK20 appendiceal tumor cells (green) in co-culture with CD8 T-cells (red) and lack of granzyme B expression, with some increased caspase 3 expression.
C: Nivolumab, results in a significant decrease in CK20 positive appendiceal tumor cells (low green expression) compared to untreated iPTOs (Panel B). The decrease in epithelial cells is correlating with release of granzyme B (red) and increased cleaved caspase 3 (red) by CD8 T-cells after treatment with Nivolumab. Post-treatment viability was 8%.
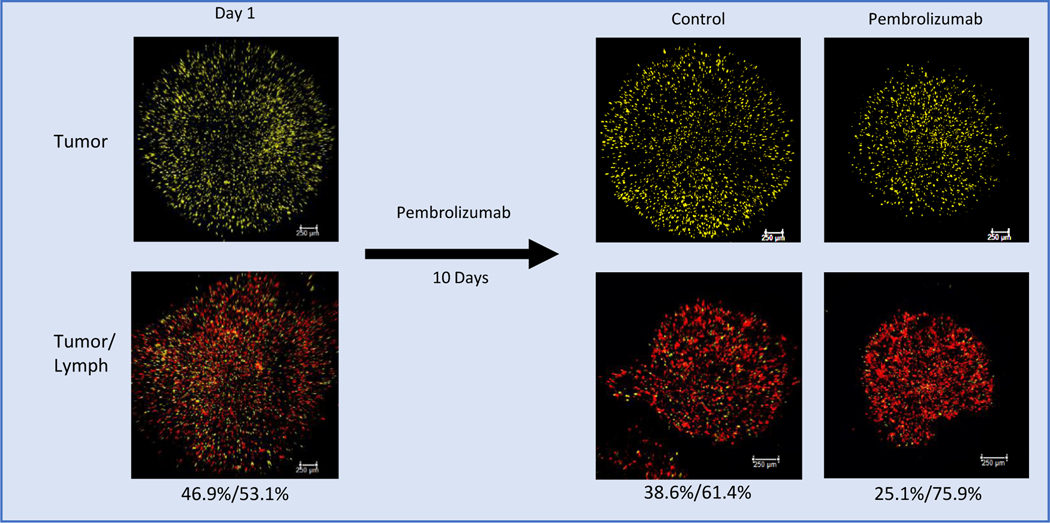
Viability of PTOs and iPTOs was also qualitatively evaluated with LIVE/DEAD staining on days 1 and 10 demonstrating stable CK20 cellular populations in the absence of immunotherapy response (Fig. 3). Cell membrane tagging, performed for Patient 21, demonstrated the presence of immune and tumor cell populations throughout the course of the study (Fig. 4). In pembrolizumab treated organoids, tagged tumor cell populations demonstrated a decrease in the ratio of tumor cells to immune cells, as a result of CPI application (25.1% vs 38.6% of total cells, Fig. 4). In addition to the decrease in the proportion of tumor cells within the organoid, there was also a decrease in the organoid size itself when compared to day 1, suggesting ECM remodeling by the imported cellular lymph node component.
