Abstract
Age is a major risk factor for cataract (ARC). However, the influence of aging on the lens transcriptome is under studied. Lens epithelial (LEC) and fiber cells (LFC) were isolated from young (3 month) and aged (24 month) old C57BL/6J mice, and the transcriptome elucidated via RNAseq. EdgeR estimated differential gene expression in pairwise contrasts, and Advaita’s Ipathway guide and custom R scripts were used to evaluate the potential biological significance of differentially expressed genes (DEGs). This analysis revealed age-dependent decreases in lens differentiation marker expression in both LECs and LFCs, with gamma crystallin transcripts downregulating nearly 50 fold in aged LFCs. The expression of the transcription factors Hsf4 and Maf, which are known to activate lens fiber cell preferred genes, are downregulated, while FoxE3, which represses gamma crystallin expression, is upregulated in aged fibers. Aged LECs upregulate genes controlling the immune response, complement pathways, and cellular stress responses, including glutathione peroxidase 3 (Gpx3). Aged LFCs exhibit broad changes in the expression of genes regulating cell communication, and upregulate genes involved in antigen processing/presentation and cholesterol metabolism, while changes in the expression of mitochondrial respiratory chain genes are consistent with mitochondrial stress, including upregulation of NDufa4l2, which encodes an alternate electron transport chain protein. However, age did not profoundly affect the response of LECs to injury as both young and aged LECs upregulate inflammatory gene signatures at 24 hours post injury to similar extents. These RNAseq profiles provide a rich data set that can be mined to understand the genetic regulation of lens aging and how this impinges on the pathophysiology of age related cataract.
Introduction
Aging is a complex process where genes and environment collaborate to yield progressive tissue dysfunction that first hampers the quality of life, then an organism’s survival (da Costa et al., 2016; Longo et al., 2015). While all tissues exhibit age related changes, the ocular lens is a particularly good model to study tissue aging, as its major disease, cataract, is recognized to sharply increase with advanced age (Chilibeck et al., 2020; Flaxman et al., 2017; Rink, 1987). Many studies have described age-related changes in the ocular lens including alterations in lipid composition (Borchman and Yappert, 2010), decreases in antioxidants (Barnes and Quinlan, 2017), and increases in post translational protein modifications including de-amidation, amino acid isomerization and proteolysis (Ray, 2015). These processes likely collaborate to drive the elevations in protein aggregation and membrane damage which are recognized to drive the pathophysiology of age-related cataract (ARC) (Harding, 2002; Michael and Bron, 2011; Truscott and Friedrich, 2019; Uwineza et al., 2019).
While nuclear ARC is a disorder of the lens nucleus which consists of metabolically inactive cells whose components were largely synthesized during fetal development/early childhood (Augusteyn, 2010), cortical cataract is a disorder of fiber cells produced later in life. As fiber cells are produced from epithelial cells throughout the lifespan, it has been hypothesized that cortical cataract could result from acquired genetic or age-related changes in the lens epithelium which would then propagate into fiber cells (Mesa et al., 2016; Pendergrass et al., 2001; Wang et al., 2020; Worgul et al., 1989). Further, as the lens has an internal circulation that delivers anti-oxidants and other protective molecules to the lens nucleus and removes their “spent” derivatives (Mathias et al., 2007), age-related changes in the biology of the lens epithelium have been hypothesized to have indirect effects on the transparency of the lens cortex and nucleus (Fan et al., 2017; Wang et al., 2017). While many laboratories have explored the idea that lens epithelial cells change their biology with age, upon oxidative stress, or coincident with ARC via “candidate gene” investigations (Periyasamy and Shinohara, 2017), the effect of aging on global gene expression in the lens is understudied.
Cataracts of all types are treated by surgery, most often consisting of an anterior capsulotomy, followed by removal of the lens fibers by phacoemulsification, then implantation of an intraocular lens (IOL) prosthetic to restore vision (Olson, 2018). While this is a very successful clinical intervention, it results in ocular inflammation (Juthani et al., 2017), which, if uncontrolled, can result in negative sequelae such as macular edema and retinal detachment (Kato et al., 2019; Shihan et al., 2019). Later, remnant lens epithelial cells undergo a wound healing response where they proliferate and migrate while either attempting to regenerate the lens or transdifferentiate into myofibroblasts (Wormstone et al., 2009). While current surgical approaches and intraocular lens designs are generally effective in keeping these cells out of the visual axis short term; longer term, they can escape their sequestration at the capsular bag periphery and migrate into the visual axis leading to high rates of posterior capsular opacification (PCO) by 5–10 years post cataract surgery (PCS) (Apple et al., 2011; Lindholm et al., 2020; Ronbeck and Kugelberg, 2014). While younger cataract patients develop more aggressive PCO than older ones (Elkin et al., 2016; Wu et al., 2018), which has been related to differences in proliferative potential and cell signaling efficiency of LEC (Dawes et al., 2013; Wormstone et al., 1997), the global mechanisms underlying these observations have not been studied.
While numerous factors are known to influence aging, it is often difficult to disentangle the relative contributions of environment, intrinsic aging mechanisms and genetic variation among individuals in “free living” organisms such as humans. Many of these complexities can be overcome by the study of aging in inbred laboratory mice as they are essentially genetically identical to each other and are housed in controlled environmental conditions (Ackert-Bicknell et al., 2015). Inbred C57BL/6 mice are commonly used in aging studies, as it is one of two strains routinely maintained by the National Institute of Aging and are used by the Nathan Shock Centers for investigations on the effects of senolytics on aging (Xu et al., 2018). Here, we use RNAseq to compare the global transcriptome of lens epithelial and fiber cells freshly isolated from either young (3 months) or old (24 month) old C57BL/6J strain mice, and investigate how age affects the acute response of lens epithelial cells to lens fiber cell removal which models modern cataract surgery.
Materials and methods:
Mice
All studies using mice comply with the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Vision Research and were approved by the University of Delaware Institutional Animal Care and Use Committee. Twenty four month old C57BL/6NIA mice (10 males and 10 females) were obtained from the National Institute on Aging Biological Resources Colony in October of 2018. These animals are derived from C57BL/6J foundation stock obtained from the Jackson Laboratory in 2016. Ten week old C57BL/6J mice (10 males and 10 females, Stock # 000664) were obtained from the Jackson Laboratory in October of 2018. In both cases, animals were housed at the University of Delaware animal facility under a 14/10 hour light-dark cycle for two weeks prior to tissue isolation. The eyes from all mice used in this study were of normal size and did not manifest signs of the sporadic eye defects that have been reported in this strain (Smith et al., 1994). The lenses from the 12 week old mice studied were transparent, while most of the aged lenses used in this study showed refractive discontinuities consistent with “nuclear sclerosis” and/or mild lens opacities as has been reported for 24 month old mice of this strain (Wolf et al., 2005; Wolf et al., 2000).
Mouse cataract surgery model and tissue isolation
Surgical removal of lens fiber cells was performed on adult mice to mimic human cataract surgery as previously described (Desai et al., 2010; Manthey et al., 2014b). Briefly, two weeks after arrival at the University of Delaware, mice were anesthetized, a central corneal incision made, and the entire lens fiber cell mass was removed from one eye by a sharp forceps, leaving behind an intact lens capsule. The cornea was sutured and the eye restored to normal shape with balanced saline solution. Twenty four hours later, mice were re- anesthetized, and the surgery repeated on the other eye. Mice were then immediately sacrificed and lens capsular bags isolated by dissection.
For RNA sequencing, lens capsular bags from either 24 hours post cataract surgery (PCS) or zero hours PCS were pooled from five animals to make a single biological replicate, while lens fiber masses from two independent animals were pooled per replicate. Four biological replicates were created for each condition (3 month old versus 24 month old at zero hours, 24 hours, and lens fiber cells) and flash frozen on dry ice. Of these four replicates, two were isolated from male animals and two from female animals in order to disentangle the effect of sex as a biological variable in the analyses (Faranda et al., EER submitted).
Next generation RNA sequencing and bioinformatic analysis
Lens epithelial cell RNA was harvested using the RNeasy Mini Kit from Qiagen (Cat No./ID: 74104), and lens fiber cell RNA isolated using the SV Total RNA Isolation System (Promega- Catalog number- Z3100). RNA libraries were prepared for sequencing using the SMARTer® Stranded Total RNA-Seq Kit-Pico Input Mammalian (Takara Bio USA, Inc., Mountain View, CA, USA) and sequenced by DNA Link, USA (901 Morena Blvd. Ste 730 San Diego CA 92117, USA) on a NovaSeq 6000 (San Diego, CA, USA). Read pairs (101 nucleotides long) were aligned to the Ensembl primary assembly of the mouse GRCm38 genome (Yates et al., 2020) using Hisat2 with its default parameters (Kim et al., 2019). Read pairs aligning to genomic features in the Ensembl Mouse version 101 GTF file were quantified as gene level counts, using HTSeq-Count in union mode (Anders et al., 2014). Length normalized abundance estimates (Fragments per Kilobase-Million (FPKM)) were calculated from gene level counts using the total length of all known exons for a given gene, after merging overlapping exons.
Samples were partitioned for TMM (Trimmed Median of Means) scaling (Phipson et al., 2016; Robinson and Oshlack, 2010) and differential expression analyses performed based on the objective of a particular contrast. For contrasts evaluating differences between epithelial cells and fiber cells, and age effects in un-injured tissues, all samples collected at 0 hours post-surgery were grouped together. For contrasts evaluating LEC injury responses, all LECs samples were grouped together.
The “exactTest” method from the edgeR statistical package (version 3.30.3) was used to estimate the magnitude and statistical significance of differential gene expression, with robust dispersion estimates (Phipson et al., 2016; Robinson et al., 2010). Genes with at least 10 mapped reads in at least four samples were considered to have “detectable” levels of expression. Genes failing “detectable” criteria were removed prior to running the “exactTest”, using edgeR’s “filterByExpr” function (Chen et al., 2016). Biologically significant differentially expressed genes (DEGs) were defined as those exhibiting a statistically significant difference in expression using Storey’s Q value to adjust for False Discovery Rate (FDR ≤ 0.05; (Storey and Tibshirani, 2003)), a difference in expression level greater than 2 FPKM between conditions, Fold Change (FC) greater than 2 in either the positive or negative direction and expressed at a level greater than 2 FPKM. (Manthey et al., 2014a).
Pathway analyses
Pathway analysis was performed on all statistically significant DEGs defined as those exhibiting a fold change ≥ |2| and FDR ≤ 0.05 using iPathwayGuide (Advaita Bioinformatics, Plymouth Michigan, USA). This software package uses Impact Analysis, an approach that considers and the directed interactions of DEG within a given pathway (as defined by the Kyoto Encyclopedia of Genes and Genomes, KEGG, (Kanehisa et al., 2017), Release 96.0+/11–21, Nov 20) and also whether more pathway participants are observed in the DEG list than would be expected by chance (Ahsan and Draghici, 2017; Draghici et al., 2007; Tarca et al., 2009). Gene ontology comparisons were made against the October 14, 2020 release of the Gene Ontology Consortium database (Ashburner et al., 2000).
Results
Tissue dysfunction associated with aging is a biological process influenced by the environment, genetic background, and the passage of time (da Costa et al., 2016). In this study, the effects of age are largely isolated from genetic variation as inbred C57BL/6 J mice, which are expected to be nearly genetically identical except for sex chromosomes (Taft et al., 2006), were used for study, while the environment was controlled by housing animals at environmentally controlled animal facilities. The young lenses studied were isolated from three month old mice, an age chosen because these animals are sexually mature adults who have completed eye development and exhibit a crystallin profile consistent with the adult lens (Ueda et al., 2002). The aged lenses were isolated from 24 month old animals, an age that 60–80% of animals from this strain can attain (Whitehead et al., 2014). Comparisons of phenotypic hallmarks associated with age-related frailty suggest that 24 month old C57Bl/6J mice are physiologically similar to 70 year old humans (Whitehead et al., 2014). All raw and processed transcriptome comparisons are available from the Gene Expression Omnibus under accession number GSE166619. RNAsequencing statistics for all samples including sequencing depth and read mapping can be viewed in supplementary table1.
Effect of aging on the lens epithelial cell transcriptome
Comparison between the young and aged LEC transcriptome revealed 226 genes to be significantly differentially expressed (differentially expressed genes, DEGs) by at least two fold (false discovery rate (FDR) corrected P value ≤ 0.05), with 83 of these downregulated and 143 upregulated (Figure 1A). Filtering this list further for genes that meet previously described criteria for likely “biological significance” (minimum expression level of 2 FPKM in either condition, at least 2 FPKM absolute change in expression level, (Manthey et al., 2014a)) revealed 111 DEGs (see supplemental Table 2).
Figure 1.
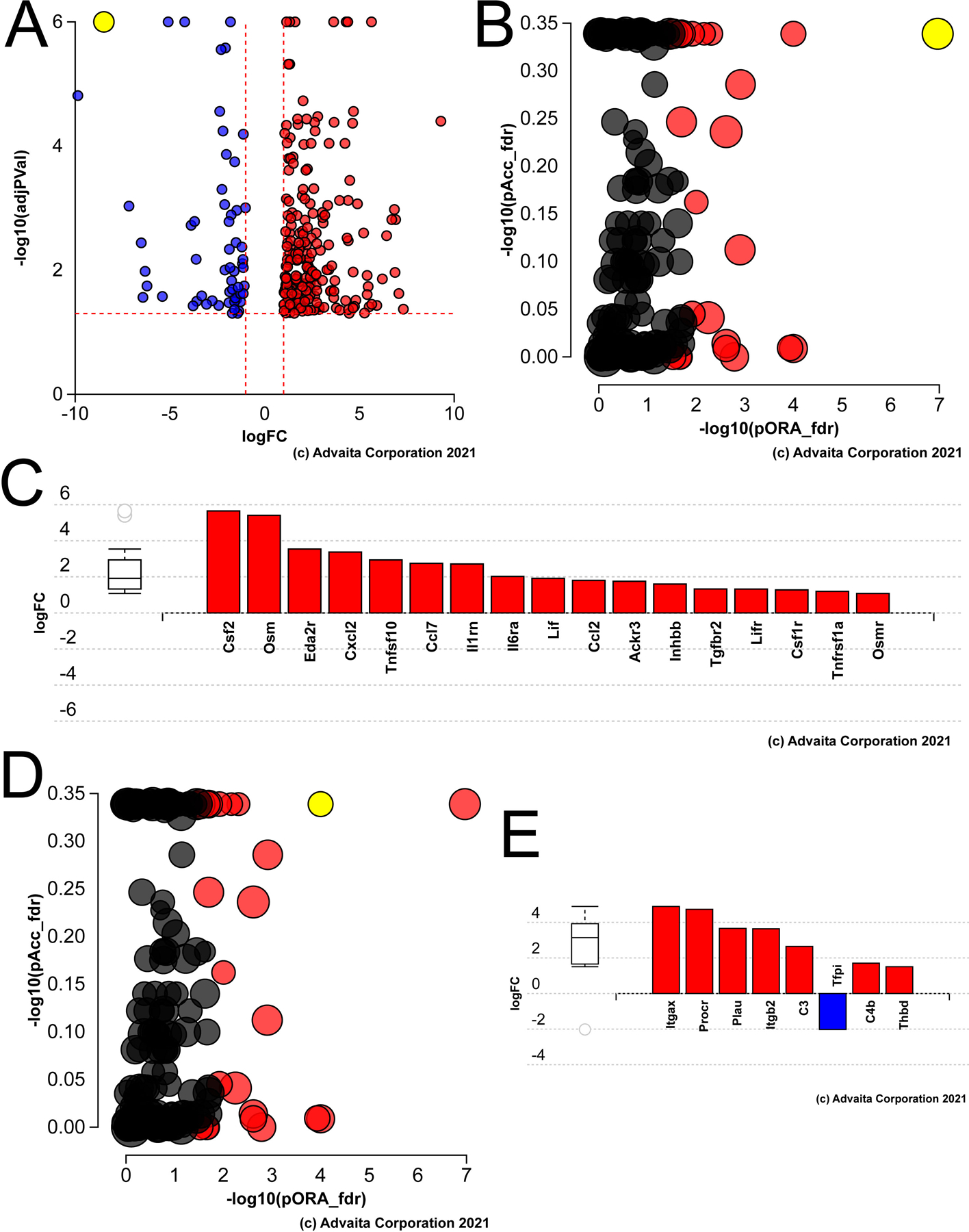
Pathway analysis of genes differentially expressed in aged versus young mouse lens epithelial cells A) Volcano plot of the genes whose expression was statistically different between aged versus young LECs, yellow dot represents γF-crystallin B) Impact analysis of the DEGs suggest that the KEGG pathway map “cytokine-cytokine receptors” (yellow dot) is likely to be the most significantly impacted pathway in the aged lens epithelium. C) Bar graph showing the cytokine-cytokine receptor genes that are differentially expressed in the aged lens epithelium. D) Impact analysis showing that the second significant pathway in the aged lens epithelium represents genes involved in the complement pathway (yellow dot). E) Bar graph showing the complement pathway genes differentially expressed in the aged mouse lens epithelium.
