Abstract
Background:
The prognosis of exercise-induced premature ventricular contractions (PVCs) in asymptomatic individuals is unclear.
Objectives:
To investigate if high-grade PVCs during stress testing predict mortality in asymptomatic individuals.
Methods:
5,486 asymptomatic individuals who took part in the Lipid Research Clinics prospective cohort had baseline interview, physical examination, blood tests, and underwent Bruce protocol treadmill testing. Adjusted Cox survival models evaluated the association of exercise-induced high-grade PVCs (defined as either frequent (>10/min), multifocal, R-on-T type, or ≥ 2 PVCs in a row) with all-cause and cardiovascular mortality.
Results:
Mean baseline age was 45.4 ± 10.8 years; 42% were women. During a mean follow-up of 20.2 ± 3.9 years, 840 deaths occurred, including 311 cardiovascular deaths. High-grade PVCs occurred during exercise in 1.8% of individuals, during recovery in 2.4%, and during both in 0.8%. After adjusting for age, sex, diabetes, hypertension, lipids, smoking, body mass index, and family history of premature coronary disease, high-grade PVCs during recovery were associated with cardiovascular mortality (HR=1.82, 95% CI [1.19–2.79], p=0.006) which remained significant after further adjusting for exercise duration, heart rate recovery, achieving target heart rate, and ST depression (HR=1.68, 95% CI [1.09–2.60], p=0.020). Results were similar by clinical subgroups. High-grade PVCs occurring during the exercise phase were not associated with increased risk. Recovery PVCs did not improve 20-year cardiovascular mortality risk discrimination beyond clinical variables.
Conclusion:
High-grade PVCs occurring during recovery were associated with long-term risk of cardiovascular mortality in asymptomatic individuals, while PVCs occurring only during exercise were not associated with increased risk.
Keywords: ventricular ectopy, premature ventricular contraction, cardiac arrhythmia, stress test, cardiovascular disease, heart disease
Condensed Abstract
In order to study the prognostic role of exercise-induced high-grade premature ventricular contractions (PVCs) in asymptomatic individuals, we analyzed data from a prospective cohort of 5,486 individuals followed up for a mean duration of 20 years. High-grade PVCs were defined as frequent (>10/min), multifocal, R-on-T type, or ≥ 2 PVCs in a row. Only high-grade PVCs during recovery and not during exercise were associated with cardiovascular mortality. Risk was increased 1.7-fold. This increased risk was independent of clinical or exercise risk factors.
Introduction
Exercise stress testing is commonly ordered in the inpatient and outpatient settings. Exercise test parameters (e.g. exercise duration or ST segment changes) have been shown to predict cardiovascular risk and mortality independently of clinical risk factors, in both symptomatic and asymptomatic patients (1–4). Whether the presence of premature ventricular contractions (PVCs) during exercise treadmill testing has important prognostic implications remains controversial, especially in asymptomatic individuals (5–8).
PVCs are classified according to the Lown criteria into low-grade (monomorphic and infrequent) or high-grade (frequent, multifocal, repetitive including ventricular tachycardia and R-on-T type PVCs).
In patients with suspected or known coronary heart disease, exercise-induced PVCs have been associated with an increase in cardiovascular risk (9,10), especially if they were high-grade and occurred during recovery from stress testing (11–13). The suggested mechanism is that PVCs occurring during recovery are due to insufficient vagal reactivation following exercise (12,14), which in itself is associated with an increase in mortality (2,15).
In asymptomatic individuals not suspected of having heart disease, high-grade exercise-induced PVCs were also found to be associated with mortality (5,7,16). However, whether differential PVC timing (i.e. during exercise or recovery) carries differential prognostic value has not been shown to date in asymptomatic individuals.
Knowing the prognostic implications of exercise-induced PVC timing in patients free of heart disease is important for two reasons. One reason is clinical, as it would help more accurately identify patients at future risk who would otherwise be considered to have no to low risk. Another important reason is that there is conflicting evidence about the relation of exercise-induced PVCs to coronary heart disease (7,12,17,18); therefore, individuals without coronary artery disease provide a unique opportunity to study the pathophysiology of exercise-induced PVCs. If recovery PVCs carry worse prognosis than exercise PVCs, this would provide additional evidence in support of the hypothesis that PVC timing is an important predictor of cardiovascular risk tied to the degree of vagal activity and independent of coronary heart disease.
Using patient information from the Lipid Research Clinics Program (19) with 20-year follow-up, we aimed to investigate if exercise or recovery high-grade PVCs differentially associate with cardiovascular and all-cause mortality in asymptomatic individuals.
Methods
Study Participants
Participants took part in the Lipid Research Clinics Prevalence Study, a prospective cohort of North American individuals across 10 geographic locations and diverse socioeconomic and occupational groups, as previously described (16,19,20). The populations fell into three broad categories: occupational groups, household or residential groups, and parents of school children. At their first visit (between 1972–1976), individuals were screened from 10 primary care centers in North America. A sample consisting of all visit 1 participants with elevated lipid levels, as well as an additional random sample of 15% of visit 1 participants were invited back for visit 2. In total, the random sample constituted 58% of the total study participants.
During visit 2, participants underwent a medical interview, physical examination, fasting blood studies and a treadmill exercise test. A participant was not eligible for exercise testing if they had resting systolic blood pressure < 90 mmHg or > 200 mmHg, resting diastolic blood pressure > 120 mmHg, had significant cardiovascular disease, had R-on-T type PVCs or ventricular tachycardia at rest, or if deemed unable to complete the stress test by a study physician. The exercise protocol used was the Bruce or modified Bruce protocol. Altogether, 8,652 of 13,852 individuals screened underwent baseline exercise tests.
In order to ensure an asymptomatic cohort, we excluded participants if they had angina, claudication, left ventricular hypertrophy (using the Estes criteria on a resting ECG), a history of myocardial infarction, stroke, cardiac surgery, vascular surgery (other than for varicose veins), or if they were taking digoxin or other antiarrhythmics (except beta-blockers). Participants were additionally excluded from this analysis if they had no stress test performed, if exercise duration was less than 1 min or missing, if they underwent a modified Bruce protocol, if they were younger than 20 or older than 80 years, if they were lost to follow-up (3 participants) or if they had missing ECG rhythm code information during stress testing (20 participants). This resulted in 5,486 asymptomatic participants.