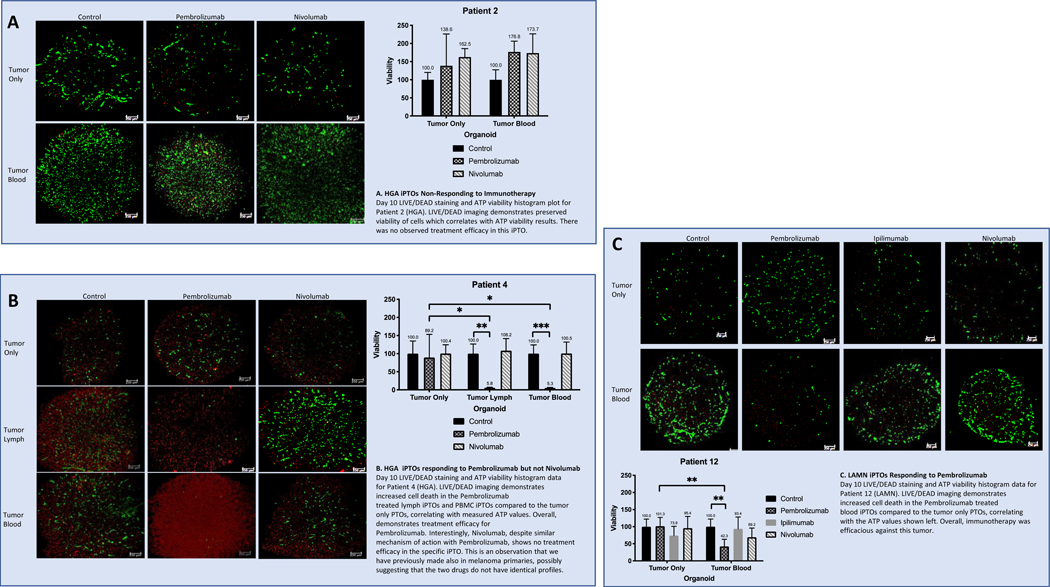
Fig 3. Viability Charts and LIVE/DEAD Imaging Analysis for Patients 2, 4, and 12.
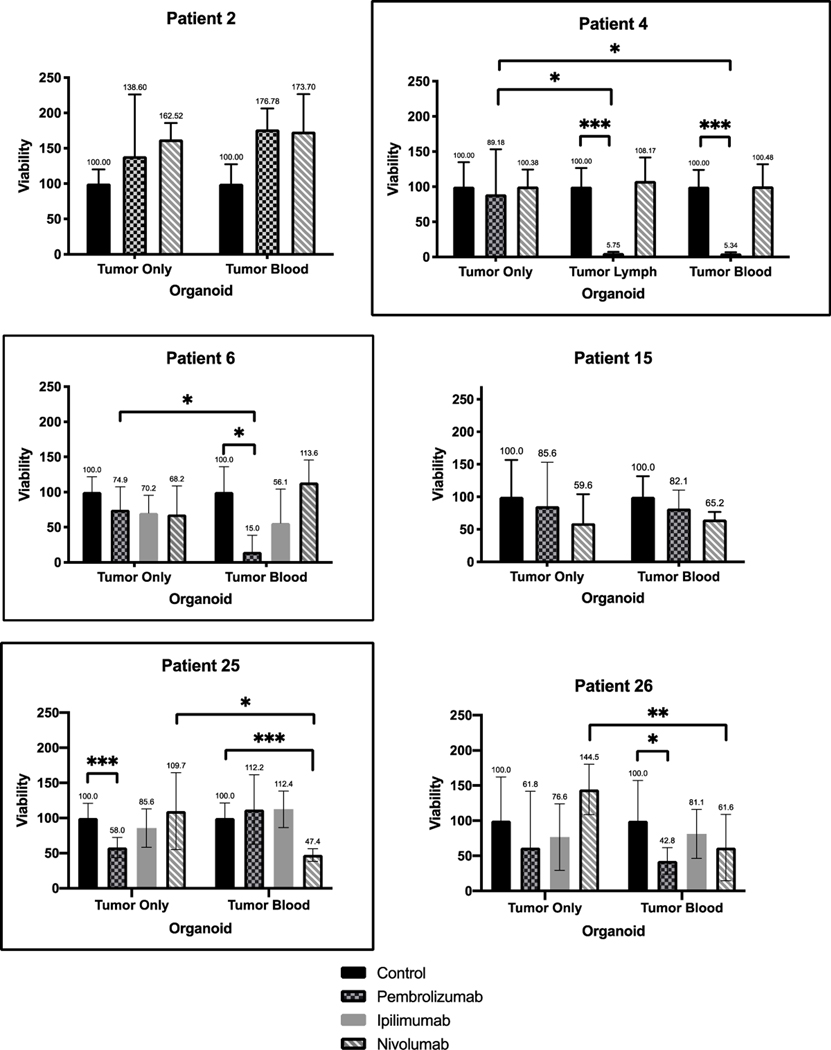
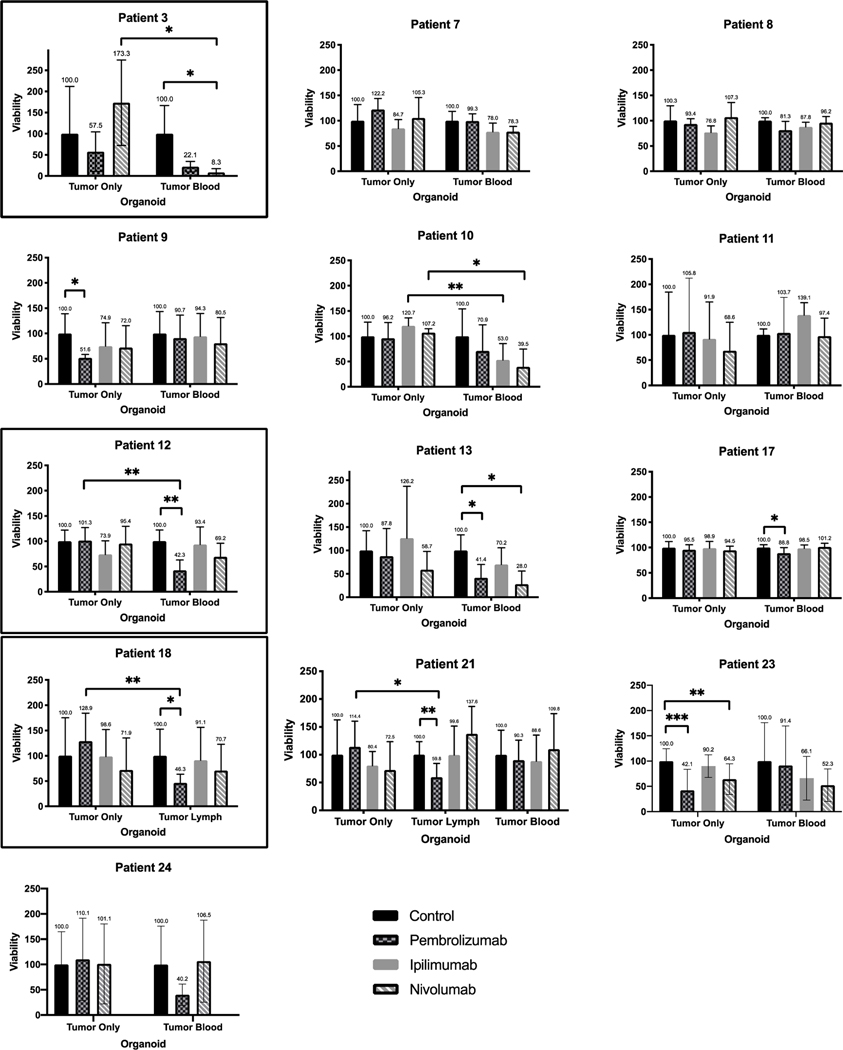
Green signal represents live, intact cells, and the red signal represents non-viable cells with damaged membranes. Scale bars indicate 250 μm. Y-axis represents viability, normalized to a scale of 100 for each control. X-axis represents the organoid condition—tumor only for non-immune enhanced PTOs, and tumor blood for immune-enhanced organoids (iPTOs). One-way error bars represent standard deviation for each treatment condition, with the mean value above each error bar.
Fig 4. Tumor Epithelial Cell/CD8 ratio within the iPTO decrease as a result to Pembrolizumab.