Inspection of the DEG list revealed that the mRNA levels of several β- and γ−crystallins downregulate, with the most dramatic changes (85–348 fold) seen in the mRNAs encoding the gamma-crystallins (γB, γC, γD, γE, γF) which are encoded by the linked genes of the mouse γ−crystallin cluster residing on Chromosome 1 (Duncan et al., 2004; Graw, 2009). As other genes known to exhibit lens preferred expression such as MIP (Bassnett et al., 2009) were also downregulated in aged lens epithelial cells, the gene list was compared to data residing in iSyTE, a bioinformatics tool capable of assessing whether genes exhibit lens-preferred expression (Kakrana et al., 2018; Lachke et al., 2012). This analysis revealed that 24 of the 111 biologically significant DEGs (17 of the DEGs downregulating with aging) exhibit lens preferred expression in 56 day old mice (Table 1).
Table 1.
Lens preferred genes differentially expressed in aged lens epithelium
| SYMBOL | DESCRIPTION | Aged epi versus Young epi fold change | Aged epi versus Young epi FDR | Young epi FPKM | Aged epi FPKM | P56 fold lens enrichment |
|---|---|---|---|---|---|---|
| Crygf | crystallin, gamma F | −348.2 | 3.7E-3 | 10.3 | 0.0 | 271.9 |
| Crygb | crystallin, gamma B | −141.5 | 3.2E-3 | 150.0 | 1.1 | 1644.0 |
| Crygc | crystallin, gamma C | −90.3 | 2.2E-3 | 161.5 | 1.8 | 1644.0 |
| Crygd | crystallin, gamma D | −84.9 | 1.2E-2 | 119.2 | 1.4 | 1640.6 |
| Pgam2 | phosphoglycerate mutase 2 | −9.5 | 9.5E-3 | 6.1 | 0.6 | 7.8 |
| Crygn | crystallin, gamma N | −4.8 | 3.5E-8 | 71.8 | 15.0 | 191.3 |
| Crybb3 | crystallin, beta B3 | −3.7 | 2.0E-6 | 625.4 | 171.1 | 316.5 |
| Mypn | myopalladin | −3.5 | 4.8E-8 | 4.9 | 1.4 | 34.8 |
| Cryba1 | crystallin, beta A1 | −3.1 | 3.3E-5 | 1807.5 | 590.9 | 993.9 |
| Cryba4 | crystallin, beta A4 | −2.9 | 1.3E-2 | 1087.6 | 378.3 | 1083.9 |
| Aldh1a7 | aldehyde dehydrogenase family 1, subfamily A7 | −2.8 | 1.4E-3 | 13.4 | 4.9 | 45.8 |
| Mip | major intrinsic protein of lens fiber | −2.7 | 1.5E-4 | 400.0 | 145.8 | 1282.7 |
| Crybb1 | crystallin, beta B1 | −2.7 | 1.1E-3 | 769.0 | 288.8 | 386.7 |
| Crygs | crystallin, gamma S | −2.4 | 3.0E-3 | 2255.2 | 940.2 | 1525.4 |
| Npnt | nephronectin | −2.2 | 2.8E-7 | 35.9 | 16.7 | 6.9 |
| Add2 | adducin 2 (beta) | −2.1 | 2.9E-5 | 8.1 | 4.0 | 8.9 |
| Cryba2 | crystallin, beta A2 | −2.0 | 6.8E-3 | 2030.8 | 1011.5 | 828.3 |
| Ptgds | prostaglandin D2 synthase (brain) | 2.0 | 6.8E-8 | 110.4 | 225.2 | 59.9 |
| Ephx1 | epoxide hydrolase 1, microsomal | 2.2 | 4.6E-9 | 14.5 | 31.7 | 28.9 |
| Slc4a5 | solute carrier family 4, member 5 | 2.2 | 2.9E-2 | 2.9 | 6.5 | 10.8 |
| Dgkg | diacylglycerol kinase, gamma | 2.5 | 1.8E-14 | 1.5 | 3.7 | 5.7 |
| 2310043M15Rik | RIKEN cDNA 2310043M15 gene | 2.6 | 1.7E-2 | 5.2 | 13.4 | 13.8 |
| Serpinb6b | serine (or cysteine) peptidase inhibitor, B6b | 3.2 | 4.3E-21 | 4.9 | 15.7 | 33.8 |
| Npvf | neuropeptide VF precursor | 5.6 | 1.2E-2 | 0.8 | 4.3 | 2.0 |
A prior study assessed age-associated changes in histone H3 lysine 4 tri-methylation (H3K4me3), a marker of open chromatin, in the mouse lens and identified 613 promoter peaks that either decrease or increase in H3K4me3 in 800 day old mouse lenses (Zheng et al., 2015). Comparison of these peaks with the list of 111 genes exhibiting “biologically significant” differences in expression in aging lens epithelial cells in the present study revealed 20 genes in common. For 18 of these, the direction of their expression change correspond to that predicted from the change in H3K4me3 of the gene’s promoter (Table 2).
Table 2.
Comparison between DEGs observed in the aging lens epithelium with those previously found to exhibit differential histone H3K4 trimethylation in the aging lens (Zheng et al., 2015). Italicized genes represent those where the expected direction of change in mRNA levels and H3K4 trimethylation is discordant
| SYMBOL | DESCRIPTION | Young epi FPKM | Aged epi FPKM | Aged epi versus Young epi fold change expression | Aged epi versus Young epi expression FDR | Aged epi versus Young epi fold change methylation | Aged epi versus Young epi Methylation FDR |
|---|---|---|---|---|---|---|---|
| Crygn | crystallin, gamma | 71.8 | 15.0 | −4.8 | 3.5E-08 | −5.3 | 4.1E-04 |
| Pdgfra | platelet derived growth factor receptor, alpha polypeptide | 16.5 | 3.9 | −4.3 | 2.6E-02 | −5.0 | 1.1E-30 |
| B230312C02Rik | RIKEN cDNA B230312C02 gene | 3.0 | 0.8 | −3.6 | 2.8E-04 | −2.0 | 3.1E-02 |
| Spock2 | sparc/osteonectin, cwcv and kazal-like domains proteoglycan 2 | 4.8 | 1.3 | −3.6 | 8.9E-08 | −2.8 | 5.9E-12 |
| Sema6a | semaphorin 6A | 3.6 | 1.0 | −3.5 | 4.9E-07 | −2.6 | 4.0E-22 |
| Timeless | timeless circadian clock 1 | 3.4 | 1.2 | −2.8 | 5.9E-06 | 2.0 | 1.0E-02 |
| Crybb1 | crystallin, beta B1 | 769.0 | 288.8 | −2.7 | 1.1E-03 | 1.6 | 1.8E-05 |
| Mfap2 | microfibrillar-associated protein 2 | 13.0 | 5.5 | −2.4 | 2.3E-02 | −2.2 | 9.5E-06 |
| Nr1d1 | nuclear receptor subfamily 1, group D, member 1 | 6.8 | 14.4 | 2.1 | 2.8E-04 | 2.3 | 2.1E-23 |
| Ephx1 | epoxide hydrolase 1, microsomal | 14.5 | 31.7 | 2.2 | 4.6E-09 | 2.1 | 4.2E-04 |
| Cd82 | CD82 antigen | 7.0 | 15.8 | 2.2 | 8.1E-09 | 2.7 | 3.6E-11 |
| Pcdhb22 | protocadherin beta 22 | 2.2 | 5.0 | 2.3 | 1.0E-05 | 7.1 | 7.4E-12 |
| Egfr | epidermal growth factor receptor | 2.3 | 5.2 | 2.3 | 1.0E-07 | 2.3 | 2.9E-09 |
| Trp53i11 | transformation related protein 53 inducible protein 11 [ | 1.6 | 3.7 | 2.3 | 6.1E-03 | 1.8 | 4.9E-02 |
| Trim47 | tripartite motif-containing 47 | 3.3 | 7.8 | 2.3 | 5.2E-04 | 3.4 | 1.2E-18 |
| Dgkg | diacylglycerol kinase, gamma | 1.5 | 3.7 | 2.5 | 1.8E-14 | 2.0 | 6.3E-10 |
| Nceh1 | neutral cholesterol ester hydrolase 1 | 1.2 | 3.3 | 2.6 | 2.7E-02 | 1.8 | 2.7E-02 |
| Serpinb6b | serine (or cysteine) peptidase inhibitor, clade B, member 6b | 4.9 | 15.7 | 3.2 | 4.3E-21 | 4.5 | 3.9E-12 |
| Klf4 | Kruppel-like factor 4 (gut) | 2.7 | 9.9 | 3.7 | 2.2E-03 | 1.7 | 1.9E-03 |
| Ndufa4l2 | Ndufa4, mitochondrial complex associated like 2 | 6.9 | 36.6 | 5.3 | 6.7E-03 | 3.9 | 5.1E-08 |
Ipathway guide analysis of the DEGs identified in aged LECs using DEG lists for which normalization by TMM scaling was done based on all epithelial samples did not reveal strong signals for enriched pathways, although they included complement/coagulation (p=1.5 X 10−5) and cytokine/cytokine receptor interactions (p=3 X 10−3), (data not shown). Determination of genes differentially expressed in aged LECs using unnormalized pairwise comparisons revealed that the most impacted KEGG pathways included cytokine-cytokine receptor (Figure 1B, 1C; p=1.5 X 10−6) and complement/coagulation (Figure 1D, 1E; p=9.4 X 10−7), while many DEGs map to the gene ontology term “immune system process”(not shown; p=1 X 10−16).
Effect of aging on the lens fiber cell transcriptome
Comparison between the young and aged LFC transcriptome revealed 2145 genes to be significantly differentially expressed by at least two fold (false discovery rate (FDR) corrected P value ≤ 0.05), with 832 of these downregulated and 1313 upregulated. Filtering this list further for genes that meet previously described criteria for likely “biological significance” (minimum expression level of 2 FPKM in either condition, at least 2 FPKM absolute change in expression level) revealed 703 DEGs, (178 upregulated genes, 525 downregulated; Figure 2A; see supplemental Table 3 for list).
Figure 2.
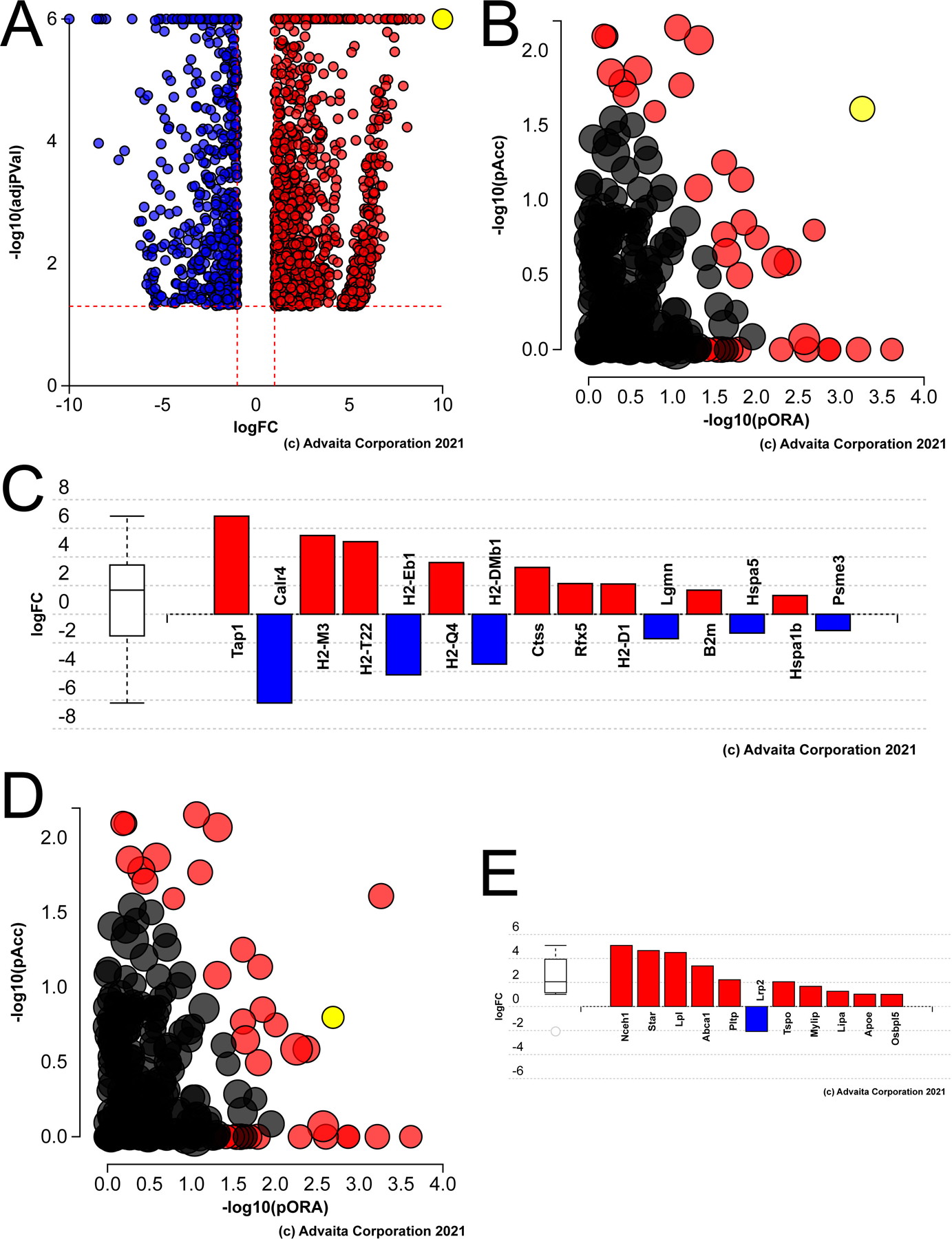
Pathway analysis of genes differentially expressed in aged versus young mouse lens fiber cells A) Volcano plot of the genes whose expression was statistically different between aged versus young LECs, yellow dot represents cdkn1a (P21) B) Impact analysis of the DEGs suggest that the KEGG pathway map “antigen processing and presentation” (yellow dot) is likely to be the most significantly impacted pathway in the aged lens fibers. C) Bar graph showing the antigen processing and presentation genes that are differentially expressed in the aged lens fibers. D) Impact analysis showing that the another significantly impacted pathway in the aged lens fibers represents genes involved in cholesterol metabolism (yellow dot). E) Bar graph showing the cholesterol metabolism genes differentially expressed in the aged mouse lens fibers.
Similar to the lens epithelium, the expression of all six genes of the gamma-crystallin cluster (cryga-crygf) found on mouse chromosome 1 are profoundly downregulated (44–56 fold) in aged lens fiber cells. Comparison of these 703 DEGs with the iSyTE database (Kakrana et al., 2018; Lachke et al., 2012) revealed that 82 of the genes downregulated and 19 of genes upregulated with aging are predicted to exhibit preferential expression in the lens at 56 days postnatal (Table 3).
Table 3.