Follow-up was until death or December 31, 1995. The primary outcome of this study was cardiovascular mortality. From 1976 to 1988, participant deaths were reported by mail or phone and then confirmed with either death certificates, family member or witness interviews, or via medical records. Reason of death was determined by cardiologists who were blinded to the identity of the departed. From 1988 to 1995, deaths were reported from death certificates by trained nosologists who used the National Death Index from 1988 to 1991 and then the Epidemiology Research Index from 1992 to 1995. All participants gave written informed consent at study enrollment. The Brigham and Women’s Hospital and the American University of Beirut Institutional Review Boards approved the present study. This research was considered exempt.
Exercise Test ECGs
Systolic and diastolic blood pressures and ECGs were recorded at rest, at the end of each of the stages of exercise, and then immediately and at 2, 4, and 6 minutes after exercise. The test was terminated when a target heart rate of 90% of the maximum predicted heart rate for age and physical fitness was achieved (20). The test was also terminated if the participant developed angina, hypotension, ECG changes of significant arrhythmia or ischemia, or was unable or refused to complete the test.
ECGs at each exercise stage and during recovery were read in duplicate by trained coders and checked by a supervisor. The visual coding system used had an internal quality control system at a central coding center. ECGs were additionally computer-analyzed, and any visual versus computer coding and inter-coder discrepancy was resolved by the supervisor and one of two cardiologists (16).
Exercise Test Parameters
A PVC was defined on ECG as a QRS-T complex with QRS duration ≥ 0.12 second not preceded at least 0.12 second by a P wave. High-grade PVCs were defined as frequent (>10/min), multifocal, 2 or more PVCs in a row (including ventricular tachycardia), or R-on-T type PVCs (Central Illustration). ST-segment depressions were defined as at least 1.0 mm horizontal or down-sloping depressions in the lead with the greatest abnormality during the last stage of exercise or recovery. Heart rate recovery was calculated as maximal heart rate achieved during exercise minus heart rate at 2 min after exercise (21).
Central Illustration. Prognostic value of exercise-induced PVCs in asymptomatic individuals.
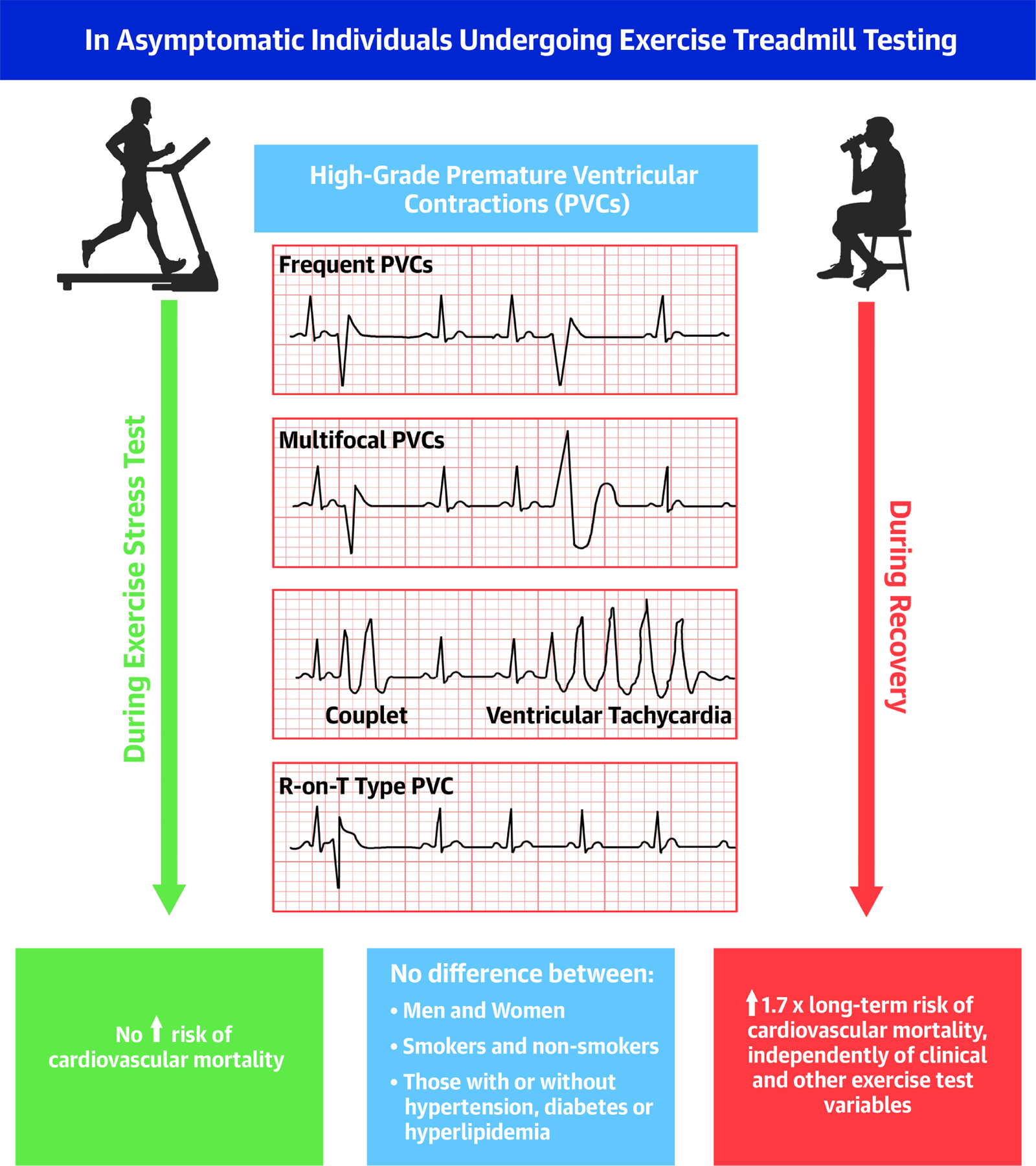
This figure summarizes how high-grade PVCs were defined in this study. It shows that in asymptomatic individuals, only high-grade PVCs during recovery and not the exercise phase independently associate with cardiovascular mortality. The arrows indicate the prognostic role of PVCs timing (during exercise versus recovery).