Cell membrane tagged fluorescent images of lymph iPTOs from Patient 21 (LAMN) at day 1 and day 10 of culture. Relative percentages of tumor cells (yellow) and lymph node-derived immunocompetent cells (red) shown, adding up to 100%. A relative decrease in tumor cell proportion is seen in day 10 controls compared to day 1 (38.6% vs 46.9%) possibly suggesting baseline immunogenicity of the tumor eliciting an immune response even in the absence of CPIs. Cell membrane tagging was performed for this patient only. Pembrolizumab treated iPTOs exhibited further reduction in tumor cell proportion (25.1% control vs 38.6% Pembrolizumab). Percentages listed as (tumor %)/(lymph %) and calculated using ImageJ Fiji analysis.
iPTO response to Immunotherapy
After 7 days of culture, 72-hour long drug screens were performed on appendiceal PTOs and iPTOs with Pembrolizumab, Ipilimumab or Nivolumab. CD8+ T-cells were 96.3% viable at 1 week in co-culture with tumor cells as demonstrated by flow cytometry (Supplementary Fig. 1). Drug screens were deemed successful if the control condition for PTO and iPTO demonstrated adequate ATP viability after 10 days of culture. Successful drug screen studies were conducted in 19/26 (73.1%) patients, specifically 6/10 (60%) in HGA patients and 13/16 (81.3%) in LGA patients (Table 1).
Six patients (6/19, 31.6%) demonstrated iPTO response to immunotherapy that was defined as at least 50% cellular death post-treatment for the purposes of the study (Table 1). Overall, response was observed in 3/6 (50%) HGA and 3/13 (23.1%) LGA iPTOs. Pembrolizumab was effective in 4/19 (21.1%) iPTOs with an average post treatment viability of 22.9% (Fig. 5A). Nivolumab was effective in 2/19 (10.5%) iPTOs, demonstrating an average post treatment viability of 27.7% (Fig. 5B). Ipilimumab was not found to be effective in any of the examined specimens. A comparative set of blood iPTOs and lymph iPTOs were fabricated for one patient (Patient 4, HGA) for which Pembrolizumab was equally effective (5.3% vs 5.8% post treatment viability, respectively) (Fig. 3B). We have noticed an increased cell viability in organoids made from Patient 2 (Fig. 3A) and treated with CPI. Although it is possible anti-PD-1 antibodies may bind to cell surface receptors (PD-1 or other) that trigger signaling pathways leading to enhanced metabolic activity, or there is a transient increased metabolism response to cytotoxic conditions, this does not likely represent in-vivo response conditions.
Fig 5A. Personalized Immunotherapy Response of High Grade Appendiceal Primaries iPTOs.
ATP viability histogram plots for HGA tumors exhibiting personalized treatment effect to CPIs. Y-axis represents viability, normalized to a scale of 100 for each control. X-axis represents the organoid condition—tumor only for non-immune enhanced PTOs, and tumor blood and/or tumor lymph for immune-enhanced organoids (iPTOs). Patients 4, 6 and 25 iPTOs were deemed to have a treatment response, outlined. One-way error bars represent standard deviation for each treatment condition, with the mean value above each error bar. * indicates p < 0.05. ** indicates p < 0.01. *** indicates p < 0.001.
Fig 5B. Personalized Immunotherapy Response of Low Grade Appendiceal Primaries iPTOs.
ATP viability histogram plots for LGA tumors exhibiting personalized treatment effect to CPIs. Y-axis represents viability, normalized to a scale of 100 for each control. X-axis represents the organoid condition—tumor only for non-immune enhanced PTOs, and tumor blood(PBMC) and/or tumor lymph for immune-enhanced organoids (iPTOs). Patient 3, 12 and 18 iPTOs met criteria for treatment response, outlined. One-way error bars represent standard deviation for each treatment condition, with the mean value above each error bar. * indicates p < 0.05. ** indicates p < 0.01. *** indicates p < 0.001.