Lens preferred genes differentially expressed in aged lens fibers
| SYMBOL | DESCRIPTION | Aged fibers versus Young fibers fold change | Aged fibers versus Young fibers FDR | Young fibers FPKM | Aged fibers FPKM | P56 fold lens enrichment |
|---|---|---|---|---|---|---|
| Wdfy4 | WD repeat and FYVE domain containing 4 | −99.3 | 6.2E-12 | 3.0 | 0.0 | 13.6 |
| Crygf | crystallin, gamma F | −56.0 | 7.3E-3 | 6469.7 | 115.6 | 271.9 |
| Crygb | crystallin, gamma B | −50.9 | 3.7E-3 | 25498.4 | 501.1 | 1644.0 |
| Cryga | crystallin, gamma A | −49.7 | 2.2E-2 | 255.2 | 5.1 | 595.9 |
| Crygd | crystallin, gamma D | −46.7 | 5.9E-3 | 22784.3 | 487.7 | 1640.6 |
| Crygc | crystallin, gamma C | −43.8 | 1.7E-3 | 23495.1 | 535.8 | 1644.0 |
| Chrng | cholinergic receptor, nicotinic, gamma | −34.2 | 5.8E-19 | 4.7 | 0.1 | 2.3 |
| Gp2 | glycoprotein 2 (zymogen granule membrane) | −33.2 | 3.0E-5 | 5.0 | 0.1 | 13.4 |
| Pgam2 | phosphoglycerate mutase 2 | −30.0 | 1.6E-6 | 335.5 | 11.2 | 7.8 |
| 1700020N01Rik | RIKEN cDNA 1700020N01 gene | −14.9 | 1.2E-6 | 12.7 | 0.8 | 65.2 |
| Ceacam10 | carcinoembryonic antigen-related cell adhesion molecule 10 | −14.4 | 2.8E-4 | 8.4 | 0.6 | 33.8 |
| Tcp11 | t-complex protein 11 | −13.7 | 7.6E-12 | 148.8 | 10.9 | 39.7 |
| Crybb3 | crystallin, beta B3 | −11.8 | 6.4E-23 | 10176.2 | 864.0 | 316.5 |
| Zfp354b | zinc finger protein 354B | −11.2 | 7.1E-8 | 8.2 | 0.7 | 14.2 |
| E130119H09Rik | RIKEN cDNA E130119H09 gene | −11.1 | 4.6E-6 | 52.6 | 4.7 | 16.5 |
| Pgap2 | post-GPI attachment to proteins 2 | −10.7 | 6.0E-64 | 19.9 | 1.9 | 22.2 |
| Snx22 | sorting nexin 22 | −9.5 | 1.1E-8 | 76.0 | 8.0 | 16.4 |
| Birc7 | baculoviral IAP repeat-containing 7 (livin) | −9.2 | 1.1E-5 | 295.7 | 32.0 | 66.5 |
| C920006O11Rik | RIKEN cDNA C920006O11 gene | −8.8 | 1.5E-7 | 7.1 | 0.8 | 43.3 |
| Ankrd24 | ankyrin repeat domain 24 | −8.5 | 6.6E-25 | 20.1 | 2.4 | 42.8 |
| Hspb1 | heat shock protein 1 | −8.1 | 4.8E-8 | 2679.5 | 330.3 | 34.7 |
| Crygn | crystallin, gamma N | −8.0 | 3.9E-15 | 157.4 | 19.9 | 191.3 |
| Ces5a | carboxylesterase 5A | −7.5 | 1.6E-4 | 60.5 | 8.0 | 26.8 |
| Rnf180 | ring finger protein 180 | −7.1 | 1.1E-25 | 29.0 | 4.1 | 87.1 |
| Rsph10b | radial spoke head 10 homolog B (Chlamydomonas) | −7.0 | 4.0E-53 | 44.5 | 6.3 | 114.4 |
| Pla2g7 | platelet-activating factor acetylhydrolase, plasma | −6.6 | 1.8E-9 | 8.2 | 1.3 | 3.2 |
| Stxbp6 | syntaxin binding protein 6 (amisyn) | −6.0 | 4.3E-11 | 5.2 | 0.9 | 2.8 |
| Gm4850 | predicted pseudogene 4850 | −5.8 | 4.2E-2 | 10.8 | 1.8 | 24.5 |
| Metrnl | meteorin, glial cell differentiation regulator-like | −5.7 | 1.7E-43 | 133.2 | 23.5 | 61.8 |
| Cela1 | chymotrypsin-like elastase family, member 1 | −5.7 | 5.1E-24 | 34.7 | 6.1 | 90.4 |
| Stx11 | syntaxin 11 | −5.4 | 7.2E-10 | 12.8 | 2.4 | 20.2 |
| Lgi2 | leucine-rich repeat LGI family, member 2 | −5.2 | 4.2E-22 | 5.6 | 1.1 | 5.3 |
| Clic5 | chloride intracellular channel 5 | −5.0 | 7.1E-8 | 31.2 | 6.2 | 63.1 |
| Crybb1 | crystallin, beta B1 | −4.9 | 1.1E-10 | 10608.6 | 2162.6 | 386.7 |
| Lim2 | lens intrinsic membrane protein 2 | −4.7 | 1.8E-2 | 12.3 | 2.6 | 350.2 |
| Ngef | neuronal guanine nucleotide exchange factor | −4.7 | 7.3E-12 | 68.7 | 14.7 | 38.6 |
| Cryba4 | crystallin, beta A4 | −4.4 | 8.6E-6 | 19980.3 | 4564.6 | 1083.9 |
| Aldh1a3 | aldehyde dehydrogenase family 1, subfamily A3 | −4.2 | 1.4E-5 | 2.8 | 0.7 | 3.0 |
| Hmox1 | heme oxygenase 1 | −4.1 | 6.3E-5 | 698.6 | 170.7 | 33.1 |
| Crygs | crystallin, gamma S | −4.1 | 2.9E-9 | 23770.5 | 5861.1 | 1525.4 |
| Bfsp1 | beaded filament structural protein 1, in lens-CP94 | −4.0 | 1.9E-15 | 12416.0 | 3101.2 | 507.8 |
| Slc7a5 | solute carrier family 7 member 5 | −3.9 | 8.8E-24 | 19.0 | 4.9 | 2.2 |
| Lrrc66 | leucine rich repeat containing 66 | −3.7 | 9.0E-14 | 8.9 | 2.4 | 13.7 |
| Gadd45b | growth arrest and DNA-damage-inducible 45 beta | −3.7 | 5.4E-12 | 24.3 | 6.6 | 6.9 |
| Cryba1 | crystallin, beta A1 | −3.5 | 1.4E-7 | 13744.0 | 3952.3 | 993.9 |
| Cryba2 | crystallin, beta A2 | −3.4 | 9.96E-10 | 14290.4 | 4219.9 | 828.3 |
| Pappa | pregnancy-associated plasma protein A | −3.2 | 4.9E-17 | 7.9 | 2.5 | 5.9 |
| Cryaa | crystallin, alpha A | −3.1 | 2.1E-12 | 57208.0 | 18646.8 | 867.1 |
| Eif5b | eukaryotic translation initiation factor 5B | −3.1 | 3.1E-20 | 289.0 | 94.7 | 20.0 |
| Crybb2 | crystallin, beta B2 | −3.0 | 3.9E-9 | 90716.5 | 30042.2 | 651.2 |
| Fas | Fas (TNF receptor superfamily member 6) | −3.0 | 5.9E-13 | 27.7 | 9.3 | 30.6 |
| Sh3bgr | SH3-binding domain glutamic acid-rich protein | −2.8 | 1.5E-10 | 29.0 | 10.3 | 13.5 |
| Cryab | crystallin, alpha B | −2.8 | 2.0E-15 | 13162.1 | 4685.7 | 176.6 |
| Hsf4 | heat shock transcription factor 4 | −2.8 | 2.0E-5 | 149.0 | 53.2 | 8.1 |
| Grifin | galectin-related inter-fiber protein | −2.8 | 3.6E-13 | 1143.0 | 408.7 | 288.4 |
| Entpd1 | ectonucleoside triphosphate diphosphohydrolase 1 | −2.8 | 4.3E-6 | 36.6 | 13.3 | 28.4 |
| Fgfrl1 | fibroblast growth factor receptor-like 1 | −2.7 | 2.0E-15 | 26.5 | 9.8 | 3.4 |
| Mip | major intrinsic protein of lens fiber | −2.7 | 2.2E-5 | 3103.0 | 1150.6 | 1282.7 |
| Fxyd1 | FXYD domain-containing ion transport regulator 1 | −2.6 | 2.4E-16 | 43.7 | 16.6 | 14.3 |
| Casp7 | caspase 7 | −2.6 | 7.3E-12 | 13.0 | 4.9 | 3.8 |
| Ninj1 | ninjurin 1 | −2.6 | 1.4E-7 | 17.8 | 6.9 | 8.1 |
| Pcbd2 | pterin 4 alpha carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 alpha (TCF1) 2 | −2.6 | 1.9E-11 | 212.5 | 83.1 | 14.3 |
| P4ha1 | procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), alpha 1 polypeptide | −2.5 | 2.4E-16 | 105.8 | 41.6 | 9.7 |
| Sned1 | sushi, nidogen and EGF-like domains 1 | −2.5 | 1.3E-4 | 3.3 | 1.3 | 5.7 |
| Mypn | myopalladin | −2.5 | 1.8E-5 | 51.3 | 20.4 | 34.8 |
| Pacsin3 | protein kinase C and casein kinase substrate in neurons 3 | −2.5 | 1.0E-11 | 121.5 | 48.6 | 22.9 |
| Ing2 | inhibitor of growth family, member 2 | −2.5 | 3.2E-13 | 100.7 | 40.3 | 13.6 |
| Dmrta2 | doublesex and mab-3 related transcription factor like A2] | −2.4 | 1.9E-13 | 35.5 | 14.5 | 15.4 |
| Zfp385a | zinc finger protein 385A | −2.4 | 1.5E-10 | 75.7 | 31.5 | 2.8 |
| Bfsp2 | beaded filament structural protein 2, phakinin | −2.3 | 2.1E-6 | 5196.8 | 2218.4 | 181.0 |
| Snta1 | syntrophin, acidic 1 | −2.3 | 6.7E-18 | 63.5 | 27.3 | 20.1 |
| Caprin2 | caprin family member 2 | −2.3 | 9.0E-4 | 13.0 | 5.6 | 14.7 |
| Ell2 | elongation factor RNA polymerase II 2 | −2.3 | 1.4E-10 | 24.2 | 10.6 | 11.5 |
| Nlrc5 | NLR family, CARD domain containing 5 | −2.2 | 1.8E-4 | 6.0 | 2.7 | 5.8 |
| Aldh1a7 | aldehyde dehydrogenase family 1, subfamily A7 | −2.2 | 9.9E-3 | 5.5 | 2.5 | 45.8 |
| S100a6 | S100 calcium binding protein A6 (calcyclin) | −2.2 | 4.0E-17 | 255.2 | 115.5 | 42.6 |
| Tom1 | target of myb1 trafficking protein | −2.2 | 1.1E-7 | 22.8 | 10.3 | 5.8 |
| Smco3 | single-pass membrane protein with coiled-coil domains 3 | −2.1 | 2.7E-4 | 65.6 | 31.1 | 199.5 |
| Tdrd7 | tudor domain containing 7 | −2.1 | 6.6E-6 | 557.9 | 265.3 | 92.8 |
| Dhx32 | DEAH (Asp-Glu-Ala-His) box polypeptide 32 | −2.1 | 8.3E-11 | 152.6 | 74.0 | 32.1 |
| Hopx | HOP homeobox | −2.0 | 1.1E-4 | 24.3 | 11.9 | 8.8 |
| Slc24a4 | solute carrier family 24 member 4 | −2.0 | 2.4E-3 | 44.3 | 21.8 | 20.3 |
| Stx3 | syntaxin 3 | 2.1 | 1.3E-4 | 1.9 | 4.1 | 4.7 |
| Neat1 | nuclear paraspeckle assembly transcript 1 | 2.2 | 1.0E-7 | 2.6 | 5.8 | 16.2 |
| Bmper | BMP-binding endothelial regulator | 2.4 | 2.9E-5 | 2.7 | 6.5 | 2.3 |
| Gstm1 | glutathione S-transferase, mu 1 | 2.4 | 2.7E-4 | 25.1 | 59.7 | 17.7 |
| Necab1 | N-terminal EF-hand calcium binding protein 1 | 2.4 | 9.8E-7 | 2.1 | 5.1 | 32.5 |
| 2310030G06Rik | RIKEN cDNA 2310030G06 gene | 2.9 | 3.4E-8 | 2.9 | 8.6 | 46.7 |
| Scel | sciellin | 3.5 | 4.3E-6 | 2.3 | 7.9 | 38.8 |
| Cyp26a1 | cytochrome P450, family 26, subfamily a, polypeptide 1 | 3.5 | 7.4E-6 | 3.4 | 12.0 | 4.1 |
| Serpinb6b | serine (or cysteine) peptidase inhibitor, clade B, member 6b | 3.8 | 1.6E-28 | 20.5 | 77.3 | 33.8 |
| Chrdl1 | chordin-like 1 | 4.2 | 7.7E-5 | 0.7 | 2.9 | 17.2 |
| Foxe3 | forkhead box E3 | 4.2 | 1.3E-3 | 3.8 | 16.3 | 4.0 |
| Ephx1 | epoxide hydrolase 1, microsomal [ | 4.7 | 1.3E-32 | 9.4 | 44.4 | 28.9 |
| Folr1 | folate receptor 1 (adult) | 4.8 | 1.2E-2 | 1.3 | 6.4 | 56.5 |
| Cdh1 | cadherin 1 | 5.0 | 1.9E-8 | 2.0 | 10.2 | 6.0 |
| Arsi | arylsulfatase i | 6.1 | 1.5E-2 | 0.8 | 5.1 | 9.5 |
| Cabp5 | calcium binding protein 5 | 6.2 | 1.9E-6 | 0.6 | 4.0 | 2.8 |
| Cdkn2b | cyclin dependent kinase inhibitor 2B | 6.4 | 5.8E-13 | 3.7 | 23.9 | 7.5 |
| Aldh3a1 | aldehyde dehydrogenase family 3, subfamily A1 | 9.2 | 3.0E-9 | 10.7 | 98.5 | 347.8 |
| Tinag | tubulointerstitial nephritis antigen | 11.6 | 1.3E-17 | 1.2 | 13.4 | 21.5 |
Comparison of the 613 genes previously reported to exhibit age-related changes in H3K4me3 in the lens(Zheng et al., 2015) with the list of 703 genes exhibiting “biologically significant” differences in expression in aging lens fibers in the present study revealed 54 genes in common. For 48 of these, the direction of their expression change corresponds to that predicted from the change in H3K4me3 of the gene’s promoter (Table 4).
Table 4.
Comparison between DEGs observed in the aging lens fibers with those previously found to exhibit differential histone H3K4 trimethylation in the aging lens (Zheng et al., 2015). Italicized genes represent those where the expected direction of change in mRNA levels and H3K4 trimethylation is discordant
| SYMBOL | DESCRIPTION | Young fibers FPKM | Aged fibers FPKM | Aged fibers versus Young fibers fold change expression | Aged fibers versus Young fibers FDR expression | Aged fibers versus Young lens fold change methylation | Aged fibers versus Young lens methylation FDR |
|---|---|---|---|---|---|---|---|
| Igf2bp2 | insulin-like growth factor 2 mRNA binding protein 2 | 2.0 | 0.0 | −1042.2 | 4.7E-29 | −14.5 | 4.0E-67 |
| Eva1a | eva-1 homolog A (C. elegans) | 15.6 | 0.7 | −22.6 | 4.0E-19 | −1.6 | 4.6E-02 |
| B230312C02Rik | RIKEN cDNA B230312C02 gene | 6.4 | 0.6 | −10.7 | 2.5E-12 | −2.0 | 3.1E-02 |
| Snx22 | sorting nexin 22 | 76.0 | 8.0 | −9.5 | 1.1E-08 | −1.7 | 2.1E-03 |
| Crygn | crystallin, gamma N | 157.4 | 19.9 | −8.0 | 3.9E-15 | −5.3 | 4.1E-04 |
| 1700101I11Rik | RIKEN cDNA 1700101I11 gene | 8.2 | 1.1 | −7.8 | 1.2E-08 | −1.6 | 1.6E-03 |
| Rasgrp1 | RAS guanyl releasing protein 1 | 2.5 | 0.4 | −6.6 | 1.8E-03 | −1.8 | 4.9E-02 |
| Stxbp6 | syntaxin binding protein 6 (amisyn) | 5.2 | 0.9 | −6.0 | 4.3E-11 | −2.1 | 1.6E-03 |
| Zfyve28 | zinc finger, FYVE domain containing 28 | 11.0 | 1.8 | −5.9 | 3.7E-09 | −2.8 | 1.9E-07 |
| Lhfp | lipoma HMGIC fusion partner | 3.4 | 0.6 | −5.5 | 4.7E-04 | −1.6 | 2.1E-02 |
| Lgi2 | leucine-rich repeat LGI family, member 2 | 5.6 | 1.1 | −5.2 | 4.2E-22 | −2.1 | 4.8E-03 |
| Crybb1 | crystallin, beta B1 | 10608.6 | 2162.6 | −4.9 | 1.1E-10 | 1.6 | 1.8E-05 |
| Wnk2 | WNK lysine deficient protein kinase 2 | 13.2 | 2.8 | −4.8 | 3.2E-27 | −1.8 | 1.1E-04 |
| Stambpl1 | STAM binding protein like 1 | 17.4 | 3.9 | −4.4 | 9.7E-04 | −1.7 | 1.2E-03 |
| Gck | glucokinase | 4.2 | 1.0 | −4.3 | 6.0E-04 | −2.5 | 2.4E-05 |
| Gng2 | guanine nucleotide binding protein (G protein), gamma 2 | 9.8 | 2.5 | −3.9 | 2.5E-17 | −1.9 | 1.6E-02 |
| Slc7a5 | solute carrier family 7 (cationic amino acid transporter, y+ system), member 5 | 19.0 | 4.9 | −3.9 | 8.8E-24 | −2.3 | 2.4E-11 |
| Chchd7 | coiled-coil-helix-coiled-coil-helix domain containing 7 | 13.7 | 3.6 | −3.8 | 4.1E-16 | −1.9 | 9.5E-11 |
| Galnt14 | polypeptide N-acetylgalactosaminyltransferase 14 | 3.7 | 1.0 | −3.7 | 3.1E-04 | −3.0 | 1.4E-02 |
| Gabarapl1 | gamma-aminobutyric acid (GABA) A receptor-associated protein-like 1 | 87.3 | 26.5 | −3.3 | 3.0E-27 | −1.6 | 1.6E-03 |
| Itpk1 | inositol 1,3,4-triphosphate 5/6 kinase | 16.0 | 5.0 | −3.2 | 6.2E-13 | −1.6 | 3.2E-04 |
| Cryaa | crystallin, alpha A | 57208.0 | 18646.8 | −3.1 | 2.1E-12 | 1.5 | 5.9E-03 |
| Ube2o | ubiquitin-conjugating enzyme E2O | 106.9 | 37.7 | −2.8 | 3.5E-19 | −1.7 | 1.6E-04 |
| Timeless | timeless circadian clock 1 | 14.2 | 5.0 | −2.8 | 4.3E-07 | 2.0 | 1.0E-02 |
| Fxyd1 | FXYD domain-containing ion transport regulator 1 | 43.7 | 16.6 | −2.6 | 2.4E-16 | 2.1 | 2.4E-02 |
| S100a6 | S100 calcium binding protein A6 (calcyclin) | 255.2 | 115.5 | −2.2 | 4.0E-17 | 3.3 | 1.6E-34 |
| Fam210b | family with sequence similarity 210, member B | 6.7 | 3.1 | −2.2 | 7.4E-06 | −1.5 | 2.9E-03 |
| D630045J12Rik | RIKEN cDNA D630045J12 gene | 7.9 | 3.6 | −2.2 | 2.9E-08 | −1.5 | 7.2E-03 |
| Neat1 | nuclear paraspeckle assembly transcript 1 | 2.6 | 5.8 | 2.2 | 1.0E-07 | 1.8 | 6.8E-12 |
| Jup | junction plakoglobin | 3.5 | 8.2 | 2.4 | 7.1E-10 | 2.8 | 2.5E-12 |
| S100a13 | S100 calcium binding protein A13 | 1.6 | 4.4 | 2.8 | 3.4E-02 | 2.9 | 9.9E-05 |
| Scarf2 | scavenger receptor class F, member 2 | 1.2 | 3.4 | 2.9 | 8.1E-04 | 2.1 | 7.0E-05 |
| Tjp2 | tight junction protein 2 | 1.4 | 4.2 | 3.0 | 1.7E-16 | 1.6 | 1.5E-03 |
| Scel | sciellin | 2.3 | 7.9 | 3.5 | 4.3E-06 | 1.7 | 6.1E-04 |
| Rtn1 | reticulon 1 | 0.9 | 3.2 | 3.5 | 7.1E-10 | 4.2 | 1.2E-19 |
| Cyp26a1 | cytochrome P450, family 26, subfamily a, polypeptide 1 | 3.4 | 12.0 | 3.5 | 7.4E-06 | 2.1 | 7.3E-09 |
| Phlda3 | pleckstrin homology like domain, family A, member 3 | 6.9 | 25.3 | 3.6 | 7.1E-09 | 2.5 | 4.3E-14 |
| E130102H24Rik | RIKEN cDNA E130102H24 gene | 1.1 | 4.1 | 3.8 | 2.9E-02 | 1.6 | 5.5E-05 |
| Serpinb6b | serine (or cysteine) peptidase inhibitor, clade B, member 6b | 20.5 | 77.3 | 3.8 | 1.6E-28 | 4.5 | 3.9E-12 |
| Dmpk | dystrophia myotonica-protein kinase | 3.8 | 15.7 | 4.2 | 1.8E-24 | 2.3 | 1.9E-04 |
| Esyt3 | extended synaptotagmin-like protein 3 | 0.8 | 3.3 | 4.3 | 8.0E-19 | 1.5 | 4.1E-04 |
| Ephx1 | epoxide hydrolase 1, microsomal | 9.4 | 44.4 | 4.7 | 1.3E-32 | 2.1 | 4.1E-04 |
| Trim47 | tripartite motif-containing 47 | 1.0 | 4.9 | 4.8 | 1.6E-10 | 3.4 | 1.2E-18 |
| Pdpn | podoplanin | 2.7 | 15.2 | 5.6 | 2.6E-09 | 1.7 | 1.6E-05 |
| R3hdml | R3H domain containing-like | 7.3 | 41.4 | 5.6 | 5.0E-05 | 2.1 | 7.0E-07 |
| Cd9 | CD9 antigen | 1.3 | 7.4 | 5.8 | 3.5E-18 | 2.5 | 2.9E-10 |
| Cdkn2b | cyclin dependent kinase inhibitor 2B | 3.7 | 23.9 | 6.4 | 5.8E-13 | 2.3 | 5.5E-06 |
| Cnksr1 | connector enhancer of kinase suppressor of Ras 1 | 0.4 | 2.7 | 6.8 | 8.7E-09 | 4.6 | 2.3E-09 |
| Hmgcs2 | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 | 2.0 | 14.9 | 7.3 | 1.3E-03 | 5.9 | 4.0E-10 |
| Ddit4l | DNA-damage-inducible transcript 4-like | 0.5 | 3.7 | 7.5 | 3.2E-07 | 2.0 | 1.3E-03 |
| Ntn4 | netrin 4 | 0.7 | 6.0 | 8.8 | 2.3E-26 | 2.4 | 4.2E-11 |
| Igfbp5 | insulin-like growth factor binding protein 5 | 0.6 | 5.8 | 10.2 | 4.4E-06 | −1.9 | 2.2E-03 |
| Ifi27 | interferon, alpha-inducible protein 27 | 0.1 | 2.6 | 20.3 | 5.4E-11 | 2.2 | 1.2E-04 |
| Ndufa4l2 | Ndufa4, mitochondrial complex associated like 2 | 0.3 | 6.8 | 21.0 | 3.3E-07 | 3.9 | 5.1E-08 |
Notably, the list of lens enriched genes whose expression is altered in aging lens fibers included three transcription factors with known roles in regulating genes important for lens phenotype. Maf, which is a transcription factor that is necessary for the initial stages of fiber cell differentiation, is downregulated 1.8 fold (p=3.8 X 10−8), and HSF4, a transcription factor important for the later steps in lens fiber cell differentiation, including gamma crystallin expression (Cui et al., 2013; Fujimoto et al., 2004; Min et al., 2004), was downregulated 2.8 fold (p=2X10−5) in aging lens fibers. Conversely, FoxE3, a transcription factor important for lens epithelial maintenance (Blixt et al., 2000; Medina-Martinez et al., 2005), while being implicated in the repression of gamma crystallin expression (Landgren et al., 2008), was 4.2 fold upregulated (p=1.3X10−3) in aging lens fiber cells.