PVCs: Premature ventricular contractions
Clinical Parameters
Participants were considered hypertensive at baseline if they took antihypertensives, had a resting systolic blood pressure ≥ 140 mmHg or resting diastolic blood pressure ≥ 90 mmHg. Diabetes was defined as taking antidiabetic medications or having a fasting glucose ≥ 126 mg/dL at visit 2. Lipids were obtained after an overnight fast. Hyperlipidemia was defined as total-cholesterol ≥ 240 mg/dL, LDL-cholesterol ≥ 160 mg/dL, or triglycerides ≥ 200 mg/dL. A participant had a positive family history of premature coronary artery disease if they had a father, mother, or sibling with the disease manifestation before the age of 60 years. Participants were considered smokers if they had ever smoked cigarettes.
Statistical Methods
Baseline clinical and exercise test characteristics were compared between groups with and without high-grade PVCs using the t-test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables.
In order to study the association of high-grade PVCs with subsequent cardiovascular and all-cause mortality, age- and sex-adjusted cumulative probability curves were constructed and survival analyses using Cox models were used. We also constructed models that adjusted for clinical variables (age, sex, hypertension, diabetes, LDL- and HDL-cholesterol, triglycerides, smoking, body mass index, family history of premature coronary artery disease) and further adjusted for exercise test variables (exercise duration, ST depression ≥ 1mm, achieving target heart rate, heart rate recovery). The association between high-grade PVCs and cardiovascular mortality was also assessed using a competing endpoints analysis (Supplemental Table S5).
In a sensitivity analysis where the missing values for diabetes (452 patients, 8%) and family history of premature coronary artery disease (343 patients, 6%) were added as missing indicators or were imputed, the results were generally similar. (Supplemental tables S2 and S3).
The proportional hazards assumption was tested for and met by including an interaction term between ln (follow-up time) and presence of high-grade PVCs (p>0.05) (Supplemental table S6). Subgroup analyses were performed according to categories of sex, diabetes, hypertension, and hyperlipidemia.
Harrell’s C-index, the net reclassification index and the integrated discrimination index were used to evaluate the improvement in predicting cardiovascular mortality gained by adding high-grade PVC data to clinical variables.
All reported p-values were 2-sided. A p<0.05 was considered statistically significant. Statistical analyses were performed using Statistical Analysis System (SAS) version 9.
Results
There were 5,486 asymptomatic individuals (mean baseline age 45.4 ± 10.8 years, 42% women, 50% with hyperlipidemia) who were followed up for a mean duration of 20.2 ± 3.9 years. There were 840 (15.3%) deaths due to any cause, including 311 cardiovascular deaths (37.0% of deaths). High-grade PVCs occurred in 101 (1.8%) asymptomatic individuals during exercise, in 133 (2.4%) during recovery and in 42 (0.8%) during both exercise and recovery.
Individuals who experienced high-grade PVCs during exercise or recovery were older and more likely to have diabetes and hypertension (Table 1). On exercise stress test, they were more likely to have evidence of ischemia (ST depression ≥ 1 mm), had significantly lower exercise duration and heart rate recovery, and were less likely to achieve their predefined target heart rate. Women were also more likely to experience PVCs during recovery (Table 1).
Table 1.
Baseline clinical and exercise test characteristics.
| Full cohort | High-grade PVCs during exercise | High-grade PVCs during recovery | |||||
|---|---|---|---|---|---|---|---|
| n=5,486 | No (n=5,385) | Yes (n=101) | p-value | No (n=5,353) | Yes (n=133) | p-value | |
| Clinical variables | |||||||
| Age (years) | 45.4 ± 10.8 | 45.1 ± 10.6 | 60.1 ± 11.8 | <.001 | 45.0 ± 10.5 | 61.4 ± 12.0 | <.001 |
| Women | 2,302 (42.0) | 2,252 (41.8) | 50 (49.5) | 0.121 | 2,232 (41.7) | 70 (52.6) | 0.012 |
| White race | 5,261 (95.9) | 5,162 (95.9) | 99 (98.0) | 0.779 | 5,128 (95.8) | 133 (100.0) | 0.212 |
| Diabetes | 173 (3.2) | 165 (3.3) | 8 (9.1) | 0.003 | 165 (3.4) | 8 (7.2) | 0.027 |
| Hypertension | 2,376 (43.3) | 2,317 (43.1) | 59 (58.4) | 0.002 | 2.293 (42.9) | 83 (62.4) | <.001 |
| Smoking | 3,541 (64.6) | 3,479 (64.6) | 62 (61.4) | 0.503 | 3,462 (64.7) | 79 (59.4) | 0.209 |
| Total cholesterol ≥ 240 mg/dL | 1,810 (33.0) | 1,768 (32.9) | 42 (41.6) | 0.067 | 1,761 (33.0) | 49 (36.8) | 0.352 |
| LDL-cholesterol ≥ 160 mg/dL | 1,878 (34.2) | 1,833 (34.2) | 45 (44.6) | 0.030 | 1,832 (34.4) | 46 (34.6) | 0.963 |
| HDL-cholesterol < 40 mg/dL | 1,440 (26.3) | 1,425 (26.6) | 15 (14.9) | 0.008 | 1,420 (26.7) | 20 (15.0) | 0.003 |
| Triglycerides ≥ 200 mg/dL | 1,155 (21.1) | 1,337 (24.8) | 24 (23.8) | 0.806 | 1,334 (24.9) | 27 (20.3) | 0.223 |
| Body mass index ≥ 30 kg/m2 | 763 (13.9) | 751 (14.0) | 12 (11.9) | 0.548 | 747 (14.0) | 16 (12.0) | 0.521 |
| Family history of premature coronary artery disease | 1,235 (22.5) | 1,213 (24.0) | 22 (25.0) | 0.827 | 1,206 (24.0) | 29 (23.0) | 0.791 |
| Exercise test variables | |||||||
| Exercise duration (minutes) | 8.5 ± 2.6 | 8.5 ± 2.6 | 5.4 ± 2.9 | <.001 | 8.6 ± 2.6 | 4.7 ± 2.7 | <.001 |
| Exercise duration ≥ median | 2,984 (54.4) | 2,967 (55.1) | 17 (16.8) | <.001 | 2,970 (55.5) | 14 (10.5) | <.001 |
| Heart rate recovery (bpm) | 55.0 ± 12.8 | 55.1 ± 12.7 | 51.1 ± 17.6 | 0.002 | 55.1 ± 12.6 | 52.2 ± 20.2 | 0.011 |
| Heart rate recovery ≥ median | 2,792 (50.9) | 2,754 (51.3) | 38 (37.6) | 0.007 | 2,736 (51.2) | 56 (42.4) | 0.046 |
| Target heart rate achieved | 3,953 (72.1) | 3,917 (72.7) | 36 (35.6) | <.001 | 3,913 (73.1) | 40 (30.1) | <.001 |
| ST depression ≥ 1 mm | 215 (3.9) | 202 (3.8) | 13 (13.0) | <.001 | 202 (3.8) | 13 (9.8) | <.001 |
Continuous variables (age, exercise duration, heart rate recovery) are presented as mean ± standard deviation, categorical variables (remaining variables) are presented as number (valid %).