Organoid correlation with patient clinical response
At this time, two patients (Patients 2 & 17) have received immunotherapy in the clinical setting and were both correlating with the organoid response. The first patient was administered Pembrolizumab due to PD-L1 expression. The patient did not respond to immunotherapy, which correlated with lack of response of their organoids. The second patient underwent Pembrolizumab treatment for a TMB > 10 without radiologic response or clinical response, similar to the corresponding appendiceal organoids.
Discussion
The rarity of appendiceal cancer is a major limitation for the identification of new therapies through recruitment in clinical trials. We have previously shown the versatility of PTOs in studying the personalized efficacy of systemic as well as intraperitoneal chemotherapy in a variety of rare cancers, including appendiceal neoplasms, sarcomas and peritoneal mesothelioma5,6.
Immunotherapy is an approved therapy for solid tumors with microsatellite instability and increased mutational burden, based on the assumption that increased mutational load will result in increased tumor antigenicity and therefore activation of adaptive immunity mechanisms. It is well documented that not all patients with MSI or increased TMB will respond to immunotherapy, while it is highly unlikely that these two indications are the only scenarios where immunotherapy will be beneficial to a cancer patient. In addition, the cost of immunotherapy treatment is not insignificant to be applied based on generalized indications. Therefore, there is an unmet need for an approach to explore the possibility of ex-vivo identification of tumors that will respond to immunotherapy at the level of the individual patient and outside the framework of current sequencing indications. The aim of this study is to demonstrate feasibility of an iPTO platform in generating preclinical immunotherapy efficacy data in a rare primary such as appendiceal cancer where level I clinical data are lacking.
Organoids recapitulate the tumor microenvironment by incorporating not only tumor cells but also cells from tumor associated stroma. Immunotherapy works through activation of a patient’s own immune system, therefore adding a patient matched immune system within the organoid is necessary to test CPI efficacy. Herein, PTOs have been co-cultured with patient matched spleen, lymph nodes and/or peripheral blood mononuclear cells (PBMCs) from peripheral blood. The power in the current study is not sufficient to identify variations in construct behavior, but from our earlier experience with melanoma immunocompetent organoids, addition of PBMCs suffices when the objective of the enrichment is limited to evaluation of immunotherapy response4. In addition, it simplifies the logistics of co-culturing, without the need of additional tissue processing.
The relationship between PD-L1 and response to immunotherapy is unclear, with the majority of data not supporting a reliably predictive role in other primaries such gastric and gastroesophageal junction cancers17. Literature review at the time of this manuscript revealed a single case report of immunotherapy efficacy in an AC patient that did not express PD-L1 and was microsatellite stable15. Similarly, we identified a patient with no PD-L1 expression that exhibited significant treatment efficacy to Pembrolizumab, as well as patients who did not respond to any immunotherapy drug despite PD-L1 ≥ 1%. The organoid observed response rate in our cohort is higher than what would be expected from non MSI-H GI malignancies, but it is likely the two groups are not comparable. The group of patients with peritoneal dissemination have significant volume of disease presenting with multiple, often more than 30–50 distinct peritoneal lesions and a multiclonal make up that directly increases antigenicity. We believe that it is this antigenicity that drives the observed immune responses and not the PD-L1 status, TMB-H, or MSI that in AC is determined to be very low at 2.8, 2.2 and 2.2 percent, respectively18. The above demonstrates the pitfalls of using isolating sequencing results in treatment decisions, that with few exceptions, cannot take into consideration interactions between variable existing genomic pathways within the entire tumor.
Interestingly, similarly to our prior melanoma work4, we observed differential treatment efficacies for Pembrolizumab and Nivolumab in iPTOs derived from the same patient. While studies currently support the clinical interchangeability of these two agents19, it seems at least at an iPTO level, the efficacy of these two drugs was not equal for the same patient. It is unknown if this can be explained by structural properties differences such as binding affinities for PD-1 receptors or epitope binding within the PD-1 loop that is dominated by interactions with the N segment for Nivolumab and CD segment for Pembrolizumab20.