Comparison of genes differentially regulated in the newborn HSF4 null lenses (He et al., 2010), with the genes differentially expressed in aged lens fibers revealed that 63 of the downregulated DEGs in the aging lens were also downregulated in the HSF4 null lens, while only 1 of the upregulated genes was upregulated in the HSF4 null lens (Table 5). Comparisons between genes previously reported as differentially regulated in newborn lens fibers upon FoxE3 upregulation (Landgren et al., 2008) and DEGs in aged lens fibers revealed that these sets have 71 genes in common (Table 6), 64 of which are downregulated in both aged lens fibers, and young lens fibers that over express FoxE3.
Table 5.
Genes differentially expressed in aged lens fibers which have previously be found to be differentially expressed in HSF4 null lenses
| SYMBOL | DESCRIPTION | Aged versus young fibers fold change | Aged versus young fibers FDR | Young fibers FPKM | Aged fibers FPKM | HSF4 null lens fold change | HSF4vWT FDR |
|---|---|---|---|---|---|---|---|
| Tnfaip6 | tumor necrosis factor alpha induced protein 6 | −40.9 | 1.4E-06 | 6.0 | 0.2 | −3.5 | 3.3E-03 |
| Ceacam10 | carcinoembryonic antigen-related cell adhesion molecule 10 | −14.4 | 2.8E-04 | 8.4 | 0.6 | −69.8 | 1.3E-04 |
| Tcp11 | t-complex protein 11 | −13.7 | 7.6E-12 | 148.8 | 10.9 | −27.3 | 2.3E-05 |
| Dgat2 | diacylglycerol O-acyltransferase 2 | −12.3 | 6.7E-06 | 2.3 | 0.2 | −3.3 | 5.9E-04 |
| Pgap2 | post-GPI attachment to proteins 2 | −10.7 | 6.0E-64 | 19.9 | 1.9 | −3.0 | 1.3E-03 |
| Snx22 | sorting nexin 22 | −9.5 | 1.1E-08 | 76.0 | 8.0 | −1.6 | 1.0E-02 |
| Hspb1 | heat shock protein 1 | −8.1 | 4.8E-08 | 2679.5 | 330.3 | −17.8 | 1.1E-04 |
| Chrna4 | cholinergic receptor, nicotinic, alpha polypeptide 4 | −6.4 | 5.3E-40 | 26.5 | 4.1 | 2.1 | 2.3E-02 |
| Cela1 | chymotrypsin-like elastase family, member 1 | −5.7 | 5.1E-24 | 34.7 | 6.1 | −2.4 | 3.4E-03 |
| Kifc3 | kinesin family member C3 | −4.8 | 4.3E-33 | 28.0 | 5.9 | −2.5 | 1.4E-03 |
| Gpsm2 | G-protein signalling modulator 2 (AGS3-like, C. elegans) | −4.6 | 9.7E-07 | 22.2 | 4.8 | −2.4 | 1.7E-02 |
| Med7 | mediator complex subunit 7 | −4.5 | 3.6E-18 | 16.6 | 3.7 | −1.9 | 4.2E-03 |
| Stambpl1 | STAM binding protein like 1 | −4.4 | 9.7E-04 | 17.4 | 3.9 | −4.7 | 1.1E-04 |
| Zfp428 | zinc finger protein 428 | −4.2 | 2.4E-06 | 15.8 | 3.8 | −2.0 | 1.1E-02 |
| Trim26 | tripartite motif-containing 26 | −4.2 | 3.7E-42 | 22.1 | 5.3 | −1.8 | 5.4E-03 |
| Btf3l4 | basic transcription factor 3-like 4 | −4.1 | 2.4E-46 | 46.9 | 11.4 | −2.3 | 1.1E-03 |
| Hmox1 | heme oxygenase 1 | −4.1 | 6.3E-05 | 698.6 | 170.7 | −5.7 | 8.0E-05 |
| Bfsp1 | beaded filament structural protein 1, in lens-CP94 | −4.0 | 1.9E-15 | 12416.0 | 3101.2 | −3.1 | 1.7E-04 |
| Ttc27 | tetratricopeptide repeat domain 27 | −4.0 | 2.7E-12 | 16.3 | 4.1 | −2.3 | 9.5E-03 |
| Gng2 | guanine nucleotide binding protein (G protein), gamma 2 | −3.9 | 2.5E-17 | 9.8 | 2.5 | −1.6 | 2.2E-02 |
| Usp45 | ubiquitin specific peptidase 45 | −3.8 | 8.4E-10 | 8.4 | 2.2 | −1.6 | 4.5E-02 |
| Hspe1 | heat shock protein 1 (chaperonin 10) | −3.7 | 3.3E-32 | 117.3 | 31.5 | −1.7 | 1.2E-02 |
| Txndc12 | thioredoxin domain containing 12 (endoplasmic reticulum) | −3.7 | 4.4E-29 | 38.1 | 10.4 | −2.0 | 2.8E-03 |
| Ddt | D-dopachrome tautomerase | −3.5 | 7.0E-04 | 14.4 | 4.1 | −2.4 | 1.2E-03 |
| Smap2 | small ArfGAP 2 | −3.5 | 3.6E-24 | 51.3 | 14.7 | −2.7 | 6.1E-04 |
| Sugp2 | SURP and G patch domain containing 2 | −3.5 | 3.2E-24 | 4.9 | 1.4 | −1.6 | 2.4E-02 |
| Inpp5a | inositol polyphosphate-5-phosphatase A | −3.5 | 2.1E-30 | 35.4 | 10.2 | −1.6 | 2.7E-02 |
| Gabarapl1 | gamma-aminobutyric acid (GABA) A receptor-associated protein-like 1 | −3.3 | 3.0E-27 | 87.3 | 26.5 | −1.6 | 3.1E-02 |
| Spns2 | spinster homolog 2 | −3.2 | 2.4E-10 | 25.8 | 8.0 | −5.8 | 1.3E-03 |
| Zfand5 | zinc finger, AN1-type domain 5 | −3.2 | 1.8E-26 | 12.9 | 4.0 | −1.5 | 1.5E-02 |
| Sin3b | transcriptional regulator, SIN3B (yeast) | −3.1 | 5.0E-27 | 52.1 | 16.7 | −1.5 | 1.8E-02 |
| Tex9 | testis expressed gene 9 | −3.1 | 2.1E-25 | 8.8 | 2.8 | −2.2 | 5.3E-03 |
| Washc3 | WASH complex subunit 3 | −3.1 | 1.6E-14 | 27.9 | 9.1 | −2.0 | 1.4E-03 |
| Eif5b | eukaryotic translation initiation factor 5B | −3.1 | 3.1E-20 | 289.0 | 94.7 | −9.5 | 1.5E-05 |
| Fas | Fas (TNF receptor superfamily member 6) | −3.0 | 5.9E-13 | 27.7 | 9.3 | −3.7 | 1.8E-03 |
| Nif3l1 | Ngg1 interacting factor 3-like 1 (S. pombe) | −2.9 | 4.4E-06 | 3.5 | 1.2 | −4.4 | 1.1E-04 |
| Ahsa1 | AHA1, activator of heat shock protein ATPase 1 | −2.9 | 5.2E-16 | 15.7 | 5.4 | −1.8 | 9.7E-03 |
| Oaf | out at first homolog | −2.9 | 1.0E-28 | 62.8 | 21.8 | −2.3 | 1.2E-02 |
| Sh3bgr | SH3-binding domain glutamic acid-rich protein | −2.8 | 1.5E-10 | 29.0 | 10.3 | −8.7 | 1.0E-03 |
| Ube2o | ubiquitin-conjugating enzyme E2O | −2.8 | 3.5E-19 | 106.9 | 37.7 | −6.0 | 1.1E-04 |
| Timeless | timeless circadian clock 1 | −2.8 | 4.3E-07 | 14.2 | 5.0 | −3.0 | 6.4E-03 |
| Hsf4 | heat shock transcription factor 4 | −2.8 | 2.0E-05 | 149.0 | 53.2 | −39.5 | 1.5E-05 |
| Gtf2f2 | general transcription factor IIF, polypeptide 2 | −2.8 | 4.5E-14 | 54.7 | 19.8 | −2.6 | 3.4E-03 |
| Dipk2a | divergent protein kinase domain 2A | −2.7 | 4.2E-20 | 31.2 | 11.7 | −3.9 | 1.5E-04 |
| Casp7 | caspase 7 | −2.6 | 7.3E-12 | 13.0 | 4.9 | −1.6 | 2.0E-02 |
| Ankrd46 | ankyrin repeat domain 46 | −2.6 | 7.7E-20 | 23.5 | 9.1 | −1.8 | 2.7E-03 |
| Paics | phosphoribosylaminoimidazole carboxylase | −2.5 | 3.1E-25 | 167.9 | 66.0 | −3.4 | 1.5E-04 |
| Skap2 | src family associated phosphoprotein 2 | −2.5 | 2.7E-09 | 4.7 | 1.9 | −7.0 | 2.5E-04 |
| Slc20a2 | solute carrier family 20, member 2 | −2.4 | 1.5E-14 | 11.0 | 4.5 | −2.1 | 2.8E-03 |
| Tubb6 | tubulin, beta 6 class V | −2.4 | 3.2E-15 | 98.1 | 40.8 | −1.9 | 2.7E-02 |
| Zfp385a | zinc finger protein 385A | −2.4 | 1.5E-10 | 75.7 | 31.5 | −2.0 | 3.1E-02 |
| Scamp5 | secretory carrier membrane protein 5 | −2.4 | 2.0E-13 | 19.0 | 7.9 | −2.9 | 8.1E-04 |
| Dnajb1 | DnaJ heat shock protein family (Hsp40) member B1 | −2.4 | 5.5E-14 | 178.4 | 75.9 | −4.4 | 1.2E-04 |
| Caprin2 | caprin family member 2 | −2.3 | 9.0E-04 | 13.0 | 5.6 | −2.0 | 1.4E-03 |
| Hexb | hexosaminidase B | −2.3 | 7.7E-08 | 16.3 | 7.2 | 1.6 | 6.9E-03 |
| Bckdhb | branched chain ketoacid dehydrogenase E1, beta polypeptide | −2.3 | 2.2E-05 | 11.1 | 4.9 | −2.1 | 2.3E-03 |
| Tmem9b | TMEM9 domain family, member B | −2.3 | 1.2E-08 | 28.3 | 12.5 | −2.0 | 3.0E-03 |
| Pdlim1 | PDZ and LIM domain 1 (elfin) | −2.2 | 1.1E-07 | 25.2 | 11.2 | −8.8 | 1.1E-04 |
| Sash1 | SAM and SH3 domain containing 1 | −2.2 | 2.8E-17 | 16.2 | 7.4 | −1.6 | 1.5E-02 |
| Smad1 | SMAD family member 1 | −2.2 | 1.1E-08 | 9.1 | 4.1 | −1.7 | 1.6E-02 |
| Mrps26 | mitochondrial ribosomal protein S26 | −2.2 | 9.5E-06 | 11.5 | 5.3 | −2.1 | 7.5E-03 |
| Dynll2 | dynein light chain LC8-type 2 | −2.1 | 2.0E-13 | 139.7 | 65.9 | −2.2 | 6.9E-03 |
| Tdrd7 | tudor domain containing 7 | −2.1 | 6.6E-06 | 557.9 | 265.3 | −2.5 | 9.5E-04 |
| Hsdl2 | hydroxysteroid dehydrogenase like 2 | −2.1 | 3.2E-05 | 19.1 | 9.2 | −1.6 | 2.5E-02 |
| Dhx32 | DEAH (Asp-Glu-Ala-His) box polypeptide 32 | −2.1 | 8.3E-11 | 152.6 | 74.0 | 1.8 | 2.7E-03 |
| Trafd1 | TRAF type zinc finger domain containing 1 | −2.0 | 1.3E-13 | 61.5 | 30.1 | −4.2 | 5.8E-04 |
| Hopx | HOP homeobox | −2.0 | 1.1E-04 | 24.3 | 11.9 | 3.0 | 9.0E-04 |
| Cgnl1 | cingulin-like 1 | 2.7 | 1.0E-06 | 2.4 | 6.6 | −1.5 | 1.9E-02 |
| Olfml3 | olfactomedin-like 3 | 3.1 | 1.2E-02 | 2.0 | 6.1 | 1.7 | 2.7E-02 |
| Serpinb6b | serine (or cysteine) peptidase inhibitor, clade B, member 6b | 3.8 | 1.6E-28 | 20.5 | 77.3 | −2.7 | 1.4E-02 |
Table 6.