Individuals with high-grade PVCs during exercise had higher rates of cardiovascular mortality (19.8% versus 5.4%, p<0.001) and all-cause mortality (48.5% versus 14.7%, p<0.001) compared to those who did not experience high-grade PVCs during exercise. Similarly, individuals with high-grade PVCs during recovery had higher rates of cardiovascular mortality (27.1% versus 5.1%, p<0.001) and all-cause mortality (52.6% versus 14.4%, p<0.001) compared to those who did not experience high-grade PVCs during recovery.
Age- and sex-adjusted cumulative probability curves of cardiovascular and all-cause mortality according to presence of high-grade PVCs seem to diverge somewhat more for high-grade PVCs during recovery rather than those during exercise, and more so for cardiovascular mortality rather than all-cause mortality (Figure 1).
Figure 1. Cumulative probability curves of cardiovascular and all-cause mortality.
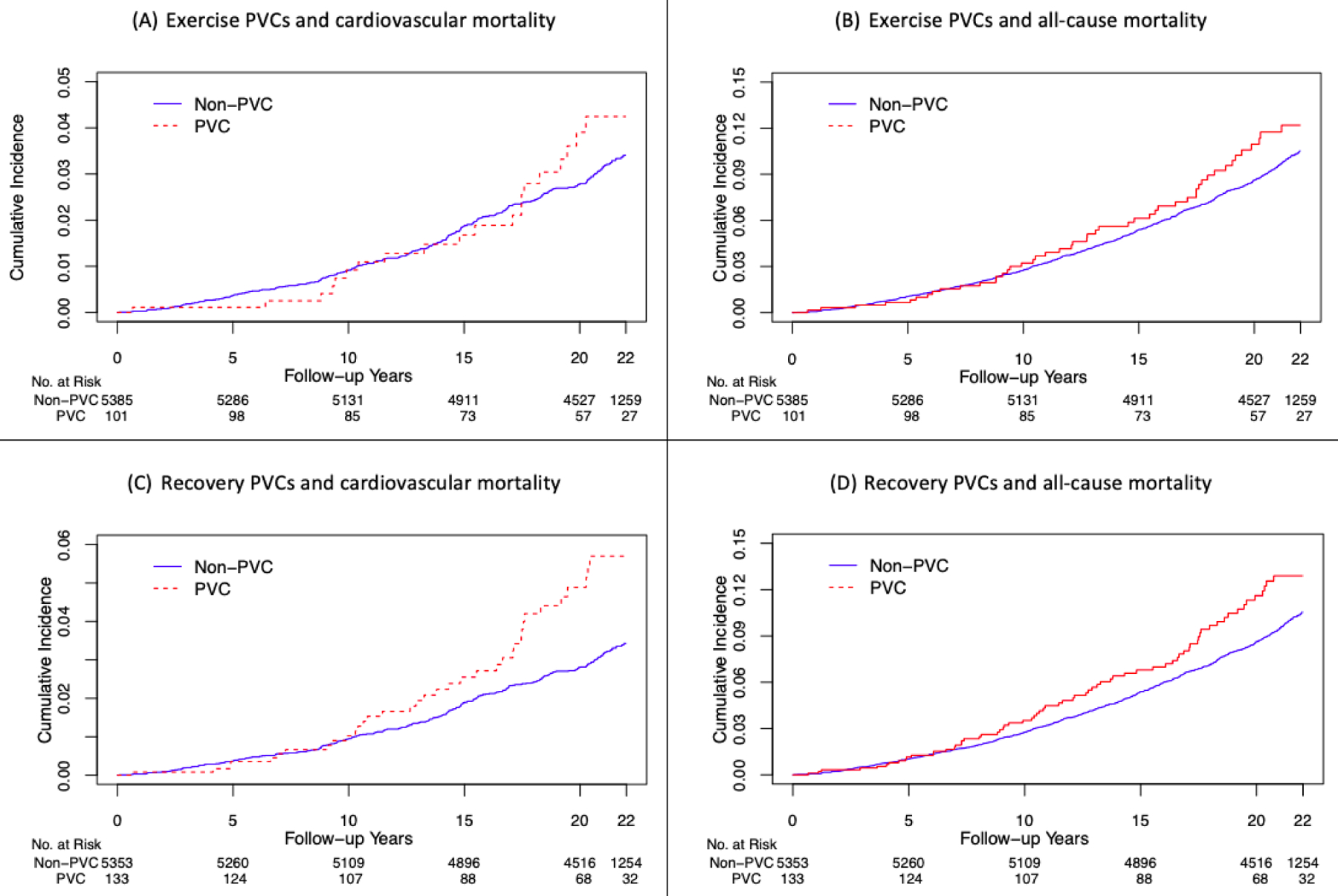
The shown curves are age- and sex-adjusted. Exercise-induced PVCs seem to confer a long-term (as opposed to short-term) mortality risk as shown by more pronounced red and blue curves separations as follow-up time increases. The curves seem to diverge somewhat more for high-grade PVCs during recovery rather than those during exercise, and more so for cardiovascular mortality rather than all-cause mortality.