We have previously shown lack of response to chemotherapy for organoids derived from low grade appendiceal primaries5. However, though often indolent, low grade AC carries significant morbidity in patients with a volume of disease that is not amenable to CRS/HIPEC or has recurred after prior multiple cytoreductions. The efficacy of immunotherapy for 23.1% of LGA patients (3/13) in this study could open opportunities for new applications of CPIs. Currently, there is only a single US-based Phase II clinical trial examining responses to Ipilimumab and Nivolumab for the treatment of mucinous appendiceal and colorectal tumors21. Interestingly, we did not identify AC iPTOs that responded to Ipilimumab within the examined specimens. PTOs have shown tremendous value and promise in ex-vivo characterization of a variety of malignant GI tumors and correlation with clinical response8. The potential to deliver personalized results for each tumor can spare patients from harmful side effects of treatments for which they will obtain no benefit.
Limitations of the study include the modest power as well as the fact that immunotherapy is not currently considered as a routine treatment option in AC patients with peritoneal dissemination. Therefore, correlation data between the organoid response and the clinical response of the corresponding patient to CPI treatment cannot be generated as we have previously demonstrated for melanoma iPTOs where the iPTO response to immunotherapy was similar to specimen clinical response in 85% (6/7) of patients4. In addition, we remove mucin during the processing stage to preserve organoid integrity. Thus, our model does not account for the possibility of excessive mucin interference on drug delivery. Nevertheless, this the only available preclinical study on immunotherapy efficacy on AC primaries, suggesting that CPIs may be a promising option in a subpopulation of AC patients that can be identified through development of immune enhanced PTOs within two weeks from tissue procurement. Current enrollment in clinical trials is predominantly guided by tumor type with limited armamentarium in incorporating information relevant to cohort heterogeneity. The implications of a platform that can generate preclinical data for enrollment of the most suitable patients to the appropriate therapeutic schemas and agents may have a significant impact on understanding and management of rare diseases. Only upon validation from clinical studies, these methods may be potentially useful in personalized application of immunotherapy or possibly target selection of optimal candidates for clinical trials.
In conclusion, immunotherapy exhibits unexpected cytotoxic efficacy in a subset of appendiceal cancer immune enhanced organoids from both low and high grade primaries possibly offering an opportunity for a more targeted approach in clinical trial design. Organoid technology could potentially identify immunotherapy responders at the level of the individual patient outside the current indications of microsatellite instability and increased tumor mutation burden.
Supplementary Material
Translational Relevance.
Clinical trial accrual in rare diseases is limited by low incidence and lack of research models. Herein, we apply patient-derived tumor organoids, enhanced with autologous immune system to study the efficacy of checkpoint inhibitors in appendiceal cancer. Appendiceal cancer is an orphan primary, with limited research models, and historically resistant to systemic chemotherapy. We explored the concept of using appendiceal cancer immunocompetent organoids as a preclinical companion platform, for the ex vivo study of the interaction between tumor and host’s immune system, possibly optimizing selection of clinical trial candidates, at the level of the individual patient.
Acknowledgements:
We graciously acknowledge the work of Libby McWilliams and Kathleen Perry for their assistance in tissue procurement and data management. The authors would also like to acknowledge their funding for the project which was supported by the Wake Forest Dean’s Hero Award, the Appendix Cancer Pseudomyxoma Peritonei Foundation (ACPMP), and the National Organization of Rare Diseases (NORD), and the National Institute of Health R01CA249087. Steven D. Forsythe is supported by a grant from the National Institute of Health (T32CA247819).