Genes differentially expressed in aged lens fibers which have previously be found to be differentially expressed in newborn lenses that over express Foxe3 in lens fibers
| SYMBOL | DESCRIPTION | Aged versus young fibers fold change | Aged fibers versus Young fibers FDR | Young Fibers FPKM | Aged Fibers FPKM | FoxE3 transgenic lens fold change | FoxE3 transgenic lens PValue |
|---|---|---|---|---|---|---|---|
| Crygf | crystallin, gamma F | −56.0 | 7.3E-03 | 6469.7 | 115.6 | −1.7 | 3.8E-04 |
| Cryga | crystallin, gamma A | −49.7 | 2.2E-02 | 255.2 | 5.1 | −3.3 | 4.5E-03 |
| Chrng | cholinergic receptor, nicotinic, gamma polypeptide | −34.2 | 5.8E-19 | 4.7 | 0.1 | −2.2 | 2.7E-03 |
| Ermap | erythroblast membrane-associated protein | −25.2 | 5.8E-05 | 6.0 | 0.2 | −5.9 | 3.7E-03 |
| Ceacam10 | carcinoembryonic antigen-related cell adhesion molecule 10 | −14.4 | 2.8E-04 | 8.4 | 0.6 | −4.8 | 3.0E-05 |
| Tcp11 | t-complex protein 11 | −13.7 | 7.6E-12 | 148.8 | 10.9 | −3.4 | 1.0E-04 |
| H19 | H19, imprinted maternally expressed transcript | −11.6 | 2.6E-02 | 2.4 | 0.2 | 4.1 | 5.7E-05 |
| Snx22 | sorting nexin 22 | −9.5 | 1.1E-08 | 76.0 | 8.0 | −1.9 | 1.3E-02 |
| Birc7 | baculoviral IAP repeat-containing 7 (livin) | −9.2 | 1.1E-05 | 295.7 | 32.0 | −3.2 | 1.5E-05 |
| Hspb1 | heat shock protein 1 | −8.1 | 4.8E-08 | 2679.5 | 330.3 | −5.2 | 2.6E-03 |
| Rnf180 | ring finger protein 180 | −7.1 | 1.0E-25 | 29.0 | 4.1 | −7.3 | 4.8E-06 |
| Dhcr7 | 7-dehydrocholesterol reductase | −7.1 | 2.0E-21 | 7.0 | 1.0 | −1.6 | 6.3E-03 |
| Fabp5 | fatty acid binding protein 5, epidermal | −6.0 | 1.4E-07 | 4619.2 | 774.7 | −4.0 | 1.5E-05 |
| Hmgn3 | high mobility group nucleosomal binding domain 3 | −5.8 | 4.0E-70 | 490.7 | 85.1 | −2.0 | 5.5E-05 |
| Metrnl | meteorin, glial cell differentiation regulator-like | −5.7 | 1.7E-43 | 133.2 | 23.5 | −2.0 | 3.8E-03 |
| Stx11 | syntaxin 11 | −5.4 | 7.2E-10 | 12.8 | 2.4 | −8.2 | 1.9E-04 |
| Clic5 | chloride intracellular channel 5 | −5.0 | 7.1E-08 | 31.2 | 6.2 | −2.2 | 2.6E-03 |
| Kctd12 | potassium channel tetramerisation domain containing 12 | −4.9 | 2.0E-18 | 29.7 | 6.1 | −1.8 | 2.0E-03 |
| Ngef | neuronal guanine nucleotide exchange factor | −4.7 | 7.3E-12 | 68.7 | 14.7 | −1.9 | 1.7E-03 |
| Trim26 | tripartite motif-containing 26 | −4.2 | 3.7E-42 | 22.1 | 5.3 | −1.7 | 4.8E-03 |
| Btf3l4 | basic transcription factor 3-like 4 | −4.1 | 2.4E-46 | 46.9 | 11.4 | −2.3 | 1.4E-05 |
| Hmox1 | heme oxygenase 1 | −4.1 | 6.3E-05 | 698.6 | 170.7 | −5.0 | 1.3E-04 |
| Bfsp1 | beaded filament structural protein 1, in lens-CP94 | −4.0 | 1.9E-15 | 12416.0 | 3101.2 | −3.3 | 4.9E-05 |
| Ttc27 | tetratricopeptide repeat domain 27 | −4.0 | 2.7E-12 | 16.3 | 4.1 | −1.7 | 4.6E-03 |
| Usp45 | ubiquitin specific petidase 45 | −3.8 | 8.4E-10 | 8.4 | 2.2 | −1.7 | 1.9E-02 |
| Hspe1 | heat shock protein 1 (chaperonin 10) | −3.7 | 3.3E-32 | 117.3 | 31.5 | −1.5 | 1.0E-03 |
| Klhdc8b | kelch domain containing 8B | −3.7 | 1.2E-22 | 6.4 | 1.7 | 1.7 | 2.1E-04 |
| Gadd45b | growth arrest and DNA-damage-inducible 45 beta | −3.7 | 5.4E-12 | 24.3 | 6.6 | −5.3 | 3.5E-04 |
| Smyd3 | SET and MYND domain containing 3 | −3.5 | 3.1E-20 | 7.9 | 2.2 | −2.5 | 2.6E-05 |
| Lgmn | legumain | −3.3 | 1.8E-37 | 92.8 | 28.4 | −1.7 | 1.7E-04 |
| Gabarapl1 | gamma-aminobutyric acid (GABA) A receptor-associated protein-like 1 | −3.3 | 3.0E-27 | 87.3 | 26.5 | −1.8 | 2.3E-03 |
| Zfand5 | zinc finger, AN1-type domain 5 | −3.2 | 1.8E-26 | 12.9 | 4.0 | −2.2 | 1.6E-04 |
| Pappa | pregnancy-associated plasma protein A | −3.2 | 4.9E-17 | 7.9 | 2.5 | −3.5 | 3.7E-04 |
| Card6 | caspase recruitment domain family, member 6 | −3.2 | 1.6E-05 | 5.1 | 1.6 | −2.4 | 1.3E-03 |
| Shc1 | src homology 2 domain-containing transforming protein C1 | −3.1 | 1.2E-22 | 27.1 | 8.6 | −1.8 | 1.1E-02 |
| Eif5b | eukaryotic translation initiation factor 5B | −3.1 | 3.1E-20 | 289.0 | 94.7 | −5.1 | 8.3E-04 |
| Ahsa1 | AHA1, activator of heat shock protein ATPase 1 | −2.9 | 5.2E-16 | 15.7 | 5.4 | −1.9 | 9.3E-04 |
| Nif3l1 | Ngg1 interacting factor 3-like 1 (S. pombe) | −2.9 | 4.4E-06 | 3.5 | 1.2 | −2.4 | 5.7E-05 |
| Ube2o | ubiquitin-conjugating enzyme E2O | −2.8 | 3.5E-19 | 106.9 | 37.7 | −2.1 | 1.6E-02 |
| Gtf2f2 | general transcription factor IIF, polypeptide 2 | −2.8 | 4.5E-14 | 54.7 | 19.8 | −2.0 | 5.0E-03 |
| Sh3bgr | SH3-binding domain glutamic acid-rich protein [ | −2.8 | 1.5E-10 | 29.0 | 10.3 | −5.8 | 4.2E-04 |
| Tigd2 | tigger transposable element derived 2 | −2.8 | 4.4E-09 | 11.0 | 3.9 | −1.8 | 6.6E-04 |
| Timeless | timeless circadian clock 1 | −2.8 | 4.3E-07 | 14.2 | 5.0 | −2.3 | 1.0E-02 |
| Entpd1 | ectonucleoside triphosphate diphosphohydrolase 1 | −2.8 | 4.3E-06 | 36.6 | 13.3 | −2.3 | 1.2E-03 |
| Gpd1l | glycerol-3-phosphate dehydrogenase 1-like | −2.7 | 5.7E-21 | 20.3 | 7.4 | −2.0 | 4.2E-03 |
| Ankrd46 | ankyrin repeat domain 46 | −2.6 | 7.7E-20 | 23.5 | 9.1 | −1.5 | 1.2E-03 |
| Casp7 | caspase 7 | −2.6 | 7.3E-12 | 13.0 | 4.9 | −4.1 | 3.5E-04 |
| Stac2 | SH3 and cysteine rich domain 2 | −2.6 | 3.9E-05 | 12.5 | 4.9 | 1.6 | 3.0E-02 |
| Paics | phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoribosylaminoimidazole, succinocarboxamide synthetase | −2.5 | 3.1E-25 | 167.9 | 66.0 | −1.8 | 3.6E-04 |
| Ing2 | inhibitor of growth family, member 2 | −2.5 | 3.2E-13 | 100.7 | 40.3 | −2.6 | 3.0E-04 |
| Slc20a2 | solute carrier family 20, member 2 | −2.4 | 1.5E-14 | 11.0 | 4.5 | −2.2 | 6.1E-03 |
| Scamp5 | secretory carrier membrane protein 5 | −2.4 | 2.0E-13 | 19.0 | 7.9 | −1.6 | 1.1E-02 |
| Lrp12 | low density lipoprotein-related protein 12 | −2.4 | 2.0E-12 | 18.4 | 7.7 | −1.8 | 2.5E-04 |
| Mxd1 | MAX dimerization protein 1 | −2.3 | 9.6E-18 | 26.5 | 11.5 | −2.1 | 1.0E-03 |
| Tacc1 | transforming, acidic coiled-coil containing protein 1 | −2.3 | 8.9E-17 | 11.4 | 5.0 | −1.5 | 1.4E-02 |
| Uhrf2 | ubiquitin-like, containing PHD and RING finger domains 2 | −2.3 | 3.2E-13 | 5.6 | 2.4 | −2.2 | 3.7E-04 |
| Bckdhb | branched chain ketoacid dehydrogenase E1, beta polypeptide | −2.3 | 2.2E-05 | 11.1 | 4.9 | −2.6 | 4.9E-05 |
| Smad1 | SMAD family member 1 | −2.2 | 1.1E-08 | 9.1 | 4.1 | −1.9 | 4.5E-03 |
| D630045J12Rik | RIKEN cDNA D630045J12 gene | −2.2 | 2.9E-08 | 7.9 | 3.6 | −2.1 | 9.5E-03 |
| Pdlim1 | PDZ and LIM domain 1 (elfin) | −2.2 | 1.1E-07 | 25.2 | 11.2 | 1.7 | 3.9E-04 |
| Klhl25 | kelch-like 25 | −2.2 | 1.8E-07 | 17.7 | 8.0 | −1.6 | 6.7E-04 |
| Prdm16 | PR domain containing 16 | −2.2 | 5.5E-07 | 23.9 | 11.1 | −5.3 | 4.6E-04 |
| Mrps26 | mitochondrial ribosomal protein S26 | −2.2 | 9.5E-06 | 11.5 | 5.3 | −1.6 | 1.3E-04 |
| Tdrd7 | tudor domain containing 7 | −2.1 | 6.6E-06 | 557.9 | 265.3 | −2.7 | 1.7E-02 |
| Hsdl2 | hydroxysteroid dehydrogenase like 2 | −2.1 | 3.2E-05 | 19.1 | 9.2 | −2.2 | 3.7E-03 |
| Trafd1 | TRAF type zinc finger domain containing 1 | −2.0 | 1.3E-13 | 61.5 | 30.1 | −3.1 | 5.1E-04 |
| Gatad2a | GATA zinc finger domain containing 2A | −2.0 | 2.0E-12 | 50.8 | 25.3 | −1.5 | 4.2E-03 |
| Nfkbib | nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, beta | −2.0 | 1.9E-03 | 12.4 | 6.2 | −1.6 | 5.2E-03 |
| Ccng1 | cyclin G1 | 2.4 | 8.8E-09 | 186.1 | 450.7 | −2.4 | 1.3E-05 |
| B2m | beta-2 microglobulin | 3.2 | 1.1E-02 | 1.0 | 3.3 | 1.6 | 4.6E-02 |
| Foxe3 | forkhead box E3 | 4.2 | 1.3E-03 | 3.8 | 16.3 | 24.1 | 6.4E-08 |
Ipathway guide analysis of the DEGs identified in aged lens fibers revealed that the most impacted KEGG pathways included antigen processing and presentation (Figure 2B,C), P= 1.6 X 10−4) and cholesterol metabolism (Figure 2D, E, P= 0.003) while cataract is the disease most strongly associated with the DEGs (not shown, FDR corrected P value= 2.8 X 10−7). The DEGs in aged lens fibers map to numerous KEGG or gene ontology terms, with ones potentially significant to the biology of the aging lens including cellular calcium ion homeostasis (p=0.001; Figure 3A), cellular senescence (p=0.023; Figure 3B), the respiratory chain complex (p=0.03; Figure 3C) and glycolysis/gluconeogenesis (p=0.056; Figure 3D).
Figure 3.
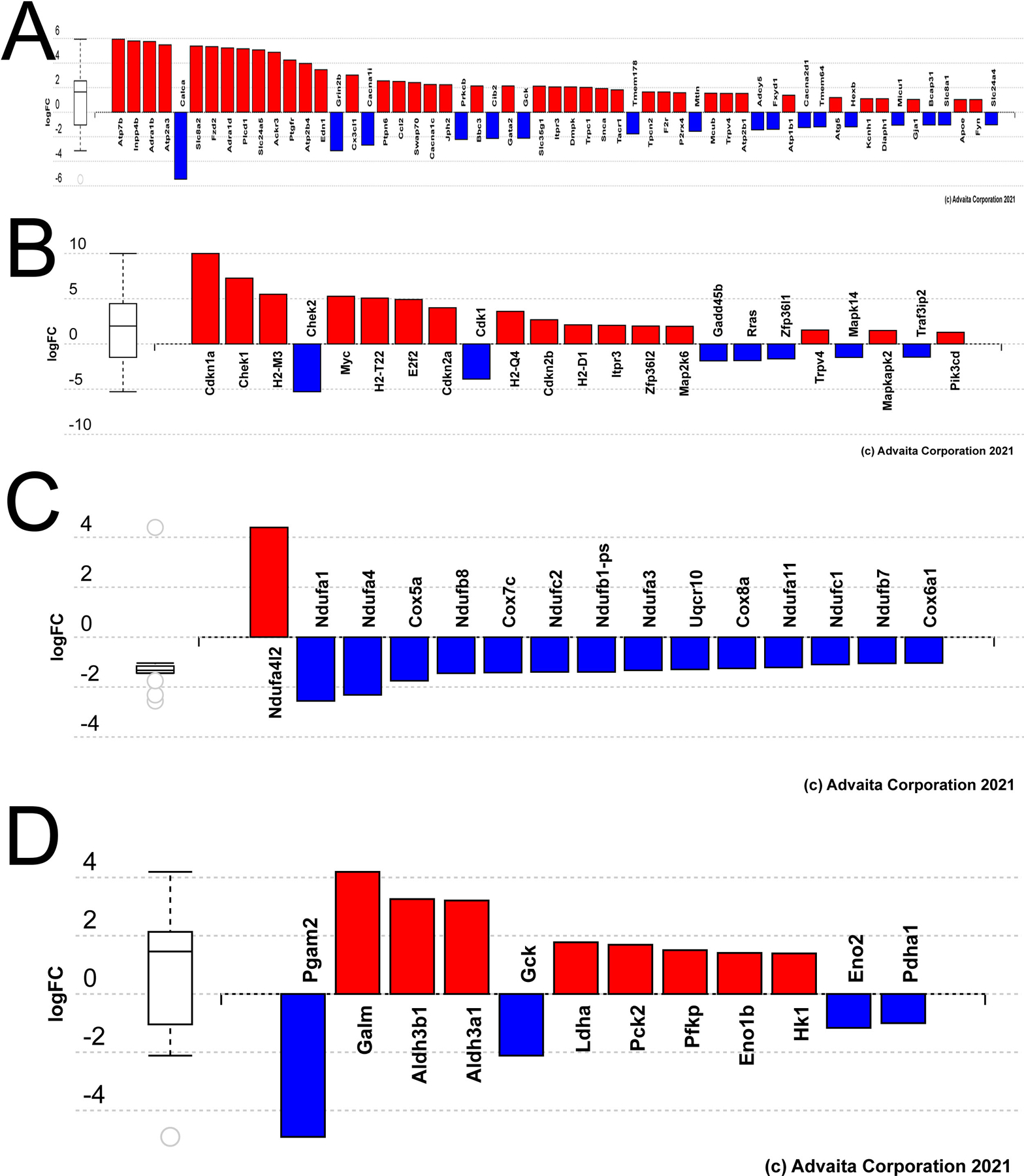
Genes differentially expressed in aged versus young mouse lens fiber cells grouped by gene ontology (GO) terms or Kegg pathways potentially relevant to age-related changes in lens biology A) Bar graph representing the DEGs in aged lens fibers mapping to the Kegg pathway cellular calcium homeostasis. B) Bar graph representing the DEGs in aged lens fibers mapping to the Kegg pathway cellular senescence. C) Bar graph representing the DEGs in aged lens fibers mapping to the GO term respiratory chain complex. D) Bar graph representing the DEGs in aged lens fibers mapping to the Kegg pathway glycolysis/gluconeogenesis.