PVC: Premature ventricular contraction
After adjusting for age and sex, high-grade PVCs during recovery were associated with cardiovascular mortality (adjusted HR=1.59, 95% CI [1.09–2.30], p=0.015) while those during exercise were not significant (adjusted HR=1.16, 95% CI [0.73–1.85], p=0.527) (Table 2). After adjusting for age and sex, high-grade PVCs during both exercise and recovery were associated with all-cause mortality (adjusted HR=1.65, 95% CI [1.11–2.45], p=0.014) (Table 2). Longer exercise duration, higher heart rate recovery values, and achieving target heart rate were associated with a decreased risk of cardiovascular and all-cause mortality and the presence of ST depression ≥ 1 mm was associated with increased risk of cardiovascular mortality (Supplemental table S1).
Table 2.
Adjusted hazard ratios (HRs) of cardiovascular and all-cause mortality according to presence of high-grade PVCs.
| Cardiovascular mortality (311 out of 5,486) | All-cause mortality (840 out of 5,486) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Adjusted for age and sex | ||||
| Presence of high-grade PVCs during exercise | 1.16 (0.73–1.85) | 0.527 | 1.21 (0.90–1.62) | 0.217 |
| Presence of high-grade PVCs during recovery | 1.59 (1.09–2.30) | 0.015 | 1.28 (0.99–1.65) | 0.065 |
| Presence of high-grade PVCs during both exercise and recovery | 1.49 (0.79–2.83) | 0.222 | 1.65 (1.11–2.45) | 0.014 |
| Presence of high-grade PVCs during exercise or recovery | 1.42 (1.01–1.99) | 0.046 | 1.17 (0.93–1.47) | 0.189 |
| Adjusted for clinical variables * | ||||
| Presence of high-grade PVCs during exercise | 1.38 (0.82–2.34) | 0.224 | 1.26 (0.88–1.80) | 0.200 |
| Presence of high-grade PVCs during recovery | 1.82 (1.19–2.79) | 0.006 | 1.30 (0.96–1.75) | 0.092 |
| Presence of high-grade PVCs during both exercise and recovery | 1.49 (0.72–3.06) | 0.281 | 1.49 (0.93–2.40) | 0.101 |
| Presence of high-grade PVCs during exercise or recovery | 1.72 (1.17–2.53) | 0.006 | 1.24 (0.95–1.63) | 0.112 |
| Adjusted for clinical* and exercise test variables† | ||||
| Presence of high-grade PVCs during exercise | 1.34 (0.79–2.26) | 0.278 | 1.18 (0.83–1.69) | 0.356 |
| Presence of high-grade PVCs during recovery | 1.68 (1.09–2.60) | 0.020 | 1.15 (0.85–1.56) | 0.377 |
| Presence of high-grade PVCs during both exercise and recovery | 1.36 (0.66–2.80) | 0.405 | 1.31 (0.81–2.12) | 0.268 |
| Presence of high-grade PVCs during exercise or recovery | 1.63 (1.10–2.42) | 0.015 | 1.13 (0.86–1.49) | 0.375 |
Among those who experienced high-grade PVCs during exercise (N=101), 49 died from any cause, including 20 from cardiovascular causes.
Among those who experienced high-grade PVCs during recovery (N=133), 70 died from any cause, including 36 from cardiovascular causes.
Among those who experienced high-grade PVCs during both exercise and recovery (N=42), 26 died from any cause, including 10 from cardiovascular causes.
Among those who experienced high-grade PVCs during exercise or recovery (N=192), 93 died from any cause, including 46 from cardiovascular causes.
Clinical variables: age, sex, diabetes, hypertension, smoking, LDL- and HDL-cholesterol, triglycerides, body mass index, family history of premature coronary artery disease
Exercise test variables: exercise duration (in minutes), heart rate recovery (in beats per minute), achieving target heart rate, ST depression ≥ 1mm
After adjusting for clinical variables (age, sex, diabetes, hypertension, LDL- and HDL-cholesterol, triglycerides, smoking, body mass index and family history of premature coronary artery disease) (adjusted HR=1.82, 95% CI [1.19–2.79], p=0.006) and both clinical and exercise test variables (exercise duration, heart rate recovery, achieving target heart rate, and ST depression ≥ 1mm) (adjusted HR=1.68, 95% CI [1.09–2.60], p=0.020), high-grade PVCs during recovery remained significantly associated with cardiovascular mortality (Table 2). Individuals who experienced high-grade PVCs during both exercise and recovery were few in number and the results did not reach statistical significance (adjusted HR=1.36, 95% CI [0.66–2.80], p=0.405). Those who experienced high-grade PVCs during either phase of exercise stress testing were at increased risk of cardiovascular mortality, driven by the risk of recovery PVCs (Table 2).
Subgroup analyses were performed according to categories of sex, diabetes, hypertension and hyperlipidemia. There was no significant difference in the ability of recovery high-grade PVCs to predict cardiovascular mortality among those with and without baseline diabetes, hyperlipidemia or hypertension, or between men and women, as evidenced by p-interaction >0.05 (Figure 2).
Figure 2. Adjusted HRs of cardiovascular mortality for recovery high-grade PVCs.