Grant Support:
1. Wake Forest Tumor Tissue and Pathology Shared Resource NCI CCSG P30CA012197.
2. National Organization of Rare Diseases (NORD).
3. The Appendix Cancer Pseudomyxoma Peritonei Research Foundation (ACPMP)
4. National Institutes of Health, T32CA247819
5. National Institutes of Health R01CA249087
References
- 1.Marmor S, Portschy PR, Tuttle TM, Virnig BA. The Rise in Appendiceal Cancer Incidence: 2000–2009. J Gastrointest Surg. 2015;19(4):743–750. doi: 10.1007/s11605-014-2726-7 [DOI] [PubMed] [Google Scholar]
- 2.Chicago Consensus Working Group. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Appendiceal Neoplasms. Ann Surg Oncol. 2020;27(6):1753–1760. doi: 10.1245/s10434-020-08316-w [DOI] [PubMed] [Google Scholar]
- 3.Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Journal of Surgical Oncology. 2014;109(7):740–745. doi: 10.1002/jso.23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Votanopoulos KI, Forsythe S, Sivakumar H, et al. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann Surg Oncol. 2020;27(6):1956–1967. doi: 10.1245/s10434-019-08143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Votanopoulos KI, Mazzocchi A, Sivakumar H, et al. Appendiceal Cancer Patient-Specific Tumor Organoid Model for Predicting Chemotherapy Efficacy Prior to Initiation of Treatment: A Feasibility Study. Ann Surg Oncol. 2019;26(1):139–147. doi: 10.1245/s10434-018-7008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsythe SD, Sasikumar S, Moaven O, et al. Personalized Identification of Optimal HIPEC Perfusion Protocol in Patient-Derived Tumor Organoid Platform. Ann Surg Oncol. 2020;27(13):4950–4960. doi: 10.1245/s10434-020-08790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzocchi AR, Rajan SAP, Votanopoulos KI, Hall AR, Skardal A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Scientific Reports. 2018;8(1):2886. doi: 10.1038/s41598-018-21200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–926. doi: 10.1126/science.aao2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke AJ, Skelton WP IV, Starr JS, et al. Immunotherapy for Colorectal Cancer: A Review of Current and Novel Therapeutic Approaches. JNCI: Journal of the National Cancer Institute. 2019;111(11):1131–1141. doi: 10.1093/jnci/djz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 12.Research C for DE and. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA. Published online June 17, 2020. Accessed November 27, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors [Google Scholar]
- 13.Research C for DE and. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA. Published online February 9, 2019. Accessed November 27, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication [Google Scholar]
- 14.Research C for DE and. FDA approves pembrolizumab for first-line treatment of MSI-H/dMMR colorectal cancer. FDA. Published online June 30, 2020. Accessed January 6, 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer [Google Scholar]
- 15.Al Attar L, Truong P. The Effect of Pembrolizumab in Absence of Programmed Death 1 Receptor. Cureus. 2018;10(6):e2896. doi: 10.7759/cureus.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FoundationOne CDx | Foundation Medicine. Accessed November 26, 2020. https://www.foundationmedicine.com/test/foundationone-cdx
- 17.Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokunaga R, Xiu J, Johnston C, et al. Molecular Profiling of Appendiceal Adenocarcinoma and Comparison with Right-sided and Left-sided Colorectal Cancer. Clin Cancer Res. 2019;25(10):3096–3103. doi: 10.1158/1078-0432.CCR-18-3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad V, Kaestner V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol. 2017;44(2):132–135. doi: 10.1053/j.seminoncol.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Fessas P, Lee H, Ikemizu S, Janowitz T. A molecular and preclinical comparison of the PD-1–targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol. 2017;44(2):136–140. doi: 10.1053/j.seminoncol.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramson Cancer Center of the University of Pennsylvania. A Phase II Study of Nivolumab and Ipilimumab in Mucinous Colorectal and Appendiceal Tumors. clinicaltrials.gov; 2020. Accessed January 4, 2021. https://clinicaltrials.gov/ct2/show/study/NCT03693846
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.