The effect of aging on the response of lens epithelial cells to a surgery modeling extracapsular cataract extraction
We have previously demonstrated that young adult lens epithelial cells robustly upregulate the expression of proinflammatory cytokines and receptors within 24 hours post surgery (post cataract surgery, PCS) in a mouse model of posterior capsular opacification (PCO) (Jiang et al., 2018). Here, similar to the results obtained from C57BL/6Hsd mice, young C57BL/6J LECs dramatically reprogram their transcriptome by 24 hours PCS (Supplemental table 4) with iPathway guide analysis revealing that the cytokine/cytokine receptor pathway as being the most impacted (p=1.1 X 10−8). As the prevalence of PCO is higher in younger patients than older (Elkin et al., 2016; Wu et al., 2018), the difference in gene expression between 0 and 24 hours PCS was also evaluated in aged LECs (Supplemental table 5). The primary elements of the injury response were preserved in aging LECs, with cytokine-cytokine receptor pathways still significantly impacted (p=1.1 X 10−8).
There were 997 genes where a biologically significant difference in expression between 0 and 24 hours PCS was observed in both young LEC and in aged LEC. Of these 997 intersecting genes, all 653 that upregulate 24 hours after injury in young LEC also upregulate in aged LEC. Likewise, the remaining 344 genes down regulate after injury in both age groups. There were no biologically significant genes where the 24 hour injury response in young LEC contradicted that of aged LEC. (653 genes were upregulated and 344 downregulated at 24 hours PCS, Supplemental Table 6.
Comparison between gene expression levels in young and aged LECs at 24 hours PCS revealed that only 73 genes (35 upregulated and 38 downregulated) met the thresholds indicative of “biologically significant” changes in expression (Supplemental table 7). Of these, 8 of the genes with lower expression in aged 24 hour PCS LECs were also downregulated in uninjured aged LECs, while 8 other genes were upregulated in both 0 hour and 24 hour PCS aged LECs.
Impact analysis identified few pathways with likely biological relevance as differentially regulated in aged versus young LECs at 24 hours PCS (Figure 4A) although some genes involved in PI3K/Akt pathways (p=0.004; Figure 4B) and human papilloma virus infection (p=0.002; Figure 4C) were significantly impacted. However, a significant proportion of these DEGs map to the gene ontology terms “regulation of cell motility” (p= 8.5 X 10−6, Figure 4D) and “cell population proliferation” (p= 1.0 X 10−5; Figure 4E), cell behaviors likely to be relevant to PCO pathogenesis.
Figure 4.
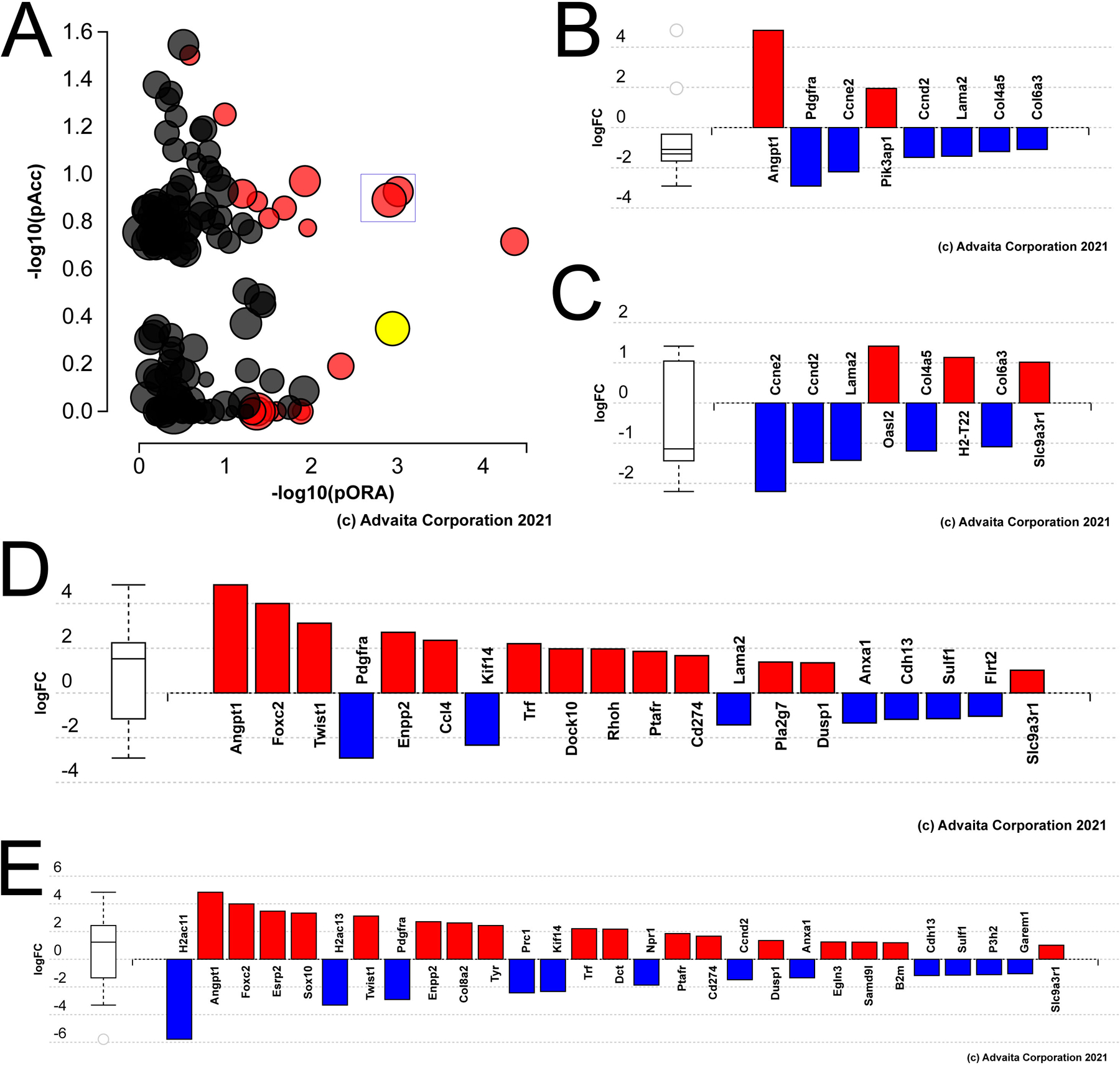
Genes differentially expressed in aged versus young mouse lens epithelial cells at 24 hours PCS. A) Impact analysis of the 24 hour PCS DEGs with the yellow dot representing Akt signaling; boxed dots represent “human papilloma virus infection” and “alcoholism” B) Bar graph representing the DEGs in aged LECs at 24 hours PCS known to be involved in “Akt signaling”. C) Bar graph representing the DEGs in aged LECs at 24 hours PCS known to be involved in “human papilloma virus infection”. D) Bar graph representing the DEGs in aged LECs at 24 hours PCS mapped to the gene ontology term “cell motility”. D) Bar graph representing the DEGs in aged LECs at 24 hours PCS the gene ontology term “cell proliferation”.
Discussion
The increased risk of cataract development with age was recognized in antiquity and was first rigorously documented in a large patient cohort by Edward Jackson in 1898 (Jackson, 1898). The biochemical, metabolic, and structural changes that the lens undergoes with aging have been studied since the mid-twentieth century (Green and Solomon, 1957; Heydt, 1930; Lerman and Zigman, 1965). Subsequently, the effects of aging on the structure of lens proteins (Lampi et al., 2014; Ozaki and Mizuno, 1992; Ray, 2015) and lipids (Borchman and Yappert, 2010), as well as oxidative stress responses in the lens (Brennan et al., 2012), have been intensely studied. However, while aging has been recognized to influence the transcriptome of many tissues (Aging Atlas, 2021; Srivastava et al., 2020), this has been less studied in the lens, and the Gene Expression Omnibus contained no publically available aging lens transcriptome comparisons prior to this study. The RNAseq study presented here provides global insights into the effects of age on gene expression in the lens, and may reveal some underlying mechanisms for previously documented age-related changes in lens physiology and wound healing responses.
The aging lens downregulates the mRNA levels of many genes exhibiting “lens preferred” expression.
Comparisons between the transcriptomes of young adult and aged LECs and fibers revealed that genes of the linked gamma crystallin cluster on mouse chromosome 1 were the most profoundly affected by age, with decreases in expression ranging from 40 to 340 fold. While this was initially surprising, this corresponds to a prior report that found significant decreases in mRNAs derived from these linked gamma crystallin genes in the Swiss CF mouse lens over the first year of life (Treton et al., 1988). As little gamma-crystallin protein was detected in cortical fibers of adult human and bovine lenses as well (Anderson et al., 2020), this suggests that the profound loss of gamma crystallin mRNA from the aging adult lens is a general feature of mammalian lens aging. In addition to the gamma crystallin mRNAs, aged LECs also express lower levels of other mRNAs encoding fiber cell markers including beta-crystallins and MIP. A prior report also found that adult mouse LECs express modest amounts of these mRNAs, although they are apparently not translated efficiently (Wang et al., 2004), so their loss from LECs may not affect LEC function. However, prior proteomic analysis of adult human lens epithelium did detect high levels of both alpha- and beta-crystallin proteins in these cells leading the authors to speculate that they have important functions in LECs (Wang-Su et al., 2003).
Aged lens fibers also profoundly downregulate the expression of mRNAs encoding numerous genes known to be important for lens physiology and function, including most crystallins, MIP (Bassnett et al., 2009; Chepelinsky, 2009), Bfsp1 (Song et al., 2009), Lim2 (Irum et al., 2016), and Grifin (Ogden et al., 1998), as well as Birc7 (De Maria and Bassnett, 2015) and Hopx (Vasiliev et al., 2007), two markers of late lens fiber differentiation. This suggests that the cortical fibers differentiating in late life may have a profoundly different protein composition than the fibers comprising the remainder of the lens due to changes in protein expression, not just post-translational modification. Notably, lens fiber protein composition has been previously shown to be dependent on cellular birthdate as βB2- and γS-crystallin are not appreciable components of primary and secondary fibers produced during embryonic development of rodents, but become major components of postnatal cortical fibers (Carper et al., 1986; Ueda et al., 2002), a pattern that was also recently reported in the human lens (Anderson et al., 2020). The functional consequences of lens fibers produced in old age undergoing such a profound downregulation of lens preferred gene expression are unclear though as these cells would not be expected to contribute directly to the refractive power of the lens as they reside behind the iris.
Some genes differentially expressed in aged LECs and fibers DEGs were previously found to undergo changes in H3K4me3 methylation the aging lens
The ability of a gene to be transcribed depends on both the presence of transcription factors able to influence the activity of the basal transcription machinery, and the gene’s promoter being in a region of “open” chromatin which allows transcription factor access to their DNA binding sites. A prior study investigated whether the distribution of “open” chromatin changes in the aging mouse lens using patterns of H3K4 trimethylation as a marker (Zheng et al., 2015) since this modification has been reported to mark active transcriptional start sites, particularly of genes important for cell and tissue identity (Benayoun et al., 2014). Comparison of the resulting 613 H3K4me3 promoter peaks genes, with the genes differentially expressed in LECs and fibers during aging revealed that 20 LEC and 54 fiber DEGs also exhibit age-dependent changes in H3K4me3, the vast majority of which occur in the direction expected if this methylation is a mark of transcriptionally active promoters. However, we also found that the mRNA levels for many of the other genes reported to have changed H3K4me3 in the aging lens were very low, suggesting that they were not appreciably transcribed in the adult lens, while the mRNA levels for others did not change during aging. This is not necessarily unexpected as steady state mRNA levels are regulated by multiple mechanisms, only one of which is chromatin accessibility.
Known regulators of lens development are differentially expressed in aging lens fibers
As the gene regulatory networks responsible for lens fiber cell phenotype are among the best characterized in vertebrate development (Anand and Lachke, 2017; Cvekl and Zhang, 2017), the DEGs in aged lens fibers were interrogated for genes known to regulate lens fiber cell biology. FGF signaling is the best characterized pathway regulating lens fiber cell differentiation as the deletion of FGFR1-FGFR3 expression from the lens abolishes fiber cell differentiation (Zhao et al., 2008). While the expression levels of these canonical receptors are not altered, the expression of lctl, which encodes a klotho family member that may allow lens cells to respond to endocrine FGFs, is downregulated 3 fold in the aging lens. Notably, clic5, the only gene whose expression is profoundly affected by deletion of lctl from the mouse lens (Fan et al., 2018), downregulates 5 fold in the aging lens as well. Similarly, fgfrl1, which encodes the protein FGF receptor-like 1, that may facilitate ligand independent FGFR signaling (Silva et al., 2013), downregulates three fold in the aging lens. As lctl and fgfrl1 downregulation in the Prox1 null lens is correlated with downregulation in ERK signaling and defects in fiber cell preferred gene expression (Audette et al., 2016), this suggests that diminished FGF signaling could contribute to the downregulation of lens fiber preferred genes with aging.
Inspection of the DEGs in aging lens fibers for key transcription factors regulating lens development revealed that the mRNA encoding Hsf4, a protein that regulates lens development/homeostasis from late embryonic development into adulthood (Fujimoto et al., 2004; Min et al., 2004), was downregulated in aged lens fibers. Notably, comparisons between the DEGs of aging fibers with those previously found to be differentially expressed in newborn lenses lacking HSF4 (He et al., 2010) revealed numerous common genes, including validated HSF4 target genes such as fas (Gao et al., 2017), γS-crystallin (Shi et al., 2009), and Hmox1 (Liao et al., 2018). In addition, the downregulation of Maf, a transcription factor essential for lens fiber cell differentiation and crystallin expression (Kawauchi et al., 1999; Kim et al., 1999), correlates with the downregulation of many crystallin genes in the aging lens. Conversely, FoxE3, a transcription factor critical for maintenance of the undifferentiated state of lens epithelial cells (Blixt et al., 2000; Medina-Martinez et al., 2005), upregulates in aged lens fibers, while numerous DEGs in aged fibers overlap with those previously reported in lens fibers overexpressing FoxE3 (Blixt et al., 2000; Landgren et al., 2008). These data imply that that the downregulation of Hsf4 and Maf coincident with the upregulation of FoxE3 expression could drive the observed downregulation of fiber cell marker mRNA levels in aging lens fibers.
However, the levels of mRNAs encoding transcription factors that bind to anti-oxidant response elements (AREs), such as Nrf2, Bach2, and the small mafs, did not make the cutoff to be considered “biologically significant” DEGs in aging lenses. The small mafs, MafA, and Mafk do upregulate 13 fold and 2.3 fold in aging lens fibers respectively (FDR ≤0.01 and ≤0.02), however, their expression levels are very low even after upregulation (0.6 and 0.9 FPKM) suggesting that they are not made at sufficient concentrations to affect the biology of aging lens fibers, while the major small Maf of the lens (Mafg) which, in concert with Mafk, is known to regulate oxidative stress genes in the lens (Agrawal et al., 2015) was not differentially expressed in the aged versus young lens. While these results were surprising in light of the hypothesis that aging lens is less able to deal with cellular stress due to loss of anti-oxidative responses mediate via AREs (Liu et al., 2017), it is possible that life under conditions where environmental stress is minimized (such as experienced in an animal facility) allow for more “protective reserve” than free living animals/people experience (Epel, 2020). Alternatively, it is also possible that the loss of anti-oxidant response in the lens with age is controlled post-translationally, a circumstance that may not manifest in transcriptomic changes.
Lens transcriptome alterations may reflect the known changes in energy metabolism in the aging lens
Lens epithelial cells from young adult rabbits produce about half of their ATP via oxidative phosphorylation, while lens fibers appear to generate most of their ATP anaerobically via glycolysis (Mandel and Klethi, 1962; Winkler and Riley, 1991). During aging, whole lenses increase their ability to produce ATP via anaerobic glycolysis leading to lactate as a byproduct (Green and Solomon, 1957). Further, oxidative damage to mitochondria is common in aging lenses and has been proposed to contribute to age related cataract (Babizhayev and Yegorov, 2016; Brennan et al., 2012), while aged tissues are recognized to develop imbalances in oxidative phosphorylation (Kwong and Sohal, 2000). Consistent with these observations, aged lens fibers downregulate mRNAs encoding many enzymes of the respiratory chain complex, while aging lens epithelial and fiber cells both upregulate the expression of Ndufa4l2, an alternate respiratory chain component that upregulates in stressed mitochondria to slow electron transport in order to protect mitochondria from further damage (Li et al., 2017). Notably, the Ndufa4l2 promoter also exhibits increased H3K4me3 in the aging lens (Zheng et al., 2015) suggesting that its upregulation is controlled by transcriptional mechanisms.
Conversely, aged lens fibers upregulate mRNAs encoding some components of the glycolytic cascade. However, aged lens fibers also profoundly downregulate their expression of Pgam2 mRNA which encodes phosphoglycerate mutase 2, which is best known as a muscle specific form of the enzyme that catalyzes the conversion of 3-phosphoglycerate to 2-phosphoglycerate during glycolysis. However, Pgam2 expression is “lens preferred” via iSyTE, and its levels in young adult lens fibers are much higher than that of other glycolytic enzymes. As mutations in Pgam2 lead to glycogen storage disease in the muscle (Tsujino et al., 1993), high Pgam2 expression in young lens fibers may help these cells utilize their glycogen stores (Hockwin, 1973) for glycolysis. As Pgam2 overexpression in the heart increased oxidative stress in mitochondria (Okuda et al., 2013), the profound (40 fold) downregulation of the expression of this gene in lens fibers may be protective to the aging lens.