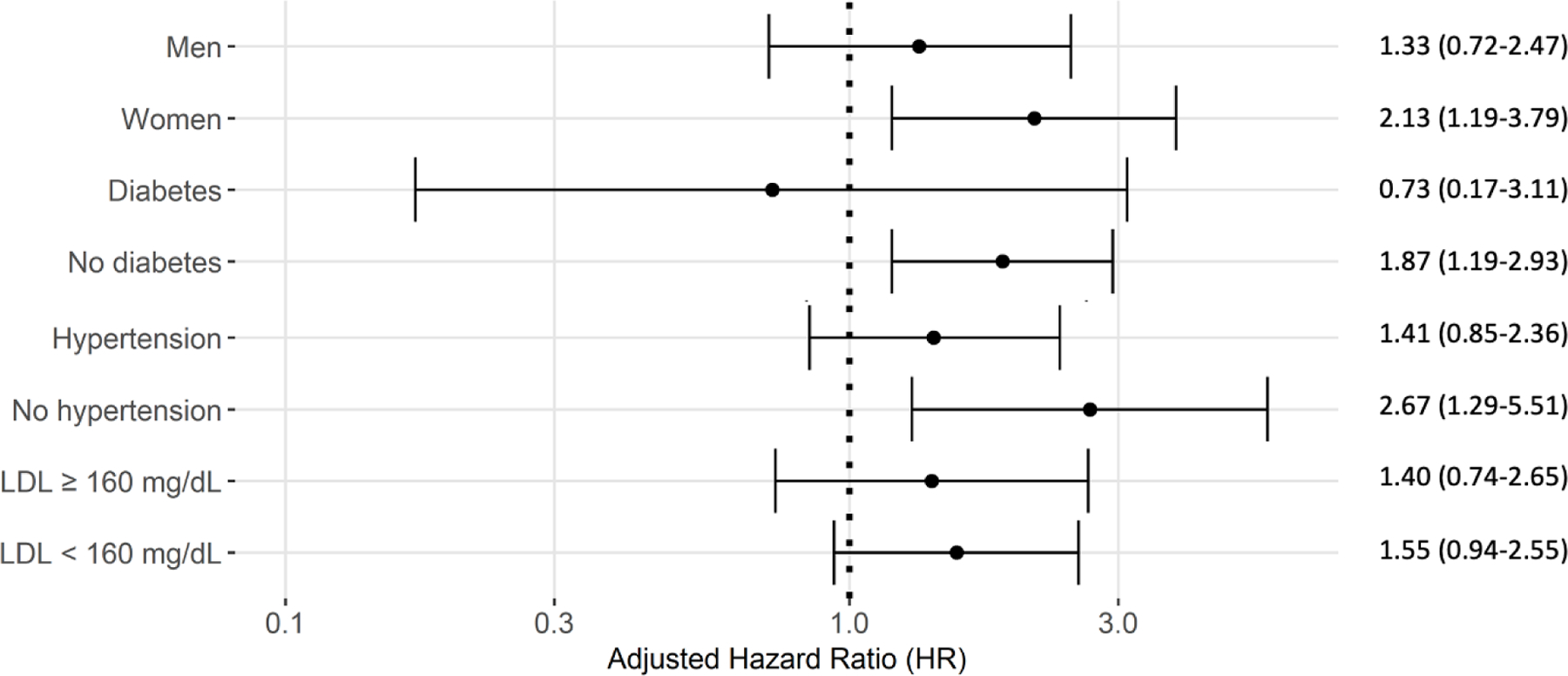
Results are shown by clinical subgroups (sex, hypertension, diabetes, LDL category). HRs were adjusted for both clinical (age, sex, diabetes, hypertension, smoking, LDL- and HDL-cholesterol, triglycerides, body mass index, family history of premature coronary artery disease) and exercise test variables (exercise duration (in minutes), heart rate recovery (in beats per minute), achieving target heart rate, ST depression ≥ 1mm). As shown by p-interactions > 0.05, there were no significant differences between subgroups.
HRs: Hazard Ratios
PVCs: Premature ventricular contractions
Note: p-interaction with high-grade PVCs during recovery was p=0.261 for sex, p=0.220 for diabetic status, p=0.147 for hypertensive status, p=0.801 for LDL category.
Adding recovery high-grade PVCs to a model that included the mentioned clinical variables did not result in statistically significant improvement in the 20-year cardiovascular mortality risk discrimination as indicated by Harrell’s C-index, the integrated discrimination improvement index, and the category-free net reclassification index (Table 3).
Table 3.
Discrimination and reclassification statistics for recovery high-grade PVCs with 20-year risk of cardiovascular mortality.
| Clinical variables* only | Clinical variables* + Recovery PVCs | |
|---|---|---|
| Harrell’s C-index (95% CI) | 0.8304 (0.8063, 0.8545) | 0.8322 (0.8063, 0.8563) |
| Difference in C-indices (95% CI), p-value | 0.0018 (−0.0008, 0.0045), p=0.178 | |
| IDI † (95% CI), p-value | Reference | 0.0050 (− 0.0026, 0.0144), p=0.239 |
| NRI ‡, 10% cutoff (95% CI), p-value | Reference | −0.0054 (− 0.0276, 0.0124), p=0.629 |
Clinical variables: age, sex, diabetes, hypertension, smoking, LDL- and HDL-cholesterol, triglycerides, body mass index, family history of premature coronary artery disease
IDI: Integrated discrimination index
NRI: Net reclassification index
Discussion
In the present study, we show that high-grade PVCs during recovery from stress testing and not during the exercise period were associated with long-term cardiovascular mortality in asymptomatic individuals, independently of clinical and exercise test variables. To our knowledge, this is the first study to assess the prognostic implications of PVC timing in asymptomatic patients not suspected of having heart disease. Studies that have previously assessed the prognostic value of PVC timing were performed in individuals suspected or known to have coronary heart disease who were referred for symptom-limited exercise testing (11–13). These have shown that only recovery high-grade PVCs associate with mortality, and their findings were additionally supported by recent meta-analyses (22,23). Although the present cohort is not a population-based cohort per se (as it was enriched at baseline with patient with hyperlipidemia), the indication for stress testing was not clinical.
The number of studies investigating the association between exercise-induced high-grade PVCs and mortality in asymptomatic individuals remains very limited (5–8,16,17). One study showed that in a non-clinical cohort of asymptomatic men who were employed by the Paris Civil Service, 2 or more consecutive PVCs or PVCs constituting > 10% of ventricular depolarizations during a 30-second recording during exercise predicted cardiovascular mortality after 23 years of follow-up (adjusted RR=2.53, 95% CI [1.65–3.88], p<0.001) (5). Another study using the Lipid Research Clinics cohort but limiting the analysis to asymptomatic women reported that exercise-induced ventricular arrhythmia (defined as multifocal PVCs, or at least 10% PVCs in the last stage of exercise or recovery, or if the test was terminated due to ventricular tachycardia) predicted cardiovascular mortality after 20 years of follow-up (adjusted HR=1.69, 95% CI [1.11–2.58], p=0.02) (16).