The aging lens undergoes transcriptomic changes similar to those seen in other aging tissues
Changes in tissue transcriptomes with age are commonly unique to each tissue, so it is not unusual for different aging tissues to share few DEGs (Barth et al., 2019; Srivastava et al., 2020). That said, there are still some common pathways reported. Aging tissues often upregulate genes with functions in inflammation, a hallmark of the “inflammaging” that contributes to the age-related decline of mammals (Fulop et al., 2018). Consistent with this, “cytokine and cytokine receptor pathways” were calculated as being the most impacted KEGG pathway in the aging lens epithelium. As many of these genes are also upregulated in LECs by 24 hours following cataract surgery (Jiang et al., 2018), this suggests that the aged lens epithelium is primed towards an injured phenotype. The second most impacted pathway in aged LECs corresponds to the complement pathway, another biological pathway commonly affected in aging that appears to drive some age-related pathologies (Propson et al., 2021).
The mRNAs encoding many proteins involved in cholesterol metabolism upregulate in aged lens fibers which may be functionally significant to lens aging as lens fibers are very cholesterol rich (Subczynski et al., 2012) and defects in cholesterol synthesis pathways lead to cataractogenesis (Aleo et al., 2019; Jira, 2013; Widomska and Subczynski, 2019). These changes may be regulated by Srebf1, a transcription factor that regulates genes with sterol responsive elements (Sato, 2010), as its mRNA levels are 4 fold upregulated in aging lens fibers. Interestingly, the protein encoded by one of the upregulated cholesterol transport genes, apoE, is a major component of pseudoexfoliation material (Sharma et al., 2009), an aberrant extracellular matrix material deposited on the lens capsule and other ocular structures during the pathogenesis of the age-related disease, pseudoexfoliation syndrome (Schlotzer-Schrehardt and Khor, 2021). It is also notable that upregulation of complement genes, mitochondrial dysfunction, and elevated ApoE levels are shared in between the aging lens and AMD (Tan et al., 2020), suggesting related mechanisms.
Aged fiber cell DEGs are also enriched in genes mapping to the KEGG pathway “cellular senescence”, which is a pathway that is recognized to contribute to age related decline (Si et al., 2021) and is thus a target for the development of anti-aging drugs (Davan-Wetton et al., 2021). Interestingly, the upregulated “senescence” genes include cdkn1a, which encodes P21, a cell cycle regulator whose upregulation was previously reported to promote cataract formation in progeroid mice (Baker et al., 2013). Despite this, mRNAs encoding the classic senescence regulators, the FoxO genes (Brown and Webb, 2018), were generally not differentially expressed in aging lens cells although we did find that both young and aged LECs express FoxO3 (8–9 FPKM) and FoxO1 (4–5 FPKM) with FoxO6 and FoxO4 present at lower levels. This lack of FoxO regulation at the mRNA level in the aging lens may not be surprising though as many studies suggest that their ability to control pathways controlling to cellular stress/aging mechanisms is often regulated post-translationally (Tia et al., 2018).
Other gene expression changes seen in aged lens cells that could influence the development of age-related cataract
Age related cataract appears to develop when genetic pre-dispositions are influenced by diverse age-related and environmental stressors that contribute to “cataractogenic load” (Uwineza et al., 2019). Among the best characterized inducers of cataract is oxidative stress, and the nucleus of aged lenses and those with cataract have elevated levels of oxidized glutathione (Beebe et al., 2010). Notably, while aging LECs exhibited few gene expression changes compared to young LECs, young LECs express abundant Gpx3 mRNA, and these levels further increase in aging LECs. While Gpx3 function in the lens has not been intensely studied, this gene encodes a secreted isoform of glutathione peroxidase that binds to basement membranes and detoxifies hydrogen peroxide in biological fluids (Baez-Duarte et al., 2014; Olson et al., 2010). As Gpx3 has been proposed to be protective against LEC cell death (Tu et al., 2019), the detected elevation in Gpx3 expression in aged LECs may protect the lens and other ocular tissues from elevated oxidative stress during aging.
Aging lens fibers downregulate the mRNA levels of Hmox1, which encodes heme oxygenase 1, an enzyme that protects cells from oxidative stress by detoxifying free heme (Chen et al., 2019). As Hmox1 mRNA is very abundant in young lens fibers, and these levels decrease in aged lenses, it is possible that Hmox1 downregulation with aging contributes to ARC as it was recently reported that expression of a dominant-negative mutant of Hmox1 in the lens leads to early onset cataract (Huang et al., 2021). Notably, Hmox1 levels in lens fibers have been reported to be regulated by the transcription factors Maf (Si et al., 2019) and Hsf4 (Liao et al., 2018), which both downregulated in aging lens fibers.
Further, the mRNA for Romo1 (reactive oxygen species modulator 1), which encodes a ion channel of the inner mitochondrial membrane, is also downregulated in aged lens fibers. While this protein has not been previously studied in the lens, its mRNA is abundant in young lens fibers, and in other systems, Romo1 limits the production of mitochondrial ROS and protects mitochondrial integrity (Norton et al., 2014), leading to the possibility that Romo1 downregulation in lens fibers with aging could contribute to the mitochondrial defects reported in aged lens fibers and ARC. However, the steady state levels of reactive oxygen species in the aged lens may be low as few genes associated with the gene ontology term “DNA repair” are induced in the aging lens which may be expected as aging is a slow, chronic condition, not acute oxidative damage.
The effect of aging on the response of LECs to an injury modeling cataract surgery
Lens epithelial cells remaining behind on the capsular bag after cataract surgery respond by increasing their proliferation rate and migrating along any bare lens capsule that they can reach. These cells also shift phenotype, either differentiating into cells with lens fiber cell character or transdifferenting into myofibroblasts capable of producing fibrotic ECM. When these cells migrate into the optical axis they prevent the transmission of light leading to Posterior capsular opacification, the most prevalent side effect of cataract surgery (Shihan et al., 2019; Wormstone et al., 2009). While PCO is prevalent in patients of all ages by 10 years post surgery (Apple et al., 2011; Ronbeck and Kugelberg, 2014; Sen et al., 2019), age is known to influence the risk of developing PCO (Wu et al., 2018), with children at such a high risk that prophalytic posterior capsulotomy is routinely performed at the time of surgery (Sukhija et al., 2014).
It is likely that the aberrant lens fibers produced during PCO arise from the same pathways that drive normal lens development, while it is well established that TGFβ signaling plays a crucial role in myofibroblast formation. We previously reported that young adult LECs quickly upregulate the expression of numerous inflammatory cytokines after lens fiber cell removal in a mouse model of cataract surgery, and this response precedes the activation of TGFβ pathways by 1–2 days (Jiang et al., 2018). Here, we found that both young and old LECs responded similarly to lens fiber cell removal, with the most impacted pathway in both cases being cytokine/cytokine interactions. A more fine grained comparison of the transcriptome of young versus old LECs at 24 hours PCS revealed fewer DEGs than were observed between the young versus old uninjured lens epithelium. This appears to result from the massive reprogramming of the LEC transcriptome by 24 hours PCS swamping out most effects due to aging. However, bioinformatic comparison of these DEGs revealed a correlation with the downregulation of pathways linked to AKT signaling, with a 7.5 fold downregulation of Pdgfra mRNA levels in aged LECs compared to young LECs at 24 hours PCS. As platelet derived growth factor signaling is important for lens differentiation (Li et al., 2019), this downregulation could both contribute to altered lens marker expression observed in aged lens epithelial cells, and the reduced rate of PCO in aged cataract patients, as PDGF stimulated Akt signaling induces the migration of lens epithelial cells (Xiong et al., 2010).
Conclusion
This study revealed that mouse lenses profoundly remodel their transcriptome with aging, with aged lens fibers in particular downregulating the expression of many genes known to regulate lens fiber cell structure and physiology. These changes, along with alterations in genes regulating mitochondrial function and detoxification of reactive oxygen species may either be induced in response to age-related oxidative stress and/or are primary protective mechanisms against the development of pathology in the aging lens. Despite these changes in gene expression observed during lens aging, aged mouse lens epithelial cells respond similarly to lens injury as young LECs, although it is intriguing to speculate that downregulation of Pdgfra expression in aged LECs could contribute to the reduced risk of PCO development with age. The major age-related effects on the mouse lens transcriptome are summarized in Figure 5.
Figure 5.

Summary of the major effects of age on the mouse lens transcriptome
Overall, this publically available data expands our understanding of the changes that the lens undergoes with aging, and will be a useful resource for researchers interested in diverse aspects of lens biology. It is still possible though that the alterations in the lens transcriptome with age detected here will not be reflected in the lens proteome, or that protein levels could be changing even for genes whose mRNA levels do not alter with aging. Thus, future proteomic analyses of isolated lens epithelial cells and nucleated cortical fibers would be valuable to gain further insight into how the lens adapts its gene expression profile as it ages.
Supplementary Material
Funding:
This study was funded by grants from the National Eye Institute (EY028597 and EY028597-S1). The 24 month old C57BL/6/Nia mice used in this study were a gift from the United States National Institute on Aging, Division of Aging Biology, Biological Resources Branch under exception approval Duncan091818. Support from the University of Delaware CBCB Bioinformatics Core Facility and use of the BIOMIX compute cluster was made possible through funding from Delaware INBRE (NIH NIGMS P20 GM103446), the State of Delaware, and the Delaware Biotechnology Institute.
References
- Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, Peters LL, 2015. Aging Research Using Mouse Models. Curr Protoc Mouse Biol 5, 95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aging Atlas C, 2021. Aging Atlas: a multi-omics database for aging biology. Nucleic Acids Res 49, D825–D830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SA, Anand D, Siddam AD, Kakrana A, Dash S, Scheiblin DA, Dang CA, Terrell AM, Waters SM, Singh A, Motohashi H, Yamamoto M, Lachke SA, 2015. Compound mouse mutants of bZIP transcription factors Mafg and Mafk reveal a regulatory network of non-crystallin genes associated with cataract. Hum Genet 134, 717–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan S, Draghici S, 2017. Identifying Significantly Impacted Pathways and Putative Mechanisms with iPathwayGuide. Curr Protoc Bioinformatics 57, 7 15 11–17 15 30. [DOI] [PubMed] [Google Scholar]
- Aleo MD, Doshna CM, Baltrukonis D, Fortner JH, Drupa CA, Navetta KA, Fritz CA, Potter DM, Verdugo ME, Beierschmitt WP, 2019. Lens cholesterol biosynthesis inhibition: A common mechanism of cataract formation in laboratory animals by pharmaceutical products. J Appl Toxicol 39, 1348–1361. [DOI] [PubMed] [Google Scholar]
- Anand D, Lachke SA, 2017. Systems biology of lens development: A paradigm for disease gene discovery in the eye. Exp Eye Res 156, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W, 2014. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Nye-Wood MG, Rose KL, Donaldson PJ, Grey AC, Schey KL, 2020. MALDI imaging mass spectrometry of beta- and gamma-crystallins in the ocular lens. J Mass Spectrom 55, e4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple DJ, Escobar-Gomez M, Zaugg B, Kleinmann G, Borkenstein AF, 2011. Modern cataract surgery: unfinished business and unanswered questions. Surv Ophthalmol 56, S3–53. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G, 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette DS, Anand D, So T, Rubenstein TB, Lachke SA, Lovicu FJ, Duncan MK, 2016. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 143, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC, 2010. On the growth and internal structure of the human lens. Exp Eye Res 90, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev MA, Yegorov YE, 2016. Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract--A Novel Platform of Mitochondria-Targeted Antioxidants With Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. Am J Ther 23, e98–117. [DOI] [PubMed] [Google Scholar]
- Baez-Duarte BG, Mendoza-Carrera F, Garcia-Zapien A, Flores-Martinez SE, Sanchez-Corona J, Zamora-Ginez I, Torres-Rasgado E, Leon-Chavez BA, Perez-Fuentes R, Multidisciplinary Research Group on Diabetes of the Instituto Mexicano del Seguro, S., 2014. Glutathione peroxidase 3 serum levels and GPX3 gene polymorphisms in subjects with metabolic syndrome. Arch Med Res 45, 375–382. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Weaver RL, van Deursen JM, 2013. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep 3, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Quinlan RA, 2017. Small molecules, both dietary and endogenous, influence the onset of lens cataracts. Exp Eye Res 156, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth E, Srivastava A, Stojiljkovic M, Frahm C, Axer H, Witte OW, Marz M, 2019. Conserved aging-related signatures of senescence and inflammation in different tissues and species. Aging (Albany NY) 11, 8556–8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL, 2009. The membrane proteome of the mouse lens fiber cell. Mol Vis 15, 2448–2463. [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Holekamp NM, Shui YB, 2010. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res 44, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Ucar D, Mahmoudi S, Karra K, Wong ED, Devarajan K, Daugherty AC, Kundaje AB, Mancini E, Hitz BC, Gupta R, Rando TA, Baker JC, Snyder MP, Cherry JM, Brunet A, 2014. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 158, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P, 2000. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev 14, 245–254. [PMC free article] [PubMed] [Google Scholar]
- Borchman D, Yappert MC, 2010. Lipids and the ocular lens. J Lipid Res 51, 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, McGreal RS, Kantorow M, 2012. Oxidative stress defense and repair systems of the ocular lens. Front Biosci (Elite Ed) 4, 141–155. [DOI] [PubMed] [Google Scholar]
- Brown AK, Webb AE, 2018. Regulation of FOXO Factors in Mammalian Cells. Current topics in developmental biology 127, 165–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper D, Smith-Gill SJ, Kinoshita JH, 1986. Immunocytochemical localization of the 27Kbeta-crystallin polypeptide in the mouse lens duringdevelopment using a specific monoclonal antibody:implications for cataract formation in the Philly mouse. Dev. Biol 113, 104–109. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang X, Nisar MF, Lin M, Zhong JL, 2019. Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress. Oxid Med Cell Longev 2019, 5416728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lun A, Smyth G, 2016. From reads to genes to pathways: differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline [version 2; peer review: 5 approved]. F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky AB, 2009. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb Exp Pharmacol, 265–297. [DOI] [PubMed]
- Chilibeck C, Mathan JJ, Ng SG, McKelvie J, 2020. Cataract surgery in New Zealand: access to surgery, surgical intervention rates and visual acuity. N Z Med J 133, 40–49. [PubMed] [Google Scholar]
- Cui X, Wang L, Zhang J, Du R, Liao S, Li D, Li C, Ke T, Li DW, Huang H, Yin Z, Tang Z, Liu M, 2013. HSF4 regulates DLAD expression and promotes lens de-nucleation. Biochim Biophys Acta 1832, 1167–1172. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Zhang X, 2017. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet 33, 677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T, 2016. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev 29, 90–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davan-Wetton CSA, Pessolano E, Perretti M, Montero-Melendez T, 2021. Senescence under appraisal: hopes and challenges revisited. Cell Mol Life Sci [DOI] [PMC free article] [PubMed]
- Dawes LJ, Duncan G, Wormstone IM, 2013. Age-related differences in signaling efficiency of human lens cells underpin differential wound healing response rates following cataract surgery. Invest Ophthalmol Vis Sci 54, 333–342. [DOI] [PubMed] [Google Scholar]
- De Maria A, Bassnett S, 2015. Birc7: A Late Fiber Gene of the Crystalline Lens. Invest Ophthalmol Vis Sci 56, 4823–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai VD, Wang Y, Simirskii VN, Duncan MK, 2010. CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury. Differentiation 79, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R, 2007. A systems biology approach for pathway level analysis. Genome Res 17, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Cvekl A, Kantorow M, Piatigorsky J, 2004. Lens crystallins, in: Lovicu FJ, Robinson ML (Eds.), Development of the Ocular Lens Cambridge University Press, p. 416pp. [Google Scholar]
- Elkin ZP, Piluek WJ, Fredrick DR, 2016. Revisiting secondary capsulotomy for posterior capsule management in pediatric cataract surgery. J AAPOS 20, 506–510. [DOI] [PubMed] [Google Scholar]
- Epel ES, 2020. The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev 63, 101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Lerner J, Wyatt MK, Cai P, Peterson K, Dong L, Wistow G, 2018. The klotho-related protein KLPH (lctl) has preferred expression in lens and is essential for expression of clic5 and normal lens suture formation. Exp Eye Res 169, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Monnier VM, Whitson J, 2017. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp Eye Res 156, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease, S., 2017. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 5, e1221–e1234. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S, Kato K, Yonemura S, Inouye S, Nakai A, 2004. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J 23, 4297–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Witkowski JM, Olivieri F, Larbi A, 2018. The integration of inflammaging in age-related diseases. Semin Immunol 40, 17–35. [DOI] [PubMed] [Google Scholar]