Important additional contributions from our study include the prognostic value of PVC timing, a more inclusive definition for high-grade PVCs, the inclusion of both men and women and subgroup analyses by sex and lipid status (as half of patients had hyperlipidemia at baseline) and the assessment of the improvement in risk discrimination gained by adding recovery PVC data to clinical variables.
Pathophysiological Rationale
Proper cardiac rhythm and function rest upon the critical balance between the sympathetic and parasympathetic arms of the autonomic nervous system (24). PVCs during exercise are believed to result from the heightened catecholaminergic state during physical activity (5,25). Such depolarizations seem therefore to be a physiological response to exercise. This is additionally supported by their lack of association with cardiovascular mortality. PVCs during recovery, similarly to insufficient heart rate recovery after exercise, have been linked to insufficient vagal reactivation following exercise (12,14,26). Importantly, and as shown in this asymptomatic cohort, this mechanism seems independent of coronary heart disease.
In the current study, recovery PVCs carried worse prognosis even after accounting for heart rate recovery after exercise (a surrogate for parasympathetic function). An important question that arises is whether additional mechanisms would explain the occurrence of recovery PVCs. One mechanism could be age-related given the older age of patients experiencing exercise-induced PVCs (Table 1) and would possibly be linked to the presence of pathological myocardial substrates.
Clinical Implications
The 2008 American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death states that high-grade ventricular ectopy during recovery has been linked to mortality risk in patients with and without heart failure and/or coronary artery diseases (27). Of note, the two studies supporting this statement (12,13) had populations either with clinically documented disease or referred to testing for clinical indications. It is therefore reasonable to believe that expanding this concept to patients without apparent cardiac disease could therefore help identify patients at risk of cardiac death who would otherwise be missed. However, the addition of exercise-induced PVC data to a comprehensive set of clinical variables did not improve the long-term cardiovascular death risk discrimination in our asymptomatic cohort. This was reflected in the low magnitude and the lack of statistical significance of the three traditional measures of incremental prognostic utility. This shows that in asymptomatic individuals and to date, the evidence in favor of the routine incorporation of exercise-induced PVC data into exercise tolerance test results seems limited. However, our findings may have important clinical implications at the individual patient level. For instance, during review of exercise stress tests for asymptomatic patients, healthcare providers could identify individuals at increased risk of cardiovascular mortality after noting high-grade PVCs during the recovery phase of exercise tests. This could prompt the clinician to schedule more frequent follow-up and to intensify efforts to reduce this risk. Given the older age and higher likelihood of diabetes and hypertension in asymptomatic patients with exercise-induced high-grade PVCs (Table 1), defining the cardiovascular risk and instituting appropriate prevention and/or treatment strategies is very likely to benefit the patient.
Study Strengths
One major advantage provided by the Lipid Research Clinics cohort is the degree of detail recorded about exercise test parameters. This allowed us to use a most comprehensive definition for high-grade PVCs. It also enabled us for the first time to study the prognostic implications of PVC timing in a population free of cardiac disease, and to adjust for an important number of additional exercise test parameters. Another additional advantage is the extensive length of follow-up, as we could have failed to find a significant association between exercise-induced PVCs and cardiovascular mortality in asymptomatic participants if the follow-up duration were shorter, as informed by the cumulative probability curves (Figure 1). One important point to mention is that while the characteristics or behaviors of the study cohort at baseline might somehow be different from more modern behaviors (specifically relating to smoking), exercise treadmill protocols have hardly changed.
Study Limitations
One limitation of our study was that baseline data with regards to behaviors and comorbidities were recorded initially and not followed up throughout the study duration. Notable advancements since study start have been the sharp decrease in smoking (although we adjusted for smoking status) and the common use of statin therapy for the management of hyperlipidemia. Although we found no significant association between hyperlipidemia and presence of exercise-induced high-grade PVCs, whether the use of statins might significantly affect the cardiovascular risk tied to high-grade PVCs remains an important question to answer. One additional future avenue of research is whether there is any effect modification by beta-blockers (only 8 patients were on beta-blockers in our asymptomatic cohort) or any significant differences among races (the majority of participants were white). Moreover, an important question that we were not able to address is if there is differential performance on exercise test and/or prognosis for patients who had documented high-grade PVCs on resting ECG, as they were very few (n= 4). In a sensitivity analysis where these patients were excluded, the results were very similar (Supplemental table S4). With the currently increasing use of heart rhythm monitoring devices, the identification of asymptomatic patients with PVCs at rest who are brought to the attention of their healthcare providers is on the rise, and the need to understand how these PVCs associate with cardiac risk is essential.
The fact that half of participants had hyperlipidemia at baseline might limit the generalizability of our results. However, as indicated by our subgroup analysis, there was no significant difference in how recovery high-grade PVCs predicted cardiovascular mortality in individuals with and without hyperlipidemia.
Finally, additional data collected about intermediate outcomes (e.g. arrhythmia-induced cardiomyopathy) or about sudden cardiac death specifically (as opposed to cardiovascular death) would have provided a more solid mechanistic link for the association between exercise-induced PVCs and cardiovascular mortality.
Conclusion
Only high-grade PVCs during recovery and not during exercise treadmill testing independently associated with long-term cardiovascular mortality in asymptomatic individuals. The presence of high-grade PVCs during recovery may be independent of coronary heart disease and instead potentially linked to insufficient vagal reactivation following exercise.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
In asymptomatic individuals without cardiac disease, high-grade PVCs during recovery from and not during exercise testing predict long-term cardiovascular mortality, independently of clinical and other exercise test variables.
Translational Outlook:
Current understanding is that high-grade PVCs during recovery are due to insufficient vagal reactivation following exercise, but future research should investigate other mechanisms underlying post-exertional ventricular ectopy.
Acknowledgments:
The authors would like to acknowledge the Scholars in HeAlth Research Program (SHARP) faculty, staff, and founding director, Dr. Ghada El-Hajj Fuleihan MD MPH, at the American University of Beirut for providing trainees with the necessary quantitative skills to analyze large clinical datasets. The authors would also like to thank the investigators, staff and participants in the Lipid Research Clinics Program.
Funding:
Dr. Gharios was supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health under Award Number D43 TW009118 via the Scholars in HeAlth Research Program (SHARP) at the American University of Beirut. Dr. Mora was supported by NHLBI K24HL 136852. The funders had no role in the design or conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Abbreviations list:
- PVC
Premature ventricular contraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Mora has served as a consultant to Pfizer and Quest Diagnostics for work unrelated to the current study. Other co-authors have no relationship with industry to disclose.
Twitter handle: @SamiaMoraMD
Tweet: In asymptomatic individuals, high-grade PVCs during recovery from exercise associate with cardiovascular mortality whereas those during exercise do not.
References
- 1.Bruce RA, DeRouen TA, Hossack KF. Value of maximal exercise tests in risk assessment of primary coronary heart disease events in healthy men. Five years’ experience of the Seattle heart watch study. Am J Cardiol 1980;46:371–8. [DOI] [PubMed] [Google Scholar]
- 2.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341:1351–7. [DOI] [PubMed] [Google Scholar]
- 3.Gordon DJ, Ekelund LG, Karon JM et al. Predictive value of the exercise tolerance test for mortality in North American men: the Lipid Research Clinics Mortality Follow-up Study. Circulation 1986;74:252–61. [DOI] [PubMed] [Google Scholar]
- 4.Rywik TM, Zink RC, Gittings NS et al. Independent prognostic significance of ischemic ST-segment response limited to recovery from treadmill exercise in asymptomatic subjects. Circulation 1998;97:2117–22. [DOI] [PubMed] [Google Scholar]
- 5.Jouven X, Zureik M, Desnos M, Courbon D, Ducimetière P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000;343:826–33. [DOI] [PubMed] [Google Scholar]
- 6.Marine JE, Shetty V, Chow GV et al. Prevalence and prognostic significance of exercise-induced nonsustained ventricular tachycardia in asymptomatic volunteers: BLSA (Baltimore Longitudinal Study of Aging). J Am Coll Cardiol 2013;62:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morshedi-Meibodi A, Evans JC, Levy D, Larson MG, Vasan RS. Clinical correlates and prognostic significance of exercise-induced ventricular premature beats in the community: the Framingham Heart Study. Circulation 2004;109:2417–22. [DOI] [PubMed] [Google Scholar]
- 8.Busby MJ, Shefrin EA, Fleg JL. Prevalence and long-term significance of exercise-induced frequent or repetitive ventricular ectopic beats in apparently healthy volunteers. J Am Coll Cardiol 1989;14:1659–65. [DOI] [PubMed] [Google Scholar]
- 9.Califf RM, McKinnis RA, McNeer JF et al. Prognostic value of ventricular arrhythmias associated with treadmill exercise testing in patients studied with cardiac catheterization for suspected ischemic heart disease. J Am Coll Cardiol 1983;2:1060–7. [DOI] [PubMed] [Google Scholar]
- 10.Udall JA, Ellestad MH. Predictive implications of ventricular premature contractions associated with treadmill stress testing. Circulation 1977;56:985–9. [DOI] [PubMed] [Google Scholar]
- 11.Dewey FE, Kapoor JR, Williams RS et al. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med 2008;168:225–34. [DOI] [PubMed] [Google Scholar]
- 12.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003;348:781–90. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill JO, Young JB, Pothier CE, Lauer MS. Severe frequent ventricular ectopy after exercise as a predictor of death in patients with heart failure. J Am Coll Cardiol 2004;44:820–6. [DOI] [PubMed] [Google Scholar]
- 14.Imai K, Sato H, Hori M et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994;24:1529–35. [DOI] [PubMed] [Google Scholar]
- 15.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. Jama 2000;284:1392–8. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Redberg RF, Cui Y et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. Jama 2003;290:1600–7. [DOI] [PubMed] [Google Scholar]
- 17.Dzikowicz DJ, Carey MG. Exercise-Induced Premature Ventricular Contractions Are Associated With Myocardial Ischemia Among Asymptomatic Adult Male Firefighters: Implications for Enhanced Risk Stratification. Biol Res Nurs 2020;22:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meine TJ, Patel MR, Shaw LK, Borges-Neto S. Relation of ventricular premature complexes during recovery from a myocardial perfusion exercise stress test to myocardial ischemia. Am J Cardiol 2006;97:1570–2. [DOI] [PubMed] [Google Scholar]
- 19.Plasma lipid distributions in selected North American populations: the Lipid Research Clinics Program Prevalence Study. The Lipid Research Clinics Program Epidemiology Committee. Circulation 1979;60:427–39. [DOI] [PubMed] [Google Scholar]
- 20.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988;319:1379–84. [DOI] [PubMed] [Google Scholar]
- 21.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000;132:552–5. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Kwon M, Chang J et al. Meta-Analysis of Prognostic Implications of Exercise-Induced Ventricular Premature Complexes in the General Population. Am J Cardiol 2016;118:725–32. [DOI] [PubMed] [Google Scholar]
- 23.Lee V, Perera D, Lambiase P. Prognostic significance of exercise-induced premature ventricular complexes: a systematic review and meta-analysis of observational studies. Heart Asia 2017;9:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 2015;116:2005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleg JL, Tzankoff SP, Lakatta EG. Age-related augmentation of plasma catecholamines during dynamic exercise in healthy males. J Appl Physiol (1985) 1985;59:1033–9. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Touchard A, Somers VK, Kara T et al. Ventricular ectopy during REM sleep: implications for nocturnal sudden cardiac death. Nat Clin Pract Cardiovasc Med 2007;4:284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberger JJ, Cain ME, Hohnloser SH et al. American Heart Association/american College of Cardiology Foundation/heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Heart Rhythm 2008;5:e1–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