- Gao M, Huang Y, Wang L, Huang M, Liu F, Liao S, Yu S, Lu Z, Han S, Hu X, Qu Z, Liu X, Assefa Yimer T, Yang L, Tang Z, Li DW, Liu M, 2017. HSF4 regulates lens fiber cell differentiation by activating p53 and its downstream regulators. Cell death & disease 8, e3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J, 2009. Genetics of crystallins: cataract and beyond. Exp Eye Res 88, 173–189. [DOI] [PubMed] [Google Scholar]
- Green H, Solomon SA, 1957. The effect of age upon lens metabolism. AMA Arch Ophthalmol 58, 23–36. [DOI] [PubMed] [Google Scholar]
- Harding JJ, 2002. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev 1, 465–479. [DOI] [PubMed] [Google Scholar]
- He S, Pirity MK, Wang WL, Wolf L, Chauhan BK, Cveklova K, Tamm ER, Ashery-Padan R, Metzger D, Nakai A, Chambon P, Zavadil J, Cvekl A, 2010. Chromatin remodeling enzyme Brg1 is required for mouse lens fiber cell terminal differentiation and its denucleation. Epigenetics Chromatin 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydt RVD, 1930. Lens changes after middle age. Arch Opthalmol 4, 188–193. [Google Scholar]
- Hockwin O, 1973. The presence of glycogen in lenses of different species. Exp Eye Res 15, 235–244. [DOI] [PubMed] [Google Scholar]
- Huang Y, Ye Z, Yin Y, Ma T, Zhang Q, Shang K, Chen W, Li Z, 2021. Cataract formation in transgenic HO-1 G143H mutant mice: Involvement of oxidative stress and endoplasmic reticulum stress. Biochem Biophys Res Commun 537, 43–49. [DOI] [PubMed] [Google Scholar]
- Irum B, Khan SY, Ali M, Kaul H, Kabir F, Rauf B, Fatima F, Nadeem R, Khan AO, Al Obaisi S, Naeem MA, Nasir IA, Khan SN, Husnain T, Riazuddin S, Akram J, Eghrari AO, Riazuddin SA, 2016. Mutation in LIM2 Is Responsible for Autosomal Recessive Congenital Cataracts. PLoS One 11, e0162620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E, 1898. Influence of age in causing opacity of the crystallin lens, and the proper use of the word “cataract” JAMA XXXI, 698–701. [Google Scholar]
- Jiang J, Shihan MH, Wang Y, Duncan MK, 2018. Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery. Invest Ophthalmol Vis Sci 59, 4986–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jira P, 2013. Cholesterol metabolism deficiency. Handb Clin Neurol 113, 1845–1850. [DOI] [PubMed] [Google Scholar]
- Juthani VV, Clearfield E, Chuck RS, 2017. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev 7, CD010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA, 2018. iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res 46, D875–D885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K, 2017. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45, D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Miyake K, Hirano K, Kondo M, 2019. Management of Postoperative Inflammation and Dry Eye After Cataract Surgery. Cornea 38 Suppl 1, S25–S33. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M, 1999. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem 274, 19254–19260. [DOI] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL, 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH, 1999. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A 96, 3781–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS, 2000. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Archives of biochemistry and biophysics 373, 16–22. [DOI] [PubMed] [Google Scholar]
- Lachke SA, Ho JW, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML, Park PJ, Maas RL, 2012. iSyTE: integrated Systems Tool for Eye gene discovery. Invest Ophthalmol Vis Sci 53, 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR, David LL, 2014. Lens beta-crystallins: The role of deamidation and related modifications in aging and cataract. Progress in biophysics and molecular biology 115, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren H, Blixt A, Carlsson P, 2008. Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci 49, 4269–4277. [DOI] [PubMed] [Google Scholar]
- Lerman S, Zigman S, 1965. The Metabolism of the Lens as Related to Aging and Experimental Cataractogenesis. Invest Ophthalmol 4, 643–666. [PubMed] [Google Scholar]
- Li H, Mao Y, Bouaziz M, Yu H, Qu X, Wang F, Feng GS, Shawber C, Zhang X, 2019. Lens differentiation is controlled by the balance between PDGF and FGF signaling. PLoS Biol 17, e3000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bai C, Guo J, Liang W, Long J, 2017. NDUFA4L2 protects against ischaemia/reperfusion-induced cardiomyocyte apoptosis and mitochondrial dysfunction by inhibiting complex I. Clin Exp Pharmacol Physiol 44, 779–786. [DOI] [PubMed] [Google Scholar]
- Liao S, Qu Z, Li L, Zhou B, Gao M, Huang M, Li D, 2018. HSF4 transcriptional regulates HMOX-1 expression in HLECs. Gene 655, 30–34. [DOI] [PubMed] [Google Scholar]
- Lindholm JM, Laine I, Tuuminen R, 2020. Intraocular Lens Power, Myopia, and the Risk of Nd:YAG Capsulotomy after 15,375 Cataract Surgeries. J Clin Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD, 2017. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 16, 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L, 2015. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 14, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel P, Klethi J, 1962. Pathways of adenosine triphosphate production by calf lens homogenates. Nature 195, 306–307. [DOI] [PubMed] [Google Scholar]
- Manthey AL, Terrell AM, Lachke SA, Polson SW, Duncan MK, 2014a. Development of novel filtering criteria to analyze RNA-sequencing data obtained from the murine ocular lens during embryogenesis. Genom Data 2, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey AL, Terrell AM, Wang Y, Taube JR, Yallowitz AR, Duncan MK, 2014b. The Zeb proteins δEF1 and Sip1 may have distinct functions in lens cells following cataract surgery. Invest Ophthalmol Vis Sci 55, 5445–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P, 2007. The lens circulation. J Membr Biol 216, 1–16. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M, 2005. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol 25, 8854–8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa R, Tyagi M, Harocopos G, Vollman D, Bassnett S, 2016. Somatic Variants in the Human Lens Epithelium: A Preliminary Assessment. Invest Ophthalmol Vis Sci 57, 4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R, Bron AJ, 2011. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci 366, 1278–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Zhang Y, Moskophidis D, Mivechi NF, 2004. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis 40, 205–217. [DOI] [PubMed] [Google Scholar]
- Norton M, Ng AC, Baird S, Dumoulin A, Shutt T, Mah N, Andrade-Navarro MA, McBride HM, Screaton RA, 2014. ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics. Sci Signal 7, ra10. [DOI] [PubMed] [Google Scholar]
- Ogden AT, Nunes I, Ko K, Wu S, Hines CS, Wang AF, Hegde RS, Lang RA, 1998. GRIFIN, a novel lens-specific protein related to the galectin family. J Biol Chem 273, 28889–28896. [DOI] [PubMed] [Google Scholar]
- Okuda J, Niizuma S, Shioi T, Kato T, Inuzuka Y, Kawashima T, Tamaki Y, Kawamoto A, Tanada Y, Iwanaga Y, Narazaki M, Matsuda T, Adachi S, Soga T, Takemura G, Kondoh H, Kita T, Kimura T, 2013. Persistent overexpression of phosphoglycerate mutase, a glycolytic enzyme, modifies energy metabolism and reduces stress resistance of heart in mice. PLoS One 8, e72173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Whitin JC, Hill KE, Winfrey VP, Motley AK, Austin LM, Deal J, Cohen HJ, Burk RF, 2010. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am J Physiol Renal Physiol 298, F1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RJ, 2018. Cataract Surgery From 1918 to the Present and Future-Just Imagine! Am J Ophthalmol 185, 10–13. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Mizuno A, 1992. Molecular aging of lens crystallins and the life expectancy of the animal. Age-related protein structural changes studied in situ by Raman spectroscopy. Biochim Biophys Acta 1121, 245–251. [DOI] [PubMed] [Google Scholar]
- Pendergrass WR, Penn PE, Li J, Wolf NS, 2001. Age-related telomere shortening occurs in lens epithelium from old rats and is slowed by caloric restriction. Exp Eye Res 73, 221–228. [DOI] [PubMed] [Google Scholar]
- Periyasamy P, Shinohara T, 2017. Age-related cataracts: Role of unfolded protein response, Ca(2+) mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog Retin Eye Res 60, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipson B, Lee S, Majewski IJ, Alexander WS, Smyth GK, 2016. Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. The Annals of Applied Statistics 10, 946–963, 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propson NE, Gedam M, Zheng H, 2021. Complement in Neurologic Disease. Annu Rev Pathol 16, 277–298. [DOI] [PubMed] [Google Scholar]
- Ray NJ, 2015. Biophysical chemistry of the ageing eye lens. Biophys Rev 7, 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink H, 1987. Cataractogenic risk factors. Dev Ophthalmol 15, 66–76. [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK, 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A, 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronbeck M, Kugelberg M, 2014. Posterior capsule opacification with 3 intraocular lenses: 12-year prospective study. J Cataract Refract Surg 40, 70–76. [DOI] [PubMed] [Google Scholar]
- Sato R, 2010. Sterol metabolism and SREBP activation. Archives of biochemistry and biophysics 501, 177–181. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Khor CC, 2021. Pseudoexfoliation syndrome and glaucoma: from genes to disease mechanisms. Curr Opin Ophthalmol 32, 118–128. [DOI] [PubMed] [Google Scholar]
- Sen P, Kshetrapal M, Shah C, Mohan A, Jain E, Sen A, 2019. Posterior capsule opacification rate after phacoemulsification in pediatric cataract: Hydrophilic versus hydrophobic intraocular lenses. J Cataract Refract Surg 45, 1380–1385. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chataway T, Burdon KP, Jonavicius L, Klebe S, Hewitt AW, Mills RA, Craig JE, 2009. Identification of LOXL1 protein and Apolipoprotein E as components of surgically isolated pseudoexfoliation material by direct mass spectrometry. Exp Eye Res 89, 479–485. [DOI] [PubMed] [Google Scholar]
- Shi X, Cui B, Wang Z, Weng L, Xu Z, Ma J, Xu G, Kong X, Hu L, 2009. Removal of Hsf4 leads to cataract development in mice through down-regulation of gamma S-crystallin and Bfsp expression. BMC Mol Biol 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihan MH, Novo SG, Duncan MK, 2019. Cataract surgeon viewpoints on the need for novel preventative anti-inflammatory and anti-posterior capsular opacification therapies. Curr Med Res Opin 35, 1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si N, Song Z, Meng X, Li X, Xiao W, Zhang X, 2019. A novel MAF missense mutation leads to congenital nuclear cataract by impacting the transactivation of crystallin and noncrystallin genes. Gene 692, 113–118. [DOI] [PubMed] [Google Scholar]
- Si Z, Sun L, Wang X, 2021. Evidence and perspectives of cell senescence in neurodegenerative diseases. Biomed Pharmacother 137, 111327. [DOI] [PubMed] [Google Scholar]
- Silva PN, Altamentova SM, Kilkenny DM, Rocheleau JV, 2013. Fibroblast growth factor receptor like-1 (FGFRL1) interacts with SHP-1 phosphatase at insulin secretory granules and induces beta-cell ERK1/2 protein activation. J Biol Chem 288, 17859–17870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Roderick TH, Sundberg JP, 1994. Microphthalmia and associated abnormalities in inbred black mice. Lab Anim Sci 44, 551–560. [PubMed] [Google Scholar]
- Song S, Landsbury A, Dahm R, Liu Y, Zhang Q, Quinlan RA, 2009. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest 119, 1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Barth E, Ermolaeva MA, Guenther M, Frahm C, Marz M, Witte OW, 2020. Tissue-specific Gene Expression Changes Are Associated with Aging in Mice. Genomics Proteomics Bioinformatics [DOI] [PMC free article] [PubMed]
- Storey JD, Tibshirani R, 2003. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100, 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski WK, Raguz M, Widomska J, Mainali L, Konovalov A, 2012. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J Membr Biol 245, 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhija J, Ram J, Gupta N, Sawhney A, Kaur S, 2014. Long-term results after primary intraocular lens implantation in children operated less than 2 years of age for congenital cataract. Indian J Ophthalmol 62, 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft RA, Davisson M, Wiles MV, 2006. Know thy mouse. Trends Genet 22, 649–653. [DOI] [PubMed] [Google Scholar]
- Tan LX, Germer CJ, La Cunza N, Lakkaraju A, 2020. Complement activation, lipid metabolism, and mitochondrial injury: Converging pathways in age-related macular degeneration. Redox Biol 37, 101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarca AL, Draghici S, Khatri P, Hassan SS, Mittal P, Kim JS, Kim CJ, Kusanovic JP, Romero R, 2009. A novel signaling pathway impact analysis. Bioinformatics 25, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia N, Singh AK, Pandey P, Azad CS, Chaudhary P, Gambhir IS, 2018. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene 648, 97–105. [DOI] [PubMed] [Google Scholar]
- Treton JA, Jacquemin E, Courtois Y, 1988. Variation in the relative abundance of gamma-crystallin gene transcripts during development and ageing. Exp Eye Res 46, 405–413. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Friedrich MG, 2019. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Invest Ophthalmol Vis Sci 60, 5007–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino S, Shanske S, Sakoda S, Fenichel G, DiMauro S, 1993. The molecular genetic basis of muscle phosphoglycerate mutase (PGAM) deficiency. Am J Hum Genet 52, 472–477. [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Li L, Qin B, Wu J, Cheng T, Kang L, Guan H, 2019. Long noncoding RNA glutathione peroxidase 3-antisense inhibits lens epithelial cell apoptosis by upregulating glutathione peroxidase 3 expression in age-related cataract. Mol Vis 25, 734–744. [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Duncan MK, David LL, 2002. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci 43, 205–215. [PubMed] [Google Scholar]
- Uwineza A, Kalligeraki AA, Hamada N, Jarrin M, Quinlan RA, 2019. Cataractogenic load - A concept to study the contribution of ionizing radiation to accelerated aging in the eye lens. Mutat Res 779, 68–81. [DOI] [PubMed] [Google Scholar]
- Vasiliev O, Rhodes SJ, Beebe DC, 2007. Identification and expression of Hop, an atypical homeobox gene expressed late in lens fiber cell terminal differentiation. Mol Vis 13, 114–124. [PMC free article] [PubMed] [Google Scholar]
- Wang-Su ST, McCormack AL, Yang S, Hosler MR, Mixon A, Riviere MA, Wilmarth PA, Andley UP, Garland D, Li H, David LL, Wagner BJ, 2003. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci 44, 4829–4836. [DOI] [PubMed] [Google Scholar]
- Wang XH, Garcia CM, Shui YB, Beebe DC, 2004. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Investigative Ophthalmology & Visual Science 45, 3608–3619. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Z, Huang C, Zhao L, Jiang X, Liu Y, Liu Y, Wan Y, Chou Y, Li X, 2020. Analysis of lens epithelium telomere length in age-related cataract. Exp Eye Res 201, 108279. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang G, Kang L, Guan H, 2017. Expression Profiling of DNA Methylation and Transcriptional Repression Associated Genes in Lens Epithelium Cells of Age-Related Cataract. Cell Mol Neurobiol 37, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE, 2014. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 69, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widomska J, Subczynski WK, 2019. Why Is Very High Cholesterol Content Beneficial for the Eye Lens but Negative for Other Organs? Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Riley MV, 1991. Relative contributions of epithelial cells and fibers to rabbit lens ATP content and glycolysis. Invest Ophthalmol Vis Sci 32, 2593–2598. [PubMed] [Google Scholar]
- Wolf N, Penn P, Pendergrass W, Van Remmen H, Bartke A, Rabinovitch P, Martin GM, 2005. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res 81, 276–285. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Li Y, Pendergrass W, Schmeider C, Turturro A, 2000. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res 70, 683–692. [DOI] [PubMed] [Google Scholar]
- Worgul BV, Merriam GR Jr., Medvedovsky C, 1989. Cortical cataract development--an expression of primary damage to the lens epithelium. Lens Eye Toxic Res 6, 559–571. [PubMed] [Google Scholar]
- Wormstone IM, Liu CS, Rakic JM, Marcantonio JM, Vrensen GF, Duncan G, 1997. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci 38, 396–404. [PubMed] [Google Scholar]
- Wormstone IM, Wang L, Liu CS, 2009. Posterior capsule opacification. Exp Eye Res 88, 257–269. [DOI] [PubMed] [Google Scholar]
- Wu S, Tong N, Pan L, Jiang X, Li Y, Guo M, Li H, 2018. Retrospective Analyses of Potential Risk Factors for Posterior Capsule Opacification after Cataract Surgery. J Ophthalmol 2018, 9089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cheng BH, Jia SB, Tang LS, 2010. Involvement of the PI3K/Akt signaling pathway in platelet-derived growth factor-induced migration of human lens epithelial cells. Curr Eye Res 35, 389–401. [DOI] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, Azov AG, Bennett R, Bhai J, Billis K, Boddu S, Marugan JC, Cummins C, Davidson C, Dodiya K, Fatima R, Gall A, Giron CG, Gil L, Grego T, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, Kay M, Lavidas I, Le T, Lemos D, Martinez JG, Maurel T, McDowall M, McMahon A, Mohanan S, Moore B, Nuhn M, Oheh DN, Parker A, Parton A, Patricio M, Sakthivel MP, Abdul Salam AI, Schmitt BM, Schuilenburg H, Sheppard D, Sycheva M, Szuba M, Taylor K, Thormann A, Threadgold G, Vullo A, Walts B, Winterbottom A, Zadissa A, Chakiachvili M, Flint B, Frankish A, Hunt SE, G II, Kostadima M, Langridge N, Loveland JE, Martin FJ, Morales J, Mudge JM, Muffato M, Perry E, Ruffier M, Trevanion SJ, Cunningham F, Howe KL, Zerbino DR, Flicek P, 2020. Ensembl 2020. Nucleic Acids Res 48, D682–D688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML, 2008. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol 318, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Yue S, Chen H, Weber B, Jia J, Zheng Y, 2015. Low-Cell-Number Epigenome Profiling Aids the Study of Lens Aging and Hematopoiesis. Cell Rep 13, 1505–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


